User login
Study provides cancer risk estimates among women undergoing morcellation
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
FROM JAMA
Key clinical point: A study that provides an estimate of malignancies specifically among women undergoing morcellation and minimally invasive hysterectomy provides valuable information on the risk overall, and risk stratified by age, that can be used in patient counseling.
Major finding: The prevalence of uterine cancer among women who underwent morcellation and a minimally invasive hysterectomy was 27/10,000 – about 1 in 370 women – a risk that significantly increased with age.
Data source: The study identified women who had a minimally invasive hysterectomy with morcellation from 2006 to 2012 in a national insurance database of over 500 hospitals, representing about 15% of hospitalizations.
Disclosures: The authors had no disclosures. Dr. Wright and one of the other authors are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
Study provides cancer risk estimates among women undergoing morcellation
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
An estimated 1 in 370 women who undergo electric power morcellation during a minimally invasive hysterectomy have uterine cancer, with the risk of cancer and endometrial hyperplasia markedly increasing with age, according to an analysis using an insurance database of more than 500 U.S. hospitals.
The estimate, published online in JAMA on July 22, is close to the Food and Drug Administration’s estimate that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and is higher than historical estimates provided in the literature. The FDA’s estimate, first reported when the agency issued a safety communication in April discouraging the use of laparoscopic power morcellators (LPMs) during a hysterectomy or myomectomy because of the risk of disseminating cancerous tissue and upstaging disease, was based on 9 U.S. and international studies of women treated from 1983 to 2011. The risk of an unsuspected leiomyosarcoma was about 1 in 500.
But unlike the FDA analysis, the most recent analysis, conducted by Dr. Jason Wright and his associates at Columbia University, New York, specifically addressed the risk associated with morcellation. The estimate was based on the records of 232,882 women who underwent a minimally invasive hysterectomy from 2006 to 2012 obtained from a database that represents about 15% of U.S. hospitalizations. Morcellators were used in almost 16% (36,470) of the women, and there were 99 cases of uterine cancers, for a prevalence of 27/10,000 – about one in 370.
Among the women who underwent morcellation, the strongest risk factor for abnormal pathology, either for cancer or any of the precancerous changes, was advanced age. Compared with women under age 40 years, the prevalence ratio for uterine malignancy was 1.42 among those aged 40-44 and 2.55 for those aged 45-49, increasing to 4.97 among those aged 50-54 years, 19.37 among those aged 55-59 years, 21.36 among those aged 60-64 years – and 35.97 among those aged 65 and older.
The researchers also identified cases of endometrial hyperplasia, other gynecologic cancers, and smooth muscle tumors of uncertain malignant potential. The risk of endometrial hyperplasia also increased significantly with age, compared with women under age 40, with prevalence ratios of 1.17 among those aged 40-44 (not statistically significant) and 1.71 among those aged 45-49, to 4.07 among those aged 50-54 years, to 10.21 among those aged 65 years and older. The results are reported in a research letter (doi:10.1001/jama.2014.9005)
Despite the availability of power morcellators for 20 years, "few studies have described the prevalence of unexpected pathology at the time of hysterectomy," Dr. Wright and his associates wrote. While the analysis had limitations, including the lack of long-term follow-up and not being able to verify the pathology results, they concluded that patients who may be undergoing morcellation "should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure."
One of the strengths and unique aspects of this study was being able to identify a large population of women who specifically had morcellation and a hysterectomy, providing a population-based estimate of the prevalence of cancer in this group, Dr. Wright said in an interview. This is slightly different than other estimates and studies, which were not specific to morcellation, including those that looked at the incidence of sarcoma among women who had a hysterectomy. "We could not separate out epithelial endometrial cancers from uterine sarcomas, so this is an estimate of any malignancy within the uterus," so "probably a high proportion of women who underwent morcellation underwent the procedure for fibroid uterus, so the chance of sarcomas is probably higher in these patients than in the general population."
The study did not allow evaluation of whether the use of morcellation increased the risk of dissemination of cancer, which "certainly warrants further study," added Dr. Wright, chief of the division of gynecologic oncology at Columbia.
The lack of data has been one of the major problems surrounding morcellation, with very few studies specifically looking at data that can be used to help guide patients and clinicians. Dr. Wright and his associates hope that their results can help guide patients and clinicians.
"There’s undoubtedly a risk of cancer and precancerous changes in women who undergo morcellation ... and [patients and clinicians] need to weigh that risk," he said. "But certainly morcellation may allow some women to undergo a minimally invasive surgery who otherwise require laparotomy, and the complications and recovery are much easier with minimally invasive surgery, when it’s feasible."
Dr. Charles E. Miller, director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., described these data as "compelling," with well-presented information that stratifies risk by age that can be used to help properly counsel patients.
The marked difference in risk among women under age 40 is particularly important, he said. Dr. Miller performs about 250 morcellations per year and has had only one patient with a sarcoma, a woman in her mid-40s, which he said reflects the younger age of his patients.
"If we can better identify age groups where that risk is higher and do the treatment that is appropriate in that age group, then I think we’ve come a long, long way," he said in an interview. There are always outliers, and unfortunately, women in younger age groups develop sarcomas, but there are also the risks of more invasive surgery that should be considered, he added.
"There’s always going to be risk, and there’s always going to be decision making with surgery," and while there is a need for better ways to identify those patients at risk, currently, "all we can hang our hat on now is stratifying [risk] with age," Dr. Miller said.
On July 10-11, the FDA held a meeting of its Obstetrics and Gynecology Devices panel, to review the safety of LPMs during uterine surgery for fibroids, Among the questions the panel was asked was whether there were characteristics of patients – such as age, physical exam findings, and imaging test results – that could help identify patients who might be at a higher risk of a sarcoma.
The FDA is currently reviewing the safety of LPMs in women undergoing surgery for presumably benign fibroids, an issue that has received widespread attention this year and resulted in the FDA’s safety communication in April – largely due to the case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage IV leiomyosarcoma after undergoing a hysterectomy with morcellation at age 40 last year. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign to highlight these risks, including a petition on change.org calling for a halt to morcellation during minimally invasive and robotic-assisted hysterectomy and myomectomy.
The authors of the JAMA report had no disclosures. Dr. Wright and one of the other authors, are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
FROM JAMA
Key clinical point: A study that provides an estimate of malignancies specifically among women undergoing morcellation and minimally invasive hysterectomy provides valuable information on the risk overall, and risk stratified by age, that can be used in patient counseling.
Major finding: The prevalence of uterine cancer among women who underwent morcellation and a minimally invasive hysterectomy was 27/10,000 – about 1 in 370 women – a risk that significantly increased with age.
Data source: The study identified women who had a minimally invasive hysterectomy with morcellation from 2006 to 2012 in a national insurance database of over 500 hospitals, representing about 15% of hospitalizations.
Disclosures: The authors had no disclosures. Dr. Wright and one of the other authors are recipients of National Cancer Institute (NCI) grants; another author is a recipient of an NCI fellowship. Dr. Miller disclosed that he is a consultant to Ethicon Endo-Surgery. Ethicon is a morcellator manufacturer.
