User login
Which patients might benefit from platelet-rich plasma?
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2
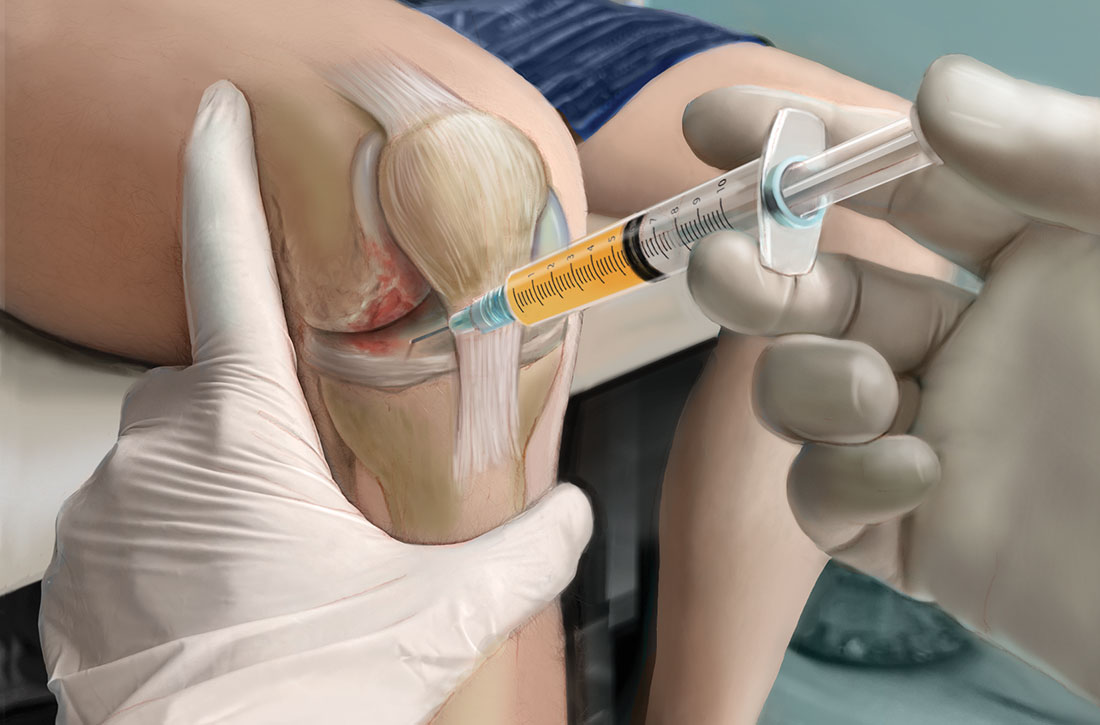
When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
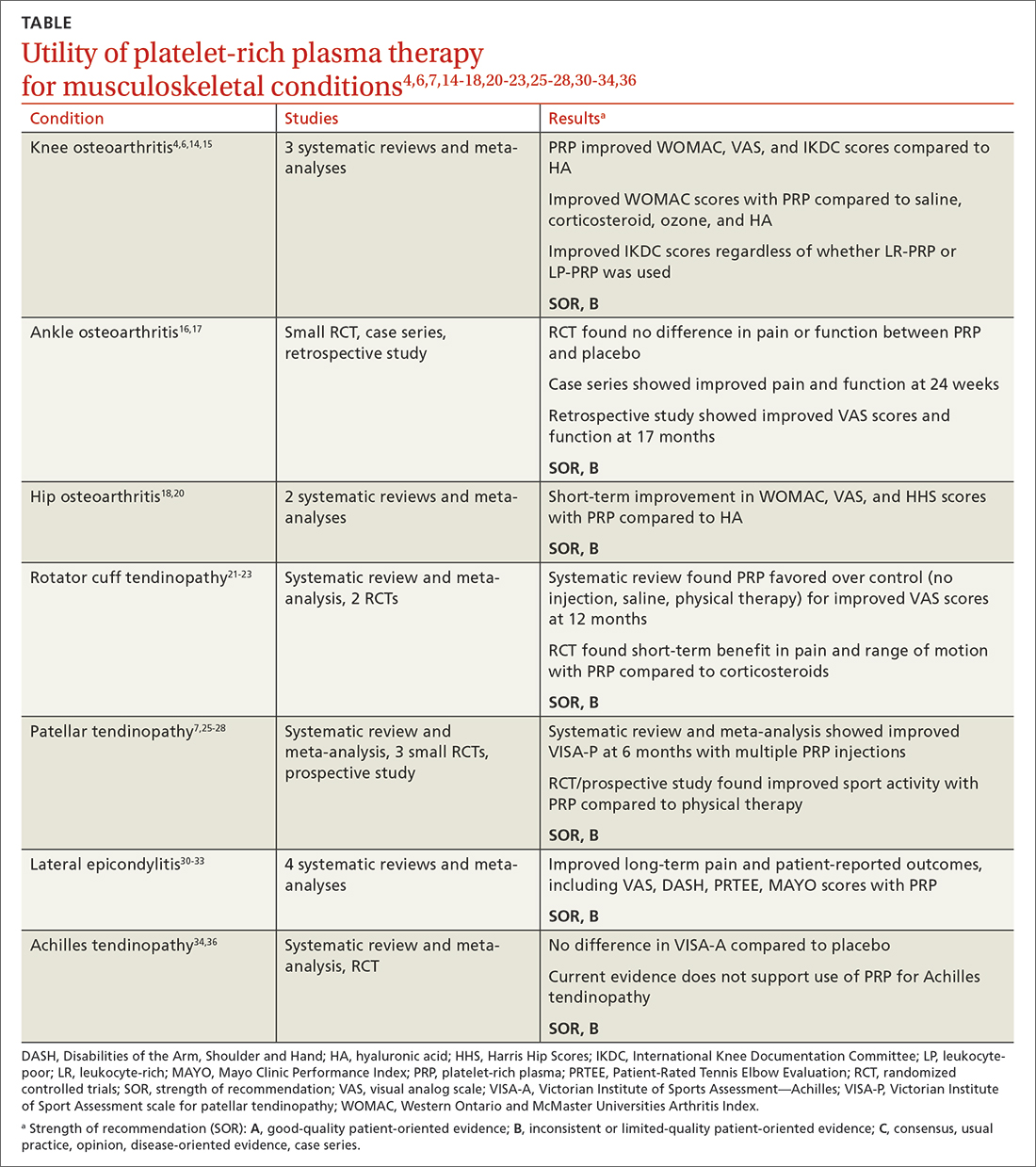
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).
Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
PRACTICE RECOMMENDATIONS
› Consider plateletrich plasma (PRP) for conservative management of knee osteoarthritis and lateral epicondylitis. B
› Consider giving multiple injections of PRP for longterm pain relief and expedited return to sport in patellar tendinopathy. B
› Do not use PRP for Achilles tendinopathy due to a lack of clinical evidence. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Exercise and empathy can help back pain patients in primary care
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
FDA OKs spinal cord stimulation devices for chronic back pain
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the indication for Abbott Laboratories’ spinal cord stimulation (SCS) devices to include treatment of chronic back pain in patients who have not had, or are not eligible for, back surgery, the company has announced.
The new indication spans all of Abbott’s SCS devices in the United States, which include the recharge-free Proclaim SCS family and the rechargeable Eterna SCS platform.
The devices feature the company’s proprietary, low-energy BurstDR stimulation waveform, a form of stimulation therapy that uses bursts of mild electrical energy without causing an abnormal tingling sensation to help disrupt pain signals before they can reach the brain, the company explained.
The expanded indication was supported by results from the DISTINCT study, which enrolled 270 adults suffering from severe, disabling chronic back pain for an average of more than 12 years and who were not eligible for surgery.
