User login
FDA advisory panel decides tivozanib falls short for advanced renal cell carcinoma
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).
"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
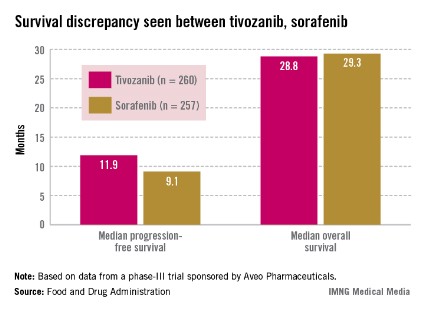
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
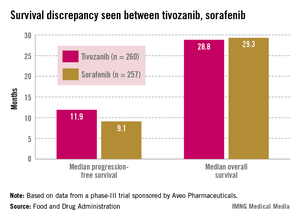
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
SILVER SPRING, MD. – Tivozanib’s risk-benefit profile fell short in a trial comparing the drug to sorafenib in patients with advanced renal cell carcinoma.
Citing a lower overall survival among patients in the tivozanib arm, despite a significant benefit in progression-free survival, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 13 to 1 at a May 2 meeting that the risk-benefit profile for tivozanib was not favorable.
Tivozanib appeared to be active in renal cell carcinoma (RCC) and had a manageable safety profile, the panelists said. But they had reservations about the design and patient demographics of the study; 88% of those enrolled in the trial were from Central and Eastern Europe. Only 8% of the patients in the study were enrolled in the United States and Western Europe, and only one black patient was enrolled, raising questions about the relevance of outcomes for a diversity of patients. Further, the trial’s comparator drug was sorafenib rather than a newer first-line tyrosine kinase inhibitor (TKI).

"While we are disappointed with the outcome of the ODAC vote, we remain confident in the efficacy, safety, and tolerability of tivozanib in RCC patients," Tuan Ha-Ngoc, president and chief executive officer of Aveo, the maker of tivozanib, said in a statement. "We are committed to the RCC patient community and will work closely with the FDA to address the issues discussed by the panel today as the agency continues its ongoing review of the New Drug Application for tivozanib."
Tivozanib, an oral capsule taken once a day, is a highly selective vascular endothelial growth factor (VEGF) TKI, according to Aveo. The company presented the results of the pivotal phase III study, the TIVO-1 (Tivozanib Versus Sorafenib in First-Line Advanced RCC) trial, which compared treatment with tivozanib in 260 patients with sorafenib in 257 patients. In 2005 sorafenib (Nexavar) became the first TKI approved for advanced RCC; several others have been approved since that time. In the United States, those newer drugs are considered before prescribing sorafenib in treating RCC.
Median progression-free survival (PFS), the primary endpoint of the study, was 11.9 months among those treated with tivozanib and 9.1 months among those on sorafenib, a significant difference that represented a 20% reduced risk (hazard ratio, 0.80).
Overall survival was poorer, however: 28.8 months in patients on tivozanib and 29.3 months in those on sorafenib (HR, 1.25). Overall, the safety profile of tivozanib was comparable to the profile of other VEGF inhibitors. Hypertension and dysphonia were more common with tivozanib, and diarrhea and plantar-palmar dysesthesia (hand-foot syndrome) were more common with sorafenib.
Aveo could not identify a safety signal to explain the lower overall survival. Company officials at the meeting said that more patients in the sorafenib arm (63%) crossed over to treatment with a targeted therapy, which in most cases was tivozanib. In the tivozanib arm, 16% crossed over to treatment with another therapy. The study’s protocol initially allowed crossover only in patients on sorafenib.
While there was a 20% improvement in PFS, there was "a potential 25% increase in the risk of death" compared with sorafenib, noted Dr. Jonathan Jarow, of the FDA’s Office of Hematology and Oncology Products. The company’s hypothesis regarding the crossover effect could not be proven with a post hoc analysis of the data "and remains just a hypothesis."
Approving a drug with a possible 25% increase in the risk of death was the FDA’s major concern and would set a precedent for approval of an oncology drug. The seven drugs approved for treating advanced RCC are thought to work by inhibiting VEGF or its receptor. Most of these approvals, except for temsirolimus, were based on improvements in PFS. Temsirolimus was additionally shown to improve overall survival in patients with a poor prognosis, according to the FDA.
Dr. Mikkael Sekeres, of the department of hematologic oncology and blood disorders at the Cleveland Clinic Taussig Cancer Institute, and the chair of the advisory panel to the FDA, expressed concern about the ethics of the study, which initially allowed crossover to another treatment in only one arm of the study and was conducted primarily in countries where other active RCC therapies are not readily available.
During the meeting, oncologists pointed out that treatments administered sequentially are associated with improved outcomes for patients with RCC.
"I cannot picture how I would be able to sit and talk with a patient about treating her or him with a drug that would allow that person to live without progression longer but possibly to die faster than if I treated that person with another available renal carcinoma drug, so I voted no," he added.
Aveo is developing tivozanib with Astellas Pharma. It is also being studied in combination with other targeted treatments or with chemotherapy regimens in patients with RCC, breast cancer, and colorectal cancer, according to Aveo. It has not been approved in any country.
The FDA usually follows the recommendations of its advisory panels. The deadline for the FDA review to be completed is July 28, according to Aveo. Panelists have been cleared of potential conflicts of interest related to the topic of the meeting. Occasionally, a panelist may be given a waiver, but not at the tivozanib meeting.
AT AN FDA ADVISORY PANEL MEETING
Obesity linked to prostatic intraepithelial neoplasia
Obesity was associated with an increased risk of precursor lesions among men with an initial benign prostate biopsy result, according to a nested study of nearly 500 prostate cancer cases and matched cancer-free controls.
Further, obese men also were more likely to have benign findings on initial core needle biopsy or transurethral resection of the prostate (TURP) and to then go on to develop prostate cancer within 4 years after their procedure.
"This is one of the first studies to assess associations between obesity and PIN (prostatic intraepithelial neoplasia)," reported Dr. Andrew Rundle of Columbia University, New York, and colleagues. The research was published in the April 23 online issue of Cancer Epidemiology, Biomarkers & Prevention (doi: 10.1158/1055-9965.EPI-12-0965). With approximately 1 million prostate biopsies conducted annually in the United States – two-thirds of which return negative results – obesity may be a factor to consider in the follow-up of individuals after an initial benign procedure.
In the study, obese men were more than twice as likely to have PIN detected in their initial benign specimens (OR = 2.17; 95% CI 1.13–4.15).
After adjustment for factors such as family history and prostate-specific antigen (PSA) levels, obesity at the time of the initial procedure was also associated with a significant increase in the incidence of prostate cancer, but only within 1,538 days of the initial procedure, which was the median duration of follow-up (OR = 1.95; 95% CI, 1.09-3.48).
Overall, a higher PSA value at the initial procedure and a family history of prostate cancer were associated with prostate cancer incidence, as was the number of PSA tests performed during follow-up. The association between obesity and prostate cancer incidence was confined to diagnoses occurring within less than 1,538 days, which was the median duration of follow-up after the initial benign procedure.
The findings emerged from a study that recruited 494 prostate cancer cases and matched cancer-free controls from a historical cohort of nearly 6,700 men followed up after a needle core biopsy or TURP. All had a benign prostate specimen collected between January 1990 and December 2002 and were followed up to December 2007 at the Henry Ford Health System in Detroit. The incidence of prostate cancer within this high-risk cohort was approximately twice that of the general Detroit Surveillance Epidemiology and End Results (SEER) population, although the ratio of African American to white cases in the cohort was similar to that in the overall SEER data.
All subjects had a recorded PSA level within a year of their initial benign procedure cohort entry and no history of a previous prostate cancer diagnosis. Patients diagnosed with prostate cancer less than 1 year from the date of their initial benign procedure were ineligible for the study. Controls were randomly selected from among those cohort members who were free of prostate cancer at a follow-up duration greater than or equal to the time between cohort entry and diagnosis of the matched case.
Body mass index was calculated from medical record data on height and weight measured within 115 days of the benign procedure. Overweight was a BMI of more than 25 and less than 30 and obese was a BMI of 30 or more. Surgical specimens were reviewed for the presence of PIN by a single urological pathologist who was blinded to prostate cancer outcomes at the time of review. Advanced stage disease was defined as pathologic or clinical stage T3a and higher.
In this study, in contrast to earlier cohort studies, obesity was not associated with high Gleason grade tumors, and obesity seemed to be more likely to be associated with low-grade tumors. "If the missed tumors were small and low grade, then they may have still been of lower grade when diagnosed in the first few years after the initial biopsy with high-grade prostate cancer and unassociated with or perhaps even protective for localized or low-grade disease," the researchers reported.
Obesity has been thought to reduce the sensitivity of PSA testing and, thus, may delay referral for biopsy. Obesity may also reduce the diagnostic efficiency of needle biopsies, as obese men have larger prostates and small tumors may be more likely to go undetected until they grow and are detected in subsequent biopsies during follow-up, the authors wrote.
The study was supported by a grant from the National Institute of Environmental Health Sciences, and the authors did not declare any potential conflicts of interest.
Obesity was associated with an increased risk of precursor lesions among men with an initial benign prostate biopsy result, according to a nested study of nearly 500 prostate cancer cases and matched cancer-free controls.
Further, obese men also were more likely to have benign findings on initial core needle biopsy or transurethral resection of the prostate (TURP) and to then go on to develop prostate cancer within 4 years after their procedure.
"This is one of the first studies to assess associations between obesity and PIN (prostatic intraepithelial neoplasia)," reported Dr. Andrew Rundle of Columbia University, New York, and colleagues. The research was published in the April 23 online issue of Cancer Epidemiology, Biomarkers & Prevention (doi: 10.1158/1055-9965.EPI-12-0965). With approximately 1 million prostate biopsies conducted annually in the United States – two-thirds of which return negative results – obesity may be a factor to consider in the follow-up of individuals after an initial benign procedure.
