User login
2017 Update on pelvic floor dysfunction
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.
If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.
If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The International Continence Society (ICS) defines overactive bladder (OAB) as a syndrome of "urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence (UUI), in the absence of urinary tract infection [UTI] or obvious pathology."1 The Agency for Healthcare Research and Quality (AHRQ) reported OAB prevalence to be 15% in US women, with 11% reporting UUI.2 OAB represents a significant health care burden that impacts nearly every aspect of life, including physical, emotional, and psychological domains.3,4 The economic impact is notable; the projected cost is estimated to reach $82.6 billion annually by 2020.5
The American Urological Association (AUA) and the Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (SUFU) have endorsed an algorithm for use in the evaluation of idiopathic OAB (FIGURE).6 If the patient's symptoms are certain, minimal evaluation is needed and it is reasonable to proceed with first-line therapy, which includes fluid management (decreasing caffeine intake and limiting evening fluid intake), bladder retraining drills such as timed voiding, and improving pelvic floor muscles with the use of biofeedback and functional electrical stimulation.6,7 Pelvic floor muscle training can be facilitated with a referral to a physical therapist trained in pelvic floor muscle education.
If treatment goals are not met with first-line strategies, second-line therapy may be initiated with anticholinergic or β3-adrenergic receptor agonist medications. If symptoms persist after 4 to 8 weeks of pharmacologic therapy, clinicians are encouraged to reassess or refer the patient to a specialist. Further evaluation may include a bladder diary in which the patient documents voided volumes, voiding frequency, and number of incontinent episodes; symptom-specific questionnaires; and/or urodynamic testing.
Related article:
The latest treatments for urinary and fecal incontinence: Which hold water?
Based on that evaluation, the patient may be a candidate for third-line therapy with either intradetrusor onabotulinumtoxinA, posterior tibial nerve stimulation (PTNS), or sacral neuromodulation.
There is a paucity of information comparing third-line therapies. In this Update, we focus on 4 randomized clinical trials that compare third-line treatment options for idiopathic OAB.
Read about how anticholinergic medication and onabotulinumtoxinA compare for treating UUI.
Anticholinergic therapy and onabotulinumtoxinA produce equivalent reductions in the frequency of daily UUI episodes
Visco AG, Brubaker L, Richter HE, et al; for the Pelvic Floor Disorders Network. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367(19):1803-1813.
In a double-blind, double-placebo-controlled randomized trial, Visco and colleagues compared anticholinergic medication with onabotulinumtoxinA 100 U for the treatment of women with UUI.
Details of the study
Two hundred forty-one women with moderate to severe UUI received either 6 months of oral anticholinergic therapy (solifenacin 5 mg daily with the option of dose escalation to 10 mg daily or change to trospium XR 60 mg daily based on the Patient Global Symptom Control score) plus a single intradetrusor injection of saline, or a single intradetrusor injection of onabotulinumtoxinA 100 U plus a 6-month oral placebo regimen.
Inclusion criteria were 5 or more UUI episodes on a 3-day diary, insufficient resolution of symptoms after 2 medications, or being drug naive. Exclusions included a postvoid residual (PVR) urine volume greater than 150 mL or previous therapy with onabotulinumtoxinA.
Participants were scheduled for follow up every 2 to 6 months post randomization, at which time all study medications were discontinued. The primary outcome was reduction from baseline in the mean number of UUI episodes per day over the 6-month period, as recorded in the monthly 3-day bladder diaries. Secondary outcomes included the proportion of participants with complete resolution of UUI, the proportion of participants with 75% or more reduction in UUI episodes, Overactive Bladder Questionnaire Short Form (OABq-SF) scores, other symptom-specific questionnaire scores, and adverse events.
Related article:
Which treatments for pelvic floor disorders are backed by evidence?
Both treatments significantly reduced UUI episodes
At baseline, participants reported a mean (SD) of 5.0 (2.7) UUI episodes per day, and 41% of participants were drug naive. Both treatment groups experienced significant reductions compared with baseline in mean UUI episodes, and the reductions were similar between the 2 groups (reduction of 3.4 episodes per day in the anticholinergic group, reduction of 3.3 episodes in the onabotulinumtoxinA group; P = .81). Complete resolution of UUI was more common in the onabotulinumtoxinA group (27%) as compared with the anticholinergic group (13%) (P = .003). There were no differences in improvement in OABq-SF scores (37.05 in the anticholinergic group vs 37.13 in the onabotulinumtoxinA group; P = .98) or other quality-of-life measures.
Adverse events. The anticholinergic group experienced a higher rate of dry mouth compared with the onabotulinumtoxinA group (46% vs 31%; P = .02) but had lower rates of intermittent catheterization use at 2 months (0% vs 5%, P = .01) and UTIs (13% vs 33%, P<.001).
Strengths and limitations. This was a well-designed, multicenter, randomized double-blind, double placebo-controlled trial. The study design allowed for dose escalation and change to another medication for inadequate symptom control and included drug-naive participants, which increases the generalizability of the results. However, current guidelines recommend reserving onabotulinumtoxinA therapy for third-line therapy, thus deterring this treatment's use in the drug-naive population. Additionally, the lack of a pure placebo arm makes it difficult to interpret the extent to which a placebo effect contributed to observed improvements in clinical symptoms.
Through 6 months, both a single intradetrusor injection of onabotulinumtoxinA 100 U and anticholinergic therapy reduce UUI episodes and improve quality-of-life measures in women who have failed medications or are drug naive. Use of onabotulinumtoxinA, however, more likely will lead to complete resolution of UUI, although with an increased risk of transient urinary retention and UTI. Even given the study findings supporting the use of onabotulinumtoxinA over anticholinergic therapy for complete resolution of UUI, it is most appropriate to align with current practice, which includes a trial of pharmacotherapy before proceeding with third-line onabotulinumtoxinA.
Read: onabotulinumtoxinA vs PTNS for OAB.
OnabotulinumtoxinA has greater 9-month durability for OAB symptoms compared with12 weeks of PTNS
Sherif H, Khalil M, Omar R. Management of refractory idiopathic overactive bladder: intradetrusor injection of botulinum toxin type A versus posterior tibial nerve stimulation. Can J Urol. 2017;24(3):8838-8846.
In this randomized clinical trial, Sherif and colleagues compared the safety and efficacy of a single intradetrusor injection of onabotulinumtoxinA 100 U with that of PTNS for OAB.
Details of the study
Sixty adult men and women with OAB who did not respond to medical therapy were randomly assigned to treatment with either onabotulinumtoxinA 100 U or PTNS. Criteria for exclusion were current UTI, PVR urine volume of more than 150 mL, previous radiation therapy or chemotherapy, previous incontinence surgery or bladder malignancy, or presence of mixed urinary incontinence.
At baseline, participants completed a 3-day bladder diary, an OAB symptom score (OABSS) questionnaire, and urodynamic testing. The OABSS questionnaire included 7 questions (scoring range, 0-28), with higher scores indicating worse symptoms, and included subscales for urgency and quality-of-life measures. Total OABSS, urgency score, quality-of-life score, bladder diary records, and urodynamic testing parameters were assessed at 6, 12, 24, and 36 weeks, along with adverse events.
OnabotulinumtoxinA injections were performed under spinal anesthesia. If PVR urine volume was greater than 200 mL at any follow-up visit, participants were instructed to begin clean intermittent self-catheterization. PTNS was administered as weekly 30-minute sessions for 12 consecutive weeks.
Participants' baseline demographics and symptoms were similar. Average age was 45 years. Averages (SD) for duration of anticholinergic use was 13 (0.8) weeks, UUI episode score was 4.5 (1) on 3-day bladder diary, and OABSS was 22 (2.7). Nine-month data were available for 29 participants in the onabotulinumtoxinA group and for 8 in the PTNS group.
Related article:
Update on pelvic floor dysfunction: Focus on urinary incontinence
OnabotulinumtoxinA treatment benefits sustained for 9 months
Through 6 months, compared with baseline assessments, both treatment groups had significant improvements in clinical symptoms and OABSS total score, as well as urgency and quality-of-life subscales. At 3 months, urodynamic study parameters were similarly improved from baseline in both groups.
At 9 months, however, only the onabotulinumtoxinA group, compared with the PTNS group, maintained the significant improvement from baseline in 3-day bladder diary voiding episodes (average [SD], 10.7 [1.01] vs 11.6 [1.09]; P = .009), 3-day bladder diary nocturia episodes (average [SD], 3.8 [1.09] vs 4.4 [0.8]; P = .02), and average [SD] UUI episodes over 3 days (3.5 [1.2] vs 4.2 [1.04]; P = .02). Similarly, onabotulinumtoxinA-treated participants, compared with those treated with PTNS, maintained improvements at 9 months in average (SD): OABSS total score (19.2 [2.4] vs 20.4 [1.7]; P = .03), urgency scores (10.9 [1.3] vs 11.8 [1.4]; P = .009), urine volume at first desire (177.8 [9.2] vs 171.8 [7.7]), maximum cystometric capacity (304 [17.6] vs 290 [13.1]), and Qmax (mL/sec) (20.7 [1.6] vs 22.2 [1.2]).
Adverse events. Average PVR urine volumes were higher in the onabotulinumtoxinA group compared with the PTNS group (36.8 [2.7] vs 32.4 [3.03]; P = .0001) at all time points, and self-catheterization was required in 6.6% of onabotulinumtoxinA-treated participants. Urinary tract infection occurred in 6.6% of participants in the onabotulinumtoxinA group and in none of the PTNS group. In the PTNS group, few experienced pain and minor bleeding at the needle site.
Strengths and limitations. This randomized, open-label trial comparing treatment with onabotulinumtoxinA 100 U and PTNS included both men and women with idiopathic OAB symptoms. The participants were assessed at regular intervals with various measures, and follow-up adherence was good. The sample size was small, so the study may not have been powered to see differences prior to 9 months.
Although at 9 months only the onabotulinumtoxinA group maintained significant improvement over baseline levels, the improvement was diminished, and therefore the clinical meaningfulness is uncertain. Further, participants in the PTNS group did not undergo monthly maintenance therapy after 3 months, which is recommended for those with a 12-week therapeutic response; this may have affected 9-month outcomes in this group. Since the one-time onabotulinumtoxinA 100 U injection was performed under spinal anesthesia, cost comparisons should be considered, since future onabotulinumtoxinA injections would be necessary.
A one-time onabotulinumtoxinA 100 U injection and 12 weeks of PTNS therapy are reasonable short-term options for symptomatic OAB relief after unsuccessful therapy with medications. OnabotulinumtoxinA injection may provide more durable OAB symptom control at 9 months but with a risk of UTI and need for self-catheterization.
Read about using different doses of onabotulinumtoxinA for OAB.
OnabotulinumtoxinA 200-U injection provides longer OAB symptom improvement than 100-U injection
Abdelwahab O, Sherif H, Soliman T, Elbarky I, Eshazly A. Efficacy of botulinum toxin type A 100 units versus 200 units for treatment of refractory idiopathic overactive bladder. Int Braz J Urol. 2015;41(6):1132-1140.
Abdelwahab and colleagues conducted a single-center, randomized clinical trial to investigate the safety and efficacy of a single injection of intradetrusor onabotulinumtoxinA in 2 different doses (100 U and 200 U) for treatment of OAB.
Details of the study
Eighty adults (63 women, 17 men) who did not benefit from anticholinergic medication during the previous 3 months were randomly assigned to receive either a 100-U (n = 40) or a 200-U (n = 40) injection of onabotulinumtoxinA. Exclusion criteria were PVR urine volume greater than 150 mL and previous radiation therapy or chemotherapy.
Initial assessments -- completed at baseline and at 1, 3, 6, and 9 months -- included the health-related quality-of-life (HR-QOL) questionnaire (maximum score, 100; higher score indicates better quality of life), an abbreviated OABSS questionnaire (4 questions; score range, 0-15; higher score indicates more severe symptoms), and urodynamic evaluation. Outcomes included OABSS, HR-QOL score, and urodynamic parameters at the various time points.
Related article:
Is there a link between impaired mobility and urinary incontinence in elderly, community-dwelling women?
