User login
The Impact of End of Life Chemotherapy on Quality of Life and Life Expectancy
Background: A majority of end-stage cancer patients are receiving chemotherapy, regardless of performance status, in the last months of life. Oncologists continue to prescribe disease targeted therapy for patients enrolled in palliative or hospice care in an attempt to alleviate symptoms and extend life expectancy. Research indicates that chemotherapy at end of life does not accomplish either of these goals of care, and may actually do more harm than good.
Methods: An evidence based research review indicates that > 50% of all cancer patients are receiving chemotherapy during their last 4-6 months of life. Data supports a decreased quality of life and decreased life expectancy for patients receiving chemotherapy during the last months of life. There is an increase in visits to the emergency department, hospitalizations, and death in the hospital when cancer patients are actively receiving chemotherapy during end of life.
Overutilization of chemotherapy treatment is poor quality care leading to adverse patient outcomes. The intent of hospice care is to meet the physical, emotional, social, and spiritual needs of a dying patient and their family. The focus of care is on comfort, not cure. The intent of palliative care focuses on symptom control and may include a combination of comfort measures and curative interventions. This review supports further investigation into the practice of chemotherapy administration in terminally ill patients.
Background: A majority of end-stage cancer patients are receiving chemotherapy, regardless of performance status, in the last months of life. Oncologists continue to prescribe disease targeted therapy for patients enrolled in palliative or hospice care in an attempt to alleviate symptoms and extend life expectancy. Research indicates that chemotherapy at end of life does not accomplish either of these goals of care, and may actually do more harm than good.
Methods: An evidence based research review indicates that > 50% of all cancer patients are receiving chemotherapy during their last 4-6 months of life. Data supports a decreased quality of life and decreased life expectancy for patients receiving chemotherapy during the last months of life. There is an increase in visits to the emergency department, hospitalizations, and death in the hospital when cancer patients are actively receiving chemotherapy during end of life.
Overutilization of chemotherapy treatment is poor quality care leading to adverse patient outcomes. The intent of hospice care is to meet the physical, emotional, social, and spiritual needs of a dying patient and their family. The focus of care is on comfort, not cure. The intent of palliative care focuses on symptom control and may include a combination of comfort measures and curative interventions. This review supports further investigation into the practice of chemotherapy administration in terminally ill patients.
Background: A majority of end-stage cancer patients are receiving chemotherapy, regardless of performance status, in the last months of life. Oncologists continue to prescribe disease targeted therapy for patients enrolled in palliative or hospice care in an attempt to alleviate symptoms and extend life expectancy. Research indicates that chemotherapy at end of life does not accomplish either of these goals of care, and may actually do more harm than good.
Methods: An evidence based research review indicates that > 50% of all cancer patients are receiving chemotherapy during their last 4-6 months of life. Data supports a decreased quality of life and decreased life expectancy for patients receiving chemotherapy during the last months of life. There is an increase in visits to the emergency department, hospitalizations, and death in the hospital when cancer patients are actively receiving chemotherapy during end of life.
Overutilization of chemotherapy treatment is poor quality care leading to adverse patient outcomes. The intent of hospice care is to meet the physical, emotional, social, and spiritual needs of a dying patient and their family. The focus of care is on comfort, not cure. The intent of palliative care focuses on symptom control and may include a combination of comfort measures and curative interventions. This review supports further investigation into the practice of chemotherapy administration in terminally ill patients.
Integrated Outpatient Palliative Care for Patients with Advanced Cancer: A Systematic Review and Meta-Analysis
Background: Despite increasing emphasis on integration of palliative care with disease-directed care for advanced cancer, the nature of this integration and its effects on patient and caregiver outcomes are not well understood.
Methods: We evaluated the effects of integrated outpatient palliative and oncology care for advanced cancer on patient and caregiver outcomes. Following a standard protocol (PROSPERO: CRD42017057541), investigators independently screened reports to identify randomized controlled trials or quasi-experimental studies that evaluated the effect of integrated outpatient palliative and oncology care interventions on quality of life, survival, and healthcare utilization among adults with advanced cancer. Data sources were English-language peer-reviewed publications in PubMed, CINAHL, and Cochrane Central through November 2016. We subsequently updated our PubMed search through July 2018. Data were synthesized using random-effects meta-analyses, supplemented with qualitative methods when necessary.
Results: Eight randomized controlled and two cluster randomized trials were included. Most patients had multiple advanced cancers, with median time from diagnosis or recurrence to enrollment ranging from eight to 12 weeks. All interventions included a multidisciplinary team, were classified as “moderately integrated,” and addressed physical and psychological symptoms. In a meta-analysis, short-term quality of life improved; symptom burden improved; and all-cause mortality decreased. Qualitative analyses revealed no association between integration elements, palliative care intervention elements, and intervention impact. Utilization and caregiver outcomes were often not reported.
Conclusion: Moderately integrated palliative and oncology outpatient interventions had positive effects on short-term quality of life, symptom burden, and survival. Evidence for effects on healthcare utilization and caregiver outcomes remains sparse.
Background: Despite increasing emphasis on integration of palliative care with disease-directed care for advanced cancer, the nature of this integration and its effects on patient and caregiver outcomes are not well understood.
Methods: We evaluated the effects of integrated outpatient palliative and oncology care for advanced cancer on patient and caregiver outcomes. Following a standard protocol (PROSPERO: CRD42017057541), investigators independently screened reports to identify randomized controlled trials or quasi-experimental studies that evaluated the effect of integrated outpatient palliative and oncology care interventions on quality of life, survival, and healthcare utilization among adults with advanced cancer. Data sources were English-language peer-reviewed publications in PubMed, CINAHL, and Cochrane Central through November 2016. We subsequently updated our PubMed search through July 2018. Data were synthesized using random-effects meta-analyses, supplemented with qualitative methods when necessary.
Results: Eight randomized controlled and two cluster randomized trials were included. Most patients had multiple advanced cancers, with median time from diagnosis or recurrence to enrollment ranging from eight to 12 weeks. All interventions included a multidisciplinary team, were classified as “moderately integrated,” and addressed physical and psychological symptoms. In a meta-analysis, short-term quality of life improved; symptom burden improved; and all-cause mortality decreased. Qualitative analyses revealed no association between integration elements, palliative care intervention elements, and intervention impact. Utilization and caregiver outcomes were often not reported.
Conclusion: Moderately integrated palliative and oncology outpatient interventions had positive effects on short-term quality of life, symptom burden, and survival. Evidence for effects on healthcare utilization and caregiver outcomes remains sparse.
Background: Despite increasing emphasis on integration of palliative care with disease-directed care for advanced cancer, the nature of this integration and its effects on patient and caregiver outcomes are not well understood.
Methods: We evaluated the effects of integrated outpatient palliative and oncology care for advanced cancer on patient and caregiver outcomes. Following a standard protocol (PROSPERO: CRD42017057541), investigators independently screened reports to identify randomized controlled trials or quasi-experimental studies that evaluated the effect of integrated outpatient palliative and oncology care interventions on quality of life, survival, and healthcare utilization among adults with advanced cancer. Data sources were English-language peer-reviewed publications in PubMed, CINAHL, and Cochrane Central through November 2016. We subsequently updated our PubMed search through July 2018. Data were synthesized using random-effects meta-analyses, supplemented with qualitative methods when necessary.
Results: Eight randomized controlled and two cluster randomized trials were included. Most patients had multiple advanced cancers, with median time from diagnosis or recurrence to enrollment ranging from eight to 12 weeks. All interventions included a multidisciplinary team, were classified as “moderately integrated,” and addressed physical and psychological symptoms. In a meta-analysis, short-term quality of life improved; symptom burden improved; and all-cause mortality decreased. Qualitative analyses revealed no association between integration elements, palliative care intervention elements, and intervention impact. Utilization and caregiver outcomes were often not reported.
Conclusion: Moderately integrated palliative and oncology outpatient interventions had positive effects on short-term quality of life, symptom burden, and survival. Evidence for effects on healthcare utilization and caregiver outcomes remains sparse.
The Impact of Registered Dietitian Staffing and Nutrition Practices in High-Risk Cancer Patients Across the Veterans Health Administration
Background: Malnutrition in cancer patients has a significant correlation with disability, dysfunction and death, as well as increased patient care costs, neutropenia, reduced quality of life, fall risk, fractures, nosocomial infections, and longer treatment durations 1-3. Registered dietitian (RD) involvement early on may increase recognition of malnutrition for at-risk patients. Guidelines for nutrition staffing in cancer centers is illdefined in the literature, with few existing recommendations.
Methods: In Phase 1, a survey of RDs across VHA was conducted to determine current referral and staffing practices surrounding nutrition care and services in outpatient oncology clinics. The survey was administered to RDs who devote some or all of their time to oncology nutrition in the outpatient setting and participate on 1 of 2 popular VHA listservs: a nutrition support listserv, and an oncology nutrition listserv.
Phase 2 will be a multi-site, retrospective, chart analysis among 20 VA facilities who treat cancer patients in the outpatient setting. Site investigators, divided into proactive vs. reactive nutrition practices based on Phase 1 survey results, will be instructed to obtain a list of patients diagnosed with high nutrition risk cancers during 2016 and 2017.
Primary outcomes measured will include weight loss, percent maximum weight change over speci ed timeframes, diagnosis of malnutrition, and reported breaks in treatment. Secondary outcomes include overall survival and disease-free survival. For all comparisons, P < 0.05 will be considered statistically signifcant.
Discussion: The data from 46 sites completing the national survey show that RD staffing practices vary widely across VA cancer centers. Few centers staff full time or dedicated oncology RDs independent of patient caseload, with the median oncology dedicated RD FTE being 0.5. Consult and referral practices dictating nutrition intervention were found to be reported as 17% proactive, 25% reactive, and 58% a combination of both practices. Phase 2 results seek to compare patient outcomes with RD staffing and nutrition care practices to determine much needed guidelines for effective nutrition delivery in VHA cancer centers across the U.S.
Background: Malnutrition in cancer patients has a significant correlation with disability, dysfunction and death, as well as increased patient care costs, neutropenia, reduced quality of life, fall risk, fractures, nosocomial infections, and longer treatment durations 1-3. Registered dietitian (RD) involvement early on may increase recognition of malnutrition for at-risk patients. Guidelines for nutrition staffing in cancer centers is illdefined in the literature, with few existing recommendations.
Methods: In Phase 1, a survey of RDs across VHA was conducted to determine current referral and staffing practices surrounding nutrition care and services in outpatient oncology clinics. The survey was administered to RDs who devote some or all of their time to oncology nutrition in the outpatient setting and participate on 1 of 2 popular VHA listservs: a nutrition support listserv, and an oncology nutrition listserv.
Phase 2 will be a multi-site, retrospective, chart analysis among 20 VA facilities who treat cancer patients in the outpatient setting. Site investigators, divided into proactive vs. reactive nutrition practices based on Phase 1 survey results, will be instructed to obtain a list of patients diagnosed with high nutrition risk cancers during 2016 and 2017.
Primary outcomes measured will include weight loss, percent maximum weight change over speci ed timeframes, diagnosis of malnutrition, and reported breaks in treatment. Secondary outcomes include overall survival and disease-free survival. For all comparisons, P < 0.05 will be considered statistically signifcant.
Discussion: The data from 46 sites completing the national survey show that RD staffing practices vary widely across VA cancer centers. Few centers staff full time or dedicated oncology RDs independent of patient caseload, with the median oncology dedicated RD FTE being 0.5. Consult and referral practices dictating nutrition intervention were found to be reported as 17% proactive, 25% reactive, and 58% a combination of both practices. Phase 2 results seek to compare patient outcomes with RD staffing and nutrition care practices to determine much needed guidelines for effective nutrition delivery in VHA cancer centers across the U.S.
Background: Malnutrition in cancer patients has a significant correlation with disability, dysfunction and death, as well as increased patient care costs, neutropenia, reduced quality of life, fall risk, fractures, nosocomial infections, and longer treatment durations 1-3. Registered dietitian (RD) involvement early on may increase recognition of malnutrition for at-risk patients. Guidelines for nutrition staffing in cancer centers is illdefined in the literature, with few existing recommendations.
Methods: In Phase 1, a survey of RDs across VHA was conducted to determine current referral and staffing practices surrounding nutrition care and services in outpatient oncology clinics. The survey was administered to RDs who devote some or all of their time to oncology nutrition in the outpatient setting and participate on 1 of 2 popular VHA listservs: a nutrition support listserv, and an oncology nutrition listserv.
Phase 2 will be a multi-site, retrospective, chart analysis among 20 VA facilities who treat cancer patients in the outpatient setting. Site investigators, divided into proactive vs. reactive nutrition practices based on Phase 1 survey results, will be instructed to obtain a list of patients diagnosed with high nutrition risk cancers during 2016 and 2017.
Primary outcomes measured will include weight loss, percent maximum weight change over speci ed timeframes, diagnosis of malnutrition, and reported breaks in treatment. Secondary outcomes include overall survival and disease-free survival. For all comparisons, P < 0.05 will be considered statistically signifcant.
Discussion: The data from 46 sites completing the national survey show that RD staffing practices vary widely across VA cancer centers. Few centers staff full time or dedicated oncology RDs independent of patient caseload, with the median oncology dedicated RD FTE being 0.5. Consult and referral practices dictating nutrition intervention were found to be reported as 17% proactive, 25% reactive, and 58% a combination of both practices. Phase 2 results seek to compare patient outcomes with RD staffing and nutrition care practices to determine much needed guidelines for effective nutrition delivery in VHA cancer centers across the U.S.
Impact of Integrated Oncology- Palliative Care Outpatient Model on Trends of Palliative Care and Hospice Care Referrals From Oncology
Background: A commonly voiced concern of oncologists, regarding the introduction of palliative care, is that patients might be immediately steered to hospice care and away from oncology care.
Objective: To assess the impact of oncology-palliative care collaboration on trends of referrals to palliative and hospice care.
Methods: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
- Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
- Shared palliative care and oncology clinic appointments;
- Introduction of palliative care for every new oncology clinic patient, for advance care planning;
- Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management; and
- Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
Measurements: We examined the following metrics for FY15, FY16, FY17, and FY18.
- Total number of palliative care consults;
- Number of palliative care consults from oncology;
- Percentage palliative care consults from oncology [(item 2 × 100) / item 1];
- Total number of referrals to hospice care;
- Number of referrals to hospice care from oncology; and
- Percentage hospice care referrals from oncology [(item 5 × 100) / item 4].
Results: During the period of FY15 to FY18, there was a consistent increase in total palliative care consults (355, 394, 549, and 570 respectively). There also was a consistent increase in percentage palliative care consults from oncology (24%, 34%, 38%, and 40% respectively) without an increase in percentage hospice care referrals from oncology.
Conclusion: A common concern is that palliative care in oncology care will result in patients being immediately steered to hospice care and away from continued oncology care. Although it was limited to a single clinical setting, our intervention resulted in increased palliative care consults from oncology without a proportionate increase in hospice care referrals from oncology during the same time-period, suggesting that earlier access to palliative care did not result in immediate transition to hospice care. Palliative care offers opportunities for goals of care conversations and symptom management in oncology care, prior to transition to hospice care. Future implications include robust studies to further test these findings, review of structure and training of oncology-palliative care teams, and systems redesign to develop dyad or shared clinic models.
Background: A commonly voiced concern of oncologists, regarding the introduction of palliative care, is that patients might be immediately steered to hospice care and away from oncology care.
Objective: To assess the impact of oncology-palliative care collaboration on trends of referrals to palliative and hospice care.
Methods: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
- Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
- Shared palliative care and oncology clinic appointments;
- Introduction of palliative care for every new oncology clinic patient, for advance care planning;
- Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management; and
- Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
Measurements: We examined the following metrics for FY15, FY16, FY17, and FY18.
- Total number of palliative care consults;
- Number of palliative care consults from oncology;
- Percentage palliative care consults from oncology [(item 2 × 100) / item 1];
- Total number of referrals to hospice care;
- Number of referrals to hospice care from oncology; and
- Percentage hospice care referrals from oncology [(item 5 × 100) / item 4].
Results: During the period of FY15 to FY18, there was a consistent increase in total palliative care consults (355, 394, 549, and 570 respectively). There also was a consistent increase in percentage palliative care consults from oncology (24%, 34%, 38%, and 40% respectively) without an increase in percentage hospice care referrals from oncology.
Conclusion: A common concern is that palliative care in oncology care will result in patients being immediately steered to hospice care and away from continued oncology care. Although it was limited to a single clinical setting, our intervention resulted in increased palliative care consults from oncology without a proportionate increase in hospice care referrals from oncology during the same time-period, suggesting that earlier access to palliative care did not result in immediate transition to hospice care. Palliative care offers opportunities for goals of care conversations and symptom management in oncology care, prior to transition to hospice care. Future implications include robust studies to further test these findings, review of structure and training of oncology-palliative care teams, and systems redesign to develop dyad or shared clinic models.
Background: A commonly voiced concern of oncologists, regarding the introduction of palliative care, is that patients might be immediately steered to hospice care and away from oncology care.
Objective: To assess the impact of oncology-palliative care collaboration on trends of referrals to palliative and hospice care.
Methods: In January 2015, we implemented an integrated oncology-palliative care clinic model with the following elements:
- Pre-clinic “huddle” among palliative care and oncology staff to identify palliative care needs for patients;
- Shared palliative care and oncology clinic appointments;
- Introduction of palliative care for every new oncology clinic patient, for advance care planning;
- Concurrent oncology and palliative care follow-up for all high-risk patients (aggressive histology, progressing disease, etc.) for goals of care discussions and symptom management; and
- Palliative care and oncology staff co-managing oncology patients enrolled in hospice care.
Measurements: We examined the following metrics for FY15, FY16, FY17, and FY18.
- Total number of palliative care consults;
- Number of palliative care consults from oncology;
- Percentage palliative care consults from oncology [(item 2 × 100) / item 1];
- Total number of referrals to hospice care;
- Number of referrals to hospice care from oncology; and
- Percentage hospice care referrals from oncology [(item 5 × 100) / item 4].
Results: During the period of FY15 to FY18, there was a consistent increase in total palliative care consults (355, 394, 549, and 570 respectively). There also was a consistent increase in percentage palliative care consults from oncology (24%, 34%, 38%, and 40% respectively) without an increase in percentage hospice care referrals from oncology.
Conclusion: A common concern is that palliative care in oncology care will result in patients being immediately steered to hospice care and away from continued oncology care. Although it was limited to a single clinical setting, our intervention resulted in increased palliative care consults from oncology without a proportionate increase in hospice care referrals from oncology during the same time-period, suggesting that earlier access to palliative care did not result in immediate transition to hospice care. Palliative care offers opportunities for goals of care conversations and symptom management in oncology care, prior to transition to hospice care. Future implications include robust studies to further test these findings, review of structure and training of oncology-palliative care teams, and systems redesign to develop dyad or shared clinic models.
Fatal Drug-Resistant Invasive Pulmonary Aspergillus fumigatus in a 56-Year-Old Immunosuppressed Man (FULL)
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
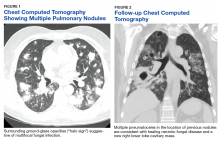
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
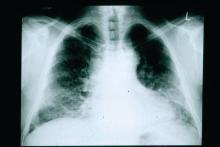
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
Historically, aspergillosis in patients with hematopoietic stem cell transplantation (HSCT) has carried a high mortality rate. However, recent data demonstrate a dramatic improvement in outcomes for patients with HSCT: 90-day survival increased from 22% before 2000 to 45% over the past 15 years.1 Improved outcomes coincide with changes in transplant immunosuppression practices, use of cross-sectional imaging for early disease identification, galactomannan screening, and the development of novel treatment options.
Voriconazole is an azole drug that blocks the synthesis of ergosterol, a vital component of the cellular membrane of fungi. Voriconazole was approved in 2002 after a clinical trial demonstrated an improvement in 50% of patients with invasive aspergillosis in the voriconazole arm vs 30% in the amphotericin B arm at 12 weeks.2 Amphotericin B is a polyene antifungal drug that binds with ergosterol, creating leaks in the cell membrane that lead to cellular demise. Voriconazole quickly became the first-line therapy for invasive aspergillosis and is recommended by both the Infectious Disease Society of American (IDSA) and the European Conference on Infections in Leukemia.3
Case Presentation
A 55-year-old man with high-risk chronic myelogenous leukemia (CML) underwent a 10 of 10 human leukocyte antigen allele and antigen-matched peripheral blood allogeneic HSCT with a myeloablative-conditioning regimen of busulfan and cyclophosphamide, along with prophylactic voriconazole, sulfamethoxazole/trimethoprim, and acyclovir. After successful engraftment (without significant neutropenia), his posttransplant course was complicated by grade 2 graft vs host disease (GVHD) of the skin, eyes, and liver, which responded well to steroids and tacrolimus. Voriconazole was continued for 5 months until immunosuppression was minimized (tacrolimus 1 mg twice daily). Two months later, the patient’s GVHD worsened, necessitating treatment at an outside hospital with high-dose prednisone (2 mg/kg/d) and cyclosporine (300 mg twice daily). Voriconazole prophylaxis was not reinitiated at that time.
One year later, at a routine follow-up appointment, the patient endorsed several weeks of malaise, weight loss, and nonproductive cough. The patient’s immunosuppression recently had been reduced to 1 mg/kg/d of prednisone and 100 mg of cyclosporine twice daily. A chest X-ray demonstrated multiple pulmonary nodules; follow-up chest computed tomography (CT) confirmed multiple nodular infiltrates with surrounding ground-glass opacities suspicious with a fungal infection (Figure 1).
Treatment with oral voriconazole (300 mg twice daily) was initiated for probable pulmonary aspergillosis. Cyclosporine (150 mg twice daily) and prednisone (1 mg/kg/d) were continued throughout treatment out of concern for hepatic GVHD. The patient’s symptoms improved over the next 10 days, and follow-up chest imaging demonstrated improvement.
Two weeks after initiation of voriconazole treatment, the patient developed a new productive cough and dyspnea, associated with fevers and chills. Repeat imaging revealed right lower-lobe pneumonia. The serum voriconazole trough level was checked and was 3.1 mg/L, suggesting therapeutic dosing. The patient subsequently developed acute respiratory distress syndrome and required intubation and mechanical ventilation. Repeat BAL sampling demonstrated multidrug-resistant Escherichia coli, a BAL galactomannan level of 2.0 ODI, and negative fungal cultures. The patient’s hospital course was complicated by profound hypoxemia, requiring prone positioning and neuromuscular blockade. He was treated with meropenem and voriconazole. His immunosuppression was reduced, but he rapidly developed acute liver injury from hepatic GVHD that resolved after reinitiation of cyclosporine and prednisone at 0.75 mg/kg/d.
The patient improved over the next 3 weeks and was successfully extubated. Repeat chest CT imaging demonstrated numerous pneumatoceles in the location of previous nodules, consistent with healing necrotic fungal disease, and a new right lower-lobe cavitary mass (Figure 2). Two days after transferring out of the intensive care unit, the patient again developed hypoxemia and fevers to 39° C. Bronchoscopy with BAL of the right lower lobe revealed positive A fumigatus and Rhizopus sp polymerase chain reaction (PCR) assays, although fungal cultures were positive only for A fumigatus. Liposomal amphotericin B (5 mg/kg) was added to voriconazole therapy to treat mucormycosis and to provide a second active agent against A fumigatus.
Unfortunately, the patient’s clinical status continued to deteriorate with signs of progressive respiratory failure and infection despite empiric, broad-spectrum antibiotics and dual antifungal therapy. His serum voriconazole level continued to be therapeutic at 1.9 mg/L. The patient declined reintubation and invasive mechanical ventilation, and he ultimately transitioned to comfort measures and died with his family at the bedside.
Autopsy demonstrated widely disseminated Aspergillus infection as the cause of death, with evidence of myocardial, neural, and vascular invasion of A fumigatus (Figures 3 and 4).
Discussion
This case of fatal, progressive, invasive, pulmonary aspergillosis demonstrates several important factors in the treatment of patients with this disease. Treatment failure usually relates to any of 4 possible factors: host immune status, severity or burden of disease, appropriate dosing of antifungal agents, and drug resistance. This patient’s immune system was heavily suppressed for a prolonged period. Attempts at reducing immunosuppression to the minimal required dosage to prevent a GVHD flare were unsuccessful and became an unmodifiable risk factor, a major contributor to his demise.
The risks of continuous high-dose immunosuppression in steroid-refractory GVHD is well understood and has been previously demonstrated to have up to 50% 4-year nonrelapse mortality, mainly due to overwhelming bacterial, viral, and fungal infections.4 All attempts should be made to cease or reduce immunosuppression in the setting of a severe infection, although this is sometimes impossible as in this case.
The patient’s disease burden was significant as evidenced by the bilateral, multifocal pulmonary nodules seen on chest imaging and the disseminated disease found at postmortem examination. His initial improvement in symptoms with voriconazole and the evolution of his images (with many of his initial pulmonary nodules becoming pneumatoceles) suggested a temporary positive immune response. The authors believe that the Rhizopus in his sputum represents noninvasive colonization of one of his pneumatoceles, because postmortem examination failed to reveal Rhizopus at any other location.
Voriconazole has excellent pulmonary and central nervous system penetration: In this patient serum levels were well within the therapeutic range. His peculiar drug resistance pattern (sensitivity to azoles and resistance to amphotericin) is unusual. Azole resistance in leukemia and patients with HSCT is more common than is amphotericin resistance, with current estimates of azole resistance close to 5%, ranging between 1% and 30%.5,6 Widespread use of antifungal prophylaxis with azoles likely selects for azole resistance.6
Despite this concern of azole resistance, current IDSA guidelines recommend against routine susceptibility testing of Aspergillus to azole therapy because of the current lack of consensus between the European Committee on Antibiotic Susceptibility Testing and Clinical and Laboratory Standards Institute on break points for resistance patterns.3,7 This is an area of emerging research, and proposed cut points for declaration of resistance do exist in the literature even if not globally agreed on.8
Combination antifungal therapy is an option for treatment in cases of possible drug resistance. Nonetheless, a recent randomized, double-blind, placebo-controlled, multicenter trial comparing voriconazole monotherapy with the combination of voriconazole and anidulafungin failed to demonstrate an overall mortality benefit in the primary analysis, although secondary analysis showed a mortality benefit with combination therapy in patients at highest risk for death.9
Despite the lack of unified standards with susceptibility testing, it may be reasonable to perform such tests in patients with demonstrating progressive disease. In this patient’s case, amphotericin B was added to treat the Rhizopus species found in his sputum, and while not the combination studied in the previously mentioned study, the drug should have provided an additional active agent for Aspergillus should this patient have had azole resistance.
Surprisingly, subsequent testing demonstrated the Aspergillus species to be resistant to amphotericin B. De novo amphotericin B-resistant A fumigates is extremely rare, with an expected incidence of 1% or less.10 The authors believe the patient may have demonstrated induction of amphotericin-B resistance through activation of fungal stress pathways by prior treatment with voriconazole. This has been demonstrated in vitro and should be considered should combination salvage therapy be required for the treatment of a refractory Aspergillus infection especially if patients have received prior treatment with voriconazole.11
Conclusion
This fatal case of invasive pulmonary aspergillosis illustrates the importance of considering the 4 main causes of treatment failure in an infection. Although the patient had a high burden of disease with a rare resistance pattern, he was treated with appropriate and well-dosed therapy. Ultimately, his unmodifiable immunosuppression was likely the driving factor leading to treatment failure and death. The indication for and number of bone marrow transplants continues to increase, thus exposure to and treatment of invasive fungal infections will increase accordingly. As such, providers should ensure that all causes of treatment failure are considered and addressed.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
1. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531-540.
2. Herbrecht R, Denning DW, Patterson TF, et al; Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408-415.
3. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Disease Society of America. Clin Infect Dis. 2016;63(4):e1-e60.
4. García-Cadenas I, Rivera I, Martino R, et al. Patterns of infection and infection-related mortality in patients with steroid-refractory acute graft versus host disease. Bone Marrow Transplant. 2017;52(1):107-113.
5. Vermeulen E, Maertens J, De Bel A, et al. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob Agents Chemother. 2015;59(8):4569-4576.
6. Wiederhold NP, Patterson TF. Emergence of azole resistance in Aspergillus. Semin Respir Crit Care Med. 2015;36(5):673-680.
7. Cuenca-Estrella M, Moore CB, Barchiesi F, et al; AFST Subcommittee of the European Committee on Antimicrobial Susceptibility Testing. Multicenter evaluation of the reproducibility of the proposed antifungal susceptibility testing method for fermentative yeasts of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin Microbiol Infect. 2003;9(6):467-474.
8. Pfaller MA, Diekema DJ, Ghannoum MA, et al; Clinical and Laboratory Standards Institute Antifungal Testing Subcommittee. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by Clinical and Laboratory Standards Institute for broth microdilution methods. J Clin Microbiol. 2009;47(10):3142-3146.
9. Marr KA, Schlamm HT, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81-89.
10. Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56(1):584-587.
11. Rajendran R, Mowat E, Jones B, Williams C, Ramage G. Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms. Int J Antimicrob Agents. 2015;46(3):342-345.
Survey: Palliative care blocked by many barriers in end-stage liver disease
results of a recent survey show.
Cultural factors, unrealistic expectations of the patient, lack of reimbursement, and competing demands for physicians’ time were some of the barriers to palliative care cited most frequently in the survey, said the researchers, in their report on the survey results that appears in Clinical Gastroenterology and Hepatology.
Moreover, most responding physicians said they felt end-of-life advance care planning discussions take place too late in the course of illness, according to Nneka N. Ufere, MD, of the Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Boston, and co-authors of the report.
“Multiple interventions targeted at patients, caregivers, institutions, and clinicians are needed to overcome barriers to improve the delivery of high-quality palliative and end-of-life care for patients with end-stage liver disease,” the researchers said.
Specialty palliative care can improve quality of life for patients with life-limiting conditions such as end-stage liver disease, which is associated with poor quality of life and a median survival of just two years without liver transplant, the authors said.
Advance care planning, in which patients discuss goals and care preferences in light of the expected course of illness, was a “critical component” of palliative care that can improve the quality of end-of-life care, Dr. Ufere and co-authors said.
Unfortunately, palliative care planning services are underutilized in end-stage liver disease, studies show, while rates of timely advance care planning discussions are low.
To find out why, Dr. Ufere and colleagues asked 1,238 physicians to fill out a web-based questionnaire designed to assess their perceptions of barriers to use of palliative care and barriers to timely advance care planning discussions. A total of 396 physicians (32%) completed the survey between February and April 2018.
Sixty percent were transplant hepatologists, and 79% of the survey participants said they worked in a teaching hospital, according to Dr. Ufere and co-authors, who added that no respondents had formal palliative care training.
Almost all respondents (95%) agreed that centers providing care for end-stage liver disease patients should have palliative care services, and most (86%) said they thought such patients would benefit from palliative care earlier in the course of disease.
While most (84%) agreed that a hepatologist was the best provider to discuss advance care planning with the patient, only about one-quarter (27%) said the hepatologist was best suited to provide palliative care, while most (88%) said the palliative care specialist was best for that role.
When asked about patient and caregiver barriers, nearly all respondents (95%) agreed that cultural factors that influenced palliative care perception was an issue, while 93% said patients’ unrealistic expectations was an issue.
Clinician barriers that respondents perceived included competing demands for clinicians’ time, cited by 91%, fear that palliative care might destroy the patient’s hope, cited by 82%, and the misperception that palliative care starts when active treatment ends, cited by 81%.
One potential solution to the competing demands on clinicians’ time would be development of “collaborative care models” between palliative care and hepatology services, according to Dr. Ufere and co-authors.
“Outpatient specialty palliative care visits, ideally temporally coordinated with the hepatology visits, can play a role not only in attending to symptom assessment and ACP, but also in addressing important psychosocial aspects of care, such as patient coping and well-being,” they said in their report on the survey.
Institutional barriers of note included limited reimbursement for time spent providing palliative care, cited by 76% and lack of a palliative care service, cited by nearly half (46%).
Some of the most commonly affirmed barriers to timely advance care planning discussions included insufficient training in end-of-life communication issues, and insufficient training in cultural competency issues related to the discussions.
