User login
From sweet to belligerent in the blink of an eye
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
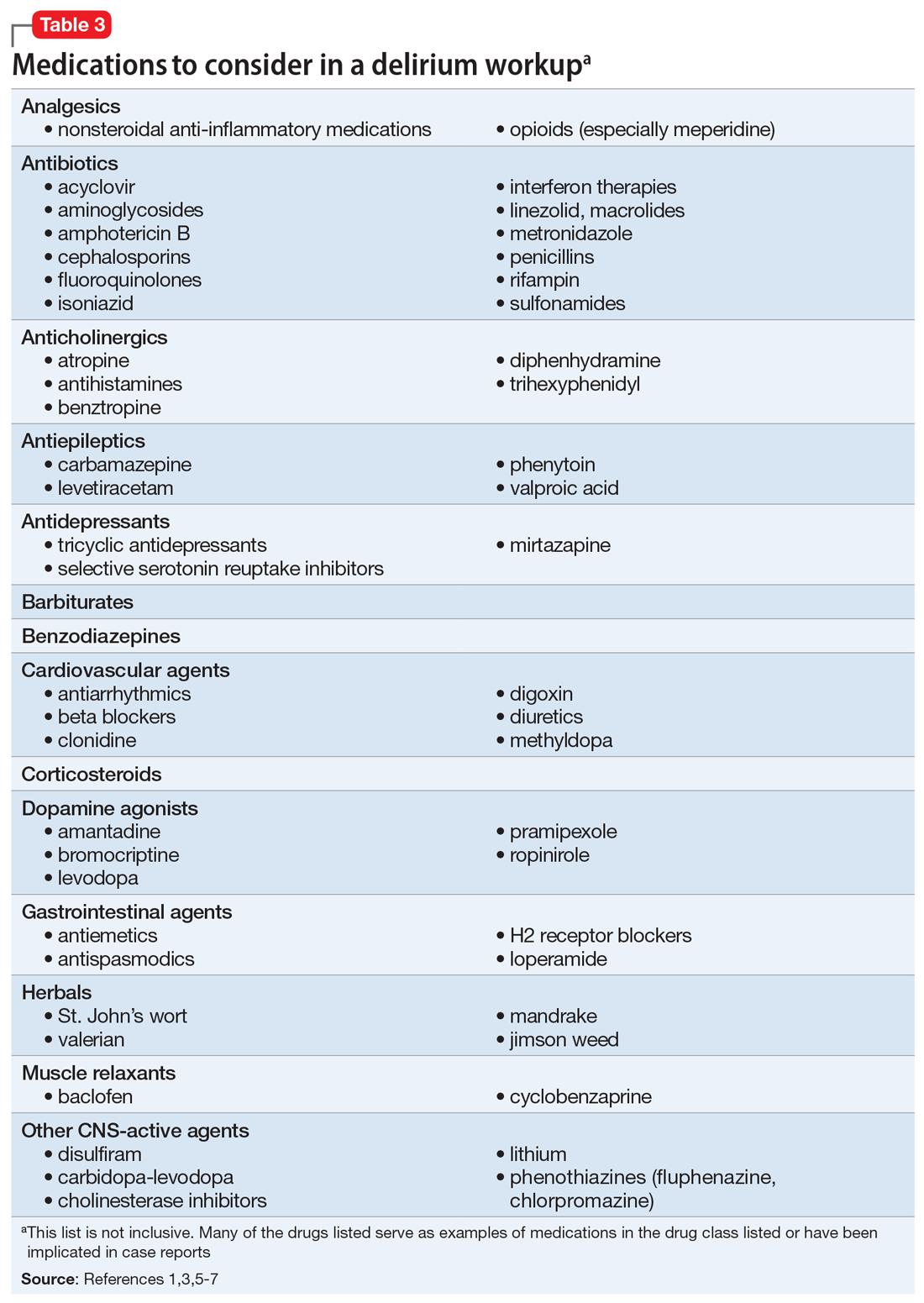
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
CASE Combative and agitated
Ms. P, age 87, presents to the emergency department (ED) with her caregiver, who says Ms. P has new-onset altered mental status, agitation, and combativeness.
Ms. P resides at a long-term care (LTC) facility, where according to the nurses she normally is pleasant, well-oriented, and cooperative. Ms. P’s medical history includes major depressive disorder, generalized anxiety disorder, hypertension, chronic kidney disease (CKD) stage III, peptic ulcer disease, gastroesophageal reflux disease, coronary artery disease with 2 past myocardial infarctions requiring stents, chronic obstructive pulmonary disease, hyperlipidemia, bradycardia requiring a pacemaker, paroxysmal atrial fibrillation, asthma, aortic stenosis, peripheral vascular disease, esophageal stricture requiring dilation, deep vein thrombosis, and migraines.
Mr. P’s medication list includes acetaminophen, 650 mg every 6 hours; ipratropium/albuterol nebulized solution, 3 mL 4 times a day; aspirin, 81 mg/d; atorvastatin, 40 mg/d; calcitonin, 1 spray nasally at bedtime; clopidogrel, 75 mg/d; ezetimibe, 10 mg/d; fluoxetine, 20 mg/d; furosemide, 20 mg/d; isosorbide dinitrate, 120 mg/d; lisinopril, 15 mg/d; risperidone, 0.5 mg/d; magnesium oxide, 800 mg/d; pantoprazole, 40 mg/d; polyethylene glycol, 17 g/d; sotalol, 160 mg/d; olanzapine, 5 mg IM every 6 hours as needed for agitation; and tramadol, 50 mg every 8 hours as needed for headache.
Seven days before coming to the ED, Ms. P was started on ceftriaxone, 1 g/d, for suspected community-acquired pneumonia. At that time, the nursing staff noticed behavioral changes. Soon after, Ms. P began refusing all her medications. Two days before presenting to the ED, Ms. P was started on nitrofurantoin, 200 mg/d, for a suspected urinary tract infection, but it was discontinued because of an allergy.
Her caregiver reports that while at the LTC facility, Ms. P’s behavioral changes worsened. Ms. P claimed to be Jesus Christ and said she was talking to the devil; she chased other residents around the facility and slapped medications away from the nursing staff. According to caregivers, this behavior was out of character.
Shortly after arriving in the ED, Ms. P is admitted to the psychiatric unit.
[polldaddy:10332748]
The authors’ observations
Delirium is a complex, acute alteration in a patient’s mental status compared with his/her baseline functioning1 (Table 12). The onset of delirium is quick, happening within hours to days, with fluctuations in mental function. Patients might present with hyperactive, hypoactive, or mixed delirium.3 Patients with hyperactive delirium often have delusions and hallucinations; these patients might be agitated and could become violent with family and caregivers.3 Patients with hypoactive delirium are less likely to experience hallucinations and more likely to show symptoms of sedation.3 Patients with hypoactive delirium can be difficult to diagnose because it is challenging to interview them and understand what might be the cause of their sedated state. Patients also can exhibit a mixed delirium in which they fluctuate between periods of hyperactivity and hypoactivity.3
Continue to: Suspected delirium...
