User login
VIDEO: Study highlights progress, challenges in nosocomial infections
One in 25 hospitalized patients on any given day has an infection acquired from health care, and as many as 1 in 9 of those will die.
At a press briefing, Dr. Michael Bell discussed new data from a prevalence study conducted by the Centers for Disease Control and Prevention, where he is the deputy director for the division of health care quality promotion. Hospitals, doctors, and patients all have a role to play in decreasing the risks of these infections, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
This article was updated 3/28/2014.
One in 25 hospitalized patients on any given day has an infection acquired from health care, and as many as 1 in 9 of those will die.
At a press briefing, Dr. Michael Bell discussed new data from a prevalence study conducted by the Centers for Disease Control and Prevention, where he is the deputy director for the division of health care quality promotion. Hospitals, doctors, and patients all have a role to play in decreasing the risks of these infections, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
This article was updated 3/28/2014.
One in 25 hospitalized patients on any given day has an infection acquired from health care, and as many as 1 in 9 of those will die.
At a press briefing, Dr. Michael Bell discussed new data from a prevalence study conducted by the Centers for Disease Control and Prevention, where he is the deputy director for the division of health care quality promotion. Hospitals, doctors, and patients all have a role to play in decreasing the risks of these infections, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
This article was updated 3/28/2014.
FROM THE CDC
Smoking bans see 10% declines in preterm births, asthma flares in children
A systematic review and meta-analysis from North America and Europe has found that the introduction of antismoking legislation covering workplaces and public spaces is associated with significant reductions of about 10% in both preterm births and pediatric hospital visits for asthma.
The findings, published online March 27 in the Lancet, also suggest that significant health benefits and associated public-health cost savings from smoking bans may be reaped beginning in the first year after antismoking legislation takes hold (doi:10.1016/S0140-6736[14]60082-9).
For their research, Jasper V. Been, Ph.D., of Maastricht University, the Netherlands, and his colleagues, evaluated 11 studies (5 from North America and 6 from European countries) published during 2008 and 2013. The included studies captured 247,168 million asthma exacerbations and about 2.5 million births. In these study areas, which fell within either local or national bans on smoking in the workplace, public places, and/or restaurants and bars, bans were followed by significant drops in preterm births (–10.4%) and childhood emergency department visits and hospital attendances (–10.1%).
Low birth weight was not seen as significantly changed in Dr. Been and his colleagues’ study (–1.7%; P = .31), but the researchers did see about a 5% decline in children born very small for their gestational age.
For several of the included studies, the drops were immediate. Significant drops in preterm births of 3% or more were seen in the first year following the implementation of some bans, with additional gradual declines occurring in subsequent years. With hospital visits for pediatric asthma, some studies saw drops as high as 20% per year after bans.
In an editorial comment accompanying the study, Dr. Sara Kalkhoran and Stanton A. Glantz, Ph.D., of the University of California, San Francisco, wrote that the findings add to a growing body of evidence that "smoke-free laws bring rapid health benefits and improved lives, whilst, at the same time, reducing medical costs by avoiding emergency department visits and admissions to hospitals."
Dr. Been and his colleagues’ study was funded by the Thrasher Fund, Lung Foundation Netherlands, the International Pediatric Research Fund, Commonwealth Fund, and Maastricht University. None of the study authors or editorial writers declared conflicts of interest.
A systematic review and meta-analysis from North America and Europe has found that the introduction of antismoking legislation covering workplaces and public spaces is associated with significant reductions of about 10% in both preterm births and pediatric hospital visits for asthma.
The findings, published online March 27 in the Lancet, also suggest that significant health benefits and associated public-health cost savings from smoking bans may be reaped beginning in the first year after antismoking legislation takes hold (doi:10.1016/S0140-6736[14]60082-9).
For their research, Jasper V. Been, Ph.D., of Maastricht University, the Netherlands, and his colleagues, evaluated 11 studies (5 from North America and 6 from European countries) published during 2008 and 2013. The included studies captured 247,168 million asthma exacerbations and about 2.5 million births. In these study areas, which fell within either local or national bans on smoking in the workplace, public places, and/or restaurants and bars, bans were followed by significant drops in preterm births (–10.4%) and childhood emergency department visits and hospital attendances (–10.1%).
Low birth weight was not seen as significantly changed in Dr. Been and his colleagues’ study (–1.7%; P = .31), but the researchers did see about a 5% decline in children born very small for their gestational age.
