User login
FDA okays serum AMH assay to determine menopause status
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
The PicoAMH Elisa diagnostic test measures circulating levels of anti-Müllerian hormone (AMH), a granulosa cell product in ovaries that is present only until menopause. In research settings, AMH levels have been used to predict menopause and to confirm the occurrence of menopause; levels have been shown to track well with antral follicle count (J Clin Endocrinol Metab. 2011 Aug 1;96[8]:2532-9).
“Diagnostic results about a woman’s menopausal status may prompt discussions about preventative care for women experiencing menopausal symptoms,” Courtney H. Lias, PhD, director of the division of chemistry and toxicology devices in the FDA’s Center for Devices and Radiological Health, said in a press release announcing the marketing permission. “This test, when used in conjunction with other clinical assessments and laboratory findings, can help inform discussions about preventative care, such as ways to help prevent loss in bone mineral density or to address cardiovascular disease, both of which are known to increase after menopause.”
As Dr. Lias emphasized, the new test is designed to be used along with a thorough clinical assessment and other laboratory tests. Having a reliable test for circulating AMH for clinical use allows measurement of a hormone that, unlike follicle stimulating hormone and luteinizing hormone, does not fluctuate throughout the menstrual cycle for premenopausal women.
JoAnn Pinkerton, MD, executive director of the North American Menopause Society, provided clinical context about the utility of the new assay. “AMH levels appear to provide valuable information about timing of menopause. While not needed for women undergoing a natural menopause at age 51, it will be very helpful for women at risk of early ovarian failure, such as following chemotherapy for cancer or genetic or endocrine reasons,” said Dr. Pinkerton. “Women desiring pregnancy who are skipping periods can be more reassured if their AMH is normal, as studies suggest, that AMH is highly predictive of timing of menopause.”*
In permitting marketing of the PicoAMH Elisa assay, the FDA looked at data drawn from the Study of Women’s Health Across the Nation. For 690 women aged 42-62 years, “the PicoAMH Elisa test performed reasonably well at determining levels of AMH in the blood,” the FDA said in the press release. The test also was able to identify women who had already had their last menstrual period, and to determine women who were at least 5 years away from stopping menstruation, according to the longitudinal study.
The PicoAMH Elisa test will be marketed by Ansh Labs. Since the device’s review went through a de novo premarket pathway designed for novel devices of low to medium risk, there will be an additional set of criteria, called special controls, put in place to monitor the safety and effectiveness of the test.
*This article was updated on 10/26/2018.
What infectious disease should parents be most worried about?
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
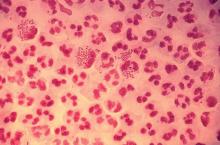
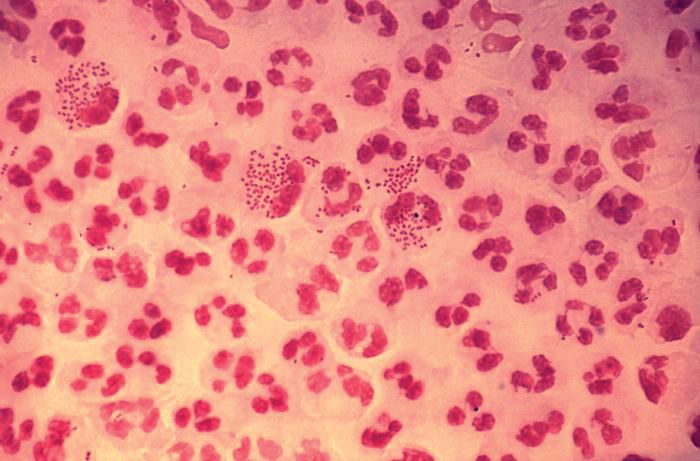
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Are women seeking short-acting contraception satisfied with LARC after giving it a try?
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
Surgical quality: How do we measure something so difficult to define?
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Quality in medicine is a peculiar thing. It is clearly apparent, and yet, can be very difficult to measure and quantify. Surgery, a performance art of sorts, can be even more challenging to qualify or rate. However, as a means to elevate the quality of care for all patients, hospital systems and care providers have aggressively made attempts to do so. This is a noble objective.
