User login
Brown fat, white fat. Is one better than the other?
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
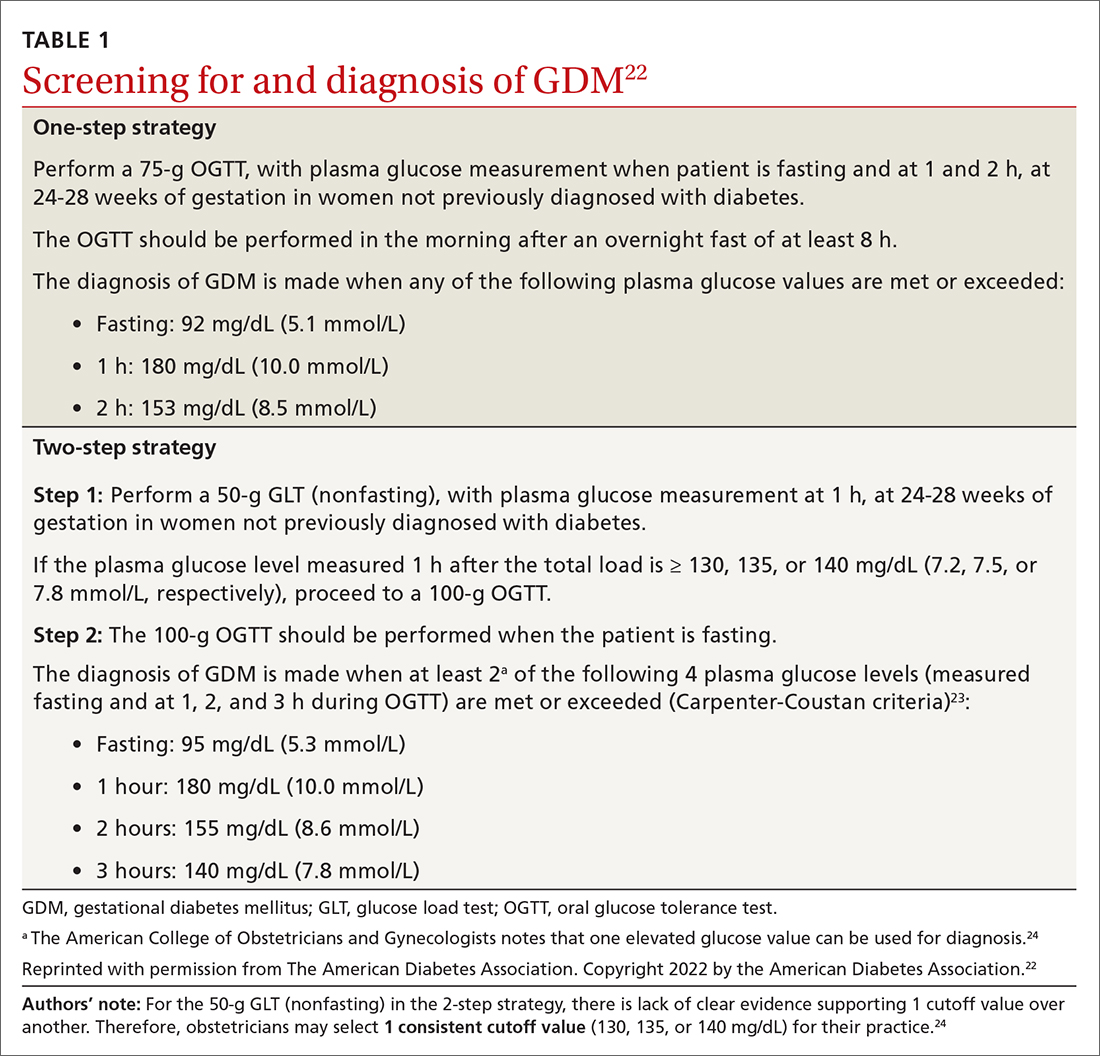
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.
If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49
CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; connie.ha@tu.edu
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
One-third of psoriatic arthritis patients could have metabolic syndrome, data analysis finds
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
of 724 individuals, as did approximately 23%-63% of patients across multiple studies, investigators from Spain report.
Previous studies of people with PsA in particular suggest they are at an increased risk of cardiovascular disease and have a higher prevalence of metabolic syndrome, prompting recommendations on cardiovascular risk management for patients with PsA, wrote the authors, Ana Urruticoechea-Arana, MD, of the department of rheumatology, Hospital Can Misses, Ibiza, Spain, and colleagues.
However, assessing the prevalence of metabolic syndrome remains a challenge because the definition varies across studies, they noted.
For a more thorough assessment of the prevalence of metabolic syndrome in this population, the researchers conducted a study using two sources: a systematic literature review of 18 studies published up to March 2019, and data on patients with PsA enrolled in the CARMA (Spanish Cardiovascular in Rheumatology) project, a longitudinal cohort observational study of adults with inflammatory diseases in Spain. The findings were published March 1 in the Journal of Clinical Rheumatology.
The literature review included a total of a total of 2,452 patients with PsA, with a mean age between 42 and 59 years, and a mean disease duration ranging from 3 to 14 years.
The definitions of metabolic syndrome varied; the most common was the definition from the National Cholesterol Education Program (NECP ATP III). Other definitions used in the studies included those issued by the International Diabetes Federation, the World Health Organization, and the American Heart Association.
Across these studies, the rate of metabolic syndrome ranged from 23.5% to 62.9%. Prevalence was similar between men and women. One study that included patients with a PsA disease duration of only 3 years showed a prevalence of 38%, similar to the average prevalence overall. Another study showed a significantly higher prevalence of metabolic syndrome in patients with PsA and cutaneous psoriasis, compared with those without psoriasis (40.8% vs. 13.16%; P = .006).
The CARMA study included 724 patients with PsA; 45.4% were women and 21.8% were smokers. The mean age of the population in this study was 51 years, and the mean disease duration was 9 years. Overall, 222 patients (30.7%) met at least three criteria for metabolic syndrome, based on the NCEP ATP III definition. The most common abnormal findings for traditional cardiovascular risk factors in the CARMA cohort were high blood pressure (66.8%), hyperglycemia (42.6%), and hypertriglyceridemia (30.6%).
Despite the variation in prevalence of metabolic syndrome, depending on the definition used, the authors wrote, “It can be stated that the rate of [metabolic syndrome] in patients with PsA is in general very high, especially if we take into account the mean age of patients included in the studies.”
“These findings support the hypotheses that this increase in the inflammatory pathway in PsA may contribute a higher risk of cardiovascular events and [metabolic syndrome] in patients with PsA than patients with psoriasis alone, the risk being even higher in severe PsA,” and that insulin resistance, metabolic syndrome, and atherosclerotic events “may have a common inflammatory basis,” the researchers wrote in their discussion of the results.
The study findings were limited by several factors, most importantly the variation in definitions of metabolic syndrome in the literature review, which limits the generalizability of the results, the researchers said. Limitations of the CARMA study include the focus only on patients who were being cared for in hospitals, which might yield an overestimation of metabolic syndrome, they added.
However, the results support findings from previous studies and highlight the need for proper assessment of body weight and cardiovascular risk factors in patients with PsA at the onset of disease, they said.
“Furthermore, it is necessary to conduct more research to standardize (and modify as appropriate) the definition of [metabolic syndrome] and establish the best strategy for managing it in these patients,” they concluded.
The study was funded by an independent grant from UCB Pharma. One author disclosed receiving grants from Pfizer, Abbvie, Novartis, Roche, UCB, Sanofi, BMS, Lilly, MSD, and Janssen. Lead author Dr. Urruticoechea-Arana and the other authors had no disclosures.
FROM JOURNAL OF CLINICAL RHEUMATOLOGY
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN
Body fat linked to lower bone density, particularly in men
an analysis of data from a large, nationally representative sample has found.
Much previous research has suggested that obesity protects against fractures and loss of BMD for a variety of reasons, including the beneficial effects of weight-bearing on the skeleton and hormonal factors linked to body fat. But the new findings should prompt a reconsideration of the relationship between obesity and fracture risk, according to the investigators, whose study appears in the Journal of Clinical Endocrinology & Metabolism.
“While higher BMI [body mass index] is generally associated with higher bone density, our study demonstrates that lean and fat mass affect bone density differently and that obesity is not a guarantee against osteoporosis,” Rajesh K. Jain, MD, of the University of Chicago said in an interview.
