User login
Hearing Loss, Hearing Aids, and Dementia Risk: What to Tell Your Patients
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Is Vision Loss a New Dementia Risk Factor? What Do the Data Say?
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
In 2019, 57 million people worldwide were living with dementia, a figure expected to soar to 153 million by 2050. A recent Lancet Commission report suggests that nearly half of dementia cases could be prevented or delayed by addressing 14 modifiable risk factors, including impaired vision.
The report’s authors recommend that vision-loss screening and treatment be universally available. But are these recommendations warranted? What is the evidence? What is the potential mechanism? And what are the potential implications for clinical practice?
Worldwide, the prevalence of avoidable vision loss and blindness in adults aged 50 years or older is estimated to hover around 13%.
“There is now overwhelming evidence that vision impairment in later life is associated with more rapid cognitive decline and an increased risk of dementia,” said Joshua Ehrlich, MD, MPH, associate professor in ophthalmology and visual sciences, the Institute for Social Research at the University of Michigan, Ann Arbor.
The evidence includes a meta-analysis of 14 prospective cohort studies with roughly 6.2 million older adults who were cognitively intact at baseline. Over the course of up to 14 years, 171,888 developed dementia. Vision loss was associated with a pooled relative risk (RR) for dementia of 1.47.
A separate meta-analysis also identified an increased risk for dementia (RR, 1.38) with visual loss. When broken down into different eye conditions, an increased dementia risk was associated with cataracts and diabetic retinopathy but not with glaucoma or age-related macular degeneration.
A US study that followed roughly 3000 older adults with cataracts and normal cognition at baseline for more than 20 years found that those who had cataract extraction had significantly reduced risk for dementia compared with those who did not have cataract extraction (hazard ratio, 0.71), after controlling for age, race, APOE genotype, education, smoking, and an extensive list of comorbidities.
Causation or Coincidence?
The mechanisms behind these associations might be related to underlying illness, such as diabetes, which is a risk factor for dementia; vision loss itself, as might be suggested by a possible effect of cataract surgery; or shared neuropathologic processes in the retina and the brain.
A longitudinal study from Korea that included roughly 6 million adults showed that dementia risk increased with severity of visual loss, which supports the hypothesis that vision loss in itself might be causal or that there is a dose-response effect to a shared causal factor.
“Work is still needed to sort out” exactly how visual deficits may raise dementia risk, although several hypotheses exist, Dr. Ehrlich said.
For example, “decreased input to the brain via the visual pathways may directly induce brain changes. Also, consequences of vision loss, like social isolation, physical inactivity, and depression, are themselves risk factors for dementia and may explain the pathways through which vision impairment increases risk,” he said.
Is the link causal? “We’ll never know definitively because we can’t randomize people to not get cataract surgery versus getting cataract surgery, because we know that improving vision improves quality of life, so we’d never want to do that. But the new evidence that’s come in over the last 5 years or so is pretty promising,” said Esme Fuller-Thomson, PhD, director of the Institute for Life Course and Aging and professor, Department of Family and Community Medicine and Faculty of Nursing, at the University of Toronto, Ontario, Canada.
She noted that results of two studies that have looked at this “seem to indicate that those who have cataract surgery are not nearly at as high risk of dementia as those who have cataracts but don’t have the surgery. That’s leaning towards causality.”
A study published in July suggests that cataracts increase dementia risk through vascular and non–Alzheimer’s disease mechanisms.
Clear Clinical Implications
Dr. Ehrlich said that evidence for an association between untreated vision loss and dementia risk and potential modification by treatment has clear implications for care.
“Loss of vision impacts so many aspects of people’s lives beyond just how they see the world and losing vision in later life is not a normal part of aging. Thus, when older adults experience vision loss, this should be a cause for concern and prompt an immediate referral to an eye care professional,” he noted.
Dr. Fuller-Thomson agrees. “Addressing vision loss will certainly help people see better and function at a higher level and improve quality of life, and it seems probable that it might decrease dementia risk so it’s a win-win,” she said.
In her own research, Dr. Fuller-Thomson has found that the combination of hearing loss and vision loss is linked to an eightfold increased risk for cognitive impairment.
