User login
Paraneoplastic leukemoid reaction – poor prognostic marker in urothelial bladder carcinoma
Certain cancers have been observed to cause symptoms called paraneoplastic syndromes that are not directly attributed to tumor invasion or compression. This phenomenon is believed to be secondary to a tumor’s secretion of functional hormones, peptides, cytokines, or its immune cross-reactivity. One such variant is a paraneoplastic leukemoid reaction (PLR), defined as a leukocytosis level of >50 x 103 cells/mm³, where the white blood cell (WBC) count differential exhibits a neutrophilia or left-shift, in which a predominance of early neutrophil precursors is observed. A PLR is believed to be incited by the tumor cell’s production of its own growth factors such as granulocyte-colony stimulating factor (G-CSF) and a number of different cytokines. These reactions may at first be mistaken for infectious processes, and it is only after an infection has been ruled out or when a leukocytosis is disproportionately high in the setting of a treated infection, that a paraneoplastic leukemoid reaction (PLR) is considered and an oncologic work-up pursued.
PLR has been previously described in a variety of malignancies including lung, esophageal, nasopharyngeal and laryngeal, gastric, cholangiocarcinoma, melanoma, multiple myeloma, renal, prostate, and hepatocellular carcinoma, but has rarely been described in urothelial carcinoma.1 Leukemoid reactions and autocrine growth induced by paraneoplastic production of G-CSF have rarely been associated with urothelial carcinoma of the bladder,2 as in the case we present here of a 67-year-old white man with invasive high-grade urothelial carcinoma of the bladder. The case highlights PLR as a negative prognostic marker, secondary to urothelial bladder cancer cells’ presumed production of G-CSF, rarely reported as in the literature previously.
Case presentation and summary
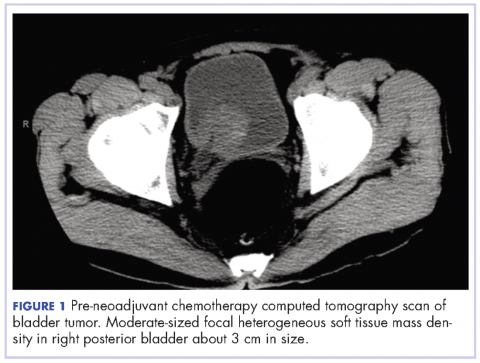
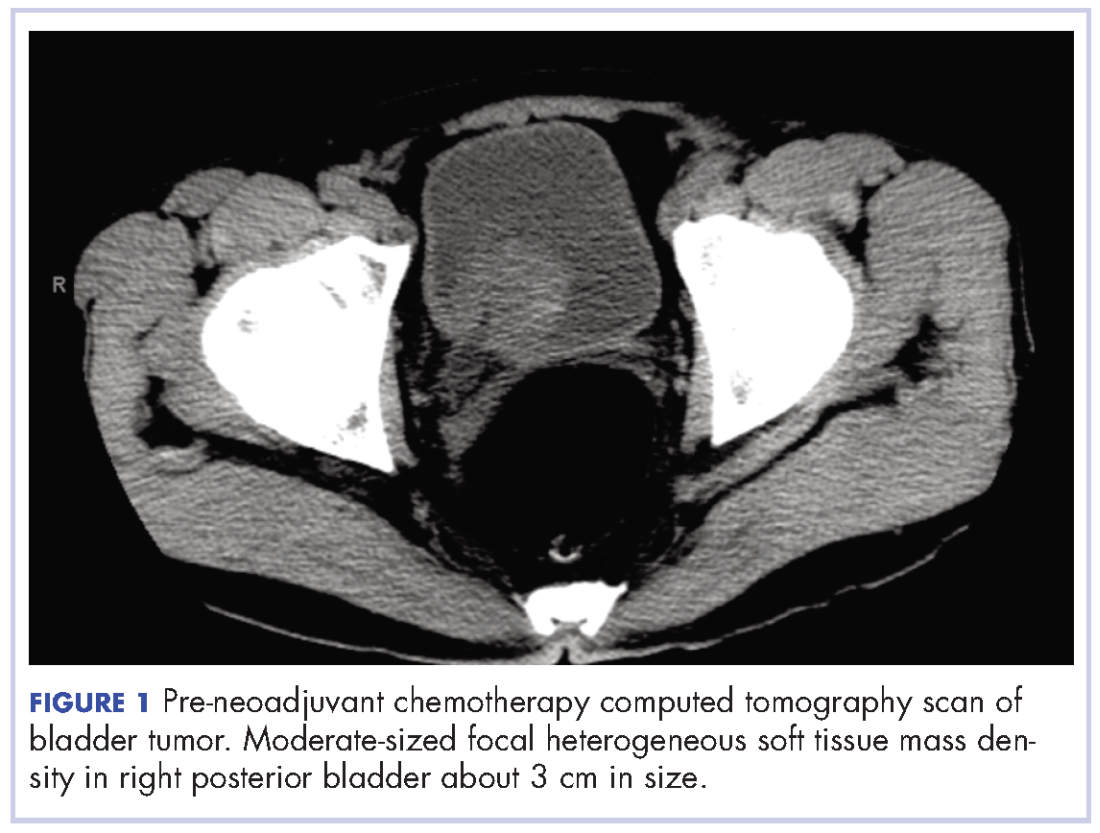
A 67-year-old white man was diagnosed with clinical stage III (T3N0M0), invasive high-grade urothelial carcinoma of the bladder (Figure 1). He received neoadjuvant chemotherapy with the standard gemcitabine-cisplatin combination (1,000 mg/m2 of gemcitabine on days 1, 8, and 15 with 70 mg/m2 of cisplatin on day 1 of a 28-day cycle for 3 cycles), and had less than partial response at the end of a 3-month course. A computed tomography scan of his pelvis obtained at treatment completion revealed persistent disease with noted enhancement of the right distal ureter and a right posterior bladder mass at the ureterovesical junction measuring 1.7 x 2.6 x 2.6 cm (0.6 x 1 x 1 in), for which, a cystoprostatectomy was recommended to remove remaining disease. The patient was seen in routine follow-up 3 weeks after his last chemotherapy treatment, when his WBC count was noted to be within normal limits at a value of 8.4 x 103 cells/mm³ (normal range, 4.5-11 x 103 cells/mm³).
One week later (a month after treatment completion), the patient presented to the emergency department with complaints of dysuria, urinary frequency, and suprapubic pain. He was found to have leukocytosis, with a WBC count of 47 x 103 cells/mm³, (normal range, 4.5-11 x 103 cells/mm³), with an elevated neutrophil count of 82.7% (normal range 40%-60%), without clinical signs of systemic infection (fevers, chills, or rigors). A urinalysis revealed pyuria with 25-50 WBC/high power field, negative nitrite, positive leukocyte esterase, and moderate bacteria, consistent with what was presumed to be a urinary tract infection. The patient was discharged home with a 1-week course of the antibiotic levofloxacin and the alpha-blocker tamsulosin to make urination easier. Of note, the final results of the urine culture, which returned 48 hours after discharge, showed no growth.
One week prior to surgery, the patient underwent a cystoscopy for ureteral stent exchange, which revealed a necrotic tumor at the right bladder base surrounding the ureteral orifice and stent. Renal pelvis urine, sampled during stent exchange, revealed >100,000 CFU/ml (colony forming units; normal value, <10³) Candida albicans, for which the patient was started on intravenous fluconazole for fungal infection. We consulted with our colleagues in the infectious disease department and continued to follow the patient throughout his hospital course, which included several antibiotic regimens, a comprehensive hematological work-up and eventual urologic surgery. Work-ups for myeloproliferative disorders, leukemia, JAK-2, and BCR-ABL were all negative. A peripheral blood smear analysis showed mostly neutrophils, no immature cells, and occasional hypersegmented neutrophils, but was overall inconsistent with myeloproliferative disease. Despite the patient’s persistent leukocytosis, he remained completely asymptomatic and his neutrophilia was attributed to his malignancy.
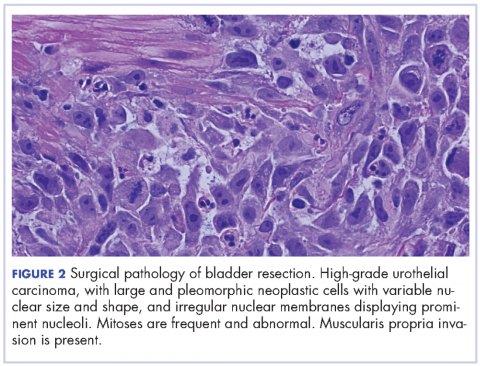
The patient subsequently underwent a cystoprostatectomy with ileal conduit. The surgical pathologic analysis showed a high-grade, invasive urothelial (transitional cell) carcinoma measuring 5.2 x 5.0 x 4.5 cm (2 x 1.9 x 1.8 in) with squamous differentiation, extensive tumor necrosis, lymphovascular invasion, invasion into the adjacent seminal vesicle and prostatic stroma, and negative margins (pT4a pN0 pMX; Figure 2). On the day of surgical resection, the patient’s leukocytosis was 70 x 103 cells/mm³.
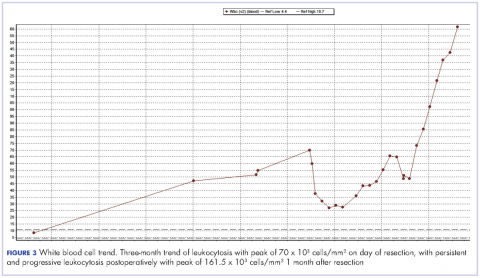
Despite a transient improvement in his leukocytosis to 37.7 x 103 cells/mm³ on postoperative day 1, the patient remained in the medical intensive care unit for uncontrolled pain and management of his leukocytosis. It is worth noting that the patient remained afebrile throughout his entire 45-day hospitalization, with negative culture data, and despite receiving an extensive broad spectrum antibiotic regimen (levofloxacin, piperacillin-tazobactam, cefazolin, metronidazole, ceftriaxone and fluconazole), his leukocytosis continued to progress, peaking at 161.5 x 103 cells/mm³ less than a month after his surgery (Figure 3).
The patient continued to deteriorate rapidly, with a progressive leukocytosis, and developing metastases to the lung, perineum, and penis. He died a month after surgery (2 months after completion of neoadjuvant chemotherapy). The leukocytosis exhibited in this patient and the aggressive tumor cell growth are believed to have been secondary to a paraneoplastic leukemoid reaction incited by the urothelial bladder cancer cells’ presumed production of G-CSF, which has been rarely reported in the literature previously.
Discussion
We report here on the rare occurrence of PLR in a urothelial bladder cancer. Several mechanisms have been proposed to explain the pathophysiology of PLR. The levels of IL-1[alpha and beta], IL-3, G-CSF, GM-CSF, IL-6, and TNF-[alpha] have all been reported to be elevated in various solid tumors and suggested to contribute to an elevated leukocyte count.3 With previous reports that receptors for G-CSF have been found on cell surfaces of several nonhematopoietic cell types, Tachibana and Murai have proposed the mechanism of a cancer cell’s simultaneous acquisition of the ligand promotion and its receptor expression conferring an autocrine growth advantage.4 They have also reported on the capability of bladder cancer cells to induce a leukemoid reaction in their host through the stimulation of leukocyte production, which has been associated with aggressive tumor cell growth and a poor clinical outcome. In addition, He and colleagues have also described the correlation between PLR and high degree of malignancy, high probability of metastasis, recurrence, and poor prognosis.5
We observed the leukocytosis of 70 x 103 cells/mm³ on the day of resection with a slight drop postoperatively, peaking at 161.5 x 103 cells/mm³ less than a month after resection of the tumor. There is no clear understanding of the cause of the persistent and rapid progression of leukocytosis seen in this patient postoperatively. There is also a dearth of literature describing similar occurrences, with even fewer attempting to explain the pathophysiology of this occurrence.
When faced with similar occurrences in patients, clinicians usually treat for occult infection. Once infections and myeloproliferative diseases have been ruled out, clinicians may consider obtaining a patient’s serum G-CSF level or performing an immunohistochemistry analysis of urothelial cells for overexpression of G-CSF, when available.5 However, despite any efforts to diagnose earlier, there is little clinicians currently have to offer these patients as treatment.
