User login
For MD-IQ use only
Stay tuned for CSI: Olive oil
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
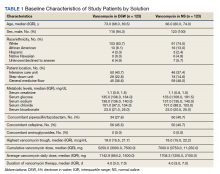
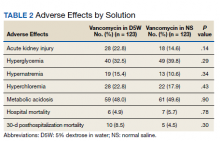
Fecal microbiota transplants may improve resistance to melanoma immunotherapy
In the fall of 2020, Hassane M. Zarour, MD, and colleagues began to pore over raw data from their
Preclinical mouse studies have demonstrated that the gut microbiota could influence the response of tumors to anti–PD-1 immunotherapy, but FMT had not been previously evaluated in human patients with malignant melanoma whose disease persisted or progressed after medical therapy. Only 30%-40% of melanoma patients respond to anti–PD-1 immunotherapy, so the researchers’ sense of anticipation was palpable. “It’s a high-risk, high-reward study, so you never know,” Dr. Zarour, a dermatologist and immunologist who is coleader of the melanoma program at the University of Pittsburgh Medical Center’s Hillman Cancer Center, said in an interview.
For the study, which was funded by the National Institutes of Health and published in Science, Dr. Zarour and a team of colleagues, including Diwakar Davar, MD, a medical oncologist/hematologist at UPMC and Giorgio Trinchieri, MD, head of the cancer immunology section at the National Cancer Institute, enrolled 16 patients with advanced melanoma whose disease had persisted or progressed with anti-PD-1 drugs; donors were 7 patients with advanced melanoma who had responded to pembrolizumab, 4 with a complete response and 3 with a partial response, with a median progression-free survival of 56 months.
After donors and patients underwent serial stool sampling and studies to stamp out the potential for transmitting infectious agents, the researchers administered the donor-derived FMT to patients via colonoscopy every 14 days for 3 weeks, followed by pembrolizumab. To their delight, 6 of the 15 evaluable recipients responded to treatment, with a reduction in tumor or long-term disease stabilization. Moreover, responders also showed increased abundance of taxa that were previously associated with response to immunotherapy, increased activation of CD8+ T cells, and decreased frequency of interleukin-8–expressing myeloid cells.
“This opens new doors for the future,” Dr. Zarour said. “It’s very encouraging, but I don’t want to overstate the data. It’s a small, nonrandomized trial, but one has to keep in mind that people were skeptical about this work; they didn’t think FMT would work. Now we see many people coming into the field to investigate the role of the microbiome as a therapeutic tool, which is great.”
Teri Greiling, MD, characterized the finding as a key development in understanding the microbiome’s potential to influence the course of melanoma and other diseases. “What’s emerging over the last decade of research is that our immune system has a close, back-and-forth relationship with our microbiota,” said Dr. Greiling, associate professor of dermatology at Oregon Health & Science University, Portland. “From day 1 of birth, we’re colonized by microbes that train our immune system how to function. In response, your immune system keeps those microbes in check and shapes which ones are allowed to colonize, and which ones are a target for attack. Thus, inflammatory responses are generated. Similarly, the goal of immunotherapy is to activate the immune system to fight cancer. This study shows that the immune system continues to need the colonizing microbes in our body to function optimally.”
Immunotherapy with checkpoint inhibitors was not an option for malignant melanoma patients until 2011, she noted, so the potential for FMT to further improve outcomes is welcome news for patients and their families. “We went from a less than 5% chance of survival with metastatic melanoma to now, with the right combination of checkpoint inhibitors, we’re up over 50%, which is amazing in a decade,” Dr. Greiling said. “Still, we’re losing half of our patients. If [FMT provides] a 30% improvement over that, that would be great, but it’s hard to extrapolate from such small numbers.”
Positive results in an Israeli study
Results from a similar, smaller phase 1 trial of 2 FMT donors and 10 recipients with metastatic melanoma who had progressed on anti-PD-1 therapy, from the Ella Lemelbaum Institute for Immuno-Oncology at Sheba Medical Center in Tel HaShomer, Israel, yielded similar results. The FMT protocol in this study included colonoscopy and oral stool capsules, followed by the reintroduction of anti–PD-1 therapy with nivolumab. The two FMT donors had previously been treated with anti–PD-1 monotherapy for metastatic melanoma and had achieved a clinical response for at least 1 year. Of the 10 FMT recipients, 1 had a complete response and 2 had a partial response.
“We expected changes in the immune system but did not expect that 3 out of the 10 patients in our study would be turned from nonresponders to responders,” the study’s lead author, Erez N. Baruch, MD, PhD, told this news organization. “Since this was a first-in-human study, we were aiming to assess safety and not clinical responses. [We found] that microbiota modulation can change the immune infiltration within melanoma tumors and by this affect response to immunotherapy.”
Dr. Baruch, an internal medicine resident in the physician-scientist track program at the University of Texas, Houston, said that the findings create a potential new therapeutic paradigm, or a new “playing ground” for drug development that can support existing immunotherapies. “It is important for dermatologists to understand that disruptions of the gut microbiota, mainly by antibiotics, may be harmful to melanoma patients,” he said. “Antibiotics in cancer patients should be used judiciously but of course should not be avoided when there’s an indication.”
As for next steps, Dr. Zarour and colleagues are recruiting more patients to boost their sample size and conducting sequential analysis of the microbiome of study participants “to better determine what the good and bad bugs are,” he said. “There are so many variables, including diet and geography. We need more data.” The hope is to develop a “microbiome signature” to identify patients likely to respond to FMT, and maybe one day, a probiotic capsule that patients take to optimize their response to immunotherapy.
“We don’t want to say that the microbiome is responsible for everything, but it’s responsible for some of the response and some of the resistance to treatment,” Dr. Zarour said. “So, we want to identify what candidate nonresponders are more likely to respond to FMT and be able to stick the right stool in the donor. This goes to better education of the microbiome signature. We are working hard on that.”
Dr. Baruch added that performing FMT for melanoma patients requires tight collaborations between oncologists, dermatologists, GI, and infectious disease experts. “These usually can be done in the setting of large cancer centers and will probably not be available in any hospital,” he said. “This is why understanding the mechanisms and developing an FMT-like drug is important. We are focusing on studying the mechanisms behind the clinical effect in order to develop a drug with an FMT-like effect without the safety and logistic issues related to FMTs.”
Tamia A. Harris-Tryon, MD, PhD, whose lab at the University of Texas Southwestern Medical Center at Dallas is studying how diet and the microbiota impact skin immunity, underscored the importance of evaluating the characteristics of the diet of patients as trials of FMT in melanoma patients carry on. “We know that the diet impacts the repertoire of microbes that colonize the gut,” said Dr. Harris-Tryon, assistant professor in the department of dermatology at the medical center. “The diet of the recipient likely has an impact” on the success of donor FMT.
She also noted that other skin conditions have been linked to a disrupted gut microbiome, such as psoriasis. “Given the safety of FMT in both of these studies, trials of FMT in psoriasis and other systemic skin conditions should be considered,” she said.
According to Dr. Zarour, mounting data from separate studies show that some gut microbiota play a role in adverse events experienced by melanoma patients on immunotherapy. “That is very important, especially with combination therapy,” he said. “There are also microbes involved in resistance to treatment, so the idea would be to identify these microbes.”
Studies raise more questions
In the opinion of Dr. Greiling, results from these two studies raise more questions than they answer. “The big question ... is why and how does FMT work, and how can we make the response better?” she said. “Is there one particular gene product from one microbe that is the key magic ingredient, and we can harness this as a drug? More likely it’s a complex interplay between multiple bacterial species needed to direct the immune response. Is there a group of microbes that is the same from person to person, or is it more complex?”
Then there are pending regulatory concerns. “We know that FMT works for [Clostridioides] difficile colitis but it’s not officially [Food and Drug Administration] approved,” Dr. Greiling said. “The FDA is really struggling with how to approve or regulate using bacteria as a drug. Where is that crossover? That inhibits things moving forward, for good reason. You want to balance safety with live microbes.”
The UPMC clinical trial was supported by Merck. Dr. Zarour disclosed that he is supported by grants from the National Cancer Institute and the James W. and Frances G. McGlothlin Chair in Melanoma Immunotherapy Research at UPMC. The Israeli study was funded by the Ella Lemelbaum Institute for Immuno-Oncology. Dr. Baruch was supported by the Allen Berg Fund for Excellence in Immuno-Oncology Research. Dr. Greiling and Dr. Harris-Tryon reported having no relevant financial disclosures.
In the fall of 2020, Hassane M. Zarour, MD, and colleagues began to pore over raw data from their
Preclinical mouse studies have demonstrated that the gut microbiota could influence the response of tumors to anti–PD-1 immunotherapy, but FMT had not been previously evaluated in human patients with malignant melanoma whose disease persisted or progressed after medical therapy. Only 30%-40% of melanoma patients respond to anti–PD-1 immunotherapy, so the researchers’ sense of anticipation was palpable. “It’s a high-risk, high-reward study, so you never know,” Dr. Zarour, a dermatologist and immunologist who is coleader of the melanoma program at the University of Pittsburgh Medical Center’s Hillman Cancer Center, said in an interview.
For the study, which was funded by the National Institutes of Health and published in Science, Dr. Zarour and a team of colleagues, including Diwakar Davar, MD, a medical oncologist/hematologist at UPMC and Giorgio Trinchieri, MD, head of the cancer immunology section at the National Cancer Institute, enrolled 16 patients with advanced melanoma whose disease had persisted or progressed with anti-PD-1 drugs; donors were 7 patients with advanced melanoma who had responded to pembrolizumab, 4 with a complete response and 3 with a partial response, with a median progression-free survival of 56 months.
After donors and patients underwent serial stool sampling and studies to stamp out the potential for transmitting infectious agents, the researchers administered the donor-derived FMT to patients via colonoscopy every 14 days for 3 weeks, followed by pembrolizumab. To their delight, 6 of the 15 evaluable recipients responded to treatment, with a reduction in tumor or long-term disease stabilization. Moreover, responders also showed increased abundance of taxa that were previously associated with response to immunotherapy, increased activation of CD8+ T cells, and decreased frequency of interleukin-8–expressing myeloid cells.
“This opens new doors for the future,” Dr. Zarour said. “It’s very encouraging, but I don’t want to overstate the data. It’s a small, nonrandomized trial, but one has to keep in mind that people were skeptical about this work; they didn’t think FMT would work. Now we see many people coming into the field to investigate the role of the microbiome as a therapeutic tool, which is great.”
Teri Greiling, MD, characterized the finding as a key development in understanding the microbiome’s potential to influence the course of melanoma and other diseases. “What’s emerging over the last decade of research is that our immune system has a close, back-and-forth relationship with our microbiota,” said Dr. Greiling, associate professor of dermatology at Oregon Health & Science University, Portland. “From day 1 of birth, we’re colonized by microbes that train our immune system how to function. In response, your immune system keeps those microbes in check and shapes which ones are allowed to colonize, and which ones are a target for attack. Thus, inflammatory responses are generated. Similarly, the goal of immunotherapy is to activate the immune system to fight cancer. This study shows that the immune system continues to need the colonizing microbes in our body to function optimally.”
Immunotherapy with checkpoint inhibitors was not an option for malignant melanoma patients until 2011, she noted, so the potential for FMT to further improve outcomes is welcome news for patients and their families. “We went from a less than 5% chance of survival with metastatic melanoma to now, with the right combination of checkpoint inhibitors, we’re up over 50%, which is amazing in a decade,” Dr. Greiling said. “Still, we’re losing half of our patients. If [FMT provides] a 30% improvement over that, that would be great, but it’s hard to extrapolate from such small numbers.”
Positive results in an Israeli study
Results from a similar, smaller phase 1 trial of 2 FMT donors and 10 recipients with metastatic melanoma who had progressed on anti-PD-1 therapy, from the Ella Lemelbaum Institute for Immuno-Oncology at Sheba Medical Center in Tel HaShomer, Israel, yielded similar results. The FMT protocol in this study included colonoscopy and oral stool capsules, followed by the reintroduction of anti–PD-1 therapy with nivolumab. The two FMT donors had previously been treated with anti–PD-1 monotherapy for metastatic melanoma and had achieved a clinical response for at least 1 year. Of the 10 FMT recipients, 1 had a complete response and 2 had a partial response.
“We expected changes in the immune system but did not expect that 3 out of the 10 patients in our study would be turned from nonresponders to responders,” the study’s lead author, Erez N. Baruch, MD, PhD, told this news organization. “Since this was a first-in-human study, we were aiming to assess safety and not clinical responses. [We found] that microbiota modulation can change the immune infiltration within melanoma tumors and by this affect response to immunotherapy.”
Dr. Baruch, an internal medicine resident in the physician-scientist track program at the University of Texas, Houston, said that the findings create a potential new therapeutic paradigm, or a new “playing ground” for drug development that can support existing immunotherapies. “It is important for dermatologists to understand that disruptions of the gut microbiota, mainly by antibiotics, may be harmful to melanoma patients,” he said. “Antibiotics in cancer patients should be used judiciously but of course should not be avoided when there’s an indication.”
As for next steps, Dr. Zarour and colleagues are recruiting more patients to boost their sample size and conducting sequential analysis of the microbiome of study participants “to better determine what the good and bad bugs are,” he said. “There are so many variables, including diet and geography. We need more data.” The hope is to develop a “microbiome signature” to identify patients likely to respond to FMT, and maybe one day, a probiotic capsule that patients take to optimize their response to immunotherapy.
“We don’t want to say that the microbiome is responsible for everything, but it’s responsible for some of the response and some of the resistance to treatment,” Dr. Zarour said. “So, we want to identify what candidate nonresponders are more likely to respond to FMT and be able to stick the right stool in the donor. This goes to better education of the microbiome signature. We are working hard on that.”
Dr. Baruch added that performing FMT for melanoma patients requires tight collaborations between oncologists, dermatologists, GI, and infectious disease experts. “These usually can be done in the setting of large cancer centers and will probably not be available in any hospital,” he said. “This is why understanding the mechanisms and developing an FMT-like drug is important. We are focusing on studying the mechanisms behind the clinical effect in order to develop a drug with an FMT-like effect without the safety and logistic issues related to FMTs.”
Tamia A. Harris-Tryon, MD, PhD, whose lab at the University of Texas Southwestern Medical Center at Dallas is studying how diet and the microbiota impact skin immunity, underscored the importance of evaluating the characteristics of the diet of patients as trials of FMT in melanoma patients carry on. “We know that the diet impacts the repertoire of microbes that colonize the gut,” said Dr. Harris-Tryon, assistant professor in the department of dermatology at the medical center. “The diet of the recipient likely has an impact” on the success of donor FMT.
She also noted that other skin conditions have been linked to a disrupted gut microbiome, such as psoriasis. “Given the safety of FMT in both of these studies, trials of FMT in psoriasis and other systemic skin conditions should be considered,” she said.
According to Dr. Zarour, mounting data from separate studies show that some gut microbiota play a role in adverse events experienced by melanoma patients on immunotherapy. “That is very important, especially with combination therapy,” he said. “There are also microbes involved in resistance to treatment, so the idea would be to identify these microbes.”
Studies raise more questions
In the opinion of Dr. Greiling, results from these two studies raise more questions than they answer. “The big question ... is why and how does FMT work, and how can we make the response better?” she said. “Is there one particular gene product from one microbe that is the key magic ingredient, and we can harness this as a drug? More likely it’s a complex interplay between multiple bacterial species needed to direct the immune response. Is there a group of microbes that is the same from person to person, or is it more complex?”
Then there are pending regulatory concerns. “We know that FMT works for [Clostridioides] difficile colitis but it’s not officially [Food and Drug Administration] approved,” Dr. Greiling said. “The FDA is really struggling with how to approve or regulate using bacteria as a drug. Where is that crossover? That inhibits things moving forward, for good reason. You want to balance safety with live microbes.”
The UPMC clinical trial was supported by Merck. Dr. Zarour disclosed that he is supported by grants from the National Cancer Institute and the James W. and Frances G. McGlothlin Chair in Melanoma Immunotherapy Research at UPMC. The Israeli study was funded by the Ella Lemelbaum Institute for Immuno-Oncology. Dr. Baruch was supported by the Allen Berg Fund for Excellence in Immuno-Oncology Research. Dr. Greiling and Dr. Harris-Tryon reported having no relevant financial disclosures.
In the fall of 2020, Hassane M. Zarour, MD, and colleagues began to pore over raw data from their
Preclinical mouse studies have demonstrated that the gut microbiota could influence the response of tumors to anti–PD-1 immunotherapy, but FMT had not been previously evaluated in human patients with malignant melanoma whose disease persisted or progressed after medical therapy. Only 30%-40% of melanoma patients respond to anti–PD-1 immunotherapy, so the researchers’ sense of anticipation was palpable. “It’s a high-risk, high-reward study, so you never know,” Dr. Zarour, a dermatologist and immunologist who is coleader of the melanoma program at the University of Pittsburgh Medical Center’s Hillman Cancer Center, said in an interview.
For the study, which was funded by the National Institutes of Health and published in Science, Dr. Zarour and a team of colleagues, including Diwakar Davar, MD, a medical oncologist/hematologist at UPMC and Giorgio Trinchieri, MD, head of the cancer immunology section at the National Cancer Institute, enrolled 16 patients with advanced melanoma whose disease had persisted or progressed with anti-PD-1 drugs; donors were 7 patients with advanced melanoma who had responded to pembrolizumab, 4 with a complete response and 3 with a partial response, with a median progression-free survival of 56 months.
After donors and patients underwent serial stool sampling and studies to stamp out the potential for transmitting infectious agents, the researchers administered the donor-derived FMT to patients via colonoscopy every 14 days for 3 weeks, followed by pembrolizumab. To their delight, 6 of the 15 evaluable recipients responded to treatment, with a reduction in tumor or long-term disease stabilization. Moreover, responders also showed increased abundance of taxa that were previously associated with response to immunotherapy, increased activation of CD8+ T cells, and decreased frequency of interleukin-8–expressing myeloid cells.
“This opens new doors for the future,” Dr. Zarour said. “It’s very encouraging, but I don’t want to overstate the data. It’s a small, nonrandomized trial, but one has to keep in mind that people were skeptical about this work; they didn’t think FMT would work. Now we see many people coming into the field to investigate the role of the microbiome as a therapeutic tool, which is great.”
Teri Greiling, MD, characterized the finding as a key development in understanding the microbiome’s potential to influence the course of melanoma and other diseases. “What’s emerging over the last decade of research is that our immune system has a close, back-and-forth relationship with our microbiota,” said Dr. Greiling, associate professor of dermatology at Oregon Health & Science University, Portland. “From day 1 of birth, we’re colonized by microbes that train our immune system how to function. In response, your immune system keeps those microbes in check and shapes which ones are allowed to colonize, and which ones are a target for attack. Thus, inflammatory responses are generated. Similarly, the goal of immunotherapy is to activate the immune system to fight cancer. This study shows that the immune system continues to need the colonizing microbes in our body to function optimally.”
Immunotherapy with checkpoint inhibitors was not an option for malignant melanoma patients until 2011, she noted, so the potential for FMT to further improve outcomes is welcome news for patients and their families. “We went from a less than 5% chance of survival with metastatic melanoma to now, with the right combination of checkpoint inhibitors, we’re up over 50%, which is amazing in a decade,” Dr. Greiling said. “Still, we’re losing half of our patients. If [FMT provides] a 30% improvement over that, that would be great, but it’s hard to extrapolate from such small numbers.”
Positive results in an Israeli study
Results from a similar, smaller phase 1 trial of 2 FMT donors and 10 recipients with metastatic melanoma who had progressed on anti-PD-1 therapy, from the Ella Lemelbaum Institute for Immuno-Oncology at Sheba Medical Center in Tel HaShomer, Israel, yielded similar results. The FMT protocol in this study included colonoscopy and oral stool capsules, followed by the reintroduction of anti–PD-1 therapy with nivolumab. The two FMT donors had previously been treated with anti–PD-1 monotherapy for metastatic melanoma and had achieved a clinical response for at least 1 year. Of the 10 FMT recipients, 1 had a complete response and 2 had a partial response.
“We expected changes in the immune system but did not expect that 3 out of the 10 patients in our study would be turned from nonresponders to responders,” the study’s lead author, Erez N. Baruch, MD, PhD, told this news organization. “Since this was a first-in-human study, we were aiming to assess safety and not clinical responses. [We found] that microbiota modulation can change the immune infiltration within melanoma tumors and by this affect response to immunotherapy.”
Dr. Baruch, an internal medicine resident in the physician-scientist track program at the University of Texas, Houston, said that the findings create a potential new therapeutic paradigm, or a new “playing ground” for drug development that can support existing immunotherapies. “It is important for dermatologists to understand that disruptions of the gut microbiota, mainly by antibiotics, may be harmful to melanoma patients,” he said. “Antibiotics in cancer patients should be used judiciously but of course should not be avoided when there’s an indication.”
As for next steps, Dr. Zarour and colleagues are recruiting more patients to boost their sample size and conducting sequential analysis of the microbiome of study participants “to better determine what the good and bad bugs are,” he said. “There are so many variables, including diet and geography. We need more data.” The hope is to develop a “microbiome signature” to identify patients likely to respond to FMT, and maybe one day, a probiotic capsule that patients take to optimize their response to immunotherapy.
“We don’t want to say that the microbiome is responsible for everything, but it’s responsible for some of the response and some of the resistance to treatment,” Dr. Zarour said. “So, we want to identify what candidate nonresponders are more likely to respond to FMT and be able to stick the right stool in the donor. This goes to better education of the microbiome signature. We are working hard on that.”
Dr. Baruch added that performing FMT for melanoma patients requires tight collaborations between oncologists, dermatologists, GI, and infectious disease experts. “These usually can be done in the setting of large cancer centers and will probably not be available in any hospital,” he said. “This is why understanding the mechanisms and developing an FMT-like drug is important. We are focusing on studying the mechanisms behind the clinical effect in order to develop a drug with an FMT-like effect without the safety and logistic issues related to FMTs.”
Tamia A. Harris-Tryon, MD, PhD, whose lab at the University of Texas Southwestern Medical Center at Dallas is studying how diet and the microbiota impact skin immunity, underscored the importance of evaluating the characteristics of the diet of patients as trials of FMT in melanoma patients carry on. “We know that the diet impacts the repertoire of microbes that colonize the gut,” said Dr. Harris-Tryon, assistant professor in the department of dermatology at the medical center. “The diet of the recipient likely has an impact” on the success of donor FMT.
She also noted that other skin conditions have been linked to a disrupted gut microbiome, such as psoriasis. “Given the safety of FMT in both of these studies, trials of FMT in psoriasis and other systemic skin conditions should be considered,” she said.
According to Dr. Zarour, mounting data from separate studies show that some gut microbiota play a role in adverse events experienced by melanoma patients on immunotherapy. “That is very important, especially with combination therapy,” he said. “There are also microbes involved in resistance to treatment, so the idea would be to identify these microbes.”
Studies raise more questions
In the opinion of Dr. Greiling, results from these two studies raise more questions than they answer. “The big question ... is why and how does FMT work, and how can we make the response better?” she said. “Is there one particular gene product from one microbe that is the key magic ingredient, and we can harness this as a drug? More likely it’s a complex interplay between multiple bacterial species needed to direct the immune response. Is there a group of microbes that is the same from person to person, or is it more complex?”
Then there are pending regulatory concerns. “We know that FMT works for [Clostridioides] difficile colitis but it’s not officially [Food and Drug Administration] approved,” Dr. Greiling said. “The FDA is really struggling with how to approve or regulate using bacteria as a drug. Where is that crossover? That inhibits things moving forward, for good reason. You want to balance safety with live microbes.”
The UPMC clinical trial was supported by Merck. Dr. Zarour disclosed that he is supported by grants from the National Cancer Institute and the James W. and Frances G. McGlothlin Chair in Melanoma Immunotherapy Research at UPMC. The Israeli study was funded by the Ella Lemelbaum Institute for Immuno-Oncology. Dr. Baruch was supported by the Allen Berg Fund for Excellence in Immuno-Oncology Research. Dr. Greiling and Dr. Harris-Tryon reported having no relevant financial disclosures.
CrossFit enters primary care with fitness-minded docs, data
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
with the launch of its newest service, CrossFit Precision Care.
Developed by family medicine physician Julie Foucher, MD, and other CrossFit-trained doctors, the new service aims to help CrossFit members build plans to protect and improve their health, according to a statement by the company.
CrossFit Precision Care plans to meet this goal through utilizing doctors who understand the CrossFit philosophy, individualized care, data-driven recommendations, proactive lifestyle changes, and continual health optimization. Informing these plans and changes are CrossFit Precision Care’s analysis through a few different methods.
CrossFit’s partner in the endeavor, Wild Health, will provide genomic testing to determine a patient’s genetic predispositions to help optimize the health plans. Blood testing reveals many things that may affect a person’s health, such as hormone status, lipid levels, thyroid function, and cardiovascular risks. An overall lifestyle review includes exercise routines, eating habits, social life, and other patterns or behaviors.
Connecting with doctors who understand CrossFit
Dr. Foucher is no stranger to CrossFit. She has competed in the CrossFit Games four times and discusses the sport regularly on Twitter and Instagram. Now, she works directly with CrossFit to help it provide users with individualized data-driven plans.
“I met Eric Roza last July,” Dr. Foucher says of CrossFit’s CEO. “We talked and saw a lot of potential for CrossFit and health care providers to work together, so we started brainstorming.”
When Dr. Foucher and Mr. Roza got to know Wild Health, specifically, two of its physician cofounders, it was a natural fit, she said. Dr. Foucher says that many who train in CrossFit or go to CrossFit-affiliated gyms feel a disconnect with their family doctors: “[CrossFit is] a pretty polarizing topic, but there are also a lot of doctors who know that people are having health improvements with these programs,” she said.
Through use of Wild Health’s precision services and algorithms, CrossFit Precision Care plans to connect its users with CrossFit-trained health care practitioners. This personalized approach allows health care practitioners to build closer relationships with users of the program, who may feel more comfortable working with doctors who understand their lifestyle. Wild Health’s precision medicine approach, with trackable data such as biomarker status and risk scores, gives doctors a more complete picture of a patient’s needs and history, according to a statement on the partnership.
A better use of data
“To me,” Dr. Foucher says of family medicine, “that was the best option coming out of residency. It was consistent with my morals.” She says much of the current health care system is algorithm based. If a patient is experiencing certain symptoms, treatment is recommended on the basis of whatever yields the best results from the data – but this doesn’t always factor in a patient’s full history and genetics. It can be difficult for doctors to build trusting and personal relationships with patients. “In our current system, there’s not a lot of time or great tools to do that,” she says.
With the approach Wild Health and CrossFit Precision Care both use, however, Dr. Foucher says she sees a huge opportunity for optimizing patient and health care practitioner relationships.
“I see huge potential here, and I really think that this should be the standard for primary care going forwards,” Dr. Foucher explains. “The nice thing about [this approach] is that it has a really quick learning curve and is relatively easy to implement with patients. Before Wild Health optimized it, the tech and data would take about 10 hours per patient to put together. But now, we can incorporate things that work with wearable tech and track results over time and allow the patient and doctor to use this platform to create relationships. And this is something that can scale to many more patients.”
According to its website, CrossFit Precision Care is currently launching an invite-only beta test version of the program in eight states ahead of an expected national release.
A version of this article first appeared on Medscape.com.
Resident physician work-hour regulations associated with improved physician safety and health
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Background: In 2011, the Accreditation Council for Graduate Medical Education (ACGME) enacted a consecutive work-hour restriction of 16 hours for first-year residents. Reports of these changes have focused on patient safety, resident education, and resident well-being. The impact on resident safety had not been addressed.
Study design: Prospective cohort study.
Setting: U.S. Academic institutions training resident physicians.
Synopsis: This study compared first-year resident physicians from 2002 to 2007 (pre-implementation) and 2014 to 2017 (post-implementation). In all, 5,680 pre-implementation residents and 9,596 post-implementation residents consented to the study. With the 2011 ACGME restriction, the risk of motor vehicle crash decreased 24% (relative risk [RR] .76; .67-.85), and percutaneous injury risk decreased more than 40% (RR .54; .48-.61). Although weekly work hours were significantly higher pre-implementation, self-reported hours involved in patient care were similar for both groups.
While this large, well-powered study suggests extended work-hour restrictions for resident physicians improve their safety, the study is limited by self-reporting of resident physicians. As the ACGME has re-introduced extended duration shifts for first-year resident physicians, hospitalists should advocate for objective physician safety studies in relation to extended-hour shifts.
Bottom line: The 2011 ACGME work-hour reform for first-year physicians improved their safety and health.
Citation: Weaver MD et al. The association between resident physician work-hour regulations and physician safety and health. Am J Med. 2020 July;133(7):e343-54.
Dr. Fletcher is a hospitalist at the Lexington (Ky.) VA Health Care System.
Product News October 2021
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Opzelura FDA Approved for Atopic Dermatitis Incyte
Corporation announces US Food and Drug Administration (FDA) approval of Opzelura (ruxolitinib) cream 1.5% for the short-term and noncontinuous chronic treatment of mild to moderate atopic dermatitis (AD) in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Opzelura is formulated with ruxolitinib, a selective Janus kinase (JAK) 1/JAK2 inhibitor, to target key cytokine signals believed to contribute to itch and inflammation. For more information, visit www.opzelurahcp.com/.
Twyneo FDA Approved for Acne Vulgaris
Sol-Gel Technologies, Ltd, announces US Food and Drug Administration (FDA) approval of Twyneo (tretinoin 0.1% /benzoyl peroxide 3%) cream for the treatment of acne vulgaris in adult and pediatric patients 9 years and older. Tretinoin and benzoyl peroxide are widely prescribed separately for acne vulgaris; however, benzoyl peroxide causes degradation of the tretinoin molecule, thereby potentially reducing its effectiveness if used at the same time or combined in the same formulation. The formulation of Twyneo uses silica (silicon dioxide) core shell structures to separately microencapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the 2 active ingredients in the cream. For more information, visit www.sol-gel.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Synthetic chemical in consumer products linked to early death, study says
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Community mourns nurse knocked over, killed in Times Square
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
Comparison of Adverse Events With Vancomycin Diluted in Normal Saline vs Dextrose 5%
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Vancomycin is a widely used IV antibiotic due to its broad-spectrum of activity, bactericidal nature, and low rates of resistance; however, adverse effects (AEs), including nephrotoxicity, are commonly associated with its use.1 The vancomycin therapeutic monitoring guidelines recognize the incidence of nephrotoxicity and suggest strategies for reducing the risk, including area under the curve/mean inhibitory concentration (AUC/MIC) monitoring rather than trough-only monitoring. Vancomycin-associated acute kidney injury (AKI) has been defined as an increase in serum creatinine (SCr) over a 48-hour period of ≥ 0.3 mg/dL or a percentage increase of ≥ 50%, which is consistent with the Acute Kidney Injury Network (AKIN) guidelines.2,3 Vancomycin-associated AKI is a common AE, with its incidence reported in previous studies ranging from 10 to 20%.4,5
The most common crystalloid fluid administered to patients in the United States is 0.9% sodium chloride (NaCl), also known as normal saline (NS), and recent trials have explored its potential to cause AEs.6-8 Balanced crystalloid solutions, such as Plasma-Lyte and lactated Ringer’s solution (LR), contain buffering agents and lower concentrations of sodium and chloride compared with that of NS. Trials in the intensive care unit (ICU) and emergency department, such as the SMART-MED, SMART-SURG, and SALT-ED have reported a significantly lower rate of AKI when using balanced crystalloids compared with NS due to the concentration of sodium and chloride in NS being supraphysiologic to normal serum concentrations.6,7 Alternatively, the SPLIT trial evaluated the use of NS compared with Plasma-Lyte for ICU fluid therapy and did not find a statistically significant difference in AKI.8 Furthermore, some studies have reported increased risk for hyperchloremia when using NS compared with dextrose 5% in water (D5W) or balanced crystalloids, which can result in metabolic acidosis.6,7,9,10 These studies have shown how the choice of fluid can have a large effect on the incidence of AEs; bringing into question whether these effects could be additive when combined with the nephrotoxicity associated with vancomycin.6-9
Vancomycin is physically and chemically stable if diluted in D5W, NS, 5% dextrose in NS, LR, or 5% dextrose in LR.1 It is not known whether the selection of diluent has an effect on nephrotoxicity or other AEs of vancomycin therapy. Furthermore, clinicians may be unaware or unable to specify which diluent to use. There are currently no practice guidelines that favor one diluent over another for vancomycin; however, trials showing higher rates of AKI and hyperchloremia using NS for fluid resuscitation may indicate an increased potential for vancomycin-associated AKI when using NS as a diluent.6,7,9 This study was performed to evaluate whether the type of crystalloid used (D5W vs NS) can influence adverse outcomes for patients. While many factors may contribute to these AEs, the potential to reduce the risk of negative adverse outcomes for hospitalized patients is a significant area of exploration.
The primary outcome of this study was the incidence of AKI, defined using AKIN guidelines where the increase in SCr occurred at least 24 hours after starting vancomycin and within 36 hours of receiving the last vancomycin dose.3 AKI was staged using the AKIN guidelines (stage 1: increase in SCr of ≥ 0.3 mg/dL or by 50 to 99%; stage 2: increase in SCr by 100 to 199%; stage 3: increase in SCr by > 200%) based on changes in SCr from baseline during vancomycin therapy or within 36 hours of stopping vancomycin therapy.3 Secondary outcomes included the incidence of hyperglycemia, hyperchloremia, metabolic acidosis, hypernatremia, mortality in hospital, and mortality within 30 days from hospital discharge.
Methods
This single-center, retrospective study of veterans who received IV vancomycin within the North Florida/South Georgia Veterans Health System (NF/SGVHS) in Gainesville, Florida, from July 1, 2015 to June 30, 2020, compared veterans who received vancomycin diluted in NS with those who received vancomycin diluted in D5W to assess for differences in AEs, including AKI, metabolic acidosis (serum bicarbonate level < 23 mmol/L), hyperchloremia (serum chloride levels > 108 mmol/L), hypernatremia (serum sodium > 145 mmol/L), and hyperglycemia (blood glucose > 180 mg/dL). The endpoint values were defined using the reference ranges determined by the local laboratory. At NF/SGVHS, vancomycin is diluted in D5W or NS based primarily on factors such as product availability and cost.
Study Criteria
Veterans were included if they received IV vancomycin between July 1, 2015 and June 30, 2020. The cohorts were grouped into those receiving vancomycin doses diluted in NS and those receiving vancomycin doses diluted in D5W. Veterans were excluded if they received < 80% of vancomycin doses diluted in their respective fluid, if they were on vancomycin for < 48 hours, or if they did not have laboratory results collected both before and after vancomycin therapy to assess a change. There were more patients receiving vancomycin in D5W, so a random sample was selected to have an equal size comparison group with those receiving NS. A sample size calculation was performed with an anticipated AKI incidence of 14%.5 To detect a 10% difference in the primary outcome with an α of 0.05 and 75% power, 226 patients (113 in each cohort) were needed for inclusion.
Data were collected using the Data Access Request Tracker tool through the US Department of Veterans Affairs (VA) Informatics and Computing Infrastructure. Data collected included demographics, laboratory data at baseline and during vancomycin therapy, characteristics of antibiotic therapy, and mortality data. Of note, all laboratory values assessed in this study were obtained while the veteran was receiving vancomycin or within 36 hours of receiving the last vancomycin dose to appropriately assess any changes.
Statistical analysis of categorical data were analyzed using a χ2 test on the GraphPad online program. This study received institutional review board approval from the University of Florida and was conducted in accordance with protections for human subjects.
Results
A total of 792 veterans received IV vancomycin NF/SGVHS in the defined study period. Of these, 381 veterans were excluded, including having < 80% of doses in a single solution (213 veterans), receiving IV vancomycin for < 48 hours (149 veterans), and not having necessary laboratory data available to assess a change in kidney function (19 veterans). An additional 165 veterans were randomly excluded from the D5W cohort in order to have an equal comparison group to the NS cohort; therefore, a total of 246 veterans were included in the final assessment (123 veterans in each cohort). The median patient age was 73 years (IQR, 68.0, 80.5) in the D5W group and 66 years (IQR, 60.0, 74.0) in the NS group; 83.7% of veterans in the D5W group and 74% veterans in the NS group were white; 94.3% of the D5W group and 100% of the NS group were male (Table 1).
The percentage of AKI in the D5W group was 22.8% compared with 14.6% in the NS group (P = .14), and all cases were classified as stage 1 AKI. Baseline cases of hyperglycemia, hypernatremia, hyperchloremia, or metabolic acidosis were not included in the reported rates of each in order to determine a change during vancomycin therapy (Table 2).
The percentage of patients with hyperglycemia in the D5W group was 32.5% compared with 39.8% in the NS group (P = .29). The percentage of patients with hypernatremia in the D5W group was 15.4% compared with 10.6% in the NS group (P = .34). The percentage of patients with hyperchloremia in the D5W group was 22.8% compared with 17.9% in the NS group (P = .43). The percentage of patients with metabolic acidosis in the D5W group was 48.0% compared with 49.6% in the NS group (P = .90).
There were no significant differences in either in-hospital or posthospital mortality between the D5W and NS groups (in-hospital: 4.9% vs 5.7%, respectively; P = .78; 30-day posthospitalization: 8.5% vs 4.5%, respectively; P = .30).
Discussion
This retrospective cohort study comparing the AEs of vancomycin diluted in NS and vancomycin diluted with D5W showed no statistically significant differences in the incidence of AKI or any metabolic AEs. Although these results did not show an association between the incidence of AEs and the dilution fluid for vancomycin, other factors may contribute to the overall incidence of AEs. Factors such as cumulative vancomycin dose, duration of therapy, and presence of concomitant nephrotoxins have been known to increase the incidence of AKI and may have a greater impact on this incidence than the fluid used in administering the vancomycin.
These results specifically the incidence of AKI were not consistent with previous trials evaluating the AEs of NS. Based on previous trials, we expected the vancomycin in the NS cohort to have a significantly higher incidence of hypernatremia, hyperchloremia, and AKI. Our results may indicate that the volume of crystalloid received played a greater role on the incidence of AEs. Our study assessed the effect of a diluent for one IV medication that may have been only a few hundred milliliters of fluid per day. The total volume of IV fluid received from vancomycin was not assessed; thus, it is not known how the volume of fluid may have impacted the results.
One consideration with this study is the method used for monitoring vancomycin levels. Most of the patients included in this study were admitted prior to the release of the updated vancomycin guidelines, which advocated for the transition from traditional trough-only monitoring to AUC/MIC. In September 2019, NF/SGVHS ICUs made the transition to this new method of monitoring with a hospital-wide transition following the study end date. The D5W group had a slightly higher percentage of patients admitted to the ICU, thus were more likely to be monitored using AUC/MIC during this period. Literature has shown the AUC/MIC method of monitoring can result in a decreased daily dose, decreased trough levels, and decreased incidence of nephrotoxicity.11-14 Although the method for monitoring vancomycin has the potential to affect the incidence of AKI, the majority of patients were monitored using the traditional trough-only method with similar trough levels reported in both groups.
Limitations
This study is limited by its retrospective nature, the potential introduction of biases, and the inability to control for confounders that may have influenced the incidence of AEs. Potential confounders present in this study included the use of concomitant nephrotoxic medications, vancomycin dose, and underlying conditions, as these could have impacted the overall incidence of AEs.
The combination of piperacillin/tazobactam plus vancomycin has commonly been associated with an increased risk of nephrotoxicity. Previous studies have identified this nephrotoxic combination to have a significantly increased risk of AKI compared with vancomycin alone or when used in combination with alternative antibiotics such as cefepime or meropenem.15,16 In our study, there was a higher percentage of patients in the NS group with concomitant piperacillin/tazobactam, so this difference between the groups may have influenced the incidence of AKI. Nephrotoxic medications other than antibiotics were not assessed in this study; however, these also could have impacted our results significantly. While the vancomycin duration of therapy and highest trough levels were similar between groups, the NS group had a larger average daily dose and overall cumulative dose. Studies have identified the risk of nephrotoxicity increases with a vancomycin daily dose of 4 g, troughs > 15 mg/mL, and a duration of therapy > 7 days.15,16 In our study, the daily doses in both groups were < 4 g, so it is likely the average daily vancomycin dose had little impact on the incidence of AKI.
Another potential confounder identified was assessment of underlying conditions in the patients. Due to the limitations associated with the data extraction method, we could not assess for underlying conditions that may have impacted the results. Notably, the potential nephrotoxicity of NS has mostly been shown in critically ill patients. Therefore, the mixed acutely ill patient sample in this study may have been less likely to develop AKI from NS compared with an exclusively critically ill patient sample.
Selection bias and information bias are common with observational studies. In our study, selection bias may have been present since prospective randomization of patient samples was not possible. Since all data were extracted from the medical health record, information bias may have been present with the potential to impact the results. Due to the single-center nature of this study with a predominantly older, white male veteran patient sample, generalizability to other patient populations may be limited. We would expect the results of this study to be similar among other patient populations of a similar age and demographic; however, the external validity of this study may be weak among other populations. Although this study included enough patients based on sample size estimate, a larger sample size could have allowed for detection of smaller differences between groups and decreased the chance for type II error.
Conclusions
Overall, the results of this study do not suggest that the crystalloid used to dilute IV vancomycin is associated with differences in nephrotoxicity or other relevant AEs. Future studies evaluating the potential for AEs from medication diluent are warranted and would benefit from a prospective, randomized design. Further studies are both necessary and crucial for enhancing the quality of care to minimize the rates of AEs of commonly used medications.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
1. Vancomycin hydrochloride intravenous injection, pharmacy bulk package. Package insert. Schaumburg, IL: APP Pharmaceuticals, LLC; 2011.
2. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-System Pharm. 2020;77(11):835-864. doi:10.1093/ajhp/zxaa036
3. Mehta RL, Kellum JA, Shah SV, et al; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
4. Elaysi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations–a literature review. Eur J Clin Pharmacol. 2012;68(9):1243-1255. doi:10.1007/s00228-012-1259-9
5. Gyamlani G, Potukuchi PK, Thomas F, et al. Vancomycin-associated acute kidney injury in a large veteran population. Am J Nephrol. 2019;49(2):133-142. doi:10.1159/000496484
6. Semler MW, Self WH, Wanderer JB, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in critically ill adults. N Engl Med. 2018;378(9):829-839. doi:10.1056/NEJMoa1711584
7. Self WH, Semler MW, Wanderer JP, et al; SMART Investigators and the Pragmatic Critical Care Research Group. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(20):819-828. doi:10.1056/NEJMc1804294
8. Young P, Bailey M, Beasley R, et al; SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA. 2015;314(16):1701-1710. doi:10.1001/jama.2015.12334
9. Magee CA, Bastin ML, Bastin T, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. doi:10.1097/CCM.0000000000003191
10. Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal. 2014;2014:627673. doi:10.1155/2014/627673
11. Mcgrady KA, Benton M, Tart S, Bowers R. Evaluation of traditional vancomycin dosing versus utilizing an electronic AUC/MIC dosing program. Pharm Pract (Granada). 2020;18(3):2024. doi:10.18549/PharmPract.2020.3.2024
12. Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented staphylococcal infections. Ther Drug Monit. 2019;41(4):483-488. doi:10.1097/FTD.0000000000000622
13. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. doi:10.1128/AAC.02042-17
14. Aljefri DM, Avedissian SN, Youn G, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881-1887. doi:10.1128/AAC.02042-17
15. Molina KC, Barletta JF, Hall ST, Yazdani C, Huang V. The risk of acute kidney injury in critically ill patients receiving concomitant vancomycin with piperacillin-tazobactam or cefepime. J Intensive Care Med. 2019;35(12):1434-1438. doi:10.1177/0885066619828290
16. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014; 34(7):670-676. doi:10.1002/phar.1442
Enhancing Access to Yoga for Older Male Veterans After Cancer: Examining Beliefs About Yoga
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
Yoga is an effective clinical intervention for cancer survivors. Studies indicate a wide range of benefits, including improvements in physical functioning, emotional well-being and overall quality of life.1-7 Two-thirds of National Cancer Institute designated comprehensive cancer centers offer yoga on-site.8 Yoga is endorsed by the National Comprehensive Cancer Network and American Society of Clinical Oncology for managing symptoms, such as cancer-related anxiety and depression and for improving overall quality of life.9,10
Although the positive effects of yoga on cancer patients are well studied, most published research in this area reports on predominantly middle-aged women with breast cancer.11,12 Less is known about the use of yoga in other groups of cancer patients, such as older adults, veterans, and those from diverse racial or ethnic backgrounds. This gap in the literature is concerning considering that the majority of cancer survivors are aged 60 years or older, and veterans face unique risk factors for cancer associated with herbicide exposure (eg, Agent Orange) and other military-related noxious exposures.13,14 Older cancer survivors may have more difficulty recovering from treatment-related adverse effects, making it especially important to target recovery efforts to older adults.15 Yoga can be adapted for older cancer survivors with age-related comorbidities, similar to adaptations made for older adults who are not cancer survivors but require accommodations for physical limitations.16-20 Similarly, yoga programs targeted to racially diverse cancer survivors are associated with improved mood and well-being in racially diverse cancer survivors, but studies suggest community engagement and cultural adaptation may be important to address the needs of culturally diverse cancer survivors.21-23
Yoga has been increasingly studied within the Veterans Health Administration (VHA) for treatment of posttraumatic stress disorder (PTSD) and has been found effective in reducing symptoms through the use of trauma-informed and military-relevant instruction as well as a military veteran yoga teacher.24-26 This work has not targeted older veterans or cancer survivors who may be more difficult to recruit into such programs, but who would nevertheless benefit.
Clinically, the VHA whole health model is providing increased opportunities for veterans to engage in holistic care including yoga.27 Resources include in-person yoga classes (varies by facility), videos, and handouts with practices uniquely designed for veterans or wounded warriors. As clinicians increasingly refer veterans to these programs, it will be important to develop strategies to engage older veterans in these services.
One important strategy to enhancing access to yoga for older veterans is to consider beliefs about yoga. Beliefs about yoga or general expectations about the outcomes of yoga may be critical to consider in expanding access to yoga in underrepresented groups. Beliefs about yoga may include beliefs about yoga improving health, yoga being difficult or producing discomfort, and yoga involving specific social norms.28 For example, confidence in one’s ability to perform yoga despite discomfort predicted class attendance and practice in a sample of 32 breast cancer survivors.29 Relatedly, positive beliefs about the impact of yoga on health were associated with improvements in mood and quality of life in a sample of 66 cancer survivors.30
The aim of this study was to examine avenues to enhance access to yoga for older veterans, including those from diverse backgrounds, with a focus on the role of beliefs. In the first study we investigate the association between beliefs about and barriers to yoga in a group of older cancer survivors, and we consider the role of demographic and clinical variables in such beliefs and how education may alter beliefs. In alignment with the whole health model of holistic health, we posit that yoga educational materials and resources may contribute to yoga beliefs and work to decrease these barriers. We apply these findings in a second study that enrolled older veterans in yoga and examining the impact of program participation on beliefs and the role of beliefs in program outcomes. In the discussion we return to consider how to increase access to yoga to older veterans based on these findings.
Methods
Study 1 participants were identified from VHA tumor registries. Eligible patients had head and neck, esophageal, gastric, or colorectal cancers and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed a face-to-face semistructured interview at 6, 12, and 18 months after their cancer diagnosis with a trained interviewer. Complete protocol methods, including nonresponder information, are described elsewhere.31
Questions about yoga were asked at the 12 month postdiagnosis interview. Participants were read the following: “Here is a list of services some patients use to recover from cancer. Please tell me if you have used any of these.” The list included yoga, physical therapy, occupational therapy, exercise, meditation, or massage therapy. Next participants were provided education about yoga via the following description: “Yoga is a practice of stress reduction and exercise with stretching, holding positions and deep breathing. For some, it may improve your sleep, energy, flexibility, anxiety, and pain. The postures are done standing, sitting, or lying down. If needed, it can be done all from a chair.” We then asked whether they would attend if yoga was offered at the VHA hospital (yes, no, maybe). Participants provided brief responses to 2 open-ended questions: (“If I came to a yoga class, I …”; and “Is there anything that might make you more likely to come to a yoga class?”) Responses were transcribed verbatim and entered into a database for qualitative analysis. Subsequently, participants completed standardized measures of health-related quality of life and beliefs about yoga as described below.
Study 2 participants were identified from VHA tumor registries and a cancer support group. Eligible patients had a diagnosis of cancer (any type except basil cell carcinoma) within the previous 3 years and were excluded if they were in hospice care, had dementia, or had a psychotic spectrum disorder. Participants completed face-to-face semistructured interviews with a trained interviewer before and after participation in an 8-week yoga group that met twice per week. Complete protocol methods are described elsewhere.16 This paper focuses on 28 of the 37 enrolled patients for whom we have complete pre- and postclass interview data. We previously reported on adaptations made to yoga in our pilot group of 14 individuals, who in this small sample did not show statistically significant changes in their quality of life from before to after the class.16 This analysis includes those 14 individuals and 14 who participated in additional classes, focusing on beliefs, which were not previously reported.
Measures
Participants reported their age, gender, ethnicity (Hispanic/Latino or not), race, and level of education. Information about the cancer diagnosis, American Joint Committee on Cancer (AJCC) cancer stage, and treatments was obtained from the medical record. The Physical Function and Anxiety Subscales from the Patient-Reported Outcomes Measurement Information System were used to measure health-related quality of life (HRQoL).32-34 Items are rated on a Likert scale from 1 (not at all) to 5 (very much).
The Beliefs About Yoga Scale (BAYS) was used to measure beliefs about the outcomes of engaging in yoga.28 The 11-item scale has 3 factors: expected health benefits (5 items), expected discomfort (3 items), and expected social norms (3 items). Items from the expected discomfort and expected social norms are reverse scored so that a higher score indicates more positive beliefs. To reduce participant burden, in study 1 we selected 1 item from each factor with high factor loadings in the original cross-validation sample.28 It would improve my overall health (Benefit, factor loading = .89); I would have to be more flexible to take a class (Discomfort, factor loading = .67); I would be embarrassed in a class (Social norms, factor loading = .75). Participants in study 2 completed the entire 11-item scale. Items were summed to create subscales and total scales.
Analysis
Descriptive statistics were used in study 1 to characterize participants’ yoga experience and interest. Changes in interest pre- and posteducation were evaluated with χ2 comparison of distribution. The association of beliefs about yoga with 3 levels of interest (yes, no, maybe) was evaluated through analysis of variance (ANOVA) comparing the mean score on the summed BAYS items among the 3 groups. The association of demographic (age, education, race) and clinical factors (AJCC stage, physical function) with BAYS was determined through multivariate linear regression.
For analytic purposes, due to small subgroup sample sizes we compared those who identified as non-Hispanic White adults to those who identified as African American/Hispanic/other persons. To further evaluate the relationship of age to yoga beliefs, we examined beliefs about yoga in 3 age groups (40-59 years [n = 24]; 60-69 years [n = 58]; 70-89 years [n = 28]) using ANOVA comparing the mean score on the summed BAYS items among the 3 groups. In study 2, changes in interest before and after the yoga program were evaluated with paired t tests and repeated ANOVA, with beliefs about yoga prior to class as a covariate. The association of demographic and clinical factors with BAYS was determined as in the first sample through multivariate linear regression, except the variable of race was not included due to small sample size (ie, only 3 individuals identified as persons of color).
Thematic analysis in which content-related codes were developed and subsequently grouped together was applied to the data of 110 participants who responded to the open-ended survey questions in study 1 to further illuminate responses to closed-ended questions.35 Transcribed responses to the open-ended questions were transferred to a spreadsheet. An initial code book with code names, definitions, and examples was developed based on an inductive method by one team member (EA).35 Initially, coding and tabulation were conducted separately for each question but it was noted that content extended across response prompts (eg, responses to question 2 “What might make you more likely to come?” were spontaneously provided when answering question 1), thus coding was collapsed across questions. Next, 2 team members (EA, KD) coded the same responses, meeting weekly to discuss discrepancies. The code book was revised following each meeting to reflect refinements in code names and definitions, adding newly generated codes as needed. The process continued until consensus and data saturation was obtained, with 90% intercoder agreement. Next, these codes were subjected to thematic analysis by 2 team members (EA, KD) combining codes into 6 overarching themes. The entire team reviewed the codes and identified 2 supra themes: positive beliefs or facilitators and negative beliefs or barriers.
Consistent with the concept of reflexivity in qualitative research, we acknowledge the influence of the research team members on the qualitative process.36 The primary coding team (EA, KD) are both researchers and employees of Veterans Affairs Boston Healthcare System who have participated in other research projects involving veterans and qualitative analyses but are not yoga instructors or yoga researchers.
Results
Study 1
The sample of 110 military veterans was mostly male (99.1%) with a mean (SD) age of 64.9 (9.4) years (range, 41-88)(Table 1). The majority (70.9%) described their race/ethnicity as White, non-Hispanic followed by Black/African American (18.2%) and Hispanic (8.2%) persons; 50.0% had no more than a high school education. The most common cancer diagnoses were colorectal (50.9%), head and neck (39.1%), and esophageal and gastric (10.0%) and ranged from AJCC stages I to IV.
When first asked, the majority of participants (78.2%) reported that they were not interested in yoga, 16.4% reported they might be interested, and 5.5% reported they had tried a yoga class since their cancer diagnosis. In contrast, 40.9% used exercise, 32.7% used meditation, 14.5% used physical or occupational therapy, and 11.8% used massage therapy since their cancer diagnosis.
After participants were provided the brief scripted education about yoga, the level of interest shifted: 46.4% not interested, 21.8% interested, and 31.8% definitely interested, demonstrating a statistically significant shift in interest following education (χ2 = 22.25, P < .001) (Figure 1). Those with the most positive beliefs about yoga were most likely to indicate interest. Using the BAYS 3-item survey, the mean (SD) for the definitely interested, might be interested, and not interested groups was 15.1 (3.2), 14.1 (3.2), and 12.3 (2.5), respectively (F = 10.63, P < .001).
A multivariable regression was run to examine possible associations between participants’ demographic characteristics, clinical characteristics, and beliefs about yoga as measured by the 3 BAYS items (Table 2). Higher expected health benefits of yoga was associated with identifying as
Six themes were identified in qualitative analysis of semistructured interviews reflecting older veterans’ beliefs about yoga, which were grouped into the following suprathemes of positive vs negative beliefs (Figure 2). Exemplar responses appear in Table 3.
Study 2 Intervention Sample
This sample of 28 veterans was mostly male (96.4%) with a mean (SD) age of 69.2 (10.9) years (range, 57-87). The majority (89.3%) described their race as White, followed by Black/African American (10.7%); no participants self-identified in other categories for race/ethnicity. Twelve veterans (42.9%) had no more than a high school education. The most common cancer diagnosis was genitourinary (35.7%) and the AJCC stage ranged from I to IV.
We employed information learned in study 1 to enhance access in study 2. We mailed letters to 278 veterans diagnosed with cancer in the previous 3 years that provided education about yoga based on study 1 findings. Of 207 veterans reached by phone, 133 (64%) stated they were not interested in coming to a yoga class; 74 (36%) were interested, but 30 felt they were unable to attend due to obstacles such as illness or travel. Ultimately 37 (18%) veterans agreed and consented to the class, and 28 (14%) completed postclass surveys.
In multivariate regression, higher expected health benefits of yoga were associated with higher physical function, lower concern about expected discomfort was also associated with higher physical function as well as higher education; similarly, lower concern about expected social norms was associated with higher physical function. Age was not associated with any of the BAYS factors.
Beliefs about yoga improved from before to after class for all 3 domains with greater expected benefit and lower concerns about discomfort or social norms:
Discussion
Yoga is an effective clinical intervention for addressing some long-term adverse effects in cancer survivors, although the body of research focuses predominantly on middle aged, female, White, college-educated breast cancer survivors. There is no evidence to suggest yoga would be less effective in other groups, but it has not been extensively studied in survivors from diverse subgroups. Beliefs about yoga are a factor that may enhance interest in yoga interventions and research, and measures aimed at addressing potential beliefs and fears may capture information that can be used to support older cancer survivors in holistic health. The aims of this study were to examine beliefs about yoga in 2 samples of older cancer survivors who received VHA care. The main findings are (1) interest in yoga was initially low and lower than that of other complementary or exercise-based interventions, but increased when participants were provided brief education about yoga; (2) interest in yoga was associated with beliefs about yoga with qualitative comments illuminating these beliefs; (3) demographic characteristics (education, race) and physical function were associated with beliefs about yoga; and (4) positive beliefs about yoga increased following a brief yoga intervention and was associated with improvements in physical function.
Willingness to consider a class appeared to shift for some older veterans when they were presented brief information about yoga that explained what is involved, how it might help, and that it could be done from a chair if needed. These findings clearly indicated that when trying to enhance participation in yoga in clinical or research programs, it will be important that recruitment materials provide such information. This finding is consistent with the qualitative findings that reflected a lack of knowledge or skepticism about benefits of yoga among some participants. Given the finding that physical function was associated with beliefs about yoga and was also a prominent theme in qualitative analyses,
Age was not associated with beliefs about yoga in either study. Importantly, in a more detailed study 1 follow-up analysis, beliefs about yoga were equivalent for aged > 70 years compared with those aged 40 to 69 years. It is not entirely clear why older adults have been underrepresented in studies of yoga in cancer survivors. However, older adults are vastly underrepresented in clinical trials for many health conditions, even though they are more likely to experience many diseases, including cancer.37 A new National Institutes of Health policy requires that individuals of all ages, including older adults, must be included in all human subjects research unless there are scientific reasons not to include them.38 It is therefore imperative to consider strategies to address underrepresentation of older adults.