“The idea is that vision and/or hearing loss makes it harder for you to be physically active, to be socially engaged, to be mentally stimulated. They are equally important in terms of social isolation, which could lead to loneliness, and we know that loneliness is not good for dementia,” she said.
“With dual sensory impairment, you don’t have as much information coming in — your brain is not engaged as much — and having an engaged brain, doing hobbies, having intellectually stimulating conversation, all of those are factors are associated with lowering risk of dementia,” Dr. Fuller-Thomson said.
The latest Lancet Commission report noted that treatment for visual loss is “effective and cost-effective” for an estimated 90% of people. However, across the world, particularly in low- and middle-income countries, visual loss often goes untreated.
the report concluded.
Dr. Ehrlich and Dr. Fuller-Thomson have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FDA ‘Recalls’ Often Leave Targeted Medical Devices in Use
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
In 2016, medical device giant Abbott issued a recall for its MitraClip cardiac device — “a Class I recall, the most serious type,” the FDA said.
“Use of this device may cause serious injuries or death,” an FDA notice about the recall said.
But neither the manufacturer nor the FDA actually recalled the device or suspended its use. They allowed doctors to continue implanting the clips in leaky heart valves in what has become a common procedure.
In a notice, the manufacturer explained, “Abbott is not removing product from commercial distribution.” Rather, Abbott revised instructions for use and required doctors who implant the clips to undergo training.
“It’s very oxymoronic,” said Rita Redberg, a cardiologist at the University of California-San Francisco and former editor-in-chief of the journal JAMA Internal Medicine. “A recall makes it sound like it’s recalled. But that is not actually what it means.”
Though the FDA and federal regulations call these actions recalls, they might be described more aptly as “non-recalls.” And they have happened repeatedly in recent years. For instance, in addition to other Abbott devices, products made by Medtronic, Abiomed, and Getinge have had recalls that left them in use.
Safeguarding the Public
Recalls that leave what the FDA identifies as potentially dangerous products in the marketplace can raise the question: Do they do enough to protect the public?
There are other ways to handle recalls. In announcements about products as varied as crib bumpers, pool drain covers, bicycle helmets, and coffee mugs, the Consumer Product Safety Commission routinely alerts consumers to stop using recalled products and contact the manufacturers for refunds, repairs, or replacements. The National Highway Traffic Safety Administration regularly advises consumers to bring recalled cars back to the dealer to have them fixed. When the U.S. Department of Agriculture and the FDA announce food recalls, they routinely tell consumers to return or discard the food.
In some cases, a medical device that is the subject of a recall can be kept on the market safely because there is a simple fix, said Sanket Dhruva, a cardiologist and an associate professor at UCSF who has studied FDA oversight of devices. In other cases, recalls that don’t remove devices from the market can provide unwarranted reassurance and leave the public at risk, Dhruva said.
From 2019 through 2023, there were 338 Class I medical device recalls, 164 of which were corrections and 174 of which were removals, FDA spokesperson Amanda Hils said.
Some products undergo recall after recall while they remain on the market. Products in the MitraClip line have been the subject of three rounds of recalls, none of which removed devices from use.
“When deciding whether a recall warrants device removal from the field, the FDA considers the frequency and severity of adverse events, effectiveness of the corrective actions that have been executed, and the benefits and risks of preserving patient access to the device,” FDA spokesperson Audra Harrison said.
Where recalled devices have already been implanted, “removal” doesn’t necessarily mean removing them from patients’ bodies. “When an implanted device has the potential to fail unexpectedly, companies often tell doctors to contact their patients to discuss the risk of removing the device compared to the risk of leaving it in place,” the FDA website says.
The FDA allowed the recalled MitraClip devices to remain in use “because the agency believed that the overall benefits of the device continued to outweigh the risks and the firm’s recall strategy was appropriate and adequate,” Harrison said.
The FDA reviews the recall strategies that manufacturers propose and often provides input to ensure the public will be protected, Hils said. The agency also monitors the effectiveness of recalls and, before terminating them, makes sure the strategy was carried out, Hils said.
Abbott, the maker of MitraClip, said the device has been proven safe and effective “based on more than 20 years of clinical evidence and has profoundly improved the lives of people living with mitral regurgitation,” a condition in which blood flows backward through the heart’s mitral valve. The condition can lead to heart failure and death.
