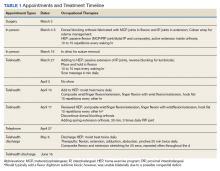
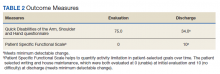
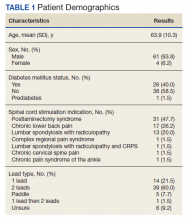
User login
Naloxone Dispensing in Patients at Risk for Opioid Overdose After Total Knee Arthroplasty Within the Veterans Health Administration
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
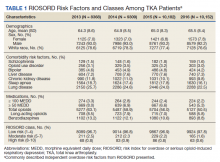
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
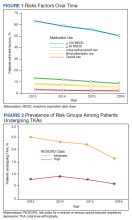
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
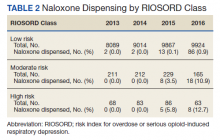
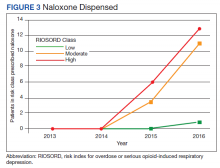
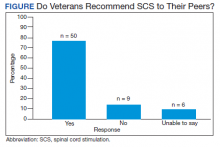
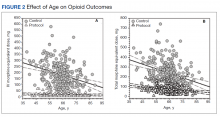
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
Opioid overdose is a major public health challenge, with recent reports estimating 41 deaths per day in the United States from prescription opioid overdose.1,2 Prescribing naloxone has increasingly been advocated to reduce the risk of opioid overdose for patients identified as high risk. Naloxone distribution has been shown to decrease the incidence of opioid overdoses in the general population.3,4 The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Chronic Pain recommends considering naloxone prescription for patients with a history of overdose or substance use disorder, opioid dosages ≥ 50 morphine equivalent daily dose (MEDD), and concurrent use of benzodiazepines.5
Although the CDC guidelines are intended for primary care clinicians in outpatient settings, naloxone prescribing is also relevant in the postsurgical setting.5 Many surgical patients are at risk for opioid overdose and data from the Veterans Health Administration (VHA) has shown that risk of opioid overdose is 11-fold higher in the 30 days following discharge from a surgical admission, when compared with the subsequent calendar year.6,7 This likely occurs due to new prescriptions or escalated doses of opioids following surgery. Overdose risk may be particularly relevant to orthopedic surgery as postoperative opioids are commonly prescribed.8 Patients undergoing total knee arthroplasty (TKA) may represent a vulnerable population to overdose as it is one of the most commonly performed surgeries for the treatment of chronic pain, and is frequently performed in older adults with medical comorbidities.9,10
Identifying patients at high risk for opioid overdose is important for targeted naloxone dispensing.5 A risk index for overdose or serious opioid-induced respiratory depression (RIOSORD) tool has been developed and validated in veteran and other populations to identify such patients.11 The RIOSORD tool classifies patients by risk level (1-10) and predicts probability of overdose or serious opioid-induced respiratory depression (OSORD). A patient’s level of risk is based on a weighted combination of the 15 independent risk factors most highly associated with OSORD, including comorbid conditions, prescription drug use, and health care utilization.12 Using the RIOSORD tool, the VHA Opioid Education and Naloxone Distribution (OEND) program is a risk mitigation initiative that aims to decrease opioid-related overdose morbidity and mortality. This is achieved via opioid overdose education for prevention, recognition, and response and includes outpatient naloxone prescription.13,14
Despite the comprehensive OEND program, there exists very little data to guide postsurgical naloxone prescribing. The prevalence of known risk factors for overdose in surgical patients remains unknown, as does the prevalence of perioperative naloxone distribution. Understanding overdose risk factors and naloxone prescribing patterns in surgical patients may identify potential targets for OEND efforts. This study retrospectively estimated RIOSORD scores for TKA patients between 2013 to 2016 and described naloxone distribution based on RIOSORD scores and risk factors.
Methods
We identified patients who had undergone primary TKA at VHA hospitals using Current Procedural Terminology (CPT), International Classification of Diseases, Ninth Revision (ICD-9) procedure codes, and data extracted from the VHA Corporate Data Warehouse (CDW) of electronic health records (EHRs). Our study was granted approval with exemption from informed consent by the Durham Veteran Affairs Healthcare System Institutional Review Board.
