User login
Oral minoxidil improves anticancer treatment–induced alopecia in women with breast cancer
Topical minoxidil is widely used to treat hair loss, but new findings suggest that
In a retrospective cohort study of women with breast cancer and anticancer therapy–induced alopecia, researchers found that combining low-dose oral minoxidil (LDOM) and topical minoxidil achieved better results than topical minoxidil alone and that the treatment was well tolerated. A total of 5 of the 37 patients (13.5%) in the combination therapy group achieved a complete response, defined as an improvement of alopecia severity from grade 2 to grade 1, compared with none of the 19 patients in the topical therapy–only group.
In contrast, none of the patients in the combination group experienced worsening of alopecia, compared with two (10.5%) in the topical monotherapy group.
The study was published online in the Journal of the American Academy of Dermatology. Topical minoxidil is approved by the Food and Drug Administration to treat androgenetic alopecia. Oral minoxidil is not approved for treating hair loss but has been receiving increased attention as an adjunctive therapy for hair loss, particularly for women. Oral minoxidil is approved for treating hypertension but at much higher doses.
An increasing number of studies have been conducted on the use of oral minoxidil for the treatment of female pattern hair loss, dating back to a pilot study in 2017, with promising results. The findings suggest that LDOM might be more effective than topical therapy, well tolerated, and more convenient for individuals to take.
Hypothesis generating
In a comment, Kai Johnson, MD, a medical oncologist who specializes in treating patients with breast cancer at the Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, noted that the study, like most small-scale retrospective studies, is hypothesis generating. However, “I’d be hesitant to broadly recommend this practice of dual therapy – oral and topical minoxidil together – until we see a placebo-controlled prospective study performed demonstrating clinically meaningful benefits for patients.”
Another factor is the study endpoints. “While there was a statistically significant benefit documented with dual therapy in this study, it’s important to have study endpoints that are more patient oriented,” Dr. Johnson said. The most important endpoint for patients would be improvements “in the actual alopecia grade, which did occur in 5 of the 37 of dual-therapy patients, versus 0 topical minoxidil patients.”
George Cotsarelis, MD, chair of the department of dermatology and professor of dermatology at the University of Pennsylvania, Philadelphia, also weighed in. He questioned whether adding the topical therapy to oral minoxidil actually improved the results. “What was missing was a study arm that used the oral alone,” he said in an interview. “So we don’t know how effective the oral therapy would be by itself and if combining it with the topical is really adding anything.”
Oral minoxidil as a treatment for hair loss is gaining traction, and it’s clear that it is effective. However, the risk of side effects is higher, he said. “The risk isn’t that high with the low dose, but it can grow hair on places other than the scalp, and that can be disconcerting.” In this study, two women who took the oral drug reported edema, and one reported headache and dizziness. Hypertrichosis was reported by five patients who received the combination.
Study details
In the study, Jeewoo Kang, MD, and colleagues from the Seoul National University evaluated the efficacy of LDOM in 100 patients with breast cancer who had been diagnosed with persistent chemotherapy-induced alopecia (pCIA) and endocrine therapy–induced alopecia (EIA) at a dermatology clinic.
They conducted an analysis of medical records, standardized clinical photographs, and trichoscopic images to evaluate the alopecia pattern, severity, treatment response, and posttreatment changes in vertex hair density and thickness.
Compared with those with EIA alone, patients with pCIA were significantly more likely to have diffuse alopecia (P < .001), and they were more likely to have more severe alopecia, although this difference was not significant (P = .058). Outcomes were evaluated for 56 patients who were treated with minoxidil (19 with topical minoxidil alone and 37 with both LDOM and topical minoxidil) and for whom clinical and trichoscopic photos were available at baseline and at the last follow-up (all patients were scheduled for follow-up at 3-month intervals).
The results showed that those treated with 1.25-5.0 mg/d of oral minoxidil and 5% topical minoxidil solution once a day had better responses (P = .002) and a higher percentage increase in hair density from baseline (P = .003), compared with those who received topical minoxidil monotherapy.
However, changes in hair thickness after treatment were not significantly different between the two groups (P = .540).
In addition to the five (13.5%) cases of hypertrichosis, two cases of edema (5.4%), and one case of headache/dizziness (2.7%) among those who received the combination, there was also one report of palpitations (2.7%). Palpitations were reported in one patient (5%) who received topical monotherapy, the only adverse event reported in this group.
Dr. Johnson noted that, at his institution, a dermatologist is conducting a clinical trial with oncology patients post chemotherapy and endocrine therapy. “She is looking at a similar question, although she is comparing oral minoxidil to topical minoxidil directly rather than in combination.” There is also an active clinical trial at Northwestern University, Chicago, of LDOM alone for patients with chemotherapy-induced alopecia.
“So there is a lot of momentum surrounding this concept, and I feel we will continue to see it come up as a possible treatment option, but more data are needed at this time before it can become standard of care,” Dr. Johnson added.
No funding for the study was reported. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Topical minoxidil is widely used to treat hair loss, but new findings suggest that
In a retrospective cohort study of women with breast cancer and anticancer therapy–induced alopecia, researchers found that combining low-dose oral minoxidil (LDOM) and topical minoxidil achieved better results than topical minoxidil alone and that the treatment was well tolerated. A total of 5 of the 37 patients (13.5%) in the combination therapy group achieved a complete response, defined as an improvement of alopecia severity from grade 2 to grade 1, compared with none of the 19 patients in the topical therapy–only group.
In contrast, none of the patients in the combination group experienced worsening of alopecia, compared with two (10.5%) in the topical monotherapy group.
The study was published online in the Journal of the American Academy of Dermatology. Topical minoxidil is approved by the Food and Drug Administration to treat androgenetic alopecia. Oral minoxidil is not approved for treating hair loss but has been receiving increased attention as an adjunctive therapy for hair loss, particularly for women. Oral minoxidil is approved for treating hypertension but at much higher doses.
An increasing number of studies have been conducted on the use of oral minoxidil for the treatment of female pattern hair loss, dating back to a pilot study in 2017, with promising results. The findings suggest that LDOM might be more effective than topical therapy, well tolerated, and more convenient for individuals to take.
Hypothesis generating
In a comment, Kai Johnson, MD, a medical oncologist who specializes in treating patients with breast cancer at the Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, noted that the study, like most small-scale retrospective studies, is hypothesis generating. However, “I’d be hesitant to broadly recommend this practice of dual therapy – oral and topical minoxidil together – until we see a placebo-controlled prospective study performed demonstrating clinically meaningful benefits for patients.”
Another factor is the study endpoints. “While there was a statistically significant benefit documented with dual therapy in this study, it’s important to have study endpoints that are more patient oriented,” Dr. Johnson said. The most important endpoint for patients would be improvements “in the actual alopecia grade, which did occur in 5 of the 37 of dual-therapy patients, versus 0 topical minoxidil patients.”
George Cotsarelis, MD, chair of the department of dermatology and professor of dermatology at the University of Pennsylvania, Philadelphia, also weighed in. He questioned whether adding the topical therapy to oral minoxidil actually improved the results. “What was missing was a study arm that used the oral alone,” he said in an interview. “So we don’t know how effective the oral therapy would be by itself and if combining it with the topical is really adding anything.”
Oral minoxidil as a treatment for hair loss is gaining traction, and it’s clear that it is effective. However, the risk of side effects is higher, he said. “The risk isn’t that high with the low dose, but it can grow hair on places other than the scalp, and that can be disconcerting.” In this study, two women who took the oral drug reported edema, and one reported headache and dizziness. Hypertrichosis was reported by five patients who received the combination.
