User login
FDA Approves IL-31 Inhibitor for Atopic Dermatitis
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
according to a press release from the manufacturer, Galderma.
Nemolizumab (Nemluvio), a monoclonal antibody administered subcutaneously, targets the interleukin (IL)–31 receptor. IL-31 is known to promote itching and inflammation in atopic dermatitis, according to the company.
Approval was based on data from the phase 3 ARCADIA 1 and ARCADIA 2 clinical trials, recently published in The Lancet, which included 1728 patients aged 12 years and older with moderate to severe atopic dermatitis and pruritus who had an inadequate response to topical steroids.
At week 16, significantly more patients randomized to nemolizumab every 4 weeks met the co-primary endpoints, compared with those taking placebo. The co-primary endpoints were an Investigator Global Assessment (IGA) score of 0 (clear skin) or 1 (almost clear skin), with an improvement of at least 2 points from baseline to 16 weeks, and an improvement of at least 75% on the Eczema Area and Severity Index score from baseline to 16 weeks (EASI-75 response). All patients in both trials also received background treatment with topical corticosteroids and/or topical calcineurin inhibitors.
At 16 weeks, 36% and 38% of patients taking nemolizumab met the IGA criteria in ARCADIA 1 and ARCADIA 2, respectively, compared with 25% and 26% of those taking placebo. Similarly, 44% and 42% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, achieved EASI-75, compared with 29% and 30% of those taking placebo. Differences between treatment and placebo groups were significant in both studies.
In addition, patients reported significant improvement in all key secondary endpoints, including itch, as early as week 1, and improvement in sleep by week 16, according to the study findings.
Safety profiles were similar between the treatment and placebo groups in both studies; the most common adverse reactions (reported by at least 1% of patients in each group) were headache (5% vs 4%), followed by arthralgia, urticaria, and myalgia (2% or less). In ARCADIA 1 and ARCADIA 2, 50% and 41% of patients taking nemolizumab reported at least one treatment-emergent adverse event, similar to the placebo groups (45% and 44%, respectively).
Serious treatment-emergent adverse events occurred in 1% and 3% of those taking nemolizumab in ARCADIA 1 and ARCADIA 2, respectively, and 1% in the placebo groups in both studies. Ten serious treatment-emergent adverse events potentially related to nemolizumab were reported in five patients in ARCADIA 2. No deaths were reported in either study.
According to the prescribing information, safety profiles were similar between treatment and placebo groups in the subset of adolescents aged 12-17 years.
In August 2024, the FDA approved nemolizumab for the treatment of prurigo nodularis in adults. Authorization applications for nemolizumab for atopic dermatitis and prurigo nodularis are under review by regulatory authorities in Australia, Singapore, Switzerland, Canada, Brazil, and South Korea, according to Galderma.
ARCADIA is funded by Galderma.
A version of this article first appeared on Medscape.com.
Wound Healing: Dermatologist’s Toolbox Requires Frequent Updates
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
NEW YORK CITY — Instructions on wound healing often involve disturbing photographs of severe diabetic ulcers, angry autoimmune blistering, and oozing lesions produced by uncommon genetic disorders, but whether or not they are dramatic, day-to-day dermatologic wound care relies on both the basics as well as novel approaches, according to a well-known wound treatment expert.
, director of the Wound Clinic at Jackson Memorial Hospital and chair of the Department of Dermatology and Cutaneous Surgery at the University of Miami, Florida.
“We as a specialty make and repair more wounds than any other specialty,” said Kirsner, who provided data to make his point. In a table he showed, the number of wound repairs made annually by dermatologists was several-fold higher than surgeons, the next highest group, and the numbers declined rapidly from there.
Speaking at the 27th Annual Winter Symposium – Advances in Medical and Surgical Dermatology (MSWS) 2024, Kirsner offered an array of clinical pearls, reinforced some basics, and pointed to well-supported strategies he believes are too often overlooked.