This retrospective cohort study included all veterans who underwent elective primary TKA from January 1, 2013 through December 31, 2016. We excluded patients who died before discharge.
Outcomes
Our primary outcome was being dispensed an outpatient naloxone prescription following TKA. Naloxone dispensing was identified by examining CDW outpatient pharmacy records with a final dispense date from 1 year before surgery through 7 days after discharge following TKA. To exclude naloxone administration that may have been given in a clinic, prescription data included only records with an outpatient prescription copay. Naloxone dispensing in the year before surgery was chosen to estimate likely preoperative possession of naloxone which could be available in the postoperative period. Naloxone dispensing until 7 days after discharge was chosen to identify any new dispensing that would be available in the postoperative period. These outcomes were examined over the study time frame on an annual basis.
Patient Factors
Demographic variables included age, sex, and race/ethnicity. Independent risk factors for overdose from RIOSORD were identified for each patient.15 These risk factors included comorbidities (opioid use disorder, schizophrenia, bipolar disorder, liver disease, chronic kidney disease, sleep apnea, or lung disease) and prescription drug use (use of opioids, benzodiazepines, long-acting opioids, ≥ 50 MEDD or ≥ 100 MEDD). ICD-9 and ICD-10 diagnosis codes were used to identify comorbidities. Risk classes on day of surgery were identified using a RIOSORD algorithm code. Consistent with the display of RIOSORD risk classes on the VHA Academic Detailing Service OEND risk report, patients were grouped into 3 groups based on their RIOSORD score: classes 1 to 4 (low risk), 5 to 7 (moderate risk), and 8 to 10 (high risk).
Descriptive statistics were used to summarize data on patient demographics, RIOSORD risk factors, overdose events, and naloxone dispensing over time.
Results
The study cohort included 38,011 veterans who underwent primary TKA in the VHA between January 1, 2013 and December 30, 2016. In this cohort, the mean age was 65 years, 93% were male, and 77% were White patients (Table 1). The most common comorbidities were lung disease in 9170 (24.1%) patients, sleep apnea in 6630 (17.4%) patients, chronic kidney disease in 4036 (10.6%) patients, liver disease in 2822 (7.4%) patients, and bipolar disorder in 1748 (4.6%) patients.
In 2013, 63.1% of patients presenting for surgery were actively prescribed opioids. By 2016, this decreased to 50.5%. Benzodiazepine use decreased from 13.2 to 8.8% and long-acting opioid use decreased from 8.5 to 5.8% over the same period. Patients taking ≥ 50 MEDD decreased from 8.0 to 5.3% and patients taking ≥ 100 MEDD decreased from 3.3 to 2.2%. The prevalence of moderate-risk patients decreased from 2.5 to 1.6% and high-risk patients decreased from 0.8 to 0.6% (Figure 1). Cumulatively, the prevalence of presenting with either moderate or high risk of overdose decreased from 3.3 to 2.2% between 2013 to 2016.
Naloxone Dispensing
In 2013, naloxone was not dispensed to any patients at moderate or high risk for overdose between 365 days prior to surgery until 7 days after discharge (Table 2 and Figure 2). Low-risk group naloxone dispensing increased to 2 (0.0%) in 2014, to 13 (0.1%), in 2015, and to 86 (0.9%) in 2016. Moderate-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 8 (3.5%) in 2015, and to 18 (10.9%) in 2016. High-risk group naloxone dispensing remained at 0 (0.0%) in 2014, but increased to 5 (5.8%) in 2015, and to 8 (12.7%) in 2016 (Figure 3).
