User login
Beware of invasive H influenzae disease among pregnant women
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
FDA panel considers human studies of modified oocytes for preventing disease
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
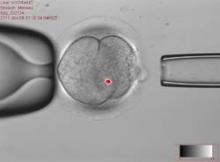
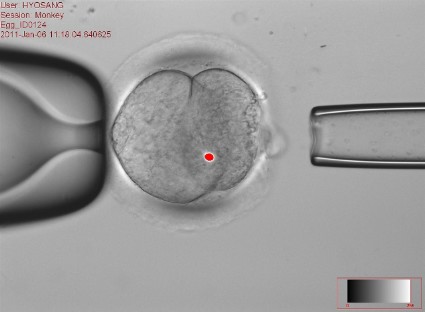
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
AT AN FDA ADVISORY COMMITTEE MEETING
VIDEO: Oocyte modification might prevent mitochondrial diseases
Clinical trials using genetically modified oocytes to prevent the transmission of mitochondrial diseases in humans may be soon become a reality. But the potentially promising approach to prevent conditions such as Leigh disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes) is not without controversy.
In an interview, Dr. Salvatore DiMauro, the Lucy G. Moses Professor of Neurology at Columbia University Medical Center, outlined the impact that mitochondrial DNA–related diseases have on women’s and children’s lives, and he explained why genetically modified oocytes may offer new hope for those affected by these diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical trials using genetically modified oocytes to prevent the transmission of mitochondrial diseases in humans may be soon become a reality. But the potentially promising approach to prevent conditions such as Leigh disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes) is not without controversy.
In an interview, Dr. Salvatore DiMauro, the Lucy G. Moses Professor of Neurology at Columbia University Medical Center, outlined the impact that mitochondrial DNA–related diseases have on women’s and children’s lives, and he explained why genetically modified oocytes may offer new hope for those affected by these diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Clinical trials using genetically modified oocytes to prevent the transmission of mitochondrial diseases in humans may be soon become a reality. But the potentially promising approach to prevent conditions such as Leigh disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes) is not without controversy.
In an interview, Dr. Salvatore DiMauro, the Lucy G. Moses Professor of Neurology at Columbia University Medical Center, outlined the impact that mitochondrial DNA–related diseases have on women’s and children’s lives, and he explained why genetically modified oocytes may offer new hope for those affected by these diseases.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AN FDA ADVISORY COMMITTEE MEETING
Aspirin for Preeclampsia Prevention?
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Draft recommendations back aspirin for preeclampsia prevention
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
Prophylactic low-dose aspirin – 81 mg per day – should be started after 12 weeks’ gestation in women at high risk for developing preeclampsia, according to a draft recommendation issued by the U.S. Preventive Services Task Force in April.
The recommendation applies to asymptomatic pregnant women at increased risk for preeclampsia who have no contraindications to using low-dose aspirin and have not experienced adverse effects associated with aspirin previously.
"The USPSTF found adequate evidence of a reduction in preeclampsia, preterm birth, and IUGR [intrauterine growth restriction] in women at increased risk for preeclampsia who received low-dose aspirin, thus demonstrating substantial benefit," the recommendations state. In a review of clinical trials, low-dose aspirin (at doses of 50-160 mg per day) reduced the risk of preeclampsia by 24%, the risk of preterm birth by 14%, and the risk of IUGR by 20%. There also was "adequate evidence" that the risks of placental abruption, postpartum hemorrhage, and fetal intracranial bleeding were not increased with low-dose aspirin, the USPSTF statement said.
The draft recommendations were based on a review of data on low-dose aspirin and preeclampsia in 23 studies of women at high or average risk of preeclampsia, which was published online April 8 in Annals of Internal Medicine (doi: 10.7326/M13-2844).
The recommendation is considered a "B" recommendation, defined as one that has a "high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial." The statement includes a table to help identify patients who are at an increased risk of preeclampsia.
The last statement about low-dose aspirin and preeclampsia, issued by the USPSTF in 1996, concluded that there was not enough evidence to support a recommendation for or against the use of aspirin for preventing preeclampsia. The American College of Obstetricians and Gynecologists recommends low-dose aspirin, starting late in the first trimester, in women with a history of early-onset preeclampsia and preterm delivery before 34 weeks’ gestation, or a history of preeclampsia in more than one previous pregnancy.
The USPSTF is an independent panel of nonfederal experts in prevention and evidence-based medicine, which includes ob.gyns., pediatricians, family physicians, nurses, and health behavior specialists, according to the USPSTF website.
The draft recommendations are available here. Comments on the recommendations can be submitted via the website until May 5, 2014, at 5 p.m. EST.
CDC: Young teens’ birth rates drop 67%
Despite a 67% drop in the birth rate among teens aged 15-17 years over the past 2 decades, there is still plenty of room for improvement, according to a report issued April 8 by the Centers for Disease Control and Prevention.
The rate of births per 1,000 teens aged 15-17 years fell from 51.9 in 1991 to 17.0 in 2012, a 67% drop. In 2012, 86,423 teens aged 15-17 years gave birth, which accounted for 28.3% of all births among teens aged 15-19 years. That’s down from 36% of all births to teens 15-19 years in 1991.
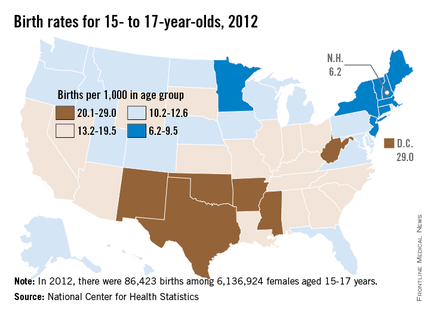
The rates among 15- to 17-year-olds varied widely by state and by ethnic and racial groups, with the highest rates among Hispanics (25.5 births per 1,000 teens) and non-Hispanic blacks (21.9), followed by American Indians/Alaska Natives (17.0). The lowest rates were among non-Hispanic whites and Asian/Pacific Islanders, at 8.4 and 4.1 births per 1,000 teens aged 15-17 years, respectively.
The researchers found no significant racial or ethnic differences in the percentage of female teens 15-17 years old who were sexually experienced or currently sexually active.
Across the United States, the rates among teens aged 15-17 years were the lowest in New Hampshire – 6.2 per 1,000 – and were highest in the District of Columbia – 29 per 1,000.
Among the report’s other findings:
• Between 2006 and 2010, 18% of female teens aged 15-17 years were sexually active.
• 90.9% of female teens aged 15-17 years had received formal sex education on either birth control or how to say no to sex, but only 61.3% received information on both topics.
• 23.6% had not discussed with their parents either saying no to sex or how to use contraceptives.
• 51.8% of the sexually active female 15- to 17-year-olds relied on less effective contraceptives, usually condoms, while 7.8% used no contraception.
• The most effective contraceptives, long-acting reversible contraceptives such as an intrauterine device, were used by 1% of sexually active females aged 15-17 years.
Previous research suggested the 55% drop in pregnancies among 15- to 17-year-olds from 1995 through 2002 was because of changes in contraceptive use (accounting for 77% of the decline) and a decrease in sexual activity (responsible for 23% of the decline), the report’s authors noted. Therefore, they recommended that "efforts to prevent pregnancy in this population should take a multifaceted approach, including the promotion of delayed sexual initiation and the use of more effective contraceptive methods."
The report’s findings "are a reminder that we as health professionals have a special duty to give young people the necessary knowledge, the skills, and the encouragement to make healthy decisions and engage in healthy behaviors," explained Ileana Arias, Ph.D., principal deputy director of the Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry, during a telephone briefing.
The report is based on the CDC’s analysis of data on births from the National Vital Statistics System from 1991 to 2012 and on teen health behaviors from the National Survey of Family Growth from 2006 to 2010. The results were published in Morbidity and Mortality Weekly Report (2014;63:1-7).
Dr. Arias pointed out that the ages of 15, 16, and 17 years "are a critical time when a teen, especially a young woman, could jeopardize her future if she can’t complete high school or go to college."
While it is encouraging that adolescent birth rates
continue to decline, the United
States continues to have one of the highest
adolescent birth rates of any developed country in the world. It is
particularly concerning that Hispanic and non-Hispanic black adolescents
continue to have about three times the birth rates of non-Hispanic white adolescents.
Equally alarming is that the majority of adolescents
report no formal sex education before their first sexual encounter. As a
community, we need to better prepare our young adolescents with evidence-based
information about sexual activity, contraception methods, and pregnancy. Just
as we vaccinate our community’s children to prevent illness, we need to talk to
our youth about sexual risk behaviors and what they can do to protect
themselves from pregnancy, sexually transmitted infections, and exploitation,
and to negotiate sexual relationships in a healthy manner. Parents and
educators need to have these conversations prior to sexual activity, so that
our youth can be prepared and protected, just as we do with vaccinations.