The study showed that significantly more patients who were treated with SCS achieved significant improvements in back pain, function, quality of life, and psychological status than peers treated with conservative medical management.
“To date, we have struggled with how to treat people who weren’t considered a good surgical candidate because we didn’t have clear, data-driven treatment options for non-surgical back pain,” Timothy Deer, MD, president and CEO of the Spine and Nerve Centers of the Virginias in Charleston, W.Va., said in a news release.
“This new indication for Abbott’s SCS devices, together with BurstDR stimulation, allows physicians the ability to identify and treat a new group of people, providing them with relief from chronic back pain,” Dr. Deer said.
A version of this article first appeared on Medscape.com.
Preventing breaks and falls in older adults
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
AT AGS 2023
Musculoskeletal disorders prevalent in orchestra musicians
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
PARIS – For orchestra musicians, performance is everything. So, it’s no wonder that musculoskeletal disorders – a reality for so many of these professionals – are not openly discussed. Physical pain is often pushed aside, unexpressed, until one day the suffering gets to be too much, the ability to play is impacted, and all the effort to keep things under wraps and under control culminates in burnout.
Anne Maugue was one of the speakers at the French College of General Medicine’s 16th Congress of General Medicine. Ms. Maugue is a postdoctoral researcher at Côte d’Azur University, Nice, France. She also plays flute in the Monte-Carlo Philharmonic Orchestra. Through her presentation to the physicians, she sought to raise awareness about MSDs in professional musicians, as well as the associated psychosocial risk factors. “If caught early enough, this pain can often be successfully treated.”
High prevalence
“You’re a violinist in a major symphony orchestra. It’s Sunday night, 8 o’clock, and you’ve just come off the stage. A few minutes ago, you felt a sharp pain in your right arm – a pain that is now, already, overwhelming. The conductor accused you of not being focused, of not concentrating. You know that you have another rehearsal in just a few hours, Monday morning. So, what do you do – other than hope that the pain goes away by then? Where can you turn to get help?”
With this opening scenario, Ms. Maugue was able to immediately orient the attendees to the realities that professional musicians face.
Pain is far from anecdotal. In professional orchestras, its prevalence over 12 months is between 41% and 93%. “An elite athlete has a full training staff they can turn to. An elite musician, on the other hand, usually only has their general practitioner – and that’s assuming the musician even reaches out to get treatment to begin with.
“The fact is that most of the time musicians only care about the pain when it becomes chronic, when it causes discomfort that affects their playing,” said Ms. Maugue.
How, then, does one evaluate this problem? In a Danish study, musicians rated the musculoskeletal problems they had experienced in the preceding 7 days. When the researchers compared those reports with findings from a clinical examination, they found that the examiners were not able to identify which musicians had reported problems. Why? Because a diagnosis does not reflect the severity or the impact, both of which are subjective.
“When faced with pain, the musician’s initial reaction is denial,” said Ms. Maugue. “The pain is often attributed to something other than the physicality of playing their instrument. They then turn to self-care, to colleagues. It’s only much later that they consult a medical professional.”
As a result, the physician is seldom aware of the musician’s psychological distress and has no sense of how long it’s been since the pain first started.
Work environment
Carrying around an instrument all the time and maintaining nonergonomic postures for extended periods are just two of the factors that put professional musicians at risk of physical pain. Not to be forgotten, Ms. Maugue added, are the work-related pressures. Musicians are not immune to issues with their work environment. They can feel like they aren’t getting the resources they need, proper recognition from their leaders, or support from their colleagues. In the end, such feelings can engender a sense of unfairness – and that acts as a stressor that can give rise to MSDs.
Evidence of this phenomenon can be found in the results of a study that Ms. Maugue conducted. Out of 440 French orchestra musicians (44% women), 64% said they had experienced MSD-related pain in the preceding 12 months and 61% in the preceding 7 days.
Using industrial and organizational psychology scales of measurement, Ms. Maugue was able to show, through hierarchical regression, that “emotional exhaustion and MSD-related pain occur when the environment in which people work causes them to feel a sense of unfairness.”
Early detection
Finally, Ms. Maugue encouraged general practitioners to ask every patient whether he or she plays a musical instrument. If the answer is yes, get an idea about any pain that he or she may have been feeling in the back, neck, and upper extremities so that prompt treatment can be given.
“There are other studies underway that are looking to better characterize instrumental activity and to enable more effective management by sports medicine departments,” said Ms. Maugue. “But back to patients with MSDs. It’s important to understand everything about their playing. Where do they practice? How often do they practice? What’s their posture like when they play? What’s the tempo of the music they’re working on? Because what we see in professional musicians is likely to be seen in amateur musicians as well – particularly in young people who study at a conservatory,” where not much is being done to prevent MSDs.
“If professional musicians are given treatment early on, half of them can be permanently cured,” she concluded. “And then, just like elite athletes, they’ll be able to get right back to playing.”
This article was translated from Medscape’s French edition and a version appeared on Medscape.com.
Answers sought for mental health challenges in pediatric rheumatology patients
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
NEW ORLEANS – Pediatric patients with rheumatologic diseases experience a particularly high prevalence of psychological distress and depression and anxiety symptoms, according to research presented at the Pediatric Rheumatology Symposium. Although this finding is not necessarily surprising, the extent to which depression and psychological distress impacts these young patients’ quality of life has led to greater research and innovation in seeking ways to identify, address, and treat depression and anxiety in children and adolescents with diseases such as juvenile idiopathic arthritis (JIA) or systemic lupus erythematosus (SLE).
Accordingly, other studies presented at the conference examined more efficient ways to screen adolescent patients for depression and assessed programs designed to improve symptoms. In fact, the American College of Rheumatology award for the top Quality, Health Services, and Education Research abstract at this year’s symposium went to Lauren Harper, MD, a pediatric rheumatology fellow at Nationwide Children’s Hospital, Columbus, whose research examined the effects of automating depression screening during check-in for adolescent patients with SLE. Her findings revealed that automation of screening increased detection of depression and suicidality, thereby increasing interventions and ultimately resulting in a reduction in depression prevalence.
“The key clinical takeaway is that mental health screening is really important – it affects our patients in so many different ways – and it’s very doable in your rheumatology clinic,” Dr. Harper said in an interview. “It’s also important because they’re coming to us very frequently, but they don’t see their PCP [primary care provider] very often, so we can’t leave screening to the PCPs.”
Two other studies assessed the effectiveness of a 6-week cognitive-behavioral intervention for youth called Treatment and Education Approach for Childhood-Onset Lupus (TEACH). One study found that remote delivery of TEACH resulted in improved mood symptoms and reduced fatigue, and another found the program particularly effective in improving mood for patients deemed “high risk” because of greater depression and fatigue symptoms.
The impact of growing mental health problems has been enormous both in the pediatric rheumatology population and society at large, Daria Sosna, MSc, a research coordinator at the University of Calgary (Alta.), said in an interview as she visited the research posters related to psychological stress and depression.
“We need to do something,” said Ms. Sosna, whose department is currently applying for funding to develop a research project to improve mental health outcomes in adolescents with lupus. “This population, specifically, has higher numbers than anyone else does because they have chronic illness” – and those issues need to be addressed.
High psychological stress levels
The study looking at psychological stress in pediatric rheumatology patients, led by Natalie Rosenwasser, MD, of Seattle Children’s Hospital, relied on cross-sectional data from patients enrolled in two Childhood Arthritis and Rheumatology Research Alliance sites, one in Utah and one in Seattle. The average age of the 71 patients who completed the surveys was 13, and the researchers reported the findings in two separate age groups: those aged 13-17, who completed the surveys themselves, and those aged 8-12, whose parents completed the surveys. Nearly all the patients (94.4%) had JIA, but one had lupus and three had juvenile dermatomyositis.
The participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) for psychological stress, physical stress, and depressive symptoms. They also filled out the National Institutes of Health–Toolbox Perceived Stress survey, the 9-item Patient Health Questionnaire (PHQ-9), the Screen for Child Anxiety Related Disorders (SCARED), a visual analog scale for COVID-related distress, and a questionnaire asking about how receptive they were to mental health screening. The researchers determined that a score 1 standard deviation above the mean on the PROMIS and NIH-Toolbox assessments qualified as a high level of psychological stress.
“There are data that suggest that psychological stress can be a precursor to depression and anxiety, which raises the concern that not every patient who’s experiencing mental health symptoms is going to be picked up on traditional measures that meet that clinical threshold, but they may really need interventions to protect their mental health,” presenter Erin Treemarcki, DO, an assistant professor of pediatric rheumatology at the University of Utah, Salt Lake City, said in an interview. “Not every patient may necessarily need referral to a mental health specialist, but there are still potential interventions that we can do in the clinical setting to address mental health, which in turn can improve outcomes, including medication compliance and knowing how patients are feeling.”
More than one-third of the patients (39%) reported a high level of psychological stress, and 43% had elevated physical stress. Broken down by age, 26% of the teens and 15% of the younger patients reported high levels of perceived stress. The PROMIS only identified increased depressive symptoms in 26% of the participants, whereas more than half (54%) had a positive PHQ-9 depression screen. Furthermore, half the patients had SCARED scores (50%) that likely indicated anxiety disorder. Only 6% of patients reported severe stress specifically related to the pandemic, but most reported mild distress from the pandemic.
“Psychological stress was highly correlated with physical stress, perceived stress, depressive symptoms [PROMIS and PHQ-9], and anxiety,” the authors reported (P < .05). The authors next plan to expand their assessment to a third CARRA site and then explore the interaction between psychological distress and sociodemographic factors.
“There’s such an increase in mental health disorders right now, and we’re overwhelmed in general,” Ms. Sosna said in an interview. “There have to be interventions that approach this. We can use pharmacological approaches, we can use CBT, we can use a lot of these things that are very well established, and they’re absolutely fantastic, but we don’t necessarily have the resources or capabilities to do that all the time.”
Benefits of automated depression screening
To reduce the likelihood of depression screenings falling through the cracks during visits, Dr. Harper’s study assessed the impact of automating screens in an adolescent population. In her presentation, she noted previous research finding that nearly half of youth with lupus (47%) had depression, compared with 24% of adults with lupus. Pediatric patients have nearly three times the odds of depression and more than five times the odds of suicidal ideation, she told attendees. These mood disorders are correlated with greater physical disability, higher cardiovascular risk, more disease activity, higher risk of premature death, and decreased educational attainment, medication compliance, and quality of life.
Despite recommendations for depression screening from the U.S. Preventive Services Task Force and the American Academy of Pediatrics, only 2% of pediatric rheumatology patients are routinely screened for depression with a validated instrument, and only 7% of those with depressive symptoms are screened, according to a 2016 study that Dr. Harper cited. Yet the same study found that nearly all pediatric rheumatologists (95%) supported routine depression screening every 6-12 months. Hence her team’s decision to test whether automating screening improved their screening rates.
Their population included lupus patients aged 12 and older seen at Nationwide Children’s Hospital between 2014 and 2022. Initially, patients completed the PHQ-9 on paper, which was then transcribed into the electronic health record. The process became automated and administered on an iPad at every visit in 2022. Positive screens – those endorsing suicidality or with a score of at least 10 – caused an alert to pop up for clinicians during their workflow so that they would talk to the mental health team about the patient’s needs.
A total of 149 patients completed 529 screenings during the study’s 8 years. Only 1 patient completed a PHQ-9 in 2014, which increased to just 17 patients in 2017. Automation resulted in 225 screens (P < .01). Subsequently, positive screens increased from 0% in 2014 to 25%-30% in 2018-2021, but then fell to 12% in 2022 (P < .01). The median PHQ-9 score was 3; overall scores decreased as screening increased.
The overall incidence of positive screens during the study period was 20% and prevalence was 38%, the authors reported. Of the 10 automated alerts triggered by positive screens, 90% resulted in a meeting with a psychologist or social worker, and 90% completed a suicide risk assessment. The intrusive alert for clinicians requires them to acknowledge the alert, agreeing to initiate a risk assessment, before they can enter data into the patient’s chart.
The study findings reveal “that you can successfully screen a high-risk population using an automated, seamless process, and you can alert providers without too much disruption to their typical clinic flow,” Dr. Harper told attendees. “And all of these processes have led to sustainability for routine depression screening in our lupus clinic.”
Dr. Harper’s team next plans to expand the automated screenings to populations with other diseases, to add an automated screening for anxiety, and to explore how PHQ-9 scores correlate with disease activity.
Treating patients’ mental health
Another two other abstracts at the symposium looked at another option, the 6-week cognitive-behavioral TEACH program. Deborah Levy, MD, MS, an associate professor of pediatrics at the University of Toronto and the clinical director of rheumatology at The Hospital for Sick Children, and colleagues assessed the program’s success when delivered remotely to adolescent patients with lupus. Pilot testing with TEACH had already shown improvements in fatigue and mood, Dr. Levy told attendees, but barriers to in-person delivery limited its utility even before the pandemic, so this study aimed to determine a remote version’s feasibility and effects, compared with treatment as usual.
The randomized, controlled trial, led by Natoshia Cunningham, PhD, from Michigan State University, Grand Rapids, included 57 participants, aged 12-22, from seven U.S. and Canadian rheumatology sites. All had been diagnosed with childhood-onset SLE by age 18 and had elevated symptoms in fatigue, pain, or depression. A PROMIS Fatigue T score of 60 or greater indicated elevated fatigue scores, whereas a high pain score was at least a 3/10 on a visual analog scale, and a high depression T score was at least a 60 but not higher than 80 on the Children’s Depression Inventory–2 or the Beck Depression Inventory–II (depending on the patient’s age).
Patients with other chronic medical conditions, developmental delays, or untreated major psychiatric illness were excluded from the study, as were patients who were receiving overlapping treatment, such as cognitive-behavioral therapy for pain or mood. Thirty patients were randomly assigned to receive treatment as usual while 27 patients were assigned to participate in the remote TEACH program.
Nearly all the patients (94%) were female, but they were racially diverse, with 42% White, 28% Asian, 19% Black, 19% Hispanic, and 4% multiracial. The patients were an average 16 years old and had been diagnosed for a median 5 years. Three of the intervention’s six modules involved the caregivers or, for older patients, their partners if desired. The communication strategies taught in the program were also tailored to patients’ ages.
“All of these strategies are educational, cognitive, behavioral, mindfulness strategies that target fatigue [and] pain, and they also developed web content for participants to use on their own,” Dr. Levy told attendees.
The researchers had complete postassessment data from 88% of participants, but they also reported some of the statements made during qualitative interviews about the program’s feasibility.
“I think it makes people more aware of themselves to become a better version of themselves, whether that’s in their normal life or in handling a lupus kind of life,” one participant said about the program’s benefits. Another appreciated the “alternative ways of thinking,” including “being more mindful of my thoughts and how those kind of aggravate my stress.”
The quantitative findings revealed a statistically significant reduction in depressive symptoms and fatigue for TEACH participants, compared with treatment as usual. Mood scores fell by an average 13.7 points in the TEACH group, compared with a drop of 2.4 points in the treatment as usual group (P < .001). Scores for fatigue fell 9.16 points in the TEACH group and 2.93 in the control group (P = .003). No statistically significant difference showed up in pain scores between the groups, although pain, medication adherence, and disease activity did improve slightly more in the TEACH group.
