User login
Data Trends 2024: Cardiology
- Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015–2018. Natl Health Stat Rep. 2021;(153):1-13. Accessed March 15, 2024. https://www.cdc.gov/nchs/data/nhsr/nhsr153-508.pdf
- Army troops have worse heart health than civilian population, study says. American Heart Association News. June 5, 2019. Accessed March 15, 2024. https://www.heart.org/en/news/2019/06/05/army-troops-have-worse-heart-health-than-civilian-population-study-says
- Haira RS, Kataruka A, Akeroyd JM, et al. Association of Body Mass Index with Risk Factor Optimization and Guideline-Directed Medical Therapy in US Veterans with Cardiovascular Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e004817 doi:10.1161/CIRCOUTCOMES.118.004817
- Merschel M. Gulf War illness may increase risk for heart disease or stroke. American Heart Association News. September 29, 2023. Accessed March 15, 2024. https://www.heart.org/en/news/2023/09/29/gulf-war-illness-may-increase-risk-for-heart-disease-or-stroke
- Women veterans and heart health. American Heart Association: Go Red for Women. Accessed March 14, 2024. https://www.goredforwomen.org/en/about-heart-disease-in-women/facts/women-veterans-and-heart-health
- Heart disease and stroke statistics - 2023 Update. American Heart Association Professional Heart Daily. January 25, 2023. Accessed March 14, 2024. https://professional.heart.org/en/science-news/heart-disease-and-stroke-statistics-2023-update
- Ebrahimi R. Sumner J, Lynch K, et al. Women veterans with PTSD have higher rate of heart disease. American Heart Association Scientific Sessions 2020, Presentation 314 - P12702. American Heart Association News. November 9, 2020. Accessed March 14, 2024. https://newsroom.heart.org/news/women-veterans-with-ptsd-have-higher-rate-of-heart-disease
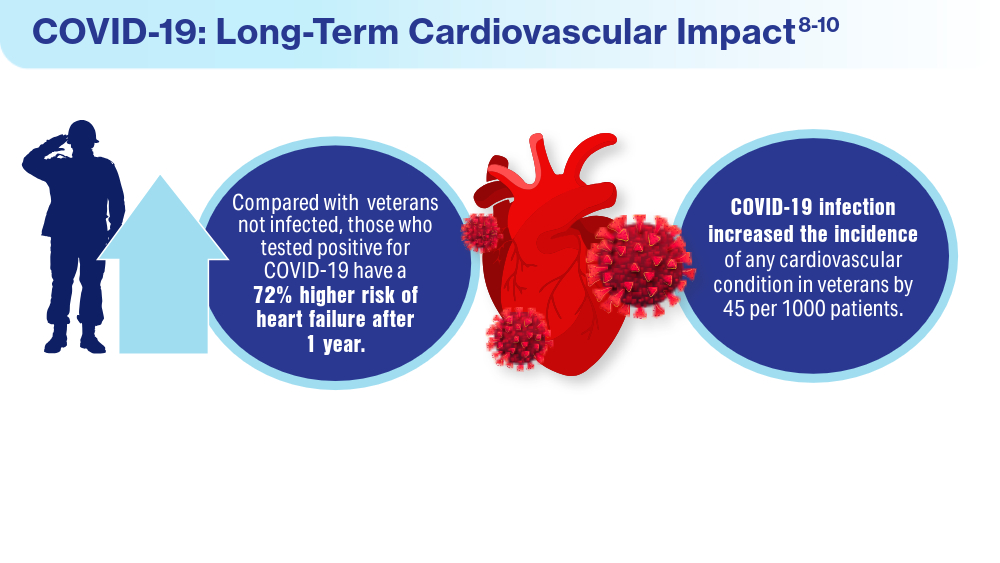
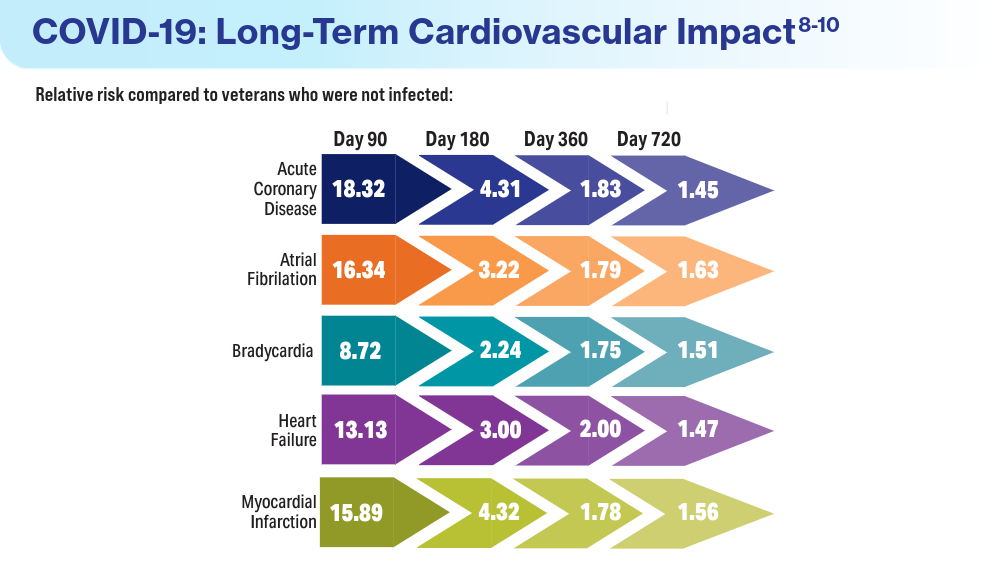
- Wadman M. COVID-19 takes serious toll on heart health—a full year after recovery. Science. Updated February 13, 2022. Accessed March 14, 2024. https://www.science.org/content/article/covid-19-takes-serious-toll-heart-health-full-year-after-recovery
- Bowe B, Xie Y, Al-Aly Z. Postacute sequale of COVID-19 at 2 years. Nature Medicine. 2023;29:2347-2357. doi:10.1038/s41591-023-02521-2
- Offord C. COVID-19 boosts risks of health problems 2 years later, giant study of veterans says. Science. August 21, 2023. Accessed March 13, 2024. https://www.science.org/content/article/covid-19-boosts-risks-health-problems-2-years-later-giant-study-veterans-says
- Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015–2018. Natl Health Stat Rep. 2021;(153):1-13. Accessed March 15, 2024. https://www.cdc.gov/nchs/data/nhsr/nhsr153-508.pdf
- Army troops have worse heart health than civilian population, study says. American Heart Association News. June 5, 2019. Accessed March 15, 2024. https://www.heart.org/en/news/2019/06/05/army-troops-have-worse-heart-health-than-civilian-population-study-says
- Haira RS, Kataruka A, Akeroyd JM, et al. Association of Body Mass Index with Risk Factor Optimization and Guideline-Directed Medical Therapy in US Veterans with Cardiovascular Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e004817 doi:10.1161/CIRCOUTCOMES.118.004817
- Merschel M. Gulf War illness may increase risk for heart disease or stroke. American Heart Association News. September 29, 2023. Accessed March 15, 2024. https://www.heart.org/en/news/2023/09/29/gulf-war-illness-may-increase-risk-for-heart-disease-or-stroke
- Women veterans and heart health. American Heart Association: Go Red for Women. Accessed March 14, 2024. https://www.goredforwomen.org/en/about-heart-disease-in-women/facts/women-veterans-and-heart-health
- Heart disease and stroke statistics - 2023 Update. American Heart Association Professional Heart Daily. January 25, 2023. Accessed March 14, 2024. https://professional.heart.org/en/science-news/heart-disease-and-stroke-statistics-2023-update