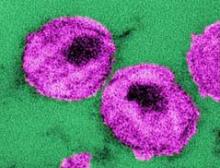
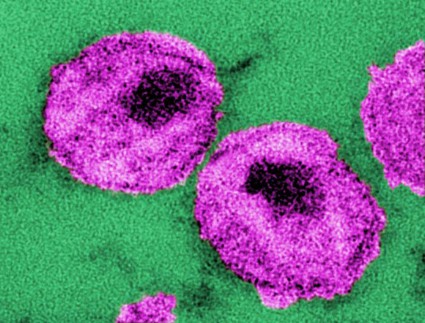
Stem cell transplantation achieved temporary HIV remission
Allogeneic hematopoietic stem cell transplantation from HIV-naive individuals to HIV-1–positive individuals may achieve temporary antiretroviral-free remission of infection and loss of detectable HIV-1, a study showed.
Two men with chronic HIV-1 infection received allogeneic hematopoietic stem cell transplants (HSCTs) from susceptible donors to treat Hodgkin’s and non-Hodgkin’s lymphoma, and achieved temporary remission of HIV despite stopping antiretroviral therapy (ART), with the virus undetectable in both blood and rectal mucosa.
However, both experienced viral rebound – one at 12 weeks after stopping ART and one at 32 weeks – with both developing the usual symptoms of acute retroviral syndrome, according to a paper published online July 22 in the Annals of Internal Medicine.
"In summary, our results suggest that allogeneic HSCT with CCR5 wild-type donor cells may lead to loss of detectable HIV-1 from blood and rectal mucosa, but viral rebound may nevertheless occur after ART interruption despite a significant reduction in reservoir size," wrote Dr. Timothy J. Henrich of Brigham and Women’s Hospital, Boston, and his colleagues.
The researchers had previously reported the reduction in peripheral blood HIV-1 reservoirs in these two patients (Ann. Intern. Med. 2014 July 22 [doi:10.7326/M14-1027]).
"However, extensive sampling of tissues and large numbers of peripheral blood mononuclear cells for the presence of HIV-1 is necessary to understand the full effect of allogeneic HSCT on HIV-1 persistence," they wrote, arguing that treatment interruption was also necessary to establish if the virus was in remission.
Antiretroviral-free remission had previously been achieved in a patient who received an HSCT from a donor with a homozygous 32–base pair deletion in the gene encoding CCR5, a coreceptor for HIV-1. In this patient – known as "the Berlin patient" – remission has been maintained for more than 7 years, representing the only known functional cure of HIV infection.
The authors of the study suggested that while allogeneic HSCT may lead to significant and sustained reductions in the HIV-1 reservoir, the virus appears to persist in infected tissue or bound into cells, and those small numbers of infected cells were enough to restart HIV-1 replication.
Although both patients did experience rebound infection, that occurred much slower than it would have under normal circumstances, the authors said.
"Despite frequent sampling, neither of our patients had detectable HIV-1 in [peripheral blood mononuclear cells] or plasma for several months after ART discontinuation before viral rebound," they wrote.
Both patients received treatment for graft versus host disease after the transplant.
The study was supported by the Foundation for AIDS Research and the National Institute of Allergy and Infectious Diseases. Dr. Henrich had no disclosures. Some of the study’s other authors, as well as the editorial author, Dr. Lewin, declared grant support, speakers fees, and consulting fees from agencies and pharmaceutical companies.
The study showed it was possible to significantly reduce the number of long-lived, latently infected T cells persisting in patients receiving ART, and that this was associated with a delay in viral rebound after cessation of ART, Sharon R. Lewin, Ph.D., said.
"More tractable and scalable approaches than HSCT and very early ART are clearly needed for the 35 million persons already infected with HIV who will all eventually require lifelong treatment," wrote Dr. Lewin. "The amount of residual infectious virus left after ART and an effective immune response are both likely to be key in achieving long-term HIV remission."
Dr. Lewin is with Alfred Health in Melbourne. Her comments were taken from an accompanying editorial.
The study showed it was possible to significantly reduce the number of long-lived, latently infected T cells persisting in patients receiving ART, and that this was associated with a delay in viral rebound after cessation of ART, Sharon R. Lewin, Ph.D., said.
"More tractable and scalable approaches than HSCT and very early ART are clearly needed for the 35 million persons already infected with HIV who will all eventually require lifelong treatment," wrote Dr. Lewin. "The amount of residual infectious virus left after ART and an effective immune response are both likely to be key in achieving long-term HIV remission."
Dr. Lewin is with Alfred Health in Melbourne. Her comments were taken from an accompanying editorial.
The study showed it was possible to significantly reduce the number of long-lived, latently infected T cells persisting in patients receiving ART, and that this was associated with a delay in viral rebound after cessation of ART, Sharon R. Lewin, Ph.D., said.
"More tractable and scalable approaches than HSCT and very early ART are clearly needed for the 35 million persons already infected with HIV who will all eventually require lifelong treatment," wrote Dr. Lewin. "The amount of residual infectious virus left after ART and an effective immune response are both likely to be key in achieving long-term HIV remission."
Dr. Lewin is with Alfred Health in Melbourne. Her comments were taken from an accompanying editorial.
Allogeneic hematopoietic stem cell transplantation from HIV-naive individuals to HIV-1–positive individuals may achieve temporary antiretroviral-free remission of infection and loss of detectable HIV-1, a study showed.
Two men with chronic HIV-1 infection received allogeneic hematopoietic stem cell transplants (HSCTs) from susceptible donors to treat Hodgkin’s and non-Hodgkin’s lymphoma, and achieved temporary remission of HIV despite stopping antiretroviral therapy (ART), with the virus undetectable in both blood and rectal mucosa.
However, both experienced viral rebound – one at 12 weeks after stopping ART and one at 32 weeks – with both developing the usual symptoms of acute retroviral syndrome, according to a paper published online July 22 in the Annals of Internal Medicine.
"In summary, our results suggest that allogeneic HSCT with CCR5 wild-type donor cells may lead to loss of detectable HIV-1 from blood and rectal mucosa, but viral rebound may nevertheless occur after ART interruption despite a significant reduction in reservoir size," wrote Dr. Timothy J. Henrich of Brigham and Women’s Hospital, Boston, and his colleagues.
The researchers had previously reported the reduction in peripheral blood HIV-1 reservoirs in these two patients (Ann. Intern. Med. 2014 July 22 [doi:10.7326/M14-1027]).
"However, extensive sampling of tissues and large numbers of peripheral blood mononuclear cells for the presence of HIV-1 is necessary to understand the full effect of allogeneic HSCT on HIV-1 persistence," they wrote, arguing that treatment interruption was also necessary to establish if the virus was in remission.
