User login
AD biomarker not tied to increased interest in physician-assisted death
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
The fascinating thing about this study is that the idea for it arose when some of the individuals spontaneously mentioned assisted suicide during their initial interview, Annette L. Hanson, MD, said in an interview.
“Would these subjects have thought of suicide in the absence of the Brittany Maynard publicity campaign? I doubt it.”
Dr. Hanson, a forensic psychiatrist, is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
Being diagnosed with an elevated amyloid-beta biomarker that indicates greater risk of Alzheimer’s disease did not lead to increased consideration of physician-assisted death (PAD), according to an analysis of patients interviewed during clinical trials on cognitive decline.
“Our findings suggest that learning one’s amyloid imaging result does not change baseline attitudes regarding the acceptability of PAD,” wrote Emily A. Largent, PhD, of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and coauthors. The study was published as a research letter in JAMA Neurology.
Participants were recruited from two ongoing clinical trials, one of which included patients with elevated amyloid-beta (n = 50), whereas the other did not (n = 30). All participants completed an interview 4-12 weeks after receiving their biomarker results; 47 and 30 participants, respectively, also completed a follow-up interview at 12 months.
When asked whether they had considered PAD, nearly two-thirds of interviewees with the Alzheimer’s disease biomarker stated that they neither had nor would. Roughly one in five from that group said they would pursue PAD if they began to suffer from cognitive impairment or became a burden on others. Interviewees who did not have elevated amyloid beta, when asked whether a reversed result would have led to PAD or suicide, showed interest in roughly similar proportion to their at-risk counterparts.
The coauthors acknowledged the limitations of their study, including not asking about other end-of-life preferences or perceived quality of life for people with dementia. They also noted that, although their sample mirrors the populations of the two studies they drew from, “its homogeneity limits generalizability.” As such, they stressed that
The study was supported by grants from the Alzheimer’s Association and the National Institute on Aging. One author reported receiving grants from those two organizations during the study; another reported receiving grants from Lilly and Novartis. No other conflicts of interest were reported.
SOURCE: Largent EA et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0797.
FROM JAMA NEUROLOGY
Medical cannabis relieved pain, decreased opioid use in elderly
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
results of a recent retrospective chart review suggest. Treatment with medical cannabis improved pain, sleep, anxiety, and neuropathy in patients aged 75 years of age and older, and was associated with reduced use of opioids in about one-third of cases, according to authors of the study, which will be presented at the annual meeting of the American Academy of Neurology.
“Our findings are promising and can help fuel further research into medical marijuana as an additional option for this group of people who often have chronic conditions,” said lead investigator Laszlo Mechtler, MD, of Dent Neurologic Institute in Buffalo, N.Y., in a news release. However, additional randomized, placebo-controlled studies are needed to confirm results of this study, Dr. Mechtler added.
The chart review focused on 204 elderly patients who participated in New York State’s medical marijuana program and were followed in a neurologic outpatient setting. The cohort included 129 female and 75 male patients, ranging in age from 75 to 102 years, with a mean age of 81 years. The medical marijuana was taken by mouth as a liquid extract tincture, capsule, or in an electronic vaporizer.
With an average exposure time of 16.8 weeks, 69% of patients experienced symptomatic benefit, according to patient self-report. The most commonly reported benefit was relief of chronic pain in 49%, while improvements in sleep, neuropathy, and anxiety were reported in 18%, 15%, and 10%, respectively. Reductions in opioid pain medication were noted in about one-third of cases, they found.
While 34% of patients had adverse effects on medical marijuana, only 21% reported adverse effects after cannabinoid doses were adjusted, investigators said. Adverse effects led to discontinuation of medical cannabis in seven patients, or 3.4% of the overall cohort. Somnolence, disequilibrium, and gastrointestinal disturbance were the most common adverse effects, occurring in 13%, 7%, and 7% of patients, respectively. Euphoria was reported in 3% of patients.
Among patients who had no reported adverse effects, the most commonly used formulation was a balanced 1:1 tincture of tetrahydrocannabinol to cannabidiol, investigators said.
Further trials could explore optimal dosing of medical cannabis in elderly patients and shed more light on adverse effects such as somnolence and disequilibrium, according to Dr. Mechtler and colleagues.
The study was supported by the Dent Family Foundation.
SOURCE: Bargnes V et al. AAN 2019, Abstract P4.1-014.
FROM AAN 2019
New sleep apnea guidelines offer evidence-based recommendations
New guidelines on treating obstructive sleep apnea with positive airway pressure include recommendations for using positive airway pressure (PAP) versus no therapy, using either continuous PAP (CPAP) or automatic PAP (APAP) for ongoing treatment, and providing educational interventions to patients starting PAP. The complete guidelines, issued by the American Academy of Sleep Medicine, were published in the Journal of Clinical Sleep Medicine.
The guidelines were driven by improvements in PAP adherence and device technology, wrote lead author Susheel P. Patil, MD, of Johns Hopkins University, Baltimore, and his colleagues.
The guidelines begin with a pair of Good Practice Statements to ensure effective and appropriate management of obstructive sleep apnea (OSA) in adults. First, “Treatment of OSA with PAP therapy should be based on a diagnosis of OSA established using objective sleep apnea testing.” Second, “Adequate follow-up, including troubleshooting and monitoring of objective efficacy and usage data to ensure adequate treatment and adherence, should occur following PAP therapy initiation and during treatment of OSA.”
The nine recommendations, approved by the AASM board of directors, include four strong recommendations that clinicians should follow under most circumstances, and five conditional recommendations that are suggested but lack strong clinical support for their appropriateness for all patients in all circumstances.
The first of the strong recommendations, for using PAP versus no therapy to treat adults with OSA and excessive sleepiness, was based on a high level of evidence from a meta-analysis of 38 randomized, controlled trials and the conclusion that the benefits of PAP outweighed the harms.
The second strong recommendation for using either CPAP or APAP for ongoing treatment was based on data from 26 trials that showed no clinically significant difference between the two. The third strong recommendation that PAP therapy be initiated using either APAP at home or in-laboratory PAP titration in adults with OSA and no significant comorbidities was supported by a meta-analysis of 10 trials that showed no clinically significant difference between at-home and laboratory initiation, and that each option has its benefits. The authors noted that “the majority of well-informed adult patients with OSA and without significant comorbidities would prefer initiation of PAP using the most rapid, convenient, and cost-effective strategy.” This comment supports the fourth strong recommendation for providing educational interventions to patients starting PAP.
The conditional recommendations include using PAP versus no therapy for adults with OSA and impaired quality of life related to poor sleep, such as insomnia, snoring, morning headaches, and daytime fatigue. Other conditional recommendations include using PAP versus no therapy for adults with OSA and comorbid hypertension, choosing CPAP or APAP over bilateral PAP for routine treatment of OSA in adults, providing behavioral interventions or troubleshooting during patients’ initial use of PAP, and using telemonitoring-guided interventions to monitor patients during their initial use of PAP.
