User login
Probiotics with Lactobacillus reduce loss in spine BMD for postmenopausal women
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
, according to recent research published in The Lancet Rheumatology.
“The menopausal and early postmenopausal lumbar spine bone loss is substantial in women, and by using a prevention therapy with bacteria naturally occurring in the human gut microbiota we observed a close to complete protection against lumbar spine bone loss in healthy postmenopausal women,” Per-Anders Jansson, MD, chief physician at the University of Gothenburg (Sweden), and colleagues wrote in their study.
Dr. Jansson and colleagues performed a double-blind trial at four centers in Sweden in which 249 postmenopausal women were randomized during April-November 2016 to receive probiotics consisting of three Lactobacillus strains or placebo once per day for 12 months. Participants were healthy women, neither underweight nor overweight, and were postmenopausal, which was defined as being 2-12 years or less from last menstruation. The Lactobacillus strains, L. paracasei 8700:2 (DSM 13434), L. plantarum Heal 9 (DSM 15312), and L. plantarum Heal 19 (DSM 15313), were equally represented in a capsule at a dose of 1 x 1010 colony-forming unit per capsule. The researchers measured the lumbar spine bone mineral density (LS-BMD) at baseline and at 12 months, and also evaluated the safety profile of participants in both the probiotic and placebo groups.
Overall, 234 participants (94%) had data available for analysis at the end of the study. There was a significant reduction in LS-BMD loss for participants who received the probiotic treatment, compared with women in the control group (mean difference, 0.71%; 95% confidence interval, 0.06%-1.35%), while there was a significant loss in LS-BMD for participants in the placebo group (percentage change, –0.72%; 95% CI, –1.22% to –0.22%) compared with loss in the probiotic group (percentage change, –0.01%; 95% CI, –0.50% to 0.48%). Using analysis of covariance, the researchers found the probiotic group had reduced LS-BMD loss after adjustment for factors such as study site, age at baseline, BMD at baseline, and number of years from menopause (mean difference, 7.44 mg/cm2; 95% CI, 0.38 to 14.50).
In a subgroup analysis of women above and below the median time since menopause at baseline (6 years), participants in the probiotic group who were below the median time saw a significant protective effect of Lactobacillus treatment (mean difference, 1.08%; 95% CI, 0.20%-1.96%), compared with women above the median time (mean difference, 0.31%; 95% CI, –0.62% to 1.23%).
Researchers also examined the effects of probiotic treatment on total hip and femoral neck BMD as secondary endpoints. Lactobacillus treatment did not appear to affect total hip (–1.01%; 95% CI, –1.65% to –0.37%) or trochanter BMD (–1.13%; 95% CI, –2.27% to 0.20%), but femoral neck BMD was reduced in the probiotic group (–1.34%; 95% CI, –2.09% to –0.58%), compared with the placebo group (–0.88%; 95% CI, –1.64% to –0.13%).
Limitations of the study included examining only one dose of Lactobacillus treatment and no analysis of the effect of short-chain fatty acids on LS-BMD. The researchers noted that “recent studies have shown that short-chain fatty acids, which are generated by fermentation of complex carbohydrates by the gut microbiota, are important regulators of both bone formation and resorption.”
The researchers also acknowledged that the LS-BMD effect size for the probiotic treatment over the 12 months was a lower magnitude, compared with first-line treatments for osteoporosis in postmenopausal women using bisphosphonates. “Further long-term studies should be done to evaluate if the bone-protective effect becomes more pronounced with prolonged treatment with the Lactobacillus strains used in the present study,” they said.
In a related editorial, Shivani Sahni, PhD, of Harvard Medical School, Boston, and Connie M. Weaver, PhD, of Purdue University, West Lafayette, Ind., reiterated that the effect size of probiotics is “of far less magnitude” than such treatments as bisphosphonates and expressed concern about the reduction of femoral neck BMD in the probiotic group, which was not explained in the study (Lancet Rheumatol. 2019 Nov;1[3]:e135-e137. doi: 10.1016/S2665-9913(19)30073-6). There is a need to learn the optimum dose of probiotics as well as which Lactobacillus strains should be used in future studies, as the strains chosen by Jansson et al. were based on results in mice.
In the meantime, patients might be better off choosing dietary interventions with proven bone protection and no documented negative effects on the hip, such as prebiotics like soluble corn fiber and dried prunes, in tandem with drug therapies, Dr. Sahni and Dr. Weaver said.
“Although Jansson and colleagues’ results are important, more work is needed before such probiotics are ready for consumers,” they concluded.
This study was funded by Probi, which employs two of the study’s authors. Three authors reported being coinventors of a patent involving the effects of probiotics in osteoporosis treatment, and one author is listed as an inventor on a pending patent application on probiotic compositions and uses. Dr. Sahni reported receiving grants from Dairy Management. Dr. Weaver reported no relevant conflicts of interest.
SOURCE: Jansson P-A et al. Lancet Rheumatol. 2019 Nov;1(3):e154-e162. doi: 10.1016/S2665-9913(19)30068-2
FROM THE LANCET RHEUMATOLOGY
Evaluating a Veterans Affairs Home-Based Primary Care Population for Patients at High Risk of Osteoporosis
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
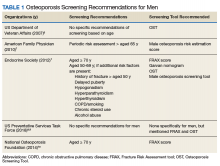
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
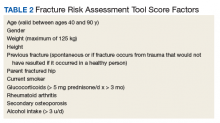
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
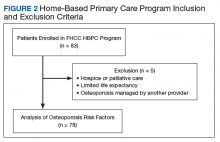
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
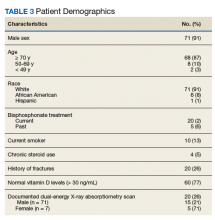
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
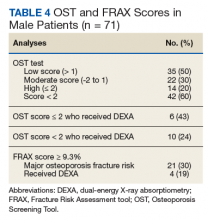
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
Osteoporosis is a disease characterized by the loss of bone density.1 Bone is normally porous and is in a state of flux due to changes in regeneration caused by osteoclast or osteoblast activity. However, age and other factors can accelerate loss in bone density and lead to decreased bone strength and an increased risk of fracture. In men, bone mineral density (BMD) can begin to decline as early as age 30 to 40 years. By age 80 years, 25% of total bone mass may be lost.2
Of the 44 million Americans with low BMD or osteoporosis, 20% are men.1 This group accounts for up to 40% of all osteoporotic fractures. About 1 in 4 men aged ≥ 50 years may experience a lifetime fracture. Fractures may lead to chronic pain, disability, increased dependence, and potentially death. These complications cause expenditures upward of $4.1 billion annually in North America alone.3,4 About 80,000 US men will experience a hip fracture each year, one-third of whom will die within that year. This constitutes a mortality rate 2 to 3 times higher than that of women. Osteoporosis often goes undiagnosed and untreated due to a lack of symptoms until a fracture occurs, underlining the potential benefit of preemptive screening.