Discussion
Our data demonstrate that patients presenting for TKA between 2013 and 2016 routinely had individual risk factors for overdose related to either prescription drug use or comorbidities. We also show that, although the number of patients at moderate and high risk for opioid overdose is decreasing, 2.2% of TKA patients remain at moderate or high risk for opioid overdose based on a weighted combination of these individual risk factors using RIOSORD. As demand for primary TKA is projected to grow to 3.5 million procedures by 2030, using prevalence from 2016, we estimate that 76,560 patients may present for TKA across the US with moderate or high risk for opioid overdose.9 Following discharge, this risk may be even higher as this estimate does not yet account for postoperative opioid use. We demonstrate that through a VHA OEND initiative, naloxone distribution increased and appeared to be targeted to those most at risk using a simple validated tool like RIOSORD.
Presence of an individual risk factor for overdose was present in as many as 63.1% of patients presenting for TKA, as was seen in 2013 with preoperative opioid use. The 3 highest scoring prescription use–related risk factors in RIOSORD are use of opioids ≥ 100 MEDD (16 points), ≥ 50 MEDD (9 points), and long-acting formulations (9 points). All 3 decreased in prevalence over the study period but by 2016 were still seen in 2.2% for ≥ 100 MEDD, 5.3% for ≥ 50 MEDD, and 5.8% for long-acting opioids. This decrease was not surprising given implementation of a VHA-wide opioid safety initiative and the OEND program, but this could also be related to changes in patient selection for surgery in the context of increased awareness of the opioid epidemic. Despite the trend toward safer opioid prescribing, by 2016 over half of patients (50.5%) who presented for TKA were already taking opioids, with 10.6% (543 of 5127) on doses ≥ 50 MEDD.
We observed a decrease in RIOSORD risk each year, consistent with decreasing prescription-related risk factors over time. This was most obvious in the moderate-risk group. It is unclear why a similar decrease was not as obvious in the high-risk group, but this in part may be due to the already low numbers of patients in the high-risk group. This may also represent the high-risk group being somewhat resistant to the initiatives that shifted moderate-risk patients to the low-risk group. There were proportionately more patients in the moderate- and high-risk groups in the original RIOSORD population than in our surgical population, which may be attributed to the fewer comorbidities seen in our surgical population, as well as the higher opioid-prescribing patterns seen prior to the VA OEND initiative.12
Naloxone prescribing was rare prior to the OEND initiative and increased from 2013 to 2016. Increases were most marked in those in moderate- and high-risk groups, although naloxone prescribing also increased among the low-risk group. Integration of RIOSORD stratification into the OEND initiative likely played a role in targeting increased access to naloxone among those at highest risk of overdose. Naloxone dispensing increased for every group, although a significant proportion of moderate- and high-risk patients, 89.1% and 87.3%, respectively, were still not dispensed naloxone by 2016. Moreover, our estimates of perioperative naloxone access were likely an overestimate by including patients dispensed naloxone up to 1 year before surgery until 7 days after surgery. The aim was to include patients who may not have been prescribed naloxone postoperatively because of an existing naloxone prescription at home. Perioperative naloxone access estimates would have been even lower if a narrower window had been used to approximate perioperative access. This identifies an important gap between those who may benefit from naloxone dispensing and those who received naloxone. This in part may be because OEND has not been implemented as routinely in surgical settings as other settings (eg, primary care). OEND efforts may more effectively increase naloxone prescribing among surgical patients if these efforts were targeted at surgical and anesthesia departments. Given that the Comprehensive Addiction and Recovery Act of 2016 requires an assessment of patient risk prior to opioid prescribing and VHA efforts to increase utilization of tools like the Stratification Tool for Opioid Risk Mitigation (STORM), which estimates patient risk when initiating an opioid prescription and includes naloxone as one of many risk mitigation strategies, we anticipate that rates of naloxone prescribing will increase over time.