In September 2018, the Committee of Gynecologic Practice of the American College of Obstetricians and Gynecologists released ACOG Committee Opinion Number 750, titled, “Perioperative Pathways: Enhanced Recovery After Surgery.”1
The goals of this committee opinion were to advocate for gynecologic surgeons using the “ERAS” pathways in their perioperative care as part of an evidenced-based approach to quality improvement. ERAS pathways have been previously discussed in this column and feature bundled perioperative pathways that incorporate various concepts such as avoidance of prolonged preoperative fasting, early postoperative feeding, multimodal analgesia (with an avoidance of opiates), and inclusion of antibiotic and antiembolic prophylaxis, among other elements.
What was alarming upon closer review of this ACOG Committee Opinion was its omission of the randomized controlled trial by Dickson et al., the only randomized trial published in gynecologic surgery evaluating ERAS pathways.2 This trial compared the length of stay for patients receiving laparotomy for gynecologic cancer surgery who received perioperative care according the ERAS pathway versus those who received standard perioperative care. They found no difference in length of stay – the primary outcome – between the two groups, an impressive 3 days for both. The secondary outcome of postoperative pain was improved for the ERAS group for some of the time points. It was likely that the excellent outcomes in both groups resulted from a Hawthorne effect in which the behavior of study participants is influenced by the fact that they were being observed, in addition to the fact that the physicians involved in the study already were practicing high quality care as part of their “standard” regimen. It simply may be that the act of trying to improve quality is what improves outcomes, not a specific pathway. As senior author, Dr. Peter A. Argenta, explained to me, many of the ERAS elements are “simply good medicine.”
ERAS pathways are an example of process measures of quality. They include elements of care or processes in the delivery of care that are thought to be associated with improved outcomes. Prescription of antibiotics or venous thromboembolism (VTE) prophylaxis are other examples of process measures thought to be associated with improved surgical quality. Rather than rating surgeons’ outcomes (surgical site infection), surgeons are rated on their compliance with a process (the rate of appropriate perioperative antibiotic prescription). However, high compliance with these processes is not automatically associated with improved observed outcomes. For example, hospitals that meet the definition of high quality by virtue of structural measures (such as procedural volume and use of hospital-level quality initiatives) are associated with worse risk-adjusted VTE rates despite demonstrating higher adherence to VTE prophylaxis.3 This is felt to be a function of surveillance bias and the fact that these same hospitals have better capabilities to capture events as part of a feedback mechanism built into their quality initiatives.
What ERAS has favorably done for surgical care is to shine a glaring light on and challenge the unnecessary, old-fashioned, and non–patient-centric interventions that were considered dogma by many. For example, minimizing preoperative fasting is most certainly a patient-friendly adjustment that should absolutely be embraced, regardless of whether or not it speeds up time to discharge. Multimodal approaches to analgesia consistently have been shown to preserve or improve postoperative pain levels with a focus on minimizing opiate use, once again a noble and patient-centered objective.
However, all too many surgical quality interventions focus on their ability to reduce postoperative length of stay. Length of stay is an important driver of health care cost, and an indirect measure of perioperative complications; however, it is not a patient-centered outcome. So long as patients recover from their surgery quickly with respect to pain and function, the location of that recovery (home versus hospital) is less of a focus for most patients. In addition, in the pursuit of shorter hospital stays and less perioperative morbidity, we may encourage practices with unintentional adverse patient-centered outcomes. For example, to preserve a surgeon’s quality metrics, patients who are at high risk for complications may not be offered surgery at all. Long-term ovarian cancer outcomes, such as survival, can be negatively impacted when surgeons opt for less morbid, less radical surgical approaches which have favorable short-term morbidity such as surgical complications and readmissions.4
Ultimately we are most likely to see improvement in quality with a complex, nuanced approach to metrics, not simplistic interventions or pathways. We should recognize interventions that are consistently associated with better outcomes such as high procedural volume, consolidating less common procedures to fewer surgeons, data ascertainment, and reporting data to surgeons.5 Physicians need to take ownership and involvement in the quality metrics that are created to assess the care we provide. Hospital administrators may not fully understand the confounders, such as comorbidities, that contribute to outcomes, which can lead to mischaracterization, cause unfair comparisons between surgeons, or create unintentional incentives that are not patient-centered.6
We all need to understand the epidemiologic science behind evidence-based medicine and to be sophisticated in our ability to review and appraise data so that we can be sensible in what interventions we promote as supported by good evidence. If we fail to correctly identify and characterize what is truly good quality, if we miss the point of what is driving outcomes, or overstate the value of certain interventions, we miss the opportunity to intervene in ways that actually do make a meaningful difference.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at obnews@mdedge.com.