In 2007, Shekell and colleagues outlined how the US Department of Veterans Affairs (VA) screened men for osteoporosis.5 At the time, 95% of the VA population was male, though it has since dropped to 91%.6 Shekell and colleagues estimated that about 200,0000 to 400,0000 male veterans had osteoporosis.5 Osteoporotic risk factors deemed specific to veterans were excessive alcohol use, spinal cord injury and lack of weight-bearing exercise, prolonged corticosteroid use, and androgen deprivation therapy in prostate cancer. Different screening techniques were assessed, and the VA recommended the Osteoporosis Self-Assessment Tool (OST).5 Many organizations have developed clinical guidance, including who should be screened; however, screening for men remains a controversial area due to a lack of any strong recommendations (Table 1).
Endocrine Society screening guidelines for men are the most specific: testing BMD in men aged ≥ 70 years, or if aged 50 to 69 years with an additional risk factor (eg, low body weight, smoking, chronic obstructive pulmonary disease, chronic steroid use).1 The Fracture Risk Assessment tool (FRAX) score is often cited as a common screening tool. It is a free online questionnaire that provides a 10-year probability risk of hip or major osteoporotic fracture.11 However, this tool is limited by age, weight, and the assumption that all questions are answered accurately. Some of the information required includes the presence of a number of risk factors, such as alcohol use, glucocorticoids, and medical history of rheumatoid arthritis, among others (Table 2). The OST score, on the other hand, is a calculation that does not take into account other risk factors (Figure 1). This tool categorizes the patient into low, moderate, or high risk for osteoporosis.8
In a study of 4,000 men aged ≥ 70 years,
A 2017 VA Office of Rural Health study examined the utility of OST to screen referred patients aged > 50 years to receive DEXA scans in patient aligned care team (PACT) clinics at 3 different VA locations.13 The study excluded patients who had been screened previously or treated for osteoporosis, were receiving hospice care; 1 site excluded patients aged > 88 years. Two of the sites also reviewed the patient’s medications to screen for agents that may contribute to increased fracture risk. Veterans identified as high risk were referred for education and offered a DEXA scan and treatment. In total, 867 veterans were screened; 19% (168) were deemed high risk, and 6% (53) underwent DEXA scans. The study noted that only 15 patients had reportable DEXA scans and 10 were positive for bone disease.
As there has been documented success in the PACT setting in implementing standardized protocols for screening and treating veterans, it is reasonable to extend the concept into other VA services. The home-based primary care (HBPC) population is especially vulnerable due to the age of patients, limited weight-bearing exercise to improve bone strength, and limited access to DEXA scans due to difficulty traveling outside of the home. Despite these issues, a goal of the HBPC service is to provide continual care for veterans and improve their health so they may return to the community setting. As a result, patients are followed frequently, providing many opportunities for interventions. This study aims to determine the proportion of HBPC patients who are at high risk for osteoporosis and can receive a DEXA scan for evaluation.
Methods
This study was a retrospective chart analysis using descriptive statistics. It was reviewed and approved by the institutional review board at Captain James A. Lovell Federal Health Care Center (FHCC). Patients were included in the study if they were enrolled in the HBPC program at FHCC. Patients were excluded if they were receiving hospice or palliative care, had a limited life expectancy per the HBPC provider, or had a diagnosis of osteoporosis that was being managed by a VA endocrinologist, rheumatologist, or non-VA provider.
The study was conducted from February 1, 2018, through November 30, 2018. All chart reviews were done through the FHCC electronic health record. A minimum of 80 and maximum of 150 charts were reviewed as this was the typical patient volume in the HBPC program. Basic demographic information was collected and analyzed by calculating FRAX and OST scores. With the results, patients were classified as low or high risk of developing osteoporosis, and whether a DEXA scan should be recommended.
Results
After chart review, 83 patients were enrolled in the FHCC HBPC program during the study period. Out of these, 5 patients were excluded due to hospice or palliative care status, limited life expectancy, or had their osteoporosis managed by another non-HBPC provider. As a result, 78 patients were analyzed to determine their risk of osteoporosis (Figure 2). Most of the patients were white males with a median age of 82 years. A majority of the patients did not have any current or previous treatment with bisphosphonates, 77% had normal vitamin D levels, and only 13% (10) were current smokers; of the male patients only 21% (15) had a previous DEXA scan (Table 3).
The FRAX and OST scores for each male patient were calculated (Table 4). Half the patients were low risk for osteoporosis. Just 20% (14) of the patients were at high risk for osteoporosis, and only 6 of those had DEXA scans. However, if expanding the criteria to OST scores of < 2, then only 24% (10) received DEXA scans. When calculating FRAX scores, 30% (21) had ≥ 9.3% for major osteoporotic fracture risk, and only 19% (4) had received a DEXA scan.
Discussion
Based on the collected data, many of the male HBPC patients have not had an evaluation for osteoporosis despite being in a high-risk population and meeting some of the screening guidelines by various organizations.1 Based on Diem and colleagues and the 2007 VA report, utilizing OST scores could help capture a subset of patients that would be referred for DEXA scans.5,12 Of the 60% (42) of patients that met OST scores of < 2, 76% (32) of them could have been referred for DEXA scans for osteoporosis evaluation. However, at the time of publication of this article, 50% (16) of the patients have been discharged from the service without interventions. Of the remaining 16 patients, only 2 were referred for a DEXA scan, and 1 patient had confirmed osteoporosis. Currently, these results have been reviewed by the HBPC provider, and plans are in place for DEXA scan referrals for the remaining patients. In addition, for new patients admitted to the program and during annual reviews, the plan is to use OST scores to help screen for osteoporosis.