“The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources,” the authors noted.
“When implementing the recommendations, providers should consider additional strategies that will maximize the individual patient’s comfort and adherence such as nasal/intranasal over oronasal mask interface and heated humidification,” they added.
The guidelines were developed by a task force commissioned by the AASM that included board-certified sleep specialists and experts in PAP use, and will be reviewed and updated as new information surfaces, the authors wrote.
Dr. Patil reported no financial conflicts; several coauthors reported conflicts that were managed by their not voting on guidelines related to those conflicts.
SOURCE: Patil SP et al. J Clin Sleep Med. 2018 Feb 15;15(2):335-43.
Octavian C. Ioachimescu, MD, FCCP, comments: The last guidelines and practice parameters for the use of positive airway pressure (PAP) as therapy for adult patients with obstructive sleep apnea, were published in 2006 and 2008, respectively. Since then, new technological advances, an ever-growing body of literature, and shifting practice patterns led to an acute need for a thorough reassessment, a comprehensive update of the previous recommendations, and the potential of issuing new ones for emerging areas. As such, the American Academy of Sleep Medicine commissioned a task force of content experts to review the existing evidence, to issue new guidelines and to publish an associated systematic review and a meta-analysis of the literature on this topic.
A welcome recommendation is the endorsement by the task force of the use of telemedicine capabilities in monitoring patients’ adherence to PAP therapy. Another interesting aspect is that, while our literature is represented by a mix of both randomized and nonrandomized controlled trials, occasionally there seems to be an interesting dichotomy in the results: Randomized trials tend to point in one direction, while nonrandomized studies pooled in the meta-analysis seem to point to the contrary or to give the impression of more definitive effects. While this is clearly not the place to make an extensive analysis of the strengths and the potential pitfalls of randomized versus nonrandomized studies, this clearly raises some issues. One is that our randomized studies are typically small, underpowered, and hence with nonconvincing risk or hazard reduction assessments. Second, the dichotomy in the results may be driven by publication bias, expense, and difficulty in performing adequately-powered, long-term trials that essentially may be studying small effects.
Guidelines are not intended to be used in an Occam’s razor approach, but in a fashion that would allow individualization of therapy while critically appraising the existing evidence for various interventions in specific conditions and maintaining a very stringent and critical view on generalizability, expected results, and adequate management of reasonable expectations. In addition, the areas that are unclear, with conflicting evidence or in which the guidelines allow “too much” latitude to the treating clinician, may be seen as either an invitation to remain “creative,” or one for abstaining from action in the name of equipoise. I would advise that both extremes are to be avoided.
Octavian C. Ioachimescu, MD, FCCP, comments: The last guidelines and practice parameters for the use of positive airway pressure (PAP) as therapy for adult patients with obstructive sleep apnea, were published in 2006 and 2008, respectively. Since then, new technological advances, an ever-growing body of literature, and shifting practice patterns led to an acute need for a thorough reassessment, a comprehensive update of the previous recommendations, and the potential of issuing new ones for emerging areas. As such, the American Academy of Sleep Medicine commissioned a task force of content experts to review the existing evidence, to issue new guidelines and to publish an associated systematic review and a meta-analysis of the literature on this topic.
A welcome recommendation is the endorsement by the task force of the use of telemedicine capabilities in monitoring patients’ adherence to PAP therapy. Another interesting aspect is that, while our literature is represented by a mix of both randomized and nonrandomized controlled trials, occasionally there seems to be an interesting dichotomy in the results: Randomized trials tend to point in one direction, while nonrandomized studies pooled in the meta-analysis seem to point to the contrary or to give the impression of more definitive effects. While this is clearly not the place to make an extensive analysis of the strengths and the potential pitfalls of randomized versus nonrandomized studies, this clearly raises some issues. One is that our randomized studies are typically small, underpowered, and hence with nonconvincing risk or hazard reduction assessments. Second, the dichotomy in the results may be driven by publication bias, expense, and difficulty in performing adequately-powered, long-term trials that essentially may be studying small effects.
Guidelines are not intended to be used in an Occam’s razor approach, but in a fashion that would allow individualization of therapy while critically appraising the existing evidence for various interventions in specific conditions and maintaining a very stringent and critical view on generalizability, expected results, and adequate management of reasonable expectations. In addition, the areas that are unclear, with conflicting evidence or in which the guidelines allow “too much” latitude to the treating clinician, may be seen as either an invitation to remain “creative,” or one for abstaining from action in the name of equipoise. I would advise that both extremes are to be avoided.
Octavian C. Ioachimescu, MD, FCCP, comments: The last guidelines and practice parameters for the use of positive airway pressure (PAP) as therapy for adult patients with obstructive sleep apnea, were published in 2006 and 2008, respectively. Since then, new technological advances, an ever-growing body of literature, and shifting practice patterns led to an acute need for a thorough reassessment, a comprehensive update of the previous recommendations, and the potential of issuing new ones for emerging areas. As such, the American Academy of Sleep Medicine commissioned a task force of content experts to review the existing evidence, to issue new guidelines and to publish an associated systematic review and a meta-analysis of the literature on this topic.
A welcome recommendation is the endorsement by the task force of the use of telemedicine capabilities in monitoring patients’ adherence to PAP therapy. Another interesting aspect is that, while our literature is represented by a mix of both randomized and nonrandomized controlled trials, occasionally there seems to be an interesting dichotomy in the results: Randomized trials tend to point in one direction, while nonrandomized studies pooled in the meta-analysis seem to point to the contrary or to give the impression of more definitive effects. While this is clearly not the place to make an extensive analysis of the strengths and the potential pitfalls of randomized versus nonrandomized studies, this clearly raises some issues. One is that our randomized studies are typically small, underpowered, and hence with nonconvincing risk or hazard reduction assessments. Second, the dichotomy in the results may be driven by publication bias, expense, and difficulty in performing adequately-powered, long-term trials that essentially may be studying small effects.
Guidelines are not intended to be used in an Occam’s razor approach, but in a fashion that would allow individualization of therapy while critically appraising the existing evidence for various interventions in specific conditions and maintaining a very stringent and critical view on generalizability, expected results, and adequate management of reasonable expectations. In addition, the areas that are unclear, with conflicting evidence or in which the guidelines allow “too much” latitude to the treating clinician, may be seen as either an invitation to remain “creative,” or one for abstaining from action in the name of equipoise. I would advise that both extremes are to be avoided.
New guidelines on treating obstructive sleep apnea with positive airway pressure include recommendations for using positive airway pressure (PAP) versus no therapy, using either continuous PAP (CPAP) or automatic PAP (APAP) for ongoing treatment, and providing educational interventions to patients starting PAP. The complete guidelines, issued by the American Academy of Sleep Medicine, were published in the Journal of Clinical Sleep Medicine.
The guidelines were driven by improvements in PAP adherence and device technology, wrote lead author Susheel P. Patil, MD, of Johns Hopkins University, Baltimore, and his colleagues.
The guidelines begin with a pair of Good Practice Statements to ensure effective and appropriate management of obstructive sleep apnea (OSA) in adults. First, “Treatment of OSA with PAP therapy should be based on a diagnosis of OSA established using objective sleep apnea testing.” Second, “Adequate follow-up, including troubleshooting and monitoring of objective efficacy and usage data to ensure adequate treatment and adherence, should occur following PAP therapy initiation and during treatment of OSA.”
The nine recommendations, approved by the AASM board of directors, include four strong recommendations that clinicians should follow under most circumstances, and five conditional recommendations that are suggested but lack strong clinical support for their appropriateness for all patients in all circumstances.
The first of the strong recommendations, for using PAP versus no therapy to treat adults with OSA and excessive sleepiness, was based on a high level of evidence from a meta-analysis of 38 randomized, controlled trials and the conclusion that the benefits of PAP outweighed the harms.
The second strong recommendation for using either CPAP or APAP for ongoing treatment was based on data from 26 trials that showed no clinically significant difference between the two. The third strong recommendation that PAP therapy be initiated using either APAP at home or in-laboratory PAP titration in adults with OSA and no significant comorbidities was supported by a meta-analysis of 10 trials that showed no clinically significant difference between at-home and laboratory initiation, and that each option has its benefits. The authors noted that “the majority of well-informed adult patients with OSA and without significant comorbidities would prefer initiation of PAP using the most rapid, convenient, and cost-effective strategy.” This comment supports the fourth strong recommendation for providing educational interventions to patients starting PAP.
The conditional recommendations include using PAP versus no therapy for adults with OSA and impaired quality of life related to poor sleep, such as insomnia, snoring, morning headaches, and daytime fatigue. Other conditional recommendations include using PAP versus no therapy for adults with OSA and comorbid hypertension, choosing CPAP or APAP over bilateral PAP for routine treatment of OSA in adults, providing behavioral interventions or troubleshooting during patients’ initial use of PAP, and using telemonitoring-guided interventions to monitor patients during their initial use of PAP.
“The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources,” the authors noted.
“When implementing the recommendations, providers should consider additional strategies that will maximize the individual patient’s comfort and adherence such as nasal/intranasal over oronasal mask interface and heated humidification,” they added.
The guidelines were developed by a task force commissioned by the AASM that included board-certified sleep specialists and experts in PAP use, and will be reviewed and updated as new information surfaces, the authors wrote.
Dr. Patil reported no financial conflicts; several coauthors reported conflicts that were managed by their not voting on guidelines related to those conflicts.
SOURCE: Patil SP et al. J Clin Sleep Med. 2018 Feb 15;15(2):335-43.
New guidelines on treating obstructive sleep apnea with positive airway pressure include recommendations for using positive airway pressure (PAP) versus no therapy, using either continuous PAP (CPAP) or automatic PAP (APAP) for ongoing treatment, and providing educational interventions to patients starting PAP. The complete guidelines, issued by the American Academy of Sleep Medicine, were published in the Journal of Clinical Sleep Medicine.
The guidelines were driven by improvements in PAP adherence and device technology, wrote lead author Susheel P. Patil, MD, of Johns Hopkins University, Baltimore, and his colleagues.
The guidelines begin with a pair of Good Practice Statements to ensure effective and appropriate management of obstructive sleep apnea (OSA) in adults. First, “Treatment of OSA with PAP therapy should be based on a diagnosis of OSA established using objective sleep apnea testing.” Second, “Adequate follow-up, including troubleshooting and monitoring of objective efficacy and usage data to ensure adequate treatment and adherence, should occur following PAP therapy initiation and during treatment of OSA.”
The nine recommendations, approved by the AASM board of directors, include four strong recommendations that clinicians should follow under most circumstances, and five conditional recommendations that are suggested but lack strong clinical support for their appropriateness for all patients in all circumstances.
The first of the strong recommendations, for using PAP versus no therapy to treat adults with OSA and excessive sleepiness, was based on a high level of evidence from a meta-analysis of 38 randomized, controlled trials and the conclusion that the benefits of PAP outweighed the harms.
The second strong recommendation for using either CPAP or APAP for ongoing treatment was based on data from 26 trials that showed no clinically significant difference between the two. The third strong recommendation that PAP therapy be initiated using either APAP at home or in-laboratory PAP titration in adults with OSA and no significant comorbidities was supported by a meta-analysis of 10 trials that showed no clinically significant difference between at-home and laboratory initiation, and that each option has its benefits. The authors noted that “the majority of well-informed adult patients with OSA and without significant comorbidities would prefer initiation of PAP using the most rapid, convenient, and cost-effective strategy.” This comment supports the fourth strong recommendation for providing educational interventions to patients starting PAP.
The conditional recommendations include using PAP versus no therapy for adults with OSA and impaired quality of life related to poor sleep, such as insomnia, snoring, morning headaches, and daytime fatigue. Other conditional recommendations include using PAP versus no therapy for adults with OSA and comorbid hypertension, choosing CPAP or APAP over bilateral PAP for routine treatment of OSA in adults, providing behavioral interventions or troubleshooting during patients’ initial use of PAP, and using telemonitoring-guided interventions to monitor patients during their initial use of PAP.
“The ultimate judgment regarding any specific care must be made by the treating clinician and the patient, taking into consideration the individual circumstances of the patient, available treatment options, and resources,” the authors noted.
“When implementing the recommendations, providers should consider additional strategies that will maximize the individual patient’s comfort and adherence such as nasal/intranasal over oronasal mask interface and heated humidification,” they added.
The guidelines were developed by a task force commissioned by the AASM that included board-certified sleep specialists and experts in PAP use, and will be reviewed and updated as new information surfaces, the authors wrote.
Dr. Patil reported no financial conflicts; several coauthors reported conflicts that were managed by their not voting on guidelines related to those conflicts.
SOURCE: Patil SP et al. J Clin Sleep Med. 2018 Feb 15;15(2):335-43.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Geroscience brings bench science to the real-world problems of aging
NEW ORLEANS – Patients ask their doctors whether dietary manipulation can extend lifespan and promote healthy aging. Right now, basic scientists and clinicians from many disciplines are teaming up under the broad umbrella of the field of geroscience to try to answer these and other concerns relevant to an aging population.
“The idea here is that, instead of going after each disease one at a time, as we do ... [we] instead go after disease vulnerability – and this is something that is shared, as a function of age,” Rozalyn Anderson, PhD, said of this new discipline. The work touches on disparate diseases such as cancer, dementia, and diabetes, she pointed out during a video interview at the annual meeting of the Endocrine Society.