- Ebrahimi R. Sumner J, Lynch K, et al. Women veterans with PTSD have higher rate of heart disease. American Heart Association Scientific Sessions 2020, Presentation 314 - P12702. American Heart Association News. November 9, 2020. Accessed March 14, 2024. https://newsroom.heart.org/news/women-veterans-with-ptsd-have-higher-rate-of-heart-disease
- Wadman M. COVID-19 takes serious toll on heart health—a full year after recovery. Science. Updated February 13, 2022. Accessed March 14, 2024. https://www.science.org/content/article/covid-19-takes-serious-toll-heart-health-full-year-after-recovery
- Bowe B, Xie Y, Al-Aly Z. Postacute sequale of COVID-19 at 2 years. Nature Medicine. 2023;29:2347-2357. doi:10.1038/s41591-023-02521-2
- Offord C. COVID-19 boosts risks of health problems 2 years later, giant study of veterans says. Science. August 21, 2023. Accessed March 13, 2024. https://www.science.org/content/article/covid-19-boosts-risks-health-problems-2-years-later-giant-study-veterans-says
- Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015–2018. Natl Health Stat Rep. 2021;(153):1-13. Accessed March 15, 2024. https://www.cdc.gov/nchs/data/nhsr/nhsr153-508.pdf
- Army troops have worse heart health than civilian population, study says. American Heart Association News. June 5, 2019. Accessed March 15, 2024. https://www.heart.org/en/news/2019/06/05/army-troops-have-worse-heart-health-than-civilian-population-study-says
- Haira RS, Kataruka A, Akeroyd JM, et al. Association of Body Mass Index with Risk Factor Optimization and Guideline-Directed Medical Therapy in US Veterans with Cardiovascular Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e004817 doi:10.1161/CIRCOUTCOMES.118.004817
- Merschel M. Gulf War illness may increase risk for heart disease or stroke. American Heart Association News. September 29, 2023. Accessed March 15, 2024. https://www.heart.org/en/news/2023/09/29/gulf-war-illness-may-increase-risk-for-heart-disease-or-stroke
- Women veterans and heart health. American Heart Association: Go Red for Women. Accessed March 14, 2024. https://www.goredforwomen.org/en/about-heart-disease-in-women/facts/women-veterans-and-heart-health
- Heart disease and stroke statistics - 2023 Update. American Heart Association Professional Heart Daily. January 25, 2023. Accessed March 14, 2024. https://professional.heart.org/en/science-news/heart-disease-and-stroke-statistics-2023-update
- Ebrahimi R. Sumner J, Lynch K, et al. Women veterans with PTSD have higher rate of heart disease. American Heart Association Scientific Sessions 2020, Presentation 314 - P12702. American Heart Association News. November 9, 2020. Accessed March 14, 2024. https://newsroom.heart.org/news/women-veterans-with-ptsd-have-higher-rate-of-heart-disease
- Wadman M. COVID-19 takes serious toll on heart health—a full year after recovery. Science. Updated February 13, 2022. Accessed March 14, 2024. https://www.science.org/content/article/covid-19-takes-serious-toll-heart-health-full-year-after-recovery
- Bowe B, Xie Y, Al-Aly Z. Postacute sequale of COVID-19 at 2 years. Nature Medicine. 2023;29:2347-2357. doi:10.1038/s41591-023-02521-2
- Offord C. COVID-19 boosts risks of health problems 2 years later, giant study of veterans says. Science. August 21, 2023. Accessed March 13, 2024. https://www.science.org/content/article/covid-19-boosts-risks-health-problems-2-years-later-giant-study-veterans-says
A Racing Heart Signals Trouble in Chronic Kidney Disease
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A higher resting heart rate, even within the normal range, is linked to an increased risk for mortality and cardiovascular events in patients with non–dialysis-dependent chronic kidney disease (CKD).
METHODOLOGY:
- An elevated resting heart rate is an independent risk factor for all-cause mortality and cardiovascular events in the general population; however, the correlation between heart rate and mortality in patients with CKD is unclear.