Limitations
The HBPC population is often in flux due to discharges as patients pass away, become eligible for long-term care, advance to hospice or palliative care status, or see an improvement in their condition to transition back into the community. Along with patients who are bed-bound, have poor prognosis, and barriers to access (eg, transportation issues), interventions for DEXA scan referrals are often not clinically indicated. During calculations of the FRAX score, documentation is often missing from a patient’s medical chart, making it difficult to answer all questions on the questionnaire. This does increase the utility of the OST score as the calculation is much easier and does not rely on other osteoporotic factors. Despite these restrictions for offering DEXA scans, the HBPC service has a high standard of excellence in preventing falls, a major contributor to fractures. Physical therapy services are readily available, nursing visits are frequent and as clinically indicated, vitamin D levels are maintained within normal limits via supplementation, and medication management is performed at least quarterly among other interventions.
Conclusions
The retrospective chart review of patients in the HBPC program suggests that there may be a lack of standardized screening for osteoporosis in the male patient population. As seen within the data, there is great potential for interventions as many of the patients would be candidates for screening based on the OST score. The tool is easy to use and readily accessible to all health care providers and staff. By increasing screening of eligible patients, it also increases the identification of those who would benefit from osteoporosis treatment. While the HBPC population has access limitations (eg, homebound, limited life expectancy), the implementation of a protocol and extension of concepts from this study can be extrapolated into other PACT clinics at VA facilities. Osteoporosis in the male population is often overlooked, but screening procedures can help reduce health care expenditures.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
1. Watts NB, Adler RA, Bilezikian JP, et al; Endocrine Society. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822.
2. Holt G, Smith R, Duncan K, Hutchison JD, Gregori A. Gender differences in epidemiology and outcome after hip fracture: evidence from the Scottish Hip Fracture Audit. J Bone Joint Surg Br. 2008;90(4):480-483.
3. Ackman JM, Lata PF, Schuna AA, Elliott ME. Bone health evaluation in a veteran population: a need for the Fracture Risk Assessment tool (FRAX). Ann Pharmacother. 2014;48(10):1288-1293.
4. International Osteoporosis Foundation. Osteoporosis in men: why change needs to happen. http://share.iofbone-health.org/WOD/2014/thematic-report/WOD14-Report.pdf. Published 2014. Accessed September 16, 2019.
5. Shekell P, Munjas B, Liu H, et al. Screening Men for Osteoporosis: Who & How. Evidence-based Synthesis Program. Washington, DC: Department of Veterans Affairs; 2007.
6. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. https://www.va.gov/vetdata/Veteran_Population.asp. Accessed September 16, 2019.
7. Rao SS, Budhwar N, Ashfaque A. Osteoporosis in men. Am Fam Physician. 2010;82(5):503-508.
8. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(24):2521-2531.
9. Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551.
10. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381.
11. Centre for Metabolic Bone Diseases, University of Sheffield, UK. FRAX Fracture Risk Assessment Tool. http://www.sheffield.ac.uk/FRAX/tool.aspx?country=9. Accessed September 16, 2019.
12. Diem SJ, Peters KW, Gourlay ML, et al; Osteoporotic Fractures in Men Research Group. Screening for osteoporosis in older men: operating characteristics of proposed strategies for selecting men for BMD testing. J Gen Intern Med. 2017;32(11):1235-1241.
13. US Department of Veterans Affairs, Office of Rural Health. Osteoporosis risk assessment using Osteoporosis Self-Assessment Tool (OST) and other interventions at rural facilities. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_OST_Issue%20Brief_v2.pdf. Published February 7, 2019. Accessed September 16, 2019.
Get ready for changes in polypharmacy quality ratings
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
REPORTING FROM AMCP NEXUS 2019
Geriatric Nutritional Risk Index predicts long-term outcomes in PAD
PARIS – The Geriatric Nutritional Risk Index proved to be an independent predictor of 5-year overall survival as well as the composite of major adverse cardiovascular and limb events in a prospective cohort study of 1,219 patients with peripheral artery disease, Yae Matsuo, MD, reported at the annual congress of the European Society of Cardiology.
The Geriatric Nutritional Risk Index (GNRI) is a score calculated with a formula based upon a patient’s height, serum albumin, and the ratio between ideal and actual body weight (Am J Clin Nutr. 2005 Oct;82(4):777-83). The GNRI tool has been shown to be an accurate prognosticator for clinical outcomes in patients on hemodialysis and those with heart failure. However, it’s predictive accuracy hasn’t been evaluated in patients with PAD, according to Dr. Matsuo, a cardiologist at Kitakanto Cardiovascular Hospital in Shibukawa, Japan.
“The Geriatric Nutritional Risk Index is simple to calculate – so easy – and I think it’s a better predictor than BMI,” she said.
Fifty-six percent of the PAD patients had a GNRI score greater than 98, indicative of no increased risk of malnutrition and nutritional deficiencies. Their 5-year overall survival rate was 81%, compared with 62% in patients with a score of 92-98, 40% in those with a score of 82-91, and 23% with a score of less than 82. Other independent predictors of overall survival in multivariate analysis were age, estimated glomerular filtration rate, ankle brachial index, and C-reactive protein level.
A GNRI score above 98 was also predictive of significantly lower 5-year risk of both major adverse cardiovascular events and the composite of major adverse cardiovascular and limb events than in patients with a score of 98 or less.
The key remaining unanswered question is whether providing timely nutritional support to PAD patients with a low GNRI score will result in improved overall and limb survival and other outcomes.
Dr. Matsuo reported having no financial conflicts.
SOURCE: Matsuo Y. ESC CONGRESS 2019. Abstract P1956.
PARIS – The Geriatric Nutritional Risk Index proved to be an independent predictor of 5-year overall survival as well as the composite of major adverse cardiovascular and limb events in a prospective cohort study of 1,219 patients with peripheral artery disease, Yae Matsuo, MD, reported at the annual congress of the European Society of Cardiology.
The Geriatric Nutritional Risk Index (GNRI) is a score calculated with a formula based upon a patient’s height, serum albumin, and the ratio between ideal and actual body weight (Am J Clin Nutr. 2005 Oct;82(4):777-83). The GNRI tool has been shown to be an accurate prognosticator for clinical outcomes in patients on hemodialysis and those with heart failure. However, it’s predictive accuracy hasn’t been evaluated in patients with PAD, according to Dr. Matsuo, a cardiologist at Kitakanto Cardiovascular Hospital in Shibukawa, Japan.