As presented in this report, PLR portends a worse prognosis for patients because of its ability to not only masquerade as an infection leading to a delay in the proper treatment, but also because of its association with a more aggressive tumor cell behavior and growth, making it critically important for clinicians to be able to identify these patients early on. With further investigation into immune regulation and G-CSF receptor signaling, there may be future discoveries of novel methods to diagnose this condition and also advancements in the treatment options made available to these patients.
1. Chakraborty S, Keenportz B, Woodward S, Anderson J, Colan D. Paraneoplastic leukemoid reaction in solid tumors. Am J Clin Oncol. 2015;38(3):326-330.
2. Kumar AK, Satyan MT, Holzbeierlein J, Mirza M, Van Veldhuizen P. Leukemoid reaction and autocrine growth of bladder cancer induced by paraneoplastic production of granulocyte colony-stimulating factor-a potential neoplastic marker: a case report and review of the literature. J Med Case Rep. 2014;8(1):147.
3. Azuma T, Sakai I, Matsumoto T, et al. Leukemoid reaction in association with bone marrow necrosis due to metastatic prostate cancer. Intern Med. 2005;44(10):1093-1096.
4. Tachibana M, Murai M. G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther. 1998;4(2):113-120.
5. He H, Zhang Z, Ge J, Zhou W. Leukemoid reaction associated with transitional cell carcinoma: a case report and literature review. Niger J Clin Pract. 2014;17(3):391-394.
Certain cancers have been observed to cause symptoms called paraneoplastic syndromes that are not directly attributed to tumor invasion or compression. This phenomenon is believed to be secondary to a tumor’s secretion of functional hormones, peptides, cytokines, or its immune cross-reactivity. One such variant is a paraneoplastic leukemoid reaction (PLR), defined as a leukocytosis level of >50 x 103 cells/mm³, where the white blood cell (WBC) count differential exhibits a neutrophilia or left-shift, in which a predominance of early neutrophil precursors is observed. A PLR is believed to be incited by the tumor cell’s production of its own growth factors such as granulocyte-colony stimulating factor (G-CSF) and a number of different cytokines. These reactions may at first be mistaken for infectious processes, and it is only after an infection has been ruled out or when a leukocytosis is disproportionately high in the setting of a treated infection, that a paraneoplastic leukemoid reaction (PLR) is considered and an oncologic work-up pursued.
PLR has been previously described in a variety of malignancies including lung, esophageal, nasopharyngeal and laryngeal, gastric, cholangiocarcinoma, melanoma, multiple myeloma, renal, prostate, and hepatocellular carcinoma, but has rarely been described in urothelial carcinoma.1 Leukemoid reactions and autocrine growth induced by paraneoplastic production of G-CSF have rarely been associated with urothelial carcinoma of the bladder,2 as in the case we present here of a 67-year-old white man with invasive high-grade urothelial carcinoma of the bladder. The case highlights PLR as a negative prognostic marker, secondary to urothelial bladder cancer cells’ presumed production of G-CSF, rarely reported as in the literature previously.
Case presentation and summary
A 67-year-old white man was diagnosed with clinical stage III (T3N0M0), invasive high-grade urothelial carcinoma of the bladder (Figure 1). He received neoadjuvant chemotherapy with the standard gemcitabine-cisplatin combination (1,000 mg/m2 of gemcitabine on days 1, 8, and 15 with 70 mg/m2 of cisplatin on day 1 of a 28-day cycle for 3 cycles), and had less than partial response at the end of a 3-month course. A computed tomography scan of his pelvis obtained at treatment completion revealed persistent disease with noted enhancement of the right distal ureter and a right posterior bladder mass at the ureterovesical junction measuring 1.7 x 2.6 x 2.6 cm (0.6 x 1 x 1 in), for which, a cystoprostatectomy was recommended to remove remaining disease. The patient was seen in routine follow-up 3 weeks after his last chemotherapy treatment, when his WBC count was noted to be within normal limits at a value of 8.4 x 103 cells/mm³ (normal range, 4.5-11 x 103 cells/mm³).
One week later (a month after treatment completion), the patient presented to the emergency department with complaints of dysuria, urinary frequency, and suprapubic pain. He was found to have leukocytosis, with a WBC count of 47 x 103 cells/mm³, (normal range, 4.5-11 x 103 cells/mm³), with an elevated neutrophil count of 82.7% (normal range 40%-60%), without clinical signs of systemic infection (fevers, chills, or rigors). A urinalysis revealed pyuria with 25-50 WBC/high power field, negative nitrite, positive leukocyte esterase, and moderate bacteria, consistent with what was presumed to be a urinary tract infection. The patient was discharged home with a 1-week course of the antibiotic levofloxacin and the alpha-blocker tamsulosin to make urination easier. Of note, the final results of the urine culture, which returned 48 hours after discharge, showed no growth.
One week prior to surgery, the patient underwent a cystoscopy for ureteral stent exchange, which revealed a necrotic tumor at the right bladder base surrounding the ureteral orifice and stent. Renal pelvis urine, sampled during stent exchange, revealed >100,000 CFU/ml (colony forming units; normal value, <10³) Candida albicans, for which the patient was started on intravenous fluconazole for fungal infection. We consulted with our colleagues in the infectious disease department and continued to follow the patient throughout his hospital course, which included several antibiotic regimens, a comprehensive hematological work-up and eventual urologic surgery. Work-ups for myeloproliferative disorders, leukemia, JAK-2, and BCR-ABL were all negative. A peripheral blood smear analysis showed mostly neutrophils, no immature cells, and occasional hypersegmented neutrophils, but was overall inconsistent with myeloproliferative disease. Despite the patient’s persistent leukocytosis, he remained completely asymptomatic and his neutrophilia was attributed to his malignancy.
The patient subsequently underwent a cystoprostatectomy with ileal conduit. The surgical pathologic analysis showed a high-grade, invasive urothelial (transitional cell) carcinoma measuring 5.2 x 5.0 x 4.5 cm (2 x 1.9 x 1.8 in) with squamous differentiation, extensive tumor necrosis, lymphovascular invasion, invasion into the adjacent seminal vesicle and prostatic stroma, and negative margins (pT4a pN0 pMX; Figure 2). On the day of surgical resection, the patient’s leukocytosis was 70 x 103 cells/mm³.
Despite a transient improvement in his leukocytosis to 37.7 x 103 cells/mm³ on postoperative day 1, the patient remained in the medical intensive care unit for uncontrolled pain and management of his leukocytosis. It is worth noting that the patient remained afebrile throughout his entire 45-day hospitalization, with negative culture data, and despite receiving an extensive broad spectrum antibiotic regimen (levofloxacin, piperacillin-tazobactam, cefazolin, metronidazole, ceftriaxone and fluconazole), his leukocytosis continued to progress, peaking at 161.5 x 103 cells/mm³ less than a month after his surgery (Figure 3).
The patient continued to deteriorate rapidly, with a progressive leukocytosis, and developing metastases to the lung, perineum, and penis. He died a month after surgery (2 months after completion of neoadjuvant chemotherapy). The leukocytosis exhibited in this patient and the aggressive tumor cell growth are believed to have been secondary to a paraneoplastic leukemoid reaction incited by the urothelial bladder cancer cells’ presumed production of G-CSF, which has been rarely reported in the literature previously.
Discussion
We report here on the rare occurrence of PLR in a urothelial bladder cancer. Several mechanisms have been proposed to explain the pathophysiology of PLR. The levels of IL-1[alpha and beta], IL-3, G-CSF, GM-CSF, IL-6, and TNF-[alpha] have all been reported to be elevated in various solid tumors and suggested to contribute to an elevated leukocyte count.3 With previous reports that receptors for G-CSF have been found on cell surfaces of several nonhematopoietic cell types, Tachibana and Murai have proposed the mechanism of a cancer cell’s simultaneous acquisition of the ligand promotion and its receptor expression conferring an autocrine growth advantage.4 They have also reported on the capability of bladder cancer cells to induce a leukemoid reaction in their host through the stimulation of leukocyte production, which has been associated with aggressive tumor cell growth and a poor clinical outcome. In addition, He and colleagues have also described the correlation between PLR and high degree of malignancy, high probability of metastasis, recurrence, and poor prognosis.5
We observed the leukocytosis of 70 x 103 cells/mm³ on the day of resection with a slight drop postoperatively, peaking at 161.5 x 103 cells/mm³ less than a month after resection of the tumor. There is no clear understanding of the cause of the persistent and rapid progression of leukocytosis seen in this patient postoperatively. There is also a dearth of literature describing similar occurrences, with even fewer attempting to explain the pathophysiology of this occurrence.
When faced with similar occurrences in patients, clinicians usually treat for occult infection. Once infections and myeloproliferative diseases have been ruled out, clinicians may consider obtaining a patient’s serum G-CSF level or performing an immunohistochemistry analysis of urothelial cells for overexpression of G-CSF, when available.5 However, despite any efforts to diagnose earlier, there is little clinicians currently have to offer these patients as treatment.
As presented in this report, PLR portends a worse prognosis for patients because of its ability to not only masquerade as an infection leading to a delay in the proper treatment, but also because of its association with a more aggressive tumor cell behavior and growth, making it critically important for clinicians to be able to identify these patients early on. With further investigation into immune regulation and G-CSF receptor signaling, there may be future discoveries of novel methods to diagnose this condition and also advancements in the treatment options made available to these patients.
Certain cancers have been observed to cause symptoms called paraneoplastic syndromes that are not directly attributed to tumor invasion or compression. This phenomenon is believed to be secondary to a tumor’s secretion of functional hormones, peptides, cytokines, or its immune cross-reactivity. One such variant is a paraneoplastic leukemoid reaction (PLR), defined as a leukocytosis level of >50 x 103 cells/mm³, where the white blood cell (WBC) count differential exhibits a neutrophilia or left-shift, in which a predominance of early neutrophil precursors is observed. A PLR is believed to be incited by the tumor cell’s production of its own growth factors such as granulocyte-colony stimulating factor (G-CSF) and a number of different cytokines. These reactions may at first be mistaken for infectious processes, and it is only after an infection has been ruled out or when a leukocytosis is disproportionately high in the setting of a treated infection, that a paraneoplastic leukemoid reaction (PLR) is considered and an oncologic work-up pursued.
PLR has been previously described in a variety of malignancies including lung, esophageal, nasopharyngeal and laryngeal, gastric, cholangiocarcinoma, melanoma, multiple myeloma, renal, prostate, and hepatocellular carcinoma, but has rarely been described in urothelial carcinoma.1 Leukemoid reactions and autocrine growth induced by paraneoplastic production of G-CSF have rarely been associated with urothelial carcinoma of the bladder,2 as in the case we present here of a 67-year-old white man with invasive high-grade urothelial carcinoma of the bladder. The case highlights PLR as a negative prognostic marker, secondary to urothelial bladder cancer cells’ presumed production of G-CSF, rarely reported as in the literature previously.
Case presentation and summary
A 67-year-old white man was diagnosed with clinical stage III (T3N0M0), invasive high-grade urothelial carcinoma of the bladder (Figure 1). He received neoadjuvant chemotherapy with the standard gemcitabine-cisplatin combination (1,000 mg/m2 of gemcitabine on days 1, 8, and 15 with 70 mg/m2 of cisplatin on day 1 of a 28-day cycle for 3 cycles), and had less than partial response at the end of a 3-month course. A computed tomography scan of his pelvis obtained at treatment completion revealed persistent disease with noted enhancement of the right distal ureter and a right posterior bladder mass at the ureterovesical junction measuring 1.7 x 2.6 x 2.6 cm (0.6 x 1 x 1 in), for which, a cystoprostatectomy was recommended to remove remaining disease. The patient was seen in routine follow-up 3 weeks after his last chemotherapy treatment, when his WBC count was noted to be within normal limits at a value of 8.4 x 103 cells/mm³ (normal range, 4.5-11 x 103 cells/mm³).