For several of the included studies, the drops were immediate. Significant drops in preterm births of 3% or more were seen in the first year following the implementation of some bans, with additional gradual declines occurring in subsequent years. With hospital visits for pediatric asthma, some studies saw drops as high as 20% per year after bans.
In an editorial comment accompanying the study, Dr. Sara Kalkhoran and Stanton A. Glantz, Ph.D., of the University of California, San Francisco, wrote that the findings add to a growing body of evidence that "smoke-free laws bring rapid health benefits and improved lives, whilst, at the same time, reducing medical costs by avoiding emergency department visits and admissions to hospitals."
Dr. Been and his colleagues’ study was funded by the Thrasher Fund, Lung Foundation Netherlands, the International Pediatric Research Fund, Commonwealth Fund, and Maastricht University. None of the study authors or editorial writers declared conflicts of interest.
A systematic review and meta-analysis from North America and Europe has found that the introduction of antismoking legislation covering workplaces and public spaces is associated with significant reductions of about 10% in both preterm births and pediatric hospital visits for asthma.
The findings, published online March 27 in the Lancet, also suggest that significant health benefits and associated public-health cost savings from smoking bans may be reaped beginning in the first year after antismoking legislation takes hold (doi:10.1016/S0140-6736[14]60082-9).
For their research, Jasper V. Been, Ph.D., of Maastricht University, the Netherlands, and his colleagues, evaluated 11 studies (5 from North America and 6 from European countries) published during 2008 and 2013. The included studies captured 247,168 million asthma exacerbations and about 2.5 million births. In these study areas, which fell within either local or national bans on smoking in the workplace, public places, and/or restaurants and bars, bans were followed by significant drops in preterm births (–10.4%) and childhood emergency department visits and hospital attendances (–10.1%).
Low birth weight was not seen as significantly changed in Dr. Been and his colleagues’ study (–1.7%; P = .31), but the researchers did see about a 5% decline in children born very small for their gestational age.
For several of the included studies, the drops were immediate. Significant drops in preterm births of 3% or more were seen in the first year following the implementation of some bans, with additional gradual declines occurring in subsequent years. With hospital visits for pediatric asthma, some studies saw drops as high as 20% per year after bans.
In an editorial comment accompanying the study, Dr. Sara Kalkhoran and Stanton A. Glantz, Ph.D., of the University of California, San Francisco, wrote that the findings add to a growing body of evidence that "smoke-free laws bring rapid health benefits and improved lives, whilst, at the same time, reducing medical costs by avoiding emergency department visits and admissions to hospitals."
Dr. Been and his colleagues’ study was funded by the Thrasher Fund, Lung Foundation Netherlands, the International Pediatric Research Fund, Commonwealth Fund, and Maastricht University. None of the study authors or editorial writers declared conflicts of interest.
FROM THE LANCET
Prenatal visits to non-ob.gyn. providers more common in younger women
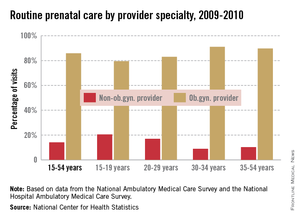
Women aged 15-29 years are more likely than older women to see a non-ob.gyn. for their routine prenatal care, the National Center for Health Statistics reported.
In 2009-2010, women aged 15-19 years saw a non-ob.gyn. provider for 20.5% of their routine prenatal care visits, and women aged 20-29 years went to a non-ob.gyn. provider for 17% of such visits. In comparison, only 8.9% of visits by women aged 30-34 years and 10.3% of visits by women 35-54 involved a non-ob.gyn. provider, the NCHS said.
Overall, women aged 15-54 years saw a non-ob.gyn. provider for 14.1% of their routine prenatal care visits, according to data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey.
Women aged 15-29 years are more likely than older women to see a non-ob.gyn. for their routine prenatal care, the National Center for Health Statistics reported.
In 2009-2010, women aged 15-19 years saw a non-ob.gyn. provider for 20.5% of their routine prenatal care visits, and women aged 20-29 years went to a non-ob.gyn. provider for 17% of such visits. In comparison, only 8.9% of visits by women aged 30-34 years and 10.3% of visits by women 35-54 involved a non-ob.gyn. provider, the NCHS said.
Overall, women aged 15-54 years saw a non-ob.gyn. provider for 14.1% of their routine prenatal care visits, according to data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey.

Women aged 15-29 years are more likely than older women to see a non-ob.gyn. for their routine prenatal care, the National Center for Health Statistics reported.