“The Geriatric Nutritional Risk Index is simple to calculate – so easy – and I think it’s a better predictor than BMI,” she said.
Fifty-six percent of the PAD patients had a GNRI score greater than 98, indicative of no increased risk of malnutrition and nutritional deficiencies. Their 5-year overall survival rate was 81%, compared with 62% in patients with a score of 92-98, 40% in those with a score of 82-91, and 23% with a score of less than 82. Other independent predictors of overall survival in multivariate analysis were age, estimated glomerular filtration rate, ankle brachial index, and C-reactive protein level.
A GNRI score above 98 was also predictive of significantly lower 5-year risk of both major adverse cardiovascular events and the composite of major adverse cardiovascular and limb events than in patients with a score of 98 or less.
The key remaining unanswered question is whether providing timely nutritional support to PAD patients with a low GNRI score will result in improved overall and limb survival and other outcomes.
Dr. Matsuo reported having no financial conflicts.
SOURCE: Matsuo Y. ESC CONGRESS 2019. Abstract P1956.
PARIS – The Geriatric Nutritional Risk Index proved to be an independent predictor of 5-year overall survival as well as the composite of major adverse cardiovascular and limb events in a prospective cohort study of 1,219 patients with peripheral artery disease, Yae Matsuo, MD, reported at the annual congress of the European Society of Cardiology.
The Geriatric Nutritional Risk Index (GNRI) is a score calculated with a formula based upon a patient’s height, serum albumin, and the ratio between ideal and actual body weight (Am J Clin Nutr. 2005 Oct;82(4):777-83). The GNRI tool has been shown to be an accurate prognosticator for clinical outcomes in patients on hemodialysis and those with heart failure. However, it’s predictive accuracy hasn’t been evaluated in patients with PAD, according to Dr. Matsuo, a cardiologist at Kitakanto Cardiovascular Hospital in Shibukawa, Japan.
“The Geriatric Nutritional Risk Index is simple to calculate – so easy – and I think it’s a better predictor than BMI,” she said.
Fifty-six percent of the PAD patients had a GNRI score greater than 98, indicative of no increased risk of malnutrition and nutritional deficiencies. Their 5-year overall survival rate was 81%, compared with 62% in patients with a score of 92-98, 40% in those with a score of 82-91, and 23% with a score of less than 82. Other independent predictors of overall survival in multivariate analysis were age, estimated glomerular filtration rate, ankle brachial index, and C-reactive protein level.
A GNRI score above 98 was also predictive of significantly lower 5-year risk of both major adverse cardiovascular events and the composite of major adverse cardiovascular and limb events than in patients with a score of 98 or less.
The key remaining unanswered question is whether providing timely nutritional support to PAD patients with a low GNRI score will result in improved overall and limb survival and other outcomes.
Dr. Matsuo reported having no financial conflicts.
SOURCE: Matsuo Y. ESC CONGRESS 2019. Abstract P1956.
REPORTING FROM THE ESC CONGRESS 2019
Severe hypoglycemia, poor glycemic control fuels fracture risk in older diabetic patients
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
Patients with type 2 diabetes and poor glycemic control or severe hypoglycemia may be at greater risk for fracture, according to recent research from a Japanese cohort of older men and postmenopausal women.
“The impacts of severe hypoglycemia and poor glycemic control on fractures appeared to be independent,” noted Yuji Komorita, MD, PhD, of the department of medicine and clinical science, Graduate School of Medical Sciences at Kyushu University, and colleagues. “This study suggests that the glycemic target to prevent fractures may be HbA1c <75 mmol/mol, which is far higher than that used to prevent microvascular complications, and higher than that for older adults with type 2 diabetes.”
Dr. Komorita and colleagues performed a prospective analysis of fracture incidence for 2,755 men and 1,951 postmenopausal women with type 2 diabetes in the Fukuoka Diabetes Registry who were mean 66 years old between April 2008 and October 2010. At the start of the study, the researchers assessed patient diabetes duration, previous fracture history, physical activity, alcohol and smoking status, whether patients were treated for diabetic retinopathy with laser photocoagulation, and their history of coronary artery disease or stroke. Patients were followed for a median 5.3 years, during which fractures were assessed through an annual self-administered questionnaire, with the results stratified by glycemic control and hypoglycemia.
Overall, there were 249 men and 413 women who experienced fractures during the study period, with a follow-up rate of 97.6%. In a multivariate analysis, patients with a higher risk of fracture included those with two or more episodes of severe hypoglycemia (hazard ratio, 2.25; 95% confidence interval, 1.57-3.22) and one episode of severe hypoglycemia (HR, 1.57; 95% CI, 1.11-2.20). In patients without severe hypoglycemic episodes, there was an increased risk of fracture in those with baseline hemoglobin A1c (HbA1c) level of 53 to less than 64 mmol/mol (7% to less than 8%; HR, 1.14; 0.94-1.39), 64 to less than 75 mmol/mol (8% to less than 9%; HR, 1.11; 95% CI, 0.86-1.43), and at least 75 mmol/mol (at least 9%; HR, 1.45; 95% CI, 1.06-1.98).
Compared with postmenopausal women, the unadjusted risk of fracture was higher in men with multiple severe hypoglycemic episodes (HR, 3.46; 95% CI, 2.05-5.85) and one episode of hypoglycemia (HR, 2.81; 95% CI, 1.74-4.56). These higher risks in older men persisted after adjustment for age, multivariate factors, and HbA1c.
“The association between severe hypoglycemia, poor glycemic control, and fracture risk at any anatomic site seems to be stronger in men than in postmenopausal women, although the interaction between men and postmenopausal women for fracture risk was not significant,” the researchers said. “The higher incidence rate of fractures in postmenopausal women, compared with men, was attributed to drastic changes in sex hormones after menopause, which may reduce the apparent impacts of hyperglycemia and severe hypoglycemia on postmenopausal women.”
Researchers said they did not consider potential external factors for fracture incidence, nor did they measure incident falls or other markers of bone health, such as bone mineral density and 25-hydroxyvitamin D levels. They also noted among the limitations of the study the self-reported nature of fracture reporting, and the lack of generalizability of the results.
