User login
Buprenorphine proves effective for fentanyl users in the ED
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
based on data from nearly 900 individuals.
California EDs include a facilitation program known as CA Bridge for the treatment of opioid use disorder. Guidelines for CA Bridge call for high-dose buprenorphine to treat patients in drug withdrawal, with doses starting at 8-16 mg, Hannah Snyder, MD, of the University of California, San Francisco, and colleagues wrote.
“Buprenorphine has been repeatedly shown to save lives and prevent overdoses,” Dr. Snyder said in an interview. “We know that emergency department–initiated buprenorphine is an essential tool for increasing access. In the era of fentanyl, both patients and providers have expressed concerns that buprenorphine may not work as well as it did when patients were more likely to be using heroin or opioid pills.
“This retrospective cohort study provides additional information about emergency department buprenorphine as fentanyl becomes increasingly prevalent.”
In a research letter published in JAMA Network Open, the investigators reviewed data from the electronic health records of 896 patients who presented with opioid use disorder (OUD) at 16 CA Bridge EDs between Jan. 1, 2020, and April 30, 2020. All patients with OUD were included regardless of chief concern, current treatment, treatment desires, or withdrawal. A total of 87 individuals reported fentanyl use; if no fentanyl use was reported, the patient was classified as not using fentanyl. The median age of the patients was 35 years, two thirds were male, approximately 46% were White and non-Hispanic, and 30% had unstable housing.
The primary outcome was follow-up engagement at 7-14 days and 25-37 days.
A total of 492 patients received buprenorphine, including 44 fentanyl users, and 439 initiated high doses of 8-32 mg. At a 30-day follow-up, eight patients had precipitated withdrawal, including two cases in fentanyl users; none of these cases required hospital admission.
The follow-up engagement was similar for both groups, with adjusted odds ratios of 0.60 for administered buprenorphine at the initial ED encounter, 1.09 for 7-day follow-up, and 1.33 for 30-day follow-up.
The findings were limited by the retrospective design and use of clinical documentation, which likely resulted in underreporting of fentanyl use and follow-up, the researchers noted. However, the results supported the effectiveness of buprenorphine for ED patients in withdrawal with a history of fentanyl exposure.
“We were pleased to see that precipitated withdrawal was relatively uncommon in this study, and that patients who did and did not use fentanyl followed up at similar rates,” said Dr. Snyder. “This aligns with our clinical experience and prior research showing that emergency department buprenorphine starts continue to be an essential tool.”
The message for clinicians: “If a patient presents to the emergency department in objective opioid withdrawal and desires buprenorphine, they should be offered treatment in that moment,” Dr. Snyder said. “Treatment protocols used by hospitals in this study are available online. Emergency departments can offer compassionate and evidence-based treatment initiation 24 hours a day, 7 days a week, 365 days a year.”
More data needed on dosing strategies
“We need additional research to determine best practices for patients who use fentanyl and want to start buprenorphine, but are not yet in withdrawal,” Dr. Snyder said. “Doses of buprenorphine like those in this study are only appropriate for patients who are in withdrawal with objective signs, so some patients may struggle to wait long enough after their last use to go into sufficient withdrawal.”
Precipitated withdrawal does occur in some cases, said Dr. Snyder. “If it does, the emergency department is a very good place to manage it. We need additional research to determine best practices in management to make patients as comfortable as possible, including additional high-dose buprenorphine as well as additional adjunctive agents.”
Findings support buprenorphine
“The classic approach to buprenorphine initiation, which emerged from psychiatry outpatient office visits, is to start with very small doses of buprenorphine [2-4 mg] and titrate up slowly,” Reuben J. Strayer, MD, said in an interview.
“This dose range turns out to be the ‘sour spot’ most likely to cause the most important complication around buprenorphine initiation–precipitated withdrawal,” said Dr. Strayer, the director of addiction medicine in the emergency medicine department at Maimonides Medical Center, New York.
“One of the current focus areas of OUD treatment research is determining how to initiate buprenorphine without entailing a period of spontaneous withdrawal and without causing precipitated withdrawal,” Strayer explained. “The two primary strategies are low-dose buprenorphine initiation [LDBI, less than 2 mg, sometimes called microdosing] and high-dose [HDBI, ≥ 16 mg] buprenorphine initiation. HDBI is attractive because the primary treatment of buprenorphine-precipitated withdrawal is more buprenorphine.
“Additionally, using a high dose up front immediately transitions the patient to therapeutic blood levels, which protects the patient from withdrawal, cravings, and overdose from dangerous opioids (heroin, fentanyl, oxycodone).”
However, “the contamination and now replacement of heroin with fentanyl in the street drug supply has challenged buprenorphine initiation, because fentanyl, when used chronically, accumulates in the body and leaks into the bloodstream slowly over time, preventing the opioid washout that is required to eliminate the risk of precipitated withdrawal when buprenorphine is administered,” said Dr. Strayer.
The current study demonstrates that patients who are initiated with a first dose of 8-16 mg buprenorphine are unlikely to experience precipitated withdrawal and are successfully transitioned to buprenorphine maintenance and clinic follow-up, Dr. Snyder said, but he was surprised by the low rate of precipitated withdrawal in the current study, “which is discordant with what is being anecdotally reported across the country.”
However, the take-home message for clinicians is the support for the initiation of buprenorphine in emergency department settings at a starting dose of 8-16 mg, regardless of reported fentanyl use, he said. “Given the huge impact buprenorphine therapy has on OUD-related mortality, clinicians should make every effort to initiate buprenorphine for OUD patients at every opportunity, and precipitated withdrawal is very unlikely in appropriately selected patients.
“Many clinicians remain reluctant to initiate buprenorphine in ED settings for unfamiliarity with the drug, fear of precipitated withdrawal, or concerns around the certainty of outpatient follow-up,” Dr. Snyder said. “Education, encouragement, systems programming, such as including decision support within the electronic health record, and role-modeling from local champions will promote wider adoption of this lifesaving practice.”
Looking ahead, “more research, including prospective research, is needed to refine best practices around buprenorphine administration,” said Dr. Snyder. Questions to address include which patients are most at risk for precipitated withdrawal and whether there are alternatives to standard initiation dosing that are sufficiently unlikely to cause precipitated withdrawal. “Possibly effective alternatives include buprenorphine initiation by administration of long-acting injectable depot buprenorphine, which accumulates slowly, potentially avoiding precipitated withdrawal, as well as a slow intravenous buprenorphine infusion such as 9 mg given over 12 hours.”
The study received no outside funding. Dr. Snyder disclosed grants from the Substance Abuse and Mental Health Services Administration and the California Department of Health Care Services during the study. Dr. Strayer reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Once-daily stimulant for ADHD safe, effective at 1 year
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
new research shows.
Results from a phase 3, multicenter, dose-optimization, open-label safety study of Azstarys (KemPharm) found that most treatment-emergent adverse events (TEAEs) were mild to moderate.
“This data show that Azstarys remains safe and effective for the treatment of ADHD when given for up to a year,” lead investigator Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine, Las Vegas, said in an interview.
The study was published online in the Journal of Child and Adolescent Psychopharmacology.
Safety at 1 year
The drug is a combination of extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate, coformulated with immediate-release d-MPH.
SDX is converted to d-MPH after it is absorbed in the gastrointestinal tract. The d-MPH is released gradually throughout the day, providing quick symptom control with the d-MPH and extended control with SDX.
Azstarys was approved by the Food and Drug Administration in 2021 on the basis of results from a laboratory classroom phase 3 trial, which showed significant improvement in ADHD symptoms, compared with placebo.
For this study, the second phase 3 trial of Azstarys, investigators analyzed data from 282 children aged 6-12 years in the United States, including 70 who participated in an earlier 1-month efficacy trial.
After screening and a 3-week dose-optimization phase for new participants, patients received once-daily treatment with doses of 26.1 mg/5.2 mg, 39.2 mg/7.8 mg, or 52.3 mg/10.4 mg of SDX/d-MPH.
After 1 year of treatment, 60.1% of participants reported at least one TEAE, the majority of which were moderate. Twelve patients reported severe TEAEs. Six children (2.5%) discontinued the study because of a TEAE during the treatment phase.
The investigators also measured growth and changes in sleep with the Children’s Sleep Habits Questionnaire during the 12-month study. Sleep improved on most measures and the impact on growth was mild.