Higher dose, greater symptom improvement and higher adverse event rate
At baseline, participants (average age, 31 years) had an average (SD) OABSS of 1.7 (1.6). OnabotulinumtoxinA treatment with both a 100-U and a 200-U dose resulted in significant improvements (compared with baseline levels) in frequency, nocturia, UUI episodes, OABSS, and urodynamic parameters throughout the 9 months. At 9 months, however, the group treated with the 200-U dose had greater improvements, compared with the group who received a 100-U dose, in urinary frequency symptom scores (mean [SD], 0.32 [0.47] vs 1.1 [0.51]; P<.05), nocturia symptom scores (mean [SD], 0.13 [0.34] vs 0.36 [0.49]; P<.05), UUI symptom scores (mean [SD], 0.68 [0.16] vs 1.26 [1.1]; P<.05), and mean (SD) total OABSS (2.6 [2.31] vs 5.3 [2.11]; P<.05). Similarly, at 9 months the 200-U dose resulted in greater improvements in volume at first desire (mean [SD], 291.8 [42.8] vs 246.8 [53.8] mL; P<.05), volume at strong desire (mean [SD], 392.1 [37.3] vs 313.1 [67.4] mL; P<.05), detrusor pressure (mean [SD], 10.4 [4.0] vs 19.2 [7.8] cm H2O; P<.05), and maximum cystometric capacity (mean [SD], 430.5 [34.2] vs 350 [69.1] mL; P<.05) compared with the 100-U dose.
Adverse events. No participant had a PVR urine volume greater than 100 mL at any follow-up visit. Postoperative hematuria occurred in 23% of the group treated with onabotulinumtoxinA 200 U versus in 15% of those treated with a 100-U dose. Similarly, UTIs occurred in 17.5% of the 200-U dose group and in 7.5% of the 100-U dose group. Dysuria was reported in 37.5% and 15% of the 200-U and 100-U dose groups, respectively.
Strengths and limitations. This randomized, open-label trial comparing a single injection of 100 U versus 200 U of onabotulinumtoxinA included mostly women. OAB symptoms and urodynamic parameters improved after treatment with both dose levels, but a longer duration of improvement was seen with the 200-U dose. The cohort had a low baseline OAB severity, based on the OABSS questionnaire, and a young average age of participants, which limits the generalizability of the study results to a population with refractory OAB. The 0% rate of clean intermittent self-catheterization postinjection might be based on the study's criteria for requiring clean intermittent catheterization. In addition, the initial postinjection visit occurred at 1 month, possibly missing participants who had symptoms of retention soon after injection.
Two dose levels (100 U and 200 U) of a single injection of onabotulinumtoxinA are associated with comparable OAB symptom and urodyanamic improvements. The benefits of a longer duration of effect with the 200-U dose must be weighed against the possible higher risks of transient hematuria, dysuria, and UTI.
Read: onabotulinumtoxinA vs sacral neuromodulation therapy for UUI.
Treatment with onabotulinumtoxinA may control UUI symptoms better than sacral neuromodulation therapy
Amundsen CL, Richter HE, Menefee SA, et al; Pelvic Floor Disorders Network. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316(13):1366-1374.
In this multicenter open-label randomized trial, Amundsen and colleagues compared the efficacy and safety of onabotulinumtoxinA 200 U with that of sacral neuromodulation.
Details of the study
Three hundred sixty-four women with UUI had data available for primary analysis at 6 months. Women were considered eligible for the study if they had 6 or more UUI episodes on a 3-day bladder diary, persistent symptoms despite anticholinergic therapy, a PVR urine volume of less than 150 mL, and had never previously received either study treatment.
There were no differences in baseline characteristics of the participants. The average (SD) age of the study population was 63 (11.6) years, with an average (SD) daily number of UUI episodes of 5.3 (2.8). The average (SD) body mass index was 32 (8) kg/m2.
Participants were randomly assigned to undergo either sacral neuromodulation (n = 174) or intradetrusor injection of onabotulinumtoxinA 200 U (n = 190). The primary outcome was change from baseline in mean number of daily UUI episodes averaged over 6 months as recorded on a monthly 3-day bladder diary. Secondary outcomes included complete resolution of urgency incontinence, 75% or more reduction in UUI episodes, the Overactive Bladder Questionnaire Short Form (SF) score (range, 0-100; higher score indicates higher symptom severity), the Overactive Bladder Satisfaction of Treatment questionnaire (range, 0-100; higher score indicates better satisfaction), other quality-of-life measures, and adverse events.
Related article:
2015 Update on pelvic floor dysfunction: Bladder pain syndrome
Greater symptom bother improvement, treatment satisfaction with onabotulinumtoxinA 200 U
Participants treated with onabotulinumtoxinA had a greater mean reduction of 3.9 UUI episodes per day than the sacral neuromodulation group's reduction of 3.3 UUI episodes per day (mean difference, 0.63; 95% confidence interval [CI], 0.13-1.14; P = .01). In addition, complete UUI resolution was higher in the onabotulinumtoxinA group as compared with the sacral neuromodulation group (20% vs 4%; P<.001). The onabotulinumtoxinA group also had higher rates of 75% or more reduction of UUI episodes compared with the sacral neuromodulation group (46% vs 26%; P<.001). Over 6 months, both groups had improvements in all quality-of-life measures, but the onabotulinumtoxinA group had greater improvement in symptom bother compared with the sacral neuromodulation group (-46.7 vs -38.6; mean difference, 8.1; 95% CI, 3.0-13.3; P = .002). Furthermore, the onabotulinumtoxinA group had greater treatment satisfaction compared with the sacral neuromodulation group (mean difference, 7.8; 95% CI, 1.6-14.1; P = .01).
Adverse events. Six women (3%) underwent sacral neuromodulation device revision or removal. Approximately 8% of onabotulinumtoxinA-treated participants required intermittent self-catheterization at 1 month, 4% at 3 months, and 2% at 6 months. The risk of UTI was higher in the onabotulinumtoxinA group compared with the sacral neuromodulation group (35% vs 11%; risk difference, 23%; 95% CI, -33% to -13%; P<.001).
Strengths and limitations. This is a well-designed randomized clinical trial comparing clinical outcomes and adverse events after treatment with onabotulinumtoxinA 200-U versus sacral neuromodulation. The interventions were standardized across investigators at multiple sites, and the study design required close follow-up to assess efficacy and adverse events. The study used a 200-U dose based on reported durability of effect at that time and findings of equivalency between onabotulinumtoxinA 100 U and anticholinergic therapy. The US Food and Drug Administration's recommendation to use a 100-U dose in all patients with idiopathic OAB might dissuade clinicians from considering the higher dose of onabotulinumtoxinA. The study was limited by the lack of a placebo group.
Both onabotulinumtoxinA 200 U and sacral neuromodulation provide significant improvement in UUI episodes and quality of life over 6 months. However, while treatment with onabotulinumtoxinA has a likelihood of complete UUI resolution, greater improvements in symptom bother and treatment satisfaction, these benefits must be weighed against the risks of transient catheterization and UTI.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
- Haylen BT, de Ridder D, Freeman RM, et al; International Urogynecological Association; International Continence Society. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4-20.
- Hartmann KE, McPheeters ML, Biller DH, et al. Treatment of overactive bladder in women. Evid Rep Technol Assess (Full Rep). 2009;187:1-120.
- Reynolds,WS, Fowke J, Dmochowski, R. The burden of overactive bladder on US public health. Curr Bladder Dysfunct Rep. 2016;11(1):8-13.
- Willis-Gray MG, Dieter AA, Geller EJ. Evaluation and management of overactive bladder: strategies for optimizing care. Res Rep Urol. 2016;8:113-122.
- Ganz ML, Smalarz AM, Krupski TL, et al. Economic costs of overactive bladder in the United States. Urology. 2010;75(3):526-532.
- Gormley EA, Lightner DJ, Faraday M, Vasavada SP; American Urological Association; Society of Urodyndamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193(5):1572-1580.
- Gormley EA, Lightner DJ, Burgio KL, et al; American Urological Association; Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188(6 suppl):2455-2463.
Top translator apps can help you communicate with patients who have limited English proficiency
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
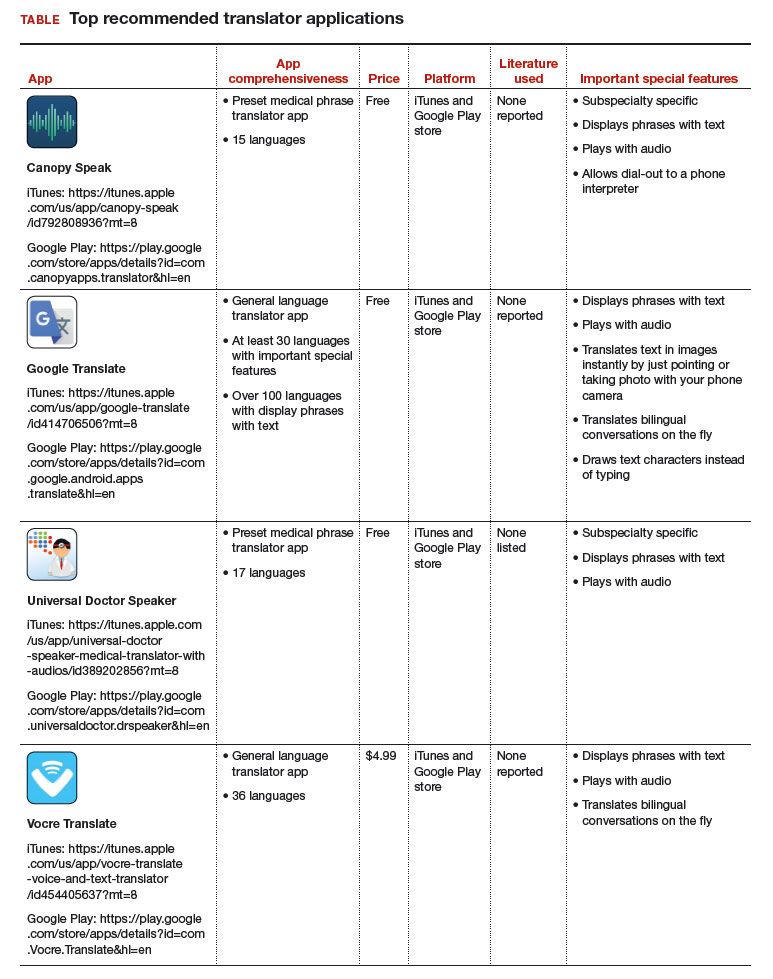
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
As the population of patients with limited English proficiency increases throughout English-speaking countries, health care providers often need translator services. Medical translator smartphone applications (apps) are useful tools that can provide ad hoc translator services.
According to the US Census Bureau in 2015, more than 60 million individuals — about 19% of Americans — reported speaking a language other than English at home, and more than 25 million said that they speak English “less than very well.”1,2 The top 5 non-English languages spoken at home were Spanish, French, Chinese, Tagalog, and Vietnamese, encompassing 72% of non-English speakers.
In the health care sector, translator services are essential for providing accurate and culturally competent care. Current options for translator services include face-to-face interpreters, phone-based translator services, and translator apps on mobile devices. In settings where face-to-face interpreters or phone-based translator services are not available, translator apps may provide reasonable alternatives. My colleagues, Dr. Amrin Khander and Dr. Sara Farag, and I identified and evaluated medical translator apps that are available from the Apple iTunes and Google Play stores to aid clinicians in using such apps during clinical encounters.3
Three types of translator apps
Preset medical phrase translator apps require the user to search for or find a question or statement in order to facilitate a conversation. With these types of apps, a health care provider can choose fully conjugated sentences, which then can be played or read back to the patient in the chosen translated language. Within this group of apps, Canopy Speak and Universal Doctor Speaker are highly accessible, since both apps are available from the Apple iTunes and Google Play stores and both are free.
Medical dictionary apps require the user to search for a medical term in one language to receive a translation in another language. These apps are less useful, but they can help providers find and define specific terms in a given language.
General language translator apps require the user to enter a term, statement, or question in one language and then provide a translation in another language. Google Translate and Vocre Translate are examples.
The top recommended translator apps are listed in the TABLE alphabetically and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 I hope the apps described here will help you enhance communication with your patients who have limited English proficiency.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
- United States Census Bureau. Detailed language spoken at home and ability to speak English for the population 5 years and over: 2009–2013. http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published October 2015. Accessed August 31, 2017.
- United States Census Bureau. US population world clock. http://www.census.gov/popclock/?intcmp=home_pop. Accessed August 31, 2017.
- Khander A, Farag S, Chen KT. Identification and rating of medical translator mobile applications using the APPLICATIONS scoring system [abstract 321]. Obstet Gynecol. 2017;129(5 suppl):101S. doi:10.1097/01.AOG.0000514971.96123.20
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125(6):1478–1483.
Breast density and optimal screening for breast cancer
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.