This study was funded in part by grants from The Japan Society for the Promotion of Science KAKENHI from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Junior Scientist Development Grant supported by the Japan Diabetes Society; and the Lilly Research Grant Program for Bone & Mineral Research. The authors reported no relevant conflicts of interest.
SOURCE: Komorita Y et al. Diabet Med. 2019 Sep 25. doi: 10.1111/dme.14142.
FROM DIABETIC MEDICINE
Dr. Paul Aisen Q&A: Aducanumab for Alzheimer’s
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.
In the wake of Biogen and Eisai’s Oct. 22 announcement about plans to apply to the Food and Drug Administration next year for the regulatory approval of the investigational monoclonal antibody aducanumab as a treatment for Alzheimer’s disease, we spoke with Paul Aisen, MD, the founding director of the Alzheimer’s Therapy Research Institute at the University of Southern California, Los Angeles, for his views on the news. He has been a consultant for Biogen and is a member of the aducanumab steering committee.
Q: What was your first reaction when you heard about the plan to submit an application for aducanumab to the FDA?
A: My initial reaction is that this provides terrific support for the amyloid hypothesis, and is consistent with the early aducanumab studies showing significant reductions in brain amyloid with resulting clinical improvement.
My next thought was that these data are going to be very, very challenging to analyze because both of these trials were stopped early, and one was clearly negative. We really need to scrutinize the data, but even at this point I would say this strongly supports targeting amyloid. The scrutiny will begin in detail at the Clinical Trials in Alzheimer’s Disease conference in December, when Biogen will likely release detailed data. A lot of people will analyze it, and I think that’s great. It’s beneficial to bring different perspectives.
We have had a terribly frustrating series of disappointments in the field. After the futility analysis of aducanumab and the multiple failures of BACE [beta-secretase] inhibitors, many were convinced we were barking up the wrong tree. I think these results, although complicated, should resurrect the enthusiasm for targeting amyloid.
Q: What is different about aducanumab from other antibodies tested – and rejected – in Alzheimer’s drug development?
A: There are lots of antibodies that have been tested in clinical trials. They all differ in terms of their affinity for amyloid beta. Some target monomers of the protein. Some target dimers. Some target fibrils. Some tie up amyloid and some reduce it. Aducanumab directly attacks brain plaques, reducing the plaque load in the brain. It carries a liability of amyloid-related imaging abnormalities [ARIA], but it also allows us to assess the impact that removing plaques might have on downstream events, including biomarkers. Overall, these data show that aducanumab did remove brain plaques and that removing them had a beneficial effect on cognition and function, and also a favorable effect on downstream biomarkers.
But again, we must be cautious because this is a complex data set taken from a post hoc analysis of two different terminated trials.
Q: We see some statistically significant differences in cognitive and functional outcomes. What would that mean for patients on an everyday basis?
A: Well, everyone is different, so that’s hard to say. A 25% slowing of functional decline on the Clinical Dementia Rating Scale sum of boxes (CDR-SB) might mean that, at the end of a year, there’s not a significant change in memory, or that there’s better social function. If both trials had been completed and if people had 18 months of high-dose aducanumab, the slowing of functional decline on the CDR-SB might in fact be greater than reported. Again, we’re having to draw conclusions from interrupted trials.
Q: This suggestion you make of a potentially continuous slowing of decline – are you suggesting that aducanumab might slow decline to the point of stopping it altogether? If an elderly patient has little or no progression until death would that, in effect, be considered a “cure?”
A: I don’t think it is possible to cure AD once the disease is clinically evident. These are studies of people with early AD, late mild cognitive impairment, and mild dementia. At that stage, there’s already a loss of synapses that won’t come back, and these studies don’t suggest that aducanumab can cure that. But what if people took it earlier, when the brain is still functioning normally? Some of us have argued for many years that earlier intervention is the way to go. And since we can now identify people [with brain plaques] before they become symptomatic, there is the possibility that if we removed them, we could stop progression.
Q: Are there any plans to study aducanumab as a preventive agent?
A: A grant has been awarded for this, but it was put on hold after the futility analysis. I don’t know when or if that will go forward.
(Editor’s note: The National Institutes of Health previously awarded Banner Health a $32 million, 5-year grant to examine this. The 2-year prevention study of aducanumab is aimed at cognitively unimpaired 65- to 80-year-old patients with PET-confirmed amyloid brain plaques. It was to be a multicenter, double-blind, placebo-controlled trial using Alzheimer’s biomarker endpoints as primary outcomes, along with cognitive and clinical changes, safety, and tolerability. The study was put on hold after Biogen discontinued the aducanumab development program in March. Investigators are considering whether to resurrect plans considering the new data. The study is intended to be a public-private partnership, with additional unspecified funding from Biogen plus $10 million from philanthropic sources. It has three intended goals: To find an approved prevention therapy as early as 2023, ahead of the National Plan to Address Alzheimer’s Disease’s goal of an effective prevention strategy by 2025; to advance the use of surrogate biomarkers to rapidly test and support accelerated approval of prevention therapies in almost everyone at biomarker or genetic risk, even in earlier preclinical Alzheimer’s stages when some treatments may have their greatest benefit; and to help make it possible to conduct prevention trials in at-risk persons even before they have extensive amyloid plaques, when some treatments may have their greatest benefit.)
Q: It seems like rolling this out to an enormous population of patients is going to be difficult, if not impossible. Are people really going to be able to commit to what could be a lifetime of monthly intravenous infusions of a medicine that could be expensive, as therapeutic antibodies generally are?
A: I would say, nothing about this disease is easy. It’s devastating and horrible. And if someone is diagnosed at this stage, I would think that individual would embrace any opportunity to treat it. My hope is that we will be able to prescreen people with an effective blood test for amyloid that would be part of a regular testing protocol once they reach a certain age. Those with positive results would be referred for more testing, including amyloid brain imaging.
ACIP plans flu review for older adults
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
according to data presented at a meeting of the Centers for Disease Control and Prevention’s ACIP.
Lynette Brammer of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD) presented a surveillance update of the flu season in the United States so far. Overall, the influenza A(H3N2) viruses are predominant, although dominance varies in different regions of the country, and it is too soon to predict what strain will dominate later in the season.
“While two of the four vaccine components were updated for the Southern Hemisphere, the components selected for the 2019-2020 Northern Hemisphere vaccine, at this time, look appropriate for the season,” she said.
