User login
With type 1 diabetes delay possible, focus now on screening
The recent approval of teplizumab-mzwv (Tzield, Provention Bio) for the delay of type 1 diabetes by the Food and Drug Administration is expected to advance efforts to increase screening to cost effectively identify those at risk for the condition who would be eligible to receive the new treatment.
The anti-CD3 monoclonal antibody was approved Nov. 17 as the first disease-modifying therapy for impeding progression of type 1 diabetes. In a clinical trial, teplizumab delayed the onset of clinical (stage 3) type 1 diabetes by approximately 2 years, and longer in some cases.
It is administered by intravenous infusion once daily for 14 consecutive days and is expected to cost in the region of $200,000 for the course of treatment.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In stage 2 type 1 diabetes, the individual has two or more islet autoantibodies and abnormal glycemia but is as yet asymptomatic. It is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
Currently, most people who are screened for type 1 diabetes autoantibodies are first-degree relatives of those with the condition through TrialNet, other local programs, or more recently, a $55 test offered by the research and advocacy organization JDRF.
But because 85%-90% of people who develop type 1 diabetes don’t have first-degree relatives with the condition, broader population screening will be necessary to identify eligible candidates for teplizumab.
During an investor call on Nov. 18, Provention Bio chief commercial officer Jason Hoitt said that among the company’s “strategic initiatives” were “advancing awareness and screening for autoantibodies in at-risk individuals, and ultimately, routine screening during pediatric well visits for the general population,” as well as “[health care provider] belief in teplizumab and desire to prescribe it for their patients.”
Without broad population-based screening, first-degree relatives of people with type 1 diabetes are likely to be the first to be screened and those with stage 2 identified for receipt of teplizumab. Today, that population is estimated at about 30,000 in the United States, Mr. Hoitt said, adding, “with this approval we hope that more stage 2 patients can be readily identified so the course of the disease can be changed.”
During the call, Mr. Hoitt also announced that the wholesale acquisition cost of Tzield would be $13,850 per vial, which translates to $193,900 per 14-vial continuous regimen, anticipated to be a sufficient dose for most patients. The company also launched a program called COMPASS to help patients navigate insurance reimbursement, as well as provide some with financial assistance.
Cost aside, JDRF CEO Aaron Kowalski, PhD, said in an interview that clinicians shouldn’t doubt the value of delaying type 1 diabetes onset, even if not completely preventing it. “This is the first drug ever to treat the underlying disease. There is this undercurrent that insulin is enough. Why would you undertake an additional risk of an immunotherapy? Type 1 is hard to live with. I think sometimes the clinical community doesn’t appreciate that insulin is not enough. It’s very difficult, and opening this door is important. ... We believe very strongly that the delay of onset of type 1 diabetes is clinically meaningful. We hear that from every family we’ve talked to. Clinicians should appreciate this and not discount it.”
How would screening happen?
While the path to universal screening for type 1 diabetes risk isn’t yet clear, quite a bit of thought and research has gone into it even before teplizumab and other immune-modulating agents showed promise in forestalling the condition.
Data from a universal screening program of schoolchildren implemented in Bavaria, Germany, and a screening program in Denver, suggest that even without such an intervention, identifying people at high risk for developing type 1 diabetes could be cost effective by allowing for education of the individual and family members about the signs of type 1 diabetes, thereby reducing the likelihood that the person would progress to developing diabetic ketoacidosis (DKA) prior to diagnosis.
Another study that used data from the United States and Western Europe, found that screening children for type 1 diabetes–associated islet autoantibodies at ages 2 and 6 years would identify most of those who go on to develop the disease by midadolescence.
However, using a genetic risk score at birth to identify those who would go on to autoantibody testing is potentially a more cost-effective approach, William A. Hagopian, MD, PhD, director of diabetes programs, Pacific Northwest Research Institute, Seattle, said in an interview.
The score – based on human leukocyte antigen haplotypes and their interactions as well as non-HLA genes – can stratify nearly 80% of childhood type 1 diabetes within the top 10% of all newborns. Thus, only the top 10% would then go on to receive the more expensive autoantibody testing.
“I’ve been working with U.K. colleagues for the past 3-4 years to develop a strategy using genetic risk scores followed by autoantibody screening. I feel strongly that that’s the cost-effective way to go. It’s relatively inexpensive, scalable, and can be applied commercially in newborn screening labs. To be successful an approach must be cost effective. Payors are willing to pay for newborn screening, but not so much on testing 100% of kids for antibodies,” Dr. Hagopian said.
He is now working with Washington State newborn screening labs to demonstrate feasibility of the approach using dried blood samples from actual neonatal screening after obtaining informed consent from the mothers in postpartum wards in several hospitals. Those found to be at high risk using the genetic risk score are contacted for follow-up with autoantibody screening. The program will continue for another year and a half. “I think it actually has a chance of being accepted into their regular program,” he said.
And then, he hopes, other states will follow, and eventually, the strategy will be added to the Recommended Uniform Screening Panel for universal newborn screening programs, as recommended by the Department of Health & Human Services.
“New newborn screenings for additional diseases are implemented regularly,” Dr. Hagopian said. “Most are far less common than type 1 diabetes. So even if our approach is less than 100% sensitive, this condition is a lot more common than the many inborn errors of metabolism, so we’re still going to be identifying a lot of cases. ... This is my hope for how universal type 1 diabetes screening will unfold. I see a way this may work quite well.”
A two-pronged approach to screening could work best
Meanwhile, JDRF, which supported the teplizumab research as well as others working in the space, is focusing on both genetic and autoantibody screening, Dr. Kowalski said.
“JDRF is working on both pathways – testing kids at birth for genetic predisposition and also antibody screening. We have huge programs focused on general population antibody screening.”
Dr. Kowalski said that, while the two-pronged approach certainly is worth exploring – and JDRF is doing that – he also thinks that universal autoantibody screening could be cost effective if done efficiently, such as with less expensive assays than the one used in TrialNet.
“We have programs where you do the genetic screening and keep an eye on people. We also have programs, like the one we’re funding in Germany, that are doing broad autoantibody screening of all kids. We’re hopeful that will be very cost effective if we move to cheaper assays.”
He noted that the proportion of children with new-onset type 1 diabetes who present in DKA rose from 40% pre–COVID-19 to 50% during the early days of the pandemic. On the other hand, “With screening you can get that to near zero, like they did in Bavaria. Here [in the United States], one ICU visit for DKA [costs] $100,000.”
While JDRF and others have been working on this for years, the new availability of teplizumab will be “multifold in helping things along. ... I think you’re going to see a lot of work on the cost-effectiveness of teplizumab. I think the case will be pretty straightforward that there’s huge upside to delaying the disease from a near-term and a long-term cost perspective. This is the first time we’ve had a drug out there with a price attached to it.”
But it may not happen quickly, Kowalski cautioned. “I feel there’s a ... series of events that has to happen to drive towards universal screening. Here in the U.S. it’s complicated because we have a very discrepant health care system with all these different payers, public and private.”
During the investor call, Mr. Hoitt said that Provention Bio is also exploring use of Tzield in younger patients and newly diagnosed patients, and the potential benefit of redosing or combining with other treatments.
Mr. Hoitt is an employee of Provention Bio. Dr. Kowalski is an employee of JDRF. Dr. Hagopian has reported receiving study funding from Janssen.
A version of this article first appeared on Medscape.com.
The recent approval of teplizumab-mzwv (Tzield, Provention Bio) for the delay of type 1 diabetes by the Food and Drug Administration is expected to advance efforts to increase screening to cost effectively identify those at risk for the condition who would be eligible to receive the new treatment.
The anti-CD3 monoclonal antibody was approved Nov. 17 as the first disease-modifying therapy for impeding progression of type 1 diabetes. In a clinical trial, teplizumab delayed the onset of clinical (stage 3) type 1 diabetes by approximately 2 years, and longer in some cases.
It is administered by intravenous infusion once daily for 14 consecutive days and is expected to cost in the region of $200,000 for the course of treatment.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In stage 2 type 1 diabetes, the individual has two or more islet autoantibodies and abnormal glycemia but is as yet asymptomatic. It is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
Currently, most people who are screened for type 1 diabetes autoantibodies are first-degree relatives of those with the condition through TrialNet, other local programs, or more recently, a $55 test offered by the research and advocacy organization JDRF.
But because 85%-90% of people who develop type 1 diabetes don’t have first-degree relatives with the condition, broader population screening will be necessary to identify eligible candidates for teplizumab.
During an investor call on Nov. 18, Provention Bio chief commercial officer Jason Hoitt said that among the company’s “strategic initiatives” were “advancing awareness and screening for autoantibodies in at-risk individuals, and ultimately, routine screening during pediatric well visits for the general population,” as well as “[health care provider] belief in teplizumab and desire to prescribe it for their patients.”