One week later (a month after treatment completion), the patient presented to the emergency department with complaints of dysuria, urinary frequency, and suprapubic pain. He was found to have leukocytosis, with a WBC count of 47 x 103 cells/mm³, (normal range, 4.5-11 x 103 cells/mm³), with an elevated neutrophil count of 82.7% (normal range 40%-60%), without clinical signs of systemic infection (fevers, chills, or rigors). A urinalysis revealed pyuria with 25-50 WBC/high power field, negative nitrite, positive leukocyte esterase, and moderate bacteria, consistent with what was presumed to be a urinary tract infection. The patient was discharged home with a 1-week course of the antibiotic levofloxacin and the alpha-blocker tamsulosin to make urination easier. Of note, the final results of the urine culture, which returned 48 hours after discharge, showed no growth.
One week prior to surgery, the patient underwent a cystoscopy for ureteral stent exchange, which revealed a necrotic tumor at the right bladder base surrounding the ureteral orifice and stent. Renal pelvis urine, sampled during stent exchange, revealed >100,000 CFU/ml (colony forming units; normal value, <10³) Candida albicans, for which the patient was started on intravenous fluconazole for fungal infection. We consulted with our colleagues in the infectious disease department and continued to follow the patient throughout his hospital course, which included several antibiotic regimens, a comprehensive hematological work-up and eventual urologic surgery. Work-ups for myeloproliferative disorders, leukemia, JAK-2, and BCR-ABL were all negative. A peripheral blood smear analysis showed mostly neutrophils, no immature cells, and occasional hypersegmented neutrophils, but was overall inconsistent with myeloproliferative disease. Despite the patient’s persistent leukocytosis, he remained completely asymptomatic and his neutrophilia was attributed to his malignancy.
The patient subsequently underwent a cystoprostatectomy with ileal conduit. The surgical pathologic analysis showed a high-grade, invasive urothelial (transitional cell) carcinoma measuring 5.2 x 5.0 x 4.5 cm (2 x 1.9 x 1.8 in) with squamous differentiation, extensive tumor necrosis, lymphovascular invasion, invasion into the adjacent seminal vesicle and prostatic stroma, and negative margins (pT4a pN0 pMX; Figure 2). On the day of surgical resection, the patient’s leukocytosis was 70 x 103 cells/mm³.
Despite a transient improvement in his leukocytosis to 37.7 x 103 cells/mm³ on postoperative day 1, the patient remained in the medical intensive care unit for uncontrolled pain and management of his leukocytosis. It is worth noting that the patient remained afebrile throughout his entire 45-day hospitalization, with negative culture data, and despite receiving an extensive broad spectrum antibiotic regimen (levofloxacin, piperacillin-tazobactam, cefazolin, metronidazole, ceftriaxone and fluconazole), his leukocytosis continued to progress, peaking at 161.5 x 103 cells/mm³ less than a month after his surgery (Figure 3).
The patient continued to deteriorate rapidly, with a progressive leukocytosis, and developing metastases to the lung, perineum, and penis. He died a month after surgery (2 months after completion of neoadjuvant chemotherapy). The leukocytosis exhibited in this patient and the aggressive tumor cell growth are believed to have been secondary to a paraneoplastic leukemoid reaction incited by the urothelial bladder cancer cells’ presumed production of G-CSF, which has been rarely reported in the literature previously.
Discussion
We report here on the rare occurrence of PLR in a urothelial bladder cancer. Several mechanisms have been proposed to explain the pathophysiology of PLR. The levels of IL-1[alpha and beta], IL-3, G-CSF, GM-CSF, IL-6, and TNF-[alpha] have all been reported to be elevated in various solid tumors and suggested to contribute to an elevated leukocyte count.3 With previous reports that receptors for G-CSF have been found on cell surfaces of several nonhematopoietic cell types, Tachibana and Murai have proposed the mechanism of a cancer cell’s simultaneous acquisition of the ligand promotion and its receptor expression conferring an autocrine growth advantage.4 They have also reported on the capability of bladder cancer cells to induce a leukemoid reaction in their host through the stimulation of leukocyte production, which has been associated with aggressive tumor cell growth and a poor clinical outcome. In addition, He and colleagues have also described the correlation between PLR and high degree of malignancy, high probability of metastasis, recurrence, and poor prognosis.5
We observed the leukocytosis of 70 x 103 cells/mm³ on the day of resection with a slight drop postoperatively, peaking at 161.5 x 103 cells/mm³ less than a month after resection of the tumor. There is no clear understanding of the cause of the persistent and rapid progression of leukocytosis seen in this patient postoperatively. There is also a dearth of literature describing similar occurrences, with even fewer attempting to explain the pathophysiology of this occurrence.
When faced with similar occurrences in patients, clinicians usually treat for occult infection. Once infections and myeloproliferative diseases have been ruled out, clinicians may consider obtaining a patient’s serum G-CSF level or performing an immunohistochemistry analysis of urothelial cells for overexpression of G-CSF, when available.5 However, despite any efforts to diagnose earlier, there is little clinicians currently have to offer these patients as treatment.
As presented in this report, PLR portends a worse prognosis for patients because of its ability to not only masquerade as an infection leading to a delay in the proper treatment, but also because of its association with a more aggressive tumor cell behavior and growth, making it critically important for clinicians to be able to identify these patients early on. With further investigation into immune regulation and G-CSF receptor signaling, there may be future discoveries of novel methods to diagnose this condition and also advancements in the treatment options made available to these patients.
1. Chakraborty S, Keenportz B, Woodward S, Anderson J, Colan D. Paraneoplastic leukemoid reaction in solid tumors. Am J Clin Oncol. 2015;38(3):326-330.
2. Kumar AK, Satyan MT, Holzbeierlein J, Mirza M, Van Veldhuizen P. Leukemoid reaction and autocrine growth of bladder cancer induced by paraneoplastic production of granulocyte colony-stimulating factor-a potential neoplastic marker: a case report and review of the literature. J Med Case Rep. 2014;8(1):147.
3. Azuma T, Sakai I, Matsumoto T, et al. Leukemoid reaction in association with bone marrow necrosis due to metastatic prostate cancer. Intern Med. 2005;44(10):1093-1096.
4. Tachibana M, Murai M. G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther. 1998;4(2):113-120.
5. He H, Zhang Z, Ge J, Zhou W. Leukemoid reaction associated with transitional cell carcinoma: a case report and literature review. Niger J Clin Pract. 2014;17(3):391-394.
1. Chakraborty S, Keenportz B, Woodward S, Anderson J, Colan D. Paraneoplastic leukemoid reaction in solid tumors. Am J Clin Oncol. 2015;38(3):326-330.
2. Kumar AK, Satyan MT, Holzbeierlein J, Mirza M, Van Veldhuizen P. Leukemoid reaction and autocrine growth of bladder cancer induced by paraneoplastic production of granulocyte colony-stimulating factor-a potential neoplastic marker: a case report and review of the literature. J Med Case Rep. 2014;8(1):147.
3. Azuma T, Sakai I, Matsumoto T, et al. Leukemoid reaction in association with bone marrow necrosis due to metastatic prostate cancer. Intern Med. 2005;44(10):1093-1096.
4. Tachibana M, Murai M. G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther. 1998;4(2):113-120.
5. He H, Zhang Z, Ge J, Zhou W. Leukemoid reaction associated with transitional cell carcinoma: a case report and literature review. Niger J Clin Pract. 2014;17(3):391-394.
Ultrasound, cystoscopy combo tops CT for asymptomatic microscopic hematuria
Combining renal ultrasound and bladder cystoscopy is the most cost-effective approach for the initial evaluation of asymptomatic microscopic hematuria, even among patients at risk for genitourinary malignancy, according to a report published online April 17 in JAMA Internal Medicine.
“The superiority of this approach over the use of CT plus cystoscopy is driven primarily by higher costs of CT and the associated complications, albeit rare,” said Joshua A. Halpern, MD, of the department of urology, CornellUniversity, New York, and his associates. “Given the low prevalence of upper-tract malignant abnormalities in patients with asymptomatic microscopic hematuria, the small advantage in the sensitivity of CT imaging does not compensate for the significant additional costs.”
Every year, hundreds of thousands of patients undergo urinalysis for a variety of indications, and an estimated 40% are found to have microscopic hematuria in the absence of any urinary symptoms. This finding requires further evaluation because of one particular possible cause: a genitourinary malignancy. An estimated 11% of people with asymptomatic microscopic hematuria are found to have malignant abnormalities, the investigators said.
They assessed the cost-effectiveness of four common follow-up evaluations by creating a decision-analysis model to simulate the rates of cancer detection in adults with no history of cancer and with negative urine cultures that ruled out UTI as the cause of the hematuria.
The model was based on data from real-world experience in the medical literature and incorporated information on cancer incidence, diagnostic test accuracy, and complications.
The four approaches they examined were CT plus cystoscopy, which is considered the preferred method of diagnostic work-up by the American Urological Association; renal ultrasound plus cystoscopy, which many clinicians in the United States and other countries use instead; cystoscopy alone; and CT alone.
Compared with no follow-up evaluation, CT alone detected the fewest cancers (221 per 10,000 patients) at the highest cost ($9,300,000 per 10,000 patients). Cystoscopy alone detected 222 cancers per 10,000 at a cost of $10,287 per 10,000. Ultrasound plus cystoscopy detected 23 additional cancers per 10,000 patients at a relatively low cost of $53,810 per 10,000. Replacing ultrasound with CT detected just one additional cancer but cost an additional $6,480,484 per 10,000 patients.
The findings were similar in several sensitivity analyses, as well as in a subgroup analysis involving only higher-risk patients – men, smokers, and patients aged 50 years and older, the investigators noted (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamaintenmed.2017.0739).
Dr. Halpern and his associates also applied their results to nationwide 2012 statistics for 485,222 patient visits to urologists to assess microscopic hematuria. If all urologists complied with AUA guidelines and used CT instead of ultrasound plus cystoscopy to assess these patients, they would have detected only 60 additional cancers, at an additional cost of $389,914,648.
Given these findings, renal ultrasound plus bladder cystoscopy should be considered the first-line assessment for these patients, Dr. Halpern and his associates said. Rewriting practice guidelines accordingly “will substantially reduce national expenditures associated with asymptomatic microscopic hematuria evaluation by up to $390 million.”
Moreover, recommending ultrasound rather than CT might have the unintended but beneficial consequence of improving compliance with further evaluation, because many primary care physicians are reluctant to refer these patients for radiocontrast CT studies, the researchers noted.
No sponsor was cited for this study. Dr. Halpern and his associates reported having no relevant financial disclosures.
The substantial differences between ultrasound and CT in cost per cancer detected, combined with the harm from CT-related contrast reactions and radiation exposure, strongly support renal ultrasound plus cystoscopy as the preferred first-line approach to assessing asymptomatic microscopic hematuria.
According to Halpern et al., this approach would cost approximately $54,000 per cancer detected. Replacing ultrasound with CT would detect just 1 additional cancer per 10,000 assessments, at an incremental cost of $6.5 million.
Leslee L. Subak, MD, and Deborah Grady, MD, are in the departments of obstetrics, gynecology, and reproductive sciences; urology; and epidemiology and biostatistics at the University of California, San Francisco. Dr. Subak reported receiving funding from Astellas to research urinary incontinence. Dr. Subak and Dr. Grady made these remarks in an invited commentary accompanying Dr. Halpern’s report (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamainternmed.2017.0758).
The substantial differences between ultrasound and CT in cost per cancer detected, combined with the harm from CT-related contrast reactions and radiation exposure, strongly support renal ultrasound plus cystoscopy as the preferred first-line approach to assessing asymptomatic microscopic hematuria.
According to Halpern et al., this approach would cost approximately $54,000 per cancer detected. Replacing ultrasound with CT would detect just 1 additional cancer per 10,000 assessments, at an incremental cost of $6.5 million.
Leslee L. Subak, MD, and Deborah Grady, MD, are in the departments of obstetrics, gynecology, and reproductive sciences; urology; and epidemiology and biostatistics at the University of California, San Francisco. Dr. Subak reported receiving funding from Astellas to research urinary incontinence. Dr. Subak and Dr. Grady made these remarks in an invited commentary accompanying Dr. Halpern’s report (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamainternmed.2017.0758).
