User login
Cutaneous Eruption in an Immunocompromised Patient
The Diagnosis: Secondary Syphilis
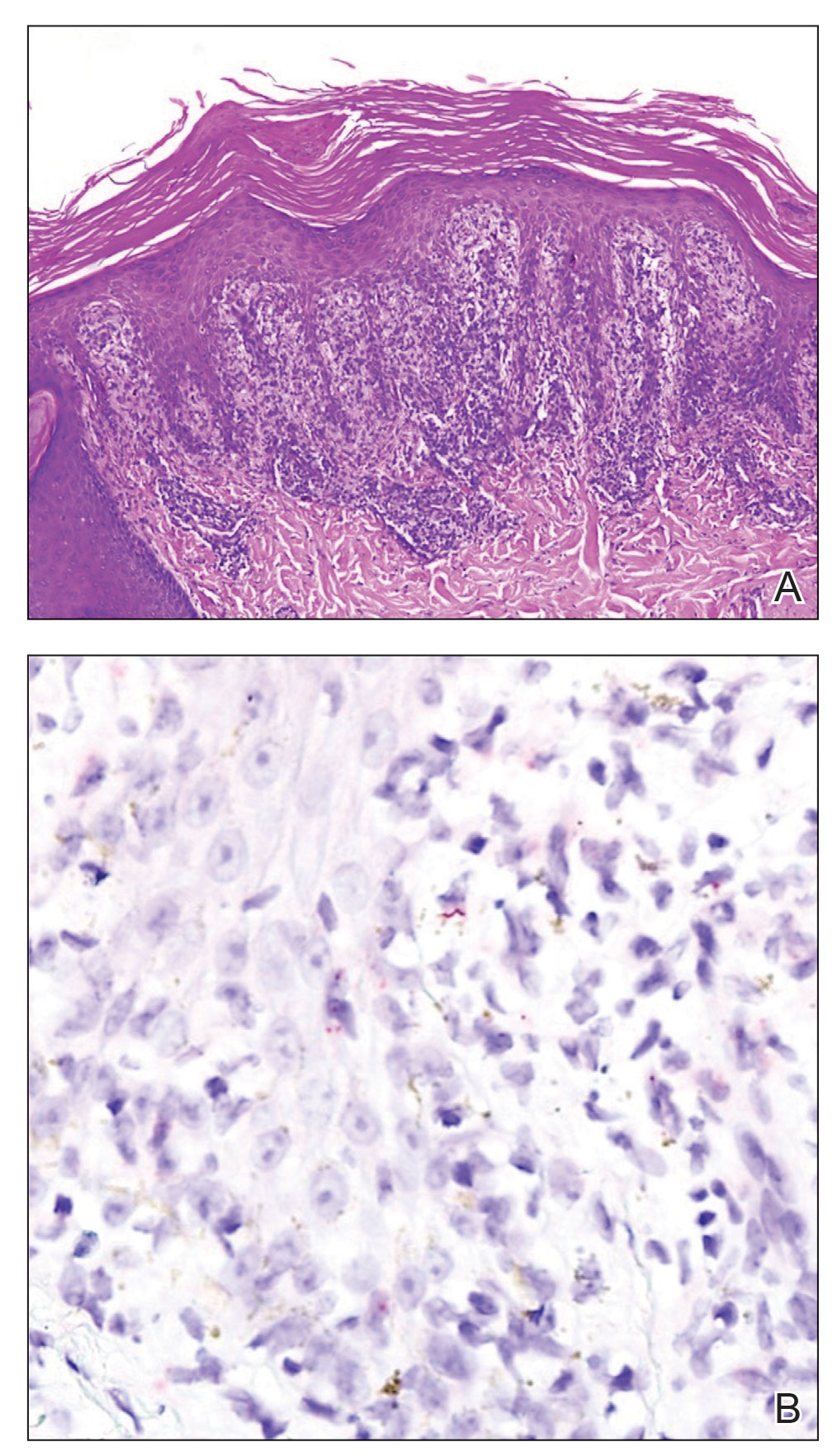
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.
