User login
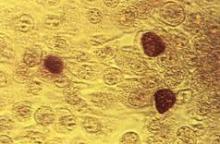
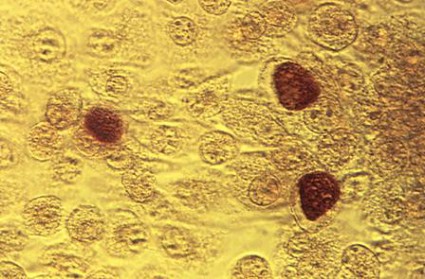
No blood clot risk found with HPV vaccination
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
The quadrivalent human papillomavirus vaccine does not increase the risk of venous thromboembolism, a study showed.
Two previous studies finding an association, one based on the Vaccine Adverse Event Reporting System and the other on the Vaccine Safety Datalink, reported few vaccinated cases, many of whom had known venous thromboembolism (VTE) risk factors, Nikolai Madrid Scheller and his colleagues at Statens Serum Institut in Copenhagen reported in a research letter (JAMA 2014;312:187-8).
The team used the self-controlled case series method with the main risk period for a first diagnosis of VTE set at 1-42 days after vaccination. They excluded women who were likely pregnant at the time of the VTE or had undergone major surgery in the previous 4 weeks or had a cancer diagnosis in the previous year from the VTE.
Women with VTE aged 10-44 years were followed until the end of the study or until emigration, death, or age 45 years during the study period from Oct. 1, 2006, to July 31, 2013. Among the 1.6 million women in the population cohort, 31% had received the quadrivalent HPV vaccine, and 4,375 women had VTE, 20% of whom had been vaccinated and served as the self-controlled cases.
The researchers found no association between the quadrivalent HPV vaccine and VTE (incidence ratio, 0.77). The lack of association remained in subsequent analyses adjusting for age and oral contraceptive use, and using only cases of VTE in which the women were receiving anticoagulants 4 weeks after diagnosis.
The study did not report external funding. The authors reported no disclosures.
FROM JAMA
Key clinical point: There appears to be no increased risk of VTE linked with HPV vaccination.
Major finding: The quadrivalent HPV vaccine is not associated with venous thromboembolism (incidence ratio, 0.77).
Data source: The findings are based on a self-controlled case series analysis of 4,375 Danish women from a population cohort of 1.6 million women, aged 10-44 years, who had a venous thromboembolism during Oct. 1, 2006, to July 31, 2013.
Disclosures: The study did not report external funding. The authors reported no disclosures.
Abortion costs, gestational age limits remain steady in 2008-2012
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
From Women’s Health Issues
Abortion costs, gestational age limits remain steady in 2008-2012
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
Secondary measures of access to abortions in the United States – cost, gestational age limits, and harassment – remained fairly stable from 2008 through 2012, according to an analysis from the Guttmacher Institute.
The researchers found that in 2008 and 2012, virtually all facilities offering abortions provided them at 8 weeks’ gestation. In 2012, 72% provided abortions at 12 weeks; 34%, at 20 weeks; and 16%, at 24 weeks. But few nonspecialized clinics and physician offices performed abortions after 9 weeks’ gestation (Womens Health Issues 2014;24:e419-24).
Similarly, costs remained about the same as in 2009 after adjustment for inflation, the researchers found. In 2011 and 2012, women paid $480, on average, for a surgical abortion at 10 weeks and $504 for an early medication abortion. The inflation-adjusted charge was $503 for a surgical abortion and $524 for an early medication abortion in 2009.
However, the stable cost may be based on providers’ efforts to keep services affordable as the cost of health care rose, the researchers noted.
The incidence of harassing behaviors at abortion facilities increased some between 2008 and 2011, climbing from 75% to 80% of clinics. In the recent survey, the most common form of harassment was exposure to picketing, followed by the harassing phone calls to facilities. In 2011, about a quarter of facilities also reported picketing that involved physical contact or blocking of patients.
The Guttmacher Institute, which conducts research into reproductive health issues and advocates for abortion access, analyzed data from its 16th census of all known abortion facilities in the United States. The data set included gestational age limits in 2012, average charges for abortion services in 2011 and 2012, and information on seven forms of harassment reported in 2011.
While the secondary factors affecting abortion access have remained "relatively stable" in recent years, the researchers noted that states have been active since the study period in passing additional abortion restrictions. For instance, in 2012, 42 laws restricting abortions were enacted around the country, with another 70 enacted in 2013.
mschneider@frontlinemedcom.com
On Twitter @MaryEllenNY
From Women’s Health Issues
Enhanced education, customized follow-up options improve STD retesting rates
ATLANTA – Educating patients treated for chlamydia or gonorrhea about reinfection and retesting, and providing customized options for follow-up care, increased patient retest return rates by 15% in a prospective cohort study.
The return rate at 1-6 months after treatment among 1,454 patients who received enhanced educational information about reinfection and the importance of retesting during the first phase of the InTOUCH study was 59%, and the return rate among 575 patients who received that educational information along with customized reminders and/or a mailed-in home testing kit in a second phase was 62%, compared with a return rate of 54% among 2,696 historical controls, Holly Howard reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The increases in return rates were statistically significant, said Ms. Howard of the California Department of Public Health, Richmond.
The initial education phase of the multicenter study occurred in 2010 and 2011 at six geographically diverse California Title X clinics. Participants were clients of the California Family Planning, Access, Care, and Treatment Program, which provides care to more than 2 million low-income women each year.
The patients were counseled about the risks and dangers of re-infection and the importance of retesting, and were given tips for remembering to return for retesting. Additionally, educational materials were updated to improve readability and user friendliness.
During 2011 and 2012, patients from the educational phase who tested positive for chlamydia or gonorrhea, and who were treated for the infections, were officially enrolled in the second phase of the study, during which they were offered the option of receiving retest reminders via postcard, text, and/or e-mail, as well as the option of retesting with a home test sent to their address 3 months after treatment.
Most patients (90%) opted to receive retest reminders, and most of those chose text and e-mail reminders. Only 5% chose to use the home test kit.
The findings have implications for improving the notoriously low return rates among women who test positive for chlamydia and gonorrhea infection. These infections are common and are associated with serious reproductive health sequelae, including an increased risk of pelvic inflammatory disease and ectopic pregnancy, Ms. Howard noted.
Routine retesting within a few months of treatment allows for early identification of reinfection, and for retreatment that can reduce the risk of adverse outcomes.
For more than a decade, national guidelines have recommended retesting of patients with chlamydia or gonorrhea, but retesting rates have remained stubbornly below 50%. Effective strategies for increasing patient retest return rates have been elusive, she noted.
In the year leading up to the InTOUCH study, only 44% of clinic patients overall were retested, and that was found to be a result both of the low (62%) return rate and low (69%) retesting rate among those who did return, she said.
The overall retesting rate improved to nearly 60% during the course of the study.
"So with a very moderate increase in patient return rates and in clinic retesting rates among returning patients, you can see that we cumulatively increased our overall retesting rates by more than 30%," she said, noting that given the consistently low return rates in prior years, this was "a very exciting result."
The current findings suggest that addressing the factors that contribute to low return rates, including lack of information about the importance of returning, forgetting to return, and difficulties getting to the clinic, can lead to significant improvement in return rates, Ms. Howard said.
"Improving is dependent on addressing both patient and clinic level causes. Through a combination of these very feasible interventions, we were able to increase the overall retesting rates by 32%," she said.
The InTOUCH study was funded by a grant from the Office of Population Affairs as a Title X Service Delivery Improvement Research Project. Ms. Howard reported having no other disclosures.
ATLANTA – Educating patients treated for chlamydia or gonorrhea about reinfection and retesting, and providing customized options for follow-up care, increased patient retest return rates by 15% in a prospective cohort study.
The return rate at 1-6 months after treatment among 1,454 patients who received enhanced educational information about reinfection and the importance of retesting during the first phase of the InTOUCH study was 59%, and the return rate among 575 patients who received that educational information along with customized reminders and/or a mailed-in home testing kit in a second phase was 62%, compared with a return rate of 54% among 2,696 historical controls, Holly Howard reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The increases in return rates were statistically significant, said Ms. Howard of the California Department of Public Health, Richmond.
The initial education phase of the multicenter study occurred in 2010 and 2011 at six geographically diverse California Title X clinics. Participants were clients of the California Family Planning, Access, Care, and Treatment Program, which provides care to more than 2 million low-income women each year.
The patients were counseled about the risks and dangers of re-infection and the importance of retesting, and were given tips for remembering to return for retesting. Additionally, educational materials were updated to improve readability and user friendliness.