Dr. Jain and a colleague, Tamara Vokes, MD, used multivariant modeling to examine the relationship between BMD and body composition of 10,814 men and women aged 20-59 years from the National Health and Nutrition Examination Survey (NHANES) 2011-2018. All underwent total body dual-energy x-ray absorptiometry scans.
Participants were stratified into sex-specific quartiles based on lean mass index (LMI; lean mass divided by height squared) and fat mass index (FMI; fat mass divided by height squared). Lean mass had a strong positive association with bone density, whereas fat mass had a moderate negative effect, the researchers found.
An additional kg/m2 of FMI was associated with a 0.10 lower T score, the number of standard deviations from the expected bone density of a young adult (P < .001). The negative effect was greater in men, who had a 0.13 lower T score per additional 1 kg/m2 of FMI, compared with 0.08 lower in women (P < .001). The effect was most pronounced in people in the highest FMI quartile.
Body composition is not a routine clinical measurement, Dr. Jain and Dr. Vokes noted. Prior studies of the effect of body composition on bone density have been limited by small patient numbers, referral bias, lack of racial or ethnic diversity, and the use of estimates rather than true measures of fat and lean tissue. NHANES is designed to mirror the U.S. population.
The researchers say when it comes to patients with obesity, the findings “should not dissuade clinicians from assessing bone density, particularly if other risk factors are present.”
Useful clinical proxies for body composition
Clinicians have no routine way to measure body composition in an office setting. As a result, Dr. Jain advised clinicians to look at factors that correlate with high body fat, such as the presence of diabetes, or with low lean mass, such as poor performance on physical activity measures like grip strength, when deciding whether to consider osteoporosis screening. Patients with obesity should undergo recommended bone density screening, especially if they have other risk factors such as older age, previous fracture, steroid use, or a family history of fracture.
Although some extra weight may have a beneficial loading effect, too much extra weight can lead to metabolic problems and restrict movement, according to Rodrigo J. Valderrábano, MD, medical director of clinical research for the Research Program in Men’s Health: Aging and Metabolism, Brigham and Women’s Hospital in Boston. “There’s a general sense that the extra weight is only good for your bones if you can carry it around,” said Dr. Valderrábano, who was not involved in the study.
More research is needed to understand why fat affects men and women differently, Dr. Jain noted. The researchers found that testosterone and estradiol values did not fully explain the variation.
Adipokines released by fat cells may be important in driving bone loss but were not measured in the study, Peter R. Ebeling, MD, president of the American Society of Bone and Mineral Research, said in an interview. Distribution of fractures in obesity suggests that a high FMI may preferentially affect cortical bone instead of trabecular bone, but further studies using high-resolution peripheral quantitative CT are required to confirm the difference.
Dr. Ebeling, who was not involved in the new study, agreed that the positive relationship between BMI and BMD has led to false reassurance that people with obesity may be protected from fragility fractures. “The take-home message for clinicians is that we should not neglect bone health in our patients with obesity, both male and female.”
Dr. Jain has reported receiving grant support from the Amgen Foundation and being a consultant for Radius Health. Dr. Vokes has reported being an investigator, consultant, and speaker for Radius Health, investigator and consultant for Takeda Pharmaceutical, and investigator for Ascendis Pharma. Dr. Valderrábano and Dr. Ebeling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
an analysis of data from a large, nationally representative sample has found.
Much previous research has suggested that obesity protects against fractures and loss of BMD for a variety of reasons, including the beneficial effects of weight-bearing on the skeleton and hormonal factors linked to body fat. But the new findings should prompt a reconsideration of the relationship between obesity and fracture risk, according to the investigators, whose study appears in the Journal of Clinical Endocrinology & Metabolism.
“While higher BMI [body mass index] is generally associated with higher bone density, our study demonstrates that lean and fat mass affect bone density differently and that obesity is not a guarantee against osteoporosis,” Rajesh K. Jain, MD, of the University of Chicago said in an interview.
Dr. Jain and a colleague, Tamara Vokes, MD, used multivariant modeling to examine the relationship between BMD and body composition of 10,814 men and women aged 20-59 years from the National Health and Nutrition Examination Survey (NHANES) 2011-2018. All underwent total body dual-energy x-ray absorptiometry scans.
Participants were stratified into sex-specific quartiles based on lean mass index (LMI; lean mass divided by height squared) and fat mass index (FMI; fat mass divided by height squared). Lean mass had a strong positive association with bone density, whereas fat mass had a moderate negative effect, the researchers found.
An additional kg/m2 of FMI was associated with a 0.10 lower T score, the number of standard deviations from the expected bone density of a young adult (P < .001). The negative effect was greater in men, who had a 0.13 lower T score per additional 1 kg/m2 of FMI, compared with 0.08 lower in women (P < .001). The effect was most pronounced in people in the highest FMI quartile.
Body composition is not a routine clinical measurement, Dr. Jain and Dr. Vokes noted. Prior studies of the effect of body composition on bone density have been limited by small patient numbers, referral bias, lack of racial or ethnic diversity, and the use of estimates rather than true measures of fat and lean tissue. NHANES is designed to mirror the U.S. population.
The researchers say when it comes to patients with obesity, the findings “should not dissuade clinicians from assessing bone density, particularly if other risk factors are present.”
Useful clinical proxies for body composition
Clinicians have no routine way to measure body composition in an office setting. As a result, Dr. Jain advised clinicians to look at factors that correlate with high body fat, such as the presence of diabetes, or with low lean mass, such as poor performance on physical activity measures like grip strength, when deciding whether to consider osteoporosis screening. Patients with obesity should undergo recommended bone density screening, especially if they have other risk factors such as older age, previous fracture, steroid use, or a family history of fracture.
Although some extra weight may have a beneficial loading effect, too much extra weight can lead to metabolic problems and restrict movement, according to Rodrigo J. Valderrábano, MD, medical director of clinical research for the Research Program in Men’s Health: Aging and Metabolism, Brigham and Women’s Hospital in Boston. “There’s a general sense that the extra weight is only good for your bones if you can carry it around,” said Dr. Valderrábano, who was not involved in the study.
More research is needed to understand why fat affects men and women differently, Dr. Jain noted. The researchers found that testosterone and estradiol values did not fully explain the variation.
Adipokines released by fat cells may be important in driving bone loss but were not measured in the study, Peter R. Ebeling, MD, president of the American Society of Bone and Mineral Research, said in an interview. Distribution of fractures in obesity suggests that a high FMI may preferentially affect cortical bone instead of trabecular bone, but further studies using high-resolution peripheral quantitative CT are required to confirm the difference.
Dr. Ebeling, who was not involved in the new study, agreed that the positive relationship between BMI and BMD has led to false reassurance that people with obesity may be protected from fragility fractures. “The take-home message for clinicians is that we should not neglect bone health in our patients with obesity, both male and female.”
Dr. Jain has reported receiving grant support from the Amgen Foundation and being a consultant for Radius Health. Dr. Vokes has reported being an investigator, consultant, and speaker for Radius Health, investigator and consultant for Takeda Pharmaceutical, and investigator for Ascendis Pharma. Dr. Valderrábano and Dr. Ebeling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
an analysis of data from a large, nationally representative sample has found.
Much previous research has suggested that obesity protects against fractures and loss of BMD for a variety of reasons, including the beneficial effects of weight-bearing on the skeleton and hormonal factors linked to body fat. But the new findings should prompt a reconsideration of the relationship between obesity and fracture risk, according to the investigators, whose study appears in the Journal of Clinical Endocrinology & Metabolism.
“While higher BMI [body mass index] is generally associated with higher bone density, our study demonstrates that lean and fat mass affect bone density differently and that obesity is not a guarantee against osteoporosis,” Rajesh K. Jain, MD, of the University of Chicago said in an interview.
Dr. Jain and a colleague, Tamara Vokes, MD, used multivariant modeling to examine the relationship between BMD and body composition of 10,814 men and women aged 20-59 years from the National Health and Nutrition Examination Survey (NHANES) 2011-2018. All underwent total body dual-energy x-ray absorptiometry scans.
Participants were stratified into sex-specific quartiles based on lean mass index (LMI; lean mass divided by height squared) and fat mass index (FMI; fat mass divided by height squared). Lean mass had a strong positive association with bone density, whereas fat mass had a moderate negative effect, the researchers found.