- Researchers analyzed the longitudinal data of patients with non–dialysis-dependent CKD enrolled in the Fukushima CKD Cohort Study to investigate the association between resting heart rate and adverse clinical outcomes.
- The patient cohort was stratified into four groups on the basis of resting heart rates: < 70, 70-79, 80-89, and ≥ 90 beats/min.
- The primary and secondary outcomes were all-cause mortality and cardiovascular events, respectively, the latter category including myocardial infarction, angina pectoris, and heart failure.
TAKEAWAY:
- Researchers enrolled 1353 patients with non–dialysis-dependent CKD (median age, 65 years; 56.7% men; median estimated glomerular filtration rate, 52.2 mL/min/1.73 m2) who had a median heart rate of 76 beats/min.
- During the median observation period of 4.9 years, 123 patients died and 163 developed cardiovascular events.
- Compared with patients with a resting heart rate < 70 beats/min, those with a resting heart rate of 80-89 and ≥ 90 beats/min had an adjusted hazard ratio of 1.74 and 2.61 for all-cause mortality, respectively.
- Similarly, the risk for cardiovascular events was higher in patients with a heart rate of 80-89 beats/min than in those with a heart rate < 70 beats/min (adjusted hazard ratio, 1.70).
IN PRACTICE:
“The present study supported the idea that reducing heart rate might be effective for CKD patients with a heart rate ≥ 70/min, since the lowest risk of mortality was seen in patients with heart rate < 70/min,” the authors concluded.
SOURCE:
This study was led by Hirotaka Saito, Department of Nephrology and Hypertension, Fukushima Medical University, Fukushima City, Japan. It was published online in Scientific Reports.
LIMITATIONS:
Heart rate was measured using a standard sphygmomanometer or an automated device, rather than an electrocardiograph, which may have introduced measurement variability. The observational nature of the study precluded the establishment of cause-and-effect relationships between heart rate and clinical outcomes. Additionally, variables such as lifestyle factors, underlying health conditions, and socioeconomic factors were not measured, which could have affected the results.
DISCLOSURES:
Some authors received research funding from Chugai Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, and other sources. They declared having no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Remission or Not, Biologics May Mitigate Cardiovascular Risks of RA
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
, suggesting that biologics may reduce cardiovascular risk in RA even if remission is not achieved.
METHODOLOGY:
- Studies reported reduced cardiovascular risk in patients with RA who respond to tumor necrosis factor inhibitors but not in nonresponders, highlighting the importance of controlling inflammation for cardiovascular protection.
- Researchers assessed whether bDMARDs modify the impact of disease activity and systemic inflammation on cardiovascular risk in 4370 patients (mean age, 55 years) with RA without cardiovascular disease from a 10-country observational cohort.
- The severity of RA disease activity was assessed using C-reactive protein (CRP) levels and 28-joint Disease Activity Score based on CRP (DAS28-CRP).
- Endpoints were time to first MACE — a composite of cardiovascular death, myocardial infarction, and stroke — and time to first ischemic cardiovascular event (iCVE) — a composite of MACE plus revascularization, angina, transient ischemic attack, and peripheral arterial disease.
TAKEAWAY:
- The interaction between use of bDMARD and DAS28-CRP (P = .017) or CRP (P = .011) was significant for MACE.
- Each unit increase in DAS28-CRP increased the risk for MACE in bDMARD nonusers (hazard ratio [HR], 1.21; P = .002) but not in users.
- The per log unit increase in CRP was associated with a risk for MACE in bDMARD nonusers (HR, 1.16; P = .009) but not in users.
- No interaction was observed between bDMARD use and DAS28-CRP or CRP for the iCVE risk.
IN PRACTICE:
“This may indicate additional bDMARD-specific benefits directly on arterial wall inflammation and atherosclerotic plaque anatomy, stability, and biology, independently of systemic inflammation,” the authors wrote.
SOURCE:
The study, led by George Athanasios Karpouzas, MD, The Lundquist Institute, Torrance, California, was published online in RMD Open.
LIMITATIONS:
Patients with a particular interest in RA-associated cardiovascular disease were included, which may have introduced referral bias and affected the generalizability of the findings. Standard definitions were used for selected outcomes; however, differences in the reporting of outcomes may be plausible. Some patients were evaluated prospectively, while others were evaluated retrospectively, leading to differences in surveillance.
DISCLOSURES:
The study was supported by Pfizer. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Xanthelasma Not Linked to Heart Diseases, Study Finds
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Xanthelasma palpebrarum, characterized by yellowish plaques on the eyelids, is not associated with increased rates of dyslipidemia or cardiovascular disease.
METHODOLOGY:
- Researchers conducted a case-control study at a single tertiary care center in Israel and analyzed data from 35,452 individuals (mean age, 52.2 years; 69% men) who underwent medical screening from 2001 to 2020.
- They compared 203 patients with xanthelasma palpebrarum with 2030 individuals without the disease (control).
- Primary outcomes were prevalence of dyslipidemia and cardiovascular disease between the two groups.
TAKEAWAY:
- Lipid profiles were similar between the two groups, with no difference in total cholesterol, high- and low-density lipoprotein, and triglyceride levels (all P > .05).
- The prevalence of dyslipidemia was similar for patients with xanthelasma palpebrarum and controls (46% vs 42%, respectively; P = .29), as was the incidence of cardiovascular disease (8.9% vs 10%, respectively; P = .56).
- The incidence of diabetes (P = .13), cerebrovascular accidents (P > .99), ischemic heart disease (P = .73), and hypertension (P = .56) were not significantly different between the two groups.
IN PRACTICE:
“Our study conducted on a large population of individuals undergoing comprehensive ophthalmic and systemic screening tests did not find a significant association between xanthelasma palpebrarum and an increased prevalence of lipid abnormalities or cardiovascular disease,” the authors wrote.
SOURCE:
The study was led by Yael Lustig, MD, of the Goldschleger Eye Institute at Sheba Medical Center, in Ramat Gan, Israel. It was published online on August 5, 2024, in Ophthalmology.