Antiretroviral-free remission had previously been achieved in a patient who received an HSCT from a donor with a homozygous 32–base pair deletion in the gene encoding CCR5, a coreceptor for HIV-1. In this patient – known as "the Berlin patient" – remission has been maintained for more than 7 years, representing the only known functional cure of HIV infection.
The authors of the study suggested that while allogeneic HSCT may lead to significant and sustained reductions in the HIV-1 reservoir, the virus appears to persist in infected tissue or bound into cells, and those small numbers of infected cells were enough to restart HIV-1 replication.
Although both patients did experience rebound infection, that occurred much slower than it would have under normal circumstances, the authors said.
"Despite frequent sampling, neither of our patients had detectable HIV-1 in [peripheral blood mononuclear cells] or plasma for several months after ART discontinuation before viral rebound," they wrote.
Both patients received treatment for graft versus host disease after the transplant.
The study was supported by the Foundation for AIDS Research and the National Institute of Allergy and Infectious Diseases. Dr. Henrich had no disclosures. Some of the study’s other authors, as well as the editorial author, Dr. Lewin, declared grant support, speakers fees, and consulting fees from agencies and pharmaceutical companies.
Allogeneic hematopoietic stem cell transplantation from HIV-naive individuals to HIV-1–positive individuals may achieve temporary antiretroviral-free remission of infection and loss of detectable HIV-1, a study showed.
Two men with chronic HIV-1 infection received allogeneic hematopoietic stem cell transplants (HSCTs) from susceptible donors to treat Hodgkin’s and non-Hodgkin’s lymphoma, and achieved temporary remission of HIV despite stopping antiretroviral therapy (ART), with the virus undetectable in both blood and rectal mucosa.
However, both experienced viral rebound – one at 12 weeks after stopping ART and one at 32 weeks – with both developing the usual symptoms of acute retroviral syndrome, according to a paper published online July 22 in the Annals of Internal Medicine.
"In summary, our results suggest that allogeneic HSCT with CCR5 wild-type donor cells may lead to loss of detectable HIV-1 from blood and rectal mucosa, but viral rebound may nevertheless occur after ART interruption despite a significant reduction in reservoir size," wrote Dr. Timothy J. Henrich of Brigham and Women’s Hospital, Boston, and his colleagues.
The researchers had previously reported the reduction in peripheral blood HIV-1 reservoirs in these two patients (Ann. Intern. Med. 2014 July 22 [doi:10.7326/M14-1027]).
"However, extensive sampling of tissues and large numbers of peripheral blood mononuclear cells for the presence of HIV-1 is necessary to understand the full effect of allogeneic HSCT on HIV-1 persistence," they wrote, arguing that treatment interruption was also necessary to establish if the virus was in remission.
Antiretroviral-free remission had previously been achieved in a patient who received an HSCT from a donor with a homozygous 32–base pair deletion in the gene encoding CCR5, a coreceptor for HIV-1. In this patient – known as "the Berlin patient" – remission has been maintained for more than 7 years, representing the only known functional cure of HIV infection.
The authors of the study suggested that while allogeneic HSCT may lead to significant and sustained reductions in the HIV-1 reservoir, the virus appears to persist in infected tissue or bound into cells, and those small numbers of infected cells were enough to restart HIV-1 replication.
Although both patients did experience rebound infection, that occurred much slower than it would have under normal circumstances, the authors said.
"Despite frequent sampling, neither of our patients had detectable HIV-1 in [peripheral blood mononuclear cells] or plasma for several months after ART discontinuation before viral rebound," they wrote.
Both patients received treatment for graft versus host disease after the transplant.
The study was supported by the Foundation for AIDS Research and the National Institute of Allergy and Infectious Diseases. Dr. Henrich had no disclosures. Some of the study’s other authors, as well as the editorial author, Dr. Lewin, declared grant support, speakers fees, and consulting fees from agencies and pharmaceutical companies.
FROM ANNALS OF INTERNAL MEDICINE
Major finding: Two men with chronic HIV-1 infection achieved 12-week and 32-week antiretroviral-free remission following allogeneic hematopoietic stem cell transplants from HIV-susceptible donors, with the virus undetectable in blood and rectal mucosa.
Data source: Two case studies.
Disclosures: The study was supported by the Foundation for AIDS Research and the National Institute of Allergy and Infectious Diseases. Dr. Henrich had no disclosures. Some of the study’s other authors, as well as the editorial author, Dr. Lewin, declared grant support, speakers fees, and consulting fees from agencies and pharmaceutical companies.
ACA: How will the recent Supreme Court decision impact contraception?
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault
It may be months, even years before it’s clear whether access to and payment for contraception will be limited in the wake of the U.S. Supreme Court’s decision that for-profit companies can forego health insurance coverage of birth control methods that violate their religious belief.
At this point, only the two plaintiffs in the case – Hobby Lobby and Conestoga Wood Specialties – can change their health insurance coverage of contraception, based on the June 30 decision by the U.S. Supreme Court.
In the meantime, there is a lot of confusion among patients – and uncertainty among doctors -- about what the ruling means.
"We’re all scratching our heads and saying, ‘Now what?’ " said Dr. Anne Davis, medical director of Physicians for Reproductive Health. "All of us are very used to hurdles with coverage, but this is a new one."
Now, ob.gyns and other physicians may have to figure out if a patient works for an employer whose insurance will not cover certain types of contraception, or perhaps all birth control, said Dr. Davis of the department of clinical obstetrics and gynecology at Columbia University, New York.
The Supreme Court ruling is "a huge step backward," said Dr. Eve Espey, chair of obstetrics and gynecology at the University of New Mexico, Albuquerque. For physicians, a key concern is whether they will be paid for inserting an IUD or providing another service; for patients, it’s whether they can get the contraception and if they can afford it, she said.
When concerns mount, "those services tend to not happen," Dr. Espey said.
Ob.gyns. also expressed concern that the ruling had opened the door to interference in the physician-patient relationship.
Clinicians need the freedom to select the birth control method that makes the most sense for the patient, said Dr. Davis. "It’s going to be really unfortunate if you and the patient agree, and it’s the right thing and you find out that her employer has decided it’s not the right kind of birth control because of their beliefs," she said.
Dr. Jeanne Conry, immediate past president of the American Congress of Obstetricians and Gynecologists (ACOG), said that if she has decided that an IUD is best for a 34-year-old diabetic patient, but her employer says it won’t pay for that method, "that’s not in her best interest."
"They’ve just interfered with my best treatment for the patient," said Dr. Conry, an ob.gyn with Kaiser Permanente in Roseville, Calif.
Dr. Mitchell Creinin, chair of the obstetrics and gynecology department at University of California, Davis, said that the ruling could return contraception to a means-tested environment, in which only those who have the means will get the most effective methods, like the IUD. Instead, he said, it should be a benefit for all. Preventing pregnancy "is a public health issue and we’ve got to look at it that way," Dr. Creinin said.
Just after the ruling, ACOG President John Jennings agreed, expressing dismay that the Court was overlooking what had been firmly established. "The value of family planning – including contraception – has been clearly demonstrated," he said, in a statement.
