User login
HPV infection in pregnancy higher among women living with HIV
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROME.
Is another COVID-19 booster really needed?
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Death of son reinforces flu vaccination message
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
Malaria vaccine gets special delivery by tiny health personnel
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Children and COVID: Weekly cases dropped by 57% in September
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.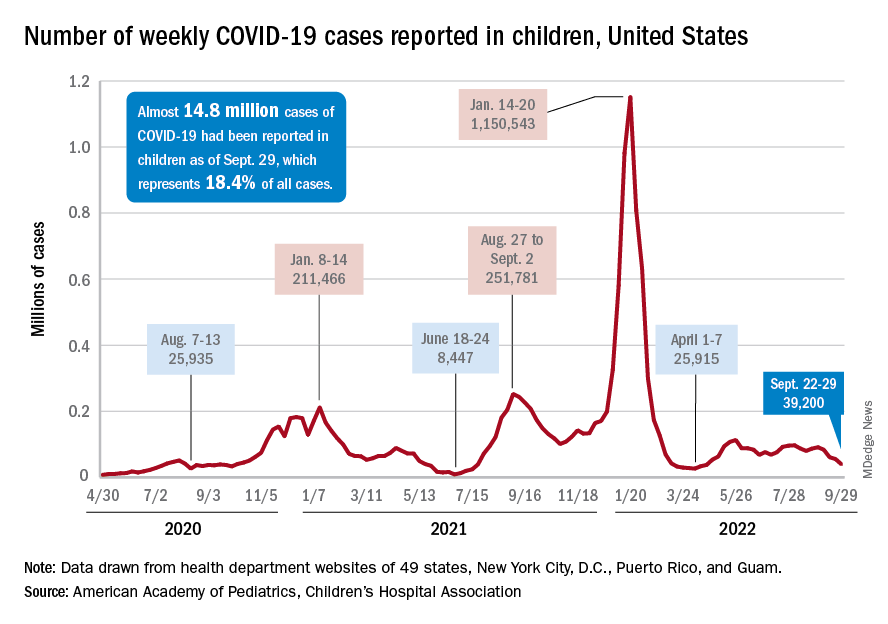
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Gardasil 9 HPV vaccine advised for MSM living with HIV
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Men who have sex with men (MSM) living with HIV, especially those who are young or who’ve had gonorrhea, should get the human papillomavirus (HPV) 9-valent vaccine (Gardasil 9), findings of a newly published study in the Journal of Acquired Immune Deficiency Syndromes suggest.
According to the World Health Organization, only 30% of the target population worldwide has received the HPV vaccine. Despite increased risk for HPV anal infection (an estimated three out of four MSM develop an anal infection from any HPV genotype in their lifetime, epidemiological studies in MSM have been lacking, leaving gaps in data in terms of prevalence rates and prevention.
To help characterize which MSM subgroups benefit the most from early 9-valent HPV vaccination, researchers from Vita-Salute San Raffaele University in Milan determined the prevalence of anal HPV genotypes in MSM who’d been living with HIV for 5 years, and they analyzed the risk factors for HPV anal infection.
Of the 1,352 study participants, 12% were not infected by any HPV genotypes, and the maximum number of genotypes infecting one person (six) was detected in 0.4% (six) people. The prevalence of HR-HPV genotypes or those present in the vaccine remained stable over time.
“Our findings suggest ... that all MSM with HIV would benefit from Gardasil 9 immunization, particularly the youngest and those with a prior gonococcal infection,” wrote Elena Bruzzesi, MD, of Vita-Salute San Raffaele University, and her coauthors.
To determine prevalence of HPV genotypes at anal sites and risk factors, the authors conducted a time-trend, monocentric study on participants who self-identified as MSM who engaged in anal intercourse. The participants underwent one or more anoscopies for HPV genotyping at one academic hospital in Milan between 2015 and 2019.
Swab specimens were collected from the anal canal mucosa, then soaked in thin-layer liquid medium, and sent for molecular analysis.
For detection of HPV phenotypes, the specimens were processed by multiplex real-time polymerase chain reaction.
Findings showed that:
- The overall prevalence of MSM with at least one anal HPV genotype was 88%, with prevalence ranging from 77% to 84%, and no trend difference over the 5-year period.
- Seventy-nine percent of participants were exposed to at least one high-risk (HR)-HPV genotype, and 67.4% by at least one low-risk (LR)-HPV genotype.
- HPV-53, in 27%, was the most prevalent genotype. HPV-6, 11, 16, and 18 prevalence was 22%, 13%, 23%, and 11%, respectively. Of the HR genotypes, HPV-16 and HPV-18 are most often linked with squamous cell cancers and adenocarcinomas, and in the study, prevalence did not change over time.
- Seventy-one percent of participants carried at least one genotype covered by the vaccine, with no change over time.
- On multivariable analysis, the risk of carrying at least one high-risk HPV genotype was linked with younger age (adjusted odds ratio [aOR] for 30 years or younger compared with older than 45 years 2.714; 95% confidence interval [CI], 1.484-4.961), and with having had gonorrhea (aOR, 2.118; 95% CI, 1.100-4.078).
- Also on multivariable analysis, the risk of having one or more genotypes targeted by the 9-valent vaccine was linked with younger age (aOR, 1.868; 95% CI, 1.141-3.060) and with having had gonorrhea (aOR, 1.785; 95% CI, 1.056-3.018).
Mehri S. McKellar, MD, an infectious disease specialist at Duke Health in Durham, N.C., told this news organization.
“This powerful study provides important data on HPV genotype prevalence in the MSM HIV+ population, validating that Gardasil 9 will greatly help these individuals,” said Dr. McKellar, who was not involved in the study.
Robert Salata, MD, infectious disease specialist and professor at Case Western Reserve University, Cleveland, also encourages MSM to get the vaccine.
“It is important to understand that the prevalence of anal HPV in men who have sex with men is very high, that the prevalence, including high-risk genotypes, has remained stable, and that the 9-valent vaccine is clearly indicated, especially in younger men and those with known gonorrhea and other STDs,” Dr. Salata (who was also not involved in the study) told this news organization.
“This is an important reminder for us to continue promoting and providing the vaccine to our patients, especially to HIV+ men who have sex with men, who have the highest rates of anal infection with HPV,” Dr. McKellar advised.
The authors, Dr. McKellar, and Dr. Salata report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROMES
FDA approves HIV-1 treatment ibalizumab for 30-second IV push
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
How to handle pesky molluscum contagiosum lesions
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
.
“If you don’t treat them, they’re going to spread,” Dr. Smith, who practices dermatology in Fort Mill, S.C., said at Medscape Live’s annual Coastal Dermatology Symposium. “They’re going to be itchy, they can spread on the patient themselves and then to others, and they can cause scarring. The prevalence is anywhere from 5% to 11%. That means there are 6 million patients out there, just waiting to come into your clinics.”
To date, no treatment has been approved by the Food and Drug Administration for MC, although a laundry list of agents have been tried, including cantharidin; cryotherapy; curettage with and without imiquimod; sinecatechins ointment, 15%; imiquimod; and retinoids. And there are several treatments that are being investigated.
A 2017 Cochrane review of 22 studies involving 1,650 patients demonstrated that no single intervention has been consistently effective in treating MC. “Most of the studies were actually very low quality,” said Dr. Smith, who was not involved with the analysis. “The one high quality study showed that imiquimod did not work any better than its vehicle.”
Investigational treatments
One of the products in the pipeline is VP-102, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been evaluated in phase 3 studies of patients with molluscum aged 2 years and older. It features a visualization agent so that the person applying the drug can see which lesions have been treated. It also contains a bittering agent to mitigate oral ingestion by children.
VP-102, which is being developed by Verrica Pharmaceuticals, is applied once every 21 days in up to 4 applications, and multiple lesions can be treated with one applicator. “It’s a stable concentration with a good shelf life, and two phase 3 randomized studies have shown about a 50% complete clearance of new and existing lesions at day 84,” Dr. Smith said. Those studies enrolled children and adults.
A separate analysis of the same data presented at a meeting in 2019 showed that 77% of patients treated with VP-102 achieved greater than 75% clearance, while 65.8% achieved more than 90% clearance.
The new kid on the block is a gel formulation of a nitric oxide–releasing medication, berdazimer 10.3%, a first-in-class topical treatment being developed by Novan, which can be applied at home. In a multicenter study published in JAMA Dermatology, researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years and participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment.
At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
More recently, investigators evaluated autoinoculation vs. 35% trichloroacetic acid (TCA) for the treatment of MC. Autoinoculation involves puncturing the perilesional and lesional skin 5-7 times with an insulin syringe. “This gets a little bit of the virus into the dermis, and you hope to elicit an immune response,” explained Dr. Smith, who was not involved with the study. At 3 months, 80% of patients in the autoinoculation group achieved complete clearance, compared with 62% of those in the TCA group, while recurrence at 6 months was 3% vs. 40%, respectively.
Manual extraction of MC lesions is another option. “I love to pop the cores out with my thumbs,” Dr. Smith said. “You have to pick the patients who can tolerate this, and the MC lesions need to be ripe and ready.”
For ophthalmic lesions, watchful waiting is advisable unless the MC lesions are symptomatic or bothersome or large lesions form on the lid margin, which may cause ocular irritation or even a corneal abrasion. “If a patient presents with a multisite infection that includes ocular lesions, treat lesions on other parts of the body and keep your fingers crossed that a systemic immune response occurs,” she said.
The desired immune response is known as the “BOTE” sign (the beginning of the end), which heralds the clearance of the molluscum infection. This often appears as reddening of all the MC lesions and occasionally as a granulomatous “id-like” reaction especially on the extensor elbows and knees. “When this happens, it often scares the patients,” Dr. Smith said. But she explains that this is a positive development, and that “this means that the lesions are about to self-resolve.”
Dr. Smith disclosed that she serves as a speaker or a member of the speakers bureau for Amgen, CeraVe, EPI, Galderma, InCyte, Lilly, Pfizer, Regeneron, Sanofi Genzyme, and Sun. She also serves as an advisor or consultant for Janssen, Lilly, Regeneron, and Sanofi Genzyme.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Strong link found between enterovirus and type 1 diabetes
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Enterovirus infection appears to be strongly linked to both type 1 diabetes and islet cell autoantibodies, new research suggests.
The strength of the relationship, particularly within the first month of type 1 diabetes diagnosis, “further supports the rationale for development of enterovirus-targeted vaccines and antiviral therapy to prevent and reduce the impact of type 1 diabetes,” according to lead investigator Sonia Isaacs, MD, of the department of pediatrics and child health at the University of New South Wales, Sydney, Australia.
Enteroviruses are a large family of viruses responsible for many infections in children. These live in the intestinal tract but can cause a wide variety of illnesses. There are more than 70 different strains, which include the group A and group B coxsackieviruses, the polioviruses, hepatitis A virus, and several strains that just go by the name enterovirus.
Dr. Isaacs presented the data, from a meta-analysis of studies using modern molecular techniques, at the annual meeting of the European Association for the Study of Diabetes.
The findings raise the question of whether people should be routinely tested for enterovirus at the time of type 1 diabetes diagnosis, she said during her presentation.
Asked by this news organization about the implications for first-degree relatives of people with type 1 diabetes, Dr. Isaacs said that they are “definitely a population to watch out for,” with regard to enteroviral infections. “Type 1 diabetes is very diverse and has different endotypes. Different environmental factors may be implicated in these different endotypes, and it may be that the enteroviruses are quite important in the first-degree relative group.”
Asked to comment, session moderator Kamlesh Khunti, MD, PhD, told this news organization that the data were “compelling,” particularly in the short term after type 1 diabetes diagnosis. “It seems that there may be plausibility for enterovirus associated with the development of type 1 diabetes ... Are there methods by which we can reduce this risk with either antivirals or vaccinations? I think that needs to be tested.”
And in regard to first-degree relatives, “I think that’s the group to go for because the association is so highly correlated. I think that’s the group worth testing with any interventions,” said Dr. Khunti, professor of primary care diabetes and vascular medicine at the University of Leicester, England.
Link stronger a month after diagnosis, in close relatives, in Europe
The new meta-analysis is an update to a prior review published in 2011 by Dr. Isaacs’ group, which found that people with islet cell autoimmunity were more than four times as likely as were controls to have an enterovirus infection, and people with type 1 diabetes were almost 10 times as likely.
This new analysis focuses on studies using more modern molecular techniques for detecting viruses, including high throughput sequencing and single-cell technologies.
The analysis identified 60 studies with a total of 12,077 participants, of whom 900 had islet autoimmunity, 5,081 had type 1 diabetes, and 6,096 were controls. Thirty-five of the studies were from Europe, while others were from the United States, Asia, and the Middle East.
Of 16 studies examining enterovirus infection in islet autoimmunity, cases with islet autoimmunity were twice as likely to have an enterovirus infection at any time point compared to controls, a significant difference (odds ratio [OR], 2.07, P = .002.)
Among 48 studies reporting enterovirus infection in type 1 diabetes, those with type 1 diabetes were eight times as likely to have an enterovirus infection compared with controls (OR, 8.0, P < .00001).
In 25 studies including 2,977 participants with onset of type 1 diabetes within the prior month, those individuals were more than 16 times more likely to present with an enterovirus infection (OR, 16.2, P < .00001).
“The strength of this is association is greater than previously reported by both us and others,” Dr. Isaacs noted.
The association between enterovirus infection and islet autoimmunity was greater in individuals who later progressed to type 1 diabetes, with odds ratio 5.1 vs. 2.0 for those who didn’t. The association was most evident at or shortly after seroconversion (5.1), was stronger in Europe (3.2) than in other regions (1.9), and was stronger among those with a first-degree relative with type 1 diabetes (9.8) than those recruited via a high-risk human leukocyte antigen (HLA), in whom the relationship wasn’t significant.
Having multiple or consecutive enteroviral infections was also associated with islet autoimmunity (2.0).
With type 1 diabetes, the relationship with enterovirus was greater in children (9.0) than in adults (4.1), and was greater for type 1 diabetes onset within 1 year (13.8) and within 1 month (16.2) than for those with established type 1 diabetes (7.0). Here, too, the relationship was stronger in Europe (10.2) than outside Europe (7.5).
The link with type 1 diabetes and enterovirus was particularly strong for those with both a first-degree relative and a high-risk HLA (141.4).
The relationship with type 1 diabetes was significant for enterovirus species A (3.7), B (12.7) and C (13.8), including coxsackie virus genotypes, but not D.
“Future studies should focus on characterizing enterovirus genomes in at-risk cohorts rather than just the presence or absence of the virus,” Dr. Isaacs said.
However, she added, “type 1 diabetes is such a heterogenous condition, viruses may be implicated more in one type than another. It’s important that we start to look into this.”
Dr. Isaacs reports no relevant financial relationships. Dr. Khunti disclosed ties with AstraZeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp.
A version of this article first appeared on Medscape.com.
AT EASD 2022