In the study, obese men were more than twice as likely to have PIN detected in their initial benign specimens (OR = 2.17; 95% CI 1.13–4.15).
After adjustment for factors such as family history and prostate-specific antigen (PSA) levels, obesity at the time of the initial procedure was also associated with a significant increase in the incidence of prostate cancer, but only within 1,538 days of the initial procedure, which was the median duration of follow-up (OR = 1.95; 95% CI, 1.09-3.48).
Overall, a higher PSA value at the initial procedure and a family history of prostate cancer were associated with prostate cancer incidence, as was the number of PSA tests performed during follow-up. The association between obesity and prostate cancer incidence was confined to diagnoses occurring within less than 1,538 days, which was the median duration of follow-up after the initial benign procedure.
The findings emerged from a study that recruited 494 prostate cancer cases and matched cancer-free controls from a historical cohort of nearly 6,700 men followed up after a needle core biopsy or TURP. All had a benign prostate specimen collected between January 1990 and December 2002 and were followed up to December 2007 at the Henry Ford Health System in Detroit. The incidence of prostate cancer within this high-risk cohort was approximately twice that of the general Detroit Surveillance Epidemiology and End Results (SEER) population, although the ratio of African American to white cases in the cohort was similar to that in the overall SEER data.
All subjects had a recorded PSA level within a year of their initial benign procedure cohort entry and no history of a previous prostate cancer diagnosis. Patients diagnosed with prostate cancer less than 1 year from the date of their initial benign procedure were ineligible for the study. Controls were randomly selected from among those cohort members who were free of prostate cancer at a follow-up duration greater than or equal to the time between cohort entry and diagnosis of the matched case.
Body mass index was calculated from medical record data on height and weight measured within 115 days of the benign procedure. Overweight was a BMI of more than 25 and less than 30 and obese was a BMI of 30 or more. Surgical specimens were reviewed for the presence of PIN by a single urological pathologist who was blinded to prostate cancer outcomes at the time of review. Advanced stage disease was defined as pathologic or clinical stage T3a and higher.
In this study, in contrast to earlier cohort studies, obesity was not associated with high Gleason grade tumors, and obesity seemed to be more likely to be associated with low-grade tumors. "If the missed tumors were small and low grade, then they may have still been of lower grade when diagnosed in the first few years after the initial biopsy with high-grade prostate cancer and unassociated with or perhaps even protective for localized or low-grade disease," the researchers reported.
Obesity has been thought to reduce the sensitivity of PSA testing and, thus, may delay referral for biopsy. Obesity may also reduce the diagnostic efficiency of needle biopsies, as obese men have larger prostates and small tumors may be more likely to go undetected until they grow and are detected in subsequent biopsies during follow-up, the authors wrote.
The study was supported by a grant from the National Institute of Environmental Health Sciences, and the authors did not declare any potential conflicts of interest.
Obesity was associated with an increased risk of precursor lesions among men with an initial benign prostate biopsy result, according to a nested study of nearly 500 prostate cancer cases and matched cancer-free controls.
Further, obese men also were more likely to have benign findings on initial core needle biopsy or transurethral resection of the prostate (TURP) and to then go on to develop prostate cancer within 4 years after their procedure.
"This is one of the first studies to assess associations between obesity and PIN (prostatic intraepithelial neoplasia)," reported Dr. Andrew Rundle of Columbia University, New York, and colleagues. The research was published in the April 23 online issue of Cancer Epidemiology, Biomarkers & Prevention (doi: 10.1158/1055-9965.EPI-12-0965). With approximately 1 million prostate biopsies conducted annually in the United States – two-thirds of which return negative results – obesity may be a factor to consider in the follow-up of individuals after an initial benign procedure.
In the study, obese men were more than twice as likely to have PIN detected in their initial benign specimens (OR = 2.17; 95% CI 1.13–4.15).
After adjustment for factors such as family history and prostate-specific antigen (PSA) levels, obesity at the time of the initial procedure was also associated with a significant increase in the incidence of prostate cancer, but only within 1,538 days of the initial procedure, which was the median duration of follow-up (OR = 1.95; 95% CI, 1.09-3.48).
Overall, a higher PSA value at the initial procedure and a family history of prostate cancer were associated with prostate cancer incidence, as was the number of PSA tests performed during follow-up. The association between obesity and prostate cancer incidence was confined to diagnoses occurring within less than 1,538 days, which was the median duration of follow-up after the initial benign procedure.
The findings emerged from a study that recruited 494 prostate cancer cases and matched cancer-free controls from a historical cohort of nearly 6,700 men followed up after a needle core biopsy or TURP. All had a benign prostate specimen collected between January 1990 and December 2002 and were followed up to December 2007 at the Henry Ford Health System in Detroit. The incidence of prostate cancer within this high-risk cohort was approximately twice that of the general Detroit Surveillance Epidemiology and End Results (SEER) population, although the ratio of African American to white cases in the cohort was similar to that in the overall SEER data.
All subjects had a recorded PSA level within a year of their initial benign procedure cohort entry and no history of a previous prostate cancer diagnosis. Patients diagnosed with prostate cancer less than 1 year from the date of their initial benign procedure were ineligible for the study. Controls were randomly selected from among those cohort members who were free of prostate cancer at a follow-up duration greater than or equal to the time between cohort entry and diagnosis of the matched case.
Body mass index was calculated from medical record data on height and weight measured within 115 days of the benign procedure. Overweight was a BMI of more than 25 and less than 30 and obese was a BMI of 30 or more. Surgical specimens were reviewed for the presence of PIN by a single urological pathologist who was blinded to prostate cancer outcomes at the time of review. Advanced stage disease was defined as pathologic or clinical stage T3a and higher.
In this study, in contrast to earlier cohort studies, obesity was not associated with high Gleason grade tumors, and obesity seemed to be more likely to be associated with low-grade tumors. "If the missed tumors were small and low grade, then they may have still been of lower grade when diagnosed in the first few years after the initial biopsy with high-grade prostate cancer and unassociated with or perhaps even protective for localized or low-grade disease," the researchers reported.
Obesity has been thought to reduce the sensitivity of PSA testing and, thus, may delay referral for biopsy. Obesity may also reduce the diagnostic efficiency of needle biopsies, as obese men have larger prostates and small tumors may be more likely to go undetected until they grow and are detected in subsequent biopsies during follow-up, the authors wrote.
The study was supported by a grant from the National Institute of Environmental Health Sciences, and the authors did not declare any potential conflicts of interest.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Major finding: Obese men were more than twice as likely to have PIN detected in their initial benign prostate biopsy specimens (OR = 2.17; 95% CI 1.13-4.15).
Data source: Nested case-control cohort study of 494 prostate cancer cases and matched controls.
Disclosures: The research was funded by the National Institute of Environmental Health Sciences. The authors did not declare any conflicts of interest.
DNA changes predict prostate cancer death
Patients whose localized prostate cancer contained a loss of the phosphatase and tensin homolog gene and amplification of the v-myc myelocytomatosis viral oncogene homolog gene were 53 times as likely to die from the disease, compared with similar patients without the genetic changes.
The increased risk was seen despite similarly staged tumors and similar levels of prostate-specific antigen (PSA) at the time of diagnosis in analyses of 458 patients in multiple cohorts, according to Wennuan Liu, Ph.D., and his associates.
If the findings are confirmed, they could help clinicians decide which patients would best benefit from aggressive treatment with prostatectomy or radiation and which might be fine with conservative management.
The investigators first assayed DNA samples from 125 patients who underwent prostatectomy for localized prostate cancer at Johns Hopkins Hospital, in Baltimore between 1988 and 2004. They found 20 significant regions of chromosomal copy number alterations in the cancer cells, including four copy number alterations and the target genes within those alterations that had not been identified before (Cancer 2013 April 22 [doi:10.1002/cncr.27954]).
Alterations in the chromosomal copy numbers of the phosphatase and tensin homolog (PTEN) and v-myc myelocytomatosis viral oncogene homolog (MYC) genes, when occurring together, were associated with a 53-fold increased risk of death from prostate cancer in a multivariate analysis. That prognostic information was independent of information gleaned from pathologic stage, Gleason score, and initial PSA level, reported Dr. Liu of Wake Forest University’s Center for Cancer Genomics and Center for Genomics and Personalized Medicine Research, in Winston-Salem, N.C.
Independently, loss of PTEN was associated with a sevenfold increased risk of death from prostate cancer, while gain in MYC was associated with an eightfold increased risk of disease-related death. The prognostic information from the chromosomal copy number alterations was most relevant for patients whose tumors had a pathologic Gleason score of 7 or less.
Many of the patients in the initial cohort had aggressive prostate cancer, with a Gleason score of 8 or greater in 34% of patients, a pathologic stage of T3b or higher in 33%, and a pretreatment PSA level higher than 10 ng/mL in 44% of patients. Twenty-two patients in this cohort died of prostate cancer (18%); the rest are alive or died of other causes.
The investigators confirmed the relationship between fatal prostate cancer and copy number alterations of PTEN and MYC in analyses of three subsequent cohorts.
Among 103 patients who underwent prostatectomy at the Karolinska University Hospital in Sweden, most had less-aggressive prostate cancer, with a pathologic Gleason score of 7 or lower in 85% of patients, a pathologic stage less than T3 in 82%, and a pretreatment PSA no greater than 10 ng/mL in 64%. Four of these patients died of prostate cancer (4%). They also analyzed publicly available clinicopathologic and survival information for 216 patients treated at Memorial Sloan-Kettering Cancer Center in New York, 4 of whom died of prostate cancer (2%).
When the Karolinska and Memorial Sloan-Kettering cohorts were combined, only 1 of 201 patients who lacked the chromosomal copy number alterations in PTEN and MYC died of prostate cancer (0.5%), compared with death from prostate cancer in 6% of the remaining patients, whose tumors did have one or both of the prognostic markers, Dr. Liu reported.