During 2011 and 2012, patients from the educational phase who tested positive for chlamydia or gonorrhea, and who were treated for the infections, were officially enrolled in the second phase of the study, during which they were offered the option of receiving retest reminders via postcard, text, and/or e-mail, as well as the option of retesting with a home test sent to their address 3 months after treatment.
Most patients (90%) opted to receive retest reminders, and most of those chose text and e-mail reminders. Only 5% chose to use the home test kit.
The findings have implications for improving the notoriously low return rates among women who test positive for chlamydia and gonorrhea infection. These infections are common and are associated with serious reproductive health sequelae, including an increased risk of pelvic inflammatory disease and ectopic pregnancy, Ms. Howard noted.
Routine retesting within a few months of treatment allows for early identification of reinfection, and for retreatment that can reduce the risk of adverse outcomes.
For more than a decade, national guidelines have recommended retesting of patients with chlamydia or gonorrhea, but retesting rates have remained stubbornly below 50%. Effective strategies for increasing patient retest return rates have been elusive, she noted.
In the year leading up to the InTOUCH study, only 44% of clinic patients overall were retested, and that was found to be a result both of the low (62%) return rate and low (69%) retesting rate among those who did return, she said.
The overall retesting rate improved to nearly 60% during the course of the study.
"So with a very moderate increase in patient return rates and in clinic retesting rates among returning patients, you can see that we cumulatively increased our overall retesting rates by more than 30%," she said, noting that given the consistently low return rates in prior years, this was "a very exciting result."
The current findings suggest that addressing the factors that contribute to low return rates, including lack of information about the importance of returning, forgetting to return, and difficulties getting to the clinic, can lead to significant improvement in return rates, Ms. Howard said.
"Improving is dependent on addressing both patient and clinic level causes. Through a combination of these very feasible interventions, we were able to increase the overall retesting rates by 32%," she said.
The InTOUCH study was funded by a grant from the Office of Population Affairs as a Title X Service Delivery Improvement Research Project. Ms. Howard reported having no other disclosures.
ATLANTA – Educating patients treated for chlamydia or gonorrhea about reinfection and retesting, and providing customized options for follow-up care, increased patient retest return rates by 15% in a prospective cohort study.
The return rate at 1-6 months after treatment among 1,454 patients who received enhanced educational information about reinfection and the importance of retesting during the first phase of the InTOUCH study was 59%, and the return rate among 575 patients who received that educational information along with customized reminders and/or a mailed-in home testing kit in a second phase was 62%, compared with a return rate of 54% among 2,696 historical controls, Holly Howard reported at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
The increases in return rates were statistically significant, said Ms. Howard of the California Department of Public Health, Richmond.
The initial education phase of the multicenter study occurred in 2010 and 2011 at six geographically diverse California Title X clinics. Participants were clients of the California Family Planning, Access, Care, and Treatment Program, which provides care to more than 2 million low-income women each year.
The patients were counseled about the risks and dangers of re-infection and the importance of retesting, and were given tips for remembering to return for retesting. Additionally, educational materials were updated to improve readability and user friendliness.
During 2011 and 2012, patients from the educational phase who tested positive for chlamydia or gonorrhea, and who were treated for the infections, were officially enrolled in the second phase of the study, during which they were offered the option of receiving retest reminders via postcard, text, and/or e-mail, as well as the option of retesting with a home test sent to their address 3 months after treatment.
Most patients (90%) opted to receive retest reminders, and most of those chose text and e-mail reminders. Only 5% chose to use the home test kit.
The findings have implications for improving the notoriously low return rates among women who test positive for chlamydia and gonorrhea infection. These infections are common and are associated with serious reproductive health sequelae, including an increased risk of pelvic inflammatory disease and ectopic pregnancy, Ms. Howard noted.
Routine retesting within a few months of treatment allows for early identification of reinfection, and for retreatment that can reduce the risk of adverse outcomes.
For more than a decade, national guidelines have recommended retesting of patients with chlamydia or gonorrhea, but retesting rates have remained stubbornly below 50%. Effective strategies for increasing patient retest return rates have been elusive, she noted.
In the year leading up to the InTOUCH study, only 44% of clinic patients overall were retested, and that was found to be a result both of the low (62%) return rate and low (69%) retesting rate among those who did return, she said.
The overall retesting rate improved to nearly 60% during the course of the study.
"So with a very moderate increase in patient return rates and in clinic retesting rates among returning patients, you can see that we cumulatively increased our overall retesting rates by more than 30%," she said, noting that given the consistently low return rates in prior years, this was "a very exciting result."
The current findings suggest that addressing the factors that contribute to low return rates, including lack of information about the importance of returning, forgetting to return, and difficulties getting to the clinic, can lead to significant improvement in return rates, Ms. Howard said.
"Improving is dependent on addressing both patient and clinic level causes. Through a combination of these very feasible interventions, we were able to increase the overall retesting rates by 32%," she said.
The InTOUCH study was funded by a grant from the Office of Population Affairs as a Title X Service Delivery Improvement Research Project. Ms. Howard reported having no other disclosures.
AT THE 2014 STD PREVENTION CONFERENCE
Key clinical point: Education about retesting and customizing reminders appear to be the keys to improving retesting rates for chlamydia.
Major finding: The overall retesting rate improved by 32%.
Data source: A prospective cohort study (InTOUCH) of 4,725 patients.
Disclosures: The InTOUCH study was funded by a grant from the Office of Population Affairs as a Title X Service Delivery Improvement Research Project. Ms. Howard reported having no other disclosures.
2014 Update on infectious disease
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
This year I focus on four interesting and clinically relevant studies:
- an article by Huang and colleagues addressing the important issue of how best to reduce the frequency of methicillin-resistant Staphylococcus aureus (MRSA) infection in critically ill patients hospitalized in the intensive care unit (ICU)
- a study by Duggal and colleagues assessing the value of perioperative oxygen supplementation to reduce the frequency of postcesarean infection
- an investigation of diagnostic criteria for urinary tract infection (UTI) by Hooton and colleagues
- an exploration of the association between intra-amniotic inflammation, as distinct from bacterial colonization, and adverse fetal outcomes.
For ICU patients, universal decolonization reduces nosocomial infection more than targeted decolonization
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265.
Infection in general, and nosocomial infection in particular, is common among patients hospitalized in the ICU. Such patients often are severely immunosuppressed and debilitated. They are likely to have multiple indwelling catheters and to require mechanical ventilation—interventions that predispose to life-threatening infection. The longer the duration of care in the ICU, the greater the risk of infection, especially infection caused by organisms that have acquired resistance to multiple antibiotics.
In this cluster-randomized trial, Huang and colleagues compared targeted and universal decolonization of patients treated in an ICU to determine which approach was more effective at preventing nosocomial infection, particularly MRSA infection. They found universal decolonization to be superior to targeted decolonization in reducing these infections.
Details of the studyInvestigators conducted their study in 74 ICUs in 43 hospitals. Each hospital was randomly assigned to one of three interventions:
- Group 1: MRSA screening followed by isolation of colonized patients
- Group 2: MRSA screening followed by isolation and decolonization of MRSA carriers
- Group 3: Universal decolonization (no screening).
The decolonization regimen consisted of twice-daily administration of intranasal mupirocin for 5 days and daily bathing with chlorhexidine-impregnated cloths for the duration of the ICU stay.
The study’s two endpoints were 1) the modeled hazard ratios for MRSA clinical isolates and 2) the hazard ratios for bloodstream infection with any pathogen.
During the intervention period, fewer MRSA isolates were found in the universal decolonization group, compared with the other two groups (P<.01). In addition, the number of bloodstream infections in the universal decolonization group was significantly lower than in the other two groups (P<.001). Fifty-four patients (number needed to treat) needed to undergo decolonization to prevent one bloodstream infection.
What this EVIDENCE means for practiceThe relevance of this investigation for those of us in the field of obstetrics and gynecology is simple and clear: If we have to transfer a patient to an ICU (such as an HIV-infected patient with a serious postcesarean infection, or an oncology patient with a badly infected surgical wound), she should immediately be started on a regimen of twice-daily nasal mupirocin and daily bathing with chlorhexidine. This straightforward intervention will be of great value in reducing the incidence of bacteremia caused by a particularly dangerous pathogen.
Related article: Update on infectious disease. Patrick Duff, MD (July 2013)
The jury is still out on supplemental oxygen to reduce surgical site infection
Duggal N, Poddatorri V, Noroozkhani S, Siddik-Ahman RI, Caughey AB. Perioperative oxygen supplementation and surgical site infection after cesarean delivery. Obstet Gynecol. 2013;122(1):79–84.