An additional kg/m2 of FMI was associated with a 0.10 lower T score, the number of standard deviations from the expected bone density of a young adult (P < .001). The negative effect was greater in men, who had a 0.13 lower T score per additional 1 kg/m2 of FMI, compared with 0.08 lower in women (P < .001). The effect was most pronounced in people in the highest FMI quartile.
Body composition is not a routine clinical measurement, Dr. Jain and Dr. Vokes noted. Prior studies of the effect of body composition on bone density have been limited by small patient numbers, referral bias, lack of racial or ethnic diversity, and the use of estimates rather than true measures of fat and lean tissue. NHANES is designed to mirror the U.S. population.
The researchers say when it comes to patients with obesity, the findings “should not dissuade clinicians from assessing bone density, particularly if other risk factors are present.”
Useful clinical proxies for body composition
Clinicians have no routine way to measure body composition in an office setting. As a result, Dr. Jain advised clinicians to look at factors that correlate with high body fat, such as the presence of diabetes, or with low lean mass, such as poor performance on physical activity measures like grip strength, when deciding whether to consider osteoporosis screening. Patients with obesity should undergo recommended bone density screening, especially if they have other risk factors such as older age, previous fracture, steroid use, or a family history of fracture.
Although some extra weight may have a beneficial loading effect, too much extra weight can lead to metabolic problems and restrict movement, according to Rodrigo J. Valderrábano, MD, medical director of clinical research for the Research Program in Men’s Health: Aging and Metabolism, Brigham and Women’s Hospital in Boston. “There’s a general sense that the extra weight is only good for your bones if you can carry it around,” said Dr. Valderrábano, who was not involved in the study.
More research is needed to understand why fat affects men and women differently, Dr. Jain noted. The researchers found that testosterone and estradiol values did not fully explain the variation.
Adipokines released by fat cells may be important in driving bone loss but were not measured in the study, Peter R. Ebeling, MD, president of the American Society of Bone and Mineral Research, said in an interview. Distribution of fractures in obesity suggests that a high FMI may preferentially affect cortical bone instead of trabecular bone, but further studies using high-resolution peripheral quantitative CT are required to confirm the difference.
Dr. Ebeling, who was not involved in the new study, agreed that the positive relationship between BMI and BMD has led to false reassurance that people with obesity may be protected from fragility fractures. “The take-home message for clinicians is that we should not neglect bone health in our patients with obesity, both male and female.”
Dr. Jain has reported receiving grant support from the Amgen Foundation and being a consultant for Radius Health. Dr. Vokes has reported being an investigator, consultant, and speaker for Radius Health, investigator and consultant for Takeda Pharmaceutical, and investigator for Ascendis Pharma. Dr. Valderrábano and Dr. Ebeling reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Limited benefits of early gestational diabetes screening
Screening pregnant women with obesity for gestational diabetes before 20 weeks of pregnancy did not lead to any improved maternal or neonatal outcomes compared with doing routine screening between 24 and 28 weeks, according to research presented Feb. 4 at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There is increasing evidence that early screening does not reduce the risk of adverse perinatal outcomes,” Jennifer Thompson, MD, associate professor of ob.gyn. at Vanderbilt University, Nashville, Tenn., said in an interview. “The increasing number of studies that have demonstrated no benefit in reducing adverse perinatal outcomes leads to consideration to revise recommendations for early screening.”
However, she did note that early screening may be helpful in identifying patients with undiagnosed preexisting diabetes.
Michael Richley, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said catching those previously undiagnosed cases is one of the goals of earlier screening with the expectation that earlier management will lead to better outcomes.
“If a patient then obtains the diagnosis of type 2 diabetes, introducing nutritional counseling and possible medical management early can lead to better outcomes,” said Dr. Richley, who attended the presentation but was not involved in the research. ”While catching undiagnosed type 2 diabetes is not common, it is becoming increasingly common lately.”
Obesity is a known risk factor for impaired glucose metabolism and for gestational diabetes, explained presenter Christopher A. Enakpene, MD, an ob.gyn. from Midland, Tex., who completed the study while completing his maternal-fetal medicine fellowship at the University of Illinois in Chicago. Dr. Enakpene reminded attendees that the American College of Obstetricians and Gynecologists (ACOG) currently recommends early screening for gestational diabetes in patients with certain risk factors, including obesity, a history of first-degree relatives with diabetes, or a history of gestational diabetes, impaired glucose tolerance, poor pregnancy outcomes, fetal demise, congenital abnormalities, or birth of an infant large for gestational age.
The researchers screened 7,126 patients for enrollment in the study from March 2017 through February 2019 and identified 600 who met the criteria: An adult with a singleton pregnancy and body mass index (BMI) of at least 30 kg/m2. Patients were excluded if they had preexisting diabetes, elevated blood glucose or impaired glucose tolerance, a history of gestational diabetes, any chromosomal anomalies or abnormalities in the pregnancy, or were past 20 weeks of pregnancy.
The prospective randomized controlled trial was open label and included 296 patients who were randomly assigned to early screening with a 1-hour glucose challenge test (GCT) and hemoglobin A1c before 20 weeks, followed by a 3-hour oral glucose tolerance test if the GCT result was between 140 and 200 mg/dL with an HbA1c of less than 6.5%. The other 304 patients were screened with a 1-hour GCT between 24 and 28 weeks but also had an HbA1c test before 20 weeks.
The primary outcome was macrosomia, defined as a birth weight at least 4,000 g, with various maternal and neonatal secondary outcomes. The only significant difference between the groups at baseline was a higher proportion of Hispanic participants in the early screening group (22.4%) compared to the routine screening group (13.7%).
The groups had no significant differences in birth weight or macrosomia, which occurred in 2.8% of the early screening group and 3.4% of the routine screening group (P = .7). There were no significant differences in gestational age at delivery, preeclampsia, polyhydramnios, shoulder dystocia, cesarean delivery, or NICU admission. However, the rate of gestational diabetes was significantly higher in the early screening group (22.5%) than in the routine screening group (15.7%; P < .05). In addition, more participants with gestational diabetes in the early screening group used insulin (34.4%) compared with those in the routine screening group (15.6%; P < .05).
Dr. Enakpene noted several reasons that the perinatal outcomes may have been similar between the groups, such as the increased rate of gestational diabetes requiring treatment in the early screening group or a higher proportion of participants using insulin in the early screening group.
“Hence, the similarity in adverse perinatal outcomes between the groups despite a higher proportion of gestational diabetes in the early group might be due to more utilization of insulin,” Dr. Enakpene said.
Dr. Richley was not surprised by the findings and hypothesized that the reason for not seeing a difference in outcomes might relate to using a 20-week cutoff for testing when type 2 diabetes would be evident at any stage of pregnancy.
“It would be interesting to have a study look at diabetes testing exclusively in the first trimester for high-risk patients that looks at neonatal outcomes and see if that would show a difference between the two groups,” Dr. Richley said.
Dr. Thompson was similarly interested in whether 20 weeks was an early enough time for early screening.
”I would also like to know the differences in management between the two groups and if the knowledge of early diagnosis impacted their management, such as timing of medication start, amount of medication required, and how that differed from the standard group,” Dr. Thompson said. ”Since patients with a hemoglobin A1c > 6.5% or glucose tolerance test > 200 [mg/dL] were excluded, I’m interested in the number of patients that were excluded since they likely have undiagnosed preexisting diabetes, which are the patients that may benefit most from early screening.”
Dr. Richley pointed out that the potential clinical implications of the study are limited right now.
“While their secondary outcomes of neonatal hypoglycemia, method of delivery, and other common obstetrical measures were not different, we cannot draw conclusions from this as the study was not powered to evaluate these findings,” Dr. Richley said. “I do still see a role in early screening for patients with risk factors but favor doing so at the first prenatal visit, whenever that is, as opposed to as late as mid-second trimester, though this is often when a patient’s first interaction with a health care system will be within their pregnancy.”
Dr. Enakpene, Dr. Thompson, and Dr. Richley reported no disclosures. External funding for the study was not noted.