Study details
In the study, Jeewoo Kang, MD, and colleagues from the Seoul National University evaluated the efficacy of LDOM in 100 patients with breast cancer who had been diagnosed with persistent chemotherapy-induced alopecia (pCIA) and endocrine therapy–induced alopecia (EIA) at a dermatology clinic.
They conducted an analysis of medical records, standardized clinical photographs, and trichoscopic images to evaluate the alopecia pattern, severity, treatment response, and posttreatment changes in vertex hair density and thickness.
Compared with those with EIA alone, patients with pCIA were significantly more likely to have diffuse alopecia (P < .001), and they were more likely to have more severe alopecia, although this difference was not significant (P = .058). Outcomes were evaluated for 56 patients who were treated with minoxidil (19 with topical minoxidil alone and 37 with both LDOM and topical minoxidil) and for whom clinical and trichoscopic photos were available at baseline and at the last follow-up (all patients were scheduled for follow-up at 3-month intervals).
The results showed that those treated with 1.25-5.0 mg/d of oral minoxidil and 5% topical minoxidil solution once a day had better responses (P = .002) and a higher percentage increase in hair density from baseline (P = .003), compared with those who received topical minoxidil monotherapy.
However, changes in hair thickness after treatment were not significantly different between the two groups (P = .540).
In addition to the five (13.5%) cases of hypertrichosis, two cases of edema (5.4%), and one case of headache/dizziness (2.7%) among those who received the combination, there was also one report of palpitations (2.7%). Palpitations were reported in one patient (5%) who received topical monotherapy, the only adverse event reported in this group.
Dr. Johnson noted that, at his institution, a dermatologist is conducting a clinical trial with oncology patients post chemotherapy and endocrine therapy. “She is looking at a similar question, although she is comparing oral minoxidil to topical minoxidil directly rather than in combination.” There is also an active clinical trial at Northwestern University, Chicago, of LDOM alone for patients with chemotherapy-induced alopecia.
“So there is a lot of momentum surrounding this concept, and I feel we will continue to see it come up as a possible treatment option, but more data are needed at this time before it can become standard of care,” Dr. Johnson added.
No funding for the study was reported. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Topical minoxidil is widely used to treat hair loss, but new findings suggest that
In a retrospective cohort study of women with breast cancer and anticancer therapy–induced alopecia, researchers found that combining low-dose oral minoxidil (LDOM) and topical minoxidil achieved better results than topical minoxidil alone and that the treatment was well tolerated. A total of 5 of the 37 patients (13.5%) in the combination therapy group achieved a complete response, defined as an improvement of alopecia severity from grade 2 to grade 1, compared with none of the 19 patients in the topical therapy–only group.
In contrast, none of the patients in the combination group experienced worsening of alopecia, compared with two (10.5%) in the topical monotherapy group.
The study was published online in the Journal of the American Academy of Dermatology. Topical minoxidil is approved by the Food and Drug Administration to treat androgenetic alopecia. Oral minoxidil is not approved for treating hair loss but has been receiving increased attention as an adjunctive therapy for hair loss, particularly for women. Oral minoxidil is approved for treating hypertension but at much higher doses.
An increasing number of studies have been conducted on the use of oral minoxidil for the treatment of female pattern hair loss, dating back to a pilot study in 2017, with promising results. The findings suggest that LDOM might be more effective than topical therapy, well tolerated, and more convenient for individuals to take.
Hypothesis generating
In a comment, Kai Johnson, MD, a medical oncologist who specializes in treating patients with breast cancer at the Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, noted that the study, like most small-scale retrospective studies, is hypothesis generating. However, “I’d be hesitant to broadly recommend this practice of dual therapy – oral and topical minoxidil together – until we see a placebo-controlled prospective study performed demonstrating clinically meaningful benefits for patients.”
Another factor is the study endpoints. “While there was a statistically significant benefit documented with dual therapy in this study, it’s important to have study endpoints that are more patient oriented,” Dr. Johnson said. The most important endpoint for patients would be improvements “in the actual alopecia grade, which did occur in 5 of the 37 of dual-therapy patients, versus 0 topical minoxidil patients.”
George Cotsarelis, MD, chair of the department of dermatology and professor of dermatology at the University of Pennsylvania, Philadelphia, also weighed in. He questioned whether adding the topical therapy to oral minoxidil actually improved the results. “What was missing was a study arm that used the oral alone,” he said in an interview. “So we don’t know how effective the oral therapy would be by itself and if combining it with the topical is really adding anything.”
Oral minoxidil as a treatment for hair loss is gaining traction, and it’s clear that it is effective. However, the risk of side effects is higher, he said. “The risk isn’t that high with the low dose, but it can grow hair on places other than the scalp, and that can be disconcerting.” In this study, two women who took the oral drug reported edema, and one reported headache and dizziness. Hypertrichosis was reported by five patients who received the combination.
Study details
In the study, Jeewoo Kang, MD, and colleagues from the Seoul National University evaluated the efficacy of LDOM in 100 patients with breast cancer who had been diagnosed with persistent chemotherapy-induced alopecia (pCIA) and endocrine therapy–induced alopecia (EIA) at a dermatology clinic.
They conducted an analysis of medical records, standardized clinical photographs, and trichoscopic images to evaluate the alopecia pattern, severity, treatment response, and posttreatment changes in vertex hair density and thickness.
Compared with those with EIA alone, patients with pCIA were significantly more likely to have diffuse alopecia (P < .001), and they were more likely to have more severe alopecia, although this difference was not significant (P = .058). Outcomes were evaluated for 56 patients who were treated with minoxidil (19 with topical minoxidil alone and 37 with both LDOM and topical minoxidil) and for whom clinical and trichoscopic photos were available at baseline and at the last follow-up (all patients were scheduled for follow-up at 3-month intervals).
The results showed that those treated with 1.25-5.0 mg/d of oral minoxidil and 5% topical minoxidil solution once a day had better responses (P = .002) and a higher percentage increase in hair density from baseline (P = .003), compared with those who received topical minoxidil monotherapy.
However, changes in hair thickness after treatment were not significantly different between the two groups (P = .540).
In addition to the five (13.5%) cases of hypertrichosis, two cases of edema (5.4%), and one case of headache/dizziness (2.7%) among those who received the combination, there was also one report of palpitations (2.7%). Palpitations were reported in one patient (5%) who received topical monotherapy, the only adverse event reported in this group.
Dr. Johnson noted that, at his institution, a dermatologist is conducting a clinical trial with oncology patients post chemotherapy and endocrine therapy. “She is looking at a similar question, although she is comparing oral minoxidil to topical minoxidil directly rather than in combination.” There is also an active clinical trial at Northwestern University, Chicago, of LDOM alone for patients with chemotherapy-induced alopecia.
“So there is a lot of momentum surrounding this concept, and I feel we will continue to see it come up as a possible treatment option, but more data are needed at this time before it can become standard of care,” Dr. Johnson added.
No funding for the study was reported. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Adverse events linked to better survival with ICIs in melanoma
Among Survival is further improved if the immunotherapy is continued after the adverse event develops, a new study confirms.
“In the largest clinical cohort to date, our data support a positive association with overall survival for patients who develop clinically significant immune-related adverse events while receiving combination immune checkpoint blockade, in keeping with other reported series,” the authors wrote.
The study was published online in JAMA Network Open.
Immune-related adverse events are common with these drugs. Severe events of grade 3 or higher occur in 59% of trial patients who receive combination ICI therapy.
The adverse events have increasingly been positively associated with survival. However, the effects for patients with metastatic melanoma, in particular, are less clear. There is little research on the effects in relation to combination therapy with ipilimumab and nivolumab, which is the standard of care for many patients with metastatic melanoma.
