User login
Crusted Scabies Presenting as Erythroderma in a Patient With Iatrogenic Immunosuppression for Treatment of Granulomatosis With Polyangiitis
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.
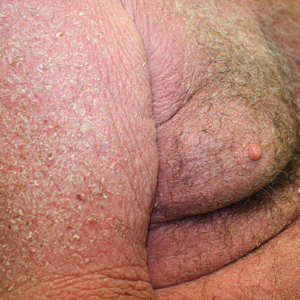
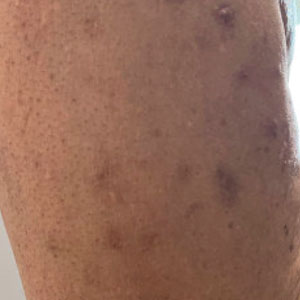
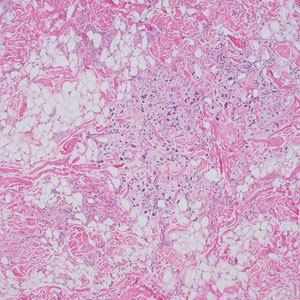
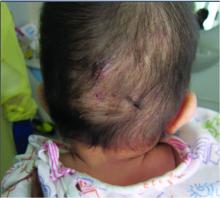
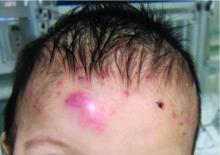
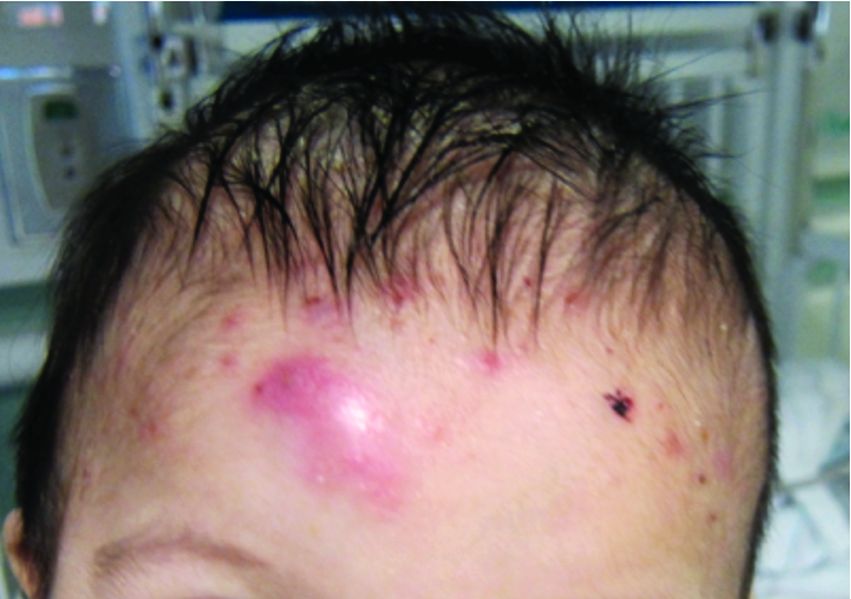
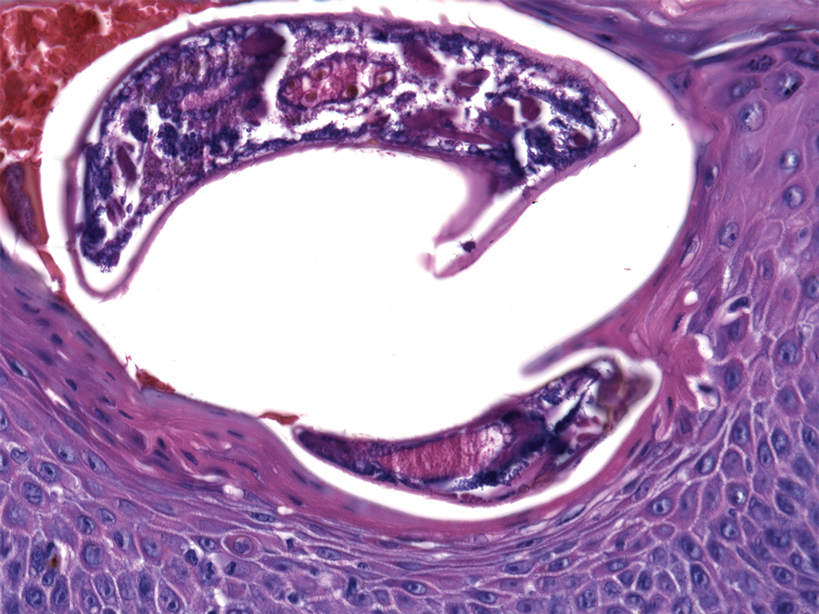
At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.
The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
Scabies is caused by cutaneous ectoparasitic infection by the mite Sarcoptes scabiei var hominis. The infection is highly contagious via direct skin-to-skin contact or indirectly through infested bedding, clothing or fomites.1,2 Scabies occurs at all ages, in all ethnic groups, and at all socioeconomic levels.1 Analysis by the Global Burden of Disease estimates that 200 million individuals have been infected with scabies worldwide. The World Health Organization has declared scabies a neglected tropical disease.3
Crusted scabies is a severe and rare form of scabies, with hyperinfestation of thousands to millions of mites, and more commonly is associated with immunosuppressed states, including HIV and hematologic malignancies.1,2,4 Crusted scabies has a high mortality rate due to sepsis when left untreated.3,5
Occasionally, iatrogenic immunosuppression contributes to the development of crusted scabies.1,2 Iatrogenic immunosuppression leading to crusted scabies most commonly occurs secondary to immunosuppression after bone marrow or solid organ transplantation.6 Less often, crusted scabies is caused by iatrogenic immunosuppression from other clinical scenarios.1,2
We describe a patient with iatrogenic immunosuppression due to azathioprine-induced myelosuppression for the treatment of granulomatosis with polyangiitis (GPA) who developed crusted scabies that clinically presented as erythroderma. Crusted scabies should be included in the differential diagnosis of erythroderma, especially in the setting of iatrogenic immunosuppression, for timely and appropriate management.
Case Report
An 84-year-old man presented with worsening pruritus, erythema, and thick yellow scale that progressed to erythroderma over the last 2 weeks. He was diagnosed with GPA 6 months prior to presentation and was treated with azathioprine 150 mg/d, prednisone 10 mg/d, and sulfamethoxazole 800 mg plus trimethoprim 160 mg twice weekly for prophylaxis against Pneumocystis jirovecii pneumonia.
Three weeks prior to presentation, the patient was hospitalized for pancytopenia attributed to azathioprine-induced myelosuppression (hemoglobin, 6.1 g/dL [reference range, 13.5–18.0 g/dL]; hematocrit, 17.5% [reference range, 42%–52%]; white blood cell count, 1.66×103/μL [reference range, 4.0–10.5×103/μL]; platelet count, 146×103/μL [reference range, 150–450×103/μL]; absolute neutrophil count, 1.29×103/μL [reference range, 1.4–6.5×103/μL]). He was transferred to a skilled nursing facility after discharge and referred to dermatology for evaluation of the worsening pruritic rash.

At the current presentation, the patient denied close contact with anyone who had a similar rash at home or at the skilled nursing facility. Physical examination revealed diffuse erythroderma with yellow scale on the scalp, trunk, arms, and legs (Figure 1). The palms showed scattered 2- to 3-mm pustules. The mucosal surfaces did not have lesions. A punch biopsy of a pustule from the right arm revealed focal spongiosis, parakeratosis, and acanthosis, as well as a perivascular and interstitial mixed inflammatory infiltrate with lymphocytes and eosinophils. Organisms morphologically compatible with scabies were found in the stratum corneum (Figure 2). Another punch biopsy of a pustule from the right arm was performed for direct immunofluorescence (DIF) and was negative for immunoglobulin deposition. Mineral oil preparation from pustules on the palm was positive for mites.

The patient was treated with permethrin cream 5% and oral ivermectin 200 μg/kg on day 1 and day 10. The prednisone dosage was increased from 10 mg/d to 50 mg/d and tapered over 2 weeks to treat the symptomatic rash and GPA. He remains on maintenance rituximab for GPA, without recurrence of scabies.
Comment
Pathogenesis—As an obligate parasite, S scabiei spends its entire life cycle within the host. Impregnated female mites burrow into the epidermis after mating and lay eggs daily for 1 to 2 months. Eggs hatch 2 or 3 days later. Larvae then migrate to the skin surface; burrow into the stratum corneum, where they mature into adults; and then mate on the skin surface.1,4
Clinical Presentation and Sequelae—Typically, scabies presents 2 to 6 weeks after initial exposure with generalized and intense itching and inflammatory pruritic papules on the finger webs, wrists, elbows, axillae, buttocks, umbilicus, genitalia, and areolae.1 Burrows are specific for scabies but may not always be present. Often, there are nonspecific secondary lesions, including excoriations, dermatitis, and impetiginization.
Complications of scabies can be severe, with initial colonization and infection of the skin resulting in impetigo and cellulitis. Systematic sequelae from local skin infection include post-streptococcal glomerulonephritis, rheumatic fever, and sepsis. Mortality from sepsis in scabies can be high.3,5
Classic Crusted Scabies and Other Variants—Crusted scabies presents with psoriasiform hyperkeratotic plaques involving the hands and feet with potential nail involvement that can become more generalized.1 Alterations in CD4+ T-cell function have been implicated in the development of crusted scabies, in which an excessive helper T cell (TH2) response is elicited against the ectoparasite, which may help explain the intense pruritus of scabies.6 Occasionally, iatrogenic immunosuppression contributes to development of crusted scabies,1 as was the case with our patient. However, it is rare for crusted scabies to present with erythroderma.7
Other atypical presentations of scabies include a seborrheic dermatitis–like presentation in infants, nodular lesions in the groin and axillae in more chronic scabies, and vesicles or bullous lesions.1
Diagnosis—Identification of mites, eggs, or feces is necessary for definitive diagnosis of scabies.8 These materials can be obtained through skin scrapings with mineral oil and observed under light microscopy or direct dermoscopy. Multiple scrapings on many lesions should be performed because failure to identify mites can be common and does not rule out scabies. Dermoscopic examination of active lesions under low power also can be helpful, given that identification of dark brown triangular structures can correspond to visualization of the pigmented anterior section of the mite.9-11 A skin biopsy can help identify mites, but histopathology often shows a nonspecific hypersensitivity reaction.12 Therefore, empiric treatment often is necessary.
Differential Diagnosis—The differential diagnosis of erythroderma is broad and includes a drug eruption; Sézary syndrome; and pre-existing skin diseases, including psoriasis, atopic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, and bullous pemphigoid. Histopathology is critical to differentiate these diagnoses. Bullous pemphigoid and pemphigus foliaceus are immunobullous diseases that typically are positive for immunoglobulin deposition on DIF. In rare cases, scabies also can present with bullae and positive DIF test results.13
Treatment—First-line treatment of crusted scabies in the United States is permethrin cream 5%, followed by oral ivermectin 200 μg/kg.4,5,14,15 Other scabicides include topicals such as benzyl benzoate 10% to 25%; precipitated sulfur 2% to 10%; crotamiton 10%; malathion 0.5%; and lindane 1%.5 The association of neurotoxicity with lindane has considerably reduced the drug’s use.1
During treatment of scabies, it is important to isolate patients to mitigate the possibility of spread.4 Pruritus can persist for a few weeks after completion of therapy.5 Patients should be closely monitored to ensure that this symptom is secondary to skin inflammation and not incomplete treatment.