LIMITATIONS:
The retrospective nature of the study and the single-center design may have limited the generalizability of the findings. The study population was self-selected, potentially introducing selection bias. Lack of histopathologic examination could have affected the accuracy of the diagnosis.
DISCLOSURES:
No funding sources were disclosed for this study. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
New Study Links Sweetener to Heart Risk: What to Know
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
Is going sugar free really good advice for patients with cardiometabolic risk factors?
That’s the question raised by new Cleveland Clinic research, which suggests that consuming erythritol, a sweetener widely found in sugar-free and keto food products, could spur a prothrombotic response.
In the study, published in Arteriosclerosis, Thrombosis, and Vascular Biology, 10 healthy participants ate 30 grams of erythritol. Thirty minutes later, their blood showed enhanced platelet aggregation and increased markers of platelet responsiveness and activation.
Specifically, the researchers saw enhanced stimulus-dependent release of serotonin (a marker of platelet dense granules) and CXCL4 (a platelet alpha-granule marker).
“ With every single person, you see a prothrombotic effect with every single test that we did,” said study author Stanley Hazen, MD, PhD, chair of the Department of Cardiovascular & Metabolic Sciences at Cleveland Clinic in Ohio. By contrast, participants who ate 30 grams of glucose saw no such effect.
The erythritol itself does not activate the platelets, Dr. Hazen said, rather it lowers the threshold for triggering a response. This could make someone more prone to clotting, raising heart attack and stroke risk over time.
Though the mechanism is unknown, Dr. Hazen has an idea.
“There appears to be a receptor on platelets that is recognizing and sensing these sugar alcohols,” Dr. Hazen said, “much in the same way your taste bud for sweet is a receptor for recognizing a glucose or sugar molecule.”
“We’re very interested in trying to figure out what the receptor is,” Dr. Hazen said, “because I think that then becomes a very interesting potential target for further investigation and study into how this is linked to causing heart disease.”
The Past and Future of Erythritol Research
In 2001, the Food and Drug Administration classified erythritol as a “generally recognized as safe” food additive. A sugar alcohol that occurs naturally in foods like melon and grapes, erythritol is also manufactured by fermenting sugars. It’s about 70% as sweet as table sugar. Humans also produce small amounts of erythritol naturally: Our blood cells make it from glucose via the pentose phosphate pathway.
Previous research from Dr. Hazen’s group linked erythritol to a risk for major adverse cardiovascular events and clotting.
“Based on their previous study, I think this was a really important study to do in healthy individuals,” said Martha Field, PhD, assistant professor in the Division of Nutritional Sciences at Cornell University, Ithaca, New York, who was not involved in the study.
The earlier paper analyzed blood samples from participants with unknown erythritol intake, including some taken before the sweetener, and it was as widespread as it is today. That made disentangling the effects of eating erythritol vs naturally producing it more difficult.
By showing that eating erythritol raises markers associated with thrombosis, the new paper reinforces the importance of thinking about and developing a deeper understanding of what we put into our bodies.
“This paper was conducted in healthy individuals — might this be particularly dangerous for individuals who are at increased risk of clotting?” asked Dr. Field. “There are lots of genetic polymorphisms that increase your risk for clotting disorders or your propensity to form thrombosis.”
Field would like to see similar analyses of xylitol and sorbitol, other sugar alcohols found in sugar-free foods. And she called for more studies on erythritol that look at lower erythritol consumption over longer time periods.
Registered dietitian nutritionist Valisa E. Hedrick, PhD, agreed: Much more work is needed in this area, particularly in higher-risk groups, such as those with prediabetes and diabetes, said Dr. Hedrick, an associate professor in the Department of Human Nutrition, Foods, and Exercise at Virginia Tech, Blacksburg, who was not involved in the study.
“Because this study was conducted in healthy individuals, the impact of a small dose of glucose was negligible, as their body can effectively regulate blood glucose levels,” she said. “Because high blood glucose concentrations have also been shown to increase platelet reactivity, and consequently increase thrombosis potential, individuals who are not able to regulate their blood glucose levels, such as those with prediabetes and diabetes, could potentially see a similar effect on the body as erythritol when consuming large amounts of sugar.”
At the same time, “individuals with diabetes or prediabetes may be more inclined to consume erythritol as an alternative to sugar,” Dr. Hedrick added. “It will be important to design studies that include these individuals to determine if erythritol has an additive adverse effect on cardiac event risk.”
Criticism and Impact
Critics have suggested the 30-gram dose of erythritol ingested by study participants is unrealistic. Dr. Hazen said that it’s not.
Erythritol is often recommended as a one-to-one sugar replacement. And you could top 30 grams with a few servings of erythritol-sweetened ice cream or soda, Dr. Hazen said.
“The dose that we used, it’s on the high end, but it’s well within a physiologically relevant level,” he said.
Still others say the results are only relevant for people with preexisting heart trouble. But Dr. Hazen said they matter for the masses.
“I think there’s a significant health concern at a population level that this work is underscoring,” he said.
After all, heart disease risk factors like obesity, hypertension, diabetes, and smoking are common and quickly add up.
“If you look at middle-aged America, most people who experience a heart attack or stroke do not know that they have coronary artery disease, and the first recognition of it is that event,” Dr. Hazen said.
For now, Dr. Hazen recommends eating real sugar in moderation. He hopes future research will reveal a nonnutritive sweetener that doesn’t activate platelets.
The Bigger Picture
The new research adds yet another piece to the puzzle of whether nonnutritive sweeteners are better than sugar.
“I think these results are concerning,” said JoAnn E. Manson, MD, chief of the Division of Preventive Medicine at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts. They “ may help explain the surprising results in some observational studies that artificial sweeteners are linked to an increased risk of cardiovascular disease.”
Dr. Manson, who was not involved in the new study, has conducted other research linking artificial sweetener use with stroke risk.