Suspected delirium should be considered a medical emergency because the outcome could be fatal.1 It is important to uncover and treat the underlying cause(s) of delirium rather than solely administering antipsychotics, which might mask the presenting symptoms. In an older study, Francis and Kapoor4 reported that 56% of geriatric patients with delirium had a single definite or probable etiology, while the other 44% had about 2.8 etiologies per patient on average. Delirium risk factors, causes, and factors to consider during patient evaluation are listed in Table 21,3,5-7 and Table 3.1,3,5-7
A synergistic relationship between comorbidities, environment, and medications can induce delirium.5 Identifying irreversible and reversible causes is the key to treating delirium. After the cause has been identified, it can be addressed and the patient could return to his/her previous level of functioning. If the delirium is the result of multiple irreversible causes, it could become chronic.
[polldaddy:10332749]
EVALUATION Cardiac dysfunction
Ms. P undergoes laboratory testing. The results include: white blood cell count, 5.9/µL; hemoglobin, 13.6 g/dL; hematocrit, 42.6%; platelets, 304 × 103/µL; sodium,143 mEq/L; potassium, 3.2 mEq/L; chloride, 96 mEq/L; carbon dioxide, 23 mEq/L; blood glucose, 87 mg/dL; creatinine, 1.2 mg/dL; estimated creatinine clearance (eCrCl) level of 33 mL/min/1.73 m2; calcium, 9.5 mg/dL; albumin, 3.6 g/dL; liver enzymes within normal limits; thyroid-stimulating hormone, 0.78 mIU/L; vitamin B12, 995 pg/mL; folic acid, 16.6 ng/mL; vitamin D, 31 pg/mL; and rapid plasma reagin: nonreactive. Urinalysis is unremarkable, and no culture is performed. Urine drug screening/toxicology is positive for the benzodiazepines that she received in the ED (oral alprazolam 0.25 mg given once and oral lorazepam 0.5 mg given once).
Electrocardiogram (ECG) shows atrial flutter/tachycardia with rapid ventricular response, marked left axis deviation, nonspecific ST- and T-wave abnormality, QT/QTC of 301/387 ms, and ventricular rate 151 beats per minute. A CT scan of the head and brain without contrast shows mild atrophy and chronic white matter changes and no acute intracranial abnormality. A two-view chest radiography shows no acute cardiopulmonary findings. Her temperature is 98.4°F; heart rate is 122 beats per minute; respiratory rate is 20 breaths per minute; blood pressure is 161/98 mm Hg; and oxygen saturation is 86% on room air.
Based on this data, Ms. P’s cardiac condition seems to be worsening, which is thought to be caused by her refusal of furosemide, lisinopril, isosorbide, sotalol, clopidogrel, and aspirin. The treatment team plans to work on compliance to resolve these cardiac issues and places Ms. P on 1:1 observation with a sitter and music in attempt to calm her.
Continue to: The authors' observations
The authors’ observations
Many factors can contribute to behavioral or cognitive changes in geriatric patients. Often, a major change noted in an older patient can be attributed to new-onset dementia, dementia with behavioral disturbances, delirium, depression, or acute psychosis. These potential causes should be considered and ruled out in a step-by-step progression. Because patients are unreliable historians during acute distress, a complete history from family or caregivers and exhaustive workup is paramount.
TREATMENT Medication adjustments
In an attempt to resolve Ms. P’s disruptive behaviors, her risperidone dosage is changed to 0.5 mg twice daily. Ms. P is encouraged to use the provided oxygen to raise her saturation level.
On hospital Day 3, a loose stool prompts a Clostridium difficile test as a possible source of delirium; however, the results are negative.
On hospital Day 4, Ms. P is confused and irritable overnight, yelling profanities at staff, refusing care, inappropriately disrobing, and having difficulty falling asleep and staying asleep. Risperidone is discontinued because it appears to have had little or no effect on Ms. P’s disruptive behaviors. Olanzapine, 10 mg/d, is initiated with mirtazapine, 7.5 mg/d, to help with mood, appetite, and sleep. Fluoxetine is also discontinued because of a possible interaction with clopidogrel.
On hospital Days 6 to 8, Ms. P remains upset and unable to follow instructions. Melatonin is initiated to improve her sleep cycle. On Day 9, she continues to decline and is cursing at hospital staff; haloperidol is initiated at 5 mg every morning, 10 mg at bedtime, and 5 mg IM as needed for agitation. Her sleep improves with melatonin and mirtazapine. IV hydration also is initiated. Ms. P has a slight improvement in medication compliance. On Day 11, haloperidol is increased to 5 mg in the morning, 5 mg in the afternoon, and 10 mg at bedtime. On Day 12, haloperidol is changed to 7.5 mg twice daily; a slight improvement in Ms. P’s behavior is noted.
Continue to: On hospital Day 13...
On hospital Day 13, Ms. P’s behavior declines again. She screams profanities at staff and does not recognize the clinicians who have been providing care to her. The physician initiates valproic acid, 125 mg, 3 times a day, to target Ms. P’s behavioral disturbances. A pharmacist notes that the patient’s sotalol could be contributing to Ms. P’s psychiatric presentation, and that based on her eCrCl level of 33 mL/min/1.73 m2, a dosage adjustment or medication change might be warranted.
On Day 14, Ms. P displays erratic behavior and intermittent tachycardia. A cardiac consultation is ordered. A repeat ECG reveals atrial fibrillation with rapid rate and a QT/QTc of 409/432 ms. Ms. P is transferred to the telemetry unit, where the cardiologist discontinues sotalol because the dosage is not properly renally adjusted. Sotalol hydrochloride has been associated with life-threatening ventricular tachycardia.8 Diltiazem, 30 mg every 6 hours is initiated to replace sotalol.
By Day 16, the treatment team notes improved cognition and behavior. On Day 17, the cardiologist reports that Ms. P’s atrial fibrillation is controlled. An ECG reveals mild left ventricular hypertrophy, an ejection fraction of 50% to 55%, no stenosis in the mitral or tricuspid valves, no valvular pulmonic stenosis, and moderate aortic sclerosis. Cardiac markers also are evaluated (creatinine phosphokinase: 105 U/L; creatinine kinase–MB fraction: 2.6 ng/mL; troponin: 0.01 ng/mL; pro-B-type natriuretic peptide: 2,073 pg/mL); and myocardial infarction is ruled out.
On Day 19, Ms. P’s diltiazem is consolidated to a controlled-delivery formulation, 180 mg/d, along with the addition of metoprolol, 12.5 mg twice daily. Ms. P is transferred back to the psychiatric unit.
OUTCOME Gradual improvement
On Days 20 to 23, Ms. P shows remarkable progress, and her mental status, cognition, and behavior slowly return to baseline. Haloperidol and valproic acid are tapered and discontinued. Ms. P is observed to be healthy and oriented to person, place, and time.