Analyses of tumor samples obtained at autopsy for 14 separate patients seen at Johns Hopkins who died of progressive prostate cancer found chromosomal copy number alterations in one of the genes in 100% of patients, and alterations in both genes in 57%. In comparison, 58% of the original cohort of 125 patients treated for localized disease at Johns Hopkins had alterations in one of the genes, and 10% had alterations in both.
The findings are supported by previous in-vitro and animal studies, the investigators noted, but more studies are needed because of the relatively small number of deaths in the Karolinska and Memorial Sloan-Kettering cohorts.
The study was limited by the possibility of mutations influencing tumor phenotype or the selection of specific copy number alterations. The median clinical follow-up of 5-7 years may not be predictive of long-term mortality risk.
Further research into the lethal tumor phenotype’s effect on tumor evolution may lead to the design of therapies to interrupt disease progression, Dr. Liu noted.
Prostate cancer is the most common cancer among men. Approximately 28,000 U.S. men die from the cancer each year. Prostatectomy improves 15-year mortality rates modestly, compared with conservative management – 15% vs. 21%, respectively – and 1 in 7 patients who undergo prostatectomy relapse and die of prostate cancer. These statistics have propelled efforts to identify better prognostic markers and therapies.
The study was funded by the National Institutes of Health and the U.S. Department of Defense. Dr. Liu and his associates made no disclosures regarding potential conflicts of interest.
On Twitter @sherryboschert
Patients whose localized prostate cancer contained a loss of the phosphatase and tensin homolog gene and amplification of the v-myc myelocytomatosis viral oncogene homolog gene were 53 times as likely to die from the disease, compared with similar patients without the genetic changes.
The increased risk was seen despite similarly staged tumors and similar levels of prostate-specific antigen (PSA) at the time of diagnosis in analyses of 458 patients in multiple cohorts, according to Wennuan Liu, Ph.D., and his associates.
If the findings are confirmed, they could help clinicians decide which patients would best benefit from aggressive treatment with prostatectomy or radiation and which might be fine with conservative management.
The investigators first assayed DNA samples from 125 patients who underwent prostatectomy for localized prostate cancer at Johns Hopkins Hospital, in Baltimore between 1988 and 2004. They found 20 significant regions of chromosomal copy number alterations in the cancer cells, including four copy number alterations and the target genes within those alterations that had not been identified before (Cancer 2013 April 22 [doi:10.1002/cncr.27954]).
Alterations in the chromosomal copy numbers of the phosphatase and tensin homolog (PTEN) and v-myc myelocytomatosis viral oncogene homolog (MYC) genes, when occurring together, were associated with a 53-fold increased risk of death from prostate cancer in a multivariate analysis. That prognostic information was independent of information gleaned from pathologic stage, Gleason score, and initial PSA level, reported Dr. Liu of Wake Forest University’s Center for Cancer Genomics and Center for Genomics and Personalized Medicine Research, in Winston-Salem, N.C.
Independently, loss of PTEN was associated with a sevenfold increased risk of death from prostate cancer, while gain in MYC was associated with an eightfold increased risk of disease-related death. The prognostic information from the chromosomal copy number alterations was most relevant for patients whose tumors had a pathologic Gleason score of 7 or less.
Many of the patients in the initial cohort had aggressive prostate cancer, with a Gleason score of 8 or greater in 34% of patients, a pathologic stage of T3b or higher in 33%, and a pretreatment PSA level higher than 10 ng/mL in 44% of patients. Twenty-two patients in this cohort died of prostate cancer (18%); the rest are alive or died of other causes.
The investigators confirmed the relationship between fatal prostate cancer and copy number alterations of PTEN and MYC in analyses of three subsequent cohorts.
Among 103 patients who underwent prostatectomy at the Karolinska University Hospital in Sweden, most had less-aggressive prostate cancer, with a pathologic Gleason score of 7 or lower in 85% of patients, a pathologic stage less than T3 in 82%, and a pretreatment PSA no greater than 10 ng/mL in 64%. Four of these patients died of prostate cancer (4%). They also analyzed publicly available clinicopathologic and survival information for 216 patients treated at Memorial Sloan-Kettering Cancer Center in New York, 4 of whom died of prostate cancer (2%).
When the Karolinska and Memorial Sloan-Kettering cohorts were combined, only 1 of 201 patients who lacked the chromosomal copy number alterations in PTEN and MYC died of prostate cancer (0.5%), compared with death from prostate cancer in 6% of the remaining patients, whose tumors did have one or both of the prognostic markers, Dr. Liu reported.
Analyses of tumor samples obtained at autopsy for 14 separate patients seen at Johns Hopkins who died of progressive prostate cancer found chromosomal copy number alterations in one of the genes in 100% of patients, and alterations in both genes in 57%. In comparison, 58% of the original cohort of 125 patients treated for localized disease at Johns Hopkins had alterations in one of the genes, and 10% had alterations in both.
The findings are supported by previous in-vitro and animal studies, the investigators noted, but more studies are needed because of the relatively small number of deaths in the Karolinska and Memorial Sloan-Kettering cohorts.
The study was limited by the possibility of mutations influencing tumor phenotype or the selection of specific copy number alterations. The median clinical follow-up of 5-7 years may not be predictive of long-term mortality risk.
Further research into the lethal tumor phenotype’s effect on tumor evolution may lead to the design of therapies to interrupt disease progression, Dr. Liu noted.
Prostate cancer is the most common cancer among men. Approximately 28,000 U.S. men die from the cancer each year. Prostatectomy improves 15-year mortality rates modestly, compared with conservative management – 15% vs. 21%, respectively – and 1 in 7 patients who undergo prostatectomy relapse and die of prostate cancer. These statistics have propelled efforts to identify better prognostic markers and therapies.
The study was funded by the National Institutes of Health and the U.S. Department of Defense. Dr. Liu and his associates made no disclosures regarding potential conflicts of interest.
On Twitter @sherryboschert
Patients whose localized prostate cancer contained a loss of the phosphatase and tensin homolog gene and amplification of the v-myc myelocytomatosis viral oncogene homolog gene were 53 times as likely to die from the disease, compared with similar patients without the genetic changes.
The increased risk was seen despite similarly staged tumors and similar levels of prostate-specific antigen (PSA) at the time of diagnosis in analyses of 458 patients in multiple cohorts, according to Wennuan Liu, Ph.D., and his associates.
If the findings are confirmed, they could help clinicians decide which patients would best benefit from aggressive treatment with prostatectomy or radiation and which might be fine with conservative management.
The investigators first assayed DNA samples from 125 patients who underwent prostatectomy for localized prostate cancer at Johns Hopkins Hospital, in Baltimore between 1988 and 2004. They found 20 significant regions of chromosomal copy number alterations in the cancer cells, including four copy number alterations and the target genes within those alterations that had not been identified before (Cancer 2013 April 22 [doi:10.1002/cncr.27954]).
Alterations in the chromosomal copy numbers of the phosphatase and tensin homolog (PTEN) and v-myc myelocytomatosis viral oncogene homolog (MYC) genes, when occurring together, were associated with a 53-fold increased risk of death from prostate cancer in a multivariate analysis. That prognostic information was independent of information gleaned from pathologic stage, Gleason score, and initial PSA level, reported Dr. Liu of Wake Forest University’s Center for Cancer Genomics and Center for Genomics and Personalized Medicine Research, in Winston-Salem, N.C.
Independently, loss of PTEN was associated with a sevenfold increased risk of death from prostate cancer, while gain in MYC was associated with an eightfold increased risk of disease-related death. The prognostic information from the chromosomal copy number alterations was most relevant for patients whose tumors had a pathologic Gleason score of 7 or less.
Many of the patients in the initial cohort had aggressive prostate cancer, with a Gleason score of 8 or greater in 34% of patients, a pathologic stage of T3b or higher in 33%, and a pretreatment PSA level higher than 10 ng/mL in 44% of patients. Twenty-two patients in this cohort died of prostate cancer (18%); the rest are alive or died of other causes.
The investigators confirmed the relationship between fatal prostate cancer and copy number alterations of PTEN and MYC in analyses of three subsequent cohorts.
Among 103 patients who underwent prostatectomy at the Karolinska University Hospital in Sweden, most had less-aggressive prostate cancer, with a pathologic Gleason score of 7 or lower in 85% of patients, a pathologic stage less than T3 in 82%, and a pretreatment PSA no greater than 10 ng/mL in 64%. Four of these patients died of prostate cancer (4%). They also analyzed publicly available clinicopathologic and survival information for 216 patients treated at Memorial Sloan-Kettering Cancer Center in New York, 4 of whom died of prostate cancer (2%).
When the Karolinska and Memorial Sloan-Kettering cohorts were combined, only 1 of 201 patients who lacked the chromosomal copy number alterations in PTEN and MYC died of prostate cancer (0.5%), compared with death from prostate cancer in 6% of the remaining patients, whose tumors did have one or both of the prognostic markers, Dr. Liu reported.
Analyses of tumor samples obtained at autopsy for 14 separate patients seen at Johns Hopkins who died of progressive prostate cancer found chromosomal copy number alterations in one of the genes in 100% of patients, and alterations in both genes in 57%. In comparison, 58% of the original cohort of 125 patients treated for localized disease at Johns Hopkins had alterations in one of the genes, and 10% had alterations in both.
The findings are supported by previous in-vitro and animal studies, the investigators noted, but more studies are needed because of the relatively small number of deaths in the Karolinska and Memorial Sloan-Kettering cohorts.
The study was limited by the possibility of mutations influencing tumor phenotype or the selection of specific copy number alterations. The median clinical follow-up of 5-7 years may not be predictive of long-term mortality risk.
Further research into the lethal tumor phenotype’s effect on tumor evolution may lead to the design of therapies to interrupt disease progression, Dr. Liu noted.
Prostate cancer is the most common cancer among men. Approximately 28,000 U.S. men die from the cancer each year. Prostatectomy improves 15-year mortality rates modestly, compared with conservative management – 15% vs. 21%, respectively – and 1 in 7 patients who undergo prostatectomy relapse and die of prostate cancer. These statistics have propelled efforts to identify better prognostic markers and therapies.