Providers and educators
can better prepare parents to have these discussions with young adolescents. There are many pregnancy prevention
programs, but not all of them have been proven to be effective. It is critical
that we use our limited resources in the employment of proven pregnancy
prevention programs.
This report highlights the adolescent birth trend from
1991 to 2012. There are many more effective contraceptive methods available
now, as compared to 1991. However, only 1% of adolescents are using the most
effective methods, which include intrauterine and implant contraception. Providers
can contribute to future declines in adolescent birth rates by educating
themselves about these contraceptive methods and counseling our youth about the
efficacy, safety, and satisfaction of intrauterine and implant contraceptives.
Dr. Amanda Jacobs is a pediatrician in the division of
adolescent medicine at the Children’s Hospital at Montefiore, Bronx, N.Y. Dr. Jacobs had no relevant disclosures.
sex education
While it is encouraging that adolescent birth rates
continue to decline, the United
States continues to have one of the highest
adolescent birth rates of any developed country in the world. It is
particularly concerning that Hispanic and non-Hispanic black adolescents
continue to have about three times the birth rates of non-Hispanic white adolescents.
Equally alarming is that the majority of adolescents
report no formal sex education before their first sexual encounter. As a
community, we need to better prepare our young adolescents with evidence-based
information about sexual activity, contraception methods, and pregnancy. Just
as we vaccinate our community’s children to prevent illness, we need to talk to
our youth about sexual risk behaviors and what they can do to protect
themselves from pregnancy, sexually transmitted infections, and exploitation,
and to negotiate sexual relationships in a healthy manner. Parents and
educators need to have these conversations prior to sexual activity, so that
our youth can be prepared and protected, just as we do with vaccinations.
Providers and educators
can better prepare parents to have these discussions with young adolescents. There are many pregnancy prevention
programs, but not all of them have been proven to be effective. It is critical
that we use our limited resources in the employment of proven pregnancy
prevention programs.
This report highlights the adolescent birth trend from
1991 to 2012. There are many more effective contraceptive methods available
now, as compared to 1991. However, only 1% of adolescents are using the most
effective methods, which include intrauterine and implant contraception. Providers
can contribute to future declines in adolescent birth rates by educating
themselves about these contraceptive methods and counseling our youth about the
efficacy, safety, and satisfaction of intrauterine and implant contraceptives.
Dr. Amanda Jacobs is a pediatrician in the division of
adolescent medicine at the Children’s Hospital at Montefiore, Bronx, N.Y. Dr. Jacobs had no relevant disclosures.
While it is encouraging that adolescent birth rates
continue to decline, the United
States continues to have one of the highest
adolescent birth rates of any developed country in the world. It is
particularly concerning that Hispanic and non-Hispanic black adolescents
continue to have about three times the birth rates of non-Hispanic white adolescents.
Equally alarming is that the majority of adolescents
report no formal sex education before their first sexual encounter. As a
community, we need to better prepare our young adolescents with evidence-based
information about sexual activity, contraception methods, and pregnancy. Just
as we vaccinate our community’s children to prevent illness, we need to talk to
our youth about sexual risk behaviors and what they can do to protect
themselves from pregnancy, sexually transmitted infections, and exploitation,
and to negotiate sexual relationships in a healthy manner. Parents and
educators need to have these conversations prior to sexual activity, so that
our youth can be prepared and protected, just as we do with vaccinations.
Providers and educators
can better prepare parents to have these discussions with young adolescents. There are many pregnancy prevention
programs, but not all of them have been proven to be effective. It is critical
that we use our limited resources in the employment of proven pregnancy
prevention programs.
This report highlights the adolescent birth trend from
1991 to 2012. There are many more effective contraceptive methods available
now, as compared to 1991. However, only 1% of adolescents are using the most
effective methods, which include intrauterine and implant contraception. Providers
can contribute to future declines in adolescent birth rates by educating
themselves about these contraceptive methods and counseling our youth about the
efficacy, safety, and satisfaction of intrauterine and implant contraceptives.
Dr. Amanda Jacobs is a pediatrician in the division of
adolescent medicine at the Children’s Hospital at Montefiore, Bronx, N.Y. Dr. Jacobs had no relevant disclosures.
Despite a 67% drop in the birth rate among teens aged 15-17 years over the past 2 decades, there is still plenty of room for improvement, according to a report issued April 8 by the Centers for Disease Control and Prevention.
The rate of births per 1,000 teens aged 15-17 years fell from 51.9 in 1991 to 17.0 in 2012, a 67% drop. In 2012, 86,423 teens aged 15-17 years gave birth, which accounted for 28.3% of all births among teens aged 15-19 years. That’s down from 36% of all births to teens 15-19 years in 1991.

The rates among 15- to 17-year-olds varied widely by state and by ethnic and racial groups, with the highest rates among Hispanics (25.5 births per 1,000 teens) and non-Hispanic blacks (21.9), followed by American Indians/Alaska Natives (17.0). The lowest rates were among non-Hispanic whites and Asian/Pacific Islanders, at 8.4 and 4.1 births per 1,000 teens aged 15-17 years, respectively.
The researchers found no significant racial or ethnic differences in the percentage of female teens 15-17 years old who were sexually experienced or currently sexually active.
Across the United States, the rates among teens aged 15-17 years were the lowest in New Hampshire – 6.2 per 1,000 – and were highest in the District of Columbia – 29 per 1,000.
Among the report’s other findings:
• Between 2006 and 2010, 18% of female teens aged 15-17 years were sexually active.
• 90.9% of female teens aged 15-17 years had received formal sex education on either birth control or how to say no to sex, but only 61.3% received information on both topics.
• 23.6% had not discussed with their parents either saying no to sex or how to use contraceptives.
• 51.8% of the sexually active female 15- to 17-year-olds relied on less effective contraceptives, usually condoms, while 7.8% used no contraception.
• The most effective contraceptives, long-acting reversible contraceptives such as an intrauterine device, were used by 1% of sexually active females aged 15-17 years.
Previous research suggested the 55% drop in pregnancies among 15- to 17-year-olds from 1995 through 2002 was because of changes in contraceptive use (accounting for 77% of the decline) and a decrease in sexual activity (responsible for 23% of the decline), the report’s authors noted. Therefore, they recommended that "efforts to prevent pregnancy in this population should take a multifaceted approach, including the promotion of delayed sexual initiation and the use of more effective contraceptive methods."
The report’s findings "are a reminder that we as health professionals have a special duty to give young people the necessary knowledge, the skills, and the encouragement to make healthy decisions and engage in healthy behaviors," explained Ileana Arias, Ph.D., principal deputy director of the Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry, during a telephone briefing.
The report is based on the CDC’s analysis of data on births from the National Vital Statistics System from 1991 to 2012 and on teen health behaviors from the National Survey of Family Growth from 2006 to 2010. The results were published in Morbidity and Mortality Weekly Report (2014;63:1-7).
Dr. Arias pointed out that the ages of 15, 16, and 17 years "are a critical time when a teen, especially a young woman, could jeopardize her future if she can’t complete high school or go to college."
Despite a 67% drop in the birth rate among teens aged 15-17 years over the past 2 decades, there is still plenty of room for improvement, according to a report issued April 8 by the Centers for Disease Control and Prevention.
The rate of births per 1,000 teens aged 15-17 years fell from 51.9 in 1991 to 17.0 in 2012, a 67% drop. In 2012, 86,423 teens aged 15-17 years gave birth, which accounted for 28.3% of all births among teens aged 15-19 years. That’s down from 36% of all births to teens 15-19 years in 1991.

The rates among 15- to 17-year-olds varied widely by state and by ethnic and racial groups, with the highest rates among Hispanics (25.5 births per 1,000 teens) and non-Hispanic blacks (21.9), followed by American Indians/Alaska Natives (17.0). The lowest rates were among non-Hispanic whites and Asian/Pacific Islanders, at 8.4 and 4.1 births per 1,000 teens aged 15-17 years, respectively.
The researchers found no significant racial or ethnic differences in the percentage of female teens 15-17 years old who were sexually experienced or currently sexually active.
Across the United States, the rates among teens aged 15-17 years were the lowest in New Hampshire – 6.2 per 1,000 – and were highest in the District of Columbia – 29 per 1,000.
Among the report’s other findings:
• Between 2006 and 2010, 18% of female teens aged 15-17 years were sexually active.
