User login
No vascular benefit of testosterone over exercise in aging men
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).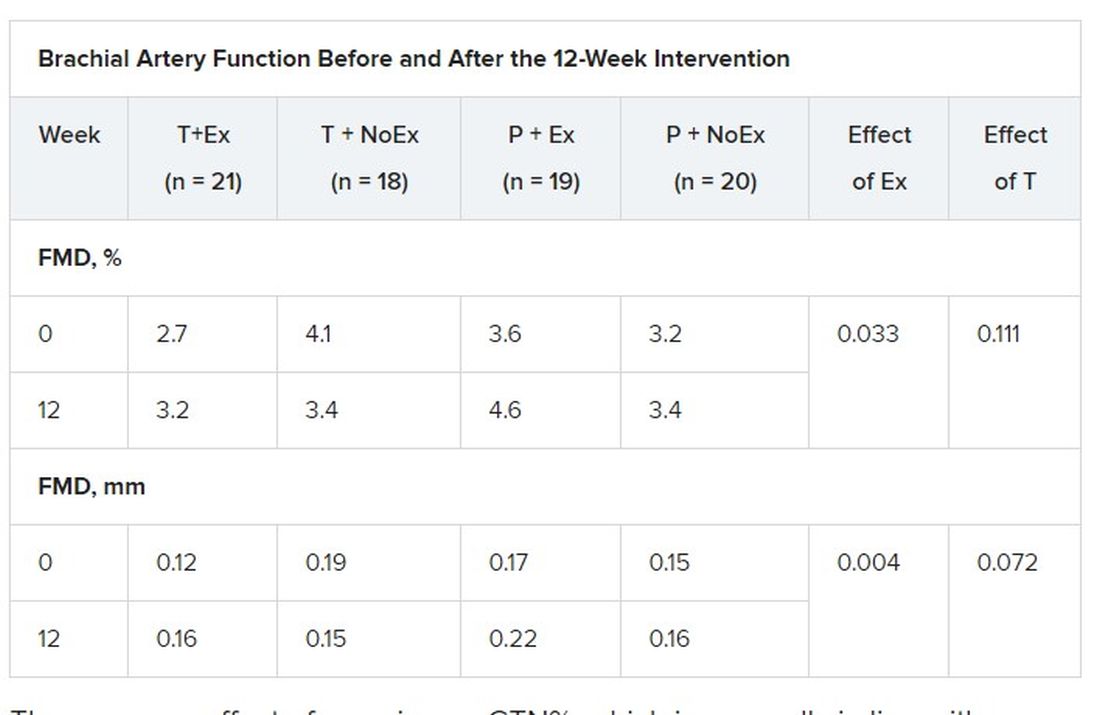
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise training – but not testosterone therapy – improved vascular health in aging men with widening midsections and low to normal testosterone, new research suggests.
“Previous studies have suggested that men with higher levels of testosterone, who were more physically active, might have better health outcomes,” Bu Beng Yeap, MBBS, PhD, University of Western Australia, Perth, said in an interview. “We formulated the hypothesis that the combination of testosterone treatment and exercise training would improve the health of arteries more than either alone.”
To test this hypothesis, the investigators randomly assigned 80 men, aged 50-70 years, to 12 weeks of 5% testosterone cream 2 mL applied daily or placebo plus a supervised exercise program that included machine-based resistance and aerobic (cycling) exercises two to three times a week or no additional exercise.
The men (mean age, 59 years) had low-normal testosterone (6-14 nmol/L), a waist circumference of at least 95 cm (37.4 inches), and no known cardiovascular disease (CVD), type 1 diabetes, or other clinically significant illnesses. Current smokers and men on testosterone or medications that would alter testosterone levels were also excluded.
High-resolution ultrasound of the brachial artery was used to assess flow-mediated dilation (FMD) and sublingual glyceryl trinitrate (GTN) responses. FMD has been shown to be predictive of CVD risk, with a 1% increase in FMD associated with a 9%-13% decrease in future CVD events.
Based on participants’ daily dairies, testosterone adherence was 97.6%. Exercise adherence was 96.5% for twice-weekly attendance and 80.0% for thrice-weekly attendance, with no between-group differences.
As reported Feb. 22, 2021, in Hypertension, testosterone levels increased, on average, 3.0 nmol/L in both testosterone groups by week 12 (P = .003). In all, 62% of these men had levels of the hormone exceeding 14 nmol/L, compared with 29% of those receiving placebo.
Testosterone levels improved with exercise training plus placebo by 0.9 nmol/L, but fell with no exercise and placebo by 0.9 nmol/L.
In terms of vascular function, exercise training increased FMD when expressed as both the delta change (mm; P = .004) and relative rise from baseline diameter (%; P = .033).
There was no effect of exercise on GTN%, which is generally in line with exercise literature indicating that shear-mediated adaptations in response to episodic exercise occur largely in endothelial cells, the authors noted.
Testosterone did not affect any measures of FMD nor was there an effect on GTN response, despite previous evidence that lower testosterone doses might enhance smooth muscle function.
“Our main finding was that testosterone – at this dose over this duration of treatment – did not have a beneficial effect on artery health, nor did it enhance the effect of exercise,” said Dr. Yeap, who is also president of the Endocrine Society of Australia. “For middle-aged and older men wanting to improve the health of their arteries, exercise is better than testosterone!”
Shalender Bhasin, MBBS, director of research programs in men’s health, aging, and metabolism at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said the study is interesting from a mechanistic perspective and adds to the overall body of evidence on how testosterone affects performance, but was narrowly focused.
“They looked at very specific markers and what they’re showing is that this is not the mechanism by which testosterone improves performance,” he said. “That may be so, but it doesn’t negate the finding that testosterone improves endurance and has other vascular effects: it increases capillarity, increases blood flow to the tissues, and improves myocardial function.”
Although well done, the study doesn’t get at the larger question of whether testosterone increases cardiovascular risk, observed Dr. Bhasin. “None of the randomized studies have been large enough or long enough to determine the effect on cardiovascular events rates. There’s a lot of argument on both sides but we need some data to address that.”
The 6,000-patient TRAVERSE trial is specifically looking at long-term major cardiovascular events with topical testosterone, compared with placebo, in hypogonadal men aged 45-80 years age who have evidence of or are at increased risk for CVD. The study, which is set to be completed in April 2022, should also provide information on fracture risk in these men, said Dr. Bhasin, one of the trial’s principal investigators and lead author of the Endocrine Society’s 2018 clinical practice guideline on testosterone therapy for hypogonadism in men.
William Evans, MD, adjunct professor of human nutrition, University of California, Berkley, said in an interview that the positive effects of testosterone occur at much lower doses in men and women who are hypogonadal but, in this particular population, exercise is the key and the major recommendation.
“Testosterone has been overprescribed and overadvertised for essentially a lifetime of sedentary living, and it’s advertised as a way to get all that back without having to work for it,” he said. “Exercise has a profound and positive effect on control of blood pressure, function, and strength, and testosterone may only affect in people who are sick, people who have really low levels.”