In an upcoming randomized clinical study, her team is comparing head-to-head sugar-sweetened beverages, drinks sweetened with calorie-free substitutes, and water to determine which is best for a range of cardiometabolic outcomes.
“We need more research on this question,” she said, “because these artificial sweeteners are commonly used, and many people are assuming that their health outcomes will be better with the artificial sweeteners than with sugar-sweetened products.”
A version of this article first appeared on Medscape.com.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Did Statin Decision-Making Just Get Harder?
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The new American Heart Association Predicting Risk of cardiovascular disease EVENTs (PREVENT) equation outperforms the standard pooled cohort equation (PCE). But there is a problem. A big one, actually.
The new score incorporates kidney function and social situation, and it eliminates race from the estimate. It was derived from larger, more modern datasets and can be applied to younger adults.
Two luminaries in preventive cardiology recently called the PREVENT calculator a “substantial improvement over the PCE in terms of accuracy and precision of risk estimates over the entire population and within demographic subgroups.”
Now to the Problem of PREVENT vs PCE
A recent study comparing PREVENT and PCE found that the PREVENT equation would assign lower 10-year risks to millions of US adults.
The authors estimated that the more accurate calculator would result in an estimated 14 million adults no longer reaching the statin eligibility risk threshold of 7.5% over 10 years. Nearly 3 million adults would also not reach the threshold for blood pressure therapy.
Because statins and blood pressure drugs reduce cardiac events, the authors further estimated that more than 100,000 excess myocardial infarctions (MIs) would occur if the PREVENT equation was used along with the current risk thresholds for statin eligibility.
The change in eligibility induced by PREVENT would affect more men than women and a greater proportion of Black adults than White adults.
The Tension of Arbitrary Thresholds
Modern cardiac therapeutics are amazing, but it’s still better to prevent an event than to treat it.
Statin drugs reduce cardiac risk by about 20%-25% at all absolute risks. American experts chose a 10-year risk of 7.5% as the threshold where statin benefit exceed risk. The USPSTF chose 10%. But the thresholds are arbitrary and derived only by opinion.
If your frame is population health, the more patients who take statins, the fewer cardiac events there will be. Anything that reduces statin use increases cardiac events.
The tension occurs because a more accurate equation decreases the number of people who meet eligibility for primary prevention therapy and therefore increases the number of cardiac events.
I write from the perspective of both a clinician and a possible patient. As a clinician, patients often ask me whether they should take a statin. (Sadly, most have not had a risk-based discussion with their clinician. But that is another column.)
The incidence of MI or stroke in a population has no effect on either of these scenarios. I see three broad categories of patients: minimizers, maximizers, and those in between.
I am a minimizer. I don’t worry much about heart disease. First, I won’t ignore symptoms, and I know that we have great treatments. Second, my wife, Staci, practiced hospice and palliative care medicine, and this taught me that worrying about one specific disease is folly. In the next decade, I, like anyone my age, could have many other bad things happen: cancer, trauma, infection, etc. Given these competing risks for serious disease, a PREVENT-calculated risk of 4% or a PCE-calculated risk of 8% makes no difference. I don’t like pills, and, with risks in this range, I decline statin drugs.
Then there are the maximizers. This person wants to avoid heart disease. Maybe they have family or friends who had terrible cardiac events. This person will maximize everything to avoid heart disease. The calculated 10-year risk makes little difference to a maximizer. Whether it is 4% or 8% matters not. They will take a statin or blood pressure drugs to reduce risk to as low as possible.
There are people between minimizers and maximizers. I am not sure that there are that many truly undecided people, but I challenge you to translate a difference of a few percent over a decade to them. I feel comfortable with numbers but struggle to sort out these small absolute differences over such a long time frame.
Other Issues With Risk-Based Decisions
Venk Murthy, MD, PhD, from the University of Michigan, wrote on X about two other issues with a risk-based decision. One is that it does not consider life-years lost. If a 50-year-old person has a fatal MI, that counts as one event. But in life-years lost, that one event is much worse than a fatal MI in a 79-year-old. Cardiac prevention, therefore, may have a greater effect in lower-risk younger people.
Another point Dr. Murthy made is that risk and benefit are driven by many different preferences and rare events. Minimizers and maximizers come to the decision with widely disparate preferences. Risk-based decisions treat patients as if they were automatons who make decisions based simply on calculated probabilities. Clinicians know how untrue that is.
Conclusion
If you carry forward the logic of being disturbed by the estimate of more MIs using the PREVENT score, then you could justify putting statins in the water — because that would reduce population estimates of MIs.
I am not disturbed by the PREVENT score. Clinicians treat individuals, not populations. Individuals want a more accurate score. They don’t need expert-based thresholds. Clinician and patient can discuss the evidence and come up with an agreeable decision, one that is concordant with a person’s goals. The next patient may have a different decision despite seeing the same evidence.
The tension created by this comparative study exposes the gap between population health and basic clinical care. I don’t think clinicians need to worry about populations.
Dr. Mandrola, a clinical electrophysiologist at Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
On Second Thought: The Truth About Beta-Blockers
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Giving patients a beta-blocker after a myocardial infarction is standard of care. It’s in the guidelines. It’s one of the performance measures used by the American College of Cardiology (ACC) and the American Heart Association (AHA). If you aren’t putting your post–acute coronary syndrome (ACS) patients on a beta-blocker, the ACC and the AHA both think you suck.
They are very disappointed in you, just like your mother was when you told her you didn’t want to become a surgeon because you don’t like waking up early, your hands shake when you get nervous, it’s not your fault, there’s nothing you can do about it, so just leave me alone!
The data on beta-blockers are decades old. In the time before stents, statins, angiotensin-converting enzyme inhibitors, and dual antiplatelet therapy, when patients either died or got better on their own, beta-blockers showed major benefits. Studies like the Norwegian Multicenter Study Group, the BHAT trial, and the ISIS-1 trial proved the benefits of beta blockade. These studies date back to the 1980s, when you could call a study ISIS without controversy.