The study was funded by the National Institutes of Health and the U.S. Department of Defense. Dr. Liu and his associates made no disclosures regarding potential conflicts of interest.
On Twitter @sherryboschert
FROM CANCER
Major finding: Death from prostate cancer was 50 times more likely in patients whose localized tumors contained a loss of the PTEN gene and an amplification of the MYC gene, compared with similar patients without these genetic changes.
Data source: Assays of DNA samples from 125 patients who underwent prostatectomy were correlated with clinicopathologic features and outcomes. The findings were confirmed in 333 patients in three subsequent cohorts.
Disclosures: The study was funded by the National Institutes of Health and the U.S. Department of Defense. Dr. Liu and his associates made no disclosures regarding potential conflicts of interest.
Abdominal, thoracic CT scans reliably detect incidental low lumbar BMD
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Dr. Pickhardt and his associates "have laid all the groundwork needed to justify using conventional CT imaging to detect incidental osteoporosis," said Dr. Sumit R. Majumdar and Dr. William D. Leslie.
Given the large number of such CT scans performed every year, "the idea of extracting more information from imaging data collected for other purposes holds merit," they said.
"It is now up to the rest of us to safely and cost-effectively translate this new knowledge into everyday clinical practice," Dr. Majumdar and Dr. Leslie said.
Dr. Majumdar is with the University of Alberta, Edmonton. Dr. Leslie is with the University of Manitoba, Winnipeg. Neither reported any financial conflicts of interest. These remarks were taken from their editorial, which accompanied Dr. Pickhardt’s report (Ann. Intern. Med. 2013;158:630-1).
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Abdominal and thoracic CT scans obtained for a variety of reasons, such as to assess pain, GI symptoms, or urinary tract complaints, also can be used "opportunistically" to examine lumbar bone mineral density and screen for occult osteoporosis, according to a report in the April 16 issue of the Annals of Internal Medicine.
Abdominal and thoracic CT scans done in routine practice happen to include imaging of the L1 level, which can easily be identified because it is the first non–rib-bearing vertebra. Such scans readily yield data on lumbar bone mineral density (BMD), which is a clinically useful way to diagnose or rule out osteoporosis, said Dr. Perry J. Pickhardt of the department of radiology and his associates at the University of Wisconsin, Madison.
It is important not to confuse this standard CT scanning with quantitative CT (QCT) scanning. QCT "is more labor-intensive; requires an imaging phantom or angle-corrected [region-of-interest] measurement of bone, muscle, and fat at multiple levels; and involves additional money, time, and radiation exposure," they explained.
Unlike dual-energy x-ray absorptiometry (DXA) screening or QCT assessment, "the method that we used requires a negligible amount of training and time; could be applied prospectively by the interpreting radiologist or retrospectively by a radiologist or even nonradiologist; adds no cost; and requires no additional patient time, equipment, software, or radiation exposure," the investigators wrote.
Such incidental CT scans can be assessed retrospectively because they are almost always stored indefinitely in electronic medical records, they noted.
Dr. Pickhardt and his colleagues evaluated CT-derived BMD assessment and compared it against DXA scanning of the hips and spine by identifying 1,867 adults who had undergone the two types of scanning within a 6-month period during the 10-year study interval. They retrieved and reviewed the images, paying particular attention to obvious moderate or severe compression deformities on the CT images, rather than to milder ones, "to avoid ambiguity related to more subjective borderline or mild compression deformities."
The study subjects had a total of 2,063 pairs of CT and DXA assessments that had been performed a median of 67 days apart. A total of 81% of these subjects were women, and the mean age was 59 years.
These patients had undergone abdominal or thoracic CT for a variety of clinical indications, most often for a suspected mass or an oncologic work-up (414 subjects), genitourinary problems (402 subjects), gastrointestinal symptoms (398 subjects), and/or unexplained abdominal pain or symptoms (374 subjects).
Approximately 55% of the CT scans involved intravenous contrast. The use of contrast had no effect on the interpretation of lumbar data on the scans.
The DXA screening identified 22.9% of the study subjects as osteoporotic, 44.8% as osteopenic, and 32.3% as having normal BMD. The CT scans were significantly more sensitive than DXA at distinguishing these three states.
In particular, CT scans identified 119 patients as having osteoporosis, with readily identifiable moderate or severe vertebral fractures, when DXA had classified 62 of these patients as having normal BMD (12 subjects) or only osteopenia (50 subjects).
"Our observations are consistent with prior studies documenting that many patients without osteoporosis diagnosed by DXA will sustain fragility fractures, and suggest that CT attenuation may be a more accurate risk predictor," Dr. Pickhardt and his associates wrote (Ann. Intern. Med. 2013:158:588-95).
If their findings are confirmed in other studies, it may become routine for all abdominal and thoracic CT scans performed for any reason to be used for lumbar BMD assessment as well. "In the future, it may even be possible to incorporate CT ... data into fracture risk assessment tools," they added.
This should result in substantial savings in health care costs since osteoporosis will be diagnosed and treated earlier, before fractures occur, and since it also will reduce the number of costly DXA studies performed.
More than 80 million CT scans were performed in the United States in 2011, "most of which carry potentially useful information about BMD," the researchers noted.
The investigators are now turning their attention to using pelvic CT scans that were obtained for various clinical indications to assess hip BMD. "We are currently investigating the potential for deriving a DXA-equivalent T-score for the hips from standard pelvic CT scans by using a dedicated software tool," Dr. Pickhardt and his associates said.
This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Major Finding: Conventional abdominal and CT scans performed for various indications were more accurate than DXA at assessing lumbar BMD and identifying occult osteoporosis.
Data Source: A 10-year cross-sectional study comparing incidental spine imaging on conventional CT scans against DXA screening in 1,867 adults from the general population.
Disclosures: This study was funded by the National Institutes of Health. None of the investigators reported having any financial conflicts of interest.
Axitinib eyed as first-line therapy for mRCC
ORLANDO – Compared with sorafenib, axitinib as first-line therapy was associated with a 3.6 month improvement in progression-free survival among patients with metastatic renal cell carcinoma.
The median PFS was 10.1 months in the axitinib arm and 6.5 months in the sorafenib arm. (hazard ratio, 0.77; one-sided P = .038). The results did not reach the predetermined significance level of 0.025, and hence the trial didn’t achieve its primary endpoint, said Dr. Thomas E. Hutson at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
No differences in overall survival data have been noted to date in the ongoing phase III trial. Dr. Hutson of Texas Oncology – Baylor Charles A. Sammons Cancer Center, Dallas, and the study’s lead author, said that researchers are waiting for the survival data to mature.
Axitinib is a vascular endothelial growth factor receptor inhibitor that is approved for advanced renal cell carcinoma (RCC) after one prior systemic therapy has failed.
To examine the value of axitinib as a first-line therapy, researchers took into account results from previous clinical trials (J. Clin. Oncol. 2009; 27:1280-9) and amended an ongoing randomized trial of second-line axitinib vs. sorafenib to include a population of first-line therapy patients.
The Pfizer-sponsored multicenter, randomized, open-label, phase III trial included 288 untreated patients with clear-cell mRCC who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were randomized 2:1 to axitinib (5 mg, twice daily) or to sorafenib (400 mg, twice daily.) The randomization was stratified by ECOG performance status.
The primary endpoint was progression-free survival (PFS) as determined by an independent radiology committee. The study had a 90% power to detect a 78% improvement in PFS.
Secondary endpoints included overall survival, objective response rate, safety, duration of response, kidney cancer–specific symptoms, and health-related quality of life.
Patients’ baseline characteristics were well balanced between the two arms, and median patient age was 58 years old. ECOG performance status was 0 for 57% and 1 for 43%. Nephrectomies were performed in 85% of patients in the axitinib arm and 90% in the sorafenib arm. About 14% of the patients were from North America.
Patients who had a nephrectomy and an ECOG performance status of 0 faired better if they were in the axitinib arm. Among patients who had a nephrectomy, axitinib-treated patients had a median PFS of 10.3 months and sorafenib-treated patients had a median PFS of 6.4 months (HR, 0.67*; one-sided P = .009). Patients with an ECOG performance status of 0 had a median PFS of 13.7 months in the axitinib arm and 6.6 months in the sorafenib arm (HR, 0.644; one-sided P =.022). Patients with an ECOG performance status of 1 had similar median PFS – 6.5 months in the axitinib arm and 6.4 months in sorafenib arm.
Objective response rate was 32.3% with axitinib and 14.6% with sorafenib.
Patients in the axitinib arm had more hypertension and diarrhea (49% vs. 29% with sorafenib, and 50% vs. 40% with sorafenib.) Palmar-plantar erythrodysesthesia was more common with sorafenib (39% vs. 26% with axitinib).
Dr. Hutson has been a consultant or adviser for, and has received honoraria and research funding from AVEO, Bayer, GlaxoSmithKline, Novartis, and Pfizer, the maker of axitinib.
*Correction, 4/17/2013: An earlier version of this story misstated the HR value in patients who had nephrectomy.
ORLANDO – Compared with sorafenib, axitinib as first-line therapy was associated with a 3.6 month improvement in progression-free survival among patients with metastatic renal cell carcinoma.
The median PFS was 10.1 months in the axitinib arm and 6.5 months in the sorafenib arm. (hazard ratio, 0.77; one-sided P = .038). The results did not reach the predetermined significance level of 0.025, and hence the trial didn’t achieve its primary endpoint, said Dr. Thomas E. Hutson at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
No differences in overall survival data have been noted to date in the ongoing phase III trial. Dr. Hutson of Texas Oncology – Baylor Charles A. Sammons Cancer Center, Dallas, and the study’s lead author, said that researchers are waiting for the survival data to mature.
Axitinib is a vascular endothelial growth factor receptor inhibitor that is approved for advanced renal cell carcinoma (RCC) after one prior systemic therapy has failed.