Treatment of crusted scabies may require repeated treatments to decrease the notable mite burden as well as the associated crusting and scale. Adding a keratolytic such as 5% to 10% salicylic acid in petrolatum to the treatment regimen may be useful for breaking up thick scale.5
Immunosuppression—With numerous immunomodulatory drugs for treating autoimmunity comes an increased risk for iatrogenic immunosuppression that may contribute to the development of crusted scabies.16 In a number of autoimmune diseases such as rheumatoid arthritis,17-19 psoriasis,20,21 pemphigus vulgaris,22 systemic lupus erythematosus,23 systemic sclerosis,22,24 bullous pemphigoid,25,26 and dermatomyositis,27 patients have developed crusted scabies secondary to treatment-related immunosuppression. These immunosuppressive therapies include systemic steroids,22-24,26-31 methotrexate,23 infliximab,18 adalimumab,21 toclizumab,19 and etanercept.20 In a case of drug-induced Stevens-Johnson syndrome, the patient developed crusted scabies during long-term use of oral steroids.22
Patients with a malignancy who are being treated with chemotherapy also can develop crusted scabies.28 Crusted scabies has even been associated with long-term topical steroid32-34 and topical calcineurin inhibitor use.16
Iatrogenic immunosuppression in our patient resulted from treatment of GPA with azathioprine, an immunosuppressive drug that acts as an antagonist of the breakdown of purines, leading to inhibition of DNA, RNA, and protein synthesis.35 On occasion, azathioprine can induce immunosuppression in the form of myelosuppression and resulting pancytopenia, as was the case with our patient.
Conclusion
Although scabies is designated as a neglected tropical disease by the World Health Organization, it still causes a notable burden worldwide, regardless of the economics. Our case highlights an unusual presentation of scabies as erythroderma in the setting of iatrogenic immunosuppression from azathioprine use. Dermatologists should consider crusted scabies in the differential diagnosis of erythroderma, especially in immunocompromised patients, to avoid delays in diagnosis and treatment. Immunosuppressive therapy is an important mainstay in the treatment of many conditions, but it is important to consider that these medications can place patients at an increased risk for rare opportunistic infections. Therefore, patients receiving such treatment should be closely monitored.
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
- Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006;354:1718-1727. doi:10.1056/NEJMcp052784
- Salgado F, Elston DM. What’s eating you? scabies in the developing world. Cutis. 2017;100:287-289.
- Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247-1254. doi:10.1016/S1473-3099(17)30483-8
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Wang X-D, Shen H, Liu Z-H. Contagious erythroderma. J Emerg Med. 2016;51:180-181. doi:10.1016/j.jemermed.2016.05.027
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622. doi:10.1136/bmj.331.7517.619
- Micali G, Lacarrubba F, Massimino D, et al. Dermatoscopy: alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135-1146. doi:10.1016/j.jaad.2010.03.010
- Bollea Garlatti LA, Torre AC, Bollea Garlatti ML, et al.. Dermoscopy aids the diagnosis of crusted scabies in an erythrodermic patient. J Am Acad Dermatol. 2015;73:E93-E95. doi:10.1016/j.jaad.2015.04.061
- Tang J, You Z, Ran Y. Simple methods to enhance the diagnosis of scabies. J Am Acad Dermatol. 2019;80:E99-E100. doi:10.1016/j.jaad.2017.07.038
- Falk ES, Eide TJ. Histologic and clinical findings in human scabies. Int J Dermatol. 1981;20:600-605. doi:10.1111/j.1365-4362.1981.tb00844.x
- Shahab RKA, Loo DS. Bullous scabies. J Am Acad Dermatol. 2003;49:346-350. doi:10.1067/s0190-9622(03)00876-4
- Strong M, Johnstone P. Interventions for treating scabies. Cochrane Database Syst Rev. 2007:CD000320. doi:10.1002/14651858.CD000320.pub2
- Rosumeck S, Nast A, Dressler C. Evaluation of ivermectin vs permethrin for treating scabies—summary of a Cochrane Review. JAMA Dermatol. 2019;155:730-732. doi:10.1001/jamadermatol.2019.0279
- Ruiz-Maldonado R. Pimecrolimus related crusted scabies in an infant. Pediatr Dermatol. 2006;23:299-300. doi:10.1111/j.1525-1470.2006.00241.x
- Bu X, Fan J, Hu X, et al. Norwegian scabies in a patient treated with Tripterygium glycoside for rheumatoid arthritis. An Bras Dermatol. 2017;92:556-558. doi:10.1590/abd1806-4841.20174946
- Pipitone MA, Adams B, Sheth A, et al. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol. 2005;52:719-720. doi:10.1016/j.jaad.2004.12.039
- Baccouche K, Sellam J, Guegan S, et al. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine. 2011;78:402-404. doi:10.1016/j.jbspin.2011.02.008
- Saillard C, Darrieux L, Safa G. Crusted scabies complicates etanercept therapy in a patient with severe psoriasis. J Am Acad Dermatol. 2013;68:E138-E139. doi:10.1016/j.jaad.2012.09.049
- Belvisi V, Orsi GB, Del Borgo C, et al. Large nosocomial outbreakassociated with a Norwegian scabies index case undergoing TNF-α inhibitor treatment: management and control. Infect Control Hosp Epidemiol. 2015;36:1358-1360. doi:10.1017/ice.2015.188
- Nofal A. Variable response of crusted scabies to oral ivermectin: report on eight Egyptian patients. J Eur Acad Dermatol Venereol. 2009;23:793-797. doi:10.1111/j.1468-3083.2009.03177.x
- Yee BE, Carlos CA, Hata T. Crusted scabies of the scalp in a patient with systemic lupus erythematosus. Dermatol Online J. 2014;20:13030/qt9dm891gd.
- Bumb RA, Mehta RD. Crusted scabies in a patient of systemic sclerosis. Indian J Dermatol Venereol Leprol. 2000;66:143-144.
- Hylwa SA, Loss L, Grassi M. Crusted scabies and tinea corporis after treatment of presumed bullous pemphigoid. Cutis. 2013;92:193-198.
- Svecova D, Chmurova N, Pallova A, et al. Norwegian scabies in immunosuppressed patient misdiagnosed as an adverse drug reaction. Epidemiol Mikrobiol Imunol. 2009;58:121-123.
- Dourmishev AL, Serafimova DK, Dourmishev LA, et al. Crusted scabies of the scalp in dermatomyositis patients: three cases treated with oral ivermectin. Int J Dermatol. 1998;37:231-234. doi:10.1046/j.1365-4362.1998.00330.x
- Mortazavi H, Abedini R, Sadri F, et al. Crusted scabies in a patient with brain astrocytoma: report of a case. Int J Infect Dis. 2010;14:E526-E527. doi:10.1016/j.ijid.2009.06.011
- Lima FCDR, Cerqueira AMM, MBS, et al. Crusted scabies due to indiscriminate use of glucocorticoid therapy in infant. An Bras Dermatol. 2017;92:383-385. doi:10.1590/abd1806-4841.20174433
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2010;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Ohtaki N, Taniguchi H, Ohtomo H. Oral ivermectin treatment in two cases of scabies: effective in crusted scabies induced by corticosteroid but ineffective in nail scabies. J Dermatol. 2003;30:411-416. doi:10.1111/j.1346-8138.2003.tb00408.x
- Bilan P, Colin-Gorski AM, Chapelon E, et al. Crusted scabies induced by topical corticosteroids: a case report [in French]. Arch Pediatr. 2015;22:1292-1294. doi:10.1016/j.arcped.2015.09.004
- Marlière V, Roul S, C, et al. Crusted (Norwegian) scabies induced by use of topical corticosteroids and treated successfully with ivermectin. J Pediatr. 1999;135:122-124. doi:10.1016/s0022-3476(99)70342-2
- Jaramillo-Ayerbe F, J. Ivermectin for crusted Norwegian scabies induced by use of topical steroids. Arch Dermatol. 1998;134:143-145. doi:10.1001/archderm.134.2.143
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41-47. doi:10.1126/science.2649979
Practice Points
- Crusted scabies is a highly contagious, severe cutaneous ectoparasitic infection that can present atypically in the form of erythroderma.
- Immunomodulatory drugs for the treatment of autoimmune disease can predispose patients to infection, including ectoparasitic infection.
- Dermatologists should be familiar with the full scope of the clinical presentations of scabies and should especially consider this condition in the differential diagnosis of patients who present in an immunosuppressed state.
Primary Effusion Lymphoma: An Infiltrative Plaque in a Patient With HIV
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.