It was a simpler time, when all you had to worry about was the Cold War, apartheid, and the global AIDS pandemic. It was a time when doctors smoked in their offices, and patients had bigger infarcts that caused large scars and systolic dysfunction. That world is no longer our world, except for the war, the global pandemic, and the out-of-control gas prices.
The reality is that, before troponins, we probably missed most small heart attacks. Now, most infarcts are small, and most patients walk away from their heart attacks with essentially normal hearts. Do beta-blockers still matter? If you’re a fan of Cochrane reviews, the answer is yes.
In 2021, Cochrane published a review of beta-blockers in patients without heart failure after myocardial infarction (MI). The authors of that analysis concluded, after the usual caveats about heterogeneity, potential bias, and the whims of a random universe, that, yes, beta-blockers do reduce mortality. The risk ratio for max all-cause mortality was 0.81.
What does that mean practically? The absolute risk was reduced from 10.9% to 8.7%, a 2.2–percentage point absolute decrease and about a 20% relative drop. A little math gives us a third number: 46. That’s the number needed to treat. If you think about how many patients you admit during a typical week of critical care unit with an MI, a number needed to treat of 46 is a pretty good trade-off for a fairly inexpensive medication with fairly minimal side effects.
Of course, these are the same people who claim that masks don’t stop the spread of COVID-19. Sure, were they the only people who thought that handwashing was the best way to stop a respiratory virus? No. We all believed that fantasy for far longer than we should have. Not everybody can bat a thousand, if by batting a thousand, you mean reflecting on how your words will impact on a broader population primed to believe misinformation because of the increasingly toxic social media environment and worsening politicization and radicalization of our politics.
By the way, if any of you want to come to Canada, you can stay with me. Things are incrementally better here. In this day and age, incrementally better is the best we can hope for.
Here’s the wrinkle with the Cochrane beta-blocker review: Many of the studies took place before early revascularization became the norm and before our current armamentarium of drugs became standard of care.
Back in the day, bed rest and the power of positive thinking were the mainstays of cardiac treatment. Also, many of these studies mixed together ST-segment MI (STEMI) and non-STEMI patients, so you’re obviously going to see more benefits in STEMI patients who are at higher risk. Some of them used intravenous (IV) beta-blockers right away, whereas some were looking only at oral beta-blockers started days after the infarct.
We don’t use IV beta-blockers that much anymore because of the risk for shock.
Also, some studies had short-term follow-up where the benefits were less pronounced, and some studies used doses and types of beta-blockers rarely used today. Some of the studies had a mix of coronary and heart failure patients, which muddies the water because the heart failure patients would clearly benefit from being on a beta-blocker.
Basically, the data are not definitive because they are old and don’t reflect our current standard of care. The data contain a heterogeneous mix of patients that aren’t really relevant to the question that we’re asking. The question we’re asking is, should you put all your post-MI patients on a beta-blocker routinely, even if they don’t have heart failure?
The REDUCE-AMI trial is the first of a few trials testing, or to be more accurate, retesting, whether beta-blockers are useful after an MI. BETAMI, REBOOT, DANBLOCK— you’ll be hearing these names in the next few years, either because the studies get published or because they’re the Twitter handles of people harassing you online. Either/or. (By the way, I’ll be cold in my grave before I call it X.)
For now, REDUCE-AMI is the first across the finish line, and at least in cardiology, finishing first is a good thing. This study enrolled patients with ACS, both STEMI and non-STEMI, with a post-MI ejection fraction ≥ 50%, and the result was nothing. The risk ratio for all-cause mortality was 0.94 and was not statistically significant.
In absolute terms, that’s a reduction from 4.1% to 3.9%, or a 0.2–percentage point decrease; this translates into a number needed to treat of 500, which is 10 times higher than what the Cochrane review found. That’s if you assume that there is, in fact, a small benefit amidst all the statistical noise, which there probably isn’t.
Now, studies like this can never rule out small effects, either positive or negative, so maybe there is a small benefit from using beta-blockers. If it’s there, it’s really small. Do beta-blockers work? Well, yes, obviously, for heart failure and atrial fibrillation — which, let’s face it, are not exactly rare and often coexist in patients with heart disease. They probably aren’t that great as blood pressure pills, but that’s a story for another day and another video.
Yes, beta-blockers are useful pills, and they are standard of care, just maybe not for post-MI patients with normal ejection fractions because they probably don’t really need them. They worked in the pre-stent, pre-aspirin, pre-anything era.
That’s not our world anymore. Things change. It’s not the 1980s. That’s why I don’t have a mullet, and that’s why you need to update your kitchen.
Dr. Labos, a cardiologist at Kirkland Medical Center, Montreal, Quebec, Canada, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Non-Prescription Semaglutide Purchased Online Poses Risks
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
Semaglutide products sold online without a prescription may pose multiple risks to consumers, new research found.
Of six test purchases of semaglutide products offered online without a prescription, only three were actually received. The other three vendors demanded additional payment. Of the three delivered, one was potentially contaminated, and all three contained higher concentrations of semaglutide than indicated on the label, potentially resulting in an overdose.
“Semaglutide products are actively being sold without prescription by illegal online pharmacies, with vendors shipping unregistered and falsified products,” wrote Amir Reza Ashraf, PharmD, of the University of Pécs, Hungary, and colleagues in their paper, published online on August 2, 2024, in JAMA Network Open.
The study was conducted in July 2023, but its publication comes a week after the US Food and Drug Administration (FDA) issued an alert about dosing errors in compounded semaglutide, which typically does require a prescription.
Study coauthor Tim K. Mackey, PhD, told this news organization, “Compounding pharmacies are another element of this risk that has become more prominent now but arguably have more controls if prescribed appropriately, while the traditional ‘no-prescription’ online market still exists and will continue to evolve.”