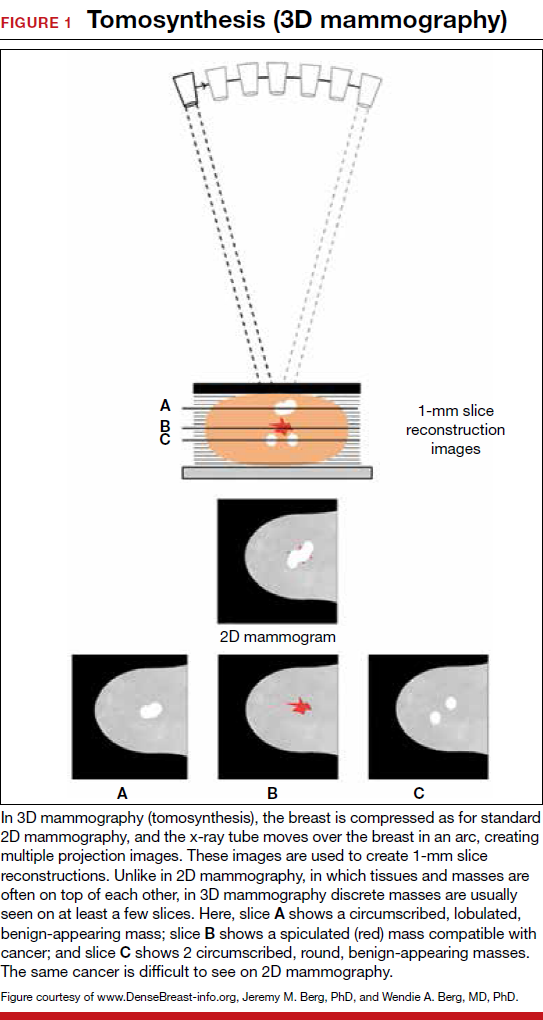
Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).
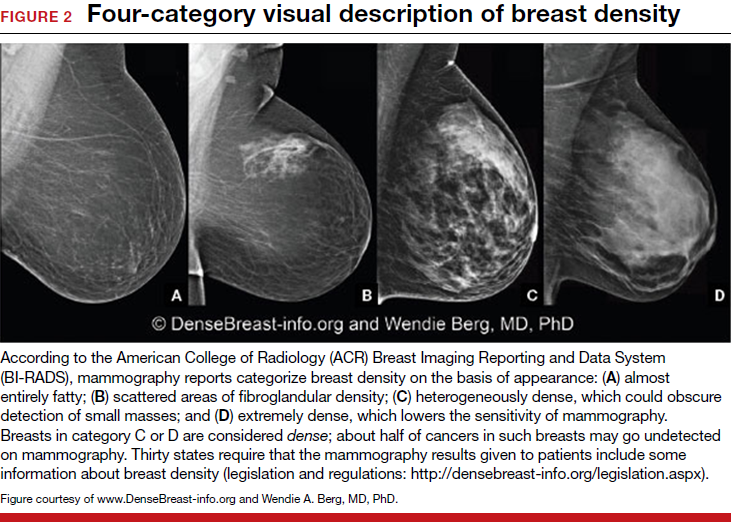
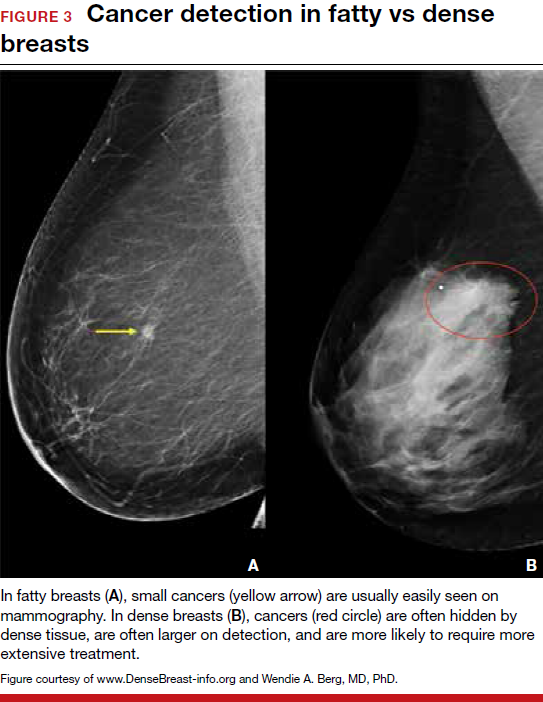
3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts
MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
MY STORY: Prologue
My aunt received a breast cancer diagnosis at age 40, and she died at age 60, in 1970. Then, in 1975, my mother’s breast cancer was found at age 55, but only after she was examined for nipple retraction; on mammography, the cancer had been obscured by dense breast tissue. Mom had 2 metastatic nodes but participated in the earliest clinical trials of chemotherapy and lived free of breast cancer for another 41 years. Naturally I thought that, were I to develop this disease, I would want it found earlier. Ironically, it was, but only because I had spent my career trying to understand the optimal screening approaches for women with dense breasts—women like me.
Cancers are masked on mammography in dense breasts
For women, screening mammography is an important step in reducing the risk of dying from breast cancer. The greatest benefits are realized by those who start annual screening at age 40, or 45 at the latest.1 As it takes 9 to 10 years to see a benefit from breast cancer screening at the population level, it is not logical to continue this testing when life expectancy is less than 10 years, as is the case with women age 85 or older, even those in the healthiest quartile.2–4 However, despite recent advances, the development of 3D mammography (tomosynthesis) (FIGURE 1) in particular, cancers can still be masked by dense breast tissue. Both 2D and 3D mammograms are x-rays; both dense tissue and cancers absorb x-rays and appear white.

Breast density is determined on mammography and is categorized as fatty, scattered fibroglandular, heterogeneously dense, or extremely dense (FIGURE 2).5 Tissue in the heterogeneous and extreme categories is considered dense. More than half of women in their 40s have dense breasts; with some fatty involution occurring around menopause, the proportion drops to 25% for women in their 60s.6 About half of breast cancers have calcifications, which on mammography are usually easily visible even in dense breasts. The problem is with noncalcified invasive cancers that can be hidden by dense tissue (FIGURE 3).


3D mammography improves cancer detection but is of minimal benefit in extremely dense breasts
Although 3D mammography improves cancer detection in most women, any benefit is minimal in women with extremely dense breasts, as there is no inherent soft-tissue contrast.7 Masked cancers are often only discovered because of a lump after a normal screening mammogram, as so-called “interval cancers.” Compared with screen-detected cancers, interval cancers tend to be more biologically aggressive, to have spread to lymph nodes, and to have worse prognoses. However, even some small screen-detected cancers are biologically aggressive and can spread to lymph nodes quickly, and no screening test or combination of screening tests can prevent this occurrence completely, regardless of breast density.
Related article:
Get smart about dense breasts

MRI provides early detection across all breast densities
In all tissue densities, contrast-enhanced magnetic resonance imaging (MRI) is far better than mammography in detecting breast cancer.8 Women at high risk for breast cancer caused by mutations in BRCA1, BRCA2, p53, and other genes have poor outcomes with screening mammography alone—up to 50% of cancers are interval cancers. Annual screening MRI reduces this percentage significantly, to 11% in women with pathogenic BRCA1 mutations and to 4% in women with BRCA2 mutations.9 Warner and colleagues found a decrease in late-stage cancers in high-risk women who underwent annual MRI screenings compared to high-risk women unable to have MRI.10
The use of MRI for screening is limited by availability, patient tolerance,11 and high cost. Research is being conducted to further validate approaches using shortened screening MRI times (so-called “abbreviated” or “fast” MRI) and, thereby, improve access, tolerance, and reduce associated costs; several investigators already have reported promising results, and a few centers offer this modality directly to patients willing to pay $300 to $350 out of pocket.12,13 Even in normal-risk women, MRI significantly increases detection of early breast cancer after a normal mammogram and ultrasound, and the cancer detection benefit of MRI is seen across all breast densities.14
Most health insurance plans cover screening MRI only for women who meet defined risk criteria, including women who have a known disease-causing mutation—or are suspected of having one, given a family history of breast cancer with higher than 20% to 25% lifetime risk by a model that predicts mutation carrier status—as well as women who had chest radiation therapy before age 30, typically for Hodgkin lymphoma, and at least 8 years earlier.15 In addition, MRI can be considered in women with atypical breast biopsy results or a personal history of lobular carcinoma in situ (LCIS).16
Screening MRI should start by age 25 in women with disease-causing mutations, or at the time of atypical or LCIS biopsy results, and should be performed annually unless the woman is pregnant or has a metallic implant, renal insufficiency, or another contraindication to MRI. MRI can be beneficial in women with a personal history of cancer, although annual mammography remains the standard of care.17–19
MRI and mammography can be performed at the same time or on an alternating 6-month basis, with mammography usually starting only after age 30 because of the small risk that radiation poses for younger women. There are a few other impediments to having breast MRI: The woman must lie on her stomach within a confined space (tunnel), the contrast that is injected may not be well tolerated, and insurance does not cover the test for women who do not meet the defined risk criteria.11
Read why mammography supplemented by US is best for women with dense breasts.
Ultrasonography supplements mammography
Mammography supplemented with ultrasonography (US) has been studied as a “Goldilocks” or best-fit solution for the screening of women with dense breasts, as detection of invasive cancers is improved with the 2 modalities over mammography alone, and US is less invasive, better tolerated, and lower in cost than the more sensitive MRI.
In women with dense breasts, US has been found to improve cancer detection over mammography alone, and early results suggest a larger cancer detection benefit from US than from 3D mammography, although research is ongoing.20 Adding US reduces the interval cancer rate in women with dense breasts to less than 10% of all cancers found—similar to results for women with fatty breasts.17,21,22
US can be performed by a trained technologist or a physician using a small transducer, which usually provides diagnostic images (so that most callbacks would be for a true finding), or a larger transducer and an automated system can be used to create more than a thousand images for radiologist review.23,24 Use of a hybrid system, a small transducer with an automated arm, has been validated as well.25 Screening US is not available universally, and with all these approaches optimal performance requires trained personnel. Supplemental screening US usually is covered by insurance but is nearly always subject to a deductible/copay.
Related article:
Educate patients about dense breasts and cancer risk
Reducing false-positives, callbacks, and additional testing
Mammography carries a risk of false-positives. On average, 11% to 12% of women are called back for additional testing after a screening mammogram, and in more than 95% of women brought back for extra testing, no cancer is found.26 Women with dense breasts are more likely than those with less dense breasts to be called back.27 US and MRI improve cancer detection and therefore yield additional positive, but also false-positive, findings. Notably, callbacks decrease after the first round of screening with any modality or combination of tests, as long as prior examinations are available for comparison.
One advantage of 3D over 2D mammography is a decrease in extra testing for areas of asymmetry, which are often recognizable on 3D mammography as representing normal superimposed tissue.28–30 Architectural distortion, which is better seen on 3D mammography and usually represents either cancer or a benign radial scar, can lead to false-positive biopsies, although the average biopsy rate is no higher for 3D than for 2D alone.31 Typically, the 3D and 2D examinations are performed together (slightly more than doubling the radiation dose), or synthetic 2D images can be created from the 3D slices (resulting in a total radiation dose almost the same as standard 2D alone).
Most additional cancers seen on 3D mammography or US are lower-grade invasive cancers with good prognoses. Some aggressive high-grade breast cancers go undetected even when mammography is supplemented with US, either because they are too small to be seen or because they resemble common benign masses and may not be recognized. MRI is particularly effective in depicting high-grade cancers, even small ones.
The TABLE summarizes the relative rates of cancer detection and additional testing by various breast screening tests or combinations of tests. Neither clinical breast examination by a physician or other health care professional nor routine breast self-examination reduces the number of deaths caused by breast cancer. Nevertheless, women should monitor any changes in their breasts and report these changes to their clinician. A new lump, skin or nipple retraction, or a spontaneous clear or bloody nipple discharge merits diagnostic breast imaging even if a recent screening mammogram was normal.
FIGURE 4 is an updated decision support tool that suggests strategies for optimizingcancer detection with widely available screening methods.
Read how to take advantage of today’s technology for breast density screening
MY STORY: Epilogue
My annual 3D mammograms were normal, even the year my cancer was present. In 2014, I entered my family history into the IBIS Breast Cancer Risk Evaluation Tool (Tyrer-Cuzick model of breast cancer risk) (http://www.ems-trials.org/riskevaluator/) and calculated my lifetime risk at 19.7%. That is when I decided to have a screening MRI. My invasive breast cancer was easily seen on MRI and then on US. The cancer was node-negative, easily confirmed with needle biopsy, and treated with lumpectomy and radiation. There was no need for chemotherapy.
