User login
The ever-evolving scope of hospitalists’ clinical services
More care ‘beyond the walls’ of the hospital
The 2018 State of Hospital Medicine (SoHM) Report provides indispensable data about the scope of clinical services routinely provided by adult and pediatric hospitalists. This year’s SoHM report reveals that a growing number of Hospital Medicine Groups (HMGs) serving adults are involved in roles beyond the inpatient medical wards, including various surgical comanagement programs, outpatient care, and post-acute care services.
The survey also compares services provided by academic and nonacademic HMGs, which remain markedly different in some areas. As the landscape of health care continues to evolve, hospitalists transform their scope of services to meet the needs of the institutions and communities they serve.
In the previous three SoHM reports, it was well established that more than 87% of adult hospital medicine groups play some role in comanaging surgical patients. In this year’s SoHM report, that role was further stratified to capture the various subspecialties represented, and to identify whether the hospitalists generally served as admitting/attending physician or consultant.
Hospitalists’ roles in comanagement are most prominent for care of orthopedic and general surgery patients, but more than 50% of surveyed HMGs reported being involved in comanagement in some capacity with neurosurgery, obstetrics, and cardiovascular surgery. Additionally, almost 95% of surveyed adult HMGs reported that they provided comanagement services for at least one other surgical specialty that was not listed in the survey.
The report also displays comanagement services provided to various medical subspecialties, including neurology, GI/liver, oncology, and more. Of the medical subspecialties represented, adult HMGs comanaged GI/liver (98.2%) and oncology (97.7%) services more often than others.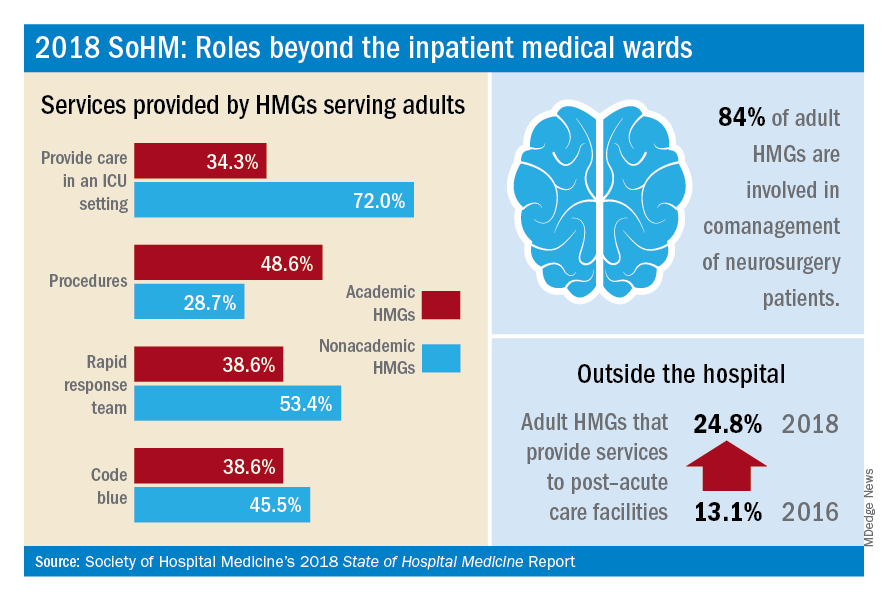
Interestingly, more HMGs are providing care for patients beyond the walls of the hospital. In the 2018 SoHM report, over 17% of surveyed HMG respondents reported providing care in an outpatient setting, representing an increase of 6.5 percentage points over 2016. Most strikingly, from 2016 to 2018, there was a 12 percentage point increase in adult HMGs reporting services provided to post-acute care facilities (from 13.1% to 24.8%).
These trends were most notable in the Midwest region where nearly 28% of HMGs provide patient care in an outpatient setting and up to 34% in post-acute care facilities. In part, this trend may result from the increased emphasis on improving transitions of care, by providing prehospital preoperative services, postdischarge follow-up encounters, or offering posthospitalization extensivist care.
Within the hospital itself, there remain striking differences in certain services provided by academic and nonacademic HMGs serving adults. Nonacademic HMGs are far more likely to cover patients in an ICU than their academic counterparts (72.0% vs. 34.3%). In contrast, academic hospitalist groups were significantly more inclined to perform procedures. However, the report also showed that there was an overall downtrend of percentage of HMGs that cover patients in an ICU or perform procedures.
As the scope of hospitalist services continues to change over time, should there be concern for scope creep? It depends on how one might view the change. As health care becomes ever more complex, high-functioning HMGs are needed to navigate it, both within and beyond the hospital. Some might consider scope evolution to be a reflection of hospitalists being recognized for their ability to provide high-quality, efficient, and comprehensive care. Hospital medicine groups will likely continue to evolve to meet the needs of an ever-changing health care environment.
Dr. Kurian is chief of the academic division of hospital medicine at Northwell Health in New York. She is a member of the SHM Practice Analysis Committee.
More care ‘beyond the walls’ of the hospital
More care ‘beyond the walls’ of the hospital
The 2018 State of Hospital Medicine (SoHM) Report provides indispensable data about the scope of clinical services routinely provided by adult and pediatric hospitalists. This year’s SoHM report reveals that a growing number of Hospital Medicine Groups (HMGs) serving adults are involved in roles beyond the inpatient medical wards, including various surgical comanagement programs, outpatient care, and post-acute care services.
The survey also compares services provided by academic and nonacademic HMGs, which remain markedly different in some areas. As the landscape of health care continues to evolve, hospitalists transform their scope of services to meet the needs of the institutions and communities they serve.
In the previous three SoHM reports, it was well established that more than 87% of adult hospital medicine groups play some role in comanaging surgical patients. In this year’s SoHM report, that role was further stratified to capture the various subspecialties represented, and to identify whether the hospitalists generally served as admitting/attending physician or consultant.
Hospitalists’ roles in comanagement are most prominent for care of orthopedic and general surgery patients, but more than 50% of surveyed HMGs reported being involved in comanagement in some capacity with neurosurgery, obstetrics, and cardiovascular surgery. Additionally, almost 95% of surveyed adult HMGs reported that they provided comanagement services for at least one other surgical specialty that was not listed in the survey.
The report also displays comanagement services provided to various medical subspecialties, including neurology, GI/liver, oncology, and more. Of the medical subspecialties represented, adult HMGs comanaged GI/liver (98.2%) and oncology (97.7%) services more often than others.
Interestingly, more HMGs are providing care for patients beyond the walls of the hospital. In the 2018 SoHM report, over 17% of surveyed HMG respondents reported providing care in an outpatient setting, representing an increase of 6.5 percentage points over 2016. Most strikingly, from 2016 to 2018, there was a 12 percentage point increase in adult HMGs reporting services provided to post-acute care facilities (from 13.1% to 24.8%).
These trends were most notable in the Midwest region where nearly 28% of HMGs provide patient care in an outpatient setting and up to 34% in post-acute care facilities. In part, this trend may result from the increased emphasis on improving transitions of care, by providing prehospital preoperative services, postdischarge follow-up encounters, or offering posthospitalization extensivist care.
Within the hospital itself, there remain striking differences in certain services provided by academic and nonacademic HMGs serving adults. Nonacademic HMGs are far more likely to cover patients in an ICU than their academic counterparts (72.0% vs. 34.3%). In contrast, academic hospitalist groups were significantly more inclined to perform procedures. However, the report also showed that there was an overall downtrend of percentage of HMGs that cover patients in an ICU or perform procedures.
As the scope of hospitalist services continues to change over time, should there be concern for scope creep? It depends on how one might view the change. As health care becomes ever more complex, high-functioning HMGs are needed to navigate it, both within and beyond the hospital. Some might consider scope evolution to be a reflection of hospitalists being recognized for their ability to provide high-quality, efficient, and comprehensive care. Hospital medicine groups will likely continue to evolve to meet the needs of an ever-changing health care environment.
Dr. Kurian is chief of the academic division of hospital medicine at Northwell Health in New York. She is a member of the SHM Practice Analysis Committee.
The 2018 State of Hospital Medicine (SoHM) Report provides indispensable data about the scope of clinical services routinely provided by adult and pediatric hospitalists. This year’s SoHM report reveals that a growing number of Hospital Medicine Groups (HMGs) serving adults are involved in roles beyond the inpatient medical wards, including various surgical comanagement programs, outpatient care, and post-acute care services.
The survey also compares services provided by academic and nonacademic HMGs, which remain markedly different in some areas. As the landscape of health care continues to evolve, hospitalists transform their scope of services to meet the needs of the institutions and communities they serve.
In the previous three SoHM reports, it was well established that more than 87% of adult hospital medicine groups play some role in comanaging surgical patients. In this year’s SoHM report, that role was further stratified to capture the various subspecialties represented, and to identify whether the hospitalists generally served as admitting/attending physician or consultant.
Hospitalists’ roles in comanagement are most prominent for care of orthopedic and general surgery patients, but more than 50% of surveyed HMGs reported being involved in comanagement in some capacity with neurosurgery, obstetrics, and cardiovascular surgery. Additionally, almost 95% of surveyed adult HMGs reported that they provided comanagement services for at least one other surgical specialty that was not listed in the survey.
The report also displays comanagement services provided to various medical subspecialties, including neurology, GI/liver, oncology, and more. Of the medical subspecialties represented, adult HMGs comanaged GI/liver (98.2%) and oncology (97.7%) services more often than others.
Interestingly, more HMGs are providing care for patients beyond the walls of the hospital. In the 2018 SoHM report, over 17% of surveyed HMG respondents reported providing care in an outpatient setting, representing an increase of 6.5 percentage points over 2016. Most strikingly, from 2016 to 2018, there was a 12 percentage point increase in adult HMGs reporting services provided to post-acute care facilities (from 13.1% to 24.8%).
These trends were most notable in the Midwest region where nearly 28% of HMGs provide patient care in an outpatient setting and up to 34% in post-acute care facilities. In part, this trend may result from the increased emphasis on improving transitions of care, by providing prehospital preoperative services, postdischarge follow-up encounters, or offering posthospitalization extensivist care.
Within the hospital itself, there remain striking differences in certain services provided by academic and nonacademic HMGs serving adults. Nonacademic HMGs are far more likely to cover patients in an ICU than their academic counterparts (72.0% vs. 34.3%). In contrast, academic hospitalist groups were significantly more inclined to perform procedures. However, the report also showed that there was an overall downtrend of percentage of HMGs that cover patients in an ICU or perform procedures.
As the scope of hospitalist services continues to change over time, should there be concern for scope creep? It depends on how one might view the change. As health care becomes ever more complex, high-functioning HMGs are needed to navigate it, both within and beyond the hospital. Some might consider scope evolution to be a reflection of hospitalists being recognized for their ability to provide high-quality, efficient, and comprehensive care. Hospital medicine groups will likely continue to evolve to meet the needs of an ever-changing health care environment.
Dr. Kurian is chief of the academic division of hospital medicine at Northwell Health in New York. She is a member of the SHM Practice Analysis Committee.
Palliative care has improved for critically ill children, but challenges remain
SAN DIEGO – and is more common among older children, female children, and those with government insurance or at a high risk of mortality. The findings come from a retrospective analysis of data from 52 hospitals, which included ICU admissions (except neonatal ICU) during 2007-2018.
The good news is that palliative care consultations have increased, with consultations in less than 1% of cases at the start of the study and rising quickly to more than 7% in 2018.
“In the adult world, palliative care has expanded in recent decades, and I think now that it’s coming to the pediatric world, it’ll just continue to go up,” said Siobhan O’Keefe, MD, in an interview. Dr. O’Keefe is with Children’s Hospital Colorado, Aurora. She presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
More work needs to be done, she said. “We are not uniformly using palliative care for critically ill children in the U.S., and it varies across institutions. That’s probably not the ideal situation,” said Dr. O’Keefe. The study did not track palliative care versus the presence of board-certified palliative care physicians or palliative care fellowships, but she suspects they would correlate.
Dr. O’Keefe called for physicians to think beyond the patient, to family members and caregivers. “We need to focus on family outcomes, how they are taking care of children with moderate disability, and incorporate that into our outcomes,” she said. Previous research has shown family members to be at risk of anxiety, depression, unemployment, and financial distress.
The researchers analyzed data from 740,890 patients with 1,024,666 hospitalizations (82% had one hospitalization). They divided subjects into three cohorts, one of which was a category of patients with criteria for palliative care based on previous research (PC-ICU). The PC-ICU cohort included patients with an expected length of stay more than 2 weeks, patients receiving extracorporeal membrane oxygenation (ECMO), severe brain injuries, acute respiratory failure with serious comorbidity, hematologic or oncologic disease, metabolic disease, renal failure that required continuous renal replacement therapy, hepatic failure, or serious chromosomal abnormality. A second cohort included chronic complex conditions not found in the PC-ICU cohort (additional criteria), and a third cohort had no criteria for palliative care.
Thirty percent of hospitalizations met the PC-ICU cohort criteria, 40% met the additional cohort criteria, and 30% fell in the no criteria cohort. The PC-ICU group had the highest mortality, at 8.03%, compared with 1.08% in the additional criteria group and 0.34% in the no criteria group (P less than .00001).
Palliative care consultations occurred more frequently in 5-12 year olds (odds ratio 1.06; 95% confidence interval, 1.01-1.13) and in those aged 13 years or older (OR, 1.38; 95% CI, 1.3-1.46), in females (OR, 1.13; 95% CI, 1.06-1.15), and in patients with government insurance (OR, 1.23; 95% CI, 1.17-1.29). Compared with those in the no criteria cohort, PC-ICU patients were more likely to receive a palliative care consult (OR, 75.5; 95% CI, 60.4-94.3), as were those in the additional criteria group (OR, 19.1; 95% CI, 15.3-23.9).
Cross-institutional palliative care frequency varied widely among patients in the PC-ICU group, ranging from 0% to 44%. The frequency ranged from 0% to 12% across institutions for patients in the additional criteria group.
SOURCE: O’Keefe S et al. Critical Care Congress 2019, Abstract 418.
SAN DIEGO – and is more common among older children, female children, and those with government insurance or at a high risk of mortality. The findings come from a retrospective analysis of data from 52 hospitals, which included ICU admissions (except neonatal ICU) during 2007-2018.
The good news is that palliative care consultations have increased, with consultations in less than 1% of cases at the start of the study and rising quickly to more than 7% in 2018.
“In the adult world, palliative care has expanded in recent decades, and I think now that it’s coming to the pediatric world, it’ll just continue to go up,” said Siobhan O’Keefe, MD, in an interview. Dr. O’Keefe is with Children’s Hospital Colorado, Aurora. She presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
More work needs to be done, she said. “We are not uniformly using palliative care for critically ill children in the U.S., and it varies across institutions. That’s probably not the ideal situation,” said Dr. O’Keefe. The study did not track palliative care versus the presence of board-certified palliative care physicians or palliative care fellowships, but she suspects they would correlate.
Dr. O’Keefe called for physicians to think beyond the patient, to family members and caregivers. “We need to focus on family outcomes, how they are taking care of children with moderate disability, and incorporate that into our outcomes,” she said. Previous research has shown family members to be at risk of anxiety, depression, unemployment, and financial distress.