Overall, said Dr. Mackey, professor of global health at the University of California San Diego and director of the Global Health Policy and Data Institute,
He advises clinicians to actively discuss with their patients the risks associated with semaglutide and, specifically, the dangers of buying it online. “Clinicians can act as a primary information source for patient safety information by letting their patients know about these risks ... and also asking where patients get their medications in case they are concerned about reports of adverse events or other patient safety issues.”
Buyer Beware: Online Semaglutide Purchases Not as They Seem
The investigators began by searching online for websites advertising semaglutide without a prescription. They ordered products from six online vendors that showed up prominently in the searches. Of those, three offered prefilled 0.25 mg/dose semaglutide injection pens, while the other three sold vials of lyophilized semaglutide powder to be reconstituted to solution for injection. Prices for the smallest dose and quantity ranged from $113 to $360.
Only three of the ordered products — all vials — actually showed up. The advertised prefilled pens were all nondelivery scams, with requests for an extra payment of $650-$1200 purportedly to clear customs. This was confirmed as fraudulent by customs agencies, the authors noted.
The three vial products were received and assessed physically, of both the packaging and the actual product, by liquid chromatography-mass spectrometry to determine purity and peptide concentration, and microbiologically, to examine sterility.
Using a checklist from the International Pharmaceutical Federation, Dr. Ashraf and colleagues found “clear discrepancies in regulatory registration information, accurate labeling, and evidence products were likely unregistered or unlicensed.”
Quality testing showed that one sample had an elevated presence of endotoxin suggesting possible contamination. While all three actually did contain semaglutide, the measured content exceeded the labeled amount by 29%-39%, posing a risk that users could receive up to 39% more than intended per injection, “particularly concerning if a consumer has to reconstitute and self-inject,” Dr. Mackey noted.
At least one of these sites in this study, “semaspace.com,” was subsequently sent a warning letter by the FDA for unauthorized semaglutide sale, Mackey noted.
Unfortunately, he told this news organization, these dangers are likely to persist. “There is a strong market opportunity to introduce counterfeit and unauthorized versions of semaglutide. Counterfeiters will continue to innovate with where they sell products, what products they offer, and how they mislead consumers about the safety and legality of what they are offering online. We are likely just at the beginning of counterfeiting of semaglutide, and it is likely that these false products will become endemic in our supply chain.”
The research was supported by the Hungarian Scientific Research Fund. The authors had no further disclosures.
A version of this article appeared on Medscape.com.
Fruits and Vegetables May Promote Kidney and Cardiovascular Health in Hypertensive Patients
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
Progression of chronic kidney disease (CKD) and cardiovascular disease risk in hypertensive adults was significantly slower among those who consumed more fruits and vegetables or oral sodium bicarbonate, compared with controls who received usual care.
A primary focus on pharmacologic strategies has failed to reduced hypertension-related CKD and cardiovascular disease mortality, Nimrit Goraya, MD, of Texas A&M Health Sciences Center College of Medicine, Temple, and colleagues wrote. High-acid diets (those with greater amounts of animal-sourced foods) have been associated with increased incidence and progression of CKD and with increased risk of cardiovascular disease.
Diets high in fruits and vegetables are associated with reduced CKD and cardiovascular disease but are not routinely used as part of hypertension treatment. The researchers hypothesized that dietary acid reduction could slow kidney disease progression and reduce cardiovascular disease risk.
In a study published in The American Journal of Medicine, the researchers randomized 153 adults aged 18-70 years with hypertension and CKD to fruits and vegetables, oral sodium bicarbonate (NaHCO3), or usual care; 51 to each group. The fruit and vegetable group received 2-4 cups daily of base-producing food items including apples, apricots, oranges, peaches, pears, raisins, strawberries, carrots, cauliflower, eggplant, lettuce, potatoes, spinach, tomatoes, and zucchini. Participants were not instructed how to incorporate these foods into their diets. The sodium bicarbonate group received an average of four to five NaHCO3 tablets daily (650 mg), divided into two doses.
The mean age of the participants was 48.8 years, 51% were female, and 47% were African American. The primary outcome was CKD progression and cardiovascular disease risk over 5 years. All participants met criteria at baseline for macroalbuminuria (a urine albumin to creatinine ratio of at least 200 mg/g) and were considered at increased risk for CKD progression.
Over the 5-year follow-up, CKD progression was significantly slower in the groups receiving fruits and vegetables and oral sodium bicarbonate, compared with usual care, based on trajectories showing a lower decline of estimated glomerular filtration rates (mean declines of 1.08 and 1.17 for fruits/vegetables and NaHCO3, respectively, vs 19.4 for usual care, P < .001 for both).
However, systolic blood pressure and subsequent cardiovascular disease risk indicators were lower only in the fruit and vegetable group, compared with both the NaHCO3 or usual-care groups over the long term. “Specifically, with fruits and vegetables, systolic blood pressure, plasma LDL and Lp(a) cholesterol, and body mass index decreased from baseline, consistent with better cardiovascular disease protection,” the researchers wrote. The protection against cardiovascular disease in the fruits and vegetables group occurred with lower doses of antihypertensive and statin medications and was not affected by baseline differences in medication doses.
The findings were limited by several factors, including the lack of data on compliance with the NaHCO3 supplements, although urine net acid excretion in this group suggested increased alkali intake similar to that provided by fruits and vegetables, the researchers noted. Other limitations included the focus only on individuals with very high albuminuria.
More basic science studies are needed to explore how the potential vascular injury suggested by albuminuria affects CKD progression and cardiovascular disease, and clinical studies are needed to assess the impact of dietary acid reduction on patients with lower levels of albuminuria that the current study, the researchers said.
However, the results suggest that consuming fruits and vegetables, rather than NaHCO3, is the preferred strategy for dietary acid reduction for patients with primary hypertension and CKD, they concluded. The findings also support routine measurement of urine albumin-to-creatinine ratios in hypertensive patients to identify CKD and assess risk for progression and subsequent cardiovascular disease.
