User login
Self-fitted and audiologist-fitted hearing aids equal for mild to moderate hearing loss
Self-fitted over-the-counter (OTC) hearing aids may be an effective option for individuals with mild to moderate hearing loss, a small randomized effectiveness trial reports. OTC devices yielded 6-week patient-perceived and clinical outcomes comparable to those with audiologist-fitted hearing aids, In fact, at week 2, the self-fitted group had a small but meaningful advantage on two of the four study outcome measures.
“After support and fine-tuning were provided to the self-fitting (remote support) and audiologist-fitted groups, no clinically meaningful differences were evident in any outcome measures at the end of the 6-week trial,” wrote researchers led by Karina C. De Sousa, PhD, a postdoctoral researcher in the department of speech-language pathology and audiology at the University of Pretoria, South Africa.
Their findings appear in JAMA Otolaryngology–Head & Neck Surgery.
Hearing aid uptake is low even in populations with adequate access to audiological resources, the authors noted, with hearing aid use in U.S. adults who could benefit estimated at about 20%. Currently, an estimated 22.9 million older Americans with audiometric hearing loss do not use hearing aids.
Major barriers have been access and affordability, Dr. De Sousa and associates wrote, and until recently, people with hearing loss could obtain hearing aids only after consultation with a credentialed dispenser. “The World Health Organization estimates that over 2.5 billion people will experience some degree of hearing loss by 2050,” Dr. De Sousa said in an interview. “This new category of self-fitting hearing aids opens up newer care pathways for people with mild to moderate hearing loss.”
The study
From April to August 2022 the trial recruited 68 participants (51.6% men) with mild-to-moderate self-reported hearing loss, a mean age of 63.6 years, and no ear disease within the past 90 days. They were randomized to a self-fitted commercially available device (Lexi Lumen), with instructional material on set-up and remote support, or to the same unit fitted by an audiologist. The majority in both arms were new users and were similar in age and baseline hearing scores.
The primary outcome measure was patient-reported hearing aid benefit, measured by the Abbreviated Profile of Hearing Aid Benefit (APHAB) questionnaire. This scale evaluates auditory acuity before and after amplification by such criteria as ease of communication, background noise, reverberation, aversiveness, and global hearing status.
Secondary measures included the International Outcome Inventory for Hearing Aids (IOI-HA) and speech recognition in noise measured using an abbreviated speech-in-noise test and a digits-in-noise test. Measures were taken at baseline, week 2, and week 6 after fitting. After the 2-week field trial, the self-fitting arm had an initial advantage on the self-reported APHAB: difference, Cohen d = −.5 (95% confidence interval [CI], −1.0 to 0). It also fared better on the IOI-HA: effect size, r = 0.3 (95% CI, .0 to –.5), but not on speech recognition in noise.
One member of the self-fitting arm withdrew owing to an unrelated middle-ear infection.
“While these results are promising, it is essential to note that OTC hearing aids are not a one-size-fits-all approach,” Dr. De Sousa said. “If a person has ear disease symptoms or hearing loss that is too severe, they have to consult a trained hearing health care professional.” She added that proper use of a self-fitted OTC hearing aid requires a degree of digital proficiency, as many devices are set up using a smartphone.
This study was supported by the National Institutes of Health and by the hearX Group, which provided the Lexie Lumen devices and software support for data collection. Dr. De Sousa reported nonfinancial support from hearX as well as consulting fees from hearX outside of the submitted work. A coauthor reported grant support from the UK’s National Institute for Health and Care Research Manchester Biomedical Research Centre, and fees from hearX during and outside of the study. Another coauthor disclosed fees, equity, and grant support from hearX during the conduct of the study.
Self-fitted over-the-counter (OTC) hearing aids may be an effective option for individuals with mild to moderate hearing loss, a small randomized effectiveness trial reports. OTC devices yielded 6-week patient-perceived and clinical outcomes comparable to those with audiologist-fitted hearing aids, In fact, at week 2, the self-fitted group had a small but meaningful advantage on two of the four study outcome measures.
“After support and fine-tuning were provided to the self-fitting (remote support) and audiologist-fitted groups, no clinically meaningful differences were evident in any outcome measures at the end of the 6-week trial,” wrote researchers led by Karina C. De Sousa, PhD, a postdoctoral researcher in the department of speech-language pathology and audiology at the University of Pretoria, South Africa.
Their findings appear in JAMA Otolaryngology–Head & Neck Surgery.
Hearing aid uptake is low even in populations with adequate access to audiological resources, the authors noted, with hearing aid use in U.S. adults who could benefit estimated at about 20%. Currently, an estimated 22.9 million older Americans with audiometric hearing loss do not use hearing aids.
Major barriers have been access and affordability, Dr. De Sousa and associates wrote, and until recently, people with hearing loss could obtain hearing aids only after consultation with a credentialed dispenser. “The World Health Organization estimates that over 2.5 billion people will experience some degree of hearing loss by 2050,” Dr. De Sousa said in an interview. “This new category of self-fitting hearing aids opens up newer care pathways for people with mild to moderate hearing loss.”
The study
From April to August 2022 the trial recruited 68 participants (51.6% men) with mild-to-moderate self-reported hearing loss, a mean age of 63.6 years, and no ear disease within the past 90 days. They were randomized to a self-fitted commercially available device (Lexi Lumen), with instructional material on set-up and remote support, or to the same unit fitted by an audiologist. The majority in both arms were new users and were similar in age and baseline hearing scores.
The primary outcome measure was patient-reported hearing aid benefit, measured by the Abbreviated Profile of Hearing Aid Benefit (APHAB) questionnaire. This scale evaluates auditory acuity before and after amplification by such criteria as ease of communication, background noise, reverberation, aversiveness, and global hearing status.
Secondary measures included the International Outcome Inventory for Hearing Aids (IOI-HA) and speech recognition in noise measured using an abbreviated speech-in-noise test and a digits-in-noise test. Measures were taken at baseline, week 2, and week 6 after fitting. After the 2-week field trial, the self-fitting arm had an initial advantage on the self-reported APHAB: difference, Cohen d = −.5 (95% confidence interval [CI], −1.0 to 0). It also fared better on the IOI-HA: effect size, r = 0.3 (95% CI, .0 to –.5), but not on speech recognition in noise.
One member of the self-fitting arm withdrew owing to an unrelated middle-ear infection.
“While these results are promising, it is essential to note that OTC hearing aids are not a one-size-fits-all approach,” Dr. De Sousa said. “If a person has ear disease symptoms or hearing loss that is too severe, they have to consult a trained hearing health care professional.” She added that proper use of a self-fitted OTC hearing aid requires a degree of digital proficiency, as many devices are set up using a smartphone.
This study was supported by the National Institutes of Health and by the hearX Group, which provided the Lexie Lumen devices and software support for data collection. Dr. De Sousa reported nonfinancial support from hearX as well as consulting fees from hearX outside of the submitted work. A coauthor reported grant support from the UK’s National Institute for Health and Care Research Manchester Biomedical Research Centre, and fees from hearX during and outside of the study. Another coauthor disclosed fees, equity, and grant support from hearX during the conduct of the study.
Self-fitted over-the-counter (OTC) hearing aids may be an effective option for individuals with mild to moderate hearing loss, a small randomized effectiveness trial reports. OTC devices yielded 6-week patient-perceived and clinical outcomes comparable to those with audiologist-fitted hearing aids, In fact, at week 2, the self-fitted group had a small but meaningful advantage on two of the four study outcome measures.
“After support and fine-tuning were provided to the self-fitting (remote support) and audiologist-fitted groups, no clinically meaningful differences were evident in any outcome measures at the end of the 6-week trial,” wrote researchers led by Karina C. De Sousa, PhD, a postdoctoral researcher in the department of speech-language pathology and audiology at the University of Pretoria, South Africa.
Their findings appear in JAMA Otolaryngology–Head & Neck Surgery.
Hearing aid uptake is low even in populations with adequate access to audiological resources, the authors noted, with hearing aid use in U.S. adults who could benefit estimated at about 20%. Currently, an estimated 22.9 million older Americans with audiometric hearing loss do not use hearing aids.
Major barriers have been access and affordability, Dr. De Sousa and associates wrote, and until recently, people with hearing loss could obtain hearing aids only after consultation with a credentialed dispenser. “The World Health Organization estimates that over 2.5 billion people will experience some degree of hearing loss by 2050,” Dr. De Sousa said in an interview. “This new category of self-fitting hearing aids opens up newer care pathways for people with mild to moderate hearing loss.”
The study
From April to August 2022 the trial recruited 68 participants (51.6% men) with mild-to-moderate self-reported hearing loss, a mean age of 63.6 years, and no ear disease within the past 90 days. They were randomized to a self-fitted commercially available device (Lexi Lumen), with instructional material on set-up and remote support, or to the same unit fitted by an audiologist. The majority in both arms were new users and were similar in age and baseline hearing scores.
The primary outcome measure was patient-reported hearing aid benefit, measured by the Abbreviated Profile of Hearing Aid Benefit (APHAB) questionnaire. This scale evaluates auditory acuity before and after amplification by such criteria as ease of communication, background noise, reverberation, aversiveness, and global hearing status.