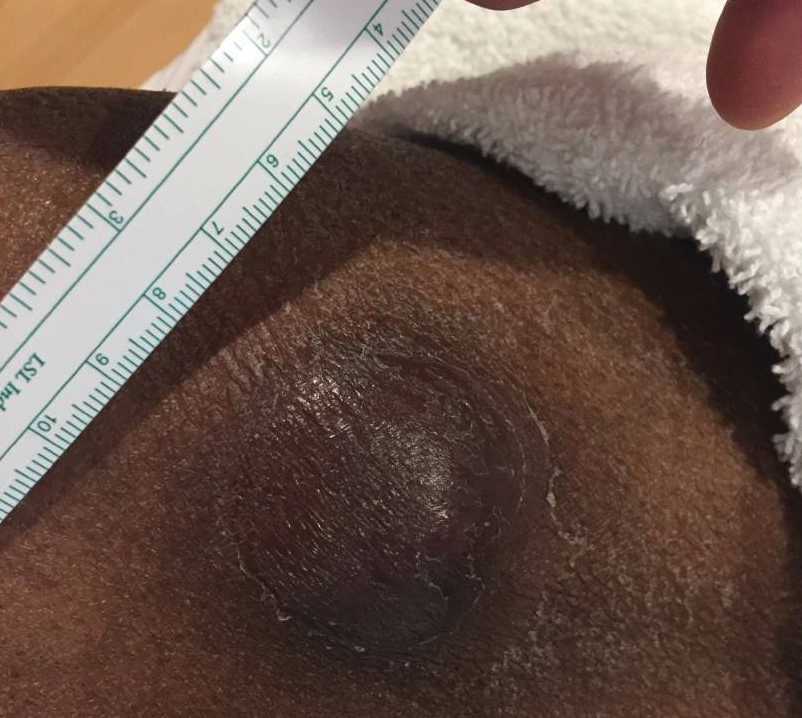
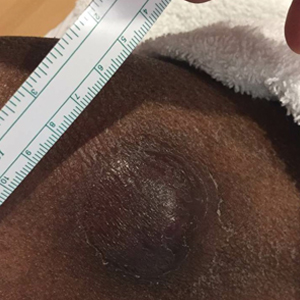
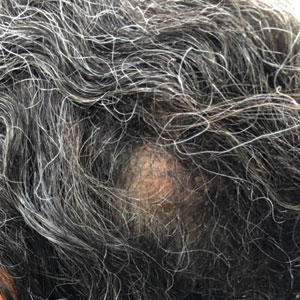
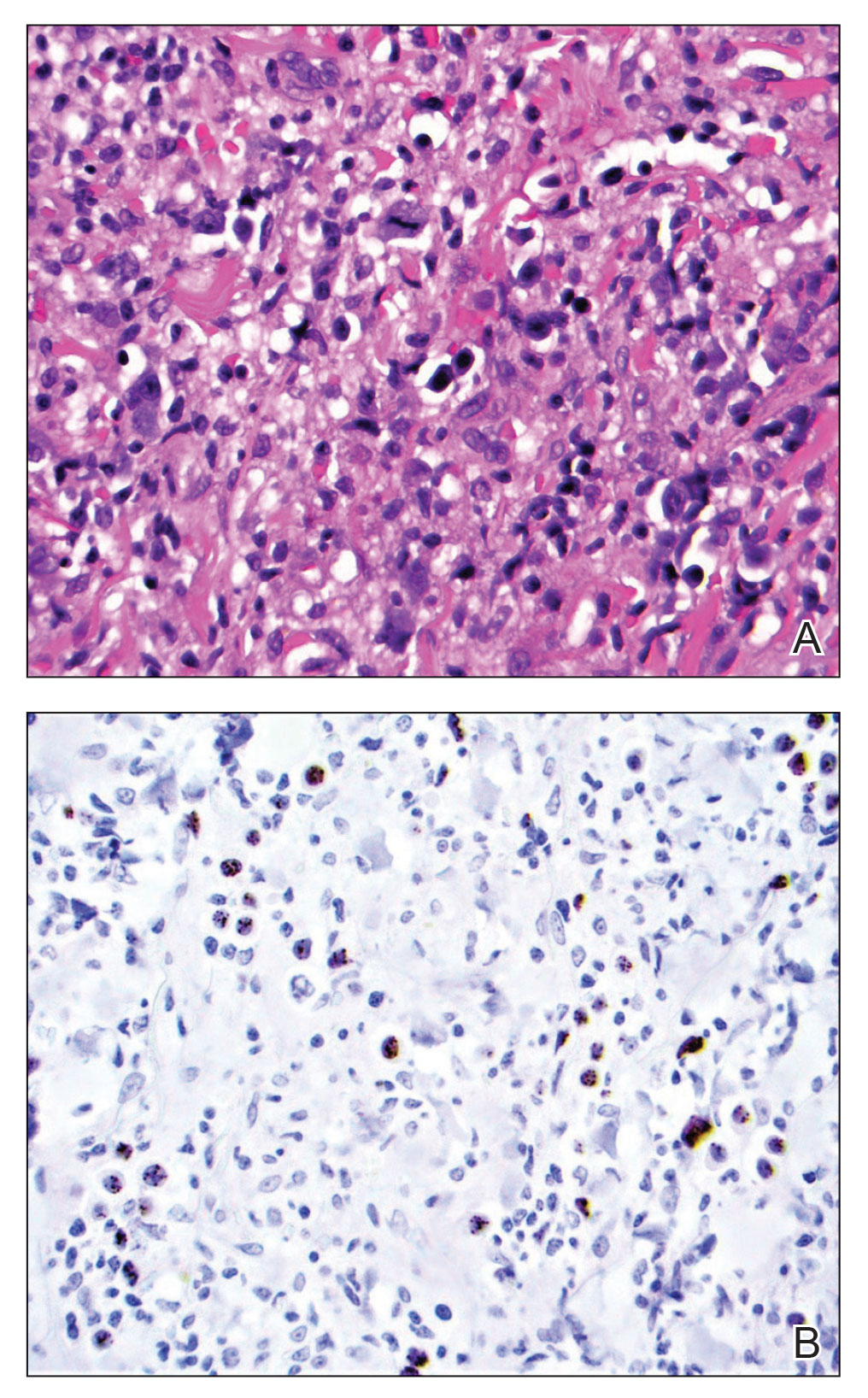
At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.
Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.
Papular Acneform Eruption With Mucositis
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.
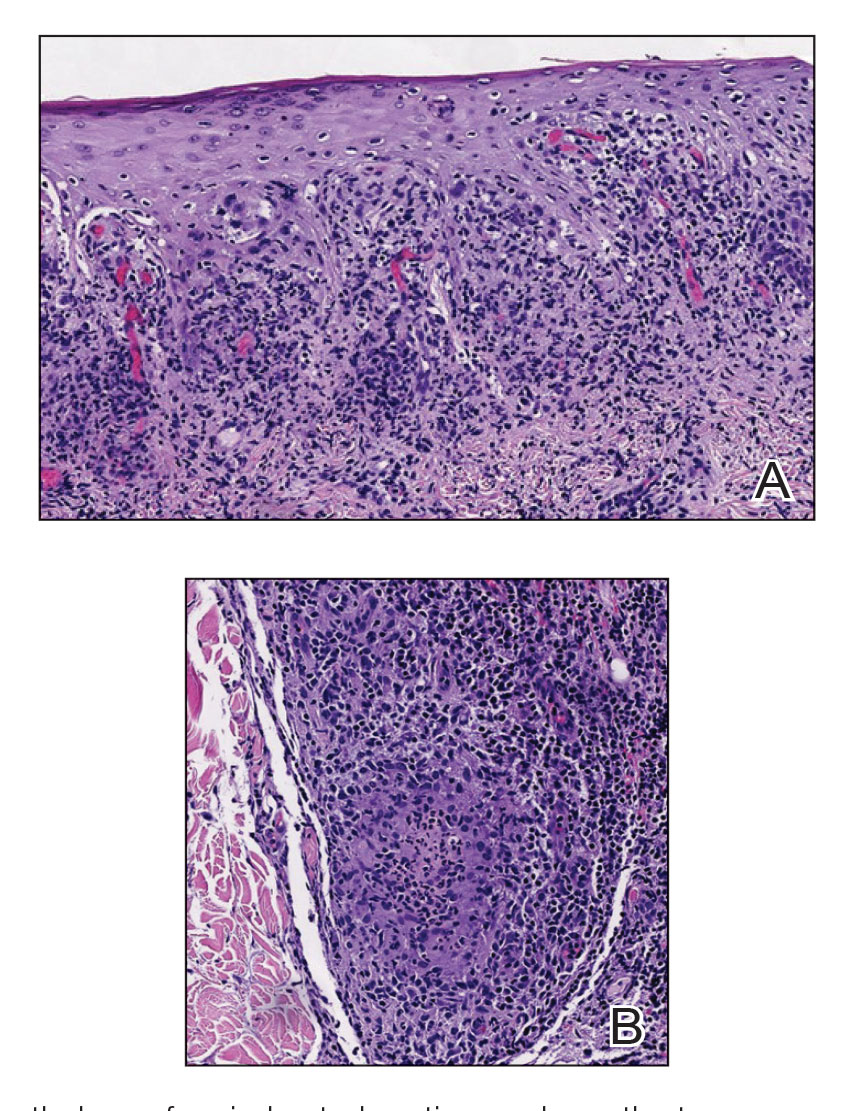
Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
A 48-year-old man with a history of ulcerative colitis that was well-controlled with adalimumab presented with a generalized acneform eruption involving the face, chest (top) and back, as well as a well-defined ovoid ulcer on the anterior aspect of the tongue (bottom) of 2 months’ duration. Prior treatment with prednisone 60 mg daily for 14 days resulted in no improvement. He denied unintentional weight loss, cyclic fever, or arthritis. A complete blood cell count with differential showed mild anemia (hemoglobin, 11.6 g/dL [reference range, 13.2–16.6 g/dL]) with a differential cell count that was within reference range for each cell type. The erythrocyte sedimentation rate was elevated at 44 mm/h (reference range, 0–22 mm/h). A 4-mm punch biopsy specimen of an indurated cystic papule on the torso was obtained.
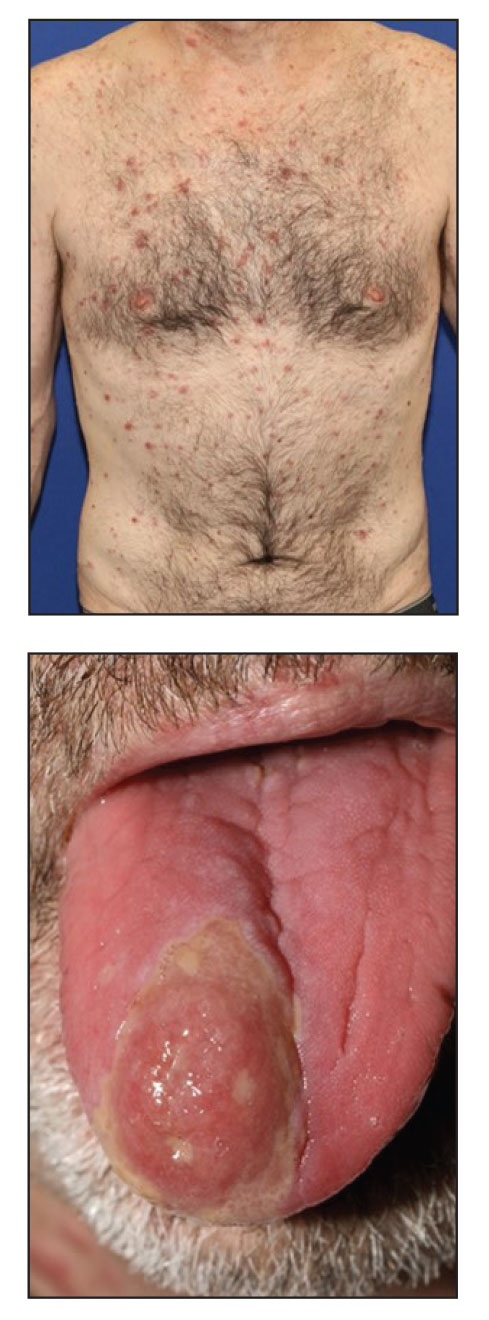
Expunging ‘penicillin allergy’: Your questions answered
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Papular Reticulated Rash
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).