In a widely read study published in 2000 in the New England Journal of Medicine, Greif and colleagues demonstrated that, in patients undergoing colorectal surgery, the rate of postoperative wound infection was significantly reduced from 11.2% in patients given 30% supplemental oxygen during surgery to 5.2% in those given 80% supplemental oxygen.1 The oxygen was continued for 2 hours after surgery.
In a later study among general surgery patients, Pryor and colleagues were unable to replicate this finding.2 It was in this setting that Duggal and colleagues undertook their investigation among women undergoing cesarean delivery. These investigators, too, were unable to replicate the 2000 finding of Greif and colleagues.
Related article: Update: Infectious Disease. Patrick Duff, MD (June 2012)
Details of the studyOver 4 years, from 2006 to 2010, Duggal and colleagues conducted a prospective, randomized, double-blinded controlled trial among patients undergoing scheduled, urgent, or emergent cesarean delivery. All patients were given prophylactic antibiotics, usually cefazolin 2 g intravenously after the infant’s umbilical cord was clamped. Surgical technique was reasonably well standardized and included closure of the deep subcutaneous layer of tissue using 2-0 plain gut sutures.
Patients were randomly assigned to receive supplemental oxygen via face mask, at 30% or 80% concentration, during surgery and for 1 hour postoperatively. They were evaluated postoperatively at 2 and 6 weeks. The primary outcome measure was a composite of surgical site infection, endometritis, or both.
A total of 415 women received 30% oxygen and 416 were given 80% oxygen. The two groups were well matched for important confounding variables such as age, race, parity, body mass index, number of prior cesarean deliveries, diabetes, cardiopulmonary disease, anemia, smoking, and chronic steroid use.
The groups did not differ in the frequency of surgical site infection or endometritis, which occurred at a rate of 2.4% in the group receiving 30% oxygen, compared with 2.9% in the group given 80% oxygen.
Rationale for oxygen supplementationAdequate tissue oxygenation has been observed to enhance the bactericidal function of neutrophils. So why were Duggal and colleagues unable to demonstrate a beneficial effect for oxygen therapy?
The most likely explanations:
- Their obstetric patients were less seriously ill than the general surgery patients undergoing colorectal surgery in the study by Greif and colleagues.
- Given the low overall rate of infection, their sample size may have been too small to show a statistically significant difference in outcome (Type II statistical error).
In point of fact, more than 80% of patients in both groups had scheduled cesarean deliveries, presumably prior to the onset of labor and ruptured membranes. The outcome may have been different had the groups included a majority of patients undergoing surgery after labor and ruptured membranes.
What this EVIDENCE means for practiceUntil additional studies are performed, I cannot recommend routine use of perioperative hyperoxygenation as a method of reducing the rate of surgical site infection and/or endometritis. However, we have very good scientific evidence indicating that the following measures significantly reduce the rate of endometritis after both scheduled and unscheduled cesarean delivery:
• administration of prophylactic antibiotics prior to the start of surgery
• removal of the placenta by gentle traction on the umbilical cord rather than by manual extraction.3,4
Similarly, we have sound evidence demonstrating that the following measures significantly reduce the rate of surgical site infection:
• clipping, rather than shaving, the hair at the surgical site just prior to the incision
• preoperative cleansing of the surgical area with chlorhexidine
• administration of prophylactic antibiotics prior to the start of surgery closure of the lower half of the subcutaneous tissue (if it exceeds 2 cm in thickness) using a relatively noninflammatory suture such as polyglactin or polyglycolic acid.
The presence of E coli in a midstream urine specimen is highly predictive of UTI
Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891.
Urinary tract infections (UTI) are among the most common infections experienced by women of all ages. Asymptomatic bacteriuria affects 5% to 10% of all sexually active women. During the course of their lifetime, at least 50% of women develop some form of UTI.
Pyelonephritis is not nearly as common as asymptomatic bacteriuria or cystitis, but this infection can be especially dangerous in older, debilitated women who reside in nursing homes and require indwelling catheters.
The most common organisms that cause UTIs in women are the aerobic gram-negative bacilli, principally Escherichia coli, Klebsiella species, and Proteus species. Other Gram-negative bacilli such as Pseudomonas species, Serratia, or Enterobacter are not common uropathogens except in immunosuppressed hosts or patients who have long-term indwelling catheters. Gram-positive organisms such as group B streptococci, enterococci, and staphylococcal species are occasional pathogens but, as Hooton and colleagues demonstrate in this study, perhaps not quite as important as we once thought.
Related articles:
• Update on infectious disease. Alan T. N. Tita, MD, PhD (June 2011)
• Have you tried these innovative alternatives to antibiotics for UTI prevention? Patrick A. Nosti, MD; Kate C. Arnold; Cheryl B. Iglesia, MD (February 2013)
Details of the studyUsing an elegantly simple design, the Hooton team studied women aged 18 to 49 years who had symptoms suggestive of acute cystitis. They collected two urine specimens from each woman for culture—one was collected using the midstream, clean-catch technique and the other by catheterization. They then compared microbial species and colony counts in the paired specimens to determine the positive and negative predictive values of midstream culture results, using the catheterized culture results as the reference standard.
The 226 women in the study experienced 236 clinical episodes suggestive of acute cystitis. One hundred forty-two (70%) of the catheterized specimens were positive for infection; of these, four specimens yielded more than one uropathogen. One hundred fifty-seven (78%) of the midstream specimens were positive for infection.
The presence of E coli in the midstream culture was highly predictive of a positive culture for E coli by catheterization, even when the cutoff was only 100 colonies/mL on the midstream specimen (positive predictive value, 93%). However, neither the presence of enterococci nor the presence of group B streptococci, at any colony count, was predictive of a positive culture by catheterization. Interestingly, among 41 patients who had either enterococci or group B streptococci in their midstream culture, E coli was present in the catheterizedculture in 61% of cases, suggesting that infection with E coli may be the more important cause of the patient’s symptoms.
Hooton and colleagues concluded that the presence of E coli on a midstream culture, even in low colony counts, is predictive of true bladder infection, as determined by catheterization. However, enterococci and group B streptococci were more likely to be vaginal contaminants or associated with coinfection with E coli, or bot.
What this EVIDENCE means for practiceThe findings of Hooton and colleagues have several key implications for practicing clinicians:
• When either a pregnant or nonpregnant patient experiences her first episode of acute cystitis, the overwhelming probability is that E coli is the infecting pathogen. We can reduce costs by empirically treating the initial infection, thereby avoiding the expense of a urine culture.
• For patients with recurrent infections or for immunocompromised patients, a culture and sensitivity test should be performed because other uropathogens are more likely to be involved and may have less predictable antibiotic susceptibility patterns.
• Contamination of supposed “clean-catch” specimens is very common, and the cultures resulting from these specimens can mislead us in our decisions about antibiotic therapy. Enterococci and group B streptococci are more likely than not to be contaminants from the vaginal flora rather than true infecting pathogens. When they are present in the bladder, they are usually associated with E coli. Accordingly, E coli should be the principal target of antibiotic therapy.
• To avoid concerns about contamination of specimens in acutely symptomatic patients, obtain the urine specimen by catheter. In the catheterized specimen, the cutoff for true bladder infection should be ≥100 colonies/mL. The cutoff of ≥100,000 colonies/mLis applicable only for clean-catch specimens obtained from asymptomatic patients.
• Clinical laboratories should embrace the new cutoff and report even seemingly low colony counts when the urine sample has been obtained by catheterization.
In preterm labor, amniotic fluid infection without inflammation does not necessarily predict a poor fetal outcome
Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125.e1–e15.
In this very important clinical investigation, Combs and colleagues collected amniotic fluid from 305 women with preterm labor. They then measured the amniotic fluid concentration of interleukin-6 (IL-6) and assessed for the presence of microbial invasion of the amniotic cavity (MIAC) by either culture or detection of microbial 16S ribosomal DNA. Based on these test results, investigators divided the patients into five groups:
- Infection—defined as positive MIAC and IL-6 >11.3 ng/mL
- Severe inflammation—negative MIAC and IL-6 >11.3 ng/mL
- Mild inflammation—no MIAC and IL-6 from 2.6 to 11.2 ng/mL
- Colonization—positive MIAC and IL-6 <2.6 ng/mL
- Negative—no MIAC and IL-6 <2.6 ng/mL.
The end points of the investigation were latency period and composite perinatal morbidity and mortality. Perinatal morbidity included respiratory distress syndrome, grade 3 or 4 intraventricular hemorrhage, necrotizing enterocolitis, and culture-proven neonatal sepsis.
Related article: Does treating asymptomatic bacterial vaginosis reduce preterm delivery? Hyagriv N. Simhan, MD, MSCR (Examining the Evidence; April 2008)
Interestingly, the infection and severe inflammation groups had similar short latency periods (median of <1 and 2 days, respectively) and similar rates of composite perinatal morbidity and mortality (81% and 72%, respectively).