• 90.9% of female teens aged 15-17 years had received formal sex education on either birth control or how to say no to sex, but only 61.3% received information on both topics.
• 23.6% had not discussed with their parents either saying no to sex or how to use contraceptives.
• 51.8% of the sexually active female 15- to 17-year-olds relied on less effective contraceptives, usually condoms, while 7.8% used no contraception.
• The most effective contraceptives, long-acting reversible contraceptives such as an intrauterine device, were used by 1% of sexually active females aged 15-17 years.
Previous research suggested the 55% drop in pregnancies among 15- to 17-year-olds from 1995 through 2002 was because of changes in contraceptive use (accounting for 77% of the decline) and a decrease in sexual activity (responsible for 23% of the decline), the report’s authors noted. Therefore, they recommended that "efforts to prevent pregnancy in this population should take a multifaceted approach, including the promotion of delayed sexual initiation and the use of more effective contraceptive methods."
The report’s findings "are a reminder that we as health professionals have a special duty to give young people the necessary knowledge, the skills, and the encouragement to make healthy decisions and engage in healthy behaviors," explained Ileana Arias, Ph.D., principal deputy director of the Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry, during a telephone briefing.
The report is based on the CDC’s analysis of data on births from the National Vital Statistics System from 1991 to 2012 and on teen health behaviors from the National Survey of Family Growth from 2006 to 2010. The results were published in Morbidity and Mortality Weekly Report (2014;63:1-7).
Dr. Arias pointed out that the ages of 15, 16, and 17 years "are a critical time when a teen, especially a young woman, could jeopardize her future if she can’t complete high school or go to college."
sex education
sex education
FROM A CDC TELEBRIEFING
Major finding: The rate of births per 1,000 teens aged 15-17 years fell from 51.9 in 1991 to 17.0 in 2012, a 67% drop.
Data source: The Centers for Disease Control and Prevention (CDC) analyzed data on teen births from the National Vital Statistics System from 1991 to 2012 and teen health behaviors, including contraceptive use, from the National Survey of Family Growth from 2006 to 2010.
Disclosures: This is a CDC report.
Review of studies finds lower preeclampsia risk with prenatal aspirin
The use of low-dose aspirin during pregnancy was associated with a reduced risk of preeclampsia in women at high risk, and did not appear to be associated with harmful effects, in a systematic review of 23 studies of low-dose aspirin in women at high or average risk of preeclampsia, the authors reported.
In women at high risk for preeclampsia, "available evidence indicates modest effects but important benefits of daily low-dose aspirin for prevention of the condition and consequent illness," concluded Jillian Henderson, Ph.D., of Kaiser Permanente Northwest, Portland, Ore., and her associates.
However, the review had limitations and could not rule out rare or long-term risks of treatment, they said. In addition, more studies are needed, including more in black women, who are disproportionately affected by preeclampsia in the United States.
The study of the effects of low-dose aspirin on the risk of preeclampsia and other maternal and fetal outcomes is being published online April 8 in Annals of Internal Medicine (www.annals.org).
The review, funded by the Agency for Healthcare Research and Quality (AHRQ), was conducted for the U.S. Preventive Services Task Force. The USPSTF will post a draft recommendation, based on this review, as an update to the USPSTF 1996 recommendation (which is not active).
The authors reviewed 23 studies of low-dose aspirin in women at high or average risk of preeclampsia with a mean age of 20-31 years; these included randomized controlled studies and observational studies. Treatment was not started before 12 weeks in any of the studies, but was started before 16 weeks in 8 studies. With the exception of six studies, treatment was continued until delivery. In most of the studies, daily aspirin doses ranged from 60 to 100 mg.
The use of aspirin was associated with a 24% reduced risk of preeclampsia in the studies of high-risk women. The dosage or timing (started before or after 16 weeks) did not have an effect on risk. The reduced risk was greater in the studies that used doses higher than 75 mg, but this difference was not significant, the authors reported.
The use of aspirin was associated with a 20% reduced risk of intrauterine growth restriction (IUGR) and a 14% reduced risk of preterm birth, they reported, adding that the addition of negative studies could make these associations nonsignificant.
In the discussion, the authors said that their confidence in the magnitude of these results was "tempered by evidence of small-study effects and modest findings" in the two largest studies. Therefore, a more conservative estimate for the reduced risk of these outcomes – preeclampsia, IUGR, and preterm birth – was "closer to 10%," they wrote.
They did not identify any harmful effects of aspirin to the baby or mother, but "potential rare or long-term harms could not be ruled out," they concluded. There was some evidence of a possible increased risk in placental abruption, but the associations were not statistically significant. There was no evidence that perinatal mortality was increased, and the data suggested there was "possibly a benefit" on mortality favoring low-dose aspirin.
Their analyses also did not find evidence that low-dose aspirin increased the risk of postpartum hemorrhage or increased mean blood loss or the risk of intracranial hemorrhage in newborns.
The long-term follow-up data on infants (up to age 18 months) available in one study did not show differences in hospital visits, gross motor development, or height and weight between infants exposed to aspirin and those not exposed to aspirin.
Among the limitations of the study was the fact that only studies considered "fair to good quality" were included, and that the smaller studies did not capture any rare events such as perinatal death or placental abruption.
The review was conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center, under a contract to the AHRQ. One author was paid by the USPSTF to conduct the review; two authors reported receiving AHRQ grants during this study (one author) or outside of the study (one author); the rest had no disclosures.
The use of low-dose aspirin during pregnancy was associated with a reduced risk of preeclampsia in women at high risk, and did not appear to be associated with harmful effects, in a systematic review of 23 studies of low-dose aspirin in women at high or average risk of preeclampsia, the authors reported.
In women at high risk for preeclampsia, "available evidence indicates modest effects but important benefits of daily low-dose aspirin for prevention of the condition and consequent illness," concluded Jillian Henderson, Ph.D., of Kaiser Permanente Northwest, Portland, Ore., and her associates.
However, the review had limitations and could not rule out rare or long-term risks of treatment, they said. In addition, more studies are needed, including more in black women, who are disproportionately affected by preeclampsia in the United States.
The study of the effects of low-dose aspirin on the risk of preeclampsia and other maternal and fetal outcomes is being published online April 8 in Annals of Internal Medicine (www.annals.org).
The review, funded by the Agency for Healthcare Research and Quality (AHRQ), was conducted for the U.S. Preventive Services Task Force. The USPSTF will post a draft recommendation, based on this review, as an update to the USPSTF 1996 recommendation (which is not active).
The authors reviewed 23 studies of low-dose aspirin in women at high or average risk of preeclampsia with a mean age of 20-31 years; these included randomized controlled studies and observational studies. Treatment was not started before 12 weeks in any of the studies, but was started before 16 weeks in 8 studies. With the exception of six studies, treatment was continued until delivery. In most of the studies, daily aspirin doses ranged from 60 to 100 mg.
The use of aspirin was associated with a 24% reduced risk of preeclampsia in the studies of high-risk women. The dosage or timing (started before or after 16 weeks) did not have an effect on risk. The reduced risk was greater in the studies that used doses higher than 75 mg, but this difference was not significant, the authors reported.
The use of aspirin was associated with a 20% reduced risk of intrauterine growth restriction (IUGR) and a 14% reduced risk of preterm birth, they reported, adding that the addition of negative studies could make these associations nonsignificant.
In the discussion, the authors said that their confidence in the magnitude of these results was "tempered by evidence of small-study effects and modest findings" in the two largest studies. Therefore, a more conservative estimate for the reduced risk of these outcomes – preeclampsia, IUGR, and preterm birth – was "closer to 10%," they wrote.
They did not identify any harmful effects of aspirin to the baby or mother, but "potential rare or long-term harms could not be ruled out," they concluded. There was some evidence of a possible increased risk in placental abruption, but the associations were not statistically significant. There was no evidence that perinatal mortality was increased, and the data suggested there was "possibly a benefit" on mortality favoring low-dose aspirin.
Their analyses also did not find evidence that low-dose aspirin increased the risk of postpartum hemorrhage or increased mean blood loss or the risk of intracranial hemorrhage in newborns.
The long-term follow-up data on infants (up to age 18 months) available in one study did not show differences in hospital visits, gross motor development, or height and weight between infants exposed to aspirin and those not exposed to aspirin.
Among the limitations of the study was the fact that only studies considered "fair to good quality" were included, and that the smaller studies did not capture any rare events such as perinatal death or placental abruption.
The review was conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center, under a contract to the AHRQ. One author was paid by the USPSTF to conduct the review; two authors reported receiving AHRQ grants during this study (one author) or outside of the study (one author); the rest had no disclosures.