To examine the value of axitinib as a first-line therapy, researchers took into account results from previous clinical trials (J. Clin. Oncol. 2009; 27:1280-9) and amended an ongoing randomized trial of second-line axitinib vs. sorafenib to include a population of first-line therapy patients.
The Pfizer-sponsored multicenter, randomized, open-label, phase III trial included 288 untreated patients with clear-cell mRCC who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were randomized 2:1 to axitinib (5 mg, twice daily) or to sorafenib (400 mg, twice daily.) The randomization was stratified by ECOG performance status.
The primary endpoint was progression-free survival (PFS) as determined by an independent radiology committee. The study had a 90% power to detect a 78% improvement in PFS.
Secondary endpoints included overall survival, objective response rate, safety, duration of response, kidney cancer–specific symptoms, and health-related quality of life.
Patients’ baseline characteristics were well balanced between the two arms, and median patient age was 58 years old. ECOG performance status was 0 for 57% and 1 for 43%. Nephrectomies were performed in 85% of patients in the axitinib arm and 90% in the sorafenib arm. About 14% of the patients were from North America.
Patients who had a nephrectomy and an ECOG performance status of 0 faired better if they were in the axitinib arm. Among patients who had a nephrectomy, axitinib-treated patients had a median PFS of 10.3 months and sorafenib-treated patients had a median PFS of 6.4 months (HR, 0.67*; one-sided P = .009). Patients with an ECOG performance status of 0 had a median PFS of 13.7 months in the axitinib arm and 6.6 months in the sorafenib arm (HR, 0.644; one-sided P =.022). Patients with an ECOG performance status of 1 had similar median PFS – 6.5 months in the axitinib arm and 6.4 months in sorafenib arm.
Objective response rate was 32.3% with axitinib and 14.6% with sorafenib.
Patients in the axitinib arm had more hypertension and diarrhea (49% vs. 29% with sorafenib, and 50% vs. 40% with sorafenib.) Palmar-plantar erythrodysesthesia was more common with sorafenib (39% vs. 26% with axitinib).
Dr. Hutson has been a consultant or adviser for, and has received honoraria and research funding from AVEO, Bayer, GlaxoSmithKline, Novartis, and Pfizer, the maker of axitinib.
*Correction, 4/17/2013: An earlier version of this story misstated the HR value in patients who had nephrectomy.
ORLANDO – Compared with sorafenib, axitinib as first-line therapy was associated with a 3.6 month improvement in progression-free survival among patients with metastatic renal cell carcinoma.
The median PFS was 10.1 months in the axitinib arm and 6.5 months in the sorafenib arm. (hazard ratio, 0.77; one-sided P = .038). The results did not reach the predetermined significance level of 0.025, and hence the trial didn’t achieve its primary endpoint, said Dr. Thomas E. Hutson at the annual Gastrointestinal Cancers Symposium sponsored by the American Society of Clinical Oncology.
No differences in overall survival data have been noted to date in the ongoing phase III trial. Dr. Hutson of Texas Oncology – Baylor Charles A. Sammons Cancer Center, Dallas, and the study’s lead author, said that researchers are waiting for the survival data to mature.
Axitinib is a vascular endothelial growth factor receptor inhibitor that is approved for advanced renal cell carcinoma (RCC) after one prior systemic therapy has failed.
To examine the value of axitinib as a first-line therapy, researchers took into account results from previous clinical trials (J. Clin. Oncol. 2009; 27:1280-9) and amended an ongoing randomized trial of second-line axitinib vs. sorafenib to include a population of first-line therapy patients.
The Pfizer-sponsored multicenter, randomized, open-label, phase III trial included 288 untreated patients with clear-cell mRCC who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients were randomized 2:1 to axitinib (5 mg, twice daily) or to sorafenib (400 mg, twice daily.) The randomization was stratified by ECOG performance status.
The primary endpoint was progression-free survival (PFS) as determined by an independent radiology committee. The study had a 90% power to detect a 78% improvement in PFS.
Secondary endpoints included overall survival, objective response rate, safety, duration of response, kidney cancer–specific symptoms, and health-related quality of life.
Patients’ baseline characteristics were well balanced between the two arms, and median patient age was 58 years old. ECOG performance status was 0 for 57% and 1 for 43%. Nephrectomies were performed in 85% of patients in the axitinib arm and 90% in the sorafenib arm. About 14% of the patients were from North America.
Patients who had a nephrectomy and an ECOG performance status of 0 faired better if they were in the axitinib arm. Among patients who had a nephrectomy, axitinib-treated patients had a median PFS of 10.3 months and sorafenib-treated patients had a median PFS of 6.4 months (HR, 0.67*; one-sided P = .009). Patients with an ECOG performance status of 0 had a median PFS of 13.7 months in the axitinib arm and 6.6 months in the sorafenib arm (HR, 0.644; one-sided P =.022). Patients with an ECOG performance status of 1 had similar median PFS – 6.5 months in the axitinib arm and 6.4 months in sorafenib arm.
Objective response rate was 32.3% with axitinib and 14.6% with sorafenib.
Patients in the axitinib arm had more hypertension and diarrhea (49% vs. 29% with sorafenib, and 50% vs. 40% with sorafenib.) Palmar-plantar erythrodysesthesia was more common with sorafenib (39% vs. 26% with axitinib).
Dr. Hutson has been a consultant or adviser for, and has received honoraria and research funding from AVEO, Bayer, GlaxoSmithKline, Novartis, and Pfizer, the maker of axitinib.
*Correction, 4/17/2013: An earlier version of this story misstated the HR value in patients who had nephrectomy.
AT THE ASCO GENITOURINARY CANCERS SYMPOSIUM
Major finding: Median progression-free survival was 10.1 months in the axitinib arm and 6.5 months in the sorafenib arm. (HR, 0.77; one-sided P = .038).
Data source: A multicenter, randomized, open-label trial of 288 untreated patients with clear-cell mRCC.
Disclosures: Dr. Hutson has been a consultant or advisor for, and has received honoraria and research funding from several drug makers including Pfizer, the maker of axitinib.
Dexamethasone eases end-of-life cancer-related fatigue
NEW ORLEANS – Dexamethasone was more effective than was placebo in relieving cancer-related fatigue in a double-blind randomized trial of patients with advanced cancer.
After 14 days of treatment, scores on the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue subscale improved by nearly 6 points in the dexamethasone group (9.0 vs. 3.1; P = .008).
"[Treatment] duration is very important in our patient population because when they are referred to us, it’s very late. They typically have a survival of just 28 to 7 days," Dr. Sriram Yennu said at the annual meeting of the American Academy of Hospice and Palliative Medicine.
Although 20%-50% of palliative care patients receive some form of corticosteroid, no steroid study to date has used cancer-related fatigue (CRF) as a primary outcome or assessed CRF with a validated outcome measure, he said. Fatigue is ubiquitous, however, contributing up to one-third of symptom distress in patients with advanced cancer.
The study enrolled 132 outpatients with a life expectancy of at least 4 weeks with three or more cancer-related symptoms (fatigue, pain, nausea, loss of appetite, depression, anxiety, or sleep disturbance), and randomly assigned them to oral dexamethasone 4 mg twice daily or placebo for 14 days.
The most common diagnosis was head and neck/lung cancer in 45 patients, followed by gastrointestinal cancer in 39, breast cancer in 13, and genitourinary in 10. Median patient age was 60; 81 patients were white, and the average FACIT fatigue score was 19.6, where 52 denotes no fatigue and 0 is severe fatigue.
Among 84 evaluable patients, total scores on FACIT favored the dexamethasone group (18.16 vs. 7.87; P = .03), as did scores on its physical subscale (5.25 vs. 1.32; P = .002), said Dr. Yennu of the department of palliative care and rehabilitation medicine, University of Texas MD Anderson Cancer Center, Houston.
Scores on the physical domain of the Edmonton Symptom Assessment Scale (ESAS) were better in the dexamethasone group than in the placebo group (–10.15 vs. –5.39; P = .04), according to the study, which earned Dr. Yennu a young investigator award.
Notably, scores were similar between the dexamethasone and placebo groups on the emotional subscale of FACIT (1.85 vs. 1.18; P = .49) and the ESAS psychological subscale (–1.48 vs. –2.08; P = .76). The emotional domain of the FACIT-F is measured by six items using a 0-4 scale where 0 is "not at all" and 4 includes statements like "I am losing hope in the fight against my illness." The finding suggests that the improvement in fatigue was likely not just a euphoric effect, as observed before in other steroid trials, Dr. Yennu said.
He expressed concern that corticosteroid use would increase toxicity, particularly insomnia, but no significant differences were observed between the dexamethasone and placebo groups regarding insomnia (3 vs. 4), overall adverse events (41 vs. 44) or serious adverse events (17 vs. 11).
Larger, long-term safety and efficacy studies are needed to address steroid dose and duration, and whether dexamethasone should be coupled with interventions targeting the psychological domain, he said. A 3-point difference in the FACIT is considered clinically important, but research in press in the Journal of Clinical Oncology, by Dr. Yennu and his colleagues, suggests a 10-point difference is more meaningful.
The American Cancer Society supported the study. Dr. Yennu reported having no financial disclosures.
NEW ORLEANS – Dexamethasone was more effective than was placebo in relieving cancer-related fatigue in a double-blind randomized trial of patients with advanced cancer.
After 14 days of treatment, scores on the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue subscale improved by nearly 6 points in the dexamethasone group (9.0 vs. 3.1; P = .008).
"[Treatment] duration is very important in our patient population because when they are referred to us, it’s very late. They typically have a survival of just 28 to 7 days," Dr. Sriram Yennu said at the annual meeting of the American Academy of Hospice and Palliative Medicine.
Although 20%-50% of palliative care patients receive some form of corticosteroid, no steroid study to date has used cancer-related fatigue (CRF) as a primary outcome or assessed CRF with a validated outcome measure, he said. Fatigue is ubiquitous, however, contributing up to one-third of symptom distress in patients with advanced cancer.