Drugs Repurposed for Wound Healing
Of the clinical pearls, he spoke of the repurposing of several agents for wound care. His first example was the monoclonal antibody dupilumab, which inhibits interleukin-4 (IL-4) and IL-13 signaling, to heal selected patients with leg ulcers. The potential of this drug for wound healing was based on a patient with a leg ulcer who presented with concomitant prurigo nodularis and biliary cirrhosis. When offered for the comorbidities, dupilumab provided a “dramatic” benefit with regard to the wound, according to Kirsner.
The explanation for the response is that IL-4 and IL-13 have been found to be upregulated in some patients with leg ulcers. Based on numerous cases, Kirsner spoke of a phenotype of nonhealing leg ulcers from which elevated IL-4 and IL-13 can be isolated; these are the candidates for adding dupilumab to wound care, he said.
Topical beta-blockade is another example of a therapy repurposed for wound healing, according to Kirsner. He said beta-blockers are already a standard of care for burn wounds, but the mechanism is relevant in other wound types.
Several studies have looked at this phenomenon, with experimental studies showing that skin healing is impaired when beta-2 receptors are agonized but accelerated when blocked.
Beta-Blockade Accelerates Wound Healing
A recent review of these mechanisms in soft-tissue wound healing pointed to an anti-inflammatory effect, acceleration of keratinocyte migration, pro-reepithelization effects, and inhibition of bacterial virulence. Beta-blockers were first implicated as mediators of wound healing more than a decade ago, but Kirsner indicated that there is now more attention to this therapy within a comprehensive approach in difficult cases.
Although not specific to wound healing, the potential for teprotumumab to improve control of pretibial myxedema is another example of a repurposed therapy for a challenging skin disease. Teprotumumab, a monoclonal antibody that targets the insulin-like growth factor-1 (IGF-1) receptor, is approved for active thyroid eye disease, but Kirsner cited data showing compelling evidence of benefit in this cutaneous complication of Graves disease.
As for basics, Kirsner devoted some time to emphasizing the importance of compression therapy for improving leg vascularization. This is not something to just consider; rather, he thinks it is part of standard practice. “Compress all leg ulcers,” was Kirsner’s simple message.
Citing encouraging work in identifying targetable molecular events in wound healing, Kirsner suggested that treatment might be increasingly guided by biomarkers. He pointed to ongoing work to characterize wound exudate as a source of biomarkers.
“The discarded dressing contains a wealth of information,” he said, referring to cell types and proteins, such as growth factors. He thinks that the ongoing studies of exudate, which have shown that molecular processes detected at the periphery are often different than those at the focal site of injury, have substantial promise for identifying new treatment targets.
Virtual Reality to Address Pain
From a practical standpoint, Kirsner looked to a well-studied but still underused adjunct to wound debridement and surgical repair: the distraction offered by relatively low-priced virtual reality systems. He described it as a simple way to help patients keep their minds off the pain. It is not a new idea and has been studied for this use numerous times, and the evidence of benefit is essentially uniform, according to Kirsner.
He said effective and sophisticated systems can now be purchased for just hundreds of dollars, and no training is needed. Indeed, he said pediatric patients can typically explain how the system works if the clinician does not know.
“If you can enhance their experience [during wound repair], you can make their lives and your life better,” he said.
Joshua Zeichner, MD, associate professor of dermatology at Mount Sinai Hospital in New York City, concurred that the evidence supports this approach and is easy to do. “I am in favor of anything that improves the experience of the patient,” said Zeichner, who chaired the portion of the meeting during which Kirsner spoke.
Kirsner said he practices what he preaches. “I routinely employ virtual reality for simple surgical procedures or processes that patients might find unpleasant,” he said. He acknowledged that clinicians might have heard this message before, but he believes those who have not yet introduced this into their practice should consider it.
Kirsner has reported no relevant financial relationships. Zeichner has reported serving as a consultant for Beiersdorf.
A version of this article first appeared on Medscape.com.
FROM MSWS 2024
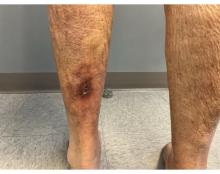
An 81-Year-Old White Woman Presented With a 2-Week History of a Painful Lesion on Her Left Calf
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
An 81-year-old White woman with a medical history significant for end stage renal disease (ESRD) on dialysis, diabetes, and a cerebrovascular accident presented with a 2-week history of a very painful lesion on her left calf. Upon physical exam, she was also noted to have tender subcutaneous nodules on her left anterolateral thigh that had been present for several weeks.
