User login
BP screening nearly universal among Medicare enrollees
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
and just under 90% saw a physician during the year, according to new data released by the Centers for Medicare & Medicaid Services.

The latest edition of Medicare Beneficiaries at a Glance takes a look at some of the services provided in 2017, and BP checks were high on the list, with 96% of enrollees getting screened. BP was also prominent on another list featured in the Medicare snapshot for 2017, as hypertension was the most common chronic condition among beneficiaries with a prevalence of 58%, the CMS said.
A second glance at the report shows that 41% of enrollees had high cholesterol that year, making it the next-most common chronic condition, with arthritis third at 33%, the CMS said. Diabetes was fourth and heart disease was fifth, but rounding gives them the same prevalence of 27%.
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Enough Fuss; She Wants Lunch!
ANSWER
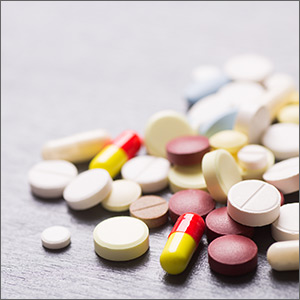
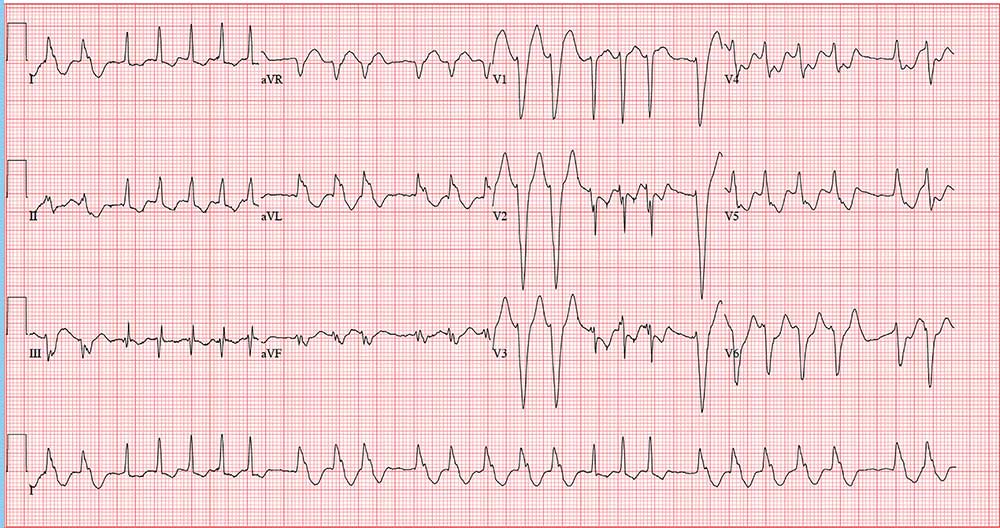
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
During morning rounds at a skilled nursing facility (SNF), a 74-year-old woman is found to have a rapid heart rate. She is placed on telemetry, which reveals a wide complex tachycardia. Concerned about possible ventricular tachycardia, the charge nurse contacts the on-call physician, who recommends calling 911. The patient is transferred via ACLS ambulance to your facility.
When you see her, she seems embarrassed by all the attention she’s receiving and expresses her desire to return to the SNF before she misses lunch. She is in no pain or discomfort, is not particularly short of breath, and does not feel dizzy or lightheaded. According to reports, she was friendly and conversive with both the nursing staff at the SNF and the paramedics during transport.
History is remarkable for several transient ischemic attacks with no residual sequelae, hypertension (under good control), and hypothyroidism (treatedwith medication). Surgical history includes a hyster-ectomy, a cholecystectomy, and an open reduction and metal plate fixation of a high (right) ankle break—all of which were performed more than 10 years ago.
Her medications include warfarin, hydrochlorothiazide, atorvastatin, and levothyroxine. She has no known drug allergies.
The patient is a retired junior high school principal. Her husband died of lung cancer 4 years ago. She has 3 adult children who are all in good health. She has never smoked but does enjoy a daily nightcap. She denies alcohol abuse or illicit drug use.
Family history reveals her parents died in a train accident and her paternal grandparents died of tuberculosis. She does not know her maternal grandparents’ medical history.
Review of systems is positive for chronic constipation and chronic hip and knee discomfort. Vital signs include a blood pressure of 124/88 mm Hg; pulse, 140 beats/min; respiratory rate, 14 breaths/min; and temperature, 97.6°F. Her weight is 158 lb, and her height is not measured.
Physical exam reveals a pleasant elderly woman in no distress. She is dressed appropriately, her hair is styled, and she is wearing makeup as she usually does. The HEENT exam reveals hearing aids and corrective lenses. Her neck has no jugular venous distention, carotid bruits, or thyromegaly.
Her lungs are clear in all fields. Her heart has a rapid and questionably irregular rhythm. There are no appreciable murmurs or rubs. Her abdominal exam is normal, with the exception of well-healed surgical scars. There is no peripheral edema, and all pulses are equal bilaterally in both upper and lower extremities. The neurologic exam is grossly normal with normal affect and mood.