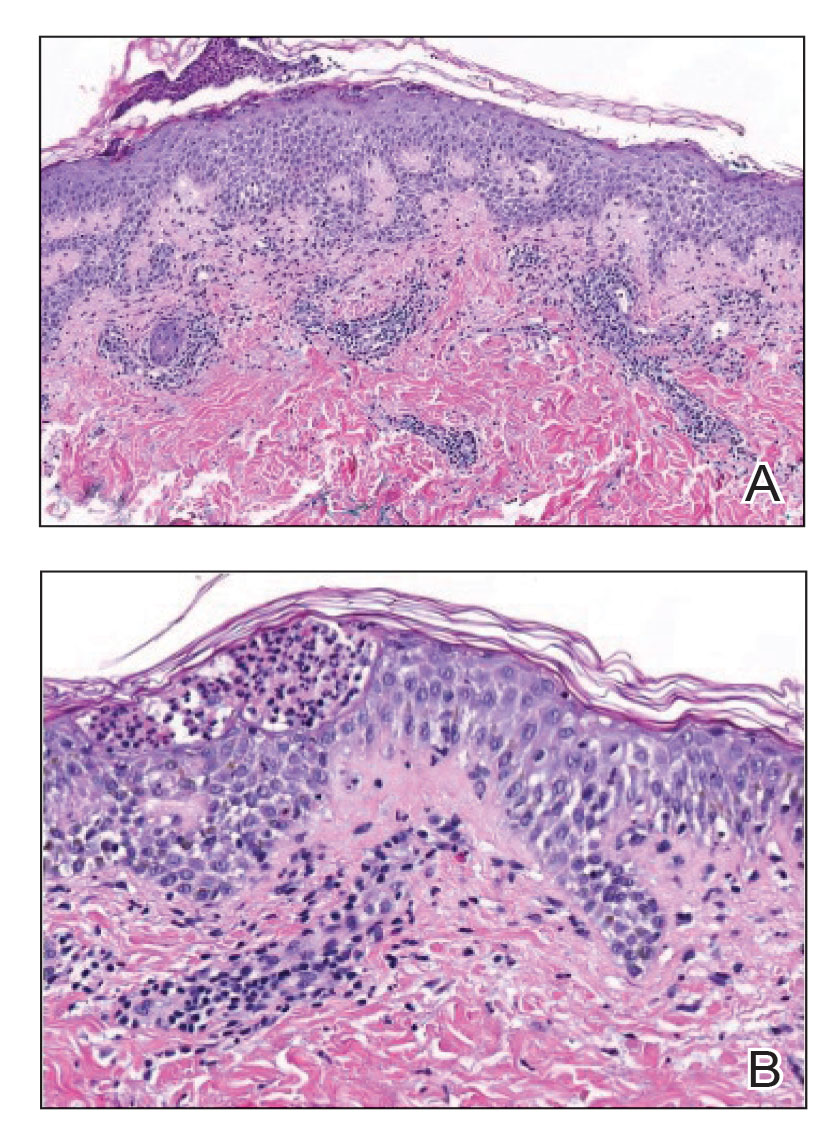
Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
An otherwise healthy 22-year-old woman presented with a painful eruption with burning and pruritus that had been slowly worsening as it spread over the last 4 weeks. The rash first appeared on the lower chest and inframammary folds (top) and spread to the upper chest, neck, back (bottom), arms, and lower face. Physical examination revealed multiple illdefined, erythematous papules, patches, and plaques on the chest, back, neck, and upper abdomen. Individual lesions coalesced into plaques that displayed a reticular configuration. There were no lesions in the axillae. The patient had been following a low-carbohydrate diet for 4 months. A punch biopsy was performed.
Miliarial Gout in an Immunocompromised Patient
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.
A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.
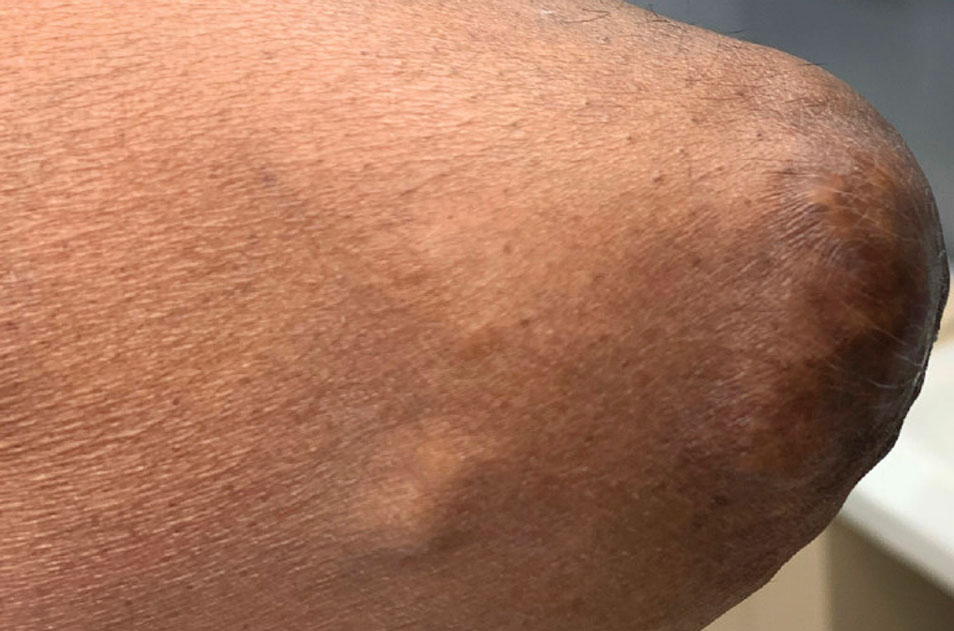
He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.
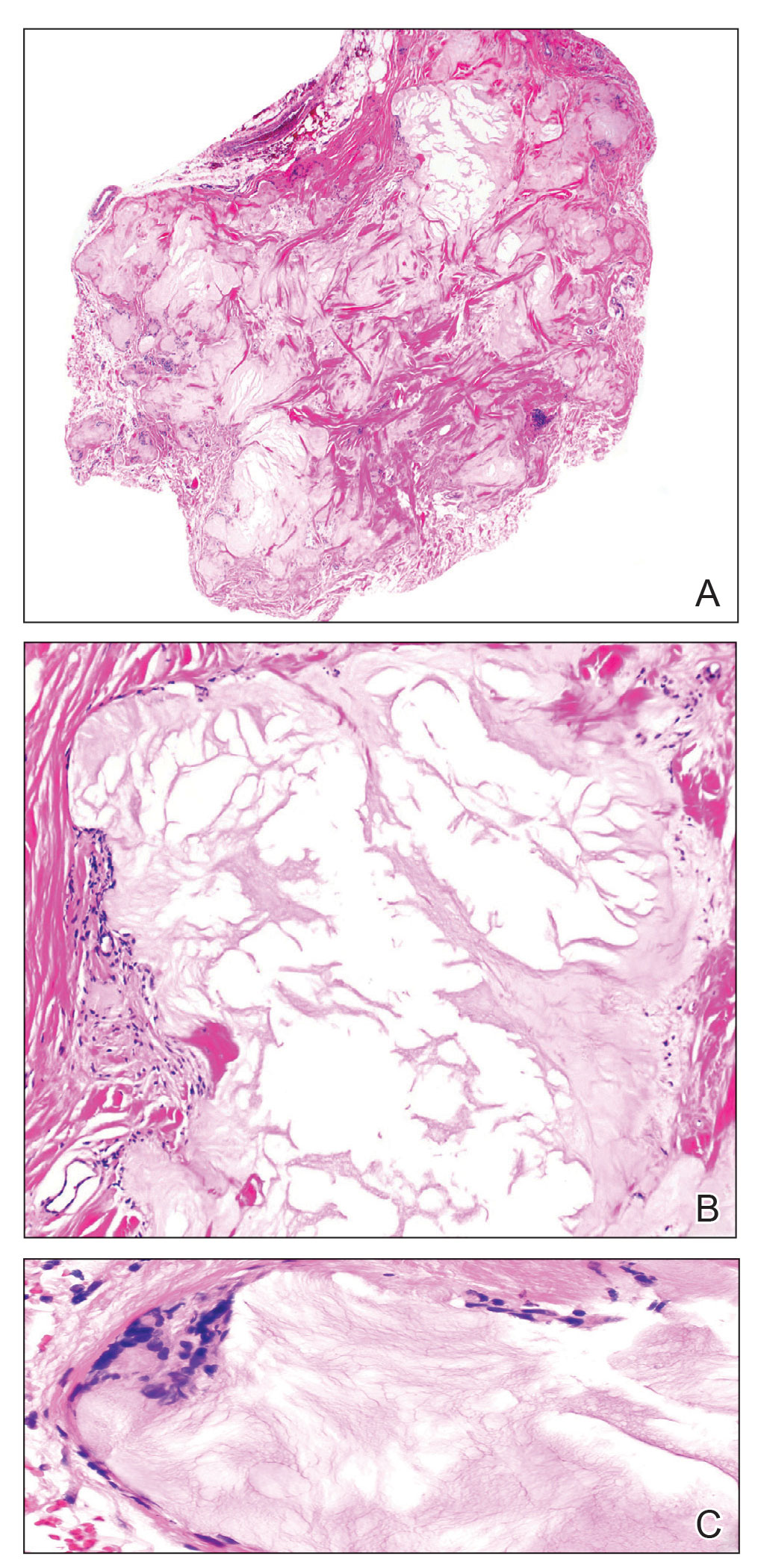
Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
Practice Points
- Miliarial gout is a rare intradermal manifestation of tophaceous gout and often presents as multiple small papules containing a white- to cream-colored substance.
- Immunocompromised status may be a risk factor for miliarial gout, especially in patients with a history of gout or hyperuricemia.
- Effective treatments for miliarial gout include allopurinol and colchicine.