In addition to the significant improvements in mood and fatigue, therefore, “completion of TEACH may be associated with improved medication adherence and disease activity versus treatment as usual,” Dr. Levy said.
A much smaller study authored by some of the same researchers also assessed TEACH’s impact not in remote form but in terms of its value specifically for adolescent patients with SLE and elevated depression and fatigue scores. Comparison of 6 high-risk patients with 10 low-risk patients who underwent TEACH suggested that the program was especially effective for improving depression in high-risk patients since these patients had a statistically significantly greater improvement in mood. Fatigue, pain, anxiety, quality of life, and disease activity scores did not statistically differ between the groups.
Authors of the automated depression screening study reported no disclosures or outside funding. The study assessing psychological distress was funded by a CARRA–Arthritis Foundation grant, and the authors reported no disclosures. The remote TEACH study was funded by a CARRA–Arthritis Foundation grant, and all but one author reported no disclosures. One author had disclosures with Janssen, Roche, and Sobi. The high-risk TEACH study was also funded by a CARRA grant, and the authors had no disclosures.
AT PRSYM 2023
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
Pretransfer visits with pediatric and adult rheumatologists smooth adolescent transition
NEW ORLEANS – Implementing a pediatric transition program in which a patient meets with both their pediatric and soon-to-be adult rheumatologist during a visit before formal transition resulted in less time setting up the first adult visit, according to research presented at the Pediatric Rheumatology Symposium.
The presentation was one of two that focused on ways to improve the transition from pediatric to adult care for rheumatology patients. The other, a poster from researchers at Baylor College of Medicine, Houston, took the first steps toward learning what factors can help predict a successful transition.
“This period of transitioning from pediatric to adult care, both rheumatology specific and otherwise, is a high-risk time,” John M. Bridges, MD, a fourth-year pediatric rheumatology fellow at the University of Alabama at Birmingham, told attendees. “There are changes in insurance coverage, employment, geographic mobility, and shifting responsibilities between parents and children in the setting of a still-developing frontal lobe that contribute to the risk of this period. Risks include disease flare, and then organ damage, as well as issues with decreasing medication and therapy, adherence, unscheduled care utilization, and increasing loss to follow-up.”
Dr. Bridges developed a structured transition program called the Bridge to Adult Care from Childhood for Young Adults with Rheumatic Disease (BACC YARD) aimed at improving the pediatric transition period. The analysis he presented focused specifically on reducing loss to follow-up by introducing a pretransfer visit with both rheumatologists. The patient first meets with their pediatric rheumatologist.
During that visit, the adult rheumatologist attends and discusses the patient’s history and current therapy with the pediatric rheumatologist before entering the patient’s room and having “a brief introductory conversation, a sort of verbal handoff and handshake, in front of the patient,” Dr. Bridges explained. “Then I assume responsibility for this patient and their next visit is to see me, both proverbially and literally down the street at the adulthood rheumatology clinic, where this patient becomes a part of my continuity cohort.”
Bridges entered patients from this BACC YARD cohort into an observational registry that included their dual provider pretransfer visit and a posttransfer visit, occurring between July 2020 and May 2022. He compared these patients with a historical control cohort of 45 patients from March 2018 to March 2020, who had at least two pediatric rheumatology visits prior to their transfer to adult care and no documentation of outside rheumatology visits during the study period. Specifically, he examined at the requested and actual interval between patients’ final pediatric rheumatology visit and their first adult rheumatology visit.
The intervention cohort included 86 patients, mostly female (73%), with a median age of 20. About two-thirds were White (65%) and one-third (34%) were Black. One patient was Asian, and 7% were Hispanic. Just over half the patients had juvenile idiopathic arthritis (58%), and 30% had lupus and related connective tissue diseases. The other patients had vasculitis, uveitis, inflammatory myopathy, relapsing polychondritis, morphea, or syndrome of undifferentiated recurrent fever.
A total of 8% of these patients had previously been lost to follow-up at Children’s of Alabama before they re-established rheumatology care at UAB, and 3.5% came from a pediatric rheumatologist from somewhere other than Children’s of Alabama but established adult care at UAB through the BACC YARD program. Among the remaining patients, 65% (n = 56) had both a dual provider pretransfer visit and a posttransfer visit.
The BACC YARD patients requested their next rheumatology visit (the first adult one) a median 119 days after their last pediatric visit, and the actual time until that visit was a median 141 days (P < .05). By comparison, the 45 patients in the historical control group had a median 261 days between their last pediatric visit and their first adult visit (P < .001). The median days between visits was shorter for those with JIA (129 days) and lupus (119 days) than for patients with other conditions (149 days).
Bridges acknowledged that the study was limited by the small size of the cohort and potential contextual factors related to individual patients’ circumstances.
“We’re continuing to make iterative changes to this process to try to continue to improve the transition and its outcomes in this cohort,” Dr. Bridges said.
Aimee Hersh, MD, an associate professor of pediatric rheumatology and division chief of pediatric rheumatology at the University of Utah and Primary Children’s Hospital, both in Salt Lake City, attended the presentation and noted that the University of Utah has a very similar transfer program.
“I think one of the challenges of that model, and our model, is that you have to have a very specific type of physician who is both [medical-pediatrics] trained and has a specific interest in transition,” Dr. Hersh said in an interview. She noted that the adult rheumatologist at her institution didn’t train in pediatric rheumatology but did complete a meds-peds residency. “So if you can find an adult rheumatologist who can do something similar, can see older adolescent patients and serve as that transition bridge, then I think it is feasible.”
For practices that don’t have the resources for this kind of program, Dr. Hersh recommended the Got Transition program, which provides transition guidance that can be applied to any adolescent population with chronic illness.
The other study, led by Kristiana Nasto, BS, a third-year medical student at Baylor College of Medicine, reported on the findings from one aspect of a program also developed to improve the transition from pediatric to adult care for rheumatology patients. It included periodic self-reported evaluation using the validated Adolescent Assessment of Preparation for Transition (ADAPT) survey. As the first step to better understanding the factors that can predict successful transition, the researchers surveyed returning patients with any rheumatologic diagnosis, aged 14 years and older, between July 2021 and November 2022.
Since the survey was automated through the electronic medical record, patients and their caregivers could respond during in-person or virtual visit check-in. The researchers calculated three composite scores out of 100 for self-management, prescription management, and transfer planning, using responses from the ADAPT survey. Among 462 patients who returned 670 surveys, 87% provided surveys that could be scored for at least one composite score. Most respondents were female (75%), White (69%), non-Hispanic (64%), English speaking (90%), and aged 14-17 years (83%).
The overall average score for self-management from 401 respondents was 35. For prescription management, the average score was 59 from 288 respondents, and the average transfer planning score was 17 from 367 respondents. Self-management and transfer planning scores both improved with age (P = .0001). Self-management scores rose from an average of 20 at age 14 to an average of 64 at age 18 and older. Transfer planning scores increased from an average of 1 at age 14 to an average of 49 at age 18 and older. Prescription management scores remained high across all ages, from an average of 59 at age 14 to an average score of 66 at age 18 and older (P = .044). Although the scores did not statistically vary by age or race, Hispanic patients did score higher in self-management with an average of 44.5, compared with 31 among other patients (P = .0001).
Only 21% of patients completed two surveys, and 8.4% completed all three surveys. The average time between the first and second surveys was 4 months, during which there was no statistically significant change in self-management or prescription management scores, but transfer planning scores did increase from 14 to 21 (P = .008) among the 90 patients who completed those surveys.