My personal experience prompted me to join JoAnn Pushkin and Cindy Henke-Sarmento, RT(R)(M), BA, in developing a website, www.DenseBreast-info.org, to give women and their physicians easy access to information on making decisions about screening in dense breasts.
My colleagues and I are often asked what is the best way to order supplemental imaging for a patient who may have dense breasts. Even in cases in which a mammogram does not exist or is unavailable, the following prescription can be implemented easily at centers that offer US: “2D plus 3D mammogram if available; if dense, perform ultrasound as needed.”
Related article:
DenseBreast-info.org: What this resource can offer you, and your patients
Breast density screening: Take advantage of today’s technology
Breast screening and diagnostic imaging have improved significantly since the 1970s, when many of the randomized trials of mammography were conducted. Breast density is one of the most common and important risk factors for development of breast cancer and is now incorporated into the Breast Cancer Surveillance Consortium model (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm) and the Tyrer-Cuzick model (see also http://densebreast-info.org/explanation-of-dense-breast-risk-models.aspx).32 Although we continue to validate newer approaches, women should take advantage of the improved methods of early cancer detection, particularly if they have dense breasts or are at high risk for breast cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361(9367):1405–1410.
- Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441.
- Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756.
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: D’Orsi CJ, Sickles EA, Mendelson EB, et al, eds. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System. 5th ed. Reston, VA: American College of Radiology; 2013.
- Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10).
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315(16):1784–1786.
- Berg WA. Tailored supplemental screening for breast cancer: what now and what next? AJR Am J Roentgenol. 2009;192(2):390–399.
- Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening—MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1458–1468.
- Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669.
- Berg WA, Blume JD, Adams AM, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology. 2010;254(1):79–87.
- Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–2310.
- Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat. 2017;162(2):283–295.
- Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283(2):361–370.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- National Comprehensive Cancer Network. NCCN guidelines for detection, prevention, and risk reduction: breast cancer screening and diagnosis. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- Berg WA, Zhang Z, Lehrer D, et al; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394–1404.
- Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195(2):510–516.
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst. 2016;108(3).
- Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial [published online ahead of print March 9, 2016]. J Clin Oncol. JCO634147.
- Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer. 2011;47(7):1021–1026.
- Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-Cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387(10016):341–348.
- Berg WA, Mendelson EB. Technologist-performed handheld screening breast US imaging: how is it performed and what are the outcomes to date? Radiology. 2014;272(1):12–27.
- Brem RF, Tabár L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2015;274(3):663–673.
- Kelly KM, Dean J, Comulada WS, Lee SJ. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010;20(3):734–742.
- Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the Breast Cancer Surveillance Consortium. Radiology. 2017;283(1):49–58.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816.
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267(1):47–56.
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
- Bahl M, Lamb LR, Lehman CD. Pathologic outcomes of architectural distortion on digital 2D versus tomosynthesis mammography [published online ahead of print August 23, 2017]. AJR Am J Roentgenol. doi:10.2214/AJR.17.17979.
- Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K; Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer [published online ahead of print February 2, 2017]. JAMA Oncol. doi:10.1001/jamaoncol.2016.6326.
Woman dies following cervical cone biopsy: $4.25M award
Woman dies following cervical cone biopsy: $4.25M award
A 46-year-old woman underwent a cervical cone biopsy at a Veterans Administration (VA) hospital on July 18. Following the test, significant bleeding occurred. The gynecologic surgeon attempted to control the hemorrhage by injecting fe
ESTATE'S CLAIM:
The surgeon’s actions were negligent. She removed too much tissue during the biopsy, injured the vaginal and uterine walls, and failed to timely diagnose and appropriately treat the injuries. The ferric subsulfate solution entered the abdominal cavity via the perforation, causing peritonitis and bowel injuries. A pathology report from the bowel resection surgery informed the surgeon that the bowel was not properly reconnected after the damaged portion was removed, but this condition was neither detected intraoperatively nor treated postoperatively.
DEFENDANTS' DEFENSE:
The surgeon moved for summary judgment, countering that, as a federal employee, she was exempt from personal liability for the services performed as an employee of the VA. That motion was denied. She then argued that injury to the vaginal/uterine wall is a known complication of the biopsy procedure.
VERDICT:
A $4.25 million Illinois verdict was returned in federal court.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Needle stick not reported to patient
A woman delivered a baby assisted by an on-call ObGyn. When the baby developed fetal tachycardia, the ObGyn recommended expediting delivery and discussed various options and the risks of each option. The mother chose a vaginal forceps delivery. During the procedure, the mother experienced a 3rd-degree perineal laceration and a few minor lacerations, which were repaired. The mother was in pain, so the ObGyn performed a revision repair. During the procedure, the ObGyn accidentally stuck himself with a clean needle. He replaced the needle and changed his glove. The mother reported instant pain relief following revision and was discharged. After the needle incident, the ObGyn’s thumb became red and swollen, so he took antibiotics.
Two days after discharge, the patient reported to the ObGyn’s office with fever, pain, and a foul odor emanating from the surgery site. She was given the diagnosis of pelvic incisional cellulitis and was taken to the operating room for exploration and debridement. The patient developed septic shock and necrotizing fasciitis. She was placed on a ventilator and underwent 13 surgeries.
PATIENTS' CLAIM:
The ObGyn was negligent. The patient claimed breach of duty: the ObGyn did not disclose that his thumb was swollen and that he took antibiotics.
PHYSICIANS' DEFENSE:
There was no breach of duty. He did not feel the need to concern the patient about an injury to himself that did not affect her.
VERDICT:
A Kansas defense verdict was returned.
Related article:
2017 Update on infectious disease
Catheter removal, air embolism: $3.5M settlement
A 44-year-old woman underwent gynecologic surgery on April 22. She developed a rectovaginal fistula and other complications. Intravenous antibiotics were required and parenteral nutrition was delivered through a central venous catheter. On May 22, after a hospital nurse removed the catheter, an air embolism developed, causing a brain injury. The patient has a mental disability and residual leg tremors.
PATIENTS' CLAIM:
Because of the surgeon’s negligence during surgery, a fistula developed. The nurse negligently removed the catheter, causing the embolism.
DEFENDANTS' DEFENSE:
The case settled during the trial.
VERDICT:
A $3.5 million Illinois settlement was reached, including payments of $1 million from the surgeon and $2.5 million from the hospital.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Woman dies following cervical cone biopsy: $4.25M award
A 46-year-old woman underwent a cervical cone biopsy at a Veterans Administration (VA) hospital on July 18. Following the test, significant bleeding occurred. The gynecologic surgeon attempted to control the hemorrhage by injecting fe
ESTATE'S CLAIM:
The surgeon’s actions were negligent. She removed too much tissue during the biopsy, injured the vaginal and uterine walls, and failed to timely diagnose and appropriately treat the injuries. The ferric subsulfate solution entered the abdominal cavity via the perforation, causing peritonitis and bowel injuries. A pathology report from the bowel resection surgery informed the surgeon that the bowel was not properly reconnected after the damaged portion was removed, but this condition was neither detected intraoperatively nor treated postoperatively.
DEFENDANTS' DEFENSE:
The surgeon moved for summary judgment, countering that, as a federal employee, she was exempt from personal liability for the services performed as an employee of the VA. That motion was denied. She then argued that injury to the vaginal/uterine wall is a known complication of the biopsy procedure.
VERDICT:
A $4.25 million Illinois verdict was returned in federal court.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Needle stick not reported to patient
A woman delivered a baby assisted by an on-call ObGyn. When the baby developed fetal tachycardia, the ObGyn recommended expediting delivery and discussed various options and the risks of each option. The mother chose a vaginal forceps delivery. During the procedure, the mother experienced a 3rd-degree perineal laceration and a few minor lacerations, which were repaired. The mother was in pain, so the ObGyn performed a revision repair. During the procedure, the ObGyn accidentally stuck himself with a clean needle. He replaced the needle and changed his glove. The mother reported instant pain relief following revision and was discharged. After the needle incident, the ObGyn’s thumb became red and swollen, so he took antibiotics.
Two days after discharge, the patient reported to the ObGyn’s office with fever, pain, and a foul odor emanating from the surgery site. She was given the diagnosis of pelvic incisional cellulitis and was taken to the operating room for exploration and debridement. The patient developed septic shock and necrotizing fasciitis. She was placed on a ventilator and underwent 13 surgeries.
PATIENTS' CLAIM:
The ObGyn was negligent. The patient claimed breach of duty: the ObGyn did not disclose that his thumb was swollen and that he took antibiotics.
PHYSICIANS' DEFENSE:
There was no breach of duty. He did not feel the need to concern the patient about an injury to himself that did not affect her.
VERDICT:
A Kansas defense verdict was returned.
Related article:
2017 Update on infectious disease
Catheter removal, air embolism: $3.5M settlement
A 44-year-old woman underwent gynecologic surgery on April 22. She developed a rectovaginal fistula and other complications. Intravenous antibiotics were required and parenteral nutrition was delivered through a central venous catheter. On May 22, after a hospital nurse removed the catheter, an air embolism developed, causing a brain injury. The patient has a mental disability and residual leg tremors.
PATIENTS' CLAIM:
Because of the surgeon’s negligence during surgery, a fistula developed. The nurse negligently removed the catheter, causing the embolism.
DEFENDANTS' DEFENSE:
The case settled during the trial.
VERDICT:
A $3.5 million Illinois settlement was reached, including payments of $1 million from the surgeon and $2.5 million from the hospital.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Woman dies following cervical cone biopsy: $4.25M award
A 46-year-old woman underwent a cervical cone biopsy at a Veterans Administration (VA) hospital on July 18. Following the test, significant bleeding occurred. The gynecologic surgeon attempted to control the hemorrhage by injecting fe
ESTATE'S CLAIM:
The surgeon’s actions were negligent. She removed too much tissue during the biopsy, injured the vaginal and uterine walls, and failed to timely diagnose and appropriately treat the injuries. The ferric subsulfate solution entered the abdominal cavity via the perforation, causing peritonitis and bowel injuries. A pathology report from the bowel resection surgery informed the surgeon that the bowel was not properly reconnected after the damaged portion was removed, but this condition was neither detected intraoperatively nor treated postoperatively.
DEFENDANTS' DEFENSE:
The surgeon moved for summary judgment, countering that, as a federal employee, she was exempt from personal liability for the services performed as an employee of the VA. That motion was denied. She then argued that injury to the vaginal/uterine wall is a known complication of the biopsy procedure.
VERDICT:
A $4.25 million Illinois verdict was returned in federal court.
Related article:
Reducing maternal mortality in the United States—Let’s get organized!
Needle stick not reported to patient
A woman delivered a baby assisted by an on-call ObGyn. When the baby developed fetal tachycardia, the ObGyn recommended expediting delivery and discussed various options and the risks of each option. The mother chose a vaginal forceps delivery. During the procedure, the mother experienced a 3rd-degree perineal laceration and a few minor lacerations, which were repaired. The mother was in pain, so the ObGyn performed a revision repair. During the procedure, the ObGyn accidentally stuck himself with a clean needle. He replaced the needle and changed his glove. The mother reported instant pain relief following revision and was discharged. After the needle incident, the ObGyn’s thumb became red and swollen, so he took antibiotics.
Two days after discharge, the patient reported to the ObGyn’s office with fever, pain, and a foul odor emanating from the surgery site. She was given the diagnosis of pelvic incisional cellulitis and was taken to the operating room for exploration and debridement. The patient developed septic shock and necrotizing fasciitis. She was placed on a ventilator and underwent 13 surgeries.
PATIENTS' CLAIM:
The ObGyn was negligent. The patient claimed breach of duty: the ObGyn did not disclose that his thumb was swollen and that he took antibiotics.
PHYSICIANS' DEFENSE:
There was no breach of duty. He did not feel the need to concern the patient about an injury to himself that did not affect her.
VERDICT:
A Kansas defense verdict was returned.
Related article:
2017 Update on infectious disease
Catheter removal, air embolism: $3.5M settlement
A 44-year-old woman underwent gynecologic surgery on April 22. She developed a rectovaginal fistula and other complications. Intravenous antibiotics were required and parenteral nutrition was delivered through a central venous catheter. On May 22, after a hospital nurse removed the catheter, an air embolism developed, causing a brain injury. The patient has a mental disability and residual leg tremors.