Screening pregnant women with obesity for gestational diabetes before 20 weeks of pregnancy did not lead to any improved maternal or neonatal outcomes compared with doing routine screening between 24 and 28 weeks, according to research presented Feb. 4 at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There is increasing evidence that early screening does not reduce the risk of adverse perinatal outcomes,” Jennifer Thompson, MD, associate professor of ob.gyn. at Vanderbilt University, Nashville, Tenn., said in an interview. “The increasing number of studies that have demonstrated no benefit in reducing adverse perinatal outcomes leads to consideration to revise recommendations for early screening.”
However, she did note that early screening may be helpful in identifying patients with undiagnosed preexisting diabetes.
Michael Richley, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said catching those previously undiagnosed cases is one of the goals of earlier screening with the expectation that earlier management will lead to better outcomes.
“If a patient then obtains the diagnosis of type 2 diabetes, introducing nutritional counseling and possible medical management early can lead to better outcomes,” said Dr. Richley, who attended the presentation but was not involved in the research. ”While catching undiagnosed type 2 diabetes is not common, it is becoming increasingly common lately.”
Obesity is a known risk factor for impaired glucose metabolism and for gestational diabetes, explained presenter Christopher A. Enakpene, MD, an ob.gyn. from Midland, Tex., who completed the study while completing his maternal-fetal medicine fellowship at the University of Illinois in Chicago. Dr. Enakpene reminded attendees that the American College of Obstetricians and Gynecologists (ACOG) currently recommends early screening for gestational diabetes in patients with certain risk factors, including obesity, a history of first-degree relatives with diabetes, or a history of gestational diabetes, impaired glucose tolerance, poor pregnancy outcomes, fetal demise, congenital abnormalities, or birth of an infant large for gestational age.
The researchers screened 7,126 patients for enrollment in the study from March 2017 through February 2019 and identified 600 who met the criteria: An adult with a singleton pregnancy and body mass index (BMI) of at least 30 kg/m2. Patients were excluded if they had preexisting diabetes, elevated blood glucose or impaired glucose tolerance, a history of gestational diabetes, any chromosomal anomalies or abnormalities in the pregnancy, or were past 20 weeks of pregnancy.
The prospective randomized controlled trial was open label and included 296 patients who were randomly assigned to early screening with a 1-hour glucose challenge test (GCT) and hemoglobin A1c before 20 weeks, followed by a 3-hour oral glucose tolerance test if the GCT result was between 140 and 200 mg/dL with an HbA1c of less than 6.5%. The other 304 patients were screened with a 1-hour GCT between 24 and 28 weeks but also had an HbA1c test before 20 weeks.
The primary outcome was macrosomia, defined as a birth weight at least 4,000 g, with various maternal and neonatal secondary outcomes. The only significant difference between the groups at baseline was a higher proportion of Hispanic participants in the early screening group (22.4%) compared to the routine screening group (13.7%).
The groups had no significant differences in birth weight or macrosomia, which occurred in 2.8% of the early screening group and 3.4% of the routine screening group (P = .7). There were no significant differences in gestational age at delivery, preeclampsia, polyhydramnios, shoulder dystocia, cesarean delivery, or NICU admission. However, the rate of gestational diabetes was significantly higher in the early screening group (22.5%) than in the routine screening group (15.7%; P < .05). In addition, more participants with gestational diabetes in the early screening group used insulin (34.4%) compared with those in the routine screening group (15.6%; P < .05).
Dr. Enakpene noted several reasons that the perinatal outcomes may have been similar between the groups, such as the increased rate of gestational diabetes requiring treatment in the early screening group or a higher proportion of participants using insulin in the early screening group.
“Hence, the similarity in adverse perinatal outcomes between the groups despite a higher proportion of gestational diabetes in the early group might be due to more utilization of insulin,” Dr. Enakpene said.
Dr. Richley was not surprised by the findings and hypothesized that the reason for not seeing a difference in outcomes might relate to using a 20-week cutoff for testing when type 2 diabetes would be evident at any stage of pregnancy.
“It would be interesting to have a study look at diabetes testing exclusively in the first trimester for high-risk patients that looks at neonatal outcomes and see if that would show a difference between the two groups,” Dr. Richley said.
Dr. Thompson was similarly interested in whether 20 weeks was an early enough time for early screening.
”I would also like to know the differences in management between the two groups and if the knowledge of early diagnosis impacted their management, such as timing of medication start, amount of medication required, and how that differed from the standard group,” Dr. Thompson said. ”Since patients with a hemoglobin A1c > 6.5% or glucose tolerance test > 200 [mg/dL] were excluded, I’m interested in the number of patients that were excluded since they likely have undiagnosed preexisting diabetes, which are the patients that may benefit most from early screening.”
Dr. Richley pointed out that the potential clinical implications of the study are limited right now.
“While their secondary outcomes of neonatal hypoglycemia, method of delivery, and other common obstetrical measures were not different, we cannot draw conclusions from this as the study was not powered to evaluate these findings,” Dr. Richley said. “I do still see a role in early screening for patients with risk factors but favor doing so at the first prenatal visit, whenever that is, as opposed to as late as mid-second trimester, though this is often when a patient’s first interaction with a health care system will be within their pregnancy.”
Dr. Enakpene, Dr. Thompson, and Dr. Richley reported no disclosures. External funding for the study was not noted.
Screening pregnant women with obesity for gestational diabetes before 20 weeks of pregnancy did not lead to any improved maternal or neonatal outcomes compared with doing routine screening between 24 and 28 weeks, according to research presented Feb. 4 at the Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
“There is increasing evidence that early screening does not reduce the risk of adverse perinatal outcomes,” Jennifer Thompson, MD, associate professor of ob.gyn. at Vanderbilt University, Nashville, Tenn., said in an interview. “The increasing number of studies that have demonstrated no benefit in reducing adverse perinatal outcomes leads to consideration to revise recommendations for early screening.”
However, she did note that early screening may be helpful in identifying patients with undiagnosed preexisting diabetes.
Michael Richley, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, said catching those previously undiagnosed cases is one of the goals of earlier screening with the expectation that earlier management will lead to better outcomes.
“If a patient then obtains the diagnosis of type 2 diabetes, introducing nutritional counseling and possible medical management early can lead to better outcomes,” said Dr. Richley, who attended the presentation but was not involved in the research. ”While catching undiagnosed type 2 diabetes is not common, it is becoming increasingly common lately.”
Obesity is a known risk factor for impaired glucose metabolism and for gestational diabetes, explained presenter Christopher A. Enakpene, MD, an ob.gyn. from Midland, Tex., who completed the study while completing his maternal-fetal medicine fellowship at the University of Illinois in Chicago. Dr. Enakpene reminded attendees that the American College of Obstetricians and Gynecologists (ACOG) currently recommends early screening for gestational diabetes in patients with certain risk factors, including obesity, a history of first-degree relatives with diabetes, or a history of gestational diabetes, impaired glucose tolerance, poor pregnancy outcomes, fetal demise, congenital abnormalities, or birth of an infant large for gestational age.
The researchers screened 7,126 patients for enrollment in the study from March 2017 through February 2019 and identified 600 who met the criteria: An adult with a singleton pregnancy and body mass index (BMI) of at least 30 kg/m2. Patients were excluded if they had preexisting diabetes, elevated blood glucose or impaired glucose tolerance, a history of gestational diabetes, any chromosomal anomalies or abnormalities in the pregnancy, or were past 20 weeks of pregnancy.
The prospective randomized controlled trial was open label and included 296 patients who were randomly assigned to early screening with a 1-hour glucose challenge test (GCT) and hemoglobin A1c before 20 weeks, followed by a 3-hour oral glucose tolerance test if the GCT result was between 140 and 200 mg/dL with an HbA1c of less than 6.5%. The other 304 patients were screened with a 1-hour GCT between 24 and 28 weeks but also had an HbA1c test before 20 weeks.
The primary outcome was macrosomia, defined as a birth weight at least 4,000 g, with various maternal and neonatal secondary outcomes. The only significant difference between the groups at baseline was a higher proportion of Hispanic participants in the early screening group (22.4%) compared to the routine screening group (13.7%).
The groups had no significant differences in birth weight or macrosomia, which occurred in 2.8% of the early screening group and 3.4% of the routine screening group (P = .7). There were no significant differences in gestational age at delivery, preeclampsia, polyhydramnios, shoulder dystocia, cesarean delivery, or NICU admission. However, the rate of gestational diabetes was significantly higher in the early screening group (22.5%) than in the routine screening group (15.7%; P < .05). In addition, more participants with gestational diabetes in the early screening group used insulin (34.4%) compared with those in the routine screening group (15.6%; P < .05).