In 2009-2010, women aged 15-19 years saw a non-ob.gyn. provider for 20.5% of their routine prenatal care visits, and women aged 20-29 years went to a non-ob.gyn. provider for 17% of such visits. In comparison, only 8.9% of visits by women aged 30-34 years and 10.3% of visits by women 35-54 involved a non-ob.gyn. provider, the NCHS said.
Overall, women aged 15-54 years saw a non-ob.gyn. provider for 14.1% of their routine prenatal care visits, according to data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey.

The optimal time for scheduled repeat cesarean may be less than 39 weeks
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
VIDEO: Don’t be afraid to treat acne in pregnant patients
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
DENVER – When it comes to treating pregnant women with acne, Dr. Jonette Keri’s goal is to prevent scarring. Her main message: there are several safe treatment options for women before, during, and after pregnancy, so don’t be afraid to use them.
Dr. Keri, associate professor of dermatology at the University of Miami Miller School of Medicine and chief of the dermatology service at Miami VA Hospital, shares her practice pearls in this video interview.
On Twitter @naseemmiller
AT THE AAD ANNUAL MEETING
Treatment Decisions Complex for Pregnant, Postpartum Women With Bipolar Disorder
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
FROM JOURNAL OF AFFECTIVE DISORDERS
Treatment decisions complex for pregnant, postpartum women with bipolar disorder
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
The clinical features of perinatal women with bipolar disorder are much more severe than those of women seeking care for other psychiatric conditions, including greater history of suicidal behavior and substance abuse, and more difficulties during childbirth and while breastfeeding, a retrospective study of 334 pregnant and postpartum women suggests.
Cynthia L. Battle, Ph.D., of Butler Hospital in Providence, R.I., and her colleagues reviewed the clinical records of the women, who sought treatment at a specialized partial hospitalization program that serves patients during pregnancy and the first postpartum year. Their ages ranged from 15 to 43 years, and they came from a range of ethnic backgrounds. Less than half of the women in the sample were either married or had partners (J. Affect. Disord. 2014;158:97-100). Two-thirds were postpartum, and one-third of them were pregnant.
The investigators asked the patients to complete the Edinburgh Postnatal Depression Scale and the facility’s Day Hospital Screener for self-reporting of psychiatric disorders, including bipolar disorder.
About 10% of women had a bipolar disorder diagnosis, including 19 with bipolar I disorder, 10 with bipolar II disorder, and 5 with bipolar not otherwise specified. Twenty-six percent reported bipolar disorder symptoms of elation, and 76% reported symptoms of irritability lasting 4 or more days within the previous month.
"Pregnant and postpartum women with [bipolar disorder] reported more extensive mental health histories, including prior use of pharmacotherapy and psychotherapy, as well as higher rates of prior substance abuse," the authors noted. Women with bipolar disorder were significantly more likely than those with other Axis I disorders to report prior suicidal behavior and attempts. A higher proportion of expectant mothers with bipolar disorder took psychotropics than did pregnant women with other disorders. Among postpartum women, mothers with bipolar disorder were more likely to report delivery complications and difficulties breastfeeding their babies.
Although analyses were limited to data recorded in the charts, the current findings "shed light on the clinical and demographic features associated with perinatal bipolar disorder," including a high level of functional impairment experienced by these women, the authors wrote. The high level of self-reported symptoms of elation and irritability "underscore the importance of consistently assessing for mania and hypomania during pregnancy and postpartum."
While bipolar disorder guidelines recommend maintenance pharmacotherapy and adjunctive psychotherapy, the authors say, "tailored psychosocial interventions have not yet been developed for this population. ... Patient-centered decision support and development of tailored adjunctive psychotherapies for perinatal [bipolar disorder] may play a key role in helping women with [bipolar disorder] remain engaged in treatment during pregnancy and postpartum."
Dr. Battle and her colleagues noted several limitations of the study. For example, because the methodology used was retrospective, their analyses were limited to data that had been recorded in the charts.
This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
FROM JOURNAL OF AFFECTIVE DISORDERS
Major finding: Perinatal women with bipolar disorder have a greater history of suicidal behavior and substance abuse, and report more difficulties during delivery and while breastfeeding, than do women with other psychiatric disorders.
Data source: A chart review of 334 women who sought treatment at a specialized partial hospitalization program serving perinatal women with psychiatric conditions. A third of the women were pregnant; two-thirds were postpartum.
Disclosures: This study was unfunded; one author was supported in part through a National Institutes of Health mentored career development award. The authors reported no relevant financial conflicts of interest.