An ECG reveals a ventricular rate of 152 beats/min; PR interval, 128 ms; QRS duration, 88 ms; QT/QTc interval, 280/445 ms; P axis, 27°; R axis, 23°; and T axis, 232°. What is your interpretation?
Higher Step Volume Is Associated with Lower Mortality in Older Women
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
Osteoporosis remains a costly burden to older U.S. adults
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
ORLANDO – The burden of osteoporosis and fragility fractures in the United States remains high, particularly in older women and minorities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
For non-Hispanic Asian, non-Hispanic white, and Hispanic patients of various ethnic groups, as well as in women and older patients, osteoporosis and fragility fractures continue to be a problem, said Nicole C. Wright, PhD, MPH, of the department of epidemiology at the University of Alabama at Birmingham.
“It remains costly; it remains associated with more health care utilization,” Dr. Wright said. “We may be seeing some declines in some fragility fractures, but [we] are seeing increases in hip fractures.”
As part of the fourth edition of the U.S. Bone and Joint Initiative publication, “The Burden of Musculoskeletal Diseases in the United States,” Dr. Wright and colleagues examined the changes in osteoporosis burden between the third and fourth editions of the publication. They used data from the National Inpatient Sample (NIS) in 2013 and 2014 as well as the National Emergency Department Sample (NEDS) of national ED visits regardless of hospital admission status. In both databases, researchers analyzed data from adults aged 50 years or older where the primary discharge ICD-9 or ICD-10 code was a diagnosis of fracture.
Using National Health and Nutrition Examination Survey data, the researchers estimated an 11.0% osteoporosis prevalence for adults aged 50 years or older overall, a 16.5% prevalence in women, and a 5.1% prevalence in men as assessed by femoral neck and lumbar spine bone mineral density. Osteoporosis was most prevalent in Asian women (40.0%) and Asian men (7.5%), while there was a difference in prevalence in patients of Hispanic race depending on their origin; for example, Puerto Rican men had a higher prevalence of osteoporosis at 8.6%, compared with Hispanic men (2.3%) and non-Hispanic white men of other races (3.9%).
Of 19.5 million hospitalizations in the NIS database between 2013 and 2014, there were approximately 540,000 fragility fractures (2.8%), of which about 300,000 were hip fractures and about 100,000 discharges were for spine fractures, Dr. Wright said. In the NEDS database, the estimate of fragility fracture prevalence was 0.9% of 46.7 million ED visits between 2013 and 2014. Fracture prevalence was increased in women and in older age, with patients aged 80 years or older and those of non-Hispanic white race having the highest prevalence of hip fracture. However, she noted that NEDS data also showed higher prevalences of wrist and humerus fractures, which are not normally fractures that a patient visits the hospital as an inpatient for. “We need both data sets to ascertain fractures in the United States,” she said.
When examining fracture site trends over time, Dr. Wright and colleagues found hip fracture prevalence increased by 3.5% between 2010 and 2014, while there was a decrease of 11.9% in the prevalence of spine fractures over the same time period.
According to data from the Medical Expenditures Panel Survey, the direct cost of osteoporosis in aggregate was $73.6 billion between 2012 and 2014, which was 118% higher than between 1998 and 2000 when the costs were $28.1 billion. The costs were spread across ambulatory care, inpatient, and prescription costs equally, the researchers said.
Although the study was limited by examining fracture prevalence rather than incidence, the potential for missing some fractures based on methodology, and limited patient characteristics and follow-up information, the goal of the presentation was to highlight the new osteoporosis prevalence data and the continued burden of the disease.
“We hope that these new prevalence estimates continue to increase the awareness of osteoporosis and prevention,” she said.
Dr. Wright reported receiving grants from Amgen and serving as an expert witness for the law firm Norton Rose Fulbright and Pfizer.
SOURCE: Wright NC et al. ASBMR 2019, Abstract 1079.
REPORTING FROM ASBMR 2019
GRECC Connect: Geriatrics Telehealth to Empower Health Care Providers and Improve Management of Older Veterans in Rural Communities
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
How does alcohol intake affect dementia risk in older adults?
Mild cognitive impairment (MCI) may influence the relationship between alcohol consumption and dementia risk, a study of more than 3,000 adults suggests. In addition, , according to the study, which was published in JAMA Network Open.
“The associations of self-reported alcohol consumption with dementia risk and cognitive decline were more consistently adverse among individuals with MCI than those with normal cognition,” reported Manja Koch, PhD, a researcher in the department of nutrition at Harvard T.H. Chan School of Public Health in Boston and colleagues. “This was particularly true for the subset of individuals [with MCI] who drank more than 14.0 servings per week, whose rate of cognitive decline and risk of dementia were the highest of any subgroup.”
Among older adults with normal cognition, the results generally were consistent with those of a recent meta-analysis that found a U-shaped relationship between drinking and dementia, the researchers said (Eur J Epidemiol. 2017 Jan;32[1]:31-42.).