Dr. Enakpene noted several reasons that the perinatal outcomes may have been similar between the groups, such as the increased rate of gestational diabetes requiring treatment in the early screening group or a higher proportion of participants using insulin in the early screening group.
“Hence, the similarity in adverse perinatal outcomes between the groups despite a higher proportion of gestational diabetes in the early group might be due to more utilization of insulin,” Dr. Enakpene said.
Dr. Richley was not surprised by the findings and hypothesized that the reason for not seeing a difference in outcomes might relate to using a 20-week cutoff for testing when type 2 diabetes would be evident at any stage of pregnancy.
“It would be interesting to have a study look at diabetes testing exclusively in the first trimester for high-risk patients that looks at neonatal outcomes and see if that would show a difference between the two groups,” Dr. Richley said.
Dr. Thompson was similarly interested in whether 20 weeks was an early enough time for early screening.
”I would also like to know the differences in management between the two groups and if the knowledge of early diagnosis impacted their management, such as timing of medication start, amount of medication required, and how that differed from the standard group,” Dr. Thompson said. ”Since patients with a hemoglobin A1c > 6.5% or glucose tolerance test > 200 [mg/dL] were excluded, I’m interested in the number of patients that were excluded since they likely have undiagnosed preexisting diabetes, which are the patients that may benefit most from early screening.”
Dr. Richley pointed out that the potential clinical implications of the study are limited right now.
“While their secondary outcomes of neonatal hypoglycemia, method of delivery, and other common obstetrical measures were not different, we cannot draw conclusions from this as the study was not powered to evaluate these findings,” Dr. Richley said. “I do still see a role in early screening for patients with risk factors but favor doing so at the first prenatal visit, whenever that is, as opposed to as late as mid-second trimester, though this is often when a patient’s first interaction with a health care system will be within their pregnancy.”
Dr. Enakpene, Dr. Thompson, and Dr. Richley reported no disclosures. External funding for the study was not noted.
FROM THE PREGNANCY MEETING
LGBTQ parents fare worse giving birth
Members of the LGBTQ community who give birth appear to have a greater risk of hypertensive disorders of pregnancy and postpartum hemorrhage, according to new research presented at the annual meeting sponsored by the Society for Maternal-Fetal Medicine.
“Our study found that birthing patients in likely sexual and gender minority partnerships experienced disparities in clinical outcomes,” Stephanie Leonard, PhD, an epidemiology and biostatistics instructor at the Stanford (Calif.) University division of maternal-fetal medicine and obstetrics, told attendees at the meeting. The disparities are likely because of various social determinants and possibly higher use of assisted reproductive technology (ART). The findings establish “how these are significant disparities that have been largely overlooked and set the groundwork for doing further research on maybe ways that we can improve the inclusivity of obstetric care.”
Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, who attended the presentation but was not involved in the research, said the findings were “overall unfortunate but not surprising given the existing studies looking at LGBTQ patients and their poorer health outcomes, largely due to lack of access to health care and discrimination in the health care setting.”
Dr. Leonard described the societal, interpersonal, and individual factors that can contribute to health disparities among gender and sexual minority patients.
“At the societal level, there are expectations of what it means to be pregnant, to give birth, and to be a parent. At the community level, there’s the clinical care environment, and at the interpersonal level, there’s an obstetrician’s relationship with the patient,” Dr. Leonard said. “At the individual level, most notably is minority stress, the biological effects of the chronic experience of discrimination.”
It has historically been difficult to collect data on this patient population, but a change in the design of the California birth certificate made it possible to gather more data than previously possible. The updated California birth certificate, issued in 2016, allows the parent not giving birth to check off whether they are the child’s mother, father, parent, or “not specified” instead of defaulting to “father.” In addition, the parent giving birth can select mother, father, parent or not specified instead of being “mother” by default.
The researchers classified sexual and gender minority (SGM) partnerships as those in which the parent giving birth was identified as the father and those where both parents were identified as mothers. Non-SGM minority partnerships were those in which the birthing parent was identified as the mother and the nonbirthing parent was identified as the father.
The population-based cohort study included data from all live birth hospitalizations from 2016-2019 in California, whose annual births represent one in eight babies born each year in the United States. The population of SGM patients different significantly from the non-SGM population in nearly every demographic and clinical factor except rates of pre-existing diabetes. For example, 42% of the SGM birthing patients were age 35 or older, compared with 23% of the non-SGM patients.
SGM patients were more likely to be born in the United States, were more likely to be White, and were less likely to be Asian or Hispanic. SGM patients had higher education levels and were more likely to have private insurance. They were also more likely to be nulliparous and have chronic hypertension. Average body mass index for SGM patients was 33 kg/m2, compared with 30 for non-SGM patients. SGM patients were also much more likely to have multifetal gestation: 7.1% of SGM patients versus 1.5% of non-SGM patients.
In terms of clinical outcomes, 14% of SGM patients had hypertensive disorders of pregnancy, compared with 8% of non-SGM patients. Before adjustment for potential confounders, SGM patients were also twice as likely to have postpartum hemorrhage (8% vs. 4% in non-SGM patients) and postterm birth at 42-44 weeks (0.6% vs 0.3% in non-SGM patients).
“Having increased postterm birth is a matter of declining induction of labor, as it is recommended to have an induction by 41 weeks of gestation in general,” Dr. Mei said in an interview. “It is also possible this patient cohort faces more barriers in access to care and possible discrimination as sexual/gender minority patients.”
Rates of severe preeclampsia, induction of labor, cesarean delivery, preterm birth, low birth weight, and a low Apgar score were also higher among SGM patients, but these associations were no longer significant after adjustment for age, education, payment method, parity, prepregnancy weight, comorbidities, and multifetal gestation. The difference in hypertensive disorders of pregnancy, postpartum hemorrhage, and postterm birth remained statistically significant after adjustment.
Past research has shown that only about a third of cisgender female same-sex marriages used ART, so the disparities cannot be completely explained by ART use, Dr. Leonard said.
“I think the main drivers are structural disparities,” Dr. Leonard said. “Every obstetric clinic is focused in a way that’s about mother-father, and many people who don’t feel like they fit into that paradigm feel excluded and disengage with health care.”
Elliott Main, MD, a clinical professor of obstetrics and gynecology at Stanford University and coauthor of the study noted that discrimination and stigma likely play a substantial role in the disparities.
“Sexual and/or gender minority people face this discrimination at structural and interpersonal levels on a regular basis, which can lead to chronic stress and its harmful physical effects as well as lower-quality health care,” Dr. Main said in an interview.
Another coauthor, Juno Obedin-Maliver, MD, an assistant professor of obstetrics and gynecology at Stanford, emphasized how much room for improvement exists in care for SGM obstetric patients.
“We hope that this study brings needed attention to the disparities in perinatal health experienced by sexual and/or gender minority people,” Dr. Obedin-Maliver said. “There is much we can do to better understand the family building goals of sexual and/or gender minority people and help those to be achieved with healthy outcomes for parents and their children.”
One limitation of the study is that it’s possible to misclassify individuals using the birth certificate data, and not everyone may be comfortable selecting the box that accurately represents their identity, particularly if they aren’t “out” or fear discrimination or stigma, so the population may underrepresent the actual numbers of sexual and gender minority individuals giving birth. Dr. Mei added that it would be helpful to see data on neonatal ICU admissions and use of ART.
It’s difficult to say how generalizable the findings are, Dr. Mei said. “It is possible the findings would be more exaggerated in the rest of the country outside of California, if we assume there is potentially lower health access and more stigma.” The fact that California offers different gender options for the birthing and nonbirthing parent is, by itself, an indication of a potentially more accepting social environment than might be found in other states.
”The take-home message is that this patient population is higher risk, likely partially due to baseline increased risk factors, such as older maternal age and likely use of ART, and partially due to possible lack of health access and stigma,” Dr. Mei said. “Health care providers should be notably cognizant of these increased risks, particularly in the psychosocial context and make efforts to reduce those burdens as much as possible.”
The research was funded by the Stanford Maternal and Child Health Research Institute. Dr. Obedin-Maliver has consulted for Sage Therapeutics, Ibis Reproductive Health, and Hims. Dr. Mei and the other authors had no disclosures.