Continue to: On Day 25...
On Day 25, she is discharged from the hospital, and returns to the LTC facility.
The authors’ observations
Ms. P’s delirium was a combination of her older age, non-renally adjusted sotalol, and CKD. At admission, the hospital treatment team first thought that pneumonia or antibiotic use could have caused delirium. However, Ms. P’s condition did not improve after antibiotics were stopped. In addition, several chest radiographs found no evidence of pneumonia. It is important to check for any source of infection because infection is a common source of delirium in older patients.1 Urine samples revealed no pathogens, a C. difficile test was negative, and the patient’s white blood cell counts remained within normal limits. Physicians began looking elsewhere for potential causes of Ms. P’s delirium.
Ms. P’s vital signs ruled out a temperature irregularity or hypertension as the cause of her delirium. She has a slightly low oxygen saturation when she first presented, but this quickly returned to normal with administration of oxygen, which ruled out hypoxemia. Laboratory results concluded that Ms. P’s glucose levels were within a normal range and she had no electrolyte imbalances. A head CT scan showed slight atrophy of white matter that is consistent with Ms. P’s age. The head CT scan also showed that Ms. P had no acute condition or head trauma.
In terms of organ function, Ms. P was in relatively healthy condition other than paroxysmal atrial fibrillation and CKD. Chronic kidney disease can interrupt the normal pharmacokinetics of medications. Reviewing Ms. P’s medication list, several agents could have induced delirium, including antidepressants, antipsychotics, cardiovascular medications (beta blocker/antiarrhythmic [sotalol]), and opioid analgesics such as tramadol.5 Ms. P’s condition did not improve after discontinuing fluoxetine, risperidone, or olanzapine, although haloperidol was started in their place. Ms. P scored an 8 on the Naranjo Adverse Drug Reaction Probability Scale, indicating this event was a probable adverse drug reaction.9
Identifying a cause
This was a unique case where sotalol was identified as the culprit for inducing Ms. P’s delirium, because her age and CKD are irreversible. It is important to note that antiarrhythmics can induce arrhythmias when present in high concentrations or administered without appropriate renal dose adjustments. Although Ms. P’s serum levels of sotalol were not evaluated, because of her renal impairment, it is possible that toxic levels of sotalol accumulated and lead to arrhythmias and delirium. Of note, a cardiologist was consulted to safely change Ms. P to a calcium channel blocker so she could undergo cardiac monitoring. With the addition of diltiazem and metoprolol, the patient’s delirium subsided and her arrhythmia was controlled. Once the source of Ms. P’s delirium had been identified, antipsychotics were no longer needed.
Continue to: Bottom Line
Bottom Line
Delirium is a complex disorder that often has multiple causes, both reversible and irreversible. A “process of elimination” approach should be used to accurately identify and manage delirium. If a patient with delirium has little to no response to antipsychotic medications, the underlying cause or causes likely has not yet been addressed, and the evaluation should continue.
Related Resources
- Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377:1456-1466.
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922.
Drug Brand Names
Acyclovir • Zovirax
Alprazolam • Niravam, Xanax
Amantadine • Symmetrel
Amphotericin B • Abelcet
Atorvastatin • Lipitor
Atropine • Atropen
Baclofen • EnovaRX-Baclofen
Benztropine • Cogentin
Bromocriptine • Cycloset
Calcitonin • Miacalcin
Carbamazepine • Tegretol
Carbidopa-levodopa • Duopa
Ceftriaxone • Rocephin
Chlorpromazine • Thorazine
Clonidine • Catapres
Clopidogrel • Plavix
Cyclobenzaprine • Amrix
Digoxin • Lanoxin
Diltiazem • Cardizem
Disulfiram • Antabuse
Ezetimibe • Zetia
Fluoxetine • Prozac
Fluphenazine • Prolixin
Furosemide • Lasix
Haloperidol • Haldol
Ipratropium/albuterol nebulized solution • Combivent Respimat
Isoniazid • Isotamine
Isosorbide nitrate • Dilatrate
Levetiracetam • Keppra
Levodopa • Stalevo
Linezolid • Zyvox
Lisinopril • Zestril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Magnesium Oxide • Mag-200
Meperidine • Demerol
Methyldopa • Aldomet
Metoprolol • Lopressor
Metronidazole • Flagyl
Mirtazapine • Remeron
Nitrofurantoin • Macrobid
Olanzapine • Zyprexa
Pantoprazole • Protonix
Phenytoin • Dilantin
Pramipexole • Mirapex
Rifampin • Rifadin
Risperidone • Risperdal
Ropinirole • Requip
Sotalol hydrochloride • Betapace AF
Tramadol • Ultram
Trihexyphenidyl • Trihexane
Valproic acid • Depakote
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
1. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention, and treatment. Nat Rev Neurol. 2009;5(4):210-220.
2. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
3. American Psychiatric Association. Practice guideline for the treatment of patients with delirium. Am J Psychiatry. 1999;156(suppl 5):1-20.
4. Francis J, Kapoor WN. Delirium in hospitalized elderly. J Gen Intern Med. 1990;5(1):65-79.
5. Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388-393.
6. Cook IA. Guideline watch: practice guideline for the treatment of patients with delirium. Arlington, VA: American Psychiatric Publishing; 2004.
7. Bourgeois J, Ategan A, Losier B. Delirium in the hospital: emphasis on the management of geriatric patients. Current Psychiatry. 2014;13(8):29,36-42.
8. Betapace AF [package insert]. Zug, Switzerland: Covis Pharma; 2016.
9. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
Coding variants in apolipoprotein B may be associated with early-onset Alzheimer’s disease
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
This important study provides the first evidence that rare genetic coding variants of apolipoprotein B may contribute to the risk of early-onset Alzheimer’s disease, Makoto Ishii, MD, PhD, wrote in an accompanying editorial (JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0212).
But the study by Wingo et al. doesn’t tell the entire tale, he wrote.
The results from this study “found that there are likely to be additional contributing factors independent of APOB and APOE. These may include rare variants in other genes involved directly in LDL cholesterol metabolism, such as the LDL receptor and proprotein convertase subtilisin/kexin type 913 or factors known to modulate circulating LDL cholesterol levels, such as thyroid hormones.”
Although intriguing, “Clearly, additional studies looking at these factors are needed to fully elucidate the association between LDL cholesterol and EOAD. Furthermore, as the authors of this study note, it is not known if there are protective variants of APOB that would decrease the risk for developing EOAD. Identifying such a protective coding variant of APOB would greatly strengthen the link between APOB and AD pathogenesis.”
Prior studies of circulating APOB levels in humans have reached disparate conclusions. A large population-based study found no association between APOB levels and incident dementia or Alzheimer’s, he noted.
“Therefore, whether these findings can be verified in individuals with late-onset AD remains to be determined.”
Dr. Ishii is with the Feil Family Brain and Mind Research Institute in the department of neurology at Cornell University, New York. He has no relevant disclosures.