There were no life-threatening TEAEs and no deaths reported during the study.
The most common TEAEs during the treatment phase were decreased appetite, upper respiratory tract infection, nasopharyngitis, decreased weight, irritability, and increased weight.
Efficacy at 1 year
ADHD symptoms improved considerably after 1 month of treatment, with responses continuing at 1 year.
At baseline, participants’ mean ADHD Rating Scale–5 score was 41.5. After 1 month of treatment, scores averaged 16.1, a decline of –25.3 (P < .001).
The mean score stabilized in the 12-15 range for the remainder of the study. After 1 year of treatment, ADHD symptoms had decreased approximately 70% from baseline.
Investigators found similar results in clinical severity. After 1 month of treatment, the average Clinical Global Impressions–Severity (CGI-S) scale score was 2.5, a decline of –2.2 (P < .0001).
CGI-S scale scores remained in the 2.2-2.4 range for the remainder of the study.
These results, combined with the results of the original classroom trial, suggest Azstarys may offer advantages over other ADHD drugs, Dr. Childress said.
“In the laboratory classroom trial, subjects taking Azstarys completed significantly more math problems than subjects taking placebo beginning at 30 minutes and up to 13 hours after dosing,” Dr. Childress said. “No other methylphenidate extended-release product currently marketed in the United States has a 13-hour duration of effect.”
‘Reassuring data’
Commenting on the findings, Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, said that the study suggests the drug may be a valuable addition to ADHD treatment options for pediatric patients.
“The study provides reassuring data on the safety of stimulants in patients without significant history of cardiac events or blood pressure changes, which are usual concerns among patients and clinicians despite the evidence supporting safety, said Dr. Pawar, who was not part of the study.
“Additionally, the 1-year data on efficacy and safety of a new stimulant medication is valuable for clinicians looking for sustained relief for their patients, despite the limitations of an open-label trial,” she added.
Overall, the safety data reported in the study are fairly consistent with the safety profile of other methylphenidates used for treating ADHD, Dr. Pawar said.
However, she noted, the study does have some limitations, including its open-label design and lack of blinding. The research also excluded children with autism, disruptive mood dysregulation disorders, and other common comorbidities of ADHD, which may limit the generalizability of the results.
“These comorbidities often require stimulants as a part of treatment, and yet have a higher risk of side effects,” Dr. Pawar said. “Future studies with a broader population may be needed to better understand treatment effectiveness and potential risks.”
The study was funded by KemPharm. Dr. Childress serves as consultant for Aardvark, Arbor, Attentive, Cingulate, Ironshore, Neos Therapeutics, Neurocentria, Otsuka, Purdue, Rhodes, Sunovion, Tris Pharma, KemPharm, Supernus, Jazz, Corium, Tulex, and Lumos.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CHILD AND ADOLESCENT PSYCHOPHARMACOLOGY
Who can sue docs for wrongful death? Some states are trying to expand that group
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
In addition, the types of emotional damage that physicians can be sued for is expanding in pockets across the nation. The latest effort to expand the capacity to sue, a bill in New York state, failed when it was not signed by the governor – but a toned-down bill is in the works.
The impact of New York’s proposed expansion of wrongful death lawsuits would have been widespread. The New York legislation would have expanded the definition of “close family members” to include spouses, domestic partners, children, parents, stepparents, siblings, grandparents, and perhaps more. Additionally, lawsuits could have allowed juries to determine “close family members” of the deceased patient on the basis of specific circumstances of the person’s relationship with the decedent.
Currently, every state allows a wrongful death claim to be filed by immediate family members. If the patient who died was married, a surviving spouse could bring the lawsuit. If the patient had been unmarried, an adult child could bring the lawsuit in some states. A parent typically brings a lawsuit if their minor child has died from alleged wrongful death. In some states, one member of a civil union or domestic partnership may bring a wrongful death lawsuit. And if a single adult has no children or spouse/partner, more distant family members, including aunts, uncles, siblings, or grandparents, may file the suit.
The New York bill would also have expanded compensable damages to include loss of affection and companionship, and it would have expanded emotional damages, which are not currently included in New York. It would also have extended the statute of limitations of a wrongful death claim from 2 years to 3.5 years.
In general, in states that allow emotional distress to be included in wrongful death lawsuits, attorneys must demonstrate that survivors have suffered mental harm, such as depression, loss of sleep, fear, and anger, says Russ Haven, JD, general counsel for the New York Public Interest Research Group. While mental harm is not particularly easy to prove, attorneys must show that survivors have ongoing distress that is the direct result of the loss of the loved one and that the distress is significant enough to severely affect their quality of life.
Mr. Haven gives an example of emotional distress: “We worked with a woman who lost her fiancé in a motor vehicle accident,” he says. “The funeral ended up on the day she had scheduled her wedding dress fitting. A situation like that causes a good deal of lasting emotional distress.”
Expanding family members who can bring the lawsuit
The fact that a fiancé could be included in a wrongful death settlement is another aspect of the New York bill that was central to arguments both for and against the expansion of family members who can make claims. “We think a modern society includes unmarried partners, grandparents, siblings, and others,” says Mr. Haven.
“The language of who is a close family member might seem clear, but to a defense attorney, it isn’t,” says Tom Stebbins, executive director of the Lawsuit Reform Alliance of New York. “This could end up being a situation where someone has 40 grandchildren, and all could be considered close family members.”
Many states currently allow damages for claims of grief and mental anguish resulting from a wrongful death.
In her recent veto of the Grieving Families Act, New York Gov. Kathy Hochul took fire for her choices. The bill represented years of effort by the state legislature to expand the qualifiers for wrongful death lawsuits. Those supporting what ultimately became Senate Bill S74A believed they finally had the law over the finish line. Those opposed breathed a sigh of relief when the bill was vetoed.
Had Gov. Hochul signed Bill 274A, the effect on costs would have been enormous for physicians. New York already has the highest cumulative medical liability payouts in the nation, according to the Medical Society of the State of New York.
The MSSNY was among many parties that fought against the law. The Greater New York Hospital Association, insurance companies, the Defense Association of New York, and the New York Conference of Mayors all joined in lobbying against the bill.
“Gov. Hochul, in her veto message, correctly noted that the proposed New York legislation represented an extraordinary departure from New York’s wrongful death jurisprudence,” says Remi Stone, director of government relations at The Doctors Company, part of the TDC Group. “I would add that while there are some other states that allow grief damages, none are as wide-ranging as the proposed legislation.”
The NYPIRG, the AARP, and the New York Immigration Coalition supported the bill. In a statement following the veto, the New York State Trial Lawyers Association said: “By vetoing the Grieving Families Act, Gov. Hochul has sided with insurance companies, the health care industry, big corporations, and anyone else who doesn’t want to be held accountable for the negligent killing of a person. This bill passed with overwhelming bipartisan support and would rectify over a century of injustice.”
Following Gov. Hochul’s veto, the bill’s proponents and the state legislature vowed to return to the drawing board and construct a bill that the governor would eventually approve. For now, however, the controversial legislation has been put to rest.
Mr. Haven and the NYPIRG argue that New York lags behind many other states in allowing survivors to claim loss for their emotional distress. “When there is relationship loss, it has a great impact on your life,” Mr. Haven says, “and this goes beyond simply the financial impact.”
“The bill was well intended but completely vague on who could bring lawsuits and would have increased medical malpractice insurance by far too much,” says MSSNY President Parag Mehta, MD. “For safety net hospitals, one lawsuit would halt their ability to provide many programs aimed at underserved populations.”
Peter Kolbert, JD, senior vice president of claim and litigation services at Healthcare Risk Advisors (part of the TDC Group), had this to say: “The current ‘recoverable’ damages in New York in a wrongful death case include loss of guidance and support for minor children of a decedent. Those damages have been sustained at $2 million per child. It is rationally very challenging, if not impossible, to distinguish between those damages and the proposed damages that the very same people would have been entitled to under the proposed statute.”
What will happen in the future?
While the veto has stalled New York’s wrongful death expansion for now, supporters in and out of the legislature remain determined to continue their fight. “Advocates argue that the bill would have brought the state in line with wrongful death law in others,” says Brian Whitelaw, JD, a partner at Michigan’s Foley, Baron, Metzger & Juip. “But if the bill had become law as written, the economic impact would have been substantial.”
Mr. Whitelaw says that such wide-ranging lawsuits can have consequences that extend far beyond physicians’ insurance premiums. “This could impact the average person on the street’s ability to obtain the medical care they need, because doctors will go elsewhere to practice,” he says. “Beyond impacting the health care system, it can hurt small businesses as well.”
Mr. Haven says supporters of the expansion are far from finished with their efforts. “New York’s current law dates back to 1847, and it was cutting edge then,” he says. “It was designed for an agrarian society where if the husband died, his widow and children wouldn’t become destitute. Now, 175 years later, we realize that the law has biases, and tort law has evolved. The state needs to evolve as well.”
For his part, Dr. Mehta is open to a dialogue with lawmakers to revise the law in a manner agreeable to all parties. “We want to work together to make the system right,” he says. “The liability system in New York needs an overall holistic change, and we are available at any time to have discussions. The vetoed bill was a Band-Aid and didn’t address the main, underlying issues in the state.”
Mr. Stebbins, too, says he would like to continue the debate over how an expansion should look. “We hope to go through a discussion on caps to these suits,” he explains. “We have already seen the cap of $10 million broken four times in the past few years through nuclear verdicts. That’s something we need to address.”
Given the legislature’s overwhelming support for the bill, some version of it will likely make another appearance in the coming session. Whether or not it can strike the middle ground that will make all parties happy – including the governor – is yet to be seen. “Is it wrong to seek compensation for pain and suffering from a wrongful death?” asks Mr. Whitelaw. “No. But there must be limits to such laws, or where does it end?”
A version of this article first appeared on Medscape.com.
Tips for treating patients with late-life depression
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; steven.lippmann@louisville.edu.
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; steven.lippmann@louisville.edu.
Late-life depression is the onset of a major depressive disorder in an individual ≥ 60 years of age. Depressive illness compromises quality of life and is especially troublesome for older people. The prevalence of depression among individuals > 65 years of age is about 4% in women and 3% in men.1 The estimated lifetime prevalence is approximately 24% for women and 10% for men.2 Three factors account for this disparity: women exhibit greater susceptibility to depression; the illness persists longer in women than it does in men; and the probability of death related to depression is lower in women.2
Beyond its direct mental and emotional impacts, depression takes a financial toll; health care costs are higher for those with depression than for those without depression.3 Unpaid caregiver expense is the largest indirect financial burden with late-life depression.4 Additional indirect costs include less work productivity, early retirement, and diminished financial security.4
Many individuals with depression never receive treatment. Fortunately, there are many interventions in the primary care arsenal that can be used to treat older patients with depression and dramatically improve mood, comfort, and function.
The interactions of emotional and physical health
The pathophysiology of depression remains unclear. However, numerous factors are known to contribute to, exacerbate, or prolong depression among elderly populations. Insufficient social engagement and support is strongly associated with depressive mood.5 The loss of independence in giving up automobile driving can compromise self-confidence.6 Sleep difficulties predispose to, and predict, the emergence of a mood disorder, independent of other symptoms.7 Age-related hearing deficits also are associated with depression.8
There is a close relationship between emotional and physical health.9 Depression adds to the likelihood of medical illness, and somatic pathology increases the risk for mood disorders.9 Depression has been linked with obesity, frailty, diabetes, cognitive impairment, and terminal illness.9
Inflammatory markers and depression may also be related. Plasma levels of interleukin-6 and C-reactive protein were measured in a longitudinal aging study.14 A high level of interleukin-6, but not C-reactive protein, correlated with an increased prevalence of depression in older people.
Chronic cerebral ischemia can result in a “vascular depression”13 in which disruption of prefrontal systems by ischemic lesions is hypothesized to be an important factor in developing despair. Psychomotor retardation, executive dysfunction, severe disability, and a heightened risk for relapse are common features of vascular depression.15 Poststroke depression often follows a cerebrovascular episode16; the exact pathogenic mechanism is unknown.17
Continue to: A summation of common risk factors
A summation of common risk factors. A personal or family history of depression increases the risk for late-life depression. Other risk factors are female gender, bereavement, sleep disturbance, and disability.18 Poor general health, chronic pain, cognitive impairment, poor social support, and medical comorbidities with impaired functioning increase the likelihood of resultant mood disorders.18
Somatic complaints may overshadow diagnostic symptoms
Manifestations of depression include disturbed sleep and reductions in appetite, concentration, activity, and energy for daily function.19 These features, of course, may accompany medical disorders and some normal physiologic changes among elderly people. We find that while older individuals may report a sad mood, disturbed sleep, or other dysfunctions, they frequently emphasize their somatic complaints much more prominently than their emotions. This can make it difficult to recognize clinical depression.
For a diagnosis of major depression, 5 of the following 9 symptoms must be present for most of the day or nearly every day over a period of at least 2 weeks19: depressed mood; diminished interest in most activities; significant weight loss or decreased appetite; insomnia or hypersomnia; agitation or retardation; fatigue or loss of energy; feelings of worthlessness or guilt; diminished concentration; and recurrent thoughts of death or suicide.19
Planning difficulties, apathy, disability, and anhedonia frequently occur. Executive dysfunction and inefficacy of antidepressant pharmacotherapy are related to compromised frontal-striatal-limbic pathways.20 Since difficulties with planning and organization are associated with suboptimal response to antidepressant medications, a psychotherapeutic focus on these executive functions can augment drug-induced benefit.
Rule out these alternative diagnoses
Dementias can manifest as depression. Other brain pathologies, particularly Parkinson disease or stroke, also should be ruled out. Overmedication can simulate depression, so be sure to review the prescription and over-the-counter agents a patient is taking. Some medications can occasionally precipitate a clinical depression; these include stimulants, steroids, methyldopa, triptans, chemotherapeutic agents, and immunologic drugs, to name a few.19
Continue to: Pharmacotherapy, Yes, but first, consider these factors
Pharmacotherapy, Yes, but first, consider these factors
Maintaining a close patient–doctor relationship augments all therapeutic interventions. Good eye contact when listening to and counseling patients is key, as is providing close follow-up appointments.
Encourage social interactions with family and friends, which can be particularly productive. Encouraging spiritual endeavors, such as attendance at religious services, can be beneficial.21
Recommend exercise. Physical exercise yields positive outcomes22; it can enhance mood, improve sleep, and help to diminish anxiety. Encourage patients with depression to take a daily walk during the day; doing so can enhance emotional outlook, health, and even socialization.
What treatment will best serve your patient?
It’s important when caring for patients with depression to assess and address suicidal ideation. Depression with a previous suicide attempt is a strong risk factor for suicide. Inquire about suicidal intent or death wishes, access to guns, and other life-ending behaviors. Whenever suicide is an active issue, immediate crisis management is required. Psychiatric referral is an option, and hospitalization may be indicated. Advise family members to remove firearms or restrict access, be with the patient as much as possible, and assist at intervention planning and implementation.
It is worth mentioning, here, the connection between chronic pain and suicidal ideation. Pain management reduces suicidal ideation, regardless of depression severity.23
Continue to: Psychotherapy and pharmacotherapies...
Psychotherapy and pharmacotherapies offered for the treatment of depression in geriatric practices are both effective, without much difference seen in efficacy.24 Psychotherapy might include direct physician and family support to the patient or referral to a mental health professional. Base treatment choices on clinical access, patient preference, and medical contraindications and other illnesses.
Pros and cons of various pharmacotherapies
Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed first for elderly patients with depression.25 Escitalopram is often better tolerated than paroxetine, which exhibits muscarinic antagonism and enzyme inhibition of cytochrome P450-2D6.26 Escitalopram also has fewer pharmaceutical interactions compared with sertraline.26
Generally, when prescribing an antidepressant drug, stay with the initial choice, gradually increasing the dose as clinically needed to its maximum limit. Suicidal ideation may be worsened by too quickly switching from one antidepressant to another or by co-prescribing anxiolytic or hypnotic medicines. Benzodiazepines have addictive and disinhibiting properties and should be avoided, if possible.27 For patients withinsomnia, consider initially selecting a sedating antidepressant medication such as paroxetine or mirtazapine to augment sleep.
Alternatives to SSRIs. Nonselective serotonin reuptake inhibitors have similar efficacy as SSRIs. However, escitalopram is as effective as venlafaxine (a selective serotonin and norepinephrine reuptake inhibitor [SSNRI]) and is better tolerated.28 Duloxetine, another SSNRI, improves mood and often diminishes chronic pain.29 Mirtazapine, an alpha-2 antagonist, might cause fewer drug-drug interactions and is effective, well tolerated, and especially helpful for patients with anxiety or insomnia.30 Dry mouth, sedation, and weight gain are common adverse effects of mirtazapine. Obesity precautions are often necessary during mirtazapine therapy; this includes monitoring body weight and metabolic profiles, instituting dietary changes, and recommending an exercise regimen. In contrast to SSRIs, mirtazapine might induce less sexual dysfunction.31
Tricyclic antidepressant drugs can also be effective but may worsen cardiac conduction abnormalities, prostatic hypertrophy, or narrow angle glaucoma. Tricyclic antidepressants may be useful in patients without cardiac disease who have not responded to an SSRI or an SSNRI.
Continue to: The role of aripiprazole
The role of aripiprazole. Elderly patients not achieving remission from depression with antidepressant agents alone may benefit from co-prescribing aripiprazole.32 As an adjunct, aripiprazole is effective in achieving and sustaining remission
Minimize risks and maximize benefits of antidepressants by following these recommendations:
- Ascertain whether any antidepressant treatments have worked well in the past.
- Start with an SSRI if no other antidepressant treatment has worked in the past.
- Counsel patients about the need for treatment adherence. Antidepressants may take 2 weeks to 2 months to provide noticeable improvement.
- Prescribe up to the maximum drug dose if needed to enhance benefit.
- Use a mood measurement tool (eg, the Patient Health Questionnaire-9) to help evaluate treatment response.
Try a different class of drugs for patients who do not respond to treatment. For patients who have a partial response, augment with bupropion XL, mirtazapine, aripiprazole, or quetiapine.33 Sertraline and nortriptyline are similarly effective on a population-wide basis, with sertraline having less-problematic adverse effects.34 Trial-and-error treatments in practice may find one patient responding only to sertraline and another patient only to nortriptyline.
Combinations of different drug classes may provide benefit for patients not responding to a single antidepressant. In geriatric patients, combined treatment with methylphenidate and citalopram enhances mood and well-being.35 Compared with either drug alone, the combination yielded an augmented clinical response profile and a higher rate of remission. Cognitive functioning, energy, and mood improve even with methylphenidate alone, especially when fatigue is an issue. However, addictive properties limit its use to cases in which conventional antidepressant medications are not effective or indicated, and only when drug refills are closely monitored.
The challenges of advancing age. Antidepressant treatment needs increase with advanced age.36 As mentioned earlier, elderly people often have medical illnesses complicating their depression and frequently are dealing with pain from the medical illness. When dementia coexists with depression, the efficacy of pharmacotherapies is compromised.
Continue to: When drug-related interventions fail
When drug-related interventions fail, therapy ought to be more psychologically focused.37 Psychotherapy is usually helpful and is particularly indicated when recovery is suboptimal. Counseling might come from the treating physician or referral to a psychotherapist.
Nasal esketamine can be efficacious when supplementing antidepressant pharmacotherapy among older patients with treatment-resistant depression.38 Elderly individuals responding to antidepressants do not benefit from adjunctive donepezil to correct mild cognitive impairment.39 There is no advantage to off-label cholinesterase inhibitor prescribing for patients with both depression and dementia.
Other options. Electroconvulsive therapy (ECT) does not cause long-term cognitive problems and is reserved for treatment-resistant cases.40 Patients with depression who also have had previous cognitive impairment often improve in mental ability following ECT.41
A promising new option. Transcranial magnetic stimulation (TMS) is a promising, relatively new therapeutic option for treating refractory cases of depressive mood disorders. In TMS, an electromagnetic coil that creates a magnetic field is placed over the left dorsolateral prefrontal cortex (which is responsible for mood regulation). Referral for TMS administration may offer new hope for older patients with treatment-resistant depression.42
Keep comorbidities in mind as you address depression
Coexisting psychiatric illnesses worsen emotions. Geriatric patients are susceptible to psychiatric comorbidities that include substance abuse, obsessive-compulsive characteristics, dysfunctional eating, and panic disorder.19 Myocardial and cerebral infarctions are detrimental to mental health, especially soon after such events.43 Poststroke depression magnifies the risk for disability and mortality,16,17 yet antidepressant pharmacotherapy often enhances prognoses. Along with early intervention algorithm-based plans and inclusion of a depression care manager, antidepressants often diminish poststroke depression severity.44 Even when cancer is present, depression care reduces mortality.44 So with this in mind, persist with antidepressant treatment, which will often benefit an elderly individual with depression.
Continue to: When possible, get ahead of depression before it sets in
When possible, get ahead of depression before it sets in
Social participation and employment help to sustain an optimistic, euthymic mood.45 Maintaining good physical health, in part through consistent activity levels (including exercise), can help prevent depression. Since persistent sleep disturbance predicts depression among those with a depression history, optimizing sleep among geriatric adults can avoid or alleviate depression.46
Sleep hygiene education for patients is also helpful. A regular waking time often promotes a better sleeping schedule. Restful sleep also is more likely when an individual avoids excess caffeine, exercises during the day, and uses the bed only for sleeping (not for listening to music or watching television).
Because inflammation may precede depression, anti-inflammatory medications have been proposed as potential treatment, but such pharmacotherapies are often ineffective. Older adults generally do not benefit from low-dose aspirin administration to prevent depression.47 Low vitamin D levels can contribute to depression, yet vitamin D supplementation may not improve mood.48
Offering hope. Tell your patients that if they are feeling depressed, they should make an appointment with you, their primary care physician, because there are medications they can take and counseling they can avail themselves of that could help.
CORRESPONDENCE
Steven Lippmann, MD, University of Louisville-Psychiatry, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; steven.lippmann@louisville.edu.
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
1. Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psych. 2000;57:601-607. doi: 10.1001/ archpsyc.57.6.601
2. Barry LC, Allore HG, Guo Z, et al. Higher burden of depression among older women: the effect of onset, persistence, and mortality over time. Arch Gen Psych. 2008;65:172-178. doi: 10.1001/archgenpsychiatry.2007.17
3. Katon WJ, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psych. 2003;60:897-903. doi: 10.1001/archpsyc.60.9.897
4. Snow CE, Abrams RC. The indirect costs of late-life depression in the United States: a literature review and perspective. Geriatrics. 2016;1,30. doi.org/10.3390/geriatrics/1040030
5. George LK, Blazer DG, Hughes D, et al. Social support and the outcome of major depression. Br J Psych. 1989;154:478-485. doi: 10.1192/bjp.154.4.478
6. Fonda SJ, Wallace RB, Herzog AR. Changes in driving patterns and worsening depressive symptoms among older adults. J Gerontol Psychol Soc Sci. 2001;56:S343-S351. doi: 10.1093/geronb/56.6.s343
7. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community dwelling older adults—a prospective study. Am J Psych. 2008;165:1543-1550. doi: 10.1176/appi.ajp.2008.07121882
8. Golub JS, Brewster KK, Brickman AM, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. 2020;28:545-556. doi: 10.1016/j.jagp.2019.12.008
9. Alexopoulos GS. Mechanisms and treatment of late-life depression. Focus (Am Psychiatr Publ). 2021;19:340-354. doi: 10.1176/appi.focus.19304
10. Starkstein SE, Preziosi TJ, Bolduc PL, et al. Depression in Parkinson’s disease. J Nerv Ment Disord. 1990;178:27-31. doi: 10.1097/00005053-199001000-00005
11. Gilman SE, Abraham HE. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alco Depend. 2001;63:277-286. doi: 10.1016/s0376-8716(00)00216-7
12. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol. 1991;46:P15-P21. doi: 10.1093/geronj/46.1.p15
13. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psych. 1997;54:915-922. doi: 10.1001/archpsyc.1997.01830220033006
14. Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249-255. doi: 10.1016/j.jad.2007.07.002
15. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psych. 2013;18:963-974. doi: 10.1038/mp.2013.20
16. Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psych. 2016;173:221-231. doi: 10.1176/appi.ajp.2015.15030363
17. Cai W, Mueller C, Li YJ, et al. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102-109. doi: 10.1016/ j.arr.2019.01.013
18. Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psych. 2003;160:1147-1156. doi: 10.1176/appi.ajp.160.6.1147
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). 2013:160-168.
20. Pimontel MA, Rindskopf D, Rutherford BR, et al. A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. Am J Geriatr Psych. 2016;24:31-34. doi: 10.1016/j.jagp.2015.05.010
21. Koenig HG, Cohen HJ, Blazer DG, et al. Religious coping and depression in elderly hospitalized medically ill men. Am J Psychiatry. 1992;149:1693-1700. doi: 10.1176/ajp.149.12.1693
22. Blake H, Mo P, Malik S, et al. How effective are physical activity interventions for alleviating depressive symptoms in older people? A systematic review. Clin Rehabil. 2009;10:873-887. doi: 10.1177/0269215509337449
23. Bruce ML, Ten Have TR, Reynolds CF, et al. Reducing suicidal and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081-1091. doi: 10.1001/jama.291.9.1081
24. Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493-1501. doi: 10.1176/ajp.2006.163.9.1493
25. Solai LK, Mulsant BH, Pollack BG. Selective serotonin reuptake inhibitors for late-life depression: a comparative review. Drugs Aging. 2001;18:355-368. doi: 10.2165/00002512-200118050-00006
26. Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline. Are they all alike? Int Clin Psychopharmacol. 2014;29:185-196. doi: 10.1097/YIC.0000000000000023
27. Hedna K, Sundell KA, Hamidi A, et al. Antidepressants and suicidal behaviour in late life: a prospective population-based study of use patterns in new users aged 75 and above. Eur J Clin Pharmacol. 2018;74:201-208. doi: 10.1007/s00228-017-2360-x
28. Bielski RJ, Ventura D, Chang CC. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1190-1196. doi: 10.4088/jcp.v65n0906
29. Robinson M, Oakes TM, Raskin J, et al. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34-45. doi: 10.1016/ j.jagp.2013.01.019
30. Holm KJ, Markham A. Mirtazapine: a review of its use in major depression. Drugs. 1999;57:607-631. doi: 10.2165/00003495-199957040-00010
31. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7:249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x
32. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised double-blind, placebo-controlled trial. Lancet. 2015;386:2404-2412. doi: 10.1016/S0140-6736(15)00308-6
33. Lenze EJ, Oughli HA. Antidepressant treatment for late-life depression: considering risks and benefits. J Am Geriatr Soc. 2019;67:1555-1556. doi: 10.1111/jgs.15964
34. Bondareff W, Alpert M, Friedhoff AJ, et al: Comparison of sertraline and nortriptyline in the treatment of major depressive disorder in late life. Am J Psychiatry. 2000;157:729-736. doi: 10.1176/appi.ajp.157.5.729
35. Lavretsky H, Reinlieb M, St Cyr N. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo controlled trial. Am J Psych. 2015;72:561-569. doi: 10.1176/appi.ajp.2014.14070889
36. Arthur A, Savva GM, Barnes LE, et al. Changing prevalence and treatment of depression among older people over two decades. Br J Psychiatry. 2020;21:49-54. doi: 10.1192/bjp.2019.193
37. Zuidersma M, Chua K-C, Hellier J, et al. Sertraline and mirtazapine versus placebo in subgroups of depression in dementia: findings from the HTA-SADD randomized controlled trial. Am J Geriatr Psychiatry. 2019;27:920-931. doi: 10.1016/ j.jagp.2019.03.021
38. Ochs-Ross R, Wajs E, Daly EJ, et al. Comparison of long-term efficacy and safety of esketamine nasal spray plus oral antidepressant in younger versus older patients with treatment-resistant depression: post-hoc analysis of SUSTAIN-2, a long-term open-label phase 3 safety and efficacy study. Am J Geriatr Psychiatry. 2022;30:541-556. doi: 10.1016/j.jagp.2021.09.014
39. Devanand DP, Pelton GH, D’Antonio K, et al. Donepezil treatment in patients with depression and cognitive impairment on stable antidepressant treatment: a randomized controlled trial. Am J Geriatr Psychiatry. 2018;26:1050-1060. doi: 10.1016/ j.jagp.2018.05.008
40. Obbels J, Vansteelandt K, Verwijk E, et al. MMSE changes during and after ECT in late life depression: a prospective study. Am J Geriatr Psychiatry. 2019;27:934-944. doi: 10.1016/ j.jagp.2019.04.006
41. Wagenmakers MJ, Vansteelandt K, van Exel E, et al. Transient cognitive impairment and white matter hyperintensities in severely depressed older patients treated with electroconvulsive therapy. Am J Geriatr Psychiatry. 2021:29:1117-1128. doi: 10.1016/j.jagp.2020.12.028
42. Trevizol AP, Goldberger KW, Mulsant BH, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Ger Psychiatry. 2019;34:822-827. doi: 10.1002/gps.5091
43. Aben I, Verhey F, Stik J, et al. A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry. 2003;74:581-585. doi: 10.1136/jnnp.74.5.581
44. Gallo JJ, Bogner HR, Morales KH, et al. The effect of a primary care practice-based depression intervention on mortality in older adults: a randomized trial. Ann Intern Med. 2007;146:689-698. doi: 10.7326/0003-4819-146-10-200705150-00002
45. Lee J, Jang SN, Cho SL. Gender differences in the trajectories and the risk factors of depressive symptoms in later life. Int Psychogeriatr. 2017;29:1495-1505. doi: 10.1017/S1041610217000709
46. Lee E, Cho HJ, Olmstead R, et al. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685-1691. doi: 10.5665/sleep.3128
47. Berk M, Woods RL, Nelson MR, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. J Am Med A Psych. 2020;77:1012-1020. doi: 10.1001/jamapsychiatry.2020.1214
48. Okereke OI, Reynolds CF, Mischoulon D, et al. Effect of long-term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: a randomized clinical trial. JAMA. 2020;324:471-480. doi: 10.1001/jama.2020.10224
PRACTICE RECOMMENDATIONS
› Begin treatment with a selective serotonin reuptake inhibitor (SSRI) unless another antidepressant has worked well in the past. A
› Consider augmenting therapy with bupropion XL, mirtazapine, aripiprazole, or quetiapine for any patient who responds only partially to an SSRI. C
› Add psychotherapy to antidepressant pharmacotherapy, particularly for patients who have difficulties with executive functions such as planning and organization. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Depressive symptoms tied to higher stroke risk, worse outcomes
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Telehealth doctor indicted on health care fraud, opioid distribution charges
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Sangita Patel, MD, 50, practiced at Advance Medical Home Physicians in Troy.
According to court documents, between July 2020 and June 2022 Patel was responsible for submitting Medicare claims for improper telehealth visits she didn’t conduct herself.
Dr. Patel, who accepted patients who paid in cash as well as those with Medicare and Medicaid coverage, billed approximately $3.4 million to Medicare between 2018 and 2022, according to court documents. An unusual number of these visits were billed using complex codes, an indication of health care fraud. The investigation also found that on many days, Dr. Patel billed for more than 24 hours of services. During this period, according to the document, 76% of Dr. Patel’s Medicare reimbursements were for telehealth.
Prosecutors say that Dr. Patel prescribed Schedule II controlled substances to more than 90% of the patients in these telehealth visits. She delegated her prescription authority to an unlicensed medical assistant. Through undercover visits and cell site search warrant data, the investigation found that Dr. Patel directed patients to contact, via cell phone, this assistant, who then entered electronic prescriptions into the electronic medical records system. Dr. Patel then signed the prescriptions and sent them to the pharmacies without ever interacting with the patients. Prosecutors also used text messages, obtained by search warrant, between Dr. Patel and her assistant and between the assistant and undercover informers to build their case.
Dr. Patel is also accused of referring patients to other providers, who in turn billed Medicare for claims associated with those patients. Advance Medical received $143,000 from these providers, potentially in violation of anti-kickback laws, according to bank records obtained by subpoena.
If convicted, Dr. Patel could be sentenced to up to 10 years in federal prison.
A version of this article first appeared on Medscape.com.
Migraine after concussion linked to worse outcomes
researchers have found.