Limitations
Our study captures a large number of patients across VHA hospitals of varying size nationwide, including a mix of those with and without academic medical center affiliations. This veteran population may not represent the US commercially insured population (CIP). Zedler and colleagues highlighted the differences in prevalence of individual risk factors: notably, the CIP had a substantially higher proportion of females and younger patients.11 VHA had a greater prevalence of common chronic conditions associated with older age. The frequency of opioid dependence was similar among CIP and VHA. However, substance abuse and nonopioid substance dependence diagnoses were 4-fold more frequent among VHA controls as CIP controls. Prescribing of all opioids, except morphine and methadone, was substantially greater in CIP than in VHA.11 Despite a difference in individual risk factors, a CIP-specific RIOSORD has been validated and can be used outside of the VHA to obviate the limitations of the VHA-specific RIOSORD.11
Other limitations include our estimation of naloxone access. We do not know whether naloxone was administered or have a reliable estimate of overdose incidence in this postoperative TKA population. Also, it is important to note that RIOSORD was not developed for surgical patients. The use of RIOSORD in a postoperative population likely underestimates risk of opioid overdose due to the frequent prescriptions of new opioids or escalation of existing MEDD to the postoperative patient. Our study was also retrospective in nature and reliant on accurate coding of patient risk factors. It is possible that comorbidities were not accurately identified by EHR and therefore subject to inconsistency.
Conclusions
Veterans presenting for TKA routinely have risk factors for opioid overdose. We observed a trend toward decreasing overdose risk which coincided with the Opioid Safety and OEND initiatives within the VHA. We also observed an increase in naloxone prescription for moderate- and high-risk patients undergoing TKA, although most of these patients still did not receive naloxone as of 2016. More research is needed to refine and validate the RIOSORD score for surgical populations. Expanding initiatives such as OEND to include surgical patients presents an opportunity to improve access to naloxone for postoperative patients that may help reduce opioid overdose in this population.
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. Published 2016 Dec 30. doi:10.15585/mmwr.mm655051e1
2. Wilson N, Kariisa M, Seth P, Smith H, Davis NL. Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi:10.15585/mmwr.mm6911a4
3. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. Jan 30 2013;346:f174. doi:10.1136/bmj.f174
4. McClellan C, Lambdin BH, Ali MM, et al. Opioid-overdose laws association with opioid use and overdose mortality. Addict Behav. 2018;86:90-95. doi:10.1016/j.addbeh.2018.03.014
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
7. Mudumbai SC, Lewis ET, Oliva EM, et al. Overdose risk associated with opioid use upon hospital discharge in Veterans Health Administration surgical patients. Pain Med. 2019;20(5):1020-1031. doi:10.1093/pm/pny150
8. Hsia HL, Takemoto S, van de Ven T, et al. Acute pain is associated with chronic opioid use after total knee arthroplasty. Reg Anesth Pain Med. 2018;43(7):705-711. doi:10.1097/AAP.0000000000000831
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222
10. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630. doi:10.2106/JBJS.M.00285
11. Zedler BK, Saunders WB, Joyce AR, Vick CC, Murrelle EL. Validation of a screening risk index for serious prescription opioid-induced respiratory depression or overdose in a US commercial health plan claims database. Pain Med. 2018;19(1):68-78. doi:10.1093/pm/pnx009
12. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans Health Administration patients. Pain Med. 2015;16(8):1566-79. doi:10.1111/pme.12777
13. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
14. Oliva EM, Christopher MLD, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003). 2017;57(2S):S168-S179.e4. doi:10.1016/j.japh.2017.01.022
15. Noël PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev. 2010;47(8):739-750. doi:10.1682/jrrd.2009.08.0110
Evaluating the Impact of a Urinalysis to Reflex Culture Process Change in the Emergency Department at a Veterans Affairs Hospital
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
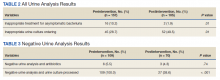
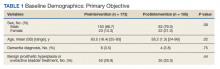
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
Automated urine cultures (UCs) following urinalysis (UA) are often used in emergency departments (EDs) to identify urinary tract infections (UTIs). The fast-paced environment of the ED makes this method of proactive collection and facilitation of UC favorable. However, results are often reported as no organism growth or the growth of clinically insignificant organisms, leading to the overdetection and overtreatment of asymptomatic bacteriuria (ASB).1-3 An estimated 30 to 60% of patients with ASB receive unwarranted antibiotic treatment, which is associated with an increased risk of developing Clostridioides difficile infection and contributes to the development of antimicrobial resistance.4-10 The costs associated with UC are an important consideration given the use of resources, the time and effort required to collect and process large numbers of negative cultures, and further efforts devoted to the follow-up of ED culture results.