To investigate, Alexander S. Watson, MD, and colleagues evaluated data on 492 patients with metastatic melanoma who had been treated with one or more doses of an anti–programmed death 1 agent as single or combination immune checkpoint blockade in the multicenter Alberta Immunotherapy Database from August 2013 to May 2020.
Of these 492 patients, 198 patients (40%) developed immune-related adverse events. The mean age of the patients who developed adverse events was 61.8 years; of those who did not develop adverse events, the mean age was 65.5 years. Men made up 69.2% and 62.2%, respectively.
A total of 288 patients received pembrolizumab as their first ICI therapy, 80 received nivolumab, and 124 received combination blockade with ipilimumab-nivolumab.
Overall, with a median follow-up of 36.6 months, among patients who experienced clinically significant immune-related adverse events, defined as requiring systemic corticosteroids and/or a treatment delay, median overall survival was significantly improved, at 56.3 months, compared with 18.5 months among those who did not experience immune-related adverse events (P < .001).
In addition, among those who received combination ICI treatment, the median overall survival was 56.2 months for those who experienced adverse events versus 19.0 months for those who did not (P < .001).
There were no significant differences in overall survival between those who were and those who were not hospitalized for their immune-related adverse events (P = .53).
For patients who resumed their ICI therapy following the adverse events, overall survival was longer, compared with those who did not resume the therapy (median, 56.3 months vs. 31.5 months; P = .009).
The improvements in overall survival seen with immune-related adverse events remained consistent after adjustment in a multivariable analysis (hazard ratio for death, 0.382; P < .001).
There were no significant differences in the median number of cycles of ICIs between those with and those without the adverse events.
The risk of recurrence of immune-related adverse events following the reintroduction of therapy after initial events was a concern, so the improved overall survival among those patients is encouraging, although further investigation is needed, commented lead author Dr. Watson, from the department of oncology, University of Calgary (Alta.).
“It may be, for certain patients with immune-related adverse events, that continued immune-priming is safe and optimizes anticancer response,” he told this news organization. “However, in a retrospective analysis such as ours, selection bias can have an impact.”
“Confirming this finding and better identifying patients who may benefit from resumption will be an area for future investigation,” he said.
Patients who developed immune-related adverse events were more likely to be younger than 50 years (21.8% vs. 13.9%), have normal albumin levels (86.4% vs. 74.8%), and have a more robust Eastern Cooperative Oncology Group status, which is consistent with other studies that have shown survival benefits among those who experience adverse events.
“We, and others, speculate this could be due to such groups having immune systems more ready to respond strongly to immunotherapy,” Dr. Watson explained.
After controlling for age and performance status in the multivariable analysis, however, “immune-related adverse events remained strongly associated with survival, potentially [indicating] that robust responses to immunotherapy lead to both cancer control and immune-related adverse events,” he said.
Overall, “we feel these findings will help clinicians in discussions with patients and in clinical decision-making after adverse events develop,” Dr. Watson said.
Dr. Watson has received personal fees from Apobiologix Canada.
A version of this article first appeared on Medscape.com.
Among Survival is further improved if the immunotherapy is continued after the adverse event develops, a new study confirms.
“In the largest clinical cohort to date, our data support a positive association with overall survival for patients who develop clinically significant immune-related adverse events while receiving combination immune checkpoint blockade, in keeping with other reported series,” the authors wrote.
The study was published online in JAMA Network Open.
Immune-related adverse events are common with these drugs. Severe events of grade 3 or higher occur in 59% of trial patients who receive combination ICI therapy.
The adverse events have increasingly been positively associated with survival. However, the effects for patients with metastatic melanoma, in particular, are less clear. There is little research on the effects in relation to combination therapy with ipilimumab and nivolumab, which is the standard of care for many patients with metastatic melanoma.
To investigate, Alexander S. Watson, MD, and colleagues evaluated data on 492 patients with metastatic melanoma who had been treated with one or more doses of an anti–programmed death 1 agent as single or combination immune checkpoint blockade in the multicenter Alberta Immunotherapy Database from August 2013 to May 2020.
Of these 492 patients, 198 patients (40%) developed immune-related adverse events. The mean age of the patients who developed adverse events was 61.8 years; of those who did not develop adverse events, the mean age was 65.5 years. Men made up 69.2% and 62.2%, respectively.
A total of 288 patients received pembrolizumab as their first ICI therapy, 80 received nivolumab, and 124 received combination blockade with ipilimumab-nivolumab.
Overall, with a median follow-up of 36.6 months, among patients who experienced clinically significant immune-related adverse events, defined as requiring systemic corticosteroids and/or a treatment delay, median overall survival was significantly improved, at 56.3 months, compared with 18.5 months among those who did not experience immune-related adverse events (P < .001).
In addition, among those who received combination ICI treatment, the median overall survival was 56.2 months for those who experienced adverse events versus 19.0 months for those who did not (P < .001).
There were no significant differences in overall survival between those who were and those who were not hospitalized for their immune-related adverse events (P = .53).
For patients who resumed their ICI therapy following the adverse events, overall survival was longer, compared with those who did not resume the therapy (median, 56.3 months vs. 31.5 months; P = .009).
The improvements in overall survival seen with immune-related adverse events remained consistent after adjustment in a multivariable analysis (hazard ratio for death, 0.382; P < .001).
There were no significant differences in the median number of cycles of ICIs between those with and those without the adverse events.
The risk of recurrence of immune-related adverse events following the reintroduction of therapy after initial events was a concern, so the improved overall survival among those patients is encouraging, although further investigation is needed, commented lead author Dr. Watson, from the department of oncology, University of Calgary (Alta.).
“It may be, for certain patients with immune-related adverse events, that continued immune-priming is safe and optimizes anticancer response,” he told this news organization. “However, in a retrospective analysis such as ours, selection bias can have an impact.”
“Confirming this finding and better identifying patients who may benefit from resumption will be an area for future investigation,” he said.
Patients who developed immune-related adverse events were more likely to be younger than 50 years (21.8% vs. 13.9%), have normal albumin levels (86.4% vs. 74.8%), and have a more robust Eastern Cooperative Oncology Group status, which is consistent with other studies that have shown survival benefits among those who experience adverse events.
“We, and others, speculate this could be due to such groups having immune systems more ready to respond strongly to immunotherapy,” Dr. Watson explained.
After controlling for age and performance status in the multivariable analysis, however, “immune-related adverse events remained strongly associated with survival, potentially [indicating] that robust responses to immunotherapy lead to both cancer control and immune-related adverse events,” he said.
Overall, “we feel these findings will help clinicians in discussions with patients and in clinical decision-making after adverse events develop,” Dr. Watson said.
Dr. Watson has received personal fees from Apobiologix Canada.
A version of this article first appeared on Medscape.com.
Among Survival is further improved if the immunotherapy is continued after the adverse event develops, a new study confirms.
“In the largest clinical cohort to date, our data support a positive association with overall survival for patients who develop clinically significant immune-related adverse events while receiving combination immune checkpoint blockade, in keeping with other reported series,” the authors wrote.
The study was published online in JAMA Network Open.
Immune-related adverse events are common with these drugs. Severe events of grade 3 or higher occur in 59% of trial patients who receive combination ICI therapy.
The adverse events have increasingly been positively associated with survival. However, the effects for patients with metastatic melanoma, in particular, are less clear. There is little research on the effects in relation to combination therapy with ipilimumab and nivolumab, which is the standard of care for many patients with metastatic melanoma.