The substantial differences between ultrasound and CT in cost per cancer detected, combined with the harm from CT-related contrast reactions and radiation exposure, strongly support renal ultrasound plus cystoscopy as the preferred first-line approach to assessing asymptomatic microscopic hematuria.
According to Halpern et al., this approach would cost approximately $54,000 per cancer detected. Replacing ultrasound with CT would detect just 1 additional cancer per 10,000 assessments, at an incremental cost of $6.5 million.
Leslee L. Subak, MD, and Deborah Grady, MD, are in the departments of obstetrics, gynecology, and reproductive sciences; urology; and epidemiology and biostatistics at the University of California, San Francisco. Dr. Subak reported receiving funding from Astellas to research urinary incontinence. Dr. Subak and Dr. Grady made these remarks in an invited commentary accompanying Dr. Halpern’s report (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamainternmed.2017.0758).
Combining renal ultrasound and bladder cystoscopy is the most cost-effective approach for the initial evaluation of asymptomatic microscopic hematuria, even among patients at risk for genitourinary malignancy, according to a report published online April 17 in JAMA Internal Medicine.
“The superiority of this approach over the use of CT plus cystoscopy is driven primarily by higher costs of CT and the associated complications, albeit rare,” said Joshua A. Halpern, MD, of the department of urology, CornellUniversity, New York, and his associates. “Given the low prevalence of upper-tract malignant abnormalities in patients with asymptomatic microscopic hematuria, the small advantage in the sensitivity of CT imaging does not compensate for the significant additional costs.”
Every year, hundreds of thousands of patients undergo urinalysis for a variety of indications, and an estimated 40% are found to have microscopic hematuria in the absence of any urinary symptoms. This finding requires further evaluation because of one particular possible cause: a genitourinary malignancy. An estimated 11% of people with asymptomatic microscopic hematuria are found to have malignant abnormalities, the investigators said.
They assessed the cost-effectiveness of four common follow-up evaluations by creating a decision-analysis model to simulate the rates of cancer detection in adults with no history of cancer and with negative urine cultures that ruled out UTI as the cause of the hematuria.
The model was based on data from real-world experience in the medical literature and incorporated information on cancer incidence, diagnostic test accuracy, and complications.
The four approaches they examined were CT plus cystoscopy, which is considered the preferred method of diagnostic work-up by the American Urological Association; renal ultrasound plus cystoscopy, which many clinicians in the United States and other countries use instead; cystoscopy alone; and CT alone.
Compared with no follow-up evaluation, CT alone detected the fewest cancers (221 per 10,000 patients) at the highest cost ($9,300,000 per 10,000 patients). Cystoscopy alone detected 222 cancers per 10,000 at a cost of $10,287 per 10,000. Ultrasound plus cystoscopy detected 23 additional cancers per 10,000 patients at a relatively low cost of $53,810 per 10,000. Replacing ultrasound with CT detected just one additional cancer but cost an additional $6,480,484 per 10,000 patients.
The findings were similar in several sensitivity analyses, as well as in a subgroup analysis involving only higher-risk patients – men, smokers, and patients aged 50 years and older, the investigators noted (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamaintenmed.2017.0739).
Dr. Halpern and his associates also applied their results to nationwide 2012 statistics for 485,222 patient visits to urologists to assess microscopic hematuria. If all urologists complied with AUA guidelines and used CT instead of ultrasound plus cystoscopy to assess these patients, they would have detected only 60 additional cancers, at an additional cost of $389,914,648.
Given these findings, renal ultrasound plus bladder cystoscopy should be considered the first-line assessment for these patients, Dr. Halpern and his associates said. Rewriting practice guidelines accordingly “will substantially reduce national expenditures associated with asymptomatic microscopic hematuria evaluation by up to $390 million.”
Moreover, recommending ultrasound rather than CT might have the unintended but beneficial consequence of improving compliance with further evaluation, because many primary care physicians are reluctant to refer these patients for radiocontrast CT studies, the researchers noted.
No sponsor was cited for this study. Dr. Halpern and his associates reported having no relevant financial disclosures.
Combining renal ultrasound and bladder cystoscopy is the most cost-effective approach for the initial evaluation of asymptomatic microscopic hematuria, even among patients at risk for genitourinary malignancy, according to a report published online April 17 in JAMA Internal Medicine.
“The superiority of this approach over the use of CT plus cystoscopy is driven primarily by higher costs of CT and the associated complications, albeit rare,” said Joshua A. Halpern, MD, of the department of urology, CornellUniversity, New York, and his associates. “Given the low prevalence of upper-tract malignant abnormalities in patients with asymptomatic microscopic hematuria, the small advantage in the sensitivity of CT imaging does not compensate for the significant additional costs.”
Every year, hundreds of thousands of patients undergo urinalysis for a variety of indications, and an estimated 40% are found to have microscopic hematuria in the absence of any urinary symptoms. This finding requires further evaluation because of one particular possible cause: a genitourinary malignancy. An estimated 11% of people with asymptomatic microscopic hematuria are found to have malignant abnormalities, the investigators said.
They assessed the cost-effectiveness of four common follow-up evaluations by creating a decision-analysis model to simulate the rates of cancer detection in adults with no history of cancer and with negative urine cultures that ruled out UTI as the cause of the hematuria.
The model was based on data from real-world experience in the medical literature and incorporated information on cancer incidence, diagnostic test accuracy, and complications.
The four approaches they examined were CT plus cystoscopy, which is considered the preferred method of diagnostic work-up by the American Urological Association; renal ultrasound plus cystoscopy, which many clinicians in the United States and other countries use instead; cystoscopy alone; and CT alone.
Compared with no follow-up evaluation, CT alone detected the fewest cancers (221 per 10,000 patients) at the highest cost ($9,300,000 per 10,000 patients). Cystoscopy alone detected 222 cancers per 10,000 at a cost of $10,287 per 10,000. Ultrasound plus cystoscopy detected 23 additional cancers per 10,000 patients at a relatively low cost of $53,810 per 10,000. Replacing ultrasound with CT detected just one additional cancer but cost an additional $6,480,484 per 10,000 patients.
The findings were similar in several sensitivity analyses, as well as in a subgroup analysis involving only higher-risk patients – men, smokers, and patients aged 50 years and older, the investigators noted (JAMA Intern Med. 2017 Apr 17. doi: 10.1001/jamaintenmed.2017.0739).
Dr. Halpern and his associates also applied their results to nationwide 2012 statistics for 485,222 patient visits to urologists to assess microscopic hematuria. If all urologists complied with AUA guidelines and used CT instead of ultrasound plus cystoscopy to assess these patients, they would have detected only 60 additional cancers, at an additional cost of $389,914,648.
Given these findings, renal ultrasound plus bladder cystoscopy should be considered the first-line assessment for these patients, Dr. Halpern and his associates said. Rewriting practice guidelines accordingly “will substantially reduce national expenditures associated with asymptomatic microscopic hematuria evaluation by up to $390 million.”
Moreover, recommending ultrasound rather than CT might have the unintended but beneficial consequence of improving compliance with further evaluation, because many primary care physicians are reluctant to refer these patients for radiocontrast CT studies, the researchers noted.
No sponsor was cited for this study. Dr. Halpern and his associates reported having no relevant financial disclosures.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Combining renal ultrasound and bladder cystoscopy is the most cost-effective approach for the initial evaluation of asymptomatic microscopic hematuria.
Major finding: If all urologists complied with AUA guidelines and used CT instead of ultrasound plus cystoscopy to assess the 485,222 patients who were seen for asymptomatic microscopic hematuria in 2012, they would have detected only 60 additional cancers, at an additional cost of $389,914,648.
Data source: Decision-analysis modeling of four common approaches to assessing asymptomatic microscopic hematuria.
Disclosures: No sponsor was cited for this study. Dr. Halpern and his associates reported having no relevant financial disclosures.
Metformin linked with better survival in RCC patients with diabetes
Metformin use was associated with better survival for patients with renal cell carcinoma and diabetes in a meta-analysis, investigators report.
Yang Li, MD, and associates at Chongqing (China) Medical University, performed a pooled analysis of data from 254,329 patients with both localized and metastatic renal cell carcinoma, and found the risk of mortality was reduced in patients exposed to metformin (hazard ratio, 0.41; P less than .001).
However, there was significant heterogeneity among the eight eligible studies included in the meta-analysis, Dr. Li and associates reported (Int Urol Nephrol. 2017 Mar 7. doi: 10.1007/s11255-017-1548-4).
In a subgroup analysis, the association held in patients with localized disease, but was not significant in those with metastatic disease.
The current meta-analysis suggests that the use of metformin could improve the survival of kidney cancer patients, particularly those with localized disease; however, further studies are needed, the investigators conclude.
The authors declared that they had no conflicts of interest.
Metformin use was associated with better survival for patients with renal cell carcinoma and diabetes in a meta-analysis, investigators report.
Yang Li, MD, and associates at Chongqing (China) Medical University, performed a pooled analysis of data from 254,329 patients with both localized and metastatic renal cell carcinoma, and found the risk of mortality was reduced in patients exposed to metformin (hazard ratio, 0.41; P less than .001).
However, there was significant heterogeneity among the eight eligible studies included in the meta-analysis, Dr. Li and associates reported (Int Urol Nephrol. 2017 Mar 7. doi: 10.1007/s11255-017-1548-4).
In a subgroup analysis, the association held in patients with localized disease, but was not significant in those with metastatic disease.
The current meta-analysis suggests that the use of metformin could improve the survival of kidney cancer patients, particularly those with localized disease; however, further studies are needed, the investigators conclude.
The authors declared that they had no conflicts of interest.
Metformin use was associated with better survival for patients with renal cell carcinoma and diabetes in a meta-analysis, investigators report.
Yang Li, MD, and associates at Chongqing (China) Medical University, performed a pooled analysis of data from 254,329 patients with both localized and metastatic renal cell carcinoma, and found the risk of mortality was reduced in patients exposed to metformin (hazard ratio, 0.41; P less than .001).
However, there was significant heterogeneity among the eight eligible studies included in the meta-analysis, Dr. Li and associates reported (Int Urol Nephrol. 2017 Mar 7. doi: 10.1007/s11255-017-1548-4).
In a subgroup analysis, the association held in patients with localized disease, but was not significant in those with metastatic disease.
The current meta-analysis suggests that the use of metformin could improve the survival of kidney cancer patients, particularly those with localized disease; however, further studies are needed, the investigators conclude.
The authors declared that they had no conflicts of interest.
Key clinical point:
Major finding: In a pooled analysis of data from eight studies, the risk of mortality was reduced in patients exposed to metformin (hazard ratio, 0.41; P less than .001).
Data source: A meta-analysis of eight studies including 254,329 patients with renal cell carcinoma.
Disclosures: The authors declared that they had no conflicts of interest.