References
1. Obstet Gynecol 2018;132:e120-e30.
2. Obstet Gynecol. 2017 Feb;129(2):355-62.
3. JAMA. 2013 Oct 9;310(14):1482-9.
4. Gynecol Oncol. 2017 Dec;147(3):607-11.
5. J Am Coll Surg. 2004 Apr;198(4):626-32.
6. Gynecol Oncol. 2018 Oct;151(1):141-4.
Diagnosis is an ongoing concern in endometriosis
according to a new survey by Health Union, a family of online health communities.
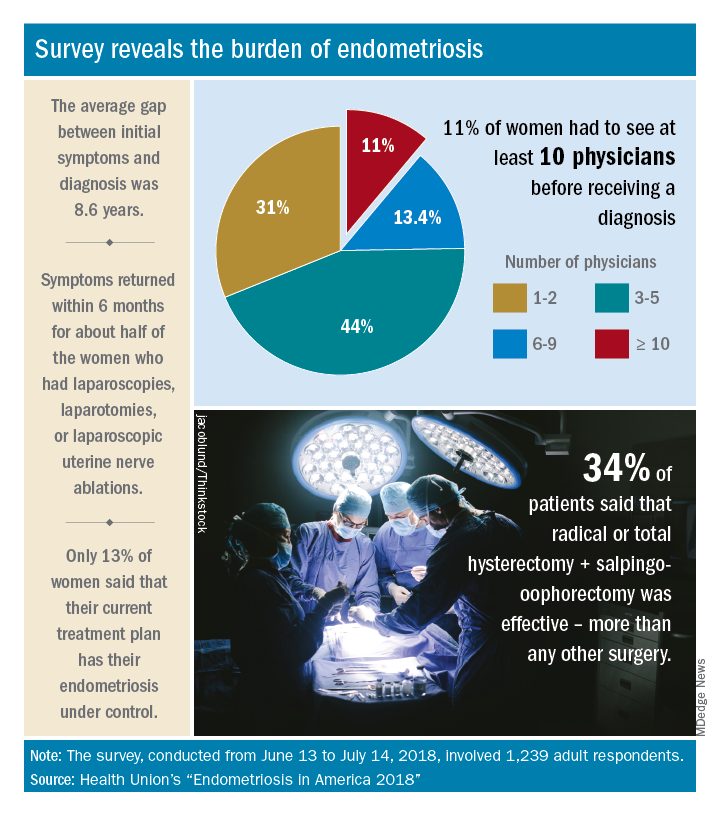
Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
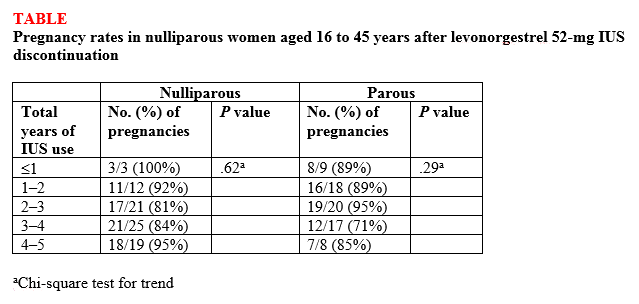
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Consider different etiologies in patients with vaginal pruritus
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
EXPERT ANALYSIS FROM SUMMER AAD 2018
No signal for CV, breast effects with bioidentical vaginal estrogen for dyspareunia
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
FROM NAMS 2018
Key clinical point: Safety data from clinical trials of a bioidentical vaginal estrogen for dyspareunia in menopausal women showed no signs of CV or breast risks.
Major finding: There were no cardiovascular events or thrombotic episodes among menopausal women with dyspareunia treated with TX-004HR.
Study details: Randomized, double-blind, placebo-controlled trial of 784 menopausal women with moderate to severe dyspareunia.
Disclosures: The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin reported financial relationships with several pharmaceutical companies, including TherapeuticsMD.