Changes in traditional testing involving testing of both a UA and UC to reflex testing where urine specimens undergo culture only if they meet certain criteria have been described.11-14 This change in traditional testing aims to reduce the number of potentially unnecessary cultures performed without compromising clinical care. Leukocyte quantity in the UA has been shown to be a reliable predictor of true infection.11,15 Fok and colleagues demonstrated that reflex urine testing in ambulatory male urology patients in which cultures were done on only urine specimens with > 5 white blood cells per high-power field (WBC/HPF) would have missed only 7% of positive UCs, while avoiding 69% of cultures.11
At the Edward Hines, Jr Veterans Affairs Hospital (Hines VA), inappropriate UC ordering and treatment for ASB has been identified as an area needing improvement. An evaluation was conducted at the facility to determine the population of inpatient veterans with a positive UC who were appropriately managed. Of the 113 study patients with a positive UC included in this review, 77 (68%) had a diagnosis of ASB, with > 80% of patients with ASB (and no other suspected infections) receiving antimicrobial therapy.8 A subsequent evaluation was conducted at the Hines VA ED to evaluate UTI treatment and follow-up. Of the 173 ED patients included, 23% received antibiotic therapy for an ASB and 60% had a UA and UC collected but did not report symptoms.9 Finally, a review by the Hines VA laboratory showed that in May 2017, of 359 UCs sent from various locations of the hospital, 38% were obtained in the setting of a negative UA.
A multidisciplinary group with representation from primary care, infectious diseases, pharmacy, nursing, laboratory, and informatics was created with a goal to improve the workup and management of UTIs. In addition to periodic education for the clinicians regarding appropriate use and interpretation of UA and UC along with judicious use of antimicrobials especially in the setting of ASB, a UA to reflex culture process change was implemented. This allowed for automatic cancellation of a UC in the setting of a negative UA, which was designed to help facilitate appropriate UC ordering.
Methods
The primary objective of this study was to compare the frequency of inappropriate UC use and inappropriate antibiotic prescribing pre- and postimplementation of this UA to reflex culture process change. An inappropriate UC was defined as a UC ordered despite a negative UA in asymptomatic patients. Inappropriate antibiotic prescribing was defined as treatment of patients with ASB. The secondary objective evaluated postintervention data to assess the frequency of outpatient, ED, and hospital visits for UTI-related symptoms in the group of patients that had a UC cancelled as a result of the new process change (within a 7-day period of the initial UA) to determine whether patients with true infections were missed due to the process change.
Study Design and Setting
This pre-post quality improvement (QI) study analyzed the UC-ordering practices for UTIs sent from the ED at the Hines VA. This VA is a 483-bed tertiary care hospital in Chicago, Illinois, and serves > 57,000 veterans and about 23,000 ED visits annually. This study was approved by the Edward Hines, Jr VA Institutional Review Board as a quality assurance/QI proposal prior to data collection.
Patient Selection
All patients who
When comparing postintervention data with preintervention data for the primary study objective, the same exclusion criteria from the 2015 study were applied to the present study, which excluded ED patients who were admitted for inpatient care, concurrent antibiotic therapy for a non-UTI indication, duplicate cultures, and use of chronic bladder management devices. All patients identified as receiving a UA during the specified postintervention study period were included for evaluation of the secondary study objective.
Interventions
After physician education, an ED process change was implemented on October 3, 2017. This process change involved the creation of new order sets in the EHR that allowed clinicians to order a UA only, a UA with culture that would be cancelled by laboratory personnel if the UA did not result in > 5 WBC/HPF, and a UA with culture designated as do not cancel, where the UC was processed regardless of the UA results. The scenarios in which the latter option was considered appropriate were listed on the ordering screen and included pregnancy, a genitourinary procedure with necessary preoperative culture, and neutropenia.