PATIENTS' CLAIM:
Because of the surgeon’s negligence during surgery, a fistula developed. The nurse negligently removed the catheter, causing the embolism.
DEFENDANTS' DEFENSE:
The case settled during the trial.
VERDICT:
A $3.5 million Illinois settlement was reached, including payments of $1 million from the surgeon and $2.5 million from the hospital.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Surviving ovarian cancer: Is there an association between hospital volume and quality of care?
The relationship between procedure volume and outcomes has long been recognized: studies have concluded that patients operated on by high-volume surgeons at high-volume hospitals have improved outcomes.1,2 This paradigm is also associated with ovarian cancer outcomes. But what affect does adherence to evidence-based guidelines have on these statistics?
Jason D. Wright, MD, and colleagues at the Columbia University College of Physicians and Surgeons, in New York City, sought to determine whether strict adherence to quality metrics by hospitals could explain the association between hospital volume and ovarian cancer survival.3
Details of the study
Using the National Cancer Database (NCD), the research team identified 100,725 patients at 1,268 hospitals who were treated for invasive epithelial ovarian cancer between 2004 and 2013. Hospitals were stratified by annual case volume into quintiles: low-volume (≤2 cases; n = 299 [23.6%]), low-intermediate–volume (2.01–5 cases; n = 465 [36.7%]), intermediate-volume (5.01–9 cases; n = 157 [12.4%]), high-intermediate–volume (9.01–19.9 cases; n = 194 [15.3%]), and high-volume (≥20 cases; n = 153 [12.1%]).3
To measure quality, the authors defined hospital-level rates of 5 metrics based on clinical guidelines3:
- lymph node dissection performed for patients with stage I–IIIB tumors
- performance of omentectomy or cytoreduction for patients with advanced stage tumors
- use of chemotherapy among patients with early-stage, high-risk tumors
- omission of chemotherapy for women with early-stage, low-risk tumors
- use of chemotherapy (either neoadjuvant or adjuvant) for women with advanced-stage disease.
For each metric, the authors determined the rate of hospital-level compliance for all study-eligible patients. Then a composite variable of overall quality was derived using all 5 metrics. Based on the overall quality metric, hospitals were stratified into quartiles: low-quality, medium-low–quality, medium-high–quality, and high-quality.3
Hospital-level adjusted 2- and 5-year survival rates were compared based on volume and adherence to quality metrics.3
Related article:
2017 Update on ovarian cancer
Trends and conclusions
Researchers found that compliance with quality metrics generally increased with hospital volume. Trends of increased compliance were observed with lymph node dissection for early-stage tumors, cytoreduction for advanced-stage tumors, and use of chemotherapy for advanced-stage tumors. No trends were evident for use of chemotherapy for high-risk, early-stage tumors. By contrast, a trend for higher-volume hospitals to administer chemotherapy for low-risk, early-stage tumors was discovered. Adherence with the composite overall quality metric was noted in 64.2% of low-volume centers and increased with each volume category to 82.2% at the highest-volume hospitals.3
Study results indicated that survival increased with increasing hospital volume and with adherence to the quality metrics. The association between volume and quality was then examined. For each volume category, survival increased with increasing adherence to the quality metrics. In the highest-volume group, 2-year adjusted survival rose from 75.5% (95% confidence interval [CI], 73.2%–77.8%) at the lowest-quality hospitals, to 78.6% (95% CI, 78.0%–79.1%) at the highest-quality hospitals. Similar trends were found for intermediate-volume hospitals and for 5-year survival. However, the relationship between adherence to quality metrics and survival was less consistent for the low-, low-intermediate–, and high-intermediate–volume hospitals.3
The authors concluded that both hospital volume and adherence to quality metrics are associated with survival for ovarian cancer. Even though survival rates are improved at low-volume hospitals that are highly adherent to quality metrics, their survival rates are still lower than high-volume hospitals.
Read about the pros and cons of regionalization for high-risk ovarian cancer surgery.
Study limitations
The authors cite several limitations to this study:
- chosen quality metrics focused on care during initial treatment. Women with ovarian cancer are often treated for many years.
- the NCD lacks information on aspects like hospital infrastructure and staffing; there are probably other confounders that influence treatment and outcomes
- although NCD data have been validated, misclassification of a small number of patients may exist. Also, some hospitals did not treat patients who might be eligible for a quality metric and therefore were not included in this analysis.
- some study participants (13.7%) received treatment at multiple hospitals
- the volume cutpoints chosen by the research team were based on prior studies; there could be outcome variation within a volume strata.
Should high-risk surgeries be regionalized?
The association between higher surgical volume and improved outcomes has led to efforts to regionalize the care for high-risk operations to high-volume centers, say the authors. They conclude that this may be a reasonable strategy for some procedures. However, they suggest that regionalization presents practical difficulties:
- patients prefer to receive local care and are often unwilling to or cannot travel
- regionalization can worsen inequalities in access to care and may adversely affect low-volume hospitals
- high-volume centers do not exist in some areas of the country. A recent report suggested that 9% of the US female population had geographic barriers to receiving care from a gynecologic oncologist.4
Can low-volume facilities attain the same outcomes as high-volume centers?
The authors pose an important question: Can lower-volume facilities that deliver high-quality care achieve the same outcomes as higher-volume centers? With the difficulties associated with regionalization, many advocates seek strategies to raise the quality of care at low-volume centers, they say. The authors note that, “although outcomes improve at low-volume centers that are highly compliant with the quality metrics examined, survival at these centers is still lower than at high-volume centers.”3 The authors suggest that there are factors other than adherence to guidelines that play a role in how hospital volume affects ovarian cancer outcomes.3
Practice considerations
“Because the best outcomes appear to be achieved at high-volume hospitals, efforts to promote volume-based referral for women with ovarian cancer are reasonable,” the authors conclude.3 However, in practicality, many women will not be able to receive care at high-volume centers, they concede. “For low-volume centers, targeted quality improvement efforts and strict adherence to quality guidelines may help to optimize outcomes for women with ovarian cancer.”3
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Birkmeyer JC, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Eng J Med. 2002;346(15):1128–1137.
- Birkmeyer JC, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. New Engl J Med. 2003;349(22):2117–2127.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130(3):545–553.
- Shalowitz DI, Vinograd AM, Giuntoli RL II. Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138(1):115–120.
The relationship between procedure volume and outcomes has long been recognized: studies have concluded that patients operated on by high-volume surgeons at high-volume hospitals have improved outcomes.1,2 This paradigm is also associated with ovarian cancer outcomes. But what affect does adherence to evidence-based guidelines have on these statistics?
Jason D. Wright, MD, and colleagues at the Columbia University College of Physicians and Surgeons, in New York City, sought to determine whether strict adherence to quality metrics by hospitals could explain the association between hospital volume and ovarian cancer survival.3
Details of the study
Using the National Cancer Database (NCD), the research team identified 100,725 patients at 1,268 hospitals who were treated for invasive epithelial ovarian cancer between 2004 and 2013. Hospitals were stratified by annual case volume into quintiles: low-volume (≤2 cases; n = 299 [23.6%]), low-intermediate–volume (2.01–5 cases; n = 465 [36.7%]), intermediate-volume (5.01–9 cases; n = 157 [12.4%]), high-intermediate–volume (9.01–19.9 cases; n = 194 [15.3%]), and high-volume (≥20 cases; n = 153 [12.1%]).3
To measure quality, the authors defined hospital-level rates of 5 metrics based on clinical guidelines3:
- lymph node dissection performed for patients with stage I–IIIB tumors
- performance of omentectomy or cytoreduction for patients with advanced stage tumors
- use of chemotherapy among patients with early-stage, high-risk tumors
- omission of chemotherapy for women with early-stage, low-risk tumors
- use of chemotherapy (either neoadjuvant or adjuvant) for women with advanced-stage disease.
For each metric, the authors determined the rate of hospital-level compliance for all study-eligible patients. Then a composite variable of overall quality was derived using all 5 metrics. Based on the overall quality metric, hospitals were stratified into quartiles: low-quality, medium-low–quality, medium-high–quality, and high-quality.3
Hospital-level adjusted 2- and 5-year survival rates were compared based on volume and adherence to quality metrics.3
Related article:
2017 Update on ovarian cancer
Trends and conclusions
Researchers found that compliance with quality metrics generally increased with hospital volume. Trends of increased compliance were observed with lymph node dissection for early-stage tumors, cytoreduction for advanced-stage tumors, and use of chemotherapy for advanced-stage tumors. No trends were evident for use of chemotherapy for high-risk, early-stage tumors. By contrast, a trend for higher-volume hospitals to administer chemotherapy for low-risk, early-stage tumors was discovered. Adherence with the composite overall quality metric was noted in 64.2% of low-volume centers and increased with each volume category to 82.2% at the highest-volume hospitals.3
Study results indicated that survival increased with increasing hospital volume and with adherence to the quality metrics. The association between volume and quality was then examined. For each volume category, survival increased with increasing adherence to the quality metrics. In the highest-volume group, 2-year adjusted survival rose from 75.5% (95% confidence interval [CI], 73.2%–77.8%) at the lowest-quality hospitals, to 78.6% (95% CI, 78.0%–79.1%) at the highest-quality hospitals. Similar trends were found for intermediate-volume hospitals and for 5-year survival. However, the relationship between adherence to quality metrics and survival was less consistent for the low-, low-intermediate–, and high-intermediate–volume hospitals.3
The authors concluded that both hospital volume and adherence to quality metrics are associated with survival for ovarian cancer. Even though survival rates are improved at low-volume hospitals that are highly adherent to quality metrics, their survival rates are still lower than high-volume hospitals.
Read about the pros and cons of regionalization for high-risk ovarian cancer surgery.
Study limitations
The authors cite several limitations to this study:
- chosen quality metrics focused on care during initial treatment. Women with ovarian cancer are often treated for many years.
- the NCD lacks information on aspects like hospital infrastructure and staffing; there are probably other confounders that influence treatment and outcomes
- although NCD data have been validated, misclassification of a small number of patients may exist. Also, some hospitals did not treat patients who might be eligible for a quality metric and therefore were not included in this analysis.
- some study participants (13.7%) received treatment at multiple hospitals
- the volume cutpoints chosen by the research team were based on prior studies; there could be outcome variation within a volume strata.
Should high-risk surgeries be regionalized?
The association between higher surgical volume and improved outcomes has led to efforts to regionalize the care for high-risk operations to high-volume centers, say the authors. They conclude that this may be a reasonable strategy for some procedures. However, they suggest that regionalization presents practical difficulties:
- patients prefer to receive local care and are often unwilling to or cannot travel
- regionalization can worsen inequalities in access to care and may adversely affect low-volume hospitals
- high-volume centers do not exist in some areas of the country. A recent report suggested that 9% of the US female population had geographic barriers to receiving care from a gynecologic oncologist.4
Can low-volume facilities attain the same outcomes as high-volume centers?
The authors pose an important question: Can lower-volume facilities that deliver high-quality care achieve the same outcomes as higher-volume centers? With the difficulties associated with regionalization, many advocates seek strategies to raise the quality of care at low-volume centers, they say. The authors note that, “although outcomes improve at low-volume centers that are highly compliant with the quality metrics examined, survival at these centers is still lower than at high-volume centers.”3 The authors suggest that there are factors other than adherence to guidelines that play a role in how hospital volume affects ovarian cancer outcomes.3
Practice considerations
“Because the best outcomes appear to be achieved at high-volume hospitals, efforts to promote volume-based referral for women with ovarian cancer are reasonable,” the authors conclude.3 However, in practicality, many women will not be able to receive care at high-volume centers, they concede. “For low-volume centers, targeted quality improvement efforts and strict adherence to quality guidelines may help to optimize outcomes for women with ovarian cancer.”3
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The relationship between procedure volume and outcomes has long been recognized: studies have concluded that patients operated on by high-volume surgeons at high-volume hospitals have improved outcomes.1,2 This paradigm is also associated with ovarian cancer outcomes. But what affect does adherence to evidence-based guidelines have on these statistics?