Liquid gold: blood-based biopsies make headway
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
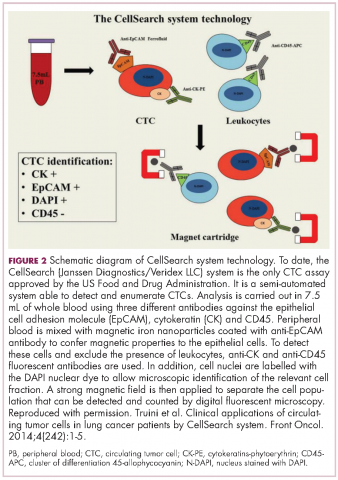
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
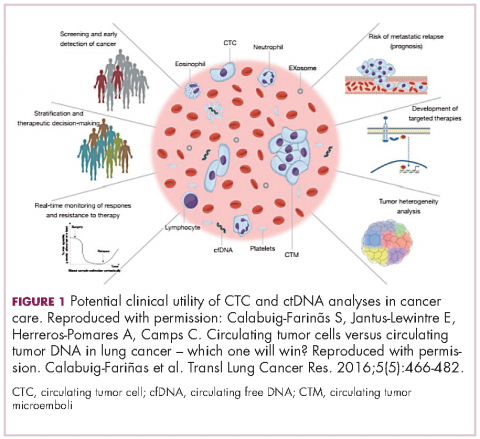
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
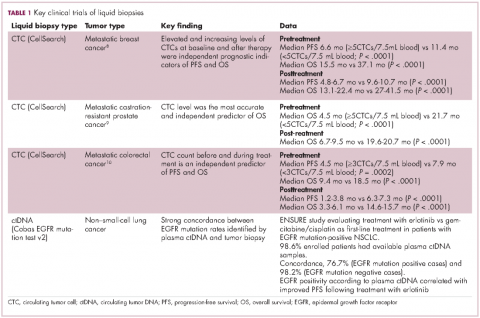
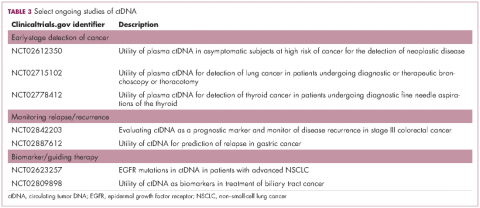
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
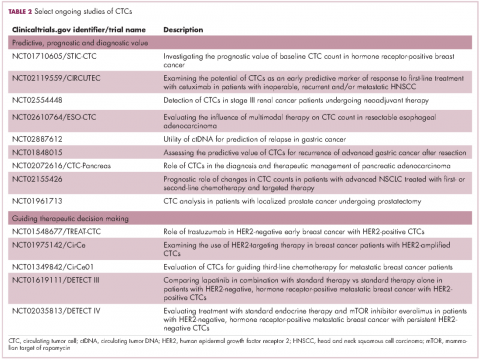
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
Pathologic and, increasingly, molecular analysis of tumor tissue biopsies is the gold standard in initial diagnosis of cancer, but liquid biopsies, which analyze tumor-derived material circulating in the bloodstream are gaining traction. Here, we discuss the current state of development of this complementary and potentially alternative approach to tumor analysis.
Liquid biopsy gaining traction
Biopsies enable oncologists to gather information about a potential or established tumor, including confirmation of the presence of cancerous tissue and determination of its histological characteristics, such as tumor grade and stage, as well as its molecular features, such as the presence of certain gene mutations. Ultimately, this information can be put to use in determining the most appropriate course of treatment.
The current gold standard is a tissue biopsy that typically involves an invasive procedure to permit the collection of a piece of tumor tissue. Yet, tissue biopsies are not always feasible because of the location of the tumor or the poor performance status of many patients with advanced disease. They also provide only a snapshot of the disease at the time at which they were taken and don’t necessarily reflect the genetic heterogeneity or evolution of a tumor over time.
The detection of components that are derived from the tumor circulating in the blood of cancer patients had fueled the idea of blood-based diagnostics in oncology – so-called liquid biopsies. These have rapidly gained traction in the past several decades as a less expensive (the cost of performing genomic analyses on blood samples is at least an order of magnitude less than on tissue samples), less invasive (requiring only a simple blood draw) alternative source of information about tumors.1
As researchers have refined the ability to exploit liquid biopsies, commercial interest has been piqued. More than 35 companies within the United States alone are developing liquid biopsies, and it’s easy to see why with a market projected to be in the many billions of dollars.2
Seeking out tumor clues in the blood
Liquid biopsies consist of a 10-15 mL blood sample drawn into a tube that contains an anticoagulant and it can contain several different types of tumor-associated material. Thus far, two components – circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) – have formed the cornerstone of liquid biopsies. At present, it is not clear whether these components are released randomly, as a by-product of tumor cell death or if they are released as part of a specific biologic process, such as for the colonization of metastatic sites. It reality, it may be a little of both, and active dissemination may be particularly relevant for CTCs, among which are postulated to be a population of cancer stem cells that can initiate distant metastases.3,4
The discovery of CTCs dates back to the 1860s, when cells that were morphologically identical to the tumor were identified in the blood of a patient with metastatic cancer. Their potential significance was not fully realized until a few decades ago, when they were found to exist from early on in the course of disease.3,4
CTCs, which can be either single cells or clusters of cells known as microemboli, have a short half-life in the bloodstream – less than 2 ½ hours – and are also extremely rare (1 mL of blood contains 1-10 CTCs) against a background of many millions of normal cells. Thus the detection and isolation of CTCs presents a significant challenge. More than 40 different platforms are being developed for the isolation and enrichment of CTCs. For the most part, these use a method called positive selection to pick out CTCs.1,3,4
Positive selection exploits the biological or physical properties that are specific to CTCs and absent in normal cells, for example, the presence of a specific tumor-associated antigen on their surface or differences in size, density or electric charge. The limitations of this method are that, not only do you need to know something about CTCs to begin to understand what makes them truly unique and ensure only isolation of CTCs, but their phenotype is also thought to be continually changing.1,3,4
In recent years, the focus has shifted toward technologies that use negative depletion, meaning that they target the other types of cells in the blood sample and filter those away until only the CTCs are left behind. The most advanced are devices that use microfluidic technology to sort the cells, such as the CTC-iChip system being developed by researchers at Massachusetts General Hospital in Boston.5
ctDNA consists of small fragments of nucleic acids that are not contained within a cell or associated with cell fragments and is thought to be present in 50%-90% of patients, depending on the type of cancer they have. ctDNA has a similarly short half-life in the circulation to CTCs and, like CTCs, ctDNA is present at very low levels in the bloodstream. Although levels of ctDNA have been shown to increase with increasing tumor burden, it is still often obscured by the presence of other cell-free DNA derived from non-tumor cells.
ctDNA can be distinguished from other cell-free DNA by the presence of somatic mutations and a number of highly sensitive methods have been developed to detect them, including the amplification-refractory mutation system (ARMS); digital polymerase chain reaction; and the beads, emulsification, amplification, and magnetics (BEAMing) system. Next-generation sequencing technologies, including tagged-amplicon deep sequencing (TAm-Seq), the Safe-Sequencing System (Safe-SeqS), and cancer personalized profiling by deep sequencing (CAPP-seq), can also be used and the race for ever more sensitive analytical tools is ongoing.1,3,4,6
Applying liquid biopsies now and in the future
There are a plethora of potential applications for liquid biopsies3,7 (Figure 1), and probably the most exciting among them is the potential for screening for and early detection of cancer. The fact that ctDNA and CTCs have both been shown to be present from the earliest stages of disease has sparked interest in the possibility of developing simple blood tests to identify tumors before they become detectable by other methods and at a point at which they may be curable.
Given that both are present at such low levels within the circulation and are particularly sparse at earlier stages of disease, current technologies may lack the specificity and sensitivity for this application at present. However, numerous clinical trials are ongoing.
For CTCs, simple enumeration has been the most extensively investigated application to date. Numerous studies have shown that the number of CTCs in the bloodstream has prognostic significance in various different tumor types. Three such studies led to the first regulatory approval for a CTC detection system (Table 1 and Table 2).8-10
One area in which liquid biopsies could really come into their own is in providing more real-time analysis of tumors. This is something that has proven particularly challenging with tissue biopsies because repeating these invasive procedures is problematic. But the ease of repeat blood draws means that serial liquid biopsies could be performed and might offer the possibility of monitoring disease progression and evolution over the course of disease and particularly in response to treatment.
Indeed, studies have shown that in addition to baseline CTC counts, changes in CTC number during treatment are also prognostic. There was improved survival among patients whose CTC counts decreased below a threshold value during treatment and vice versa. This is also an approved use for CellSearch though at present it is not widely clinically implemented.12
Clinical utility remains elusive
The ultimate goal would be for liquid biopsies to have an impact on treatment decisions, allowing oncologists to change management strategy based on predicted sensitivity or resistance to therapy, so-called clinical utility. Thus far, clinical utility has proved elusive, though liquid biopsies using ctDNA to evaluate tumor genotype have come closest.
The Cobas EGFR Mutation Test v2 recently became the first ctDNA-based liquid biopsy to receive regulatory approval. It was approved as a companion diagnostic to identify patients with advanced non–small-cell lung cancer (NSCLC) who have specific mutations in the epidermal growth factor receptor (EGFR) gene and are therefore eligible for treatment with the EGFR inhibitor erlotinib.13
Approval was based on comparison of EGFR mutation identification rates using plasma ctDNA samples and tumor tissue samples from patients enrolled in the phase 3 ENSURE trial, which compared the efficacy of erlotinib with chemotherapy as first-line therapy in patients with advanced NSCLC. Of the 217 patients enrolled in the trial, 98.6% of patients had both tumor biopsy and plasma ctDNA samples available for testing. Concordance between the two types of biopsy in identifying patients with EGFR mutations was high and patients with EGFR positivity according to liquid biopsy results demonstrated improved progression-free survival when treated with erlotinib.14
The results of a large-scale genomic analysis of various different types of tumors using ctDNA were also recently presented at the 2016 American Society of Clinical Oncology meeting. Blood samples from more than 15,000 patients with 50 different tumor types, including advanced lung cancer (37%), breast cancer (14%), and CRC (10%), were collected and compared with either available tumor biopsy samples from the same cases (n = 398) or, in the majority of cases, with The Cancer Genome Atlas database, which uses tumor biopsies to perform genome-wide sequencing studies. Both types of biopsy revealed very similar mutation patterns when the Guardant360 next-generation sequencing test, which targets 70 genes, was applied. In particular, when EGFR, BRAF, KRAS, ALK, RET, and ROS1 mutations were identified by tumor tissue biopsy, the same mutations were reported in 94%-100% of plasma samples.15
Studies of the clinical utility of ctDNA and CTCs are among ongoing clinical trials of liquid biopsies (Tables 2 and 3). The potential for using CTCs to guide treatment decisions has become particularly relevant in breast cancer in light of results showing that patients with primary tumors that are negative for human epidermal growth factor receptor 2 (HER2) amplification, an important biomarker in breast cancer, may have CTCs that are HER2-positive, in up to 30% of cases. These patients may therefore still benefit from HER2-targeted therapy.16
The DETECT studies are the first phase 3 trials in which treatment decisions are being based on the phenotypic characteristics of CTCs. DETECT III (NCT01619111) is comparing lapatinib in combination with standard therapy with standard therapy alone in patients with HER2-negative metastatic breast cancer who have HER2-positive CTCs, whereas DETECT IV (NCT02035813) is enrolling patients with HER2-negative, hormone receptor-positive metastatic breast cancer and persistent HER2-negative CTCs to receive standard endocrine therapy and the mammalian target of rapamycin inhibitor everolimus.
Other targets and sources for liquid biopsy
Another approach to liquid biopsies that is also beginning to take off is to collect tumor-derived exosomes from the bloodstream. Exosomes are tiny, fluid-filled, membrane-bound sacks that bud off from the surface of a cell to expel waste or to transport cargo from one cell to another. DNA, RNA, and protein can be extracted from tumor-derived exosomes and could also serve as molecular biomarkers relating to the cancer cells from which they came.6,7
Exosome Diagnostics is bringing the first exosome-based diagnostic tests to the market and recently teamed up with Amgen for the development of these liquid biopsies.17 In January 2016, they launched ExoDx Lung (ALK), for detection of EML4-ALK gene fusions in patients with NSCLC, using a proprietary platform for the isolation of RNA from exosomes. Data that was presented at several different conferences in 2015 demonstrated a sensitivity of 88% and specificity of 100% for this diagnostic when compared with tissue ALK status in NSCLC patients receiving a second-generation ALK inhibitor following progression on prior ALK inhibitor therapy.18
In September, they subsequently announced the launch of a test that analyses genetic information from exosomes collected from a urine sample taken from prostate cancer patients. Using a 3-gene signature, in combination with a proprietary algorithm, this diagnostic generates a score assessing a prostate cancer patient’s risk for higher grade, more aggressive disease. It is designed to complement the prostate-specific antigen score and has demonstrated accuracy in ruling out the presence of high-grade cancer before an initial biopsy in more than 1,
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
1. Lennon NK, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112.
2. MIT Technology Review website. Liquid biopsy: fast DNA-sequencing machines are leading to simple blood tests for cancer. https://www.technologyreview.com/s/534991/liquid-biopsy/. Published 2015. Accessed December 19, 2016.
3. Alix-Panabières C and Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479-491.
4. Calabuig-Farinãs S, Jantus-Lewintre E, Herreros-Pomares A, Camps C. Circulating tumor cells versus circulating tumor DNA in lung cancer – which one will win? Transl Lung Cancer Res. 2016;5(5):466-482.