Without broad population-based screening, first-degree relatives of people with type 1 diabetes are likely to be the first to be screened and those with stage 2 identified for receipt of teplizumab. Today, that population is estimated at about 30,000 in the United States, Mr. Hoitt said, adding, “with this approval we hope that more stage 2 patients can be readily identified so the course of the disease can be changed.”
During the call, Mr. Hoitt also announced that the wholesale acquisition cost of Tzield would be $13,850 per vial, which translates to $193,900 per 14-vial continuous regimen, anticipated to be a sufficient dose for most patients. The company also launched a program called COMPASS to help patients navigate insurance reimbursement, as well as provide some with financial assistance.
Cost aside, JDRF CEO Aaron Kowalski, PhD, said in an interview that clinicians shouldn’t doubt the value of delaying type 1 diabetes onset, even if not completely preventing it. “This is the first drug ever to treat the underlying disease. There is this undercurrent that insulin is enough. Why would you undertake an additional risk of an immunotherapy? Type 1 is hard to live with. I think sometimes the clinical community doesn’t appreciate that insulin is not enough. It’s very difficult, and opening this door is important. ... We believe very strongly that the delay of onset of type 1 diabetes is clinically meaningful. We hear that from every family we’ve talked to. Clinicians should appreciate this and not discount it.”
How would screening happen?
While the path to universal screening for type 1 diabetes risk isn’t yet clear, quite a bit of thought and research has gone into it even before teplizumab and other immune-modulating agents showed promise in forestalling the condition.
Data from a universal screening program of schoolchildren implemented in Bavaria, Germany, and a screening program in Denver, suggest that even without such an intervention, identifying people at high risk for developing type 1 diabetes could be cost effective by allowing for education of the individual and family members about the signs of type 1 diabetes, thereby reducing the likelihood that the person would progress to developing diabetic ketoacidosis (DKA) prior to diagnosis.
Another study that used data from the United States and Western Europe, found that screening children for type 1 diabetes–associated islet autoantibodies at ages 2 and 6 years would identify most of those who go on to develop the disease by midadolescence.
However, using a genetic risk score at birth to identify those who would go on to autoantibody testing is potentially a more cost-effective approach, William A. Hagopian, MD, PhD, director of diabetes programs, Pacific Northwest Research Institute, Seattle, said in an interview.
The score – based on human leukocyte antigen haplotypes and their interactions as well as non-HLA genes – can stratify nearly 80% of childhood type 1 diabetes within the top 10% of all newborns. Thus, only the top 10% would then go on to receive the more expensive autoantibody testing.
“I’ve been working with U.K. colleagues for the past 3-4 years to develop a strategy using genetic risk scores followed by autoantibody screening. I feel strongly that that’s the cost-effective way to go. It’s relatively inexpensive, scalable, and can be applied commercially in newborn screening labs. To be successful an approach must be cost effective. Payors are willing to pay for newborn screening, but not so much on testing 100% of kids for antibodies,” Dr. Hagopian said.
He is now working with Washington State newborn screening labs to demonstrate feasibility of the approach using dried blood samples from actual neonatal screening after obtaining informed consent from the mothers in postpartum wards in several hospitals. Those found to be at high risk using the genetic risk score are contacted for follow-up with autoantibody screening. The program will continue for another year and a half. “I think it actually has a chance of being accepted into their regular program,” he said.
And then, he hopes, other states will follow, and eventually, the strategy will be added to the Recommended Uniform Screening Panel for universal newborn screening programs, as recommended by the Department of Health & Human Services.
“New newborn screenings for additional diseases are implemented regularly,” Dr. Hagopian said. “Most are far less common than type 1 diabetes. So even if our approach is less than 100% sensitive, this condition is a lot more common than the many inborn errors of metabolism, so we’re still going to be identifying a lot of cases. ... This is my hope for how universal type 1 diabetes screening will unfold. I see a way this may work quite well.”
A two-pronged approach to screening could work best
Meanwhile, JDRF, which supported the teplizumab research as well as others working in the space, is focusing on both genetic and autoantibody screening, Dr. Kowalski said.
“JDRF is working on both pathways – testing kids at birth for genetic predisposition and also antibody screening. We have huge programs focused on general population antibody screening.”
Dr. Kowalski said that, while the two-pronged approach certainly is worth exploring – and JDRF is doing that – he also thinks that universal autoantibody screening could be cost effective if done efficiently, such as with less expensive assays than the one used in TrialNet.
“We have programs where you do the genetic screening and keep an eye on people. We also have programs, like the one we’re funding in Germany, that are doing broad autoantibody screening of all kids. We’re hopeful that will be very cost effective if we move to cheaper assays.”
He noted that the proportion of children with new-onset type 1 diabetes who present in DKA rose from 40% pre–COVID-19 to 50% during the early days of the pandemic. On the other hand, “With screening you can get that to near zero, like they did in Bavaria. Here [in the United States], one ICU visit for DKA [costs] $100,000.”
While JDRF and others have been working on this for years, the new availability of teplizumab will be “multifold in helping things along. ... I think you’re going to see a lot of work on the cost-effectiveness of teplizumab. I think the case will be pretty straightforward that there’s huge upside to delaying the disease from a near-term and a long-term cost perspective. This is the first time we’ve had a drug out there with a price attached to it.”
But it may not happen quickly, Kowalski cautioned. “I feel there’s a ... series of events that has to happen to drive towards universal screening. Here in the U.S. it’s complicated because we have a very discrepant health care system with all these different payers, public and private.”
During the investor call, Mr. Hoitt said that Provention Bio is also exploring use of Tzield in younger patients and newly diagnosed patients, and the potential benefit of redosing or combining with other treatments.
Mr. Hoitt is an employee of Provention Bio. Dr. Kowalski is an employee of JDRF. Dr. Hagopian has reported receiving study funding from Janssen.
A version of this article first appeared on Medscape.com.
The recent approval of teplizumab-mzwv (Tzield, Provention Bio) for the delay of type 1 diabetes by the Food and Drug Administration is expected to advance efforts to increase screening to cost effectively identify those at risk for the condition who would be eligible to receive the new treatment.
The anti-CD3 monoclonal antibody was approved Nov. 17 as the first disease-modifying therapy for impeding progression of type 1 diabetes. In a clinical trial, teplizumab delayed the onset of clinical (stage 3) type 1 diabetes by approximately 2 years, and longer in some cases.
It is administered by intravenous infusion once daily for 14 consecutive days and is expected to cost in the region of $200,000 for the course of treatment.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In stage 2 type 1 diabetes, the individual has two or more islet autoantibodies and abnormal glycemia but is as yet asymptomatic. It is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
Currently, most people who are screened for type 1 diabetes autoantibodies are first-degree relatives of those with the condition through TrialNet, other local programs, or more recently, a $55 test offered by the research and advocacy organization JDRF.
But because 85%-90% of people who develop type 1 diabetes don’t have first-degree relatives with the condition, broader population screening will be necessary to identify eligible candidates for teplizumab.
During an investor call on Nov. 18, Provention Bio chief commercial officer Jason Hoitt said that among the company’s “strategic initiatives” were “advancing awareness and screening for autoantibodies in at-risk individuals, and ultimately, routine screening during pediatric well visits for the general population,” as well as “[health care provider] belief in teplizumab and desire to prescribe it for their patients.”
Without broad population-based screening, first-degree relatives of people with type 1 diabetes are likely to be the first to be screened and those with stage 2 identified for receipt of teplizumab. Today, that population is estimated at about 30,000 in the United States, Mr. Hoitt said, adding, “with this approval we hope that more stage 2 patients can be readily identified so the course of the disease can be changed.”
During the call, Mr. Hoitt also announced that the wholesale acquisition cost of Tzield would be $13,850 per vial, which translates to $193,900 per 14-vial continuous regimen, anticipated to be a sufficient dose for most patients. The company also launched a program called COMPASS to help patients navigate insurance reimbursement, as well as provide some with financial assistance.
Cost aside, JDRF CEO Aaron Kowalski, PhD, said in an interview that clinicians shouldn’t doubt the value of delaying type 1 diabetes onset, even if not completely preventing it. “This is the first drug ever to treat the underlying disease. There is this undercurrent that insulin is enough. Why would you undertake an additional risk of an immunotherapy? Type 1 is hard to live with. I think sometimes the clinical community doesn’t appreciate that insulin is not enough. It’s very difficult, and opening this door is important. ... We believe very strongly that the delay of onset of type 1 diabetes is clinically meaningful. We hear that from every family we’ve talked to. Clinicians should appreciate this and not discount it.”