What's your diagnosis?
Melanoma: Study Addresses Impact of Indoor Tanning on Tumor Mutational Burden
TOPLINE:
in a retrospective cohort study. Higher TMB was linked to older age, head and neck tumors, and a history of nonmelanoma skin cancer (NMSC).
METHODOLOGY:
- Researchers conducted a retrospective cohort study at a tertiary care cancer center between 2013 and 2022.
- A total of 617 patients (median age at diagnosis, 61 years; 62.9% men) with melanoma who had next-generation sequencing data and indoor tanning bed exposure history available were included.
- Analysis involved multivariable modeling to evaluate the association between tanning bed use and TMB.
- Patients’ demographics, pathologic staging, TMB, and dermatologic history, including Fitzpatrick skin type, history of exposure to ultraviolet (UV) light, indoor tanning, NMSC, atypical nevi, and blistering sunburns, were considered for the analysis.
TAKEAWAY:
- About 22% of participants had an indoor tanning history. Indoor tanning exposure showed no association with TMB after adjustment for all possible predictors.
- A significant association was found between TMB and age at diagnosis, primary melanoma site, and history of NMSC (P < .001 for all).
- Patients with a history of atypical nevi demonstrated a significantly lower TMB than those without (log2 TMB, 3.89 vs 4.15; P = .01).
- Tumors of the head and neck exhibited a significantly higher TMB than those occurring in other primary sites, while skin-localized melanomas at diagnosis showed a significantly higher TMB than node-positive or metastatic stage III or IV tumors (log2 TMB, 3.88 vs 3.48; P = .005).
IN PRACTICE:
“Despite the known association between indoor tanning and melanoma risk,” the study did not find an association between indoor tanning and melanoma TMB, which “suggests that cumulative lifetime sun exposure may be a greater primary driver of TMB than intermittent radiation during indoor tanning,” the authors of the study wrote.
SOURCE:
The study was led by Grace B. Hanrahan, BA, of the Center for Melanoma Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, and was published online on December 11 in JAMA Dermatology.
LIMITATIONS:
The study was conducted at a tertiary referral center, potentially representing a higher-risk subset with more advanced disease than the broader population. Additionally, the retrospective collection of UV exposure history, including indoor tanning and blistering sunburns, may have introduced recall bias.
DISCLOSURES:
The authors did not disclose any funding information. No conflicts of interest were reported.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective cohort study. Higher TMB was linked to older age, head and neck tumors, and a history of nonmelanoma skin cancer (NMSC).
METHODOLOGY:
- Researchers conducted a retrospective cohort study at a tertiary care cancer center between 2013 and 2022.
- A total of 617 patients (median age at diagnosis, 61 years; 62.9% men) with melanoma who had next-generation sequencing data and indoor tanning bed exposure history available were included.
- Analysis involved multivariable modeling to evaluate the association between tanning bed use and TMB.
- Patients’ demographics, pathologic staging, TMB, and dermatologic history, including Fitzpatrick skin type, history of exposure to ultraviolet (UV) light, indoor tanning, NMSC, atypical nevi, and blistering sunburns, were considered for the analysis.
TAKEAWAY:
- About 22% of participants had an indoor tanning history. Indoor tanning exposure showed no association with TMB after adjustment for all possible predictors.
- A significant association was found between TMB and age at diagnosis, primary melanoma site, and history of NMSC (P < .001 for all).
- Patients with a history of atypical nevi demonstrated a significantly lower TMB than those without (log2 TMB, 3.89 vs 4.15; P = .01).
- Tumors of the head and neck exhibited a significantly higher TMB than those occurring in other primary sites, while skin-localized melanomas at diagnosis showed a significantly higher TMB than node-positive or metastatic stage III or IV tumors (log2 TMB, 3.88 vs 3.48; P = .005).