Secondary measures included the International Outcome Inventory for Hearing Aids (IOI-HA) and speech recognition in noise measured using an abbreviated speech-in-noise test and a digits-in-noise test. Measures were taken at baseline, week 2, and week 6 after fitting. After the 2-week field trial, the self-fitting arm had an initial advantage on the self-reported APHAB: difference, Cohen d = −.5 (95% confidence interval [CI], −1.0 to 0). It also fared better on the IOI-HA: effect size, r = 0.3 (95% CI, .0 to –.5), but not on speech recognition in noise.
One member of the self-fitting arm withdrew owing to an unrelated middle-ear infection.
“While these results are promising, it is essential to note that OTC hearing aids are not a one-size-fits-all approach,” Dr. De Sousa said. “If a person has ear disease symptoms or hearing loss that is too severe, they have to consult a trained hearing health care professional.” She added that proper use of a self-fitted OTC hearing aid requires a degree of digital proficiency, as many devices are set up using a smartphone.
This study was supported by the National Institutes of Health and by the hearX Group, which provided the Lexie Lumen devices and software support for data collection. Dr. De Sousa reported nonfinancial support from hearX as well as consulting fees from hearX outside of the submitted work. A coauthor reported grant support from the UK’s National Institute for Health and Care Research Manchester Biomedical Research Centre, and fees from hearX during and outside of the study. Another coauthor disclosed fees, equity, and grant support from hearX during the conduct of the study.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Urban green and blue spaces linked to less psychological distress
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
The findings of the study, which was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology, build on a growing understanding of the relationship between types and qualities of urban environments and dementia risk.
Adithya Vegaraju, a student at Washington State University, Spokane, led the study, which looked at data from the Washington State Behavioral Risk Factor Surveillance System to assess prevalence of serious psychological distress among 42,980 Washington state residents aged 65 and over.
The data, collected between 2011 and 2019, used a self-reported questionnaire to determine serious psychological distress, which is defined as a level of mental distress considered debilitating enough to warrant treatment.
Mr. Vegaraju and his coauthor Solmaz Amiri, DDes, also of Washington State University, used ZIP codes, along with U.S. census data, to approximate the urban adults’ proximity to green and blue spaces.
After controlling for potential confounders of age, sex, ethnicity, education, and marital status, the investigators found that people living within half a mile of green or blue spaces had a 17% lower risk of experiencing serious psychological distress, compared with people living farther from these spaces, the investigators said in a news release.
Implications for cognitive decline and dementia?
Psychological distress in adults has been linked in population-based longitudinal studies to later cognitive decline and dementia. One study in older adults found the risk of dementia to be more than 50% higher among adults aged 50-70 with persistent depression. Blue and green spaces have also been investigated in relation to neurodegenerative disease among older adults; a 2022 study looking at data from some 62 million Medicare beneficiaries found those living in areas with more vegetation saw lower risk of hospitalizations for Alzheimer’s disease and related dementias.
“Since we lack effective prevention methods or treatments for mild cognitive impairment and dementia, we need to get creative in how we look at these issues,” Dr. Amiri commented in a press statement about her and Mr. Vegaraju’s findings. “Our hope is that this study showing better mental health among people living close to parks and water will trigger other studies about how these benefits work and whether this proximity can help prevent or delay mild cognitive impairment and dementia.”
The investigators acknowledged that their findings were limited by reliance on a self-reported measure of psychological distress.
A bidirectional connection with depression and dementia
In a comment, Anjum Hajat, PhD, an epidemiologist at University of Washington School of Public Health in Seattle who has also studied the relationship between green space and dementia risk in older adults, noted some further apparent limitations of the new study, for which only an abstract was available at publication.
“It has been shown that people with depression are at higher risk for dementia, but the opposite is also true,” Dr. Hajat commented. “Those with dementia are more likely to develop depression. This bidirectionality makes this study abstract difficult to interpret since the study is based on cross-sectional data: Individuals are not followed over time to see which develops first, dementia or depression.”
Additionally, Dr. Hajat noted, the data used to determine proximity to green and blue spaces did not allow for the calculation of precise distances between subjects’ homes and these spaces.
Mr. Vegaraju and Dr. Amiri’s study had no outside support, and the investigators declared no conflicts of interest. Dr. Hajat declared no conflicts of interest.
FROM AAN 2023
New Medicare rule streamlines prior authorization in Medicare Advantage plans
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
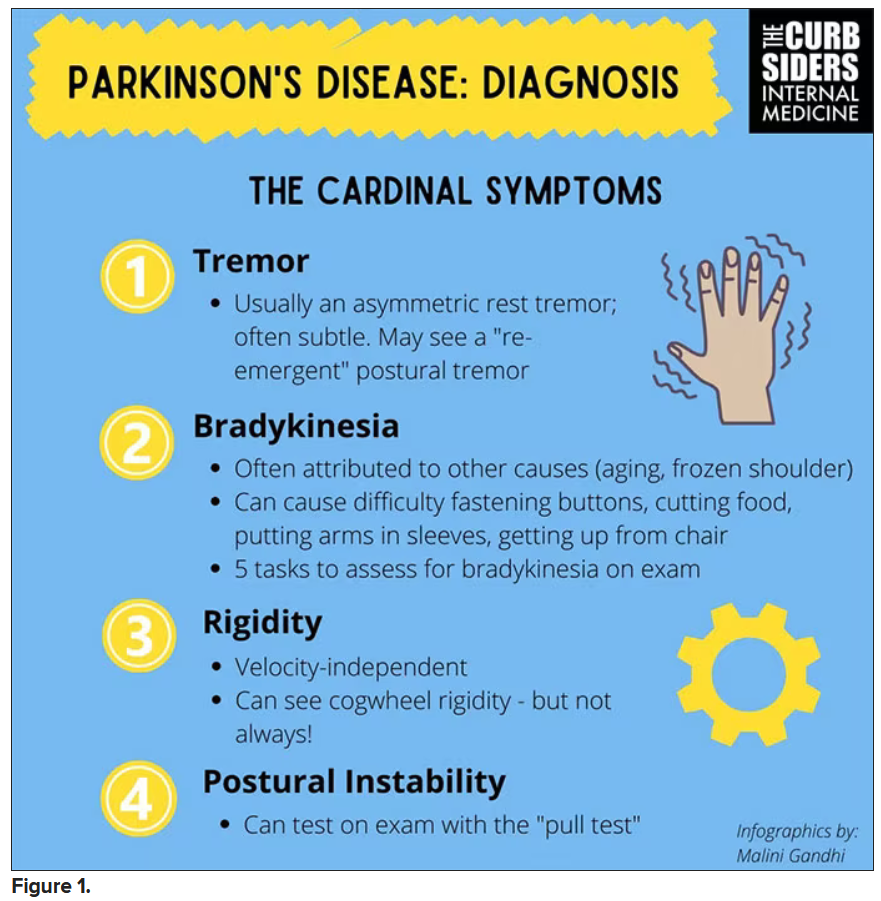
Picking up the premotor symptoms of Parkinson’s
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
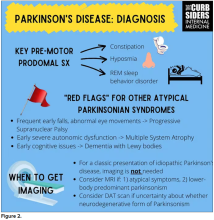
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
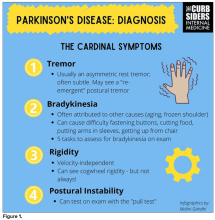
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
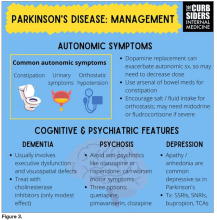
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Parkinson’s disease: What’s trauma got to do with it?
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Kathrin LaFaver, MD: Hello. I’m happy to talk today to Dr. Indu Subramanian, clinical professor at University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education and Clinical Center in Los Angeles. I am a neurologist in Saratoga Springs, New York, and we will be talking today about Indu’s new paper on childhood trauma and Parkinson’s disease. Welcome and thanks for taking the time.
Indu Subramanian, MD: Thank you so much for letting us highlight this important topic.
Dr. LaFaver: There are many papers published every month on Parkinson’s disease, but this topic stands out because it’s not a thing that has been commonly looked at. What gave you the idea to study this?
Neurology behind other specialties
Dr. Subramanian: Kathrin, you and I have been looking at things that can inform us about our patients – the person who’s standing in front of us when they come in and we’re giving them this diagnosis. I think that so much of what we’ve done [in the past] is a cookie cutter approach to giving everybody the standard treatment. [We’ve been assuming that] It doesn’t matter if they’re a man or woman. It doesn’t matter if they’re a veteran. It doesn’t matter if they may be from a minoritized population.
We’ve also been interested in approaches that are outside the box, right? We have this integrative medicine and lifestyle medicine background. I’ve been going to those meetings and really been struck by the mounting evidence on the importance of things like early adverse childhood events (ACEs), what zip code you live in, what your pollution index is, and how these things can affect people through their life and their health.
I think that it is high time neurologists pay attention to this. There’s been mounting evidence throughout many disease states, various types of cancers, and mental health. Cardiology is much more advanced, but we haven’t had much data in neurology. In fact, when we went to write this paper, there were just one or two papers that were looking at multiple sclerosis or general neurologic issues, but really nothing in Parkinson’s disease.