Measurements
Postimplementation, all UAs were reviewed and grouped as follows: (1) positive UA with subsequent UC; (2) negative UA, culture cancelled; (3) only UA ordered (no culture); or (4) do not cancel UC ordered. Of the UAs that were analyzed, the following data were collected: demographics, comorbidities, concurrent medications for benign prostatic hyperplasia (BPH) and/or overactive bladder (OAB), documented allergies/adverse drug reactions to antibiotics, date of ED visit, documented UTI signs/symptoms (defined as frequency, urgency, dysuria, fever, suprapubic pain, or altered mental status in patients unable to verbalize urinary symptoms), UC results and susceptibilities, number of UCs repeated within 7 days after initial UA, requirement of antibiotic for UTI within 7 days of initial UA, antibiotic prescribed, duration of antibiotic therapy, and outpatient visits, ED visits, or need for hospital admission within 7 days of the initial UA for UTI-related symptoms. Other relevant UA and UC data that could not be obtained from the EHR were collected by generating a report using the Veterans Information Systems and Technology Architecture (VistA).
Analysis
Statistical analysis was performed using SAS v9.4. Independent t tests and Fisher exact tests were used to describe difference pre- and postintervention. Statistical significance was considered for P < .05. Based on results from the previous study conducted at this facility in addition to a literature review, it was determined that 92 patients in each group (pre- and postintervention) would be necessary to detect a 15% increase in percentage of patients appropriately treated for a UTI.
Results
There were 684 UAs evaluated from ED visits, 429 preintervention and 255 postintervention. The 255 patients were evaluated for the secondary objective of the study. Of the 255 patients with UAs identified postintervention, 150 were excluded based on the predefined exclusion criteria, and the remaining 105 were compared with the 173 patients from the preintervention group and were included in the analysis for the primary objective (Figure 1).
Patients in the postintervention group were younger than those in the preintervention group (P < .02): otherwise the groups were similar (Table 1). Inappropriate antibiotics for ASB decreased from 10.2% preintervention to 1.9% postintervention (odds ratio, 0.17; P = .01) (Table 2). UC processing despite a negative UA significantly decreased from 100% preintervention to 38.6% postintervention (P < .001) (Table 3). In patients with a negative UA, antibiotic prescribing decreased by 25.3% postintervention, but this difference was not statistically significant.
Postintervention, of 255 UAs evaluated, 95 (37.3%) were positive with a processed UC and 95 (37.3%) were negative with UC cancelled, 43 (16.9%) were ordered as DNC, and 22 (8.6%) were ordered without a UC (Figure 2). Twenty-eight of the 95 (29.5%) UAs with processed UCs did not meet the criteria for a positive UA and were not designated as DNC. When the UCs of this subgroup of patients were further analyzed, we found that 2 of the cultures were positive of which 1 patient was symptomatic and required antibiotic therapy.
Of the 95 patients with a negative UA, 69 (72.6%) presented without any UTI-related symptoms. In this group, there were no reports of outpatient visits, ED visits, or hospital admissions within 7 days of initial UA for UTI-related symptoms. None of the UCs ordered as DNC had a supporting reason identified. Nonetheless, the UC results from this patient subgroup also were analyzed further and resulted in 4 patients with negative UA and positive subsequent UC, 1 was symptomatic and required antibiotic therapy.
Discussion
A simple process change at the Hines VA resulted in benefits related to antimicrobial stewardship without conferring adverse outcomes on patient safety. Both UC processing despite a negative UA and inappropriate antibiotic prescribing for ASB were reduced significantly postintervention. This process change was piloted in the ED where UCs are often included as part of the initial diagnostic testing in patients who may not report UTI-related symptoms but for whom a UC is often bundled with other infectious workup, depending on the patient presentation.