In terms of timeliness, only 17% said advance care planning discussions happen at the right time, while 81% said they happen too late, investigators found.
Funding for the research came from the National Institutes of Health. The authors had no disclosures or conflicts of interest related to the report.
SOURCE: Ufere NN, et al. Clin Gastroenterol Hepatol. 2019 Mar 15. doi: 10.1016/j.cgh.2019.03.022.
results of a recent survey show.
Cultural factors, unrealistic expectations of the patient, lack of reimbursement, and competing demands for physicians’ time were some of the barriers to palliative care cited most frequently in the survey, said the researchers, in their report on the survey results that appears in Clinical Gastroenterology and Hepatology.
Moreover, most responding physicians said they felt end-of-life advance care planning discussions take place too late in the course of illness, according to Nneka N. Ufere, MD, of the Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Boston, and co-authors of the report.
“Multiple interventions targeted at patients, caregivers, institutions, and clinicians are needed to overcome barriers to improve the delivery of high-quality palliative and end-of-life care for patients with end-stage liver disease,” the researchers said.
Specialty palliative care can improve quality of life for patients with life-limiting conditions such as end-stage liver disease, which is associated with poor quality of life and a median survival of just two years without liver transplant, the authors said.
Advance care planning, in which patients discuss goals and care preferences in light of the expected course of illness, was a “critical component” of palliative care that can improve the quality of end-of-life care, Dr. Ufere and co-authors said.
Unfortunately, palliative care planning services are underutilized in end-stage liver disease, studies show, while rates of timely advance care planning discussions are low.
To find out why, Dr. Ufere and colleagues asked 1,238 physicians to fill out a web-based questionnaire designed to assess their perceptions of barriers to use of palliative care and barriers to timely advance care planning discussions. A total of 396 physicians (32%) completed the survey between February and April 2018.
Sixty percent were transplant hepatologists, and 79% of the survey participants said they worked in a teaching hospital, according to Dr. Ufere and co-authors, who added that no respondents had formal palliative care training.
Almost all respondents (95%) agreed that centers providing care for end-stage liver disease patients should have palliative care services, and most (86%) said they thought such patients would benefit from palliative care earlier in the course of disease.
While most (84%) agreed that a hepatologist was the best provider to discuss advance care planning with the patient, only about one-quarter (27%) said the hepatologist was best suited to provide palliative care, while most (88%) said the palliative care specialist was best for that role.
When asked about patient and caregiver barriers, nearly all respondents (95%) agreed that cultural factors that influenced palliative care perception was an issue, while 93% said patients’ unrealistic expectations was an issue.
Clinician barriers that respondents perceived included competing demands for clinicians’ time, cited by 91%, fear that palliative care might destroy the patient’s hope, cited by 82%, and the misperception that palliative care starts when active treatment ends, cited by 81%.
One potential solution to the competing demands on clinicians’ time would be development of “collaborative care models” between palliative care and hepatology services, according to Dr. Ufere and co-authors.
“Outpatient specialty palliative care visits, ideally temporally coordinated with the hepatology visits, can play a role not only in attending to symptom assessment and ACP, but also in addressing important psychosocial aspects of care, such as patient coping and well-being,” they said in their report on the survey.
Institutional barriers of note included limited reimbursement for time spent providing palliative care, cited by 76% and lack of a palliative care service, cited by nearly half (46%).
Some of the most commonly affirmed barriers to timely advance care planning discussions included insufficient training in end-of-life communication issues, and insufficient training in cultural competency issues related to the discussions.
In terms of timeliness, only 17% said advance care planning discussions happen at the right time, while 81% said they happen too late, investigators found.
Funding for the research came from the National Institutes of Health. The authors had no disclosures or conflicts of interest related to the report.
SOURCE: Ufere NN, et al. Clin Gastroenterol Hepatol. 2019 Mar 15. doi: 10.1016/j.cgh.2019.03.022.
results of a recent survey show.
Cultural factors, unrealistic expectations of the patient, lack of reimbursement, and competing demands for physicians’ time were some of the barriers to palliative care cited most frequently in the survey, said the researchers, in their report on the survey results that appears in Clinical Gastroenterology and Hepatology.
Moreover, most responding physicians said they felt end-of-life advance care planning discussions take place too late in the course of illness, according to Nneka N. Ufere, MD, of the Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Boston, and co-authors of the report.
“Multiple interventions targeted at patients, caregivers, institutions, and clinicians are needed to overcome barriers to improve the delivery of high-quality palliative and end-of-life care for patients with end-stage liver disease,” the researchers said.
Specialty palliative care can improve quality of life for patients with life-limiting conditions such as end-stage liver disease, which is associated with poor quality of life and a median survival of just two years without liver transplant, the authors said.
Advance care planning, in which patients discuss goals and care preferences in light of the expected course of illness, was a “critical component” of palliative care that can improve the quality of end-of-life care, Dr. Ufere and co-authors said.
Unfortunately, palliative care planning services are underutilized in end-stage liver disease, studies show, while rates of timely advance care planning discussions are low.
To find out why, Dr. Ufere and colleagues asked 1,238 physicians to fill out a web-based questionnaire designed to assess their perceptions of barriers to use of palliative care and barriers to timely advance care planning discussions. A total of 396 physicians (32%) completed the survey between February and April 2018.
Sixty percent were transplant hepatologists, and 79% of the survey participants said they worked in a teaching hospital, according to Dr. Ufere and co-authors, who added that no respondents had formal palliative care training.
Almost all respondents (95%) agreed that centers providing care for end-stage liver disease patients should have palliative care services, and most (86%) said they thought such patients would benefit from palliative care earlier in the course of disease.
While most (84%) agreed that a hepatologist was the best provider to discuss advance care planning with the patient, only about one-quarter (27%) said the hepatologist was best suited to provide palliative care, while most (88%) said the palliative care specialist was best for that role.
When asked about patient and caregiver barriers, nearly all respondents (95%) agreed that cultural factors that influenced palliative care perception was an issue, while 93% said patients’ unrealistic expectations was an issue.
Clinician barriers that respondents perceived included competing demands for clinicians’ time, cited by 91%, fear that palliative care might destroy the patient’s hope, cited by 82%, and the misperception that palliative care starts when active treatment ends, cited by 81%.
One potential solution to the competing demands on clinicians’ time would be development of “collaborative care models” between palliative care and hepatology services, according to Dr. Ufere and co-authors.
“Outpatient specialty palliative care visits, ideally temporally coordinated with the hepatology visits, can play a role not only in attending to symptom assessment and ACP, but also in addressing important psychosocial aspects of care, such as patient coping and well-being,” they said in their report on the survey.
Institutional barriers of note included limited reimbursement for time spent providing palliative care, cited by 76% and lack of a palliative care service, cited by nearly half (46%).
Some of the most commonly affirmed barriers to timely advance care planning discussions included insufficient training in end-of-life communication issues, and insufficient training in cultural competency issues related to the discussions.
In terms of timeliness, only 17% said advance care planning discussions happen at the right time, while 81% said they happen too late, investigators found.
Funding for the research came from the National Institutes of Health. The authors had no disclosures or conflicts of interest related to the report.
SOURCE: Ufere NN, et al. Clin Gastroenterol Hepatol. 2019 Mar 15. doi: 10.1016/j.cgh.2019.03.022.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Gastric outlet obstruction: A red flag, potentially manageable
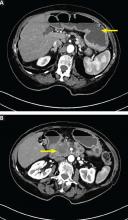
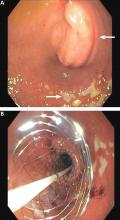
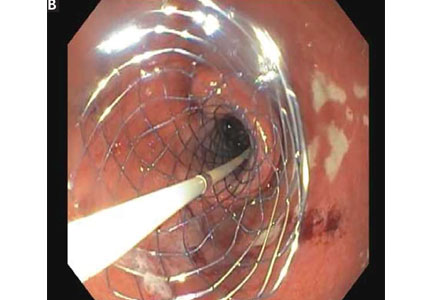
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
KEY POINTS
- Causes of gastric outlet obstruction fall into 2 categories: benign and malignant. The cause should be presumed to be malignant until proven otherwise.
- Peptic ulcer disease, a benign cause, used to account for most cases of gastric outlet obstruction. It is still common but has declined in frequency with the development of acid-suppressing drugs.
- Gastric cancer used to be the most common malignant cause but has declined in frequency in Western countries with treatment for Helicobacter pylori infection. Now, pancreatic cancer predominates.
- Endoscopic stenting is an effective, minimally invasive treatment for patients with malignant gastric outlet obstruction and poor prognosis, allowing resumption of oral intake and improving quality of life.
More Medicare beneficiaries receiving hospice care services than in previous years
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Background: Studies abound on the accelerated cost and health care activities of patients toward the end of life. Previous analyses of Medicare trends of medical care at the time of death have been compiled in 2000, 2005, 2009, and 2011; this study reexamines recent trends.
Study design: Retrospective cohort of a random sample of Medicare Fee-for-Service and Medicare Advantage decedents during 2000-2015.
Setting: Medicare patients in acute care hospitals, home/community, hospice inpatient care units, or nursing homes.
Synopsis: Approximately 1.4 million Medicare Fee-for-Service decedents and 870,000 Medicare Advantage decedents were studied in a random sample that included 20% of Medicare Fee-for-Service recipients in the years 2000, 2005, 2009, 2011, and 2015 and 100% of Medicare Advantage patients in the years 2011 and 2015. Deaths of Medicare Fee-for-Service recipients occurring in acute care hospitals and nursing homes decreased from 32.6% (95% confidence interval, 32.4%-32.8%) in 2000 to 19.8% (95% CI, 19.6%-20.0%) in 2015. Patients who died while receiving hospice services increased from 21.6% (95% CI, 21.5%-21.8%) in 2000 to 50.4% (95% CI, 50.2%-50.6%) in 2015. Review of Medicare Advantage data demonstrated similar shifts.
Although there are concerns about the accuracy of reported location of community deaths and these results may not be generalizable to other, non-Medicare populations, the study overall adds statistical data on death trends and suggests an improvement in the use of palliative and hospice care services.
Bottom line: Compared with previous years, fewer Medicare beneficiaries are dying in acute care settings, and more beneficiaries are receiving hospice care in other settings.
Citation: Teno J et al. Site of death, place of care, and health care transitions among U. S. Medicare beneficiaries between 2000-2015. JAMA. 2018;320(3):264-71.
Dr. Smith is an assistant professor of medicine in the division of hospital medicine at Emory University, Atlanta.
Don’t delay palliative care for IPF patients
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
and indicates that early, integrated palliative care should be a priority, according to the finding of a survey study.
“Patients with IPF suffer from exceptionally low [health-related quality of life] together with severe breathlessness and fatigue already two years before death. In addition, physical and emotional well-being further deteriorates near death concurrently with escalating overall symptom burden,” wrote Kaisa Rajala, MD, and her colleagues at Helsinki University Hospital.
They conducted a substudy of patients in the larger FinnishIPF study to assess health-related quality of life (HRQOL) and symptom burden in the period before death. Among 300 patients invited to participate, 247 agreed. Patient disease and sociodemographic data were collected from the FinnishIPF records and the study group completed questionnaires five times at 6 month intervals. The study began in April 2015 and continued until August 2017, by which time 92 (37%) of the patients had died (BMC Pulmonary Medicine 2018;18:172; doi: 0.1186/s12890-018-0738-x).
The investigators used self-reporting tools to look at HRQOL and symptom burden: RAND 36-item Health Survey (RAND-36), the Modified Medical Research and Council Dyspnea Scale (MMRC), the Modified Edmonton Symptom Assessment Scale (ESAS), and the Numeric Rating Scale (NRS).
About 35% of these patients were being treated with antifibrotic medication. Most of the patients had comorbidities, with cardiovascular disease being the most common.
The dimensions of HRQOL studied were physical function, general health, vitality, mental health, social function, and bodily pain. These patients experienced a gradual impairment in HRQOL similar to that of patients with chronic obstructive pulmonary disease, but with a pronounced, rapid deterioration beginning in the last 2 years of life.
The symptom burden also intensified in the last 2 years of life and ramped up significantly in the last 6 months before death. NRS scores are on a scale of 0-10, from no symptoms to worst symptoms. In most clinical situations, NRS scores equal to greater than 4 trigger more comprehensive symptom assessment. The scores for symptoms for these patients during the last 6 months were dyspnea, 7.1 (standard deviation 2.8); tiredness, 6.0 (SD 2.5), cough, 5.0 (SD 3.5), pain with movement, 3.9 (SD 3.1), insomnia, 3.9 (SD 2.9), anxiety, 3.9 (SD 2.9), and depression, 3.6 (SD 3.1).
Investigators noted the steep change in the proportion of patients with MMRC scores greater than or equal to 3 (needing to stop walking after approximately 100 m or a few minutes because of breathlessness) beginning in the last 2 years of life.
The study limitations are its relatively small size, the self-reported data, and the lack of lung function measurements in most patients in the last 6 months of life.
The findings point to the urgent need for early palliative care in IPF patients, the investigators concluded. They noted that the sharp decline in HRQOL is similar to that seen in lung cancer patients, in contrast to the more gradual trend seen in COPD patients.
But there are common benefits of an early palliative program for all of these patients, they stressed. “Early integrated palliative care for patients with lung cancer has shown substantial benefits, such as lower depression scores, higher HRQOL, better communication of end-of-life care preferences, less aggressive care at the end of life, and longer overall survival. Similarly, a randomized trial demonstrated better control of dyspnea and a survival benefit with integrated palliative care in patients with COPD and interstitial lung disease. In addition to cancer patients, early integrated palliative care may reduce end-of-life acute care utilization, and allow patients with IPF to die in their preferred locations. Integrated palliative care in IPF patients seems to lower respiratory-related emergency room visits and hospitalizations and may allow more patients to die at home.”
The study was funded by The Academy of Finland and various Finnish nonprofit organizations funded the study.
SOURCE: Rajala K et al. BMC Pulm Med. 2018;18:172. doi: 0.1186/s12890-018-0738-x.
FROM BMC PULMONARY MEDICINE
Advance care planning codes not being used
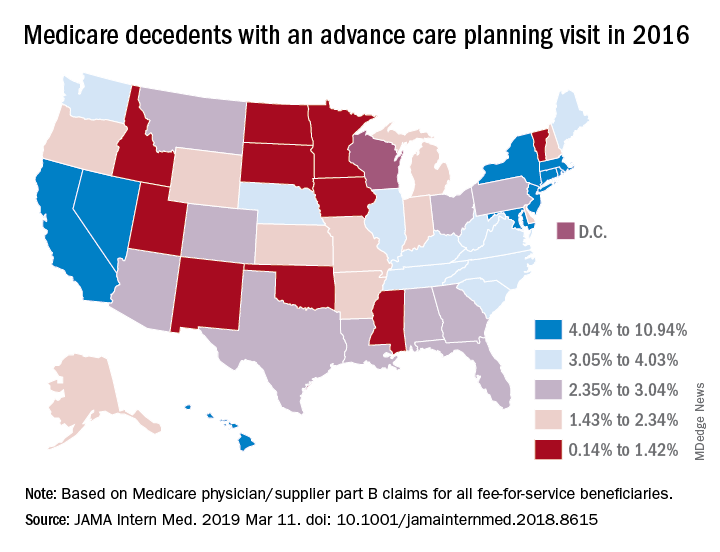
Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”

Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”

Starting in 2016, the Centers for Medicare & Medicaid Services began paying physicians for advance care planning discussions with the approval of two new codes: 99497 and 99498. The codes pay about $86 for the first 30 minutes of a face-to-face conversation with a patient, family member, and/or surrogate and about $75 for additional sessions. Services can be furnished in both inpatient and ambulatory settings, and payment is not limited to particular physician specialties.
In 2016, health care professionals in New England (Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island, and Vermont) billed Medicare 26,522 times for the advance care planning (ACP) codes for a total of 24,536 patients, which represented less than 1% of Medicare beneficiaries in New England at the time, according to Kimberly Pelland, MPH, of Healthcentric Advisors, Providence, R.I., and her colleagues. Most claims were billed in the office, followed by in nursing homes, and in hospitals; 40% of conversations occurred during an annual wellness visit (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8107).
Internists billed Medicare the most for ACP claims (65%), followed by family physicians (22%) gerontologists (5%), and oncologist/hematologists (0.3%), according to the analysis based on 2016 Medicare claims data and Census Bureau data. A greater proportion of patients with ACP claims were female, aged 85 years or older, enrolled in hospice, and died in the study year. Patients had higher odds of having an ACP claim if they were older and had lower income, and if they had cancer, heart failure, stroke, chronic kidney disease, or dementia. Male patients who were Asian, black, and Hispanic had lower chances of having an ACP claim.
In a related study, Emmanuelle Belanger, PhD, of Brown University, Providence, R.I., and her colleagues examined national Medicare data from 2016 to the third quarter of 2017. Across the United States, 2% of Medicare patients aged 65 years and older received advance care planning services that were billed under the ACP codes (JAMA Intern Med. 2019 March 11. doi: 10.1001/jamainternmed.2018.8615). Visits billed under the ACP codes increased from 538,275 to 633,214 during the same time period. Claim rates were higher among patients who died within the study period, reaching 3% in 2016 and 6% in 2017. The percentage of decedents with an ACP billed visit varied strongly across states, with states such as North Dakota, South Dakota, and Wyoming having the fewest ACP visits billed and states such as California and Nevada having the most. ACP billed visits increased in all settings in 2017, but primarily in hospitals and nursing homes. Nationally, internists billed the codes most (48%), followed by family physicians (28%).
While the two studies indicate low usage of the ACP codes, many physicians are discussing advance care planning with their patients, said Mary M. Newman, MD, an internist based in Lutherville, Md., and former American College of Physicians adviser to the American Medical Association Relative Scale Value Update Committee (RUC).
“What cannot be captured by tracking under Medicare claims data are those shorter conversations that we have frequently,” Dr. Newman said in an interview. “If we have a short conversation about advance care planning, it gets folded into our evaluation and management visit. It’s not going to be separately billed.”
At the same time, some patients are not ready to discuss end-of-life options and decline the discussions when asked, Dr. Newman said. Particularly for healthier patients, end of life care is not a primary focus, she noted.
“Not everybody’s ready to have an advance care planning [discussion] that lasts 16-45 minutes,” she said. “Many people over age 65 are not ready to deal with advance care planning in their day-to-day lives, and it may not be what they wish to discuss. I offer the option to patients and some say, ‘Yes, I’d love to,’ and others decline or postpone.”
Low usage of the ACP codes may be associated with lack of awareness, uncertainty about appropriate code use, or associated billing that is not part of the standard workflow, Ankita Mehta, MD, of Mount Sinai in New York wrote an editorial accompanying the studies (JAMA Intern Med. 2019 March 11. doi:10.1001/jamainternmed.2018.8105).
“Regardless, the low rates of utilization of ACP codes is alarming and highlights the need to create strategies to integrate ACP discussions into standard practice and build ACP documentation and billing in clinical workflow,” Dr. Mehta said.
Dr. Newman agreed that more education among physicians is needed.
“The amount of education clinicians have received varies tremendously across the geography of the country,” she said. “I think the codes are going to be slowly adopted. The challenge to us is to make sure we’re all better educated on palliative care as people age and get sick and that we are sensitive to our patients explicit and implicit needs for these discussions.”