We know that Parkinson’s disease is not only a motor disease that affects mental health, but that it also affects nonmotor issues. Childhood adversity may affect how people progress or how quickly they may get a disease, and we were interested in how it may manifest in a disease like Parkinson’s disease.
That was the framework going to meetings. As we wrote this paper and were in various editing stages, there was a beautiful paper that came out by Nadine Burke Harris and team that really was a call to action for neurologists and caring about trauma.
Dr. LaFaver: I couldn’t agree more. It’s really an underrecognized issue. With my own background, being very interested in functional movement disorders, psychosomatic disorders, and so on, it becomes much more evident how common a trauma background is, not only for people we were traditionally asking about.
Why don’t you summarize your findings for us?
Adverse childhood events
Dr. Subramanian: This is a web-based survey, so obviously, these are patient self-reports of their disease. We have a large cohort of people that we’ve been following over 7 years. I’m looking at modifiable variables and what really impacts Parkinson’s disease. Some of our previous papers have looked at diet, exercise, and loneliness. This is the same cohort.
We ended up putting the ACEs questionnaire, which is 10 questions looking at whether you were exposed to certain things in your household below the age of 18. This is a relatively standard questionnaire that’s administered one time, and you get a score out of 10. This is something that has been pushed, at least in the state of California, as something that we should be checking more in all people coming in.
We introduced the survey, and we didn’t force everyone to take it. Unfortunately, there was 20% or so of our patients who chose not to answer these questions. One has to ask, who are those people that didn’t answer the questions? Are they the ones that may have had trauma and these questions were triggering? It was a gap. We didn’t add extra questions to explore why people didn’t answer those questions.
We have to also put this in context. We have a patient population that’s largely quite affluent, who are able to access web-based surveys through their computer, and largely Caucasian; there are not many minoritized populations in our cohort. We want to do better with that. We actually were able to gather a decent number of women. We represent women quite well in our survey. I think that’s because of this online approach and some of the things that we’re studying.
In our survey, we broke it down into people who had no ACEs, one to three ACEs, or four or more ACEs. This is a standard way to break down ACEs so that we’re able to categorize what to do with these patient populations.
What we saw – and it’s preliminary evidence – is that people who had higher ACE scores seemed to have more symptom severity when we controlled for things like years since diagnosis, age, and gender. They also seem to have a worse quality of life. There was some indication that there were more nonmotor issues in those populations, as you might expect, such as anxiety, depression, and things that presumably ACEs can affect separately.
There are some confounders, but I think we really want to use this as the first piece of evidence to hopefully pave the way for caring about trauma in Parkinson’s disease moving forward.
Dr. LaFaver: Thank you so much for that summary. You already mentioned the main methodology you used.
What is the next step for you? How do you see these findings informing our clinical care? Do you have suggestions for all of the neurologists listening in this regard?
PD not yet considered ACE-related
Dr. Subramanian: Dr. Burke Harris was the former surgeon general in California. She’s a woman of color and a brilliant speaker, and she had worked in inner cities, I think in San Francisco, with pediatric populations, seeing these effects of adversity in that time frame.
You see this population at risk, and then you’re following this cohort, which we knew from the Kaiser cohort determines earlier morbidity and mortality across a number of disease states. We’re seeing things like more heart attacks, more diabetes, and all kinds of things in these populations. This is not new news; we just have not been focusing on this.
In her paper, this call to action, they had talked about some ACE-related conditions that currently do not include Parkinson’s disease. There are three ACE-related neurologic conditions that people should be aware of. One is in the headache/pain universe. Another is in the stroke universe, and that’s understandable, given cardiovascular risk factors . Then the third is in this dementia risk category. I think Parkinson’s disease, as we know, can be associated with dementia. A large percentage of our patients get dementia, but we don’t have Parkinson’s disease called out in this framework.
What people are talking about is if you have no ACEs or are in this middle category of one to three ACEs and you don’t have an ACE-related diagnosis – which Parkinson’s disease is not currently – we just give some basic counseling about the importance of lifestyle. I think we would love to see that anyway. They’re talking about things like exercise, diet, sleep, social connection, getting out in nature, things like that, so just general counseling on the importance of that.
Then if you’re in this higher-risk category, and so with these ACE-related neurologic conditions, including dementia, headache, and stroke, if you had this middle range of one to three ACEs, they’re getting additional resources. Some of them may be referred for social work help or mental health support and things like that.
I’d really love to see that happening in Parkinson’s disease, because I think we have so many needs in our population. I’m always hoping to advocate for more mental health needs that are scarce and resources in the social support realm because I believe that social connection and social support is a huge buffer for this trauma.
ACEs are just one type of trauma. I take care of veterans in the Veterans [Affairs Department]. We have some information now coming out about posttraumatic stress disorder, predisposing to certain things in Parkinson’s disease, possibly head injury, and things like that. I think we have populations at risk that we can hopefully screen at intake, and I’m really pushing for that.
Maybe it’s not the neurologist that does this intake. It might be someone else on the team that can spend some time doing these questionnaires and understand if your patient has a high ACE score. Unless you ask, many patients don’t necessarily come forward to talk about this. I really am pushing for trying to screen and trying to advocate for more research in this area so that we can classify Parkinson’s disease as an ACE-related condition and thus give more resources from the mental health world, and also the social support world, to our patients.
Dr. LaFaver: Thank you. There are many important points, and I think it’s a very important thing to recognize that it may not be only trauma in childhood but also throughout life, as you said, and might really influence nonmotor symptoms of Parkinson’s disease in particular, including anxiety and pain, which are often difficult to treat.
I think there’s much more to do in research, advocacy, and education. We’re going to educate patients about this, and also educate other neurologists and providers. I think you mentioned that trauma-informed care is getting its spotlight in primary care and other specialties. I think we have catching up to do in neurology, and I think this is a really important work toward that goal.
Thank you so much for your work and for taking the time to share your thoughts. I hope to talk to you again soon.
Dr. Subramanian: Thank you so much, Kathrin.
Dr. LaFaver has disclosed no relevant financial relationships. Dr. Subramanian disclosed ties with Acorda Therapeutics.
A version of this article originally appeared on Medscape.com.
Cervical screening often stops at 65, but should it?
“Did you love your wife?” asks a character in “Rose,” a book by Martin Cruz Smith.
“No, but she became a fact through perseverance,” the man replied.
Medicine also has such relationships, it seems – tentative ideas that turned into fact simply by existing long enough.
Age 65 as the cutoff for cervical screening may be one such example. It has existed for 27 years with limited science to back it up. That may soon change with the launch of a $3.3 million study that is being funded by the National Institutes of Health (NIH). The study is intended to provide a more solid foundation for the benefits and harms of cervical screening for women older than 65.
It’s an important issue: 20% of all cervical cancer cases are found in women who are older than 65. Most of these patients have late-stage disease, which can be fatal. In the United States, 35% of cervical cancer deaths occur after age 65. But women in this age group are usually no longer screened for cervical cancer.
Back in 1996, the U.S. Preventive Services Task Force recommended that for women at average risk with adequate prior screening, cervical screening should stop at the age of 65. This recommendation has been carried forward year after year and has been incorporated into several other guidelines.
For example, current guidelines from the American Cancer Society, the American College of Obstetricians and Gynecologists, and the USPSTF recommend that cervical screening stop at aged 65 for patients with adequate prior screening.
“Adequate screening” is defined as three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative co-tests within the prior 10 years, with the most recent screening within 5 years and with no precancerous lesions in the past 25 years.
This all sounds reasonable; however, for most women, medical records aren’t up to the task of providing a clean bill of cervical health over many decades.
Explained Sarah Feldman, MD, an associate professor in obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston: “You know, when a patient says to me at 65, ‘Should I continue screening?’ I say, ‘Do you have all your results?’ And they’ll say, ‘Well, I remember I had a sort of abnormal pap 15 years ago,’ and I say, ‘All right; well, who knows what that was?’ So I’ll continue screening.”
According to George Sawaya, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, up to 60% of women do not meet the criteria to end screening at age 65. This means that each year in the United States, approximately 1.7 million women turn 65 and should, in theory, continue to undergo screening for cervical cancer.
Unfortunately, the evidence base for the harms and benefits of cervical screening after age 65 is almost nonexistent – at least by the current standards of evidence-based medicine.
“We need to be clear that we don’t really know the appropriateness of the screening after 65,” said Dr. Sawaya, “which is ironic, because cervical cancer screening is probably the most commonly implemented cancer screening test in the country because it starts so early and ends so late and it’s applied so frequently.”
Dr. Feldman agrees that the age 65 cutoff is “somewhat arbitrary.” She said, “Why don’t they want to consider it continuing past 65? I don’t really understand, I have to be honest with you.”
So what’s the scientific evidence backing up the 27-year-old recommendation?
In 2018, the USPSTF’s cervical-screening guidelines concluded “with moderate certainty that the benefits of screening in women older than 65 years who have had adequate prior screening and are not otherwise at high risk for cervical cancer do not outweigh the potential harms.”
This recommendation was based on a new decision model commissioned by the USPSTF. The model was needed because, as noted by the guidelines’ authors, “None of the screening trials enrolled women older than 65 years, so direct evidence on when to stop screening is not available.”
In 2020, the ACS carried out a fresh literature review and published its own recommendations. The ACS concluded that “the evidence for the effectiveness of screening beyond age 65 is limited, based solely on observational and modeling studies.”