“I separate these things out into ‘front-end’ and ‘back-end,’ work,” said Dr. Anderson of the University of Wisconsin-Madison’s aging and caloric restriction program. She explained that the caloric restriction she researches is back-end work to support the rapidly evolving field of nutritional modulation of aging.
When the basic science builds the framework, physicians and scientists can turn to front-end research, looking at humans to see which dietary manipulations are effective – and which are achievable.
“Take a paradigm that works, and then try to understand how it works,” said Dr. Anderson. “So [for example], we have this paradigm, and it’s tremendously effective in rodents. It’s effective in flies, in worms, in yeast, in spiders, in dogs – and in nonhuman primates.” Then, she and her team try to pull out clues “about the biology of aging itself, and what creates disease vulnerability as a function of age,” she said.
“The most important thing of all is that we can modify aging. This is not a foregone conclusion – no one would have believed it. But even in a primate species, we can change how they age. And the way in which we change is through nutrition.”
Dr Anderson added that “the paradigm of caloric restriction is tremendously effective, but [in reality], people are not going to do it.” It’s simply not practical to ask individuals to restrict calories by 30% or more over a lifespan, so “.”
Dr. Anderson reported no relevant conflicts of interest or disclosures.
NEW ORLEANS – Patients ask their doctors whether dietary manipulation can extend lifespan and promote healthy aging. Right now, basic scientists and clinicians from many disciplines are teaming up under the broad umbrella of the field of geroscience to try to answer these and other concerns relevant to an aging population.
“The idea here is that, instead of going after each disease one at a time, as we do ... [we] instead go after disease vulnerability – and this is something that is shared, as a function of age,” Rozalyn Anderson, PhD, said of this new discipline. The work touches on disparate diseases such as cancer, dementia, and diabetes, she pointed out during a video interview at the annual meeting of the Endocrine Society.
“I separate these things out into ‘front-end’ and ‘back-end,’ work,” said Dr. Anderson of the University of Wisconsin-Madison’s aging and caloric restriction program. She explained that the caloric restriction she researches is back-end work to support the rapidly evolving field of nutritional modulation of aging.
When the basic science builds the framework, physicians and scientists can turn to front-end research, looking at humans to see which dietary manipulations are effective – and which are achievable.
“Take a paradigm that works, and then try to understand how it works,” said Dr. Anderson. “So [for example], we have this paradigm, and it’s tremendously effective in rodents. It’s effective in flies, in worms, in yeast, in spiders, in dogs – and in nonhuman primates.” Then, she and her team try to pull out clues “about the biology of aging itself, and what creates disease vulnerability as a function of age,” she said.
“The most important thing of all is that we can modify aging. This is not a foregone conclusion – no one would have believed it. But even in a primate species, we can change how they age. And the way in which we change is through nutrition.”
Dr Anderson added that “the paradigm of caloric restriction is tremendously effective, but [in reality], people are not going to do it.” It’s simply not practical to ask individuals to restrict calories by 30% or more over a lifespan, so “.”
Dr. Anderson reported no relevant conflicts of interest or disclosures.
NEW ORLEANS – Patients ask their doctors whether dietary manipulation can extend lifespan and promote healthy aging. Right now, basic scientists and clinicians from many disciplines are teaming up under the broad umbrella of the field of geroscience to try to answer these and other concerns relevant to an aging population.
“The idea here is that, instead of going after each disease one at a time, as we do ... [we] instead go after disease vulnerability – and this is something that is shared, as a function of age,” Rozalyn Anderson, PhD, said of this new discipline. The work touches on disparate diseases such as cancer, dementia, and diabetes, she pointed out during a video interview at the annual meeting of the Endocrine Society.
“I separate these things out into ‘front-end’ and ‘back-end,’ work,” said Dr. Anderson of the University of Wisconsin-Madison’s aging and caloric restriction program. She explained that the caloric restriction she researches is back-end work to support the rapidly evolving field of nutritional modulation of aging.
When the basic science builds the framework, physicians and scientists can turn to front-end research, looking at humans to see which dietary manipulations are effective – and which are achievable.
“Take a paradigm that works, and then try to understand how it works,” said Dr. Anderson. “So [for example], we have this paradigm, and it’s tremendously effective in rodents. It’s effective in flies, in worms, in yeast, in spiders, in dogs – and in nonhuman primates.” Then, she and her team try to pull out clues “about the biology of aging itself, and what creates disease vulnerability as a function of age,” she said.
“The most important thing of all is that we can modify aging. This is not a foregone conclusion – no one would have believed it. But even in a primate species, we can change how they age. And the way in which we change is through nutrition.”
Dr Anderson added that “the paradigm of caloric restriction is tremendously effective, but [in reality], people are not going to do it.” It’s simply not practical to ask individuals to restrict calories by 30% or more over a lifespan, so “.”
Dr. Anderson reported no relevant conflicts of interest or disclosures.
REPORTING FROM ENDO 2019
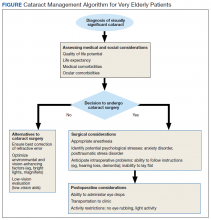
A Primary Care Provider’s Guide to Cataract Surgery in the Very Elderly
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
Cataract surgery is the most commonly performed surgical procedure in the US, including within the Veterans Health Administration (VHA).1,2 As the risk of surgical complications has decreased with improved techniques and instrumentation, the threshold for performing surgery has lowered.3 A substantial number of patients do not develop clinically significant cataracts until they are “very elderly,” defined as aged ≥ 85 years by the World Health Organization and National Institute of Aging.4
Should the general approach to cataract evaluation and surgery differ in this subset of patients? Advanced age is associated with a variety of systemic and ocular comorbidities that theoretically increase the risk of cataract surgery and reduce the potential visual benefit it might yield. However, the impact of age on the outcomes of cataract surgery differs even among the very elderly. There are no universally acknowledged guidelines that address the perioperative evaluation and management of cataracts in the very elderly, whose systemic and ocular health have greater variability than those of their younger counterparts. For very elderly patients who are found to have visually significant cataracts by their ophthalmologists, input from the primary care provider (PCP), who has insight into a patient’s health and well-being, is vital for formulating a management plan. Herein, we provide a framework for PCPs to assist very elderly patients and their ophthalmologists in making an informed decision regarding cataract surgery and in planning for perioperative care.
Cataract Surgery
Cataract surgeons recommend surgical extraction when there is a clinically significant lens opacity that imposes functional impairment, such as inability to read, perform near work, watch television, or drive.4 The standard of care for a clinically significant cataract is surgical removal of the crystalline lens and replacement with an artificial intraocular lens (IOL). At times, the onset of vision loss from a cataract is insidious such that patients may not be aware of their declining vision or the deterioration in quality of life (QOL) that it causes.