Hospital physician orders increased postpartum Tdap vaccination rates
Changing in-hospital ordering procedures for postpartum Tdap vaccination considerably increased vaccination rates in birth mothers, investigators reported in the March issue of the American Journal of Obstetrics & Gynecology.
Tdap vaccinations protect against tetanus, diphtheria, and pertussis (whooping cough), the latter of which has increased in recent years and causes significant morbidity for adults and children and mortality in infants.
ACIP recommended postpartum Tdap vaccination in birth mothers in 2006 and then updated their recommendations in 2011 to administer the Tdap during pregnant women’s second or third trimester each pregnancy. Yet a 2012 survey showed update of the Tdap during pregnancy as low as 2.6%, and postpartum vaccination remaining limited.
Dr. Sylvia Yeh of the University of California, Los Angeles, and her colleagues reported on an intervention at a Los Angeles private community hospital with a 0% baseline rate of postpartum Tdap vaccination (Am. J. Obstet. Gynecol. 2014;210:237.e1-6).
For completed deliveries between October 2009 and July 2010, the researchers reviewed 658 charts for birth mothers at that hospital and compared them with 606 women’s charts at a hospital with no procedure changes. The comparison hospital, also with a 0% baseline vaccination rate, was located 18 miles from the intervention hospital and served a relatively similar demographic population.
Implementing physician opt-in orders for postpartum Tdap vaccination at the intervention hospital in November 2009 led to an increase in the vaccination rate to 18.8% (P less than .001). The hospital then implemented standing orders in February 2010, allowing nurses to deliver the vaccines without an additional physician order. The postpartum Tdap vaccination rate then climbed again to 62.7% (P less than .001). The comparison hospital’s rate for postpartum Tdap vaccination remained at 0% throughout the same period.
The researchers identified no differences in demographic characteristics among the women who received in-hospital postpartum Tdap vaccination and those who did not.
The study was funded by the Centers for Disease Control and Prevention. No disclosures were reported.
Changing in-hospital ordering procedures for postpartum Tdap vaccination considerably increased vaccination rates in birth mothers, investigators reported in the March issue of the American Journal of Obstetrics & Gynecology.
Tdap vaccinations protect against tetanus, diphtheria, and pertussis (whooping cough), the latter of which has increased in recent years and causes significant morbidity for adults and children and mortality in infants.
ACIP recommended postpartum Tdap vaccination in birth mothers in 2006 and then updated their recommendations in 2011 to administer the Tdap during pregnant women’s second or third trimester each pregnancy. Yet a 2012 survey showed update of the Tdap during pregnancy as low as 2.6%, and postpartum vaccination remaining limited.
Dr. Sylvia Yeh of the University of California, Los Angeles, and her colleagues reported on an intervention at a Los Angeles private community hospital with a 0% baseline rate of postpartum Tdap vaccination (Am. J. Obstet. Gynecol. 2014;210:237.e1-6).
For completed deliveries between October 2009 and July 2010, the researchers reviewed 658 charts for birth mothers at that hospital and compared them with 606 women’s charts at a hospital with no procedure changes. The comparison hospital, also with a 0% baseline vaccination rate, was located 18 miles from the intervention hospital and served a relatively similar demographic population.
Implementing physician opt-in orders for postpartum Tdap vaccination at the intervention hospital in November 2009 led to an increase in the vaccination rate to 18.8% (P less than .001). The hospital then implemented standing orders in February 2010, allowing nurses to deliver the vaccines without an additional physician order. The postpartum Tdap vaccination rate then climbed again to 62.7% (P less than .001). The comparison hospital’s rate for postpartum Tdap vaccination remained at 0% throughout the same period.
The researchers identified no differences in demographic characteristics among the women who received in-hospital postpartum Tdap vaccination and those who did not.
The study was funded by the Centers for Disease Control and Prevention. No disclosures were reported.
Changing in-hospital ordering procedures for postpartum Tdap vaccination considerably increased vaccination rates in birth mothers, investigators reported in the March issue of the American Journal of Obstetrics & Gynecology.
Tdap vaccinations protect against tetanus, diphtheria, and pertussis (whooping cough), the latter of which has increased in recent years and causes significant morbidity for adults and children and mortality in infants.
ACIP recommended postpartum Tdap vaccination in birth mothers in 2006 and then updated their recommendations in 2011 to administer the Tdap during pregnant women’s second or third trimester each pregnancy. Yet a 2012 survey showed update of the Tdap during pregnancy as low as 2.6%, and postpartum vaccination remaining limited.