As a result, the ACS assigned a “qualified recommendation” to the age-65 moratorium (defined as “less certainty about the balance of benefits and harms or about patients’ values and preferences”).
Most recently, the 2021 Updated Cervical Cancer Screening Guidelines, published by the American College of Obstetricians and Gynecologists, endorsed the recommendations of the USPSTF.
Dr. Sawaya said, “The whole issue about screening over 65 is complicated from a lot of perspectives. We don’t know a lot about the safety. We don’t really know a lot about patients’ perceptions of it. But we do know that there has to be an upper age limit after which screening is just simply imprudent.”
Dr. Sawaya acknowledges that there exists a “heck-why-not” attitude toward cervical screening after 65 among some physicians, given that the tests are quick and cheap and could save a life, but he sounds a note of caution.
“It’s like when we used to use old cameras: the film was cheap, but the developing was really expensive,” Dr. Sawaya said. “So it’s not necessarily about the tests being cheap, it’s about the cascade of events [that follow].”
Follow-up for cervical cancer can be more hazardous for a postmenopausal patient than for a younger woman, explained Dr. Sawaya, because the transformation zone of the cervix may be difficult to see on colposcopy. Instead of a straightforward 5-minute procedure in the doctor’s office, the older patient may need the operating room simply to provide the first biopsy.
In addition, treatments such as cone biopsy, loop excision, or ablation are also more worrying for older women, said Dr. Sawaya, “So you start thinking about the risks of anesthesia, you start thinking about the risks of bleeding and infection, etc. And these have not been well described in older people.”
To add to the uncertainty about the merits and risks of hunting out cervical cancer in older women, a lot has changed in women’s health since 1996.
Explained Dr. Sawaya, “This stake was put in the ground in 1996, ... but since that time, life expectancy has gained 5 years. So a logical person would say, ‘Oh, well, let’s just say it should be 70 now, right?’ [But] can we even use old studies to inform the current cohort of women who are entering this 65-year-and-older age group?”
To answer all these questions, a 5-year, $3.3 million study funded by the NIH through the National Cancer Institute is now underway.
The project, named Comparative Effectiveness Research to Validate and Improve Cervical Cancer Screening (CERVICCS 2), will be led by Dr. Sawaya and Michael Silverberg, PhD, associate director of the Behavioral Health, Aging and Infectious Diseases Section of Kaiser Permanente Northern California’s Division of Research.
It’s not possible to conduct a true randomized controlled trial in this field of medicine for ethical reasons, so CERVICCS 2 will emulate a randomized study by following the fate of approximately 280,000 women older than 65 who were long-term members of two large health systems during 2005-2022. – both before and after the crucial age 65 cutoff.
The California study will also look at the downsides of diagnostic procedures and surgical interventions that follow a positive screening result after the age of 65 and the personal experiences of the women involved.
Dr. Sawaya and Dr. Silverberg’s team will use software that emulates a clinical trial by utilizing observational data to compare the benefits and risks of screening continuation or screening cessation after age 65.
In effect, after 27 years of loyalty to a recommendation supported by low-quality evidence, medicine will finally have a reliable answer to the question, Should we continue to look for cervical cancer in women over 65?
Dr. Sawaya concluded: “There’s very few things that are packaged away and thought to be just the truth. And this is why we always have to be vigilant. ... And that’s what keeps science so interesting and exciting.”
Dr. Sawaya has disclosed no relevant financial relationships. Dr. Feldman writes for UpToDate and receives several NIH grants.
A version of this article first appeared on Medscape.com.
“Did you love your wife?” asks a character in “Rose,” a book by Martin Cruz Smith.
“No, but she became a fact through perseverance,” the man replied.
Medicine also has such relationships, it seems – tentative ideas that turned into fact simply by existing long enough.
Age 65 as the cutoff for cervical screening may be one such example. It has existed for 27 years with limited science to back it up. That may soon change with the launch of a $3.3 million study that is being funded by the National Institutes of Health (NIH). The study is intended to provide a more solid foundation for the benefits and harms of cervical screening for women older than 65.
It’s an important issue: 20% of all cervical cancer cases are found in women who are older than 65. Most of these patients have late-stage disease, which can be fatal. In the United States, 35% of cervical cancer deaths occur after age 65. But women in this age group are usually no longer screened for cervical cancer.
Back in 1996, the U.S. Preventive Services Task Force recommended that for women at average risk with adequate prior screening, cervical screening should stop at the age of 65. This recommendation has been carried forward year after year and has been incorporated into several other guidelines.
For example, current guidelines from the American Cancer Society, the American College of Obstetricians and Gynecologists, and the USPSTF recommend that cervical screening stop at aged 65 for patients with adequate prior screening.
“Adequate screening” is defined as three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative co-tests within the prior 10 years, with the most recent screening within 5 years and with no precancerous lesions in the past 25 years.
This all sounds reasonable; however, for most women, medical records aren’t up to the task of providing a clean bill of cervical health over many decades.
Explained Sarah Feldman, MD, an associate professor in obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston: “You know, when a patient says to me at 65, ‘Should I continue screening?’ I say, ‘Do you have all your results?’ And they’ll say, ‘Well, I remember I had a sort of abnormal pap 15 years ago,’ and I say, ‘All right; well, who knows what that was?’ So I’ll continue screening.”
According to George Sawaya, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, up to 60% of women do not meet the criteria to end screening at age 65. This means that each year in the United States, approximately 1.7 million women turn 65 and should, in theory, continue to undergo screening for cervical cancer.
Unfortunately, the evidence base for the harms and benefits of cervical screening after age 65 is almost nonexistent – at least by the current standards of evidence-based medicine.
“We need to be clear that we don’t really know the appropriateness of the screening after 65,” said Dr. Sawaya, “which is ironic, because cervical cancer screening is probably the most commonly implemented cancer screening test in the country because it starts so early and ends so late and it’s applied so frequently.”
Dr. Feldman agrees that the age 65 cutoff is “somewhat arbitrary.” She said, “Why don’t they want to consider it continuing past 65? I don’t really understand, I have to be honest with you.”
So what’s the scientific evidence backing up the 27-year-old recommendation?
In 2018, the USPSTF’s cervical-screening guidelines concluded “with moderate certainty that the benefits of screening in women older than 65 years who have had adequate prior screening and are not otherwise at high risk for cervical cancer do not outweigh the potential harms.”
This recommendation was based on a new decision model commissioned by the USPSTF. The model was needed because, as noted by the guidelines’ authors, “None of the screening trials enrolled women older than 65 years, so direct evidence on when to stop screening is not available.”
In 2020, the ACS carried out a fresh literature review and published its own recommendations. The ACS concluded that “the evidence for the effectiveness of screening beyond age 65 is limited, based solely on observational and modeling studies.”
As a result, the ACS assigned a “qualified recommendation” to the age-65 moratorium (defined as “less certainty about the balance of benefits and harms or about patients’ values and preferences”).
Most recently, the 2021 Updated Cervical Cancer Screening Guidelines, published by the American College of Obstetricians and Gynecologists, endorsed the recommendations of the USPSTF.
Dr. Sawaya said, “The whole issue about screening over 65 is complicated from a lot of perspectives. We don’t know a lot about the safety. We don’t really know a lot about patients’ perceptions of it. But we do know that there has to be an upper age limit after which screening is just simply imprudent.”
Dr. Sawaya acknowledges that there exists a “heck-why-not” attitude toward cervical screening after 65 among some physicians, given that the tests are quick and cheap and could save a life, but he sounds a note of caution.
“It’s like when we used to use old cameras: the film was cheap, but the developing was really expensive,” Dr. Sawaya said. “So it’s not necessarily about the tests being cheap, it’s about the cascade of events [that follow].”
Follow-up for cervical cancer can be more hazardous for a postmenopausal patient than for a younger woman, explained Dr. Sawaya, because the transformation zone of the cervix may be difficult to see on colposcopy. Instead of a straightforward 5-minute procedure in the doctor’s office, the older patient may need the operating room simply to provide the first biopsy.
In addition, treatments such as cone biopsy, loop excision, or ablation are also more worrying for older women, said Dr. Sawaya, “So you start thinking about the risks of anesthesia, you start thinking about the risks of bleeding and infection, etc. And these have not been well described in older people.”
To add to the uncertainty about the merits and risks of hunting out cervical cancer in older women, a lot has changed in women’s health since 1996.
Explained Dr. Sawaya, “This stake was put in the ground in 1996, ... but since that time, life expectancy has gained 5 years. So a logical person would say, ‘Oh, well, let’s just say it should be 70 now, right?’ [But] can we even use old studies to inform the current cohort of women who are entering this 65-year-and-older age group?”
To answer all these questions, a 5-year, $3.3 million study funded by the NIH through the National Cancer Institute is now underway.
The project, named Comparative Effectiveness Research to Validate and Improve Cervical Cancer Screening (CERVICCS 2), will be led by Dr. Sawaya and Michael Silverberg, PhD, associate director of the Behavioral Health, Aging and Infectious Diseases Section of Kaiser Permanente Northern California’s Division of Research.
It’s not possible to conduct a true randomized controlled trial in this field of medicine for ethical reasons, so CERVICCS 2 will emulate a randomized study by following the fate of approximately 280,000 women older than 65 who were long-term members of two large health systems during 2005-2022. – both before and after the crucial age 65 cutoff.