How would screening happen?
While the path to universal screening for type 1 diabetes risk isn’t yet clear, quite a bit of thought and research has gone into it even before teplizumab and other immune-modulating agents showed promise in forestalling the condition.
Data from a universal screening program of schoolchildren implemented in Bavaria, Germany, and a screening program in Denver, suggest that even without such an intervention, identifying people at high risk for developing type 1 diabetes could be cost effective by allowing for education of the individual and family members about the signs of type 1 diabetes, thereby reducing the likelihood that the person would progress to developing diabetic ketoacidosis (DKA) prior to diagnosis.
Another study that used data from the United States and Western Europe, found that screening children for type 1 diabetes–associated islet autoantibodies at ages 2 and 6 years would identify most of those who go on to develop the disease by midadolescence.
However, using a genetic risk score at birth to identify those who would go on to autoantibody testing is potentially a more cost-effective approach, William A. Hagopian, MD, PhD, director of diabetes programs, Pacific Northwest Research Institute, Seattle, said in an interview.
The score – based on human leukocyte antigen haplotypes and their interactions as well as non-HLA genes – can stratify nearly 80% of childhood type 1 diabetes within the top 10% of all newborns. Thus, only the top 10% would then go on to receive the more expensive autoantibody testing.
“I’ve been working with U.K. colleagues for the past 3-4 years to develop a strategy using genetic risk scores followed by autoantibody screening. I feel strongly that that’s the cost-effective way to go. It’s relatively inexpensive, scalable, and can be applied commercially in newborn screening labs. To be successful an approach must be cost effective. Payors are willing to pay for newborn screening, but not so much on testing 100% of kids for antibodies,” Dr. Hagopian said.
He is now working with Washington State newborn screening labs to demonstrate feasibility of the approach using dried blood samples from actual neonatal screening after obtaining informed consent from the mothers in postpartum wards in several hospitals. Those found to be at high risk using the genetic risk score are contacted for follow-up with autoantibody screening. The program will continue for another year and a half. “I think it actually has a chance of being accepted into their regular program,” he said.
And then, he hopes, other states will follow, and eventually, the strategy will be added to the Recommended Uniform Screening Panel for universal newborn screening programs, as recommended by the Department of Health & Human Services.
“New newborn screenings for additional diseases are implemented regularly,” Dr. Hagopian said. “Most are far less common than type 1 diabetes. So even if our approach is less than 100% sensitive, this condition is a lot more common than the many inborn errors of metabolism, so we’re still going to be identifying a lot of cases. ... This is my hope for how universal type 1 diabetes screening will unfold. I see a way this may work quite well.”
A two-pronged approach to screening could work best
Meanwhile, JDRF, which supported the teplizumab research as well as others working in the space, is focusing on both genetic and autoantibody screening, Dr. Kowalski said.
“JDRF is working on both pathways – testing kids at birth for genetic predisposition and also antibody screening. We have huge programs focused on general population antibody screening.”
Dr. Kowalski said that, while the two-pronged approach certainly is worth exploring – and JDRF is doing that – he also thinks that universal autoantibody screening could be cost effective if done efficiently, such as with less expensive assays than the one used in TrialNet.
“We have programs where you do the genetic screening and keep an eye on people. We also have programs, like the one we’re funding in Germany, that are doing broad autoantibody screening of all kids. We’re hopeful that will be very cost effective if we move to cheaper assays.”
He noted that the proportion of children with new-onset type 1 diabetes who present in DKA rose from 40% pre–COVID-19 to 50% during the early days of the pandemic. On the other hand, “With screening you can get that to near zero, like they did in Bavaria. Here [in the United States], one ICU visit for DKA [costs] $100,000.”
While JDRF and others have been working on this for years, the new availability of teplizumab will be “multifold in helping things along. ... I think you’re going to see a lot of work on the cost-effectiveness of teplizumab. I think the case will be pretty straightforward that there’s huge upside to delaying the disease from a near-term and a long-term cost perspective. This is the first time we’ve had a drug out there with a price attached to it.”
But it may not happen quickly, Kowalski cautioned. “I feel there’s a ... series of events that has to happen to drive towards universal screening. Here in the U.S. it’s complicated because we have a very discrepant health care system with all these different payers, public and private.”
During the investor call, Mr. Hoitt said that Provention Bio is also exploring use of Tzield in younger patients and newly diagnosed patients, and the potential benefit of redosing or combining with other treatments.
Mr. Hoitt is an employee of Provention Bio. Dr. Kowalski is an employee of JDRF. Dr. Hagopian has reported receiving study funding from Janssen.
A version of this article first appeared on Medscape.com.
Both potatoes and beans reduced insulin resistance, weight in controlled study
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
Low energy–density diets that are based either on potatoes or beans similarly reduced insulin resistance in adults with poor blood glucose control, according to a controlled feeding study in 36 individuals.
Potatoes have gotten a bad rap for their high glycemic index, but they have little fat and a low energy density, wrote the study investigators. In fact, “cooling of gelatinized potatoes generates appreciable levels of slowly digested starch (resistant starch type 3) and substantially lowers the blood glucose response that potatoes elicit.”
“There is a view that potatoes are a less healthy plant food, but there is very little empirical data from randomized trials to support this view,” senior investigator John P. Kirwan, PhD, said in an interview.
Dry beans and peas (known as pulses) also contain resistant starch that improves insulin sensitivity and glucose tolerance, and multiple studies support pulses as part of a low-glycemic diet to improve glucose control in adults, the researchers explained, but because the density of food often guides how much people eat, they hypothesized that potatoes could substitute for beans and provide similar glucose control benefits.
In a study published in the Journal of Medicinal Food, the researchers randomized 36 adults aged 18-60 years with insulin resistance to 8 weeks of a low energy–density diet (1 kcal/g) high in either potatoes or beans. The baseline body mass index ranged from 25 to 40 kg/m2. Insulin resistance was defined using the homeostatic model assessment of insulin resistance (HOMA-IR) with a score greater than 2.
The controlled diet consisted of 50%-55% carbohydrates, 30%-35% fats, and 15%-20% protein. Each meal in the potato group included a side of potatoes, and each meal in the bean group included a side of beans.
The primary outcome was the mean change in blood glucose concentration; the researchers also assessed weight loss.
A total of 14 individuals in the potato group and 17 in the bean group completed the study; but data from the 18 individuals in each group were included in an intent-to-treat analysis.
Among study completers, HOMA-IR in the bean group showed an average decrease of 1.4 from baseline (P = .02 ); a similar decrease of 1.3 occurred in the potato group (P < .05) with no significant difference between the two diets.
Overall compliance with both diets was roughly 88%. Body weight reductions were similar in both groups and significantly reduced from baseline over the study period, with average reductions in intent-to-treat analysis of 5.82 kg in the potato group and 4.0 kg in the bean group. BMI also was significantly reduced from baseline in both potato and bean groups (2.04 kg/m2 and 1.35 kg/m2, respectively). Although baseline differences were not significant, “BMI at baseline was higher and the reduction in response to the treatment was significantly greater in the potato diet compared with the bean diet,” the researchers noted. The effect on blood glucose response was not significantly different between the two groups or from baseline, they said.
The findings were limited by several factors including the small size, relatively short study period, and controlled nature of the study diet, the researchers noted. “The addition of a typical Western diet would have enhanced our understanding of the effect of low energy–dense diets on metabolic outcomes,” they noted in their discussion.
However, both diets led to a reduction in body weight, and the low energy density of both potato and bean diets promoted weight loss without affecting appetite or requiring calorie restriction, the researchers explained. Therefore, “this weight loss if sustained over time could have a substantial impact on body weight,” they said.
“We hypothesized that there would be equivalence between the potato and bean diet and this hypothesis proved to be correct,” said Dr. Kirwan, of the Pennington Biomedical Research Center, Baton Rouge, La., in an interview.
The take-home message for clinicians is that, though small, the study was very well-controlled, Dr. Kirwan emphasized. “Clinicians ought to consider the health benefits of the potato when it is cooked and served appropriately.”
Looking ahead, larger randomized controlled trials with additional control arms, longer time of at least 12 weeks, and different patient populations are needed, Dr. Kirwan added.
Findings mitigate food myths
The debate continues about whether there are foods that are “good” or “evil;” or foods that one “should not eat” or “should eat,” said Amy Rothberg, MD, associate professor of internal medicine and of nutritional sciences at the University of Michigan, Ann Arbor, in an interview.
“This study dispels the myth that incorporating a small portion of potato into the diet (although these are not potatoes that are fried, or are topped with cheese, bacon, sour cream, etc.) results in deleterious metabolic outcomes when compared to a diet that is comprised of beans (pulses) as part of a low energy–dense diet,” she explained.