The colonization and negative groups also had similar latency periods (median of 23.5 and 25 days, respectively) and similar rates of composite morbidity and mortality (21% and 25%, respectively).
The mild inflammation group had intermediate outcomes.
When Combs and colleagues used multivariate analysis to adjust for gestational age at enrollment, amniotic fluid IL-6 concentrations greater than 11.3 ng/mL and in the range of 2.6 to 11.3 ng/mL—but not MIAC—were associated with increased composite perinatal morbidity and mortality.
What this EVIDENCE means for practiceThis study offers several critically important take-home messages:
• Bacterial colonization of the amniotic fluid, without actual inflammation, is not necessarily associated with an ominous outcome for the fetus
• Varying degrees of inflammation exist
• The more intense the inflammation, the worse the outcome for the baby
• The logical clinical application of this investigation is to modify our practice so that, when we perform an amniocentesis for patients with preterm labor, we look not only for bacterial growth but for the presence of key inflammatory mediators in the amniotic fluid, such as IL-6
• A rapidly available, inexpensive, and easy-to-perform assay for IL-6 would be invaluable in improving our ability to assess patients for subclinical infection and inflammation
• An important question, of course, is whether early implementation of specific anti-inflammatory therapy could alter the prognosis for the fetus in selected cases.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
1. Greif R, Akca O, Horn EP, Kurz A, Sessler DI; Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342(3):161–167.
2. Pryor KO, Fahey TJ III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgery population. JAMA. 2004;291(1):79–87.
3. Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
4. Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
Supreme Court strikes down abortion clinic buffer zones
WASHINGTON – A Massachusetts law that established a 35-foot protest-free buffer zone around abortion clinics is unconstitutional, the Supreme Court ruled June 26.
The buffer zone represents a "dramatic intrusion on First Amendment rights," said Chief Justice John Roberts Jr., in reading the opinion from the bench.
The Justices were unanimous that the law was a First Amendment violation, but varied on how they formulated their opinions. Chief Justice Roberts Jr. was joined by Justice Ruth Bader Ginsburg, Justice Stephen G. Breyer, Justice Sonia Sotomayor and Justice Elena Kagan. Justice Antonin Scalia was joined by Justice Anthony M. Kennedy and Justice Clarence Thomas, who all agreed that the law was unconstitutional on its face, and that there were no acceptable alternatives to barring protesters from the clinics. Justice Samuel A. Alito Jr. agreed in a separate concurring opinion.
The 2007 law prohibited most people – with the exception of employees and their agents, patients, and people passing by the clinics – from standing within 35 feet of an abortion clinic. Those buffer zones were delineated by painted lines.
In McCullen v. Coakley, Eleanor McCullen said that the law violated her First Amendment right to talk with women going into the clinics, in an effort to counsel them and persuade them not to have an abortion.
The Chief Justice and his five colleagues agreed and said that though the buffer zones had helped the state maintain public safety and preserve access to the clinics, it had done so at the expense of the First Amendment. Ms. McCullen and her colleagues are not "protesters," the Justices said. Instead, "they seek not merely to express their opposition to abortion, but to engage in personal, caring, consensual conversations with women about various alternatives."
And, they said, "It is not enough for Massachusetts simply to say that other approaches have not worked."
Pro-life groups praised the ruling. "The Massachusetts law impermissibly discriminated against and censored pro-life Americans," said Dr. Charmaine Yoest, president and chief executive officer of Americans United for Life, in a statement. "The pro-abortion position could be represented in the zone, while the pro-life view point was strictly prohibited under threat of criminal sanctions."
The Pro-Life Action League issued a statement following the decision that, "The pro-life movement scored a huge victory at the Supreme Court today."
Pro-choice groups, however, said they were disappointed. Ilyse Hogue, president of NARAL Pro-Choice America, noted that there has been a long history of violence against women who patronize clinics, and physicians and other clinic workers, with 8 murders and 17 attempted murders since 1991.
"Let’s be clear: Today’s decision puts women and health care providers at greater risk," Ms. Hogue said in a statement.
Megan Amundson, executive director of NARAL Pro-Choice Massachusetts, said that the ruling "turns back the clock to when women were too intimidated to seek medical care," adding that "women’s health will suffer because of it."
Planned Parenthood echoed that position. "This decision shows a troubling level of disregard for American women, who should be able to make carefully considered, private medical decisions without running a gauntlet of harassing and threatening protesters," Cecile Richards, president of Planned Parenthood Federation of America, said in a statement.
"We are taking a close look at this ruling, as well as patient protection laws around the country, to ensure that women can continue to make their own health care decisions without fear of harassment or intimidation," she said.
On Twitter @aliciaault
WASHINGTON – A Massachusetts law that established a 35-foot protest-free buffer zone around abortion clinics is unconstitutional, the Supreme Court ruled June 26.
The buffer zone represents a "dramatic intrusion on First Amendment rights," said Chief Justice John Roberts Jr., in reading the opinion from the bench.
The Justices were unanimous that the law was a First Amendment violation, but varied on how they formulated their opinions. Chief Justice Roberts Jr. was joined by Justice Ruth Bader Ginsburg, Justice Stephen G. Breyer, Justice Sonia Sotomayor and Justice Elena Kagan. Justice Antonin Scalia was joined by Justice Anthony M. Kennedy and Justice Clarence Thomas, who all agreed that the law was unconstitutional on its face, and that there were no acceptable alternatives to barring protesters from the clinics. Justice Samuel A. Alito Jr. agreed in a separate concurring opinion.
The 2007 law prohibited most people – with the exception of employees and their agents, patients, and people passing by the clinics – from standing within 35 feet of an abortion clinic. Those buffer zones were delineated by painted lines.
In McCullen v. Coakley, Eleanor McCullen said that the law violated her First Amendment right to talk with women going into the clinics, in an effort to counsel them and persuade them not to have an abortion.
The Chief Justice and his five colleagues agreed and said that though the buffer zones had helped the state maintain public safety and preserve access to the clinics, it had done so at the expense of the First Amendment. Ms. McCullen and her colleagues are not "protesters," the Justices said. Instead, "they seek not merely to express their opposition to abortion, but to engage in personal, caring, consensual conversations with women about various alternatives."
And, they said, "It is not enough for Massachusetts simply to say that other approaches have not worked."
Pro-life groups praised the ruling. "The Massachusetts law impermissibly discriminated against and censored pro-life Americans," said Dr. Charmaine Yoest, president and chief executive officer of Americans United for Life, in a statement. "The pro-abortion position could be represented in the zone, while the pro-life view point was strictly prohibited under threat of criminal sanctions."
The Pro-Life Action League issued a statement following the decision that, "The pro-life movement scored a huge victory at the Supreme Court today."
Pro-choice groups, however, said they were disappointed. Ilyse Hogue, president of NARAL Pro-Choice America, noted that there has been a long history of violence against women who patronize clinics, and physicians and other clinic workers, with 8 murders and 17 attempted murders since 1991.
"Let’s be clear: Today’s decision puts women and health care providers at greater risk," Ms. Hogue said in a statement.
Megan Amundson, executive director of NARAL Pro-Choice Massachusetts, said that the ruling "turns back the clock to when women were too intimidated to seek medical care," adding that "women’s health will suffer because of it."
Planned Parenthood echoed that position. "This decision shows a troubling level of disregard for American women, who should be able to make carefully considered, private medical decisions without running a gauntlet of harassing and threatening protesters," Cecile Richards, president of Planned Parenthood Federation of America, said in a statement.
"We are taking a close look at this ruling, as well as patient protection laws around the country, to ensure that women can continue to make their own health care decisions without fear of harassment or intimidation," she said.
On Twitter @aliciaault
WASHINGTON – A Massachusetts law that established a 35-foot protest-free buffer zone around abortion clinics is unconstitutional, the Supreme Court ruled June 26.
The buffer zone represents a "dramatic intrusion on First Amendment rights," said Chief Justice John Roberts Jr., in reading the opinion from the bench.
The Justices were unanimous that the law was a First Amendment violation, but varied on how they formulated their opinions. Chief Justice Roberts Jr. was joined by Justice Ruth Bader Ginsburg, Justice Stephen G. Breyer, Justice Sonia Sotomayor and Justice Elena Kagan. Justice Antonin Scalia was joined by Justice Anthony M. Kennedy and Justice Clarence Thomas, who all agreed that the law was unconstitutional on its face, and that there were no acceptable alternatives to barring protesters from the clinics. Justice Samuel A. Alito Jr. agreed in a separate concurring opinion.