Dr. Sylvia Yeh of the University of California, Los Angeles, and her colleagues reported on an intervention at a Los Angeles private community hospital with a 0% baseline rate of postpartum Tdap vaccination (Am. J. Obstet. Gynecol. 2014;210:237.e1-6).
For completed deliveries between October 2009 and July 2010, the researchers reviewed 658 charts for birth mothers at that hospital and compared them with 606 women’s charts at a hospital with no procedure changes. The comparison hospital, also with a 0% baseline vaccination rate, was located 18 miles from the intervention hospital and served a relatively similar demographic population.
Implementing physician opt-in orders for postpartum Tdap vaccination at the intervention hospital in November 2009 led to an increase in the vaccination rate to 18.8% (P less than .001). The hospital then implemented standing orders in February 2010, allowing nurses to deliver the vaccines without an additional physician order. The postpartum Tdap vaccination rate then climbed again to 62.7% (P less than .001). The comparison hospital’s rate for postpartum Tdap vaccination remained at 0% throughout the same period.
The researchers identified no differences in demographic characteristics among the women who received in-hospital postpartum Tdap vaccination and those who did not.
The study was funded by the Centers for Disease Control and Prevention. No disclosures were reported.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Major finding: Tdap postpartum vaccination rates in birth mothers increased from 0% to 18.8% following physician opt-in orders and then to 62.7% following standing orders at an intervention hospital, compared with a continuing 0% rate at a hospital with no procedural changes.
Data source: A prospective evaluation of in-hospital postpartum pertussis vaccination rates of 1,264 birth mothers at two hospitals from October 2009 through July 2010.
Disclosures: This study was supported by the U.S. Centers for Disease Control and Prevention. No disclosures were reported.
No VAERS safety signal with Tdap in pregnancy
No unexpected adverse events were seen with the administration of Tdap vaccine during pregnancy, although vaccine surveillance data show a shift toward later pregnancy administration.
Serious adverse events, defined as death, life threatening, hospitalization, prolonged hospitalization, and permanent disability, occurred in 6 of 132 (5%) Tdap pregnancies before the recommendation for routine Tdap vaccination during pregnancy and in 14 of 90 (16%) Tdap pregnancies after the recommendation.
The proportion of preterm births (2 vs. 5) and stillbirths (2 vs. 4) increased after the recommendation, while spontaneous abortions decreased (22 vs. 4), Dr. Pedro L. Moro reported at the winter meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
There was one major birth defect before and two after ACIP’s October 2011 recommendation for routine Tdap vaccination during pregnancy.
One of the biggest changes observed in the Vaccine Adverse Event Reporting System (VAERS) reports was the number of women receiving Tdap during the third trimester, up from just 4% to 55% now, said Dr. Moro, an epidemiologist with the CDC.
The most common non–pregnancy-related outcome in the postrecommendation cohort (Oct. 11, 2011-Jan. 31, 2014) was injection site reactions in 19, compared with 6 reported in the prerecommendation cohort (January 2005-June 2010).
"Changes in reporting patterns are likely due to the new routine Tdap recommendation, increased awareness, and differences in the trimester of vaccination," he said.
Pediatric infectious disease specialist and vaccine developer Dr. Stanley Plotkin said he was troubled that 45% of women are still receiving Tdap before the third trimester because this fails to derive maximum benefit from the vaccine in terms of transmitting passive antibodies to the infant.
"It seems to me the recommendation should be stronger for a third-trimester vaccination than for early [vaccination]," he said. "Also, the issue of confusion with congenital anomalies if the vaccine is given in the first trimester, could damage the idea of safety of vaccinations during pregnancy."
The issue of high antibody levels at birth is important, but "We shouldn’t assume that 45% of the Tdap doses are being given before the third trimester because that is not what this VAERS can tell us," commented Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases.
During his presentation, Dr. Moro emphasized that VAERS looks for safety signals and generates hypotheses, but has inconsistent data quality and completeness and was not designed to assess whether a vaccine caused an adverse event.
Dr. Moro reported having no financial disclosures.
No unexpected adverse events were seen with the administration of Tdap vaccine during pregnancy, although vaccine surveillance data show a shift toward later pregnancy administration.
Serious adverse events, defined as death, life threatening, hospitalization, prolonged hospitalization, and permanent disability, occurred in 6 of 132 (5%) Tdap pregnancies before the recommendation for routine Tdap vaccination during pregnancy and in 14 of 90 (16%) Tdap pregnancies after the recommendation.