5. Karabacak, NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710.
6. Buder A, Tomuta C, and Filipits M. The potential of liquid biopsies. Curr Opin Oncol. 2016;28:130-134.
7. Hofman P, Popper HH. Pathologists and liquid biopsies: to be or not to be? Virchows Arch. 2016;469:601-609.
8. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumor cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406-414.
9. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302-6309.
10. Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213-3221.
11. CellSearch Web site. What is the CELLSEARCH® System? https://www.cellsearchctc.com/product-systems-overview/cellsearch-system-overview. Last updated December 5th, 2016. Accessed online December 19th, 2016.
12. CellSearch Web site [advertisement]. https://www.cellsearchctc.com/clinical-applications/clinical-applications-overview. Last updated December 5, 2016. Accessed December 19, 2016.
13. US Food and Drug Administration. cobas EGFR Mutation Test v2 – P150047. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm519922.htm. Last updated September 9, 2016. Accessed December 19, 2016.
14. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883-1889.
15. Zill OA, Mortimer S, Banks KC, et al. Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl;abstr LBA11501).
16. Jordan NV, Bardia A, Wittner BS, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102-106.
17. Exosome Diagnostics. Exosome diagnostics enters agreement with Amgen. http://www.exosomedx.com/news-events/press-releases/exosome-diagnostics-enters-agreement-amgen. Published October 3, 2016. Accessed December 19, 2016.
18. Brinkman K, Emenegger J, Tannous B, et al. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients [2015 World Conference on Lung Cancer, Denver, CO; abstract 2591]. http://library.iaslc.org/search-speaker?search_speaker=30493. Accessed January 6, 2017.
19. Exosome Diagnostics website. Prostate cancer. http://www.exosomedx.com/prostate-cancer-0. Last updated 2017. Accessed online December 19, 2016.
Unavoidable, random DNA replication errors are the most common cancer drivers
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Key clinical point:
Major finding: Two-thirds (66%) of cancer drivers are replication errors, 29% are environmentally induced, and 5% are hereditary.
Data source: The researchers examined cancer mutation drivers in two cohorts that spanned 69 countries.
Disclosures: Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
Point of prostate cancer diagnosis experiences and needs of black men: the Florida CaPCaS study
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
No benefit from adjuvant sunitinib or sorafenib for clear cell renal cancer
Adjuvant sunitinib or sorafenib show no significant advantages in disease-free or overall survival over placebo in patients with high-risk clear cell renal cancer, according to secondary analysis of data from the ASSURE trial.
The primary analysis of data from the ASSURE trial, which included patients with all types of renal cell carcinoma, had failed to show a benefit in disease-free survival.
“Given recently published results of a 750-patient randomized trial, S-TRAC, (sunitinib 50 mg daily [4/2 schedule] vs placebo in clear cell predominant pT3-4 or node-positive disease) that show improved [disease-free survival], the appropriate adjuvant strategy for high-risk patients is unclear,” Naomi B. Haas, MD, and coauthors wrote (JAMA Oncol. 2017 Mar 9. doi: 10.1001/jamaoncol.2017.0076).
Therefore, the investigators focused on a subset of patients from the ASSURE trial with high-risk clear cell renal cancer to determine if there might be a benefit in this group.
The secondary analysis involved 1,069 participants with pT3 and higher or node-positive renal cancer with clear cell histology who were randomized to receive 54 weeks of sunitinib (50mg, oral daily for 28 of 42 days per cycle), sorafenib (400 mg, oral twice daily continuously), or placebo.
The 5-year disease-free survival rate was 47.7% for patients in the sunitinib arm, 49.9% for those taking sorafenib, and 50% for placebo, with no statistically significant difference between the three groups. The 5-year overall survival rate was 75.2% for the sunitinib arm, 80.2% in the sorafenib arm, and 76.5% for those on placebo, with no statistically significant differences between the groups, reported Dr. Haas of the Abramson Cancer Center of the University of Pennsylvania, Philadelphia, and colleagues.
“This high-risk population had a better 5-year recurrence-free rate (around 50%) than expected (41.9% for high-risk disease and 36.0% for node-positive disease), possibly a result of better surgical technique, more accurate staging, or unknown biologic factors,” the authors wrote.
When the researchers analyzed disease-free survival according to quartiles of total dose per 6-week cycle, they also found no differences between each quartile of average dose per cycle.
There was, however, a significantly higher rate of grade 3 or higher adverse events in the sunitinib arm (66%) and sorafenib group (72%), compared with placebo (22%).
“Based on this analysis, a rationale for adjuvant therapy in this high-risk population is not elucidated,” Dr. Haas and colleagues said.
The study was coordinated by the ECOG-ACRIN Cancer Research Group and supported by Public Health Service grants, the National Cancer Institute, National Institutes of Health, and the Department of Health & Human Services. The drugs and placebos were provided by Bayer and Pfizer through the National Cancer Institute.
Adjuvant sunitinib or sorafenib show no significant advantages in disease-free or overall survival over placebo in patients with high-risk clear cell renal cancer, according to secondary analysis of data from the ASSURE trial.
The primary analysis of data from the ASSURE trial, which included patients with all types of renal cell carcinoma, had failed to show a benefit in disease-free survival.
“Given recently published results of a 750-patient randomized trial, S-TRAC, (sunitinib 50 mg daily [4/2 schedule] vs placebo in clear cell predominant pT3-4 or node-positive disease) that show improved [disease-free survival], the appropriate adjuvant strategy for high-risk patients is unclear,” Naomi B. Haas, MD, and coauthors wrote (JAMA Oncol. 2017 Mar 9. doi: 10.1001/jamaoncol.2017.0076).
Therefore, the investigators focused on a subset of patients from the ASSURE trial with high-risk clear cell renal cancer to determine if there might be a benefit in this group.
The secondary analysis involved 1,069 participants with pT3 and higher or node-positive renal cancer with clear cell histology who were randomized to receive 54 weeks of sunitinib (50mg, oral daily for 28 of 42 days per cycle), sorafenib (400 mg, oral twice daily continuously), or placebo.
The 5-year disease-free survival rate was 47.7% for patients in the sunitinib arm, 49.9% for those taking sorafenib, and 50% for placebo, with no statistically significant difference between the three groups. The 5-year overall survival rate was 75.2% for the sunitinib arm, 80.2% in the sorafenib arm, and 76.5% for those on placebo, with no statistically significant differences between the groups, reported Dr. Haas of the Abramson Cancer Center of the University of Pennsylvania, Philadelphia, and colleagues.
“This high-risk population had a better 5-year recurrence-free rate (around 50%) than expected (41.9% for high-risk disease and 36.0% for node-positive disease), possibly a result of better surgical technique, more accurate staging, or unknown biologic factors,” the authors wrote.
When the researchers analyzed disease-free survival according to quartiles of total dose per 6-week cycle, they also found no differences between each quartile of average dose per cycle.
There was, however, a significantly higher rate of grade 3 or higher adverse events in the sunitinib arm (66%) and sorafenib group (72%), compared with placebo (22%).
“Based on this analysis, a rationale for adjuvant therapy in this high-risk population is not elucidated,” Dr. Haas and colleagues said.
The study was coordinated by the ECOG-ACRIN Cancer Research Group and supported by Public Health Service grants, the National Cancer Institute, National Institutes of Health, and the Department of Health & Human Services. The drugs and placebos were provided by Bayer and Pfizer through the National Cancer Institute.
Adjuvant sunitinib or sorafenib show no significant advantages in disease-free or overall survival over placebo in patients with high-risk clear cell renal cancer, according to secondary analysis of data from the ASSURE trial.
The primary analysis of data from the ASSURE trial, which included patients with all types of renal cell carcinoma, had failed to show a benefit in disease-free survival.
“Given recently published results of a 750-patient randomized trial, S-TRAC, (sunitinib 50 mg daily [4/2 schedule] vs placebo in clear cell predominant pT3-4 or node-positive disease) that show improved [disease-free survival], the appropriate adjuvant strategy for high-risk patients is unclear,” Naomi B. Haas, MD, and coauthors wrote (JAMA Oncol. 2017 Mar 9. doi: 10.1001/jamaoncol.2017.0076).
Therefore, the investigators focused on a subset of patients from the ASSURE trial with high-risk clear cell renal cancer to determine if there might be a benefit in this group.
The secondary analysis involved 1,069 participants with pT3 and higher or node-positive renal cancer with clear cell histology who were randomized to receive 54 weeks of sunitinib (50mg, oral daily for 28 of 42 days per cycle), sorafenib (400 mg, oral twice daily continuously), or placebo.
The 5-year disease-free survival rate was 47.7% for patients in the sunitinib arm, 49.9% for those taking sorafenib, and 50% for placebo, with no statistically significant difference between the three groups. The 5-year overall survival rate was 75.2% for the sunitinib arm, 80.2% in the sorafenib arm, and 76.5% for those on placebo, with no statistically significant differences between the groups, reported Dr. Haas of the Abramson Cancer Center of the University of Pennsylvania, Philadelphia, and colleagues.
“This high-risk population had a better 5-year recurrence-free rate (around 50%) than expected (41.9% for high-risk disease and 36.0% for node-positive disease), possibly a result of better surgical technique, more accurate staging, or unknown biologic factors,” the authors wrote.
When the researchers analyzed disease-free survival according to quartiles of total dose per 6-week cycle, they also found no differences between each quartile of average dose per cycle.
There was, however, a significantly higher rate of grade 3 or higher adverse events in the sunitinib arm (66%) and sorafenib group (72%), compared with placebo (22%).
“Based on this analysis, a rationale for adjuvant therapy in this high-risk population is not elucidated,” Dr. Haas and colleagues said.
The study was coordinated by the ECOG-ACRIN Cancer Research Group and supported by Public Health Service grants, the National Cancer Institute, National Institutes of Health, and the Department of Health & Human Services. The drugs and placebos were provided by Bayer and Pfizer through the National Cancer Institute.
FROM JAMA ONCOLOGY
Key clinical point: Adjuvant sunitinib or sorafenib show no significant benefit in disease-free or overall survival in patients with high-risk clear cell renal cancer.
Major finding: The 5-year disease-free survival rate was 47.7% for patients treated with sunitinib, 49.9% for those treated with sorafenib, and 50% for those given placebo.
Data source: Secondary analysis of data from the ASSURE trial in 1,943 patients with pT3 and higher or node-positive renal cancer with clear cell histology.
Disclosures: The study was coordinated by the ECOG-ACRIN Cancer Research Group and supported by Public Health Service grants, the National Cancer Institute, National Institutes of Health, and the Department of Health & Human Services. The drugs and placebos were provided by Bayer and Pfizer through the National Cancer Institute.
Outcomes of neoadjuvant therapy vary by subtype in bladder cancer
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer, investigators report.
Researchers classified the subtypes in 223 patients with muscle-invasive bladder cancer (luminal, basal, claudin-low, and luminal-infiltrated) and found response to neoadjuvant cisplatin-based chemotherapy varied by subtype (P = .0001).
“Neoadjuvant chemotherapy improves outcomes in muscle-invasive bladder cancer, but only at 5%-7% for overall survival at 5 years,” study author Roland Seiler, MD, of the University of British Columbia, Vancouver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “About 60% of patients still have invasive disease at cystectomy so they are therefore nonresponders, but they still suffer from unnecessary side effects.”
Dr. Seiler explained that molecular subtypes of muscle-invasive bladder cancers had been identified that were based on gene expression. They decided to investigate the impact of different subtyping methods on patient response to NAC with the goal of developing a single sample model for subtyping.
A transcriptome-wide microarray analysis was conducted, using bladder tumor transurethral resection specimens that were obtained from 223 patients prior to receipt of NAC and cystectomy. The specimens were then classified according to four published methods for molecular subtype: University of North Carolina (UNC) dataset, MD Anderson (MDA) dataset, The Cancer Genome Atlas (TCGA) dataset, and the Lund dataset.
A validation set of 82 pre-NAC specimens also was done, with the results compared to non-NAC cohorts in the public domain including 179 cases from TCGA dataset, 107 from the MDA dataset, and 190 from the Lund dataset.