To investigate, Alexander S. Watson, MD, and colleagues evaluated data on 492 patients with metastatic melanoma who had been treated with one or more doses of an anti–programmed death 1 agent as single or combination immune checkpoint blockade in the multicenter Alberta Immunotherapy Database from August 2013 to May 2020.
Of these 492 patients, 198 patients (40%) developed immune-related adverse events. The mean age of the patients who developed adverse events was 61.8 years; of those who did not develop adverse events, the mean age was 65.5 years. Men made up 69.2% and 62.2%, respectively.
A total of 288 patients received pembrolizumab as their first ICI therapy, 80 received nivolumab, and 124 received combination blockade with ipilimumab-nivolumab.
Overall, with a median follow-up of 36.6 months, among patients who experienced clinically significant immune-related adverse events, defined as requiring systemic corticosteroids and/or a treatment delay, median overall survival was significantly improved, at 56.3 months, compared with 18.5 months among those who did not experience immune-related adverse events (P < .001).
In addition, among those who received combination ICI treatment, the median overall survival was 56.2 months for those who experienced adverse events versus 19.0 months for those who did not (P < .001).
There were no significant differences in overall survival between those who were and those who were not hospitalized for their immune-related adverse events (P = .53).
For patients who resumed their ICI therapy following the adverse events, overall survival was longer, compared with those who did not resume the therapy (median, 56.3 months vs. 31.5 months; P = .009).
The improvements in overall survival seen with immune-related adverse events remained consistent after adjustment in a multivariable analysis (hazard ratio for death, 0.382; P < .001).
There were no significant differences in the median number of cycles of ICIs between those with and those without the adverse events.
The risk of recurrence of immune-related adverse events following the reintroduction of therapy after initial events was a concern, so the improved overall survival among those patients is encouraging, although further investigation is needed, commented lead author Dr. Watson, from the department of oncology, University of Calgary (Alta.).
“It may be, for certain patients with immune-related adverse events, that continued immune-priming is safe and optimizes anticancer response,” he told this news organization. “However, in a retrospective analysis such as ours, selection bias can have an impact.”
“Confirming this finding and better identifying patients who may benefit from resumption will be an area for future investigation,” he said.
Patients who developed immune-related adverse events were more likely to be younger than 50 years (21.8% vs. 13.9%), have normal albumin levels (86.4% vs. 74.8%), and have a more robust Eastern Cooperative Oncology Group status, which is consistent with other studies that have shown survival benefits among those who experience adverse events.
“We, and others, speculate this could be due to such groups having immune systems more ready to respond strongly to immunotherapy,” Dr. Watson explained.
After controlling for age and performance status in the multivariable analysis, however, “immune-related adverse events remained strongly associated with survival, potentially [indicating] that robust responses to immunotherapy lead to both cancer control and immune-related adverse events,” he said.
Overall, “we feel these findings will help clinicians in discussions with patients and in clinical decision-making after adverse events develop,” Dr. Watson said.
Dr. Watson has received personal fees from Apobiologix Canada.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
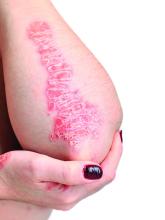
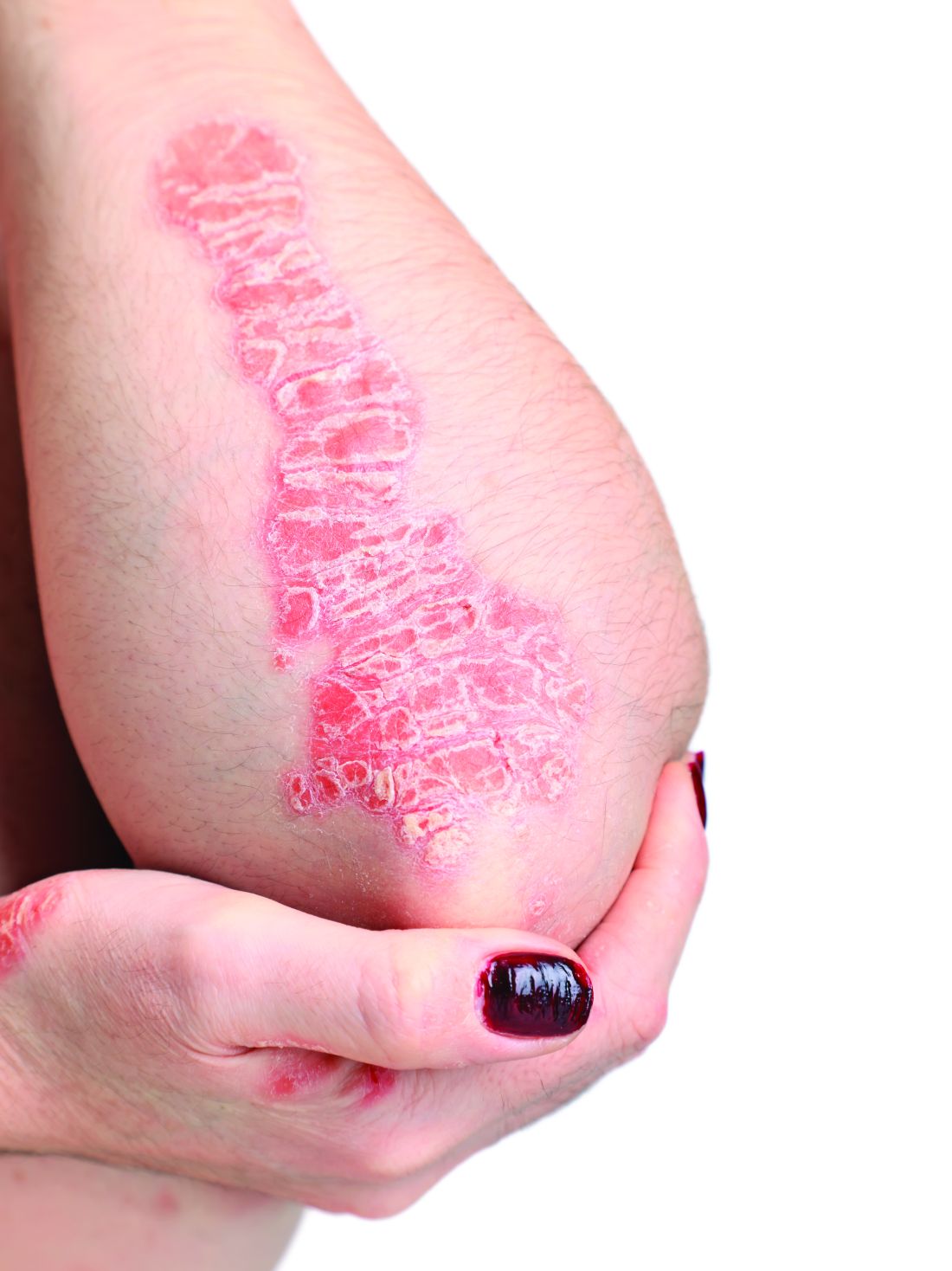
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Study evaluates features of alopecia areata in Hispanic/Latinx patients
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Cochrane Review bolsters case that emollients don’t prevent AD
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
associated with early use of emollients.
The document, published in November 2022, updates a February 2021 version, said Robert Boyle, MD, PhD, senior author of the Cochrane Review and a pediatric allergist at Imperial College London. “The differences were slight,” he told this news organization. “Mainly, we had a little more data about food allergy outcomes, which slightly strengthened the concern about a possible increase in food allergy with emollients; and we had some new genetic information, which allowed us to add some further interaction analyses and confirm that chromosome 11 intergenic variant rs2212434 doesn’t seem to impact the effect – or lack of effect – of emollient on eczema development.”