“Our results did not show significant associations and clearly do not suffice to suggest a clinical benefit from even limited alcohol use,” said Dr. Koch and colleagues. “Nonetheless, our findings provide some reassurance that alcohol consumed within recommended limits was not associated with an increased risk of dementia among older adults with normal baseline cognition.”
GEMS data
To study whether alcohol consumption is associated with the risk of dementia and cognitive decline in older adults with and without MCI, the investigators analyzed data from the Ginkgo Evaluation of Memory Study (GEMS). GEMS was a randomized controlled trial conducted between 2000 and 2008 that found no overall association between ginkgo biloba and dementia prevention. During the trial, participants completed the Modified Mini-Mental State Examination, the Clinical Dementia Rating scale, and the cognitive portion of the Alzheimer’s Disease Assessment Scale.
In the present study, the investigators analyzed data from 3,021 participants aged 72 years and older who were free of dementia at baseline and had provided information about their alcohol intake. Their median age was 78 years, and 46.2% were female. Fifty-eight percent consumed alcohol, including 45% of the participants with MCI at baseline.
During follow-up, 512 cases of dementia occurred. Among the 473 participants with MCI at baseline, the adjusted hazard ratio (HR) for dementia was 1.72 for those who consumed more than 14 drinks per week, compared with light drinkers who consumed less than 1 drink per week. For participants who consumed between 7 and 14 drinks per week, the adjusted HR for dementia was 0.63 among those without MCI and 0.93 among those with MCI, relative to light drinkers who consumed less than 1 drink per week.
Among adults with normal cognition at baseline, daily low-quantity drinking was associated with lower dementia risk, compared with infrequent higher-quantity drinking (HR, 0.45).
Trial excluded adults with excessive alcohol use
Limitations of the study include a lack of data about any changes in alcohol consumption over time. In addition, the original trial excluded people with a known history of excessive alcohol use. Furthermore, it is possible that the “long preclinical phase of dementia” and other health issues affect drinking behavior, the authors said. “At present, our findings cannot be directly translated into clinical recommendations,” the authors said. Nevertheless, the results “suggest that, while caring for older adults, physicians should carefully assess the full dimensions of drinking behavior and cognition when providing guidance to patients about alcohol consumption,” they said.
The study was supported by grants from the National Center for Complementary and Alternative Medicine; the National Institute of Neurological Disorders and Stroke; the Office of Dietary Supplements of the National Institute on Aging; the National Heart, Lung, and Blood Institute; the University of Pittsburgh Alzheimer’s Disease Research Center; the Roena Kulynych Center for Memory and Cognition Research; and Wake Forest University School of Medicine. In addition, the researchers used plasma samples from the National Cell Repository for Alzheimer’s Disease, which receives support from the National Institute on Aging. Dr. Koch had no conflicts of interest. Coauthors disclosed university and government grants and personal fees from pharmaceutical companies outside the study. One author was an employee of Genentech at the time of publication, but Genentech did not contribute to the study.
SOURCE: Koch M et al. JAMA Network Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.10319.
Mild cognitive impairment (MCI) may influence the relationship between alcohol consumption and dementia risk, a study of more than 3,000 adults suggests. In addition, , according to the study, which was published in JAMA Network Open.
“The associations of self-reported alcohol consumption with dementia risk and cognitive decline were more consistently adverse among individuals with MCI than those with normal cognition,” reported Manja Koch, PhD, a researcher in the department of nutrition at Harvard T.H. Chan School of Public Health in Boston and colleagues. “This was particularly true for the subset of individuals [with MCI] who drank more than 14.0 servings per week, whose rate of cognitive decline and risk of dementia were the highest of any subgroup.”
Among older adults with normal cognition, the results generally were consistent with those of a recent meta-analysis that found a U-shaped relationship between drinking and dementia, the researchers said (Eur J Epidemiol. 2017 Jan;32[1]:31-42.).
“Our results did not show significant associations and clearly do not suffice to suggest a clinical benefit from even limited alcohol use,” said Dr. Koch and colleagues. “Nonetheless, our findings provide some reassurance that alcohol consumed within recommended limits was not associated with an increased risk of dementia among older adults with normal baseline cognition.”
GEMS data
To study whether alcohol consumption is associated with the risk of dementia and cognitive decline in older adults with and without MCI, the investigators analyzed data from the Ginkgo Evaluation of Memory Study (GEMS). GEMS was a randomized controlled trial conducted between 2000 and 2008 that found no overall association between ginkgo biloba and dementia prevention. During the trial, participants completed the Modified Mini-Mental State Examination, the Clinical Dementia Rating scale, and the cognitive portion of the Alzheimer’s Disease Assessment Scale.
In the present study, the investigators analyzed data from 3,021 participants aged 72 years and older who were free of dementia at baseline and had provided information about their alcohol intake. Their median age was 78 years, and 46.2% were female. Fifty-eight percent consumed alcohol, including 45% of the participants with MCI at baseline.