The 2007 law prohibited most people – with the exception of employees and their agents, patients, and people passing by the clinics – from standing within 35 feet of an abortion clinic. Those buffer zones were delineated by painted lines.
In McCullen v. Coakley, Eleanor McCullen said that the law violated her First Amendment right to talk with women going into the clinics, in an effort to counsel them and persuade them not to have an abortion.
The Chief Justice and his five colleagues agreed and said that though the buffer zones had helped the state maintain public safety and preserve access to the clinics, it had done so at the expense of the First Amendment. Ms. McCullen and her colleagues are not "protesters," the Justices said. Instead, "they seek not merely to express their opposition to abortion, but to engage in personal, caring, consensual conversations with women about various alternatives."
And, they said, "It is not enough for Massachusetts simply to say that other approaches have not worked."
Pro-life groups praised the ruling. "The Massachusetts law impermissibly discriminated against and censored pro-life Americans," said Dr. Charmaine Yoest, president and chief executive officer of Americans United for Life, in a statement. "The pro-abortion position could be represented in the zone, while the pro-life view point was strictly prohibited under threat of criminal sanctions."
The Pro-Life Action League issued a statement following the decision that, "The pro-life movement scored a huge victory at the Supreme Court today."
Pro-choice groups, however, said they were disappointed. Ilyse Hogue, president of NARAL Pro-Choice America, noted that there has been a long history of violence against women who patronize clinics, and physicians and other clinic workers, with 8 murders and 17 attempted murders since 1991.
"Let’s be clear: Today’s decision puts women and health care providers at greater risk," Ms. Hogue said in a statement.
Megan Amundson, executive director of NARAL Pro-Choice Massachusetts, said that the ruling "turns back the clock to when women were too intimidated to seek medical care," adding that "women’s health will suffer because of it."
Planned Parenthood echoed that position. "This decision shows a troubling level of disregard for American women, who should be able to make carefully considered, private medical decisions without running a gauntlet of harassing and threatening protesters," Cecile Richards, president of Planned Parenthood Federation of America, said in a statement.
"We are taking a close look at this ruling, as well as patient protection laws around the country, to ensure that women can continue to make their own health care decisions without fear of harassment or intimidation," she said.
On Twitter @aliciaault
AT THE U.S. SUPREME COURT
LGBT adults more likely to use tobacco
LGBT adults are considerably more likely to use tobacco products than are heterosexual adults, according to a Centers for Disease Control and Prevention report published June 24.
In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while heterosexual adults were more likely to use smokeless tobacco (2.6% vs. 1.9%), according to the CDC report.
In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while he
LGBT adults are considerably more likely to use tobacco products than are heterosexual adults, according to a Centers for Disease Control and Prevention report published June 24.
In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while heterosexual adults were more likely to use smokeless tobacco (2.6% vs. 1.9%), according to the CDC report.

LGBT adults are considerably more likely to use tobacco products than are heterosexual adults, according to a Centers for Disease Control and Prevention report published June 24.
In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while heterosexual adults were more likely to use smokeless tobacco (2.6% vs. 1.9%), according to the CDC report.

In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while he
In 2012-2013, the prevalence of "every day" or "some day" use of any tobacco product among lesbian, gay, bisexual, or transgender adults was 30.8%, compared with 20.5% for heterosexual adults. "Every day" or "some day" cigarette use was reported by 27.7% of LGBT adults and 17.3% of heterosexual adults, according to the CDC (MMWR 2014 June 24;63:1-6).
The data from the National Adult Tobacco Survey show that LGBT adults also were more likely to use cigars/cigarillos/filtered small cigarettes (3.0% vs. 1.9%) and electronic cigarettes (4.5% vs. 1.9%), while he
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
SSTI guidelines stress diagnostic skill, careful treatment
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
Skin and soft tissue infections are one of the most common causes for patient evaluation in emergency departments and are common reasons for consultations by surgeons. SSTIs occur across a broad continuum of severity and often require only antimicrobial therapy (such as cellulitis), but they may be fulminate and life-threatening necrotizing infections that require aggressive surgical intervention. The guidelines provided by a distinguished group of clinicians from the Infectious Diseases Society of America provide an excellent organizational framework to understand this heterogenous collection of infections and provide a meaningful structure to direct management.
Several points in these guidelines deserve emphasis. First, considerable discussion in the guidelines has focused on the immunocompromised patient with SSTIs, and appropriately so. A broader consideration might have been to also include those patients with health care-associated exposure in addition to clinical immunosuppression. About 40 million hospitalizations occur annually in the United State, which makes over 3 million patients within 30 days of discharge. A larger number of patients have had recent antibiotic exposure. About 1.5 million patients are in chronic care facilities and nearly 500,000 are receiving chronic hemodialysis. Accordingly, immunocompromised and health care-associated patient exposures require that assumptions about the microbiology of SSTIs have "sensitivity" to the resistant pathogens (such as MRSA) not traditionally typical of community-acquired infections.
Second, the guidelines refer to the use of Gram stains for directing antimicrobial therapy. Although the Gram stain does not have the high-technology flare of contemporary health care, it remains a useful tool in differentiating pathogens, especially in necrotizing SSTIs.
Of the major microbiological presentations of necrotizing SSTIs, Streptococcus pyogenes is a gram-positive cocci in chains, Staphylococcus aureus is a gram-positive cocci in clusters, Clostridium perfringens is a gram-positive rod, and polymicrobial infections will have an assortment of different morphologic and gram-staining characteristics in identified bacteria. Aeromonas hydrophilia and Vibrio vulnificus will appear as gram-negative rods in those necrotizing SSTIs associated with fresh or salt-water recreational exposure. The Gram stain provides immediate direction for therapy when culture results will often be too late for a meaningful impact on patient care. Unfortunately, many hospitals have abandoned the use of Gram stains for clinical specimens.
Finally, prompt diagnosis of necrotizing SSTIs is essential. A cause of potentially preventable morbidity and deaths is a delay in the recognition of necrotizing SSTIs and the need for urgent surgical debridement. Necrotizing SSTIs are common issues in medicolegal actions because of the issue of failure to make the timely diagnosis. The hallmark of necrotizing SSTIs is pain out of proportion to the inciting injury. Trivial cutaneous injuries that are associated with an advancing perimeter of palpable tenderness and induration are necrotizing SSTIs until proven otherwise. Importantly, S. pyogenes in particular is associated with "metastatic" infection. Patients with soft-tissue contusions, joint effusions, and even fractures may have blood-borne streptococcal contamination of the injury site and yield a necrotizing infection without any cutaneous source of microbial contamination.
Because monitoring the progression of SSTIs is so important in differentiating necrotizing infections, I would only take to task the recommendation for the use of corticosteroids in treatment of cellulitic infections. Pharmacologic immunosuppression of the patient with an active SSTI in the interest of providing symptomatic relief compromises the clinical evaluation of disease progression.
In summary, the guidelines and the two algorithms for managing community-acquired and surgical incision infections are very useful for providing surgical clinicians direction in patient management. The increased incidence of S. aureus-associated necrotizing SSTIs and the emergence of community-associated MRSA over the last 20 years indicate that this is a dynamic area with changing characteristics. The changing pattern of pathogens and antimicrobial choices require a more frequent updating of these important guidelines for patient management.
Dr. Donald E. Fry is an ACS Fellow, executive vice-president for clinical outcomes management of MPA Inc. of Chicago, adjunct professor of surgery at the Northwestern University in Chicago, and professor emeritus of surgery at the University of New Mexico. He is a fellow of the Infectious Diseases Society of America, a past president of the Surgical Infection Society, and associate editor of the journal Surgical Infections.
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
New practice guidelines on skin and soft tissue infections from the Infectious Diseases Society of America stress careful clinical attention to the type of infection, the epidemiological setting in which the infection occurred, the health status of the patient, and the selection and dosage of the most appropriate treatment agents.
The guidelines, published online June 18 in Clinical Infectious Diseases (doi:10.1093/cid/ciu296), update those issued by IDSA in 2005 and cover everything from preventing infections caused by animal bites in healthy hosts to life-threatening infections in immunocompromised patients. They also emphasize accurate identification of pathogens, stressing that clinical presentations can be very similar.
"This is not one of those guidelines that boils complex issues down to a choice between a couple of different drugs or combinations of drugs," said Dr. Dennis Stevens of the Department of Veterans Affairs in Boise, Idaho, the guidelines’ lead author. "Skin and soft tissue infections [SSTIs] have multiple causes and different presentations, depending upon the immune status of the host. Here it’s much more complicated and really requires an astute physician to consider a number of things."