The study enrolled 132 outpatients with a life expectancy of at least 4 weeks with three or more cancer-related symptoms (fatigue, pain, nausea, loss of appetite, depression, anxiety, or sleep disturbance), and randomly assigned them to oral dexamethasone 4 mg twice daily or placebo for 14 days.
The most common diagnosis was head and neck/lung cancer in 45 patients, followed by gastrointestinal cancer in 39, breast cancer in 13, and genitourinary in 10. Median patient age was 60; 81 patients were white, and the average FACIT fatigue score was 19.6, where 52 denotes no fatigue and 0 is severe fatigue.
Among 84 evaluable patients, total scores on FACIT favored the dexamethasone group (18.16 vs. 7.87; P = .03), as did scores on its physical subscale (5.25 vs. 1.32; P = .002), said Dr. Yennu of the department of palliative care and rehabilitation medicine, University of Texas MD Anderson Cancer Center, Houston.
Scores on the physical domain of the Edmonton Symptom Assessment Scale (ESAS) were better in the dexamethasone group than in the placebo group (–10.15 vs. –5.39; P = .04), according to the study, which earned Dr. Yennu a young investigator award.
Notably, scores were similar between the dexamethasone and placebo groups on the emotional subscale of FACIT (1.85 vs. 1.18; P = .49) and the ESAS psychological subscale (–1.48 vs. –2.08; P = .76). The emotional domain of the FACIT-F is measured by six items using a 0-4 scale where 0 is "not at all" and 4 includes statements like "I am losing hope in the fight against my illness." The finding suggests that the improvement in fatigue was likely not just a euphoric effect, as observed before in other steroid trials, Dr. Yennu said.
He expressed concern that corticosteroid use would increase toxicity, particularly insomnia, but no significant differences were observed between the dexamethasone and placebo groups regarding insomnia (3 vs. 4), overall adverse events (41 vs. 44) or serious adverse events (17 vs. 11).
Larger, long-term safety and efficacy studies are needed to address steroid dose and duration, and whether dexamethasone should be coupled with interventions targeting the psychological domain, he said. A 3-point difference in the FACIT is considered clinically important, but research in press in the Journal of Clinical Oncology, by Dr. Yennu and his colleagues, suggests a 10-point difference is more meaningful.
The American Cancer Society supported the study. Dr. Yennu reported having no financial disclosures.
NEW ORLEANS – Dexamethasone was more effective than was placebo in relieving cancer-related fatigue in a double-blind randomized trial of patients with advanced cancer.
After 14 days of treatment, scores on the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue subscale improved by nearly 6 points in the dexamethasone group (9.0 vs. 3.1; P = .008).
"[Treatment] duration is very important in our patient population because when they are referred to us, it’s very late. They typically have a survival of just 28 to 7 days," Dr. Sriram Yennu said at the annual meeting of the American Academy of Hospice and Palliative Medicine.
Although 20%-50% of palliative care patients receive some form of corticosteroid, no steroid study to date has used cancer-related fatigue (CRF) as a primary outcome or assessed CRF with a validated outcome measure, he said. Fatigue is ubiquitous, however, contributing up to one-third of symptom distress in patients with advanced cancer.
The study enrolled 132 outpatients with a life expectancy of at least 4 weeks with three or more cancer-related symptoms (fatigue, pain, nausea, loss of appetite, depression, anxiety, or sleep disturbance), and randomly assigned them to oral dexamethasone 4 mg twice daily or placebo for 14 days.
The most common diagnosis was head and neck/lung cancer in 45 patients, followed by gastrointestinal cancer in 39, breast cancer in 13, and genitourinary in 10. Median patient age was 60; 81 patients were white, and the average FACIT fatigue score was 19.6, where 52 denotes no fatigue and 0 is severe fatigue.
Among 84 evaluable patients, total scores on FACIT favored the dexamethasone group (18.16 vs. 7.87; P = .03), as did scores on its physical subscale (5.25 vs. 1.32; P = .002), said Dr. Yennu of the department of palliative care and rehabilitation medicine, University of Texas MD Anderson Cancer Center, Houston.
Scores on the physical domain of the Edmonton Symptom Assessment Scale (ESAS) were better in the dexamethasone group than in the placebo group (–10.15 vs. –5.39; P = .04), according to the study, which earned Dr. Yennu a young investigator award.
Notably, scores were similar between the dexamethasone and placebo groups on the emotional subscale of FACIT (1.85 vs. 1.18; P = .49) and the ESAS psychological subscale (–1.48 vs. –2.08; P = .76). The emotional domain of the FACIT-F is measured by six items using a 0-4 scale where 0 is "not at all" and 4 includes statements like "I am losing hope in the fight against my illness." The finding suggests that the improvement in fatigue was likely not just a euphoric effect, as observed before in other steroid trials, Dr. Yennu said.
He expressed concern that corticosteroid use would increase toxicity, particularly insomnia, but no significant differences were observed between the dexamethasone and placebo groups regarding insomnia (3 vs. 4), overall adverse events (41 vs. 44) or serious adverse events (17 vs. 11).
Larger, long-term safety and efficacy studies are needed to address steroid dose and duration, and whether dexamethasone should be coupled with interventions targeting the psychological domain, he said. A 3-point difference in the FACIT is considered clinically important, but research in press in the Journal of Clinical Oncology, by Dr. Yennu and his colleagues, suggests a 10-point difference is more meaningful.
The American Cancer Society supported the study. Dr. Yennu reported having no financial disclosures.
AT THE AAHPM ANNUAL ASSEMBLY
Major finding: Scores on the FACIT fatigue subscale improved by an average of 6 points in patients treated with dexamethasone, compared with 3 points in patients treated with placebo.
Data source: Double-blind, randomized trial of 132 patients with advanced cancer.
Disclosures: The American Cancer Society supported the study. Dr. Yennu reported having no financial disclosures.
Androgen deprivation decreases lung tumors in men with prostate cancer
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
WASHINGTON – Men who received androgen blockade therapy as part of prostate cancer treatment were less likely to develop a secondary lung cancer than were those who didn’t receive the hormone regimen.
The significant finding suggests that estrogen may fuel non–small cell lung cancers in both men and women, Dr. Hyunseok Kang reported at the annual meeting of the American Association for Cancer Research.
"Recent literature suggests estrogen receptors and aromatase play a role in the development of non–small cell lung cancers in women with breast cancer," Dr. Kang said. "Aromatase can convert testosterone to estrogen – which could increase the same risk in men."
This recently observed association prompted Dr. Kang, a fellow at Emory University, Atlanta, and his colleagues to investigate whether a similar hormone-mediated link might exist for men with prostate cancer – and whether blocking it could also block lung cancer.
The retrospective observational study pulled data from the Veterans Affairs Central Cancer Registry. It comprised 62,589 men who were diagnosed with prostate cancer between 1999 and 2008, with follow-up through 2010.
There were some significant – and important – baseline differences between the 20,378 men who underwent androgen blockade and those who did not. Men who received the treatment were significantly older (mean age, 71 years vs. 66 years) and slightly more likely to have ever smoked tobacco (73% vs. 71%).
Prostate cancer stage at diagnosis was also significantly different, with fewer of the hormone therapy group presenting at stage II (77% vs. 92%) and more presenting at stage IV (17% vs. 2%).
Men from the VA database were significantly more likely than the general population to develop lung cancer, whether they underwent androgen blockade therapy or not (1.8/100,000 men who received treatment and 2.4/100,000 men who did not receive treatment vs. 0.80/100,000 in the general population).
Of men in the VA database who received androgen treatment, 258 developed secondary lung cancers, compared with 508 men who did not receive treatment.
In an unadjusted model, men who had treatment were 18% less likely to develop a secondary lung cancer (hazard ratio, 0.82). That finding held in a multivariate analysis that adjusted for smoking and age (HR, 0.83).
The study was sponsored by the Veterans Health Administration. Dr. Kang had no financial disclosures.
FROM THE AACR ANNUAL MEETING
Major finding: Men with prostate cancer who underwent androgen blockade were 17% less likely to develop secondary non–small cell lung cancer than were those who didn’t have the hormone treatment.
Data source: The observational study comprised 62,589 men who were diagnosed with prostate cancer during 1998-2008, with follow-up through 2010.
Disclosures: The Veterans Health Administration sponsored the study. Dr. Kang had no financial disclosures.
Novel therapies extend life in patients with metastatic prostate cancer
Community Oncology Editor-in-Chief Dr. David Henry spoke with Dr. Nicholas Vogelzang at the Oncology Practice Summit in Las Vegas about how he treats patients with metastatic prostate cancer given the new agents that are now available and growing concerns about treatment costs and patient quality of life.
The Oncology Practice Summit was the 8th annual meeting of Community Oncology, the journal of clinical issues in community practice. Dr. Henry was a co-chair of the Summit, which was hosted this year by Community Oncology as well as The Journal of Supportive Oncology and The Oncology Report.
Community Oncology Editor-in-Chief Dr. David Henry spoke with Dr. Nicholas Vogelzang at the Oncology Practice Summit in Las Vegas about how he treats patients with metastatic prostate cancer given the new agents that are now available and growing concerns about treatment costs and patient quality of life.
The Oncology Practice Summit was the 8th annual meeting of Community Oncology, the journal of clinical issues in community practice. Dr. Henry was a co-chair of the Summit, which was hosted this year by Community Oncology as well as The Journal of Supportive Oncology and The Oncology Report.
Community Oncology Editor-in-Chief Dr. David Henry spoke with Dr. Nicholas Vogelzang at the Oncology Practice Summit in Las Vegas about how he treats patients with metastatic prostate cancer given the new agents that are now available and growing concerns about treatment costs and patient quality of life.
The Oncology Practice Summit was the 8th annual meeting of Community Oncology, the journal of clinical issues in community practice. Dr. Henry was a co-chair of the Summit, which was hosted this year by Community Oncology as well as The Journal of Supportive Oncology and The Oncology Report.