During follow-up, 512 cases of dementia occurred. Among the 473 participants with MCI at baseline, the adjusted hazard ratio (HR) for dementia was 1.72 for those who consumed more than 14 drinks per week, compared with light drinkers who consumed less than 1 drink per week. For participants who consumed between 7 and 14 drinks per week, the adjusted HR for dementia was 0.63 among those without MCI and 0.93 among those with MCI, relative to light drinkers who consumed less than 1 drink per week.
Among adults with normal cognition at baseline, daily low-quantity drinking was associated with lower dementia risk, compared with infrequent higher-quantity drinking (HR, 0.45).
Trial excluded adults with excessive alcohol use
Limitations of the study include a lack of data about any changes in alcohol consumption over time. In addition, the original trial excluded people with a known history of excessive alcohol use. Furthermore, it is possible that the “long preclinical phase of dementia” and other health issues affect drinking behavior, the authors said. “At present, our findings cannot be directly translated into clinical recommendations,” the authors said. Nevertheless, the results “suggest that, while caring for older adults, physicians should carefully assess the full dimensions of drinking behavior and cognition when providing guidance to patients about alcohol consumption,” they said.
The study was supported by grants from the National Center for Complementary and Alternative Medicine; the National Institute of Neurological Disorders and Stroke; the Office of Dietary Supplements of the National Institute on Aging; the National Heart, Lung, and Blood Institute; the University of Pittsburgh Alzheimer’s Disease Research Center; the Roena Kulynych Center for Memory and Cognition Research; and Wake Forest University School of Medicine. In addition, the researchers used plasma samples from the National Cell Repository for Alzheimer’s Disease, which receives support from the National Institute on Aging. Dr. Koch had no conflicts of interest. Coauthors disclosed university and government grants and personal fees from pharmaceutical companies outside the study. One author was an employee of Genentech at the time of publication, but Genentech did not contribute to the study.
SOURCE: Koch M et al. JAMA Network Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.10319.
Mild cognitive impairment (MCI) may influence the relationship between alcohol consumption and dementia risk, a study of more than 3,000 adults suggests. In addition, , according to the study, which was published in JAMA Network Open.
“The associations of self-reported alcohol consumption with dementia risk and cognitive decline were more consistently adverse among individuals with MCI than those with normal cognition,” reported Manja Koch, PhD, a researcher in the department of nutrition at Harvard T.H. Chan School of Public Health in Boston and colleagues. “This was particularly true for the subset of individuals [with MCI] who drank more than 14.0 servings per week, whose rate of cognitive decline and risk of dementia were the highest of any subgroup.”
Among older adults with normal cognition, the results generally were consistent with those of a recent meta-analysis that found a U-shaped relationship between drinking and dementia, the researchers said (Eur J Epidemiol. 2017 Jan;32[1]:31-42.).
“Our results did not show significant associations and clearly do not suffice to suggest a clinical benefit from even limited alcohol use,” said Dr. Koch and colleagues. “Nonetheless, our findings provide some reassurance that alcohol consumed within recommended limits was not associated with an increased risk of dementia among older adults with normal baseline cognition.”
GEMS data
To study whether alcohol consumption is associated with the risk of dementia and cognitive decline in older adults with and without MCI, the investigators analyzed data from the Ginkgo Evaluation of Memory Study (GEMS). GEMS was a randomized controlled trial conducted between 2000 and 2008 that found no overall association between ginkgo biloba and dementia prevention. During the trial, participants completed the Modified Mini-Mental State Examination, the Clinical Dementia Rating scale, and the cognitive portion of the Alzheimer’s Disease Assessment Scale.
In the present study, the investigators analyzed data from 3,021 participants aged 72 years and older who were free of dementia at baseline and had provided information about their alcohol intake. Their median age was 78 years, and 46.2% were female. Fifty-eight percent consumed alcohol, including 45% of the participants with MCI at baseline.
During follow-up, 512 cases of dementia occurred. Among the 473 participants with MCI at baseline, the adjusted hazard ratio (HR) for dementia was 1.72 for those who consumed more than 14 drinks per week, compared with light drinkers who consumed less than 1 drink per week. For participants who consumed between 7 and 14 drinks per week, the adjusted HR for dementia was 0.63 among those without MCI and 0.93 among those with MCI, relative to light drinkers who consumed less than 1 drink per week.
Among adults with normal cognition at baseline, daily low-quantity drinking was associated with lower dementia risk, compared with infrequent higher-quantity drinking (HR, 0.45).
Trial excluded adults with excessive alcohol use
Limitations of the study include a lack of data about any changes in alcohol consumption over time. In addition, the original trial excluded people with a known history of excessive alcohol use. Furthermore, it is possible that the “long preclinical phase of dementia” and other health issues affect drinking behavior, the authors said. “At present, our findings cannot be directly translated into clinical recommendations,” the authors said. Nevertheless, the results “suggest that, while caring for older adults, physicians should carefully assess the full dimensions of drinking behavior and cognition when providing guidance to patients about alcohol consumption,” they said.