The use of low-dose aspirin during pregnancy was associated with a reduced risk of preeclampsia in women at high risk, and did not appear to be associated with harmful effects, in a systematic review of 23 studies of low-dose aspirin in women at high or average risk of preeclampsia, the authors reported.
In women at high risk for preeclampsia, "available evidence indicates modest effects but important benefits of daily low-dose aspirin for prevention of the condition and consequent illness," concluded Jillian Henderson, Ph.D., of Kaiser Permanente Northwest, Portland, Ore., and her associates.
However, the review had limitations and could not rule out rare or long-term risks of treatment, they said. In addition, more studies are needed, including more in black women, who are disproportionately affected by preeclampsia in the United States.
The study of the effects of low-dose aspirin on the risk of preeclampsia and other maternal and fetal outcomes is being published online April 8 in Annals of Internal Medicine (www.annals.org).
The review, funded by the Agency for Healthcare Research and Quality (AHRQ), was conducted for the U.S. Preventive Services Task Force. The USPSTF will post a draft recommendation, based on this review, as an update to the USPSTF 1996 recommendation (which is not active).
The authors reviewed 23 studies of low-dose aspirin in women at high or average risk of preeclampsia with a mean age of 20-31 years; these included randomized controlled studies and observational studies. Treatment was not started before 12 weeks in any of the studies, but was started before 16 weeks in 8 studies. With the exception of six studies, treatment was continued until delivery. In most of the studies, daily aspirin doses ranged from 60 to 100 mg.
The use of aspirin was associated with a 24% reduced risk of preeclampsia in the studies of high-risk women. The dosage or timing (started before or after 16 weeks) did not have an effect on risk. The reduced risk was greater in the studies that used doses higher than 75 mg, but this difference was not significant, the authors reported.
The use of aspirin was associated with a 20% reduced risk of intrauterine growth restriction (IUGR) and a 14% reduced risk of preterm birth, they reported, adding that the addition of negative studies could make these associations nonsignificant.
In the discussion, the authors said that their confidence in the magnitude of these results was "tempered by evidence of small-study effects and modest findings" in the two largest studies. Therefore, a more conservative estimate for the reduced risk of these outcomes – preeclampsia, IUGR, and preterm birth – was "closer to 10%," they wrote.
They did not identify any harmful effects of aspirin to the baby or mother, but "potential rare or long-term harms could not be ruled out," they concluded. There was some evidence of a possible increased risk in placental abruption, but the associations were not statistically significant. There was no evidence that perinatal mortality was increased, and the data suggested there was "possibly a benefit" on mortality favoring low-dose aspirin.
Their analyses also did not find evidence that low-dose aspirin increased the risk of postpartum hemorrhage or increased mean blood loss or the risk of intracranial hemorrhage in newborns.
The long-term follow-up data on infants (up to age 18 months) available in one study did not show differences in hospital visits, gross motor development, or height and weight between infants exposed to aspirin and those not exposed to aspirin.
Among the limitations of the study was the fact that only studies considered "fair to good quality" were included, and that the smaller studies did not capture any rare events such as perinatal death or placental abruption.
The review was conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center, under a contract to the AHRQ. One author was paid by the USPSTF to conduct the review; two authors reported receiving AHRQ grants during this study (one author) or outside of the study (one author); the rest had no disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Major finding: Prenatal use of low-dose aspirin was associated with a 24% reduced risk of preeclampsia in women at high risk.
Data source: A systematic review of 23 randomized controlled studies and cohort studies that evaluated the risks and benefits of prenatal aspirin among women at high risk of preeclampsia.
Disclosures: The review was conducted by the Kaiser Permanente Research Affiliates Evidence-based Practice Center, under a contract to AHRQ. The study was funded by the Agency for Healthcare Research and Quality (AHRQ), under a contract that supported the work of the USPSTF. One author was paid by the USPSTF to conduct the review; two authors reported receiving AHRQ grants during this study (one author) or outside of the study (one author); the rest had no disclosures.
Drop in circumcision rates prompts call for Medicaid coverage
Data suggesting that circumcision rates in neonates have declined in recent years have prompted a renewed call for universal Medicaid coverage for the procedure and inclusion of circumcision as part of public health policy, according to a report published online in the April 2 issue of Mayo Clinic Proceedings.
A review examining trends in U.S. male circumcision rates and the impact of the 2012 American Academy of Pediatrics policy has found that the overall rate of circumcision in the United States declined from 83% in 1960-1969 to 77% by 2010.
Brian Morris, Ph.D., of the University of Sydney and his colleagues suggested that the decline was partly the result of demographic changes, such as the increase in the proportion of Hispanic people, who are traditionally noncircumcising, but also was likely influenced by the withdrawal of Medicaid coverage for circumcision in 18 states.
The review was prompted by a recent report from the Centers for Disease Control and Prevention, which used data from the National Health and Nutrition Examination Surveys to estimate that total circumcision prevalence had risen from 79% to 81% over the past decade, with increases to 90.8% in non-Hispanic white, 75.7% in non-Hispanic black, and 44.0% in Mexican American males.
"Because these data are for males aged 14 to 59 years – and most circumcisions in the United States take place during the neonatal period – they largely reflect past practice," Dr. Morris and his associates wrote.
Instead, they used hospital discharge data, corrected for so-called unreported circumcisions that take place after neonates are discharged from the hospital, to reveal the six percentage point decline since the 1960s.
The authors also conducted a risk-benefit analysis of circumcision, finding that the benefits – such as a reduced risk of urinary tract infection, human papillomavirus, herpes simplex, and HIV infections – exceeded the risks by at least 100 to 1 (Mayo Clin. Proc. 2014 Apr. 2 [doi:10.1016/j.mayocp.2014.01.001]).
They concluded that, over the course of a lifetime, around one in two uncircumcised men would require treatment for some medical condition associated with retention of the foreskin.
"It would be unethical for medical practitioners not to recommend circumcision for a baby boy," Dr. Morris said in an interview. "That’s what the AAP says, and it recommends that parents be educated about the benefits and also the risks early in the pregnancy so they’ve got plenty of time to make up their minds should they have a baby boy."
The most common risk associated with circumcision was local bruising at the site of the local anesthetic injection, affecting approximately one-quarter of individuals. More serious adverse effects such as infection, bleeding, or loss of the penis were extremely rare.
Commenting on the often-quoted risk of reduced penile sensitivity and enjoyment of sex, Dr. Morris said that the overwhelming majority of studies, including those conducted in adult men undergoing circumcision, showed no difference or even an improvement in sexual pleasure.
The reviewers also argued that circumcision was extremely cost effective, citing one study of infant urinary tract infections and sexually transmitted infections suggesting that, if male circumcision rates declined to those seen in Europe – around 10% – it would result in additional medical costs exceeding $4.4 billion.
"When considered together with ethical and human rights arguments, neonatal circumcision should logically be strongly supported and encouraged as an important evidence-based intervention akin to childhood vaccination," the authors wrote.
No conflicts of interest were disclosed.
Data suggesting that circumcision rates in neonates have declined in recent years have prompted a renewed call for universal Medicaid coverage for the procedure and inclusion of circumcision as part of public health policy, according to a report published online in the April 2 issue of Mayo Clinic Proceedings.
A review examining trends in U.S. male circumcision rates and the impact of the 2012 American Academy of Pediatrics policy has found that the overall rate of circumcision in the United States declined from 83% in 1960-1969 to 77% by 2010.
Brian Morris, Ph.D., of the University of Sydney and his colleagues suggested that the decline was partly the result of demographic changes, such as the increase in the proportion of Hispanic people, who are traditionally noncircumcising, but also was likely influenced by the withdrawal of Medicaid coverage for circumcision in 18 states.
The review was prompted by a recent report from the Centers for Disease Control and Prevention, which used data from the National Health and Nutrition Examination Surveys to estimate that total circumcision prevalence had risen from 79% to 81% over the past decade, with increases to 90.8% in non-Hispanic white, 75.7% in non-Hispanic black, and 44.0% in Mexican American males.
"Because these data are for males aged 14 to 59 years – and most circumcisions in the United States take place during the neonatal period – they largely reflect past practice," Dr. Morris and his associates wrote.
Instead, they used hospital discharge data, corrected for so-called unreported circumcisions that take place after neonates are discharged from the hospital, to reveal the six percentage point decline since the 1960s.
The authors also conducted a risk-benefit analysis of circumcision, finding that the benefits – such as a reduced risk of urinary tract infection, human papillomavirus, herpes simplex, and HIV infections – exceeded the risks by at least 100 to 1 (Mayo Clin. Proc. 2014 Apr. 2 [doi:10.1016/j.mayocp.2014.01.001]).