Source: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
With more mindfulness, menopausal symptoms wane
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
An observational Furthermore, mindfulness had the greatest positive effect on menopausal symptoms for those women with the highest self-reported stress levels.
“In this cross-sectional study, mindfulness was associated with lower menopausal symptom burden. In women with higher stress, the magnitude of association between mindfulness and menopausal symptoms appeared more robust,” said Richa Sood, MD, speaking at the annual meeting of the North American Menopause Society.
Menopausal symptoms can exist alongside many other midlife issues because women often are trying to keep many balls in the air: This age group may be facing aging parents, a household with teenagers, and work-related pressures, she noted.
Thus, menopausal symptoms can be amplified by stressors. New mood problems – or worsening of preexisting ones – can interfere with work productivity and negatively affect relationships. Life satisfaction can take a steep dive during midlife for some women, said Dr. Sood of the Mayo Clinic, Rochester, Minn.
Could mindfulness be effective for stress management in this complex landscape of physiological and lifespan changes? “Mindfulness is paying attention,” said Dr. Sood. Practitioners of mindfulness focus on purposeful attention, staying in the present moment, and avoiding judgment.
Mindfulness may work as a stress-management tool for a variety of reasons, said Dr. Sood. The technique can help retrain people with tendencies for emotional reactivity in stressful situations; additionally, the focus on the present and on observation, rather than judgment, may help avoid maladaptive rumination.
To see how mindfulness in everyday life could affect the burden of menopausal symptoms, Dr. Sood and her collaborators designed an observational, cross-sectional study of 1,744 women aged 40-64 years.
The investigators used three scales in their assessments. The first, the Menopause Rating Scale (MRS), is an 11-item scale ranging from 0 to 44 that assesses psychological, somatovegetative, and urogenital domains. The second, the Perceived Stress Scale 4 (PSS-4), is a four-item scale that is a global measure of stress over the last 4 weeks, with tallied scores in the 0-16 range. Finally, the Mindful Attention Awareness Scale (MAAS) is a 15-item scale that captures how frequently respondents are in a mindful state during their daily life, with higher scores meaning more mindfulness.
The 1,744 women were seen in a women’s health clinic over the period of one year. Participants were a mean 53.4 years old (standard deviation, 6.1 years). Almost all were white (93%), most were married (82.7%), and most also had at least a 4-year college degree (64.6%) and were employed (65.3%).
The investigators mapped each scale against each of the others, which yielded three plots. In the first, higher MAAS scores were correlated with lower MRS scores (correlation, –0.49; P less than .001), which means that more time in a mindful state was associated with less severe menopausal symptoms.
In the second plot, lower MRS scores were associated with lower PSS-4 scores (correlation, 0.55; P less than .001). The last plot mapped PSS-4 scores against MAAS scores, showing that higher MAAS scores were correlated with lower PSS-4 scores, which means that more time in a mindful state was also associated with less perceived stress (correlation, –0.53; P less than .001).
Most of these associations remained statistically significant after multivariable linear regression analysis and breaking out the subscales of the MRS. Only the association between higher MAAS scores and the somatovegetative domain of the PSS-4 lost significance (P = 0.44).
Next, Dr. Sood and her collaborators probed how higher perceived stress, as measured by higher PSS-4 scores, altered the effects that mindful activity had on menopausal symptoms (as measured by the MRS).
The effect of mindfulness became stronger in the milieu of higher perceived stress. At a relatively low PSS-4 value of 4, the MRS score dropped 1.53 points for each one-point increase in the MAAS total score. However, with a PSS-4 score of 12, the decrease in MRS was 2.27 points for each one-point increase in MAAS, and with the maximum perceived stress score of 16, the MRS fell 2.64 points for each one-point increase in the MAAS score.
These findings are set against the backdrop of previous literature linking mindfulness to positive health behaviors and outcomes, she said, noting that work looking specifically at mindfulness-based stress reduction in peri- and postmenopausal women showed a halving of symptoms and improved quality of life.
Dr. Sood said that the present study was observational only, noting that it looked only at time spent in a mindful state in an untrained cohort of women in midlife. “Trait, or dispositional, mindfulness appears to be protective against stress and symptoms in midlife women,” she commented. “More mindful women may be choosing to shift their attention to more pleasant aspects of life, rather than their symptoms.”