Despite the higher burden of ocular comorbidity (eg, age-related macular degeneration, glaucoma) relative to their younger counterparts, most very elderly patients obtain functionally important improvement in their vision, QOL, and cognitive function after surgery.5-16 Cataract surgery can also reduce the risk of dementia and the risk of falls and hip fractures.6,9,12-14,16-18 Ophthalmic complications of cataract surgery in the very elderly include posterior capsule tear (< 1%-9%), vitreous loss (< 1%-8%), zonular rupture (2%-5%), and retained lens fragments (≤ 1%).5,8-11,17,19-21 There is no evidence from well-controlled studies that suggests that very elderly cataract surgery patients are at higher risk of ocular complications relative to that of their younger counterparts.22
Surgery Alternatives
In some very elderly patients, cataract surgery may not be the best option, and PCPs can aid in establishing an alternative plan. Such patients include those with a limited life expectancy, incapacitating anxiety over surgery, or those in whom the potential for visual improvement is marginal because of ocular or systemic comorbidities—eg, vision-limiting glaucoma or age-related macular degeneration, history of stroke to the visual pathway, or restriction to bed. Alternatives to cataract surgery in these instances include changing environmental conditions to improve visual function, such as enhanced lighting and contrast, and/or use of low-vision aids (referring patients to low-vision professionals often improves QOL).23 Low-vision specialists also have a variety of nonvisual aids that can expand functional capabilities: large-print and talking versions of reading materials, telephones, remote controls, clocks, scales, calculators, and glucose monitors; glare-free lights for stairs, floors, and counters; and specialty glasses that use light-emitting diode screens and live video streams to magnify sight.23-25
Medical Evaluation
For patients who decide to proceed with surgery, it can be helpful to have a medical evaluation by their PCPs to minimize potential complications during surgery. The very elderly may be at increased risk of intraoperative transient hypertension, restlessness, and electrocardiogram abnormalities.5,7,17 Systemic comorbidities that become more prevalent with age, such as diabetes mellitus (DM), hypertension, heart disease, chronic obstructive pulmonary disease, and dementia, may adversely impact the risk of sedation and/or general anesthesia. In the VHA, providers also must be aware of combat-related disorders that can confound cataract surgery, such as posttraumatic stress disorder (PTSD), anxiety, and claustrophobia.26,27
Anesthesia in cataract surgery ranges from topical to general, and the selection largely rests on patient physical and psychological comfort and cooperation. Often, intracameral (inside the anterior eye) anesthetic is used with topical anesthesia to provide additional comfort.27 Patients who have high levels of anxiety about surgery may not tolerate topical anesthesia alone.28 In these cases, retrobulbar anesthesia may be performed to block all sensation and motility of the eye. IV sedation is performed prior to the retrobulbar injection to calm patients. Although cataract surgery is typically performed with topical or retrobulbar anesthesia (reducing the potential for systemic complications), there are cases in which general anesthesia may be considered.27 Very elderly patients may become confused or disoriented in the operating room (OR), leading to surgical complications and less than optimal outcomes.5 A higher rate of intraoperative “restlessness,” which occurred in patients who had comorbid dementia, and transient hypertension were found in a study on cataract surgery in the very elderly, but well-controlled studies are lacking.5 Dementia can impose problems with intraoperative cooperation, which is vital for successful surgery in patients who undergo topical or local anesthesia. If these potential problems are thought likely preoperatively, light sedation or general anesthesia—in conjunction with input from the patient’s PCP—are options to minimize disruptive behavior in the OR.
Additional features of the VHA population may influence the selection of anesthesia. The VHA has an important educational mission, and retrobulbar anesthesia may be preferred to minimize unpredictable intraoperative behavior in cases where resident surgeons are performing surgery under attending supervision.27,29,30 The prevalence of PTSD among veterans also may impact the selection of anesthesia. Patients with PTSD have displayed greater levels of anxiety and more discomfort, requiring more sedation and longer surgical times compared with that of a control group.28 Ophthalmic comorbidities prevalent among the predominantly older male population in the VHA include the use of α-1 antagonist prostate medications, such as tamsulosin and terazosin. These medications are associated with intraoperative floppy iris syndrome, which can increase case difficulty and prolong operative time.29
Surgery Preparation
Cataract surgery induces minimal physiologic stress since most surgeries are performed under local or topical anesthesia. Unless the preoperative medical history or physical examination detects an active or unattended medical condition that needs to be addressed, preoperative laboratory testing is generally not required.31-33 Current general guidelines for preoperative testing for cataract surgery exist but do not address specific issues facing very elderly patients. The American Academy of Ophthalmology advises against preoperative medical tests for eye surgery unless there are medical indications: an electrocardiogram for patients with a history of heart disease, a blood glucose test for those with DM, and a potassium test for patients who are on diuretics.31 The direct correlation of age with these comorbidities may translate into higher rates of preoperative testing among very elderly patients. In the VHA, 45% of ophthalmology services studied routinely performed preoperative electrocardiography, chemical analysis, and complete blood counts prior to performing cataract surgery.27 Patients who live with chronic bacterial colonization from indwelling catheters, ostomies, or bed sores need to be given instructions for proper hygienic practices to minimize risks of postoperative infection.34
Some patients undergoing cataract surgery may not be candidates for topical or local anesthesia alone. Sedation is often used to reduce anxiety and discomfort of surgery, but very elderly patients have narrower margins of therapeutic safety because of advanced aged or medical comorbidities. Since patients need to follow basic commands in the OR for ideal surgical execution, general anesthesia may need to be considered for those with dementia, deafness, anxiety attacks, or language barriers.
Postsurgical Care
Although cataract surgery is a less invasive procedure than it was in the past, full postoperative recovery regularly spans a month. During this time, proper healing relies on the regular administration of eye drops and a refrain from heavy lifting, straining, and eye rubbing. Very elderly patients may need varying degrees of assistance with postsurgical care. For example, adherence to the regimen of eye drops can be complicated by decreased dexterity from arthritis and difficulty remembering the administration schedule in some patients. Reliable transportation also is an important factor as patients are routinely scheduled for postoperative visits at the 1- day, 1-week, and 1-month mark. PCPs can assist in ensuring patients have prearranged assistance for eye care and transportation to and from appointments. Additionally, very elderly patients with a history of constipation may benefit from stool softeners and/or laxatives to help prevent straining.
Conclusion
The limited literature on clinical outcomes of cataract surgery in the very elderly indicates that most have successful surgery and improved postoperative QOL.22 Much of the benefits derived from cataract surgery in the very elderly can be ascribed to thoughtful preoperative evaluation and planning with the PCP.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
1. US Census Bureau. An aging nation: the older population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html Published May 2014. Accessed March 18, 2019.
2. VA Office of Inspector General. Healthcare inspection: evaluation of cataract surgeries and outcomes in veterans health administration facilities. Report No. 11-02487-158. https://www.va.gov/oig/pubs/vaoig-11-02487-158.pdf. Published March 28, 2013. Accessed March 11, 2019.
3. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98-103.
4. American Academy of Ophthalmology. Cataract in the adult eye preferred practice pattern—2016. https://www.aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016. Published October 2016. Accessed March 19, 2019.
5. Mutoh T, Isome S, Matsumoto Y, Chikuda M. Cataract surgery in patients older than 90 years of age. Can J Ophthalmol. 2012;47(2):140-144.
6. Monestam E, Wachmeister L. Impact of cataract surgery on the visual ability of the very old. Am J Ophthalmol. 2004;137(1):145-155.
7. Lai FH, Lok JY, Chow PP, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
8. Michalska-Malecka K, Nowak M, Gos´ciniewicz P, et al. Results of cataract surgery in the very elderly population. Clin Interv Aging. 2013;8:1041-1046.
9. Syam PP, Eleftheriadis H, Casswell AG, Brittain GP, McLeod BK, Liu CS. Clinical outcome following cataract surgery in very elderly patients. Eye (Lond). 2004;18(1):59-62.
10. Rosen E, Rubowitz A, Assia EI. Visual outcome following cataract extraction in patients aged 90 years and older. Eye (Lond). 2009;23(5):1120-1124.
11. Mehmet B, Abuzer G. Results of cataract surgery in the very elderly population. J Optom. 2009;2(3):138-141.
12. To KG, Meuleners L, Bulsara M, et al. A longitudinal cohort study of the impact of first- and both-eye cataract surgery on falls and other injuries in Vietnam. Clin Interv Aging. 2014;9:743-751.
13. Song E, Sun H, Xu Y, Ma Y, Zhu H, Pan CW. Age-related cataract, cataract surgery and subsequent mortality: a systematic review and meta-analysis. PLoS One. 2014;9(11):e112054.
14. Brannan S, Dewar C, Sen J, Clarke D, Marshall T, Murray PI. A prospective study of the rate of falls before and after cataract surgery. Br J Ophthalmol. 2003;87(5):560-562.
15. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23.
16. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJ. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10):1370-1377, e1379-1380.
17. Tseng VL, Greenberg PB, Wu WC, et al. Cataract surgery complications in nonagenarians. Ophthalmology. 2011;118(7):1229-1235.
18. Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247.
19. Celebi AR. The relationship between age and the intraoperative complication rate during phacoemulsification surgery. Aging Clin Exp Res. 2014;26(2):177-181.
20. Berler DK. Intraoperative complications during cataract surgery in the very old. Trans Am Ophthalmol Soc. 2000;98:127-130; discussion 130-132.
21. Lai FHP, Lok JYC, Chow PPC, Young AL. Clinical outcomes of cataract surgery in very elderly adults. J Am Geriatr Soc. 2014;62(1):165-170.
22. Li E, Margo CE, Greenberg PB. Cataract surgery outcomes in the very elderly. J Cataract Refract Surg. 2018;44(9):1144-1149.
23. Young JS. Age-related eye diseases and recommendations for low-vision AIDS. Home Healthc Now. 2015;33(1):10-17; quiz 18-19.
24. Virgili G, Acosta R, Grover LL, Bentley SA, Giacomelli G. Reading aids for adults with low vision. Cochrane Database Syst Rev. 2013;(10):CD003303.
25. Young JS. Age-related eye diseases: a review of current treatment and recommendations for low-vision aids. Home Healthc Nurse. 2008;26(8):464-471; quiz 472-473.
26. Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans study. Prim Care Companion CNS Disord. 2017;19(3):17m02118.
27. Havnaer AG, Greenberg PB, Cockerham GC, Clark MA, Chomsky A. Cataract surgery practices in the United States Veterans Health Administration. J Cataract Refract Surg. 2017;43(4):543-551.
28. Rapoport Y, Wayman LL, Chomsky AS. The effect of post-traumatic-stress-disorder on intra-operative analgesia in a veteran population during cataract procedures carried out using retrobulbar or topical anesthesia: a retrospective study. BMC Ophthalmol. 2017;17(1):85.
29. Payal AR, Gonzalez-Gonzalez LA, Chen X, et al. Outcomes of cataract surgery with residents as primary surgeons in the Veterans Affairs Healthcare System. J Cataract Refract Surg. 2016;42(3):370-384.
30. US Department of Veterans Affairs. Mission of the office of academic affiliations. https://www.va.gov/oaa/oaa_mission.asp. Updated November 30, 2018. Accessed March 18, 2019.
31. American Academy of Ophthalmology. Choosing wisely: five things ophthalmologists and patients should question. https://www.aao.org/choosing-wisely. Published February 2013. Accessed March 18, 2019.
32. Martin SK, Cifu AS. Routine preoperative laboratory tests for elective surgery. JAMA. 2017;318(6):567-568.
33. Schein OD, Katz J, Bass EB, et al; Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168-175.
34. Margo CE. Asymptomatic bacteriuria and acute-onset endophthalmitis after cataract surgery. Can J Ophthalmol. 2015;50(4):e51-52.
35. Fukui K, Fujioka M, Yamasaki K, Yamakawa S, Matsuo H, Noguchi M. Risk factors for postoperative complications among the elderly after plastic surgery procedures performed under general anesthesia. Plast Surg Int. 2018:7053839.
What are the risks of long-term PPI use for GERD symptoms in patients > 65 years?
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
EVIDENCE SUMMARY
A 2017 meta-analysis of 16 RCTs examined the risk of cardiovascular events in 7540 adult patients taking PPIs for GERD (mean ages 45-55 years).1 The primary outcome was cardiovascular events—including acute myocardial infarction, myocardial ischemia, angina pectoris, cardiac failure, and coronary artery stenosis—and cardiac disorders.
Analysis of pooled data found that PPI use was associated with a 70% increase in cardiovascular risk (relative risk [RR] = 1.7; 95% confidence interval [CI], 1.13-2.56; number needed to harm [NNH] = 241) when compared with controls (placebo, H2 blocker, or surgery). A subgroup analysis found that PPI use for longer than 8 weeks was associated with an even higher risk of adverse cardiovascular events (6 trials, 2296 patients; RR = 2.33; 95% CI, 1.33-4.08; NNH = 67) when compared with controls. The meta-analysis wasn’t limited by heterogeneity (I2 = 0).
C difficile infection risk is higherfor PPI users
A 2016 meta-analysis of 23 observational studies (19 case-control, 4 retrospective cohort; 186,033 patients) examined the risk of hospital-acquired C difficile infections in adults prescribed PPI for any indication.2 PPI exposure varied from use at time of diagnosis or hospitalization to any use within 90 days. Of the 23 studies, 16 reported sufficient data to calculate the mean age for the patients which was 69.9 years.
The risk of C difficile infection was found to be higher with PPI use than no use (pooled odds ratio [OR] = 1.81; 95% CI, 1.52-2.14). Although a significant association was found across a large group, the results were limited by considerable heterogeneity (I2 = 82%).