But pro-life groups, including Americans United for Life and the Association of Pro-Life Obstetricians and Gynecologists, applauded the Hobby Lobby decision, saying that it helps preserve the right to practice according to conscience.
"Respect for the constitutionally guaranteed rights of conscience – whether held by business owners or medical professionals – must and should be protected," said Dr. Monique Chireau, AUL board member, with the department of ob.gyn at Duke University, Durham, N.C.
Dr. Gene Rudd, senior vice president for the Christian Medical and Dental Associations (CMDA), said that he did not think the decision would have any direct impact on ob.gyns.’ practices. However, he said, "Had the Justices allowed the government to force business owners to act against their conscience, that could establish a legal precedent by which the government might one day force physicians to act against their consciences."
The CMDA’s membership view the ACA’s mandate that women receive contraceptives free of charge as a potential infringement on their right to practice according to their beliefs, said Dr. Rudd, an ob.gyn.
In a CMDA survey, "95% of our members said they’d leave medicine before being required to violate their conscience," he said. They want "women free to pursue getting the contraceptive of their choice," but not to force either individual physicians or religiously affiliated organizations or small private employers to do things that are against their beliefs.
Who might be affected?
For now, there is no definitive answer on how many women could potentially lose coverage of contraception as a result of the Supreme Court decision. Hobby Lobby has 500 stores and 13,000 employees. Conestoga Wood Specialties has 950 employees. There are 90-100 cases challenging the contraception requirement, with about half them from nonprofit organizations and half from for-profit companies. And there’s motivation to sue: Companies that do not comply with the ACA requirement face fines of $100 per day per enrollee.
According to the Dept. of Health & Human Services, about 48 million women are eligible for preventive services because they are in "non-grandfathered" health plans.
Millions of women receive family planning services outside of employer-sponsored insurance, and thus may not be directly affected. A Guttmacher Institute report shows that 19 million women need publicly funded services and supplies because they are low income or are assumed to have a low income because they are under age 20 years. Family planning services provided through federal and state government funding helped women avoid 2.2 million unintended pregnancies in 2010, according to Guttmacher.
Reversing the decision
A handful of Democrats in the House and Senate introduced legislation July 9 to reverse the Supreme Court’s decision. The Protect Women's Health From Corporate Interference Act, introduced by Sen. Patty Murray (D-Wash.), would eliminate the loophole given by the court to companies that are not religiously affiliated.
ACOG praised the effort and applauded the legislators "for recognizing that contraceptives and other essential health care services should not be treated differently than other forms of health care.
"We look forward to working with them and other Congressional leaders to ensure that the ability of physicians to provide care for women is no longer subject to outside interference," Dr. Jennings said in a statement.
The White House said in an official Statement of Administration Policy that it strongly supported the legislation, saying that it "would prevent owners of for-profit companies from asserting their personal religious views to deny their employees federally-required health benefits."
Senate Democrats attempted to bring the bill to the floor on July 16, but failed to secure enough votes for debate. Republican Senators in the meantime introduced their own legislation to guarantee that employers could not bar "access" to contraceptives. The Preserving Religious Freedom and a Woman’s Access to Contraception Act (S. 2605) was introduced by Sen. Kelly Ayotte (R-N.H.).
Neither of the bills are expected to gain much traction in either the Senate or the House.
ACOG has asked its members to report coverage problems to their regional chapters, Dr. Conry said.
The federal government also could pursue several methods of reinstating the coverage. It could give for-profit employers the same accommodation that it has given nonprofit religiously affiliated employers like schools or charities. If these employers self-attest that paying for birth control violates their beliefs, then the employer’s insurer pays for the contraception. For self-insured employers, the exemption is not as easily navigated, because they would have to pay for the coverage.
That accommodation is being challenged by at least 45 legal cases that argue filling out the form violates plaintiffs’ religious beliefs because it means they are facilitating access to birth control.
The government could also pay directly for contraception for women whose employers receive an exemption. Supreme Court Justice Samuel Alito suggested such a possibility in his majority opinion in the Hobby Lobby case.
Dr. Davis said that, ideally, gynecologists would be able to give patients the contraception of choice on the same day of a visit and that the physician would be reimbursed for the cost of the device plus a fee for placing it. And, physicians should be able to counsel patients without thought of potential oversight from an employer or anyone else.
But, she said, "We are very far away from that right now."
On Twitter @aliciaault
International AIDS conference pays tribute to colleagues on flight MH17
MELBOURNE – Speakers at the opening plenary of the 20th International AIDS Conference struggled with their emotions as they paid tribute to colleagues – including former International AIDS Society President Dr. Joep Lange – who were killed when Malaysian Airlines flight MH17 crashed in Ukraine.
Dr. Lange was instrumental in research and implementation of mother-to-child transmission therapy, and as president of the society, showed a rare combination of enthusiasm, commitment, and perseverance, Lambert Grijns, Dutch Ambassador for Sexual and Reproductive Health and Rights and HIV/AIDS, said July 20.
Dr. Lange’s partner, Jacqueline van Tongeren, of the Amsterdam Institute for Global Health and Development, also was killed in the incident, along with Lucie van Mens, who Mr. Grijns said had advocated the cause of sex workers at a time when few other were doing so.
"She was a driving force in advocacy for the female condom, she gave the product its rightful place in the field of sexual reproductive health and rights, and her impact will continue to be felt," Mr Grijns told the packed auditorium.
Other high-profile researchers on the flight included Martine de Schutter, program manager for Bridging The Gap, who Mr. Grijns said had been a staunch defender of human rights and the right to good health; Glenn Thomas from the World Health Organization’s communications team; and Pim de Kuijer, a prominent AIDS campaigner and lobbyist for Stop AIDS Now!
Prof. Françoise Barré-Sinoussi, IAS president and the director of the regulation of retroviral infections unit at the Institut Pasteur in Paris, called for a moment’s silence in their memory, during which the audience spontaneously rose to its feet.
"Our colleagues were traveling because of their dedication to bringing an end to AIDS, and our determination to continue their work honors their commitment," she said.
MELBOURNE – Speakers at the opening plenary of the 20th International AIDS Conference struggled with their emotions as they paid tribute to colleagues – including former International AIDS Society President Dr. Joep Lange – who were killed when Malaysian Airlines flight MH17 crashed in Ukraine.
Dr. Lange was instrumental in research and implementation of mother-to-child transmission therapy, and as president of the society, showed a rare combination of enthusiasm, commitment, and perseverance, Lambert Grijns, Dutch Ambassador for Sexual and Reproductive Health and Rights and HIV/AIDS, said July 20.
Dr. Lange’s partner, Jacqueline van Tongeren, of the Amsterdam Institute for Global Health and Development, also was killed in the incident, along with Lucie van Mens, who Mr. Grijns said had advocated the cause of sex workers at a time when few other were doing so.
"She was a driving force in advocacy for the female condom, she gave the product its rightful place in the field of sexual reproductive health and rights, and her impact will continue to be felt," Mr Grijns told the packed auditorium.