The updated Cochrane Review concludes that, “based on low‐ to moderate-certainty evidence, skin care interventions such as emollients during the first year of life in healthy infants are probably not effective for preventing eczema; may increase risk of food allergy; and probably increase risk of skin infection.”
The latest publication should strengthen clinicians’ confidence in not recommending emollient use for preventing AD in at-risk infants – however, that message is being diluted by a stream of contradictory conclusions from poor-quality systematic reviews, say Dr. Boyle and two coauthors. “It’s a systematic problem of people churning out endless systematic reviews without much rigor,” explained the lead author Maeve Kelleher, MD, from Children’s Health Ireland, Crumlin. There have been “misleading systematic reviews published, often in high-ranking journals,” agreed Dr. Boyle.
“I have been an advocate of systematic reviews for the last 20 years, but they have gone completely out of control,” added Hywel Williams, MD, PhD, another of the Cochrane Review coauthors, who is professor of dermato-epidemiology and codirector of the Centre of Evidence Based Dermatology, at Nottingham (England) University Hospitals NHS Trust. In an editorial, published last year, Dr. Williams even posed the question: “Are Dermatology Systematic Reviews Spinning Out of Control?” in which he blamed “the misrepresentation of study results” – which he calls “the sin of spin” – for degrading the quality of science in dermatology.
“The field has become a ‘sausage machine’ industry that undermines the value of systematic reviews in providing a summary of the best evidence to inform patient care,” he wrote. “Fewer systematic reviews are needed in dermatology,” but “better ones” are needed, he continued, calling for all systematic reviews to be registered prospectively, and reported according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Earlier this year, in a letter to the editor, Dr. Kelleher, Dr. Boyle, Dr. Williams, and several others outlined their concerns after a systemic review and meta-analysis was published, “which came to very different conclusions” than their Cochrane Review.
“It is quite common to see non-Cochrane reviews published in leading specialty journals, which interpret data in a more positive light than Cochrane reviews, which have assessed a similar dataset/topic,” Dr. Boyle said in the interview.
Such concerns also apply to the publication of another systematic review that was recently published. “Overall, early application of emollients is an effective strategy for preventing AD development in high-risk infants,” reported senior author Xiaojing Kang, MD, PhD, from People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, China, and coauthors, who could not be reached for comment. In their discussion, the authors cite several criticisms of the Cochrane Review: that it included two meeting abstracts and two “ineligible” studies; did not do subgroup analysis of high-risk infants; did not look at different types of emollients; and did not examine the risk of food sensitization.
“A Cochrane Review can be quite a large and complex document to negotiate for those who are not very familiar with Cochrane’s methodology,” said Dr. Boyle. He dismissed the criticism, saying “we did do subgroup analysis of high risk infants, we did look at different types of emollient, and we did look at food sensitization and food allergy risk. We only included eligible studies. … Certainly we would include abstracts of trials, which are not reported in any other form, in order to capture as complete a picture.”
Ultimately, Dr. Boyle said, the discrepancy in conclusions between such systematic reviews and the Cochrane Review relates to quality of methodology. “Our Cochrane review was an individual participant data (IPD) meta-analysis, meaning that authors of the main trials in this area shared their original datasets with us,” he said in the interview. “This is the ‘gold standard’ in systematic reviews, and allowed us to check data/ query inconsistencies and to apply a single-analysis methodology across all studies. It also allowed us to undertake some analyses, which are just not possible in aggregate data analysis based on published work without IPD.”
The most recently published systematic review had no registered protocol, “so, there is no transparency about the methods used,” he noted. “It is free and simple to register a protocol – multiple websites such as PROSPERO, open science framework, and zenodo allow this,” he said “In the journal I edit, we use availability of a registered protocol as a marker of quality. We find that systematic reviews with no registered protocol are almost universally poor quality.”
Dr. Williams is a founding member and coordinating editor of the Cochrane Skin Group 1998 to 2017. Dr. Boyle was paid by Cochrane for senior editor work, until recently, and had no other relevant disclosures. Dr. Kelleher had no relevant disclosures.
FROM THE COCHRANE REVIEW
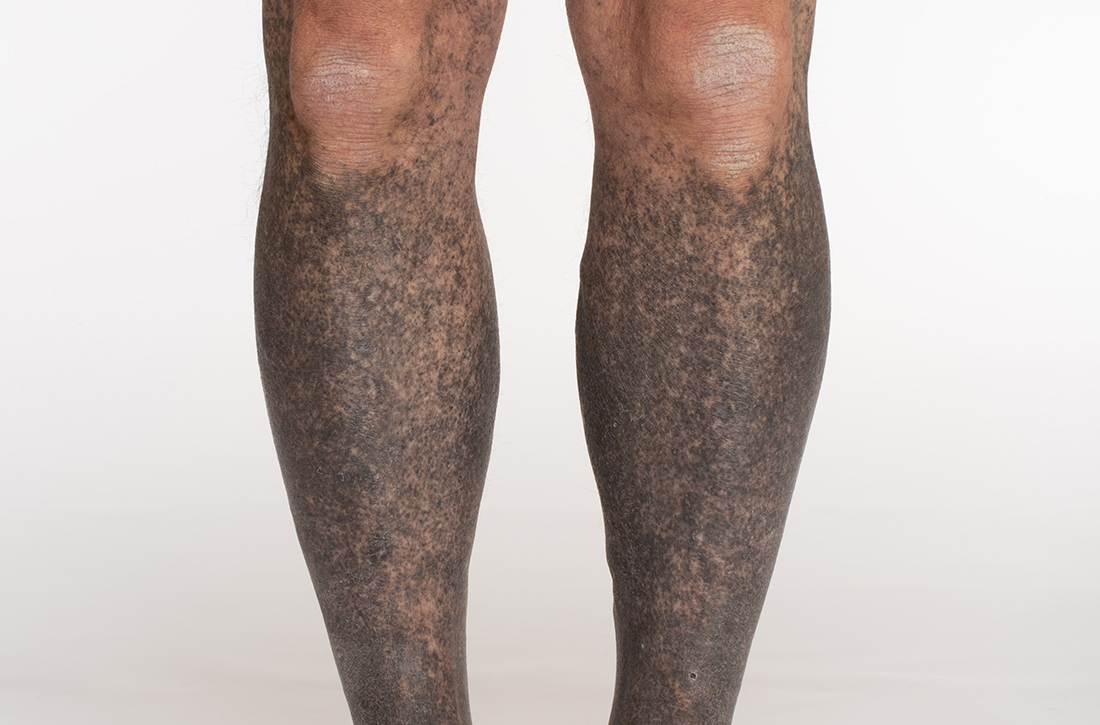
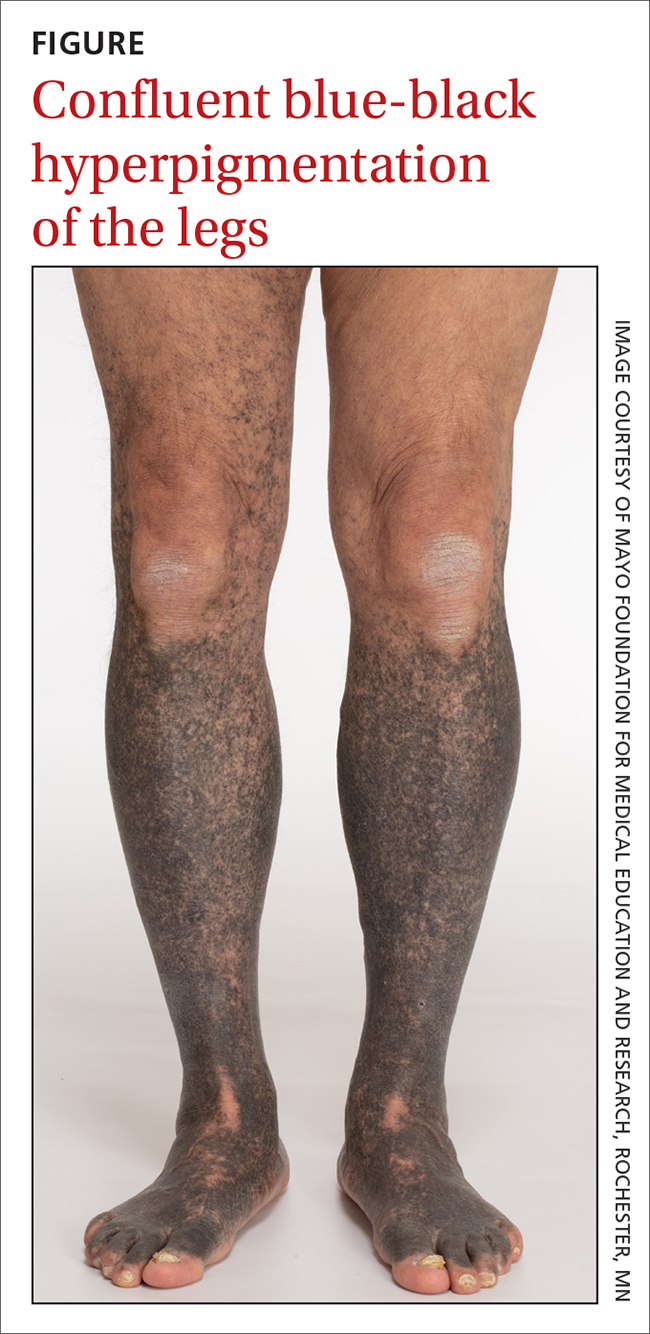
Blue-black hyperpigmentation on the extremities
A 68-year-old man with type 2 diabetes presented with progressive hyperpigmentation of the lower extremities and face over the past 3 years. Clinical examination revealed confluent, blue-black hyperpigmentation of the lower extremities (Figure), upper extremities, neck, and face. Laboratory tests and arterial studies were within normal ranges. The patient’s medication list included lisinopril 10 mg/d, metformin 1000 mg twice daily, minocycline 100 mg twice daily, and omeprazole 20 mg/d.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
Hyperpigmentation is a rare but not uncommon adverse effect of long-term minocycline use. In this case, our patient had been taking minocycline for more than 5 years. When seen in our clinic, he said he could not remember why he was taking minocycline and incorrectly assumed it was for his diabetes. Chart review of outside records revealed that it had been prescribed, and refilled annually, by his primary physician for rosacea.
Minocycline hyperpigmentation is subdivided into 3 types:
- Type I manifests with blue-black discoloration in previously inflamed areas of skin.
- Type II manifests with blue-gray pigmentation in previously normal skin areas.
- Type III manifests diffusely with muddy-brown hyperpigmentation on photoexposed skin.
Furthermore, noncutaneous manifestations may occur on the sclera, nails, ear cartilage, bone, oral mucosa, teeth, and thyroid gland.1
Diagnosis focuses on identifying the source
Minocycline is one of many drugs that can induce hyperpigmentation of the skin. In addition to history, examination, and review of the patient’s medication list, there are some clues on exam that may suggest a certain type of medication at play.
Continue to: Antimalarials
Antimalarials. Chloroquine, hydroxychloroquine, and quinacrine can cause blue-black skin hyperpigmentation in as many as 25% of patients. Common locations include the shins, face, oral mucosa, and subungual skin. This hyperpigmentation rarely fully resolves.2
Amiodarone. Hyperpigmentation secondary to amiodarone use typically is slate-gray in color and involves photoexposed skin. Patients should be counseled that pigmentation may—but does not always—fade with time after discontinuation of the drug.2
Heavy metals. Argyria results from exposure to silver, either ingested orally or applied externally. A common cause of argyria is ingestion of excessive amounts of silver-containing supplements.3 Affected patients present with diffuse slate-gray discoloration of the skin.
Other metals implicated in skin hyperpigmentation include arsenic, gold, mercury, and iron. Review of all supplements and herbal remedies in patients presenting with skin hyperpigmentation is crucial.
Bleomycin is a chemotherapeutic agent with a rare but unique adverse effect of inducing flagellate hyperpigmentation that favors the chest, abdomen, or back. This may be induced by trauma or scratching and is often transient. Hyperpigmentation can occur secondary to either intravenous or intralesional injection of the medication.2
Continue to: In addition to medication...
In addition to medication- or supplement-induced hyperpigmentation, there is a physiologic source that should be considered when a patient presents with lower-extremity hyperpigmentation:
Stasis hyperpigmentation. Patients with chronic venous insufficiency may present with hyperpigmentation of the lower extremities. Commonly due to dysfunctional venous valves or obstruction, stasis hyperpigmentation manifests with red-brown discoloration from dermal hemosiderin deposition.4
Unlike our patient, those with stasis hyperpigmentation may present symptomatically, with associated dry skin, pruritus, induration, and inflammation. Treatment involves management of the underlying venous insufficiency.4
When there’s no obvious cause, be prepared to dig deeper
At the time of initial assessment, a thorough review of systems and detailed medication history, including over-the-counter supplements, should be obtained. Physical examination revealing diffuse, generalized hyperpigmentation with no reliable culprit medication in the patient’s history warrants further laboratory evaluation. This includes ordering renal and liver studies and tests for thyroid-stimulating hormone and ferritin and cortisol levels to rule out metabolic or endocrine hyperpigmentation disorders.
Stopping the offending medication is the first step
Discontinuation of the offending medication may result in mild improvement in skin hyperpigmentation over time. Some patients may not experience any improvement. If improvement occurs, it is important to educate patients that it can take several months to years. Dermatology guidelines favor discontinuation of antibiotics for acne or rosacea after 3 to 6 months to avoid bacterial resistance.5 Worsening hyperpigmentation despite medication discontinuation warrants further work-up.
Patients who are distressed by persistent hyperpigmentation can be treated using picosecond or Q-switched lasers.6
Our patient was advised to discontinue the minocycline. Three test spots on his face were treated with pulsed-dye laser, carbon dioxide laser, and dermabrasion. The patient noted that the spots responded better to the carbon dioxide laser and dermabrasion compared to the pulsed-dye laser. He did not follow up for further treatment.
1. Wetter DA. Minocycline hyperpigmentation. Mayo Clin Proc. 2012;87:e33. doi: 10.1016/j.mayocp.2012.02.013
2. Chang MW. Chapter 67: Disorders of hyperpigmentation. In: Bolognia J, Schaffer J, Cerroni L, et al (eds). Dermatology. 4th ed. Elsevier; 2018:1122-1124.
3. Bowden LP, Royer MC, Hallman JR, et al. Rapid onset of argyria induced by a silver-containing dietary supplement. J Cutan Pathol. 2011;38:832-835. doi: 10.1111/j.1600-0560.2011.01755.x
4. Patterson J. Stasis dermatitis. In: Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier;2010: 121-153.
5. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73.e33. doi: 10.1016/j.jaad.2015.12.037
6. Barrett T, de Zwaan S. Picosecond alexandrite laser is superior to Q-switched Nd:YAG laser in treatment of minocycline-induced hyperpigmentation: a case study and review of the literature. J Cosmet Laser Ther. 2018;20:387-390. doi: 10.1080/14764172.2017.1418514
A 68-year-old man with type 2 diabetes presented with progressive hyperpigmentation of the lower extremities and face over the past 3 years. Clinical examination revealed confluent, blue-black hyperpigmentation of the lower extremities (Figure), upper extremities, neck, and face. Laboratory tests and arterial studies were within normal ranges. The patient’s medication list included lisinopril 10 mg/d, metformin 1000 mg twice daily, minocycline 100 mg twice daily, and omeprazole 20 mg/d.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
Hyperpigmentation is a rare but not uncommon adverse effect of long-term minocycline use. In this case, our patient had been taking minocycline for more than 5 years. When seen in our clinic, he said he could not remember why he was taking minocycline and incorrectly assumed it was for his diabetes. Chart review of outside records revealed that it had been prescribed, and refilled annually, by his primary physician for rosacea.