Subcutaneous Nodule on the Postauricular Neck
The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5
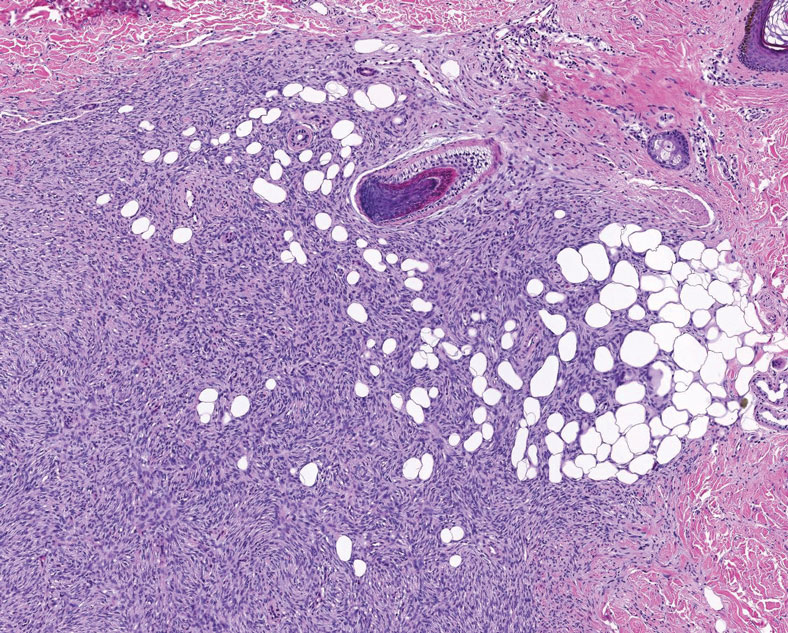
Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8
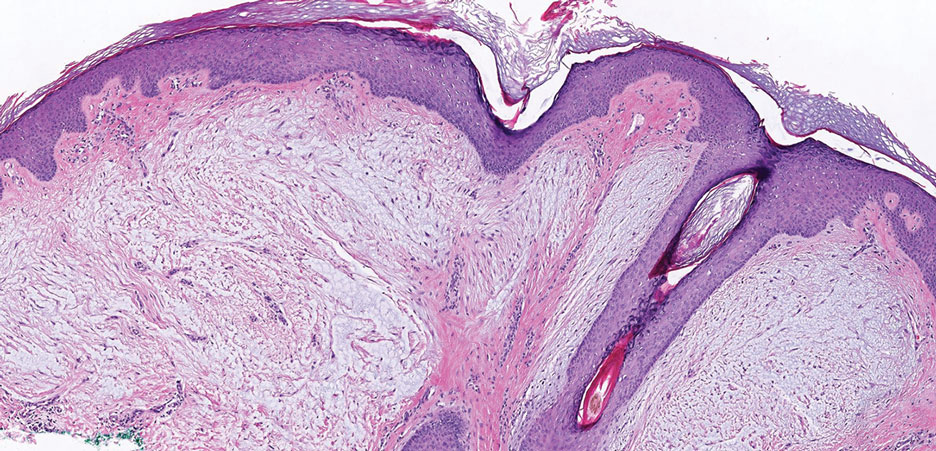
Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9
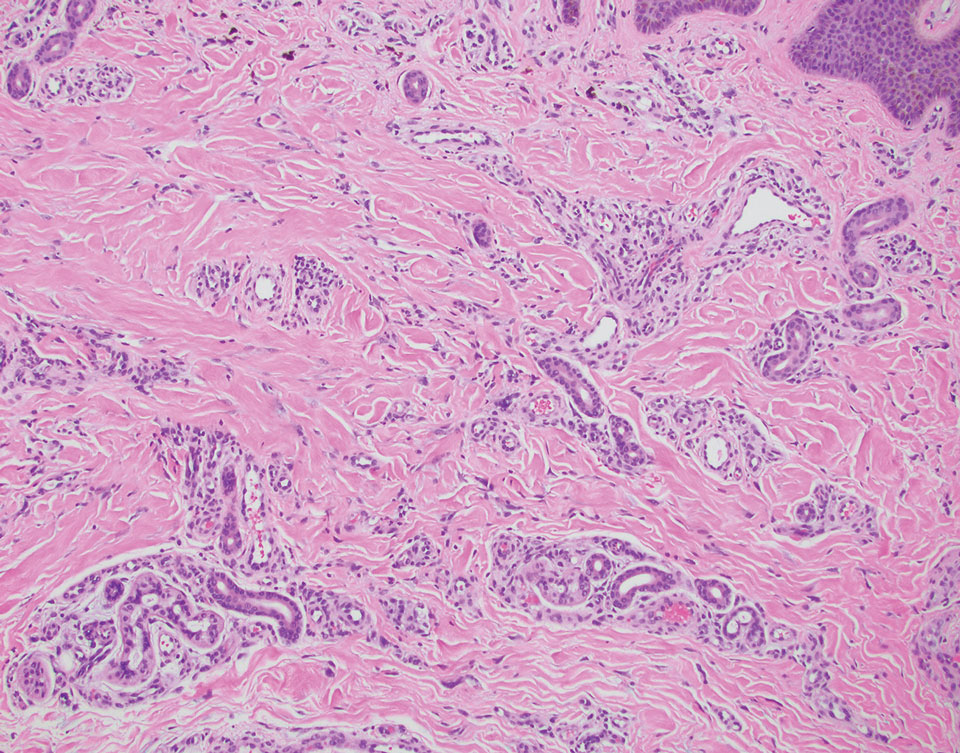
Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13
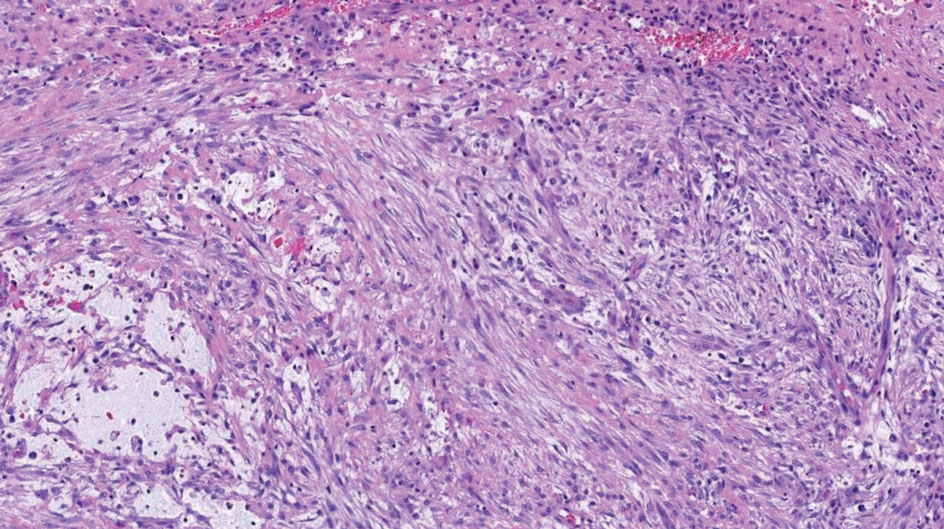
- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5

Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8

Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9

Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13

The Diagnosis: Pleomorphic Lipoma
Pleomorphic lipoma is a rare, benign, adipocytic neoplasm that presents in the subcutaneous tissues of the upper shoulder, back, or neck. It predominantly affects men aged 50 to 70 years. Most lesions are situated in the subcutaneous tissues; few cases of intramuscular and retroperitoneal tumors have been reported.1 Clinically, pleomorphic lipomas present as painless, well-circumscribed lesions of the subcutaneous tissue that often resemble a lipoma or occasionally may be mistaken for liposarcoma. Histopathologic examination of ordinary lipomas reveals uniform mature adipocytes. However, pleomorphic lipomas consist of a mixture of multinucleated floretlike giant cells, variable-sized adipocytes, and fibrous tissue (ropy collagen bundles) with some myxoid and spindled areas.1,2 The most characteristic histologic feature of pleomorphic lipoma is multinucleated floretlike giant cells. The nuclei of these giant cells appear hyperchromatic, enlarged, and disposed to the periphery of the cell in a circular pattern. Additionally, tumors frequently contain excess mature dense collagen bundles that are strongly refractile in polarized light. Numerous mast cells are present. Atypical lipoblasts and capillary networks commonly are not visible in pleomorphic lipoma.3 The spindle cells express CD34 on immunohistochemistry. Loss of Rb-1 expression is typical.4
Dermatofibrosarcoma protuberans is a slow-growing soft tissue sarcoma that commonly begins as a pink or violet plaque on the trunk or upper limbs. Involvement of the head or neck accounts for only 10% to 15% of cases.5 This tumor has low metastatic potential but is highly infiltrative of surrounding tissues. It is associated with a translocation between chromosomes 22 and 17, leading to the fusion of the platelet-derived growth factor subunit β, PDGFB, and collagen type 1α1, COL1A1, genes.5 Clinically, patients often report that the lesion was present for several years prior to presentation with general stability in size and shape. Eventually, untreated lesions progress to become nodules or tumors and may even bleed or ulcerate. Histology reveals a storiform spindle cell proliferation throughout the dermis with infiltration into subcutaneous fat, commonly appearing in a honeycomblike pattern (Figure 1). Numerous histologic variants exist, including myxoid, sclerosing, pigmented (Bednar tumor), myoid, atrophic, or fibrosarcomatous dermatofibrosarcoma protuberans, as well as a giant cell fibroblastoma variant.6 These tumor subtypes can exist independently or in association with one another, creating hybrid lesions that can closely mimic other entities such as pleomorphic lipoma. The spindle cells stain positively for CD34. Treatment of these tumors involves complete surgical excision or Mohs micrographic surgery; however, recurrence is common for tumors involving the head or neck.5

Superficial angiomyxoma is a slow-growing papule that most commonly appears on the trunk, head, or neck in middle-aged adults. Occasionally, patients with Carney complex also can develop lesions on the external ear or breast.7 Histologically, superficial angiomyxoma is a hypocellular tumor characterized by abundant myxoid stroma, thin blood vessels, and small spindled and stellate cells with minimal cytoplasm (Figure 2).8 Superficial angiomyxoma and pleomorphic lipoma present differently on histology; superficial angiomyxoma is not associated with nuclear atypia or pleomorphism, whereas pleomorphic lipoma characteristically contains multinucleated floretlike giant cells and pleomorphism. Frequently, there also is loss of normal PRKAR1A gene expression, which is responsible for protein kinase A regulatory subunit 1-alpha expression.8

Multinucleate cell angiohistiocytoma is a rare benign proliferation that presents with numerous red-violet asymptomatic papules that commonly appear on the upper and lower extremities of women aged 40 to 70 years. Lesions feature both a fibrohistiocytic and vascular component.9 Histologic examination commonly shows multinucleated cells with angular outlining in the superficial dermis accompanied by fibrosis and ectatic small-caliber vessels (Figure 3). Although both pleomorphic lipoma and multinucleate cell angiohistiocytoma have similar-appearing multinucleated giant cells, the latter has a proliferation of narrow vessels in thick collagen bundles and lacks an adipocytic component, which distinguishes it from the former.10 Multinucleate cell angiohistiocytoma also is characterized by a substantial number of factor XIIIa–positive fibrohistiocytic interstitial cells and vascular hyperplasia.9

Nodular fasciitis is a benign lesion involving the rapid proliferation of myofibroblasts and fibroblasts in the subcutaneous tissue and most commonly is encountered on the extremities or head and neck regions. Many cases appear at sites of prior trauma, especially in patients aged 20 to 40 years. However, in infants and children the lesions typically are found in the head and neck regions.11 Clinically, lesions present as subcutaneous nodules. Histology reveals an infiltrative and poorly circumscribed proliferation of spindled myofibroblasts associated with myxoid stroma and dense collagen depositions. The spindled cells are loosely associated, rendering a tissue culture–like appearance (Figure 4). It also is common to see erythrocyte extravasation adjacent to myxoid stroma.11 Positive stains include vimentin, smooth muscle actin, and CD68, though immunohistochemistry often is not necessary for diagnosis.12 There often is abundant mitotic activity in nodular fasciitis, especially in early lesions, and the differential diagnosis includes sarcoma. Although nodular fasciitis is mitotically active, it does not show atypical mitotic figures. Nodular fasciitis commonly harbors a gene translocation of the MYH9 gene’s promoter region to the USP6 gene’s coding region.13

- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
- Sakhadeo U, Mundhe R, DeSouza MA, et al. Pleomorphic lipoma: a gentle giant of pathology. J Cytol. 2015;32:201-203. doi:10.4103 /0970-9371.168904
- Shmookler BM, Enzinger FM. Pleomorphic lipoma: a benign tumor simulating liposarcoma. a clinicopathologic analysis of 48 cases. Cancer. 1981;47:126-133.
- Azzopardi JG, Iocco J, Salm R. Pleomorphic lipoma: a tumour simulating liposarcoma. Histopathology. 1983;7:511-523. doi:10.1111/j.1365-2559.1983.tb02264.x
- Jäger M, Winkelmann R, Eichler K, et al. Pleomorphic lipoma. J Dtsch Dermatol Ges. 2018;16:208-210. doi:10.1111/ddg.13422
- Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma protuberans. Dermatol Clin. 2019;37:483-488. doi:10.1016/j.det.2019.05.006
- Socoliuc C, Zurac S, Andrei R, et al. Multiple histological subtypes of dermatofibrosarcoma protuberans occurring in the same tumor. Rom J Intern Med. 2015;53:79-88. doi:10.1515/rjim-2015-0011
- Abarzúa-Araya A, Lallas A, Piana S, et al. Superficial angiomyxoma of the skin. Dermatol Pract Concept. 2016;6:47-49. doi:10.5826 /dpc.0603a09
- Hornick J. Practical Soft Tissue Pathology A Diagnostic Approach. 2nd ed. Elsevier Health Sciences; 2017.