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
Variants in the apolipoprotein B gene (APOB), which creates the main protein in low-density and very low-density cholesterol, may be associated with early-onset Alzheimer’s disease, Thomas Wingo, MD, and his colleagues have determined.
The finding may help fill out the genetic risk picture for early-onset Alzheimer’s disease (EOAD), said Dr. Wingo of the Atlanta Veterans Affairs Medical Center. The study found that the already-known genetic markers for EOAD – mutations of the presenilin (PSEN) 1 and 2 genes and amyloid precursor protein (APP) – account for just a small fraction of cases.
“To place the genetic association between APOB and EOAD in context, we note that only 3.4% of all EOAD cases in our combined data set showed a known pathogenic mutation, and we found a stronger association between EOAD and rare coding variants in APOB, compared with PSEN1 in our fully adjusted analysis,” the team wrote. However, “approximately 5.0% of patients with EOAD and 1.7% of controls were found to harbor a rare coding polymorphism in APOB that is likely to disrupt the structure, functions, or abundance of ApoB protein.”
The team conducted genetic analysis on plasma samples from 2,125 EOAD and control subjects included in several research cohorts. They first determined the association between cholesterol and EOAD, and then the frequency of variants in apolipoprotein E epsilon 4 (APOE e4), APP, PSEN1, PSEN2, and ApoB. Gene sequencing revealed that 3.4% of samples showed mutations in APP, PSEN1, or PSEN2.
“Given the strong associations between APOE e4 and EOAD and elevated circulating LDL cholesterol levels, we expected individuals with EOAD to have elevated LDL levels,” the team said. But an analysis of 267 of the samples for lipid levels found that, even after the researchers controlled for APOE e4, EOAD cases had higher total cholesterol, low-density cholesterol, and plasma ApoB, compared with controls. However, they found no association between EOAD and high-density lipoprotein or triglycerides.
“Because total cholesterol largely consists of LDL-C, and ApoB is the main lipoprotein of LDL-C, these findings are consistent with one another.
“From these data, we estimated that LDL-C explains 7.6% of the variance in liability to EOAD, independently of APOE e4 ... These results demonstrate that elevated levels of LDL-C [and ApoB] were significantly associated with increased EOAD risk, and this effect was only partially mediated by APOE e4 genotype.”
The results also raised a question: What was driving the association between LDL and EOAD? Because variants of the ApoB gene can either raise or lower LDL, the team examined variants associated with coding changes. These variants were significantly more common in EOAD cases than in controls (5.0% vs. 1.7%).
“Two affected individuals ... were compound heterozygotes, with the remainder being heterozygotes,” the researchers wrote. “Each compound heterozygote case was heterozygous for two different rare coding sites ... Of these four variants, only [one] has been previously described.”
“Our finding of a significant association between rare coding variants in APOB and EOAD independently of APOE is novel, important, and consistent with multiple genome-wide association studies that revealed strong associations between late-onset AD and common intron markers of genes involved in brain cholesterol metabolism [ABCA7, BIN1, CLU, and SORL1]. Furthermore, mice overexpressing ApoB show hyperlipidemia, neurodegeneration, increases in APP, accumulation of amyloid plaques, and cognitive impairment similar to mice overexpressing wild-type human APP. Collectively, these studies and our findings suggest an important role of cholesterol metabolism in AD pathogenesis.”
This research was supported by grants from the Veterans Health Administration, the National Institutes of Health, the To Remember Foundation, the Douglas French Alzheimer’s Foundation, and a contract with the State of California Department of Health Services. Several authors reported financial ties to pharmaceutical companies outside of this work.
SOURCE: Wingo TS et al. JAMA Neurol. 2019 May 28. doi: 10.1001/jamaneurol.2019.0648.
FROM JAMA NEUROLOGY
mTORC1 inhibitor protects elderly asthmatics from viral respiratory tract infections
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
DALLAS – A molecule that boosts innate viral immunity may protect elderly people with asthma from the root cause of most exacerbations – viral respiratory tract infections.
Dubbed RTB101, the oral medication is a selective, potent inhibitor of target of rapamycin complex 1 (TORC1). In phase 2b data presented at the American Thoracic Society’s international conference, RTB101 decreased by 52% the number of elderly subjects with severe, lab-confirmed respiratory tract infections (RTI) symptoms.
But the molecule was even more effective in patients with asthma aged 65 years and older, Joan Mannick, MD, said in an interview during the meeting. In this group, it reduced by 69% the percentage of subjects who developed RTIs and reduced the rate of infection by about 79%, compared with placebo.
“The core cause of asthma exacerbations in these patients is viral respiratory tract infection,” said Dr. Mannick, chief medical officer of resTORbio, the Boston company developing RTB101. “About 80% of the viruses detected in these infections are rhinoviruses, and there are 170 rhinovirus serotypes. We have never been able to develop a vaccine against rhinovirus, and we have no treatment other than to treat the inflammation caused by the infection.”
Centers for Disease Control and Prevention mortality records confirm the impact of viral respiratory infections on older people who experience asthma exacerbations: 6 of 10,000 will die, compared with less than 2 per 10,000 for all other age groups. Decreasing the number of these infections in older people with asthma would prevent morbidity and mortality and save considerable health care dollars.
“One of the reasons that asthmatics have such difficulty when they get respiratory infections is that they seem to have deficient antiviral immunity in the airways,” Dr. Mannick said. She pointed to a 2008 study of bronchial epithelial cells from both patients with asthma and healthy controls. When inoculated with rhinovirus, the cells from asthmatic airways were unable to mount a healthy immune response and were particularly deficient in producing interferon-beta.
By inhibiting mammalian TORC1 (mTORC1), RBT101 also inhibits sterol regulatory element binding transcription factor 2, a pathway that influences cholesterol synthesis. Cells perceive cholesterol synthesis attenuation as a threat, Dr. Mannick said, and react by up-regulating a number of immune response genes – including some specifically antiviral genes that up-regulate interferon-alpha and -beta production and immune cytokine signaling pathways.
RTB101 is not a particularly new molecule; Novartis originally investigated it as an anticancer agent. “It failed, because it was too selective for mTORC1,” Dr. Mannick said. After Novartis dropped the molecule, resTORbio, a Novartis spin-off, began to investigate it as an immunotherapy for RTIs, particularly in patients with asthma.
reSTORbio’s phase 2 studies on RTB101 comprised 264 healthy subjects aged 65 years and older, who received placebo or 10 mg RTB101 daily for 6 weeks, during cold and flu season. They were followed for a year, confirming the antiviral gene up-regulation. Treatment was also associated with a 42% reduction in the rate of respiratory tract infections.
Conversations with the Food and Drug Administration and payers collected, Dr. Mannick said. “They said that where this drug could really make a difference was if it could decrease these infections in high-risk elderly, who are expensive to treat. So, we targeted people 65 years and older with asthma, chronic obstructive pulmonary disease, and smokers, and people who are 85 years or older.”