Minocycline hyperpigmentation is subdivided into 3 types:
- Type I manifests with blue-black discoloration in previously inflamed areas of skin.
- Type II manifests with blue-gray pigmentation in previously normal skin areas.
- Type III manifests diffusely with muddy-brown hyperpigmentation on photoexposed skin.
Furthermore, noncutaneous manifestations may occur on the sclera, nails, ear cartilage, bone, oral mucosa, teeth, and thyroid gland.1
Diagnosis focuses on identifying the source
Minocycline is one of many drugs that can induce hyperpigmentation of the skin. In addition to history, examination, and review of the patient’s medication list, there are some clues on exam that may suggest a certain type of medication at play.
Continue to: Antimalarials
Antimalarials. Chloroquine, hydroxychloroquine, and quinacrine can cause blue-black skin hyperpigmentation in as many as 25% of patients. Common locations include the shins, face, oral mucosa, and subungual skin. This hyperpigmentation rarely fully resolves.2
Amiodarone. Hyperpigmentation secondary to amiodarone use typically is slate-gray in color and involves photoexposed skin. Patients should be counseled that pigmentation may—but does not always—fade with time after discontinuation of the drug.2
Heavy metals. Argyria results from exposure to silver, either ingested orally or applied externally. A common cause of argyria is ingestion of excessive amounts of silver-containing supplements.3 Affected patients present with diffuse slate-gray discoloration of the skin.
Other metals implicated in skin hyperpigmentation include arsenic, gold, mercury, and iron. Review of all supplements and herbal remedies in patients presenting with skin hyperpigmentation is crucial.
Bleomycin is a chemotherapeutic agent with a rare but unique adverse effect of inducing flagellate hyperpigmentation that favors the chest, abdomen, or back. This may be induced by trauma or scratching and is often transient. Hyperpigmentation can occur secondary to either intravenous or intralesional injection of the medication.2
Continue to: In addition to medication...
In addition to medication- or supplement-induced hyperpigmentation, there is a physiologic source that should be considered when a patient presents with lower-extremity hyperpigmentation:
Stasis hyperpigmentation. Patients with chronic venous insufficiency may present with hyperpigmentation of the lower extremities. Commonly due to dysfunctional venous valves or obstruction, stasis hyperpigmentation manifests with red-brown discoloration from dermal hemosiderin deposition.4
Unlike our patient, those with stasis hyperpigmentation may present symptomatically, with associated dry skin, pruritus, induration, and inflammation. Treatment involves management of the underlying venous insufficiency.4
When there’s no obvious cause, be prepared to dig deeper
At the time of initial assessment, a thorough review of systems and detailed medication history, including over-the-counter supplements, should be obtained. Physical examination revealing diffuse, generalized hyperpigmentation with no reliable culprit medication in the patient’s history warrants further laboratory evaluation. This includes ordering renal and liver studies and tests for thyroid-stimulating hormone and ferritin and cortisol levels to rule out metabolic or endocrine hyperpigmentation disorders.
Stopping the offending medication is the first step
Discontinuation of the offending medication may result in mild improvement in skin hyperpigmentation over time. Some patients may not experience any improvement. If improvement occurs, it is important to educate patients that it can take several months to years. Dermatology guidelines favor discontinuation of antibiotics for acne or rosacea after 3 to 6 months to avoid bacterial resistance.5 Worsening hyperpigmentation despite medication discontinuation warrants further work-up.
Patients who are distressed by persistent hyperpigmentation can be treated using picosecond or Q-switched lasers.6
Our patient was advised to discontinue the minocycline. Three test spots on his face were treated with pulsed-dye laser, carbon dioxide laser, and dermabrasion. The patient noted that the spots responded better to the carbon dioxide laser and dermabrasion compared to the pulsed-dye laser. He did not follow up for further treatment.
A 68-year-old man with type 2 diabetes presented with progressive hyperpigmentation of the lower extremities and face over the past 3 years. Clinical examination revealed confluent, blue-black hyperpigmentation of the lower extremities (Figure), upper extremities, neck, and face. Laboratory tests and arterial studies were within normal ranges. The patient’s medication list included lisinopril 10 mg/d, metformin 1000 mg twice daily, minocycline 100 mg twice daily, and omeprazole 20 mg/d.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Minocycline-induced hyperpigmentation
Hyperpigmentation is a rare but not uncommon adverse effect of long-term minocycline use. In this case, our patient had been taking minocycline for more than 5 years. When seen in our clinic, he said he could not remember why he was taking minocycline and incorrectly assumed it was for his diabetes. Chart review of outside records revealed that it had been prescribed, and refilled annually, by his primary physician for rosacea.
Minocycline hyperpigmentation is subdivided into 3 types:
- Type I manifests with blue-black discoloration in previously inflamed areas of skin.
- Type II manifests with blue-gray pigmentation in previously normal skin areas.
- Type III manifests diffusely with muddy-brown hyperpigmentation on photoexposed skin.
Furthermore, noncutaneous manifestations may occur on the sclera, nails, ear cartilage, bone, oral mucosa, teeth, and thyroid gland.1
Diagnosis focuses on identifying the source
Minocycline is one of many drugs that can induce hyperpigmentation of the skin. In addition to history, examination, and review of the patient’s medication list, there are some clues on exam that may suggest a certain type of medication at play.
Continue to: Antimalarials
Antimalarials. Chloroquine, hydroxychloroquine, and quinacrine can cause blue-black skin hyperpigmentation in as many as 25% of patients. Common locations include the shins, face, oral mucosa, and subungual skin. This hyperpigmentation rarely fully resolves.2
Amiodarone. Hyperpigmentation secondary to amiodarone use typically is slate-gray in color and involves photoexposed skin. Patients should be counseled that pigmentation may—but does not always—fade with time after discontinuation of the drug.2
Heavy metals. Argyria results from exposure to silver, either ingested orally or applied externally. A common cause of argyria is ingestion of excessive amounts of silver-containing supplements.3 Affected patients present with diffuse slate-gray discoloration of the skin.
Other metals implicated in skin hyperpigmentation include arsenic, gold, mercury, and iron. Review of all supplements and herbal remedies in patients presenting with skin hyperpigmentation is crucial.
Bleomycin is a chemotherapeutic agent with a rare but unique adverse effect of inducing flagellate hyperpigmentation that favors the chest, abdomen, or back. This may be induced by trauma or scratching and is often transient. Hyperpigmentation can occur secondary to either intravenous or intralesional injection of the medication.2
Continue to: In addition to medication...