- Rato M, Monteiro AF, Parente J, et al. Case for diagnosis. multinucleated cell angiohistiocytoma. An Bras Dermatol. 2018;93:291-293. doi:10.1590 /abd1806-4841.20186821
- Grgurich E, Quinn K, Oram C, et al. Multinucleate cell angiohistiocytoma: case report and literature review. J Cutan Pathol. 2019;46:59-61. doi:10.1111/cup.13361
- Zuber TJ, Finley JL. Nodular fasciitis. South Med J. 1994;87:842-844. doi:10.1097/00007611-199408000-00020
- Yver CM, Husson MA, Friedman O. Pathology clinic: nodular fasciitis involving the external ear [published online March 18, 2021]. Ear Nose Throat J. doi:10.1177/01455613211001958
- Erickson-Johnson M, Chou M, Evers B, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433. https://doi.org/10.1038 /labinvest.2011.118
An otherwise healthy 56-year-old man with a family history of lymphoma presented with a raised lesion on the postauricular neck. He first noticed the nodule 3 months prior and was unsure if it was still getting larger. It was predominantly asymptomatic. Physical examination revealed a 1.5×1.5-cm, mobile, subcutaneous nodule. An incisional biopsy was performed and submitted for histologic evaluation.
Collision Course of a Basal Cell Carcinoma and Apocrine Hidrocystoma on the Scalp
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.
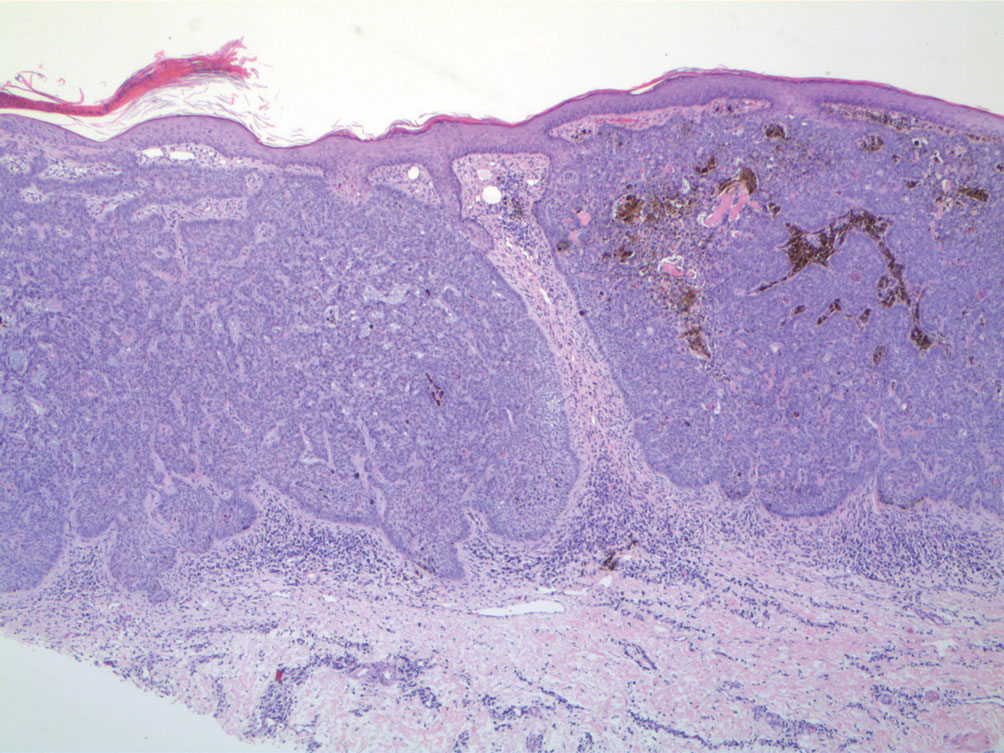
A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.
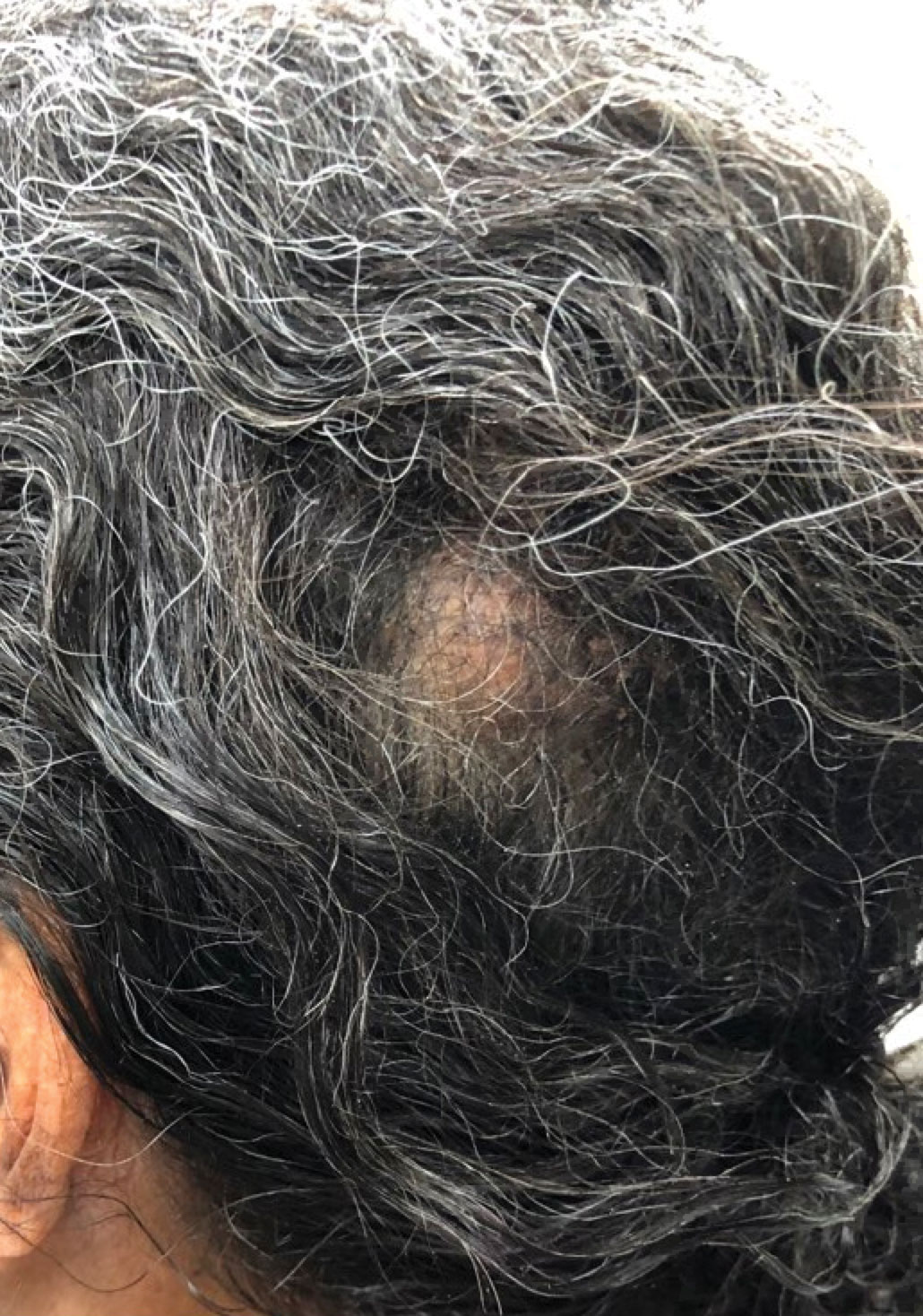
The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).
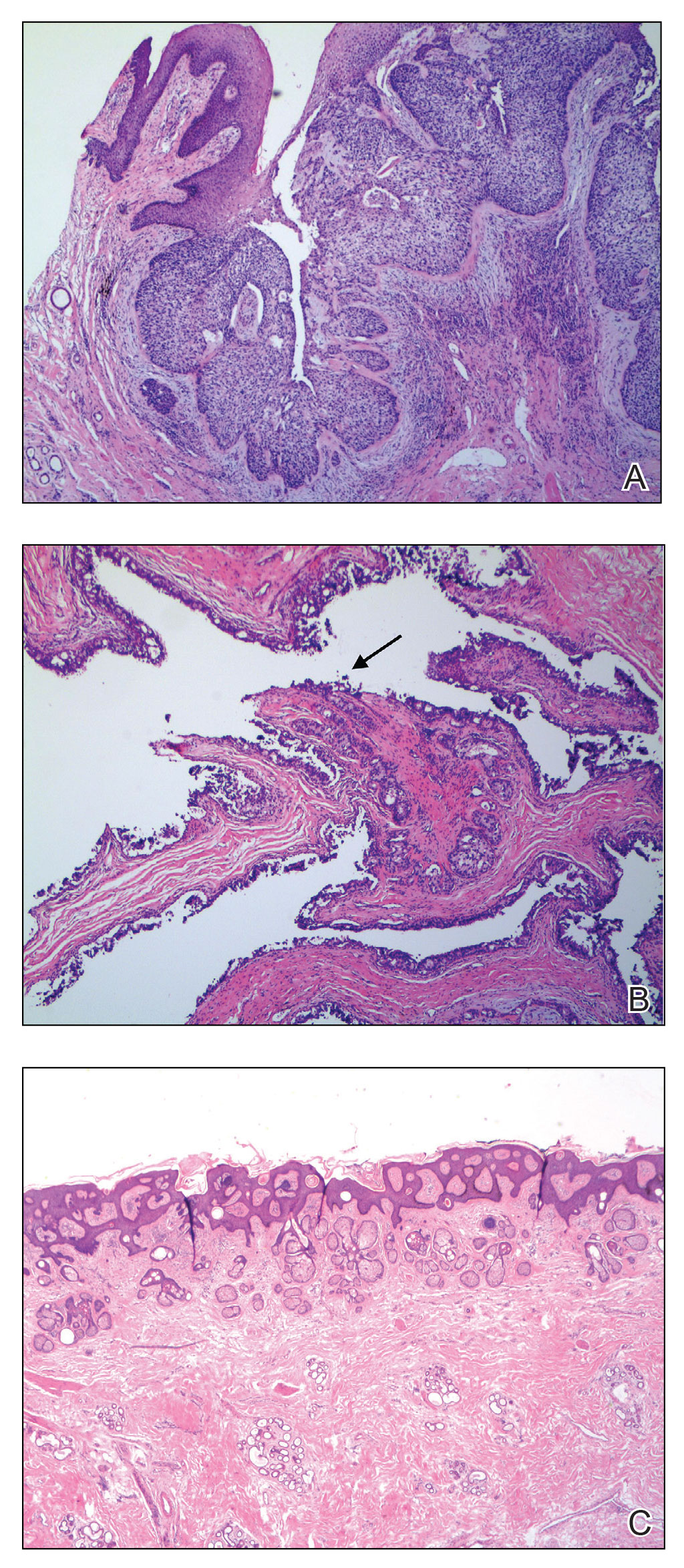
The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
To the Editor:
A collision tumor is the coexistence of 2 discrete tumors in the same neoplasm, possibly comprising a malignant tumor and a benign tumor, and thereby complicating appropriate diagnosis and treatment. We present a case of a basal cell carcinoma (BCC) of the scalp that was later found to be in collision with an apocrine hidrocystoma that might have arisen from a nevus sebaceus. Although rare, BCC can coexist with apocrine hidrocystoma. Jayaprakasam and Rene1 reported a case of a collision tumor containing BCC and hidrocystoma on the eyelid.1 We present a case of a BCC on the scalp that was later found to be in collision with an apocrine hidrocystoma that possibly arose from a nevus sebaceus.