The guidelines, drafted by a 10-member panel, offer a novel algorithm for management of nonpurulent and purulent infections that aims to define a pathway for mild, moderate, and severe infections in each category. For example, no antibiotic is recommended for a purulent infection – only incision and drainage – if the patient has no signs of systemic involvement.
For moderate cases of purulent infection with some systemic involvement, incision and drainage should be followed by culture and sensitivity testing, the guidelines say, listing two antibiotics, trimethoprim-sulfamethoxazole and doxycycline, as appropriate for empiric treatment, while trimethoprim-sulfamethoxazole is recommended if the pathogen is found to be methicillin-resistant Staphylococcus aureus (MRSA) and dicloxacillin or cephalexin if it is methicillin-susceptible S. aureus (MSSA).
The purpose of the algorithm, expressed in the guidelines in chart form, "is to make the physician think," Dr. Stevens said in an interview. "There is a huge move to try and monitor antibiotic stewardship to prevent resistance, and we’re just trying to get the clinician to think of tier 1, tier 2, and tier 3 approaches, depending not only on the bug, but on how sick the patient is. Instead of a knee-jerk approach treating everybody with highly expensive IV antibiotics, [the algorithm] provides a clear pathway to treat appropriately."
In people with an abscess who have failed antibiotic treatment, are immunocompromised, or have fever and elevated white blood cell counts or other evidence of severe infection, "we’re not going to gamble," Dr. Stevens said, adding that the guidelines recommend prompt treatment using "an antibiotic that gets all of these organisms, including resistant ones." Newly approved agents dalbavancin and tedizolid are effective in treating SSTIs caused by MRSA, the guidelines note.
The guidelines are intended for use by clinicians in emergency departments, family practice, internal medicine, general surgery, orthopedics, gynecology, dermatology, infectious disease, and oncology.
Another algorithm charted in the guidelines covers wound infections following surgeries, which can involve multiple pathogens. The algorithm provides simple clinical clues as to which require antibiotics, a simple opening of the suture line, "or a full-court press for the kind of devastating infections that occur within the first 48 hours," Dr. Stevens said. Additional recommendations address infections that can occur in individuals receiving treatment for cancer or receiving immunosuppressant medications, or those who have had an organ transplant or who have HIV/AIDS.
Immunocompromised patients, Dr. Stevens said, are among the most challenging to treat because they may have a history of extensive antibiotic exposure, are likely to have infections with resistant bacteria, and often see involvement with fungal and parasitic agents that might be considered innocuous in normal individuals. "This is the first time physicians will have some decent guidelines about how to approach the problem of skin and soft tissue infections in these kinds of patients," he noted.
The guidelines’ development was funded by the IDSA. Dr. Stevens reported no conflicts of interest. Panel member Alan L. Bisno disclosed receiving honoraria from UpToDate, while five other members – Dr. Henry F. Chambers, Dr. E. Patchen Dellinger, Dr. Ellie J. C. Goldstein, Dr. Sherwood L. Gorbach, and Dr. Sheldon L. Kaplan – disclosed financial relationships with pharmaceutical manufacturers.
FROM CLINICAL INFECTIOUS DISEASES
Safety techniques regarding morcellation
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
Power morcellation within an insufflated bag
By Tony Shibley, M.D.
Morcellating safely in laparoscopic hysterectomy or myomectomy involves not only the use of a bag, but also the creation of an artificial pneumoperitoneum inside the bag. Inflation of the bag allows us to create a safe and completely contained working environment in the abdomen, with good visualization and adequate working space.
In the past, specimen containment bags were used for morcellation, but the bags would adhere to tissue and would be easily cut or would tear or rupture. Without proper space and visualization, surgeons could not break tissue into smaller fragments without endangering the bag or the structures behind the bag. We were safely and precisely disconnecting tissue in our surgeries, but falling short with tissue removal. There were calls for more durable bags, but the problems of visualization and safe distance were not addressed. Eventually, the goal of morcellating within a bag was largely abandoned in favor of open power morcellation.
I stopped performing open power morcellation about 2 years ago and developed a new approach to enclosed morcellation. By creating an artificial pneumoperitoneum in a bag, a good working space is developed within it. This meets the needs of containment while significantly lowering the risk of tissue dissemination and also reducing the risk of bowel injury, vascular injury, and other types of morcellator-related mechanical injuries. This approach to morcellation is safer on all fronts.
My method of enclosed morcellation has been utilized in both single-port and multiport hysterectomy (total and supracervical) and myomectomy performed with either traditional laparoscopy or the robotic platform.
The morcellation process is begun after surgery is complete except for tissue removal, and the patient is hemostatic. The specimen has been placed in a location where it can be easily retrieved; I prefer the right upper quadrant.
In a single-port approach, the bag is inserted into the abdomen through an open single-port cannula at the umbilicus. I use a Steri-Drape Isolation Bag (3M), 50 cm by 50 cm, with drawstrings. (See Figure 1.) Standing across from my assistant, I tightly fan-fold the bag and wring it to purge it of air. Using an atraumatic grasper, I position the bag linearly with the opening up; it is inserted into the pelvis first, with the superior end guided upward into the mid- and then upper-abdomen.
After the bag is placed, the cannula is recapped with the multiport cap. I use the Olympus TriPort 15 (Advanced Surgical Concepts), and the abdomen is reinsufflated. A camera is inserted into one of the 5-mm ports. I use the Olympus 5-mm articulating laparoscope.
Through the other 5-mm port, I maneuver the bag to form a pocket opening by pushing the upper corners of the bag against the respective abdominal sidewalls, and the body of the bag into the pelvis. I then elevate the specimen and, with a rotational movement, I guide the specimen along the right abdominal sidewall and into the pocket opening. I then elevate the sides of the bag and ensure that the specimen is well contained. (See Figure 2.)
The drawstrings are then brought up into the port hub. The cap is removed, and the opening of the bag is pulled up through the port and out approximately 15-20 cm, as symmetrically as possible.
The port cap is then replaced within the exteriorized neck of the bag, and the bag is insufflated with traditional laparoscopic pressure of 15-18 mm, creating an artificial pneumoperitoneum. (See Figure 3.) The camera is reinserted, followed by the morcellator, into the 15-mm port of the single-site tri-port. The morcellator must be lubricated so it does not catch the bag on the way in. Morcellation is carried out under direct vision.
After the morcellation process is complete, the morcellator is removed, followed by the camera and the tri-port cap. The bag is desufflated and removed through the open cannula, and the abdomen is closed as usual.
In a multiport approach, the umbilical incision is extended to 15 mm after completion of the hysterectomy or myomectomy. The bag is inserted and placed in a manner similar to the single-port approach, except that the lateral ports may be utilized to further position the bag once it is placed about halfway in. The bag is similarly opened, the specimen contained, and the neck of the bag exteriorized. A 15-mm port is similarly lubricated and placed inside the neck of the bag and through the umbilical incision.
As the bag is insufflated, the lateral ports are backed slightly out so that they're flush with the abdominal wall, and the trocars are opened to allow the release of any residual gas in the abdomen. The laparoscope is then placed into the 15-mm umbilical trocar.
Visualization will ultimately occur from another site, however. One of the lateral 5-mm trocars is advanced at a right angle to the insufflated bag, and an introducer is placed to puncture the bag. I recommend using a blunt plastic trocar; a balloon-tip trocar also can be used. Contrary to what one might think, the bag will not leak any gas. The laparoscope is now transferred to this 5-mm lateral port, and the umbilical port is removed. A lubricated morcellator is inserted directly through the umbilical incision, and morcellation is performed under direct visualization from the side port.
These techniques for single-port or multiport enclosed power morcellation have been shown to be reproducible and successful – with all bags intact and specimens contained – in a multicenter analysis that is being prepared for publication. The largest uterus in the study was 1,481 g.
To make the approach less cumbersome, I have designed a morcellation device consisting of a specialized bag that will open automatically and assist in capturing the specimen. The device, which has closure tabs and a retrieval lanyard, has been developed with the input of other minimally invasive gynecologic surgeons and the design and engineering expertise of Advanced Surgical Concepts. It will be manufactured by the company pending Food and Drug Administration approval.
Concealed power morcellation: Our take
By Bernard Taylor, M.D.
The approach to the use of power morcellation within a bag in my practice was not specifically driven by the desire to prevent the dispersion of malignant leiomyosarcoma – an issue that is now front and center and indeed, is important – but by a broader desire to perform as complete an extraction as possible.
For years we have used isolation bags to conceal and contain small uteri, ovaries, and specimens that we debulk using a scalpel and remove through a small umbilical incision. However, this approach is cumbersome for removing larger uteri. Consequently, over the past year we refined our methods for performing power morcellation within a bag.