“The diet in both groups was of low energy density, which has been shown to result in fewer calories consumed, weight loss, and improvement in insulin resistance,” so the similarity in results was not so surprising, said Dr. Rothberg.
For the clinical takeaway, Dr. Rothberg agreed with the study authors: “Clinicians may counsel their patients that they can still consume a small potato (with the caveat above regarding cooking methods and toppings) as part of a balanced meal so long as they are keeping their overall calories low and not exceeding their metabolic requirements based on body weight/BMI,” she said.
As for additional research, studies with a longer time frame and a larger and more diverse study population are needed, including populations with common insulin resistance comorbidities such as type 2 diabetes, fatty liver disease, and cardiovascular disease, Dr. Rothberg noted.
Consumer considerations, with caveats
The key message for consumers is that, “based on this very small study of short duration, consuming a small portion of potato as part of an overall balanced, low-energy diet did not produce adverse effects on glucose or insulin when compared to a diet of pulses known to have favorable effects on glucose and insulin,” Dr. Rothberg told this news organization. However, “consumers should note that, although the results from this small study are encouraging, it would be premature to extrapolate the findings from this study to other populations,” she said. Also, keep in mind that the study was supported in part by the Alliance for Potato Research, although the authors stated that none of the funders (Alliance for Potato Research and Education and the National Institutes of Health) had any role in the design, analysis, or writing of the article, she added.
The study was supported in part by the Alliance for Potato Research and Education and the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The researchers and Dr. Rothberg had no financial conflicts to disclose.
FROM THE JOURNAL OF MEDICINAL FOOD
New genetic variant linked to maturity-onset diabetes of the young
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
A newly discovered genetic variant that is associated with type 2 diabetes (T2D) is responsible for almost 7% of all diabetes cases in Greenland, according to a whole-genome sequencing analysis of 448 Greenlandic Inuit individuals.
The variant, identified as c.1108G>T, “has the largest population impact of any previously reported variant” within the HNF1A gene – a gene that can cause maturity-onset diabetes of the young (MODY), reported senior author Torben Hansen, MD, PhD, of the University of Copenhagen, and colleagues in The Lancet Regional Health–Europe. The c.1108G>T variant does not cause MODY, but other variants within the HNF1A gene do. However, carriers of this variant, which is present in 1.9% of the Greenlandic Inuit population and has not been found elsewhere, have normal insulin sensitivity, but decreased beta-cell function and a more than fourfold risk of developing type 2 diabetes. “This adds to a previous discovery that about 11% of all diabetes in Greenlandic Inuit is explained by a mutation in the TBC1D4 variant,” Dr. Hansen told this publication. “Thus 1 in 5 patients diagnosed with type 2 diabetes in Greenland have a specific mutation explaining their diabetes. In European populations only about 1%-2% of patients diagnosed with type 2 diabetes have a known genetic etiology.”
The finding “provides new avenues to subgroup patients, detect diabetes in family members, and pursue precision treatment trials,” noted the authors, although they acknowledged that treatment choices for individuals with this variant still need to be explored. “We know from HNF1A-mutation carriers with European ancestry that they benefit from sulfonylurea treatment,” said Dr. Hansen. “However, we have not yet done treatment studies in Inuit.” The investigators noted that “it is not always the case that variants in HNF1A result in an increased insulin secretory response to sulfonylurea. ... Whether carriers of the c.1108G>T variant could benefit from treatment with sulfonylurea should be pursued within the context of a randomized clinical trial establishing both short- and long-term efficacy of sulfonylurea in these patients.”
A total of 4,497 study participants were randomly sampled from two cross-sectional cohorts in an adult Greenlandic population health survey. Among 448 participants who had whole genome sequencing, 14 known MODY genes were screened for both previously identified as well as novel variants. This identified the c.1108G>T variant, which was then genotyped in the full cohort in order to estimate an allele frequency of 1.3% in the general Greenlandic population, and 1.9% in the Inuit component. The variant was not found in genome sequences of other populations.
The researchers then tested the association of the variant with T2D and showed strong association with T2D (odds ratio, 4.35) and higher hemoglobin A1c levels.
“This is very well-conducted and exciting research that highlights the importance of studying the genetics of diverse populations,” said Miriam Udler, MD, PhD, director of the Massachusetts General Diabetes Genetics Clinic, and assistant professor at Harvard University, both in Boston. “This manuscript builds on prior work from the researchers identifying another genetic variant specific to the Greenlandic Inuit population in the gene TBC1D4,” she added. “About 3.8% of people in this population carry two copies of the TBC1D4 variant and have about a 10-fold increased risk of diabetes. Together the two variants affect 18% of Greenlanders with diabetes.”
With its fourfold increased risk of diabetes, the new variant falls into “an ever-growing category” of “intermediate risk” genetic variants, explained Dr. Udler – “meaning that they have a large impact on diabetes risk, but cannot fully predict whether someone will get diabetes. The contribution of additional risk factors is particularly important for ‘intermediate risk’ genetic variants,” she added. “Thus, clinically, we can tell patients who have variants such as HNF1A c.1108>T that they are at substantial increased risk of diabetes, but that many will not develop diabetes. And for those who do develop diabetes, we are not yet able to advise on particular therapeutic strategies.”
Still, she emphasized, the importance of studying diverse populations with specific genetic risk factors is the end-goal of precision medicine. “An active area of research is determining whether and how to return such information about ‘intermediate risk’ variants to patients who get clinical genetic testing for diabetes, since typically only variants that are very high risk ... are returned in clinical testing reports.” Dr. Udler added that “many more such “intermediate risk’ variants likely exist in all populations, but have yet to be characterized because they are less common than HNF1A c.1108>T; however, ongoing worldwide efforts to increase the sample sizes of human genetic studies will facilitate such discovery.”
The study was funded by Novo Nordisk Foundation, Independent Research Fund Denmark, and Karen Elise Jensen’s Foundation. Dr. Hansen and Dr. Udler had no disclosures.
FROM THE LANCET REGIONAL HEALTH–EUROPE
Will ICER review aid bid for Medicare to pay for obesity drugs?
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
A report from a well-respected nonprofit group may bolster efforts to have Medicare, the largest U.S. purchaser of prescription drugs, cover obesity medicines, for which there has been accumulating evidence of significant benefit.
The Institute for Clinical and Economic Review (ICER) released a report last month on obesity medicines, based on extensive review of research done to date and input from clinicians, drug-makers, and members of the public.
Of the treatments reviewed, the ICER report gave the best ratings to two Novo Nordisk products, a B+ for semaglutide (Wegovy) and a B for liraglutide (Saxenda), while also making the case for price cuts. At an annual U.S. net price estimated at $13,618, semaglutide exceeds what ICER considers typical cost-effectiveness thresholds. ICER suggested a benchmark annual price range for semaglutide of between $7,500 and $9,800.
The ICER report also directs insurers in general to provide more generous coverage of obesity medicines, with a specific recommendation for the U.S. Congress to pass a pending bill known as the Treat and Reduce Obesity Act of 2021. The bill would undo a restriction on weight-loss drugs in the Medicare Part D plans, which covered about 49 million people last year. Sen. Tom Carper (D-Del.) and Sen. Bill Cassidy, MD, (R-La.) have repeatedly introduced versions of the bill since 2013.
“In both chambers of Congress and with bipartisan support, we’ve pushed to expand Medicare coverage of additional therapies and medications to treat obesity,” Sen. Cassidy said in an email. “This report confirms what we’ve worked on for nearly a decade – our legislation will help improve lives.”
The current House version of the bill has the backing of more than a third of the members of that chamber, with 113 Democratic and 40 Republican cosponsors. The Senate version has 22 sponsors.
Changing views
The ICER report comes amid a broader change in how clinicians view obesity.
The American Academy of Pediatrics is readying a new Clinical Practice Guideline for the Evaluation and Treatment of Pediatric Obesity that will mark a major shift in approach. Aaron S. Kelly, PhD, a professor of pediatrics at the University of Minnesota, Minneapolis, described it as a “sea change,” with obesity now seen as “a chronic, refractory, relapsing disease,” for which watchful waiting is no longer appropriate.
But the field of obesity treatment looked quite different in the early 2000s when Congress worked on a plan to add a pharmacy benefit to Medicare.
The deliberate omission of obesity medicine in the Medicare Part D benefit reflected both the state of science at the time and U.S. experience with a dangerous weight-loss drug combo in the late 1990s.
