User login
Ambulatory ECG monitoring in the age of smartphones
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
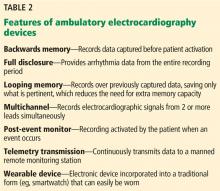
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
KEY POINTS
- Ambulatory ECG monitoring is commonly used to correlate symptoms with arrhythmia, confirm occult atrial fibrillation, and assess the efficacy of antiarrhythmic therapy.
- Devices have features such as access to the full monitoring time (“full disclosure”), extended monitoring, and telemetry, each with advantages and limitations.
- Consumer-oriented wearable devices are aimed at arrhythmia monitoring, which could lead to increased arrhythmia detection, but at the risk of more false-positive results and excessive use of healthcare resources.
A right atrial mass
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
PRIMARY HEART TUMORS ARE RARE
The most common neoplasms that metastasize to the heart are malignant melanoma, lymphoma, leukemia, breast, and lung cancers. The layers of the heart affected by malignant neoplasms in order of frequency from highest to lowest are the pericardium, epicardium, myocardium, and endocardium.3
MYXOMA: A PRIMARY CARDIAC TUMOR
The most common type of primary cardiac tumor is myxoma. Most—75% to 80%—occur in the left atrium, while 15% to 20% occur in the right atrium.5 Right atrial myxomas are usually found in the intraatrial septum at the border of the fossa ovalis.6 Myxomas can occur at any age, but are most common in women between the third and sixth decades.2
The cause of atrial myxomas is currently unknown. Most cases are sporadic. However, 10% are familial, with an autosomal-dominant pattern.7
The clinical symptoms of right atrial myxoma depend on the tumor’s size, location, and mobility and on the patient’s physical activity and body position.4 Common presenting symptoms include shortness of breath, pulmonary edema, cough, hemoptysis, and fatigue. Thirty percent of patients present with constitutional symptoms.4
Auscultation may reveal a characteristic “tumor plop” early in diastole.4,7 About 35% of patients have laboratory abnormalities such as elevations in erythrocyte sedimentation rate, C-reactive protein, and globulin levels and anemia. Our patient did not.
Embolization occurs in about 10% of cases of right-sided myxoma and can result in pulmonary artery embolism or a stroke. Pulmonary artery embolism occurs with myxoma embolization to the lungs. Strokes can occur in patients who have a patent foramen ovale or atrial septal defect, through which embolism to the systemic arterial circulation can occur.
The primary treatment for myxoma is complete resection of the tumor and its base with wide safety margins. This is particularly important to prevent recurrence of the myxoma and need for repeat operations, with their risk of surgical complications.9
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
- Dujardin KS, Click RL, Oh JK. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 2000; 13(12):1080–1083. pmid:11119275
- Jang KH, Shin DH, Lee C, Jang JK, Cheong S, Yoo SY. Left atrial mass with stalk: thrombus or myxoma? J Cardiovasc Ultrasound 2010; 18(4):154–156. doi:10.4250/jcu.2010.18.4.154
- Goldberg AD, Blankstein R, Padera RF. Tumors metastatic to the heart. Circulation 2013; 128(16):1790–1794. doi:10.1161/CIRCULATIONAHA.112.000790
- Aggarwal SK, Barik R, Sarma TC, et al. Clinical presentation and investigation findings in cardiac myxomas: new insights from the developing world. Am Heart J 2007; 154(6):1102–1107. doi:10.1016/j.ahj.2007.07.032
- Diaz A, Di Salvo C, Lawrence D, Hayward M. Left atrial and right ventricular myxoma: an uncommon presentation of a rare tumour. Interact Cardiovasc Thorac Surg 2011; 12(4):622–623. doi:10.1510/icvts.2010.255661
- Reynen K. Cardiac myxomas. N Engl J Med 1995; 333(24):1610–1617. doi:10.1056/NEJM199512143332407
- Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol 2011; 57(21):e371. doi:10.1016/j.jacc.2010.09.085
- Kassab R, Wehbe L, Badaoui G, el Asmar B, Jebara V, Ashoush R. Recurrent cerebrovascular accident: unusual and isolated manifestation of myxoma of the left atrium. J Med Liban 1999; 47(4):246–250. French. pmid:10641454
- Guhathakurta S, Riordan JP. Surgical treatment of right atrial myxoma. Tex Heart Inst J 2000; 27(1):61–63. pmid:10830633
Daily aspirin use may not improve CV outcomes in healthy elderly
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Short Takes
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Subset of patients benefits from in-hospital sleep apnea screening
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
EXPERT ANALYSIS FROM SLEEP 2019
What drives intensification of antihypertensive therapy at discharge?