They concluded that, over the course of a lifetime, around one in two uncircumcised men would require treatment for some medical condition associated with retention of the foreskin.
"It would be unethical for medical practitioners not to recommend circumcision for a baby boy," Dr. Morris said in an interview. "That’s what the AAP says, and it recommends that parents be educated about the benefits and also the risks early in the pregnancy so they’ve got plenty of time to make up their minds should they have a baby boy."
The most common risk associated with circumcision was local bruising at the site of the local anesthetic injection, affecting approximately one-quarter of individuals. More serious adverse effects such as infection, bleeding, or loss of the penis were extremely rare.
Commenting on the often-quoted risk of reduced penile sensitivity and enjoyment of sex, Dr. Morris said that the overwhelming majority of studies, including those conducted in adult men undergoing circumcision, showed no difference or even an improvement in sexual pleasure.
The reviewers also argued that circumcision was extremely cost effective, citing one study of infant urinary tract infections and sexually transmitted infections suggesting that, if male circumcision rates declined to those seen in Europe – around 10% – it would result in additional medical costs exceeding $4.4 billion.
"When considered together with ethical and human rights arguments, neonatal circumcision should logically be strongly supported and encouraged as an important evidence-based intervention akin to childhood vaccination," the authors wrote.
No conflicts of interest were disclosed.
Data suggesting that circumcision rates in neonates have declined in recent years have prompted a renewed call for universal Medicaid coverage for the procedure and inclusion of circumcision as part of public health policy, according to a report published online in the April 2 issue of Mayo Clinic Proceedings.
A review examining trends in U.S. male circumcision rates and the impact of the 2012 American Academy of Pediatrics policy has found that the overall rate of circumcision in the United States declined from 83% in 1960-1969 to 77% by 2010.
Brian Morris, Ph.D., of the University of Sydney and his colleagues suggested that the decline was partly the result of demographic changes, such as the increase in the proportion of Hispanic people, who are traditionally noncircumcising, but also was likely influenced by the withdrawal of Medicaid coverage for circumcision in 18 states.
The review was prompted by a recent report from the Centers for Disease Control and Prevention, which used data from the National Health and Nutrition Examination Surveys to estimate that total circumcision prevalence had risen from 79% to 81% over the past decade, with increases to 90.8% in non-Hispanic white, 75.7% in non-Hispanic black, and 44.0% in Mexican American males.
"Because these data are for males aged 14 to 59 years – and most circumcisions in the United States take place during the neonatal period – they largely reflect past practice," Dr. Morris and his associates wrote.
Instead, they used hospital discharge data, corrected for so-called unreported circumcisions that take place after neonates are discharged from the hospital, to reveal the six percentage point decline since the 1960s.
The authors also conducted a risk-benefit analysis of circumcision, finding that the benefits – such as a reduced risk of urinary tract infection, human papillomavirus, herpes simplex, and HIV infections – exceeded the risks by at least 100 to 1 (Mayo Clin. Proc. 2014 Apr. 2 [doi:10.1016/j.mayocp.2014.01.001]).
They concluded that, over the course of a lifetime, around one in two uncircumcised men would require treatment for some medical condition associated with retention of the foreskin.
"It would be unethical for medical practitioners not to recommend circumcision for a baby boy," Dr. Morris said in an interview. "That’s what the AAP says, and it recommends that parents be educated about the benefits and also the risks early in the pregnancy so they’ve got plenty of time to make up their minds should they have a baby boy."
The most common risk associated with circumcision was local bruising at the site of the local anesthetic injection, affecting approximately one-quarter of individuals. More serious adverse effects such as infection, bleeding, or loss of the penis were extremely rare.
Commenting on the often-quoted risk of reduced penile sensitivity and enjoyment of sex, Dr. Morris said that the overwhelming majority of studies, including those conducted in adult men undergoing circumcision, showed no difference or even an improvement in sexual pleasure.
The reviewers also argued that circumcision was extremely cost effective, citing one study of infant urinary tract infections and sexually transmitted infections suggesting that, if male circumcision rates declined to those seen in Europe – around 10% – it would result in additional medical costs exceeding $4.4 billion.
"When considered together with ethical and human rights arguments, neonatal circumcision should logically be strongly supported and encouraged as an important evidence-based intervention akin to childhood vaccination," the authors wrote.
No conflicts of interest were disclosed.
FROM MAYO CLINIC PROCEEDINGS
Major finding: Neonatal circumcision rates declined from 83% in 1960-1969 to 77% in 2010.
Data source: Review including analysis of National Health and Nutrition Examination Survey data combined with hospital discharge data.
Disclosures: None declared.
Q: Following cesarean delivery, what is the optimal oxytocin infusion duration to prevent postpartum bleeding?
CASE: DISCONTINUED OXYTOCIN LEADS TO POSTPARTUM HEMORRHAGE
You have just completed a repeat cesarean delivery for a 41-year-old woman, now G2P2. You order an infusion of oxytocin, 20 U in 1 L lactated Ringer’s solution, to run at a rate of 125 mL/hr for 8 hours. Without informing you, the recovery room nurse discontinues the bag with the oxytocin solution and starts an infusion of lactated Ringer’s solution without oxytocin.
One hour later, you are called to the recovery room because your patient is having a postpartum hemorrhage (PPH). Physical examination shows that the uterus is boggy and above the level of the umbilicus. On bedside ultrasonography, the uterine cavity is demonstrated to contain minimal blood, and Doppler sonography does not demonstrate any vascular tissue within the uterine cavity. You diagnose uterine atony and initiate treatment. You massage the uterus, rapidly infuse 1 L crystalloid solution, place misoprostol 800 µg in the rectum, and reinitiate the oxytocin infusion. The uterine bleeding slows and then stops.
The following morning, the patient’s hematocrit has decreased from a preoperative value of 37% to 21%.
Could this case of PPH have been prevented?
Cesarean delivery is one of the most commonly performed major operations in developed countries. More than 1,250,000 cesarean deliveries are performed annually in the United States. In 2012, there were 3,952,937 births and a cesarean delivery rate of 32.8%.1 It is an important goal of obstetric care providers to continuously improve our approach to cesarean delivery in order to minimize the surgical risks of this procedure. Evidence-based, standardized protocols for cesarean delivery are critical to ensuring high- reliability surgical outcomes.
A key gap in cesarean delivery protocols is the lack of a nationwide, standardized approach to reducing the risk of postoperative bleeding by maintaining a continuous infusion of oxytocin in the hours immediately following cesarean delivery.
OXYTOCIN: A CRITICAL INTERVENTION TO PREVENT PPH
More than half of all maternal deaths occur in the 24 hours following delivery, with the most common cause being PPH.2 In addition to death, serious complications of PPH include coagulopathy, shock, emergency hysterectomy, transfusion complications, respiratory distress, and pituitary necrosis. Most cases of PPH that occur within 24 hours of delivery are caused by uterine atony.3 Other causes include retained products of conception, placenta accreta, infection, coagulation defects, and amniotic fluid embolism.
Administering a uterotonic such as oxytocin at the time of delivery reduces the risk of PPH by approximately 66% and the risk of maternal blood transfusion by about 65%.4 In order to prevent uterine atony and PPH, oxytocin should be routinely administered following birth of the baby or after delivery of the placenta. Appropriate doses following vaginal delivery are oxytocin 10 U administered intramuscularly or 10 U administered as a slow intravenous (IV) infusion.5 The onset of action of oxytocin is approximately 2 to 5 minutes after an intramuscular dose and 1 minute after an IV dose.6
Related article: Routine use of oxytocin at birth: just the right amount to prevent postpartum hemorrhage Robert L. Barbieri, MD (Editorial, July 2012)
OXYTOCIN AND CESAREAN DELIVERY
Many clinical trials have reported that during a cesarean delivery, the routine administration of a uterotonic agent following birth of the baby reduces the risk of uterine atony and excessive bleeding. Three uterotonics: oxytocin, misoprostol, and carbetocin (a long-acting oxytocin analogue, see SIDEBAR), have been reported to reduce the risk of excessive bleeding during cesarean delivery.7 Oxytocin is the uterotonic most commonly used during cesarean delivery in developed countries.
Related article: A new (to the US) first-line agent for heavy menstrual bleeding Robert L. Barbieri, MD (Editorial, October 2010)
In the United States, there is no standardized oxytocin regimen for prevention of uterine atony and hemorrhage at cesarean delivery. The most common regimen is to add 10–40 U of oxytocin in 1 L crystalloid solution and initiate the oxytocin infusion following delivery of the baby. Initially, the infusion is run at a rapid rate. Once the obstetrician reports that there is adequate uterine tone, the infusion rate is slowed to one that maintains uterine tone.