In addition to medication- or supplement-induced hyperpigmentation, there is a physiologic source that should be considered when a patient presents with lower-extremity hyperpigmentation:
Stasis hyperpigmentation. Patients with chronic venous insufficiency may present with hyperpigmentation of the lower extremities. Commonly due to dysfunctional venous valves or obstruction, stasis hyperpigmentation manifests with red-brown discoloration from dermal hemosiderin deposition.4
Unlike our patient, those with stasis hyperpigmentation may present symptomatically, with associated dry skin, pruritus, induration, and inflammation. Treatment involves management of the underlying venous insufficiency.4
When there’s no obvious cause, be prepared to dig deeper
At the time of initial assessment, a thorough review of systems and detailed medication history, including over-the-counter supplements, should be obtained. Physical examination revealing diffuse, generalized hyperpigmentation with no reliable culprit medication in the patient’s history warrants further laboratory evaluation. This includes ordering renal and liver studies and tests for thyroid-stimulating hormone and ferritin and cortisol levels to rule out metabolic or endocrine hyperpigmentation disorders.
Stopping the offending medication is the first step
Discontinuation of the offending medication may result in mild improvement in skin hyperpigmentation over time. Some patients may not experience any improvement. If improvement occurs, it is important to educate patients that it can take several months to years. Dermatology guidelines favor discontinuation of antibiotics for acne or rosacea after 3 to 6 months to avoid bacterial resistance.5 Worsening hyperpigmentation despite medication discontinuation warrants further work-up.
Patients who are distressed by persistent hyperpigmentation can be treated using picosecond or Q-switched lasers.6
Our patient was advised to discontinue the minocycline. Three test spots on his face were treated with pulsed-dye laser, carbon dioxide laser, and dermabrasion. The patient noted that the spots responded better to the carbon dioxide laser and dermabrasion compared to the pulsed-dye laser. He did not follow up for further treatment.
1. Wetter DA. Minocycline hyperpigmentation. Mayo Clin Proc. 2012;87:e33. doi: 10.1016/j.mayocp.2012.02.013
2. Chang MW. Chapter 67: Disorders of hyperpigmentation. In: Bolognia J, Schaffer J, Cerroni L, et al (eds). Dermatology. 4th ed. Elsevier; 2018:1122-1124.
3. Bowden LP, Royer MC, Hallman JR, et al. Rapid onset of argyria induced by a silver-containing dietary supplement. J Cutan Pathol. 2011;38:832-835. doi: 10.1111/j.1600-0560.2011.01755.x
4. Patterson J. Stasis dermatitis. In: Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier;2010: 121-153.
5. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73.e33. doi: 10.1016/j.jaad.2015.12.037
6. Barrett T, de Zwaan S. Picosecond alexandrite laser is superior to Q-switched Nd:YAG laser in treatment of minocycline-induced hyperpigmentation: a case study and review of the literature. J Cosmet Laser Ther. 2018;20:387-390. doi: 10.1080/14764172.2017.1418514
1. Wetter DA. Minocycline hyperpigmentation. Mayo Clin Proc. 2012;87:e33. doi: 10.1016/j.mayocp.2012.02.013
2. Chang MW. Chapter 67: Disorders of hyperpigmentation. In: Bolognia J, Schaffer J, Cerroni L, et al (eds). Dermatology. 4th ed. Elsevier; 2018:1122-1124.
3. Bowden LP, Royer MC, Hallman JR, et al. Rapid onset of argyria induced by a silver-containing dietary supplement. J Cutan Pathol. 2011;38:832-835. doi: 10.1111/j.1600-0560.2011.01755.x
4. Patterson J. Stasis dermatitis. In: Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier;2010: 121-153.
5. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-73.e33. doi: 10.1016/j.jaad.2015.12.037
6. Barrett T, de Zwaan S. Picosecond alexandrite laser is superior to Q-switched Nd:YAG laser in treatment of minocycline-induced hyperpigmentation: a case study and review of the literature. J Cosmet Laser Ther. 2018;20:387-390. doi: 10.1080/14764172.2017.1418514
Incidental skin finding
This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
Macules and abdominal pain
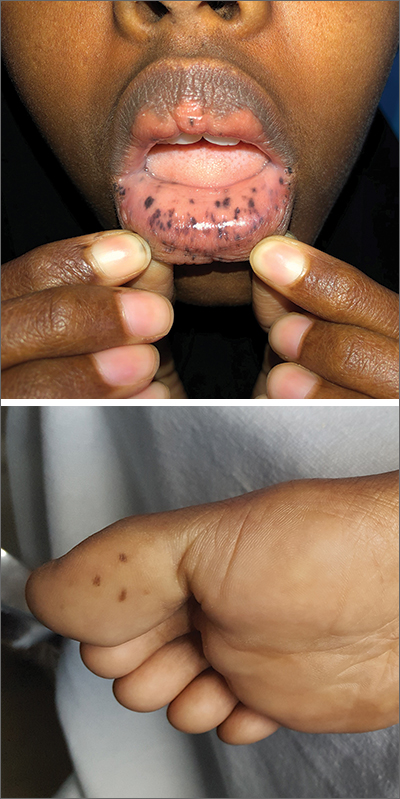
This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.

This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD

This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
FDA will review pediatric indication for roflumilast cream
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
, according to a press release from the manufacturer.
The company, Arcutis Biotherapeutics, announced the submission of a supplemental new drug application for approval of roflumilast cream (Zoryve), a topical phosphodiesterase-4 (PDE-4) inhibitor, to treat psoriasis in children aged 2-11 years. If approved, this would be the first such product for young children with plaque psoriasis, according to the press release. In July 2022, the FDA approved roflumilast cream 0.3% for the treatment of plaque psoriasis in people 12 years of age and older, including in intertriginous areas, based on data from the phase 3 DERMIS-1 and DERMIS-2 trials.
The new submission is supported by data from two 4-week Maximal Usage Systemic Exposure (MUSE) studies in children ages 2-11 years with plaque psoriasis. In these phase 2, open-label studies, one study of children aged 2-5 years and another study of children aged 6-11 years, participants were treated with roflumilast cream 0.3% once daily for 4 weeks. The MUSE studies are also intended to fulfill postmarketing requirements for roflumilast, according to the company. The MUSE results were consistent with those from DERMIS-1 and DERMIS-2, according to the company press release. In DERMIS-1 and DERMIS-2, significantly more patients randomized to roflumilast met criteria for Investigators Global Success (IGA) scores after 8 weeks of daily treatment compared with placebo patients, and significantly more achieved a 75% reduction in Psoriasis Area and Severity Index (PASI) scores compared with those on placebo.
Common adverse events associated with roflumilast include diarrhea, headache, insomnia, nausea, application site pain, upper respiratory tract infection, and urinary tract infection. None of these have been reported in more than 3% of patients, the press release noted.
FDA approves Idacio as eighth adalimumab biosimilar in U.S.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.
A biosimilar drug to the tumor necrosis factor inhibitor adalimumab, marketed as Idacio (adalimumab-aacf), has been approved by the Food and Drug Administration for use in the United States, according to a press release from manufacturer Fresenius Kabi.
Idacio is a citrate-free, low-concentration formulation of adalimumab and is now approved for use for all but three of the indications that currently apply to the reference adalimumab product (Humira): rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis, Crohn’s disease in adults and children aged 6 years or older, ulcerative colitis in adults, and plaque psoriasis in adults. It does not apply to Humira’s indications for hidradenitis suppurativa, uveitis, or ulcerative colitis in pediatric patients aged 5 years and older.
Idacio is the eighth adalimumab biosimilar to be approved in the United States. Its approval was based on evidence of a similar profile of pharmacokinetics, safety, efficacy, and immunogenicity to Humira.
Idacio was first launched in 2019 and has been marketed in more than 37 countries worldwide, according to Fresenius Kabi. The U.S. launch is scheduled for July, and Idacio will be available as a self-administered prefilled syringe or prefilled pen.
A version of this article first appeared on Medscape.com.