The study was supported by grants from the National Center for Complementary and Alternative Medicine; the National Institute of Neurological Disorders and Stroke; the Office of Dietary Supplements of the National Institute on Aging; the National Heart, Lung, and Blood Institute; the University of Pittsburgh Alzheimer’s Disease Research Center; the Roena Kulynych Center for Memory and Cognition Research; and Wake Forest University School of Medicine. In addition, the researchers used plasma samples from the National Cell Repository for Alzheimer’s Disease, which receives support from the National Institute on Aging. Dr. Koch had no conflicts of interest. Coauthors disclosed university and government grants and personal fees from pharmaceutical companies outside the study. One author was an employee of Genentech at the time of publication, but Genentech did not contribute to the study.
SOURCE: Koch M et al. JAMA Network Open. 2019 Sep 27. doi: 10.1001/jamanetworkopen.2019.10319.
FROM JAMA NETWORK OPEN
Lumbar spine BMD, bone strength benefits persist after romosozumab-to-alendronate switch
ORLANDO – Patients who took romosozumab for 12 months and then switched to alendronate continued to see benefits in bone mineral density (BMD) of the lumbar spine after 12 months of therapy with alendronate, compared with patients who began taking, and continued to take, alendronate over the same time period, according to findings from a subgroup of the ARCH study presented at the annual meeting of the American Society for Bone and Mineral Research.
“These effects occurred rapidly, as early as month 6, were sustained beyond 12 months after transitioning to alendronate, and are consistent with greater fracture-risk reduction observed in ARCH with romosozumab to alendronate versus alendronate to alendronate,” Jacques P. Brown, MD, FRCPC, of Laval University, Quebec City, said in his presentation.
In the double-blinded ARCH study, 4,093 postmenopausal women with osteoporosis and a previous fracture history were randomized to receive subcutaneous monthly romosozumab 210 mg or oral weekly alendronate 70 mg for 12 months, followed by an open-label period during which romosozumab patients received oral weekly alendronate 70 mg and alendronate patients continued to receive the same dose on the same schedule for an additional 24 months (Saag KG et al. N Eng J Med. 2017 Oct 12. doi: 10.1056/NEJMoa1708322).
Dr. Brown and colleagues performed an imaging substudy of ARCH, which included examining how the romosozumab-to-alendronate and alendronate-only groups improved lumbar spine BMD and lumbar spine bone strength. Lumbar spine BMD was assessed through quantitative CT, and lumbar spine bone strength was measured with finite element analysis. The researchers received quantitative CT images from baseline and at 6 months, 12 months, and 24 months, and determined the percentage change at each of those periods to calculate integral, trabecular, and cortical lumbar spine volumetric BMD (vBMD), and to bone mineral content (BMC). They also measured areal BMD (aBMD) at baseline, 6 months, 12 months, 18 months, and 24 months with dual-energy x-ray absorptiometry.
Overall, 49 romosozumab patients and 41 alendronate patients from the ARCH study were enrolled in the imaging substudy. Of those patients, 76 had vBMD and BMC information available at baseline and one or more time periods post baseline, and 86 patients had finite element analysis data at baseline and one or more postbaseline time periods. Patients in the romosozumab and alendronate groups had similar baseline characteristics with regard to age (73.1 years vs. 72.8 years, respectively), mean lumbar spine BMD T score (–2.82 vs. –3.38), mean total hip BMD T score (–2.65 vs. –2.75), mean femoral neck T score (–2.84 vs. –2.83), mean lumbar spine integral vBMD (130.3 mg/cm3 vs. 120.5 mg/cm3), trabecular vBMD (60.1 mg/cm3 vs. 53.7 mg/cm3) and cortical vBMD (284.6 mg/cm3 vs. 270.9 mg/cm3). Patients in both groups also had similar rates of previous osteoporotic fracture at or after aged 45 years, previous vertebral fracture, and history of hip fracture.
Beginning at 6 months, there were significant least squares mean BMD improvements in both groups, but the romosozumab group had significant improvements in aBMD percentage changes, compared with the alendronate group, which persisted until 24 months (P less than .001 at all time points). Integral, trabecular, and cortical vBMD in the romosozumab group also saw significantly greater increases from baseline, compared with the alendronate group, and those results persisted in the open-label portion of the study for patients in the romosozumab group who transitioned to alendronate and patients in the alendronate to alendronate group (P less than .001 at all time points).
“The rapid and large increases in BMD with romosozumab followed by BMD consolidation where [patients were] transitioning to alendronate, support the important role of romosozumab as a first-line therapy in treating patients who are at very high risk for fracture,” Dr. Brown said.
In regard to BMC, there were larger increases in least squares mean BMC changes from baseline in the cortical compartment than the trabecular compartment, and actual change in bone strength as measured by finite element analysis was highly correlated with integral BMC in the romosozumab group.
Dr. Brown said the study was limited to the small sample size from the imaging substudy of ARCH, and quantitative CT dictated the imaging sites for the substudy, which may have affected patient selection. However, he noted that the characteristics of the ARCH imaging substudy were similar to patients in the overall ARCH study.