In other flu news, Lisa Groskopf, MD, of the NCIRD discussed the influenza work group’s plans for a meta-analysis to assess the relative benefit of different vaccines for older adults, in light of the growing variety of products available.
Currently, no preferential recommendations have been made for a specific vaccine for a particular age group. “There’s a dearth of data comparing these vaccines to one another,” said Dr. Groskopf. She added that, because vaccine effectiveness varies by season, the generalizability of effectiveness data is another challenge.
The work group’s systematic review and meta-analysis is designed to compare the high-dose inactivated influenza vaccine (HD-IIV), the adjuvanted inactivated influenza vaccine (aIIV), and the recombinant influenza vaccine (RIV). The study will include adults aged 65 years and older who receive trivalent or quadrivalent HD-IIV, aIIV, or RIV, compared with those who receive another influenza vaccine, a noninfluenza control vaccine, placebo, or no vaccine. The outcomes will include data on safety and effectiveness of the vaccines, Dr. Groskopf said.
In addition to safety and effectiveness, manufacturers such as Sanofi Pasteur continue to collect data on the success of available vaccines and develop new ones. Lee-Jah Chang, MD, of Sanofi Pasteur presented results of a noninferiority study of the company’s investigational high-dose quadrivalent influenza vaccine (QIV-HD; including two prevailing B viruses) versus the high-dose trivalent influenza vaccine (TID-HD). The study was conducted at 35 sites in the United States and included 2,670 adults aged 65 years and older.
Overall, the reactogenicity profile for patients given QIV-HD was similar to that of TID-HD, and approximately 5% of patients in the QIV group reported an immediate adverse event, Dr. Chang said. However, no related deaths or related adverse events of special interest occurred in any of the study groups.
Sanofi plans to pursue licensure of the QIV-HD vaccine, with a Center for Biologics Evaluation and Research action date of Nov. 4, 2019, said Dr. Chang. If the vaccine is licensed, it should be available for purchase by health care providers in the first quarter of 2020.
The ACIP members had no financial conflicts to disclose.
REPORTING FROM AN ACIP MEETING
Biogen plans to submit application to FDA for Alzheimer’s drug aducanumab
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
Biogen aims to file with the Food and Drug Administration for regulatory approval of aducanumab, an antibody under investigation for Alzheimer’s disease, in 2020 following largely positive results of a secondary analysis of two failed phase 3 trials, ENGAGE and EMERGE, the company announced Oct. 22.
Biogen’s plans reverse its March 21, 2019, decision with codeveloper Eisai to discontinue work on the drug after a futility analysis of the trials determined aducanumab was unlikely to yield significant benefit. Biogen announced the plan to file a biologic drug application following a new analysis of additional data that became available after the data lock on the futility analysis. But while primary and secondary endpoints were nearly all positive for EMERGE in the secondary analysis of the larger dataset, the same could not be said for the twin trial, ENGAGE, which had negative results for most of its endpoints. However, Biogen said that “results from a subset of patients in the phase 3 ENGAGE study who received sufficient exposure to high-dose aducanumab support the findings from EMERGE.”
Both the Alzheimer’s Association and researchers interpreted the announcement with a measured tone, saying it offered a hopeful sign for a field continually stymied in its quest for an effective treatment. More than 100 clinical trials have failed over the last 20 years.
“This really is very encouraging news,” Rebecca M. Edelmayer, PhD, director of scientific engagement at the Alzheimer’s Association, said in an interview. The secondary combined analyses showed “the largest reductions in clinical and functional decline we have seen. It’s an important moment for the patients with AD and their families, and for researchers all around the world. This deserves to be discussed and considered by the research community, but we really need to dig deep into the data. We expect to see more of them at the Clinical Trials on Alzheimer’s Disease conference,” which is set for early December.
Paul Aisen, MD, a consultant for Biogen and director of the Alzheimer’s Therapeutic Research Institute at the University of Southern California, Los Angeles, was similarly measured.
“There is an enormous amount of data here and they will be very challenging to interpret, especially since both trials were stopped in a futility analysis,” he said in an interview. “We’re now interpreting data that continue to be collected after the initial data lock. But I do believe there is evidence that supports the amyloid hypothesis and the development of aducanumab.”
A deep data dive is in order before the field completely embraces aducanumab’s advancement, agreed Michael Wolfe, PhD, the Mathias P. Mertes Professor of Medicinal Chemistry at the University of Kansas, Lawrence.
“We would have to see exactly how they came up with this new data set and analysis. I have felt for many years now that these companies would try to parse and shuffle the data around until they got a statistically significant result, just to get approval. They would make billions per year but not really make a difference in people’s lives. That being said, if aducanumab truly slows the decline in activities of daily living, keeping people independent longer, that would be a worthwhile clinical result.”
Still to be considered is whether aducanumab could confer enough benefits to be worth monthly, potentially lifelong, infusions of a pricey medication that still won’t stop disease progression.
“Whether the clinical impact will be worth the anticipated cost remains to be seen,” Richard J. Caselli, MD, said in an interview. “This is likely to be a very expensive treatment for a subgroup of individuals with the hope of slowing decline, but – unless there is a huge upside surprise in data yet to be released – it is not going to halt progression and will certainly increase the cost of care dramatically.”
Dr. Caselli, clinical core director of the Alzheimer’s Disease Center at the Mayo Clinic in Phoenix, continued: “I imagine if approved, there will be a number of insurance obstacles to overcome regarding who qualifies, for how long, etc. Scientifically, this certainly supports the long-held view that beta amyloid is important in the pathophysiology of Alzheimer’s disease. But, again, unless the impact is unexpectedly huge, I don’t think this quiets those who feel there is more to the story than only a gain of beta amyloid toxicity, though this does support the idea that it plays a role, which should not surprise anyone.”
Dr. Edelmayer said the Alzheimer’s Association has raised the issue of access and cost with Biogen.
“We have had many discussions about their capacity to roll this out,” if aducanumab is approved, she said. “Those who were in the studies will be the first recipients. But Biogen is making plans to move this into the general population if approved, to all patients who meet the diagnostic criteria.”
“There is precedent out there when it comes to doing infusion medicines, and those centers are part of the planning process. In terms of pricing, this is a problem we would be happy to see, because it would mean that we have a treatment. But we’ll cross that bridge when we come to it.”