“With MitraClip, we’re addressing the needs of people with MR who often have no other options,” company spokesperson Brent Tippen said.
Speaking of the MitraClip recalls, Redberg said, “So hard to imagine these are effective actions in protecting patients.”
In 2021, for Medtronic’s StealthStation S7 cranial software, the company and the FDA sent a different message.
StealthStation is an elaborate system of screens and other equipment that guides neurosurgeons using instruments in the brain — for instance, to biopsy or cut out tumors. Drawing from CT scans, MRIs, and other imaging, it’s meant to show the location of the surgical instruments.
In connection with a Class I November 2021 recall, the FDA website said potential inaccuracies in a biopsy depth gauge could result in “life-threatening injury (such as hemorrhage, unintended tissue damage, or permanent neurological injury), which could lead to death.”
The FDA website explained what Medtronic was doing about it.
“The recalling firm will provide a warning and instructional placard to be applied to impacted systems,” the website said. “Until a software update is available, ensure you are following the instructions below to prevent the issue from occurring,” it advised doctors.
In a statement to KFF Health News, Medtronic spokesperson Erika Winkels said the safety and well-being of patients is the company’s primary concern, and certain issues “can be safely and effectively remedied with a correction on site.”
Richard Everson, a neurosurgeon and an assistant professor at UCLA, noted that the 2021 recall allowed doctors to continue using unaffected StealthStation features, a benefit for patients and facilities depending on them.
“But, I mean, then you could ask, ‘Well, why don’t they just disable the view [of the brain] that’s bugged?’” Everson said. “Why would they give you the option of looking at an inaccurate one?”
“That’s kind of a strange solution,” he said.
The FDA lists the 2021 recall as still open, explaining “not all products have been corrected or removed.”
That recall was not the last word on problems with StealthStation. Since then, the manufacturer has submitted adverse event reports to the FDA describing trouble in cases involving various versions of StealthStation.
In a September 2022 case, guidance provided by a StealthStation device was allegedly off the mark, a procedure was aborted, and, when the patient awoke, they “had almost no speech for two days,” according to a Medtronic report. In the report, Medtronic said there was “insufficient information to determine the relationship of the software to the reported issue.”
In a February 2024 case, after brain surgery, an MRI found that the operation “missed the tumor” and that other tissue was removed instead, according to a report Medtronic submitted to the FDA. In the report, Medtronic said that when a company representative tested the system, it performed as intended.
In March 2024, Medtronic recalled versions of StealthStation S8 without removing them from hospitals. The company said at the time that it would provide a software update.
“Software updates are available to correct the anomalies identified in the 2021 S7 and 2024 S8 recalls and are actively being deployed,” Medtronic’s Winkels told KFF Health News in a July email. “While the software updates for the 2021 S7 recall are complete in the US, they remain ongoing in some international regions.”
In June 2023, Abiomed issued an urgent medical device correction for its Impella 2.5 intravascular micro axial blood pump, which supports the heart. In patients with a certain type of replacement heart valve, there was a risk of “destruction of the impeller blades,” which could cause “low flow” and “embolization of the fractured impeller material,” an entry on the FDA website said.
“Clinicians are cautioned to position the Impella system carefully in patients,” the FDA website said, among other instructions.
The updated instructions “provide technical guidance to mitigate the risk of rare complications,” Abiomed spokesperson Ryan Carbain said. There were no product removals and no reports of adverse events “related to product design or manufacturing,” Carbain said.
Another set of medical devices, Cardiosave Hybrid and Rescue Intra-Aortic Balloon Pumps made by Getinge of Sweden, have failed persistently, according to FDA records.
The devices — which are placed in the aorta, a major artery, to assist the heart — were the subject of eight Class I recalls from December 2022 to July 2023. All were corrections rather than removals, a KFF Health News analysis found.
In a May 2024 letter to health care providers, the FDA said that, in the previous 12 months, it had received almost 3,000 adverse event reports related to the balloon pumps. It was referring to reports of malfunctions and cases in which the products might have caused or contributed to a death or injury. Of those, 15 reportedly involved serious injury or death, the FDA said.
During the summer of 2023, the FDA noted that “alternative treatments are limited” and said the devices could continue to be used.
But, in May, the FDA changed its stance. The agency advised health care facilities to “transition away from these devices and seek alternatives, if possible.”