Amgen, UCB Pharma, and Astellas Pharma funded the study in part. Amgen and UCB Pharma assisted in the preparation of Dr. Brown’s presentation at ASBMR 2019, including funding costs associated with its development. Dr. Brown and the other coauthors reported relationships with Amgen, UCB Pharma, and other companies in the form of consultancies, grants and research support, speaker’s bureau appointments, paid employment, and stock options.
SOURCE: Brown JP et al. ASBMR 2019, Abstract 1050.
ORLANDO – Patients who took romosozumab for 12 months and then switched to alendronate continued to see benefits in bone mineral density (BMD) of the lumbar spine after 12 months of therapy with alendronate, compared with patients who began taking, and continued to take, alendronate over the same time period, according to findings from a subgroup of the ARCH study presented at the annual meeting of the American Society for Bone and Mineral Research.
“These effects occurred rapidly, as early as month 6, were sustained beyond 12 months after transitioning to alendronate, and are consistent with greater fracture-risk reduction observed in ARCH with romosozumab to alendronate versus alendronate to alendronate,” Jacques P. Brown, MD, FRCPC, of Laval University, Quebec City, said in his presentation.
In the double-blinded ARCH study, 4,093 postmenopausal women with osteoporosis and a previous fracture history were randomized to receive subcutaneous monthly romosozumab 210 mg or oral weekly alendronate 70 mg for 12 months, followed by an open-label period during which romosozumab patients received oral weekly alendronate 70 mg and alendronate patients continued to receive the same dose on the same schedule for an additional 24 months (Saag KG et al. N Eng J Med. 2017 Oct 12. doi: 10.1056/NEJMoa1708322).
Dr. Brown and colleagues performed an imaging substudy of ARCH, which included examining how the romosozumab-to-alendronate and alendronate-only groups improved lumbar spine BMD and lumbar spine bone strength. Lumbar spine BMD was assessed through quantitative CT, and lumbar spine bone strength was measured with finite element analysis. The researchers received quantitative CT images from baseline and at 6 months, 12 months, and 24 months, and determined the percentage change at each of those periods to calculate integral, trabecular, and cortical lumbar spine volumetric BMD (vBMD), and to bone mineral content (BMC). They also measured areal BMD (aBMD) at baseline, 6 months, 12 months, 18 months, and 24 months with dual-energy x-ray absorptiometry.
Overall, 49 romosozumab patients and 41 alendronate patients from the ARCH study were enrolled in the imaging substudy. Of those patients, 76 had vBMD and BMC information available at baseline and one or more time periods post baseline, and 86 patients had finite element analysis data at baseline and one or more postbaseline time periods. Patients in the romosozumab and alendronate groups had similar baseline characteristics with regard to age (73.1 years vs. 72.8 years, respectively), mean lumbar spine BMD T score (–2.82 vs. –3.38), mean total hip BMD T score (–2.65 vs. –2.75), mean femoral neck T score (–2.84 vs. –2.83), mean lumbar spine integral vBMD (130.3 mg/cm3 vs. 120.5 mg/cm3), trabecular vBMD (60.1 mg/cm3 vs. 53.7 mg/cm3) and cortical vBMD (284.6 mg/cm3 vs. 270.9 mg/cm3). Patients in both groups also had similar rates of previous osteoporotic fracture at or after aged 45 years, previous vertebral fracture, and history of hip fracture.
Beginning at 6 months, there were significant least squares mean BMD improvements in both groups, but the romosozumab group had significant improvements in aBMD percentage changes, compared with the alendronate group, which persisted until 24 months (P less than .001 at all time points). Integral, trabecular, and cortical vBMD in the romosozumab group also saw significantly greater increases from baseline, compared with the alendronate group, and those results persisted in the open-label portion of the study for patients in the romosozumab group who transitioned to alendronate and patients in the alendronate to alendronate group (P less than .001 at all time points).
“The rapid and large increases in BMD with romosozumab followed by BMD consolidation where [patients were] transitioning to alendronate, support the important role of romosozumab as a first-line therapy in treating patients who are at very high risk for fracture,” Dr. Brown said.
In regard to BMC, there were larger increases in least squares mean BMC changes from baseline in the cortical compartment than the trabecular compartment, and actual change in bone strength as measured by finite element analysis was highly correlated with integral BMC in the romosozumab group.
Dr. Brown said the study was limited to the small sample size from the imaging substudy of ARCH, and quantitative CT dictated the imaging sites for the substudy, which may have affected patient selection. However, he noted that the characteristics of the ARCH imaging substudy were similar to patients in the overall ARCH study.
Amgen, UCB Pharma, and Astellas Pharma funded the study in part. Amgen and UCB Pharma assisted in the preparation of Dr. Brown’s presentation at ASBMR 2019, including funding costs associated with its development. Dr. Brown and the other coauthors reported relationships with Amgen, UCB Pharma, and other companies in the form of consultancies, grants and research support, speaker’s bureau appointments, paid employment, and stock options.