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Revised CMS TAVR rules expected to widen access
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
The new National Coverage Determination by Medicare for transcatheter aortic valve replacement should produce a bump in the number of U.S. programs offering the procedure, especially with the Food and Drug Administration on the cusp of approving the procedure for low-risk patients.
In the revised National Coverage Determination (NCD) by the Centers for Medicare & Medicaid Services that went into effect on June 21, 2019, the agency allowed for Medicare coverage of transcatheter aortic valve (TAVR) procedures at hospitals that perform at least 20 of these procedures annually or at least 40 every 2 years, the same volume minimums that CMS first applied to TAVR in its prior 2012 NCD. Retention of this minimum ran against the 2018 proposal of the American College of Cardiology, the Society of Thoracic Surgeons, and two other collaborating societies that called for an annual TAVR volume minimum at a hospital program of 50 procedures annually or 100 every 2 years (J Am Coll Cardiol. 2019 Jan 29;73[3]:340-74).
That change, coupled with a cut in the minimum number of annual percutaneous coronary interventions a TAVR program needs to perform – newly revised to a minimum of 300 cases/year – will likely mean more U.S. sites performing TAVR, predicted James Vavricek, director of regulatory affairs for the ACC in Washington. TAVR volume is seen as a reasonable, approximate surrogate for a more rigorous, statistically adjusted assessment of program quality. The ACC and representatives from the other societies that collaborated on the 2018 statement used a 50 case/year minimum for a TAVR program because volume at that level generates enough outcomes data to allow for a meaningful, risk-adjusted measure of performance.
The ACC does not consider the minimum of 20 TAVR cases/year the “right decision,” Mr. Vavricek said in an interview, but the ACC sees it as a compromise that accommodated the interests of multiple TAVR stakeholders. “It will be interesting to see where new TAVR programs locate,” whether they will expand access in underserved regions or mostly cluster in regions already fairly replete with TAVR access, he added. Currently, over 600 U.S. TAVR programs are in operation.
In April 2019, the president of the ACC along with the presidents of three other U.S. societies with an interest in TAVR told the CMS in a comment letter that “we are extremely concerned that the proposed volume requirements will translate into a proliferation of low-volume TAVR programs at increased risk for having suboptimal outcomes.”
Another change to procedure volume requirements in the new NCD was setting a minimum of 100 total TAVR plus surgical aortic valve replacements in a 2-year period or 50 total procedures/year for each TAVR program. Setting a minimum that bundles TAVR plus surgical valve replacements is a “forward-looking” approach as wider application of TAVR gradually erodes the volume of surgical procedures, Mr. Vavricek said.
An additional notable change in the revised NCD was elimination of the “two-surgeon” rule, which the CMS had made mandatory for TAVR decisions until now, stipulating that a patient considered for TAVR needed independent assessment by two cardiac surgeons. The final 2019 NCD calls for the TAVR decision to come from one cardiac surgeon and one interventional cardiologist working together on a care team.
“The ACC is pleased to see CMS issue updated TAVR coverage criteria that emphasizes care by an interdisciplinary heart team for these complex patients, as well as continues to mandate the collection of TAVR patient data. With the new lowered minimum yearly volume criteria set by CMS in their efforts to improve patient access, the value of the STS/ACC TVT Registry, along with ACC’s Transcatheter Valve Certification, will be critical in assuring quality of care for our patients particularly in low-volume centers,” commented Richard J. Kovacs, MD, ACC’s president.
Aggressive lowering of LDL cholesterol: Is it a good idea?
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
SAN FRANCISCO – Powerful drugs now make it possible to lower LDL cholesterol levels to dramatically low levels. But is this a good idea? There are risks, and a cardiologist urged diabetes professionals to not overdo cholesterol reduction. But a colleague argued in favor of aggressively targeting “bad” cholesterol.
“We used to say you can’t be too rich or too thin. We now say you can’t be too rich or too thin or have a too-low LDL cholesterol,” said cardiologist Steven E. Nissen, MD, chairman of cardiovascular medicine at the Cleveland Clinic Foundation, who spoke at the annual scientific sessions of the American Diabetes Association about the wisdom of extreme LDL cholesterol lowering.
Dr. Nissen faced off in a debate with cardiologist Sanket Dhruva, MD, of the University of California, San Francisco, who doesn’t support aggressive LDL cholesterol lowering.
It is fine, Dr. Dhruva said, to treat patients so their LDL cholesterol levels drop below 100 mg/dL. “I don’t think there’s any argument there.”
But Dr. Dhruva questioned whether it’s a good idea to generally decrease LDL cholesterol well below 70 mg/dL, as is now possible with the use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
He pointed to a 2010 study that found aggressively lowering LDL cholesterol led to a mean net gain of 4.1 quality-adjusted life-years in high-risk patients, but less than 1 quality-adjusted life-year in low-risk patients. According to him, the study also found that the biggest benefits in both high- and low-risk patients came from the initial lower statin dose (Arch Intern Med. 2010 Jun 28;170[12]:1037-44).
“It’s really the statin initiation that provides the most benefit to our patients with diabetes,” Dr. Dhruva said.
Also, he added, a 2016 study questioned the value of aggressively lowering LDL cholesterol. It found that, although patients on statins with LDL cholesterol levels of 70-100 mg/dL had a lower risk of adverse cardiac outcomes than did those with levels between 100 and 130 mg/dL, no additional benefit was gained by achieving an LDL cholesterol level below 70 mg/dL (JAMA Intern Med. 2016 Aug 1;176[8]:1105-13)
As for risks, Dr. Dhruva highlighted a 2016 pooled analysis of 14 trials that linked the PCSK9 inhibitor alirocumab (Praluent) and LDL cholesterol levels below 25 mg/dL to significantly higher levels of cataracts, compared with levels of at least 25 mg/dL (hazard ratio, 3.4).
There are other reasons to be cautious of aggressive LDL cholesterol lowering. For one, many patients are not on statins when they’re prescribed PCSK-9 inhibitors. “We’re sometimes missing the building blocks before getting to expensive medications,” he said.
He added that PCSK-9 inhibitors are pricey, and some patients can’t get access to them. “Lipid control is incredibly important, but what about the stress or anxiety of our patients who are told this medication will reduce their cardiac risk but they can’t afford it? That’s not good for their cardiovascular risk.”
For his part, Dr. Nissen challenged Dr. Dhruva’s concerns about the cost of the drugs. “It’s not like they’re way out of line in terms of expense,” he said, noting that their cost – several thousand dollars a year – is similar to the cost of diabetes drugs known as glucagonlike peptide–1 receptor agonists and sodium-glucose transporter 2 inhibitors.
According to Dr. Nissen, multiple studies have supported aggressive LDL cholesterol lowering. “You’re going to see this over and over again in clinical trials: Every time we lower LDL by more, we get more reductions in morbidity and mortality.”
For example, he said, the FOURIER trial of the PCSK9 inhibitor evolocumab (Repatha) found that it lowered LDL cholesterol levels to a median 30 mg/dL “and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets [N Engl J Med 2017;376:1713-22].”
Dr. Nissen pointed to another study, this one also from 2017, that reported “in individuals with 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy.”
In regard to adverse effects, he said, research has hinted at a slight uptick in blood sugar levels “that does not take away the major cardiovascular benefits of the drugs.”
Overall, he said, “compelling evidence from trials in hundreds of thousands of patients demonstrates that reducing LDL cholesterol to very low levels reduces cardiovascular events in broad populations and is extremely safe.”
Dr. Nissen reported consulting for many pharmaceutical companies and performing clinical trials for Amgen, AbbVie, AstraZeneca, Cerenis Therapeutics, Esperion Therapeutics, Lilly, Novartis, Novo Nordisk, the Medicines Company, Orexigen Therapeutics, Takeda, and Pfizer. He does not receive income for honoraria, speaking fees, or consulting fees as they are paid directly to charity.
EXPERT ANALYSIS FROM ADA 2019
No cardiovascular benefit from vitamin D supplementation
There are no benefits from vitamin D supplementation in reducing the risk of major adverse cardiovascular events or all-cause mortality, according to a meta-analysis published in JAMA Cardiology.
Researchers analyzed data from 83,291 patients enrolled in 21 randomized, placebo-controlled clinical trials of at least 1 year of vitamin D supplementation.