A 92-year-old Black woman with a biopsy-confirmed primary BCC of the left parietal scalp presented for Mohs micrographic surgery. The pathology report from an outside facility was reviewed. The initial diagnosis had been made with 2 punch biopsies from separate areas of the large nodule—one consistent with nodular and pigmented BCC (Figure 1), and the other revealed nodular ulcerated BCC. Physical examination prior to Mohs surgery revealed a mobile, flesh-colored, 6.2×6.0-cm nodule with minimal overlying hair on the left parietal scalp (Figure 2). During stage-I processing by the histopathology laboratory, large cystic structures were encountered; en face frozen sections showed a cystic tumor. Excised tissue was submitted for permanent processing to aid in diagnosis; the initial diagnostic biopsy slides were requested from the outside facility for review.

The initial diagnostic biopsy slides were reviewed and found to be consistent with nodular and pigmented BCC, as previously reported. Findings from hematoxylin and eosin staining of tissue obtained from Mohs sections were consistent with a combined neoplasm comprising BCC (Figure 3A) and apocrine hidrocystoma (Figure 3B). In addition, one section was characterized by acanthosis, papillomatosis, and sebaceous glands—similar to findings that are seen in a nevus sebaceus (Figure 3C).

The BCC was cleared after stage I; the final wound size was 7×6.6 cm. Although benign apocrine hidrocystoma was still evident at the margin, further excision was not performed at the request of the patient and her family. Partial primary wound closure was performed with pulley sutures. A xenograft was placed over the unclosed central portion. The wound was permitted to heal by second intention.
The clinical differential diagnosis of a scalp nodule includes a pilar cyst, BCC, squamous cell carcinoma, melanoma, cutaneous metastasis, adnexal tumor, atypical fibroxanthoma, and collision tumor. A collision tumor—the association of 2 or more benign or malignant neoplasms—represents a well-known pitfall in making a correct clinical and pathologic diagnosis.2 Many theories have been proposed to explain the pathophysiology of collision tumors. Some authors have speculated that they arise from involvement of related cell types.1 Other theories include induction by cytokines and growth factors secreted from one tumor that provides an ideal environment for proliferation of other cell types, a field cancerization effect of sun-damaged skin, or a coincidence.2
In our case, it is possible that the 2 tumors arose from a nevus sebaceus. One retrospective study of 706 cases of nevus sebaceus (707 specimens) found that 22.5% of cases developed secondary proliferation; of those cases, 18.9% were benign.3 Additionally, in 4.2% of cases of nevus sebaceus, proliferation of 2 or more tumors developed. The most common malignant neoplasm to develop from nevus sebaceus was BCC, followed by squamous cell carcinoma and sebaceous carcinoma. The most common benign neoplasm to develop from nevus sebaceus was trichoblastoma, followed by syringocystadenoma papilliferum.3
Our case highlights the possibility of a sampling error when performing a biopsy of any large neoplasm. Additionally, Mohs surgeons should maintain high clinical suspicion for collision tumors when encountering a large tumor with pathology inconsistent with the original biopsy. Apocrine hidrocystoma should be considered in the differential diagnosis of a large cystic mass of the scalp. Also, it is important to recognize that malignant lesions, such as BCC, can coexist with another benign tumor. Basal cell carcinoma is rare in Black patients, supporting our belief that our patient’s tumors arose from a nevus sebaceus.
It also is important for Mohs surgeons to consider any potential discrepancy between the initial pathology report and Mohs intraoperative pathology that can impact diagnosis, the aggressiveness of the tumors identified, and how such aggressiveness may affect management options.4,5 Some dermatology practices request biopsy slides from patients who are referred for Mohs micrographic surgery for internal review by a dermatopathologist before surgery is performed; however, this protocol requires additional time and adds costs for the overall health care system.4 One study found that internal review of outside biopsy slides resulted in a change in diagnosis in 2.2% of patients (N=3345)—affecting management in 61% of cases in which the diagnosis was changed.4 Another study (N=163) found that the reported aggressiveness of 50.5% of nonmelanoma cases in an initial biopsy report was changed during Mohs micrographic surgery.5 Mohs surgeons should be aware that discrepancies can occur, and if a discrepancy is discovered, the procedure may be paused until the initial biopsy slide is reviewed and further information is collected.
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
- Jayaprakasam A, Rene C. A benign or malignant eyelid lump—can you tell? an unusual collision tumour highlighting the difficulty differentiating a hidrocystoma from a basal cell carcinoma. BMJ Case Reports. 2012;2012:bcr1220115307. doi:10.1136/bcr.12.2011.5307
- Miteva M, Herschthal D, Ricotti C, et al. A rare case of a cutaneous squamomelanocytic tumor: revisiting the histogenesis of combined neoplasms. Am J Dermatopathol. 2009;31:599-603. doi:10.1097/DAD.0b013e3181a88116
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337. doi:10.1016/j.jaad.2013.10.004
- Butler ST, Youker SR, Mandrell J, et al. The importance of reviewing pathology specimens before Mohs surgery. Dermatol Surg. 2009;35:407-412. doi:10.1111/j.1524-4725.2008.01056.x
- Stiegel E, Lam C, Schowalter M, et al. Correlation between original biopsy pathology and Mohs intraoperative pathology. Dermatol Surg. 2018;44:193-197. doi:10.1097/DSS.0000000000001276
PRACTICE POINTS
- When collision tumors are encountered during Mohs micrographic surgery, review of the initial diagnostic material is recommended.
- Permanent processing of Mohs excisions may be helpful in determining the diagnosis of the occult second tumor diagnosis.
Scattered Red-Brown, Centrally Violaceous, Blanching Papules on an Infant
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.
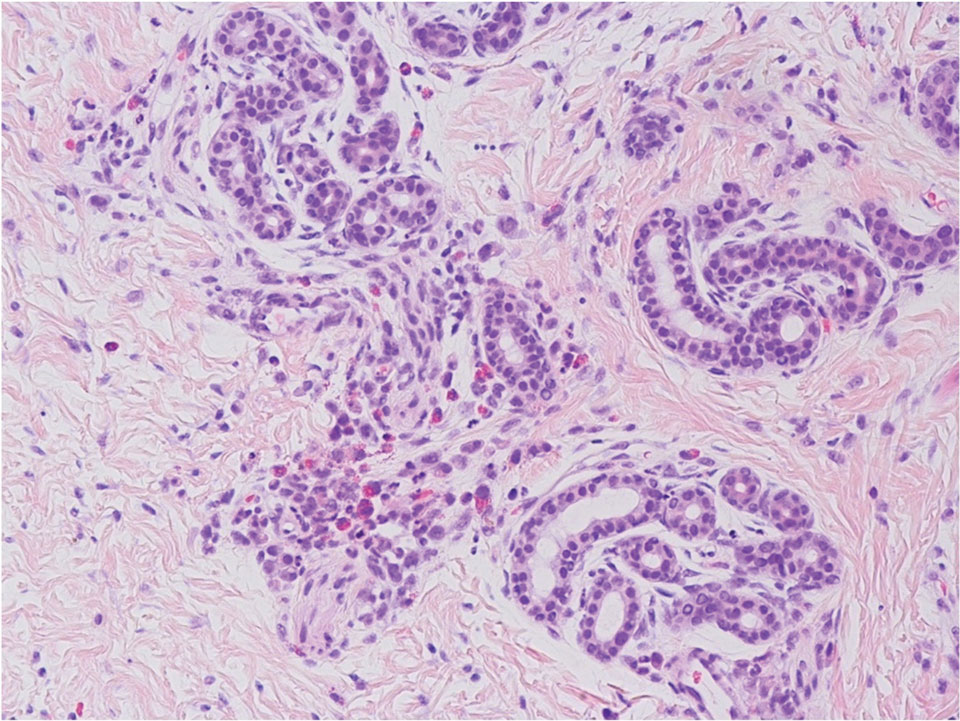
Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
The Diagnosis: Neonatal-Onset Multisystem Inflammatory Disorder (NOMID)
The punch biopsy demonstrated a predominantly deep but somewhat superficial, periadnexal, neutrophilic and eosinophilic infiltrate (Figure). The eruption resolved 3 days later with supportive treatment, including appropriate wound care. Genetic analysis revealed an autosomal-dominant NLR family pyrin domain containing 3 gene, NLRP3, de novo variant associated with neonatal-onset multisystem inflammatory disorder (NOMID). Additional workup to characterize our patient’s inflammatory profile revealed elevated IL-18, CD3, CD4, S100A12, and S100A8/A9 levels. On day 48 of life, she was started on anakinra, an IL-1 inhibitor, at a dose of 1 mg/kg subcutaneously, which eventually was titrated to 10 mg/kg at hospital discharge. Hearing screenings were within normal limits.

Cryopyrin-associated periodic syndromes (CAPS) consist of 3 rare, IL-1–associated, autoinflammatory disorders, including familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS), and NOMID (also known as chronic infantile neurologic cutaneous and articular syndrome). These conditions result from a sporadic or autosomal-dominant gain-of-function mutations in a single gene, NLRP3, on chromosome 1q44. NLRP3 encodes for cryopyrin, an important component of an IL-1 and IL-18 activating inflammasome.1 The most severe manifestation of CAPS is NOMID, which typically presents at birth as a migratory urticarial eruption, growth failure, myalgia, fever, and abnormal facial features, including frontal bossing, saddle-shaped nose, and protruding eyes.2 The illness also can manifest with hepatosplenomegaly, lymphadenopathy, uveitis, sensorineural hearing loss, cerebral atrophy, and other neurologic manifestations.3 A diagnosis of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome was less likely given that our patient remained afebrile and did not show signs of lipodystrophy and persistent violaceous eyelid swelling. Both FCAS and MWS are less severe forms of CAPS when compared to NOMID. Familial cold autoinflammatory syndrome was less likely given the absence of the typical periodic fever pattern associated with the condition and severity of our patient’s symptoms. Muckle-Wells syndrome typically presents in adolescence with symptoms of FCAS, painful urticarial plaques, and progressive sensorinueral hearing loss. Tumor necrosis factor receptor–associated periodic fever (TRAPS) usually is associated with episodic fevers, abdominal pain, periorbital edema, migratory erythema, and arthralgia.1,3,4
Diagnostic criteria for CAPS include elevated inflammatory markers and serum amyloid, plus at least 2 of the typical CAPS symptoms: urticarial rash, cold-triggered episodes, sensorineural hearing loss, musculoskeletal symptoms, chronic aseptic meningitis, and skeletal abnormalities.4 The sensitivity and specificity of these diagnostic criteria are 84% and 91%, respectively. Additional findings that can be seen but are not part of the diagnostic criteria include intermittent fever, transient joint swelling, bony overgrowths, uveitis, optic disc edema, impaired growth, and hepatosplenomegaly.5 Laboratory findings may reveal leukocytosis, eosinophilia, anemia, and/or thrombocytopenia.3,5
Genetic testing, skin biopsies, ophthalmic examinations, neuroimaging, joint radiography, cerebrospinal fluid tests, and hearing examinations can be performed for confirmation of diagnosis and evaluation of systemic complications.4 A skin biopsy may reveal a neutrophilic infiltrate. Ophthalmic examination can demonstrate uveitis and optic disk edema. Neuroimaging may reveal cerebral atrophy or ventricular dilation. Lastly, joint radiography can be used to evaluate for the presence of premature long bone ossification or osseous overgrowth.4
In summary, NOMID is a multisystemic disorder with cutaneous manifestations. Early recognition of this entity is important given the severe sequelae and available efficacious therapy. Dermatologists should be aware of these manifestations, as dermatologic consultation and a skin biopsy may aid in diagnosis.