The California study will also look at the downsides of diagnostic procedures and surgical interventions that follow a positive screening result after the age of 65 and the personal experiences of the women involved.
Dr. Sawaya and Dr. Silverberg’s team will use software that emulates a clinical trial by utilizing observational data to compare the benefits and risks of screening continuation or screening cessation after age 65.
In effect, after 27 years of loyalty to a recommendation supported by low-quality evidence, medicine will finally have a reliable answer to the question, Should we continue to look for cervical cancer in women over 65?
Dr. Sawaya concluded: “There’s very few things that are packaged away and thought to be just the truth. And this is why we always have to be vigilant. ... And that’s what keeps science so interesting and exciting.”
Dr. Sawaya has disclosed no relevant financial relationships. Dr. Feldman writes for UpToDate and receives several NIH grants.
A version of this article first appeared on Medscape.com.
“Did you love your wife?” asks a character in “Rose,” a book by Martin Cruz Smith.
“No, but she became a fact through perseverance,” the man replied.
Medicine also has such relationships, it seems – tentative ideas that turned into fact simply by existing long enough.
Age 65 as the cutoff for cervical screening may be one such example. It has existed for 27 years with limited science to back it up. That may soon change with the launch of a $3.3 million study that is being funded by the National Institutes of Health (NIH). The study is intended to provide a more solid foundation for the benefits and harms of cervical screening for women older than 65.
It’s an important issue: 20% of all cervical cancer cases are found in women who are older than 65. Most of these patients have late-stage disease, which can be fatal. In the United States, 35% of cervical cancer deaths occur after age 65. But women in this age group are usually no longer screened for cervical cancer.
Back in 1996, the U.S. Preventive Services Task Force recommended that for women at average risk with adequate prior screening, cervical screening should stop at the age of 65. This recommendation has been carried forward year after year and has been incorporated into several other guidelines.
For example, current guidelines from the American Cancer Society, the American College of Obstetricians and Gynecologists, and the USPSTF recommend that cervical screening stop at aged 65 for patients with adequate prior screening.
“Adequate screening” is defined as three consecutive normal Pap tests or two consecutive negative human papillomavirus tests or two consecutive negative co-tests within the prior 10 years, with the most recent screening within 5 years and with no precancerous lesions in the past 25 years.
This all sounds reasonable; however, for most women, medical records aren’t up to the task of providing a clean bill of cervical health over many decades.
Explained Sarah Feldman, MD, an associate professor in obstetrics, gynecology, and reproductive biology at Harvard Medical School, Boston: “You know, when a patient says to me at 65, ‘Should I continue screening?’ I say, ‘Do you have all your results?’ And they’ll say, ‘Well, I remember I had a sort of abnormal pap 15 years ago,’ and I say, ‘All right; well, who knows what that was?’ So I’ll continue screening.”
According to George Sawaya, MD, professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco, up to 60% of women do not meet the criteria to end screening at age 65. This means that each year in the United States, approximately 1.7 million women turn 65 and should, in theory, continue to undergo screening for cervical cancer.
Unfortunately, the evidence base for the harms and benefits of cervical screening after age 65 is almost nonexistent – at least by the current standards of evidence-based medicine.
“We need to be clear that we don’t really know the appropriateness of the screening after 65,” said Dr. Sawaya, “which is ironic, because cervical cancer screening is probably the most commonly implemented cancer screening test in the country because it starts so early and ends so late and it’s applied so frequently.”
Dr. Feldman agrees that the age 65 cutoff is “somewhat arbitrary.” She said, “Why don’t they want to consider it continuing past 65? I don’t really understand, I have to be honest with you.”
So what’s the scientific evidence backing up the 27-year-old recommendation?
In 2018, the USPSTF’s cervical-screening guidelines concluded “with moderate certainty that the benefits of screening in women older than 65 years who have had adequate prior screening and are not otherwise at high risk for cervical cancer do not outweigh the potential harms.”
This recommendation was based on a new decision model commissioned by the USPSTF. The model was needed because, as noted by the guidelines’ authors, “None of the screening trials enrolled women older than 65 years, so direct evidence on when to stop screening is not available.”
In 2020, the ACS carried out a fresh literature review and published its own recommendations. The ACS concluded that “the evidence for the effectiveness of screening beyond age 65 is limited, based solely on observational and modeling studies.”
As a result, the ACS assigned a “qualified recommendation” to the age-65 moratorium (defined as “less certainty about the balance of benefits and harms or about patients’ values and preferences”).
Most recently, the 2021 Updated Cervical Cancer Screening Guidelines, published by the American College of Obstetricians and Gynecologists, endorsed the recommendations of the USPSTF.
Dr. Sawaya said, “The whole issue about screening over 65 is complicated from a lot of perspectives. We don’t know a lot about the safety. We don’t really know a lot about patients’ perceptions of it. But we do know that there has to be an upper age limit after which screening is just simply imprudent.”
Dr. Sawaya acknowledges that there exists a “heck-why-not” attitude toward cervical screening after 65 among some physicians, given that the tests are quick and cheap and could save a life, but he sounds a note of caution.
“It’s like when we used to use old cameras: the film was cheap, but the developing was really expensive,” Dr. Sawaya said. “So it’s not necessarily about the tests being cheap, it’s about the cascade of events [that follow].”
Follow-up for cervical cancer can be more hazardous for a postmenopausal patient than for a younger woman, explained Dr. Sawaya, because the transformation zone of the cervix may be difficult to see on colposcopy. Instead of a straightforward 5-minute procedure in the doctor’s office, the older patient may need the operating room simply to provide the first biopsy.
In addition, treatments such as cone biopsy, loop excision, or ablation are also more worrying for older women, said Dr. Sawaya, “So you start thinking about the risks of anesthesia, you start thinking about the risks of bleeding and infection, etc. And these have not been well described in older people.”
To add to the uncertainty about the merits and risks of hunting out cervical cancer in older women, a lot has changed in women’s health since 1996.
Explained Dr. Sawaya, “This stake was put in the ground in 1996, ... but since that time, life expectancy has gained 5 years. So a logical person would say, ‘Oh, well, let’s just say it should be 70 now, right?’ [But] can we even use old studies to inform the current cohort of women who are entering this 65-year-and-older age group?”
To answer all these questions, a 5-year, $3.3 million study funded by the NIH through the National Cancer Institute is now underway.
The project, named Comparative Effectiveness Research to Validate and Improve Cervical Cancer Screening (CERVICCS 2), will be led by Dr. Sawaya and Michael Silverberg, PhD, associate director of the Behavioral Health, Aging and Infectious Diseases Section of Kaiser Permanente Northern California’s Division of Research.
It’s not possible to conduct a true randomized controlled trial in this field of medicine for ethical reasons, so CERVICCS 2 will emulate a randomized study by following the fate of approximately 280,000 women older than 65 who were long-term members of two large health systems during 2005-2022. – both before and after the crucial age 65 cutoff.
The California study will also look at the downsides of diagnostic procedures and surgical interventions that follow a positive screening result after the age of 65 and the personal experiences of the women involved.
Dr. Sawaya and Dr. Silverberg’s team will use software that emulates a clinical trial by utilizing observational data to compare the benefits and risks of screening continuation or screening cessation after age 65.
In effect, after 27 years of loyalty to a recommendation supported by low-quality evidence, medicine will finally have a reliable answer to the question, Should we continue to look for cervical cancer in women over 65?
Dr. Sawaya concluded: “There’s very few things that are packaged away and thought to be just the truth. And this is why we always have to be vigilant. ... And that’s what keeps science so interesting and exciting.”
Dr. Sawaya has disclosed no relevant financial relationships. Dr. Feldman writes for UpToDate and receives several NIH grants.
A version of this article first appeared on Medscape.com.
Osteoporosis drugs may extend life after fracture
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Long-term osteoporosis medications are associated with a reduced mortality risk following a fracture, new data suggest.
The findings, from nearly 50,000 individuals in a nationwide Taiwanese database from 2009 until 2018, suggest that alendronate/risedronate, denosumab, and zoledronic acid all result in a significantly lower mortality risk post fracture of 17%-22%, compared with raloxifene and bazedoxifene.
“Treatment for osteoporosis has the potential to minimize mortality risk in people of all ages and sexes for any type of fracture. The longer-acting treatments could lower mortality risk,” wrote Chih-Hsing Wu, MD, of the Institute of Gerontology at National Cheng Kung University, Tainan, Taiwan, and colleagues.
The findings have been published online in the Journal of Clinical Endocrinology and Metabolism.
Robert A. Adler, MD, who is chief of endocrinology at the Central Virginia Veterans Affairs Health Care System, Richmond, told this news organization that he hopes these new findings from a “really good database ... may be helpful in talking to a patient about the pros and cons of taking these drugs.”
“Patients have been made very fearful of the unusual side effects, particularly of the antiresorptive drugs,” which he notes include the rare adverse effects of jaw necrosis and atypical femoral fracture, which occur in about 1 per 10,000 patient-years.
“And because of that we have a hard time convincing people to want to take the drug in the first place or to stay on the drug once they start,” said Dr. Adler, who stressed that his viewpoints are his own and not representative of the VA.