“These recommendations are based on our continued concerns” that the manufacturer “has not sufficiently addressed the problems and risks with these recalled devices.”
Getinge sent KFF Health News written answers from Elin Frostehav, the company’s president of Acute Care Therapies.
“There is no question that we would have liked to have solved these issues in full much earlier,” she said.
As a result of the FDA’s May action, the company “immediately paused proactive marketing” of the balloon pumps in the United States, and it is selling them only to customers who have no alternatives, Frostehav said.
“We are working with the agency to finalize remediation and product update solutions,” Frostehav said.
‘Known Possible Complications’
Abbott’s MitraClip system includes tiny clips implanted in the heart’s mitral valve and the equipment used to implant them. The apparatus features a steering mechanism with hand controls and a catheter that is threaded through a major vein, typically from an incision in the groin, to place one or more clips in the heart.
Worldwide, more than 200,000 people have been treated with MitraClip, according to an Abbott website.
The 2016 MitraClip recall described cases in which “the user was unable to separate the implantable Clip from the delivery system.”
In a news release at the time, Abbott said it had “received a small number of reports” in which that happened.
Those cases “resulted in surgical interventions to remove the delivery system or replace the mitral valve, and it is expected that any future similar incidents would also require surgery to correct the problem,” the FDA said in a 2016 notice. “There was one patient death in these cases as a result of severe comorbidities following surgery.”
Years later, something similar happened.
In February 2021, a clip was implanted in an 81-year-old patient but the doctor couldn’t separate the clip from the delivery system, according to a report Abbott filed with the FDA. The patient was transferred to surgery, where the delivery system “had to be cut down in order to detach the clip.”
The patient then underwent an operation to replace the mitral valve, and, hours later, the patient was brought back to surgery to address bleeding, the report said.
The patient “coded” the next day and died from an aortic bleed, the report said.
In the report to the FDA, the manufacturer blamed “case-specific circumstances.”
“Cardiac arrest, hemorrhage and death are listed” in the device instructions “as known possible complications associated with mitraclip procedures,” the company said. “There is no indication of a product issue with respect to manufacture, design or labeling.”
The third MitraClip recall, initiated in September 2022, cited an “increase in clip locking malfunctions.”
Most of the reported malfunctions were not associated with adverse outcomes, the FDA said then. Treatment with MitraClip “remains within the anticipated risk levels,” the company told customers.
As with the two earlier recalls, the third advised doctors to follow the device’s instructions. But the 2022 recall identified a contributing factor: the way the device was made.
“Abbott has identified a contributing cause … as a change in the material properties of one of the Clip locking components,” the company said in a 2022 letter to customers.
“Abbott is working on producing new lots with updated manufacturing processing and raw material,” the company wrote. In the same letter, Abbott told doctors that, in the meantime, they could use the devices they had in stock.
Six days later, a clip opened while locked and a patient died, according to a report the manufacturer submitted to the FDA.
“There is no evidence that death was related to the device but it was likely related to the procedure,” Abbott wrote.
Now, almost two years later, the 2022 recall remains open, according to the FDA website, and “not all products have been corrected or removed.”
KFF Health News data editor Holly K. Hacker contributed to this report.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Dementia Deemed Highly Preventable: Here’s How
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
A new report on the preventability of dementia is both exciting and paradigm-shifting. The new study, published in The Lancet by the Lancet Commission on Dementia, estimates that .
This is paradigm-shifting because dementia is often perceived as an inevitable consequence of the aging process, with a major genetic component. But this study suggests that modifying these risk factors can benefit everyone, irrespective of genetic risk, and that it’s important to have a life-course approach. It’s never too early or too late to start to modify these factors.
We’ve known for a long time that many chronic diseases are highly preventable and modifiable. Some that come to mind are type 2 diabetes, coronary heart disease, and even certain forms of cancer. Modifiable risk factors include cigarette smoking, diet, physical activity, and maintaining a healthy weight. This study suggests that many of the same risk factors and more are relevant to reducing risk for dementia.