Jason D. Wright, MD, and colleagues at the Columbia University College of Physicians and Surgeons, in New York City, sought to determine whether strict adherence to quality metrics by hospitals could explain the association between hospital volume and ovarian cancer survival.3
Details of the study
Using the National Cancer Database (NCD), the research team identified 100,725 patients at 1,268 hospitals who were treated for invasive epithelial ovarian cancer between 2004 and 2013. Hospitals were stratified by annual case volume into quintiles: low-volume (≤2 cases; n = 299 [23.6%]), low-intermediate–volume (2.01–5 cases; n = 465 [36.7%]), intermediate-volume (5.01–9 cases; n = 157 [12.4%]), high-intermediate–volume (9.01–19.9 cases; n = 194 [15.3%]), and high-volume (≥20 cases; n = 153 [12.1%]).3
To measure quality, the authors defined hospital-level rates of 5 metrics based on clinical guidelines3:
- lymph node dissection performed for patients with stage I–IIIB tumors
- performance of omentectomy or cytoreduction for patients with advanced stage tumors
- use of chemotherapy among patients with early-stage, high-risk tumors
- omission of chemotherapy for women with early-stage, low-risk tumors
- use of chemotherapy (either neoadjuvant or adjuvant) for women with advanced-stage disease.
For each metric, the authors determined the rate of hospital-level compliance for all study-eligible patients. Then a composite variable of overall quality was derived using all 5 metrics. Based on the overall quality metric, hospitals were stratified into quartiles: low-quality, medium-low–quality, medium-high–quality, and high-quality.3
Hospital-level adjusted 2- and 5-year survival rates were compared based on volume and adherence to quality metrics.3
Related article:
2017 Update on ovarian cancer
Trends and conclusions
Researchers found that compliance with quality metrics generally increased with hospital volume. Trends of increased compliance were observed with lymph node dissection for early-stage tumors, cytoreduction for advanced-stage tumors, and use of chemotherapy for advanced-stage tumors. No trends were evident for use of chemotherapy for high-risk, early-stage tumors. By contrast, a trend for higher-volume hospitals to administer chemotherapy for low-risk, early-stage tumors was discovered. Adherence with the composite overall quality metric was noted in 64.2% of low-volume centers and increased with each volume category to 82.2% at the highest-volume hospitals.3
Study results indicated that survival increased with increasing hospital volume and with adherence to the quality metrics. The association between volume and quality was then examined. For each volume category, survival increased with increasing adherence to the quality metrics. In the highest-volume group, 2-year adjusted survival rose from 75.5% (95% confidence interval [CI], 73.2%–77.8%) at the lowest-quality hospitals, to 78.6% (95% CI, 78.0%–79.1%) at the highest-quality hospitals. Similar trends were found for intermediate-volume hospitals and for 5-year survival. However, the relationship between adherence to quality metrics and survival was less consistent for the low-, low-intermediate–, and high-intermediate–volume hospitals.3
The authors concluded that both hospital volume and adherence to quality metrics are associated with survival for ovarian cancer. Even though survival rates are improved at low-volume hospitals that are highly adherent to quality metrics, their survival rates are still lower than high-volume hospitals.
Read about the pros and cons of regionalization for high-risk ovarian cancer surgery.
Study limitations
The authors cite several limitations to this study:
- chosen quality metrics focused on care during initial treatment. Women with ovarian cancer are often treated for many years.
- the NCD lacks information on aspects like hospital infrastructure and staffing; there are probably other confounders that influence treatment and outcomes
- although NCD data have been validated, misclassification of a small number of patients may exist. Also, some hospitals did not treat patients who might be eligible for a quality metric and therefore were not included in this analysis.
- some study participants (13.7%) received treatment at multiple hospitals
- the volume cutpoints chosen by the research team were based on prior studies; there could be outcome variation within a volume strata.
Should high-risk surgeries be regionalized?
The association between higher surgical volume and improved outcomes has led to efforts to regionalize the care for high-risk operations to high-volume centers, say the authors. They conclude that this may be a reasonable strategy for some procedures. However, they suggest that regionalization presents practical difficulties:
- patients prefer to receive local care and are often unwilling to or cannot travel
- regionalization can worsen inequalities in access to care and may adversely affect low-volume hospitals
- high-volume centers do not exist in some areas of the country. A recent report suggested that 9% of the US female population had geographic barriers to receiving care from a gynecologic oncologist.4
Can low-volume facilities attain the same outcomes as high-volume centers?
The authors pose an important question: Can lower-volume facilities that deliver high-quality care achieve the same outcomes as higher-volume centers? With the difficulties associated with regionalization, many advocates seek strategies to raise the quality of care at low-volume centers, they say. The authors note that, “although outcomes improve at low-volume centers that are highly compliant with the quality metrics examined, survival at these centers is still lower than at high-volume centers.”3 The authors suggest that there are factors other than adherence to guidelines that play a role in how hospital volume affects ovarian cancer outcomes.3
Practice considerations
“Because the best outcomes appear to be achieved at high-volume hospitals, efforts to promote volume-based referral for women with ovarian cancer are reasonable,” the authors conclude.3 However, in practicality, many women will not be able to receive care at high-volume centers, they concede. “For low-volume centers, targeted quality improvement efforts and strict adherence to quality guidelines may help to optimize outcomes for women with ovarian cancer.”3
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Birkmeyer JC, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Eng J Med. 2002;346(15):1128–1137.
- Birkmeyer JC, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. New Engl J Med. 2003;349(22):2117–2127.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130(3):545–553.
- Shalowitz DI, Vinograd AM, Giuntoli RL II. Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138(1):115–120.
- Birkmeyer JC, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Eng J Med. 2002;346(15):1128–1137.
- Birkmeyer JC, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. New Engl J Med. 2003;349(22):2117–2127.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130(3):545–553.
- Shalowitz DI, Vinograd AM, Giuntoli RL II. Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138(1):115–120.
FDA approves single-dose, oral bacterial vaginosis treatment
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
, thanks to its approval by the Food and Drug Administration.
Symbiomix Therapeutics announced Sept. 18 that the FDA had granted approval to secnidazole (Solosec) 2 g oral granules for the treatment of bacterial vaginosis in adult women. Clinical trials of the 5-nitroimidazole antibiotic have shown it to be effective in a single dose, offering the potential for greater patient adherence to treatment over the common regimen of twice-a-day dosing for 7 days.
In a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study of 215 women with bacterial vaginosis, the clinical cure rate was 65.3% for the 2-g secnidazole group, 49.3% for the 1-g secnidazole group, and 19.4% for the placebo group (Obstet Gynecol. 2017 Aug;130[2]:379-86).
Similarly, in a phase 3 double-blind, placebo-controlled study with 189 women, clinical cure rates based on the 2016 FDA guidance were 64.0% for single-dose secnidazole 2 g versus 26.4% for placebo (Am J Obstet Gynecol. 2017 Sep 1. doi: 10.1016/j.ajog.2017.08.017).
The most common adverse events in the trials were vulvovaginal candidiasis (9.6%), headache (3.6%), nausea (3.6%), dysgeusia (3.4%), vomiting (2.5%), diarrhea (2.5%), abdominal pain (2.0%), and vulvovaginal pruritus (2.0%).
The FDA designated the drug as a qualified infectious disease product and granted it fast-track designation, making it eligible for priority review and at least 10 years of market exclusivity.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Know the best specific signs for polycystic ovary syndrome
SAN FRANCISCO – Dermatologists are often on the frontline when it comes to diagnosing polycystic ovary syndrome (PCOS), which is one reason they should be up to date and aware of the changing diagnostic criteria for the condition, according to Kanade Shinkai, MD.
About one-quarter of patients who are diagnosed with PCOS are seen first by a dermatologist. That’s because skin conditions may be more concerning than reproductive issues in young women.
“Sometimes, people don’t see [irregular menstruation] as a problem,” explained Dr. Shinkai of the department of dermatology at the University of California, San Francisco. “Maybe they’re young, or they’re not trying to get pregnant. But if their hair is falling out, they see that as a problem, or if they have bad acne, or they’re becoming hirsute, they see that as a problem. So, they present to a dermatologist.”
Early recognition of PCOS is important, because many women with the condition go on to develop diabetes, impaired glucose intolerance, hyperlipidemia, hypertension, fertility problems, and obesity.
It used to be that physicians expected patients with PCOS to have menstrual irregularities, biochemical or clinical evidence of hyperandrogenism, and evidence of polycystic ovaries on ultrasound. But just two of the three are now considered enough to warrant a diagnosis.
“Our original view of the classic patient has gone away, and it’s really a heterogeneous phenotype,” Dr. Shinkai said. “Originally, it was all three [criteria], and the patient was obese, and they all had diabetes. Now, we know that’s not true. Every woman who has PCOS has her own version of PCOS.”
Dr. Shinkai’s team conducted a study of clinical markers associated with PCOS and found that some of the classic signs of PCOS may be unreliable.
“Alopecia turns out not to be a very reliable marker,” she explained. “That’s paradigm shifting, I think, because often if patients present with hair loss in a hormonal pattern, they get worked up for PCOS, and it turns out that workup is not always fruitful.” Acne can also be misleading, given its frequency in the general population.
More reliable signs include hirsutism and acanthosis nigricans; 70%-80% of women with hirsutism have PCOS, and 53% of patients with PCOS have hirsutism, most commonly on the trunk. Acanthosis nigricans occurs in 37% of PCOS patients.
“Those are the best specific signs for PCOS,” said Dr. Shinkai. “If we see those, we should probably work the patient up.”
In preparation, the patient should be off of birth control treatment for at least 4 weeks, because hormonal treatment can interfere with test results, Dr Shinkai noted.
She also recommended a transvaginal ultrasound and a free-testosterone test. Consensus statements recommend testing of 17-hydroxyprogesterone, but Dr. Shinkai said she isn’t so sure. “That’s only going to capture about 3% of your patients with cutaneous hyperandrogenism, so it’s pretty low yield,” she said.
For treatment of cutaneous symptoms of PCOS, it’s important for the patient to understand that treatment courses will last at least 6 months. “It’s not a quick fix,” said Dr. Shinkai. Oral contraceptives are a mainstay, and are often sufficient for mild hirsutism. But moderate or severe cases call for high doses of spironolactone (150-200 mg/day). She said she usually combines spironolactone with oral contraceptives, because the drug can lead to menstrual irregularities, which birth control pills can relieve.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – Dermatologists are often on the frontline when it comes to diagnosing polycystic ovary syndrome (PCOS), which is one reason they should be up to date and aware of the changing diagnostic criteria for the condition, according to Kanade Shinkai, MD.
About one-quarter of patients who are diagnosed with PCOS are seen first by a dermatologist. That’s because skin conditions may be more concerning than reproductive issues in young women.
“Sometimes, people don’t see [irregular menstruation] as a problem,” explained Dr. Shinkai of the department of dermatology at the University of California, San Francisco. “Maybe they’re young, or they’re not trying to get pregnant. But if their hair is falling out, they see that as a problem, or if they have bad acne, or they’re becoming hirsute, they see that as a problem. So, they present to a dermatologist.”
Early recognition of PCOS is important, because many women with the condition go on to develop diabetes, impaired glucose intolerance, hyperlipidemia, hypertension, fertility problems, and obesity.
It used to be that physicians expected patients with PCOS to have menstrual irregularities, biochemical or clinical evidence of hyperandrogenism, and evidence of polycystic ovaries on ultrasound. But just two of the three are now considered enough to warrant a diagnosis.
“Our original view of the classic patient has gone away, and it’s really a heterogeneous phenotype,” Dr. Shinkai said. “Originally, it was all three [criteria], and the patient was obese, and they all had diabetes. Now, we know that’s not true. Every woman who has PCOS has her own version of PCOS.”
Dr. Shinkai’s team conducted a study of clinical markers associated with PCOS and found that some of the classic signs of PCOS may be unreliable.
“Alopecia turns out not to be a very reliable marker,” she explained. “That’s paradigm shifting, I think, because often if patients present with hair loss in a hormonal pattern, they get worked up for PCOS, and it turns out that workup is not always fruitful.” Acne can also be misleading, given its frequency in the general population.
More reliable signs include hirsutism and acanthosis nigricans; 70%-80% of women with hirsutism have PCOS, and 53% of patients with PCOS have hirsutism, most commonly on the trunk. Acanthosis nigricans occurs in 37% of PCOS patients.
“Those are the best specific signs for PCOS,” said Dr. Shinkai. “If we see those, we should probably work the patient up.”
In preparation, the patient should be off of birth control treatment for at least 4 weeks, because hormonal treatment can interfere with test results, Dr Shinkai noted.
She also recommended a transvaginal ultrasound and a free-testosterone test. Consensus statements recommend testing of 17-hydroxyprogesterone, but Dr. Shinkai said she isn’t so sure. “That’s only going to capture about 3% of your patients with cutaneous hyperandrogenism, so it’s pretty low yield,” she said.