They found the incidence of major adverse cardiovascular events was the same among patients taking vitamin D supplements and those taking placebo (risk ratio, 1; P = .85). Even stratifying by age, sex, postmenopausal status, pretreatment vitamin D levels, vitamin D dosage and formulation, chronic kidney disease, or excluding studies that used vitamin D analogues made no significant difference.
However, there was the suggestion of reduced incidence of major adverse cardiovascular events with vitamin D supplementation in individuals of advanced age, but the authors wrote that the finding should be interpreted with caution.
The analysis found no benefit from vitamin D supplementation on the secondary endpoints of MI, stroke, cardiovascular mortality, or all-cause mortality risk.
Mahmoud Barbarawi, MD, from the Hurley Medical Center at Michigan State University, East Lansing, and coauthors commented that previous observational studies have found significant associations between low vitamin D levels and cardiovascular events and all-cause mortality.
“However, observational studies are susceptible to uncontrolled confounding by outdoor physical activity, nutritional status, and prevalent chronic disease, which may influence serum 25-hydroxyvitamin D levels,” they wrote.
This updated analysis extended earlier clinical trial findings and added in some more-recent randomized trial outcomes, including the massive VITAL trial, which showed that neither daily vitamin D nor omega-3 fatty acids reduce cancer or cardiovascular event risk (N Engl J Med. 2019;380[1]:33-44).
Still, the authors noted that most of the trials included in the analysis had not prespecified cardiovascular disease as the primary endpoint and were underpowered to detect an effect on cardiovascular events. They also pointed out that few trials included data on heart failure, and a previous meta-analysis had suggested a potential benefit of supplementation in reducing the risk of this condition.
“Additional trials of higher-dose vitamin D supplementation, perhaps targeting members of older age groups and with attention to other [cardiovascular disease] endpoints such as heart failure, are of interest,” they wrote.
One author reported receiving funding from the National Institutes of Health and in-kind support from the pharmaceutical sector for a vitamin D study. No other disclosures were reported.
SOURCE: Barbarawi M et al. JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1870.
The past decade has seen a nearly 100-fold increase in vitamin D testing and supplementation, driven by a widespread fascination with the notion of vitamin D as a panacea. Vitamin D assessments alone are costing the United States an estimated $350 million annually.
Population and cohort studies have shown a clear link between vitamin D status and cardiovascular disease, but this link is complicated by the possibility that low serum 25-hydroxyvitamin D levels may be a result of, rather than the cause of, cardiovascular disease.
The findings of this meta-analysis, that vitamin D supplementation does not reduce the risk of major cardiovascular events and all-cause mortality, should support efforts to reduce unnecessary vitamin D testing and treatment in populations not at risk for deficiency or to prevent cardiovascular disease morbidity and mortality.
Arshed A. Quyyumi, MD, and Ibhar Al Mheid, MD, are from the division of cardiology at Emory University, Atlanta. The comments are adapted from an accompanying editorial (JAMA Cardiol. 2019 Jun 19. doi:10.1001/jamacardio.2019.1906). No conflicts of interest were reported.
The past decade has seen a nearly 100-fold increase in vitamin D testing and supplementation, driven by a widespread fascination with the notion of vitamin D as a panacea. Vitamin D assessments alone are costing the United States an estimated $350 million annually.
Population and cohort studies have shown a clear link between vitamin D status and cardiovascular disease, but this link is complicated by the possibility that low serum 25-hydroxyvitamin D levels may be a result of, rather than the cause of, cardiovascular disease.
The findings of this meta-analysis, that vitamin D supplementation does not reduce the risk of major cardiovascular events and all-cause mortality, should support efforts to reduce unnecessary vitamin D testing and treatment in populations not at risk for deficiency or to prevent cardiovascular disease morbidity and mortality.
Arshed A. Quyyumi, MD, and Ibhar Al Mheid, MD, are from the division of cardiology at Emory University, Atlanta. The comments are adapted from an accompanying editorial (JAMA Cardiol. 2019 Jun 19. doi:10.1001/jamacardio.2019.1906). No conflicts of interest were reported.
The past decade has seen a nearly 100-fold increase in vitamin D testing and supplementation, driven by a widespread fascination with the notion of vitamin D as a panacea. Vitamin D assessments alone are costing the United States an estimated $350 million annually.