Let’s go through the risk factors, many of which are behavioral. These risk factors include lifestyle factors such as lack of physical activity, cigarette smoking, excessive alcohol consumption, and obesity. The cardiovascular or vascular-specific risk factors include not only those behavioral factors but also hypertension, high LDL cholesterol, and diabetes. Cognitive engagement–specific risk factors include social isolation, which is a major risk factor for dementia, as well as untreated hearing or vision loss, which can exacerbate social isolation and depression, and low educational attainment, which can be related to less cognitive engagement.
They also mention traumatic brain injury from an accident or contact sports without head protection as a risk factor, and the environmental risk factor of air pollution or poor air quality.
Two of these risk factors are new since the previous report in 2020: elevated LDL cholesterol and untreated vision loss, both of which are quite treatable. Overall, these findings suggest that a lot can be done to lower dementia risk, but it requires individual behavior modifications as well as a comprehensive approach with involvement of the healthcare system for improved screening, access, and public policy to reduce air pollution.
Some of these risk factors are more relevant to women, especially the social isolation that is so common later in life in women. In the United States, close to two out of three patients with dementia are women.
So, informing our patients about these risk factors and what can be done in terms of behavior modification, increased screening, and treatment for these conditions can go a long way in helping our patients reduce their risk for dementia.
Dr. Manson is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, chief, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, and past president, North American Menopause Society, 2011-2012. She disclosed receiving study pill donation and infrastructure support from Mars Symbioscience (for the COSMOS trial).
A version of this article appeared on Medscape.com.
Data Trends 2024: Parkinson Disease
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
- Power MC, Parthasarathy V, Gianattasio KZ, et al. Investigation of the association of military employment and Parkinson’s disease with a validated Parkinson’s disease case-finding strategy. Brain Inj. 2023;37(5):383-387. doi:10.1080/02699052.2022.2158234
- Scott GD, Neilson LE, Woltjer R, Quinn JF, Lim MM. Lifelong association of disorders related to military trauma with subsequent Parkinson’s disease. Mov Disord. 2023;38(8):1483-1492. doi:10.1002/mds.29457
- Goldman SM, Weaver FM, Stroupe KT, et al. Risk of Parkinson disease among service members at Marine Corps Base Camp Lejeune. JAMA Neurol. 2023;80(7):673-681. doi:10.1001/jamaneurol.2023.1168
- Pankratz N, Cole BR, Beutel KM, Liao KP, Ashe J. Parkinson disease genetics extended to African and Hispanic ancestries in the VA Million Veteran Program. Neurol Genet. 2023;10(1):e200110. doi:10.1212/NXG.0000000000200110
Federal Health Care Data Trends 2024
Data Trends 2024: Amyotrophic Lateral Sclerosis (ALS)
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
- Mehta P, Raymond J, Zhang Y, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 21, 2023. doi:10.1080/21678421.2023.2245858
- What is amyotrophic lateral sclerosis (ALS)? Centers for Disease Control and Prevention. Updated May 13, 2022. Accessed April 15, 2024. https://www.cdc.gov/als/WhatisAmyotrophiclateralsclerosis.html
- Reimer RJ, Goncalves A, Soper B, et al. An electronic health record cohort of veterans with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. Published online August 9, 2023. doi:10.1080/21678421.2023.2239300
- Kudritzki V, Howard IM. Telehealth-based exercise in amyotrophic lateral sclerosis. Front Neurol. 2023;14:1238916. doi:10.3389/fneur.2023.1238916
- Colvin LE, Foster ZW, Stein TD, et al. Utility of the ALSFRS-R for predicting ALS and comorbid disease neuropathology: the Veterans Affairs Biorepository Brain Bank. Muscle Nerve. 2022;66(2):167-174. doi:10.1002/mus.27635
- Rabadi MH, Russell KC, Xu C. Predictors of mortality in veterans with amyotrophic lateral sclerosis: respiratory status and speech disorder at presentation. Med Sci Monit. 2024;30:e943288. doi:10.12659/MSM.943288
AHS White Paper Guides Treatment of Posttraumatic Headache in Youth
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
The guidance document, the first of its kind, covers risk factors for prolonged recovery, along with pharmacologic and nonpharmacologic management strategies, and supports an emphasis on multidisciplinary care, lead author Carlyn Patterson Gentile, MD, PhD, attending physician in the Division of Neurology at Children’s Hospital of Philadelphia in Pennsylvania, and colleagues reported.