The phase 2b trial comprised 652 of these elderly high-risk subjects randomized to the following treatment arms: RTB101 5 mg once daily (n = 61), RTB101 10 mg once daily (n = 176), RTB101 10 mg b.i.d. (n = 120), RTB101 10 mg plus everolimus 0.1 mg daily (n = 115), or matching placebo (n = 180) over 16 weeks, during the entire cold and flu season. The primary endpoint was laboratory-confirmed RTIs in all groups.
The RTB101 10-mg, once-daily group had the best results with a 30.6% reduction in the percentage of patients with lab-confirmed RTIs, compared with placebo, and a 52% reduction in the percentage with severe symptoms.
A subgroup analysis found even more benefit to those with asthma. Among these patients, RTB101 effected a 58.2% decrease in patients with RTIs, and a 66.4% decrease in the rate of infections, compared with placebo.
RTB101 was most effective against rhinoviruses, but it also prevented RTIs associated with influenza A and coronavirus OC43. It also decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
There were no safety signals, Dr. Mannick noted. Adverse events were similar in both placebo and active groups, and none were deemed related to the study drug. About 5% of each group discontinued the drug because an adverse event.
Plans for a phase 3 trial are underway. A phase 3, placebo-controlled study in the Southern Hemisphere is now ongoing, during the winter cold and flu season. The Northern Hemisphere phase 3 will commence fall and winter of 2019.
Whether RBT101 can help younger people with asthma is an open question. Elderly patients not only have the asthma-related immune deficiency, but also the general age-related immune issues. Younger patients, however, still express the same asthma-related impairment of bronchial immunity.
“We would like to investigate this in younger people and in children, but that will have to wait until our other phase 3 studies are complete,” Dr. Mannick said.
The trial was sponsored by resTORbio.
SOURCE: Mannick J et al. ATS 2019, Abstract A2623.
CORRECTION 5/24/2019 The article was corrected to state a decreased the incidence of RTIs caused by respiratory syncytial virus, parainfluenza 4, influenza B, metapneumovirus, or other coronavirus serotypes.
REPORTING FROM ATS 2019
Elderly concussion patients who used statins had lower dementia risk
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
This appears to be the first large study to explore the relationship between statin use, concussions, and the development of dementia. Although statins have anti-inflammatory properties, no trials have linked statins to reduced cognitive impairment. Considering it can be difficult to mitigate against confounding by indication in pharmacologic studies, this observational study included a large group of diverse individuals who developed concussions over a period of 20 years.
Rachel A. Whitmer, PhD, is with the division of epidemiology and department of public health sciences at the University of California, Davis. She made her remarks in a related editorial published with the study, and reported no relevant conflicts of interest.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
, compared with similar adults not taking statins.
The findings come from a population-based double cohort study of 28,815 patients in the Ontario Health Insurance Plan. Study patients were enrolled over 20 years, and had a minimum follow-up of 3 years. The study excluded patients hospitalized caused by a severe concussion, those previously diagnosed with delirium or dementia, and those who died within 90 days of their concussions.
Concussions are a common injury in older adults and dementia may be a frequent outcome years afterward, Donald A. Redelmeier, MD, of the University of Toronto and colleagues wrote in a study published in JAMA Neurology. A concussion should not be interpreted as a reason to stop statins, and a potential neuroprotective benefit may encourage medication adherence among patients who are already prescribed a statin.
Of the 28,815 patients studied, 4,727 patients (1 case per 6 patients) developed dementia over the mean follow-up period of 3.9 years. The 7,058 patients who received a statin had a 13% reduced risk of developing dementia, compared with the 21,757 patients who did not (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Even though statin use was associated with a lower risk, the subsequent incidence of dementia was still twice the population norm in statin users who had concussions, the researchers wrote. The findings indicate concussions are a common injury in older adults and dementia may be a frequent outcome years after concussions.
Statin users who had concussions continued to have a reduced risk of developing dementia after adjustment for patient characteristics, use of other cardiovascular medications, dosage, and depression risk. The statin associated with the greatest risk reduction was rosuvastatin; simvastatin was associated with the least risk reduction. With the possible exception of angiotensin II receptor blockers, no other cardiovascular or noncardiovascular medications were associated with a decreased risk of dementia after a concussion, the researchers wrote.
They also examined data for elderly patients using statins after an ankle sprain and found the risk of dementia was similar for those who did and did not receive statins after the injury.
Factors such as smoking status, exercise, drug adherence, and other unknown aspects of patient health might have influenced the results of the study, the researchers acknowledged. Additionally, a secondary analysis was not statistically powered to distinguish the relative efficacy of statin use before a concussion.
This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
SOURCE: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
FROM JAMA NEUROLOGY
Key clinical point: Older adults taking a statin within 90 days after a concussion had a lower rate of dementia.
Major finding: Statin use within 90 days of a concussion in older adults was associated with a 13% reduced risk of dementia (relative risk, 0.87; 95% confidence interval, 0.81-0.93; P less than .001).
Study details: A population-based double cohort study of 28,815 elderly patients who had a concussion between April 1993 and April 2013.
Disclosures: This study was funded in part by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, the BrightFocus Foundation, and the Comprehensive Research Experience for Medical Students at the University of Toronto. The authors reported no relevant conflicts of interest.
Source: Redelmeier DA et al. JAMA Neurol. 2019 May 20. doi: 10.1001/jamaneurol.2019.1148.
Report on newly recognized cause of dementia should be read widely
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.
Alzheimer’s disease is recognized as the most common cause of dementia, and many in the laity use the two terms almost interchangeably. However, there is increasing recognition that dementia in old age is a complex disorder, with mixed neuropathologies being the norm rather than the exception (Ann Neurol. 2018 Jan;83[1]:74-83).
Alzheimer’s disease (AD) and cerebrovascular pathologies are the most common, but another pathology is receiving increasing attention in relation to cognitive disorders in very old individuals – that related to the transactive response DNA binding protein of 43 kDa (TDP-43). This protein is expressed in most human tissues, including the brain, is localized mostly in nuclei, and binds to RNA and DNA as well as numerous proteins, with the role of regulating gene expression.
It has been known for nearly 2 decades that TDP-43 can become abnormally phosphorylated and translocated to the cytoplasm to produce a proteinopathy that forms the basis of a significant proportion of frontotemporal dementia (FTD) and the majority of amyotrophic lateral sclerosis. More recently, it has also been reported to be common in the brains of older people (over age 80 years) and associated with a cognitive disorder characterized by an amnestic picture that mimics AD. Since the protein deposition is predominantly in the limbic regions (amygdala, hippocampus, insula), it has been termed “‘limbic-predominant, age-related TDP-43 encephalopathy”, or LATE.