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
- Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31:596-609. doi:10.1016/j.berh.2017.12.001
- Hull KM, Shoham N, Jin Chae J, et al. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr Opin Rheumatol. 2003;15:61-69. doi:10.1097/00002281-200301000-00011
- Ahmadi N, Brewer CC, Zalewski C, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. 2011;145:295-302. doi:10.1177/0194599811402296
- Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76:942-947. doi:10.1136/annrheumdis-2016-209686
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrinassociated autoinflammatory diseases. Arthritis Rheum. 2002; 46:3340-3348. doi:10.1002/art.10688
A 2-week-old infant girl was transferred to a specialty pediatric hospital where dermatology was consulted for evaluation of a diffuse eruption triggered by cold that was similar to an eruption present at birth. She was born at 31 weeks and 2 days’ gestation at an outside hospital via caesarean delivery. Early delivery was prompted by superimposed pre-eclampsia with severe hypertension after administration of antenatal steroids. At birth, the infant was cyanotic and apneic and had a documented skin eruption, according to the medical record. She had thrombocytopenia, elevated C-reactive protein, and an elevated temperature without fever. Extensive septic workup, including blood, urine, and cerebrospinal fluid cultures; herpes simplex virus and cytomegalovirus screening; and Toxoplasma polymerase chain reaction were negative. Magnetic resonance imaging of the brain revealed no evidence of intracranial congenital infection. Ampicillinsulbactam was initiated for presumed culture-negative sepsis. On day 2 of hospitalization, she developed conjunctival icterus, hepatomegaly, and jaundice. Direct hyperbilirubinemia; anemia; and elevated triglycerides, ferritin, and ammonia all were present. Coagulation studies were normal. Subsequent workup, including abdominal ultrasonography and hepatobiliary iminodiacetic acid scan, was concerning for biliary atresia. Despite appropriate treatment, her condition did not improve and she was transferred. Repeat abdominal ultrasonography on day 24 of life confirmed hepatomegaly but did not demonstrate other findings of biliary atresia. At the current presentation, physical examination revealed many scattered, redbrown and centrally violaceous, blanching papules measuring a few millimeters involving the trunk, arms, buttocks, and legs. A punch biopsy was obtained.
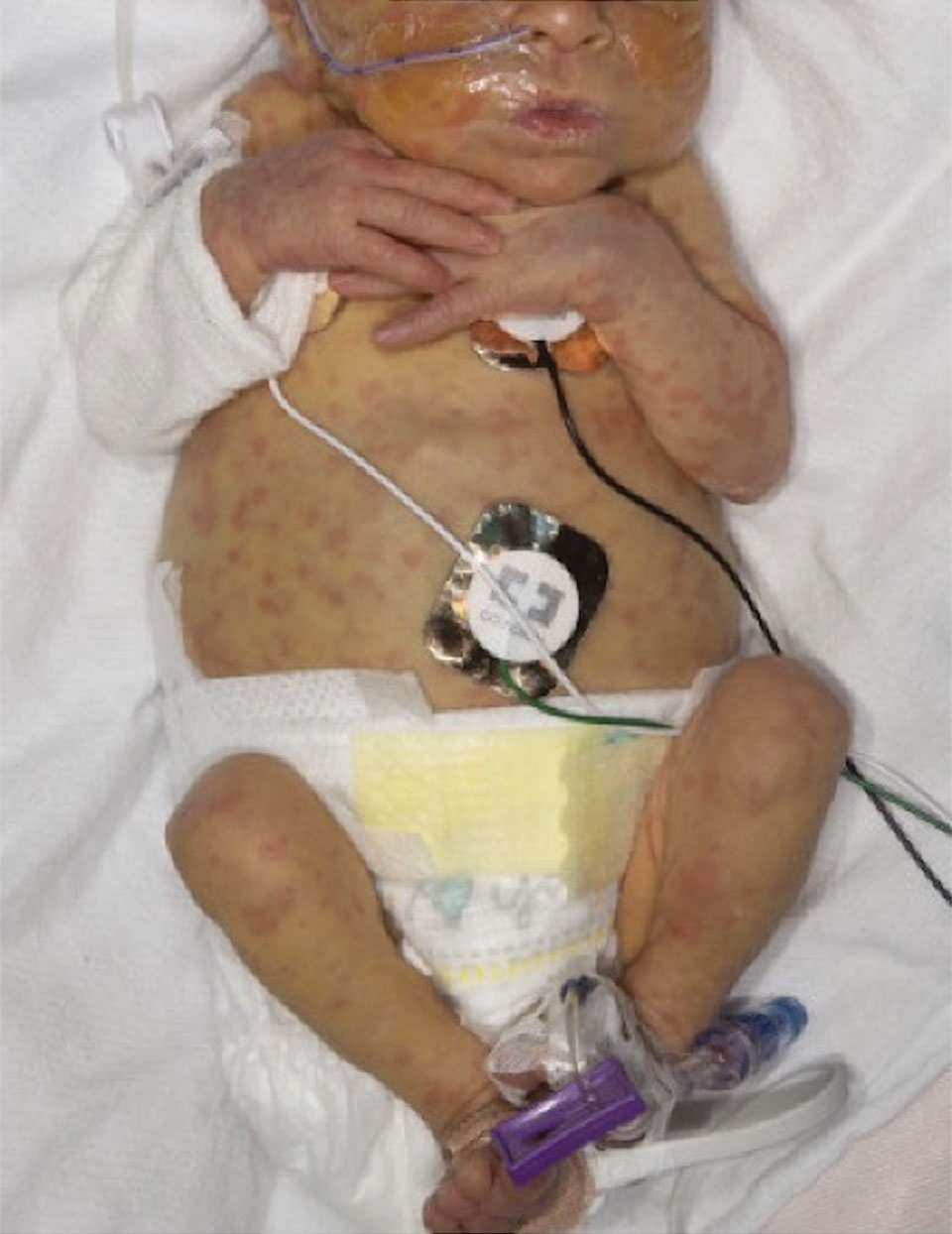
A 7-month-old male presents with pustules and inflamed papules on the scalp and extremities
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
The bacterial, fungal, and atypical mycobacterial cultures from the lesions performed at the emergency department were all negative.
Pediatric dermatology was consulted and a punch biopsy of one of the lesions was done. Histopathologic examination showed a mixed perifollicular infiltrate of predominantly eosinophils with some neutrophils and associated microabscesses. Periodic acid Schiff and Fite stains failed to reveal any organisms. CD1 immunostain was negative. Fresh tissue cultures for bacteria, fungi, and atypical mycobacteria were negative.
Given the clinical presentation of chronic recurrent sterile pustules on an infant with associated eosinophilia and the reported histopathologic findings, the patient was diagnosed with eosinophilic pustular folliculitis of infancy (EPFI).
EPFI is a rare and idiopathic cutaneous disorder present in children. About 70% of the cases reported occur in the first 6 month of life and rarely present past 3 years of age. EPF encompasses a group of conditions including the classic adult form, or Ofuji disease. EPF is seen in immunosuppressed patients, mainly HIV positive, and EPF is also seen in infants and children.
In EPFI, males are most commonly affected. The condition presents, as it did in our patient, with recurrent crops of sterile papules and pustules mainly on the scalp, but they can occur in other parts of the body. The lesions go away within a few weeks to months without leaving any scars but it can take months to years to resolve. Histopathologic analysis of the lesions show an eosinophilic infiltrate which can be follicular, perifollicular, or periadnexal with associated flame figures in about 26% of cases.
Aggressive treatment is usually not needed as lesions are self-limited. Lesions can be treated with topical corticosteroids and oral antihistamine medications like cetirizine if symptomatic.
If the lesions start to present during the neonatal period, one may consider in the differential diagnosis, neonatal rashes like transient neonatal pustular melanosis and erythema toxicum neonatorum. Both of these neonatal conditions tend to resolve in the first month of life, compared with EPFI where lesions can come and go for months to years. EPFI lesions can be described as pustules and inflammatory papules, as well as furuncles and vesicles. All of the lesions may be seen in one patient at one time, which will not be typical for transient neonatal pustular melanosis or erythema toxicum. Eosinophils can be seen in erythema toxicum but folliculitis is not present. The inflammatory infiltrate seen in transient neonatal pustular melanosis is polymorphonuclear, not eosinophilic.
Early in the presentation, infectious conditions like staphylococcal or streptococcal folliculitis, cellulitis and furunculosis, tinea capitis, atypical mycobacterial infections, herpes simplex, and parasitic infections like scabies should be considered. In young infants, empiric antibiotic treatment may be started until cultures are finalized. If there is a family history of pruritic papules and pustules, scabies should be considered. A scabies prep can be done to rule out this entity.
Langerhans cell histiocytosis can also present with pustules and papules in early infancy and also has a predilection for the scalp. When this condition is in question, a skin biopsy should be performed which shows a CD1 positive histiocytic infiltrate.
In conclusion, EPFI is a benign rare condition that can present in infants as recurrent pustules and papules, mainly on the scalp, which are self-limited and if symptomatic can be treated with topical corticosteroids and antihistamines.
References
Alonso-Castro L et al. Dermatol Online J. 2012 Oct 15;18(10):6.
Frølunde AS et al. Clin Case Rep. 2021 May 11;9(5):e04167.
Hernández-Martín Á et al. J Am Acad Dermatol. 2013 Jan;68(1):150-5.
A 7-month-old male is brought to the emergency department for evaluation of pustules and inflamed papules on the scalp and extremities for several weeks of duration. The parents report the lesions started about a month prior and he has already been treated with cephalexin, clindamycin, and sulfamethoxazole without any improvement. Cultures sent prior by the child's pediatrician did not reveal any fungus or bacteria. The parents report a low-grade fever for about 3 days.
He was born via natural vaginal delivery with no instrumentation or external monitoring. Mom had prenatal care. Besides the skin lesions, the baby has been healthy and growing well. He has no history of eczema or severe infections. He has not been hospitalized before.
On physical examination the baby was not febrile. On the scalp and forehead, he had diffusely distributed pustules, erythematous papules, and nodules. He also presented with scattered, fine, small, crusted 1-2-mm pink papules on the trunk and extremities. He had no adenopathy or hepatosplenomegaly.
At the emergency department, samples from one of the pustules were sent for bacterial, fungal, and atypical mycobacteria cultures. Laboratory test showed a normal blood count with associated eosinophilia (2.8 x 109 L), and normal liver and kidney function. A head ultrasound showed three ill-defined hypoechoic foci within the scalp.
The patient was admitted for treatment with broad-spectrum antibiotics and dermatology was consulted.