The study was funded by the Heart Foundation of Australia. Lawley Pharmaceuticals provided the study medication and placebo. Dr. Yeap has received speaker honoraria and conference support from Bayer, Eli Lilly, and Besins Healthcare; research support from Bayer, Lily, and Lawley; and served as an adviser for Lily, Besins Healthcare, Ferring, and Lawley. Dr. Shalender reports consultation or advisement for GTx, Pfizer, and TAP; grant or other research support from Solvay and GlaxoSmithKline; and honoraria from Solvay and Auxilium. Dr. Evans reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Big data ‘clinch’ link between high glycemic index diets and CVD
People who mostly ate foods with a low glycemic index had a lower likelihood of premature death and major cardiovascular disease (CVD) events, compared with those whose diet included more “poor-quality” food with a high glycemic index.
The results from the global PURE study of nearly 120,000 people provide evidence that helps cement glycemic index as a key measure of dietary health.
This new analysis from PURE (Prospective Urban and Rural Epidemiological Study) – a massive prospective epidemiologic study – shows people with a diet in the highest quintile of glycemic index had a significant 25% higher rate of combined total deaths and major CVD events during a median follow-up of nearly 10 years, compared with those with a diet in the lowest glycemic index quintile, in the report published online on Feb. 24, 2021, in the New England Journal of Medicine.
David J.A. Jenkins, MD, PhD, DSc, lead author, said people do not necessarily need to closely track the glycemic index of what they eat to follow the guidance that lower is better.
The link between lower glycemic load and fewer CVD events was even stronger among people with an established history of CVD at study entry. In this subset, which included 9% of the total cohort, people in the highest quintile for glycemic index consumption had a 51% higher rate of the composite primary endpoint, compared with those in the lowest quintile, in an analysis that adjusted for several potential confounders.
A simple but accurate and effective public health message is to follow existing dietary recommendations to eat better-quality food – more unprocessed fruits, vegetables, legumes, and whole grains – Dr. Jenkins advised. Those who prefer a more detailed approach could use the comprehensive glycemic index tables compiled by researchers at the University of Sydney.
‘All carbohydrates are not the same’
“What we’re saying is that all carbohydrates are not the same. Some seem to increase the risk for CVD, and others seem protective. This is not new, but worth restating in an era of low-carb and no-carb diets,” said Dr. Jenkins.
Low-glycemic-index foods are generally unprocessed foods in their native state, including fruits, vegetables, legumes, and unrefined whole grains. High-glycemic-index foods contain processed and refined carbohydrates that deliver jolts of glucose soon after eating, as the sugar in these carbohydrates quickly moves from the gut to the bloodstream.
An association between a diet with a lower glycemic index and better outcomes had appeared in prior reports from other studies, but not as unambiguously as in the new data from PURE, likely because of fewer study participants in previous studies.
Another feature of PURE that adds to the generalizability of the findings is the diversity of adults included in the study, from 20 countries on five continents.
“This clinches it,” Dr. Jenkins declared in an interview.
New PURE data tip the evidence balance
The NEJM article includes a new meta-analysis that adds the PURE findings to data from two large prior reports that were each less conclusive. The new calculation with the PURE numbers helps establish a clearer association between a diet with a higher glycemic index and the endpoint of CVD death, showing an overall 26% increase in the outcome.
The PURE data are especially informative because the investigators collected additional information on a range of potential confounders they incorporated into their analyses.
“We were able to include a lot of documentation on many potential confounders. That’s a strength of our data,” noted Dr. Jenkins, a professor of nutritional science and medicine at the University of Toronto.
“The present data, along with prior publications from PURE and several other studies, emphasize that consumption of poor quality carbohydrates is likely to be more adverse than the consumption of most fats in the diet,” said senior author Salim Yusuf, MD, DPhil, professor of medicine and executive director of the Population Health Research Institute at McMaster University, Hamilton, Ont.
“This calls for a fundamental shift in our thinking of what types of diet are likely to be harmful and what types neutral or beneficial,” Dr. Yusuf said in a statement from his institution.
Higher BMI associated with greater glycemic index effect
Another important analysis in the new report calculated the impact of a higher glycemic index diet among people with a body mass index (BMI) of less than 25 kg/m2 as well as higher BMIs.
Among people in the lower BMI subgroup, greater intake of high-glycemic-index foods showed slightly more incident primary outcome events. In contrast, people with a BMI of 25 or greater showed a steady increment in primary outcome events as the glycemic index of their diet increased.
People with higher BMIs in the quartile that ate the greatest amount of high-glycemic =-index foods had a significant 38% higher rate of primary outcome events, compared with people with similar BMIs in the lowest quartile for high-glycemic-index intake.
However, the study showed no impact on the primary association of high glycemic index and increased adverse outcomes by exercise habits, smoking, use of blood pressure medications, or use of statins.
The new report complements a separate analysis from PURE published just a few weeks earlier in the BMJ that established a significant association between increased consumption of whole grains and fewer CVD events, compared with people who had more refined grains in their diet, as reported by this news organization.
This prior report on whole versus refined grains, which Dr. Jenkins coauthored, looked at carbohydrate quality using a two-pronged approach, while glycemic index is a continuous variable that provides more nuance and takes into account carbohydrates from sources other than grains, Dr. Jenkins said.
PURE enrolled roughly 225,000 people aged 35-70 years at entry. The glycemic index analysis focused on 119,575 people who had data available for the primary outcome. During a median follow-up of 9.5 years, these people had 14,075 primary outcome events, including 8,780 deaths.
Analyses that looked at the individual outcomes that comprised the composite endpoint showed significant associations between a high-glycemic-index diet and total mortality, CVD death, non-CVD death, and stroke, but showed no significant link with myocardial infarction or heart failure. These findings are consistent with prior results of other studies that showed a stronger link between stroke and a high glycemic index diet, compared with other nonfatal CVD events.
Dr. Jenkins suggested that the significant excess of non-CVD deaths linked with a high-glycemic-index diet may stem from the impact of this type of diet on cancer-associated mortality.
PURE received partial funding through unrestricted grants from several drug companies. Dr. Jenkins has reported receiving gifts from several food-related trade associations and food companies, as well as research grants from two legume-oriented trade associations.
A version of this article first appeared on Medscape.com.
People who mostly ate foods with a low glycemic index had a lower likelihood of premature death and major cardiovascular disease (CVD) events, compared with those whose diet included more “poor-quality” food with a high glycemic index.
The results from the global PURE study of nearly 120,000 people provide evidence that helps cement glycemic index as a key measure of dietary health.
This new analysis from PURE (Prospective Urban and Rural Epidemiological Study) – a massive prospective epidemiologic study – shows people with a diet in the highest quintile of glycemic index had a significant 25% higher rate of combined total deaths and major CVD events during a median follow-up of nearly 10 years, compared with those with a diet in the lowest glycemic index quintile, in the report published online on Feb. 24, 2021, in the New England Journal of Medicine.
David J.A. Jenkins, MD, PhD, DSc, lead author, said people do not necessarily need to closely track the glycemic index of what they eat to follow the guidance that lower is better.
The link between lower glycemic load and fewer CVD events was even stronger among people with an established history of CVD at study entry. In this subset, which included 9% of the total cohort, people in the highest quintile for glycemic index consumption had a 51% higher rate of the composite primary endpoint, compared with those in the lowest quintile, in an analysis that adjusted for several potential confounders.
A simple but accurate and effective public health message is to follow existing dietary recommendations to eat better-quality food – more unprocessed fruits, vegetables, legumes, and whole grains – Dr. Jenkins advised. Those who prefer a more detailed approach could use the comprehensive glycemic index tables compiled by researchers at the University of Sydney.