“These data should help reinforce the advice already given in professional guidelines that their benefit outweighs any risks,” he stresses.
Dr. Adler also pointed out that both bisphosphonates included in the study, alendronate and zoledronic acid, are now available as generics and therefore inexpensive, but the latter can be subject to facility fees depending on where the infusion is delivered.
He added that hip fracture, in particular, triples the overall 1-year mortality risk in women aged 75-84 years and quadruples the risk in men. The study’s findings suggest that bisphosphonates, in particular, have pleiotropic effects beyond the bone; however, the underlying mechanisms are hard to determine.
“We don’t know all the reasons why people die after a fracture. These are older people who often have multiple medical problems, so it’s hard to dissect that out,” he said.
But whatever the mechanism for the salutary effect of the drugs, Dr. Adler said: “This is one other factor that might change people’s minds. You’re less likely to die. Well, that’s pretty good.”
‘Denosumab is a more potent antiresorptive than bisphosphonates’
Dr. Wu and colleagues analyzed data for individuals from Taiwan’s National Health Insurance Research Database. Between 2009 and 2017, 219,461 individuals had been newly diagnosed with an osteoporotic fracture. Of those, 46,729 were aged 40 and older and had been prescribed at least one anti-osteoporosis medication.
Participants were a mean age of 74.5 years, were 80% women, and 32% died during a mean follow-up of 4.7 years. The most commonly used anti-osteoporosis medications were the bisphosphonates alendronate or risedronate, followed by denosumab and the selective estrogen-receptor modulators (SERMs) daily oral raloxifene or bazedoxifene.
Patients treated with SERMs were used as the reference group because those drugs have been shown to have a neutral effect on mortality.
After adjustments, all but one of the medications had significantly lower mortality risks during follow-up, compared with raloxifene and bazedoxifene.
Compared with SERMs, at all fracture sites, the hazard ratios for mortality were 0.83 for alendronate/risedronate, 0.86 for denosumab, and 0.78 for zoledronic acid. Only ibandronate did not show the same protective effect.
Similar results were found for hip and vertebral fractures analyzed individually.
Women had a lower mortality risk than men.
Dr. Adler wrote an accompanying editorial for the article by Dr. Wu and colleagues.
Regarding the finding of benefit for denosumab, Dr. Adler notes: “I don’t know of another study that found denosumab leads to lower mortality. On the other hand, denosumab is a more potent antiresorptive than bisphosphonates.”
The study was funded by research grants from the Ministry of Science and Technology, Taiwan, partially supported by a research grant from the Taiwanese Osteoporosis Association and grants from National Cheng Kung University Hospital, Taiwan. Dr. Wu has reported receiving honoraria for lectures, attending meetings, and/or travel from Eli Lilly, Roche, Amgen, Merck, Servier, GE Lunar, Harvester, TCM Biotech, and Alvogen/Lotus. Dr. Adler has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: Residence Characteristics and Nursing Home Compare Quality Measures
Relationships Between Residence Characteristics and Nursing Home Compare Database Quality Measures
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18
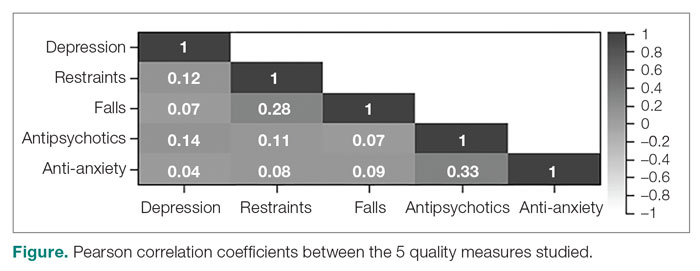
Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).
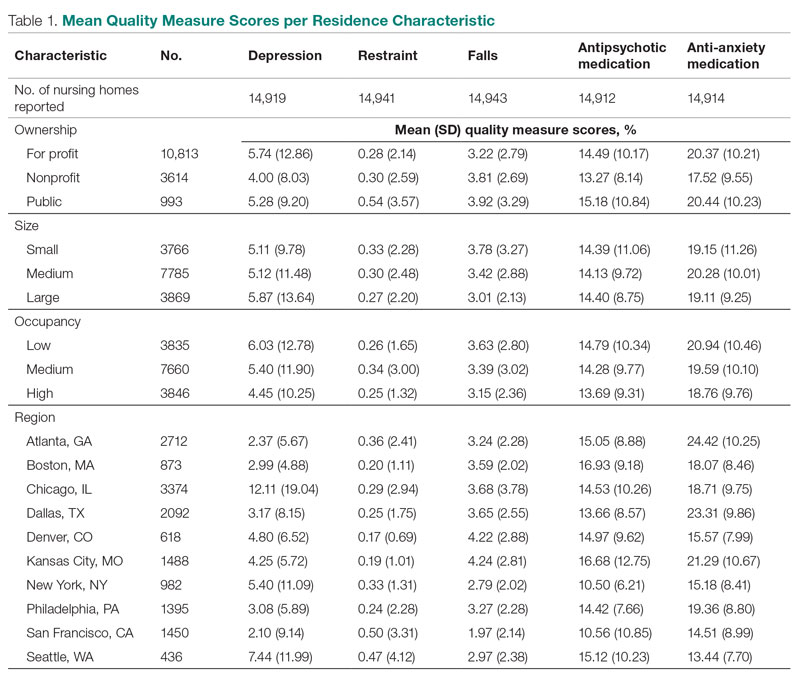
Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.
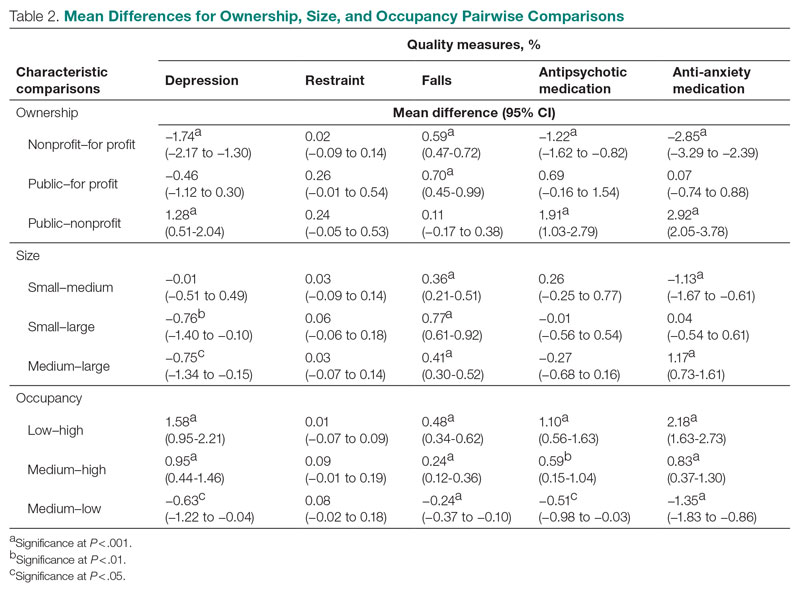
Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.
Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
From the University of Nebraska, Lincoln (Mr. Puckett and Dr. Ryherd), University of Nebraska Medical Center, Omaha (Dr. Manley), and the University of Nebraska, Omaha (Dr. Ryan).
ABSTRACT
Objective: This study evaluated relationships between physical characteristics of nursing home residences and quality-of-care measures.
Design: This was a cross-sectional ecologic study. The dependent variables were 5 Centers for Medicare & Medicaid Services (CMS) Nursing Home Compare database long-stay quality measures (QMs) during 2019: percentage of residents who displayed depressive symptoms, percentage of residents who were physically restrained, percentage of residents who experienced 1 or more falls resulting in injury, percentage of residents who received antipsychotic medication, and percentage of residents who received anti-anxiety medication. The independent variables were 4 residence characteristics: ownership type, size, occupancy, and region within the United States. We explored how different types of each residence characteristic compare for each QM.
Setting, participants, and measurements: Quality measure values from 15,420 CMS-supported nursing homes across the United States averaged over the 4 quarters of 2019 reporting were used. Welch’s analysis of variance was performed to examine whether the mean QM values for groups within each residential characteristic were statistically different.
Results: Publicly owned and low-occupancy residences had the highest mean QM values, indicating the poorest performance. Nonprofit and high-occupancy residences generally had the lowest (ie, best) mean QM values. There were significant differences in mean QM values among nursing home sizes and regions.
Conclusion: This study suggests that residence characteristics are related to 5 nursing home QMs. Results suggest that physical characteristics may be related to overall quality of life in nursing homes.
Keywords: quality of care, quality measures, residence characteristics, Alzheimer’s disease and related dementias.