Members of the LGBTQ community who give birth appear to have a greater risk of hypertensive disorders of pregnancy and postpartum hemorrhage, according to new research presented at the annual meeting sponsored by the Society for Maternal-Fetal Medicine.
“Our study found that birthing patients in likely sexual and gender minority partnerships experienced disparities in clinical outcomes,” Stephanie Leonard, PhD, an epidemiology and biostatistics instructor at the Stanford (Calif.) University division of maternal-fetal medicine and obstetrics, told attendees at the meeting. The disparities are likely because of various social determinants and possibly higher use of assisted reproductive technology (ART). The findings establish “how these are significant disparities that have been largely overlooked and set the groundwork for doing further research on maybe ways that we can improve the inclusivity of obstetric care.”
Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, who attended the presentation but was not involved in the research, said the findings were “overall unfortunate but not surprising given the existing studies looking at LGBTQ patients and their poorer health outcomes, largely due to lack of access to health care and discrimination in the health care setting.”
Dr. Leonard described the societal, interpersonal, and individual factors that can contribute to health disparities among gender and sexual minority patients.
“At the societal level, there are expectations of what it means to be pregnant, to give birth, and to be a parent. At the community level, there’s the clinical care environment, and at the interpersonal level, there’s an obstetrician’s relationship with the patient,” Dr. Leonard said. “At the individual level, most notably is minority stress, the biological effects of the chronic experience of discrimination.”
It has historically been difficult to collect data on this patient population, but a change in the design of the California birth certificate made it possible to gather more data than previously possible. The updated California birth certificate, issued in 2016, allows the parent not giving birth to check off whether they are the child’s mother, father, parent, or “not specified” instead of defaulting to “father.” In addition, the parent giving birth can select mother, father, parent or not specified instead of being “mother” by default.
The researchers classified sexual and gender minority (SGM) partnerships as those in which the parent giving birth was identified as the father and those where both parents were identified as mothers. Non-SGM minority partnerships were those in which the birthing parent was identified as the mother and the nonbirthing parent was identified as the father.
The population-based cohort study included data from all live birth hospitalizations from 2016-2019 in California, whose annual births represent one in eight babies born each year in the United States. The population of SGM patients different significantly from the non-SGM population in nearly every demographic and clinical factor except rates of pre-existing diabetes. For example, 42% of the SGM birthing patients were age 35 or older, compared with 23% of the non-SGM patients.
SGM patients were more likely to be born in the United States, were more likely to be White, and were less likely to be Asian or Hispanic. SGM patients had higher education levels and were more likely to have private insurance. They were also more likely to be nulliparous and have chronic hypertension. Average body mass index for SGM patients was 33 kg/m2, compared with 30 for non-SGM patients. SGM patients were also much more likely to have multifetal gestation: 7.1% of SGM patients versus 1.5% of non-SGM patients.
In terms of clinical outcomes, 14% of SGM patients had hypertensive disorders of pregnancy, compared with 8% of non-SGM patients. Before adjustment for potential confounders, SGM patients were also twice as likely to have postpartum hemorrhage (8% vs. 4% in non-SGM patients) and postterm birth at 42-44 weeks (0.6% vs 0.3% in non-SGM patients).
“Having increased postterm birth is a matter of declining induction of labor, as it is recommended to have an induction by 41 weeks of gestation in general,” Dr. Mei said in an interview. “It is also possible this patient cohort faces more barriers in access to care and possible discrimination as sexual/gender minority patients.”
Rates of severe preeclampsia, induction of labor, cesarean delivery, preterm birth, low birth weight, and a low Apgar score were also higher among SGM patients, but these associations were no longer significant after adjustment for age, education, payment method, parity, prepregnancy weight, comorbidities, and multifetal gestation. The difference in hypertensive disorders of pregnancy, postpartum hemorrhage, and postterm birth remained statistically significant after adjustment.
Past research has shown that only about a third of cisgender female same-sex marriages used ART, so the disparities cannot be completely explained by ART use, Dr. Leonard said.
“I think the main drivers are structural disparities,” Dr. Leonard said. “Every obstetric clinic is focused in a way that’s about mother-father, and many people who don’t feel like they fit into that paradigm feel excluded and disengage with health care.”
Elliott Main, MD, a clinical professor of obstetrics and gynecology at Stanford University and coauthor of the study noted that discrimination and stigma likely play a substantial role in the disparities.
“Sexual and/or gender minority people face this discrimination at structural and interpersonal levels on a regular basis, which can lead to chronic stress and its harmful physical effects as well as lower-quality health care,” Dr. Main said in an interview.
Another coauthor, Juno Obedin-Maliver, MD, an assistant professor of obstetrics and gynecology at Stanford, emphasized how much room for improvement exists in care for SGM obstetric patients.
“We hope that this study brings needed attention to the disparities in perinatal health experienced by sexual and/or gender minority people,” Dr. Obedin-Maliver said. “There is much we can do to better understand the family building goals of sexual and/or gender minority people and help those to be achieved with healthy outcomes for parents and their children.”
One limitation of the study is that it’s possible to misclassify individuals using the birth certificate data, and not everyone may be comfortable selecting the box that accurately represents their identity, particularly if they aren’t “out” or fear discrimination or stigma, so the population may underrepresent the actual numbers of sexual and gender minority individuals giving birth. Dr. Mei added that it would be helpful to see data on neonatal ICU admissions and use of ART.
It’s difficult to say how generalizable the findings are, Dr. Mei said. “It is possible the findings would be more exaggerated in the rest of the country outside of California, if we assume there is potentially lower health access and more stigma.” The fact that California offers different gender options for the birthing and nonbirthing parent is, by itself, an indication of a potentially more accepting social environment than might be found in other states.
”The take-home message is that this patient population is higher risk, likely partially due to baseline increased risk factors, such as older maternal age and likely use of ART, and partially due to possible lack of health access and stigma,” Dr. Mei said. “Health care providers should be notably cognizant of these increased risks, particularly in the psychosocial context and make efforts to reduce those burdens as much as possible.”
The research was funded by the Stanford Maternal and Child Health Research Institute. Dr. Obedin-Maliver has consulted for Sage Therapeutics, Ibis Reproductive Health, and Hims. Dr. Mei and the other authors had no disclosures.
Members of the LGBTQ community who give birth appear to have a greater risk of hypertensive disorders of pregnancy and postpartum hemorrhage, according to new research presented at the annual meeting sponsored by the Society for Maternal-Fetal Medicine.
“Our study found that birthing patients in likely sexual and gender minority partnerships experienced disparities in clinical outcomes,” Stephanie Leonard, PhD, an epidemiology and biostatistics instructor at the Stanford (Calif.) University division of maternal-fetal medicine and obstetrics, told attendees at the meeting. The disparities are likely because of various social determinants and possibly higher use of assisted reproductive technology (ART). The findings establish “how these are significant disparities that have been largely overlooked and set the groundwork for doing further research on maybe ways that we can improve the inclusivity of obstetric care.”
Jenny Mei, MD, a maternal-fetal medicine fellow at the University of California, Los Angeles, who attended the presentation but was not involved in the research, said the findings were “overall unfortunate but not surprising given the existing studies looking at LGBTQ patients and their poorer health outcomes, largely due to lack of access to health care and discrimination in the health care setting.”
Dr. Leonard described the societal, interpersonal, and individual factors that can contribute to health disparities among gender and sexual minority patients.
“At the societal level, there are expectations of what it means to be pregnant, to give birth, and to be a parent. At the community level, there’s the clinical care environment, and at the interpersonal level, there’s an obstetrician’s relationship with the patient,” Dr. Leonard said. “At the individual level, most notably is minority stress, the biological effects of the chronic experience of discrimination.”
It has historically been difficult to collect data on this patient population, but a change in the design of the California birth certificate made it possible to gather more data than previously possible. The updated California birth certificate, issued in 2016, allows the parent not giving birth to check off whether they are the child’s mother, father, parent, or “not specified” instead of defaulting to “father.” In addition, the parent giving birth can select mother, father, parent or not specified instead of being “mother” by default.
The researchers classified sexual and gender minority (SGM) partnerships as those in which the parent giving birth was identified as the father and those where both parents were identified as mothers. Non-SGM minority partnerships were those in which the birthing parent was identified as the mother and the nonbirthing parent was identified as the father.