Finally, a genomic classifier was trained to predict the different subtypes in a single sample model and was then validated in both independent NAC and non-NAC datasets.
Investigators found that patients with luminal tumors had the best overall survival and it was independent of NAC. Patients with tumors classified as UNC basal, MDA basal, and TCGA cluster III achieved the greatest improvement in overall survival following NAC as compared with cystectomy alone.
Patients with tumors that were classified as UNC basal, MDA basal and TCGA cluster III achieved the greatest survival benefit following NAC, compared to surgery alone. Tumors that were assigned as UNC claudin-low had the worst overall survival regardless of the type of treatment regimen (P = .005).
“Basal tumors showed the most improvement with neoadjuvant therapy and should be prioritized with this treatment,” said Dr. Seiler. “Whereas for other subtypes we may need novel therapies.”
As discussant for the presentation, Jonathan Rosenberg, MD, of Memorial Sloan Kettering Cancer Center in New York, said that cisplatin “is a toxic therapy and we would love to have a great biomarker to select the right group of patients. Currently using present data, anywhere from 10 to 20 patients need to be treated to save one life.”
He reiterated that these findings confirm that basal tumors appear to benefit significantly from platinum-based chemotherapy and this benefit appears to exist regardless of downstaging.
“However it does not automatically follow that other subtypes do not benefit, and we are not ready to change clinical practice just yet,” Dr. Rosenberg said. “We need to prospectively validate these findings before we implement them into clinical practice.”
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Clinical outcomes for neoadjuvant chemotherapy (NAC) vary by molecular subtype in muscle-invasive bladder cancer.
Major finding: Luminal tumors had the best overall survival independent of NAC and basal tumors the best response to NAC.
Data source: Experimental study that evaluated the efficacy of NAC in the molecular subtypes of muscle invasive bladder cancer.
Disclosures: The funding source is not disclosed. Dr Seiler has no disclosures. Several of his coauthors report relationships with multiple pharmaceutical companies. Dr Rosenberg reports financial ties to multiple pharmaceutical companies.
Chemo gives no boost to ADT for patients with localized prostate cancer
Mitoxantrone plus prednisone (MP) added to androgen deprivation therapy (ADT) does not improve outcomes in patients with clinically localized prostate cancer, according to the results of a large long-term multicenter clinical trial.
At a follow-up time of almost 11 years, outcomes were nearly the same whether patients had received chemotherapy and ADT or just ADT.
Overall survival was 87% in the cohort that received ADT only and 86% in the study arm that received ADT plus MP (HR 1.05 (CI 0.78, 1.42), P = .74). Recurrence-free survival was 84% in both groups (HR 0.98 (CI 0.78, 1.23), P = .83).
Disease free survival was 72% in both arms, and there was no statistical difference. Similarly, death without recurrence were also similar in both arms.
“Survival was greater than anticipated in both arms,” lead author L. Michael Glode, MD, of the University of Colorado, Denver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “There is no evidence that MP improves prostate cancer specific survival when added to 2 years of adjuvant ADT.”
There were 85 deaths in the ADT arm compared to 91 in the chemotherapy arm. Prostate cancer accounted for 18% of deaths among the patients receiving ADT only and 22% in the chemotherapy arm, but the incidence of other cancers was twice as common in the chemotherapy arm as in the ADT only arm.
“The predominant cancers were GI and lung,” said Dr. Glode, “And noncancer deaths were variable.”
Of note, MP increased the incidence of leukemia (one case in the ADT group vs. five in the chemotherapy group).
“This trial demonstrates the feasibility of doing adjuvant trials in prostate cancer post radical prostatectomy,” said Dr. Glode. “Survival was greater than anticipated in both arms.”
The assumptions of this trial were that 2 years of adjuvant ADT would improve overall survival and progression-free survival although definitive data were unavailable.
The rationale for doing a study using adjuvant therapy was based on published literature, in which findings had showed that while short-term neoadjuvant ADT prior to prostatectomy reduced positive margins, it had no effect on disease-free survival. In addition, research had shown that longer-term ADT improved outcomes for patients undergoing curative radiation therapy.
For chemotherapy, Dr. Glode pointed to data showing that adjuvant chemotherapy improved progression-free survival in patients who had undergone both prostatectomy and radiation therapy.
“We hypothesized that the addition of modestly active chemotherapy earlier in disease might improve overall survival and progression-free survival,” he said.
The primary objective was overall survival and the secondary endpoint was disease-free survival.
The S9921 trial enrolled 983 patients from October 1999 to January 2007 with clinically localized prostate cancer, before enrollment ceased because of the increased incidence of leukemia in the ADT plus MP arm.
Of this group, 22 patients were ineligible, and the remaining patients were assigned to goserelin acetate 10.8 mg plus bicalutamide 50 mg (n = 481) or the same ADT plus MP (n = 480).
The patients were stratified by stage (≤pT2, ≥pT3, N0 or N+), Gleason score, and intent to receive adjuvant radiation, and the presurgical PSA was 7.6 ng/mL. Radiation therapy was allowed in both arms at physician discretion, and 26% intended to receive radiation therapy.
In the ADT only arm, 402 completed the treatment, and 390 completed treatment in the ADT plus chemotherapy arm.
Grade 3 or higher adverse events were more common in the chemotherapy group (56%/30%, P less than .0001). “As for toxicities, the main difference was the presence of leukopenia in the chemotherapy arm,” said Dr. Glode.
The study was funded by Southwest Oncology Group’s Urologic Cancer Outreach Program, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B, Clinical Trials Support Unit, and National Cancer Institute. None of the authors had disclosures.
Mitoxantrone plus prednisone (MP) added to androgen deprivation therapy (ADT) does not improve outcomes in patients with clinically localized prostate cancer, according to the results of a large long-term multicenter clinical trial.
At a follow-up time of almost 11 years, outcomes were nearly the same whether patients had received chemotherapy and ADT or just ADT.
Overall survival was 87% in the cohort that received ADT only and 86% in the study arm that received ADT plus MP (HR 1.05 (CI 0.78, 1.42), P = .74). Recurrence-free survival was 84% in both groups (HR 0.98 (CI 0.78, 1.23), P = .83).
Disease free survival was 72% in both arms, and there was no statistical difference. Similarly, death without recurrence were also similar in both arms.
“Survival was greater than anticipated in both arms,” lead author L. Michael Glode, MD, of the University of Colorado, Denver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “There is no evidence that MP improves prostate cancer specific survival when added to 2 years of adjuvant ADT.”
There were 85 deaths in the ADT arm compared to 91 in the chemotherapy arm. Prostate cancer accounted for 18% of deaths among the patients receiving ADT only and 22% in the chemotherapy arm, but the incidence of other cancers was twice as common in the chemotherapy arm as in the ADT only arm.
“The predominant cancers were GI and lung,” said Dr. Glode, “And noncancer deaths were variable.”
Of note, MP increased the incidence of leukemia (one case in the ADT group vs. five in the chemotherapy group).
“This trial demonstrates the feasibility of doing adjuvant trials in prostate cancer post radical prostatectomy,” said Dr. Glode. “Survival was greater than anticipated in both arms.”
The assumptions of this trial were that 2 years of adjuvant ADT would improve overall survival and progression-free survival although definitive data were unavailable.
The rationale for doing a study using adjuvant therapy was based on published literature, in which findings had showed that while short-term neoadjuvant ADT prior to prostatectomy reduced positive margins, it had no effect on disease-free survival. In addition, research had shown that longer-term ADT improved outcomes for patients undergoing curative radiation therapy.
For chemotherapy, Dr. Glode pointed to data showing that adjuvant chemotherapy improved progression-free survival in patients who had undergone both prostatectomy and radiation therapy.
“We hypothesized that the addition of modestly active chemotherapy earlier in disease might improve overall survival and progression-free survival,” he said.
The primary objective was overall survival and the secondary endpoint was disease-free survival.
The S9921 trial enrolled 983 patients from October 1999 to January 2007 with clinically localized prostate cancer, before enrollment ceased because of the increased incidence of leukemia in the ADT plus MP arm.
Of this group, 22 patients were ineligible, and the remaining patients were assigned to goserelin acetate 10.8 mg plus bicalutamide 50 mg (n = 481) or the same ADT plus MP (n = 480).
The patients were stratified by stage (≤pT2, ≥pT3, N0 or N+), Gleason score, and intent to receive adjuvant radiation, and the presurgical PSA was 7.6 ng/mL. Radiation therapy was allowed in both arms at physician discretion, and 26% intended to receive radiation therapy.
In the ADT only arm, 402 completed the treatment, and 390 completed treatment in the ADT plus chemotherapy arm.
Grade 3 or higher adverse events were more common in the chemotherapy group (56%/30%, P less than .0001). “As for toxicities, the main difference was the presence of leukopenia in the chemotherapy arm,” said Dr. Glode.
The study was funded by Southwest Oncology Group’s Urologic Cancer Outreach Program, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B, Clinical Trials Support Unit, and National Cancer Institute. None of the authors had disclosures.
Mitoxantrone plus prednisone (MP) added to androgen deprivation therapy (ADT) does not improve outcomes in patients with clinically localized prostate cancer, according to the results of a large long-term multicenter clinical trial.
At a follow-up time of almost 11 years, outcomes were nearly the same whether patients had received chemotherapy and ADT or just ADT.
Overall survival was 87% in the cohort that received ADT only and 86% in the study arm that received ADT plus MP (HR 1.05 (CI 0.78, 1.42), P = .74). Recurrence-free survival was 84% in both groups (HR 0.98 (CI 0.78, 1.23), P = .83).
Disease free survival was 72% in both arms, and there was no statistical difference. Similarly, death without recurrence were also similar in both arms.
“Survival was greater than anticipated in both arms,” lead author L. Michael Glode, MD, of the University of Colorado, Denver, said in a press briefing held at the 2017 genitourinary cancers symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology. “There is no evidence that MP improves prostate cancer specific survival when added to 2 years of adjuvant ADT.”
There were 85 deaths in the ADT arm compared to 91 in the chemotherapy arm. Prostate cancer accounted for 18% of deaths among the patients receiving ADT only and 22% in the chemotherapy arm, but the incidence of other cancers was twice as common in the chemotherapy arm as in the ADT only arm.
“The predominant cancers were GI and lung,” said Dr. Glode, “And noncancer deaths were variable.”
Of note, MP increased the incidence of leukemia (one case in the ADT group vs. five in the chemotherapy group).
“This trial demonstrates the feasibility of doing adjuvant trials in prostate cancer post radical prostatectomy,” said Dr. Glode. “Survival was greater than anticipated in both arms.”
The assumptions of this trial were that 2 years of adjuvant ADT would improve overall survival and progression-free survival although definitive data were unavailable.
The rationale for doing a study using adjuvant therapy was based on published literature, in which findings had showed that while short-term neoadjuvant ADT prior to prostatectomy reduced positive margins, it had no effect on disease-free survival. In addition, research had shown that longer-term ADT improved outcomes for patients undergoing curative radiation therapy.
For chemotherapy, Dr. Glode pointed to data showing that adjuvant chemotherapy improved progression-free survival in patients who had undergone both prostatectomy and radiation therapy.
“We hypothesized that the addition of modestly active chemotherapy earlier in disease might improve overall survival and progression-free survival,” he said.
The primary objective was overall survival and the secondary endpoint was disease-free survival.
The S9921 trial enrolled 983 patients from October 1999 to January 2007 with clinically localized prostate cancer, before enrollment ceased because of the increased incidence of leukemia in the ADT plus MP arm.
Of this group, 22 patients were ineligible, and the remaining patients were assigned to goserelin acetate 10.8 mg plus bicalutamide 50 mg (n = 481) or the same ADT plus MP (n = 480).
The patients were stratified by stage (≤pT2, ≥pT3, N0 or N+), Gleason score, and intent to receive adjuvant radiation, and the presurgical PSA was 7.6 ng/mL. Radiation therapy was allowed in both arms at physician discretion, and 26% intended to receive radiation therapy.
In the ADT only arm, 402 completed the treatment, and 390 completed treatment in the ADT plus chemotherapy arm.
Grade 3 or higher adverse events were more common in the chemotherapy group (56%/30%, P less than .0001). “As for toxicities, the main difference was the presence of leukopenia in the chemotherapy arm,” said Dr. Glode.