SOURCE: Brown JP et al. ASBMR 2019, Abstract 1050.
ORLANDO – Patients who took romosozumab for 12 months and then switched to alendronate continued to see benefits in bone mineral density (BMD) of the lumbar spine after 12 months of therapy with alendronate, compared with patients who began taking, and continued to take, alendronate over the same time period, according to findings from a subgroup of the ARCH study presented at the annual meeting of the American Society for Bone and Mineral Research.
“These effects occurred rapidly, as early as month 6, were sustained beyond 12 months after transitioning to alendronate, and are consistent with greater fracture-risk reduction observed in ARCH with romosozumab to alendronate versus alendronate to alendronate,” Jacques P. Brown, MD, FRCPC, of Laval University, Quebec City, said in his presentation.
In the double-blinded ARCH study, 4,093 postmenopausal women with osteoporosis and a previous fracture history were randomized to receive subcutaneous monthly romosozumab 210 mg or oral weekly alendronate 70 mg for 12 months, followed by an open-label period during which romosozumab patients received oral weekly alendronate 70 mg and alendronate patients continued to receive the same dose on the same schedule for an additional 24 months (Saag KG et al. N Eng J Med. 2017 Oct 12. doi: 10.1056/NEJMoa1708322).
Dr. Brown and colleagues performed an imaging substudy of ARCH, which included examining how the romosozumab-to-alendronate and alendronate-only groups improved lumbar spine BMD and lumbar spine bone strength. Lumbar spine BMD was assessed through quantitative CT, and lumbar spine bone strength was measured with finite element analysis. The researchers received quantitative CT images from baseline and at 6 months, 12 months, and 24 months, and determined the percentage change at each of those periods to calculate integral, trabecular, and cortical lumbar spine volumetric BMD (vBMD), and to bone mineral content (BMC). They also measured areal BMD (aBMD) at baseline, 6 months, 12 months, 18 months, and 24 months with dual-energy x-ray absorptiometry.
Overall, 49 romosozumab patients and 41 alendronate patients from the ARCH study were enrolled in the imaging substudy. Of those patients, 76 had vBMD and BMC information available at baseline and one or more time periods post baseline, and 86 patients had finite element analysis data at baseline and one or more postbaseline time periods. Patients in the romosozumab and alendronate groups had similar baseline characteristics with regard to age (73.1 years vs. 72.8 years, respectively), mean lumbar spine BMD T score (–2.82 vs. –3.38), mean total hip BMD T score (–2.65 vs. –2.75), mean femoral neck T score (–2.84 vs. –2.83), mean lumbar spine integral vBMD (130.3 mg/cm3 vs. 120.5 mg/cm3), trabecular vBMD (60.1 mg/cm3 vs. 53.7 mg/cm3) and cortical vBMD (284.6 mg/cm3 vs. 270.9 mg/cm3). Patients in both groups also had similar rates of previous osteoporotic fracture at or after aged 45 years, previous vertebral fracture, and history of hip fracture.
Beginning at 6 months, there were significant least squares mean BMD improvements in both groups, but the romosozumab group had significant improvements in aBMD percentage changes, compared with the alendronate group, which persisted until 24 months (P less than .001 at all time points). Integral, trabecular, and cortical vBMD in the romosozumab group also saw significantly greater increases from baseline, compared with the alendronate group, and those results persisted in the open-label portion of the study for patients in the romosozumab group who transitioned to alendronate and patients in the alendronate to alendronate group (P less than .001 at all time points).
“The rapid and large increases in BMD with romosozumab followed by BMD consolidation where [patients were] transitioning to alendronate, support the important role of romosozumab as a first-line therapy in treating patients who are at very high risk for fracture,” Dr. Brown said.
In regard to BMC, there were larger increases in least squares mean BMC changes from baseline in the cortical compartment than the trabecular compartment, and actual change in bone strength as measured by finite element analysis was highly correlated with integral BMC in the romosozumab group.
Dr. Brown said the study was limited to the small sample size from the imaging substudy of ARCH, and quantitative CT dictated the imaging sites for the substudy, which may have affected patient selection. However, he noted that the characteristics of the ARCH imaging substudy were similar to patients in the overall ARCH study.
Amgen, UCB Pharma, and Astellas Pharma funded the study in part. Amgen and UCB Pharma assisted in the preparation of Dr. Brown’s presentation at ASBMR 2019, including funding costs associated with its development. Dr. Brown and the other coauthors reported relationships with Amgen, UCB Pharma, and other companies in the form of consultancies, grants and research support, speaker’s bureau appointments, paid employment, and stock options.
SOURCE: Brown JP et al. ASBMR 2019, Abstract 1050.
REPORTING FROM ASBMR 2019
Vitamin D does not improve bone density, structure in healthy patients
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
REPORTING FROM ASBMR 2019
Project ECHO helps osteoporosis specialists connect with PCPs
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
ORLANDO – The use of a teleconferencing program to share knowledge about osteoporosis has helped health care professionals learn about the disease and may potentially reduce the osteoporosis treatment gap in underserved communities, according to a speaker at the annual meeting of the American Society for Bone and Mineral Research.