More than 55 million people worldwide are living with Alzheimer’s disease and related dementias (ADRD).1 With the aging of the Baby Boomer population, this number is expected to rise to more than 78 million worldwide by 2030.1 Given the growing number of cognitively impaired older adults, there is an increased need for residences designed for the specialized care of this population. Although there are dozens of living options for the elderly, and although most specialized establishments have the resources to meet the immediate needs of their residents, many facilities lack universal design features that support a high quality of life for someone with ADRD or mild cognitive impairment. Previous research has shown relationships between behavioral and psychological symptoms of dementia (BPSD) and environmental characteristics such as acoustics, lighting, and indoor air temperature.2,3 Physical behaviors of BPSD, including aggression and wandering, and psychological symptoms, such as depression, anxiety, and delusions, put residents at risk of injury.4 Additionally, BPSD is correlated with caregiver burden and stress.5-8 Patients with dementia may also experience a lower stress threshold, changes in perception of space, and decreased short-term memory, creating environmental difficulties for those with ADRD9 that lead them to exhibit BPSD due to poor environmental design. Thus, there is a need to learn more about design features that minimize BPSD and promote a high quality of life for those with ADRD.10
Although research has shown relationships between physical environmental characteristics and BPSD, in this work we study relationships between possible BPSD indicators and 4 residence-level characteristics: ownership type, size, occupancy, and region in the United States (determined by location of the Centers for Medicare & Medicaid Services [CMS] regional offices). We analyzed data from the CMS Nursing Home Compare database for the year 2019.11 This database publishes quarterly data and star ratings for quality-of-care measures (QMs), staffing levels, and health inspections for every nursing home supported by CMS. Previous research has investigated the accuracy of QM reporting for resident falls, the impact of residential characteristics on administration of antipsychotic medication, the influence of profit status on resident outcomes and quality of care, and the effect of nursing home size on quality of life.12-16 Additionally, research suggests that residential characteristics such as size and location could be associated with infection control in nursing homes.17
Certain QMs, such as psychotropic drug administration, resident falls, and physical restraint, provide indicators of agitation, disorientation, or aggression, which are often signals of BPSD episodes. We hypothesized that residence types are associated with different QM scores, which could indicate different occurrences of BPSD. We selected 5 QMs for long-stay residents that could potentially be used as indicators of BPSD. Short-stay resident data were not included in this work to control for BPSD that could be a result of sheer unfamiliarity with the environment and confusion from being in a new home.
Methods
Design and Data Collection
This was a cross-sectional ecologic study aimed at exploring relationships between aggregate residential characteristics and QMs. Data were retrieved from the 2019 annual archives found in the CMS provider data catalog on nursing homes, including rehabilitation services.11 The dataset provides general residence information, such as ownership, number of beds, number of residents, and location, as well as residence quality metrics, such as QMs, staffing data, and inspection data. Residence characteristics and 4-quarter averages of QMs were retrieved and used as cross-sectional data. The data used are from 15,420 residences across the United States. Nursing homes located in Guam, the US Pacific Territories, Puerto Rico, and the US Virgin Islands, while supported by CMS and included in the dataset, were excluded from the study due to a severe absence of QM data.
Dependent Variables
We investigated 5 QMs that were averaged across the 4 quarters of 2019. The QMs used as dependent variables were percentage of residents who displayed depressive symptoms (depression), percentage of residents who were physically restrained (restraint), percentage of residents who experienced 1 or more falls resulting in a major injury (falls), percentage of residents who received antipsychotic medication (antipsychotic medication), and percentage of residents who received anti-anxiety or hypnotic medication (anti-anxiety medication).
A total of 2471 QM values were unreported across the 5 QM analyzed: 501 residences did not report depression data; 479 did not report restraint data; 477 did not report falls data; 508 did not report antipsychotic medication data; and 506 did not report anti-anxiety medication data. A residence with a missing QM value was excluded from that respective analysis.
To assess the relationships among the different QMs, a Pearson correlation coefficient r was computed for each unique pair of QMs (Figure). All QMs studied were found to be very weakly or weakly correlated with one another using the Evans classification for very weak and weak correlations (r < 0.20 and 0.20 < r < 0.39, respectively).18

Independent Variables
A total of 15,420 residences were included in the study. Seventy-nine residences did not report occupancy data, however, so those residences were excluded from the occupancy analyses. We categorized the ownership of each nursing home as for-profit, nonprofit, or public. We categorized nursing home size, based on quartiles of the size distribution, as large (> 127 beds), medium (64 to 126 beds), and small (< 64 beds). This method for categorizing the residential characteristics was similar to that used in previous work.19 Similarly, we categorized nursing home occupancy as high (> 92% occupancy), medium (73% to 91% occupancy), and low (< 73% occupancy) based on quartiles of the occupancy distribution. For the regional analysis, we grouped states together based on the CMS regional offices: Atlanta, Georgia; Boston, Massachusetts; Chicago, Illinois; Dallas, Texas; Denver, Colorado; Kansas City, Missouri; New York, New York; Philadelphia, Pennsylvania; San Francisco, California; and Seattle, Washington.20
Analyses
We used Levene’s test to determine whether variances among the residential groups were equal for each QM, using an a priori α = 0.05. For all 20 tests conducted (4 residential characteristics for all 5 QMs), the resulting F-statistics were significant, indicating that the assumption of homogeneity of variance was not met.
We therefore used Welch’s analysis of variance (ANOVA) to evaluate whether the groups within each residential characteristic were the same on their QM means. For example, we tested whether for-profit, nonprofit, and public residences had significantly different mean depression rates. For statistically significant differences, a Games-Howell post-hoc test was conducted to test the difference between all unique pairwise comparisons. An a priori α = 0.05 was used for both Welch’s ANOVA and post-hoc testing. All analyses were conducted in RStudio Version 1.2.5033 (Posit Software, PBC).
Results
Mean Differences
Mean QM scores for the 5 QMs investigated, grouped by residential characteristic for the 2019 year of reporting, are shown in Table 1. It should be noted that the number of residences that reported occupancy data (n = 15,341) does not equal the total number of residences included in the study (N = 15,420) because 79 residences did not report occupancy data. For all QMs reported in Table 1, lower scores are better. Table 2 and Table 3 show results from pairwise comparisons of mean differences for the different residential characteristic and QM groupings. Mean differences and 95% CI are presented along with an indication of statistical significance (when applicable).

Ownership
Nonprofit residences had significantly lower (ie, better) mean scores than for-profit and public residences for 3 QMs: resident depression, antipsychotic medication use, and anti-anxiety medication use. For-profit and public residences did not significantly differ in their mean values for these QMs. For-profit residences had a significantly lower mean score for resident falls than both nonprofit and public residences, but no significant difference existed between scores for nonprofit and public residence falls. There were no statistically significant differences between mean restraint scores among the ownership types.

Size
Large (ie, high-capacity) residences had a significantly higher mean depression score than both medium and small residences, but there was not a significant difference between medium and small residences. Large residences had the significantly lowest mean score for resident falls, and medium residences scored significantly lower than small residences. Medium residences had a significantly higher mean score for anti-anxiety medication use than both small and large residences, but there was no significant difference between small and large residences. There were no statistically significant differences between mean scores for restraint and antipsychotic medication use among the nursing home sizes.

Occupancy
The mean scores for 4 out of the 5 QMs exhibited similar relationships with occupancy rates: resident depression, falls, and antipsychotic and anti-anxiety medication use. Low-occupancy residences consistently scored significantly higher than both medium- and high-occupancy residences, and medium-occupancy residences consistently scored significantly higher than high-occupancy residences. On average, high-occupancy (≥ 92%) residences reported better QM scores than low-occupancy (< 73%) and medium-occupancy (73% to 91%) residences for all the QMs studied except physical restraint, which yielded no significant results. These findings indicate a possible inverse relationship between building occupancy rate and these 4 QMs.
Region
Pairwise comparisons of mean QM scores by region are shown in Table 3. The Chicago region had a significantly higher mean depression score than all other regions, while the San Francisco region’s score was significantly lower than all other regions, except Atlanta and Boston. The Kansas City region had a significantly higher mean score for resident falls than all other regions, with the exception of Denver, and the San Francisco region scored significantly lower than all other regions in falls. The Boston region had a significantly higher mean score for administering antipsychotic medication than all other regions, except for Kansas City and Seattle, and the New York and San Francisco regions both had significantly lower scores than all other regions except for each other. The Atlanta region reported a significantly higher mean score for administering antianxiety medication than all other regions, and the Seattle region’s score for anti-anxiety medication use was significantly lower than all other regions except for San Francisco.
Discussion
This study presented mean percentages for 5 QMs reported in the Nursing Home Compare database for the year 2019: depression, restraint, falls, antipsychotic medication use, and anti-anxiety medication use. We investigated these scores by 4 residential characteristics: ownership type, size, occupancy, and region. In general, publicly owned and low-occupancy residences had the highest scores, and thus the poorest performances, for the 5 chosen QMs during 2019. Nonprofit and high-occupancy residences generally had the lowest (ie, better) scores, and this result agrees with previous findings on long-stay nursing home residents.21 One possible explanation for better performance by high-occupancy buildings could be that increased social interaction is beneficial to nursing home residents as compared with low-occupancy buildings, where less social interaction is probable. It is difficult to draw conclusions regarding nursing home size and region; however, there are significant differences among sizes for 3 out of the 5 QMs and significant differences among regions for all 5 QMs. The analyses suggest that residence-level characteristics are related to QM scores. Although reported QMs are not a direct representation of resident quality of life, this work agrees with previous research that residential characteristics have some impact on the lives of nursing home residents.13-17 Improvements in QM reporting and changes in quality improvement goals since the formation of Nursing Home Compare exist, suggesting that nursing homes’ awareness of their reporting duties may impact quality of care or reporting tendencies.21,22 Future research should consider investigating the impacts of the COVID-19 pandemic on quality-reporting trends and QM scores.