Risk of community-acquired pneumonia also increases with PPI use
A 2015 systematic review and meta-analysis of 33 trials (18 case-control, 10 cohort, 4 RCTs, and 1 case-crossover study) examined the risk of CAP in adult patients prescribed PPI for any indication for durations ranging from less than 1 month to > 6 months.3 The systematic review was distilled to 26 studies because of overlapping study populations. These 26 studies included 226,769 cases of CAP among 6,351,656 patients. The primary outcome was development of CAP, the secondary outcome was hospitalization for CAP.
PPI use, compared with no use, was associated with an increased risk of developing CAP (pooled OR = 1.49; 95% CI, 1.16-1.92) and an increased risk of hospitalization for CAP (pooled OR = 1.61; 95% CI, 1.12-2.31).
In a subgroup analysis for age, patients older than 65 years were also found to have an increased risk of developing CAP with PPI use (11 trials, total number of patients not provided; OR = 1.33; 95% CI, 1.13-1.58). Despite the significant associations of PPI use with risk revealed in the primary, secondary, and subgroup analyses, the results were limited by marked heterogeneity, with an I2 > 99%.
Continue to: Hip and vertebral fracture risks associated with PPIs
Hip and vertebral fracture riskis associated with PPIs
A 2011 systematic review and meta-analysis investigated the risk of fracture in adult patients taking PPIs for any indication.4 The analysis included 10 observational studies (4 cohort, 6 case-control) with a total of 223,210 fracture cases. The authors examined the incidence of hip, vertebral, and wrist or forearm fractures.
No significant association was found between PPI use and wrist or forearm fracture (3 studies; pooled OR = 1.09; 95% CI, 0.95-1.24). A modest association was noted between PPI use and both hip fractures (9 trials; OR = 1.25; 95% CI, 1.14-1.37) and vertebral fractures (4 trials; OR = 1.5; 95% CI, 1.32-1.72).
Subgroup analysis didn’t reveal evidence of an effect of duration of PPI use on fracture. Investigators didn’t conduct subgroup analysis of different patient ages. Final results were limited by significant heterogeneity with an I2 of 86%.
RECOMMENDATIONS
A 2015 American Geriatrics Society Beers Criteria update recommends limiting PPI use because of increased risk of C difficile infections and fractures. It also recommends against using PPIs for longer than 8 weeks except for high-risk patients (such as patients taking oral corticosteroids or chronic nonsteroidal anti-inflammatory drug users), patients with Barrett’s esophagitis, or patients who need maintenance after failure of a drug discontinuation trial or H2 blockers (quality of evidence, high; SOR, strong).5
Editor’s take
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
1. Sun S, Cui Z, Zhou M, et al. Proton pump inhibitor monotherapy and the risk of cardiovascular events in patients with gastro-esophageal reflux disease: a meta-analysis. Neurogastroenterol Motil. 2017;29:e12926.
2. Arriola V, Tischendorf J, Musuuza J, et al. Assessing the risk of hospital-acquired clostridium difficile infection with proton pump inhibitor use: a meta-analysis. Infect Control Hosp Epidemiol. 2016;37:1408-1417.
3. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004.
4. Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106:1209-1218.
5. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
EVIDENCE-BASED ANSWER:
The use of proton pump inhibitors (PPIs) to control gastroesophageal reflux disease (GERD) is significantly associated with an increased risk of cardiovascular events such as acute myocardial infarction and myocardial ischemia, especially with treatment longer than 8 weeks (strength of recommendation [SOR]: A, systematic review of randomized, controlled trials [RCTs]). This summary is based on data extrapolated from studies on all adults because there is limited evidence that specifically addresses patients older than 65 years.
Adults taking PPIs also appear to be at increased risk of Clostridium difficile infection, community-acquired pneumonia (CAP; with use for < 30 days), and fracture (SOR: B, systematic reviews of heterogeneous prospective and retrospective observational studies).
Personalizing guideline-driven cancer screening
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
Reports of cancer date back thousands of years to Egyptian texts. Its existence baffled scientists until the 1950s, when Watson, Crick, and Franklin discovered the structure of DNA, laying the groundwork for identifying the genetic pathways leading to cancer. Currently, cancer is a leading global cause of death and the second leading cause of death in the United States.1,2
In an effort to curtail cancer and its related morbidity and mortality, population-based screening programs have been implemented with tests that identify precancerous lesions and, preferably, early-stage rather than late-stage cancer.
Screening for cancer can lead to early diagnosis and prevent death from cancer, but the topic continues to provoke controversy.
VALUE OF SCREENING QUESTIONED
In a commentary in the March 2019 Cleveland Clinic Journal of Medicine, Kim et al3 argued that cancer screening is not very effective and that we need to find the balance between the potential benefit and harm.
Using data from the US Preventive Services Task Force (USPSTF) and various studies, the authors showed that although screening can prevent some deaths from breast, colon, prostate, and lung cancer, at least 3 times as many people who are screened still die of those diseases. Given that screening does not eliminate all cancer deaths, has not been definitely shown to decrease the all-cause mortality rate, and has the potential to harm through false-positive results, overdiagnosis, and overtreatment, the authors questioned the utility of screening and encouraged us to discuss the benefits and harms with our patients.
In view of the apparently meager benefit, the USPSTF has relaxed its recommendations for screening for breast and prostate cancer in average-risk populations in recent years, a move that has evoked strong reactions from some clinicians. Proponents of screening argue that preventing late-stage cancers can save money, as the direct and indirect costs of morbidity associated with late-stage cancers are substantial, and that patients prefer screening when a test is available. Current models of screening efficacy do not take these factors into account.4
Kim et al, in defending the USPSTF’s position, suggested that the motivation for aggressive testing may be a belief that no harm is greater than the benefit of saving a life. They illustrated this through a Swiftian “modest proposal,” ie, universal prophylactic organectomy to prevent cancer. This hypothetical extreme measure would nearly eliminate the risk of cancer in the removed organs and prevent overdiagnosis and overtreatment of malignancies, but at substantial harm and cost.
In response to this proposal, we would like to point out the alternative extreme: stop all cancer screening programs. The pendulum would swing from what was previously considered a benefit—cancer prevention—to a harm, ie, cancer.