“Early assessment of headache – and whether it has migraine features – after concussion can be helpful in predicting which children are at risk for poor outcomes and identifying children who require targeted intervention,” said senior author Keith Owen Yeates, PhD, the Ronald and Irene Ward Chair in Pediatric Brain Injury Professor and head of the department of psychology at the University of Calgary (Alta.). “Posttraumatic headache, especially when it involves migraine features, is a strong predictor of persisting symptoms and poorer quality of life after childhood concussion.”
Approximately 840,000 children per year visit an emergency department in the United States after having a traumatic brain injury. As many as 90% of those visits are considered to involve a concussion, according to the investigators. Although most children recover quickly, approximately one-third continue to report symptoms a month after the event.
Posttraumatic headache occurs in up to 90% of children, most commonly with features of migraine.
The new study, published in JAMA Network Open, was a secondary analysis of the Advancing Concussion Assessment in Pediatrics (A-CAP) prospective cohort study. The study was conducted at five emergency departments in Canada from September 2016 to July 2019 and included children and adolescents aged 8-17 years who presented with acute concussion or an orthopedic injury.
Children were included in the concussion group if they had a history of blunt head trauma resulting in at least one of three criteria consistent with the World Health Organization definition of mild traumatic brain injury. The criteria include loss of consciousness for less than 30 minutes, a Glasgow Coma Scale score of 13 or 14, or at least one acute sign or symptom of concussion, as noted by emergency clinicians.
Patients were excluded from the concussion group if they had deteriorating neurologic status, underwent neurosurgical intervention, had posttraumatic amnesia that lasted more than 24 hours, or had a score higher than 4 on the Abbreviated Injury Scale (AIS). The orthopedic injury group included patients without symptoms of concussion and with blunt trauma associated with an AIS 13 score of 4 or less. Patients were excluded from both groups if they had an overnight hospitalization for traumatic brain injury, a concussion within the past 3 months, or a neurodevelopmental disorder.
The researchers analyzed data from 928 children of 967 enrolled in the study. The median age was 12.2 years, and 41.3% were female. The final study cohort included 239 children with orthopedic injuries but no headache, 160 with a concussion and no headache, 134 with a concussion and nonmigraine headaches, and 254 with a concussion and migraine headaches.
Children with posttraumatic migraines 10 days after a concussion had the most severe symptoms and worst quality of life 3 months following their head trauma, the researchers found. Children without headaches within 10 days after concussion had the best 3-month outcomes, comparable to those with orthopedic injuries alone.
The researchers said the strengths of their study included its large population and its inclusion of various causes of head trauma, not just sports-related concussions. Limitations included self-reports of headaches instead of a physician diagnosis and lack of control for clinical interventions that might have affected the outcomes.
Charles Tator, MD, PhD, director of the Canadian Concussion Centre at Toronto Western Hospital, said the findings were unsurprising.
“Headaches are the most common symptom after concussion,” Dr. Tator, who was not involved in the latest research, told this news organization. “In my practice and research with concussed kids 11 and up and with adults, those with preconcussion history of migraine are the most difficult to treat because their headaches don’t improve unless specific measures are taken.”
Dr. Tator, who also is a professor of neurosurgery at the University of Toronto, said clinicians who treat concussions must determine which type of headaches children are experiencing – and refer as early as possible for migraine prevention or treatment and medication, as warranted.
“Early recognition after concussion that migraine headaches are occurring will save kids a lot of suffering,” he said.
The study was supported by a Canadian Institute of Health Research Foundation Grant and by funds from the Alberta Children’s Hospital Foundation and the Alberta Children’s Hospital Research Institute. Dr. Tator has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
researchers have found.
“Early assessment of headache – and whether it has migraine features – after concussion can be helpful in predicting which children are at risk for poor outcomes and identifying children who require targeted intervention,” said senior author Keith Owen Yeates, PhD, the Ronald and Irene Ward Chair in Pediatric Brain Injury Professor and head of the department of psychology at the University of Calgary (Alta.). “Posttraumatic headache, especially when it involves migraine features, is a strong predictor of persisting symptoms and poorer quality of life after childhood concussion.”
Approximately 840,000 children per year visit an emergency department in the United States after having a traumatic brain injury. As many as 90% of those visits are considered to involve a concussion, according to the investigators. Although most children recover quickly, approximately one-third continue to report symptoms a month after the event.
Posttraumatic headache occurs in up to 90% of children, most commonly with features of migraine.
The new study, published in JAMA Network Open, was a secondary analysis of the Advancing Concussion Assessment in Pediatrics (A-CAP) prospective cohort study. The study was conducted at five emergency departments in Canada from September 2016 to July 2019 and included children and adolescents aged 8-17 years who presented with acute concussion or an orthopedic injury.
Children were included in the concussion group if they had a history of blunt head trauma resulting in at least one of three criteria consistent with the World Health Organization definition of mild traumatic brain injury. The criteria include loss of consciousness for less than 30 minutes, a Glasgow Coma Scale score of 13 or 14, or at least one acute sign or symptom of concussion, as noted by emergency clinicians.
Patients were excluded from the concussion group if they had deteriorating neurologic status, underwent neurosurgical intervention, had posttraumatic amnesia that lasted more than 24 hours, or had a score higher than 4 on the Abbreviated Injury Scale (AIS). The orthopedic injury group included patients without symptoms of concussion and with blunt trauma associated with an AIS 13 score of 4 or less. Patients were excluded from both groups if they had an overnight hospitalization for traumatic brain injury, a concussion within the past 3 months, or a neurodevelopmental disorder.
The researchers analyzed data from 928 children of 967 enrolled in the study. The median age was 12.2 years, and 41.3% were female. The final study cohort included 239 children with orthopedic injuries but no headache, 160 with a concussion and no headache, 134 with a concussion and nonmigraine headaches, and 254 with a concussion and migraine headaches.
Children with posttraumatic migraines 10 days after a concussion had the most severe symptoms and worst quality of life 3 months following their head trauma, the researchers found. Children without headaches within 10 days after concussion had the best 3-month outcomes, comparable to those with orthopedic injuries alone.
The researchers said the strengths of their study included its large population and its inclusion of various causes of head trauma, not just sports-related concussions. Limitations included self-reports of headaches instead of a physician diagnosis and lack of control for clinical interventions that might have affected the outcomes.
Charles Tator, MD, PhD, director of the Canadian Concussion Centre at Toronto Western Hospital, said the findings were unsurprising.
“Headaches are the most common symptom after concussion,” Dr. Tator, who was not involved in the latest research, told this news organization. “In my practice and research with concussed kids 11 and up and with adults, those with preconcussion history of migraine are the most difficult to treat because their headaches don’t improve unless specific measures are taken.”
Dr. Tator, who also is a professor of neurosurgery at the University of Toronto, said clinicians who treat concussions must determine which type of headaches children are experiencing – and refer as early as possible for migraine prevention or treatment and medication, as warranted.
“Early recognition after concussion that migraine headaches are occurring will save kids a lot of suffering,” he said.
The study was supported by a Canadian Institute of Health Research Foundation Grant and by funds from the Alberta Children’s Hospital Foundation and the Alberta Children’s Hospital Research Institute. Dr. Tator has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
researchers have found.
“Early assessment of headache – and whether it has migraine features – after concussion can be helpful in predicting which children are at risk for poor outcomes and identifying children who require targeted intervention,” said senior author Keith Owen Yeates, PhD, the Ronald and Irene Ward Chair in Pediatric Brain Injury Professor and head of the department of psychology at the University of Calgary (Alta.). “Posttraumatic headache, especially when it involves migraine features, is a strong predictor of persisting symptoms and poorer quality of life after childhood concussion.”
Approximately 840,000 children per year visit an emergency department in the United States after having a traumatic brain injury. As many as 90% of those visits are considered to involve a concussion, according to the investigators. Although most children recover quickly, approximately one-third continue to report symptoms a month after the event.
Posttraumatic headache occurs in up to 90% of children, most commonly with features of migraine.
The new study, published in JAMA Network Open, was a secondary analysis of the Advancing Concussion Assessment in Pediatrics (A-CAP) prospective cohort study. The study was conducted at five emergency departments in Canada from September 2016 to July 2019 and included children and adolescents aged 8-17 years who presented with acute concussion or an orthopedic injury.