Some clinicians administer a single bolus of oxytocin following birth of the baby. However, a bolus of oxytocin commonly causes hypotension and, less commonly, ST segment changes on the electrocardiogram (EKG) suggestive of cardiac ischemia.8–10Many experts recommend against administering one large bolus of oxytocin over a short period of time and favor a continuous infusion.
At cesarean delivery, the minimum infusion rate of oxytocin that has been reported to avoid most cases of uterine atony, as reported by the obstetrician immediately following delivery, is approximately oxytocin 0.3 U/min.11 Oxytocin infusion rates of 0.2 U/min and 0.1 U/min were associated with uterine atony rates of 21% and 40%, respectively. An infusion rate of oxytocin 0.3 U/min can be achieved by the administration of 20 U of oxytocin in 1 L crystalloid solution at a rate of 15 mL/min until uterine tone is achieved. The oxytocin dose then can be titrated to maintain adequate uterine tone. Following completion of surgery, uterine tone can be maintained with a low-dose continuous infusion of oxytocin.
4- TO 8-HOUR OXYTOCIN RULE
A key gap in our cesarean delivery protocols is a standardized recommendation concerning the duration of the oxytocin infusion following cesarean delivery. To my knowledge, no national organization has made a firm recommendation concerning the duration of oxytocin infusion following cesarean delivery.
One recent clinical trial studied PPH following cesarean delivery utilizing two oxytocin regimens: a bolus of oxytocin following delivery of the baby versus a bolus of oxytocin followed by a 4-hour IV infusion of oxytocin.12 In this trial, 2,058 women undergoing a scheduled cesarean delivery with a singleton pregnancy were randomly assigned to an oxytocin bolus alone, oxytocin 5 U administered intravenously over 1 minute, or an oxytocin bolus plus a 4-hour oxytocin infusion at a rate of 10 U/hr. The 4-hour postoperative oxytocin infusion was formulated by adding 40 U of oxytocin to 500 mL saline and infusing the solution at 125 mL/hr, equivalent to 0.167 U of oxytocin per minute. In this trial, 65% of the women were undergoing a repeat cesarean delivery and 35% were undergoing a primary cesarean delivery.
The authors reported that women who received the oxytocin bolus alone were significantly more likely to be diagnosed with uterine atony requiring additional uterotonic treatment than women who received both the bolus and the 4-hour postoperative infusion (18.4% versus 12.2%, respectively; P <.001). There was no difference in the rate of PPH between the two groups.
The rate of PPH was 16% in women receiving an oxytocin bolus alone and 15.7% in women receiving both an oxytocin bolus and the continuous oxytocin infusion. However, among less experienced surgeons, the rate of PPH was significantly greater in the group that received the oxytocin bolus alone compared with the women receiving the bolus and continuous infusion (22.2% versus 17.3%, respectively). The authors concluded that obstetricians should consider using a 4-hour infusion of oxytocin following cesarean delivery to reduce the risk of uterine atony.
In a recent evidence-based review of optimal interventions in cesarean delivery, the authors recommended an IV infusion of 10 to 40 U of oxytocin administered over 4 to 8 hours after cesarean delivery.7 Following cesarean, an IV infusion of crystalloid solution is typically maintained for at least 4 to 8 hours. Consequently, adding oxytocin (which costs approximately $1 for 10 units) to the crystalloid infusion does not add substantially to the cost of the patient’s postoperative care and may reduce the risk of uterine atony and PPH.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial, March 2012)
My bottom-line recommendation. In the United States, we should adopt a policy of maintaining a continuous infusion of oxytocin for 4 to 8 hours following a cesarean delivery. Following a 4- to 8-hour rule will decrease the rate of uterine atony and excessive bleeding, thereby improving the safety of our cesarean delivery surgery.
INSTANT POLL
How many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
If the patient is a Jehovah’s Witness and refuses the transfusion of all blood products, how many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
Tell us—at rbarbieri@frontlinemedcom.com Please include your name and city and state.
- Hamilton BE, Martin JA, Ventura SJ. National Vital Statistics Reports. Births: Preliminary Data for 2012. 2013;62(3). http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_03.pdf. Published September 6, 2013. Accessed March 18, 2014.
- AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11.
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76.
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Embrey MP. Simultaneous intramuscular injection of oxytocin and ergometrine: a tocographic study. BMJ. 1961;1(5241):1737–1738.
- Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
- Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: Findings in six cases. Int J Obstet Anesth. 2008;17(3):247–254.
- Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76–83.
- Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfourds EM. Signs of myocardial ischaemia after injection of oxytocin: A randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100(5):683–689.
- George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing cesarean delivery. Can J Anesth. 2010;57(6):578–582.
- Sheehan SR, Montgomery AA, Carey M, et al; ECSSIT Study Group. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective cesarean section: Double blind, placebo controlled, randomized trial. BMJ. 2011;343:d4661.
CASE: DISCONTINUED OXYTOCIN LEADS TO POSTPARTUM HEMORRHAGE
You have just completed a repeat cesarean delivery for a 41-year-old woman, now G2P2. You order an infusion of oxytocin, 20 U in 1 L lactated Ringer’s solution, to run at a rate of 125 mL/hr for 8 hours. Without informing you, the recovery room nurse discontinues the bag with the oxytocin solution and starts an infusion of lactated Ringer’s solution without oxytocin.
One hour later, you are called to the recovery room because your patient is having a postpartum hemorrhage (PPH). Physical examination shows that the uterus is boggy and above the level of the umbilicus. On bedside ultrasonography, the uterine cavity is demonstrated to contain minimal blood, and Doppler sonography does not demonstrate any vascular tissue within the uterine cavity. You diagnose uterine atony and initiate treatment. You massage the uterus, rapidly infuse 1 L crystalloid solution, place misoprostol 800 µg in the rectum, and reinitiate the oxytocin infusion. The uterine bleeding slows and then stops.
The following morning, the patient’s hematocrit has decreased from a preoperative value of 37% to 21%.
Could this case of PPH have been prevented?
Cesarean delivery is one of the most commonly performed major operations in developed countries. More than 1,250,000 cesarean deliveries are performed annually in the United States. In 2012, there were 3,952,937 births and a cesarean delivery rate of 32.8%.1 It is an important goal of obstetric care providers to continuously improve our approach to cesarean delivery in order to minimize the surgical risks of this procedure. Evidence-based, standardized protocols for cesarean delivery are critical to ensuring high- reliability surgical outcomes.
A key gap in cesarean delivery protocols is the lack of a nationwide, standardized approach to reducing the risk of postoperative bleeding by maintaining a continuous infusion of oxytocin in the hours immediately following cesarean delivery.
OXYTOCIN: A CRITICAL INTERVENTION TO PREVENT PPH
More than half of all maternal deaths occur in the 24 hours following delivery, with the most common cause being PPH.2 In addition to death, serious complications of PPH include coagulopathy, shock, emergency hysterectomy, transfusion complications, respiratory distress, and pituitary necrosis. Most cases of PPH that occur within 24 hours of delivery are caused by uterine atony.3 Other causes include retained products of conception, placenta accreta, infection, coagulation defects, and amniotic fluid embolism.
Administering a uterotonic such as oxytocin at the time of delivery reduces the risk of PPH by approximately 66% and the risk of maternal blood transfusion by about 65%.4 In order to prevent uterine atony and PPH, oxytocin should be routinely administered following birth of the baby or after delivery of the placenta. Appropriate doses following vaginal delivery are oxytocin 10 U administered intramuscularly or 10 U administered as a slow intravenous (IV) infusion.5 The onset of action of oxytocin is approximately 2 to 5 minutes after an intramuscular dose and 1 minute after an IV dose.6
Related article: Routine use of oxytocin at birth: just the right amount to prevent postpartum hemorrhage Robert L. Barbieri, MD (Editorial, July 2012)
OXYTOCIN AND CESAREAN DELIVERY
Many clinical trials have reported that during a cesarean delivery, the routine administration of a uterotonic agent following birth of the baby reduces the risk of uterine atony and excessive bleeding. Three uterotonics: oxytocin, misoprostol, and carbetocin (a long-acting oxytocin analogue, see SIDEBAR), have been reported to reduce the risk of excessive bleeding during cesarean delivery.7 Oxytocin is the uterotonic most commonly used during cesarean delivery in developed countries.