IN PRACTICE:
“Despite the known association between indoor tanning and melanoma risk,” the study did not find an association between indoor tanning and melanoma TMB, which “suggests that cumulative lifetime sun exposure may be a greater primary driver of TMB than intermittent radiation during indoor tanning,” the authors of the study wrote.
SOURCE:
The study was led by Grace B. Hanrahan, BA, of the Center for Melanoma Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, and was published online on December 11 in JAMA Dermatology.
LIMITATIONS:
The study was conducted at a tertiary referral center, potentially representing a higher-risk subset with more advanced disease than the broader population. Additionally, the retrospective collection of UV exposure history, including indoor tanning and blistering sunburns, may have introduced recall bias.
DISCLOSURES:
The authors did not disclose any funding information. No conflicts of interest were reported.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective cohort study. Higher TMB was linked to older age, head and neck tumors, and a history of nonmelanoma skin cancer (NMSC).
METHODOLOGY:
- Researchers conducted a retrospective cohort study at a tertiary care cancer center between 2013 and 2022.
- A total of 617 patients (median age at diagnosis, 61 years; 62.9% men) with melanoma who had next-generation sequencing data and indoor tanning bed exposure history available were included.
- Analysis involved multivariable modeling to evaluate the association between tanning bed use and TMB.
- Patients’ demographics, pathologic staging, TMB, and dermatologic history, including Fitzpatrick skin type, history of exposure to ultraviolet (UV) light, indoor tanning, NMSC, atypical nevi, and blistering sunburns, were considered for the analysis.
TAKEAWAY:
- About 22% of participants had an indoor tanning history. Indoor tanning exposure showed no association with TMB after adjustment for all possible predictors.
- A significant association was found between TMB and age at diagnosis, primary melanoma site, and history of NMSC (P < .001 for all).
- Patients with a history of atypical nevi demonstrated a significantly lower TMB than those without (log2 TMB, 3.89 vs 4.15; P = .01).
- Tumors of the head and neck exhibited a significantly higher TMB than those occurring in other primary sites, while skin-localized melanomas at diagnosis showed a significantly higher TMB than node-positive or metastatic stage III or IV tumors (log2 TMB, 3.88 vs 3.48; P = .005).
IN PRACTICE:
“Despite the known association between indoor tanning and melanoma risk,” the study did not find an association between indoor tanning and melanoma TMB, which “suggests that cumulative lifetime sun exposure may be a greater primary driver of TMB than intermittent radiation during indoor tanning,” the authors of the study wrote.
SOURCE:
The study was led by Grace B. Hanrahan, BA, of the Center for Melanoma Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, and was published online on December 11 in JAMA Dermatology.
LIMITATIONS:
The study was conducted at a tertiary referral center, potentially representing a higher-risk subset with more advanced disease than the broader population. Additionally, the retrospective collection of UV exposure history, including indoor tanning and blistering sunburns, may have introduced recall bias.
DISCLOSURES:
The authors did not disclose any funding information. No conflicts of interest were reported.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Australia Registry Study: Melanoma-Related Deaths Increase at 0.8-mm Breslow Thickness
TOPLINE:
in an Australian study that used registry data.
METHODOLOGY:
- The study analyzed 144,447 individuals (median age, 56 years, 54% men) diagnosed with thin (T1) primary invasive melanomas (Breslow thickness, ≤ 1.0 mm) between 1982 and 2014 from all eight Australian state and territory population-based cancer registries.
- The researchers evaluated the associations between Breslow thickness (< 0.8 mm vs 0.8-1.0 mm) and incidences of melanoma-related and nonmelanoma-related deaths.
- The primary endpoint was time to death attributable to a melanoma-related cause, with death by a nonmelanoma-related cause as a competing event.
TAKEAWAY:
- The 20-year cumulative incidence of melanoma-related deaths was 6.3% for the whole cohort. The incidence was higher for tumors with a thickness of 0.8-1.0 mm (11%) than for those with a thickness < 0.8 mm (5.6%).
- The overall 20-year melanoma-specific survival rate was 95.9%, with rates of 94.2% for tumors < 0.8 mm and 87.8% for tumors measuring 0.8-1.0 mm in thickness. Each 0.1-mm increase in Breslow thickness was associated with worse prognosis.