Other high-profile researchers on the flight included Martine de Schutter, program manager for Bridging The Gap, who Mr. Grijns said had been a staunch defender of human rights and the right to good health; Glenn Thomas from the World Health Organization’s communications team; and Pim de Kuijer, a prominent AIDS campaigner and lobbyist for Stop AIDS Now!
Prof. Françoise Barré-Sinoussi, IAS president and the director of the regulation of retroviral infections unit at the Institut Pasteur in Paris, called for a moment’s silence in their memory, during which the audience spontaneously rose to its feet.
"Our colleagues were traveling because of their dedication to bringing an end to AIDS, and our determination to continue their work honors their commitment," she said.
MELBOURNE – Speakers at the opening plenary of the 20th International AIDS Conference struggled with their emotions as they paid tribute to colleagues – including former International AIDS Society President Dr. Joep Lange – who were killed when Malaysian Airlines flight MH17 crashed in Ukraine.
Dr. Lange was instrumental in research and implementation of mother-to-child transmission therapy, and as president of the society, showed a rare combination of enthusiasm, commitment, and perseverance, Lambert Grijns, Dutch Ambassador for Sexual and Reproductive Health and Rights and HIV/AIDS, said July 20.
Dr. Lange’s partner, Jacqueline van Tongeren, of the Amsterdam Institute for Global Health and Development, also was killed in the incident, along with Lucie van Mens, who Mr. Grijns said had advocated the cause of sex workers at a time when few other were doing so.
"She was a driving force in advocacy for the female condom, she gave the product its rightful place in the field of sexual reproductive health and rights, and her impact will continue to be felt," Mr Grijns told the packed auditorium.
Other high-profile researchers on the flight included Martine de Schutter, program manager for Bridging The Gap, who Mr. Grijns said had been a staunch defender of human rights and the right to good health; Glenn Thomas from the World Health Organization’s communications team; and Pim de Kuijer, a prominent AIDS campaigner and lobbyist for Stop AIDS Now!
Prof. Françoise Barré-Sinoussi, IAS president and the director of the regulation of retroviral infections unit at the Institut Pasteur in Paris, called for a moment’s silence in their memory, during which the audience spontaneously rose to its feet.
"Our colleagues were traveling because of their dedication to bringing an end to AIDS, and our determination to continue their work honors their commitment," she said.
AT AIDS 2014
Survival benefit from contralateral prophylactic mastectomy small
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The decision of whether or not to undergo a contralateral prophylactic mastectomy after being treated for breast cancer is a difficult one for many women. The goal of such aggressive therapy is to lower the likelihood of a second primary carcinoma. The downsides are operative risk, impairment of the woman’s self-image, and short-term and long-term morbidities.
This is a well done analysis from an experienced group of investigators and is based on the currently available data. Given the JNCI audience, we shall refrain from niggling points about modeling. Rather, we will stick to the big picture and clinical implications. Although the survival benefit from CPM is small as demonstrated in this model, it is greater than zero, which suggests that for some patients even that small gain may be enough to make it a not unreasonable choice.
From a societal perspective, which was not addressed by Portschy et al., the associated costs of CPM, including the procedure, its complications, reconstruction, and perhaps psychotherapy, may outweigh the modest benefit CPM provides. The small denominator of the cost-effectiveness ratio, were one to be calculated, would imply that the ratio would be very high, making CPM a suboptimal use of health care dollars. Further, we suspect that adding quality of life to the analysis would diminish the benefit and well might turn it into a net harm, in particular for patients with high concern for negative impact of CPM on cosmesis, self image, and morbidity. However, in a fraction of patients who are very troubled by a 0.7% risk of a second, contralateral cancer, CPM might provide an acceptable benefit. The balance between harm and benefit depends on the patient’s preferences and highlights the importance of capturing the patient’s values and expectations before considering CPM.
Of course, these conclusions are based on analysis of women who are at average risk for a contralateral second primary. In women at substantially higher risk (based either on family history or genetics), the benefit of CPM might be far greater, and CPM might be a good choice for the patient or for society.
Dr. Stephen G. Pauker and Dr. Mohamed Alseiari are with the division of clinical decision making in the department of medicine at Tufts Medical Center, Boston. They reported no relevant financial conflicts. This was excerpted from an editorial (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju175]).
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
The absolute 20-year survival benefit from contralateral prophylactic mastectomy stands at less than 1%, regardless of age, estrogen receptor status, and cancer stage, a decision analysis demonstrated.
"Long-term survival in women with unilateral breast cancer treated with or without CPM depends upon several factors, including mortality of the primary breast cancer, risk of CBC [contralateral breast cancer], stage and mortality of the CBC, and the individual patient’s overall life expectancy," wrote Dr. Pamela R. Portschy of the University of Minnesota, Minneapolis.
The report was published July 16 in the Journal of the National Cancer Institute.
"Prospective randomized trials comparing CPM with no CPM are not feasible. Retrospective studies evaluating a potential survival benefit with CPM are limited by short follow-up, potential selection bias, and lack of important clinical information," noted Dr. Portschy and her associates.
They limited their analysis to women with stage I and II breast cancer without BRCA mutations. They developed a Markov model to simulate survival outcomes among those who did and did not have contralateral prophylactic mastectomy (CPM), and they used published studies to estimate probabilities for developing CBC, dying from CBC, dying from primary breast cancer, and age-specific mortality rates. Data were extracted from numerous sources including Surveillance, Epidemiology, and End Results (SEER), the Early Breast Cancer Trialists’ Collaborative Group, and the Oregon State Cancer Registry.
The researchers estimated the 20-year overall survival and life expectancy, but not quality of life or cost, and their analysis considered variation in age, estrogen receptor status, and cancer stage (J. Natl. Cancer Inst. 2014 July 16 [doi:10.1093/jnci/dju160]).
The predicted life expectancy gain from CPM ranged from .13 to .59 years for women with stage I breast cancer, and .08 to .29 years for those with stage II breast cancer. CPM conferred a life expectancy benefit among younger women and among those who had stage I and estrogen receptor–positive disease. "The potential benefit of CPM was consistently lower for patients with stage II breast cancer because of the worse prognosis associated with the primary breast cancer," the researchers wrote. "Similarly, the potential benefits of CPM are more modest for older women because they have relatively fewer years remaining of [life expectancy]."
Dr. Portschy and her associates could not identify any cohort of women that had a greater than 1% absolute survival difference at 20 years. In fact, the predicted 20-year survival differences ranged from .56 to .94% for women with stage I breast cancer and .36 to .61% for those with stage II breast cancer.
The researchers acknowledged limitations of the study, including the fact that the results "do not apply to BRCA gene mutation carriers with unilateral breast cancer who have a cumulative 10-year risk of CBC of approximately 30% to 40%," they wrote. "The outcomes of this analysis were limited to overall and disease-specific survival; we did not evaluate other important outcomes such as surgical complications and quality of life. Also, we assumed the mortality of CBC was the same as the mortality of the index cancer reported by SEER."
They also noted that survival is not the only potential benefit of a cancer risk reduction strategy. "Effects on cancer-related anxiety, cosmesis, and self-image are also important in the decision-making process," they wrote. "For some women, the negative impact of CPM on quality of life may outweigh a potential survival benefit. For others who are very anxious about CBC, CPM may result in a psychological benefit even if survival benefits are minimal."
They concluded that the survival estimates from their Markov model "may be useful for physicians and breast cancer patients to arrive at evidence-based informed decisions regarding CPM. Moreover, the use of accurate and easily understood decision aids may reverse some of the mastectomy trends recently observed in the United States."
The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: The long-term survival benefit of contralateral prophylactic mastectomy is small.
Major Finding: The absolute 20-year survival benefit from contralateral prophylactic mastectomy was less than 1% among all age groups, regardless of estrogen receptor status and cancer stage.
Data Source: Results from a Markov model designed to simulate 20-year survival outcomes among those who did and did not have CPM, with considerations for variation in age, estrogen receptor status, and cancer stage.
Disclosures: The researchers disclosed no relevant financial conflicts.
A teen-focused STD prevention program reduced gonorrhea incidence
ATLANTA – A communitywide condom distribution program is proving successful for expanding access to and awareness of free condoms, and for reducing the incidence of sexually transmitted diseases among teens in Philadelphia.
The program, which includes the Philadelphia Freedom Condom program and Take Control Philly, is a comprehensive, youth-targeted STD prevention initiative launched in April 2011 by the Philadelphia Department of Public Health (PDPH). A 2013 assessment, including interviews with 301 black/African American and/or Hispanic/Latino individuals aged 13-24 years showed that knowledge of at least one aspect of the campaign had almost doubled since 2011, when baseline data were collected from 300 individuals in that age group (97% vs. 52%), Matthew Prior of the PDPH reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Furthermore, 82% of respondents in 2013 vs. 18% in 2011 reported having seen information about the Freedom Condom program, 9% in 2013 vs. 1% in 2011 said they had received a free condom from a commercial business, and 70% in 2013 vs. 58% in 2011 knew where to get free condoms.
Although there was no significant change in reported condom use at last sexual encounter between 2011 and 2013, the overall number of gonorrhea cases declined significantly from approximately 1,600 cases in the first quarter of 2011 to approximately 1,400 cases in the first quarter of 2104; the greatest decline was among adolescents, who accounted for about 8% of cases in the first quarter of 2011 vs. about 5% in the first quarter of 2014, Mr. Prior found.
This followed a 38% increase in the number of gonorrhea cases among adolescents from 2009 to 2010, he noted.
The Freedom Condom program aimed to improve access to condoms, in part through a free condom mailing program, and Take Control Philly aimed to prevent STDs among adolescents through a sexual health awareness campaign and by addressing sexual risk-taking behaviors among a target population. Baseline data showed that 61% of adolescents surveyed had ever had sexual intercourse, 15% had sexual intercourse before the age of 13 years, 40.4% did not use a condom during their last sexual intercourse, and 27.2% had sexual intercourse with four or more persons during their lifetime.
"Multiple strategies were used to promote the Take Control Philly website and the Freedom Condom [program], including social media advertisement, traditional media advertisement, expanded condom distribution, community event outreach, and promotion in Philadelphia public high schools," Mr. Prior wrote.
Between April 2011 and 2014, more than 8.2 million condoms were distributed through the program. The success in promoting the program is due in part to the Philadelphia health commissioner and other city health leaders, whose support was pivotal to the planning and launch of the initiative, he noted.
Also, promotion of the program website through traditional media avenues and Facebook advertising was effective for increasing website traffic and condom mailing requests, he said.
"Communitywide condom distribution expansion is a promising approach for addressing STDs in youth," he concluded, adding that using multiple strategies for increasing program awareness is necessary for reaching youth.
Mr. Prior reported having no relevant financial disclosures.
ATLANTA – A communitywide condom distribution program is proving successful for expanding access to and awareness of free condoms, and for reducing the incidence of sexually transmitted diseases among teens in Philadelphia.
The program, which includes the Philadelphia Freedom Condom program and Take Control Philly, is a comprehensive, youth-targeted STD prevention initiative launched in April 2011 by the Philadelphia Department of Public Health (PDPH). A 2013 assessment, including interviews with 301 black/African American and/or Hispanic/Latino individuals aged 13-24 years showed that knowledge of at least one aspect of the campaign had almost doubled since 2011, when baseline data were collected from 300 individuals in that age group (97% vs. 52%), Matthew Prior of the PDPH reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Furthermore, 82% of respondents in 2013 vs. 18% in 2011 reported having seen information about the Freedom Condom program, 9% in 2013 vs. 1% in 2011 said they had received a free condom from a commercial business, and 70% in 2013 vs. 58% in 2011 knew where to get free condoms.
Although there was no significant change in reported condom use at last sexual encounter between 2011 and 2013, the overall number of gonorrhea cases declined significantly from approximately 1,600 cases in the first quarter of 2011 to approximately 1,400 cases in the first quarter of 2104; the greatest decline was among adolescents, who accounted for about 8% of cases in the first quarter of 2011 vs. about 5% in the first quarter of 2014, Mr. Prior found.
This followed a 38% increase in the number of gonorrhea cases among adolescents from 2009 to 2010, he noted.
The Freedom Condom program aimed to improve access to condoms, in part through a free condom mailing program, and Take Control Philly aimed to prevent STDs among adolescents through a sexual health awareness campaign and by addressing sexual risk-taking behaviors among a target population. Baseline data showed that 61% of adolescents surveyed had ever had sexual intercourse, 15% had sexual intercourse before the age of 13 years, 40.4% did not use a condom during their last sexual intercourse, and 27.2% had sexual intercourse with four or more persons during their lifetime.
"Multiple strategies were used to promote the Take Control Philly website and the Freedom Condom [program], including social media advertisement, traditional media advertisement, expanded condom distribution, community event outreach, and promotion in Philadelphia public high schools," Mr. Prior wrote.
Between April 2011 and 2014, more than 8.2 million condoms were distributed through the program. The success in promoting the program is due in part to the Philadelphia health commissioner and other city health leaders, whose support was pivotal to the planning and launch of the initiative, he noted.
Also, promotion of the program website through traditional media avenues and Facebook advertising was effective for increasing website traffic and condom mailing requests, he said.
"Communitywide condom distribution expansion is a promising approach for addressing STDs in youth," he concluded, adding that using multiple strategies for increasing program awareness is necessary for reaching youth.
Mr. Prior reported having no relevant financial disclosures.
ATLANTA – A communitywide condom distribution program is proving successful for expanding access to and awareness of free condoms, and for reducing the incidence of sexually transmitted diseases among teens in Philadelphia.
The program, which includes the Philadelphia Freedom Condom program and Take Control Philly, is a comprehensive, youth-targeted STD prevention initiative launched in April 2011 by the Philadelphia Department of Public Health (PDPH). A 2013 assessment, including interviews with 301 black/African American and/or Hispanic/Latino individuals aged 13-24 years showed that knowledge of at least one aspect of the campaign had almost doubled since 2011, when baseline data were collected from 300 individuals in that age group (97% vs. 52%), Matthew Prior of the PDPH reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Furthermore, 82% of respondents in 2013 vs. 18% in 2011 reported having seen information about the Freedom Condom program, 9% in 2013 vs. 1% in 2011 said they had received a free condom from a commercial business, and 70% in 2013 vs. 58% in 2011 knew where to get free condoms.
Although there was no significant change in reported condom use at last sexual encounter between 2011 and 2013, the overall number of gonorrhea cases declined significantly from approximately 1,600 cases in the first quarter of 2011 to approximately 1,400 cases in the first quarter of 2104; the greatest decline was among adolescents, who accounted for about 8% of cases in the first quarter of 2011 vs. about 5% in the first quarter of 2014, Mr. Prior found.
This followed a 38% increase in the number of gonorrhea cases among adolescents from 2009 to 2010, he noted.
The Freedom Condom program aimed to improve access to condoms, in part through a free condom mailing program, and Take Control Philly aimed to prevent STDs among adolescents through a sexual health awareness campaign and by addressing sexual risk-taking behaviors among a target population. Baseline data showed that 61% of adolescents surveyed had ever had sexual intercourse, 15% had sexual intercourse before the age of 13 years, 40.4% did not use a condom during their last sexual intercourse, and 27.2% had sexual intercourse with four or more persons during their lifetime.
"Multiple strategies were used to promote the Take Control Philly website and the Freedom Condom [program], including social media advertisement, traditional media advertisement, expanded condom distribution, community event outreach, and promotion in Philadelphia public high schools," Mr. Prior wrote.
Between April 2011 and 2014, more than 8.2 million condoms were distributed through the program. The success in promoting the program is due in part to the Philadelphia health commissioner and other city health leaders, whose support was pivotal to the planning and launch of the initiative, he noted.
Also, promotion of the program website through traditional media avenues and Facebook advertising was effective for increasing website traffic and condom mailing requests, he said.
"Communitywide condom distribution expansion is a promising approach for addressing STDs in youth," he concluded, adding that using multiple strategies for increasing program awareness is necessary for reaching youth.
Mr. Prior reported having no relevant financial disclosures.
AT THE 2014 STD PREVENTION CONFERENCE
Key clinical point: Making condoms available to youths may reduce gonorrhea incidence.
Major finding: Adolescents accounted for about 8% of cases in the first quarter of 2011 vs. about 5% in the first quarter of 2014.
Data source: A follow-up survey of 301 subjects.
Disclosures: Mr. Prior reported having no disclosures.
FDA panel recommends informed consent, labeling changes to address morcellator risk
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
emechcatie@frontlinemedcom.com
This article has been updated 7/14/2014.
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
emechcatie@frontlinemedcom.com
This article has been updated 7/14/2014.
SILVER SPRING, MD. – A requirement for informed consent outlining the risks of morcellation of unsuspected malignancies in women treated with laparoscopic power morcellators for presumably benign fibroids was among the recommendations made by a Food and Drug Administration advisory panel.
On July 11, the second day of a two-day meeting of the FDA’s Obstetrics and Gynecology Devices Advisory Committee, panelists also supported adding a boxed warning to the labels of laparoscopic power morcellators (LPMs). Other suggestions and recommendations included identifying characteristics of patients or fibroids, physical exam findings, and other features that could help determine patients who may have a sarcoma before treatment, as well as strategies to mitigate risks during treatment.
"There should be some labeling or special controls" to ensure that women who are being considered for treatment with this device and their physicians "get the message that we do believe there is an increased risk" and that the device is contraindicated in patients with a known or suspected malignancy, said Dr. Carol Brown, a gynecologic cancer surgeon at Memorial Sloan Kettering Cancer Center, New York.
The advisory panel did not vote on any issues and was not asked whether LPMs should be taken off the market or reclassified as class III medical devices, which require clinical data for approval.
During testimony from women and relatives of those who had been diagnosed with stage 4 LMS after treatment that included morcellation for what was thought to be uterine fibroids, "a recurring theme" was that they had not been told that morcellation could be part of their treatment, and if they had known, they may not have agreed to that treatment and chosen an alternative, she pointed out.
The FDA convened the meeting to discuss the benefits, risks, and clinical role of LPMs in the treatment of women with uterine fibroids. Panelists also were told to discuss strategies that could be used to reduce the risks of morcellation disseminating cancerous tissue into the pelvis and abdomen of women with an unsuspected uterine sarcoma or leiomyosarcoma (LMS).
Concerns about this risk have received widespread attention this year. An FDA safety advisory was issued in April recommending that the use of LPMs during a hysterectomy or myomectomy in women with uterine fibroids be discouraged. The case of Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 LMS after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids, also garnered media attention. She and her husband, Dr. Hooman Noorchashm, a cardiothoracic surgeon, are leading a campaign highlighting these risks, calling for a ban on the use of LPMs. They -- along with women who have had similar experiences, and husbands and sisters of women who died of disseminated LMS after undergoing morcellation for what was thought to be benign fibroids -- also spoke at the meeting. These speakers emphasized that they are aware of at least 130 such cases, despite the small number of cases reported to the FDA (21 as of June 2014).
In April, the FDA recommended that physicians discuss alternative treatment options with women who have symptomatic uterine fibroids. If power morcellation is considered the best option, the agency advised, women should be informed that their fibroids may contain cancerous cells and, if so, morcellation could significantly worsen their prognosis. Among women who undergo a hysterectomy or myomectomy for a presumed fibroid, about one in 350 have a uterine sarcoma, and about one in 500 have a leiomyosarcoma (LMS), the FDA estimates.
The currently available LPMs that are "cleared" for gynecologic indications are regulated as moderate-risk class II devices, which require little or no clinical data. The agency is considering requiring that clinical data be provided for LPMs used for gynecologic indications.
Panelists said that features and tools that could help determine whether a patient could have a sarcoma include an older age, certain symptoms, and genetic susceptibility (a history of retinoblastoma), some imaging techniques, as well as a history of pelvic radiation.
Two panelists – a surgical oncologist and a bioethicist – said that LPMs should not be used for gynecologic indications until better data are available.
"I have not seen anything in isolation or together" that could help predict whether a woman with presumed uterine fibroids has a leiomyosarcoma, said Dr. Craig Shriver, professor of surgery and director of the John P. Murtha Cancer Center at Walter Reed National Navy Military Medical Center, Bethesda, Md.
Referring to the tenets of considering all masses as cancer until proven otherwise, he said, "I have been perplexed over the last two decades watching the introduction of laparoscopic power morcellation techniques that is totally anathema to these and my core principles as a cancer surgeon," he said. Two days of testimony have "only more strongly reaffirmed my commitment and belief that at present, there is no safe way to offer laparoscopic power morcellation as part of any minimally invasive surgery," he added. They should be withdrawn from the market, reclassified as class III devices and studied in clinical trials, he recommended.