Secondary analysis results
The new analysis comprised 2,066 patients who had the opportunity to complete the full 18-month trials by March 20, 2019. The full intent-to-treat population of the trials comprised 3,285 patients with mild cognitive impairment caused by Alzheimer’s disease or mild Alzheimer’s disease dementia.
In the secondary analysis of EMERGE’s intent-to-treat population, patients who took the highest dose of aducanumab (10 mg/kg every month) showed 23% lower functional and cognitive decline on the trial’s primary endpoint of Clinical Dementia Rating–Sum of Boxes (CDR-SB) at 18 months when compared with placebo. The rate of decline was slowed by a nonsignificant 14% among users of the lower dose (6 mg/kg monthly).Secondary endpoints for the high-dose group in EMERGE showed 27% slower cognitive decline on the 13-item cognitive subscale of the AD Assessment Scale (ADAS-Cog13) and 40% lower decline in function among patients with mild cognitive impairment based on the MCI version of the AD Cooperative Study–Activities of Daily Living Inventory (ADCS-ADL-MCI).
However, data from the ENGAGE trial, which had the same primary endpoint, were not positive. CDR-SB scores worsened 2% more among high-dose aducanumab users but low-dose users slowed decline by a nonsignificant 12% when compared with placebo.“Exposure to high-dose aducanumab was important for efficacy,” the company noted in a slide set presented at an Oct. 22 investors webcast. “Differences in exposure to high-dose aducanumab largely explain the different results between the futility analysis and the new analysis of this larger dataset, as well as the different results between the two studies.”
In EMERGE, changes in secondary endpoints among patients who had the opportunity to complete the full 18-month trials included:
- Mini Mental State Exam (MMSE): Significant 23% decrease in rate of decline in the high-dose group and nonsignificant 3% increase in decline in the low-dose group.
- ADAS-Cog13: Significant 25% decrease for high-dose users and nonsignificant 10% decrease for low dose.
- ADCS-ADL-MCI: Significant decreases of 46% for high-dose and 20% for low-dose users.
The ENGAGE secondary endpoints of high- vs. low-dose patients who completed the full trials were:
- MMSE: Significant 13% increase, nonsignificant 3% decrease.
- ADAS-Cog13: Nonsignificant 2% decrease, nonsignificant 1% decrease.
- ADCS-ADL-MCI: Significant 12% declines in both dosage groups.
All of the positive cognitive and functional results tracked along with results of amyloid PET imaging and CSF biomarkers. In EMERGE, amyloid plaque binding declined about 27% in the high-dose group and about 16% in the low-dose group, reflecting plaque clearance. Phosphorylated tau in CSF decreased by about 17 pg/mL and 10 pg/mL, respectively, and total tau decreased by 160 pg/mL and 120 pg/mL. Tau decreases indicate a slowing of neuronal damage.
In ENGAGE, amyloid plaque binding decreased by about 24% in the high-dose group and by about 16% in the low-dose group. Phosphorylated tau dropped by about 10 pg/mL and 11 pg/mL, respectively. But total tau dropped more in the low-dose group than in the high-dose group (about –100 pg/mL vs. –20 pg/mL).
Biogen said the amyloid PET imaging biomarker results and CDR-SB scores in both studies were consistent with each other in a subset of patients with “sufficient exposure to 10 mg/kg,” which was defined as “10 or more uninterrupted 10-mg/kg dosing intervals at steady-state.”
The most common adverse events were amyloid related imaging abnormalities–edema (ARIA-E), which occurred in 35%, and headache in 20%. The majority of patients who experienced ARIA-E (74%) were asymptomatic; episodes generally resolved within 4-16 weeks, typically without long-term sequelae.
Dr. Aisen is a consultant for Biogen and on the aducanumab steering committee. None of the other sources in this article have any financial relationship with Biogen or Eisai.
This article was updated 10/23/19.
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at pdnews@mdedge.com.
Agitation in psychosis: Still no ‘magic bullet’
SAN DIEGO – The Food and Drug Administration has not approved a drug to treat agitation in dementia, and the absence of medication candidates is only part of the picture. As a geriatric psychiatrist explained to colleagues, the FDA has not taken the step of recognizing that the condition exists. But there are still options to treat this dangerous disorder – although none is ideal.
Research into efficacy of potential treatments for agitation is limited, variable, and “have high placebo effects,” said Marc E. Agronin, MD, of the MIND Institute and Miami Jewish Health, at the annual Psych Congress. “There is no one single magic bullet, especially since there are so many manifestations of agitation, and there are side effects of medication. This is a tough area to focus on.”
What can clinicians do? Dr. Agronin recommended starting with the steps in the DICE algorithm.
- Describe: Learn about the aspects of agitation by talking to caregivers and understanding the circumstances when symptoms develop.
- Investigate: Identify contributing factors, such as those related to illness, medication, and the environment.
- Create: Come up with a team strategy to address the contributing factors. Delirium is especially dangerous since it can lead to injury and subacute cognitive decline. And keep in mind, Dr. Agorin said, that it may be risky to do nothing or undertreat.
- Evaluate: Track the results of the strategy while realizing that there’s “not always a quick fix.” Research suggests that therapeutic approaches such as music, aromatherapy, exercise, group activities, hand massage, and thermal baths can be helpful, Dr. Agronin said.
As for medications, he advised starting with lower doses, perhaps 50%, because older people are less tolerant of medication. And beware of oversedation, dizziness, and lowered blood pressure, which can lead to falls. A hip fracture can “spiral down to someone’s demise very quickly,” he said.
Here’s a closer look at Dr. Agronin’s comments regarding specific medications.
- Antipsychotics: “Every antipsychotic has been used for agitation,” he said, “and they probably have the best efficacy,” compared with other drugs. But the risk of side effects is moderate to high, and atypical antipsychotics have a black-box warning about their use in dementia-related psychosis in elderly patients. Also, discontinuation of antipsychotics can trigger worsening symptoms in some patients. There has been tremendous controversy in recent years over the use of antipsychotics in older patients, but other drugs might be less effective than antipsychotics while still having similar side effect profiles, he said. And clinicians might be too cautious about doses even when they do use these drugs.
- Benzodiazepines: They can work quickly but come with a risk of sedation. Trazodone is an “excellent” alternative to reduce agitation in the short-term, he said.