The researchers concluded from their analysis that “participation in the transition pathway can rapidly improve transfer planning scores, [but] opportunities remain to improve readiness in all domains.” The researchers are in the process of developing Spanish-language surveys.
No external funding was noted for either study. Dr. Bridges, Dr. Hersh, and Ms. Nasto reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Implementing a pediatric transition program in which a patient meets with both their pediatric and soon-to-be adult rheumatologist during a visit before formal transition resulted in less time setting up the first adult visit, according to research presented at the Pediatric Rheumatology Symposium.
The presentation was one of two that focused on ways to improve the transition from pediatric to adult care for rheumatology patients. The other, a poster from researchers at Baylor College of Medicine, Houston, took the first steps toward learning what factors can help predict a successful transition.
“This period of transitioning from pediatric to adult care, both rheumatology specific and otherwise, is a high-risk time,” John M. Bridges, MD, a fourth-year pediatric rheumatology fellow at the University of Alabama at Birmingham, told attendees. “There are changes in insurance coverage, employment, geographic mobility, and shifting responsibilities between parents and children in the setting of a still-developing frontal lobe that contribute to the risk of this period. Risks include disease flare, and then organ damage, as well as issues with decreasing medication and therapy, adherence, unscheduled care utilization, and increasing loss to follow-up.”
Dr. Bridges developed a structured transition program called the Bridge to Adult Care from Childhood for Young Adults with Rheumatic Disease (BACC YARD) aimed at improving the pediatric transition period. The analysis he presented focused specifically on reducing loss to follow-up by introducing a pretransfer visit with both rheumatologists. The patient first meets with their pediatric rheumatologist.
During that visit, the adult rheumatologist attends and discusses the patient’s history and current therapy with the pediatric rheumatologist before entering the patient’s room and having “a brief introductory conversation, a sort of verbal handoff and handshake, in front of the patient,” Dr. Bridges explained. “Then I assume responsibility for this patient and their next visit is to see me, both proverbially and literally down the street at the adulthood rheumatology clinic, where this patient becomes a part of my continuity cohort.”
Bridges entered patients from this BACC YARD cohort into an observational registry that included their dual provider pretransfer visit and a posttransfer visit, occurring between July 2020 and May 2022. He compared these patients with a historical control cohort of 45 patients from March 2018 to March 2020, who had at least two pediatric rheumatology visits prior to their transfer to adult care and no documentation of outside rheumatology visits during the study period. Specifically, he examined at the requested and actual interval between patients’ final pediatric rheumatology visit and their first adult rheumatology visit.
The intervention cohort included 86 patients, mostly female (73%), with a median age of 20. About two-thirds were White (65%) and one-third (34%) were Black. One patient was Asian, and 7% were Hispanic. Just over half the patients had juvenile idiopathic arthritis (58%), and 30% had lupus and related connective tissue diseases. The other patients had vasculitis, uveitis, inflammatory myopathy, relapsing polychondritis, morphea, or syndrome of undifferentiated recurrent fever.
A total of 8% of these patients had previously been lost to follow-up at Children’s of Alabama before they re-established rheumatology care at UAB, and 3.5% came from a pediatric rheumatologist from somewhere other than Children’s of Alabama but established adult care at UAB through the BACC YARD program. Among the remaining patients, 65% (n = 56) had both a dual provider pretransfer visit and a posttransfer visit.
The BACC YARD patients requested their next rheumatology visit (the first adult one) a median 119 days after their last pediatric visit, and the actual time until that visit was a median 141 days (P < .05). By comparison, the 45 patients in the historical control group had a median 261 days between their last pediatric visit and their first adult visit (P < .001). The median days between visits was shorter for those with JIA (129 days) and lupus (119 days) than for patients with other conditions (149 days).
Bridges acknowledged that the study was limited by the small size of the cohort and potential contextual factors related to individual patients’ circumstances.
“We’re continuing to make iterative changes to this process to try to continue to improve the transition and its outcomes in this cohort,” Dr. Bridges said.
Aimee Hersh, MD, an associate professor of pediatric rheumatology and division chief of pediatric rheumatology at the University of Utah and Primary Children’s Hospital, both in Salt Lake City, attended the presentation and noted that the University of Utah has a very similar transfer program.
“I think one of the challenges of that model, and our model, is that you have to have a very specific type of physician who is both [medical-pediatrics] trained and has a specific interest in transition,” Dr. Hersh said in an interview. She noted that the adult rheumatologist at her institution didn’t train in pediatric rheumatology but did complete a meds-peds residency. “So if you can find an adult rheumatologist who can do something similar, can see older adolescent patients and serve as that transition bridge, then I think it is feasible.”
For practices that don’t have the resources for this kind of program, Dr. Hersh recommended the Got Transition program, which provides transition guidance that can be applied to any adolescent population with chronic illness.
The other study, led by Kristiana Nasto, BS, a third-year medical student at Baylor College of Medicine, reported on the findings from one aspect of a program also developed to improve the transition from pediatric to adult care for rheumatology patients. It included periodic self-reported evaluation using the validated Adolescent Assessment of Preparation for Transition (ADAPT) survey. As the first step to better understanding the factors that can predict successful transition, the researchers surveyed returning patients with any rheumatologic diagnosis, aged 14 years and older, between July 2021 and November 2022.
Since the survey was automated through the electronic medical record, patients and their caregivers could respond during in-person or virtual visit check-in. The researchers calculated three composite scores out of 100 for self-management, prescription management, and transfer planning, using responses from the ADAPT survey. Among 462 patients who returned 670 surveys, 87% provided surveys that could be scored for at least one composite score. Most respondents were female (75%), White (69%), non-Hispanic (64%), English speaking (90%), and aged 14-17 years (83%).
The overall average score for self-management from 401 respondents was 35. For prescription management, the average score was 59 from 288 respondents, and the average transfer planning score was 17 from 367 respondents. Self-management and transfer planning scores both improved with age (P = .0001). Self-management scores rose from an average of 20 at age 14 to an average of 64 at age 18 and older. Transfer planning scores increased from an average of 1 at age 14 to an average of 49 at age 18 and older. Prescription management scores remained high across all ages, from an average of 59 at age 14 to an average score of 66 at age 18 and older (P = .044). Although the scores did not statistically vary by age or race, Hispanic patients did score higher in self-management with an average of 44.5, compared with 31 among other patients (P = .0001).
Only 21% of patients completed two surveys, and 8.4% completed all three surveys. The average time between the first and second surveys was 4 months, during which there was no statistically significant change in self-management or prescription management scores, but transfer planning scores did increase from 14 to 21 (P = .008) among the 90 patients who completed those surveys.
The researchers concluded from their analysis that “participation in the transition pathway can rapidly improve transfer planning scores, [but] opportunities remain to improve readiness in all domains.” The researchers are in the process of developing Spanish-language surveys.
No external funding was noted for either study. Dr. Bridges, Dr. Hersh, and Ms. Nasto reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Implementing a pediatric transition program in which a patient meets with both their pediatric and soon-to-be adult rheumatologist during a visit before formal transition resulted in less time setting up the first adult visit, according to research presented at the Pediatric Rheumatology Symposium.
The presentation was one of two that focused on ways to improve the transition from pediatric to adult care for rheumatology patients. The other, a poster from researchers at Baylor College of Medicine, Houston, took the first steps toward learning what factors can help predict a successful transition.
“This period of transitioning from pediatric to adult care, both rheumatology specific and otherwise, is a high-risk time,” John M. Bridges, MD, a fourth-year pediatric rheumatology fellow at the University of Alabama at Birmingham, told attendees. “There are changes in insurance coverage, employment, geographic mobility, and shifting responsibilities between parents and children in the setting of a still-developing frontal lobe that contribute to the risk of this period. Risks include disease flare, and then organ damage, as well as issues with decreasing medication and therapy, adherence, unscheduled care utilization, and increasing loss to follow-up.”