High selenium exposure may lower incidence of advanced prostate cancer
Increased exposure to selenium was associated with a significantly reduced risk of advanced prostate cancer, according to the results of a large, prospective cohort study of men in the Netherlands.
The results of the study, conducted in men with low to moderate selenium levels, "suggest that selenium may prevent advanced, clinically relevant, prostate cancer," noted Milan Geybels, M.Sc., at the annual meeting of the American Association for Cancer Research. And since there is little evidence on risk factors that can modify prostate cancer risk, "any compound that would prevent the incidence of advanced, clinically relevant prostate cancer would have a substantial impact on public health," Mr. Geybels said in an interview.
The men were enrolled in the Netherlands Cohort Study, a study of diet and cancer in 58,279 men between the ages of 55 and 69 years at entry. Over 17 years, 898 men were diagnosed with advanced prostate cancer and were compared with 1,203 men also in the study. Using the concentration of selenium in the men’s toenails, an indication of long-term exposure, the investigators determined that the risk of advanced prostate cancer was significantly reduced as selenium levels increased. The risk for advanced prostate cancer was more than 60% lower in men with the highest levels of toenail selenium, when compared with those with the lowest levels, and the association was "more pronounced" among the men diagnosed later in the follow-up period, noted Mr. Geybels, a doctoral candidate in cancer epidemiology at Maastricht University, the Netherlands.
Previous prospective studies on the possible link between selenium and prostate cancer risk have produced conflicting results. Many of the studies included men with moderate to high selenium levels. Also, they did not exclusively study advanced prostate cancer, and "almost exclusively" used blood levels of selenium, which reflect recent exposure, he noted.
SELECT (Selenium and Vitamin E Cancer Prevention Trial) found no relationship between selenium supplementation and prostate cancer risk. However, "a possible explanation for the null finding is that selenium intake among SELECT participants was adequate ... [so], further selenium supplementation had no effect on prostate cancer incidence," he said.
The intake of selenium varies worldwide as a result of variations in soil content and the related variability in the selenium content in foods; in the Netherlands, low selenium is common.
The results need to be replicated in more prospective studies, and if confirmed, "A prevention trial of selenium and advanced prostate cancer in a low selenium population may be justified," said Mr. Geybels. Whether levels higher than those detected in the men in the study are associated with a further reduction in risk is unknown.
He and his coauthors had no disclosures to report.
Increased exposure to selenium was associated with a significantly reduced risk of advanced prostate cancer, according to the results of a large, prospective cohort study of men in the Netherlands.
The results of the study, conducted in men with low to moderate selenium levels, "suggest that selenium may prevent advanced, clinically relevant, prostate cancer," noted Milan Geybels, M.Sc., at the annual meeting of the American Association for Cancer Research. And since there is little evidence on risk factors that can modify prostate cancer risk, "any compound that would prevent the incidence of advanced, clinically relevant prostate cancer would have a substantial impact on public health," Mr. Geybels said in an interview.
The men were enrolled in the Netherlands Cohort Study, a study of diet and cancer in 58,279 men between the ages of 55 and 69 years at entry. Over 17 years, 898 men were diagnosed with advanced prostate cancer and were compared with 1,203 men also in the study. Using the concentration of selenium in the men’s toenails, an indication of long-term exposure, the investigators determined that the risk of advanced prostate cancer was significantly reduced as selenium levels increased. The risk for advanced prostate cancer was more than 60% lower in men with the highest levels of toenail selenium, when compared with those with the lowest levels, and the association was "more pronounced" among the men diagnosed later in the follow-up period, noted Mr. Geybels, a doctoral candidate in cancer epidemiology at Maastricht University, the Netherlands.
Previous prospective studies on the possible link between selenium and prostate cancer risk have produced conflicting results. Many of the studies included men with moderate to high selenium levels. Also, they did not exclusively study advanced prostate cancer, and "almost exclusively" used blood levels of selenium, which reflect recent exposure, he noted.
SELECT (Selenium and Vitamin E Cancer Prevention Trial) found no relationship between selenium supplementation and prostate cancer risk. However, "a possible explanation for the null finding is that selenium intake among SELECT participants was adequate ... [so], further selenium supplementation had no effect on prostate cancer incidence," he said.
The intake of selenium varies worldwide as a result of variations in soil content and the related variability in the selenium content in foods; in the Netherlands, low selenium is common.
The results need to be replicated in more prospective studies, and if confirmed, "A prevention trial of selenium and advanced prostate cancer in a low selenium population may be justified," said Mr. Geybels. Whether levels higher than those detected in the men in the study are associated with a further reduction in risk is unknown.
He and his coauthors had no disclosures to report.
Increased exposure to selenium was associated with a significantly reduced risk of advanced prostate cancer, according to the results of a large, prospective cohort study of men in the Netherlands.
The results of the study, conducted in men with low to moderate selenium levels, "suggest that selenium may prevent advanced, clinically relevant, prostate cancer," noted Milan Geybels, M.Sc., at the annual meeting of the American Association for Cancer Research. And since there is little evidence on risk factors that can modify prostate cancer risk, "any compound that would prevent the incidence of advanced, clinically relevant prostate cancer would have a substantial impact on public health," Mr. Geybels said in an interview.
The men were enrolled in the Netherlands Cohort Study, a study of diet and cancer in 58,279 men between the ages of 55 and 69 years at entry. Over 17 years, 898 men were diagnosed with advanced prostate cancer and were compared with 1,203 men also in the study. Using the concentration of selenium in the men’s toenails, an indication of long-term exposure, the investigators determined that the risk of advanced prostate cancer was significantly reduced as selenium levels increased. The risk for advanced prostate cancer was more than 60% lower in men with the highest levels of toenail selenium, when compared with those with the lowest levels, and the association was "more pronounced" among the men diagnosed later in the follow-up period, noted Mr. Geybels, a doctoral candidate in cancer epidemiology at Maastricht University, the Netherlands.
Previous prospective studies on the possible link between selenium and prostate cancer risk have produced conflicting results. Many of the studies included men with moderate to high selenium levels. Also, they did not exclusively study advanced prostate cancer, and "almost exclusively" used blood levels of selenium, which reflect recent exposure, he noted.
SELECT (Selenium and Vitamin E Cancer Prevention Trial) found no relationship between selenium supplementation and prostate cancer risk. However, "a possible explanation for the null finding is that selenium intake among SELECT participants was adequate ... [so], further selenium supplementation had no effect on prostate cancer incidence," he said.
The intake of selenium varies worldwide as a result of variations in soil content and the related variability in the selenium content in foods; in the Netherlands, low selenium is common.
The results need to be replicated in more prospective studies, and if confirmed, "A prevention trial of selenium and advanced prostate cancer in a low selenium population may be justified," said Mr. Geybels. Whether levels higher than those detected in the men in the study are associated with a further reduction in risk is unknown.
He and his coauthors had no disclosures to report.
FROM THE ANNUAL AACR MEETING
Major finding: The risk for advanced prostate cancer was more than 60% lower in men with the highest levels of toenail selenium, when compared with those with the lowest levels.
Data source: Men in the Netherlands Cohort Study diagnosed with advanced prostate cancer (898) were compared with 1,203 men also in the study.
Disclosures: Mr. Geybels and his colleagues had no disclosures to report.
ACP: PSA screening's harms outweigh benefits
PSA screening should not be done in men younger than 50 years or in those older than 69 years – and men between those ages should be offered the test only if they want to take it after being told that they are more likely to be hurt by it than helped, according to new prostate-specific antigen screening guidelines from the American College of Physicians.
"A man’s chances of being harmed are much greater than his chances of benefiting from the PSA test," caution the guidelines’ authors. Those harms include surgery that risks incontinence, impotency, and other problems to remove harmless tumors, the group said.
"Any absolute mortality risk reduction" from routine screening "is probably small to none," according to the guidelines. "The vast majority of prostate cancer is slow-growing and does not cause death."
That news isn’t likely to surprise many doctors – PSA testing has been suspect for years, and ACP’s guidelines mirror and, in fact, are derived from those from other groups. But it might be a surprise to the general public.
There’s still "a lot of misperception about the accuracy and utility of the test," because it’s been promoted until recently as a kind of "mammography for men," noted Dr. William Golden, a professor of medicine and public health at the University of Arkansas.
With patient demand still high and many physicians fearing malpractice risk if they don’t screen, "it’s been a whole lot easier to just check the box and order the test," Dr. Golden observed. "I think there are still a lot of PSAs being ordered [even for men] over age 75 [years].
"Statements like this from the ACP give doctors a little more ammunition to help educate patients that this is not a real strong diagnostic tool," he said.
To that end, the ACP has also published a one-page patient information sheet that explains the problems with PSA tests. The guidelines themselves include 10 talking points men need to hear before opting to be screened.
Men are open to the message, Dr. Golden said. "I say [to them], ‘Look, if you really want the test, I’ll give it to you.’ " But he lets them know first that he and many other doctors don’t get themselves screened; that the test isn’t very sensitive or specific; and that it often leads to biopsies until something is found and operations regardless of therapeutic value.
"When they hear that kind of framework, a lot of patients don’t want to go near" it, Dr. Golden said. Instead, they say, "Look, I’d rather take my chances. I’d rather not have incontinence, I’d rather have sexual function, and I’d rather not have surgery for something that may or may not make a difference to my health."
That discussion is exactly the approach ACP hopes to foster.
"The key is to talk with your patient and help determine what they value" – preserving continence, sexual function, and peace of mind, or gambling them on a tiny chance of catching a fatal tumor early, said the senior author of ACP’s guidelines, Dr. Paul Shekelle, director of the RAND Corporation’s Southern California Evidence-Based Practice Center. "Do not over-sell the screen-biopsy-surgery approach."
Practice is still "probably tilted toward screening," he said. "The ACP guideline is an attempt to steer this back."
The conversation is much the same even for high-risk men – blacks and those with strong family histories of prostate cancer. But instead of starting at age 50 years, it should come at age 45 or 40. "Screening in high-risk men has not been demonstrated to be associated with different outcomes than screening in average-risk men," the ACP guidelines note.