Related article: A new (to the US) first-line agent for heavy menstrual bleeding Robert L. Barbieri, MD (Editorial, October 2010)
In the United States, there is no standardized oxytocin regimen for prevention of uterine atony and hemorrhage at cesarean delivery. The most common regimen is to add 10–40 U of oxytocin in 1 L crystalloid solution and initiate the oxytocin infusion following delivery of the baby. Initially, the infusion is run at a rapid rate. Once the obstetrician reports that there is adequate uterine tone, the infusion rate is slowed to one that maintains uterine tone.
Some clinicians administer a single bolus of oxytocin following birth of the baby. However, a bolus of oxytocin commonly causes hypotension and, less commonly, ST segment changes on the electrocardiogram (EKG) suggestive of cardiac ischemia.8–10Many experts recommend against administering one large bolus of oxytocin over a short period of time and favor a continuous infusion.
At cesarean delivery, the minimum infusion rate of oxytocin that has been reported to avoid most cases of uterine atony, as reported by the obstetrician immediately following delivery, is approximately oxytocin 0.3 U/min.11 Oxytocin infusion rates of 0.2 U/min and 0.1 U/min were associated with uterine atony rates of 21% and 40%, respectively. An infusion rate of oxytocin 0.3 U/min can be achieved by the administration of 20 U of oxytocin in 1 L crystalloid solution at a rate of 15 mL/min until uterine tone is achieved. The oxytocin dose then can be titrated to maintain adequate uterine tone. Following completion of surgery, uterine tone can be maintained with a low-dose continuous infusion of oxytocin.
4- TO 8-HOUR OXYTOCIN RULE
A key gap in our cesarean delivery protocols is a standardized recommendation concerning the duration of the oxytocin infusion following cesarean delivery. To my knowledge, no national organization has made a firm recommendation concerning the duration of oxytocin infusion following cesarean delivery.
One recent clinical trial studied PPH following cesarean delivery utilizing two oxytocin regimens: a bolus of oxytocin following delivery of the baby versus a bolus of oxytocin followed by a 4-hour IV infusion of oxytocin.12 In this trial, 2,058 women undergoing a scheduled cesarean delivery with a singleton pregnancy were randomly assigned to an oxytocin bolus alone, oxytocin 5 U administered intravenously over 1 minute, or an oxytocin bolus plus a 4-hour oxytocin infusion at a rate of 10 U/hr. The 4-hour postoperative oxytocin infusion was formulated by adding 40 U of oxytocin to 500 mL saline and infusing the solution at 125 mL/hr, equivalent to 0.167 U of oxytocin per minute. In this trial, 65% of the women were undergoing a repeat cesarean delivery and 35% were undergoing a primary cesarean delivery.
The authors reported that women who received the oxytocin bolus alone were significantly more likely to be diagnosed with uterine atony requiring additional uterotonic treatment than women who received both the bolus and the 4-hour postoperative infusion (18.4% versus 12.2%, respectively; P <.001). There was no difference in the rate of PPH between the two groups.
The rate of PPH was 16% in women receiving an oxytocin bolus alone and 15.7% in women receiving both an oxytocin bolus and the continuous oxytocin infusion. However, among less experienced surgeons, the rate of PPH was significantly greater in the group that received the oxytocin bolus alone compared with the women receiving the bolus and continuous infusion (22.2% versus 17.3%, respectively). The authors concluded that obstetricians should consider using a 4-hour infusion of oxytocin following cesarean delivery to reduce the risk of uterine atony.
In a recent evidence-based review of optimal interventions in cesarean delivery, the authors recommended an IV infusion of 10 to 40 U of oxytocin administered over 4 to 8 hours after cesarean delivery.7 Following cesarean, an IV infusion of crystalloid solution is typically maintained for at least 4 to 8 hours. Consequently, adding oxytocin (which costs approximately $1 for 10 units) to the crystalloid infusion does not add substantially to the cost of the patient’s postoperative care and may reduce the risk of uterine atony and PPH.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial, March 2012)
My bottom-line recommendation. In the United States, we should adopt a policy of maintaining a continuous infusion of oxytocin for 4 to 8 hours following a cesarean delivery. Following a 4- to 8-hour rule will decrease the rate of uterine atony and excessive bleeding, thereby improving the safety of our cesarean delivery surgery.
INSTANT POLL
How many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
If the patient is a Jehovah’s Witness and refuses the transfusion of all blood products, how many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
Tell us—at rbarbieri@frontlinemedcom.com Please include your name and city and state.
CASE: DISCONTINUED OXYTOCIN LEADS TO POSTPARTUM HEMORRHAGE
You have just completed a repeat cesarean delivery for a 41-year-old woman, now G2P2. You order an infusion of oxytocin, 20 U in 1 L lactated Ringer’s solution, to run at a rate of 125 mL/hr for 8 hours. Without informing you, the recovery room nurse discontinues the bag with the oxytocin solution and starts an infusion of lactated Ringer’s solution without oxytocin.
One hour later, you are called to the recovery room because your patient is having a postpartum hemorrhage (PPH). Physical examination shows that the uterus is boggy and above the level of the umbilicus. On bedside ultrasonography, the uterine cavity is demonstrated to contain minimal blood, and Doppler sonography does not demonstrate any vascular tissue within the uterine cavity. You diagnose uterine atony and initiate treatment. You massage the uterus, rapidly infuse 1 L crystalloid solution, place misoprostol 800 µg in the rectum, and reinitiate the oxytocin infusion. The uterine bleeding slows and then stops.
The following morning, the patient’s hematocrit has decreased from a preoperative value of 37% to 21%.
Could this case of PPH have been prevented?
Cesarean delivery is one of the most commonly performed major operations in developed countries. More than 1,250,000 cesarean deliveries are performed annually in the United States. In 2012, there were 3,952,937 births and a cesarean delivery rate of 32.8%.1 It is an important goal of obstetric care providers to continuously improve our approach to cesarean delivery in order to minimize the surgical risks of this procedure. Evidence-based, standardized protocols for cesarean delivery are critical to ensuring high- reliability surgical outcomes.
A key gap in cesarean delivery protocols is the lack of a nationwide, standardized approach to reducing the risk of postoperative bleeding by maintaining a continuous infusion of oxytocin in the hours immediately following cesarean delivery.
OXYTOCIN: A CRITICAL INTERVENTION TO PREVENT PPH
More than half of all maternal deaths occur in the 24 hours following delivery, with the most common cause being PPH.2 In addition to death, serious complications of PPH include coagulopathy, shock, emergency hysterectomy, transfusion complications, respiratory distress, and pituitary necrosis. Most cases of PPH that occur within 24 hours of delivery are caused by uterine atony.3 Other causes include retained products of conception, placenta accreta, infection, coagulation defects, and amniotic fluid embolism.
Administering a uterotonic such as oxytocin at the time of delivery reduces the risk of PPH by approximately 66% and the risk of maternal blood transfusion by about 65%.4 In order to prevent uterine atony and PPH, oxytocin should be routinely administered following birth of the baby or after delivery of the placenta. Appropriate doses following vaginal delivery are oxytocin 10 U administered intramuscularly or 10 U administered as a slow intravenous (IV) infusion.5 The onset of action of oxytocin is approximately 2 to 5 minutes after an intramuscular dose and 1 minute after an IV dose.6
Related article: Routine use of oxytocin at birth: just the right amount to prevent postpartum hemorrhage Robert L. Barbieri, MD (Editorial, July 2012)
OXYTOCIN AND CESAREAN DELIVERY
Many clinical trials have reported that during a cesarean delivery, the routine administration of a uterotonic agent following birth of the baby reduces the risk of uterine atony and excessive bleeding. Three uterotonics: oxytocin, misoprostol, and carbetocin (a long-acting oxytocin analogue, see SIDEBAR), have been reported to reduce the risk of excessive bleeding during cesarean delivery.7 Oxytocin is the uterotonic most commonly used during cesarean delivery in developed countries.
Related article: A new (to the US) first-line agent for heavy menstrual bleeding Robert L. Barbieri, MD (Editorial, October 2010)
In the United States, there is no standardized oxytocin regimen for prevention of uterine atony and hemorrhage at cesarean delivery. The most common regimen is to add 10–40 U of oxytocin in 1 L crystalloid solution and initiate the oxytocin infusion following delivery of the baby. Initially, the infusion is run at a rapid rate. Once the obstetrician reports that there is adequate uterine tone, the infusion rate is slowed to one that maintains uterine tone.
Some clinicians administer a single bolus of oxytocin following birth of the baby. However, a bolus of oxytocin commonly causes hypotension and, less commonly, ST segment changes on the electrocardiogram (EKG) suggestive of cardiac ischemia.8–10Many experts recommend against administering one large bolus of oxytocin over a short period of time and favor a continuous infusion.