The researchers analyzed data from 740,890 patients with 1,024,666 hospitalizations (82% had one hospitalization). They divided subjects into three cohorts, one of which was a category of patients with criteria for palliative care based on previous research (PC-ICU). The PC-ICU cohort included patients with an expected length of stay more than 2 weeks, patients receiving extracorporeal membrane oxygenation (ECMO), severe brain injuries, acute respiratory failure with serious comorbidity, hematologic or oncologic disease, metabolic disease, renal failure that required continuous renal replacement therapy, hepatic failure, or serious chromosomal abnormality. A second cohort included chronic complex conditions not found in the PC-ICU cohort (additional criteria), and a third cohort had no criteria for palliative care.
Thirty percent of hospitalizations met the PC-ICU cohort criteria, 40% met the additional cohort criteria, and 30% fell in the no criteria cohort. The PC-ICU group had the highest mortality, at 8.03%, compared with 1.08% in the additional criteria group and 0.34% in the no criteria group (P less than .00001).
Palliative care consultations occurred more frequently in 5-12 year olds (odds ratio 1.06; 95% confidence interval, 1.01-1.13) and in those aged 13 years or older (OR, 1.38; 95% CI, 1.3-1.46), in females (OR, 1.13; 95% CI, 1.06-1.15), and in patients with government insurance (OR, 1.23; 95% CI, 1.17-1.29). Compared with those in the no criteria cohort, PC-ICU patients were more likely to receive a palliative care consult (OR, 75.5; 95% CI, 60.4-94.3), as were those in the additional criteria group (OR, 19.1; 95% CI, 15.3-23.9).
Cross-institutional palliative care frequency varied widely among patients in the PC-ICU group, ranging from 0% to 44%. The frequency ranged from 0% to 12% across institutions for patients in the additional criteria group.
SOURCE: O’Keefe S et al. Critical Care Congress 2019, Abstract 418.
SAN DIEGO – and is more common among older children, female children, and those with government insurance or at a high risk of mortality. The findings come from a retrospective analysis of data from 52 hospitals, which included ICU admissions (except neonatal ICU) during 2007-2018.
The good news is that palliative care consultations have increased, with consultations in less than 1% of cases at the start of the study and rising quickly to more than 7% in 2018.
“In the adult world, palliative care has expanded in recent decades, and I think now that it’s coming to the pediatric world, it’ll just continue to go up,” said Siobhan O’Keefe, MD, in an interview. Dr. O’Keefe is with Children’s Hospital Colorado, Aurora. She presented the study at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
More work needs to be done, she said. “We are not uniformly using palliative care for critically ill children in the U.S., and it varies across institutions. That’s probably not the ideal situation,” said Dr. O’Keefe. The study did not track palliative care versus the presence of board-certified palliative care physicians or palliative care fellowships, but she suspects they would correlate.
Dr. O’Keefe called for physicians to think beyond the patient, to family members and caregivers. “We need to focus on family outcomes, how they are taking care of children with moderate disability, and incorporate that into our outcomes,” she said. Previous research has shown family members to be at risk of anxiety, depression, unemployment, and financial distress.
The researchers analyzed data from 740,890 patients with 1,024,666 hospitalizations (82% had one hospitalization). They divided subjects into three cohorts, one of which was a category of patients with criteria for palliative care based on previous research (PC-ICU). The PC-ICU cohort included patients with an expected length of stay more than 2 weeks, patients receiving extracorporeal membrane oxygenation (ECMO), severe brain injuries, acute respiratory failure with serious comorbidity, hematologic or oncologic disease, metabolic disease, renal failure that required continuous renal replacement therapy, hepatic failure, or serious chromosomal abnormality. A second cohort included chronic complex conditions not found in the PC-ICU cohort (additional criteria), and a third cohort had no criteria for palliative care.
Thirty percent of hospitalizations met the PC-ICU cohort criteria, 40% met the additional cohort criteria, and 30% fell in the no criteria cohort. The PC-ICU group had the highest mortality, at 8.03%, compared with 1.08% in the additional criteria group and 0.34% in the no criteria group (P less than .00001).
Palliative care consultations occurred more frequently in 5-12 year olds (odds ratio 1.06; 95% confidence interval, 1.01-1.13) and in those aged 13 years or older (OR, 1.38; 95% CI, 1.3-1.46), in females (OR, 1.13; 95% CI, 1.06-1.15), and in patients with government insurance (OR, 1.23; 95% CI, 1.17-1.29). Compared with those in the no criteria cohort, PC-ICU patients were more likely to receive a palliative care consult (OR, 75.5; 95% CI, 60.4-94.3), as were those in the additional criteria group (OR, 19.1; 95% CI, 15.3-23.9).
Cross-institutional palliative care frequency varied widely among patients in the PC-ICU group, ranging from 0% to 44%. The frequency ranged from 0% to 12% across institutions for patients in the additional criteria group.
SOURCE: O’Keefe S et al. Critical Care Congress 2019, Abstract 418.
REPORTING FROM CCC48
A National WestlawNext Database Analysis of Malpractice Litigation in Radiation Oncology (FULL)
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
A rise in medical malpractice insurance premiums and malpractice claims has brought the issue of medical malpractice to the forefront of medicine over the past few decades.1 The VA has more than tripled the number of legal settlements it has made over the past 5 years, and it has paid more than $871 million in medical malpractice settlements over the past decade.2,3 Legislation by the federal and state governments in the U.S., collectively referred to as tort reform, has been passed to curb the rate at which malpractice claims are filed; to set caps on noneconomic damages, such as pain and suffering; to control the effect of these claims on insurance premiums; and to prevent the delivery of negligent and harmful medical care.1
An observed high prevalence of medical malpractice claims has significant consequences within the clinical setting and has given rise to the practice of defensive medicine.4-8 Even the perceived threat of possible tort action may lead to aberrant practice behaviors. These defensive medical practices may include excessive testing, unnecessary referrals to other physicians or health facilities, or even refusal to treat particular patients.4,9-11 Furthermore, physicians devote valuable time and energy engaging in lawsuits rather than in delivering quality care to their patients.12
The increasingly litigious environment has discouraged physicians from practicing medicine, leading to earlier retirement, geographic relocation, and restriction of scope of services, all limiting patients’ access to health care.13 One such figure reported in 2008 found that in the U.S., defensive medicine costs can total nearly $56 billion.14 Radiation oncology is generally considered a medium-to-low risk specialty for litigation.15,16 Its average annual indemnity payment in 2006 was $276,792 and has increased at a rate of $1,500 per year, ranking it fifth among 22 specialty groups.16 Studies revealed that the practice of defensive medicine is not strictly limited to the U.S. and has been reported in other countries.6,17-20,21
A recent study by Jena and colleagues found that nearly 10% of oncologists face a malpractice claim annually, the 10th highest among the specialties surveyed.22 Malpractice within the field of radiation oncology has been previously discussed in the literature.16,23,24 There are limited data that examine the basis for these claims, the resulting jury verdicts, and the subsequent indemnity payments associated with claims.24,25
In this study, the authors sought to describe radiation oncology malpractice claims over the past 30 years. It is hoped that this study will not only help traditional oncologists in particular, but also all other practitioners who might be included as co-defendants to be more aware of the common causes of action that plaintiffs have been using to sue.
Methods
This public and online study did not involve human subjects research and accordingly did not require institutional review board approval. The WestlawNext (Thomson Reuters, New York) online legal database was used to search retrospectively for state and federal jury verdicts and settlements related to radiation oncology and medical malpractice. The database is a collection of several thousand search engines that can locate court dockets, jury verdicts, and settlements compiled by attorney-editors. Local cases and claims that were dismissed prior to proceeding to trial or that were settled out of court were not available. All cases in the database were considered and provided this study’s sample size, spanning from January 1, 1985, to December 31, 2015.