The population-based cohort study included data from all live birth hospitalizations from 2016-2019 in California, whose annual births represent one in eight babies born each year in the United States. The population of SGM patients different significantly from the non-SGM population in nearly every demographic and clinical factor except rates of pre-existing diabetes. For example, 42% of the SGM birthing patients were age 35 or older, compared with 23% of the non-SGM patients.
SGM patients were more likely to be born in the United States, were more likely to be White, and were less likely to be Asian or Hispanic. SGM patients had higher education levels and were more likely to have private insurance. They were also more likely to be nulliparous and have chronic hypertension. Average body mass index for SGM patients was 33 kg/m2, compared with 30 for non-SGM patients. SGM patients were also much more likely to have multifetal gestation: 7.1% of SGM patients versus 1.5% of non-SGM patients.
In terms of clinical outcomes, 14% of SGM patients had hypertensive disorders of pregnancy, compared with 8% of non-SGM patients. Before adjustment for potential confounders, SGM patients were also twice as likely to have postpartum hemorrhage (8% vs. 4% in non-SGM patients) and postterm birth at 42-44 weeks (0.6% vs 0.3% in non-SGM patients).
“Having increased postterm birth is a matter of declining induction of labor, as it is recommended to have an induction by 41 weeks of gestation in general,” Dr. Mei said in an interview. “It is also possible this patient cohort faces more barriers in access to care and possible discrimination as sexual/gender minority patients.”
Rates of severe preeclampsia, induction of labor, cesarean delivery, preterm birth, low birth weight, and a low Apgar score were also higher among SGM patients, but these associations were no longer significant after adjustment for age, education, payment method, parity, prepregnancy weight, comorbidities, and multifetal gestation. The difference in hypertensive disorders of pregnancy, postpartum hemorrhage, and postterm birth remained statistically significant after adjustment.
Past research has shown that only about a third of cisgender female same-sex marriages used ART, so the disparities cannot be completely explained by ART use, Dr. Leonard said.
“I think the main drivers are structural disparities,” Dr. Leonard said. “Every obstetric clinic is focused in a way that’s about mother-father, and many people who don’t feel like they fit into that paradigm feel excluded and disengage with health care.”
Elliott Main, MD, a clinical professor of obstetrics and gynecology at Stanford University and coauthor of the study noted that discrimination and stigma likely play a substantial role in the disparities.
“Sexual and/or gender minority people face this discrimination at structural and interpersonal levels on a regular basis, which can lead to chronic stress and its harmful physical effects as well as lower-quality health care,” Dr. Main said in an interview.
Another coauthor, Juno Obedin-Maliver, MD, an assistant professor of obstetrics and gynecology at Stanford, emphasized how much room for improvement exists in care for SGM obstetric patients.
“We hope that this study brings needed attention to the disparities in perinatal health experienced by sexual and/or gender minority people,” Dr. Obedin-Maliver said. “There is much we can do to better understand the family building goals of sexual and/or gender minority people and help those to be achieved with healthy outcomes for parents and their children.”
One limitation of the study is that it’s possible to misclassify individuals using the birth certificate data, and not everyone may be comfortable selecting the box that accurately represents their identity, particularly if they aren’t “out” or fear discrimination or stigma, so the population may underrepresent the actual numbers of sexual and gender minority individuals giving birth. Dr. Mei added that it would be helpful to see data on neonatal ICU admissions and use of ART.
It’s difficult to say how generalizable the findings are, Dr. Mei said. “It is possible the findings would be more exaggerated in the rest of the country outside of California, if we assume there is potentially lower health access and more stigma.” The fact that California offers different gender options for the birthing and nonbirthing parent is, by itself, an indication of a potentially more accepting social environment than might be found in other states.
”The take-home message is that this patient population is higher risk, likely partially due to baseline increased risk factors, such as older maternal age and likely use of ART, and partially due to possible lack of health access and stigma,” Dr. Mei said. “Health care providers should be notably cognizant of these increased risks, particularly in the psychosocial context and make efforts to reduce those burdens as much as possible.”
The research was funded by the Stanford Maternal and Child Health Research Institute. Dr. Obedin-Maliver has consulted for Sage Therapeutics, Ibis Reproductive Health, and Hims. Dr. Mei and the other authors had no disclosures.
FROM THE PREGNANCY MEETING
Picture warnings on sugary drinks could help fight childhood obesity
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sugary beverages – juice, soda, decadent lattes, sports drinks, and more – are the leading source of both calories and added sugar in the American diet, according to the Harvard T. H. Chan School of Public Health. But new research published in PLoS Medicine has found that
The new study, from researchers at the University of North Carolina at Chapel Hill, found that parents were 17% less likely to buy sugary drinks for their children when the beverages had the graphical – and graphic – warnings on the products.
The researchers turned a laboratory setting into a “minimart,” and parents were told to choose one drink and snack for their children, along with one household item (to disguise the purpose of the study).
Some parents were presented with sweetened drinks with images on the products reflecting type 2 diabetes and heart damage. Others were shown sugary drinks with a barcode label and no picture warning.
Forty-five percent of parents chose sugary drinks for their children when the products had no picture warning, but only 28% of parents chose sugary beverages with the cautionary images.
“When people make choices about what food to buy, they are juggling dozens of factors, like taste, cost, and advertising, and are looking at many products at once,” said Lindsey Smith Taillie, PhD, a nutrition researcher and the senior author of the paper.
“Showing that warnings can cut through the noise of everything else that’s happening in a food store is powerful evidence that they would help reduce sugary drink purchases in the real world.”
Children are particularly prone to overindulging on sugar, largely due to companies’ frequent marketing displays of pleasurable-looking and seemingly “thirst-quenching” sweet beverages.
Drink packaging also can be misleading.
Fruits and vegetables displayed on the front of many beverages often lead parents to buy what they believe are “healthy” options, when these drinks could be packed with sugar, according to a study published online Jan. 22 in the journal Appetite.
Parents are often “doing the best with what information they have,” so more education about nutrition, through picture warning labels, for example, would make a difference, said Caroline Fausel, a paleo food blogger, podcaster, and author of “Prep, Cook, Freeze: A Paleo Meal Planning Cookbook.”
Healthier choices on the rise
The American Beverage Association, an industry trade group, shared the current steps that major companies are taking to help lower Americans’ sugar intake.
Pepsi, Coca-Cola, and Keurig Dr Pepper joined forces in 2014 to create the Balance Calories Initiative, which aims to reduce beverage calories in the national diet.
Coca-Cola now offers 250 beverages with zero to low calories, and Keurig Dr Pepper has 158 products with 40 calories or less. Pepsi sells 7.5-ounce mini-cans, along with various other sizes, to encourage portion control.
“Beverage companies are fully transparent about the calories and sugar in our products, and we are offering more choices with less sugar than ever before,” William Dermody, vice president of media and public affairs for the American Beverage Association, said in a statement. “We agree that too much sugar is not good for anyone, and clear information about beverages is most helpful to consumers.”
Other big companies also are taking strides to lower sugar content in their products.
Kraft Heinz, which owns the popular line of Capri Sun drinks, has publicly shared its efforts to ramp up the nutritional value of its products.
The company has a goal to slash 60 million pounds of total sugar in Kraft Heinz products globally by 2025.
“As more people become aware of the harm that excessive sugar can cause in the body, my hope is that they continue to choose healthier alternatives,” Ms. Fausel said.
Creating new patterns
For children who consume sweetened juices and sodas regularly, the transition to healthier options might be challenging at first.
“Change can involve tantrums and unhappiness, and right now parents are at their max living pandemic parenting life,” said Jennifer Anderson, a registered dietitian and CEO of Kids Eat in Color, LLC, a resource for improving child nutrition and health through innovative education, meal plans, and tools. “Kids can get used to having sugary drinks, and they don’t want to give them up.”
One way to help make the switch is by having only water and milk as options while children are up and about, a technique that works particularly well for younger children, she said.
“This sort of ‘quiet restriction’ helps kids learn to love the healthier option without feeling deprived,” Ms. Anderson said. “They will eventually learn about juice, soda, chocolate milk, sports drinks, and more, but you can let them learn about those foods at a slower pace when you rarely or don’t serve them at home.”
The researchers reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Anxiety in men tied to risk factors for CVD, diabetes
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among healthy middle-aged men, those who were more anxious were more likely to develop high levels of multiple biomarkers of cardiometabolic risk over a 40-year follow-up in a new study.