‘All carbohydrates are not the same’
“What we’re saying is that all carbohydrates are not the same. Some seem to increase the risk for CVD, and others seem protective. This is not new, but worth restating in an era of low-carb and no-carb diets,” said Dr. Jenkins.
Low-glycemic-index foods are generally unprocessed foods in their native state, including fruits, vegetables, legumes, and unrefined whole grains. High-glycemic-index foods contain processed and refined carbohydrates that deliver jolts of glucose soon after eating, as the sugar in these carbohydrates quickly moves from the gut to the bloodstream.
An association between a diet with a lower glycemic index and better outcomes had appeared in prior reports from other studies, but not as unambiguously as in the new data from PURE, likely because of fewer study participants in previous studies.
Another feature of PURE that adds to the generalizability of the findings is the diversity of adults included in the study, from 20 countries on five continents.
“This clinches it,” Dr. Jenkins declared in an interview.
New PURE data tip the evidence balance
The NEJM article includes a new meta-analysis that adds the PURE findings to data from two large prior reports that were each less conclusive. The new calculation with the PURE numbers helps establish a clearer association between a diet with a higher glycemic index and the endpoint of CVD death, showing an overall 26% increase in the outcome.
The PURE data are especially informative because the investigators collected additional information on a range of potential confounders they incorporated into their analyses.
“We were able to include a lot of documentation on many potential confounders. That’s a strength of our data,” noted Dr. Jenkins, a professor of nutritional science and medicine at the University of Toronto.
“The present data, along with prior publications from PURE and several other studies, emphasize that consumption of poor quality carbohydrates is likely to be more adverse than the consumption of most fats in the diet,” said senior author Salim Yusuf, MD, DPhil, professor of medicine and executive director of the Population Health Research Institute at McMaster University, Hamilton, Ont.
“This calls for a fundamental shift in our thinking of what types of diet are likely to be harmful and what types neutral or beneficial,” Dr. Yusuf said in a statement from his institution.
Higher BMI associated with greater glycemic index effect
Another important analysis in the new report calculated the impact of a higher glycemic index diet among people with a body mass index (BMI) of less than 25 kg/m2 as well as higher BMIs.
Among people in the lower BMI subgroup, greater intake of high-glycemic-index foods showed slightly more incident primary outcome events. In contrast, people with a BMI of 25 or greater showed a steady increment in primary outcome events as the glycemic index of their diet increased.
People with higher BMIs in the quartile that ate the greatest amount of high-glycemic =-index foods had a significant 38% higher rate of primary outcome events, compared with people with similar BMIs in the lowest quartile for high-glycemic-index intake.
However, the study showed no impact on the primary association of high glycemic index and increased adverse outcomes by exercise habits, smoking, use of blood pressure medications, or use of statins.
The new report complements a separate analysis from PURE published just a few weeks earlier in the BMJ that established a significant association between increased consumption of whole grains and fewer CVD events, compared with people who had more refined grains in their diet, as reported by this news organization.
This prior report on whole versus refined grains, which Dr. Jenkins coauthored, looked at carbohydrate quality using a two-pronged approach, while glycemic index is a continuous variable that provides more nuance and takes into account carbohydrates from sources other than grains, Dr. Jenkins said.
PURE enrolled roughly 225,000 people aged 35-70 years at entry. The glycemic index analysis focused on 119,575 people who had data available for the primary outcome. During a median follow-up of 9.5 years, these people had 14,075 primary outcome events, including 8,780 deaths.
Analyses that looked at the individual outcomes that comprised the composite endpoint showed significant associations between a high-glycemic-index diet and total mortality, CVD death, non-CVD death, and stroke, but showed no significant link with myocardial infarction or heart failure. These findings are consistent with prior results of other studies that showed a stronger link between stroke and a high glycemic index diet, compared with other nonfatal CVD events.
Dr. Jenkins suggested that the significant excess of non-CVD deaths linked with a high-glycemic-index diet may stem from the impact of this type of diet on cancer-associated mortality.
PURE received partial funding through unrestricted grants from several drug companies. Dr. Jenkins has reported receiving gifts from several food-related trade associations and food companies, as well as research grants from two legume-oriented trade associations.
A version of this article first appeared on Medscape.com.
People who mostly ate foods with a low glycemic index had a lower likelihood of premature death and major cardiovascular disease (CVD) events, compared with those whose diet included more “poor-quality” food with a high glycemic index.
The results from the global PURE study of nearly 120,000 people provide evidence that helps cement glycemic index as a key measure of dietary health.
This new analysis from PURE (Prospective Urban and Rural Epidemiological Study) – a massive prospective epidemiologic study – shows people with a diet in the highest quintile of glycemic index had a significant 25% higher rate of combined total deaths and major CVD events during a median follow-up of nearly 10 years, compared with those with a diet in the lowest glycemic index quintile, in the report published online on Feb. 24, 2021, in the New England Journal of Medicine.
David J.A. Jenkins, MD, PhD, DSc, lead author, said people do not necessarily need to closely track the glycemic index of what they eat to follow the guidance that lower is better.
The link between lower glycemic load and fewer CVD events was even stronger among people with an established history of CVD at study entry. In this subset, which included 9% of the total cohort, people in the highest quintile for glycemic index consumption had a 51% higher rate of the composite primary endpoint, compared with those in the lowest quintile, in an analysis that adjusted for several potential confounders.
A simple but accurate and effective public health message is to follow existing dietary recommendations to eat better-quality food – more unprocessed fruits, vegetables, legumes, and whole grains – Dr. Jenkins advised. Those who prefer a more detailed approach could use the comprehensive glycemic index tables compiled by researchers at the University of Sydney.
‘All carbohydrates are not the same’
“What we’re saying is that all carbohydrates are not the same. Some seem to increase the risk for CVD, and others seem protective. This is not new, but worth restating in an era of low-carb and no-carb diets,” said Dr. Jenkins.
Low-glycemic-index foods are generally unprocessed foods in their native state, including fruits, vegetables, legumes, and unrefined whole grains. High-glycemic-index foods contain processed and refined carbohydrates that deliver jolts of glucose soon after eating, as the sugar in these carbohydrates quickly moves from the gut to the bloodstream.
An association between a diet with a lower glycemic index and better outcomes had appeared in prior reports from other studies, but not as unambiguously as in the new data from PURE, likely because of fewer study participants in previous studies.
Another feature of PURE that adds to the generalizability of the findings is the diversity of adults included in the study, from 20 countries on five continents.
“This clinches it,” Dr. Jenkins declared in an interview.
New PURE data tip the evidence balance
The NEJM article includes a new meta-analysis that adds the PURE findings to data from two large prior reports that were each less conclusive. The new calculation with the PURE numbers helps establish a clearer association between a diet with a higher glycemic index and the endpoint of CVD death, showing an overall 26% increase in the outcome.
The PURE data are especially informative because the investigators collected additional information on a range of potential confounders they incorporated into their analyses.
“We were able to include a lot of documentation on many potential confounders. That’s a strength of our data,” noted Dr. Jenkins, a professor of nutritional science and medicine at the University of Toronto.