IN DEFENSE OF CANCER SCREENING
Observational studies, systematic reviews, meta-analyses, and modeling studies show that screening for cervical, colorectal, breast, and prostate cancer decreases disease-specific mortality.5–11
For example, in lung cancer, the National Lung Screening Trial demonstrated reductions in disease-specific and overall mortality in patients at high risk who underwent low-dose screening computed tomography.12
In breast cancer, a systematic review demonstrated decreased disease-specific mortality for women ages 50 through 79 who underwent screening mammography.13
In cervical cancer, lower rates of cancer-related death and invasive cancer have also been shown with screening.14
In colorectal cancer, great strides have been made in reducing both the incidence of and mortality from this disease over the past 30 years through fecal occult blood testing. Early detection shifts the 5-year survival rate—14% for late-stage cancer—to over 90%.15 Colorectal cancer screening has also been shown to be cost-effective, with savings in excess of $30,000 per life-year gained from screening.16
Moreover, recent data from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial17 demonstrated a 2-fold higher overall non-cancer-related mortality rate in participants who did not adhere to screening compared with those who were fully adherent to all sex-specific PLCO screening tests when adjusted for age, sex, and ethnicity. Although a possible explanation is that people who adhere to screening recommendations are also likely to have a healthier lifestyle overall, the association persisted (although it was slightly attenuated) even after adjusting for medical risk and behavioral factors.
ON THIS WE CAN AGREE
Like Kim et al, we also believe an informed discussion of screening should occur with each patient—and challenge Kim et al to design an efficient and practical approach to allow providers to do so in a busy office visit aimed to address and manage other competing diseases.
In addition, medical science needs to improve. Methods to increase the efficacy of screening and decrease risks should be explored; these include improving test and operator performance, reducing nonadherence to screening, investigating novel biomarkers or precursors of cancer and pathways that escape current detection, and devising better risk-stratification tools.
Bodies such as the USPSTF should use models that account for factors not considered previously but important when informing patients of potential benefits and harm. Examples include varying sensitivities and specificities at different rounds of testing and accounting for the variability in risk or efficacy affected by race, ethnicity, sex, and patient preferences.
We practice in the era of evidence-based medicine. Guidelines and recommendations are based on the available evidence. As more studies are published, disease mechanisms are better understood, and the effects of previous recommendations are evaluated, cancer screening programs will be further refined or replaced. The balance between benefit and harm will be further delineated.
Kim et al knocked on the door of personalized medicine, where individual screening will be based on individual risk. Until that door is opened, screening should be personalized through the risk-benefit discussions we have with our patients. Ultimately, the choice to undergo screening is the patient’s.
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016; 25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1):7–30. doi:10.3322/caac.21442
- Kim MS, Nishikawa G, Prasad V. Cancer screening: a modest proposal for prevention. Cleve Clin J Med 2019; 86(3):157–160. doi:10.3949/ccjm.86a.18092
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA 2016; 315(23):2595–2609. doi:10.1001/jama.2016.6828
- Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2:35. doi:10.1186/2046-4053-2-35
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155(10):687–697. doi:10.7326/0003-4819-155-10-201111150-00376
- Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014; 120:2893–2901. doi:10.1002/cncr.28794
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010; 116(3):544–573. doi:10.1002/cncr.24760
- Myers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review. JAMA 2015; 314(15):1615–1634. doi:10.1001/jama.2015.13183
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012; 380(9855):1778–1786. doi:10.1016/S0140-6736(12)61611-0
- Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control 2008; 19(2):175–181. doi:10.1007/s10552-007-9083-8
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5):395–409. doi:10.1056/NEJMoa1102873
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med 2016; 164(4):244–255. doi:10.7326/M15-0969
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018; 320(7):674–686. doi:10.1001/jama.2018.10897
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009; 27(22):3677–3683. doi:10.1200/JCO.2008.20.5278
- Patel S, Kilgore M. Cost effectiveness of colorectal cancer screening strategies. Cancer Control 2015; 22(2):248–258. doi:10.1177/107327481502200219
- Pierre-Victor D, Pinsky PF. Association of nonadherence to cancer screening examinations with mortality from unrelated causes: a secondary analysis of the PLCO cancer screening trial. JAMA Intern Med 2019; 179(2):196–203. doi:10.1001/jamainternmed.2018.5982
Metformin for type 2 diabetes
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
To the Editor: I enjoyed reading “Should metformin be used in every patient with type 2 diabetes” by Makin and Lansang in the January 2019 issue.1
I just wanted to point out that metformin is a frequent cause of low serum vitamin B12 levels, and serum vitamin B12 levels should be monitored intermittently in patients using metformin.
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
- Makin V, Lansang MC. Should metformin be used in every patient with type 2 diabetes? Cleve Clin J Med 2019; 86(1):17–20. doi:10.3949/ccjm.86a.18039
In reply: Metformin for type 2 diabetes
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
In Reply: We thank Dr. Moskowitz for his kind comments. We agree about the need for assessing vitamin B12 levels during chronic metformin use.
Secondary analysis of patients in the Diabetes Prevention Program Outcomes Study showed a higher incidence of combined low and low-normal vitamin B12 deficiency in users assigned to the metformin group compared with those assigned to the placebo group at the 5-year and 13-year marks after randomization.1 Post hoc analysis of patients in the Hyperinsulinemia: the Outcome of Its Metabolic Effects trial also showed lower levels of vitamin B12 and higher levels of methylmalonic acid associated with significant worsening of a validated neuropathy score in metformin users.2
The mechanism behind the development of vitamin B12 deficiency is not completely understood but could possibly be alterations in intestinal mobility, bacterial overgrowth, or calcium-dependent uptake by ileal cells of the vitamin B12-intrinsic factor complex.3
Our electronic medical record has a built-in tool that suggests checking vitamin B12 whenever a patient requests metformin refills. There are no current guidelines on the need for baseline testing of the vitamin B12 level. The American Diabetes Association recommends periodic measurement of vitamin B12 levels, possibly yearly, in metformin users and more often if there are symptoms indicative of deficiency.4
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
- Aroda VR, Edelstein SL, Goldberg RB, et al; Diabetes Prevention Program Research Group. Long-term metformin use and vitamin B12 deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab 2019; 101(4):1754–1761. doi:10.1210/jc.2015-3754
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications 2018; 32(2):171–178. doi:10.1016/j.jdiacomp.2017.11.001
- Liu KW, Dai LK, Jean W. Metformin-related vitamin B12 deficiency. Age Ageing 2006; 35(2):200–201. doi:10.1093/ageing/afj042
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019; 42(suppl 1):S90–S102. doi:10.2337/dc19-S009
Click for Credit: Suicide in Medicaid youth; persistent back pain; more
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019
Here are 5 articles from the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Back pain persists in one in five patients
To take the posttest, go to: https://bit.ly/2Uiod8N
Expires January 14, 2019
2. COPD linked to higher in-hospital death rates in patients with PAD
To take the posttest, go to: https://bit.ly/2TFCeJC
Expires January 22, 2019
3. Medicaid youth suicides include more females, younger kids, hanging deaths
To take the posttest, go to: https://bit.ly/2Uleyyp
Expires January 17, 2019
4. Potential antidepressant overprescribing found in 24% of elderly cohort
To take the posttest, go to: https://bit.ly/2HWwcSq
Expires January 24, 2019
5. Perceptions of liver transplantation for ALD are evolving
To take the posttest, go to: https://bit.ly/2OCANuA
Expires January 22, 2019