“By middle adulthood, higher anxiety levels are associated with stable differences” in biomarkers of risk for coronary artery disease (CAD), stroke, and type 2 diabetes, which “are maintained into older ages,” the researchers wrote.
Anxious individuals “may experience deteriorations in cardiometabolic health earlier in life and remain on a stable trajectory of heightened risk into older ages,” they concluded.
The study, led by Lewina Lee, PhD, was published online Jan. 24, 2022, in the Journal of the American Heart Association.
“Men who had higher levels of anxiety at the beginning of the study had consistently higher biological risk for cardiometabolic disease than less anxious men from midlife into old age,” Dr. Lee, assistant professor of psychiatry, Boston University, summarized in an email.
Clinicians may not screen for heart disease and diabetes, and/or only discuss lifestyle modifications when patients are older or have the first signs of disease, she added.
However, the study findings “suggest that worries and anxiety are associated with preclinical pathophysiological processes that tend to culminate in cardiometabolic disease” and show “the importance of screening for mental health difficulties, such as worries and anxiety, in men as early as in their 30s and 40s,” she stressed.
Since most of the men were White (97%) and veterans (94%), “it would be important for future studies to evaluate if these associations exist among women, people from diverse racial and ethnic groups, and in more socioeconomically varying samples, and to consider how anxiety may relate to the development of cardiometabolic risk in much younger individuals than those in our study,” Dr. Lee said in a press release from the American Heart Association.
“This study adds to the growing body of research that link psychological health to cardiovascular risk,” Glenn N. Levine, MD, who was not involved with this research, told this news organization in an email.
“We know that factors such as depression and stress can increase cardiac risk; this study further supports that anxiety can as well,” added Dr. Levine, chief of cardiology, Michael E. DeBakey Veterans Affairs Medical Center, Houston.
“Everyone experiences some anxiety in their life,” he added. However, “if a provider senses that a patient’s anxiety is far beyond the ‘normal’ that we all have from time to time, and it is seemingly adversely impacting both their psychological and physical health, it would be reasonable to suggest to the patient that it might be useful to speak with a mental health professional, and if the patient is receptive, to then make a formal consultation or referral,” said Dr. Levine, who was writing group chair of a recent AHA Scientific Statement on mind-heart-body connection.
Neuroticism and worry
Several studies have linked anxiety to a greater risk of cardiometabolic disease onset, Dr. Lee and colleagues wrote, but it is unclear if anxious individuals have a steadily worsening risk as they age, or if they have a higher risk in middle age, which stays the same in older age.
To investigate this, they analyzed data from 1561 men who were seen at the VA Boston outpatient clinic and did not have CAD, type 2 diabetes, stroke, or cancer when they enrolled in the Normative Aging Study.
The men had a mean age of 53 years (range, 33-84) in 1975 and were followed until 2015 or until dropout from the study or death.
At baseline, the study participants filled in the Eysenck Personality Inventory, which assesses neuroticism, and also responded to a scale indicating how much they worry about 20 issues (excluding health).
“Neuroticism,” the researchers explained, “is a tendency to perceive experiences as threatening, feel that challenges are uncontrollable, and experience frequent and disproportionately intense negative emotions,” such as fear, anxiety, sadness, and anger, “across many situations.”
“Worry refers to attempts to solve a problem where future outcome is uncertain and potentially positive or negative,” Dr. Lee noted. Although worry can be healthy and lead to constructive solutions, “it may be unhealthy, especially when it becomes uncontrollable and interferes with day-to-day functioning.”
Of note, in 1980, the American Psychiatric Association removed the term neurosis from its diagnostic manual. What was previously called neurosis is included as part of generalized anxiety disorder; GAD also encompasses excessive worry.
Cardiometabolic risk from midlife to old age
The men in the current study had on-site physical examinations every 3-5 years.
The researchers calculated the men’s cardiometabolic risk score (from 0 to 7) by assigning 1 point each for the following: systolic blood pressure greater than 130 mm Hg, diastolic blood pressure greater than 85 mm Hg, total cholesterol of at least 240 mg/dL, triglycerides of at least 150 mg/dL, body mass index of at least 30 kg/m2, glucose of at least 100 mg/dL, and erythrocyte sedimentation rate of at least 14 mm/hour.
Alternatively, patients were assigned a point each for taking medication that could affect these markers (except for body mass index).
Overall, on average, at baseline, the men had a cardiometabolic risk score of 2.9. From age 33-65, this score increased to 3.8, and then it did not increase as much later on.
That is, the cardiometabolic risk score increased by 0.8 per decade until age 65, followed by a slower increase of 0.5 per decade.
At all ages, men with higher levels of neuroticism or worry had a higher cardiometabolic risk score
Each additional standard deviation of neuroticism was associated with a 13% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up, after adjusting for age, demographics, and family history of CAD, but the relationship was attenuated after also adjusting for health behaviors (for example, smoking, alcohol consumption, physical activity, and past-year physician visit at baseline).
Similarly, each additional standard deviation of worry was associated with a 10% increased risk of having six or more of the seven cardiometabolic risk markers during follow-up after the same adjustments, and was also no longer significantly different after the same further adjustments.
The research was supported by grants from the National Institutes of Health and a Senior Research Career Scientist Award from the Office of Research and Development, Department of Veterans Affairs. The Normative Aging Study is a research component of the Massachusetts Veterans Epidemiology Research and Information Center and is supported by the VA Cooperative Studies Program/Epidemiological Research Centers. The study authors and Dr. Levine disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Lifestyle likely responsible for obesity in children, not mother’s BMI
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
Lifestyle is more likely to affect a child’s body mass index than the weight of their mother before and during pregnancy say researchers who have found that a mother’s high BMI before and during pregnancy is not a major cause of high BMI in their offspring – indicating that childhood and teen obesity is more likely to be a result of lifestyle factors.
According to UK Government figures 9.9% of reception age children (age 4-5) are obese, with a further 13.1% overweight. At age 10-11 (year 6), 21.0% are obese and 14.1% overweight.
Research from the Centre for Longitudinal Studies (CLS) at the UCL Social Research Institute, published in December 2020, showed that one in five (21%) young people were obese at age 17, and a further one in seven (14%) were overweight.
Nature or nurture
Greater maternal BMI before or during pregnancy is known to be associated with higher BMI throughout childhood, but exactly how much a mother’s weight before or during pregnancy contributes to childhood obesity, or whether it is lifestyle and environmental factors that are responsible, remains unclear.
To investigate this question researchers from the University of Bristol (England) and Imperial College London used data from the “Children of the 90s” (also known as the Avon Longitudinal Study of Parents and Children), and data from the “Born in Bradford” longitudinal study.
For their study, published in BMC Medicine, researchers used Mendelian randomisation, measuring variation in genes to determine the effect of an exposure on an outcome, along with polygenic risk scores, to investigate if associations between before and during early pregnancy BMI, and a child’s BMI from birth to adolescence, are causal.
They looked at birth weight and BMI at age 1 and 4 years in both “Children of the 90s” and “Born in Bradford” participants, and then also BMI at age 10 and 15 years in just the Children of the 90s participants.
Since the effects being explored may differ by ethnicity the authors reported that they limited analyses to two ethnic groups – White European and South Asian.
Interventions targeting everyone needed
The researchers found that there was a moderate causal effect between maternal BMI and the birth weight of children, however they said they “found no strong evidence for a causal effect of maternal BMI on offspring adiposity beyond birth”.
Tom Bond, MSc, senior research associate at the University of Bristol, explained: “We found that if women are heavier at the start of pregnancy this isn’t a strong cause of their children being heavier as teenagers.”
The authors wrote that their results suggested that “higher maternal pre-/early-pregnancy BMI is not a key driver of higher adiposity in the next generation,” something that Mr. Bond said was “important to know”.
The authors concluded that their findings “support interventions that target the whole population for reducing overweight and obesity, rather than a specific focus on women of reproductive age”.
Mr. Bond pointed out that “it isn’t enough to just focus on women entering pregnancy.” However, “there is good evidence that maternal obesity causes other health problems for mothers and babies, so prospective mothers should still be encouraged and supported to maintain a healthy weight.”
A version of this article first appeared on Medscape UK.
FROM BMC MEDICINE