The proportion of preterm births (2 vs. 5) and stillbirths (2 vs. 4) increased after the recommendation, while spontaneous abortions decreased (22 vs. 4), Dr. Pedro L. Moro reported at the winter meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
There was one major birth defect before and two after ACIP’s October 2011 recommendation for routine Tdap vaccination during pregnancy.
One of the biggest changes observed in the Vaccine Adverse Event Reporting System (VAERS) reports was the number of women receiving Tdap during the third trimester, up from just 4% to 55% now, said Dr. Moro, an epidemiologist with the CDC.
The most common non–pregnancy-related outcome in the postrecommendation cohort (Oct. 11, 2011-Jan. 31, 2014) was injection site reactions in 19, compared with 6 reported in the prerecommendation cohort (January 2005-June 2010).
"Changes in reporting patterns are likely due to the new routine Tdap recommendation, increased awareness, and differences in the trimester of vaccination," he said.
Pediatric infectious disease specialist and vaccine developer Dr. Stanley Plotkin said he was troubled that 45% of women are still receiving Tdap before the third trimester because this fails to derive maximum benefit from the vaccine in terms of transmitting passive antibodies to the infant.
"It seems to me the recommendation should be stronger for a third-trimester vaccination than for early [vaccination]," he said. "Also, the issue of confusion with congenital anomalies if the vaccine is given in the first trimester, could damage the idea of safety of vaccinations during pregnancy."
The issue of high antibody levels at birth is important, but "We shouldn’t assume that 45% of the Tdap doses are being given before the third trimester because that is not what this VAERS can tell us," commented Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases.
During his presentation, Dr. Moro emphasized that VAERS looks for safety signals and generates hypotheses, but has inconsistent data quality and completeness and was not designed to assess whether a vaccine caused an adverse event.
Dr. Moro reported having no financial disclosures.
No unexpected adverse events were seen with the administration of Tdap vaccine during pregnancy, although vaccine surveillance data show a shift toward later pregnancy administration.
Serious adverse events, defined as death, life threatening, hospitalization, prolonged hospitalization, and permanent disability, occurred in 6 of 132 (5%) Tdap pregnancies before the recommendation for routine Tdap vaccination during pregnancy and in 14 of 90 (16%) Tdap pregnancies after the recommendation.
The proportion of preterm births (2 vs. 5) and stillbirths (2 vs. 4) increased after the recommendation, while spontaneous abortions decreased (22 vs. 4), Dr. Pedro L. Moro reported at the winter meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
There was one major birth defect before and two after ACIP’s October 2011 recommendation for routine Tdap vaccination during pregnancy.
One of the biggest changes observed in the Vaccine Adverse Event Reporting System (VAERS) reports was the number of women receiving Tdap during the third trimester, up from just 4% to 55% now, said Dr. Moro, an epidemiologist with the CDC.
The most common non–pregnancy-related outcome in the postrecommendation cohort (Oct. 11, 2011-Jan. 31, 2014) was injection site reactions in 19, compared with 6 reported in the prerecommendation cohort (January 2005-June 2010).
"Changes in reporting patterns are likely due to the new routine Tdap recommendation, increased awareness, and differences in the trimester of vaccination," he said.
Pediatric infectious disease specialist and vaccine developer Dr. Stanley Plotkin said he was troubled that 45% of women are still receiving Tdap before the third trimester because this fails to derive maximum benefit from the vaccine in terms of transmitting passive antibodies to the infant.
"It seems to me the recommendation should be stronger for a third-trimester vaccination than for early [vaccination]," he said. "Also, the issue of confusion with congenital anomalies if the vaccine is given in the first trimester, could damage the idea of safety of vaccinations during pregnancy."
The issue of high antibody levels at birth is important, but "We shouldn’t assume that 45% of the Tdap doses are being given before the third trimester because that is not what this VAERS can tell us," commented Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases.
During his presentation, Dr. Moro emphasized that VAERS looks for safety signals and generates hypotheses, but has inconsistent data quality and completeness and was not designed to assess whether a vaccine caused an adverse event.
Dr. Moro reported having no financial disclosures.
AT AN ACIP MEETING
Early treatment appears to clear virus in second HIV-infected newborn
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
FROM CROI 2014
Major finding: Combined antiretroviral therapy in the first hours of life appears to clear HIV from perinatally infected infants.
Data source: Case reports of two children born with HIV infection to untreated mothers.
Disclosures: The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.