Given the boolean search functionality integrated into the Westlaw database, search parameters included “radiation oncology” and “medical malpractice” to yield the greatest number of cases (n = 223). All derived cases were manually reviewed, and files that were duplicates or associated with litigation unrelated to radiation oncology were excluded from analysis (n = 191).
Analysis
Factors that were collected and considered included the state and county in which the claim was filed, the age and sex of the litigant at the time of malpractice, the year the case was settled, co-defendant specialties, jury verdicts, award payouts, death status of the litigant and the alleged basis for the medical malpractice claim. A lack of informed consent, a failure to treat in a timely manner, a failure to order appropriate tests or to make a timely referral, misinterpretation of a test, excessive radiation, unnecessary radiation, unnecessary surgery, and procedural error all were included as alleged bases for the malpractice claim. Descriptive statistics were then compiled.
Results
A total of 32 cases were included for analysis (Tables 1, 2, and 3). Anonymized summaries of all 32 cases are provided in the Appendix. The average age of the patient was 54.6 years (range 34-83) and included 17 (54.8%) female and 14 (45.2%) male patients.
Excessive radiation (n = 11, 34.4%), unnecessary radiation (n = 8, 25%), and a failure to refer and/or order appropriate tests (n = 9, 28.1%) were the 3 most commonly alleged causes of malpractice. A lack of informed consent was implicated in less than one-seventh of cases (4; 12.5%). In 7 (21.9%) cases, the patient passed away.
Between 1985 and 2015, decisions were made in radiation oncologists’ favor in more than half of the cases. The jury ruled for the plaintiff in 11 (34.4%) cases and for the defendant in 17 (53.1%) cases. Settlements were reached in 4 (12.5%) cases, with a mean payout of $1,476,775.
Discussion
A physician’s duty is to provide medical care within the standard of care. In the courtroom, a radiation oncologist is judged on what a “reasonably prudent” radiation oncologist would do in similar circumstances.26 The plaintiff must establish the standard of care for the patient’s specific diagnosis with evidence, which is often accomplished through expert testimony. A physician is deemed negligent when deviating from this standard of care. The plaintiff must establish 4 factors to be awarded compensation for medical negligence: (1) the physician owed a professional duty to the patient such as the doctor-patient relationship; (2) the physician breeched this duty or failed to meet the standard of care; (3) proximate cause—the breach of duty by the physician directly caused the patient’s injury; and (4) the patient experienced emotional and/or physical damage while in the care of the physician.27
Reasons for Malpractice Claims
The WestlawNext search revealed 3 top theories of breach of standard of care: excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests. As a result, these theories can be interpreted as medical malpractice law in evolution. In other words, the courts still may be laying groundwork to clarify these theories.
However, a more cynical interpretation of why these 3 top theories of breech of standard of care were seen would note the practice of using expert witness testimony as “hired guns” in the U.S. legal system. Plaintiff attorneys know that use of expert witnesses can increase the attorney’s billable hours during the discovery phase and can decrease the likelihood that the case would be thrown out as lacking merit. Nevertheless, when the claim eventually does go to trial, it may lack merit, but not before plaintiff and defense attorneys complete many hours of work. This use of the legal system for financial gains can potentially confound the true reasons why the search resulted in these 3 top theories of breach of standard of care.
A lack of informed consent was not a major issue and was cited only in 4 (12.5%) cases as the cause of alleged malpractice. This finding was reassuring, as informed consent is an important issue that reinforces the physician-patient relationship and enhances patient trust. Previous studies found a perceived lack of informed consent as a basis for a malpractice claim in more than 34% of otolaryngology cases,25% of cranial nerve surgery cases,and 39% of facial plastic surgery cases.28-30 Perhaps the physician patient discussion in radiation oncology may be different compared with that of surgery, as treatments in radiation oncology are guided by large clinical trials, and patients are often referred after discussions with other specialty providers, such as surgeons and medical oncologists. Improving patients’ understanding of their radiation treatment plans is important in reducing malpractice claims relating to informed consent, and recent studies have identified areas where patient education can be improved.31,32
Settlements
Although settlements were reached in a minority of cases, the monetary value of jury verdicts favoring the plaintiff were 3-fold higher than those of out-of-court settlements. Specifically, cases that were settled had a mean payout of $1,476,775, which sharply contrasts with cases that proceeded to trial and a mean payout of $4,744,219. The highest jury award to the plaintiff was $16,000,000, involving a case where it was determined that a double dose of radiation was delivered to a patient’s shoulder. In a simple risk-reward analysis, this suggests that radiation oncologists should consider settling out of court if a malpractice guilty verdict seems possible. However, given the retrospective nature of the analysis, only limited conclusions can be drawn regarding the effectiveness of such a strategy.
Regardless, cases that were settled or judged on the plaintiff’s behalf were for a much higher value in radiation oncology compared with indemnity payment claims data in other high-risk specialties (emergency medicine, general surgery, obstetrics and gynecologic surgery, and radiology).33 It is important to highlight the magnitude of real and perceived harm that can be associated with radiation oncology. Regarding perceived harm, the public may lack an understanding of how radiation works. Interestingly, even though the perceived harm may be misplaced, the real harm is still there. Unlike other specialties where some errors can be reversed (ie, if heparin is mistakenly administered, its effects can be reversed by protamine sulfate), once radiation is delivered, it is not reversible. The harm is permanent and can cause disability.
Settlements are often lower in legal cases due to insurance policy limitations, the time line of award payout (settlement funds are paid more rapidly, as verdict awards are dependent on the conclusion of the case), and the inherent risk that an appeals court may overturn a verdict or reduce the amount of the award.34 For all the radiation oncology cases that proceeded to trial, more than half (53.1%) of the cases were in favor of the physician (Table 3). While this is positive news for radiation oncologists, it is still lower than the national average of 75% of malpractice verdicts in favor o
Geographic Locations
The concentration of cases in a few states in this analysis is likely due to a combination of factors, including the distinct legal climates in individual states and the geographic unequal distribution of radiation oncologists across the country. For instance, California’s Medical Injury Compensation Reform Act of 1975 caps limited pain, suffering, inconvenience, physical impairment, disfigurement, and other noneconomic and nonmedical damages in malpractice to $250,000.37-39 Because of this cap, plaintiffs and their attorneys may be more hesitant to file a suit.
Radiation oncologists also remain concentrated in highly populated metropolitan health service areas, likely due to the attractiveness of academic centers, the large patient base required to sustain a practice, and the large capital investment needed to obtain the radiation equipment and staff resources to establish practices.40-42
Evolving Malpractice Theories
Zaorsky and colleagues used a similar methodology to this study.24 However, the distinction between this study and the Zaorsky study is that the latter attempted to use medical malpractice cases to draw conclusions on the validity and utility of quality assurance programs, specifically the Accreditation Program for Excellence (APEx) and the Radiation Oncology Incident Learning System (RO-ILS).43-45 The APEx/RO-ILS systems report only errors and faults, and medical malpractice is based on different sets of variables, such as legal theories, litigation procedures, plaintiff/defense zealousness, and the judicial system of inclusion and exclusion of cases in the docket. It is not possible to control for these confounding variables. This study, in contrast to the Zaorsky study, distills the essence of medical malpractice in radiation oncology and draws conclusions to advance the theories of recovery of monetary damage.
Limitations
The WestlawNext database is a comprehensive source for outcomes and details in malpractice litigation and draws from multiple legal sources, but there are limitations to acknowledge. This study is a retrospective analysis and is limited by the inherent bias associated with its design. As noted in previous studies,28,46 some jurisdictions may include only cases reported by attorneys on a voluntary basis with the purpose of predicting future outcomes and awards.47 Settlements may be underrepresented in this study. Out-of-court settlements often are not filed with state or federal courts and thus do not become part of the public record. The level of detail in jury verdicts in this database also is heterogeneous, and each case has different details and varying depths emphasized.
A better source of settlements and plaintiff verdict awards may be the National Practitioner Data Bank (NPDB), an electronic repository created by the U.S. Congress. It contains information on medical malpractice payments and certain adverse actions related to health care practitioners, entities, providers, and suppliers. However, the reports are confidential and not available to the public.
This study had a low number of cases (n = 32), but the information provided is impactful given there is a lack of access to a better source. For instance, insurance companies provide claims data, but the data have been criticized because insurers may be biased in determining which data to release. As discussed previously, the NPDB is not available for public review. Therefore, it is uncertain how many of the medical malpractice cases the WestlawNext database captures.
Based on the discussion with multiple medical malpractice lawyers practicing in various jurisdictions across the country and law school reference librarians, there is a concurrence that about 70% to 90% of claims are not taken on by plaintiff attorneys because of lack of merit or for procedural legal reasons, such as when there is no standing or when the statute of limitations has expired. Of the 10% to 30% claims that proceed to trial, about 90% result in a confidential settlement. Moreover, the court can render an order or an opinion. If it is an order, the case is never recorded. If it is an opinion, the case still may not be included in the WestlawNext database. Only cases that are on appeal, with controversy, proceed through the state and federal appellate system; judges still can decide whether to publish the results from these cases. Depending on jurisdiction, these factors result in 20% to 92% of opinions not being published for any given year. However, opinions that are marked for publishing should be included in the WestlawNext database with negligible omissions and errors. The percentage of published cases in WestlawNext database of all claims could very well be only 1% to 5%.
Nevertheless, the WestlawNext database covers a large geographic area and is a comprehensive source of litigation information. The authors selected WestlawNext over other online legal databases (ie, Bloomberg Law, LexisNexis, VerdictSearch) due to its reputation, quality of case entries, and ease of navigation. WestlawNext is well known among lawyers and legal professions, and it has been validated through previous studies in other medical fields such as general surgery and its subspecialties,36,48 otolaryngology,28,46,47,49 ophthalmology,50 urology,51 dermatology,52 and plastic surgery.53
Conclusion
Litigation involving radiation oncologists were infrequent, and most verdicts were in favor of defendant radiation oncologists. Excessive radiation, unnecessary radiation, and a failure to refer and/or order appropriate tests were noted in most cases. Settlements were reached in the minority of cases, although mean payouts were more than 3 times less in these cases compared with jury verdicts. An increased awareness of radiation oncology malpractice litigation has the potential to improve physician-patient relationships and provide insight into the situations and conditions that commonly lead to litigation within the radiation oncology field.
Click here to read the digital edition.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
1. Mello MM, Studdert DM, Brennan TA. The new medical malpractice crisis. N Engl J Med. 2003;348(23):2281-2284.
2. Howard C, Blau R. Exclusive: legal settlements at Veterans Affairs more than tripled since 2011, many due to medical malpractices. http://www.nydailynews.com/amp /news/national/legal-settlements-veterans-affairs-triple -article-1.2654179. Published May 30, 2016. Accessed January 10, 2018.
3. Rosiak L. VA paid $871M in medical malpractice deals in past decade. http://amp.dailycaller.com/2015/12/17/va-has-paid-230m-in-medical-malpractice-settlements. Published December 17, 2015. Accessed January 11, 2018.
4. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617.
5. Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170(12):1081-1083.
6. Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood). 2010;29(9):1585-1592.
7. Hermer LD, Brody H. Defensive medicine, cost containment, and reform. J Gen Intern Med. 2010;25(5):470-473.
8. Rothberg MB, Class J, Bishop TF, Friderici J, Kleppel R, Lindenauer PK. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868.
9. Martello J. Basic medical legal principles. Clin Plast Surg. 1999;26(1):9-14, v.
10. Kessler DP. Evaluating the medical malpractice system and options for reform. J Econ Perspect. 2011;25(2):93-110.
11. Rosenblatt RA, Detering B. Changing patterns of obstetric practice in Washington State: the impact of tort reform. Fam Med. 1988;20(2):101-107.
12. Seabury SA, Chandra A, Lakdawalla DN, Jena AB. On average, physicians spend nearly 11 percent of their 40-year careers with an open, unresolved malpractice claim. Health Aff (Millwood). 2013;32(1):111-119.
13. Mello MM, Williams CH. Medical malpractice: impact of the crisis and effect of state tort reforms. Research Synthesis Report No. 10. Princeton, NJ: The Robert Wood Johnson Foundation; 2006.
14. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577.
15. Ramella S, Mandoliti G, Trodella L, D’Angelillo RM. The first survey on defensive medicine in radiation oncology. Radiol Med. 2015;120(5):421-429.
16. Marshall DC, Punglia RS, Fox D, Recht A, Hattangadi-Gluth JA. Medical malpractice claims in radiation oncology: a population-based study 1985-2012. Int J Radiat Oncol Biol Phys. 2015;93(2):241-250.
17. Baicker K, Fisher ES, Chandra A. Malpractice liability costs and the practice of medicine in the medicare program. Health Aff (Millwood). 2007;26(3):841-852.
18. Kessler DP, McClellan MB. How liability law affects medical productivity. J Health Econ. 2002;21(6):931-955.
19. Dubay L, Kaestner R, Waidmann T. The impact of malpractice fears on cesarean section rates. J Health Econ. 1999;18(4):491-522.
20. Lakdawalla DN, Seabury SA. The welfare effects of medical malpractice liability. Int Rev Law Econ. 2012;32(4):356-369.
21. Ortashi O, Virdee J, Hassan R, Mutrynowski T, Abu-Zidan F. The practice of defensive medicine among hospital doctors in the United Kingdom. BMC Med Ethics. 2013;14(1):42.
22. Jena AB, Seabury S, Lakdawalla D, Chandra A. Malpractice risk according to physician specialty. N Engl J Med. 2011;365(7):629-636.
23. Marshall D, Tringale K, Connor M, Punglia R, Recht A, Hattangadi-Gluth J. Nature of medical malpractice claims against radiation oncologists. Int J Radiat Oncol Biol Phys. 2017;98(1):21-30.
24. Zaorsky NG, Ricco AG, Churilla TM, Horwitz EM, Den RB. ASTRO APEx® and RO-ILS™ are applicable to medical malpractice in radiation oncology. Future Oncol. 2016;12(22):2643-2657.
25. Hattangadi J, Murphy J, Sanghvi P, Recht A, Punglia RS. A 25-year epidemiologic study of medical malpractice claims in radiation oncology. Int J Radiat Oncol Biol Phys. 2014;90(1)(suppl 9):S749.
26. Necessary elements of proof that injury resulted from failure to follow accepted standard of care. Washington State Legislature. Revised Code of Washington 7.70.040. 2011.
27. Moffett P, Moore G. The standard of care: legal history and definitions: the bad and good news. West J Emerg Med. 2011;12(1):109-112.
28. Svider PF, Husain Q, Kovalerchik O, et al. Determining legal responsibility in otolaryngology: a review of 44 trials since 2008. Am J Otolaryngol. 2013;34(6):699-705.
29. Svider PF, Sunaryo PL, Keeley BR, Kovalerchik O, Mauro AC, Eloy JA. Characterizing liability for cranial nerve injuries: a detailed analysis of 209 malpractice trials. Laryngoscope. 2013;123(5):1156-1162.
30. Svider PF, Keeley BR, Zumba O, Mauro AC, Setzen M, Eloy JA. From the operating room to the courtroom: a comprehensive characterization of litigation related to facial plastic surgery procedures. Laryngoscope. 2013;123(8):1849-1853.
31. Prabhu AV, Crihalmeanu T, Hansberry DR, et al. Online palliative care and oncology patient education resources through Google: do they meet national health literacy recommendations? Pract Radiat Oncol. 2017;7(5):306-310.
32. Prabhu AV, Hansberry DR, Agarwal N, Clump DA, Heron DE. Radiation oncology and online patient education materials: deviating from NIH and AMA recommendations. Int J Radiat Oncol Biol Phys. 2016;96(3):521-528.
33. Carroll AE, Buddenbaum JL. High and low-risk specialties experience with the U.S. medical malpractice system. BMC Health Serv Res. 2013;13:465.
34. Vidmar N. Juries and medical malpractice claims: empirical facts versus myths. Clin Orthop Relat Res. 2009;467(2):367-375.
35. Danzon PM. Medical Malpractice: Theory, Evidence, and Public Policy. Cambridge, MA: Harvard University Press; 1985.
36. Gordhan CG, Anandalwar SP, Son J, Ninan GK, Chokshi RJ. Malpractice in colorectal surgery: a review of 122 medicolegal cases. J Surg Res. 2015;199(2):351-356.
37. Code CC. Civil Code Section 3333.2. In: California So, ed1975.
38. Waters TM, Budetti PP, Claxton G, Lundy JP. Impact of state tort reforms on physician malpractice payments. Health Aff (Millwood). 2007;26(2):500-509.
39. Studdert DM, Yang YT, Mello MM. Are damages caps regressive? A study of malpractice jury verdicts in California. Health Aff (Millwood). 2004;23(4):54-67.
40. Aneja S, Smith BD, Gross CP, et al. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2012;82(5):1723-1729.
41. ASTRO Workforce Committee. 2002 Radiation Oncology Workforce Study: American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 2003;56(2):309-318.
42. Fears D. Renewed effort to lure doctors to rural areas faces obstacles. Washington Post. http://www.was hingtonpost.com/wp-dyn/content/article/2010/08/08/AR2010080802832.html. Published August 9, 2010. Accessed January 11, 2018.
43. American Society for Radiation Oncology. RO-ILS. https://www.astro.org/RO-ILS.aspx. Accessed January 12, 2018.
44. Hoopes DJ, Dicker AP, Eads NL, et al. RO-ILS: Radiation Oncology Incident Learning System: a report from the first year of experience. Pract Radiat Oncol. 2015;5(5):312-318.
45. American Society for Radiation Oncology. APEx® Program Standards. Version 1.4. https://www.astro.org/uploaded Files/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Updated February 1, 2016. Accessed January 12, 2018.
46. Svider PF, Kovalerchik O, Mauro AC, Baredes S, Eloy JA. Legal liability in iatrogenic orbital injury. Laryngoscope. 2013;123(9):2099-2103.
47. Nash JJ, Nash AG, Leach ME, Poetker DM. Medical malpractice and corticosteroid use. Otolaryngol Head Neck Surg. 2011;144(1):10-15.
48. Choudhry AJ, Haddad NN, Rivera M, et al. Medical malpractice in the management of small bowel obstruction: a 33-year review of case law. Surgery. 2016;160(4):1017-1027.
49. Ta JH, Liu YF, Krishna P. Medicolegal aspects of iatrogenic dysphonia and recurrent laryngeal nerve injury. Otolaryngol Head Neck Surg. 2016;154(1):80-86.
50. Engelhard SB, Collins M, Shah C, Sim AJ, Reddy AK. Malpractice litigation in pediatric ophthalmology. JAMA Ophthalmol. 2016;134(11):1230-1235.
51. Sunaryo PL, Svider PF, Jackson-Rosario I, Eloy JA. Expert witness testimony in urology malpractice litigation. Urology. 2014;83(4):704-708.
52. Rayess HM, Gupta A, Svider PF, et al. A critical analysis of melanoma malpractice litigation: should we biopsy everything? Laryngoscope. 2017;127(1):134-139.
53. Paik AM, Mady LJ, Sood A, Eloy JA, Lee ES. A look inside the courtroom: an analysis of 292 cosmetic breast surgery medical malpractice cases. Aesthet Surg J. 2014;34(1):79-86.
Managing malignant pleural effusion
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
KEY POINTS
- Asymptomatic pleural effusion in patients currently on chemotherapy does not require treatment but should be monitored for progression.
- Indwelling pleural catheters are best used to treat effusion with lung collapse and are increasingly used as first-line therapy in other settings.
- Chemical or mechanical pleurodesis results in filling the pleural space to prevent further fluid accumulation and can be accomplished by one of several methods.
- For patients near the end of life, simple thoracentesis, repeated as needed, is a reasonable strategy.
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
AAP guidance: How to ask about military service
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
AAN publishes position statement on brain death
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
FROM NEUROLOGY
Key clinical point: The AAN calls for uniform brain death laws, policies, and practices.
Major finding: The association published a position statement online on January 2.
Study details: The AAN’s Brain Death Working Group drafted the statement.
Disclosures: The authors reported no relevant disclosures, and the American Academy of Neurology funded their work.
Source: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
Hospice liability
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
Question: Hospice liability may exist in which of the following?
A. False claims in violation of Medicare rules regarding eligible beneficiaries.
B. False claims for continuous home care services.
C. Negligent billing practices.
D. Only A and B are correct.
E. A, B, and C are correct.
Answer: D. With an aging population and better end-of-life care, the United States has in the last decade witnessed about a 50% increase in the number of hospices. Hospice care is a Medicare-covered benefit, and most hospices operate on a for-profit basis. Although occasionally institution based, services are more often offered as an outpatient or home-care option. In 2016, hospice care reached 1.4 million beneficiaries, with total Medicare expenditure of $16.7 billion.1
There are two broad categories of legal jeopardy that hospices face: Medicare fraud and malpractice lawsuits. This article will address these two issues. In addition, hospices, like all health care institutions, face numerous other liabilities, such as negligent hiring, breach of confidentiality, premise liability, HIPAA violations, sexual harassment, vicarious liability, and many others.
Medicare fraud
The False Claims Act (FCA) is an old law enacted by Congress way back in 1863. It imposes liability for submitting a payment demand to the federal government where there is actual or constructive knowledge that the claim is false.2
Intent to defraud is not a required element. But knowing or reckless disregard of the truth or material misrepresentation are required, although negligence is insufficient to constitute a violation. Penalties include treble damages, costs and attorney fees, and fines of $11,000 per false claim – as well as possible imprisonment. The FCA is the most prominent health care antifraud statute.3 Two others are the federal Anti-Kickback Statute and the Stark Law.
A recent example of hospice fraud involved Ohio’s Chemed and Vitas Hospice Services, which were accused of knowingly billing for hospice-ineligible patients and inflated levels of care.4
The government alleged that the defendants rewarded employees with bonuses based on the number of patients receiving hospice services, irrespective of whether they were actually terminally ill or needed continuous home care services (CHCS). CHCS commands the highest Medicare daily rate and is meant only for the temporary treatment of acute symptoms constituting a medical crisis.
According to the complaint, the defendants set aggressive billing goals for CHCS without regard to whether the patients actually required such a level of service. The defendants agreed to pay $75 million to settle the lawsuit, the largest in the history of hospice false-claim settlements.
Can an alleged wrong prognosis regarding life expectancy amount to a false claim? Under Medicare rules, a physician certifying that a patient is eligible for hospice care must attest that the condition is terminal, with death expected within 6 months.
AseraCare, a hospice company, was accused of knowingly submitting false claims to Medicare by certifying patients as eligible for hospice. The government claimed that the medical records of the 123 patients at issue did not contain clinical information and other documentation that supported the medical prognosis, and thus, AseraCare’s claims for those patients were false.
AseraCare won a summary judgment defending against the $200 million lawsuit in a federal district court in Alabama. The court opined that, when hospice-certifying physicians and government medical experts look at the very same medical records and disagree about eligibility, the opinion of one medical expert alone cannot prove falsity without further evidence of an objective falsehood.5 The government, however, has appealed the decision to the Court of Appeals for the Eleventh Circuit.
Malpractice
Hospices have their share of malpractice litigation, and judgments may be substantial because of noneconomic losses such as pain and suffering, not to mention punitive damages.
For example, in 2013a Maryland jury awarded more than $950,000 to a family that alleged that the decedent’s death was caused by the excessive use of morphine and oxycodone in treating her infected ulcers. Such treatment was deemed suitable for a hospice-type situation, but in fact, the patient was not expected to die within 6 months.
Her husband and two children argued successfully that the hospital committed malpractice by misdiagnosing her need for hospice care and by performing unnecessary surgery. The bulk of the judgment was for pain and suffering and other noneconomic damages.
In another negligence suit, a 66-year-old woman died in a hospice after receiving an overdose of Dilaudid for pancreatic cancer, which an autopsy revealed she did not have. In that case, the plaintiffs were awarded $4.5 million in a wrongful death lawsuit filed against Hospice Ministries and its medical director. The jury awarded the family $4 million in monetary compensation and $500,000 in punitive damages.
The case of McGregor v. Hospice Care of Louisiana is illustrative of a malpractice action with a focus on expert testimony.6 The issue in this case was whether the testimony of the plaintiffs’ expert, Bruce Samuels, MD, was admissible and whether it correctly addressed the requisite standard of care.
The decedent had terminal metastatic prostate cancer and was under the care of an oncologist. He eventually enrolled as a patient of Hospice of Baton Rouge, whose nurses visited him in his home several times a week. They reported their findings to the attending oncologist, who prescribed a total of 40 morphine suppositories to be administered 1-2 per hour as needed for pain. However, the prescription noted that only half – that is, 20 suppositories – were to be filled, and stipulated when the remaining 20 suppositories could be released.
Believing that his father was in pain, the patient’s son demanded the early release of the remaining 20 morphine suppositories; he also refused to allow the nurse to assess the patient and exhibited threatening behavior toward her. After conferring with the oncologist on call, the hospice discharged the patient from its care. An ambulance later took the patient to a hospital, where he died that evening.
The family filed a lawsuit against the hospice, alleging negligence in failing to release the remaining 20 morphine suppositories and in abandoning the patient by discharging him. At trial, the jury rendered a verdict in favor of the hospice, after the court excluded the testimony of the plaintiffs’ expert as being outside his expertise. However, the Louisiana Supreme Court found that the trial court erred in excluding his testimony.
On remand, the appellate court affirmed the trial court’s judgment that the plaintiffs had failed to meet the burden of proof showing negligence. It found that the expert, Dr. Samuels, admitted he had never written a partial-fill prescription before and that he did not know who had the authority to authorize the pharmacist to release the remainder of the partial fill prescription in this case.
In addition, Dr. Samuels acknowledged that a nurse has the obligation to assess a patient and report her findings to the physician and follow any orders of the physician. Further, the nurse indicated that the doctor had instructed her to discharge the patient, not from the doctor’s care, but for treatment to be continued at the hospital.
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the materials have been taken from earlier columns in Internal Medicine News. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. “Medicare’s most indefensible fraud hotspot: Hospice care.” CNBC, Modern Medicine, Aug. 3, 2018.
2. 31 U.S. Code, Section 3729(a)(1)(A).
3. Tan SY. “Update on the False Claims Act.” Internal Medicine News, April 5, 2017.
4. U.S. Department of Justice, Office of Public Affairs, Oct. 30, 2017.
5. U.S. ex rel. Paradies et al. v. AseraCare Inc. et al., case number 2:12-CV-245-KOB, in the U.S. District Court for the Northern District of Alabama, March 31, 2016.
6. McGregor v. Hospice Care of Louisiana in Baton Rouge, LLC, No. 2013 CA 1979R, consolidated with No. 2013 CA 1980R. Court of Appeals of Louisiana, First Circuit, judgment rendered Sept. 21, 2015.
Telemedicine not widely used
Also today, you ought to be judicious with empiric antibiotics for febrile neutropenia, home-based exercise is better than supervised treadmill exercise for peripheral arterial disease, and brain injury in sickle cell merits more attention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, you ought to be judicious with empiric antibiotics for febrile neutropenia, home-based exercise is better than supervised treadmill exercise for peripheral arterial disease, and brain injury in sickle cell merits more attention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, you ought to be judicious with empiric antibiotics for febrile neutropenia, home-based exercise is better than supervised treadmill exercise for peripheral arterial disease, and brain injury in sickle cell merits more attention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
The Right Choice? A New Chapter
As I write this last installment of “The Right Choice?” for ACS Surgery News, a number of different emotions are going through my mind all at the same time. I am surprised at how quickly the time has passed since I wrote my first surgical ethics column for SN in 2011. In the 33 columns that I have written since then, I have tried to focus on aspects of surgical practice that emphasize the ethical dimension. I have tried to write columns that would be of interest to practicing surgeons in any setting and not only to academic surgeons that practice in urban environments such as I practice in. This is the last column and thus the end of a chapter of my life and the beginning of a new one.
Over the last 7 years, I have been flattered by the comments from fellow surgeons who report that they actually read the column. I have always said that I wrote this column with the expectation that no one would actually read them. I have to confess that this is not completely true. As I wrote each column, I did so as though I was writing them for my father to read. My father, S. Peter Angelos, MD, FACS, was a general surgeon who spent his entire career practicing in the town of Plattsburgh, N.Y., where he grew up. My father’s practice was very different from mine. I work at an urban academic medical center where I have a very narrow subspecialty practice in endocrine surgery. My father had a small-town community practice of “bread and butter” general surgery. Yet, when he and I would talk about patients, the commonality of the relationship between a surgeon and a patient transcended these differences. I realize that in many ways, I wrote this column as a way of organizing my own thoughts and then presenting them to my father in the hopes that he would find them of some value.
For several years, I would send drafts of my column to my parents, and both my father and mother would read them and give me suggestions. Many of the earlier columns were changed for the better by their comments. In recent years, my father’s health declined and he was no longer able to give me comments. Nevertheless, I continued to compose them as though writing for him. Approximately 6 weeks ago, my father passed away. It has been sad for my mother and my entire family. We all realized that it was the end of one chapter of our lives and the start of a new one without my father.
I find the concept of “beginning a new chapter” to be an important one for surgeons to reflect upon. There are certain events, such as the death of a parent, that force us to think about the end of one phase of life and the beginning of another phase. However, the division of one’s experience into phases or chapters, is somewhat arbitrary. This past summer I became a patient and had surgery myself for the first time. I cannot help but think of that operation as the start of a new chapter for me. I am convinced that although all patients may not reflect upon surgery in the same way that I did, nevertheless, an operation is a dramatic event that most people remember for a long time. In this context, many people will see their interactions with their surgeon and their operation as the end of one chapter and the beginning of a new one.
In this context, it is critical for surgeons to be fully cognizant of the great impact that we may have on our patient’s internal narratives of their lives. When we operate on someone, we run the risk of that person’s functional status changing forever. We may be the means by which our patient is cured of cancer or suffers a debilitating complication. As surgeons, we therefore, occupy a potentially significant role in the trajectory of our patients’ lives. I believe that the relationship between a surgeon and a patient is distinctive and central in the narrative that so many patients create about their lives. It is essential that surgeons continue to appreciate the value of the quality of that relationship with our patients and the impact—potentially positive or negative—that it can have upon our patients.
Throughout medicine, in general, and in surgery in particular, one cannot go a week without hearing about the problem of burnout. Although there is no single cure for burnout, I do believe that paying attention to the ethical dimension of our interactions with our patients and the impact that surgery can have on their lives will go a long way to reducing the risks of burnout among surgeons.
In an era in which we are often pushed to increase RVUs at the expense of spending time with individual patients, we must not forget how significant our relationships with our patients can be. I believe that attention to this relationship will be beneficial to patients and also to us as we help our patients start new chapters in their lives.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I write this last installment of “The Right Choice?” for ACS Surgery News, a number of different emotions are going through my mind all at the same time. I am surprised at how quickly the time has passed since I wrote my first surgical ethics column for SN in 2011. In the 33 columns that I have written since then, I have tried to focus on aspects of surgical practice that emphasize the ethical dimension. I have tried to write columns that would be of interest to practicing surgeons in any setting and not only to academic surgeons that practice in urban environments such as I practice in. This is the last column and thus the end of a chapter of my life and the beginning of a new one.
Over the last 7 years, I have been flattered by the comments from fellow surgeons who report that they actually read the column. I have always said that I wrote this column with the expectation that no one would actually read them. I have to confess that this is not completely true. As I wrote each column, I did so as though I was writing them for my father to read. My father, S. Peter Angelos, MD, FACS, was a general surgeon who spent his entire career practicing in the town of Plattsburgh, N.Y., where he grew up. My father’s practice was very different from mine. I work at an urban academic medical center where I have a very narrow subspecialty practice in endocrine surgery. My father had a small-town community practice of “bread and butter” general surgery. Yet, when he and I would talk about patients, the commonality of the relationship between a surgeon and a patient transcended these differences. I realize that in many ways, I wrote this column as a way of organizing my own thoughts and then presenting them to my father in the hopes that he would find them of some value.
For several years, I would send drafts of my column to my parents, and both my father and mother would read them and give me suggestions. Many of the earlier columns were changed for the better by their comments. In recent years, my father’s health declined and he was no longer able to give me comments. Nevertheless, I continued to compose them as though writing for him. Approximately 6 weeks ago, my father passed away. It has been sad for my mother and my entire family. We all realized that it was the end of one chapter of our lives and the start of a new one without my father.
I find the concept of “beginning a new chapter” to be an important one for surgeons to reflect upon. There are certain events, such as the death of a parent, that force us to think about the end of one phase of life and the beginning of another phase. However, the division of one’s experience into phases or chapters, is somewhat arbitrary. This past summer I became a patient and had surgery myself for the first time. I cannot help but think of that operation as the start of a new chapter for me. I am convinced that although all patients may not reflect upon surgery in the same way that I did, nevertheless, an operation is a dramatic event that most people remember for a long time. In this context, many people will see their interactions with their surgeon and their operation as the end of one chapter and the beginning of a new one.
In this context, it is critical for surgeons to be fully cognizant of the great impact that we may have on our patient’s internal narratives of their lives. When we operate on someone, we run the risk of that person’s functional status changing forever. We may be the means by which our patient is cured of cancer or suffers a debilitating complication. As surgeons, we therefore, occupy a potentially significant role in the trajectory of our patients’ lives. I believe that the relationship between a surgeon and a patient is distinctive and central in the narrative that so many patients create about their lives. It is essential that surgeons continue to appreciate the value of the quality of that relationship with our patients and the impact—potentially positive or negative—that it can have upon our patients.
Throughout medicine, in general, and in surgery in particular, one cannot go a week without hearing about the problem of burnout. Although there is no single cure for burnout, I do believe that paying attention to the ethical dimension of our interactions with our patients and the impact that surgery can have on their lives will go a long way to reducing the risks of burnout among surgeons.
In an era in which we are often pushed to increase RVUs at the expense of spending time with individual patients, we must not forget how significant our relationships with our patients can be. I believe that attention to this relationship will be beneficial to patients and also to us as we help our patients start new chapters in their lives.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.
As I write this last installment of “The Right Choice?” for ACS Surgery News, a number of different emotions are going through my mind all at the same time. I am surprised at how quickly the time has passed since I wrote my first surgical ethics column for SN in 2011. In the 33 columns that I have written since then, I have tried to focus on aspects of surgical practice that emphasize the ethical dimension. I have tried to write columns that would be of interest to practicing surgeons in any setting and not only to academic surgeons that practice in urban environments such as I practice in. This is the last column and thus the end of a chapter of my life and the beginning of a new one.
Over the last 7 years, I have been flattered by the comments from fellow surgeons who report that they actually read the column. I have always said that I wrote this column with the expectation that no one would actually read them. I have to confess that this is not completely true. As I wrote each column, I did so as though I was writing them for my father to read. My father, S. Peter Angelos, MD, FACS, was a general surgeon who spent his entire career practicing in the town of Plattsburgh, N.Y., where he grew up. My father’s practice was very different from mine. I work at an urban academic medical center where I have a very narrow subspecialty practice in endocrine surgery. My father had a small-town community practice of “bread and butter” general surgery. Yet, when he and I would talk about patients, the commonality of the relationship between a surgeon and a patient transcended these differences. I realize that in many ways, I wrote this column as a way of organizing my own thoughts and then presenting them to my father in the hopes that he would find them of some value.
For several years, I would send drafts of my column to my parents, and both my father and mother would read them and give me suggestions. Many of the earlier columns were changed for the better by their comments. In recent years, my father’s health declined and he was no longer able to give me comments. Nevertheless, I continued to compose them as though writing for him. Approximately 6 weeks ago, my father passed away. It has been sad for my mother and my entire family. We all realized that it was the end of one chapter of our lives and the start of a new one without my father.
I find the concept of “beginning a new chapter” to be an important one for surgeons to reflect upon. There are certain events, such as the death of a parent, that force us to think about the end of one phase of life and the beginning of another phase. However, the division of one’s experience into phases or chapters, is somewhat arbitrary. This past summer I became a patient and had surgery myself for the first time. I cannot help but think of that operation as the start of a new chapter for me. I am convinced that although all patients may not reflect upon surgery in the same way that I did, nevertheless, an operation is a dramatic event that most people remember for a long time. In this context, many people will see their interactions with their surgeon and their operation as the end of one chapter and the beginning of a new one.
In this context, it is critical for surgeons to be fully cognizant of the great impact that we may have on our patient’s internal narratives of their lives. When we operate on someone, we run the risk of that person’s functional status changing forever. We may be the means by which our patient is cured of cancer or suffers a debilitating complication. As surgeons, we therefore, occupy a potentially significant role in the trajectory of our patients’ lives. I believe that the relationship between a surgeon and a patient is distinctive and central in the narrative that so many patients create about their lives. It is essential that surgeons continue to appreciate the value of the quality of that relationship with our patients and the impact—potentially positive or negative—that it can have upon our patients.
Throughout medicine, in general, and in surgery in particular, one cannot go a week without hearing about the problem of burnout. Although there is no single cure for burnout, I do believe that paying attention to the ethical dimension of our interactions with our patients and the impact that surgery can have on their lives will go a long way to reducing the risks of burnout among surgeons.
In an era in which we are often pushed to increase RVUs at the expense of spending time with individual patients, we must not forget how significant our relationships with our patients can be. I believe that attention to this relationship will be beneficial to patients and also to us as we help our patients start new chapters in their lives.
Dr. Angelos is the Linda Kohler Anderson Professor of Surgery and Surgical Ethics, chief of endocrine surgery, and associate director of the MacLean Center for Clinical Medical Ethics at the University of Chicago.