Dr. Bridges developed a structured transition program called the Bridge to Adult Care from Childhood for Young Adults with Rheumatic Disease (BACC YARD) aimed at improving the pediatric transition period. The analysis he presented focused specifically on reducing loss to follow-up by introducing a pretransfer visit with both rheumatologists. The patient first meets with their pediatric rheumatologist.
During that visit, the adult rheumatologist attends and discusses the patient’s history and current therapy with the pediatric rheumatologist before entering the patient’s room and having “a brief introductory conversation, a sort of verbal handoff and handshake, in front of the patient,” Dr. Bridges explained. “Then I assume responsibility for this patient and their next visit is to see me, both proverbially and literally down the street at the adulthood rheumatology clinic, where this patient becomes a part of my continuity cohort.”
Bridges entered patients from this BACC YARD cohort into an observational registry that included their dual provider pretransfer visit and a posttransfer visit, occurring between July 2020 and May 2022. He compared these patients with a historical control cohort of 45 patients from March 2018 to March 2020, who had at least two pediatric rheumatology visits prior to their transfer to adult care and no documentation of outside rheumatology visits during the study period. Specifically, he examined at the requested and actual interval between patients’ final pediatric rheumatology visit and their first adult rheumatology visit.
The intervention cohort included 86 patients, mostly female (73%), with a median age of 20. About two-thirds were White (65%) and one-third (34%) were Black. One patient was Asian, and 7% were Hispanic. Just over half the patients had juvenile idiopathic arthritis (58%), and 30% had lupus and related connective tissue diseases. The other patients had vasculitis, uveitis, inflammatory myopathy, relapsing polychondritis, morphea, or syndrome of undifferentiated recurrent fever.
A total of 8% of these patients had previously been lost to follow-up at Children’s of Alabama before they re-established rheumatology care at UAB, and 3.5% came from a pediatric rheumatologist from somewhere other than Children’s of Alabama but established adult care at UAB through the BACC YARD program. Among the remaining patients, 65% (n = 56) had both a dual provider pretransfer visit and a posttransfer visit.
The BACC YARD patients requested their next rheumatology visit (the first adult one) a median 119 days after their last pediatric visit, and the actual time until that visit was a median 141 days (P < .05). By comparison, the 45 patients in the historical control group had a median 261 days between their last pediatric visit and their first adult visit (P < .001). The median days between visits was shorter for those with JIA (129 days) and lupus (119 days) than for patients with other conditions (149 days).
Bridges acknowledged that the study was limited by the small size of the cohort and potential contextual factors related to individual patients’ circumstances.
“We’re continuing to make iterative changes to this process to try to continue to improve the transition and its outcomes in this cohort,” Dr. Bridges said.
Aimee Hersh, MD, an associate professor of pediatric rheumatology and division chief of pediatric rheumatology at the University of Utah and Primary Children’s Hospital, both in Salt Lake City, attended the presentation and noted that the University of Utah has a very similar transfer program.
“I think one of the challenges of that model, and our model, is that you have to have a very specific type of physician who is both [medical-pediatrics] trained and has a specific interest in transition,” Dr. Hersh said in an interview. She noted that the adult rheumatologist at her institution didn’t train in pediatric rheumatology but did complete a meds-peds residency. “So if you can find an adult rheumatologist who can do something similar, can see older adolescent patients and serve as that transition bridge, then I think it is feasible.”
For practices that don’t have the resources for this kind of program, Dr. Hersh recommended the Got Transition program, which provides transition guidance that can be applied to any adolescent population with chronic illness.
The other study, led by Kristiana Nasto, BS, a third-year medical student at Baylor College of Medicine, reported on the findings from one aspect of a program also developed to improve the transition from pediatric to adult care for rheumatology patients. It included periodic self-reported evaluation using the validated Adolescent Assessment of Preparation for Transition (ADAPT) survey. As the first step to better understanding the factors that can predict successful transition, the researchers surveyed returning patients with any rheumatologic diagnosis, aged 14 years and older, between July 2021 and November 2022.
Since the survey was automated through the electronic medical record, patients and their caregivers could respond during in-person or virtual visit check-in. The researchers calculated three composite scores out of 100 for self-management, prescription management, and transfer planning, using responses from the ADAPT survey. Among 462 patients who returned 670 surveys, 87% provided surveys that could be scored for at least one composite score. Most respondents were female (75%), White (69%), non-Hispanic (64%), English speaking (90%), and aged 14-17 years (83%).
The overall average score for self-management from 401 respondents was 35. For prescription management, the average score was 59 from 288 respondents, and the average transfer planning score was 17 from 367 respondents. Self-management and transfer planning scores both improved with age (P = .0001). Self-management scores rose from an average of 20 at age 14 to an average of 64 at age 18 and older. Transfer planning scores increased from an average of 1 at age 14 to an average of 49 at age 18 and older. Prescription management scores remained high across all ages, from an average of 59 at age 14 to an average score of 66 at age 18 and older (P = .044). Although the scores did not statistically vary by age or race, Hispanic patients did score higher in self-management with an average of 44.5, compared with 31 among other patients (P = .0001).
Only 21% of patients completed two surveys, and 8.4% completed all three surveys. The average time between the first and second surveys was 4 months, during which there was no statistically significant change in self-management or prescription management scores, but transfer planning scores did increase from 14 to 21 (P = .008) among the 90 patients who completed those surveys.
The researchers concluded from their analysis that “participation in the transition pathway can rapidly improve transfer planning scores, [but] opportunities remain to improve readiness in all domains.” The researchers are in the process of developing Spanish-language surveys.
No external funding was noted for either study. Dr. Bridges, Dr. Hersh, and Ms. Nasto reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
Little change in rheumatology faculty coverage in pediatric residency programs in nearly 20 years
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – More than one-third of pediatric residency programs do not have a pediatric rheumatologist on faculty, a situation that has changed little since 2004, according to a poster presented at the Pediatric Rheumatology Symposium 2023 conference.
“This shortage has significant downstream effects,” according to author Miriah Gillispie-Taylor, MD, an assistant professor of pediatric rheumatology at Baylor College of Medicine and Texas Children’s Hospital in Houston. Without adequate education, it’s unreasonable to expect that a pediatrician will recognize the great diversity of presentations among rheumatic diseases, for example. “Without recognition, patients are not referred in a timely manner, and earlier identification and treatment of rheumatic diseases leads to improved outcomes,” Dr. Gillispie-Taylor said.
Currently, eight U.S. states do not have a board-certified pediatric rheumatologist, including Alaska. Dr. Gillispie-Taylor cited a 2006 study that found that one-third of medical schools (33%) and 40% of U.S. pediatric residency programs did not have an on-site pediatric rheumatologist in 2004.
As the long-standing workforce shortage in pediatric rheumatology continues, Dr. Gillispie-Taylor and her colleagues investigated whether increasing awareness of this problem has influenced the number of United States and Puerto Rico residency training programs with pediatric rheumatology faculty from 2004 to present.
The researchers identified 212 pediatric residency programs accredited by the Accreditation Council for Graduate Medical Education for 2022-2023 and reviewed their program website to see which ones had affiliated pediatric rheumatology faculty. After determining the faculty from the website for 85% of the programs, the researchers emailed the other programs to find out whether a pediatric rheumatologist was on faculty, filling out another 6% of the programs. Most of the remaining uncategorized programs (7%) were categorized at a meeting of the Childhood Arthritis and Rheumatology Research Alliance medical education workgroup. Only 2% of programs could not be ultimately categorized.