But Dr. Brown said that while she agreed with those principles, since fibroids are so common, banning the use of morcellation could result in “hundreds of thousands” of hysterectomies.
Specimen collection bags were used when LPMs were first introduced for gynecologic indications, but dropped over time. Use has increased recently as a result of the increased attention to the risks, but there is no evidence that bags are effective in reducing the risk of disseminating malignant cells in the peritoneal cavity, in the case of an unsuspected malignancy.
“If you are going to morcellate, and it can be done safely in a bag, then that should be encouraged,” said panelist Dr. Keith Isaacson, medical director of the Center for Minimally Invasive Gynecologic Surgery at Newton (Mass.) Wellesley Hospital. More work is needed to evaluate bags and the techniques for their use, and to determine how easy it is train clinicians in how to use them safely, he added. “I still believe that intuitively – and that’s all we have is intuition here – that it more likely mitigates the risk of upstaging a tumor if you morcellate within a containment system, such as a bag.”
The FDA usually follows the recommendations of its advisory panels. Panel members have been cleared of potential conflicts, although occasionally a panelist is given a waiver, but not at this meeting.
Adverse events related to LPMs or other medical devices should be reported to the FDA.
emechcatie@frontlinemedcom.com
This article has been updated 7/14/2014.
AT AN FDA ADVISORY COMMITTEE MEETING
ACP pelvic guidelines could lead to care variations
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
The new pelvic exam guidelines from the American College of Physicians may be a relief for women who would prefer to forgo the annual ritual, but they could also lead to variation in well-woman care, depending on which type of specialist provides that care.
The guidelines advise physicians to skip annual pelvic examinations in otherwise healthy, asymptomatic women who are not pregnant (Ann. Intern. Med. 2014;161:67-72).
The evidence-based clinical practice guidelines recommend that cervical cancer screening be limited to the visual inspection of the cervix and cervical swabs for cancer and human papillomavirus. They recommend against performing a speculum examination of the vagina and cervix, and a bimanual examination of the adnexa, uterus, ovaries, and bladder. The recommendation does not apply to using the Pap smear to screen for cervical cancer.
The diagnostic accuracy of the pelvic exam in detecting gynecologic cancer or infections is low, the ACP said, and carries the risk of false positives that can lead to unnecessary testing and procedures. The embarrassment and discomfort of the exam may also keep some women from seeking care, the ACP stated in the guidelines.
But that advice conflicts with an August 2012 policy statement from the American College of Obstetricians and Gynecologists, which recommends that pelvic exams be performed in asymptomatic adults as part of an annual well-woman visit. However, citing a lack of evidence, the opinion leaves the decision about when and how often to perform the exams in the hands of physicians and patients (Obstet. Gynecol. 2012;120:421-4).
Following the release of the ACP guidelines, ACOG issued a statement renewing its support for the pelvic exam, but saying that the ACOG policy was a "complement" to the new ACP recommendations. ACOG said that the use of the exam is "supported by the clinical experiences of gynecologists treating their patients."
It’s a position shared by Dr. Jill Rabin, professor of obstetrics and gynecology at Hofstra North Shore–LIJ School of Medicine and head of urogynecology at Long Island Jewish Medical Center in New Hyde Park, N.Y. She currently performs a full pelvic exam during the well-woman visit and plans to continue doing so.
"I want every woman to get a full exam every year and whoever does it, they should do a good job," Dr. Rabin said.
She praised the ACP guideline, saying that she agreed that there was a lack of evidence and that the exams can create anxiety and embarrassment. But pelvic exams are also essential in uncovering conditions such as pelvic floor weakness, fibroids, and vulvovaginal atrophy, Dr. Rabin said. And the exam provides a unique opportunity for women to bring up concerns that they might not raise during a history taking, she said, such as symptoms of incontinence.
As for the lack of evidence, Dr. Rabin said researchers should begin those studies even if they take decades to provide complete answers.
"There’s a lot that we do in life where we don’t have the studies," she said. "But lack of the evidence doesn’t mean that there’s lack of value."
And Dr. Rabin isn’t alone in doing a full pelvic exam. A recent survey of ob.gyns. found that nearly all perform bimanual pelvic examinations in asymptomatic women for a variety of reasons including patient reassurance, detection of ovarian cancer, or identification of benign uterine and ovarian conditions (Am. J. Obstet. Gynecol. 2013;208:109.e1-7).
Dr. Molly Cooke, the ACP’s immediate past president and a member of the group’s Clinical Practice Guidelines Committee, said that for years she performed pelvic exams in asymptomatic patients mainly out of "habit," rather than evidence. But with the ACP’s new guidelines, she plans to change her approach.
Going forward, Dr. Cooke said she will discuss the utility of the pelvic exam with patients and explain that the evidence indicates that the bimanual exam does not produce meaningful information and could lead them astray. She said she expects that most patients will agree to forgo the pelvic exam when presented with the evidence.
As for ob.gyns. who may continue to perform pelvic exams routinely in asymptomatic patients, Dr. Cooke said that they are essentially making a "faith-based" assertion about the usefulness of the exam.
Dr. Cooke said the ACP recommendations are meant to apply to all clinicians who provide well-woman care. "We don’t see any reason why the guideline isn’t as useful and applicable to a nurse practitioner, a gynecologist, a family physician, and an internist," she said.
The new ACP guidelines are being well received by some internists and family physicians who are feeling the pressure to cram more and more preventive care into a short visit.
"You really have to think about the opportunity cost here," said Dr. Giang Nguyen of the Hospital of the University of Pennsylvania, Philadelphia.
"When we take those extra minutes out of a visit, which might only be 15 minutes long, you’re preventing the patient and the provider from using that time for things that we have strong evidence for, like counseling about weight management, talking about smoking cessation, reviewing other parts of their sexual history that maybe would be useful to talk about in order to prevent future illness," he added.
Given the shortage of primary care physicians, Dr. Nguyen said spending visit time on screenings that aren’t evidence based essentially reduces access to care.
The American Academy of Family Physicians doesn’t have a recommendation for or against performing screening pelvic exams. As part of the Choosing Wisely campaign, the AAFP issued a clinical recommendation against requiring a pelvic exam or other physical exam to prescribe oral contraceptives.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
No blood clot risk found with HPV vaccination
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
FROM JAMA
Key clinical point: There appears to be no increased risk of VTE linked with HPV vaccination.
Major finding: The quadrivalent HPV vaccine is not associated with venous thromboembolism (incidence ratio, 0.77).
Data source: The findings are based on a self-controlled case series analysis of 4,375 Danish women from a population cohort of 1.6 million women, aged 10-44 years, who had a venous thromboembolism during Oct. 1, 2006, to July 31, 2013.
Disclosures: The study did not report external funding. The authors reported no disclosures.