A recently convened international working group has published consensus criteria for LATE and provided guidelines for its staging. Community-based autopsy studies suggest that 20%-50% of people aged over 80 years have the neuropathologic change associated with LATE. The clinical presentation resembles amnestic dementia syndrome, much like AD. Both LATE and AD pathologies often occur in the same individual, but the relative predominance of one or the other varies greatly between individuals. The genetic risks of LATE overlap with those for FTD and AD, and other risk factors may also be shared with AD, which remains an area for further investigation. There are at present no specific biomarkers of LATE. It is associated with hippocampal sclerosis in some cases, which may be visible on MRI, but hippocampal sclerosis itself is not specific to TDP-43 pathology.
of LATE and calls for systematic study of the causes of dementia – which may be nearly as common as AD in the very old. The report should be read widely and should remind us of the diverse pathologies that contribute to cognitive disorders, alone and in combination with one another.
Dr. Sachdev is Scientia Professor of Neuropsychiatry and codirector of the Center for Healthy Brain Aging at the University of New South Wales, Sydney; and clinical director of the Neuropsychiatric Institute at the Prince of Wales Hospital, also in Sydney. His major areas of research are drug-induced movement disorders, brain imaging, cognitive aging and dementia. Dr. Sachdev also served on the Neurocognitive Disorders Work Group of the DSM-5.
Rheumatoid arthritis treatment less aggressive, not less favorable in older adults
BIRMINGHAM, ENGLAND – Being diagnosed with rheumatoid arthritis at age 75 years or older made it less likely that patients would receive intensive therapy than their younger counterparts, but that did not mean that they were treated any less favorably overall, according to findings derived from the Early RA Network cohort.
“The claim that the elderly are treated less aggressively isn’t completely true throughout the whole treat-to-target strategy,” said Simone Howard of King’s College London at the British Society for Rheumatology annual conference. While older patients were less likely to receive intensive treatment up to 2 years after their diagnosis, there was a shorter delay between the onset of symptoms and the first outpatient visit to a rheumatology clinic.
When compared against patients who were younger than 65 years, those aged 65-74 years and 75 years and older were 11% (P = .02) and 15% (P = .02) more likely to have their first outpatient visit within 10 months.
Furthermore, no significant differences were seen between any age groups in the time to first initiation of a conventional synthetic disease-modifying antirheumatic drug (csDMARD), which averaged nearly 3 months after symptoms appeared.
Ms. Howard, who has previously worked at the European Medicines Agency, noted that, during her time at the EMA, “there was a real push to incorporate the elderly into the regulatory framework more. In parallel, there were also reports of the elderly being treated less aggressively. So the question was, where was that coming from?”
Similar therapeutic approaches are advocated for older and younger RA patients, and to look for any disparities, Ms. Howard and associates turned to the Early RA Network (ERAN) to “investigate potential treatment bias against the elderly.”
ERAN is a hospital-based inception cohort of 1,236 patients with early RA who were recruited across 23 centers in the United Kingdom and Ireland between 2002 and 2014.
Of 1,131 patients used in the analyses, 9.7% (n = 110) were 75 years or older, 21.5% (n = 243) were aged 65-74 years, and 68.8% (n = 778) were 65 years or younger. The majority (67.7%) of patients were female.
Patients aged 75 years and older were more likely to present with comorbidities than the youngest group, and they had higher health assessment questionnaire scores at baseline. However, they were no more likely to have high disease activity at the first visit, which was defined as a disease activity score in 28 joints of more than 5, and the older patients were 27% less likely to be seropositive (P = .004).
“It’s when we come to pharmacological aspects of care that we are seeing treatment biases,” Ms. Howard noted. Patients over 75 years were significantly more likely than the youngest age group to be treated with glucocorticoids or csDMARD monotherapy at 1 year, and 23% more likely to be on less aggressive therapy (P equal to or less than .0001). Aggressive therapy was defined as the use of a combination of csDMARDs or the use of biologic drugs.
At 2 years, the oldest patients were 46% more likely than those under 65 years to be on less-intensive therapies (P equal to or less than .0001), with those aged 65-74 years 19% more likely to be on glucocorticoid or csDMARD therapy (P = .005).
Factors such as patient choice and tolerance were not considered in the analyses and could be important, Ms. Howard conceded in response to a question after her presentation.
Another point raised was that perhaps the prescribing of aggressive therapy would rationally be different in someone diagnosed with RA at age 85 versus 65 because the duration of time that would be likely to be lived with accumulating joint damage would be shorter at the older age and that would be balanced against the other effects of the therapy. So, there may be important reasons in shared decision making that influenced the treatment choices other than the age of patients.
Ms. Howard agreed, noting that this demonstrated the need to be careful around the language used for defining what constituted aggressive or intensive therapy.
She and her coauthors reported no conflicts of interest.
SOURCE: Howard S et al. Rheumatology. 2019;58(suppl 3), Abstract 011.
BIRMINGHAM, ENGLAND – Being diagnosed with rheumatoid arthritis at age 75 years or older made it less likely that patients would receive intensive therapy than their younger counterparts, but that did not mean that they were treated any less favorably overall, according to findings derived from the Early RA Network cohort.
“The claim that the elderly are treated less aggressively isn’t completely true throughout the whole treat-to-target strategy,” said Simone Howard of King’s College London at the British Society for Rheumatology annual conference. While older patients were less likely to receive intensive treatment up to 2 years after their diagnosis, there was a shorter delay between the onset of symptoms and the first outpatient visit to a rheumatology clinic.
When compared against patients who were younger than 65 years, those aged 65-74 years and 75 years and older were 11% (P = .02) and 15% (P = .02) more likely to have their first outpatient visit within 10 months.
Furthermore, no significant differences were seen between any age groups in the time to first initiation of a conventional synthetic disease-modifying antirheumatic drug (csDMARD), which averaged nearly 3 months after symptoms appeared.
Ms. Howard, who has previously worked at the European Medicines Agency, noted that, during her time at the EMA, “there was a real push to incorporate the elderly into the regulatory framework more. In parallel, there were also reports of the elderly being treated less aggressively. So the question was, where was that coming from?”
Similar therapeutic approaches are advocated for older and younger RA patients, and to look for any disparities, Ms. Howard and associates turned to the Early RA Network (ERAN) to “investigate potential treatment bias against the elderly.”
ERAN is a hospital-based inception cohort of 1,236 patients with early RA who were recruited across 23 centers in the United Kingdom and Ireland between 2002 and 2014.
Of 1,131 patients used in the analyses, 9.7% (n = 110) were 75 years or older, 21.5% (n = 243) were aged 65-74 years, and 68.8% (n = 778) were 65 years or younger. The majority (67.7%) of patients were female.
Patients aged 75 years and older were more likely to present with comorbidities than the youngest group, and they had higher health assessment questionnaire scores at baseline. However, they were no more likely to have high disease activity at the first visit, which was defined as a disease activity score in 28 joints of more than 5, and the older patients were 27% less likely to be seropositive (P = .004).
“It’s when we come to pharmacological aspects of care that we are seeing treatment biases,” Ms. Howard noted. Patients over 75 years were significantly more likely than the youngest age group to be treated with glucocorticoids or csDMARD monotherapy at 1 year, and 23% more likely to be on less aggressive therapy (P equal to or less than .0001). Aggressive therapy was defined as the use of a combination of csDMARDs or the use of biologic drugs.