“There are no guidelines to inform the management of posttraumatic headache in youth, but multiple studies have been conducted over the past 2 decades,” the authors wrote in Headache. “This white paper aims to provide a thorough review of the current literature, identify gaps in knowledge, and provide a road map for [posttraumatic headache] management in youth based on available evidence and expert opinion.”
Clarity for an Underrecognized Issue
According to Russell Lonser, MD, professor and chair of neurological surgery at Ohio State University, Columbus, the white paper is important because it offers concrete guidance for health care providers who may be less familiar with posttraumatic headache in youth.
“It brings together all of the previous literature ... in a very well-written way,” Dr. Lonser said in an interview. “More than anything, it could reassure [providers] that they shouldn’t be hunting down potentially magical cures, and reassure them in symptomatic management.”
Meeryo C. Choe, MD, associate clinical professor of pediatric neurology at UCLA Health in Calabasas, California, said the paper also helps shine a light on what may be a more common condition than the public suspects.
“While the media focuses on the effects of concussion in professional sports athletes, the biggest population of athletes is in our youth population,” Dr. Choe said in a written comment. “Almost 25 million children participate in sports throughout the country, and yet we lack guidelines on how to treat posttraumatic headache which can often develop into persistent postconcussive symptoms.”
This white paper, she noted, builds on Dr. Gentile’s 2021 systematic review, introduces new management recommendations, and aligns with the latest consensus statement from the Concussion in Sport Group.
Risk Factors
The white paper first emphasizes the importance of early identification of youth at high risk for prolonged recovery from posttraumatic headache. Risk factors include female sex, adolescent age, a high number of acute symptoms following the initial injury, and social determinants of health.
“I agree that it is important to identify these patients early to improve the recovery trajectory,” Dr. Choe said.
Identifying these individuals quickly allows for timely intervention with both pharmacologic and nonpharmacologic therapies, Dr. Gentile and colleagues noted, potentially mitigating persistent symptoms. Clinicians are encouraged to perform thorough initial assessments to identify these risk factors and initiate early, personalized management plans.
Initial Management of Acute Posttraumatic Headache
For the initial management of acute posttraumatic headache, the white paper recommends a scheduled dosing regimen of simple analgesics. Ibuprofen at a dosage of 10 mg/kg every 6-8 hours (up to a maximum of 600 mg per dose) combined with acetaminophen has shown the best evidence for efficacy. Provided the patient is clinically stable, this regimen should be initiated within 48 hours of the injury and maintained with scheduled dosing for 3-10 days.
If effective, these medications can subsequently be used on an as-needed basis. Careful usage of analgesics is crucial, the white paper cautions, as overadministration can lead to medication-overuse headaches, complicating the recovery process.
Secondary Treatment Options
In cases where first-line oral medications are ineffective, the AHS white paper outlines several secondary treatment options. These include acute intravenous therapies such as ketorolac, dopamine receptor antagonists, and intravenous fluids. Nerve blocks and oral corticosteroid bridges may also be considered.
The white paper stresses the importance of individualized treatment plans that consider the specific needs and responses of each patient, noting that the evidence supporting these approaches is primarily derived from retrospective studies and case reports.
“Patient preferences should be factored in,” said Sean Rose, MD, pediatric neurologist and codirector of the Complex Concussion Clinic at Nationwide Children’s Hospital, Columbus, Ohio.
Supplements and Preventive Measures
For adolescents and young adults at high risk of prolonged posttraumatic headache, the white paper suggests the use of riboflavin and magnesium supplements. Small randomized clinical trials suggest that these supplements may aid in speeding recovery when administered for 1-2 weeks within 48 hours of injury.
If significant headache persists after 2 weeks, a regimen of riboflavin 400 mg daily and magnesium 400-500 mg nightly can be trialed for 6-8 weeks, in line with recommendations for migraine prevention. Additionally, melatonin at a dose of 3-5 mg nightly for an 8-week course may be considered for patients experiencing comorbid sleep disturbances.
Targeted Preventative Therapy
The white paper emphasizes the importance of targeting preventative therapy to the primary headache phenotype.
For instance, patients presenting with a migraine phenotype, or those with a personal or family history of migraines, may be most likely to respond to medications proven effective in migraine prevention, such as amitriptyline, topiramate, and propranolol.