- A multivariable analysis revealed that a tumor thickness of 0.8-1.0 mm was associated with both a greater absolute risk for melanoma-related deaths (subdistribution hazard ratio, 2.92) and a higher rate of melanoma-related deaths (hazard ratio, 2.98) than a tumor thickness < 0.8 mm.
- The 20-year incidence of death from nonmelanoma-related causes was 23.4%, but the risk for death from these causes showed no significant association with Breslow thickness categories.
IN PRACTICE:
“The findings of this large-scale population–based analysis suggest the separation of risk for patients with melanomas with a Breslow thickness above and below 0.8 mm,” the authors wrote, adding: “These results suggest that a change of the T1 threshold from 1.0 mm to 0.8 mm should be considered when the AJCC [American Joint Committee on Cancer] staging system is next reviewed.”
SOURCE:
The study was led by Serigne N. Lo, PhD, Melanoma Institute Australia, the University of Sydney. It was published online on December 11, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was registry-based and did not capture details such as tumor characteristics and treatment modalities. Inaccuracies in reporting the cause of death may have led to an underestimation of melanoma-specific mortality risks across all thickness groups and an overestimation of nonmelanoma mortality risks.
DISCLOSURES:
The study received funding support from Melanoma Institute Australia and two grants from the Australian National Health and Medical Research Council (NHMRC). Several authors reported receiving grants or personal fees from or having ties with various sources, including NHMRC.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in an Australian study that used registry data.
METHODOLOGY:
- The study analyzed 144,447 individuals (median age, 56 years, 54% men) diagnosed with thin (T1) primary invasive melanomas (Breslow thickness, ≤ 1.0 mm) between 1982 and 2014 from all eight Australian state and territory population-based cancer registries.
- The researchers evaluated the associations between Breslow thickness (< 0.8 mm vs 0.8-1.0 mm) and incidences of melanoma-related and nonmelanoma-related deaths.
- The primary endpoint was time to death attributable to a melanoma-related cause, with death by a nonmelanoma-related cause as a competing event.
TAKEAWAY:
- The 20-year cumulative incidence of melanoma-related deaths was 6.3% for the whole cohort. The incidence was higher for tumors with a thickness of 0.8-1.0 mm (11%) than for those with a thickness < 0.8 mm (5.6%).
- The overall 20-year melanoma-specific survival rate was 95.9%, with rates of 94.2% for tumors < 0.8 mm and 87.8% for tumors measuring 0.8-1.0 mm in thickness. Each 0.1-mm increase in Breslow thickness was associated with worse prognosis.
- A multivariable analysis revealed that a tumor thickness of 0.8-1.0 mm was associated with both a greater absolute risk for melanoma-related deaths (subdistribution hazard ratio, 2.92) and a higher rate of melanoma-related deaths (hazard ratio, 2.98) than a tumor thickness < 0.8 mm.
- The 20-year incidence of death from nonmelanoma-related causes was 23.4%, but the risk for death from these causes showed no significant association with Breslow thickness categories.
IN PRACTICE:
“The findings of this large-scale population–based analysis suggest the separation of risk for patients with melanomas with a Breslow thickness above and below 0.8 mm,” the authors wrote, adding: “These results suggest that a change of the T1 threshold from 1.0 mm to 0.8 mm should be considered when the AJCC [American Joint Committee on Cancer] staging system is next reviewed.”
SOURCE:
The study was led by Serigne N. Lo, PhD, Melanoma Institute Australia, the University of Sydney. It was published online on December 11, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was registry-based and did not capture details such as tumor characteristics and treatment modalities. Inaccuracies in reporting the cause of death may have led to an underestimation of melanoma-specific mortality risks across all thickness groups and an overestimation of nonmelanoma mortality risks.
DISCLOSURES:
The study received funding support from Melanoma Institute Australia and two grants from the Australian National Health and Medical Research Council (NHMRC). Several authors reported receiving grants or personal fees from or having ties with various sources, including NHMRC.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in an Australian study that used registry data.