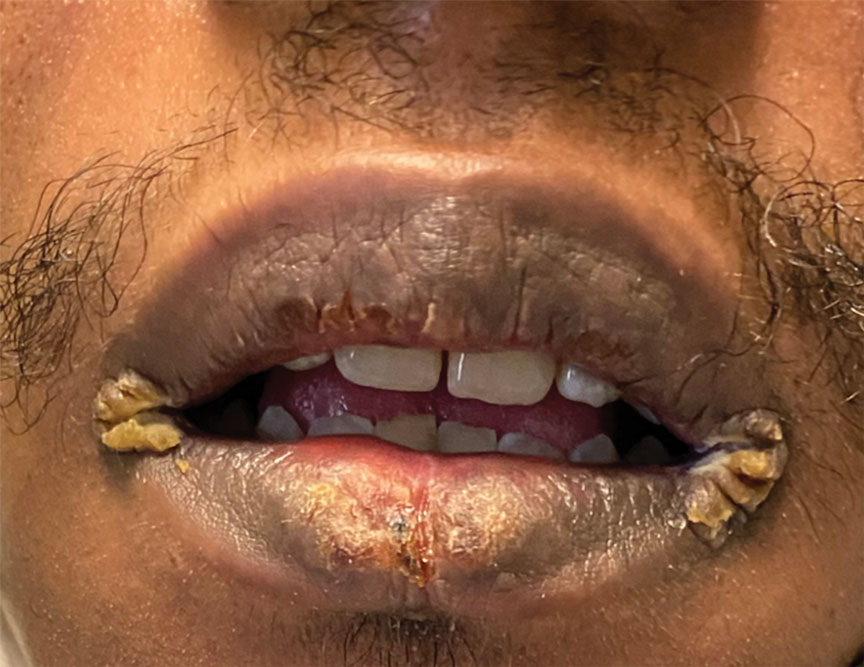
Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

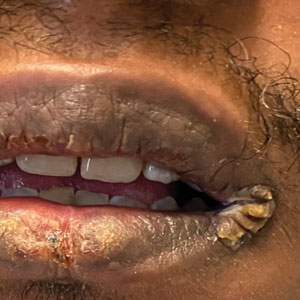
A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
A 29-year-old Black man with long-standing untreated HIV presented with mildly pruritic, scaly plaques on the palms and soles of 2 weeks’ duration. His medical history was notable for primary syphilis treated approximately 1 year prior. A review of symptoms was positive for blurry vision and floaters but negative for constitutional symptoms. Physical examination revealed well-defined scaly plaques over the palms, soles, and elbows with subungual hyperkeratosis. Patches of nonscarring alopecia over the scalp and split papules at the oral commissures also were noted. There were no palpable lymph nodes or genital involvement. Eye examination showed conjunctival injection and 20 cells per field in the vitreous humor. Laboratory evaluation revealed an HIV viral load of 31,623 copies/mL and a CD4 count of 47 cells/μL (reference range, 362–1531 cells/μL). A shave biopsy of the left elbow was performed for histopathologic evaluation.
Monkeypox features include mucocutaneous involvement in almost all cases
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
MILAN – In the current spread of monkeypox among countries outside of Africa, this zoonotic orthopox DNA virus is sexually transmitted in more than 90% of cases, mostly among men having sex with men (MSM), and can produce severe skin and systemic symptoms but is rarely fatal, according to a breaking news presentation at the annual congress of the European Academy of Dermatology and Venereology.
Synthesizing data from 185 cases in Spain with several sets of recently published data, Alba Català, MD, a dermatologist at Centro Médico Teknon, Barcelona, said at the meeting that there have been only two deaths in Spain in the current epidemic. (As of Sept. 30, after the EADV meeting had concluded, a total of three deaths related to monkeypox in Spain and one death in the United States had been reported, according to the Centers for Disease Control and Prevention).
Hospitalizations have been uncommon, and in Spain, there were only four hospitalizations, according to data collected from the beginning of May through early August, she said. Almost all cases in this Spanish series were from men having high-risk sex with men. Upon screening, 76% had another sexually transmitted disease, including 41% infected with human immunodeficiency virus.
More than 40% of patients with monkeypox have HIV
These data are consistent with several other recently published studies, such as one that evaluated 528 infections in 16 non-African countries, including those in North America, South America, Europe, the Mideast, as well as Australia. In that survey, published in the New England Journal of Medicine, and covering cases between late April and late June, 2022, 41% were HIV positive. Of those who were HIV negative, 57% were taking a pre-exposure prophylaxis regimen of antiretroviral drugs to prevent HIV infection.
However, these data do not preclude a significant risk of nonsexual transmission, according to Dr. Català, who noted that respiratory transmission and transmission through nonsexual skin contact is well documented in endemic areas.
“The virus has no preference for a sexual orientation,” Dr. Català cautioned. Despite the consistency of the data in regard to a largely MSM transmission in the epidemic so far, “these data may change with further spread of infection in the community.”
Typically, the incubation period of monkeypox lasts several days before the invasive period, which is commonly accompanied by systemic complaints, particularly fever, headache, and often lymphadenopathy. These systemic features usually but not always precede cutaneous involvement, which is seen in more than 90% of patients, according to Dr. Català. In the Spanish series, mucocutaneous involvement was recorded in 100% of patients.
Monkeypox and smallpox
“The differential diagnosis might include other vesicular eruptions, such as those caused by varicella or smallpox,” reported Dr. Català, who noted that monkeypox and smallpox are related.
Cutaneous lesions often appear first at the site of infection, such as the genitalia, but typically spread in a secondary eruption that is pruritic and may take days to resolve, according to Dr. Català. She reported that single lesions are less common but do occur. While hundreds of lesions have been reported among cases in endemic areas, most patients had 25 lesions or fewer in the Spanish epidemic and other recent series.
Even though there is a common progression in which lesions begin in a papular stage before the vesicular and pustular stages in a given area, new eruptions can occur before a prior eruption develops scabs.
“Frequently, not all the patient’s lesions are in the same stage of development,” said Dr. Català, who explained that disease activity and its complications, such as proctitis, pharyngitis, and penile edema, can take weeks to resolve. Because of the highly invasive nature of monkeypox, it is appropriate to be alert to less common manifestations, such as ocular involvement.
Many of these and other complications, such as secondary bacterial infections, will require targeted treatment, but the mainstay of therapy for the dermatologic manifestations of monkeypox is symptomatic treatment that includes nonsteroidal anti-inflammatory drugs and analgesics.
Re-epithelialization reduces transmission risk
“A clean, moist environment can mitigate transmission potential by covering infectious sores and promoting the re-epithelialization of the damaged exanthem,” Dr. Català advised. Tecovirimat (TPOXX, ST-246), an antiviral drug for smallpox, is approved for treating monkeypox in Europe but not in the United States (but it is approved for smallpox in the United States). Another antiviral drug, brincidofovir (CMX001 or Tembexa), is approved for smallpox in the United States, but not in Europe, according to Dr. Català. (In the United States, no treatment is specifically approved for treating monkeypox, but antivirals developed for smallpox “may prove beneficial against monkeypox,” according to the CDC.)
But she advised weighing the risks and benefits of using either drug in any individual patient.
The data suggest that the risk of viral shedding persists until the late stages of the disease trajectory. “A person is considered infectious from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelization has occurred,” Dr. Català said.
The prolonged period of contagion might be one reason to expect monkeypox to be transmitted more generally than it is now, according to Boghuma K. Titanji, MD, PhD, assistant professor of infectious diseases, Emory University, Atlanta.
“The longer the outbreak persists, the more likely we will see cases reported in groups other than MSM who have been most affected so far,” said Dr. Titanji, the first author of a recently published review article on monkeypox in Open Forum Infectious Diseases.
However, he acknowledged that a COVID-like spread is not expected. “The spread of monkeypox requires close and prolonged contact and is generally inefficient via fomites and droplet modes of transmission,” Dr. Titanji said in an interview. “Spread in heterosexual networks and congregate settings like crowded jails where close contact is unavoidable remains a concern that we need to educate the public about and maintain a high level of vigilance for.”
Dr. Català and Dr. Titanji report no potential conflicts of interest.
AT THE EADV CONGRESS
Severe COVID-19–related outcomes found worse in men with RA
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
A retrospective study that analyzed sex disparities in patients with COVID-19 and rheumatoid arthritis found that men had more baseline comorbidities and increased risk of COVID-19–related outcomes, compared with women.
“Differences in genetics between sex and sex steroid hormones may play a role in predisposition to COVID-19 infection as well as modulating the disease progression,” according to Xiaofeng Zhou, PhD, senior director at Pfizer, New York, and the study’s lead author.
Dr. Zhou presented her findings at The Lancet Summit on Sex and Gender in Rheumatology.
Patients with chronic rheumatic diseases treated with immunomodulatory therapies may be at higher risk for more severe COVID-19 outcomes, including hospitalization, complications, and death. Research on sex-based disparities in RA patients with COVID-19 in the United States is limited, said Dr. Zhou, who embarked on a retrospective cohort study to examine the demographic and clinical characteristics of RA patients with COVID-19 and estimate the risk of possible COVID-19 outcomes by sex.
Dr. Zhou and colleagues used U.S. COVID-19 data collected through electronic health records by Optum during 2020 to June 2021. The study included adult patients with RA and a COVID-19 diagnosis (≥ 1 diagnosis code or positive SARS-CoV-2 laboratory test) and greater than or equal to 183 days of database enrollment who received treatment with immunomodulatory therapies prior to the diagnosis date. They were stratified by sex.
Investigators used logistic regression to estimate the risk of 11 possible COVID-19–related outcomes within 30 days of the COVID-19 diagnosis (hospitalization, ICU admission, pneumonia, kidney failure, thrombotic event, heart failure, acute respiratory distress syndrome [ARDS], sepsis/septic shock, mechanical ventilation/extracorporeal membrane oxygenation [ECMO], in-hospital death, and all-cause mortality), adjusting for demographics and baseline clinical covariates.
A total of 4,476 COVID-19 patients with RA (78% female) took part in the study. Male patients trended older (64 vs. 60 years) and had lower African American representation and Medicaid enrollment than female patients, but they had more baseline comorbidities such as hypertension (55% vs. 45%), hyperlipidemia (45% vs. 33%), diabetes (25% vs. 20%), coronary artery disease (28% vs. 12%), and chronic kidney disease (20% vs. 15%).
Eight of the eleven COVID-19 outcomes were significantly more likely to occur in men than women (hospitalization: odds ratio, 1.32 [95% confidence interval (CI), 1.11-1.56]; ICU admission: OR, 1.80 [95% CI, 1.36-2.40]; mechanical ventilation/ECMO: OR, 1.48 [95% CI, 1.04-2.11]; in-hospital death: OR, 1.53 [95% CI, 1.13-2.07]; all-cause mortality: OR, 1.42 [95% CI, 1.09-1.86]; sepsis: OR, 1.55 [95% CI, 1.20-2.02]; kidney failure: OR, 1.46 [95% CI, 1.15-1.85]; ARDS: OR, 1.39 [95% CI, 1.15-1.69]).
Sex hormones factor into risk
The data illustrated that men with RA had more baseline comorbidities and increased risk of COVID-19 outcomes than women.
Sex hormones regulate virus entry into host cells, respiratory function, immune response, the cardiovascular system, and coagulation, explained Dr. Zhou.
Estrogen and progesterone in women could help develop stronger and efficient immune responses to viruses and reduce virus entry into the host cells. Also, “[the] larger number of copies of ACE2 genes in women, [which] is linked with protection in the lungs against edema, permeability, and pulmonary damage, could be associated with lower incidence of severe COVID-19 outcomes, such as respiratory-related mortality and mortality,” Dr. Zhou said.
By comparison, androgens in men may increase virus entry into the host cells and promote unfavorable immune response through the induction of cytokine production and reducing the antibody response to the virus. This could lead to severe infection, Dr. Zhou said.
Sex-based differences in steroid hormones may also explain the higher incidence of morbidity and fatality that’s been observed in other studies of male patients with other infectious diseases, such as severe acute respiratory syndrome and Middle East respiratory syndrome.
Study bolsters evidence on sex disparities
The results add real-world evidence to the limited literature on sex disparities in COVID-19 outcomes among patients with RA in the United States, Dr. Zhou said. “The differential role in sex steroid hormones among women and men may shed light on clinical management of COVID-19 patients and the need to consider sex-specific approaches in clinical trials in preventing and treating COVID-19 patients,” she said.
Considering that all patients are recommended to get COVID-19 vaccinations, “it is difficult to say how this impacts clinical practice,” said Janet Pope, MD, MPH, professor of medicine in the division of rheumatology at the University of Western Ontario, London, who was not involved with the study.
Sharing results with some patients may help to encourage vaccination, thus reducing risk of poor COVID-19 outcomes, Dr. Pope said.
In future studies, Dr. Zhou suggests using multiple databases and considering other geographies beyond the United States to further understand the etiology of sexual dimorphism in COVID-19 and expand generalizability. “In addition, future research will seek to provide insights into health equity gaps in the management of COVID-19. This may inform development of precision medicines and vaccines, especially among patients on immunosuppressive treatments,” she said.
The study was sponsored by Pfizer. Dr. Zhou and other study authors are Pfizer employees and hold Pfizer stock.
A version of this article first appeared on Medscape.com.
FROM THE LANCET SUMMIT ON SEX AND GENDER IN RHEUMATOLOGY
USPSTF: Screen at-risk, nonpregnant people for syphilis
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
People at increased risk for syphilis – including asymptomatic, nonpregnant adolescents and adults who have ever been sexually active and are at high risk for the disease – should be screened for it, according to a reaffirmation by the United States Preventive Services Task Force of its 2016 recommendation of syphilis screening for people at increased risk for infection.
“Using a reaffirmation process, the authors, led by Carol M. Mangione, MD, MSPH, of the University of California, Los Angeles, wrote in JAMA.
Reported cases in the United States of primary and secondary syphilis – a sexually transmitted infection caused by the bacterium Treponema pallidum that can damage the brain, nerves, eyes, and cardiovascular system if left untreated – increased from a low of 2.1 cases per 100,000 people in 2000 and 2001 to 11.9 cases per 100,000 in 2019, the authors reported. In 2019, men accounted for 83% of all primary and secondary syphilis cases, and men who have sex with men (MSM) accounted for 57% of all primary and secondary syphilis cases in men. Screening and follow-up treatment can cure syphilis and prevent complications.
To help them evaluate the effectiveness and safety of screening, the USPSTF authors reviewed the literature and visually displayed key questions and linkages to interventions and outcomes, Michelle L. Henninger, PhD, Sarah I. Bean, MPH, and Jennifer S. Lin, MD, MCR, of the Kaiser Permanente Evidence-based Practice Center in Portland, Ore., noted in a related evidence report of the post-2016 recommendation data.
Reaffirming its 2016 recommendation, the USPSTF now advises clinicians to:
Assess risk:
- Clinicians should know how common syphilis is in their community and assess their patient’s individual risk.
- Risk for syphilis is higher in MSM, people with HIV infection or other STIs, and those who use illicit drugs or have a history of incarceration, sex work, or military service.
Screen and confirm by testing:
- Traditional screening algorithm: Start with a nontreponemal test such as Venereal Disease Research Laborator or rapid plasma reagin. If positive, confirm result with a treponemal antibody detection test, such as T. pallidum particle agglutination.
- Reverse sequence algorithm: Screen with an initial automated treponemal test such as enzyme-linked or chemiluminescence immunoassay. If positive, confirm result with a nontreponemal test.
Consider screening interval:
- Evidence on optimal screening intervals is limited for the general population, but MSM and people with HIV may benefit from screening yearly or every 3-6 months if they remain at high risk.
The authors acknowledged that primary and secondary syphilis rates are higher in Blacks, Hispanics, Native Americans/Alaska Native, and Native Hawaiians/Pacific Islanders, and that the disparities are primarily driven by social determinants of health including differences in income, education, and access to coverage and care.
They added that differences in sexual networks also play a role in disparities and that sexually active people in communities with higher STI rates may be more likely to become infected.
More testing, treatment, and research are needed
Four experts welcomed the reaffirmation.
“It is important and necessary that the task force has chosen to reaffirm their syphilis screening recommendations, given the continued increase in sexually transmitted infections in the U.S. since the 2016 published recommendations,” Judith A. O’Donnell, MD, director of the department of infection prevention and control at Penn Presbyterian Medical Center in Philadelphia, said in an interview.
“Awareness of the ongoing incidence, understanding of the importance of screening in interrupting transmission, and getting people diagnosed and treated before serious complications are key,” she added.
Heidi Gullettt, MD, MPH, associate director of the Center for Community Health Integration at Case Western Reserve University, Cleveland, said: “The reaffirmation document authors demonstrated a comprehensive review of high-quality studies and epidemiologic data.
“Primary care clinicians rely on USPSTF recommendations to help prioritize evidence-based prevention in practice, so this reaffirmation is a critical step to remind us of the importance of regularly assessing risk and screening with a readily available screening test in the office,” she added.
Testing during office visits is not easy, Dr. Gullettt said, because of competing priorities, stigma associated with STIs, and testing and treatment costs.
“Under the Affordable Care Act, USPSTF screening recommendations are supposed to be covered without cost sharing by patients. This should be the case for syphilis screening,” Dr. Gullett pointed out. “Patients are often reluctant to do screening because of cost.”
Michael Anthony Moody, MD, director of the Collaborative Influenza Vaccine Innovation Center at Duke University, Durham, N.C., said that the true incidence and prevalence of syphilis is unknown.
“The more we test, the more accurate our data will be,” he said. “Syphilis can hide in plain sight, has symptoms that mimic many other diseases, and is usually not diagnosed. Reaffirming that testing for syphilis is important reminds providers that this is a key test for their patient’s health.”
Aniruddha Hazra, MD, medical director of the University of Chicago Medicine Sexual Wellness Clinic, noted that the United States is in a syphilis epidemic.
“Screening asymptomatic people at risk for syphilis is important, but without comprehensive education and training of primary care providers on how to address STIs and sexual health, these recommendations fall flat,” he said.
In an accompanying editorial, Susan Tuddenham, MD, MPH; and Khalil G. Ghanem, MD, PhD, of Johns Hopkins University, Baltimore, urged that funding to develop novel syphilis diagnostics be prioritized, “just as there has been for development of syphilis vaccines, which are still many years from becoming a reality.”
“Relying on emerging biomedical prevention interventions that hold promise, such as doxycycline postexposure prophylaxis, without concomitant robust screening strategies will not lead to syphilis control. Failure to modernize screening strategies for syphilis will also mean failure to control this infection,” they cautioned.
The authors of the recommendation statement and the evidence report, as well as Dr. O’Donnell, Dr. Gullettt, Dr. Moody, and Dr. Hazra, who were not involved in the study, reported no relevant financial relationships. Dr. Tuddenham reported financial relationships with the pharmaceutical and publishing industries. Dr. Ghanem reported financial relationships with the publishing industry. The research was federally funded.
A version of this article first appeared on Medscape.com.
FROM JAMA
Meet the JCOM Author with Dr. Barkoudah: Improving Inpatient COVID-19 Vaccination Rates
GERD linked to increased risk of nontuberculous mycobacterial pulmonary disease
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
Patients with gastrointestinal esophageal reflux disease (GERD) have more than three times the risk of developing nontuberculous mycobacterial pulmonary disease (NTM-PD), compared with those without GERD, according to a population-based retrospective cohort study.
“GERD is a common comorbidity of nontuberculous mycobacterial pulmonary disease [but] whether GERD is associated with an increased risk of developing NTM-PD is unknown,” Hayoung Choi, MD, PhD, Hallym University, Seoul, Republic of Korea, and colleagues reported.
Dr. Choi added in an email. “What needs to be understood is that GERD increases health care utilization in patients with NTM pulmonary disease; hence, clinicians who treat patients with NTM pulmonary disease need to be aware of the burden of GERD and treat the gastrointestinal illness simultaneously,” he added.
The study was published online in the journal CHEST.
Sample cohort
Data from the Korean National Health Insurance Service-National Sample Cohort between 2002 and 2015 were used to assess the impact of GERD on NTM-PD. The incidence and risk of NTM-PD were compared between 17,424 patients with GERD and 69,000 patients without GERD in a matched cohort. GERD was defined as patients having received more than 3 months of proton pump inhibitors (PPIs).
During a median follow-up of 5.1 years, the age- and sex-adjusted incidence of NTM-PD was significantly higher in the GERD cohort, at a rate of 34.8/100,000 person-years, than in the matched cohort, at a rate of only 10.5/100,000 person-years (P < .001), the authors reported.
As for risk factors for NTM-PD, being 60 years of age and older was associated with a 3.5-times higher risk of NTM-PD at an adjusted hazard ratio of 3.57 (95% confidence interval, 1.58-8.07), while bronchiectasis was associated with over an 18-times higher risk of NTM-PD in the GERD cohort at an adjusted HR of 18.69 (95% CI, 6.68-552.28). Those with GERD who developed NTM-PD had higher all-cause and respiratory disease–related emergency department visits or hospitalizations compared with patients with GERD who did not develop NTM-PD (P = .011), the investigators noted.
As the authors pointed out, the incidence of NTM-PD in the Korean population ranged from 6 to 19 cases/100,000 between 2008 and 2016; thus, the burden of incident NTM-PD associated with GERD appears to be considerable. As Dr. Choi explained, a combination of three factors influenced the development of NTM infections. The first is environmental, from water source, climate, or region; the second is patient influences, including such factors as immunodeficiency and comorbidities (including bronchiectasis); and the third is microbiological factors, including various NTM species.
Bile aspirating into the lung during GERD may be another possible pathway, as the authors suggested. Even if acid secretion is suppressed by PPI treatment in patients with GERD, NTM-PD may be induced or aggravated through mechanisms such as bile reflux. The fact that patients over the age of 60 were more prone to develop NTM-PD suggests that a decrease in gastric emptying and increased micro-aspiration or reflux associated with impaired swallowing (which are more common in elderly patients) may also be at play.
“Bronchiectasis is also a very well known risk factor for NTM pulmonary disease,” Dr. Choi emphasized. Thus, he recommends clinicians carefully observe clinical, radiological, and microbiological changes to detect NTM pulmonary disease when managing patients with bronchiectasis.
“The results of the present study have several potential clinical implications,” Dr. Choi and colleagues observed. First, NTM-PD should be suspected when new-onset worsening of respiratory symptoms develops during regular follow-up in patients with GERD. Second, because results indicate that older age and bronchiectasis significantly increase the risk of NTM-PD, “more active strategies (e.g., screening of symptoms and regular chest x-rays)” might be helpful in patients with GERD and these risk factors, the authors suggested. Because patients with GERD who developed NTM-PD had more respiratory disease–related ED visits and hospitalizations than those who did not develop NTM-PD, when GERD and NTM-PD are combined, clinicians should focus on the variations of respiratory symptoms, they suggested.
The authors cautioned, however, that because the study was one in a Korean population, studies in other countries and different ethnicities are needed before findings can be generalized.
More common than TB
Asked to comment on the findings, NTM-PD expert Theodore Marras, MD, clinician investigator, Krembil Research Institute, Toronto, noted that non-TB M-PD is about 10 times more common than TB and that could be an underestimate as there have been very large increases in the incidence of NTM-PD in recent years. “It’s an environmental germ – it’s in our water – and certain people are particularly susceptible to it, typically older age women who have underlying bronchiectasis,” Dr. Marras told this news organization. “And while there are ethnic differences in incidence rates between East Asian people and Black African people, immigration is not the main driver for the increase as far as we can tell,” he said.
He personally treats a lot of NTM-PD and he also believes that GERD is an important risk factor for all types of lung infections including NTM lung disease. “So without a doubt, I believe that GERD should be treated in patients with NTM-PD,” Dr. Marras emphasized. The big question is how to treat GERD, as there may be concerns with acid-suppressive agents such as proton pump inhibitors that “the reflux that comes back up may harbor more germs in it and if that reflux comes up high enough, we are at risk of aspirating some of that fluid into our lungs, especially when we’re asleep,” he said.
Some experts therefore argue in favor of using motility agents instead of PPIs. However, if Dr. Marras has a patient with heartburn, “you have to treat it,” he stressed. Similarly, if a patient has evidence of esophageal erosions, physicians need to treat those as well. However, if neither feature is present, “I tend to like the motility agents preferentially or use them in combination with a PPI,” Dr. Marras said.
Dr. Marras also thinks the study is encouraging physicians involved in treating these patients to think about controlling GERD both when they are treating patients and after they are treated to try to reduce recurrence.
The authors had no financial disclosures to make. Dr. Marras has given several talks on NTM lung disease, one sponsored by AstraZeneca and the other by Novartis.
FROM CHEST
Continued monkeypox spread can lead to viral mutations
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HIV Pre-exposure Prophylaxis (PrEP): A Survey of Dermatologists’ Knowledge and Practice Patterns
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.
Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).
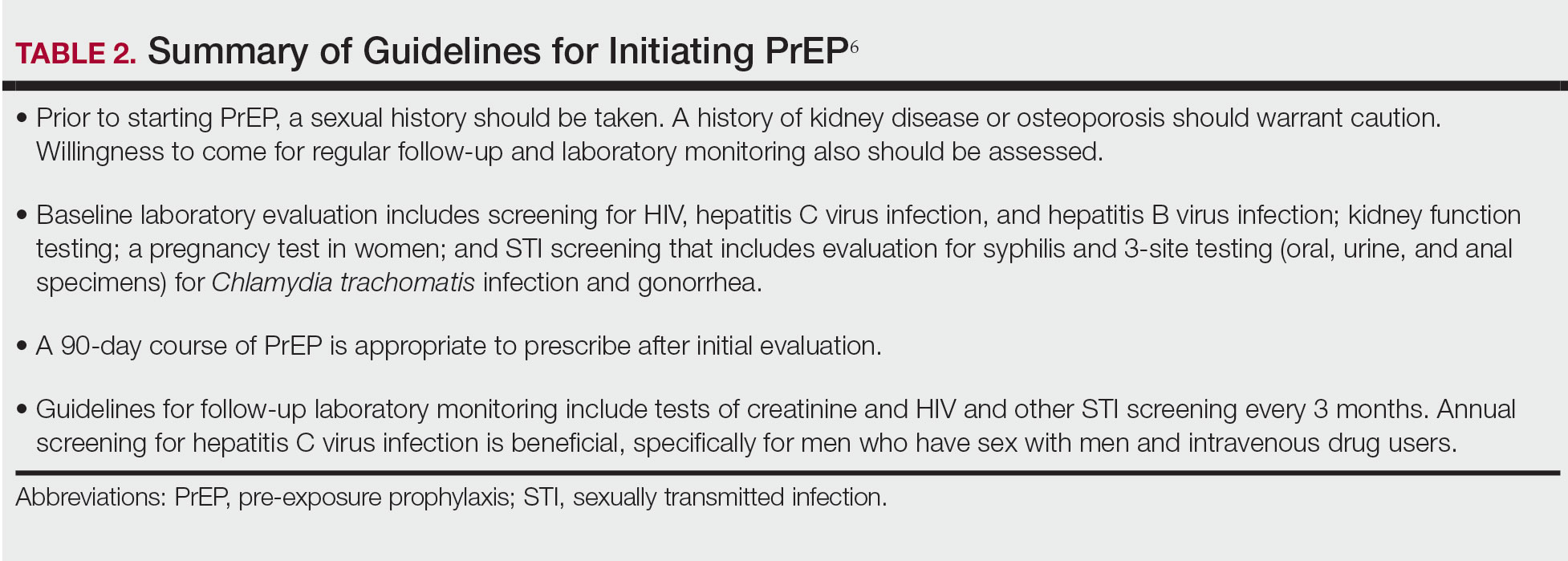
In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
Practice Points
- Sexually transmitted infections (STIs) often have skin manifestations, with patients presenting to dermatologists.
- Pre-exposure prophylaxis (PrEP) uses antiretrovirals taken prophylactically to prevent transmission of and infection with HIV. Dermatologists are aware of PrEP, but several barriers prevent them from being prescribers.
- Patients with a history of an STI should be considered for PrEP.
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.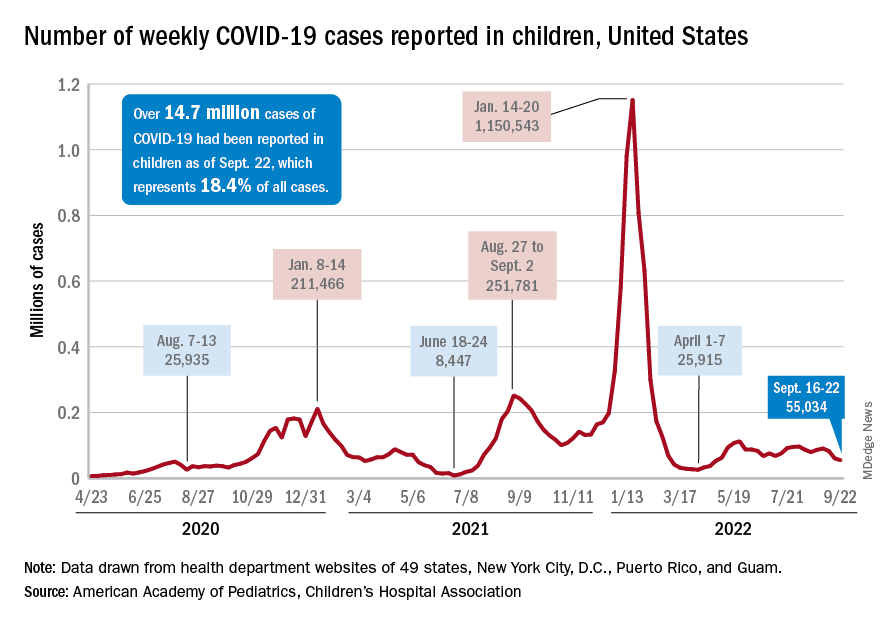
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
Meet the JCOM Author with Dr. Barkoudah: Diabetes Population Health Innovations