“Most research evidence [for treating posttraumatic headache in youth] is still based on the treatment of migraine,” Dr. Rose pointed out in a written comment.
Dr. Gentile and colleagues recommend initiating preventive therapies 4-6 weeks post injury if headaches are not improving, occur more than 1-2 days per week, or significantly impact daily functioning.
Specialist Referrals and Physical Activity
Referral to a headache specialist is advised for patients who do not respond to first-line acute and preventive therapies. Specialists can offer advanced diagnostic and therapeutic options, the authors noted, ensuring a comprehensive approach to managing posttraumatic headache.
The white paper also recommends noncontact, sub–symptom threshold aerobic physical activity and activities of daily living after an initial 24-48 hour period of symptom-limited cognitive and physical rest. Engaging in these activities may promote faster recovery and help patients gradually return to their normal routines.
“This has been a shift in the concussion treatment approach over the last decade, and is one of the most important interventions we can recommend as physicians,” Dr. Choe noted. “This is where pediatricians and emergency department physicians seeing children acutely can really make a difference in the recovery trajectory for a child after a concussion. ‘Cocoon therapy’ has been proven not only to not work, but be detrimental to recovery.”
Nonpharmacologic Interventions
Based on clinical assessment, nonpharmacologic interventions may also be considered, according to the white paper. These interventions include cervico-vestibular therapy, which addresses neck and balance issues, and cognitive-behavioral therapy, which helps manage the psychological aspects of chronic headache. Dr. Gentile and colleagues highlighted the potential benefits of a collaborative care model that incorporates these nonpharmacologic interventions alongside pharmacologic treatments, providing a holistic approach to posttraumatic headache management.
“Persisting headaches after concussion are often driven by multiple factors,” Dr. Rose said. “Multidisciplinary concussion clinics can offer multiple treatment approaches such as behavioral, physical therapy, exercise, and medication options.”
Unmet Needs
The white paper concludes by calling for high-quality prospective cohort studies and placebo-controlled, randomized, controlled trials to further advance the understanding and treatment of posttraumatic headache in children.
Dr. Lonser, Dr. Choe, and Dr. Rose all agreed.
“More focused treatment trials are needed to gauge efficacy in children with headache after concussion,” Dr. Rose said.
Specifically, Dr. Gentile and colleagues underscored the need to standardize data collection via common elements, which could improve the ability to compare results across studies and develop more effective treatments. In addition, research into the underlying pathophysiology of posttraumatic headache is crucial for identifying new therapeutic targets and clinical and biological markers that can personalize patient care.
They also stressed the importance of exploring the impact of health disparities and social determinants on posttraumatic headache outcomes, aiming to develop interventions that are equitable and accessible to all patient populations.The white paper was approved by the AHS, and supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke K23 NS124986. The authors disclosed relationships with Eli Lilly, Pfizer, Amgen, and others. The interviewees disclosed no conflicts of interest.
FROM HEADACHE
New First-Line Therapies for Migraine Prevention
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 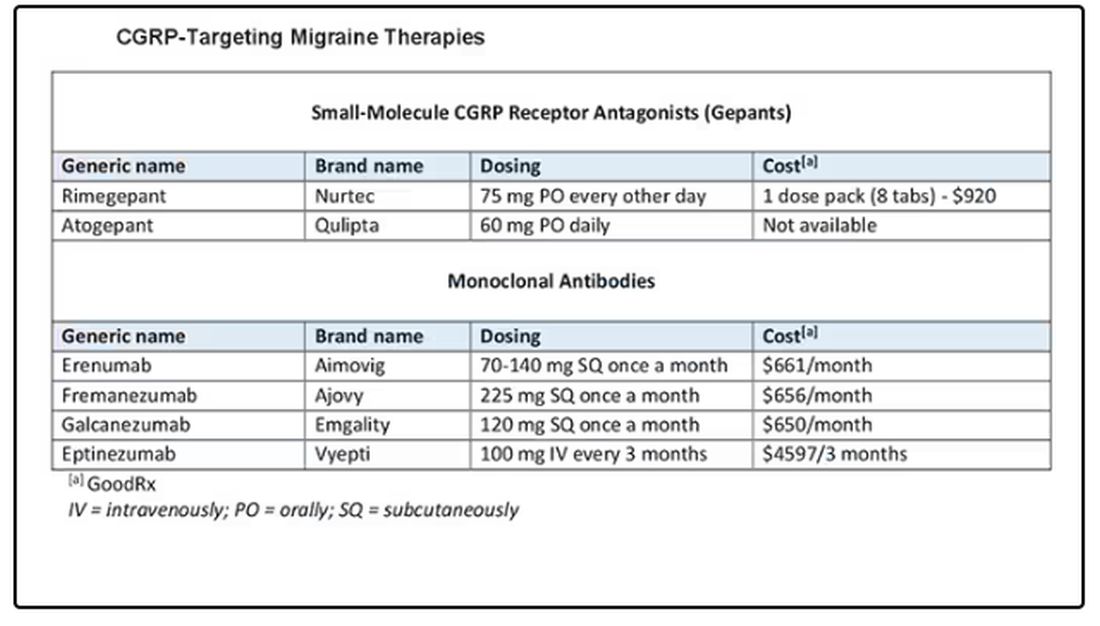