“The present data, along with prior publications from PURE and several other studies, emphasize that consumption of poor quality carbohydrates is likely to be more adverse than the consumption of most fats in the diet,” said senior author Salim Yusuf, MD, DPhil, professor of medicine and executive director of the Population Health Research Institute at McMaster University, Hamilton, Ont.
“This calls for a fundamental shift in our thinking of what types of diet are likely to be harmful and what types neutral or beneficial,” Dr. Yusuf said in a statement from his institution.
Higher BMI associated with greater glycemic index effect
Another important analysis in the new report calculated the impact of a higher glycemic index diet among people with a body mass index (BMI) of less than 25 kg/m2 as well as higher BMIs.
Among people in the lower BMI subgroup, greater intake of high-glycemic-index foods showed slightly more incident primary outcome events. In contrast, people with a BMI of 25 or greater showed a steady increment in primary outcome events as the glycemic index of their diet increased.
People with higher BMIs in the quartile that ate the greatest amount of high-glycemic =-index foods had a significant 38% higher rate of primary outcome events, compared with people with similar BMIs in the lowest quartile for high-glycemic-index intake.
However, the study showed no impact on the primary association of high glycemic index and increased adverse outcomes by exercise habits, smoking, use of blood pressure medications, or use of statins.
The new report complements a separate analysis from PURE published just a few weeks earlier in the BMJ that established a significant association between increased consumption of whole grains and fewer CVD events, compared with people who had more refined grains in their diet, as reported by this news organization.
This prior report on whole versus refined grains, which Dr. Jenkins coauthored, looked at carbohydrate quality using a two-pronged approach, while glycemic index is a continuous variable that provides more nuance and takes into account carbohydrates from sources other than grains, Dr. Jenkins said.
PURE enrolled roughly 225,000 people aged 35-70 years at entry. The glycemic index analysis focused on 119,575 people who had data available for the primary outcome. During a median follow-up of 9.5 years, these people had 14,075 primary outcome events, including 8,780 deaths.
Analyses that looked at the individual outcomes that comprised the composite endpoint showed significant associations between a high-glycemic-index diet and total mortality, CVD death, non-CVD death, and stroke, but showed no significant link with myocardial infarction or heart failure. These findings are consistent with prior results of other studies that showed a stronger link between stroke and a high glycemic index diet, compared with other nonfatal CVD events.
Dr. Jenkins suggested that the significant excess of non-CVD deaths linked with a high-glycemic-index diet may stem from the impact of this type of diet on cancer-associated mortality.
PURE received partial funding through unrestricted grants from several drug companies. Dr. Jenkins has reported receiving gifts from several food-related trade associations and food companies, as well as research grants from two legume-oriented trade associations.
A version of this article first appeared on Medscape.com.
Thirteen percent of patients with type 2 diabetes have major ECG abnormalities
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
Major ECG abnormalities were found in 13% of more than 8,000 unselected patients with type 2 diabetes, including a 9% prevalence in the subgroup of these patients without identified cardiovascular disease (CVD) in a community-based Dutch cohort. Minor ECG abnormalities were even more prevalent.
These prevalence rates were consistent with prior findings from patients with type 2 diabetes, but the current report is notable because “it provides the most thorough description of the prevalence of ECG abnormalities in people with type 2 diabetes,” and used an “unselected and large population with comprehensive measurements,” including many without a history of CVD, said Peter P. Harms, MSc, and associates noted in a recent report in the Journal of Diabetes and Its Complications.
The analysis also identified several parameters that significantly linked with the presence of a major ECG abnormality including hypertension, male sex, older age, and higher levels of hemoglobin A1c.
“Resting ECG abnormalities might be a useful tool for CVD screening in people with type 2 diabetes,” concluded Mr. Harms, a researcher at the Amsterdam University Medical Center, and coauthors.
Findings “not unexpected”
Patients with diabetes have a higher prevalence of ECG abnormalities “because of their higher likelihood of having hypertension and other CVD risk factors,” as well as potentially having subclinical CVD, said Fred M. Kusumoto, MD, so these findings are “not unexpected. The more risk factors a patient has for structural heart disease, atrial fibrillation (AFib), or stroke from AFib, the more a physician must consider whether a baseline ECG and future surveillance is appropriate,” Dr. Kusumoto said in an interview.
But he cautioned against seeing these findings as a rationale to routinely run a resting ECG examination on every adult with diabetes.
“Patients with diabetes are very heterogeneous,” which makes it “difficult to come up with a ‘one size fits all’ recommendation” for ECG screening of patients with diabetes, he said.
While a task force of the European Society of Cardiology and the European Association for the Study of Diabetes set a class I level C guideline for resting ECG screening of patients with diabetes if they also have either hypertension or suspected CVD, the American Diabetes Association has no specific recommendations on which patients with diabetes should receive ECG screening.
“The current absence of U.S. recommendations is reasonable, as it allows patients and physicians to discuss the issues and decide on the utility of an ECG in their specific situation,” said Dr. Kusumoto, director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. But he also suggested that “the more risk factors that a patient with diabetes has for structural heart disease, AFib, or stroke from AFib the more a physician must consider whether a baseline ECG and future surveillance is appropriate.”
Data from a Dutch prospective cohort
The new study used data collected from 8,068 patients with type 2 diabetes and enrolled in the prospective Hoorn Diabetes Care System cohort, which enrolled patients newly diagnosed with type 2 diabetes in the West Friesland region of the Netherlands starting in 1996. The study includes most of these patients in the region who are under regular care of a general practitioner, and the study protocol calls for an annual resting ECG examination.
The investigators used standard, 12-lead ECG readings taken for each patient during 2018, and classified abnormalities by the Minnesota Code criteria. They divided the abnormalities into major or minor groups “in accordance with consensus between previous studies who categorised abnormalities according to perceived importance and/or severity.” The major subgroup included major QS pattern abnormalities, major ST-segment abnormalities, complete left bundle branch block or intraventricular block, or atrial fibrillation or flutter. Minor abnormalities included minor QS pattern abnormalities, minor ST-segment abnormalities, complete right bundle branch block, or premature atrial or ventricular contractions.
The prevalence of a major abnormality in the entire cohort examined was 13%, and another 16% had a minor abnormality. The most common types of abnormalities were ventricular conduction defects, in 14%; and arrhythmias, in 11%. In the subgroup of 6,494 of these patients with no history of CVD, 9% had a major abnormality and 15% a minor abnormality. Within this subgroup, 23% also had no hypertension, and their prevalence of a major abnormality was 4%, while 9% had a minor abnormality.
A multivariable analysis of potential risk factors among the entire study cohort showed that patients with hypertension had nearly triple the prevalence of a major ECG abnormality as those without hypertension, and men had double the prevalence of a major abnormality compared with women. Other markers that significantly linked with a higher rate of a major abnormality were older age, higher body mass index, higher A1c levels, and moderately depressed renal function.
“While the criteria the authors used for differentiating major and minor criteria are reasonable, in an asymptomatic patient even the presence of frequent premature atrial contractions on a baseline ECG has been associated with the development of AFib and a higher risk for stroke. The presence of left or right bundle branch block could spur additional evaluation with an echocardiogram,” said Dr. Kusumoto, president-elect of the Heart Rhythm Society.
“Generally an ECG abnormality is supplemental to clinical data in deciding the choice and timing of next therapeutic steps or additional testing. Physicians should have a fairly low threshold for obtaining ECG in patients with diabetes since it is inexpensive and can provide supplemental and potentially actionable information,” he said. “The presence of ECG abnormalities increases the possibility of underlying cardiovascular disease. When taking care of patients with diabetes at initial evaluation or without prior cardiac history or symptoms referable to the heart, two main issues are identifying the likelihood of coronary artery disease and atrial fibrillation.”
Mr. Harms and coauthors, and Dr. Kusumoto, had no disclosures.
FROM THE JOURNAL OF DIABETES AND ITS COMPLICATIONS
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
How to convince patients muscle pain isn’t a statin Achilles heel: StatinWISE
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
Study: Central sleep apnea is common in ticagrelor users post ACS
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
jremaly@mdedge.com
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
jremaly@mdedge.com
The prevalence of asymptomatic central sleep apnea after acute coronary syndrome is high and may be associated with the use of ticagrelor, a new study finds.
Prior studies have suggested that ticagrelor is associated with an increased likelihood of central sleep apnea. The drug’s label notes that two respiratory conditions – central sleep apnea and Cheyne-Stokes respiration – are adverse reactions that were identified after the drug’s approval in the United States in 2011. “Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the label says.
Among 80 patients receiving ticagrelor, 24 had central sleep apnea hypopnea syndrome (CSAHS), whereas of 41 patients not taking ticagrelor, 3 had this condition (30% vs. 7.3%, P = .004), in the new study published online Jan. 20, 2021, in Sleep Medicine. A multivariable analysis included in the paper found that age and ticagrelor administration were the only two factors associated with the occurrence of CSAHS.
Findings are ‘striking’
The different rates of central sleep apnea in the study are striking, but it is not clear that asymptomatic central sleep apnea in patients taking ticagrelor is a concern, Ofer Jacobowitz, MD, PhD, associate professor of otolaryngology at Hofstra University, Hempstead, N.Y, said in an interview.
“Whether this particular drug-induced central sleep apnea is consequential” is an open question, noted Dr. Jacobowitz. “There is no evidence that shows that this is definitely harmful.”
“The different types of central sleep apnea are caused by different mechanisms and this one, we don’t know,” Dr. Jacobwitz added.
Study author continues to prescribe ticagrelor
One of the study authors, Philippe Meurin, MD, said that he continues to prescribe ticagrelor every day and that the side effect is not necessarily important.
It is possible that central sleep apnea may resolve, although further studies would need to examine central sleep apnea over time to establish the duration of the condition, he added. Nevertheless, awareness of the association could have implications for clinical practice, Dr. Meurin said.
Central sleep apnea is rare, and if doctors detect it during a sleep study, they may perform extensive tests to assess for possible neurologic diseases, for example, when the cause may be attributed to the medication, he said. In addition, if a patient who is taking ticagrelor has dyspnea, the presence of central sleep apnea may suggest that dyspnea could be related to the drug, although this possibility needs further study, he noted.
Study included patients with ACS history, but no heart failure
Dr. Meurin, of Centre de Réadaptation Cardiaque de La Brie, Les Grands Prés, Villeneuve-Saint-Denis, France, and colleagues included in their study patients between 1 week and 1 year after acute coronary syndrome who did not have heart failure or a history of sleep apnea.
After an overnight sleep study, they classified patients as normal, as having CSAHS (i.e., an apnea-hypopnea index of 15 or greater, mostly with central sleep apneas), or as having obstructive sleep apnea hypopnea syndrome (OSAHS; i.e., an apnea-hypopnea index of 15 or greater, mostly with obstructive sleep apneas).
The prospective study included 121 consecutive patients between January 2018 and March 2020. Patients had a mean age of 56.8, and 88% were men.
Switching to another P2Y12 inhibitor ‘does not seem appropriate’
“CSAHS could be promoted by the use of ticagrelor, a relatively new drug that modifies the apneic threshold,” the study authors wrote. “Regarding underlying mechanisms, the most probable explanation seems to be increased chemosensitivity to hypercapnia by a direct P2Y12 inhibitory effect on the central nervous system.”
Doctors should not overestimate the severity of the adverse reaction or consider it the same way they do OSASH, they added.
Among patients with acute coronary syndrome in the PLATO study, ticagrelor, compared with clopidogrel, “significantly reduced the rate of death from vascular causes, myocardial infarction, or stroke,” Dr. Meurin and colleagues said. “Because in this study more than 9,000 patients received ticagrelor for 12 months, CSAHS (even if it seems frequent in our study) did not seem to impair the good efficacy/tolerance balance of the drug. Therefore, in asymptomatic CSAHS patients, switching from ticagrelor to another P2Y12 inhibitor does not seem appropriate.”
A recent analysis of data from randomized, controlled trials with ticagrelor did not find excess cases of sleep apnea with the drug. But an asymptomatic adverse event such as central sleep apnea “cannot emerge from a post hoc analysis,” Dr. Meurin and colleagues said.
The analysis of randomized trial data was conducted by Marc S. Sabatine, MD, MPH, chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group at Brigham and Women’s Hospital, and coauthors. It was published in JACC: Cardiovascular Interventions in April 2020.
They “used the gold standard for medical evidence (randomized, placebo-controlled trials) and found 158 cases of sleep apnea reported, with absolutely no difference between ticagrelor and placebo,” Dr. Sabatine said in an interview. Their analysis examined clinically overt apnea, he noted.
“It is quite clear that when looking at large numbers in placebo-controlled trials, there is no excess,” Dr. Sabatine said. “Meurin et al. are examining a different outcome: the results of a lab test in what may be entirely asymptomatic patients.”
A randomized trial could confirm the association, he said.
“The association may be real, but also may be play of chance or confounded,” said Dr. Sabatine. “To convince the medical community, the next step would be for the investigators to do a randomized trial and test whether ticagrelor increases the risk of central sleep apnea.”
Dr. Meurin and the study coauthors had no disclosures. The analysis of randomized, controlled trial data by Dr. Sabatine and colleagues was funded by AstraZeneca, which distributes ticagrelor under the trade name Brilinta. Dr. Sabatine has been a consultant for AstraZeneca and received research grants through Brigham and Women’s Hospital from AstraZeneca. He has consulted for and received grants through the hospital from other companies as well. Dr. Jacobowitz had no relevant disclosures.
jremaly@mdedge.com
FROM SLEEP MEDICINE
Home devices screen for atrial fibrillation
In an ad for one of these products, KardiaMobile, a cardiologist says this device “detects atrial fibrillation, one of the major causes of stroke.” You might also have heard that the Apple Watch has an opt-in feature that constantly screens for atrial fibrillation without any effort being made by the patient, or can check on-demand for AFib if a wearer experiences palpitations or an abnormal heart beat. Both of these devices generate a standard limb–lead ECG (essentially lead I) by connecting the device to both arms and producing a 30-second rhythm strip.
KardiaMobile recently introduced a newer device. When you place this device on a bare knee and touch one electrode with fingers from the right hand and another electrode with fingers from the left hand, the device produces a six-lead ECG. These small devices send an image of the ECG to a patient’s smartphone over Bluetooth, and the results can be easily read, printed out, or sent to the doctor for further analysis. Additionally, both of KardiaMobile’s devices utilize artificial intelligence to analyze a rhythm strip in real time and let the patient know if the ECG is normal, shows AFib, or is unable to be analyzed.
The electrocardiographic technology was formerly only available in a medical setting. It required an expensive machine and could only be interpreted by someone with expertise developed through years of training. Now it is readily available to patients in their homes. But how accurate is the technology and how are we going to use it?
How effective is KardiaMobile at detecting AFib?
Studies have looked at both KardiaMobile and the Apple Watch. One study of KardiaMobile in patients with Afib who were admitted for antiarrhythmic drug initiation showed that about a quarter of readings could not be classified because of artifact and other reasons. After exclusion of unclassified recordings, the KardiaMobile interpretation had 97% sensitivity and 94% specificity for AFib detection when compared with physician-interpreted ECGs.1 In a large review of the device’s accuracy, there was about 85% sensitivity and specificity of the automated readings.2
How does the Apple Watch find AFib?
Like the KardiaMobile device, the Apple Watch can be used whenever patients notice symptoms or whenever they and their physicians decide the device would be useful. In addition, though, the Apple Watch has a function where the wearer can opt in to have the watch screen for AFib in the background whenever the watch is worn.
The watch monitors heart rate using photoplethysmography, where light-sensitive photodiodes detect blood pulses to assess heart rate variability. When an irregular heart rate is detected, the AW alerts the user of possible AFib. Once alerted, the wearer can then utilize a second function to obtain a single-lead ECG. Heart rate, rhythm, and a 30-second ECG tracing are saved in the Bluetooth-linked iPhone’s health app and can be exported for review by a physician.
In a study of over 400,000 participants, among participants notified of an irregular pulse through screening there was a positive predictive value of 84%.3 Single-lead EKGs initiated by watch wearers had a specificity for AFib of 99.6% among tracings with good wave forms, indicating very few false positives. Only 1 individual of the 263 individuals who had normal sinus rhythm on 12-lead ECG was classified as having AFib, though in 7% sinus rhythm could not be confirmed because of poor tracings.4,5
What should we do with the results?
It’s impressive that these devices deliver accurate information with very good specificity. Our hope is that detecting AFib with one of these devices will lead to an intervention being made that will decrease a patient’s risk of stroke. But it is not clear if routine screening in asymptomatic adults will accomplish this.
While more data is needed, we must acknowledge that our patients will soon be bringing us results from home. Regardless of what we think of this technology, we need to decide what to do when patients call us with results from these devices.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. William A et al. Heart Rhythm. 2018 Oct;15(10):1561-5.
2. KardiaMobile for the ambulatory detection of atrial fibrillation. NICE Medtech innovation briefing. 29 October 2020 Oct 29. www.nice.org.uk/guidance/mib232.
3. Perez MV et al. N Engl J Med. 2019; 381:1909-17.
4. Using Apple Watch for Arrhythmia Detection, December 2018. Apple. https://www.apple.com/healthcare/site/docs/Apple_Watch_Arrhythmia_Detection.pdf. Accessed 2019 Apr 5.
5. De Novo Classification Request for ECG App. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180044.pdf. Accessed 2019 Apr 29.
In an ad for one of these products, KardiaMobile, a cardiologist says this device “detects atrial fibrillation, one of the major causes of stroke.” You might also have heard that the Apple Watch has an opt-in feature that constantly screens for atrial fibrillation without any effort being made by the patient, or can check on-demand for AFib if a wearer experiences palpitations or an abnormal heart beat. Both of these devices generate a standard limb–lead ECG (essentially lead I) by connecting the device to both arms and producing a 30-second rhythm strip.
KardiaMobile recently introduced a newer device. When you place this device on a bare knee and touch one electrode with fingers from the right hand and another electrode with fingers from the left hand, the device produces a six-lead ECG. These small devices send an image of the ECG to a patient’s smartphone over Bluetooth, and the results can be easily read, printed out, or sent to the doctor for further analysis. Additionally, both of KardiaMobile’s devices utilize artificial intelligence to analyze a rhythm strip in real time and let the patient know if the ECG is normal, shows AFib, or is unable to be analyzed.
The electrocardiographic technology was formerly only available in a medical setting. It required an expensive machine and could only be interpreted by someone with expertise developed through years of training. Now it is readily available to patients in their homes. But how accurate is the technology and how are we going to use it?
How effective is KardiaMobile at detecting AFib?
Studies have looked at both KardiaMobile and the Apple Watch. One study of KardiaMobile in patients with Afib who were admitted for antiarrhythmic drug initiation showed that about a quarter of readings could not be classified because of artifact and other reasons. After exclusion of unclassified recordings, the KardiaMobile interpretation had 97% sensitivity and 94% specificity for AFib detection when compared with physician-interpreted ECGs.1 In a large review of the device’s accuracy, there was about 85% sensitivity and specificity of the automated readings.2
How does the Apple Watch find AFib?
Like the KardiaMobile device, the Apple Watch can be used whenever patients notice symptoms or whenever they and their physicians decide the device would be useful. In addition, though, the Apple Watch has a function where the wearer can opt in to have the watch screen for AFib in the background whenever the watch is worn.
The watch monitors heart rate using photoplethysmography, where light-sensitive photodiodes detect blood pulses to assess heart rate variability. When an irregular heart rate is detected, the AW alerts the user of possible AFib. Once alerted, the wearer can then utilize a second function to obtain a single-lead ECG. Heart rate, rhythm, and a 30-second ECG tracing are saved in the Bluetooth-linked iPhone’s health app and can be exported for review by a physician.
In a study of over 400,000 participants, among participants notified of an irregular pulse through screening there was a positive predictive value of 84%.3 Single-lead EKGs initiated by watch wearers had a specificity for AFib of 99.6% among tracings with good wave forms, indicating very few false positives. Only 1 individual of the 263 individuals who had normal sinus rhythm on 12-lead ECG was classified as having AFib, though in 7% sinus rhythm could not be confirmed because of poor tracings.4,5
What should we do with the results?
It’s impressive that these devices deliver accurate information with very good specificity. Our hope is that detecting AFib with one of these devices will lead to an intervention being made that will decrease a patient’s risk of stroke. But it is not clear if routine screening in asymptomatic adults will accomplish this.
While more data is needed, we must acknowledge that our patients will soon be bringing us results from home. Regardless of what we think of this technology, we need to decide what to do when patients call us with results from these devices.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. William A et al. Heart Rhythm. 2018 Oct;15(10):1561-5.
2. KardiaMobile for the ambulatory detection of atrial fibrillation. NICE Medtech innovation briefing. 29 October 2020 Oct 29. www.nice.org.uk/guidance/mib232.
3. Perez MV et al. N Engl J Med. 2019; 381:1909-17.
4. Using Apple Watch for Arrhythmia Detection, December 2018. Apple. https://www.apple.com/healthcare/site/docs/Apple_Watch_Arrhythmia_Detection.pdf. Accessed 2019 Apr 5.
5. De Novo Classification Request for ECG App. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180044.pdf. Accessed 2019 Apr 29.
In an ad for one of these products, KardiaMobile, a cardiologist says this device “detects atrial fibrillation, one of the major causes of stroke.” You might also have heard that the Apple Watch has an opt-in feature that constantly screens for atrial fibrillation without any effort being made by the patient, or can check on-demand for AFib if a wearer experiences palpitations or an abnormal heart beat. Both of these devices generate a standard limb–lead ECG (essentially lead I) by connecting the device to both arms and producing a 30-second rhythm strip.
KardiaMobile recently introduced a newer device. When you place this device on a bare knee and touch one electrode with fingers from the right hand and another electrode with fingers from the left hand, the device produces a six-lead ECG. These small devices send an image of the ECG to a patient’s smartphone over Bluetooth, and the results can be easily read, printed out, or sent to the doctor for further analysis. Additionally, both of KardiaMobile’s devices utilize artificial intelligence to analyze a rhythm strip in real time and let the patient know if the ECG is normal, shows AFib, or is unable to be analyzed.
The electrocardiographic technology was formerly only available in a medical setting. It required an expensive machine and could only be interpreted by someone with expertise developed through years of training. Now it is readily available to patients in their homes. But how accurate is the technology and how are we going to use it?
How effective is KardiaMobile at detecting AFib?
Studies have looked at both KardiaMobile and the Apple Watch. One study of KardiaMobile in patients with Afib who were admitted for antiarrhythmic drug initiation showed that about a quarter of readings could not be classified because of artifact and other reasons. After exclusion of unclassified recordings, the KardiaMobile interpretation had 97% sensitivity and 94% specificity for AFib detection when compared with physician-interpreted ECGs.1 In a large review of the device’s accuracy, there was about 85% sensitivity and specificity of the automated readings.2
How does the Apple Watch find AFib?
Like the KardiaMobile device, the Apple Watch can be used whenever patients notice symptoms or whenever they and their physicians decide the device would be useful. In addition, though, the Apple Watch has a function where the wearer can opt in to have the watch screen for AFib in the background whenever the watch is worn.
The watch monitors heart rate using photoplethysmography, where light-sensitive photodiodes detect blood pulses to assess heart rate variability. When an irregular heart rate is detected, the AW alerts the user of possible AFib. Once alerted, the wearer can then utilize a second function to obtain a single-lead ECG. Heart rate, rhythm, and a 30-second ECG tracing are saved in the Bluetooth-linked iPhone’s health app and can be exported for review by a physician.
In a study of over 400,000 participants, among participants notified of an irregular pulse through screening there was a positive predictive value of 84%.3 Single-lead EKGs initiated by watch wearers had a specificity for AFib of 99.6% among tracings with good wave forms, indicating very few false positives. Only 1 individual of the 263 individuals who had normal sinus rhythm on 12-lead ECG was classified as having AFib, though in 7% sinus rhythm could not be confirmed because of poor tracings.4,5
What should we do with the results?
It’s impressive that these devices deliver accurate information with very good specificity. Our hope is that detecting AFib with one of these devices will lead to an intervention being made that will decrease a patient’s risk of stroke. But it is not clear if routine screening in asymptomatic adults will accomplish this.
While more data is needed, we must acknowledge that our patients will soon be bringing us results from home. Regardless of what we think of this technology, we need to decide what to do when patients call us with results from these devices.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. William A et al. Heart Rhythm. 2018 Oct;15(10):1561-5.
2. KardiaMobile for the ambulatory detection of atrial fibrillation. NICE Medtech innovation briefing. 29 October 2020 Oct 29. www.nice.org.uk/guidance/mib232.
3. Perez MV et al. N Engl J Med. 2019; 381:1909-17.
4. Using Apple Watch for Arrhythmia Detection, December 2018. Apple. https://www.apple.com/healthcare/site/docs/Apple_Watch_Arrhythmia_Detection.pdf. Accessed 2019 Apr 5.
5. De Novo Classification Request for ECG App. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN180044.pdf. Accessed 2019 Apr 29.
New light cast on type 2 MI aims to sharpen diagnosis, therapy
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
The hospital and postdischarge course of patients diagnosed with type 2 myocardial infarction, triggered when myocardial oxygen demand outstrips supply, differs in telling ways from those with the more common atherothrombotic type 1 MI, suggests a new registry analysis that aims to lift a cloud of confusion surrounding their management.
The observational study of more than 250,000 patients with either form of MI, said to be the largest of its kind, points to widespread unfamiliarity with distinctions between the two, and the diagnostic and therapeutic implications of misclassification. It suggests, in particular, that type 2 MI may be grossly underdiagnosed and undertreated.
The minority of patients with type 2 MI were more likely female and to have heart failure (HF), renal disease, valve disease, or atrial fibrillation, and less likely to have a lipid disorder, compared with those with type 1 MI. They were one-fifth as likely to be referred for coronary angiography and 20 times less likely to undergo revascularization.
Indeed, only about 2% of the type 2 cohort ultimately underwent percutaneous coronary intervention (PCI) or coronary bypass surgery (CABG). Yet the analysis suggests that cardiovascular risk climbs regardless of MI type and that in patients with type 2 MI, coronary revascularization might well cut the risk of death in half over the short term.
There were also disparities in clinical outcomes in the analysis, based on data from the final 3 months of 2017 in the Nationwide Readmissions Database, which reportedly documents almost 60% of hospitalizations in the United States.
For example, those with type 1 or type 2 MI – as characterized in the then-current third Universal Definition of Myocardial Infarction and today’s UDMI-4 – were comparably at risk for both 30-day all-cause readmission and HF readmission. But type 2 patients were less likely to die in the hospital or be readmitted within 30 days for recurrent MI.
Revascularization uncertainty
Importantly, the study’s 3-month observation period immediately followed the debut of a code specifically for type 2 MI in the ICD-10-CM system.
Type 2 accounted for about 15% of MIs during that period, the percentage climbing sharply from the first to the third month. That suggests clinicians were still getting used to the code during the early weeks, “undercoding” for type-2 MI at first but less so after some experience, Cian P. McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston, said in an interview.
“I can imagine that as people become more aware of the coding, using it more often, the proportion of type 2 MI relative to the total MI cases will probably be much higher,” said McCarthy, lead author on the study published online Feb. 15, 2021, in the Journal of the American College of Cardiology.
What had been understood about type 2 MI came largely from single-center studies, he said. This “first national study of type-2 MI in the United States” sought to determine whether such findings are hospital specific or “representative of what people are doing nationally.”
The new analysis largely confirms that patients with type 2 MI are typically burdened with multiple comorbidities, Dr. McCarthy said, but also suggests that type 2 often was, and likely still is, incorrectly classified as type 1. So, it was “surprising” that they were rarely referred for angiography. “Only 1 in 50 received revascularization.”
Those diagnosed with type-2 MI were far less likely to receive coronary angiography (10.9% vs. 57.3%), PCI (1.7% vs. 38.5%), or CABG (0.4% vs. 7.8%) (P < .001 for all three differences), the report noted.
That, Dr. McCarthy said, “clearly shows that clinicians are uncertain about whether revascularization is beneficial” in type 2 MI.
Coding not in sync with UDMI
If there is confusion in practice about differentiating type 2 from type 1 MI, it likely has multiple sources, and one may be inconsistencies in how the UDMI and relevant ICD codes are applied in practice.
For example, the coding mandate is always to classify ST-segment elevation MI and non-STEMI as type 1, yet UDMI-4 itself states that a type 2 MI may be either STEMI or non-STEMI, noted Dr. McCarthy, as well as an editorial accompanying the report.
“It also can be difficult at times to distinguish type 2 MI from the diagnosis of myocardial injury,” both of which are partly defined by elevated cardiac troponin (cTn), adds the editorial, from Kristian Thygesen, MD, DSc, Aarhus (Denmark) University Hospital, Aarhus, Denmark, and Allan S. Jaffe, MD, Mayo Clinic, Rochester, Minn.