Up to a third of men are screened without their knowledge during routine physicals; that practice should end, the ACP also said.
The ACP derived its own recommendations from a review of those from the American College of Preventive Medicine, the American Cancer Society, the American Urological Association, and the U.S. Preventive Services Task Force. "ACP believes that it is more valuable to provide clinicians with a rigorous review of available guidelines, rather than develop a new guideline on the same topic when several guidelines are available," it said.
The ACP found the USPSTF’s efforts the most rigorous; that group in 2012 recommended abandoning PSA screening.
"The one nuance [ACP] added is that in the [50-69 ] age range where there is a hint of benefit from one of the large trials, even for them the evidence of harm is much stronger than the evidence of benefit. I think [ACP] has the messaging correct," said Dr. Barry Kramer, director of the National Cancer Institute’s Division of Cancer Prevention.
If they are performed, PSA tests should be conducted no more than every 4 years. "No evidence supports annual screening for prostate cancer," the ACP guidelines note.
The potential benefit of screening is vanishingly small for men older than 69 years – and for those expected to live no more than 15 years – because prostate cancer isn’t likely to cause any problems in the time they have left, the guidelines explain. For men younger than 50 years, the downstream harms "such as erectile dysfunction and urinary incontinence carry even more weight relative to any potential benefit," according to the guidelines.
Dr. Golden, Dr. Kramer, and Dr. Shekelle said they have no relevant financial disclosures.
*This story was updated 4/10/13.
PSA screening should not be done in men younger than 50 years or in those older than 69 years – and men between those ages should be offered the test only if they want to take it after being told that they are more likely to be hurt by it than helped, according to new prostate-specific antigen screening guidelines from the American College of Physicians.
"A man’s chances of being harmed are much greater than his chances of benefiting from the PSA test," caution the guidelines’ authors. Those harms include surgery that risks incontinence, impotency, and other problems to remove harmless tumors, the group said.
"Any absolute mortality risk reduction" from routine screening "is probably small to none," according to the guidelines. "The vast majority of prostate cancer is slow-growing and does not cause death."
That news isn’t likely to surprise many doctors – PSA testing has been suspect for years, and ACP’s guidelines mirror and, in fact, are derived from those from other groups. But it might be a surprise to the general public.
There’s still "a lot of misperception about the accuracy and utility of the test," because it’s been promoted until recently as a kind of "mammography for men," noted Dr. William Golden, a professor of medicine and public health at the University of Arkansas.
With patient demand still high and many physicians fearing malpractice risk if they don’t screen, "it’s been a whole lot easier to just check the box and order the test," Dr. Golden observed. "I think there are still a lot of PSAs being ordered [even for men] over age 75 [years].
"Statements like this from the ACP give doctors a little more ammunition to help educate patients that this is not a real strong diagnostic tool," he said.
To that end, the ACP has also published a one-page patient information sheet that explains the problems with PSA tests. The guidelines themselves include 10 talking points men need to hear before opting to be screened.
Men are open to the message, Dr. Golden said. "I say [to them], ‘Look, if you really want the test, I’ll give it to you.’ " But he lets them know first that he and many other doctors don’t get themselves screened; that the test isn’t very sensitive or specific; and that it often leads to biopsies until something is found and operations regardless of therapeutic value.
"When they hear that kind of framework, a lot of patients don’t want to go near" it, Dr. Golden said. Instead, they say, "Look, I’d rather take my chances. I’d rather not have incontinence, I’d rather have sexual function, and I’d rather not have surgery for something that may or may not make a difference to my health."
That discussion is exactly the approach ACP hopes to foster.
"The key is to talk with your patient and help determine what they value" – preserving continence, sexual function, and peace of mind, or gambling them on a tiny chance of catching a fatal tumor early, said the senior author of ACP’s guidelines, Dr. Paul Shekelle, director of the RAND Corporation’s Southern California Evidence-Based Practice Center. "Do not over-sell the screen-biopsy-surgery approach."
Practice is still "probably tilted toward screening," he said. "The ACP guideline is an attempt to steer this back."
The conversation is much the same even for high-risk men – blacks and those with strong family histories of prostate cancer. But instead of starting at age 50 years, it should come at age 45 or 40. "Screening in high-risk men has not been demonstrated to be associated with different outcomes than screening in average-risk men," the ACP guidelines note.
Up to a third of men are screened without their knowledge during routine physicals; that practice should end, the ACP also said.
The ACP derived its own recommendations from a review of those from the American College of Preventive Medicine, the American Cancer Society, the American Urological Association, and the U.S. Preventive Services Task Force. "ACP believes that it is more valuable to provide clinicians with a rigorous review of available guidelines, rather than develop a new guideline on the same topic when several guidelines are available," it said.
The ACP found the USPSTF’s efforts the most rigorous; that group in 2012 recommended abandoning PSA screening.
"The one nuance [ACP] added is that in the [50-69 ] age range where there is a hint of benefit from one of the large trials, even for them the evidence of harm is much stronger than the evidence of benefit. I think [ACP] has the messaging correct," said Dr. Barry Kramer, director of the National Cancer Institute’s Division of Cancer Prevention.
If they are performed, PSA tests should be conducted no more than every 4 years. "No evidence supports annual screening for prostate cancer," the ACP guidelines note.
The potential benefit of screening is vanishingly small for men older than 69 years – and for those expected to live no more than 15 years – because prostate cancer isn’t likely to cause any problems in the time they have left, the guidelines explain. For men younger than 50 years, the downstream harms "such as erectile dysfunction and urinary incontinence carry even more weight relative to any potential benefit," according to the guidelines.
Dr. Golden, Dr. Kramer, and Dr. Shekelle said they have no relevant financial disclosures.
*This story was updated 4/10/13.
PSA screening should not be done in men younger than 50 years or in those older than 69 years – and men between those ages should be offered the test only if they want to take it after being told that they are more likely to be hurt by it than helped, according to new prostate-specific antigen screening guidelines from the American College of Physicians.
"A man’s chances of being harmed are much greater than his chances of benefiting from the PSA test," caution the guidelines’ authors. Those harms include surgery that risks incontinence, impotency, and other problems to remove harmless tumors, the group said.
"Any absolute mortality risk reduction" from routine screening "is probably small to none," according to the guidelines. "The vast majority of prostate cancer is slow-growing and does not cause death."
That news isn’t likely to surprise many doctors – PSA testing has been suspect for years, and ACP’s guidelines mirror and, in fact, are derived from those from other groups. But it might be a surprise to the general public.
There’s still "a lot of misperception about the accuracy and utility of the test," because it’s been promoted until recently as a kind of "mammography for men," noted Dr. William Golden, a professor of medicine and public health at the University of Arkansas.
With patient demand still high and many physicians fearing malpractice risk if they don’t screen, "it’s been a whole lot easier to just check the box and order the test," Dr. Golden observed. "I think there are still a lot of PSAs being ordered [even for men] over age 75 [years].
"Statements like this from the ACP give doctors a little more ammunition to help educate patients that this is not a real strong diagnostic tool," he said.
To that end, the ACP has also published a one-page patient information sheet that explains the problems with PSA tests. The guidelines themselves include 10 talking points men need to hear before opting to be screened.
Men are open to the message, Dr. Golden said. "I say [to them], ‘Look, if you really want the test, I’ll give it to you.’ " But he lets them know first that he and many other doctors don’t get themselves screened; that the test isn’t very sensitive or specific; and that it often leads to biopsies until something is found and operations regardless of therapeutic value.
"When they hear that kind of framework, a lot of patients don’t want to go near" it, Dr. Golden said. Instead, they say, "Look, I’d rather take my chances. I’d rather not have incontinence, I’d rather have sexual function, and I’d rather not have surgery for something that may or may not make a difference to my health."
That discussion is exactly the approach ACP hopes to foster.
"The key is to talk with your patient and help determine what they value" – preserving continence, sexual function, and peace of mind, or gambling them on a tiny chance of catching a fatal tumor early, said the senior author of ACP’s guidelines, Dr. Paul Shekelle, director of the RAND Corporation’s Southern California Evidence-Based Practice Center. "Do not over-sell the screen-biopsy-surgery approach."
Practice is still "probably tilted toward screening," he said. "The ACP guideline is an attempt to steer this back."
The conversation is much the same even for high-risk men – blacks and those with strong family histories of prostate cancer. But instead of starting at age 50 years, it should come at age 45 or 40. "Screening in high-risk men has not been demonstrated to be associated with different outcomes than screening in average-risk men," the ACP guidelines note.
Up to a third of men are screened without their knowledge during routine physicals; that practice should end, the ACP also said.
The ACP derived its own recommendations from a review of those from the American College of Preventive Medicine, the American Cancer Society, the American Urological Association, and the U.S. Preventive Services Task Force. "ACP believes that it is more valuable to provide clinicians with a rigorous review of available guidelines, rather than develop a new guideline on the same topic when several guidelines are available," it said.
The ACP found the USPSTF’s efforts the most rigorous; that group in 2012 recommended abandoning PSA screening.
"The one nuance [ACP] added is that in the [50-69 ] age range where there is a hint of benefit from one of the large trials, even for them the evidence of harm is much stronger than the evidence of benefit. I think [ACP] has the messaging correct," said Dr. Barry Kramer, director of the National Cancer Institute’s Division of Cancer Prevention.
If they are performed, PSA tests should be conducted no more than every 4 years. "No evidence supports annual screening for prostate cancer," the ACP guidelines note.
The potential benefit of screening is vanishingly small for men older than 69 years – and for those expected to live no more than 15 years – because prostate cancer isn’t likely to cause any problems in the time they have left, the guidelines explain. For men younger than 50 years, the downstream harms "such as erectile dysfunction and urinary incontinence carry even more weight relative to any potential benefit," according to the guidelines.
Dr. Golden, Dr. Kramer, and Dr. Shekelle said they have no relevant financial disclosures.
*This story was updated 4/10/13.
FROM THE ANNALS OF INTERNAL MEDICINE