Population and cohort studies have shown a clear link between vitamin D status and cardiovascular disease, but this link is complicated by the possibility that low serum 25-hydroxyvitamin D levels may be a result of, rather than the cause of, cardiovascular disease.
The findings of this meta-analysis, that vitamin D supplementation does not reduce the risk of major cardiovascular events and all-cause mortality, should support efforts to reduce unnecessary vitamin D testing and treatment in populations not at risk for deficiency or to prevent cardiovascular disease morbidity and mortality.
Arshed A. Quyyumi, MD, and Ibhar Al Mheid, MD, are from the division of cardiology at Emory University, Atlanta. The comments are adapted from an accompanying editorial (JAMA Cardiol. 2019 Jun 19. doi:10.1001/jamacardio.2019.1906). No conflicts of interest were reported.
There are no benefits from vitamin D supplementation in reducing the risk of major adverse cardiovascular events or all-cause mortality, according to a meta-analysis published in JAMA Cardiology.
Researchers analyzed data from 83,291 patients enrolled in 21 randomized, placebo-controlled clinical trials of at least 1 year of vitamin D supplementation.
They found the incidence of major adverse cardiovascular events was the same among patients taking vitamin D supplements and those taking placebo (risk ratio, 1; P = .85). Even stratifying by age, sex, postmenopausal status, pretreatment vitamin D levels, vitamin D dosage and formulation, chronic kidney disease, or excluding studies that used vitamin D analogues made no significant difference.
However, there was the suggestion of reduced incidence of major adverse cardiovascular events with vitamin D supplementation in individuals of advanced age, but the authors wrote that the finding should be interpreted with caution.
The analysis found no benefit from vitamin D supplementation on the secondary endpoints of MI, stroke, cardiovascular mortality, or all-cause mortality risk.
Mahmoud Barbarawi, MD, from the Hurley Medical Center at Michigan State University, East Lansing, and coauthors commented that previous observational studies have found significant associations between low vitamin D levels and cardiovascular events and all-cause mortality.
“However, observational studies are susceptible to uncontrolled confounding by outdoor physical activity, nutritional status, and prevalent chronic disease, which may influence serum 25-hydroxyvitamin D levels,” they wrote.
This updated analysis extended earlier clinical trial findings and added in some more-recent randomized trial outcomes, including the massive VITAL trial, which showed that neither daily vitamin D nor omega-3 fatty acids reduce cancer or cardiovascular event risk (N Engl J Med. 2019;380[1]:33-44).
Still, the authors noted that most of the trials included in the analysis had not prespecified cardiovascular disease as the primary endpoint and were underpowered to detect an effect on cardiovascular events. They also pointed out that few trials included data on heart failure, and a previous meta-analysis had suggested a potential benefit of supplementation in reducing the risk of this condition.
“Additional trials of higher-dose vitamin D supplementation, perhaps targeting members of older age groups and with attention to other [cardiovascular disease] endpoints such as heart failure, are of interest,” they wrote.
One author reported receiving funding from the National Institutes of Health and in-kind support from the pharmaceutical sector for a vitamin D study. No other disclosures were reported.
SOURCE: Barbarawi M et al. JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1870.
There are no benefits from vitamin D supplementation in reducing the risk of major adverse cardiovascular events or all-cause mortality, according to a meta-analysis published in JAMA Cardiology.
Researchers analyzed data from 83,291 patients enrolled in 21 randomized, placebo-controlled clinical trials of at least 1 year of vitamin D supplementation.
They found the incidence of major adverse cardiovascular events was the same among patients taking vitamin D supplements and those taking placebo (risk ratio, 1; P = .85). Even stratifying by age, sex, postmenopausal status, pretreatment vitamin D levels, vitamin D dosage and formulation, chronic kidney disease, or excluding studies that used vitamin D analogues made no significant difference.
However, there was the suggestion of reduced incidence of major adverse cardiovascular events with vitamin D supplementation in individuals of advanced age, but the authors wrote that the finding should be interpreted with caution.
The analysis found no benefit from vitamin D supplementation on the secondary endpoints of MI, stroke, cardiovascular mortality, or all-cause mortality risk.
Mahmoud Barbarawi, MD, from the Hurley Medical Center at Michigan State University, East Lansing, and coauthors commented that previous observational studies have found significant associations between low vitamin D levels and cardiovascular events and all-cause mortality.