Crucially, but potentially sometimes overlooked, a diagnosis of infarction requires evidence of ischemia along with the biomarker elevation, whereas myocardial injury is defined by raised cTn without evidence of ischemia. Yet there is no ICD-10-CM code for “nonischemic myocardial injury,” Dr. Thygesen and Dr. Jaffe observed.
“Instead, the new ICD-10-CM coding includes a proxy called ‘non-MI troponin elevation due to an underlying cause,’ ” they wrote. “Unfortunately, although some have advocated using this code for myocardial injury, it is not specific for an elevated cTn value and could represent any abnormal laboratory measurements.” The code could be “misleading” and thus worsen the potential for miscoding and “misattribution of MI diagnoses.”
In the current study, 84.6% of the cohort were classified with type 1 MI, 14.8% with type 2, and 0.6% with both types. Of those with type 1 MI, 22.1% had STEMI, 76.4% had non-STEMI with the remainder “unspecified.”
“I think the introduction of ICD codes for type-2 MI is helpful in that we can study type 2 MI more broadly, across institutions, and try and get a better sense of its outcomes and how these patients are treated,” Dr. McCarthy said. But the coding system’s deficiencies may often lead to misclassification of patients. Especially, patients with type 2 STEMI may be miscoded as having type-1 STEMI, and those with only myocardial injury may be miscoded as having type 2 MI.
Most type 2 MI is a complication
A profile of patients with type 2 MI may be helpful for making distinctions. The analysis showed that, compared with patients with type 1 MI, they were slightly but significantly older and more likely to have clinical depression, alcohol or other substance abuse disorder, and to be female. They also had more heart failure (27.9% vs. 10.9%), kidney disease (35.7% vs. 25.7%), atrial fibrillation (31% vs. 21%), and anemia (26% vs. 18.9%) (P < .001 for all differences).
Type 2 patients were less likely to have CV risk factors usually associated with plaque instability and atherothrombosis, including a history of smoking, dyslipidemia, MI, PCI, or CABG (P < .001 for all differences), the group noted.
Of the 37,765 patients with type 2 MI, 91% received the diagnosis as secondary to another condition, including sepsis in 24.5%, hypertension in 16.9%, arrhythmias in 6.1%, respiratory failure in 4.3%, and pneumonia in 2.8% of cases.
In multivariate analyses, patients with type 2 MI, compared with type 1, showed lower risks of in-hospital death and readmission for MI within 30 days. Their 30-day risks of readmission from any cause and from MI were similar.
In-hospital mortality was lower for patients with type 2 MI who underwent revascularization, compared with those who did not, “but they were a very select, small proportion of the patient group. I would say there are probably unmeasured confounders,” Dr. McCarthy said.
“There’s a real kind of equipoise, so I think we desperately need a trial to guide us on whether revascularization is beneficial.”
Dr. McCarthy has disclosed no relevant financial relationships. Dr. Thygesen disclosed no relevant financial relationships. Dr. Jaffe disclosed serving as a consultant for Abbott, Roche, Siemens, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Brava, Quidel, Amgen, Novartis, and Medscape for educational activities.
A version of this article first appeared on Medscape.com.
Long-term CPAP use linked with more physical activity
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
in new research.
“The aim of this study was to determine whether long-term CPAP treatment affects self-reported physical activity among participants with moderate-severe OSA and comorbid CV disease,” wrote David Stevens, PhD, of Flinders University, Adelaide, Australia, and his colleagues. The findings were recently published in the Journal of Clinical Sleep Medicine.
Researchers conducted a secondary analysis of the Sleep apnea cardiovascular endpoints (SAVE) trial that enrolled 2,687 adults aged 45-75 years old with OSA and confirmed CVD. In the study, participants were randomized to receive either CPAP plus usual care or usual care alone.
Physical activity levels were self-reported using the Leisure-Time Exercise Questionnaire (LTEQ) at baseline and at 6-, 24-, and 48-month follow-up intervals. The physical functioning subscale of the 36-item short form questionnaire (SF-36) was used to determine if activity levels were consistent with expert recommendations and to evaluate the effects on any self-perceived limitation of physical activity.
Moderate physical activity was higher among CPAP users
After a mean follow-up duration of 3.7 years, participants in the CPAP arm had approximately 20% higher levels of moderate physical activity, compared with the control arm (adjusted mean scores]: 8.7 points vs. 7.3 points; 95% confidence interval, 7.5-9.9 vs. 6.1-8.5; P = .003).
However, no significant difference was observed between treatment arms for mild physical activity (adjusted mean scores, 14.4 points vs. 14.2 points; 95% CI, 13.5-15.3 vs. 13.3-15.1; P = 0.599) or vigorous physical activity (adjusted mean scores, 3.4 points vs. 2.9 points; 95% CI 2.6-4.2 vs. 2.1-3.7; P = .125).
In addition, participants in the CPAP group reported less limitation in physical activity (adjusted between-group difference in SF-36 physical functioning subscale score = 1.66; 95% CI, 0.87-2.45; P < .001) and were more likely to report activity levels consistent with guideline recommendations.
“We were pleasantly surprised to find that people assigned to CPAP reported more physical activity than their counterparts who received usual care, despite being given no specific exercise instructions,” Kelly A. Loffler, PhD, a coauthor of the study, said in an interview.
“While I don’t think this will result in any immediate changes to guidelines, it is a helpful reminder to clinicians who are treating such patients, that the symptomatic benefits people experience with CPAP present a window of opportunity to improve health more holistically,” Dr. Loffler explained.
The researchers acknowledged that a key limitation of the study was the use of self-reported outcome measures. In future studies, they recommended that recent technological innovations, such as the availability of activity tracking devices, should be used to measure physical activity.
They also noted that patients with excessive sleepiness and severe hypoxemia were excluded from the SAVE trial; thus, the findings may not be generalizable to all patients.
Study reinforces CPAP’s health benefits
Emerson M. Wickwire, PhD, associate professor of psychiatry and medicine at the University of Maryland, Baltimore, explained that CPAP treatment is associated with well-documented health benefits among patients with CVD, as well as enhanced quality of life.
“These results provide further evidence that treating OSA can provide direct and indirect health benefits, suggesting that increased physical activity can be a vital pathway to improved cardiovascular health and enjoyment of life,” Dr. Wickwire, who is also director of the Insomnia Program at the University of Maryland Midtown Medical Center, Baltimore, said in an interview.
Steven M. Scharf, MD, a pulmonologist who is director of the Sleep Disorders Center (Adults) at the University of Maryland, also said the study findings were consistent with previous research involving patients treated for OSA.
“It is no surprise that treatment of OSA improves patient’s daily physical functioning,” explained Dr. Scharf, who is also a clinical professor, in an interview. “These results are expected, but very welcome, and I was glad to see them.”
The study was funded by the National Health and Medical Research Council of Australia, the Respironics Sleep and Respiratory Research Foundation, and Philips Respironics. Some authors reported financial affiliations with medical device and pharmaceutical companies. Dr. Loffler, Dr. Wickwire, and Dr. Scharf reported no conflicts of interest related to this work.
FROM JOURNAL OF CLINICAL SLEEP MEDICINE