“If you allow me to speculate a bit,” Dr. Sood continued, “the underpinnings of psychological symptoms rest in threat-focused attention and emotional reactivity – so the mindfulness approach appears to fit very well to impact such a change.”
Mindfulness, she added, “might be a tool to impact the emotional component of the overall experience, thereby decreasing the total suffering.” However, she noted that what’s needed are studies with a more heterogeneous population, as well as ones designed to tease out causality. Still, “the current study adds to the wealth of data supporting the role of mindfulness in various settings for impacting positive change in health and behaviors.”
Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
SOURCE: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
FROM NAMS 2018
Key clinical point: Time spent in a mindful state was associated with fewer menopause symptoms.
Major finding: With maximum stress, each 1-point increase in mindfulness was associated with a 2.64-point drop in menopausal symptoms.
Study details: A cross-sectional, single-center study of 1,744 women aged 40-64 years.
Disclosures: Dr. Sood reported that she has ownership stake in the Global Center for Resiliency and Well-Being.
Source: Sood R et al. NAMS 2018, Top-Scoring Abstract Session.
Endocrine Society updates guidelines for congenital adrenal hyperplasia
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
recently updated by the Endocrine Society.
The guidelines are an update to the 2010 Endocrine Society Clinical Practice Guideline on congenital adrenal hyperplasia (CAH) due to steroid 21-hydroxylase deficiency. They were published in The Journal of Clinical Endocrinology and Metabolism.
Richard J. Auchus, MD, PhD, of the University of Michigan, Ann Arbor, and coauthor of the 2018 guidelines, said many of the guidelines remain the same, such as use of neonatal screening. However, neonatal diagnosis methods should use gestational age and birth weight or liquid chromatography–tandem mass spectrometry for secondary screening. The authors also noted that the addition of commercially available serum 21-deoxycortisol measurements, while untested, could potentially help identify CAH carriers.
Changes in genital reconstructive surgery were also addressed in the new guidelines, and a recent systematic review and meta-analysis found a “favorable benefit to risk ratio” for both early and late genital reconstructive surgery. Dr. Auchus said the timing of the surgery remains controversial and that there were “downsides of both approaches.”
“I wish there was a straightforward and perfect solution, but I don’t think there is,” he said in an interview.
Dexamethasone for the prenatal treatment of CAH, and prenatal therapy in general is still regarded as experimental and is not recommended, Dr. Auchus said. The authors encouraged pregnant women who are considering prenatal treatment of CAH to go through Institutional Review Board–approved centers that can obtain outcomes. Pregnant women should not receive a glucocorticoid that traverses the placenta, such as dexamethasone.
Classical CAH should be treated with hydrocortisone maintenance therapy, while nonclassic CAH patients should receive glucocorticoid treatment, such as in cases of early onset and rapid progression of pubarche or bone age in children and overt virilization in adolescents.
Dr. Auchus said the new guidelines have been reorganized so information is easier to find, with recommendations beginning at birth before transitioning into recommendations for childhood and adulthood.
“I think the pediatric endocrinologists are familiar with the management of this disease, but I think a lot of the internal medicine endocrinologists don’t get much training in fellowships, and I think it will be easy for them now to find the information,” Dr. Auchus said. “[I]n the previous set of guidelines, it would’ve been difficult for them to find the information that’s scattered throughout.”
However, Dr. Auchus noted, the guidelines were careful to avoid recommendations of specific levels for analyzing biomarkers for monitoring treatment and specific doses. “[W]e gave some general ideas about ranges: that they should be low, they should be normal, they should be not very high, but it’s okay if it’s a little bit high,” he added.
Also, the evidence for the recommendations is limited to best practice guidelines because of a lack of randomized controlled trials, he noted.
“We certainly do need additional long-term data on these patients,” Dr. Auchus said. “[I]t’s our hope that with some of the networks that have been developed for studying adrenal diseases that we can collect that information in a minimally intrusive way for the benefit of all the current and future patients.”
The guidelines were funded by the Intramural Research Program of the National Institutes of Health. The authors report various personal and organizational financial interests in the form of paid consultancies, researcher support positions, advisory board memberships and investigator roles. See the full study for a complete list of disclosures.
SOURCE: Speiser PW et al. J Clin Endocrinol Metab. 2018 Sep 27. doi: 10.1210/jc.2018-01865.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM