User login
Novel Solutions Needed to Attract Residents to Pediatric Rheumatology
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2023
Teen and young adult rheumatology patients report gaps in sexual health counseling
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2023
Patients with hypermobile Ehlers-Danlos syndrome report skin laxity, scarring
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
.
The genetic cause of hEDS, a common inherited connective tissue disorder, remains unknown, wrote Alan Snyder, MD, of the department of dermatology and dermatologic surgery at Medical University of South Carolina, Charleston, and colleagues.
Previous research suggests that changes in dermal mechanics predispose these patients to a range of skin conditions including mast cell activation disorder (MCAD) spectrum and chronic spontaneous urticaria, abnormal scars or wound healing, piezogenic papules, dyshidrosis, skin laxity or softness, easy bruising, local anesthesia resistance, keratosis pilaris, striae, and hidradenitis suppurativa, the researchers wrote.
However, data on these and other dermatologic manifestations of hEDS are limited, they said.
The diagnosis of hEDS will continue to be made more frequently and carefully, as the condition becomes more recognized and understood in the medical community, especially with anticipated capabilities of genetic testing, Dr. Snyder said in an interview.
“Being able to be aware of disease-specific comorbidities, such as those discovered in this study, allows providers to better stratify phenotypes and improve patient disease co-management,” he said.
In the study, published in the Journal of the American Academy of Dermatology, the researchers reviewed data on 1,364 patients with ICD-10 or ICD-9 codes for hEDS or EDS unspecified who were seen at a single institution between June 2005 and May 2022. Most of the patients were White (95.4%) and female (86.7%); the average age was 29.2 years.
Of the 1,364 patients included in the chart review, 497 (36.4%) had documented skin manifestations. Of these, 118 (24.2%) had disorders of follicular occlusion (12 had hidradenitis suppurativa, 32 had folliculitis, and 74 had acne); 112 (23%) had eczema or atopic dermatitis, 98 (19.7%) had mast cell disorder, 32 (6.4%) had psoriasis, and 32 (6.4%) had wound healing issues (16 had hypertrophic keloids/scarring, 5 had abscesses, 3 had abnormal bruising, and 8 had other would healing issues).
The study also included results of a multiple-choice patient survey from 1,354 individuals. In the survey, approximately two-thirds of patients reported abnormal scarring, abnormal wound healing, and cutaneous laxity (61.7%, 69.0%, and 71.0%, respectively).
The findings were limited by several factors including the retrospective study design, lack of testing to confirm hEDS diagnosis, and the potential interdisciplinary selection bias for diagnoses, the authors noted.
However, the results support previous studies showing increased rates of occlusive conditions in hEDS and higher rates of acne, folliculitis, and psoriasis, and highlight the need for clinician education to manage patients and promote better outcomes, the researchers concluded.
Data Enhance Clinical Awareness
“Given the increasingly understood relation between TH2-directed and mast-cell mediated diseases and hEDS, it was not necessarily a surprise to find the increased prevalence of atopy and mast cell disease, but rather an interesting confirmation, within the limitations that exist with retrospective chart review,” Dr. Snyder told this news organization. “While it may make some intuitive sense that certain cohorts with higher risk of HS may have a higher risk of acne, this had not been reported in the literature to date,” he noted. “Given the high levels of patient reported issues with scarring and wound healing, I was surprised that so few analogous diagnoses were physician-reported in the medical records.”
In clinical practice, “health care professionals and patients need to be aware hEDS is associated with high rates of eczematous, mast-cell mediated and follicular occlusive cutaneous disorders,” Dr. Snyder said in an interview. “There seems to be a discrepancy between patients and physician awareness of scarring or wound healing issues in this patient population,” he added.
Looking ahead, “we need to better research and characterize the various hEDS phenotypes to understand who is at highest risk for various TH2-mediated or follicular occlusive disorders,” said Dr. Snyder. “Moreover, a greater understanding is needed of the wound healing inadequacies that predispose these patients to poor outcomes during dermatologic surgery,” he said.
The study was supported by the Ehlers-Danlos Society and the Milton and Tamar Maltz Family Foundation. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Rheumatology Match Day results for 2024 follow trends of past years
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
First referral guide issued for axial spondyloarthritis
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.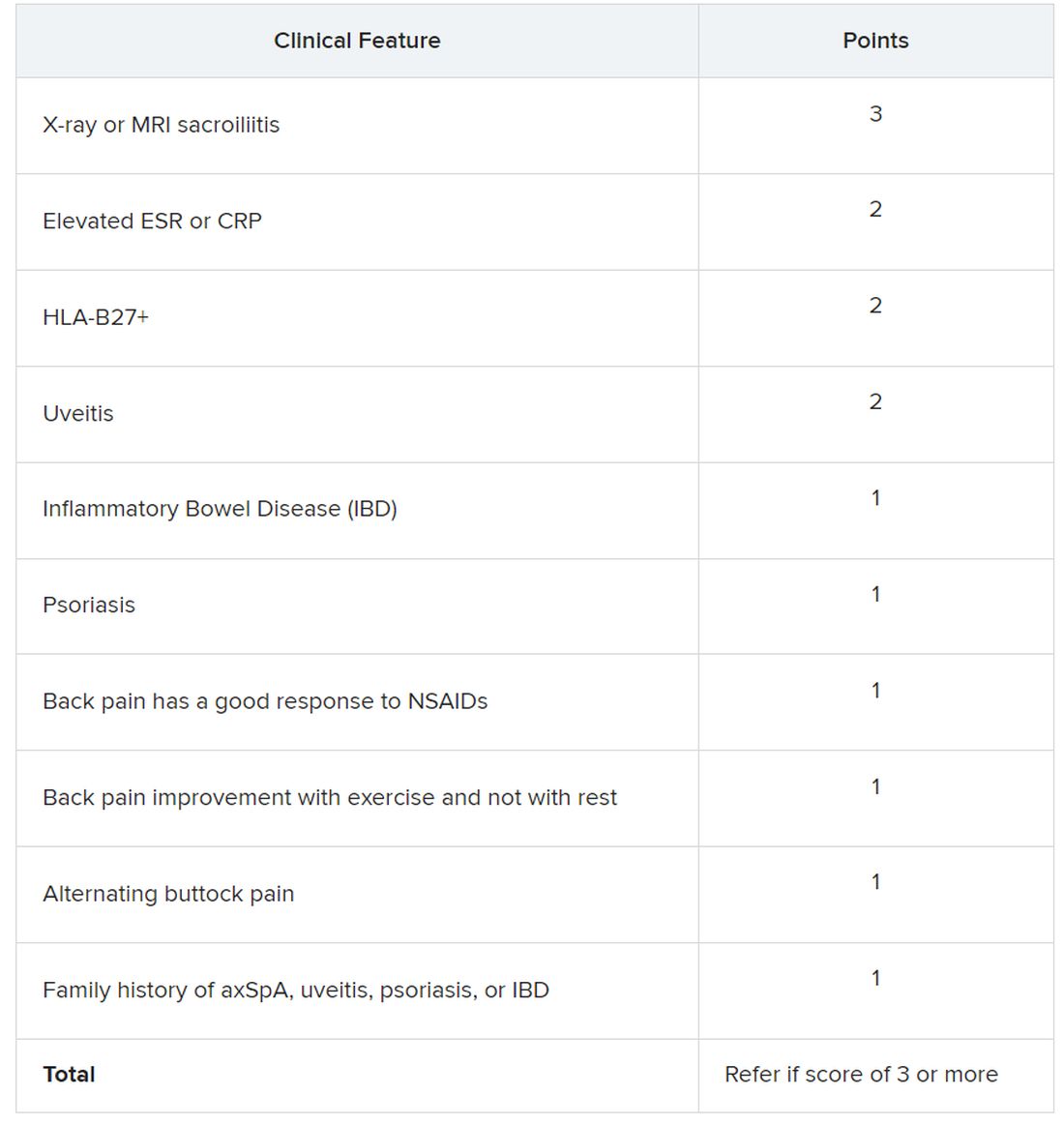
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
SAN DIEGO – The Spondyloarthritis Research and Treatment Network (SPARTAN) has created the first referral recommendations for axial spondyloarthritis (axSpA).
The draft recommendations use a points scoring system, with the goal that at least one in three patients referred would be diagnosed with axSpA, an inflammatory arthritis that affects the central skeleton and shares a genetic overlap with skin psoriasis, inflammatory bowel disease, and inflammatory eye disease.
Patients with axSpA can wait 10 years after symptom onset to be diagnosed with the condition. There are currently no guidelines to advise clinicians on when to refer to a rheumatologist, and with the rheumatology workforce shortage, “it is impossible for rheumatologists to evaluate the 20% of adults in the U.S. who have chronic back pain,” said Maureen Dubreuil, MD, a rheumatologist at Boston University. She presented the work at the annual meeting of the American College of Rheumatology.
To address this issue, Dr. Dubreuil and colleagues conducted a literature review to determine how predictive different spondyloarthritis features were of eventual axSpA diagnosis. The interdisciplinary team identified 38 studies published before March 2022, and uncovered 28 individual potential features associated with axSpA, including pain sites, family history of axSpA and related conditions, blood markers of inflammation, genetic testing, and imaging findings.
Inflammatory back pain elements had the lower predictive values, with positive likelihood ratios (LR+) ranging from 1.15 to 2.32, while imaging findings were the most predictive (LR+s from 6.40 to 10.02).
Using a Delphi exercise and discrete choice experiments, members narrowed the checklist down to 10 features. These 10 features were assigned points, with a score of 3 points qualifying for a referral of adults 45 years or younger with chronic pain (3 or more months) in the back, hip, or buttock.
Sacroiliitis seen on imaging, either by x-ray or MRI, received the highest score of 3 points. Dr. Dubreuil emphasized that imaging was not required for a referral, but if a patient has received imaging “that shows sacroiliitis, that is sufficient for referral to a rheumatologist,” she said in her presentation.
Elevated erythrocyte sedimentation rate or C-reactive protein, HLA-B27 positivity, and uveitis score 2 points. Inflammatory bowel disease; psoriasis; back pain with good response to NSAIDs; back pain improvement with exercise and not with rest; alternating buttock pain; and family history of axial spondyloarthritis, uveitis, psoriasis, or IBD score 1 point.
Dr. Dubreuil and colleagues expect that these criteria for referral will result in about one in three referred adults aged 45 years or younger with chronic back pain being diagnosed with axSpA. They also say additional research is necessary to understand if these recommendations increase probability of axSpA diagnosis and reduce diagnostic delays.
“We’re now getting to the stage where we are creating this screening tool, but [testing the] performance of the screening tool is going to be the major next step,” said Mark Hwang, MD, of UTHealth Houston in an interview with this news organization. He is a member of SPARTAN but was not involved with authoring the recommendations. “Will the screening tool enhance the ability on the back end to identify axSpA? We don’t know yet.”
Jon Chan, MD, a rheumatologist at the University of British Columbia, Vancouver, agreed that these recommendations “are a good first step,” but that more awareness about axSpA from nonrheumatologists would also be helpful in identifying new axSpA patients. He is also a member of SPARTAN and comoderated with Dr. Hwang the session where the new recommendations were presented. “I think other diseases like rheumatoid arthritis or lupus have a lot more recognition in the nonrheumatology community,” he told this news organization.
Connecting with other health professionals who see a lot of patients with back pain – physiotherapists, chiropractors, and chronic pain physicians – could also be helpful, he added. “A lot of times, patients go straight to a physio and circumvent the doctor,” he said.
Dr. Chan reports success in educating other departments. “I put up a poster in the emergency department saying, ‘If you’re young with back pain and uveitis, you need to be seen by rheumatology,’ and we’ve identified a ton of axSpA patients that way,” he said. “Maybe their uveitis was very mild, but their back pain was quite severe, and no one really clued in.”
Dr. Dubreuil disclosed financial relationships with Amgen, Pfizer, and UCB Pharma. Her abstract coauthors disclosed financial relationships with multiple pharmaceutical companies. Dr. Hwang consults for UCB and has received research support from Janssen. Dr. Chan has relationships with AbbVie/Abbott, Eli Lilly, Janssen, Novartis, and UCB.
AT ACR 2023
A new standard for treatment of torus fractures of the wrist?
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
PRACTICE CHANGER
For uncomplicated pediatric torus fractures of the distal radius, consider definitive management with soft bandage immobilization until pain resolution, rather than rigid immobilization and clinical follow-up.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial with patient-oriented outcomes.1
Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
‘Hidden’ cognitive impairments in DMD may worsen outcomes
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
A new tool from the National Institutes of Health, called NIH Toolbox, could improve that outcome, according to Mathula Thangarajh, MD, PhD, who has conducted research in the field.
“When we talk to families and parents, they are able to identify that even during infancy that [children with DMD] have delayed cognitive function. This includes speech delay, but also language and adaptive skills. We also know that those children with speech delay, which is really a very commonly reported phenotype in up to 50%, go on to have school-based needs. They may repeat [grades] in elementary years, but they also use more resources at school,” said Dr. Thangarajh, who is an assistant professor of neurology at the Children’s Hospital of Richmond at Virginia Commonwealth University, Richmond, during a talk at the 2023 annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine (AANEM).
A previous natural history study that utilized the Pediatric Quality of Life assessment also showed that DMD patients reported the lowest scores in brain health, including emotional health and school performance.
Other research has shown a correlation between cognitive function and survival in DMD. “This suggests that health maintenance may play an important role [in outcomes],” said Dr. Thangarajh. Another study found a correlation between psychomotor delay that required school-based interventions and earlier loss of ambulation, lower cardiac ejection fraction, and worse pulmonary function. The researchers also found that boys with cognitive delay were diagnosed at an earlier age, and yet had delays in diagnosis and worse motor function, pulmonary health, and cardiac health outcomes. On average, they lost ambulatory ability 2 years earlier.
A study by Dr. Thangarajh’s group showed that patients with speech delay and lower IQ had lower performance in timed tests, including 6-minute walk test distance and scored an average of 2 points lower on the North Star Ambulatory Assessment.
A tool for continuous cognitive assessment
The Centers for Disease Control and Prevention–supported DMD CARE guidelines only say that neuropsychological evaluations should be considered at diagnosis, but is essential if concerns arise about developmental progress. However, the Muscular Dystrophy Association has found barriers both in access to specialists, with an average wait time of 1-2 years, and burdensome out-of-pocket costs.
Those issues prompted Dr. Thangarajh to look for an alternative solution. At the time that she embarked on this work, the NIH was interested in technologies to assess neurobehavioral issues across different diseases. The resulting NIH Toolbox iPad app was driven largely by failed clinical trials in dementia, and the aim was to be able to provide continuous assessment over time. “It will allow for assessments across the lifespan, so you can use the same construct from age 3 to 80-plus,” said Dr. Thangarajh. It can also normalize population factors, such as annual household income and mother’s IQ.
She set out to validate the NIH Toolbox in children with DMD. The toolbox includes measures of crystalized cognition and fluid cognition. The former encompasses vocabulary and reading ability, which are strongly predicted by socioeconomic status and maternal IQ. On the other hand, fluid cognition includes cognitive features that develop across the lifespan and is directly related to academic underperformance in DMD patients.
Dr. Thangarajh’s group assessed 30 boys with DMD and found that crystallized cognition was normal, but they had a deficit in fluid cognition. They found deficits within several subdomains of fluid cognition. “This tells us that the NIH Toolbox was able to replicate what we had known in the literature, that these boys really have lower intellectual capacity, but they also have significant weakness in fluid cognition,” she said.
She also wanted to examine changes over time by testing the boys at a 1-year interview. “What we found was that they are not making as much gain in fluid cognition as we would like. They are just making marginal improvements over time. This has implications on how often we should screen them, but also not be over reliant on using school-based resources for them to get tested,” said Dr. Thangarajh.
Her group’s analysis of a dataset of 55 boys provided by PTC Therapeutics revealed a difference by age in a test of working memory. “What we found was that boys who are actually greater than 9 years, compared with those who are less than 9 years, they actually had a reversal of development-based improvement. The older they get, they were not making as much gains as you would expect,” said Dr. Thangarajh.
She went on to discuss psychosocial determinants of cognitive health in DMD. It is known that women who are carriers of the dystrophin mutation can underperform in cognitively stressful tasks, leading her to wonder if this could lead to transgenerational risk to offspring with DMD. Her group tested women who were carriers of the mutation with the NIH Toolbox and found that they had lower fluid cognition than noncarriers. They then tested 65 dyads of mothers and children, and found a correlation, but only when it came to inhibitory control, which required the individual to note the direction of an arrow while ignoring surrounding arrows pointing in various directions.
Next, the researchers examined neighborhoods and their impact on cognitive health, which can be affected by the presence of green spaces, access to public transportation and good nutrition, and other factors. There were significant deficits associated with residence zip codes. “We were pretty shocked. Someone who is not in a socially vulnerable region is scoring slightly below average, but someone who is in a very socially vulnerable neighborhood is only scoring 75 [age-adjusted score] on the NIH toolbox. So with this, we can conclude that carrier women are vulnerable in certain cognitive domains, but also children who come from socially vulnerable [situations] have poor cognitive control. This, again, has implications on how often we should screen and how much we should overly rely on school-based resources for these individuals,” said Dr. Thangarajh.
Overcoming a significant barrier
The NIH Toolbox has a lot of potential to improve DMD care, according to Dianna Quan, MD, who is the incoming president of AANEM, and professor of neurology at the University of Colorado at Denver, Aurora. “There’s this huge problem in terms of getting people in to see neuropsychologists and having formal evaluations. I think that’s a huge barrier. If we have people able to access this toolkit, which is simple and easily and universally accessible, how wonderful is that? I think that will be a really great improvement on what’s going on right now. It allows people to easily screen for these cognitive disabilities and make sure that we address them,” Dr. Quan said in an interview.
Asked how the tool could specifically improve care, Dr. Quan suggested that the first step is to understand the contributing factors to cognitive issues, whether they are biological, social, or a combination. “Some of them we can modify, potentially, through addressing the social environment. Some of those biologic factors may also be modifiable with many of the new drug studies that are coming.”
Dr. Thangarajh has received speaker honoraria from NS Pharma and PTC Therapeutics. Dr. Quan has received funding from Alnylam, Pfizer, Cytokinetics, Momenta, and Argenx.
FROM AANEM 2023
Strength training promotes knee health, lowers OA risk
TOPLINE:
Strength training at any point in life is associated with a lower risk of knee pain and osteoarthritis, contrary to persistent assumptions of adverse effects.
METHODOLOGY:
- Researchers reviewed data on strength training and knee pain from 2,607 adults. They used the Historical Physical Activity Survey Instrument to assess the impact of strength training during four periods (ages 12-18 years, 19-34 years, 35-49 years, and 50 years and older).
- The participants were enrolled in the Osteoarthritis Initiative, a multicenter, prospective, longitudinal study; 44% were male, the average age was 64.3 years, and the mean body mass index was 28.5 kg/m2.
- Strength training was defined as those exposed and not exposed, as well as divided into low, medium, and high tertiles for those exposed. A total of 818 individuals were exposed to strength training, and 1,789 were not exposed to strength training.
- The primary outcomes were frequent knee pain, radiographic OA (ROA), and symptomatic radiographic OA (SOA).
TAKEAWAY:
- The study is the first to examine the effect of strength training on knee health in a community population sample not selected for a history of elite weight lifting.
- Overall, strength training at any point in life was associated with lower incidence of frequent knee pain, ROA, and SOA, compared with no strength training (odds ratios, 0.82, 0.83, and 0.77, respectively).
- When separated by tertiles, only the high-exposure group had significantly reduced odds of frequent knee pain, ROA, and SOA, with odds ratios of 0.74, 0.70, and 0.69, respectively. A dose-response relationship appeared for all three conditions, with the lowest odds ratios in the highest strength training exposure groups.
- Findings were similar for different age ranges, but the association between strength training and less frequent knee pain, less ROA, and less SOA was strongest in the older age groups.
IN PRACTICE:
“Our findings support the idea that the medical community should proactively encourage more people to participate in strength training to help reduce their risk of osteoarthritis and other chronic conditions,” the researchers write.
SOURCE:
The study, with first author Grace H. Lo, MD, of Baylor College of Medicine, Houston, and colleagues, was published in Arthritis and Rheumatology.
LIMITATIONS:
The observational design and self-selected study population of strength training participants might bias the results, including participants’ recall of their activity level levels and changes in exercise trends over time. More research is needed to explore associations between strength training and knee OA among those who started strength training at a younger age.
DISCLOSURES:
The study was funded in part by the VA Health Services Research and Development Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston, and by donations to the Tupper Research Fund at Tufts Medical Center. The Osteoarthritis Initiative is supported by the National Institutes of Health; private funding partners include Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. Three authors report having financial relationships with multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Strength training at any point in life is associated with a lower risk of knee pain and osteoarthritis, contrary to persistent assumptions of adverse effects.
METHODOLOGY:
- Researchers reviewed data on strength training and knee pain from 2,607 adults. They used the Historical Physical Activity Survey Instrument to assess the impact of strength training during four periods (ages 12-18 years, 19-34 years, 35-49 years, and 50 years and older).
- The participants were enrolled in the Osteoarthritis Initiative, a multicenter, prospective, longitudinal study; 44% were male, the average age was 64.3 years, and the mean body mass index was 28.5 kg/m2.
- Strength training was defined as those exposed and not exposed, as well as divided into low, medium, and high tertiles for those exposed. A total of 818 individuals were exposed to strength training, and 1,789 were not exposed to strength training.
- The primary outcomes were frequent knee pain, radiographic OA (ROA), and symptomatic radiographic OA (SOA).
TAKEAWAY:
- The study is the first to examine the effect of strength training on knee health in a community population sample not selected for a history of elite weight lifting.
- Overall, strength training at any point in life was associated with lower incidence of frequent knee pain, ROA, and SOA, compared with no strength training (odds ratios, 0.82, 0.83, and 0.77, respectively).
- When separated by tertiles, only the high-exposure group had significantly reduced odds of frequent knee pain, ROA, and SOA, with odds ratios of 0.74, 0.70, and 0.69, respectively. A dose-response relationship appeared for all three conditions, with the lowest odds ratios in the highest strength training exposure groups.
- Findings were similar for different age ranges, but the association between strength training and less frequent knee pain, less ROA, and less SOA was strongest in the older age groups.
IN PRACTICE:
“Our findings support the idea that the medical community should proactively encourage more people to participate in strength training to help reduce their risk of osteoarthritis and other chronic conditions,” the researchers write.
SOURCE:
The study, with first author Grace H. Lo, MD, of Baylor College of Medicine, Houston, and colleagues, was published in Arthritis and Rheumatology.
LIMITATIONS:
The observational design and self-selected study population of strength training participants might bias the results, including participants’ recall of their activity level levels and changes in exercise trends over time. More research is needed to explore associations between strength training and knee OA among those who started strength training at a younger age.
DISCLOSURES:
The study was funded in part by the VA Health Services Research and Development Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston, and by donations to the Tupper Research Fund at Tufts Medical Center. The Osteoarthritis Initiative is supported by the National Institutes of Health; private funding partners include Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. Three authors report having financial relationships with multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
Strength training at any point in life is associated with a lower risk of knee pain and osteoarthritis, contrary to persistent assumptions of adverse effects.
METHODOLOGY:
- Researchers reviewed data on strength training and knee pain from 2,607 adults. They used the Historical Physical Activity Survey Instrument to assess the impact of strength training during four periods (ages 12-18 years, 19-34 years, 35-49 years, and 50 years and older).
- The participants were enrolled in the Osteoarthritis Initiative, a multicenter, prospective, longitudinal study; 44% were male, the average age was 64.3 years, and the mean body mass index was 28.5 kg/m2.
- Strength training was defined as those exposed and not exposed, as well as divided into low, medium, and high tertiles for those exposed. A total of 818 individuals were exposed to strength training, and 1,789 were not exposed to strength training.
- The primary outcomes were frequent knee pain, radiographic OA (ROA), and symptomatic radiographic OA (SOA).
TAKEAWAY:
- The study is the first to examine the effect of strength training on knee health in a community population sample not selected for a history of elite weight lifting.
- Overall, strength training at any point in life was associated with lower incidence of frequent knee pain, ROA, and SOA, compared with no strength training (odds ratios, 0.82, 0.83, and 0.77, respectively).
- When separated by tertiles, only the high-exposure group had significantly reduced odds of frequent knee pain, ROA, and SOA, with odds ratios of 0.74, 0.70, and 0.69, respectively. A dose-response relationship appeared for all three conditions, with the lowest odds ratios in the highest strength training exposure groups.
- Findings were similar for different age ranges, but the association between strength training and less frequent knee pain, less ROA, and less SOA was strongest in the older age groups.
IN PRACTICE:
“Our findings support the idea that the medical community should proactively encourage more people to participate in strength training to help reduce their risk of osteoarthritis and other chronic conditions,” the researchers write.
SOURCE:
The study, with first author Grace H. Lo, MD, of Baylor College of Medicine, Houston, and colleagues, was published in Arthritis and Rheumatology.
LIMITATIONS:
The observational design and self-selected study population of strength training participants might bias the results, including participants’ recall of their activity level levels and changes in exercise trends over time. More research is needed to explore associations between strength training and knee OA among those who started strength training at a younger age.