For treatment of cutaneous symptoms of PCOS, it’s important for the patient to understand that treatment courses will last at least 6 months. “It’s not a quick fix,” said Dr. Shinkai. Oral contraceptives are a mainstay, and are often sufficient for mild hirsutism. But moderate or severe cases call for high doses of spironolactone (150-200 mg/day). She said she usually combines spironolactone with oral contraceptives, because the drug can lead to menstrual irregularities, which birth control pills can relieve.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – Dermatologists are often on the frontline when it comes to diagnosing polycystic ovary syndrome (PCOS), which is one reason they should be up to date and aware of the changing diagnostic criteria for the condition, according to Kanade Shinkai, MD.
About one-quarter of patients who are diagnosed with PCOS are seen first by a dermatologist. That’s because skin conditions may be more concerning than reproductive issues in young women.
“Sometimes, people don’t see [irregular menstruation] as a problem,” explained Dr. Shinkai of the department of dermatology at the University of California, San Francisco. “Maybe they’re young, or they’re not trying to get pregnant. But if their hair is falling out, they see that as a problem, or if they have bad acne, or they’re becoming hirsute, they see that as a problem. So, they present to a dermatologist.”
Early recognition of PCOS is important, because many women with the condition go on to develop diabetes, impaired glucose intolerance, hyperlipidemia, hypertension, fertility problems, and obesity.
It used to be that physicians expected patients with PCOS to have menstrual irregularities, biochemical or clinical evidence of hyperandrogenism, and evidence of polycystic ovaries on ultrasound. But just two of the three are now considered enough to warrant a diagnosis.
“Our original view of the classic patient has gone away, and it’s really a heterogeneous phenotype,” Dr. Shinkai said. “Originally, it was all three [criteria], and the patient was obese, and they all had diabetes. Now, we know that’s not true. Every woman who has PCOS has her own version of PCOS.”
Dr. Shinkai’s team conducted a study of clinical markers associated with PCOS and found that some of the classic signs of PCOS may be unreliable.
“Alopecia turns out not to be a very reliable marker,” she explained. “That’s paradigm shifting, I think, because often if patients present with hair loss in a hormonal pattern, they get worked up for PCOS, and it turns out that workup is not always fruitful.” Acne can also be misleading, given its frequency in the general population.
More reliable signs include hirsutism and acanthosis nigricans; 70%-80% of women with hirsutism have PCOS, and 53% of patients with PCOS have hirsutism, most commonly on the trunk. Acanthosis nigricans occurs in 37% of PCOS patients.
“Those are the best specific signs for PCOS,” said Dr. Shinkai. “If we see those, we should probably work the patient up.”
In preparation, the patient should be off of birth control treatment for at least 4 weeks, because hormonal treatment can interfere with test results, Dr Shinkai noted.
She also recommended a transvaginal ultrasound and a free-testosterone test. Consensus statements recommend testing of 17-hydroxyprogesterone, but Dr. Shinkai said she isn’t so sure. “That’s only going to capture about 3% of your patients with cutaneous hyperandrogenism, so it’s pretty low yield,” she said.
For treatment of cutaneous symptoms of PCOS, it’s important for the patient to understand that treatment courses will last at least 6 months. “It’s not a quick fix,” said Dr. Shinkai. Oral contraceptives are a mainstay, and are often sufficient for mild hirsutism. But moderate or severe cases call for high doses of spironolactone (150-200 mg/day). She said she usually combines spironolactone with oral contraceptives, because the drug can lead to menstrual irregularities, which birth control pills can relieve.
Dr. Shinkai reported having no relevant financial disclosures.
AT PDA 2017
WHI hormone trials offer reassurance on long-term mortality risk
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
The findings from the Women’s Health Initiative hormone therapy trials as reported by Dr. Manson and her colleagues expand the understanding of the long-term risks and benefits of hormone therapy, which is an important issue for women around the world, Melissa A. McNeil, MD, wrote in an editorial.
The information will be helpful for counseling women considering whether to start hormone therapy, and “hopefully will alleviate concerns that many patients and physicians have about the initiation of hormone therapy,” she said, explaining that the effect of hormone therapy on cancer mortality, and especially on breast cancer mortality, has generated concern and reluctance to prescribe and take hormone therapy for troubling menopausal symptoms.
“The current report ... provides substantial reassurance for patients and physicians about this issue,” she said. “For women with troubling vasomotor symptoms, premature menopause, or early-onset osteoporosis, hormone therapy appears to be both safe and efficacious.”
While several questions remain, including about optimal duration of therapy, whether there is a difference in mortality by age and menopausal status at hormone therapy initiation, and if earlier initiation would provide additional benefits, the data “fully support the newly released 2017 hormone therapy position statement of the North American Menopause Society and are a welcome addition to current knowledge regarding hormone therapy administration,” she wrote.
Dr. McNeil is with the University of Pittsburgh. She reported having no financial disclosures. Her comments come from an accompanying editorial (JAMA. 2017 Sep;318[10]:911-13).
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
Postmenopausal women treated with conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) for a median of 5.6 years, or with CEE alone for a median of 7.2 years had no increased risk of all-cause, cardiovascular, or cancer mortality over a cumulative follow-up of 18 years, according to the latest report from the Women’s Health Initiative hormone therapy trials.
All-cause mortality among the 27,347 participants in the two randomized, double-blind, placebo-controlled trials was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99), JoAnn E. Manson, MD, Harvard Medical School, Boston, and her colleagues reported.
For those who received CEE plus MPA, the hazard ratio was 1.02, while for CEE alone the hazard ratio was 0.94 (JAMA. 2017 Sep;318[10]:927-38).
Cardiovascular mortality among the pooled cohort was 8.9% with hormone therapy and 9.0% with placebo (HR, 1.00), and total cancer mortality was 8.2% and 8.0%, respectively (HR, 1.03). Mortality from other causes was 10.0% with hormone therapy, compared with 10.7% with placebo (HR, 0.95). The results did not differ significantly between the trials, the investigators noted.
An analysis by age showed that women aged 50-59 years tended to have lower hazard ratios for mortality from cardiovascular disease, cancer, and other causes during the intervention phases of the trials, but only the difference for “other” causes in the CEE-alone trial showed a statistically significant trend with age. This was influenced in part by adverse effects of the treatment in women aged 70-79 years. During cumulative follow-up, trends related to mortality across age groups were not statistically significantly different.
The trials included women aged 50-79 years who were enrolled between 1993 and 1998 and followed through 2014. Given the hormone-therapy-related risks identified in the CEE plus MPA and CEE-alone trials – which were stopped early because of increased risk of breast cancer/overall risks exceeding benefits, and for increased stroke risk, respectively – the absence of an increase in all-cause mortality during the intervention and cumulative follow-up phases of the trials is noteworthy, the investigators wrote.
“Although these findings lend support to practice guidelines endorsing use of hormone therapy for recently menopausal women with moderate to severe symptoms, in the absence of contraindications, the attenuation of age differences with longer follow-up and potential health risks of treatment would not support use of hormone therapy for reducing chronic disease or mortality,” they wrote. “Moreover, it is unclear whether benefits would outweigh risks with longer duration of treatment.”
They added that “in clinical decision making, these considerations must be weighed against the evidence linking untreated vasomotor symptoms in midlife women to impaired health and quality of life, disrupted sleep, reduced work productivity, and increased health care expenditures.”
The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no financial disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
FROM JAMA
Key clinical point:
Major finding: All-cause mortality was 27.1% in the hormone therapy group versus 27.6% in the placebo group (hazard ratio, 0.99).
Data source: The randomized, double-blind, placebo-controlled Women’s Health Initiative hormone therapy trials of 27,347 women.
Disclosures: The Women’s Health Initiative is funded by the U.S. Department of Health & Human Services. Study drugs were donated by Wyeth Ayerst. Dr. Manson reported having no disclosures. Several of her coauthors reported receiving grants and research funding from the National Institutes of Health, and receiving personal fees, speaking fees, and honoraria from various pharmaceutical companies.
USPSTF backs away from cotesting in cervical cancer screening
Women aged 30-65 years should be offered a choice between two cervical cancer screening methods, according to draft recommendations from the U.S. Preventive Services Task Force. The recommendations were released on Sept. 12.
The Task Force continues to recommend that women in their 20s be screened every 3 years via cervical cytology, but in a change from the 2012 recommendations, the researchers now advise clinicians to offer women aged 30-65 years a choice of either cytology every 3 years or the high-risk human papillomavirus (hrHPV) test every 5 years as a method of screening for cervical cancer. Cotesting is no longer recommended.
Offering women aged 30-65 years a screening choice received an A recommendation. The draft retains the previous Task Force position and D recommendation against cervical cancer screening for certain groups, including women younger than 21 years, women aged 65 and older with a history of screening and a low risk of cervical cancer, and women who have had a hysterectomy.
The USPSTF based the draft recommendations in part on a review of four randomized, controlled trials of cotesting hrHPV and cytology that included more than 130,000 women.
“Modeling found that cotesting does not offer any benefit in terms of cancer reduction or life-years gained over hrHPV testing alone but increases the number of tests and procedures per each cancer case averted,” the Task Force members noted in the draft recommendation statement. “Therefore, the USPSTF concluded that there is convincing evidence that screening with either cytology alone or hrHPV testing alone provides substantial benefit and is preferable to cotesting” in otherwise healthy women aged 30-65 years.
The American College of Obstetricians and Gynecologists currently recommends cotesting with cytology and HPV testing every 5 years or cytology alone every 3 years in women aged 30-65 years (Obstet Gynecol. 2016;128[4]:e111-30).
The USPSTF draft recommendations do not apply to women at increased risk for cervical cancer, including those with compromised immune systems or those who have cervical intraepithelial neoplasia grade 2 or 3.
The draft recommendations are available online for public comment from Sept. 12 through Oct. 9, 2017, at the USPSTF website, www.uspreventiveservicestaskforce.org.
Women aged 30-65 years should be offered a choice between two cervical cancer screening methods, according to draft recommendations from the U.S. Preventive Services Task Force. The recommendations were released on Sept. 12.
The Task Force continues to recommend that women in their 20s be screened every 3 years via cervical cytology, but in a change from the 2012 recommendations, the researchers now advise clinicians to offer women aged 30-65 years a choice of either cytology every 3 years or the high-risk human papillomavirus (hrHPV) test every 5 years as a method of screening for cervical cancer. Cotesting is no longer recommended.
Offering women aged 30-65 years a screening choice received an A recommendation. The draft retains the previous Task Force position and D recommendation against cervical cancer screening for certain groups, including women younger than 21 years, women aged 65 and older with a history of screening and a low risk of cervical cancer, and women who have had a hysterectomy.
The USPSTF based the draft recommendations in part on a review of four randomized, controlled trials of cotesting hrHPV and cytology that included more than 130,000 women.
“Modeling found that cotesting does not offer any benefit in terms of cancer reduction or life-years gained over hrHPV testing alone but increases the number of tests and procedures per each cancer case averted,” the Task Force members noted in the draft recommendation statement. “Therefore, the USPSTF concluded that there is convincing evidence that screening with either cytology alone or hrHPV testing alone provides substantial benefit and is preferable to cotesting” in otherwise healthy women aged 30-65 years.
The American College of Obstetricians and Gynecologists currently recommends cotesting with cytology and HPV testing every 5 years or cytology alone every 3 years in women aged 30-65 years (Obstet Gynecol. 2016;128[4]:e111-30).
The USPSTF draft recommendations do not apply to women at increased risk for cervical cancer, including those with compromised immune systems or those who have cervical intraepithelial neoplasia grade 2 or 3.
The draft recommendations are available online for public comment from Sept. 12 through Oct. 9, 2017, at the USPSTF website, www.uspreventiveservicestaskforce.org.
Women aged 30-65 years should be offered a choice between two cervical cancer screening methods, according to draft recommendations from the U.S. Preventive Services Task Force. The recommendations were released on Sept. 12.
The Task Force continues to recommend that women in their 20s be screened every 3 years via cervical cytology, but in a change from the 2012 recommendations, the researchers now advise clinicians to offer women aged 30-65 years a choice of either cytology every 3 years or the high-risk human papillomavirus (hrHPV) test every 5 years as a method of screening for cervical cancer. Cotesting is no longer recommended.
Offering women aged 30-65 years a screening choice received an A recommendation. The draft retains the previous Task Force position and D recommendation against cervical cancer screening for certain groups, including women younger than 21 years, women aged 65 and older with a history of screening and a low risk of cervical cancer, and women who have had a hysterectomy.
The USPSTF based the draft recommendations in part on a review of four randomized, controlled trials of cotesting hrHPV and cytology that included more than 130,000 women.
“Modeling found that cotesting does not offer any benefit in terms of cancer reduction or life-years gained over hrHPV testing alone but increases the number of tests and procedures per each cancer case averted,” the Task Force members noted in the draft recommendation statement. “Therefore, the USPSTF concluded that there is convincing evidence that screening with either cytology alone or hrHPV testing alone provides substantial benefit and is preferable to cotesting” in otherwise healthy women aged 30-65 years.
The American College of Obstetricians and Gynecologists currently recommends cotesting with cytology and HPV testing every 5 years or cytology alone every 3 years in women aged 30-65 years (Obstet Gynecol. 2016;128[4]:e111-30).
The USPSTF draft recommendations do not apply to women at increased risk for cervical cancer, including those with compromised immune systems or those who have cervical intraepithelial neoplasia grade 2 or 3.
The draft recommendations are available online for public comment from Sept. 12 through Oct. 9, 2017, at the USPSTF website, www.uspreventiveservicestaskforce.org.
Radiofrequency devices appear to reduce vaginal laxity
SAN DIEGO – The first randomized, sham-controlled study of a radiofrequency energy-based device for vaginal laxity showed a significant and sustained effect, and likely raises the bar on vaginal rejuvenation options, Suzanne L. Kilmer, MD, said during a presentation at the annual Masters of Aesthetics Symposium.
“There are a lot of women who have vaginal laxity, vaginal atrophy, and other issues, and this is something that seems to help,” she said. No devices have yet received female rejuvenation/vaginal function indications from the Food and Drug Administration.
Current in-office procedures involve the delivery of radiofrequency (RF) energy, which appears to stimulate collagen production, and the use of fractionated lasers to target the epithelium. “RF devices tend to be easier to use,” said Dr. Kilmer, director of the Laser and Skin Surgery of Northern California, Sacramento. “They’re smaller devices, do not require as much laser training, and they tend to be less expensive. There’s no plume or odor with any of the RF devices.”
She discussed the results of the Viveve Treatment of the Vaginal Introitus to Evaluate Effectiveness (Viveve I) study, conducted at nine sites in four countries, which examined the Viveve monopolar RF device in 155 premenopausal women. The study subjects were randomized to one of two groups: the treatment group received 90 J/cm2 for five passes and the sham group received 1 J/cm2 for five passes (J Sex Med 2017;14[2]:215-25).
The researchers used the Vaginal Laxity Questionnaire (VSQ), which grades vaginal tone on a 7-point scale that ranges from very loose (0) to very tight (7), and the Female Sexual Function Index (FSFI) to collect patient-reported outcomes at one, three, and six months. Subjects had to have a VSQ score of 3 or less to participate in the study.
At 6 months, patients in the treatment group were more than 3 times as likely to have no vaginal laxity, compared with their counterparts in the sham group (P less than or equal to 0.006). In addition, more than half of patients in the treatment group moved at least 2 points on the VSQ scale toward a “tighter” vagina.
“Even a one-point increase in tightness will be significant for women,” said Dr. Kilmer, who has used the Viveve device in her practice but was not part of this study.
Based on responses to the FSFI questionnaire, the researchers observed a significant and sustained improvement in sexual function after a single treatment among patients in the treatment group, compared with those in the sham group. The placebo effect didn’t rise above “dysfunctional” at six months, and rate of treatment emergent adverse events was similar between the treatment and sham groups (11.1% vs. 12.3%, respectively), she said.
The researchers also reported the following patient tolerability variables: warmth during the procedure (96% in the treatment group vs. 19% in the sham group, respectively); cool sensation during the procedure (42% vs. 75%), and stopped procedure due to discomfort (1% in each group).
Dr. Kilmer explained that RF heating of the skin and mucosa provides immediate contraction of collagen, long-term stimulation of new collagen production, as well as increased blood flow and restoration of nerve signaling, which results in normal vaginal lubrication. “The critical RF temperature is in the 35 to 47 degree range,” she said. “Very few people can tolerate above 42 degrees.”
Similarly, good results were noted in a pilot study of the ThermiVa radiofrequency product manufactured by ThermoGen in 23 patients who underwent three treatments one month apart (Int J Laser Aesthet Med. July 2015:16-21). All patients experienced significant change with about a 50% reduction in symptoms.
“Patients are very happy with this treatment,” said Dr. Kilmer, who is also a professor of dermatology at the University of California, Davis Medical Center. “It may not be the absolute home run, but I think it’s very safe ... Most people say it lasts about six months then they’ll start to see some of their symptoms coming back.”
Dr. Kilmer reported that she is a member of the medical advisory board for Allergan, Cytrellis, Lumenis, Merz, Miramar, Sienna Labs, Syneron/Candela, Zarin, Zeltiq, and Zift. She has also received research support from those companies as well as from Cutera, Cynosure, Lutronic, R2 Derm, Solta/Valeant, and Ulthera.
dbrunk@frontlinemedcom.com
SAN DIEGO – The first randomized, sham-controlled study of a radiofrequency energy-based device for vaginal laxity showed a significant and sustained effect, and likely raises the bar on vaginal rejuvenation options, Suzanne L. Kilmer, MD, said during a presentation at the annual Masters of Aesthetics Symposium.
“There are a lot of women who have vaginal laxity, vaginal atrophy, and other issues, and this is something that seems to help,” she said. No devices have yet received female rejuvenation/vaginal function indications from the Food and Drug Administration.
Current in-office procedures involve the delivery of radiofrequency (RF) energy, which appears to stimulate collagen production, and the use of fractionated lasers to target the epithelium. “RF devices tend to be easier to use,” said Dr. Kilmer, director of the Laser and Skin Surgery of Northern California, Sacramento. “They’re smaller devices, do not require as much laser training, and they tend to be less expensive. There’s no plume or odor with any of the RF devices.”
She discussed the results of the Viveve Treatment of the Vaginal Introitus to Evaluate Effectiveness (Viveve I) study, conducted at nine sites in four countries, which examined the Viveve monopolar RF device in 155 premenopausal women. The study subjects were randomized to one of two groups: the treatment group received 90 J/cm2 for five passes and the sham group received 1 J/cm2 for five passes (J Sex Med 2017;14[2]:215-25).
The researchers used the Vaginal Laxity Questionnaire (VSQ), which grades vaginal tone on a 7-point scale that ranges from very loose (0) to very tight (7), and the Female Sexual Function Index (FSFI) to collect patient-reported outcomes at one, three, and six months. Subjects had to have a VSQ score of 3 or less to participate in the study.
At 6 months, patients in the treatment group were more than 3 times as likely to have no vaginal laxity, compared with their counterparts in the sham group (P less than or equal to 0.006). In addition, more than half of patients in the treatment group moved at least 2 points on the VSQ scale toward a “tighter” vagina.
“Even a one-point increase in tightness will be significant for women,” said Dr. Kilmer, who has used the Viveve device in her practice but was not part of this study.
Based on responses to the FSFI questionnaire, the researchers observed a significant and sustained improvement in sexual function after a single treatment among patients in the treatment group, compared with those in the sham group. The placebo effect didn’t rise above “dysfunctional” at six months, and rate of treatment emergent adverse events was similar between the treatment and sham groups (11.1% vs. 12.3%, respectively), she said.
The researchers also reported the following patient tolerability variables: warmth during the procedure (96% in the treatment group vs. 19% in the sham group, respectively); cool sensation during the procedure (42% vs. 75%), and stopped procedure due to discomfort (1% in each group).
Dr. Kilmer explained that RF heating of the skin and mucosa provides immediate contraction of collagen, long-term stimulation of new collagen production, as well as increased blood flow and restoration of nerve signaling, which results in normal vaginal lubrication. “The critical RF temperature is in the 35 to 47 degree range,” she said. “Very few people can tolerate above 42 degrees.”
Similarly, good results were noted in a pilot study of the ThermiVa radiofrequency product manufactured by ThermoGen in 23 patients who underwent three treatments one month apart (Int J Laser Aesthet Med. July 2015:16-21). All patients experienced significant change with about a 50% reduction in symptoms.
“Patients are very happy with this treatment,” said Dr. Kilmer, who is also a professor of dermatology at the University of California, Davis Medical Center. “It may not be the absolute home run, but I think it’s very safe ... Most people say it lasts about six months then they’ll start to see some of their symptoms coming back.”
Dr. Kilmer reported that she is a member of the medical advisory board for Allergan, Cytrellis, Lumenis, Merz, Miramar, Sienna Labs, Syneron/Candela, Zarin, Zeltiq, and Zift. She has also received research support from those companies as well as from Cutera, Cynosure, Lutronic, R2 Derm, Solta/Valeant, and Ulthera.
dbrunk@frontlinemedcom.com
SAN DIEGO – The first randomized, sham-controlled study of a radiofrequency energy-based device for vaginal laxity showed a significant and sustained effect, and likely raises the bar on vaginal rejuvenation options, Suzanne L. Kilmer, MD, said during a presentation at the annual Masters of Aesthetics Symposium.
“There are a lot of women who have vaginal laxity, vaginal atrophy, and other issues, and this is something that seems to help,” she said. No devices have yet received female rejuvenation/vaginal function indications from the Food and Drug Administration.
Current in-office procedures involve the delivery of radiofrequency (RF) energy, which appears to stimulate collagen production, and the use of fractionated lasers to target the epithelium. “RF devices tend to be easier to use,” said Dr. Kilmer, director of the Laser and Skin Surgery of Northern California, Sacramento. “They’re smaller devices, do not require as much laser training, and they tend to be less expensive. There’s no plume or odor with any of the RF devices.”
She discussed the results of the Viveve Treatment of the Vaginal Introitus to Evaluate Effectiveness (Viveve I) study, conducted at nine sites in four countries, which examined the Viveve monopolar RF device in 155 premenopausal women. The study subjects were randomized to one of two groups: the treatment group received 90 J/cm2 for five passes and the sham group received 1 J/cm2 for five passes (J Sex Med 2017;14[2]:215-25).
The researchers used the Vaginal Laxity Questionnaire (VSQ), which grades vaginal tone on a 7-point scale that ranges from very loose (0) to very tight (7), and the Female Sexual Function Index (FSFI) to collect patient-reported outcomes at one, three, and six months. Subjects had to have a VSQ score of 3 or less to participate in the study.
At 6 months, patients in the treatment group were more than 3 times as likely to have no vaginal laxity, compared with their counterparts in the sham group (P less than or equal to 0.006). In addition, more than half of patients in the treatment group moved at least 2 points on the VSQ scale toward a “tighter” vagina.
“Even a one-point increase in tightness will be significant for women,” said Dr. Kilmer, who has used the Viveve device in her practice but was not part of this study.
Based on responses to the FSFI questionnaire, the researchers observed a significant and sustained improvement in sexual function after a single treatment among patients in the treatment group, compared with those in the sham group. The placebo effect didn’t rise above “dysfunctional” at six months, and rate of treatment emergent adverse events was similar between the treatment and sham groups (11.1% vs. 12.3%, respectively), she said.
The researchers also reported the following patient tolerability variables: warmth during the procedure (96% in the treatment group vs. 19% in the sham group, respectively); cool sensation during the procedure (42% vs. 75%), and stopped procedure due to discomfort (1% in each group).
Dr. Kilmer explained that RF heating of the skin and mucosa provides immediate contraction of collagen, long-term stimulation of new collagen production, as well as increased blood flow and restoration of nerve signaling, which results in normal vaginal lubrication. “The critical RF temperature is in the 35 to 47 degree range,” she said. “Very few people can tolerate above 42 degrees.”
Similarly, good results were noted in a pilot study of the ThermiVa radiofrequency product manufactured by ThermoGen in 23 patients who underwent three treatments one month apart (Int J Laser Aesthet Med. July 2015:16-21). All patients experienced significant change with about a 50% reduction in symptoms.
“Patients are very happy with this treatment,” said Dr. Kilmer, who is also a professor of dermatology at the University of California, Davis Medical Center. “It may not be the absolute home run, but I think it’s very safe ... Most people say it lasts about six months then they’ll start to see some of their symptoms coming back.”
Dr. Kilmer reported that she is a member of the medical advisory board for Allergan, Cytrellis, Lumenis, Merz, Miramar, Sienna Labs, Syneron/Candela, Zarin, Zeltiq, and Zift. She has also received research support from those companies as well as from Cutera, Cynosure, Lutronic, R2 Derm, Solta/Valeant, and Ulthera.
dbrunk@frontlinemedcom.com
REPORTING FROM MOAS 2017