The study was supported by the Larry and Jane Woirhaye Memorial Endowment in Renal Research at the Texas Tech University Health Sciences Center, the University Medical Center (both in Lubbock, Texas), the Endowment, Academic Operations Division of Baylor Scott & White Health, and the Episcopal Health Foundation. The researchers had no financial conflicts to disclose.
FROM THE AMERICAN JOURNAL OF MEDICINE
Wearables May Confirm Sleep Disruption Impact on Chronic Disease
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Rapid eye movement (REM) sleep, deep sleep, and sleep irregularity were significantly associated with increased risk for a range of chronic diseases, based on a new study of > 6000 individuals.
“Most of what we think we know about sleep patterns in adults comes from either self-report surveys, which are widely used but have all sorts of problems with over- and under-estimating sleep duration and quality, or single-night sleep studies,” corresponding author Evan L. Brittain, MD, of Vanderbilt University, Nashville, Tennessee, said in an interview.
The single-night study yields the highest quality data but is limited by extrapolating a single night’s sleep to represent habitual sleep patterns, which is often not the case, he said. In the current study, published in Nature Medicine, “we had a unique opportunity to understand sleep using a large cohort of individuals using wearable devices that measure sleep duration, quality, and variability. The All of Us Research Program is the first to link wearables data to the electronic health record at scale and allowed us to study long-term, real-world sleep behavior,” Dr. Brittain said.
The timing of the study is important because the American Heart Association now recognizes sleep as a key component of heart health, and public awareness of the value of sleep is increasing, he added.
The researchers reviewed objectively measured, longitudinal sleep data from 6785 adults who used commercial wearable devices (Fitbit) linked to electronic health record data in the All of Us Research Program. The median age of the participants was 50.2 years, 71% were women, and 84% self-identified as White individuals. The median period of sleep monitoring was 4.5 years.
REM sleep and deep sleep were inversely associated with the odds of incident heart rhythm and heart rate abnormalities. A higher percentage of deep sleep was associated with reduced odds of atrial fibrillation (OR, 0.87), major depressive disorder (OR, 0.93), and anxiety disorder (OR, 0.94).
Increased irregular sleep was significantly associated with increased odds of incident obesity (OR, 1.49), hyperlipidemia (OR, 1.39), and hypertension (OR, 1.56), as well as major depressive disorder (OR, 1.75), anxiety disorder (OR, 1.55), and bipolar disorder (OR, 2.27).
The researchers also identified J-shaped associations between average daily sleep duration and hypertension (P for nonlinearity = .003), as well as major depressive disorder and generalized anxiety disorder (both P < .001).
The study was limited by several factors including the relatively young, White, and female study population. However, the results illustrate how sleep stages, duration, and regularity are associated with chronic disease development, and may inform evidence-based recommendations on healthy sleeping habits, the researchers wrote.
Findings Support Need for Sleep Consistency
“The biggest surprise for me was the impact of sleep variability of health,” Dr. Brittain told this news organization. “The more your sleep duration varies, the higher your risk of numerous chronic diseases across the entire spectrum of organ systems. Sleep duration and quality were also important but that was less surprising,” he said.
The clinical implications of the findings are that sleep duration, quality, and variability are all important, said Dr. Brittain. “To me, the easiest finding to translate into the clinic is the importance of reducing the variability of sleep duration as much as possible,” he said. For patients, that means explaining that they need to go to sleep and wake up at roughly the same time night to night, he said.
“Commercial wearable devices are not perfect compared with research grade devices, but our study showed that they nonetheless collect clinically relevant information,” Dr. Brittain added. “For patients who own a device, I have adopted the practice of reviewing my patients’ sleep and activity data which gives objective insight into behavior that is not always accurate through routine questioning,” he said.
As for other limitations, “Our cohort was limited to individuals who already owned a Fitbit; not surprisingly, these individuals differ from a random sample of the community in important ways, both demographic and behavioral, and our findings need to be validated in a more diverse population,” said Dr. Brittain.
Looking ahead, “we are interested in using commercial devices as a tool for sleep interventions to test the impact of improving sleep hygiene on chronic disease incidence, severity, and progression,” he said.
Device Data Will Evolve to Inform Patient Care
“With the increasing use of commercial wearable devices, it is crucial to identify and understand the data they can collect,” said Arianne K. Baldomero, MD, a pulmonologist and assistant professor of medicine at the University of Minnesota, Minneapolis, in an interview. “This study specifically analyzed sleep data from Fitbit devices among participants in the All of Us Research Program to assess sleep patterns and their association with chronic disease risk,” said Dr. Baldomero, who was not involved in the study.
The significant relationships between sleep patterns and risk for chronic diseases were not surprising, said Dr. Baldomero. The findings of an association between shorter sleep duration and greater sleep irregularity with obesity and sleep apnea validated previous studies in large-scale population surveys, she said. Findings from the current study also reflect data from the literature on sleep duration associated with hypertension, major depressive disorder, and generalized anxiety findings, she added.
“This study reinforces the importance of adequate sleep, typically around 7 hours per night, and suggests that insufficient or poor-quality sleep may be associated with chronic diseases,” Dr. Baldomero told this news organization. “Pulmonologists should remain vigilant about sleep-related issues, and consider further investigation and referrals to sleep specialty clinics for patients suspected of having sleep disturbances,” she said.
“What remains unclear is whether abnormal sleep patterns are a cause or an effect of chronic diseases,” Dr. Baldomero noted. “Additionally, it is essential to ensure that these devices accurately capture sleep patterns and continue to validate their data against gold standard measures of sleep disturbances,” she said.
The study was based on work that was partially funded by an unrestricted gift from Google, and the study itself was supported by National Institutes of Health. Dr. Brittain disclosed received research funds unrelated to this work from United Therapeutics. Dr. Baldomero had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.