Initial expectations for weight-loss pills were high after the Food and Drug Administration cleared dexfenfluramine HCl (Redux) in 1996, which was part of the popular fen-phen combination. “Newly Approved Diet Drug Promises to Help Millions of Obese Americans – But Is No Magic Bullet,” read a headline about the Redux approval in The Washington Post
When work began in the 2000s to create a Medicare pharmacy benefit, lawmakers and congressional staff had a pool of about $400 billion available to establish what became the Part D program, Joel White, a former House staffer who helped draft the law, told this news organization in an email exchange.
Given the state of obesity research at the time, it seemed to make sense to exclude weight-loss medications, wrote Mr. White. Mr. White is now chief executive of the consulting firm Horizon, which has clients in the drug industry including the Pharmaceutical Research and Manufacturers of America.
“Now we know that obesity is a chronic disease of epidemic proportions. Decades of research have produced a series of advances in the way we understand and treat obesity. While scientists and many who work directly with those impacted by this epidemic understand how treatments have advanced, the law lags behind,” Mr. White said.
XXXCurrent payment policies for obesity treatments are based on “outdated information and ongoing misperception,” he noted. “While Part D has been a resounding success, our Medicare approach to obesity is not.”
“In addition, it makes no sense that Medicare covers the most drastic procedure (bariatric surgery) but not less-invasive, effective treatments,” he added. “We should have long ago lifted restrictions based on advances in science and medicine.”
Overcoming the stigma
Scott Kahan, MD, MPH, agreed and hopes that the new ICER report will help more patients secure needed medications, raising a “call to arms” about the need for better coverage of obesity drugs.
Dr. Kahan is director of the National Center for Weight and Wellness, a private clinic in Washington, and chair of the clinical committee for The Obesity Society. He also served as a member of a policy roundtable that ICER convened as part of research on the report on obesity drugs. Dr. Kahan, who also serves on the faculty at the Johns Hopkins Bloomberg School of Public Health, Baltimore, has received fees from drug makers such as Eli Lilly.
The ICER report may help what Dr. Kahan described as well-founded caution about obesity treatments in general.
“When it comes to weight loss, there are all of these magical treatments that are sold on social media and traditional media. There are a lot of bad actors in terms of people calling themselves experts and gurus and promising all kinds of crazy stuff,” said Dr. Kahan.
And there are long-standing stigmas about obesity, he stressed.
“That underlies a lot of the backward policies, including poor coverage for medications and the noncoverage by Medicare,” Dr. Kahan said. “There’s a societal ingrained set of beliefs and misperceptions and biases. That takes time to unwind, and I think we’re on the way, but we’re not quite there yet.”
Lifestyle changes not enough to tackle obesity
AHIP (formerly America’s Health Insurance Plans) told this news organization its members consider ICER reports when making decisions about which products to cover. “And health plans already cover obesity treatments that they consider medically necessary,” said David Allen, an AHIP spokesperson.
“It is important to note that every treatment does not work for every patient, and many patients experience adverse events and may discontinue treatment,” he added in an email. “Health insurance providers play an important role in helping [health care] providers and patients identify the treatment options that are most likely to be effective as well as affordable.”
Separately, the nonprofit watchdog group Public Citizen cautioned against liraglutide on its Worst Pills, Best Pills website. In its view, the drug is minimally effective and has many dangerous adverse effects, which are even more frequent with the higher-dose weight-loss version (a lower-dose version is approved for type 2 diabetes).
“There is currently no medication that can be used safely to achieve weight loss effortlessly and without dangerous adverse effects,” the group said. “Rather than focus on losing weight by turning to risky drugs, overweight and obese adults seeking to achieve better health should make reasonable and sustainable changes to their lifestyle, such as eating a healthy diet and getting regular exercise.”
Yet, many people find there is little help available for making lifestyle changes, and some patients and physicians say these modifications by themselves are not enough.
“The vast majority of people with obesity cannot achieve sustained weight loss through diet and exercise alone,” said David Rind, MD, chief medical officer of ICER, in an Oct. 20 statement. “As such, obesity, and its resulting physical health, mental health, and social burdens, is not a choice or failing, but a medical condition.”
The focus should now be on assuring that effective medications “are priced in alignment with their benefits so that they are accessible and affordable across U.S. society,” Dr. Rind urges.
‘My own demise with a fork and knife’
ICER sought public feedback on a draft version of the report before finalizing it.
In their comments on ICER’s work, several pharmaceutical researchers and Novo Nordisk questioned the calculations used in making judgments about the value of obesity drugs. In a statement, Novo Nordisk told this news organization that the company’s view is that ICER’s modeling “does not adequately address the real-world complexities of obesity, and consequently underestimates the health and societal impact medical treatments can have.”
Commenters also dug into aspects of ICER’s calculations, including ones that consider quality-adjusted life-years (QALYs). ICER describes QALY as an academic standard for measuring how well all different types of medical treatments can extend or improve patients’ lives. In an explainer on its website, ICER says this metric has served as a fundamental component of cost-effectiveness analyses in the United States and around the world for more than 30 years.
ICER and drug makers have been at odds for some time, with PhRMA having criticized the nonprofit group. A 2020 Reuters article detailed public relations strategies used by firms paid by drug makers to raise questions about ICER’s work. Critics accuse it of allying with insurers.
ICER’s list of its recent financial supporters includes Blue Cross Blue Shield of Massachusetts and the Kaiser Foundation Health Plan, but also many other groups, such as the U.S. Department of Veterans Affairs, the American Academy of Neurology, and the American College of Rheumatology.
The public comments on the ICER report also include one from an unidentified woman who wrote of her past struggles to lose weight.
She said her health plan wouldn’t cover behavioral programs or semaglutide as a weight-loss drug but did cover it eventually because of signs that she had developed insulin resistance. The patient said the drug worked for her, whereas other approaches to control weight had failed.
“To put it simply, I now experience hunger and satiety in a way that I can only assume people with normal metabolism do. I am 49 years old and approaching the age where serious comorbidities associated with obesity begin to manifest,” the patient wrote.
“I no longer worry about bringing about my own demise with a fork and knife because of misfiring hunger cues.”
A version of this article first appeared on Medscape.com.
Night lights in the city link to increased risk of diabetes
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Higher levels of exposure to outdoor artificial light at night are significantly linked with markers of diabetes and impaired glucose homeostasis, in a new national, cross-sectional study from China.
The results showed a 7% significant increase in diabetes prevalence per quintile exposure to artificial light at night (prevalence ratio, 1.07), report Ruizhi Zheng, PhD, of the Shanghai (China) Jiaotong University School of Medicine, and colleagues. People living in areas with the most exposure to light at night had a 28% higher prevalence of diabetes than those living in places with the lowest exposure (PR, 1.28), the researchers found.
The study was published online in Diabetologia.
Previous animal studies have shown that exposure to light at night may interfere with circadian rhythms and affect glucose homeostasis, the study team note. Other research has demonstrated that chronic exposure to moderate indoor light during sleep elevated the prevalence of diabetes in older adults, compared with those sleeping in a dim setting, the authors add.
“Our findings contribute to the growing literature suggesting that artificial light at night is detrimental to health and demonstrate that artificial light at night may be a potential novel risk factor for diabetes,” they write.
“Considering the coexistence of the diabetes epidemic and the widespread influence of light pollution at night, the positive associations indicate an urgent need for countries and governments to develop effective prevention and intervention policies and to protect people from the adverse health effects of light pollution at night,” the study authors stress.
Gareth Nye, PhD, senior lecturer at the University of Chester, England, agreed that prior research has found an association between metabolic conditions, such as diabetes, and artificial light at night, with most theories as to the cause focusing on the body’s natural circadian cycle.
He said that internal clocks regulate a variety of bodily processes, such as metabolism and hormone synthesis. They also affect sleep patterns by interfering with synthesis of the hormone melatonin, which is essential for sound sleep, Dr. Nye told the UK Science Media Centre.
However, he stressed that much more research is needed before any link can be considered definitive.
Outdoor night light exposure linked to fasting glucose, A1c
The Chinese researchers set out to approximate the relationships between diabetes prevalence and glucose homeostasis with chronic exposure to outdoor light at night.
They assessed 98,658 participants from the China Noncommunicable Disease Surveillance Study across 162 sites. The mean age of participants was 42.7 years. Female participants comprised 49.2% of the study cohort.
Diabetes was defined based on American Diabetes Association criteria. Satellite data were used to determine exposure to outdoor light at night in 2010. The associations between light exposure at night and indicators of glucose homeostasis were investigated.
Prevalence ratios were calculated and adjusted for sex, age, smoking status, education, body mass index, physical activity, household income, family history of diabetes, rural/urban areas, drinking status, and use of lipid-lowering prescription drugs (primarily statins) or antihypertensives.