This procedure can be performed after traditional laparoscopy or robotic-assisted hysterectomy or myomectomy to extract the specimen from the abdomen. A surgical tissue bag is placed into the abdomen and is insufflated within the abdomen. Morcellation is carried out within the concealed "pseudopneumoperitoneum," and the bag is exteriorized from the abdomen, typically through the umbilical incision.
The majority of my procedures utilize a multiport approach due to the nature of my practice as a urogynecologist. After hysterectomy or myomectomy, the specimen is placed in the upper abdomen. The cuff is closed, other procedures are completed in the usual fashion, and hemostasis is reassured. In robotic procedures, we undock the robot prior to morcellation. The umbilical port is removed, and the bag is placed into the abdomen with a ring forceps or atraumatic forceps without teeth. The umbilical port is then replaced to reestablish the pneumoperitoneum for visualization.
For larger uterine specimens, I use an Isodrape isolation bag (Microtek Medical). This 18 x 18 inch bag with a drawstring opening easily accommodates uteri larger than 1,000 g. The bag is placed into the pelvis with the open end at the pelvic brim and is opened wide. I use the term "open from iliac to iliac and sacrum to bladder." Once the bag is positioned, the specimen is grasped; the patient is slowly taken out of the Trendelenburg position to allow for gravity to assist in the placement of the specimen into the bag.
The drawstrings are then grasped and taken out of the umbilical trocar with simultaneous removal of the umbilical port. At this time, the open end of the bag is exteriorized, and the specimen technically is extraperitoneal and "concealed."
To insufflate the bag at this point and create the "pseudopneumoperitoneum," a 15-mm port is lubricated and placed through the opening in the bag. The bag is then insufflated to the usual pressure (15 mm Hg). The accessory ports are opened at this time to allow for the abdominal pressure to fall and for the bag to expand and fill the abdominal cavity.
To enable visualization throughout the morcellation procedure, I use one of the previously placed lower abdomen lateral ports to place a 5-mm camera. The camera is placed after a 5-mm balloon tip trocar is introduced and advanced to perforate the bag. The balloon tip is insufflated in the usual fashion, securing the bag against the abdominal wall and preventing a gas leak.
Alternatively, I have also used a SILSport (Covidien) at the umbilicus in cases when there is a leak at the umbilical incision and the 15-mm port does not seal properly. While use of a single-port technique has been shown to be feasible for both visualization and morcellation, it can present challenges in complex cases with a larger uterus or multiple fibroids.
Either way, morcellation can take place within the concealed "pseudo-peritoneal" cavity, with the morcellator lubricated and placed either through the umbilical incision or through the Tri-Port. After morcellation is complete, the bag containing the smaller tissue fragments and blood is simply removed through the umbilicus, and closure of the abdomen is completed in the usual fashion.
I also have adopted a simplified approach to remove the smaller uteri with supracervical hysterectomy and sacral colpopexy performed laparoscopically. Despite preoperative screening with endometrial biopsy and pelvic ultrasound, several of my patients in the past have been diagnosed with either early ovarian or endometrial neoplasias on final pathology. After completing the procedure, I place the small menopausal senile uterus and adnexa into a 15-mm endoscopic bag. The bag is brought through the umbilical port, and the specimen is removed via morcellation with a scalpel or scissors.
Surgeons have asked about additional time needed to place the bag and position the specimen. Technically, specimen placement is a learned skill; once one is proficient, the case times are not any different. In fact, cases may be shorter because of the time saved by not having to retrieve uterine fragments from the abdomen and pelvis.
Theoretically, the procedure addresses concerns associated with tissue fragmentation and dissemination within the abdominal cavity. To date, there are no trials showing prevention of cancer upstaging or benign conditions such as leiomyomatosis peritonealis disseminata or endometriosis.
Enclosed vaginal morcellation for enlarged uteri
By Ceana H. Nezhat, M.D.
Removal of large uteri via minimally invasive surgery poses challenges to gynecologic surgeons. Limitations on the use of intraperitoneal electromechanical morcellation are a step forward in terms of protecting patients from undue harm, but we also must acknowledge that minimally invasive surgery has been shown to be superior to laparotomy in the majority of cases.
While safer ways to extract large specimens without risk of spreading both benign and malignant tissue are being studied and developed, gynecologic surgeons need to find alternative minimally invasive approaches for removing large specimens.
Minimally invasive approaches to extirpate uteri and myomas have been described prior to the advent of electromechanical morcellation. A natural orifice such as the vagina for gynecologic procedures is a clear choice for removing specimens. Laparoscopic-assisted myomectomy is a combination of laparoscopy and minilaparotomy for tissue extraction (JSLS 2001;5:299-303). Another alternative is extracting myoma and other tissues through a posterior colpotomy. Rates of dyspareunia and adhesions in the cul-de-sac after tissue extraction through a colpotomy are low (J. Reprod. Med. 1993;38:534-6).
Vaginal hysterectomy was chronicled and performed by the Greek physician Soranus of Ephesus in 120 A.D. (Acta Chir. Iugosl. 2011:58:9-14), and it is still the preferred route of hysterectomy when it can be performed. Large uteri can be transvaginally morcellated and vaginally extracted; however, this technique still poses the risk, although low, of spilling fragments of uterine tissue in the abdomen.
By combining total laparoscopic hysterectomy and vaginal morcellation of enlarged uteri within an enclosed specimen bag, the risk of spilling uterine fragments is reduced, in addition to avoiding potential visceral and vascular complications associated with intraperitoneal electromechanical morcellation.
Enclosed vaginal morcellation is the technique of placing the enlarged hysterectomy specimen in an endoscopic specimen retrieval bag and then transvaginally morcellating the tissue.
In the development of this technique, I have experimented with different endoscopic sacs and retractors. In my experience, the optimal laparoscopic bag is the LapSac Surgical Tissue Pouch (Cook Medical). This endoscopic, one-time use, specimen retrieval bag is flexible, durable nylon with an integral polyurethane inner coating and polypropylene drawstring (Figure 4). The bag comes in four sizes, the largest (size 8 x 10 inch, volume 1,500 mL) of which I used to assist in removing the 18-week size uterus in the accompanying video.
The other key piece of equipment for vaginal extraction of large uteri is a transvaginally-placed wound retractor. As I have previously described, the self-retaining retractor provides optimal exposure in a narrow field and minimizes trauma to surrounding tissue (J. Minim. Inv. Gynecol. 2009:16:616-7). Two wound retractors are suitable for this surgical technique: the Alexis Wound Protector/Retractor (Applied Medical) (Figure 5) and the Mobius Elastic Abdominal Retractor (Cooper Surgical) (Figure 6).
After complete laparoscopic detachment of the uterus and cervix (and adnexa), the wound retractor is placed transvaginally. One semirigid ring is pushed superiorly through the vagina and into the peritoneal cavity, where is it placed in the cul-de-sac. In this manner, a uniform, circumferential orifice is created. In narrower vaginal vaults, the ring can be compressed between the thumb and index finger to reduce its diameter and thus be introduced into the peritoneal cavity atraumatically. With the small-size retraction systems, an orifice of 2.5 to 6 cm diameter can be created.
The LapSac is folded and then inserted with ring forceps transvaginally through the wound retractor. (See Figure 7.) The bag is unfolded intraperitoneally using the integral tabs to demarcate the edges of the bag. The bag should be unfolded in the pelvis with the opening of the bag cephalad. The fundus of the hysterectomy specimen is grasped with a tenaculum and placed inside the bag. (See Figure 8.)
It is an important technical point to grab the fundus or the heaviest point of the specimen to allow weight and gravity to facilitate placing the specimen securely in the bag. If the cervix or a lighter part of the specimen is grasped, placement in the bag is more difficult and the specimen will likely slip out of the bag.
Once the entire hysterectomy specimen is in the LapSac, the bag is cinched by grasping the blue drawstrings. The opening of the bag is pulled through the vagina. The opening of the bag is externalized and the edges are attached with Allis clamps to the external edge of the wound retractor. The specimen (typically the cervix first) is grasped with a tenaculum and vaginally morcellated and cored with a scalpel (Figure 9).
Metal vaginal retractors are placed within the bag to protect against sharp injury during morcellation. I prefer to amputate the cervix and adnexa separately and en bloc to preserve as much original architecture as possible so the pathologists can properly orient and examine the tissue. After completion of the morcellation, the wound retractor is removed, the cuff closed, and the procedure completed per surgeon preference.
Dr. Shibley has been in full-time practice with Ob.Gyn. Specialists, Fairview Health Services in the Minneapolis area (Edina), for 20 years. He reported that he has a financial interest in the artificial pneumoperitoneum device under development, and that he is a consultant for Olympus.