At 2 years, the oldest patients were 46% more likely than those under 65 years to be on less-intensive therapies (P equal to or less than .0001), with those aged 65-74 years 19% more likely to be on glucocorticoid or csDMARD therapy (P = .005).
Factors such as patient choice and tolerance were not considered in the analyses and could be important, Ms. Howard conceded in response to a question after her presentation.
Another point raised was that perhaps the prescribing of aggressive therapy would rationally be different in someone diagnosed with RA at age 85 versus 65 because the duration of time that would be likely to be lived with accumulating joint damage would be shorter at the older age and that would be balanced against the other effects of the therapy. So, there may be important reasons in shared decision making that influenced the treatment choices other than the age of patients.
Ms. Howard agreed, noting that this demonstrated the need to be careful around the language used for defining what constituted aggressive or intensive therapy.
She and her coauthors reported no conflicts of interest.
SOURCE: Howard S et al. Rheumatology. 2019;58(suppl 3), Abstract 011.
BIRMINGHAM, ENGLAND – Being diagnosed with rheumatoid arthritis at age 75 years or older made it less likely that patients would receive intensive therapy than their younger counterparts, but that did not mean that they were treated any less favorably overall, according to findings derived from the Early RA Network cohort.
“The claim that the elderly are treated less aggressively isn’t completely true throughout the whole treat-to-target strategy,” said Simone Howard of King’s College London at the British Society for Rheumatology annual conference. While older patients were less likely to receive intensive treatment up to 2 years after their diagnosis, there was a shorter delay between the onset of symptoms and the first outpatient visit to a rheumatology clinic.
When compared against patients who were younger than 65 years, those aged 65-74 years and 75 years and older were 11% (P = .02) and 15% (P = .02) more likely to have their first outpatient visit within 10 months.
Furthermore, no significant differences were seen between any age groups in the time to first initiation of a conventional synthetic disease-modifying antirheumatic drug (csDMARD), which averaged nearly 3 months after symptoms appeared.
Ms. Howard, who has previously worked at the European Medicines Agency, noted that, during her time at the EMA, “there was a real push to incorporate the elderly into the regulatory framework more. In parallel, there were also reports of the elderly being treated less aggressively. So the question was, where was that coming from?”
Similar therapeutic approaches are advocated for older and younger RA patients, and to look for any disparities, Ms. Howard and associates turned to the Early RA Network (ERAN) to “investigate potential treatment bias against the elderly.”
ERAN is a hospital-based inception cohort of 1,236 patients with early RA who were recruited across 23 centers in the United Kingdom and Ireland between 2002 and 2014.
Of 1,131 patients used in the analyses, 9.7% (n = 110) were 75 years or older, 21.5% (n = 243) were aged 65-74 years, and 68.8% (n = 778) were 65 years or younger. The majority (67.7%) of patients were female.
Patients aged 75 years and older were more likely to present with comorbidities than the youngest group, and they had higher health assessment questionnaire scores at baseline. However, they were no more likely to have high disease activity at the first visit, which was defined as a disease activity score in 28 joints of more than 5, and the older patients were 27% less likely to be seropositive (P = .004).
“It’s when we come to pharmacological aspects of care that we are seeing treatment biases,” Ms. Howard noted. Patients over 75 years were significantly more likely than the youngest age group to be treated with glucocorticoids or csDMARD monotherapy at 1 year, and 23% more likely to be on less aggressive therapy (P equal to or less than .0001). Aggressive therapy was defined as the use of a combination of csDMARDs or the use of biologic drugs.
At 2 years, the oldest patients were 46% more likely than those under 65 years to be on less-intensive therapies (P equal to or less than .0001), with those aged 65-74 years 19% more likely to be on glucocorticoid or csDMARD therapy (P = .005).
Factors such as patient choice and tolerance were not considered in the analyses and could be important, Ms. Howard conceded in response to a question after her presentation.
Another point raised was that perhaps the prescribing of aggressive therapy would rationally be different in someone diagnosed with RA at age 85 versus 65 because the duration of time that would be likely to be lived with accumulating joint damage would be shorter at the older age and that would be balanced against the other effects of the therapy. So, there may be important reasons in shared decision making that influenced the treatment choices other than the age of patients.
Ms. Howard agreed, noting that this demonstrated the need to be careful around the language used for defining what constituted aggressive or intensive therapy.
She and her coauthors reported no conflicts of interest.
SOURCE: Howard S et al. Rheumatology. 2019;58(suppl 3), Abstract 011.
REPORTING FROM BSR 2019
Lonely elderly patients suffer worse health outcomes
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
WASHINGTON – More lonely elderly patients suffered from health symptoms and received very aggressive end of life care than nonlonely elderly patients, according to a study presented at the annual meeting of the Society of General Internal Medicine.
“Loneliness and social isolation are very common problems, especially in older Americans, and inflict about 30%-40% of older Americans. But while we know that this may have implications for their quality [of] life and may actually lead to premature death, we know very little about the end of life experience,” said Nauzley Abedini, MD, MSc, a hospitalist in internal medicine at the University of Michigan, Ann Arbor.
The study sought to determine the association between loneliness and end of life experience as measured by symptom burden, intensity of care, and advance care planning in adults. The pooled cohort study used data from the Health and Retirement Study (HRS) to analyze older Americans (aged 50 years or more) who died between 2004 and 2014. Investigators conducted postmortem “exit interviews” with the next of kin after each participant’s death. There were 2,896 participants included in the survey. Of these participants, 34% (942) were lonely; the remaining 1,954 of elderly adults were classified as nonlonely.
Loneliness was defined using the three-item Revised University of California, Los Angeles, Loneliness Scale score from a decedent’s last HRS interview prior to death. These items included feeling left out, feeling isolated, and lacking companionship. Investigators used this data to create a loneliness variable on previously established cutpoints for “lonely” and “nonlonely” participants. The data was used from the most recent survey prior to death.
Results showed more lonely older adults suffered from health symptoms in the last year of life, compared with nonlonely older adults (69.1% vs. 59.5%; odds ratio, 1.52; 95% confidence interval, 1.30-1.78). These symptoms included being troubled by pain, having difficulty breathing, experiencing severe fatigue, and having periodic confusion.
Patients with loneliness associated with intensity of health care at the end of life were more likely to die in a nursing home than at home, compared with nonlonely adults (OR, 1.68; 95% CI, 1.25-2.27). The lonely patients also were more likely to use life support during their last 2 years of life (OR, 1.41; 95% CI, 1.16-1.70).
“For clinicians, we need to identify end of life as an additional vulnerable time for people who are lonely. Currently, most of our interventions in terms of screening for loneliness are in the outpatient setting, but I would argue that working in hospitals, hospices, nursing homes, and community organizations, where these folks are living and dying, would be useful places to screen for this,” Dr. Abedini said.
The authors had no disclosures.