The findings showed exposure levels to outdoor light at night were positively linked with 2-hour and fasting glucose concentrations, A1c, and insulin resistance (measured using homeostatic model assessment [HOMA]), but negatively related to β-cell function (measured using HOMA).
More research needed
“We advise caution against causal interpretation of the findings and call for further studies involving direct measurement of individual exposure to light at night,” the researchers conclude.
Dr. Nye agreed.
“One issue with this study is that the areas with the highest outdoor artificial light levels are likely to be those in urban areas and bigger cities. It has been known for a long time now that living in an urbanized area increases your risk of obesity through increased access to high-fat and convenience food, less physical activity levels due to transport links, and less social activities. The authors also state this and the fact participants tended to be older,” he noted.
Large datasets are used in this investigation, however, which generally increases the reliability of the data, he observed.
But it is also “unclear as to whether the population here was selected for this study or was retrospectively analyzed, which poses reliability issues, as does the selection of the representative sample, as it is not discussed,” he noted.
Ultimately, there is no confirmed evidence of the link, and until further work is done to directly link light exposure and diabetes in humans, “the link will remain an association only,” he concluded.
A version of this article first appeared on Medscape.com.
Medical school culinary medicine programs grow despite limited funding
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”
Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”
Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”
Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
FROM ACADEMIC MEDICINE
FDA approves first-ever agent to delay type 1 diabetes onset
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
“Today’s approval of a first-in-class therapy adds an important new treatment option for certain at-risk patients,” said John Sharretts, MD, director of the Division of Diabetes, Lipid Disorders, and Obesity in the FDA’s Center for Drug Evaluation and Research. “The drug’s potential to delay clinical diagnosis of type 1 diabetes may provide patients with months to years without the burdens of disease.”
The agent, which interferes with T-cell-mediated autoimmune destruction of pancreatic beta cells, is the first disease-modifying therapy for impeding progression of type 1 diabetes. It is administered by intravenous infusion once daily for 14 consecutive days.
The specific indication is “to delay the onset of stage 3 type 1 diabetes in adults and pediatric patients 8 years and older who currently have stage 2 type 1 diabetes.” In type 1 diabetes staging, adopted in 2015, stage 1 is defined as the presence of beta cell autoimmunity with two or more islet autoantibodies with normoglycemia, stage 2 is beta-cell autoimmunity with dysglycemia yet asymptomatic, and stage 3 is the onset of symptomatic type 1 diabetes.
Stage 2 type 1 diabetes is associated with a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
The FDA had previously rejected teplizumab for this indication in July 2021, despite a prior endorsement from an advisory panel in May 2021.
Now, with the FDA approval, Provention Bio cofounder and CEO Ashleigh Palmer said in a statement, “This is a historic occasion for the T1D community and a paradigm shifting breakthrough ... It cannot be emphasized enough how precious a delay in the onset of stage 3 T1D can be from a patient and family perspective; more time to live without and, when necessary, prepare for the burdens, complications, and risks associated with stage 3 disease.”
T1D onset delayed by 2 years
In 2019, a pivotal phase 2, randomized, placebo-controlled trial involving 76 at-risk children and adults aged 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes compared with 32 who received placebo.
Those “game changer” data were presented at the American Diabetes Association (ADA) annual meeting in June 2019 and simultaneously published in the New England Journal of Medicine.
Three-year data were presented at the June 2020 ADA meeting and published in March 2021 in Science Translational Medicine, by Emily K. Sims, MD, department of pediatrics, Indiana University, Indianapolis, and colleagues.
At a median follow-up of 923 days, 50% of those randomly assigned to teplizumab remained diabetes free, compared with 22% of those who received placebo infusions (hazard ratio, 0.457; P = .01). The teplizumab group had a greater average C-peptide area under the curve compared with placebo, reflecting improved beta-cell function (1.96 vs. 1.68 pmol/mL; P = .006).
C-peptide levels declined over time in the placebo group but stabilized in those receiving teplizumab (P = .0015).
“The mid-range time from randomization to stage 3 type 1 diabetes diagnosis was 50 months for the patients who received Tzield and 25 months for those who received a placebo. This represents a statistically significant delay in the development of stage 3 type 1 diabetes,” according to the FDA statement.
The most common side effects of Tzield include lymphopenia (73% teplizumab vs. 6% placebo), rash (36% vs. 0%), leukopenia (221% vs. 0%), and headache (11% vs. 6%). Label warnings and precautions include monitoring for cytokine release syndrome, risk for serious infections, and avoidance of live, inactivated, and mRNA vaccines.
This approval is likely to accelerate discussion about universal autoantibody screening. Currently, most individuals identified as having preclinical type 1 diabetes are first-degree relatives of people with type 1 diabetes identified through the federally funded TrialNet program. In December 2020, the type 1 diabetes research and advocacy organization JDRF began offering a $55 home blood test to screen for the antibodies, and other screening programs have been launched in the United States and Europe.
Previous studies have examined cost-effectiveness of universal screening in children and the optimal ages that such screening should take place.
In October, Provention Bio announced a co-promotion agreement with Sanofi for the U.S. launch of Tzield for delay in onset of clinical T1D in at-risk individuals. Provention Bio offers financial assistance options (e.g., copay assistance) to eligible patients for out-of-pocket costs.
A version of this article first appeared on Medscape.com.
Best Practice Implementation and Clinical Inertia
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.
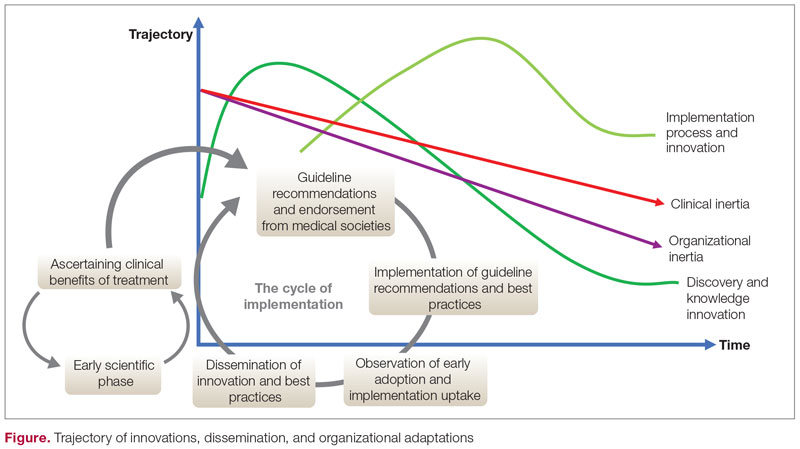
Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
Safety and Efficacy of GLP-1 Receptor Agonists and SGLT2 Inhibitors Among Veterans With Type 2 Diabetes
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR).A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR).A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
Selecting the best medication regimen for a patient with type 2 diabetes mellitus (T2DM) depends on many factors, such as glycemic control, adherence, adverse effect (AE) profile, and comorbid conditions.1 Selected agents from 2 newer medication classes, glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium-glucose cotransporter 2 inhibitors (SGLT2i), have demonstrated cardiovascular and renal protective properties, creating a new paradigm in management.
The American Diabetes Association recommends medications with proven benefit in cardiovascular disease (CVD), such as the GLP-1 RAs liraglutide, injectable semaglutide, or dulaglutide, or the SGLT2i empagliflozin or canagliflozin, as second-line after metformin in patients with established atherosclerotic CVD or indicators of high risk to reduce the risk of major adverse cardiovascular events (MACE).1 SGLT2i are preferred in patients with diabetic kidney disease, and GLP-1 RAs are next in line for selection of agents with proven nephroprotection (liraglutide, injectable semaglutide, dulaglutide). The mechanisms of these benefits are not fully understood but may be due to their extraglycemic effects. The classes likely induce these benefits by different mechanisms: SGLT2i by hemodynamic effects and GLP-1 RAs by anti-inflammatory mechanisms.2 Although there is much interest, evidence is limited regarding the cardiovascular and renal protection benefits of these agents used in combination.
The combined use of GLP-1 RA and SGLT2i agents demonstrated greater benefit than separate use in trials with nonveteran populations.3-7 These studies evaluated effects on hemoglobin A1c (HbA1c) levels, weight loss, blood pressure (BP), and estimated glomerular filtration rate (eGFR).A meta-analysis of 7 trials found that the combination of GLP-1 RA and SGLT2i reduced HbA1c levels, body weight, and systolic blood pressure (SBP).8 All of the changes were statistically significant except for body weight with combination vs SGLT2i alone. Combination therapy was not associated with increased risk of severe hypoglycemia compared with either therapy separately.