DISCLOSURES:
The study was funded in part by the VA Health Services Research and Development Center for Innovations in Quality, Effectiveness, and Safety at the Michael E. DeBakey VA Medical Center, Houston, and by donations to the Tupper Research Fund at Tufts Medical Center. The Osteoarthritis Initiative is supported by the National Institutes of Health; private funding partners include Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. Three authors report having financial relationships with multiple pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FDA OKs first ustekinumab biosimilar
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved ustekinumab-auub (Wezlana) as a biosimilar to ustekinumab (Stelara) for the treatment of multiple inflammatory conditions. This is the first approval for a ustekinumab biosimilar in the United States.
Ustekinumab-auub was also granted an interchangeability designation, meaning that, depending on state law, a pharmacist may substitute the biosimilar for the reference product without consulting the prescribing provider.
“Today’s approval exemplifies the FDA’s longstanding commitment to support a competitive marketplace for biological products,” Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars in the FDA’s Center for Drug Evaluation and Research, said in a statement. “This approval can empower patients by helping to increase access to safe, effective, and high-quality medications at potentially lower cost.”
Ustekinumab, manufactured by Johnson & Johnson, targets interleukin-12 and IL-23 and was first approved in 2009. Ustekinumab-auub was developed by Amgen.
Ustekinumab-auub is approved for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, active psoriatic arthritis, moderate to severely active Crohn’s disease, and moderate to severely active ulcerative colitis. It is also approved for pediatric patients aged 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and active psoriatic arthritis.
The approval was based on “comprehensive review of scientific evidence,” including “comparisons of the products on an analytical level using an extensive battery of chemical and biological tests and biological assays that confirmed similarity in the structural and functional features of Wezlana and Stelara (including those known to impact safety and efficacy), and comparative human pharmacokinetic data, clinical immunogenicity data, and other clinical safety and effectiveness data,” the FDA said.
Some common side effects of ustekinumab-auub include nasopharyngitis, upper respiratory tract infection, headache, fatigue, and nausea. The most severe side effect of the biosimilar, as with the reference drug ustekinumab, is infection.
The product launch of ustekinumab-auub will be delayed as a part of a settlement of Johnson & Johnson’s lawsuit against Amgen, according to Reuters. The details of the settlement are confidential, but it was stated that the biosimilar would be available by Jan. 1, 2025.
A version of this article first appeared on Medscape.com.
FDA approves ninth Humira biosimilar, with interchangeability
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has granted an interchangeability designation to adalimumab-afzb (Abrilada), according to an announcement from Pfizer.
This is the second adalimumab biosimilar granted interchangeability. The first, adalimumab-adbm (Cyltezo), became available in July.
Biosimilars introduce market competition that can help lower drug prices. Adalimumab-afzb is one of nine approved biosimilars for Humira, and the last to launch in 2023.
Adalimumab-afzb is indicated for:
- Adults with rheumatoid arthritis.
- Polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
- Adults with psoriatic arthritis.
- Adults with ankylosing spondylitis.
- Crohn’s disease in adults and children 6 years of age and older.
- Adults with ulcerative colitis.
- Adults with plaque psoriasis.
- Adults with hidradenitis suppurativa.
- Adults with noninfectious intermediate and posterior uveitis and panuveitis.
“With this designation, Abrilada is now both biosimilar to and interchangeable with Humira, reinforcing confidence among physicians and pharmacists that there is no decrease in effectiveness or increase in safety risk associated with switching between Abrilada and the reference product,” Roy Fleischmann, MD, clinical professor of medicine, University of Texas Southwestern Medical Center, Dallas, said in Pfizer’s statement.
An interchangeability designation allows pharmacists to substitute the biosimilar for the reference product without involving the prescribing clinician (according to state law). To achieve this designation, Pfizer submitted data from a phase 3 study led by Dr. Fleischmann that evaluated adalimumab-afzb in patients with RA. Patients who were switched three times between the biosimilar and the reference product had outcomes similar to those of patients continuously treated with the reference product.
Adalimumab-afzb will be available later in October at a 5% discount from Humira’s price. Later this year, the drug will launch at a second price, a 60% discount from Humira.
Full prescribing information for adalimumab-afzb is available here.
A version of this article first appeared on Medscape.com.