The concept, called “technology-enabled collaborative learning,” is intended to address the problem of there being not enough specialists to see patients who need treatment, and the ineffectiveness of educating primary care providers in how to treat complex medical conditions, E. Michael Lewiecki, MD, the director of the New Mexico Clinical Research & Osteoporosis Center said in his presentation.
“Primary care doctors are busy,” said Dr. Lewiecki. “They have limited time taking care of patients. They don’t have the time or often the skills to manage patients who have any questions or concerns about osteoporosis and treatments for osteoporosis.”
One solution, he said, is to find health care professionals in underserved communities who are already interested in and motivated to learn more about osteoporosis, turn them into near-experts on osteoporosis for their patients as well as in their own community.
Dr. Lewiecki proposed the Extension for Community Healthcare Outcomes (ECHO), or Project ECHO, an initiative out of the University of New Mexico School of Medicine, as a potential answer. Project ECHO uses videoconferencing to connect experts in a therapeutic area, with Bone Health TeleECHO focusing on raising knowledge of osteoporosis for its participants. “The idea of ECHO is to be a force multiplier to educate health care professionals, each of whom takes care of many patients, and to have many ECHO programs around the world in convenient time zones and convenient languages for people who are interested in participating,” said Dr. Lewiecki.
The idea began when a gastroenterologist at Dr. Lewiecki’s own center was frustrated that patients were not seeking treatment for hepatitis C because of time or travel issues. In response, a pilot program for Project ECHO was developed through a collaboration between the University of New Mexico Health Sciences Center and the Osteoporosis Foundation of New Mexico where gastroenterologists at University of New Mexico connected with primary care providers across the state, sharing information about hepatitis C and discussing case studies. The results of the pilot program were published in the New England Journal of Medicine and showed a similar rate of sustained viral response between patients treated at the University of New Mexico clinic (84 of 146 patients; 57.5%) and at 21 ECHO clinics (152 of 261 patients; 58.2%) (Arora S et al. N Eng J Med. 2011. doi: 10.1056/NEJMoa1009370).
“ECHO expands the capacity to deliver best practice medical care through collegial, interactive, case-based discussions with minimal disruption to the office routine,” said Dr. Lewiecki. “Patients benefit from better care, closer to home, with greater convenience and lower cost than referral to a medical center. And the potential is to reduce the osteoporosis treatment gap by having many ECHOs starting up in many places in the world.”
Today, the ECHO program is in 37 countries, with 322 ECHO hubs and 677 ECHO programs. The top three specialties are endocrinology, orthopedics, and rheumatology; 51% of ECHO participants are primary care providers, 24% are advanced care providers, and 19% are health care providers such as nutritionists, physical therapists, and other providers that have an interest in bone health.
In survey results adapted from a 2017 study from his own group, Dr. Lewiecki showed that 263 health care professionals who participated in Bone Health TeleECHO rated themselves as more confident in 20 different domains of osteoporosis treatment, such as secondary osteoporosis and anabolic therapy, after 21 months of using the ECHO program (Lewiecki EM et al. J Endocr Soc. 2017. doi: 10.1210/js.2017-00361). However, he admitted that showing fracture prevention outcomes at these ECHO centers has proven more difficult.
“Of course, we’re all interested in outcomes. The ultimate outcome here is preventing fractures, but it is extraordinarily difficult to design a study to actually show that we’re reducing fractures, but certainly self-confidence in managing osteoporosis has improved,” he said.
There have also been some misconceptions of the Project ECHO. The program is not only for beginners or primary care providers, said Dr. Lewiecki. It is also not limited to providers in rural areas, as the program has many participants at urban centers, he added.
“We are a virtual community of practice. It’s a collegial relationship,” he said. “It’s really recapitulating the way that we learned during our postgraduate training: When we see a patient, we present the case to our attending, the attending pontificates a little bit, we bounce things off of one another, and we go back and then we do some different things with our patients. And that’s exactly what we do with Echo. It makes learning fun again.”
Dr. Lewiecki challenged the attendees in the room who are already experts in osteoporosis to help share their knowledge of the disease to help other health care professionals learn more about how to better care for their patients. “If you have a passion for teaching, if you want to share knowledge and you’re willing to devote a little bit of your time to doing that and reaching out to more people, this is the way that you can do it.”
Dr. Lewiecki reports research grant support from Amgen, consulting fees from Alexion, Amgen, Radius, Shire, and Ultragenyx, speaking fees from Alexion, Radius, and Shire, and is an advisory board member with the National Osteoporosis Foundation, International Society for Clinical Densitometry, and the Osteoporosis Foundation of New Mexico.
SOURCE: Lewiecki ME. ASBMR 2019. Symposia: Cutting Edge Concepts: Novel Approaches to Reducing Fractures. Bone Health TeleECHO.
FROM ASBMR 2019