The purpose of our study was to evaluate the safety and efficacy of the combined use of GLP-1 RA and SGLT2i in a real-world, US Department of Veterans Affairs (VA) population with T2DM.
Methods
This study was a pre-post, retrospective, single-center chart review. Subjects served as their own control. The project was reviewed and approved by the VA Ann Arbor Healthcare System Institutional Review Board. Subjects prescribed both a GLP-1 RA (semaglutide or liraglutide) and SGLT2i (empagliflozin) between January 1, 2014, and November 10, 2019, were extracted from the Corporate Data Warehouse (CDW) for possible inclusion in the study.
Patients were excluded if they received < 12 weeks of combination GLP-1 RA and SGLT2i therapy or did not have a corresponding 12-week HbA1c level. Patients also were excluded if they had < 12 weeks of monotherapy before starting combination therapy or did not have a baseline HbA1c level, or if the start date of combination therapy was not recorded in the VA electronic health record (EHR). We reviewed data for each patient from 6 months before to 1 year after the second agent was started. Start of the first agent (GLP-1 RA or SGLT2i) was recorded as the date the prescription was picked up in-person or 7 days after release date if mailed to the patient. Start of the second agent (GLP-1 RA or SGLT2i) was defined as baseline and was the date the prescription was picked up in person or 7 days after the release date if mailed.
Baseline measures were taken anytime from 8 weeks after the start of the first agent through 2 weeks after the start of the second agent. Data collected included age, sex, race, height, weight, BP, HbA1c levels, serum creatinine (SCr), eGFR, classes of medications for the treatment of T2DM, and the number of prescribed antihypertensive medications. HbA1c levels, SCr, eGFR, weight, and BP also were collected at 12 weeks (within 8-21 weeks); 26 weeks (within 22-35 weeks); and 52 weeks (within 36-57 weeks) of combination therapy. We reviewed progress notes and laboratory results to determine AEs within 26 weeks before initiating second agent (baseline) and 0 to 26 weeks and 26 to 52 weeks after initiating combination therapy.
The primary objective was to determine the effect on HbA1c levels at 12 weeks when using a GLP-1 RA and SGLT2i in combination vs separately. Secondary objectives were to determine change from baseline in mean body weight, BP, SCr, and eGFR at 12, 26, and 52 weeks; change in HbA1c levels at 26 and 52 weeks; and incidence of prespecified adverse drug reactions during combination therapy vs separately.
Statistical Analysis
Assuming a SD of 1, 80% power, significance level of P < .05, 2-sided test, and a correlation between baseline and follow-up of 0.5, we determined that a sample size of 34 subjects was required to detect a 0.5% change in baseline HbA1c level at 12 weeks. A t test (or Wilcoxon signed rank test if outcome not normally distributed) was conducted to examine whether the expected change from baseline was different from 0 for continuous outcomes. Median change from baseline was reported for SCr as a nonparametric t test (Wilcoxon signed rank test) was used.
Results
We identified 110 patients for possible study inclusion and 39 met eligibility criteria. After record review, 30 patients were excluded for receiving < 12 weeks of combination therapy or no 12 week HbA1c level; 26 patients were excluded for receiving < 12 weeks of monotherapy before starting combination therapy or no baseline HbA1c level; and 15 patients were excluded for lack of documentation in the VA EHR. Of the 39 patients included, 24 (62%) were prescribed empagliflozin first and then 8 started liraglutide and 16 started semaglutide.
HbA1c levels decreased by 1% after 12 weeks of combination therapy compared with baseline (P < .001), and this reduction was sustained through the duration of the study period (Table 2).
The most common AE during the trial was hypoglycemia, which was mostly mild (level 1) (Table 3).
Discussion
This study evaluated the safety and efficacy of combined use of semaglutide or liraglutide and empagliflozin in a veteran population with T2DM. The retrospective chart review captured real-world practice and outcomes. Combination therapy was associated with a significant reduction in HbA1c levels, body weight, and SBP compared with either agent alone. No significant change was seen in DBP, SCr, or eGFR. Overall, the combination of GLP-1 RA and SGLT2i medications demonstrated a good safety profile with most patients reporting no AEs.
Several other studies have assessed the safety and efficacy of using GLP-1 RA and SGLT2i in combination. The DURATION 8 trial is the only double-blind trial to randomize subjects to receive either exenatide once weekly, dapagliflozin, or the combination of both for up to 52 weeks.3 Other controlled trials required stable background therapy with either SGLT2i or GLP-1 RA before randomization to receive the other class or placebo and had durations between 18 and 30 weeks.4-7 The AWARD 10 trial studied the combination of canagliflozin and dulaglutide, which both have proven CVD benefit.4 Other studies did not restrict SGLT2i or GLP-1 RA background therapy to agents with proven CVD benefit.5-7 The present study evaluated the combination of empagliflozin plus liraglutide or semaglutide, agents that all have proven CVD benefit.
A meta-analysis of 7 trials, including those previously mentioned, was conducted to evaluate the combination of GLP-1 RA and SGLT2i.8 The combination significantly reduced HbA1c levels by 0.61% and 0.85% compared with GLP-1 RA or SGLT2i, respectively. Our trial showed greater HbA1c level reduction of 1% with combination therapy compared with either agent separately. This may have been due in part to a higher baseline HbA1c level in our real-world veteran population. The meta-analysis found the combination decreased body weight 2.6 kg and 1.5 kg compared with GLP-1 RA or SGLT2i, respectively.8 This only reached significance with comparison vs GLP-1 RA alone. Our study demonstrated impressive weight loss of up to about 5 kg after 26 and 52 weeks of combination therapy. This is equivalent to about 5% weight loss from baseline, which is clinically significant.9 Liraglutide and semaglutide are the GLP-1 RAs associated with the greatest weight loss, which may contribute to greater weight loss efficacy seen in the present trial.1
In our trial SBP fell lower compared with the meta-analysis. Combination therapy significantly reduced SBP by 4.1 mm Hg and 2.7 mm Hg compared with GLP-1 RA or SGLT2i, respectively, in the meta-analysis.8 We observed a significant 9 to 12 mm Hg reduction in SBP after 26 to 52 weeks of combination therapy compared with baseline. This reduction occurred despite relatively controlled SBP at baseline (135 mm Hg). Each reduction of 10 mm Hg in SBP significantly reduces the risk of MACE, stroke, and heart failure, making our results clinically significant.10 Neither the meta-analysis nor present study found a significant difference in DBP or eGFR with combination therapy.
AEs were similar in this trial compared with the meta-analysis. Combination treatment with GLP-1 RA and SGLT2i did not increase the incidence of severe hypoglycemia in either study.8 Hypoglycemia was the most common AE in this study, but frequency was similar with combination and separate therapy. Both medication classes are associated with low or no risk of hypoglycemia on their own.1 Baseline medications likely contributed to episodes of hypoglycemia seen in this study: About 80% of patients were prescribed basal insulin, 15% were prescribed a sulfonylurea, and 13% were prescribed prandial insulin. There is limited overlap between the known AEs of GLP-1 RA and SGLT2i, making combination therapy a safe option for use in patients with T2DM.
Our study confirms greater reduction in HbA1c levels, weight, and SBP in veterans taking GLP-1 RA and SGLT2i medications in combination compared with separate use in a real-world setting in a veteran population. The magnitude of change seen in this population appears greater compared with previous studies.
Limitations
There were several limitations to our study. Given the retrospective nature, many patients included in the study did not have bloodwork drawn during the specified time frames. Because of this, many patients were excluded and missing data on renal outcomes limited the power to detect differences. Data regarding AEs were limited to what was recorded in the EHR, which may underrepresent the AEs that patients experienced. Finally, our study size was small, consisting primarily of a White and male population, which may limit generalizability.
Further research is needed to validate these findings in this population and should include a larger study population. The impact of combining GLP-1 RA with SGLT2i on cardiorenal outcomes is an important area of ongoing research.
ConclusionS
The combined use of GLP-1 RA and SGLT2i resulted in significant improvement in HbA1c levels, weight, and SBP compared with separate use in this real-world study of a VA population with T2DM. The combination was well tolerated overall. Awareness of these results can facilitate optimal care and outcomes in the VA population.
Acknowledgments
Serena Kelley, PharmD, and Michael Brenner, PharmD, assisted with study design and initial data collection. Julie Strominger, MS, provided statistical support.