Other physical characteristics of nursing homes, such as noise, lighting levels, and air quality, may also have an impact on QMs and possibly nursing home residents themselves. This type of data exploration could be included in future research. Additionally, future research could include a similar analysis over a longer period, rather than the 1-year period examined here, to investigate which types of residences consistently have high or low scores or how different types of residences have evolved over the years, particularly considering the impact of the COVID-19 pandemic. Information such as staffing levels, building renovations, and inspection data could be accounted for in future studies. Different QMs could also be investigated to better understand the influence of residential characteristics on quality of care.
Conclusion
This study suggests that residence-level characteristics are related to 5 reported nursing home QMs. Overall, nonprofit and high-occupancy residences had the lowest QM scores, indicating the highest performance. Although the results do not necessarily suggest that residence-level characteristics impact individual nursing home residents’ quality of life, they suggest that physical characteristics affect overall quality of life in nursing homes. Future research is needed to determine the specific physical characteristics of these residences that affect QM scores.
Corresponding author: Brian J. Puckett, puckett.brian@huskers.unl.edu.
Disclosures: None reported.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
1. Gauthier S, Rosa-Neto P, Morais JA, et al. World Alzheimer report 2021: journey through the diagnosis of dementia. Alzheimer’s Disease International; 2021.
2. Garre-Olmo J, López-Pousa S, Turon-Estrada A, et al. Environmental determinants of quality of life in nursing home residents with severe dementia. J Am Geriatr Soc. 2012;60(7):1230-1236. doi:10.1111/j.1532-5415.2012.04040.x
3. Zeisel J, Silverstein N, Hyde J, et al. Environmental correlates to behavioral health outcomes in Alzheimer’s special care units. Gerontologist. 2003;43(5):697-711. doi:10.1093/geront/43.5.697
4. Brawley E. Environmental design for Alzheimer’s disease: a quality of life issue. Aging Ment Health. 2001;5(1):S79-S83. doi:10.1080/13607860120044846
5. Joosse L. Do sound levels and space contribute to agitation in nursing home residents with dementia? Research Gerontol Nurs. 2012;5(3):174-184. doi:10.3928/19404921-20120605-02
6. Dowling G, Graf C, Hubbard E, et al. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. Western J Nurs Res. 2007;29(8):961-975. doi:10.1177/0193945907303083
7. Tartarini F, Cooper P, Fleming R, et al. Indoor air temperature and agitation of nursing home residents with dementia. Am J Alzheimers Dis Other Demen. 2017;32(5):272-281. doi:10.1177/1533317517704898
8. Miyamoto Y, Tachimori H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253. doi:10.1016/j.gerinurse.2010.01.002
9. Dementia care and the built environment: position paper 3. Alzheimer’s Australia; 2004.
10. Cloak N, Al Khalili Y. Behavioral and psychological symptoms in dementia. Updated July 21, 2022. In: StatPearls [Internet]. StatPearls Publishing; 2022.
11. Centers for Medicare & Medicaid Services. Nursing homes including rehab services data archive. 2019 annual files. Accessed January 30, 2023. https://data.cms.gov/provider-data/archived-data/nursing-homes
12. Sanghavi P, Pan S, Caudry D. Assessment of nursing home reporting of major injury falls for quality measurement on Nursing Home Compare. Health Serv Res. 2020;55(2):201-210. doi:10.1111/1475-6773.13247
13. Hughes C, Lapane K, Mor V. Influence of facility characteristics on use of antipsychotic medications in nursing homes. Med Care. 2000;38(12):1164-1173. doi:10.1097/00005650-200012000-00003
14. Aaronson W, Zinn J, Rosko M. Do for-profit and not-for-profit nursing homes behave differently? Gerontologist. 1994;34(6):775-786. doi:10.1093/geront/34.6.775
15. O’Neill C, Harrington C, Kitchener M, et al. Quality of care in nursing homes: an analysis of relationships among profit, quality, and ownership. Med Care. 2003;41(12):1318-1330. doi:10.1097/01.MLR.0000100586.33970.58
16. Allen PD, Klein WC, Gruman C. Correlates of complaints made to the Connecticut Long-Term Care Ombudsman program: the role of organizational and structural factors. Res Aging. 2003;25(6):631-654. doi:10.1177/0164027503256691
17. Abrams H, Loomer L, Gandhi A, et al. Characteristics of U.S. nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(8):1653-1656. doi:10.1111/jgs.16661
18. Evans JD. Straightforward Statistics for the Behavioral Sciences. Thomson Brooks/Cole Publishing Co; 1996.
19. Zinn J, Spector W, Hsieh L, et al. Do trends in the reporting of quality measures on the Nursing Home Compare web site differ by nursing home characteristics? Gerontologist. 2005;45(6):720-730.
20. Centers for Medicare & Medicaid Services. CMS Regional Offices. Accessed January 30, 2023. https://www.cms.gov/Medicare/Coding/ICD10/CMS-Regional-Offices
21. Mukamel DB, Weimer DL, Spector WD, et al. Publication of quality report cards and trends in reported quality measures in nursing homes. Health Serv Res. 2008;43(4):1244-1262. doi:10.1093/geront/45.6.720
22. Harris Y, Clauser SB. Achieving improvement through nursing home quality measurement. Health Care Financ Rev. 2002;23(4):5-18.
Tooth loss and diabetes together hasten mental decline
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
most specifically in those 65-74 years of age, new findings suggest.
The data come from a 12-year follow-up of older adults in a nationally representative U.S. survey.
“From a clinical perspective, our study demonstrates the importance of improving access to dental health care and integrating primary dental and medical care. Health care professionals and family caregivers should pay close attention to the cognitive status of diabetic older adults with poor oral health status,” lead author Bei Wu, PhD, of New York University, said in an interview. Dr. Wu is the Dean’s Professor in Global Health and codirector of the NYU Aging Incubator.
Moreover, said Dr. Wu: “For individuals with both poor oral health and diabetes, regular dental visits should be encouraged in addition to adherence to the diabetes self-care protocol.”
Diabetes has long been recognized as a risk factor for cognitive decline, but the findings have been inconsistent for different age groups. Tooth loss has also been linked to cognitive decline and dementia, as well as diabetes.
The mechanisms aren’t entirely clear, but “co-occurring diabetes and poor oral health may increase the risk for dementia, possibly via the potentially interrelated pathways of chronic inflammation and cardiovascular risk factors,” Dr. Wu said.
The new study, published in the Journal of Dental Research, is the first to examine the relationships between all three conditions by age group.
Diabetes, edentulism, and cognitive decline
The data came from a total of 9,948 participants in the Health and Retirement Study (HRS) from 2006 to 2018. At baseline, 5,440 participants were aged 65-74 years, 3,300 were aged 75-84, and 1,208 were aged 85 years or older.
They were assessed every 2 years using the 35-point Telephone Survey for Cognitive Status, which included tests of immediate and delayed word recall, repeated subtracting by 7, counting backward from 20, naming objects, and naming the president and vice president of the U.S. As might be expected, the youngest group scored the highest, averaging 23 points, while the oldest group scored lowest, at 18.5 points.
Participants were also asked if they had ever been told by a doctor that they have diabetes. Another question was: “Have you lost all of your upper and lower natural permanent teeth?”
The condition of having no teeth is known as edentulism.
The percentages of participants who reported having both diabetes and edentulism were 6.0%, 6.7%, and 5.0% for those aged 65-74 years, 75-84 years, and 85 years or older, respectively. The proportions with neither of those conditions were 63.5%, 60.4%, and 58.3% in those three age groups, respectively (P < .001).
Compared with their counterparts with neither diabetes nor edentulism at baseline, older adults with both conditions aged 65-74 years (P < .001) and aged 75-84 years had worse cognitive function (P < .001).
In terms of the rate of cognitive decline, compared with those with neither condition from the same age cohort, older adults aged 65-74 years with both conditions declined at a higher rate (P < .001).
Having diabetes alone led to accelerated cognitive decline in older adults aged 65-74 years (P < .001). Having edentulism alone led to accelerated decline in older adults aged 65-74 years (P < .001) and older adults aged 75-84 years (P < 0.01).
“Our study finds the co-occurrence of diabetes and edentulism led to a worse cognitive function and a faster cognitive decline in older adults aged 65-74 years,” say Wu and colleagues.
Study limitations: Better data needed
The study has several limitations, most of them due to the data source. For example, while the HRS collects detailed information on cognitive status, edentulism is its only measure of oral health. There were no data on whether individuals had replacements such as dentures or implants that would affect their ability to eat, which could influence other health factors.
“I have made repeated appeals for federal funding to collect more oral health-related information in large national surveys,” Dr. Wu told this news organization.
Similarly, assessments of diabetes status such as hemoglobin A1c were only available for small subsets and not sufficient to demonstrate statistical significance, she explained.
Dr. Wu suggested that both oral health and cognitive screening might be included in the “Welcome to Medicare” preventive visit. In addition, “Oral hygiene practice should also be highlighted to improve cognitive health. Developing dental care interventions and programs are needed for reducing the societal cost of dementia.”
The study was partially supported by the National Institutes of Health. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF DENTAL RESEARCH