Children were included in the concussion group if they had a history of blunt head trauma resulting in at least one of three criteria consistent with the World Health Organization definition of mild traumatic brain injury. The criteria include loss of consciousness for less than 30 minutes, a Glasgow Coma Scale score of 13 or 14, or at least one acute sign or symptom of concussion, as noted by emergency clinicians.
Patients were excluded from the concussion group if they had deteriorating neurologic status, underwent neurosurgical intervention, had posttraumatic amnesia that lasted more than 24 hours, or had a score higher than 4 on the Abbreviated Injury Scale (AIS). The orthopedic injury group included patients without symptoms of concussion and with blunt trauma associated with an AIS 13 score of 4 or less. Patients were excluded from both groups if they had an overnight hospitalization for traumatic brain injury, a concussion within the past 3 months, or a neurodevelopmental disorder.
The researchers analyzed data from 928 children of 967 enrolled in the study. The median age was 12.2 years, and 41.3% were female. The final study cohort included 239 children with orthopedic injuries but no headache, 160 with a concussion and no headache, 134 with a concussion and nonmigraine headaches, and 254 with a concussion and migraine headaches.
Children with posttraumatic migraines 10 days after a concussion had the most severe symptoms and worst quality of life 3 months following their head trauma, the researchers found. Children without headaches within 10 days after concussion had the best 3-month outcomes, comparable to those with orthopedic injuries alone.
The researchers said the strengths of their study included its large population and its inclusion of various causes of head trauma, not just sports-related concussions. Limitations included self-reports of headaches instead of a physician diagnosis and lack of control for clinical interventions that might have affected the outcomes.
Charles Tator, MD, PhD, director of the Canadian Concussion Centre at Toronto Western Hospital, said the findings were unsurprising.
“Headaches are the most common symptom after concussion,” Dr. Tator, who was not involved in the latest research, told this news organization. “In my practice and research with concussed kids 11 and up and with adults, those with preconcussion history of migraine are the most difficult to treat because their headaches don’t improve unless specific measures are taken.”
Dr. Tator, who also is a professor of neurosurgery at the University of Toronto, said clinicians who treat concussions must determine which type of headaches children are experiencing – and refer as early as possible for migraine prevention or treatment and medication, as warranted.
“Early recognition after concussion that migraine headaches are occurring will save kids a lot of suffering,” he said.
The study was supported by a Canadian Institute of Health Research Foundation Grant and by funds from the Alberta Children’s Hospital Foundation and the Alberta Children’s Hospital Research Institute. Dr. Tator has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.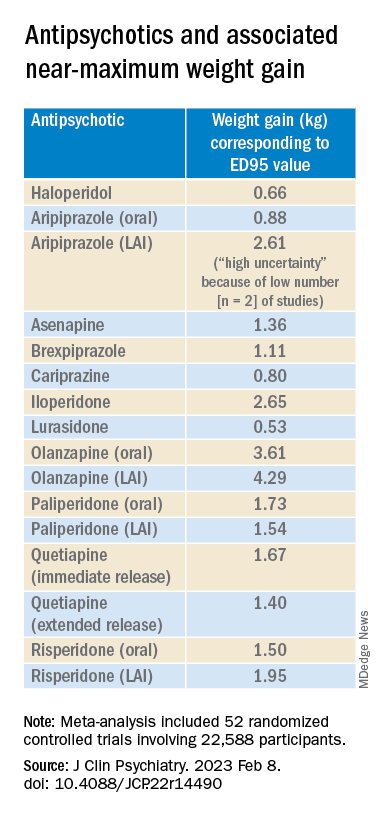
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
APA releases updated eating disorder guidelines
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
The updated guidelines focus primarily on anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED) and include recommendations for screening and treatment.
“Eating disorders often are unrecognized and untreated,” Catherine Crone, MD, chair of the guideline writing group, said in a statement from APA. “This guideline and supplementary resources are intended to serve as a practical tool for clinicians, to help with screening, diagnosis, and providing evidence-based treatment for eating disorders.”
Approximately one in five children worldwide are at risk for developing an eating disorder and U.S. medical admissions for adolescents with restrictive eating disorders more than doubled during the pandemic.
The economic cost of eating disorders in the United States from 2018 to 2019 was an estimated $64.7 billion, the report notes, with an additional $326.5 billion attributable to reductions in well-being associated with eating disorders.
The executive summary of the updated guidelines was published online in The American Journal of Psychiatry.
The practice guideline, which was approved at the 2021 APA annual meeting, features 16 recommendations for clinicians, including screening patients for eating disorders as part of an initial psychiatric evaluation and conducting comprehensive patient evaluations that incorporate laboratory tests and electrocardiograms.
Recommendations also include setting individualized weight goals for patients with anorexia and incorporating family-based therapy as part of a treatment plan for adolescents with anorexia or bulimia.
“This practice guideline aims to help clinicians improve care for their patients by reviewing current evidence and providing evidence-based statements that are intended to enhance knowledge, increase assessment, and optimize treatment of eating disorders,” the authors wrote.
A range of other resources were released with the new guidelines to provide clinicians with support to implement the recommendations, including a pocket guide for clinicians, continuing medical education activities, and slides. The association is also launching a pocket guide for patients and families and an interactive tool kit with a screening assessment calculator.
The APA guidelines follow the 2021 release by the American Academy of Pediatrics on diagnosing and managing eating disorders in children and adolescents.
The development of the guidelines was supported by a grant from the Council of Medical Specialty Societies.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Mental health risks higher among young people with IBD
, a new U.K. study suggests.
The retrospective, observational study of young people with IBD versus those without assessed the incidence of a wide range of mental health conditions in people aged 5-25 years.
“Anxiety and depression will not be a surprise to most of us. But we also saw changes for eating disorders, PTSD, and sleep changes,” said Richard K. Russell, MD, a pediatric gastroenterologist at the Royal Hospital for Sick Children, Edinburgh.
Dr. Russell presented the research at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
The findings indicate an unmet need for mental health care for young patients with IBD, he said. “All of us at ECCO need to address this gap.”
Key findings
Dr. Russell and colleagues identified 3,898 young people diagnosed with IBD in the 10-year period Jan. 1, 2010, through Jan. 1, 2020, using the Optimum Patient Care Research Database, which includes de-identified data from more than 1,000 general practices across the United Kingdom. They used propensity score matching to create a control group of 15,571 people without IBD, controlling for age, sex, socioeconomic status, ethnicity, and health conditions other than IBD.
Median follow-up was about 3 years.
The cumulative lifetime risk for developing any mental health condition by age 25 was 31.1% in the IBD group versus 25.1% in controls, a statistically significant difference.
Compared with the control group, the people with incident IBD were significantly more likely to develop the following:
- PTSD.
- Eating disorders.
- Self-harm.
- Sleep disturbance.
- Depression.
- Anxiety disorder.
- ‘Any mental health condition.’
Those most are risk included males overall, and specifically boys aged 12-17 years. Those with Crohn’s disease versus other types of IBD were also most at risk.
In a subgroup analysis, presented as a poster at the meeting, Dr. Russell and colleagues also found that mental health comorbidity in children and young adults with IBD is associated with increased IBD symptoms and health care utilization, as well as time off work.
Children and young adults with both IBD and mental health conditions should be monitored and receive appropriate mental health support as part of their multidisciplinary care, Dr. Russell said.
Dr. Russell added that the study period ended a few months before the COVID-19 pandemic began, so the research does not reflect its impact on mental health in the study population.
“The number of children and young adults we’re seeing in our clinic with mental health issues has rocketed through the roof because of the pandemic,” he said.
Dr. Russell suggested that the organization create a psychology subgroup called Proactive Psychologists of ECCO, or Prosecco for short.
Clinical implications
The study is important for highlighting the increased burden of mental health problems in young people with IBD, said session comoderator Nick Kennedy, MD, a consultant gastroenterologist and chief research information officer with the Royal Devon University Healthcare NHS Foundation Trust in England.
Dr. Kennedy, who was not affiliated with the research, is also supportive of the idea of a psychological subgroup within ECCO.
The peak age for developing mental health disorders found by the study (12-17 years) “is a unique and very sensitive time,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in London.
“These results highlight the need for development of early screening psychiatric programs starting from time of diagnosis and continuing on periodic intervals to offer the best management plan for IBD patients, especially those with childhood-onset IBD,” said Dr. Mesilhy, who was not affiliated with the research.