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S111-S124. doi.10.2337/dc21-S009
2. DeFronzo RA. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes Metab. 2017;19(10):1353-1362. doi.10.1111/dom.12982
3. Jabbour S, Frias J, Guja C, Hardy E, Ahmed A, Ohman P. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes Metab. 2018;20(6):1515-1519. doi:10.1111/dom.13206
4. Ludvik B, Frias J, Tinahones F, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(5):370-381. doi:10.1016/S2213-8587(18)30023-8
5. Blonde L, Belousova L, Fainberg U, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929-937. doi:10.1111/dom.13978
6. Fulcher G, Matthews D, Perkovic V, et al; CANVAS trial collaborative group. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):82-91. doi:10.1111/dom.12589
7. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
8. Mantsiou C, Karagiannis T, Kakotrichi P, et al. Glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter-2 inhibitors as combination therapy for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1857-1868. doi:10.1111/dom.14108
9. US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of adult overweight and obesity. Version 3.0. Accessed August 18, 2022. www.healthquality.va.gov/guidelines/CD/obesity/VADoDObesityCPGFinal5087242020.pdf
10. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2015;387(10022):957-967. doi.10.1016/S0140-6736(15)01225-8
Make room for continuous glucose monitoring in type 2 diabetes management
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.
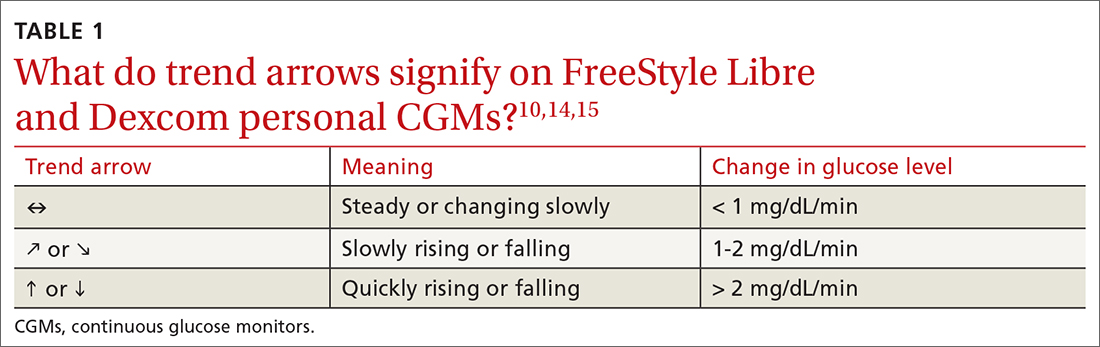
For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17
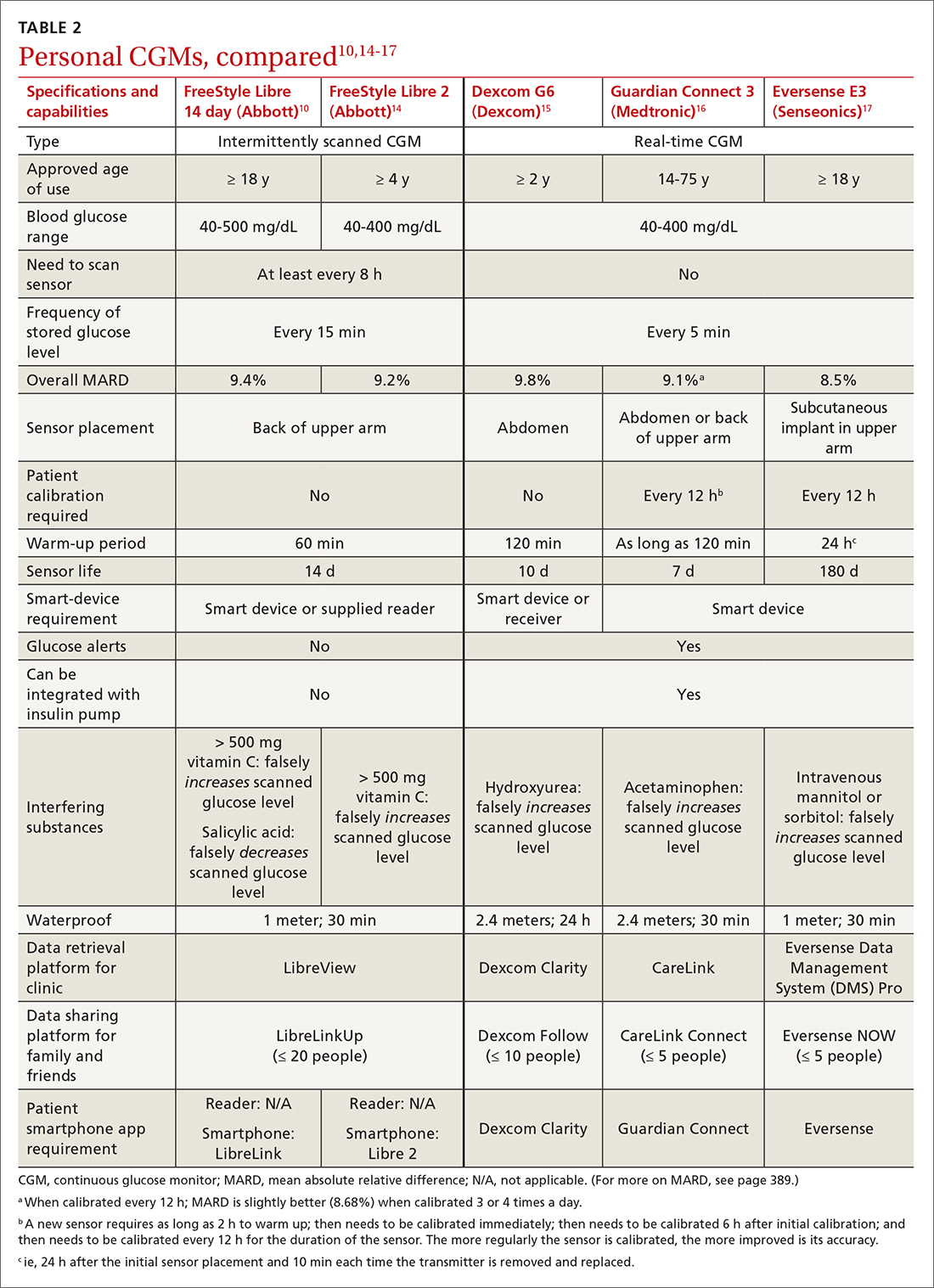
The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32
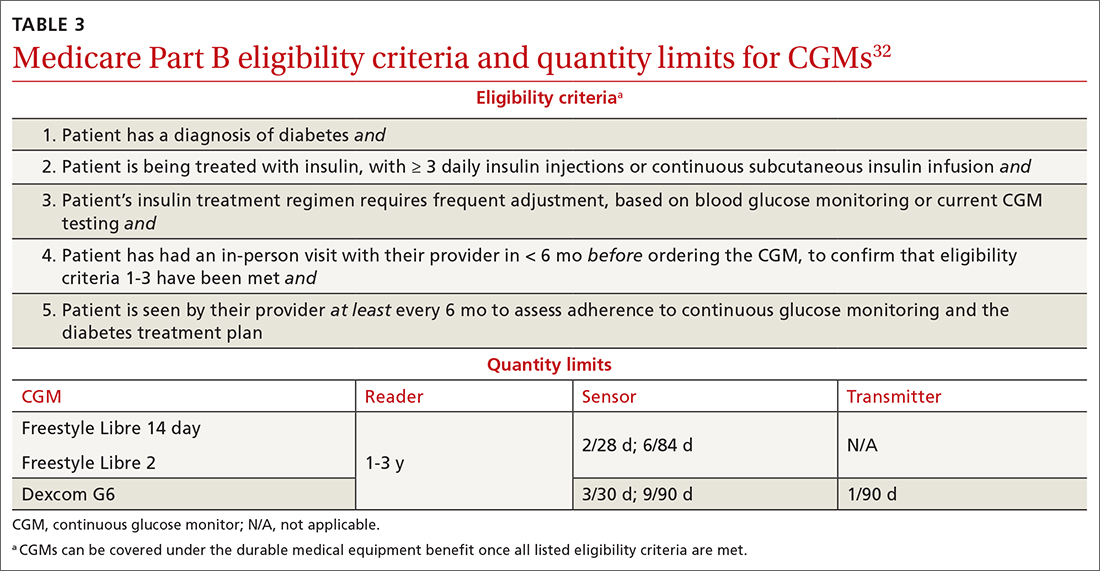
Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
PRACTICE RECOMMENDATIONS
› Initiate continuous glucose monitoring early in the disease process, based on a patient’s needs or preferences. C
› Interpret a continuous glucose monitor (CGM) report with the understanding that time within target range is the most important factor to evaluate. Minimizing or eliminating time below range is of paramount importance. B
› Advise patients who use a CGM to continue to have access to a glucometer and instruct them on appropriate times when such confirmation might be necessary. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series