At cesarean delivery, the minimum infusion rate of oxytocin that has been reported to avoid most cases of uterine atony, as reported by the obstetrician immediately following delivery, is approximately oxytocin 0.3 U/min.11 Oxytocin infusion rates of 0.2 U/min and 0.1 U/min were associated with uterine atony rates of 21% and 40%, respectively. An infusion rate of oxytocin 0.3 U/min can be achieved by the administration of 20 U of oxytocin in 1 L crystalloid solution at a rate of 15 mL/min until uterine tone is achieved. The oxytocin dose then can be titrated to maintain adequate uterine tone. Following completion of surgery, uterine tone can be maintained with a low-dose continuous infusion of oxytocin.
4- TO 8-HOUR OXYTOCIN RULE
A key gap in our cesarean delivery protocols is a standardized recommendation concerning the duration of the oxytocin infusion following cesarean delivery. To my knowledge, no national organization has made a firm recommendation concerning the duration of oxytocin infusion following cesarean delivery.
One recent clinical trial studied PPH following cesarean delivery utilizing two oxytocin regimens: a bolus of oxytocin following delivery of the baby versus a bolus of oxytocin followed by a 4-hour IV infusion of oxytocin.12 In this trial, 2,058 women undergoing a scheduled cesarean delivery with a singleton pregnancy were randomly assigned to an oxytocin bolus alone, oxytocin 5 U administered intravenously over 1 minute, or an oxytocin bolus plus a 4-hour oxytocin infusion at a rate of 10 U/hr. The 4-hour postoperative oxytocin infusion was formulated by adding 40 U of oxytocin to 500 mL saline and infusing the solution at 125 mL/hr, equivalent to 0.167 U of oxytocin per minute. In this trial, 65% of the women were undergoing a repeat cesarean delivery and 35% were undergoing a primary cesarean delivery.
The authors reported that women who received the oxytocin bolus alone were significantly more likely to be diagnosed with uterine atony requiring additional uterotonic treatment than women who received both the bolus and the 4-hour postoperative infusion (18.4% versus 12.2%, respectively; P <.001). There was no difference in the rate of PPH between the two groups.
The rate of PPH was 16% in women receiving an oxytocin bolus alone and 15.7% in women receiving both an oxytocin bolus and the continuous oxytocin infusion. However, among less experienced surgeons, the rate of PPH was significantly greater in the group that received the oxytocin bolus alone compared with the women receiving the bolus and continuous infusion (22.2% versus 17.3%, respectively). The authors concluded that obstetricians should consider using a 4-hour infusion of oxytocin following cesarean delivery to reduce the risk of uterine atony.
In a recent evidence-based review of optimal interventions in cesarean delivery, the authors recommended an IV infusion of 10 to 40 U of oxytocin administered over 4 to 8 hours after cesarean delivery.7 Following cesarean, an IV infusion of crystalloid solution is typically maintained for at least 4 to 8 hours. Consequently, adding oxytocin (which costs approximately $1 for 10 units) to the crystalloid infusion does not add substantially to the cost of the patient’s postoperative care and may reduce the risk of uterine atony and PPH.
Related article: Act fast when confronted by a coagulopathy postpartum Robert L. Barbieri, MD (Editorial, March 2012)
My bottom-line recommendation. In the United States, we should adopt a policy of maintaining a continuous infusion of oxytocin for 4 to 8 hours following a cesarean delivery. Following a 4- to 8-hour rule will decrease the rate of uterine atony and excessive bleeding, thereby improving the safety of our cesarean delivery surgery.
INSTANT POLL
How many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
If the patient is a Jehovah’s Witness and refuses the transfusion of all blood products, how many hours following cesarean delivery do you think that an oxytocin infusion should be maintained to reduce the risk of uterine atony and postpartum hemorrhage?
Tell us—at rbarbieri@frontlinemedcom.com Please include your name and city and state.
- Hamilton BE, Martin JA, Ventura SJ. National Vital Statistics Reports. Births: Preliminary Data for 2012. 2013;62(3). http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_03.pdf. Published September 6, 2013. Accessed March 18, 2014.
- AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11.
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76.
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Embrey MP. Simultaneous intramuscular injection of oxytocin and ergometrine: a tocographic study. BMJ. 1961;1(5241):1737–1738.
- Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
- Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: Findings in six cases. Int J Obstet Anesth. 2008;17(3):247–254.
- Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76–83.
- Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfourds EM. Signs of myocardial ischaemia after injection of oxytocin: A randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100(5):683–689.
- George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing cesarean delivery. Can J Anesth. 2010;57(6):578–582.
- Sheehan SR, Montgomery AA, Carey M, et al; ECSSIT Study Group. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective cesarean section: Double blind, placebo controlled, randomized trial. BMJ. 2011;343:d4661.
- Hamilton BE, Martin JA, Ventura SJ. National Vital Statistics Reports. Births: Preliminary Data for 2012. 2013;62(3). http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_03.pdf. Published September 6, 2013. Accessed March 18, 2014.
- AbouZahr C. Global burden of maternal death and disability. Br Med Bull. 2003;67:1–11.
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol. 1991;77(1):69–76.
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Embrey MP. Simultaneous intramuscular injection of oxytocin and ergometrine: a tocographic study. BMJ. 1961;1(5241):1737–1738.
- Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: An updated systematic review. Am J Obstet Gynecol. 2013;209(4):294–306.
- Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: Findings in six cases. Int J Obstet Anesth. 2008;17(3):247–254.
- Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76–83.
- Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfourds EM. Signs of myocardial ischaemia after injection of oxytocin: A randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100(5):683–689.
- George RB, McKeen D, Chaplin AC, McLeod L. Up-down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing cesarean delivery. Can J Anesth. 2010;57(6):578–582.
- Sheehan SR, Montgomery AA, Carey M, et al; ECSSIT Study Group. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective cesarean section: Double blind, placebo controlled, randomized trial. BMJ. 2011;343:d4661.
Study links number of live births to future cardiac risk
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
The number of children a woman has may affect her future risk of cardiovascular disease – and the mechanism may be subclinical atherosclerosis, data from the Dallas Heart Study suggest.
Women who had had two or three live births had the lowest prevalence of subclinical atherosclerosis, compared with women who had four or more children and with women who had no live births or one live birth, depicting "a U-shaped association between the number of children a woman gives birth to and future risk of cardiovascular events," said Dr. Monika Sanghavi, chief cardiology fellow at the University of Texas Southwestern Medical Center, Dallas.
Dr. Sanghavi summarized the study results in a webcast held prior to the annual meeting of the American College of Cardiology, where she is presenting the data.
The number of children women have has been associated with their future cardiovascular disease risk, but the mechanism has not been clearly understood. For this study of 1,644 women enrolled in the Dallas Heart Study the researchers addressed whether the mechanism could be subclinical atherosclerosis by using coronary artery calcium (CAC) scores measured with CT imaging and aortic wall thickness (AWT) measured with magnetic resonance imaging. The average age of the women was 45 years, and 55% were black.
The Dallas Heart Study is a multiethnic population–based sample of Dallas County residents.
CAC scores were positive (greater than 10) in 11% of the women who had 2-3 live births, compared with 27% in those who had at least 4 live births, and 15% of those who had 0-1 live births, all significant differences.
AWTs were abnormal (greater than the 75th percentile for age and gender) in 22% of those with had 2-3 live births, compared with 33% of those who had at least 4 live births and 25% of those who had 0-1 live births. The association persisted after adjustment for cardiovascular disease risk factors, including socioeconomic status, race and education.
"The results support the hypothesis that subclinical atherosclerosis may be the mechanism for this association," Dr. Sanghavi said. As for the two groups identified at risk, she added, "we think that increased risk on either end of the curve likely represents two different processes, such that repeated pregnancies increase risk in a different way than those women who are unable to get pregnant."
These results need to be confirmed in future studies, which will also further explore why women with one or no live births are at increased risk, Dr. Sanghavi said. Those with four or more live births may be at increased risk because of "repeated exposure to an atherogenic milieu," with higher cholesterol levels, insulin resistance and other pregnancy-related changes, or factors not accounted for in the study, she noted.
But women with 0-1 live births may be at increased risk because of an underlying systemic condition such as polycystic ovarian syndrome "or some other inflammatory condition that is preventing them from getting pregnant or carrying multiple pregnancies to term," she added.
Dr. Sanghavi emphasized that the results should not be used to recommend that women should have two or three children.
Dr. Vera Bittner, chair of the ACC Prevention of Cardiovascular Disease Committee, said the results highlight the need to take "a better reproductive history when we’re trying to judge a woman’s cardiovascular risk when they present to our office."
Dr. Sanghavi had no disclosures.
FROM ACC.14