METHODOLOGY:
- The study analyzed 144,447 individuals (median age, 56 years, 54% men) diagnosed with thin (T1) primary invasive melanomas (Breslow thickness, ≤ 1.0 mm) between 1982 and 2014 from all eight Australian state and territory population-based cancer registries.
- The researchers evaluated the associations between Breslow thickness (< 0.8 mm vs 0.8-1.0 mm) and incidences of melanoma-related and nonmelanoma-related deaths.
- The primary endpoint was time to death attributable to a melanoma-related cause, with death by a nonmelanoma-related cause as a competing event.
TAKEAWAY:
- The 20-year cumulative incidence of melanoma-related deaths was 6.3% for the whole cohort. The incidence was higher for tumors with a thickness of 0.8-1.0 mm (11%) than for those with a thickness < 0.8 mm (5.6%).
- The overall 20-year melanoma-specific survival rate was 95.9%, with rates of 94.2% for tumors < 0.8 mm and 87.8% for tumors measuring 0.8-1.0 mm in thickness. Each 0.1-mm increase in Breslow thickness was associated with worse prognosis.
- A multivariable analysis revealed that a tumor thickness of 0.8-1.0 mm was associated with both a greater absolute risk for melanoma-related deaths (subdistribution hazard ratio, 2.92) and a higher rate of melanoma-related deaths (hazard ratio, 2.98) than a tumor thickness < 0.8 mm.
- The 20-year incidence of death from nonmelanoma-related causes was 23.4%, but the risk for death from these causes showed no significant association with Breslow thickness categories.
IN PRACTICE:
“The findings of this large-scale population–based analysis suggest the separation of risk for patients with melanomas with a Breslow thickness above and below 0.8 mm,” the authors wrote, adding: “These results suggest that a change of the T1 threshold from 1.0 mm to 0.8 mm should be considered when the AJCC [American Joint Committee on Cancer] staging system is next reviewed.”
SOURCE:
The study was led by Serigne N. Lo, PhD, Melanoma Institute Australia, the University of Sydney. It was published online on December 11, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was registry-based and did not capture details such as tumor characteristics and treatment modalities. Inaccuracies in reporting the cause of death may have led to an underestimation of melanoma-specific mortality risks across all thickness groups and an overestimation of nonmelanoma mortality risks.
DISCLOSURES:
The study received funding support from Melanoma Institute Australia and two grants from the Australian National Health and Medical Research Council (NHMRC). Several authors reported receiving grants or personal fees from or having ties with various sources, including NHMRC.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Geriatric Dermatology: Q&A With Daniel C. Butler, MD
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Skin Stress Biomarker May Predict Nerve Damage in Early T2D
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Increased cutaneous carbonyl stress is linked to slower nerve conduction in patients with metabolically well-controlled, recent-onset type 2 diabetes (T2D) and can predict the development of neuropathic deficits over 5 years.
METHODOLOGY:
- Accumulation of advanced glycation end products (AGEs), which results from endogenous carbonyl stress, may be a potential target for preventing and treating the diabetic sensorimotor polyneuropathy (DSPN) that is a common complication of T2D.
- Researchers investigated novel cutaneous biomarkers for the development and progression of DSPN in 160 individuals with recent-onset T2D (diagnosed within 12 months or less) and 144 individuals with normal glucose tolerance, all recruited consecutively from the German Diabetes Study baseline cohort.
- Peripheral nerve function was assessed through nerve conduction studies, quantitative sensory testing, and clinical neuropathy scores.
- Skin biopsies were used to analyze intraepidermal nerve fiber density, endothelial integrity, cutaneous oxidative stress markers, and cutaneous carbonyl stress markers, including AGE autofluorescence and argpyrimidine area.
- Skin autofluorescence was measured noninvasively using an AGE reader device.
- A subgroup of 80 patients with T2D were reassessed after 5 years to evaluate the progression of neurophysiological deficits.
TAKEAWAY:
- Patients with recent-onset T2D had greater AGE autofluorescence and argpyrimidine area (P ≤ .05 for both) and lower nerve fiber density (P ≤ .05) than individuals with normal glucose tolerance.