Such programs would “improve the patient’s quality of life, protecting them from a lot of suffering and preventing the bad sequelae for these disorders,” said Dr. Mesilhy. “Moreover, we still need further studies to identify the most efficient monitoring and treatment protocols.”
Dr. Kennedy applauded the researchers for conducting a population-based study because it ensured an adequate cohort size and maximized identification of mental health disorders.
“It was interesting to see that there were a range of conditions where risk was increased, and that males with IBD were at particularly increased risk,” he added.
Researchers’ use of coded primary care data was a study limitation, but it was “appropriately acknowledged by the presenter,” Dr. Kennedy said.
The study was supported by Pfizer. Dr. Russell disclosed he is a consultant and member of a speakers’ bureau for Pfizer outside the submitted work. Dr. Kennedy and Dr. Mesilhy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new U.K. study suggests.
The retrospective, observational study of young people with IBD versus those without assessed the incidence of a wide range of mental health conditions in people aged 5-25 years.
“Anxiety and depression will not be a surprise to most of us. But we also saw changes for eating disorders, PTSD, and sleep changes,” said Richard K. Russell, MD, a pediatric gastroenterologist at the Royal Hospital for Sick Children, Edinburgh.
Dr. Russell presented the research at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
The findings indicate an unmet need for mental health care for young patients with IBD, he said. “All of us at ECCO need to address this gap.”
Key findings
Dr. Russell and colleagues identified 3,898 young people diagnosed with IBD in the 10-year period Jan. 1, 2010, through Jan. 1, 2020, using the Optimum Patient Care Research Database, which includes de-identified data from more than 1,000 general practices across the United Kingdom. They used propensity score matching to create a control group of 15,571 people without IBD, controlling for age, sex, socioeconomic status, ethnicity, and health conditions other than IBD.
Median follow-up was about 3 years.
The cumulative lifetime risk for developing any mental health condition by age 25 was 31.1% in the IBD group versus 25.1% in controls, a statistically significant difference.
Compared with the control group, the people with incident IBD were significantly more likely to develop the following:
- PTSD.
- Eating disorders.
- Self-harm.
- Sleep disturbance.
- Depression.
- Anxiety disorder.
- ‘Any mental health condition.’
Those most are risk included males overall, and specifically boys aged 12-17 years. Those with Crohn’s disease versus other types of IBD were also most at risk.
In a subgroup analysis, presented as a poster at the meeting, Dr. Russell and colleagues also found that mental health comorbidity in children and young adults with IBD is associated with increased IBD symptoms and health care utilization, as well as time off work.
Children and young adults with both IBD and mental health conditions should be monitored and receive appropriate mental health support as part of their multidisciplinary care, Dr. Russell said.
Dr. Russell added that the study period ended a few months before the COVID-19 pandemic began, so the research does not reflect its impact on mental health in the study population.
“The number of children and young adults we’re seeing in our clinic with mental health issues has rocketed through the roof because of the pandemic,” he said.
Dr. Russell suggested that the organization create a psychology subgroup called Proactive Psychologists of ECCO, or Prosecco for short.
Clinical implications
The study is important for highlighting the increased burden of mental health problems in young people with IBD, said session comoderator Nick Kennedy, MD, a consultant gastroenterologist and chief research information officer with the Royal Devon University Healthcare NHS Foundation Trust in England.
Dr. Kennedy, who was not affiliated with the research, is also supportive of the idea of a psychological subgroup within ECCO.
The peak age for developing mental health disorders found by the study (12-17 years) “is a unique and very sensitive time,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in London.
“These results highlight the need for development of early screening psychiatric programs starting from time of diagnosis and continuing on periodic intervals to offer the best management plan for IBD patients, especially those with childhood-onset IBD,” said Dr. Mesilhy, who was not affiliated with the research.
Such programs would “improve the patient’s quality of life, protecting them from a lot of suffering and preventing the bad sequelae for these disorders,” said Dr. Mesilhy. “Moreover, we still need further studies to identify the most efficient monitoring and treatment protocols.”
Dr. Kennedy applauded the researchers for conducting a population-based study because it ensured an adequate cohort size and maximized identification of mental health disorders.
“It was interesting to see that there were a range of conditions where risk was increased, and that males with IBD were at particularly increased risk,” he added.
Researchers’ use of coded primary care data was a study limitation, but it was “appropriately acknowledged by the presenter,” Dr. Kennedy said.
The study was supported by Pfizer. Dr. Russell disclosed he is a consultant and member of a speakers’ bureau for Pfizer outside the submitted work. Dr. Kennedy and Dr. Mesilhy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new U.K. study suggests.
The retrospective, observational study of young people with IBD versus those without assessed the incidence of a wide range of mental health conditions in people aged 5-25 years.
“Anxiety and depression will not be a surprise to most of us. But we also saw changes for eating disorders, PTSD, and sleep changes,” said Richard K. Russell, MD, a pediatric gastroenterologist at the Royal Hospital for Sick Children, Edinburgh.
Dr. Russell presented the research at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
The findings indicate an unmet need for mental health care for young patients with IBD, he said. “All of us at ECCO need to address this gap.”
Key findings
Dr. Russell and colleagues identified 3,898 young people diagnosed with IBD in the 10-year period Jan. 1, 2010, through Jan. 1, 2020, using the Optimum Patient Care Research Database, which includes de-identified data from more than 1,000 general practices across the United Kingdom. They used propensity score matching to create a control group of 15,571 people without IBD, controlling for age, sex, socioeconomic status, ethnicity, and health conditions other than IBD.
Median follow-up was about 3 years.
The cumulative lifetime risk for developing any mental health condition by age 25 was 31.1% in the IBD group versus 25.1% in controls, a statistically significant difference.
Compared with the control group, the people with incident IBD were significantly more likely to develop the following:
- PTSD.
- Eating disorders.
- Self-harm.
- Sleep disturbance.
- Depression.
- Anxiety disorder.
- ‘Any mental health condition.’
Those most are risk included males overall, and specifically boys aged 12-17 years. Those with Crohn’s disease versus other types of IBD were also most at risk.
In a subgroup analysis, presented as a poster at the meeting, Dr. Russell and colleagues also found that mental health comorbidity in children and young adults with IBD is associated with increased IBD symptoms and health care utilization, as well as time off work.
Children and young adults with both IBD and mental health conditions should be monitored and receive appropriate mental health support as part of their multidisciplinary care, Dr. Russell said.
Dr. Russell added that the study period ended a few months before the COVID-19 pandemic began, so the research does not reflect its impact on mental health in the study population.
“The number of children and young adults we’re seeing in our clinic with mental health issues has rocketed through the roof because of the pandemic,” he said.
Dr. Russell suggested that the organization create a psychology subgroup called Proactive Psychologists of ECCO, or Prosecco for short.
Clinical implications
The study is important for highlighting the increased burden of mental health problems in young people with IBD, said session comoderator Nick Kennedy, MD, a consultant gastroenterologist and chief research information officer with the Royal Devon University Healthcare NHS Foundation Trust in England.
Dr. Kennedy, who was not affiliated with the research, is also supportive of the idea of a psychological subgroup within ECCO.
The peak age for developing mental health disorders found by the study (12-17 years) “is a unique and very sensitive time,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in London.
“These results highlight the need for development of early screening psychiatric programs starting from time of diagnosis and continuing on periodic intervals to offer the best management plan for IBD patients, especially those with childhood-onset IBD,” said Dr. Mesilhy, who was not affiliated with the research.
Such programs would “improve the patient’s quality of life, protecting them from a lot of suffering and preventing the bad sequelae for these disorders,” said Dr. Mesilhy. “Moreover, we still need further studies to identify the most efficient monitoring and treatment protocols.”
Dr. Kennedy applauded the researchers for conducting a population-based study because it ensured an adequate cohort size and maximized identification of mental health disorders.
“It was interesting to see that there were a range of conditions where risk was increased, and that males with IBD were at particularly increased risk,” he added.
Researchers’ use of coded primary care data was a study limitation, but it was “appropriately acknowledged by the presenter,” Dr. Kennedy said.
The study was supported by Pfizer. Dr. Russell disclosed he is a consultant and member of a speakers’ bureau for Pfizer outside the submitted work. Dr. Kennedy and Dr. Mesilhy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECCO 2023