The region with the greatest proportion of pediatric residency programs that had a pediatric rheumatologist was the Southeast, where 95% (36 of 38 programs) of programs had one on faculty. The Southwest, comprising Texas, Oklahoma, New Mexico, and Arizona, had the lowest proportion: 43% (9 of 21 programs). For the other regions, 69% of the West/Pacific Northwest (18 of 26), 62% of the Midwest (28 of 45), and 61% of the Northeast (39 of 64) programs had a pediatric rheumatologist on faculty. Three of Puerto Rico’s four programs had one as well.
Overall, 63% of programs had a pediatric rheumatologist on faculty, and 36% did not; the state of three programs was unknown.
The large proportion of programs without a pediatric rheumatologist “limits exposure to rheumatologic conditions and learning opportunities during residency and contribute to declining fellow match rates,” the authors concluded. They noted that only 62.8% of pediatric rheumatology fellowship positions were filled in 2022, down slightly from the 69.2% filled in 2021, according to report data from the National Matching Resident Program.
The researchers acknowledged that their results could be skewed if website information was outdated for any programs, and it’s difficult to determine which programs might lack resources on the basis of only publicly available information. Though programs without pediatric rheumatologists might benefit from visiting professorships, it can be difficult to identify which ones, they added.
The authors recommend two next steps: one, establishing areas of essential knowledge in pediatric rheumatology to enable the creation of learning objectives so programs can focus their educational efforts; and two, continuing efforts to understand residents’ motivation to pursue fellowships in pediatric rheumatology for the purpose of improving recruitment.
Two medical students at Dr. Gillispie-Taylor’s institution spoke with this news organization about their thoughts on the findings and how they were approaching their own career goals in medicine in light of these findings.
Kyla Fergason, a second-year medical student at Baylor College of Medicine, said that she thinks she wants to pursue pediatrics or meds-peds. Though she’s not sure whether she specifically wants to pursue pediatric rheumatology, she is very interested in the area and said that she has learned much from the Pediatric Rheumatology Symposium conference. She found the dearth of pediatric rheumatology faculty at residency programs worrisome, particularly in states like Alaska and Hawaii because they aren’t contiguous with the rest of the United States. Only three pediatric rheumatologists are practicing in Hawaii.
“It’s really concerning that sometimes there is not any rheumatologist there to see the patient,” Ms. Fergason told this news organization. “These are diseases that affect people chronically throughout their entire lives, so it’s definitely concerning to think that, at a time when they could be helped and there could be interventions made, none are made because there’s just no one available.”
Kristiana Nasto, a third-year medical student at Baylor College of Medicine, is similarly interested in pediatrics but leaning more toward meds-peds and has an interest in rheumatology as well. She was surprised at how many programs had no pediatric rheumatologist on faculty because Baylor has a robust program.
“I was not aware of the fact that other states or other parts of Texas do not have the luxury of the great rheumatologists that we have at Baylor College of Medicine,” Ms. Nasto said. “That can definitely impact care for many patients because some of these rheumatologic diseases are so unique and challenging to treat that they require specialized care, so it makes me a bit sad that this is the case.”
Dr. Gillispie-Taylor has received an educational grant from Pfizer. Ms. Fergason and Ms. Nasto had no disclosures. No external funding was noted for the study.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
Biosimilars and patients: Discussions should address safety, cost, and anxiety about change
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.
Rheumatologist Marcus Snow, MD, is comfortable with prescribing biosimilars as a first-line, first-time biologic, and discussing them with patients.
“If a biosimilar is on the market, it has gone through rigorous study proving its effectiveness and equivalence to a bio-originator,” said Dr. Snow, a rheumatologist with the University of Nebraska Medical Center, Omaha, and chair of the American College of Rheumatology’s Committee on Rheumatologic Care.
The formulary makes a big difference in the conversation about options, he said. “The formularies dictate what we can prescribe. It may not be appropriate, but it is reality. The cost of biologics for a patient without insurance coverage makes it impossible to afford.”
He will often tell patients that he’ll fight any changes or formulary restrictions he does not agree with. “However, when I see patients in follow-up, even if there is no known change on the horizon, I may bring up biosimilars when we have a moment to chat about them to familiarize them with what may happen in the future.”
The need for patient education on biosimilars presents a barrier to realizing their potential to save money and expand choice, noted Cardinal Health in its 2023 biosimilars report. Of 103 rheumatologists who responded to a Cardinal Health survey, 85% agreed that patient education was important. But those conversations can take an uncomfortable turn if the patient pushes back against taking a biosimilar owing to cost or safety concerns.
It’s not uncommon for a patient to express some anxiety about biosimilars, especially if they’re doing well on a current treatment plan. Most patients do not want any changes that may lead to worsening disease control, Dr. Snow said.
Patients and physicians alike often don’t understand the mechanics of biosimilars. “There’s a lot of misinformation about this,” said Sameer Awsare, MD, an associate executive director for The Permanente Medical Group in Campbell, Calif. Patients should know that a biosimilar will be as clinically efficacious as the medicine they’ve been on, with the same safety profiles, said Dr. Awsare, who works with Kaiser Permanente’s pharmacy partners on biosimilars.
Insurance often drives the conversation
The global anti-inflammatory biologics market is anticipated to reach $150 billion by 2027, according to a recent CVS report. As of March 2023, the Food and Drug Administration had approved 40 biosimilars to 11 different reference products. There are 28 on the U.S. market and 100 more in development. Projected to save more than $180 billion over the next 5 years, they are anticipated to expand choice and drive competition.
Rheumatologists, dermatologists, and gastroenterologists are frequent prescribers, although their choices for immune-mediated inflammatory diseases are limited to tumor necrosis factor inhibitors (infliximab [Remicade] originator and adalimumab [Humira] originator) and anti-CD20 agents, such as rituximab (Rituxan) originator.
Benefit design or formulary usually dictates what medicine a patient receives. “Because of significantly higher out-of-pocket cost or formulary positioning, patients may end up with a generic or a biosimilar instead of a brand-name medicine or branded biologic,” said Robert Popovian, PharmD, MS, chief science policy officer of the Global Healthy Living Foundation.
Insurers rarely offer both Remicade and biosimilar infliximab, allowing the doctor to choose, said Miguel Regueiro, MD, chair of the Cleveland Clinic’s Digestive Disease & Surgery Institute, who prescribes infliximab biosimilars. Most often, the payer will choose the lower-cost biosimilar. “I am fine with the biosimilar, either as a new start or a switch from the reference product.”
However, the patient might feel differently. They can form an attachment to the reference medication if it has prevented severe illness. “They do not want to change, as they feel they are going on a ‘new’ medication that will not work as well,” Dr. Regueiro said.
This is where the education comes in: to reassure patients that a biosimilar will work just as well as the reference product. “For patients who have done well for years on a biologic, more time needs to be spent reassuring them and answering questions,” compared with a patient just starting on a biosimilar, he advised.
But not all physicians are quick to prescribe biosimilars.
Especially with psoriasis, which has so many strong options for reference drugs, a switch may be hard to justify, said dermatologist Stephanie K. Fabbro, MD, assistant professor at Northeast Ohio Medical University, Rootstown. “If I have a preference, I would rather switch a patient to a drug from a different class without a biosimilar option to reduce the possibility of pushback.”
Dr. Fabbro, part of the core faculty in the Riverside Methodist Hospital Dermatology Residency Program in Columbus, will share data from clinical trials and postmarket surveillance with patients to support her decision.
Conversations about cost
Patients may also push back if they don’t save money when switching to a biosimilar. “This dilemma raises the question of who is profiting when a biosimilar is dispensed,” Dr. Popovian said. Insurers and pharmacy benefit managers (PBMs) that take additional concessions from biopharmaceutical manufacturers in the form of rebates and fees will often pocket this money as profit instead of passing savings back to the patient to help reduce their out-of-pocket requirement, he added.