Qualitative findings here suggest it will be important to consider logistical barriers including transportation and affordability as well as adaptations requested by older adults (eg, preferences for older teachers).18
Although our sample was small, we also found that adults from diverse racial and ethnic backgrounds had more positive beliefs about yoga, such that this finding should be interpreted with caution. Similar to older adults, individuals from diverse racial and ethnic groups are also underrepresented in clinical trials and may have lower access to complementary treatments. Cultural and linguistic adaptations and building community partnerships should be considered in both recruitment and intervention delivery strategies.40We learned that education about yoga may increase interest and that it is possible to recruit older veterans to yoga class. Nevertheless, in study 2, our rate of full participation was low, with only about 1 in 10 participating. Additional efforts to enhance beliefs about yoga and to addresslogistical barriers (offering telehealth yoga) are needed to best reach older veterans.
Limitations
These findings have several limitations. First, participants were homogeneous in age, gender, race/ethnicity and veteran status, which provides a window into this understudied population but limits generalizability and our ability to control across populations. Second, the sample size limited the ability to conduct subgroup and interaction analyses, such as examining potential differential effects of cancer type, treatment, and PTSD on yoga beliefs or to consider the relationship of yoga beliefs with changes in quality of life before and after the yoga intervention in study 2. Additionally, age was not associated with beliefs about yoga in these samples that of mostly older adults. We were able to compare middle-aged and older adults but could not compare beliefs about yoga to adults aged in their 20s and 30s. Last, our study excluded people with dementia and psychotic disorders. Further research is needed to examine yoga for older cancer survivors who have these conditions.
Conclusions
Education that specifically informs potential participants about yoga practice, potential modifications, and potential benefits, as well as adaptations to programs that address physical and logistical barriers may be useful in increasing access to and participation in yoga for older Veterans who are cancer survivors.
Acknowledgments/Funding
The authors have no financial or personal relationships to disclose. This work was supported by the US Department of Veterans Affairs (VA) Rehabilitation Research and Development Service. This material is the result of work supported with resources and the use of facilities at the VA Boston Healthcare System, Bedford VA Medical Center, and Michael E. DeBakey VA Medical Center in Houston, Texas. We thank the members of the Veterans Cancer Rehabilitation Study (Vetcares) Research teams in Boston and in Houston and the veterans who have participated in our research studies and allow us to contribute to their health care.
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
1. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233-3241. doi:10.1200/JCO.2012.43.7707
2. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8(2):43-55.
3. Erratum: Primary follicular lymphoma of disguised as multiple miliary like lesions: A case report and review of literature. Indian J Pathol Microbiol. 2018;61(4):643. doi:10.4103/0377-4929.243009
4. Eyigor S, Uslu R, Apaydın S, Caramat I, Yesil H. Can yoga have any effect on shoulder and arm pain and quality of life in patients with breast cancer? A randomized, controlled, single-blind trial. Complement Ther Clin Pract. 2018;32:40-45. doi:10.1016/j.ctcp.2018.04.010
5. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. Published 2014 Jul 1. doi:10.1186/1472-6882-14-214
6. Browning KK, Kue J, Lyons F, Overcash J. Feasibility of mind-body movement programs for cancer survivors. Oncol Nurs Forum. 2017;44(4):446-456. doi:10.1188/17.ONF.446-456
7. Rosenbaum MS, Velde J. The effects of yoga, massage, and reiki on patient well-being at a cancer resource center. Clin J Oncol Nurs. 2016;20(3):E77-E81. doi:10.1188/16.CJON.E77-E81
8. Yun H, Sun L, Mao JJ. Growth of integrative medicine at leading cancer centers between 2009 and 2016: a systematic analysis of NCI-designated comprehensive cancer center websites. J Natl Cancer Inst Monogr. 2017;2017(52):lgx004. doi:10.1093/jncimonographs/lgx004
9. Sanft T, Denlinger CS, Armenian S, et al. NCCN guidelines insights: survivorship, version 2.2019. J Natl Compr Canc Netw. 2019;17(7):784-794. doi:10.6004/jnccn.2019.0034
10. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36(25):2647-2655. doi:10.1200/JCO.2018.79.2721
11. Culos-Reed SN, Mackenzie MJ, Sohl SJ, Jesse MT, Zahavich AN, Danhauer SC. Yoga & cancer interventions: a review of the clinical significance of patient reported outcomes for cancer survivors. Evid Based Complement Alternat Med. 2012;2012:642576. doi:10.1155/2012/642576
12. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979-1989. doi:10.1002/cncr.31979
13. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
14. US Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Updated June 16, 2021. Accessed September 22, 2021. https://www.publichealth.va.gov/exposures/agentorange/conditions
15. Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57(suppl 2):S289-S292. doi:10.1111/j.1532-5415.2009.02515.x
16. King K, Gosian J, Doherty K, et al. Implementing yoga therapy adapted for older veterans who are cancer survivors. Int J Yoga Therap. 2014;24:87-96.
17. Wertman A, Wister AV, Mitchell BA. On and off the mat: yoga experiences of middle-aged and older adults. Can J Aging. 2016;35(2):190-205. doi:10.1017/S0714980816000155
18. Chen KM, Wang HH, Li CH, Chen MH. Community vs. institutional elders’ evaluations of and preferences for yoga exercises. J Clin Nurs. 2011;20(7-8):1000-1007. doi:10.1111/j.1365-2702.2010.03337.x
19. Saravanakumar P, Higgins IJ, Van Der Riet PJ, Sibbritt D. Tai chi and yoga in residential aged care: perspectives of participants: A qualitative study. J Clin Nurs. 2018;27(23-24):4390-4399. doi:10.1111/jocn.14590
20. Fan JT, Chen KM. Using silver yoga exercises to promote physical and mental health of elders with dementia in long-term care facilities. Int Psychogeriatr. 2011;23(8):1222-1230. doi:10.1017/S1041610211000287
21. Taylor TR, Barrow J, Makambi K, et al. A restorative yoga intervention for African-American breast cancer survivors: a pilot study. J Racial Ethn Health Disparities. 2018;5(1):62-72. doi:10.1007/s40615-017-0342-4
22. Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387-4395. doi:10.1200/JCO.2006.06.6027
23. Smith SA, Whitehead MS, Sheats JQ, Chubb B, Alema-Mensah E, Ansa BE. Community engagement to address socio-ecological barriers to physical activity among African American breast cancer survivors. J Ga Public Health Assoc. 2017;6(3):393-397. doi:10.21633/jgpha.6.312
24. Cushing RE, Braun KL, Alden C-Iayt SW, Katz AR. Military-Tailored Yoga for Veterans with Post-traumatic Stress Disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
25. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. US Department of Veterans Affairs. Whole health. Updated September 13, 2021. Accessed September 22, 2021. https://www.va.gov/wholehealth
28. Sohl SJ, Schnur JB, Daly L, Suslov K, Montgomery GH. Development of the beliefs about yoga scale. Int J Yoga Therap. 2011;(21):85-91.
29. Cadmus-Bertram L, Littman AJ, Ulrich CM, et al. Predictors of adherence to a 26-week viniyoga intervention among post-treatment breast cancer survivors. J Altern Complement Med. 2013;19(9):751-758. doi:10.1089/acm.2012.0118
30. Mackenzie MJ, Carlson LE, Ekkekakis P, Paskevich DM, Culos-Reed SN. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496. doi:10.1155/2013/419496
31. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93. Published 2013 Mar 11. doi:10.1186/1472-6963-13-93
32. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v2.0 - Physical Function 6b. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=793&Itemid=992
33. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS Short Form v1.0 - Anxiety 6a. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=145&Itemid=992
34. Northwestern University. PROMIS Health Organization and the PROMIS Cooperative Group. PROMIS-43 Profile v2.1. Accessed September 24, 2021. https://www.healthmeasures.net/index.php?option=com_instruments&view=measure&id=858&Itemid=992
35. Todd NJ, Jones SH, Lobban FA. “Recovery” in bipolar disorder: how can service users be supported through a self-management intervention? A qualitative focus group study. J Ment Health. 2012;21(2):114-126. doi:10.3109/09638237.2011.621471
36. Finlay L. “Outing” the researcher: the provenance, process, and practice of reflexivity. Qual Health Res. 2002;12(4):531-545. doi:10.1177/104973202129120052
37. Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;10(suppl 1):S105-S112. doi:10.2105/AJPH.2009.162982
38. National Institutes of Health. Revision: NIH policy and guidelines on the inclusion of individuals across the lifespan as participants in research involving human subjects. Published December 19, 2017. Accessed September 22, 2021. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
39. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112-3124. doi:10.1200/JCO.2005.00.141
40. Vuong I, Wright J, Nolan MB, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials-a narrative review. J Cancer Educ. 2020;35(5):841-849. doi:10.1007/s13187-019-01650-y
What’s in a Name? The Problematic Term “Provider”
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
Health care has been dramatically transformed and influenced by medical and technological advances, insurance companies, state and federal legislation, and medical ethics. Amid these changes, including crises such as the ongoing coronavirus pandemic, earning the trust of patients to care for their mental and physical health remains a priority and a privilege.