Dr. Taylor is a urogynecologist who is a female pelvic medicine and reconstructive surgeon practicing at the Carolinas Medical Center–Advanced Surgical Specialties for Women in Charlotte, N.C. He reported that he has no disclosures relevant to this Master Class.
Dr. Nezhat is the current president of the AAGL, adjunct professor of obstetrics and gynecology at Emory University and program director of minimally invasive surgery at Northside Hospital, both in Atlanta, and adjunct clinical professor of obstetrics and gynecology at Stanford (Calif.) University. He reported that he is a consultant for Karl Storz Endoscopy.
Videos of these experts' individual techniques of electromechanical power morcellation within the confines of a bag, as well as that of Dr. Douglas Brown, director of the Center for Minimally Invasive Gynecologic Surgery at Massachusetts General Hospital, Boston, can be viewed at the SurgeryU website.
Chlamydia prevalence is high, many infections go undiagnosed
ATLANTA – An estimated 1 in 15 sexually active adolescent girls has a chlamydial infection, and many of these infections go undiagnosed, according to an analysis of National Health and Nutrition Examination Survey data.
According to 2007-2012 NHANES data, the overall prevalence of chlamydia among sexually active adolescents and adults aged 14-39 years is 1.7%, suggesting that there are 1.8 million infections nationally, Elizabeth Torrone, Ph.D., reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
However, only 1.4 million infections are reported annually, according to information from the CDC, suggests that approximately 400,000 infections go undetected each year.
Adolescent girls – and particularly adolescent non-Hispanic black girls – are at highest risk, according to Dr. Torrone.
The estimated overall prevalence among adolescent girls aged 14-19 years in this study was 6.4%. The estimated prevalence among white girls in this age group was 3.2%, and among black girls in this age group it was 18.6%, reported Dr. Torrone of the division of STD prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the CDC, Atlanta.
The overall prevalence was 1.4% among males, 2.0% among females, 2.4% among persons aged 14-19 years, 2.9% among those aged 20-24 years, 1.1% among those aged 25-39 years, 2.3% among Mexican Americans, 5.2% among non-Hispanic blacks, and 0.8% among whites.
Having multiple sex partners in the past year was associated with increased prevalence: 3.2% for those with two or more partners, compared with 1.4% among those with one or no partner.
NHANES respondents included in this analysis were 8,330 adolescents and adults aged 14-39 years between 2007 and 2012 who were tested for C. trachomatis using the Gen-Probe Aptima test. Prevalence estimates were based on demographics and self-reported sexual activity measured through audio, computer-assessed self-interviews.
Estimates were weighted to be nationally representative and to account for oversampling and nonresponse, and the number of infections in the population was estimated by multiplying census estimates by weighted prevalence, Dr. Torrone explained.
Chlamydia is the most commonly reported infection in the United States, and annual screening is currently recommended for all sexually active females under age 25 years, she noted.
Since the findings of this study likely represent an underestimate of the true burden of disease because the estimates do not account for nongenital infection and infections in nonsampled populations where prevalence may be higher (such as incarcerated individuals), they highlight a need for targeted interventions to reduce the impact of chlamydia among young African American women and underscore the importance of adhering to the screening recommendations.
"Health care providers should screen all sexually active young females annually and ensure that all sex partners of patients diagnosed with chlamydia are treated appropriately," Dr. Torrone concluded.
Dr. Torrone reported having no disclosures.
ATLANTA – An estimated 1 in 15 sexually active adolescent girls has a chlamydial infection, and many of these infections go undiagnosed, according to an analysis of National Health and Nutrition Examination Survey data.
According to 2007-2012 NHANES data, the overall prevalence of chlamydia among sexually active adolescents and adults aged 14-39 years is 1.7%, suggesting that there are 1.8 million infections nationally, Elizabeth Torrone, Ph.D., reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
However, only 1.4 million infections are reported annually, according to information from the CDC, suggests that approximately 400,000 infections go undetected each year.
Adolescent girls – and particularly adolescent non-Hispanic black girls – are at highest risk, according to Dr. Torrone.
The estimated overall prevalence among adolescent girls aged 14-19 years in this study was 6.4%. The estimated prevalence among white girls in this age group was 3.2%, and among black girls in this age group it was 18.6%, reported Dr. Torrone of the division of STD prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the CDC, Atlanta.
The overall prevalence was 1.4% among males, 2.0% among females, 2.4% among persons aged 14-19 years, 2.9% among those aged 20-24 years, 1.1% among those aged 25-39 years, 2.3% among Mexican Americans, 5.2% among non-Hispanic blacks, and 0.8% among whites.
Having multiple sex partners in the past year was associated with increased prevalence: 3.2% for those with two or more partners, compared with 1.4% among those with one or no partner.
NHANES respondents included in this analysis were 8,330 adolescents and adults aged 14-39 years between 2007 and 2012 who were tested for C. trachomatis using the Gen-Probe Aptima test. Prevalence estimates were based on demographics and self-reported sexual activity measured through audio, computer-assessed self-interviews.
Estimates were weighted to be nationally representative and to account for oversampling and nonresponse, and the number of infections in the population was estimated by multiplying census estimates by weighted prevalence, Dr. Torrone explained.
Chlamydia is the most commonly reported infection in the United States, and annual screening is currently recommended for all sexually active females under age 25 years, she noted.
Since the findings of this study likely represent an underestimate of the true burden of disease because the estimates do not account for nongenital infection and infections in nonsampled populations where prevalence may be higher (such as incarcerated individuals), they highlight a need for targeted interventions to reduce the impact of chlamydia among young African American women and underscore the importance of adhering to the screening recommendations.
"Health care providers should screen all sexually active young females annually and ensure that all sex partners of patients diagnosed with chlamydia are treated appropriately," Dr. Torrone concluded.
Dr. Torrone reported having no disclosures.
ATLANTA – An estimated 1 in 15 sexually active adolescent girls has a chlamydial infection, and many of these infections go undiagnosed, according to an analysis of National Health and Nutrition Examination Survey data.
According to 2007-2012 NHANES data, the overall prevalence of chlamydia among sexually active adolescents and adults aged 14-39 years is 1.7%, suggesting that there are 1.8 million infections nationally, Elizabeth Torrone, Ph.D., reported in a poster at a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
However, only 1.4 million infections are reported annually, according to information from the CDC, suggests that approximately 400,000 infections go undetected each year.
Adolescent girls – and particularly adolescent non-Hispanic black girls – are at highest risk, according to Dr. Torrone.
The estimated overall prevalence among adolescent girls aged 14-19 years in this study was 6.4%. The estimated prevalence among white girls in this age group was 3.2%, and among black girls in this age group it was 18.6%, reported Dr. Torrone of the division of STD prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the CDC, Atlanta.
The overall prevalence was 1.4% among males, 2.0% among females, 2.4% among persons aged 14-19 years, 2.9% among those aged 20-24 years, 1.1% among those aged 25-39 years, 2.3% among Mexican Americans, 5.2% among non-Hispanic blacks, and 0.8% among whites.
Having multiple sex partners in the past year was associated with increased prevalence: 3.2% for those with two or more partners, compared with 1.4% among those with one or no partner.
NHANES respondents included in this analysis were 8,330 adolescents and adults aged 14-39 years between 2007 and 2012 who were tested for C. trachomatis using the Gen-Probe Aptima test. Prevalence estimates were based on demographics and self-reported sexual activity measured through audio, computer-assessed self-interviews.
Estimates were weighted to be nationally representative and to account for oversampling and nonresponse, and the number of infections in the population was estimated by multiplying census estimates by weighted prevalence, Dr. Torrone explained.
Chlamydia is the most commonly reported infection in the United States, and annual screening is currently recommended for all sexually active females under age 25 years, she noted.
Since the findings of this study likely represent an underestimate of the true burden of disease because the estimates do not account for nongenital infection and infections in nonsampled populations where prevalence may be higher (such as incarcerated individuals), they highlight a need for targeted interventions to reduce the impact of chlamydia among young African American women and underscore the importance of adhering to the screening recommendations.
"Health care providers should screen all sexually active young females annually and ensure that all sex partners of patients diagnosed with chlamydia are treated appropriately," Dr. Torrone concluded.
Dr. Torrone reported having no disclosures.
AT THE 2014 STD PREVENTION CONFERENCE
Key clinical point: These findings highlight a need for targeted interventions to reduce the impact of chlamydia among young African American women, and they underscore the importance of adhering to the screening recommendations.
Major finding: Estimated chlamydia prevalence among all girls, white girls, and black girls aged 14-19 years is 6.4%, 3.2%, and 18.6%, respectively.
Data source: Analysis of data from 8,330 NHANES participants from 2007 to 2012.
Disclosures: Dr. Torrone reported having no disclosures.