- Antidepressants: These drugs can address underlying depression. Study results have been mixed.
- Mood stabilizers: Study results are mixed. “Unfortunately, in many situations [clinicians] get scared away from antipsychotics and use mood stabilizers, but there is less data for them in terms of efficacy, and there are a lot of side effects that have to be monitored,” he said.
Dr. Agronin is the author of “How We Age” (Da Capo Lifelong Books, 2012) and “The End of Old Age” (Da Capo Lifelong Books, 2018). He has no relevant disclosures.
SAN DIEGO – The Food and Drug Administration has not approved a drug to treat agitation in dementia, and the absence of medication candidates is only part of the picture. As a geriatric psychiatrist explained to colleagues, the FDA has not taken the step of recognizing that the condition exists. But there are still options to treat this dangerous disorder – although none is ideal.
Research into efficacy of potential treatments for agitation is limited, variable, and “have high placebo effects,” said Marc E. Agronin, MD, of the MIND Institute and Miami Jewish Health, at the annual Psych Congress. “There is no one single magic bullet, especially since there are so many manifestations of agitation, and there are side effects of medication. This is a tough area to focus on.”
What can clinicians do? Dr. Agronin recommended starting with the steps in the DICE algorithm.
- Describe: Learn about the aspects of agitation by talking to caregivers and understanding the circumstances when symptoms develop.
- Investigate: Identify contributing factors, such as those related to illness, medication, and the environment.
- Create: Come up with a team strategy to address the contributing factors. Delirium is especially dangerous since it can lead to injury and subacute cognitive decline. And keep in mind, Dr. Agorin said, that it may be risky to do nothing or undertreat.
- Evaluate: Track the results of the strategy while realizing that there’s “not always a quick fix.” Research suggests that therapeutic approaches such as music, aromatherapy, exercise, group activities, hand massage, and thermal baths can be helpful, Dr. Agronin said.
As for medications, he advised starting with lower doses, perhaps 50%, because older people are less tolerant of medication. And beware of oversedation, dizziness, and lowered blood pressure, which can lead to falls. A hip fracture can “spiral down to someone’s demise very quickly,” he said.
Here’s a closer look at Dr. Agronin’s comments regarding specific medications.
- Antipsychotics: “Every antipsychotic has been used for agitation,” he said, “and they probably have the best efficacy,” compared with other drugs. But the risk of side effects is moderate to high, and atypical antipsychotics have a black-box warning about their use in dementia-related psychosis in elderly patients. Also, discontinuation of antipsychotics can trigger worsening symptoms in some patients. There has been tremendous controversy in recent years over the use of antipsychotics in older patients, but other drugs might be less effective than antipsychotics while still having similar side effect profiles, he said. And clinicians might be too cautious about doses even when they do use these drugs.
- Benzodiazepines: They can work quickly but come with a risk of sedation. Trazodone is an “excellent” alternative to reduce agitation in the short-term, he said.
- Antidepressants: These drugs can address underlying depression. Study results have been mixed.
- Mood stabilizers: Study results are mixed. “Unfortunately, in many situations [clinicians] get scared away from antipsychotics and use mood stabilizers, but there is less data for them in terms of efficacy, and there are a lot of side effects that have to be monitored,” he said.
Dr. Agronin is the author of “How We Age” (Da Capo Lifelong Books, 2012) and “The End of Old Age” (Da Capo Lifelong Books, 2018). He has no relevant disclosures.
SAN DIEGO – The Food and Drug Administration has not approved a drug to treat agitation in dementia, and the absence of medication candidates is only part of the picture. As a geriatric psychiatrist explained to colleagues, the FDA has not taken the step of recognizing that the condition exists. But there are still options to treat this dangerous disorder – although none is ideal.
Research into efficacy of potential treatments for agitation is limited, variable, and “have high placebo effects,” said Marc E. Agronin, MD, of the MIND Institute and Miami Jewish Health, at the annual Psych Congress. “There is no one single magic bullet, especially since there are so many manifestations of agitation, and there are side effects of medication. This is a tough area to focus on.”
What can clinicians do? Dr. Agronin recommended starting with the steps in the DICE algorithm.
- Describe: Learn about the aspects of agitation by talking to caregivers and understanding the circumstances when symptoms develop.
- Investigate: Identify contributing factors, such as those related to illness, medication, and the environment.
- Create: Come up with a team strategy to address the contributing factors. Delirium is especially dangerous since it can lead to injury and subacute cognitive decline. And keep in mind, Dr. Agorin said, that it may be risky to do nothing or undertreat.
- Evaluate: Track the results of the strategy while realizing that there’s “not always a quick fix.” Research suggests that therapeutic approaches such as music, aromatherapy, exercise, group activities, hand massage, and thermal baths can be helpful, Dr. Agronin said.
As for medications, he advised starting with lower doses, perhaps 50%, because older people are less tolerant of medication. And beware of oversedation, dizziness, and lowered blood pressure, which can lead to falls. A hip fracture can “spiral down to someone’s demise very quickly,” he said.
Here’s a closer look at Dr. Agronin’s comments regarding specific medications.
- Antipsychotics: “Every antipsychotic has been used for agitation,” he said, “and they probably have the best efficacy,” compared with other drugs. But the risk of side effects is moderate to high, and atypical antipsychotics have a black-box warning about their use in dementia-related psychosis in elderly patients. Also, discontinuation of antipsychotics can trigger worsening symptoms in some patients. There has been tremendous controversy in recent years over the use of antipsychotics in older patients, but other drugs might be less effective than antipsychotics while still having similar side effect profiles, he said. And clinicians might be too cautious about doses even when they do use these drugs.
- Benzodiazepines: They can work quickly but come with a risk of sedation. Trazodone is an “excellent” alternative to reduce agitation in the short-term, he said.
- Antidepressants: These drugs can address underlying depression. Study results have been mixed.
- Mood stabilizers: Study results are mixed. “Unfortunately, in many situations [clinicians] get scared away from antipsychotics and use mood stabilizers, but there is less data for them in terms of efficacy, and there are a lot of side effects that have to be monitored,” he said.
Dr. Agronin is the author of “How We Age” (Da Capo Lifelong Books, 2012) and “The End of Old Age” (Da Capo Lifelong Books, 2018). He has no relevant disclosures.
REPORTING FROM PSYCH CONGRESS 2019