The study was funded by Southwest Oncology Group’s Urologic Cancer Outreach Program, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B, Clinical Trials Support Unit, and National Cancer Institute. None of the authors had disclosures.
FROM THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: Chemotherapy added to adjuvant androgen deprivation therapy in patients with clinically localized prostate cancer does not improve outcomes.
Major finding: Overall survival was the same in both arms of the study: 87% in the cohort that received ADT only and 86% for those receiving chemotherapy (1.05 [0.78, 1.42], P = .74).
Data source: A phase III randomized trial of 983 patients to determine the utility of adding chemotherapy to adjuvant ADT.
Disclosures: The study was funded by Southwest Oncology Group’s Urologic Cancer Outreach Program, Eastern Cooperative Oncology Group, Cancer and Leukemia Group B, Clinical Trials Support Unit, and National Cancer Institute. None of the authors had disclosures.
Genomic differences seen in mRCC during first- and second-line therapy
ORLANDO – In the largest assessment to date of circulating tumor DNA (ctDNA) in patients with metastatic renal cell carcinoma (mRCC), the majority of patients were found to have clinically relevant genomic alterations.
The most frequently occurring alterations for the entire cohort were TP53, VHL, NF1, EGFR, and ARID1A, but, importantly, the genetic profiles differed between patients receiving first-line therapy and those receiving second-line treatments.
“Compared to patients receiving first-line therapy, patients receiving post–first-line agents had increased genomic alterations in TP53, NF1, EGFR, and PIK3CA,” said lead study author Sumanta K. Pal, MD, a urologic oncologist at City of Hope, Duarte, Calif.
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal, who presented the findings of his study in a press briefing held at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology..
Several targeted therapies have been approved for mRCC, including vascular endothelial growth factor–targeted therapies, mammalian target of rapamycin inhibitors, and checkpoint inhibitors. However, treatment of mRCC is generally distinctly different for the first- and second-line settings.
Dr. Pal noted that, while “efforts such as the TCGA [The Cancer Genome Atlas] have shed some light on the tumor biology, it is important to keep in mind that these datasets reflect earlier stages of disease. Certainly, there may be an evolution of tumor biology as patients progress toward metastasis.”
Circulating tumor markers represent a practical means of serially assessing tumor biology, and ctDNA can account for tumor heterogeneity. In this study, the authors sought to determine the mutational landscape of mRCC as well as to assess changes across patients receiving first-line and subsequent therapies by using ctDNA.
Data were obtained from 224 patients who received ctDNA profiling at progression as part of routine clinical care using Guardant360, a CLIA-certified comprehensive plasma assay that evaluated 70 genes. Of this group, 64 and 56 patients were coded as receiving frontline and post–first-line agents, respectively.
Genomic alterations were pooled for the entire group, and first and second (subsequent) therapies were compared, based on conventional practice patterns (first-line regimens included sunitinib, pazopanib and bevacizumab, and second line included everolimus, axitinib, cabozantinib, and nivolumab).
Genomic alterations were found in 78.6% of patients, with an average of 3.3 genomic alterations per patient. For patients receiving first-line therapy, the average number of ctDNA alterations was 2.9, compared with 3.7 for those in the cohort who were receiving second-line therapy. The median (range) ctDNA variant allele fractions were 0.23 (0.05-9.92) in the first-line group and 0.24 (0.04-47.14) in second line.
The authors observed that there were disparities in genomic alterations between both patient cohorts, with the highest disparity seen in (second vs. first line) TP53 (49% vs. 25%), VHL (29% vs. 25%), NF1 (20% vs. 15%), EGFR (17% vs. 21%), and PIK3CA (17% vs. 8%).
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal.
He also pointed out that there were significant differences between the current dataset and other published reports, which may reflect the advanced state of the disease of the patients in this study.
Efforts are also ongoing to add detailed data on demographics and clinical outcomes to the current dataset, Dr. Pal added.
Acting as the paper’s discussant, Primo N. Lara Jr., MD, of the University of California, Davis, Comprehensive Cancer Center pointed out that as there are no validated biomarkers of drug resistance or tumor evolution and that liquid biopsy offers a potential platform.
The use of ctDNA is a “convenient technology that offers new means to assess RCC biology,” said Dr. Lara, but the caveat is that it is “still in its infancy and has no immediate clinical application.”
The current study is “hypothesis generating only.” ctDNA changes need to be related to outcome following treatment, and the functional role of genomic alterations in RCC biology must be validated, he said.
ORLANDO – In the largest assessment to date of circulating tumor DNA (ctDNA) in patients with metastatic renal cell carcinoma (mRCC), the majority of patients were found to have clinically relevant genomic alterations.
The most frequently occurring alterations for the entire cohort were TP53, VHL, NF1, EGFR, and ARID1A, but, importantly, the genetic profiles differed between patients receiving first-line therapy and those receiving second-line treatments.
“Compared to patients receiving first-line therapy, patients receiving post–first-line agents had increased genomic alterations in TP53, NF1, EGFR, and PIK3CA,” said lead study author Sumanta K. Pal, MD, a urologic oncologist at City of Hope, Duarte, Calif.
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal, who presented the findings of his study in a press briefing held at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology..
Several targeted therapies have been approved for mRCC, including vascular endothelial growth factor–targeted therapies, mammalian target of rapamycin inhibitors, and checkpoint inhibitors. However, treatment of mRCC is generally distinctly different for the first- and second-line settings.
Dr. Pal noted that, while “efforts such as the TCGA [The Cancer Genome Atlas] have shed some light on the tumor biology, it is important to keep in mind that these datasets reflect earlier stages of disease. Certainly, there may be an evolution of tumor biology as patients progress toward metastasis.”
Circulating tumor markers represent a practical means of serially assessing tumor biology, and ctDNA can account for tumor heterogeneity. In this study, the authors sought to determine the mutational landscape of mRCC as well as to assess changes across patients receiving first-line and subsequent therapies by using ctDNA.
Data were obtained from 224 patients who received ctDNA profiling at progression as part of routine clinical care using Guardant360, a CLIA-certified comprehensive plasma assay that evaluated 70 genes. Of this group, 64 and 56 patients were coded as receiving frontline and post–first-line agents, respectively.
Genomic alterations were pooled for the entire group, and first and second (subsequent) therapies were compared, based on conventional practice patterns (first-line regimens included sunitinib, pazopanib and bevacizumab, and second line included everolimus, axitinib, cabozantinib, and nivolumab).
Genomic alterations were found in 78.6% of patients, with an average of 3.3 genomic alterations per patient. For patients receiving first-line therapy, the average number of ctDNA alterations was 2.9, compared with 3.7 for those in the cohort who were receiving second-line therapy. The median (range) ctDNA variant allele fractions were 0.23 (0.05-9.92) in the first-line group and 0.24 (0.04-47.14) in second line.
The authors observed that there were disparities in genomic alterations between both patient cohorts, with the highest disparity seen in (second vs. first line) TP53 (49% vs. 25%), VHL (29% vs. 25%), NF1 (20% vs. 15%), EGFR (17% vs. 21%), and PIK3CA (17% vs. 8%).
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal.
He also pointed out that there were significant differences between the current dataset and other published reports, which may reflect the advanced state of the disease of the patients in this study.
Efforts are also ongoing to add detailed data on demographics and clinical outcomes to the current dataset, Dr. Pal added.
Acting as the paper’s discussant, Primo N. Lara Jr., MD, of the University of California, Davis, Comprehensive Cancer Center pointed out that as there are no validated biomarkers of drug resistance or tumor evolution and that liquid biopsy offers a potential platform.
The use of ctDNA is a “convenient technology that offers new means to assess RCC biology,” said Dr. Lara, but the caveat is that it is “still in its infancy and has no immediate clinical application.”
The current study is “hypothesis generating only.” ctDNA changes need to be related to outcome following treatment, and the functional role of genomic alterations in RCC biology must be validated, he said.
ORLANDO – In the largest assessment to date of circulating tumor DNA (ctDNA) in patients with metastatic renal cell carcinoma (mRCC), the majority of patients were found to have clinically relevant genomic alterations.
The most frequently occurring alterations for the entire cohort were TP53, VHL, NF1, EGFR, and ARID1A, but, importantly, the genetic profiles differed between patients receiving first-line therapy and those receiving second-line treatments.
“Compared to patients receiving first-line therapy, patients receiving post–first-line agents had increased genomic alterations in TP53, NF1, EGFR, and PIK3CA,” said lead study author Sumanta K. Pal, MD, a urologic oncologist at City of Hope, Duarte, Calif.
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal, who presented the findings of his study in a press briefing held at the 2017 Genitourinary Cancers Symposium sponsored by the American Society of Clinical Oncology, ASTRO, and the Society of Urologic Oncology..
Several targeted therapies have been approved for mRCC, including vascular endothelial growth factor–targeted therapies, mammalian target of rapamycin inhibitors, and checkpoint inhibitors. However, treatment of mRCC is generally distinctly different for the first- and second-line settings.
Dr. Pal noted that, while “efforts such as the TCGA [The Cancer Genome Atlas] have shed some light on the tumor biology, it is important to keep in mind that these datasets reflect earlier stages of disease. Certainly, there may be an evolution of tumor biology as patients progress toward metastasis.”
Circulating tumor markers represent a practical means of serially assessing tumor biology, and ctDNA can account for tumor heterogeneity. In this study, the authors sought to determine the mutational landscape of mRCC as well as to assess changes across patients receiving first-line and subsequent therapies by using ctDNA.
Data were obtained from 224 patients who received ctDNA profiling at progression as part of routine clinical care using Guardant360, a CLIA-certified comprehensive plasma assay that evaluated 70 genes. Of this group, 64 and 56 patients were coded as receiving frontline and post–first-line agents, respectively.
Genomic alterations were pooled for the entire group, and first and second (subsequent) therapies were compared, based on conventional practice patterns (first-line regimens included sunitinib, pazopanib and bevacizumab, and second line included everolimus, axitinib, cabozantinib, and nivolumab).
Genomic alterations were found in 78.6% of patients, with an average of 3.3 genomic alterations per patient. For patients receiving first-line therapy, the average number of ctDNA alterations was 2.9, compared with 3.7 for those in the cohort who were receiving second-line therapy. The median (range) ctDNA variant allele fractions were 0.23 (0.05-9.92) in the first-line group and 0.24 (0.04-47.14) in second line.
The authors observed that there were disparities in genomic alterations between both patient cohorts, with the highest disparity seen in (second vs. first line) TP53 (49% vs. 25%), VHL (29% vs. 25%), NF1 (20% vs. 15%), EGFR (17% vs. 21%), and PIK3CA (17% vs. 8%).
“These alterations underscore potential mechanisms of resistance,” said Dr. Pal.
He also pointed out that there were significant differences between the current dataset and other published reports, which may reflect the advanced state of the disease of the patients in this study.
Efforts are also ongoing to add detailed data on demographics and clinical outcomes to the current dataset, Dr. Pal added.
Acting as the paper’s discussant, Primo N. Lara Jr., MD, of the University of California, Davis, Comprehensive Cancer Center pointed out that as there are no validated biomarkers of drug resistance or tumor evolution and that liquid biopsy offers a potential platform.
The use of ctDNA is a “convenient technology that offers new means to assess RCC biology,” said Dr. Lara, but the caveat is that it is “still in its infancy and has no immediate clinical application.”
The current study is “hypothesis generating only.” ctDNA changes need to be related to outcome following treatment, and the functional role of genomic alterations in RCC biology must be validated, he said.
AT THE GENITOURINARY CANCERS SYMPOSIUM
Key clinical point: The genetic profile of tumors in mRCC differed in patients receiving first-line and second-line therapies.
Major finding: Genomic alterations were identified in 78.6% of patients, with an average of 3.3 genomic alterations per patient.
Data source: Experimental study that used circulating tumor DNA to assess the mutational landscape of metastatic renal cell carcinoma.
Disclosures: The funding source is not disclosed. Dr. Pal and his coauthors all report relationships with multiple pharmaceutical companies. Dr. Lara reports financial ties to multiple pharmaceutical companies.