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I am going to talk about the position statement from the American Headache Society (AHS) “Calcitonin gene-related peptide [CGRP]–targeting therapies are a first-line option for the prevention of migraine”. This update is of critical importance because about three fourths of people with migraine get their care from a primary care clinician, not from a neurologist or a headache specialist. CGRP-targeting therapies have transformed migraine care at the specialty level, but many in primary care are not yet familiar with this class of medicines. Until this new statement was released, CGRPs were not viewed as first-line agents for migraine. That has now changed.
Two main types of therapy for people with migraine headache are: (1) acute or abortive therapy (when a headache develops, it is treated), and (2) preventive therapy. Preventive therapy is typically used when the patient has headaches on 4 or more days per month. Preventive therapy is aimed at reducing the frequency and severity of headaches. About 40% of patients with migraine qualify for preventive therapy, but only a minority are receiving it.
The armamentarium for preventive therapy of migraines had not changed in a long time — until now. First-line preventive therapy has traditionally consisted of three classes of agents: beta-blockers, tricyclic antidepressants, and topiramate. These medicines were developed for different therapeutic purposes, yet they work for migraines. These drugs may have off-target effects that can make them difficult to tolerate.
Based on new evidence, candesartan — an angiotensin receptor blocker (ARB) — is now also a first-line drug for migraine. This is good news, because ARBs are a drug class that we have a lot of experience with, are easy to use, and could be an excellent choice for people with concomitant hypertension or chronic kidney disease. The serotonin-norepinephrine reuptake inhibitors (venlafaxine and duloxetine) are also considered first-line agents for migraine treatment.
In the AHS’s new position statement, the two main drug classes are small-molecule CGRP receptor antagonists and monoclonal antibodies.
The role of the neuropeptide CGRP in migraine was originally discovered after finding that blood levels of CGRP were elevated during migraine attacks. This led to the discovery of agents that blocked CGRP, initially for acute treatment of migraine, and then for preventive therapy. Multiple clinical studies show the CGRP targeting therapies to be as or even more effective than traditional first-line agents at decreasing the number of migraine days per month.
The efficacy and safety of these agents have been demonstrated in both randomized trials and in real-world studies. Other important positive endpoints include fewer days of migraine, reduced acute medication use, and improvements in many quality-of-life outcomes. Studies also have shown that CGRP-targeting therapies are well tolerated and safe, with very few serious adverse events.
Furthermore, studies have shown the CGRP targeting therapies are effective in individuals who have failed multiple other first-line therapies. They fit now both as first-line agents and as agents that can be used in difficult-to-treat patients as well as in patients who struggle with acute medication overuse, which is often very challenging.
To quote from the AHS statement,
Side effects are uncommon and can include hypertension, constipation, and Raynaud phenomenon.
The position statement is strong and is based on a lot of evidence and clinical experience. CGRP-targeting therapies are now first-line agents for the prevention of migraine headache. We should learn more about and begin to feel comfortable using this class of agents because they stand to benefit our patients greatly. I’d suggest looking at the table below and picking one new agent to become familiar with so that you can add that agent to your toolbox. 
Dr. Skolnik, professor, Department of Family Medicine, Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania, and associate director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, Bayer, and Teva.
A version of this article appeared on Medscape.com.