REPORTING FROM SGIM 2019
Insomnia meds get boxed warning from FDA
The Food and Drug Administration will now require that certain
Complex sleep behaviors have been seen with these medications in patients with and without a history of them, at low doses, and even after one dose of the medication. They’ve also been observed with and without concomitant use of alcohol or other CNS depressants.
Health care professionals should advise patients about these risks, even though they are rare. Patients should contact health care professionals if they either experience a complex sleep behavior while not fully awake on one of these medicines or have performed activities they don’t remember while taking the medicine.
More information about these risks and the safety warnings can be found in the FDA’s safety announcement. Other information is also available in a press announcement from the agency.
The Food and Drug Administration will now require that certain
Complex sleep behaviors have been seen with these medications in patients with and without a history of them, at low doses, and even after one dose of the medication. They’ve also been observed with and without concomitant use of alcohol or other CNS depressants.
Health care professionals should advise patients about these risks, even though they are rare. Patients should contact health care professionals if they either experience a complex sleep behavior while not fully awake on one of these medicines or have performed activities they don’t remember while taking the medicine.
More information about these risks and the safety warnings can be found in the FDA’s safety announcement. Other information is also available in a press announcement from the agency.
The Food and Drug Administration will now require that certain
Complex sleep behaviors have been seen with these medications in patients with and without a history of them, at low doses, and even after one dose of the medication. They’ve also been observed with and without concomitant use of alcohol or other CNS depressants.
Health care professionals should advise patients about these risks, even though they are rare. Patients should contact health care professionals if they either experience a complex sleep behavior while not fully awake on one of these medicines or have performed activities they don’t remember while taking the medicine.
More information about these risks and the safety warnings can be found in the FDA’s safety announcement. Other information is also available in a press announcement from the agency.
ERRATUM
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
A recent letter, “Hypoglycemia in the elderly: Watch for atypical symptoms” (J Fam Pract. 2019;68:116) provided an incomplete list of the letter’s authors. The list should have read: Jan Brož, MD, Jana Urbanová, MD, PhD, Prague, Czech Republic; Brian M. Frier, MD, BSc, Edinburgh, United Kingdom.
Experts propose new definition and recommendations for Alzheimer’s-like disorder
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
Alois Alzheimer’s original patient was 51 years old, and for roughly 70 years Alzheimer’s disease was considered a rare disease that caused presenile dementia. In the 1970s, Robert Katzman, MD, and Robert D. Terry, MD, equated the neuropathologic features of Alzheimer’s disease with the more common senile dementia, and since then we have recognized Alzheimer’s disease as the most common form of dementia. Autopsy studies of patients dying in their 80s and 90s, however, has revealed that far more common than pure Alzheimer’s disease is a mixed neuropathologic picture. In addition, with the advent of biomarker studies a substantial number of individuals have “suspected non-Alzheimer pathology.”
Interestingly, the authors identify the apolipoprotein E epsilon 4 (APOE4) allele as a predisposing factor for LATE, although given the advanced age of the LATE patient population, one could argue that a certain degree of resilience extended their lives into the LATE age range.
In contrast, in the Alzheimer’s Disease Sequencing Project, among those with autopsy confirmation, the prevalence of APOE4 in Braak stage 5-6 declines with succeeding decades so that, by the 80s and 90s, the prevalence of APOE2 is actually higher at 7.3% vs. 4.1% with APOE4 for ages 80 to younger than 85 years, 9.3% with APOE2 vs. 8.6% with APOE4 for 85 to younger than 90 years, and 16.7% with APOE2 vs. 6.9% with APOE4 for ages 90 years and above.
Our understanding of age-related cognitive decline, from the normal to the pathological ends of the spectrum, continues to evolve, and LATE is simply the latest addition to our growing knowledge base that will further inform clinical diagnosis, research, and experimental therapeutics.
Richard J. Caselli, MD, is professor of neurology at the Mayo Clinic Arizona in Scottsdale and associate director and clinical core director of the Arizona Alzheimer’s Disease Center.
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
An international group of experts has proposed a new name, staging criteria, and recommendations for a recently recognized brain disorder that mimics Alzheimer’s disease and is marked by a proteinopathy caused by malformed transactive response DNA-binding protein of 43 kDa (TDP-43).
The term limbic-predominant age-related TDP-43 encephalopathy (LATE) was coined in an effort to raise awareness and kick-start research into this “pathway to dementia,” the experts wrote in a report appearing in Brain.
“As there is currently no universally agreed-upon terminology or staging system for common age-related TDP-43 proteinopathy, this condition is understudied and not well recognized, even among investigators in the field of dementia research,” wrote the authors of the report, led by Peter T. Nelson, MD, PhD, of the University of Kentucky, Lexington.
LATE neuropathologic changes, associated with a progressive amnesia syndrome that mimics Alzheimer’s, are seen in more than 20% of individuals past the age of 80 years, according to large, community-based autopsy series. It coexists with Alzheimer’s disease in many patients, lowering the threshold for developing dementia, authors said.
The term LATE is designed to encompass several other terms related to TDP-43 pathology, including hippocampal sclerosis and cerebral age-related TDP-43 with sclerosis, Dr. Nelson and coauthors noted in their report.
The TDP-43 protein is encoded by the TARDBP gene and provides several functions related to the regulation of gene expression, the authors wrote.
Misfolded TDP-43 was known to play a causative role in amyotrophic lateral sclerosis and frontotemporal lobar degeneration, the authors noted, and then was also identified in the brains of older individuals with hippocampal sclerosis or Alzheimer’s disease neuropathologic changes.
The authors proposed a three-stage classification system for LATE neuropathologic change based on TDP-43 immunohistochemistry performed during routine autopsy evaluation of the amygdala, hippocampus, and middle frontal gyrus.
The amygdala is an area affected early in the course of the disease (Stage 1), whereas involvement of the hippocampus represents a more intermediate stage (Stage 2), and the middle frontal gyrus is more affected in advanced stages of the disease (Stage 3), according to the schema.
Five genes have been identified with risk alleles for LATE neuropathologic changes, authors said. Of note, several groups have found that the apolipoprotein E epsilon 4 (APOE4) allele, known to be a risk factor for Alzheimer’s disease neuropathologic changes and Lewy body disease, is also linked to increased risk of TDP-43 proteinopathy.
There are no established biomarkers specific to TDP-43 proteinopathy yet, which hampers development of clinical trials designed to test interventions to treat or prevent LATE, Dr. Nelson and colleagues said in their report.
LATE could also obscure the effects of potentially disease-modifying agents being tested in Alzheimer’s disease clinical trials, which can complicate the interpretation of study results, they added.
“Until there are biomarkers for LATE, clinical trials should be powered to account for TDP-43 proteinopathy,” they wrote.
Dr. Nelson and coauthors of the report in Brain reported no competing interests.
SOURCE: Nelson PT, et al. Brain. 2019 Apr 30. doi: 10.1093/brain/awz099
FROM BRAIN