“However, observational studies are susceptible to uncontrolled confounding by outdoor physical activity, nutritional status, and prevalent chronic disease, which may influence serum 25-hydroxyvitamin D levels,” they wrote.
This updated analysis extended earlier clinical trial findings and added in some more-recent randomized trial outcomes, including the massive VITAL trial, which showed that neither daily vitamin D nor omega-3 fatty acids reduce cancer or cardiovascular event risk (N Engl J Med. 2019;380[1]:33-44).
Still, the authors noted that most of the trials included in the analysis had not prespecified cardiovascular disease as the primary endpoint and were underpowered to detect an effect on cardiovascular events. They also pointed out that few trials included data on heart failure, and a previous meta-analysis had suggested a potential benefit of supplementation in reducing the risk of this condition.
“Additional trials of higher-dose vitamin D supplementation, perhaps targeting members of older age groups and with attention to other [cardiovascular disease] endpoints such as heart failure, are of interest,” they wrote.
One author reported receiving funding from the National Institutes of Health and in-kind support from the pharmaceutical sector for a vitamin D study. No other disclosures were reported.
SOURCE: Barbarawi M et al. JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1870.
FROM JAMA CARDIOLOGY
Cognitive decline sped up after CHD
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
The findings “highlight the role of cardiovascular risk factors and cardiovascular health as crucial determinants of cognitive trajectories in later life,” wrote Suvi P. Rovio, PhD; Katja Pahkala, PhD; and Olli T. Raitakari, MD, PhD. For example, accelerated declines in verbal memory might indicate a specific vulnerability to vascular changes within the medial temporal lobe and hippocampus.
The fact that cognitive decline did not accelerate immediately after coronary heart disease suggests that CHD itself does not acutely alter the brain, such as by causing microinfarcts, they commented. Instead, CHD might induce longer-term shifts in cerebral vascular function by affecting the blood-brain barrier or perfusion and oxidation in the brain. While these complex relationships need further untangling, the study suggests interventions that cut CHD risk also might help prevent cognitive decline itself and slow the rate of cognitive decline if it occurs.
Dr. Rovio, Dr. Pahkala, and Dr. Raitakari are at the University of Turku (Finland) and Turku University Hospital. These comments are adapted from an editorial accompanying the article by Xie et al. (J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.020). They reported having no relevant financial disclosures.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
according to the results of a large prospective study with a median of 12 years of follow-up.
“We found that incident CHD was significantly associated with faster post–CHD-diagnosis cognitive decline, but not pre–CHD-diagnosis or short-term cognitive decline after the event,” Wuxiang Xie, PhD, of Peking University Health Science Center, Beijing, and associates wrote in the Journal of the American College of Cardiology. Linear mixed models showed that cognitive decline sped up during the year after incident CHD.
Past research had suggested a link between accelerated cognitive decline and CHD, but the temporal pattern of the relationship was unclear. For the study, Dr. Xie and associates followed 7,888 adults from the English Longitudinal Study of Aging who were an average of 62 years old and had no history of stroke, MI, angina, or dementia (Alzheimer’s disease or otherwise). All participants underwent a baseline cognitive assessment for verbal memory, semantic fluency, and temporal orientation, plus a median of six follow-up assessments.
In all, 480 (6%) participants developed CHD during follow-up. Their rate of cognitive decline remained constant before and immediately after their CHD diagnosis, but in subsequent years, they experienced significant accelerations in loss of global cognitive function, verbal memory, and temporal orientation even after accounting for time and many demographic and clinical variables. For example, the slope representing temporal change in global cognitive score decreased by a mean of 0.039 per year, compared with the pre-CHD slope (slope difference, –0.039; 95% confidence interval, –0.063 to –0.015; P =. 002). Semantic fluency also declined faster after CHD, but the difference, compared with before CHD, did not reach statistical significance (P = .11).
Individuals without CHD showed no such accelerations in cognitive decline throughout follow-up in adjusted models, the researchers wrote. “Based on repeated cognitive measurements over a long follow-up period, this study revealed a reliable and robust trajectory of cognitive decline [after CHD]. Future studies are warranted to determine the precise mechanisms linking incident CHD to cognitive decline.”
Funders included the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Newton International Fellowship from the Academy of Medical Sciences. The researchers reported having no relevant financial disclosures.
SOURCE: Xie W et al. J Amer Coll Cardiol. 2019 Jun 17. doi: 10.1016/j.jacc.2019.04.019.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY