User login
Current Concepts in Labral Repair and Refixation: Anatomical Approach to Labral Management
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
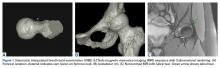
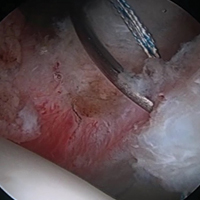
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
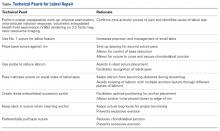
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
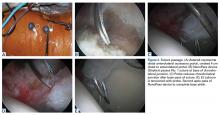
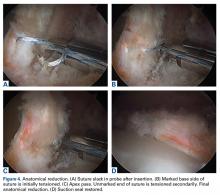
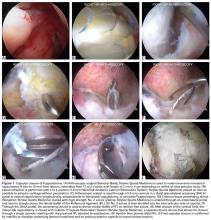
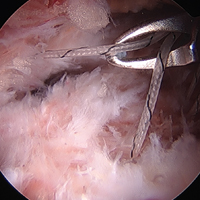
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
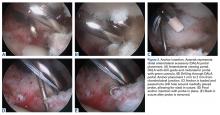
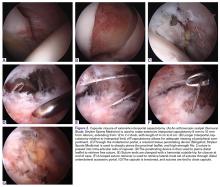
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Labral preservation is recommended when possible to ensure restoration of suction seal, stability, and contact pressure of the hip joint.
- Over 95% of labral tears can be addressed with primary repair.
- Consider using an accessory portal (ie, DALA) to allow for more anatomic placement of suture anchor.
- Mattress stitch when labrum >3 mm and looped stitch when labrum <3 mm.
- 10Control labral repair to avoid excessive inversion or eversion.
Arthroscopic labral repair and refixation have garnered much attention over the past several years. Restoration of suction seal and native labral function has been an evolving focus for achieving excellent results in hip preservation surgery.1-6 Given the superior results of labral repair, including level I evidence, repair or refixation should be pursued whenever possible.7 Authors have reported using several labral management techniques: débridement, labralization, looped suture fixation, base stitch fixation, inversion-eversion, and reconstruction.7-13 The optimal technique is yet to be determined. When possible, steps should be taken to repair the labrum to an anatomical position. Absolute indications for labral repair are a confirmed intra-articular diagnosis with symptomatic pain, joint space >2 mm with or without femoroacetabular impingement (FAI), labral tear or instability, and failed conservative management.9,11,12,14,15 More important, the surgeon must have a clear etiology of the pathologic cause of the tear and be aware of the limitations of the procedure. Labral repair is relatively contraindicated in end-stage arthritis and has failed when used alone in undiagnosed dysplasia or hip instability.16 In this article, we discuss indications for labral repair; describe Dr. Mather’s preoperative planning, labral repair technique, and postoperative care; and review published outcomes and future trends in labral repair.
Indications
At our institution, anatomical labral repair is the preferred procedure for most primary and revision hip arthroscopy procedures. We aim to restore the suction seal, re-create the contact of the labrum and the femoral head to facilitate proprioception, and restore normal stability of the labrum. Indications for primary repair are labrum width >3 mm, no more than 2 repairs, and ability to hold a suture. Our indications for reconstruction or débridement are stage 3 irreparable labral tear, calcified/cystic labrum, and multiple failed labral repairs or reconstructions. The decision to perform labral débridement or reconstruction is made on a case-by-case basis but is primarily influenced by the stability of the hip joint and the activity goals of the patient. If preoperative presentation and intraoperative examination suggest labral instability as a major component of the pathology, or if the patient wants to return to high-demand activity, we more strongly favor reconstruction over débridement. In our experience, with the technique described in this article, more than 95% of all primary labral tears can be addressed with repair.
Preoperative Planning
The goals in hip preservation surgery are to identify and address the underlying cause of the labral tear, whether it be FAI syndrome, trauma, labral instability, or all 3, and to re-create the anatomy and biomechanics of the acetabular labrum. For repair, we prefer an inversion-eversion technique with independent control of the labrum. Our initial work-up includes a thorough history and physical examination with baseline patient-reported outcome scores. Standard erect anteroposterior pelvis, Dunn lateral, and false-profile radiographs are obtained. Standard measurements of lateral center edge angle, anterior center edge angle, Tönnis angle, Tönnis grade, lateral joint space, and head extrusion indices are evaluated. Selective in-office ultrasound-guided injections are used to confirm an intra-articular source of pain. At our institution, noncontrast 3.0 Tesla magnetic resonance imaging (MRI) with volumetric interpolated breath-hold examination (VIBE) sequencing and 3-dimensional rendering is obtained for evaluation of labral and FAI morphology.17 All advanced imaging is performed without arthrogram or radiation exposure (Figures 1A-1C).
With use of the radiographs and the MRI scans, we engage the patient in an informed discussion about the labral tear, FAI, and concomitant pathology. We discuss expected outcomes of conservative or operative management given the patient’s expected functional activities, and inform the patient that primary repair is indicated for many others in similar situations. The potential for possible labral reconstruction is discussed if the patient had prior intra-articular hip surgery, has a large calcified labrum or a cystic labrum, is an athlete with failed prior surgery, or is younger than 40 years.
Labral Repair Technique
The patient is taken to the surgical suite, and a general anesthetic is administered. A peripheral nerve block is not routinely used. The patient’s feet are padded, and boots for the traction table are applied. The patient is carefully placed on a Hana table in modified supine position. Balanced traction is used to achieve proper joint distraction. The C-arm is used to verify proper distraction, assess hip stability, and achieve standard anterolateral (AL) portal placement. A midanterior portal (MAP) is created and an interportal capsulotomy is performed. Capsular suspension is performed with the InJector II Capsule Restoration System (Stryker Sports Medicine) and typically 4 or 5 high-strength No. 2 sutures (Zipline; Stryker Sports Medicine).19 Diagnostic arthroscopy is performed to identify the tear type, measure the labral width, determine the impingement area, and identify the intra-articular pathology. After the intra-articular pathology is addressed, a radiofrequency Ambient HIPVAC 50 Coblation Wand (Smith & Nephew) is used to expose the acetabular rim and subspine as indicated. Acetabuloplasty or subspine decompression is performed, and then a primary repair or refixation of the labrum is performed. We do not routinely detach the labrum for acetabular rim trimming. A crucial step here is to expose a bleeding surface to which the labrum can be repaired. If the rim is sclerotic, or the rim cannot be removed because of underlying low acetabular coverage, we prefer to obtain the bleeding surface with a microdrilling device (Stryker) that is routinely used for acetabular microfracture.
Labrum quality is used to determine which repair method to use. A hypertrophic labrum is debulked. The acetabular rim is seldom resected >3 mm, but, when it is, the newly exposed cartilage is removed. We have found that >3 mm of residual cartilage prevents refixation of the labrum directly to the bone and may interfere with anatomical positioning. When a labrum is <3 mm in width or will not hold a base technique, repair stability is the priority, and a looped method is used. A knotless anchor with No. 1 permanent suture designed for hip labral repair (CinchLock; Stryker) is our first-line anchor choice. A distal anterolateral accessory (DALA) portal is created with an outside-in technique, and anchors are drilled through this portal into zones 2 to 4 (Figures 2A-2E).
A 2.4-mm drill guide is advanced through the DALA portal and placed in the appropriate position for drilling. We aim for 1 mm to 2 mm from the chondrolabral junction. Next, the probe is placed intra-articular and medial to the anchor insertion site, and the anchor is loaded and then inserted around the probe (Figures 3A-3E).
The hip is then reduced. If indicated, a T-capsulotomy is performed for femoral osteochondroplasty.
Postoperative Care
Patients are placed in a postoperative hip brace and use a continuous passive motion machine 6 hours a day for 2 weeks, and an ice machine. They maintain 30 lb of foot-flat weight-bearing for 3 weeks, and begin a standard labral repair protocol on postoperative days 3 to 7.
Discussion
Hip labral preservation has evolved over the past 10 years, and current options for labral management include excision, débridement, labralization, repair, and reconstruction.1-13 Labral excision was studied by Miozzari and colleagues,8 who postulated on the basis of animal models that the labrum may regenerate. In their series of 9 patients treated with surgical hip dislocation and labral excision at average 4-year follow-up, repeat magnetic resonance angiography revealed no regeneration of tissue—modified Harris Hip Score was 83. The hip scores were less than those of patients treated with the same procedure with repair, and the authors concluded that defining labral débridement versus excision in the literature, and treating patients with primary repair or reconstruction techniques, may lead to better results. Their study used a small sample and was limited to an open procedure. Arthroscopic labral débridement in isolation was also a poor option for treatment of a labral tear. In a 2-year follow-up of 59 isolated labral débridement procedures, Krych and colleagues9 found 47% combined poor results.
There is level I evidence of the importance of labral repair. In 2013, Krych and colleagues7 conducted a randomized control trial of 38 female patients who underwent hip arthroscopy for FAI. At time of surgery, patients were randomly assigned to either débridement or repair. At 1-year follow-up, activities of daily living and Sports specific Hip Outcome Scores were statistically significantly superior in the repair group. On a subjective scale, 94% vs 78% of patients reported normal or near normal hips in the repair versus débridement groups respectively. Ayeni and colleagues20 performed a systematic review of 6 studies in an attempt to develop labral management recommendations. Five of the studies (N = 490 patients total) had improved results with labral repair over reconstruction. Although the studies had a low level of evidence, they found a trend toward improved results with labral repair. These studies highlight the importance of labral preservation and proper FAI management.
Techniques for labrum repair have advanced as well—from a looped suture technique to a base stitch and knotless independent tensioning.11-13 Restoration of the hip labrum function as a suction seal, fluid circulator and anatomic capsular repair is paramount to excellent results and stresses the importance of performing an anatomic labral repair.1-6 Knotless anchor repair is not novel and has been previously described. Fry and Domb12 reported on a knotless labral repair technique that uses push-lock devices (Arthrex) that do not allow for independent tensioning. Inversion-eversion was introduced to the literature by Moreira and colleagues,13 who described an independent tensioning technique that uses speed-lock anchors (Smith & Nephew). Our technique differs in that it involves a DALA portal; labral reduction and tensioning with a probe assist to ensure the second pass of the base stitch is at the apex of the labrum; and use of No. 1 instead of No. 2 suture. Although seemingly subtle, these differences allow for proper anchor placement nearer the rim, additional support in achieving precise suture placement, and less disruption of small labra. These differences are particularly relevant for smaller labra.
Evaluating repair techniques on the basis of high-evidence literature is challenging. In a matched-cohort study of 220 patients, Jackson and colleagues21 compared 2 techniques: looped and base stitch. At 2-year follow-up, patients in both groups showed improvement, and there was no statistically significant difference in patient-reported outcome measures between the groups. Sawyer and colleagues22 studied the outcomes of 326 consecutive patients who underwent looped, pierced, or combined labral repair at an average 32-month follow-up. The groups’ revision rates were comparable, each group improved in postoperative patient-reported outcomes, and the pierced group had significantly higher preoperative scores on the Western Ontario and McMaster Universities Osteoarthritis Index. These studies described a base or pierce repair that did not differ from a looped repair, though the techniques did not allow for independent tensioning to re-create an anatomical inversion-eversion repair and may have altered the reported outcomes.
Our current technique uses independent tensioning of the repair to allow control of labrum inversion-eversion to give an anatomical repair with restoration of the suction seal. Preoperative planning, addressing the FAI appropriately, proper suture-passing technique, controlling the labrum in inversion-eversion fashion, and anatomical labral repair are the elements of Dr. Mather’s preferred method for preserving the native labrum and allowing it to assume its native function.
Future Directions
As our understanding of FAI and labral function evolves, labral preservation surgery continues to advance. With surgeons continually developing new techniques and following up on previous techniques, the ability to preserve the native hip with lasting procedures evolves as well. Proper identification of the underlying cause of the labral tear and proper anatomical repair are paramount to the success of FAI surgery.
Am J Orthop. 2017;46(1):42-48. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
3. Dwyer MK, Jones HL, Hogan MG, Field RE, McCarthy JC, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42(4):812-819.
4. Greaves LL, Gilbart MK, Yung AC, Kozlowski, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43(5):858-863.
5. Freehill MT, Safran MR. The labrum of the hip: diagnosis and rationale for surgical correction. Clin Sports Med. 2011;30(2):293-315.
6. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
7. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
8. Miozzari HH, Celia M, Clark JM, Werlen S, Naal FD, Nötzli HP. No regeneration of the human acetabular labrum after excision to bone. Clin Orthop Relat Res. 2015;473(4):1349-1357.
9. Krych AJ, Kuzma SA, Kovachevich R, Hudgens JL, Stuart MJ, Levy BA. Modest mid-term outcomes after isolated arthroscopic debridement of acetabular labral tears. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):763-767.
10. Matsuda DK. Arthroscopic labralization of the hip: an alternative to labral reconstruction. Arthrosc Tech. 2014;3(1):e131-e133.
11. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
12. Fry D, Domb B. Labral base refixation in the hip: rationale and technique for an anatomic approach to labral repair. Arthroscopy. 2010;26(9 suppl):S81-S89.
13. Moreira B, Pascual-Garrido C, Chadayamurri V, Mei-Dan O. Eversion-inversion labral repair and reconstruction technique for optimal suction seal. Arthrosc Tech. 2015;4(6):e697-e700.
14. Mook WR, Briggs KK, Philippon MJ. Evidence and approach for management of labral deficiency: the role for labral reconstruction. Sports Med Arthrosc. 2015;23(4):205-212.
15. Gupta A, Suarez-Ahedo C, Redmond JM, et al. Best practices during hip arthroscopy: aggregate recommendations of high-volume surgeons. Arthroscopy. 2015;31(9):1722-1727.
16. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
17. Hash TW. Magnetic resonance imaging of the hip. In: Nho SJ, Leunig M, Larson CM, Bedi A, Kelly BT, eds. Hip Arthroscopy and Hip Joint Preservation Surgery, Vol. 1. New York, NY: Springer; 2015:65-113.
18. Sutter R, Zubler V, Hoffmann A, et al. Hip MRI: how useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014;202(1):160-169.
19. Federer AE, Karas V, Nho S, Coleman SH, Mather RC 3rd. Capsular suspension technique for hip arthroscopy. Arthrosc Tech. 2015;4(4):e317-e322.
20. Ayeni OR, Adamich J, Farrokhyar F, et al. Surgical management of labral tears during femoroacetabular impingement surgery: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):756-762.
21. Jackson TJ, Hammarstedt JE, Vemula SP, Domb BG. Acetabular labral base repair versus circumferential suture repair: a matched-paired comparison of clinical outcomes. Arthroscopy. 2015;31(9):1716-1721.
22. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
Current Techniques in Treating Femoroacetabular Impingement: Capsular Repair and Plication
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
Bariatric surgery or total joint replacement: which first?
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
NEW ORLEANS – Performing bariatric surgery prior to total knee or hip replacement instead of vice versa resulted in significantly shorter orthopedic surgical operating time and length of stay in an observational study, Emanuel E. Nearing II, MD, reported at Obesity Week 2016.
“We propose that strong consideration be given to bariatric surgery as a means of weight loss and BMI [body mass index] reduction in patients with obesity prior to total joint replacement,” he said at the meeting presented by the Obesity Society of America and the American Society for Metabolic and Bariatric Surgery.
“A common complaint of patients presenting with obesity is that their osteoarthritis has limited their mobility and that their weight gain is secondary to that reduced mobility. They believe that a new joint will help them regain their mobility and then lose weight. Interestingly, this does not appear to be the case. In fact, the majority of patients in our study actually gained weight following joint replacement. Given that, these patients need to be weight-optimized prior to total joint replacement. Bariatric surgery is a durable way to facilitate this,” he continued.
Dr. Nearing presented a retrospective observational study of 102 patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy plus a total knee or hip replacement in the Gundersen system. Sixty-six patients had their bariatric surgery first, by a mean of 4.3 years, while the other 36 had arthroplasty a mean of 4.9 years before their bariatric surgery. The two groups were similar in terms of demographics and baseline comorbid conditions.
Patients who had their total joint replacement first had a mean preoperative BMI of 43.7 kg/m2 and a mean pre–bariatric surgery BMI of 46.3 kg/m2. The patients who had bariatric surgery first had a preoperative BMI of 49.6 kg/m2 and a mean pre–orthopedic surgery BMI of 37.6 kgm2. One year after joint replacement surgery, patients who had that operation first had a mean BMI of 43.9 kg/m2, compared with 37.8 kg/m2 for those who waited until after they underwent bariatric surgery.
Mean operative time for total joint replacement when it was the first operation was 113.5 minutes and substantially less at 71 minutes when it was done after bariatric surgery. Mean hospital length of stay for total joint replacement when it followed bariatric surgery was 2.9 days, a full day less than when joint replacement came first.
Rates of complications including skin or soft tissue infection, venous thromboembolism, hematoma, need for transfusion, and periprosthetic infection at 30 and 90 days didn’t differ between the two groups. Neither did the need for late reinterventions.
Dr. Nearing noted that a working group of the American Association of Hip and Knee Surgeons has conducted a review of the orthopedic surgery literature and concluded that all patients with a BMI of 30 kg/m2 or more undergoing total knee or hip arthroplasty are at increased risk for perioperative respiratory complications, thromboembolic events, delayed wound healing, infection, and need for joint revision surgery (J Arthroplasty. 2013 May;28[5]:714-21).
He observed that a retrospective study such as his cannot shed light on the optimal time interval for total joint replacement following bariatric surgery. That key question is being addressed by the ongoing prospective SWIFT (Surgical Weight-Loss to Improve Functional Status Trajectories Following Total Knee Arthroplasty) trial. The study hypothesis is that bariatric surgery prior to the knee replacement surgery will reduce risk and improve long-term outcomes and physical function.
Several audience member commented that, based upon their experience, they would have anticipated that complication rates would have been significantly lower in total joint replacement patients when that operation followed bariatric surgery.
“We were surprised, too,” Dr. Nearing replied. “I think the explanation is that at Gundersen we have three bariatric surgeons and only a handful of orthopedic surgeons, and we use protocols and pathways. We just routinely do our operations the same way each and every time.”
John M. Morton, MD, a former American Society for Metabolic and Bariatric Surgery president, commented that the Gundersen study findings sound a call for more cross-specialty collaboration in steering obese patients with severe knee or hip osteoarthritis to bariatric surgery first in order to maximize the results of the joint replacement surgery.
“I think we’re all seeing weight loss as another form of prehabilitation for other specialties. Our orthopedic colleagues are kind of like us – surgeons – so this seems to be a great place for us to partner with them,” said Dr. Morton, chief of bariatric and minimally invasive surgery at Stanford (Calif.) University.
Dr. Nearing reported having no financial interests relevant to his study.
AT OBESITY WEEK 2016
Key clinical point:
Major finding: When total joint replacement in obese patients was performed after bariatric surgery, mean hospital length of stay was a full day less than when the orthopedic surgery preceded the bariatric surgery.
Data source: This retrospective observational study included 102 obese patients who underwent bariatric surgery and total knee or hip replacement.
Disclosures: The study presenter reported having no financial conflicts of interest.
T-Capsulotomy to Improve Visualization of the Peripheral Compartment and Repair
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Comparing Cost, Efficacy, and Safety of Intravenous and Topical Tranexamic Acid in Total Hip and Knee Arthroplasty
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) can be associated with significant blood loss that in some cases requires transfusion. The incidence of transfusion ranges from 16% to 37% in patients who undergo THA and from 11% to 21% in patients who undergo TKA.1-3 Allogeneic blood transfusions have been associated with several risks (transfusion-related acute lung injury, hemolytic reactions, immunologic reactions, fluid overload, renal failure, infections), increased cost, and longer hospital length of stay (LOS).4-7 With improved patient outcomes the ultimate goal, blood-conserving strategies designed to decrease blood loss and transfusions have been adopted as a standard in successful joint replacement programs.
Tranexamic acid (TXA), an antifibrinolytic agent, has become a major component of blood conservation management after THA and TKA. TXA stabilizes clots at the surgical site by inhibiting plasminogen activation and thereby blocking fibrinolysis.8 The literature supports intravenous (IV) TXA as effective in significantly reducing blood loss and transfusion rates in elective THA and TKA.9,10 However, data on increased risk of thrombotic events with IV TXA in both THA and TKA are conflicting.11,12 Topical TXA is thought to have an advantage over IV TXA in that it provides a higher concentration of drug at the surgical site and is associated with little systemic absorption.2,13Recent prospective randomized studies have compared the efficacy and safety of IV and topical TXA in THA and TKA.9,14 However, controversy remains because relatively few studies have compared these 2 routes of administration. In addition, healthcare–associated costs have come under increased scrutiny, and the cost of these treatments should be considered. More research is needed to determine which application is most efficacious and cost-conscious and poses the least risk to patients. Therefore, we conducted a study to compare the cost, efficacy, and safety of IV and topical TXA in primary THA and TKA.
Materials and Methods
Our Institutional Review Board approved this study. Patients who were age 18 years or older, underwent primary THA or TKA, and received IV or topical TXA between August 2013 and September 2014 were considered eligible for the study. For both groups, exclusion criteria were trauma service admission, TXA hypersensitivity, pregnancy, and concomitant use of IV and topical TXA.
We collected demographic data (age, sex, weight, height, body mass index), noted all transfusions of packed red blood cells, and recorded preoperative and postoperative hemoglobin (Hgb) levels and surgical drain outputs. We also recorded any complications that occurred within 90 days after surgery: deep vein thrombosis (DVT), pulmonary embolism (PE), cardiac events, cerebrovascular events, and wound drainage. Wound drainage was defined as readmission to hospital or return to operating room for wound drainage caused by infection or hematoma. Postoperative care (disposition, LOS, follow-up) was documented. Average cost of both IV and topical TXA administration was calculated using average wholesale price.
Use of IV TXA and use of topical TXA were compared in both THA and TKA. Patients in the IV TXA group received TXA in two 10-mg/kg doses with a maximum of 1 g per dose. The first IV dose was given before the incision, and the second was given 3 hours after the first. Patients in the topical TXA group underwent direct irrigation with 3 g of TXA in 100 mL of normal saline at the surgical site after closure of the deep fascia in THA and after closure of the knee arthrotomy in TKA. The drain remained occluded for 30 minutes after surgery. The wound was irrigated with topical TXA before wound closure in the THA group and before tourniquet release in the TKA group. TXA dosing was based on institutional formulary dosing restrictions and was consistent with best practices and current literature.3,9,14,15Primary outcomes measured for each cohort and treatment arm were Hgb levels (difference between preoperative levels and lowest postoperative levels 24 hours after surgery), blood loss, transfusion rates, and cost. Secondary outcomes were LOS and complications that occurred within 90 days after surgery (DVT, PE, cardiac events, cerebrovascular events, wound drainage).
Calculated blood loss was determined with equations described by Konig and colleagues,3 Good and colleagues,16 and Nadler and colleagues.17 Total calculated blood loss was based on the difference in Hgb levels before surgery and the lowest Hgb levels 24 hours after surgery:
Blood loss (mL) = 100 mL/dL × Hgbloss/Hgbi
Hgbloss = BV × (Hgbi – Hgbe) × 10 dL/L + Hgbt
= 0.3669 × Height3 (m) + 0.03219 × Weight (kg) + 0.6041 (for men)
= 0.3561 × Height3 (m) + 0.03308 × Weight (kg) + 0.1833 (for women)
where Hgbi is the Hgb concentration (g/dL) before surgery, Hgbe is the lowest Hgb concentration (g/dL) 24 hours after surgery, Hgbt is the total amount (g) of allogeneic Hgb transfused, and BV is the estimated total body blood volume (L).17 As Hgb concentrations after blood transfusions were compared in this study, the Hgbt variable was removed from the equation. Based on Hgb decrease data in a study that compared IV and topical TXA in TKA,14 we determined that a sample size of least 140 patients (70 in each cohort) was needed in order to have 80% power to detect a difference in Hgb decrease of 0.36 g/dL in IV and topical TXA.
All data were reported with descriptive statistics. Frequencies and percentages were reported for categorical variables. Means and standard deviations were reported for continuous variables. The groups of continuous data were compared with unpaired Student t tests and 1-way analysis of variance. Comparisons among groups of categorical data were analyzed with Fisher exact tests. Statistical significance was set at P < .05.
Results
Data were collected on 291 patients (156 THA, 135 TKA). There was a significant (P = .044) sex difference in the THA group: more men in the topical TXA subgroup and more women in the IV TXA subgroup. Other patient demographics were similarly matched with respect to age, height, weight, and body mass index (Table 1).
The secondary outcomes (differences in complications and LOS) are listed in Table 3.
Discussion
TXA, an analog of the amino acid lysine, is an antifibrinolytic agent that has been used for many years to inhibit fibrin degradation.3,18 TXA works by competitively inhibiting tissue plasminogen activation, which is elevated by the trauma of surgery, and blocking plasmin binding to fibrin.3,19 The mechanism of action is not procoagulant, as TXA prevents fibrin breakdown and supports coagulation that is underway rather than increasing clot formation. These characteristics make the drug attractive for orthopedic joint surgery—TXA reduces postoperative blood loss in patients who need fibrinolysis suppressed in order to maintain homeostasis without increasing the risk of venous thromboembolism. IV TXA has been well studied, which supports its efficacy profile for reducing blood loss and transfusions; there are no reports of increased risk of thromboembolic events.20-22 Despite these studies, the risk of adverse events is still a major concern, especially in patients with medical conditions that predispose them to venothrombotic events. Topical TXA has become a viable option, especially in high-risk patients, as studies have shown 70% lower systemic absorption relative to IV TXA plasma concentration.23 Still, too few studies have compared the efficacy, safety, and cost of IV and topical TXA in both THA and TKA.
Topical TXA costs an average of $2100 per case, primarily because standard dosing is 3 g per case. Despite repeat dosing for IV TXA (first dose at incision, second dose 3 hours after first), IV TXA costs were much lower on average: $939 less for THA and $829 less for TKA. As numerous studies have outlined results similar to ours, cost-effectiveness should be considered in decisions about treatment options.
Patel and colleagues14 reported that the efficacy of topical TXA was similar to that of IV TXA and that there were no significant differences in Hgb decrease, wound drainage, or need for transfusions after TKA. Their report conflicts with our finding significant differences favoring topical TXA for Hgb change (P = .015) and reduced calculated blood loss (P = .019) in TKA. A potential reason for these differing results is that the topical TXA doses were different (2 g in the study by Patel and colleagues,14 3 g in our study). Martin and colleagues24 compared the effects of topical TXA and placebo and found a nonsignificant difference in reduced blood loss and postoperative transfusions when the drug was dosed at 2 g. Konig and colleagues3 found that topical TXA dosed at 3 g (vs placebo) could reduce blood loss and transfusions after THA and TKA. These studies support our 3-g dose protocol for topical TXA rather than the 2-g protocol used in the study by Patel and colleagues.14 Our results are congruent with those of Seo and colleagues,25 who found topical TXA superior in decreasing blood loss in TKA. Furthermore, our study is unique in that it compared costs and found topical TXA to be more expensive by almost $1000 on average.
Wei and Wei9 concluded that IV TXA 3 g and topical TXA 3 g were equally effective in reducing total blood loss, change in hematocrit, and need for transfusion after THA. In contrast, we found a significant (P = .031) difference favoring topical TXA for Hgb change. The 2 studies differed in their dosing protocols: Wei and Wei9 infused a 3-g dose, whereas we gave a maximum of two 1-g IV doses. The higher IV dose used by Wei and Wei9 could explain why they found no difference between IV and topical TXA, whereas we did find a difference. Our study was unique in that it measured Hgb change, blood loss, and cost.
Our study included an in-depth analysis of blood loss: estimated blood loss, drain outputs, calculated blood loss, and Hgb change. The equation we used for calculated blood loss is well established and has been used in multiple studies.3,16,17 To thoroughly assess the safety of TXA, we reviewed and documented complications that occurred within 90 days after surgery and that could be attributed to TXA. This study was adequately powered and exceeded the required sample size to detect a difference in one primary outcome measure, perioperative Hgb change, as calculated by the prestudy statistical power analysis.
Our study had several limitations. First, it was a retrospective chart review; documentation could have been incomplete or missing. Second, the study was not randomized and thus subject to drug selection bias. Third, patients were selected for topical TXA on the basis of perceived risk factors, such as prior or family history of DVT, PE, cardiac events, or cerebrovascular events. It was thought that, given the decrease in systemic absorption with topical TXA, these high-risk patients would be less likely to have a thromboembolic event. Their complex past medical histories may explain why the topical TXA group had more cardiac events. Furthermore, 1 orthopedic surgeon used topical TXA exclusively, and the other 3 used it selectively, according to risk factors. In addition, unlike TKA patients, not all THA patients received drains. This study was powered to measure a difference in perioperative Hgb change but may not have been powered to detect the statistically significant difference favoring topical TXA for calculated blood loss in TKA. In the THA group, a statistically significant difference was found for reduced Hgb decrease but not for estimated or calculated blood loss. This finding reinforces some of the disparities in measurements of the effects of blood conservation strategies. The study also lacked a placebo or control group. However, several other studies have found that both IV TXA and topical TXA are superior to placebo in decreasing blood loss, Hgb change, and transfusion requirements.10,12,20,22 In addition, the effects of TXA are based on estimates of blood conservation and are not without their disparities.
Conclusion
The present study found that both IV TXA and topical TXA were effective in decreasing blood loss, Hgb levels, and need for transfusion after THA and TKA. Topical TXA appears to be more effective than IV TXA in preventing Hgb decrease during THA and TKA and calculated blood loss during TKA. This increased efficacy comes with a higher cost. Thromboembolic complications were similar between groups. More studies are needed to compare the efficacy and safety profiles of topical TXA against the routine standard of IV TXA, especially in patients with perceived contraindications to IV TXA.
Am J Orthop. 2016;45(7):E439-E443. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
1. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
2. Yue C, Kang P, Yang P, Xie J, Pei F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29(12):2452-2456.
3. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
4. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135.
5. Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78(8):1260-1270.
6. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406-3417.
7. Kumar A. Perioperative management of anemia: limits of blood transfusion and alternatives to it. Cleve Clin J Med. 2009;76(suppl 4):S112-S118.
8. Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673(1):75-85.
9. Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty. 2014;29(11):2113-2116.
10. Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742-1752.
11. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
12. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
13. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
14. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528-1531.
15. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
16. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596-599.
17. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224-232.
18. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
19. Mannucci PM. Homostatic drugs. N Engl J Med. 1998;339(4):245-253.
20. Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on transfusion rate in primary total hip arthroplasty. J Arthroplasty. 2014;29(2):387-389.
21. Dahuja A, Dahuja G, Jaswal V, Sandhu K. A prospective study on role of tranexamic acid in reducing postoperative blood loss in total knee arthroplasty and its effect on coagulation profile. J Arthroplasty. 2014;29(4):733-735.
22. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
23. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
24. Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Westendorf DS, Garton AS, Lemke JH. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29(5):889-894.
25. Seo JG, Moon YW, Park SH, Kim SM, Ko KR. The comparative efficacies of intra-articular and IV tranexamic acid for reducing blood loss during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1869-1874.
VIDEO: Biologics: Proposed guideline addresses perioperative management
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Biologic agents should be stopped prior to elective total knee or hip arthroplasty in patients with rheumatic diseases, according to a draft guideline developed by the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
The guideline, which address the perioperative management of antirheumatic medications in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, juvenile idiopathic arthritis (JIA), or lupus who are undergoing such surgery, is currently under review, Dr. Susan Goodman, MD, coprincipal investigator, reported at the annual meeting of the American College of Rheumatology.
The draft guideline was created because “guidance was needed for common clinical situations, even where data were sparse. We didn’t want to configure treatment mandates – that’s not what these are,” Dr. Goodman of Cornell University, New York, said.
The recommendations are conditional, she said, meaning that the benefits probably outweigh the harms, that the recommendations apply to most but not all patients, and that future research may lead to changes.
“They’re also preference sensitive,” she said, explaining that patients’ values and preferences should be carefully considered, as they might differ from those of the patient panel consulted during guideline development; the panel expressed greater concern about the risk of infection following surgery than about perioperative flares resulting from medication discontinuation.
Based on agreement by at least 80% of a voting panel which considered available evidence in the context of their clinical experience along with the input from the patient panel, the draft guideline states that:
• Current doses of methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued in patients with rheumatic diseases undergoing elective hip and knee replacement. This recommendation is based on an extensive literature review that showed the infection rate is decreased in patients who continue these medications, Dr. Goodman said.
• All biologics should be withheld prior to surgery in patients with inflammatory arthritis, and surgery should be planned for the end of the dosing cycle. This matter wasn’t specifically addressed in the literature; however, numerous randomized controlled trials outside of the surgical setting demonstrate an increased risk of infection associated with their use, she noted.
“All of the biologic medications were found to be associated with an increased risk of infection,” she said. “Because of this and the level of importance patients place on minimizing infection risk, we’ve recommended that biologics be withheld prior to surgery.”
• Tofacitinib, which was considered in a separate oral, targeted therapy category, should be withheld for at least 7 days prior to surgery in patients with RA, spondyloarthritis, and JIA. Data from systematic reviews and meta-analyses showed an increased risk of infection with tofacitinib, although more research is needed in order to “firm up” this recommendation, Dr. Goodman said.
• In lupus patients, rituximab and belimumab should be withheld prior to surgery, and surgery should be planned for the end of the dosing period.
“Again, this was not answered in the literature. We depended on observational studies that we reviewed that did show that patients with severe active lupus were at much higher risk for adverse events. But since rituximab isn’t approved by the [Food and Drug Administration] for use in lupus, and belimumab isn’t approved for use in severe lupus – and those seem to be the high-risk patients – we thought withholding them was more prudent,” she said.
• Patients with severe lupus should continue on current doses of methotrexate, mycophenolic acid, azathioprine, mizoribine, cyclosporine, and tacrolimus through surgery. This recommendation is based on indirect data from experience in organ transplant patients.
• All medications should be discontinued in patients whose lupus is not severe.
“Our recommendation is to withhold for 7 days to 2-5 days after surgery in the absence of any wound healing complications or any other complications,” she said, noting that the literature does not directly address this; the recommendation is based on indirect evidence in patients with either active infection or who are at risk for infection.
“We thought that careful monitoring of the patient would permit us to identify flare and intervene quickly. … and that, for mild cases of lupus, the morbidity associated with infection might not be greater than the morbidity associated with the disease flare,” she said.
• Biologics should be restarted once surgical wounds show evidence of healing and there is no clinical evidence of infection. The literature does not directly address this; the recommendation is based on the rationale for use of these medications in patients with either active infection or risk for infection.
• Current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with RA, lupus, or inflammatory arthritis. A meta-analysis and systematic review of randomized controlled trial data and observational data showed no hemodynamic difference between daily doses and stress doses.
“In addition, there are abundant observational data demonstrating an increase in infection in patients on chronic steroids greater than 15 mg, and we thought that part of the optimization of the patient would be getting them on the lowest possible steroid dose,” she said, stressing that this refers only to adults receiving glucocorticoids for their rheumatic disease, and not to those with a history of JIA who may have received steroids during development, or to those receiving glucocorticoids for primary, adrenal, or hypothalamic disease.
According to Dr. Goodman, the time is right for the introduction of these recommendations, because the increased use of disease-modifying drugs and biologics means that most patients coming in for these surgeries will be taking these medications.
Further, despite the widespread use of the medications, the rate of total knee and hip arthroplasty surgeries among patients with rheumatic diseases is about the same as it was 20 or 30 years ago – and their risk for devastating complications, including infections, remains high, she said, noting that appropriate medication management provides an opportunity to mitigate risk.
Coprincipal investigator, Bryan Springer, MD, further emphasized the importance of the guideline, noting that the 5-year survival among rheumatic disease patients who develop certain perioperative complications is lower than for many common cancers, and that the literature offers little guidance on managing medications in the perioperative period.
“We now have a document that’s based on the available evidence, and also based on expert opinion, to help us manage these patients much more thoroughly in the perioperative period,” Dr. Springer, an orthopedic surgeon in Charlotte, N.C., said during a press briefing on the guideline.
Dr. Springer highlighted the value of the unique collaboration between the ACR and the AAHKS, calling the effort a win both for patients, and for “collaborative efforts, collaborative research, which we just really don’t do enough of,” he said. “I hope this is a huge step towards that direction.”
This guideline development process was funded by the ACR and AAHKS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Anti–nerve growth factor drug has long-term OA pain benefit, but unclear safety
Treatment for a year with the human anti–nerve growth factor monoclonal antibody fulranumab provided pain relief and functional benefits to patients with moderate to severe chronic osteoarthritis knee or hip pain but continued to show signs that the biologic may contribute to rapid progression of disease in a proportion of patients who came to need joint replacement, especially when used concurrently with nonsteroidal anti-inflammatory drugs.
The findings come from the double-blind extension phase of a 12-week, phase II, placebo-controlled, double-blind, randomized study that found significant reduction in the average pain intensity score (P less than or equal to .030) for patients who took fulranumab (Pain. 2013 Oct;154[10]:1910-9). The investigators wanted to determine the long-term safety of the biologic in light of the Food and Drug Administration’s 2010 “clinical hold” on studies of anti–nerve growth factor (anti-NGF) drugs such as fulranumab after concerns emerged that the class of anti-NGF antibodies may be associated with potential treatment-emergent adverse events (TEAEs) leading to joint destruction.
The long-term safety and efficacy results in the extension phase of the study when fulranumab was given as an adjunctive therapy to standard pain therapy revealed higher rates of serious TEAEs, including joint replacement, that were associated with fulranumab. While most of those TEAEs were independently adjudicated to stem from normal progression of OA, one-fifth of the patients on fulranumab who needed joint replacement had rapid progression of OA (RPOA).
Investigators led by Panna Sanga, MD, director of clinical research at Janssen, sought to determine the effects of the anti-NGF biologic as an adjunctive therapy to standard pain therapy over a 92-week period in 401 patients who had completed the 12-week efficacy study, as well as over a 26-week posttreatment follow-up. In the current extension phase of the trial, patients continued on their randomized dose in the original trial of placebo or subcutaneous fulranumab 1 mg or 3 mg every 4 weeks or fulranumab 3 mg, 6 mg, or 10 mg every 8 weeks, but they were permitted to change their concurrent pain medications as clinically needed.
Although the study intended for patients to receive 2 years of treatment (104 weeks), the FDA’s clinical hold meant that the median duration of exposure to fulranumab in the extension study was only 365-393 days across the dosing regimens, the authors explained (Arthritis Rheumatol. 2016 Oct 16 doi: 10.1002/art.39943).
Overall, 421 (90%) of 466 intent-to-treat patients experienced at least one TEAE during both study phases, with similar incidence between those randomized to placebo (n = 69, 88%) and the fulranumab groups (n = 352, 91%).
TEAEs that occurred with a frequency of 10% or more among all fulranumab-treated patients were arthralgia (21%), OA (18%), paresthesia, and upper respiratory tract infection (13% each), while these were arthralgia (15%), OA (14%), and sinusitis (12%) among patients randomized to placebo.
A total of 109 patients (23%) reported serious TEAEs, including 13 (17%) taking placebo and 96 (25%) taking fulranumab. Serious TEAEs that were reported in 5% or more of patients in the fulranumab groups were knee arthroplasty (n = 38, 10%) and hip arthroplasty (n = 26, 7%). Overall, 81 joint replacements occurred in 71 patients (placebo: n = , 11%; fulranumab: n = 63, 89%).
An independent adjudication committee that the study sponsor, Janssen, established after the study was put on hold ruled that the majority of these joint replacements resulted from the normal progression of OA (n = 56, 79%). However, the adjudication committee determined that 15 (21%) patients in the fulranumab treatment groups had RPOA.
The study authors pointed out that the patients with RPOA regularly used NSAIDs and had a prior history of OA in the affected joint. Nevertheless, because of the small number of RPOA cases per treatment group, a drug or dose effect for RPOA could not be evaluated, they said.
“Future studies are warranted to demonstrate whether limiting the use of concomitant chronic NSAIDs and using only lower doses of fulranumab may reduce the risk of RPOA,” they wrote.
The extension study also looked at the longer-term efficacy of fulranumab. Efficacy endpoints on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and physical function subscales and the Patient Global Assessment (PGA) scores that looked at changes from baseline showed that in comparison with placebo, dosing regimens of fulranumab 3 mg every 4 weeks and 10 mg every 8 weeks gave “continued effective relief of the pain associated with knee and hip OA as early as week 4 and was maintained up to week 53,” the researchers reported.
These results were further corroborated by improvements in physical function on the WOMAC physical function subscale and PGA scale scores, which suggested “sustained efficacy of long-term fulranumab treatment,” they said.
All authors except one were employees of Janssen.
Treatment for a year with the human anti–nerve growth factor monoclonal antibody fulranumab provided pain relief and functional benefits to patients with moderate to severe chronic osteoarthritis knee or hip pain but continued to show signs that the biologic may contribute to rapid progression of disease in a proportion of patients who came to need joint replacement, especially when used concurrently with nonsteroidal anti-inflammatory drugs.
The findings come from the double-blind extension phase of a 12-week, phase II, placebo-controlled, double-blind, randomized study that found significant reduction in the average pain intensity score (P less than or equal to .030) for patients who took fulranumab (Pain. 2013 Oct;154[10]:1910-9). The investigators wanted to determine the long-term safety of the biologic in light of the Food and Drug Administration’s 2010 “clinical hold” on studies of anti–nerve growth factor (anti-NGF) drugs such as fulranumab after concerns emerged that the class of anti-NGF antibodies may be associated with potential treatment-emergent adverse events (TEAEs) leading to joint destruction.
The long-term safety and efficacy results in the extension phase of the study when fulranumab was given as an adjunctive therapy to standard pain therapy revealed higher rates of serious TEAEs, including joint replacement, that were associated with fulranumab. While most of those TEAEs were independently adjudicated to stem from normal progression of OA, one-fifth of the patients on fulranumab who needed joint replacement had rapid progression of OA (RPOA).
Investigators led by Panna Sanga, MD, director of clinical research at Janssen, sought to determine the effects of the anti-NGF biologic as an adjunctive therapy to standard pain therapy over a 92-week period in 401 patients who had completed the 12-week efficacy study, as well as over a 26-week posttreatment follow-up. In the current extension phase of the trial, patients continued on their randomized dose in the original trial of placebo or subcutaneous fulranumab 1 mg or 3 mg every 4 weeks or fulranumab 3 mg, 6 mg, or 10 mg every 8 weeks, but they were permitted to change their concurrent pain medications as clinically needed.
Although the study intended for patients to receive 2 years of treatment (104 weeks), the FDA’s clinical hold meant that the median duration of exposure to fulranumab in the extension study was only 365-393 days across the dosing regimens, the authors explained (Arthritis Rheumatol. 2016 Oct 16 doi: 10.1002/art.39943).
Overall, 421 (90%) of 466 intent-to-treat patients experienced at least one TEAE during both study phases, with similar incidence between those randomized to placebo (n = 69, 88%) and the fulranumab groups (n = 352, 91%).
TEAEs that occurred with a frequency of 10% or more among all fulranumab-treated patients were arthralgia (21%), OA (18%), paresthesia, and upper respiratory tract infection (13% each), while these were arthralgia (15%), OA (14%), and sinusitis (12%) among patients randomized to placebo.
A total of 109 patients (23%) reported serious TEAEs, including 13 (17%) taking placebo and 96 (25%) taking fulranumab. Serious TEAEs that were reported in 5% or more of patients in the fulranumab groups were knee arthroplasty (n = 38, 10%) and hip arthroplasty (n = 26, 7%). Overall, 81 joint replacements occurred in 71 patients (placebo: n = , 11%; fulranumab: n = 63, 89%).
An independent adjudication committee that the study sponsor, Janssen, established after the study was put on hold ruled that the majority of these joint replacements resulted from the normal progression of OA (n = 56, 79%). However, the adjudication committee determined that 15 (21%) patients in the fulranumab treatment groups had RPOA.
The study authors pointed out that the patients with RPOA regularly used NSAIDs and had a prior history of OA in the affected joint. Nevertheless, because of the small number of RPOA cases per treatment group, a drug or dose effect for RPOA could not be evaluated, they said.
“Future studies are warranted to demonstrate whether limiting the use of concomitant chronic NSAIDs and using only lower doses of fulranumab may reduce the risk of RPOA,” they wrote.
The extension study also looked at the longer-term efficacy of fulranumab. Efficacy endpoints on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and physical function subscales and the Patient Global Assessment (PGA) scores that looked at changes from baseline showed that in comparison with placebo, dosing regimens of fulranumab 3 mg every 4 weeks and 10 mg every 8 weeks gave “continued effective relief of the pain associated with knee and hip OA as early as week 4 and was maintained up to week 53,” the researchers reported.
These results were further corroborated by improvements in physical function on the WOMAC physical function subscale and PGA scale scores, which suggested “sustained efficacy of long-term fulranumab treatment,” they said.
All authors except one were employees of Janssen.
Treatment for a year with the human anti–nerve growth factor monoclonal antibody fulranumab provided pain relief and functional benefits to patients with moderate to severe chronic osteoarthritis knee or hip pain but continued to show signs that the biologic may contribute to rapid progression of disease in a proportion of patients who came to need joint replacement, especially when used concurrently with nonsteroidal anti-inflammatory drugs.
The findings come from the double-blind extension phase of a 12-week, phase II, placebo-controlled, double-blind, randomized study that found significant reduction in the average pain intensity score (P less than or equal to .030) for patients who took fulranumab (Pain. 2013 Oct;154[10]:1910-9). The investigators wanted to determine the long-term safety of the biologic in light of the Food and Drug Administration’s 2010 “clinical hold” on studies of anti–nerve growth factor (anti-NGF) drugs such as fulranumab after concerns emerged that the class of anti-NGF antibodies may be associated with potential treatment-emergent adverse events (TEAEs) leading to joint destruction.
The long-term safety and efficacy results in the extension phase of the study when fulranumab was given as an adjunctive therapy to standard pain therapy revealed higher rates of serious TEAEs, including joint replacement, that were associated with fulranumab. While most of those TEAEs were independently adjudicated to stem from normal progression of OA, one-fifth of the patients on fulranumab who needed joint replacement had rapid progression of OA (RPOA).
Investigators led by Panna Sanga, MD, director of clinical research at Janssen, sought to determine the effects of the anti-NGF biologic as an adjunctive therapy to standard pain therapy over a 92-week period in 401 patients who had completed the 12-week efficacy study, as well as over a 26-week posttreatment follow-up. In the current extension phase of the trial, patients continued on their randomized dose in the original trial of placebo or subcutaneous fulranumab 1 mg or 3 mg every 4 weeks or fulranumab 3 mg, 6 mg, or 10 mg every 8 weeks, but they were permitted to change their concurrent pain medications as clinically needed.
Although the study intended for patients to receive 2 years of treatment (104 weeks), the FDA’s clinical hold meant that the median duration of exposure to fulranumab in the extension study was only 365-393 days across the dosing regimens, the authors explained (Arthritis Rheumatol. 2016 Oct 16 doi: 10.1002/art.39943).
Overall, 421 (90%) of 466 intent-to-treat patients experienced at least one TEAE during both study phases, with similar incidence between those randomized to placebo (n = 69, 88%) and the fulranumab groups (n = 352, 91%).
TEAEs that occurred with a frequency of 10% or more among all fulranumab-treated patients were arthralgia (21%), OA (18%), paresthesia, and upper respiratory tract infection (13% each), while these were arthralgia (15%), OA (14%), and sinusitis (12%) among patients randomized to placebo.
A total of 109 patients (23%) reported serious TEAEs, including 13 (17%) taking placebo and 96 (25%) taking fulranumab. Serious TEAEs that were reported in 5% or more of patients in the fulranumab groups were knee arthroplasty (n = 38, 10%) and hip arthroplasty (n = 26, 7%). Overall, 81 joint replacements occurred in 71 patients (placebo: n = , 11%; fulranumab: n = 63, 89%).
An independent adjudication committee that the study sponsor, Janssen, established after the study was put on hold ruled that the majority of these joint replacements resulted from the normal progression of OA (n = 56, 79%). However, the adjudication committee determined that 15 (21%) patients in the fulranumab treatment groups had RPOA.
The study authors pointed out that the patients with RPOA regularly used NSAIDs and had a prior history of OA in the affected joint. Nevertheless, because of the small number of RPOA cases per treatment group, a drug or dose effect for RPOA could not be evaluated, they said.
“Future studies are warranted to demonstrate whether limiting the use of concomitant chronic NSAIDs and using only lower doses of fulranumab may reduce the risk of RPOA,” they wrote.
The extension study also looked at the longer-term efficacy of fulranumab. Efficacy endpoints on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and physical function subscales and the Patient Global Assessment (PGA) scores that looked at changes from baseline showed that in comparison with placebo, dosing regimens of fulranumab 3 mg every 4 weeks and 10 mg every 8 weeks gave “continued effective relief of the pain associated with knee and hip OA as early as week 4 and was maintained up to week 53,” the researchers reported.
These results were further corroborated by improvements in physical function on the WOMAC physical function subscale and PGA scale scores, which suggested “sustained efficacy of long-term fulranumab treatment,” they said.
All authors except one were employees of Janssen.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: A total of 81 joint replacements occurred in 71 patients (placebo: n = 8, 11%; fulranumab: n = 63, 89%).
Data source: Phase II randomized double-blind extension study of 401 patients with moderate to severe chronic OA knee or hip pain.
Disclosures: All authors except one were employees of Janssen, which funded the study.
Direct Anterior Versus Posterior Simultaneous Bilateral Total Hip Arthroplasties: No Major Differences at 90 Days
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
Ceramic Femoral Heads for All Patients? An Argument for Cost Containment in Hip Surgery
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) has revolutionized the practice of orthopedic surgery. The number of primary THAs performed in the United States alone is predicted to rise to 572,000 per year by 2030.1 Increasing demand requires a tighter focus on cost-effectiveness, particularly with regard to expensive postoperative complications. Trunnionosis and taper corrosion have recently emerged as problems in THA.2-7 No longer restricted to metal-on-metal bearings, these phenomena now affect an increasing number of metal-on-polyethylene THAs and are exacerbated by modularity.8 The emergence of these complications adds complexity to the diagnostic algorithm in patients who present with painful THAs. Furthermore, the diagnosis of either trunnionosis or taper corrosion calls for revision surgery. In response to the increase in these complications, a group of orthopedic professional societies developed an algorithm for managing suspected metal toxicity issues.9 However, increases in toxicity and patient morbidity, and the added costs of toxicity surveillance and revision surgery, will place a substantial economic burden on many health systems at a time when policy makers are implementing substantial changes to health delivery in an effort to contain costs while improving patient outcomes.
Although they are more expensive than cobalt-chrome heads, ceramic femoral heads make metal toxicity a nonissue and eliminate the need for toxicity surveillance protocols. Furthermore, ceramic femoral heads are thought to have longevity advantages (this relationship needs to be confirmed in long-term studies).
In this article, we provide a theoretical framework for debating whether use of ceramic femoral heads in all THA patients could represent a more cost-effective option over the long term.
Materials and Methods
Guidelines for the diagnostic algorithm for painful THA with suspected metal toxicity were obtained from a recent orthopedic professional society consensus statement.9 The cost of this work-up was obtained from the finance department at our institution (Table 1).
We created 2 metrics to analyze the cost difference between ceramic and cobalt-chrome femoral heads. The first metric was “ceramic surplus,” the extra cost of a ceramic femoral head above that of a cobalt-chrome femoral head, and the second was “maximum ceramic surplus,” the ceramic surplus cutoff value for which using ceramic femoral heads in all patients becomes more cost-effective than using cobalt-chrome heads.
The cost of a metal work-up was determined for a single round of imaging tests (stratified by MRI and US), serum tests, aspiration tests, and clinic visit. These data were then combined with the cost of revision THA (Table 1) to create a series of maximum ceramic surplus models. In all these simulations, we assumed that about 7% of patients with metal-on-polyethylene THA would present with groin pain 1 to 2 years after surgery,10 and, working on this assumption, we applied a series of theoretical incidence ratios (12.5%, 25%, 50%) to both the percentage of patients who presented with a painful THA and received a metal toxicity work-up and the percentage of those who received the toxicity work-up and eventually needed revision surgery. For example, in the best-case scenario, the model assumes that 7% of THA patients present with pain and that 12.5% of the painful cohort receives a single work-up for metal toxicity (0.875% of all THAs). The best-case scenario then assumes that 12.5% of patients who receive a work-up for metal toxicity are eventually revised (0.11% of all THAs). By contrast, in the worst-case scenario, the model continues to assume that 7% of THA patients present with pain, but it also assumes that 50% of the painful cohort receives a single work-up for metal toxicity (3.5% of all THAs).
The lowest maximum ceramic surplus values were calculated from the best-case scenario, and the highest from the worst-case scenario. These steps were taken in keeping with the fact that a lower incidence of metal toxicity work-ups and revisions would require the price difference between ceramic and cobalt-chrome heads (ceramic surplus) to be small in order for ceramic heads in all patients to be cost-effective. The inverse is true for a high incidence of metal toxicity work-ups and revisions: A larger price difference between ceramic and cobalt-chrome femoral heads would be tolerable to still be cost-effective.
Results
A single metal toxicity work-up cost $5085 with MARS-MRI and $2402 with US (Table 1). Revision THA with a 3-day inpatient stay cost $53,320, and that figure does not include the cost of surgical implants or perioperative medications and devices, all of which have highly variable cost structures (Table 1). Ceramic surplus was as low as $500 in a high-volume academic practice and as high as $1500 in a low-volume private practice (Table 2). Maximum ceramic surplus ranged from $511 to $2044 in the models integrating MARS-MRI and from $488 to $1950 in the models integrating US (Table 3).
Discussion
Trunnionosis, corrosion, and metal toxicity are of increasing concern in hip implants that incorporate a cobalt-chrome femoral head, regardless of the counterpart articulation surface (metal, ceramic, polyethylene).2-8 In response to the added diagnostic challenge raised by these phenomena, a group of orthopedic professional societies developed an algorithm that can guide surgeons in the management of suspected corrosion or metal toxicity.9 In this protocol, toxicity surveillance in conjunction with potential revision surgery for metal-associated complications has the potential to increase patient morbidity and place a significant economic burden on many health systems. Given the recent emergence of trunnionosis, epidemiologic data on this complication are lacking.10 However, there is a substantial body of evidence showing devastating complications associated with adverse reactions to metal debris.11-17
Given the potential complications specific to cobalt-chrome femoral heads, we wanted to provide a theoretical framework for debating whether use of ceramic heads in all patients has the potential to be a more cost-effective option over the long term. Ceramic femoral heads are premium implants, certainly more expensive at initial point of care. One study based on a large community registry showed premium implants (eg, ceramic femoral heads) add a surplus averaging $1000.18 In our investigation, ceramic surplus varied with practice setting, from $500 to $1500. Lower costs were discovered in high-volume practice settings, indicating that a shift to increased use of ceramic femoral heads would likely decrease ceramic surplus for most institutions.
We used a series of simulations to predict maximum ceramic surplus after manipulation of theoretical incidence ratios. The main limitation of this study was our use of 7% as the incidence of painful THA within 1- to 2-year follow-up. This point estimate was derived from a manuscript that to our knowledge provides the most realistic estimate of this complication10; with use of more complete data in upcoming studies, however, the 7% figure could certainly change. As data are also lacking on the proportion of painful THAs that receive a metal work-up and on the proportion of metal work-ups that indicate revision surgery, we modeled values of 12.5%, 25%, and 50% for each of these metrics to cover a wide range of possibilities.
It is also true the model did not incorporate scenarios to account for the law of unintended consequences, which would caution that using ceramics for all patients may bring a new set of complications. Zirconia ceramic bearings have tended to fracture, with the vast majority of fractures occurring in the liner of ceramic-on-ceramic articulations. Midterm reports and laboratory data suggest this issue has largely been solved with the advent of delta ceramics, a composite containing only a small fraction of zirconia.19,20 Nevertheless, longer term in vivo data are needed to confirm the stability, longevity, and complication profile of these materials.
A final limitation of the present study is that the cost of a single metal toxicity work-up was based on just one institution. Grossly differing cost structures in other markets could alter the economic risk–benefit analysis we have described. However, we should note that the costs of tests, procedures, and appointments at our institution were uniform across a wide variety of practice settings in multiple regions of the United States, and thus are likely similar to the costs at a majority of practices.
Although our model took some liberties by necessity, it was also quite conservative in many respects. Many patients who undergo surveillance for metal toxicity undergo serial follow-ups; for this analysis, however, we considered the cost of only a single work-up. In addition, our proposed cost of revision surgery accounts only for facility and personnel costs during a 3-day inpatient stay and does not include the costs of implants, perioperative medications and devices, follow-up care, and potentially longer hospital stays or subsequent procedures, all of which can be highly variable and add considerable cost. Had any or all of these factors been incorporated into more complex modeling, the potential economic benefits of ceramic femoral heads would have been significantly greater.
After taking all these factors into account, our model found that maximum ceramic surplus ranged from $488 to $2044, depending on theoretical incidence ratio and imaging modality (Table 3). The lowest maximum ceramic surplus values ($511 for MARS-MRI protocol, $488 for US protocol) were based on the assumption that only 12.5% of patients who present with a painful THA receive a single metal work-up (0.875% of all THAs) and that only 12.5% of those patients are eventually revised (0.11% of all THAs). This outcome suggests ceramic femoral heads could be more cost-effective than cobalt-chrome femoral heads under these conservative projections when considering ceramic surplus is already as low as $500 at some high-volume centers. This figure would likely decline further in parallel with widespread growth in demand. Further study on the epidemiology of trunnionosis, corrosion, and metal toxicity in metal-on-polyethylene THA is needed to evaluate the economic validity of this proposal. Nevertheless, the superior safety profile of ceramic femoral heads with regard to metal toxicity indicates that wholesale use in THAs may in fact provide the most economical option on a societal scale.
Am J Orthop. 2016;45(6):E362-E366. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9-18.
3. Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-1661.
4. Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95(10):865-872.
5. Jacobs JJ, Cooper HJ, Urban RM, Wixson RL, Della Valle CJ. What do we know about taper corrosion in total hip arthroplasty? J Arthroplasty. 2014;29(4):668-669.
6. Pastides PS, Dodd M, Sarraf KM, Willis-Owen CA. Trunnionosis: a pain in the neck. World J Orthop. 2013;4(4):161-166.
7. Shulman RM, Zywiel MG, Gandhi R, Davey JR, Salonen DC. Trunnionosis: the latest culprit in adverse reactions to metal debris following hip arthroplasty. Skeletal Radiol. 2015;44(3):433-440.
8. Mihalko WM, Wimmer MA, Pacione CA, Laurent MP, Murphy RF, Rider C. How have alternative bearings and modularity affected revision rates in total hip arthroplasty? Clin Orthop Relat Res. 2014;472(12):3747-3758.
9. Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96(1):e4.
10. Bartelt RB, Yuan BJ, Trousdale RT, Sierra RJ. The prevalence of groin pain after metal-on-metal total hip arthroplasty and total hip resurfacing. Clin Orthop Relat Res. 2010;468(9):2346-2356.
11. Bozic KJ, Lau EC, Ong KL, Vail TP, Rubash HE, Berry DJ. Comparative effectiveness of metal-on-metal and metal-on-polyethylene bearings in Medicare total hip arthroplasty patients. J Arthroplasty. 2012;27(8 suppl):37-40.
12. Cuckler JM. Metal-on-metal surface replacement: a triumph of hope over reason: affirms. Orthopedics. 2011;34(9):e439-e441.
13. de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: an analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287-2293.
14. Fehring TK, Odum S, Sproul R, Weathersbee J. High frequency of adverse local tissue reactions in asymptomatic patients with metal-on-metal THA. Clin Orthop Relat Res. 2014;472(2):517-522.
15. Hasegawa M, Yoshida K, Wakabayashi H, Sudo A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics. 2013;36(5):e606-e612.
16. Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty—a changing paradigm. J Arthroplasty. 2014;29(6):1285-1288.
17. Wyles CC, Van Demark RE 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):509-516.
18. Gioe TJ, Sharma A, Tatman P, Mehle S. Do “premium” joint implants add value?: Analysis of high cost joint implants in a community registry. Clin Orthop Relat Res. 2011;469(1):48-54.
19. D’Antonio JA, Capello WN, Naughton M. Ceramic bearings for total hip arthroplasty have high survivorship at 10 years. Clin Orthop Relat Res. 2012;470(2):373-381.
20. D’Antonio JA, Capello WN, Naughton M. High survivorship with a titanium-encased alumina ceramic bearing for total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):611-616.
Does Accelerated Physical Therapy After Elective Primary Hip and Knee Arthroplasty Facilitate Early Discharge?
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are among the most effective surgical procedures in modern medicine. Use of primary THA in the United States is projected to increase by 174% by 2030, to 532,000 cases annually, and the estimate for TKA is even greater.1 Hospital length of stay (LOS) accounts for a significant portion of the overall cost of these procedures. Reducing LOS to limit costs without compromising patient safety, satisfaction, and outcomes remains the goal at all joint arthroplasty centers. Rapid-recovery or fast-track clinical pathways limiting opioid use and emphasizing patient education and early (day-of-surgery) mobilization have been shown to reduce LOS without compromising patient outcomes.2-5 Factors correlated with LOS after THA include surgical approach, use of multimodal analgesia, obesity, age, and social situations or living conditions.4,6-10
Our institution recently implemented a protocol in which certified physical therapists provide accelerated (day-of-surgery) physical therapy (PT) for all total joint arthroplasty patients. For the study reported here, we hypothesized that, compared with PT started on postoperative day 1 (POD-1), PT started day of surgery (Day 0) would result in shorter LOS for unilateral primary THA and TKA patients. In addition, we wanted to evaluate any predischarge differences in function, as measured by gait distance, between the groups.
Methods
After obtaining Institutional Review Board approval, we retrospectively evaluated use of the new postoperative protocol (Day 0 PT) for primary THA and TKA patients. We reviewed all cases of primary unilateral THA or TKA performed by a single surgeon over the 12-month period immediately following initiation of the protocol. There were 116 THA cases and 126 TKA cases. Charts were reviewed for patient demographics, intraoperative data, in-hospital course, and PT session notes. Patients who had a PT session at any point on day of surgery were designated the Day 0 group, and patients who had PT starting the next day (POD-1) were designated the Non-Day 0 group. Although the medical records showed that Day 0 PT had been ordered in all cases, not all patients received PT on the day of their surgery; the most common reason was that they returned from postanesthesia care after the physical therapists’ work shift had ended. Another reason was patient noncompliance or unwillingness stemming from the prolonged effects of general anesthesia, diminished mental orientation, excess fatigue, or inadequate pain control. PT sessions after THA and TKA remained consistent over the study period, with twice daily sessions directed at patient mobility, range of motion, and gentle strengthening exercise. PT was performed only with patient consent.
Surgery
A combination of general and spinal anesthesia was used in almost all THA and TKA cases. In <5% of cases, either the patient refused spinal anesthesia, or it was unsuccessful. In addition, tranexamic acid was administered to limit blood loss in all THA and TKA cases. Of the 116 THAs performed over the study period, 3 were excluded (see below). Of the 113 patients included in the study, 88 (77.9%) used a minimally invasive posterolateral approach, 18 (15.9%) a direct anterior approach, and 7 (6.2%) an anterolateral approach. All THAs were performed with conventional instruments and uncemented components. All TKAs were performed with a standard medial parapatellar approach, conventional instruments, and a tourniquet; in each case, the patella was resurfaced, and cemented fixation was used. Drains were not used in any THA or TKA cases. A local anesthetic cocktail (100 mL of 0.25% ropivacaine, 15 mL of 0.5% ropivacaine, and 1 mL of 1:1000 epinephrine) was injected for postoperative analgesia in all THA and TKA cases.
There were 3 important intraoperative findings in the THA Day 0 group: 2 cases of incidental gluteus medius tendon tears requiring repair and 1 case of nondisplaced calcar fracture treated with a cerclage cable. The THA Non-Day 0 group and both TKA groups had no major intraoperative findings.
Physical Therapy
Day-of-surgery PT was ordered for all patients. Patients did not receive formal PT before surgery. The PT protocol consisted of subjective assessment of patient condition, expectations, and goals; lower limb strengthening exercises; and maximum gait training with use of an assistive device as tolerated. Standard hip movement restrictions were ordered for posterolateral approach patients to protect the soft-tissue repair. Continuous passive motion (CPM) was not used during this study period.
Discharge Criteria
Patients were cleared for discharge by a multidisciplinary team using several criteria: no medical condition that would require readmission, intact surgical incision without discharge or concerning erythema, adequate analgesia (oral medications), intact neurovascular examination, and PT goals achieved (independence with bed mobility, transfers, standing balance, and minimum gait distance of 150 feet). Patients who could not be discharged home because of family or occupation issues or because of problems with gait or transfer were referred to skilled nursing or home healthcare. Follow-up for wound assessment and for examination of radiographs and functional range of motion was planned for 2 to 3 weeks after surgery (all patients followed up). Two patients, 1 in the THA Non-Day 0 group and 1 in the TKA Day 0 group, had a mechanical fall 1 day before discharge, but there were no complication-related discharge delays. In addition, there were no readmissions during the first 4 weeks after surgery.
Excluded Patients
Of the 116 THA cases, 113 (63 Day 0, 50 Non-Day 0) were analyzed. To establish homogeneity between groups and remove potential confounding factors, we excluded 4 THA patients (all Non-Day 0) from analysis because of medical complications prolonging LOS. In 1 of these cases, the patient developed respiratory insufficiency and myocardial infarction on POD-3, and critical care support was required (LOS, 16 days). In another case, anticoagulation treatment led to the development of a hip hematoma on POD-9 and to treatment (evacuation) in the operating room (LOS, 14 days). The other 2 cases involved exacerbation of dysphagia from preexisting myasthenia gravis (LOS, 5 days) and Ogilvie syndrome, managed conservatively (LOS, 9 days).
Of the 126 TKA cases, 123 (97 Day 0, 26 Non-Day 0) were analyzed. Three TKA patients were excluded because of prolonged hospitalization for medical reasons: One developed a deep vein thrombosis, 1 acquired Clostridium difficile colitis (history of lung transplantation, multiple immunosuppressive drugs), and 1 developed respiratory insufficiency from asthma exacerbation.
Statistical Analysis
Power analysis (G*Power) was used to determine an appropriate sample size for comparison.11 Given a previously published mean LOS after THA of 4 days, the hypothesized mean LOS reducing that by at least 0.5 day to 3.5 days, a significance level set at 5%, a power of test set at 0.95, and an allocation ratio of 1, a minimum of 23 subjects would be needed in each group to attain a statistically significant difference using the nonparametric Mann-Whitney test. The Shapiro-Wilk test was used to assess data normality. Regarding statistical significance, the Mann-Whitney U test was used for non-normally distributed data, the 2-sided Fisher exact test and χ2 test for qualitative data and contingency, and the 2-tailed, unpaired, independent-samples Student t test for normally distributed data. Data were analyzed with SPSS Statistics for Windows Version 20 (IBM).
Results
TKA and THA patients had similar demographic profiles, types of anesthesia, operating room and surgery times, surgical approaches, and total number of PT sessions before discharge. Estimated blood loss, however, was significantly (P < .05) higher for Non-Day 0 patients than for Non-Day 0 patients (Table 1).
Mean (SD) distance ambulated during first PT session was 2-fold farther (P = .014) for Non-Day 0 patients, 84.1 (10.4) feet, than for Day 0 patients, 42.1 (6.4) feet. On POD-1, mean (SD) gait was significantly (P = .019) longer for Day 0 patients, 162.4 (12.9) feet, than for Non-Day 0 patients, 118 (11.7) feet (Figure 2).
In TKA patients, although mean (SD) distance ambulated tended to be farther for the Day 0 group than for the Non-Day 0 group—114 (12.3) feet on POD-1 and 176 (15.2) feet on POD-2 for Day 0 vs 94 (22.2) feet on POD-1 and 148 (22.1) feet on POD-2 for Non-Day 0—the differences were not statistically significant. In addition, knee arc of motion during first PT session was statistically significantly (P = .3) higher for Day 0 patients, 69.1° (18.7°), than for Non-Day 0 patients, 61.7° (18.8°).
Statistical analysis revealed no difference in LOS based on surgical approach to the hip: 2.4 days for posterolateral (2.2 days for Day 0 and 2.6 days for Non-Day 0; P = .06); 2.1 days for direct anterior (2.1 days for Day 0 and 2.0 days for Non-Day 0; P = .7); and 2.7 days for anterolateral (3.0 days for Day 0 and 2.6 days for Non-Day 0; P = .6).
Discussion
Protocols for PT after THA and TKA remain unstandardized and largely dependent on institutions and surgeons. Factors permitting successful implementation of accelerated rehabilitation include patient motivation, adequate analgesia, and adequate support by physical therapists.12 A potential risk associated with accelerated PT after THA is dislocation, which did not occur in any patient in our Day 0 group. Other risks are increased pain and swelling leading to increased risk of falling and bleeding, which were not observed in our cohort. Although Day 0 PT was ordered in all cases in this study, only 55% of THA patients and 79% of TKA patients received PT the same day as their surgery. The delay can be addressed by making physical therapists’ work shifts more flexible for cases that finish later in the day and by providing preoperative education on the importance of day-of-surgery PT. Dr. Incavo and office staff routinely discuss discharge planning with all patients before surgery, but there was no stimulus protocol or communication to discuss or emphasize LOS with patients before surgery, and there was no questionnaire or survey given to assess patient expectations about PT and discharge.
Our finding of no statistically significant reduction in mean LOS after implementation of accelerated PT for THA or TKA differs from findings in multiple other reports.4,5,13-17 Baseline or control group mean LOS tended to be higher in previous studies3,5,18-23 (3.4-11.4 days) than in our control group (2.5 days) (Table 2).
Conclusion
These results provide useful information for providers who are managing primary THA and TKA cases and seeking continual improvement in postoperative patient care and better resource allocation. Hospitals, particularly those operating in bundled-care environments, are increasingly coming under scrutiny to control costs. Our study results showed that the costs associated with Day 0 PT are justified for THA but not for TKA.
Am J Orthop. 2016;45(6):E337-E342. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32.
3. den Hartog YM, Mathijssen NM, Vehmeijer SB. Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop. 2013;84(5):444-447.
4. Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79(2):168-173.
5. Robbins CE, Casey D, Bono JV, Murphy SB, Talmo CT, Ward DM. A multidisciplinary total hip arthroplasty protocol with accelerated postoperative rehabilitation: does the patient benefit? Am J Orthop. 2014;43(4):178-181.
6. den Hartog YM, Mathijssen NM, Hannink G, Vehmeijer SB. Which patient characteristics influence length of hospital stay after primary total hip arthroplasty in a ‘fast-track’ setting? Bone Joint J. 2015;97(1):19-23.
7. Forrest G, Fuchs M, Gutierrez A, Girardy J. Factors affecting length of stay and need for rehabilitation after hip and knee arthroplasty. J Arthroplasty. 1998;13(2):186-190.
8. Foote J, Panchoo K, Blair P, Bannister G. Length of stay following primary total hip replacement. Ann R Coll Surg Engl. 2009;91(6):500-504.
9. Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467(6):1400-1411.
10. Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg Am. 2007;89(6):1153-1160.
11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
12. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. 2007;22(7 suppl 3):12-15.
13. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
14. Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82(6):679-684.
15. Husted H, Jensen CM, Solgaard S, Kehlet H. Reduced length of stay following hip and knee arthroplasty in Denmark 2000-2009: from research to implementation. Arch Orthop Trauma Surg. 2012;132(1):101-104.
16. Berger RA, Sanders SA, Thill ES, Sporer SM, Della Valle C. Newer anesthesia and rehabilitation protocols enable outpatient hip replacement in selected patients. Clin Orthop Relat Res. 2009;467(6):1424-1430.
17. Peck CN, Foster A, McLauchlan GJ. Reducing incision length or intensifying rehabilitation: what makes the difference to length of stay in total hip replacement in a UK setting? Int Orthop. 2006;30(5):395-398.
18. Isaac D, Falode T, Liu P, I’Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee. 2005;12(5):346-350.
19. Labraca NS, Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Sánchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25(6):557-566.
20. Larsen K, Hansen TB, Søballe K. Hip arthroplasty patients benefit from accelerated perioperative care and rehabilitation: a quasi-experimental study of 98 patients. Acta Orthop. 2008;79(5):624-630.
21. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761-772.
22. Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop. 2008;79(2):149-159.
23. Wellman SS, Murphy AC, Gulcynski D. Murphy SB. Implementation of an accelerated mobilization protocol following primary total hip arthroplasty: impact on length of stay and disposition. Curr Rev Musculoskelet Med. 2011;4(3):84-90.