If an originator biologic and a biosimilar are available, “as a pharmacist, I will choose the medicine that will incur the lowest out-of-pocket cost for the patient,” Dr. Popovian said.
Discussing cost – and who dictates which biosimilar is on the formulary – is an important conversation to have with patients, said Vivek Kaul, MD, Segal-Watson Professor of Medicine at the University of Rochester (N.Y.) Medical Center.
Providing equivalent clinical efficacy while saving costs is the economic reality of biosimilars, Dr. Kaul said. Third-party payers regularly evaluate how to provide the same quality of care while saving money. Physicians and patients alike “must be mindful that as time goes on, if the science on biosimilars stays robust, if the adoption is more widespread and the cost-saving proposition turns out to be true, more formularies will be attracted to replacing the reference product with the biosimilar counterpart.”
Providers and patients can weigh the options if a formulary suddenly switches to a biosimilar, Dr. Kaul continued. “You can accept the novel product on the formulary or may have to face out-of-pocket expenses as a patient.” If providers and patients have concerns about the biosimilar, they can always appeal if there’s solid scientific evidence that supports reverting back to the reference product.
“If you think the biosimilar is equally efficacious, comes at a lower cost, and is right for the patient, then the providers should tell the patient that,” he added.
Some studies have questioned whether the biosimilars will save money, compared with the reference drug, Dr. Fabbro noted. Medicare, for example, may pay only for a certain percentage of an approved biosimilar, saddling the patient with a monthly copay costing thousands of dollars. “It is unclear whether biosimilar manufacturers will have the same level of patient support programs as the reference drug companies.”
For that reason, physicians should also inform patients about the robust patient assistance and copay assistance programs many reference drug manufacturers offer, she said.
Biosimilars 101: Familiarizing patients
Safety and ease of use are other common concerns about biosimilars. Patients may ask if the application is different, or why it’s advantageous to switch to a biosimilar, Dr. Awsare said.
Sometimes the syringe or injector for a biosimilar might look different from that of the originator drug, he said.
Anecdotally, Dr. Fabbro has heard stories of patients having injection reactions that they did not experience with the reference drug or having a disease flare-up after starting a biosimilar.
As is the case with reference products, in their conversations with patients, clinicians should address the adverse event profile of biosimilars, offering data points from published studies and clinical guidelines that support the use of these products. “There should be an emphasis on patient education around efficacy and any side effects, and how the profile of the reference product compares with a proposed biosimilar,” Dr. Kaul suggested.
When Dr. Snow discusses biosimilars and generics, “I make sure to share this in an understandable way based on the patient’s scientific background, or lack thereof,” he said. If there is enough time, he also discusses how European- and U.S.-sourced biologics are slightly different.
Pharmacists should tell patients to expect the same clinical outcomes from a biosimilar, Dr. Popovian said. However, if they have any reduction in efficacy or potential safety concerns, they should communicate with their physician or pharmacist immediately.
In Dr. Regueiro’s practice, a pharmacist specializing in inflammatory bowel disease often has a one-on-one meeting with patients to educate and answer questions. “Additionally, we provide them the Crohn’s and Colitis Foundation web link on biosimilars,” said Dr. Regueiro.
A village approach to education
When biosimilars first came out, there were no formal education materials, Dr. Awsare said. Kaiser Permanente decided to create its own educational materials, not just for patients but also to help educate its primary care doctors; the rheumatologists, dermatologists, and gastroenterologists using the biosimilars; the nurses infusing patients; and the pharmacists preparing the biosimilars.
The health system also has a different approach to choosing medication. Instead of having an insurance company or PBM decide what’s in the formulary, clinicians work with the pharmacists at Kaiser to look at clinical evidence and decide which biosimilar to use. Most of its plans also provide lower copays to patients when they use the biosimilar.
This was the approach for Humira biosimilars, Dr. Awsare said. Eight will be on the market in 2023. “Our rheumatologists, dermatologists, and gastroenterologists looked at the data from Europe, looked at some real-world evidence, and then said: ‘We think this one’s going to be the best one for our patients.’ ”
Having clinicians choose the biosimilar instead of a health plan makes it a lot easier to have conversations with patients, he said. “Once we’ve moved that market share to that particular biosimilar, we give our physicians the time to have those discussions.”
Clinical pharmacists also provide educational support, offering guidance on issues such as side effects, as patients transition to the biosimilar. “We like to use the word ‘transition’ because it’s essentially the same biologic. So, you’re not actually switching,” Dr. Awsare said.
No consensus on interchangeability
Whether the conversation on interchangeability will affect patient conversations with physicians depends on who you ask.
If a biosimilar has an interchangeability designation, it means that the pharmacist can substitute it without the intervention of the clinician who prescribed the reference product. It does not relate to the quality, safety, or effectiveness of biosimilars or interchangeable biosimilar products, Dr. Popovian said.
The United States is the only country that has this designation. Even though it’s not identical to the originator drug, a biosimilar has the same clinical efficacy and safety profile. “So clinically, interchangeability is meaningless,” Dr. Awsare said.
In its report on biosimilars in the autoimmune category, CVS acknowledged that interchangeability was important but would not be a significant factor in driving adoption of biosimilars. However, in a Cardinal Health survey of 72 gastroenterologists, 38% cited the interchangeability of biosimilars as a top concern for adalimumab biosimilars, along with transitioning patients from Humira to a biosimilar (44%).
“Patient education regarding biosimilar safety, efficacy, and interchangeability appears paramount to the acceptance of these products, particularly for patients who are switched from a reference product,” Dr. Kaul noted in the Cardinal Health report.
Wherever supported by data, Dr. Kaul recommends incorporating biosimilar use and interchangeability into best practice guidelines going forward. “That will go a long way in disseminating the latest information on this topic and position this paradigm for increased adoption among providers.”
Some physicians like Dr. Snow aren’t that concerned with interchangeability. This hasn’t affected conversations with patients, he said. Multiple studies demonstrating the lack of antibody formation with multiple switches from different biosimilar drugs has eased his concern about multiple switches causing problems.
“Initially, there was a gap in demonstrating the long-term effect of multiple switches on antibody production and drug effectiveness. That gap has started to close as more data from Europe’s experience with biosimilars becomes available,” Dr. Snow said.
Resources for physicians, patients
The federal government has taken steps to advance biosimilars education and adoption. In 2021, President Biden signed the Advancing Education on Biosimilars Act into law, which directs the FDA to develop or improve continuing education programs that address prescribing of biosimilars and biological products.
The FDA provides educational materials on its website, including a comprehensive curriculum toolkit. The Accreditation Council for Medical Affairs has also created an online 40-hour curriculum for health care professionals called the Board-Certified Biologics and Biosimilars Specialist Program.
Dr. Fabbro recommended patients use the FDA page Biosimilar Basics for Patients to educate themselves on biosimilars. The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars, is another free resource for patients.
“While much has changed, the continued need for multistakeholder education, awareness, and dedicated research remains even more important as we expand into newer therapeutic areas and classes,” wrote the authors of the Cardinal Health report.
Help patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars.
Dr. Regueiro is on advisory boards and consults for AbbVie, Janssen, UCB, Takeda, Pfizer, Bristol-Myers Squibb, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET PharmaSolutions, Trellis, and Boehringer Ingelheim. Dr. Fabbro is a principal investigator for Castle Biosciences, on the speakers bureau for Valchlor, and on the advisory boards of Janssen and Bristol-Myers Squibb. Dr. Popovian, Dr. Snow, Dr. Awsare, and Dr. Kaul had no disclosures.
A version of this article originally appeared on Medscape.com.