It is troubling that federal health care agencies, in addition to hospitals, clinics, pharmacies, insurance companies, and administrators, often use the term provider when referring to clinicians on the multidisciplinary health care treatment team, which has become the predominant model for health care delivery. The word provider does not originate in the health care arena but from the world of commerce and contains no reference to professionalism or therapeutic relationships.1 Therefore, it should be replaced with more appropriate terminology that acknowledges clinicians’ roles and expertise and values our unique relationship with patients.
Why Is Provider a Problem?
First, the origin of the term provider is deplorable. During its ascent to power in the 1930s, the Nazi Party promoted the devaluation and exclusion of Jews in German society, including the medical community. Due to its eugenics campaign, the Nazi Party first targeted pediatrics, a specialty in which nearly half of its practitioners were Jewish.2 Beginning with female pediatricians, all Jewish physicians were redesignated as Behandler (provider) instead of Arzt (doctor).2 This is the first documented demeaning of physicians as providers in modern history. Jewish doctors were soon restricted to treating only Jewish patients and were further persecuted during the Holocaust. Knowing this background, what health care organization would use a term once associated with Nazi ideology?3
Second, using provider changes the treatment relationship. The nomenclature shift in the United States also seems to have originated in political and legislative circles. Although the reasons for this shift are unclear, the terminology became more pervasive after the government first used the term provider in Title XIX of the 1965 Social Security Amendments that established Medicare and Medicaid. Paydarfar and Schwartz noted it was used “in the sense of a contractor being paid for delivering any health-related products and services.”4 Ironically, a 1967 medical student health organization grant proposal discussed the role of a patient advocate in facilitating communication between “health care provider and patient.”5 A journalist for the New York Times used the word to describe a 1970 New York Senate debate surrounding the sale of Medicaid bills to collection agencies, but it is unclear whether the senators themselves used the term.6 Provider was later used in the National Health Planning and Resource Development Act of 1974.7
Ultimately, the adaptation of this terminology led to medicine being thought of only as a business, a commoditization of care, and reinforced by referring to patients as consumers, clients, or customers.3 This terminology suggests that the clinician-patient relationship is a commercial transaction based on a market concept where patients are consumers to be serviced.1,8 Emphasis is placed on following algorithms and treating symptoms rather than patients.9 Despite a goal of minimizing cost, a mismatched referral to a provider may actually compromise patient safety and cost-effectiveness due to missed diagnoses or excessive diagnostic testing.10
In addition to government, other nonclinical entities (eg, insurance companies, advocacy groups) and some clinicians may prefer the generic term provider. Besides health care commoditization, reasons may include convenience, simplifying health care nomenclature, or removing distinctions among health care professionals to reduce costs and/or increase autonomy.
However, our value as health care professionals is not simply what we can “provide.”11 We seek to know patients as people, putting their needs ahead of ours.1 We serve as confidants and advocates and not merely providers of medications, tests, or procedures.11 This personalized nature of health care depends on trust and professionalism rather than dispassionate delivery of commoditized services.1 Using traditional terminology acknowledges the true nature of the treatment relationship—one that is established not on market concepts but on medical ethics of autonomy, justice, beneficence, and nonmaleficence.
Third, provider is inaccurate and potentially disrespectful and harmful. The word doctor is derived from Latin doctus or docere, meaning to teach or instruct—a valued function in our interactions with patients, families, students, and colleagues.12,13 In contrast, provider refers to commercial transactions or the provision of shelter, food, and love within families and communities.1,14
Although there are no studies assessing the impact of this terminology on individual clinicians, the term provider may have a negative impact on both individual clinicians and on the health care system. Health care professionals may feel they are being disrespected by being portrayed as dispensers of services rather than as individuals.13,15 Furthermore, provider does not acknowledge the specialized training and qualifications of multidisciplinary treatment team members. The historical and theoretical foundation, degrees awarded, and scopes of practice for physicians, physician assistants, nurse practitioners, dentists, psychologists, optometrists, physical therapists, or social workers are different yet valuable, and their expertise and accomplishment should be recognized.
The use of this term has potential for causing moral injury and reduced self-worth, sense of purpose, and meaning in our daily work; this could threaten satisfaction and commitment and lead to demoralization and burnout.1,16 It may impair effective team dynamics, as it makes no reference to professional values and may lead patients and clinicians to place lower value on professionalism and conduct.10 It may negatively impact primary care specialties by propagating the connotation that primary care is simple care and promoting low compensation, lagging recruitment, and diminished respect.10 Finally, it is detrimental to patients by changing the nature of the relationship and failing to evoke the compassion and support that sick people (that is, patients) need and deserve.3
Last, use of this term can mislead patients. By law, a health care provider is defined as “a doctor of medicine or osteopathy who is authorized to practice medicine or surgery… or any other person determined by the Secretary [of Labor] to be capable of providing health care services,” which includes podiatrists, dentists, clinical psychologists, optometrists, chiropractors, nurse practitioners, nurse-midwives, clinical social workers, and physician assistants.17
When clinicians are categorized as providers rather than by their degrees and roles/responsibilities, patients may assume that all team members have equal training, interchangeable skills, and uniform expertise and knowledge and may conclude they can receive the same level of care from anyone.8,10 Potential for confusion is increased by the nearly ubiquitous white laboratory coat in clinical settings and doctoral degrees attainable in different health care disciplines (eg, medicine, nursing, psychology, pharmacy, physical therapy). Patients deserve to know who does what on the team of professionals who care for them and may not be fully informed when requesting or receiving treatment if they are not provided important information, such as a clinician’s title, training, and scope of practice.8,16
Reversing the Trend
Increasing awareness among patients, their families, health professions students, and health care colleagues and administrators of the importance of traditional nomenclature is a first step in reversing this trend or mitigating its impact. If an overarching generic term is required, then health care professional, clinician, or practitioner are preferred.10,12 Fifteen years ago, the Southern California Permanente Medical Group prohibited the use of the word provider to describe physicians, and its editorial style deemed it cold and institutional.16 Many, but not all, state, regional, or national medical associations and journals avoid provider in their names or titles.
I am encouraged that this journal—drawing its audience from several government health care agencies—is named Federal Practitioner rather than Federal Provider. This is reasonable and accurate, as practitioner refers to the practice of a profession, usually associated with health care.
I hope other professions can resist this trend. Lawyers are not considered legal aid providers, and teachers are not called knowledge providers.3 We do not refer to airline pilots as air transportation providers or musicians as instrument-playing melody providers. Many veterans likely would be offended if they were referred to as Constitution support and defense providers rather than by the military branch-specific titles that they earned through dedication, training, and sacrifice. The individuals in these examples demonstrate commitment to representing clients, educating students, flying passengers, playing instruments, or ensuring national defense. As health care professionals, our commitment to treating patients is equally important.4
Language matters when it comes to people feeling respected and achieving their full potential.1 I encourage government health care agencies to stop referring to us as providers and resume using traditional nomenclature. This will demonstrate genuine respect for us, transparency for the patients we serve, and recognition that caring for the sick is a calling, not a commodity.
Dedication
The author dedicates this article to his father John E. Scarff, Jr, a physician and United States Army veteran.
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).
1. Beasley JW, Roberts RG, Goroll AH. Promoting trust and morale by changing how the word provider is used: encouraging specificity and transparency. JAMA. 2021;325(23):2343-2344. doi:10.1001/jama.2021.6046
2. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
3. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Curr Psychiatr. 2020;19(2):5-7,29.
4. Paydarfar D, Schwartz WJ. A piece of my mind. Dear provider. JAMA. 2011;305(20):2046-2047. doi:10.1001/jama.2011.702
5. Student Health Organization. Grant Proposal of Student Health Organization. Summer Project of the South Bronx, 1967. Albert Einstein College of Medicine, unpublished.
6. Clines FX. Doctors face ban on sale of bills. New York Times. February 25, 1970:51
7. The National Health Planning and Resource Development Act of 1974. 42 USC § 300 (1975).
8. American Academy of Family Physicians. Provider, use of term (Position paper). Published 2018. Accessed September 22, 2021. https://www.aafp.org/about/policies/all/provider.html
9. Sanniec K, Gellis M. I am not a medical provider; I am a doctor. Aesthet Surg J. 2013;33(5):749-750. doi:10.1177/1090820X13487017
10. Goroll AH. Eliminating the term primary care “provider”: consequences of language for the future of primary care. JAMA. 2016;315(17):1833-1834. doi:10.1001/jama.2016.2329
11. Lee BY. Time to stop labeling physicians as providers. Published May 5, 2019. Accessed September 22, 2021. https://www.forbes.com/sites/brucelee/2019/05/05/time-to-stop-labeling-physicians-as-providers/?sh=7edfc865118e
12. Dhand S, Carbone WJ. Physicians are not providers: An open letter to the AMA and medical boards. Published November 30, 2015. Accessed September 22, 2021. https://www.kevinmd.com/blog/2015/11/physicians-are-not-providers-an-open-letter-to-the-ama-and-medical-boards.html
13. Al-Agba N. If you call me a provider, I will assume you are a Nazi. Published February 8, 2019. Accessed September 22, 2021. https://thedeductible.com/2019/02/08/if-you-call-me-a-provider-i-will-assume-you-are-a-nazi
14. Weiss JM. Physician or surgeon, but not “provider.” Published February 5, 2020. Accessed September 22, 2021. https://www.psychologytoday.com/us/blog/women-in-medicine/202002/physician-or-surgeon-not-provider
15. Liao L. Providers or professionals: how our conceptions of physician as machine or person lead to burnout. Med Teach. 2021;43(2):234-236. doi:10.1080/0142159X.2020.1769049
16. Weiss J. ‘Physician’ not ‘provider’ is better for doctor and patient. Published December 16, 2019. Accessed September 22, 2021. https://permanente.org/physician-not-provider-is-better-for-doctor-and-patient
17. Definition of Health Care Provider. 29 CFR § 825.125 (1993).