- In patients with T2D, AGE autofluorescence was inversely associated with nerve conduction (P = .0002, P = .002, and P = .001 for peroneal motor, median motor, and sural sensory nerve conduction velocity, respectively) and positively associated with AGE reader measurements (P < .05); no such associations were observed in those with normal glucose tolerance.
- In the prospective T2D cohort, associations were noted between cutaneous markers for AGEs and endothelial cells at baseline and changes in nerve function indices over a 5-year period.
IN PRACTICE:
“Prospective analyses revealed some predictive value of cutaneous AGEs and lower endothelial integrity for declining nerve function, supporting the role of carbonyl stress in the development and progression of DSPN, representing a potential therapeutic target,” the authors wrote.
SOURCE:
The study was led by Gidon J. Bönhof, Department of Endocrinology and Diabetology, Medical Faculty, University Hospital Düsseldorf, Heinrich Heine University Düsseldorf, Düsseldorf, Germany. It was published online in Diabetes Care.
LIMITATIONS:
The observational design of the study limited the ability to draw causal conclusions. The groups were not matched for age or body mass index. Various mechanisms related to DSPN were analyzed; however, specific pathways of AGEs were not studied in detail. The relatively low number of individuals with clinically manifested DSPN limited the exploration of different stages of the condition.
DISCLOSURES:
The study was supported by a German Center for Diabetes Research grant. The German Diabetes Study was supported by the German Diabetes Center funded by the German Federal Ministry of Health (Berlin), the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia (Düsseldorf, Germany), and grants from the German Federal Ministry of Education and Research to the German Center for Diabetes Research e.V. No relevant conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Acne Outcome Measures: Do they Incorporate LGBTQ+ Inclusive Language?
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Cutaneous Lupus Associated with Greater Risk for Atherosclerotic Cardiovascular Disease
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Childhood Atopic Dermatitis Doesn’t Delay Puberty
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators conducted a nationwide cohort study among 15,534 children in Denmark whose pubertal development was assessed every 6 months with a web-based questionnaire starting at the age of 11 years.
- The children were classified into three groups: No atopic dermatitis; self-reported doctor-diagnosed atopic dermatitis (maternal report of a doctor diagnosis at 6 months, 18 months, and/or 7 years of age); hospital-diagnosed atopic dermatitis (registry data showing it as the primary reason for hospital contact up to the age of 8 years), representing mainly severe cases.
- The main outcome was the age difference averaged across a range of pubertal milestones (attainment of Tanner stages; development of axillary hair, acne, and voice break; and occurrence of first ejaculation and menarche).
TAKEAWAY:
- Overall, 21.5% of the children had self-reported doctor-diagnosed atopic dermatitis and 0.7% had hospital-diagnosed atopic dermatitis.
- Relative to girls without atopic dermatitis, girls with self-reported doctor-diagnosed atopic dermatitis reached the milestones at the same age, with a mean difference of 0.0 months, and girls with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- Relative to boys without atopic dermatitis, boys with self-reported doctor-diagnosed atopic dermatitis reached the milestones a mean of 0.1 month later and boys with hospital-diagnosed atopic dermatitis reached them a mean of 0.3 months earlier.
- A more stringent definition of atopic dermatitis — persistent or recurrent atopic dermatitis at 7 years of age (assumed more likely to affect sleep and disrupt the skin barrier near the start of puberty) — was also not associated with delayed pubertal development.
IN PRACTICE:
“Previous studies on atopic dermatitis and puberty are limited, some suggest a link between atopic dermatitis and delayed puberty, akin to other chronic inflammatory diseases in childhood,” the authors wrote. “The results of the present study are reassuring for young patients with atopic dermatitis approaching puberty and reproductive health in adult life,” they concluded.
SOURCE:
The study was led by Camilla Lomholt Kjersgaard, MD, Aarhus University, Aarhus, Denmark, and was published online in JAAD International.
LIMITATIONS:
Limitations included a lack of information on treatment, the use of analyses that did not address missing data, and a possible misclassification of self-reported pubertal development.
DISCLOSURES:
The study was funded by the Danish Council for Independent Research; Aarhus University; and Fonden af Fam. Kjærsgaard, Sunds; and was cofunded by the European Union. The authors reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.