Reflex testing of urine specimens has been described in the literature, both in an exploratory nature where impact of a reflex UC cancellation protocol based on certain UA criteria is measured by percent reduction of UCs processed as well as results of such interventions implemented into clinical practice.11-13 A retrospective study performed at the University of North Carolina Medical Center evaluated patients who presented to the ED during a 6-month period and had both an automated UA and UC collected. UC processing was restricted to UA that was positive for nitrites, leukocyte esterase, bacteria, or > 10 WBC/HPF. Use of this reflex culture cancellation protocol could have eliminated 604 of the 1546 (39.1%) cultures processed. However, 11 of the 314 (3.5%) positive cultures could have been missed.13 This same protocol was externally validated at another large academic ED setting, where similar results were found.14
In clinical practice, there is a natural tendency to reflexively prescribe antibiotics based on the results of a positive UC due to the hesitancy in ignoring these results, despite lack of a suspicion for a true infection. Leis and colleagues explored this in a proof-of-concept study evaluating the impact of discontinuing the routine reporting of positive UC results from noncatheterized inpatients and requesting clinicians to call the laboratory for results if a UTI was suspected.16 This intervention resulted in a statistically significant reduction in treatment of ASB in noncatheterized patients from 48 to 12% pre- and postintervention. Clinicians requested culture results only 14% of the time, and there were no adverse outcomes among untreated noncatheterized patients. More recently, a QI study conducted at a large community hospital in Toronto, Ontario, Canada, implemented a 2-step model of care for urine collection.17 UC was collected but only processed by the microbiology laboratory if the ED physicians deemed it necessary after clinical assessment.
After implementation, there was a decrease in the proportion of ED visits associated with processed UC (from 6.0% to 4.7% of visits per week; P < .001), ED visits associated with callbacks for processing UC (1.8% to 1.1% of visits per month; P < .001), and antimicrobial prescriptions for urinary symptoms among hospitalized patients (from 20.6% to 10.9%; P < .001). Equally important, despite the 937 cases in which urine was collected but cultures were not processed, no evidence of untreated UTIs was identified.17
The results from the present study similarly demonstrate minimal concern for potentially undertreating these patients. As seen in the subgroup of patients included in the positive UA group, which did not meet criteria for positive UA per protocol (n = 29), only 2 of the subsequent cultures were positive, of which only 1 patient required antibiotic therapy based on the clinical presentation. In addition, in the group of negative UAs with subsequent cancellation of the UC, there were no found reports of outpatient visits, ED visits, or hospital admissions within 7 days of the initial UA for UTI-related symptoms.
Limitations
This single-center, pre-post QI study was not without limitations. Manual chart reviews were required, and accuracy of information was dependent on clinician documentation and assessment of UTI-related symptoms. The population studied was predominately older males; thus, results may not be applicable to females or young adults. Additionally, recognition of a negative UA and subsequent cancellation of the UC was dependent on laboratory personnel. As noted in the patient group with a positive UA, some of these UAs were negative and may have been overlooked; therefore, subsequent UCs were inappropriately processed. However, this occurred infrequently and confirmed the low probability of true UTI in the setting of a negative UA. Follow-up for UTI-related symptoms may not have been captured if a patient had presented to an outside facility. Last, definitions of a positive UA differed slightly between the pre- and postintervention groups. The preintervention study defined a positive UA as a WBC count > 5 WBC/HPF and positive leukocyte esterase, whereas the present study defined a positive UA with a WBC count > 5. This may have resulted in an overestimation of positive UA in the postintervention group.
Conclusions
Better selective use of UC testing may improve stewardship resources and reduce costs impacting both ED and clinical laboratories. Furthermore, benefits can include a reduction in the use of time and resources required to collect samples for culture, use of test supplies, the time and effort required to process the large number of negative cultures, and resources devoted to the follow-up of these ED culture results. The described UA to reflex culture process change demonstrated a significant reduction in the processing of inappropriate UC and unnecessary antibiotics for ASB. There were no missed UTIs or other adverse patient outcomes noted. This process change has been implemented in all departments at the Hines VA and additional data will be collected to ensure consistent outcomes.
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250
1. Chironda B, Clancy S, Powis JE. Optimizing urine culture collection in the emergency department using frontline ownership interventions. Clin Infect Dis. 2014;59(7):1038-1039. doi:10.1093/cid/ciu412
2. Nagurney JT, Brown DF, Chang Y, Sane S, Wang AC, Weiner JB. Use of diagnostic testing in the emergency department for patients presenting with non-traumatic abdominal pain. J Emerg Med. 2003;25(4):363-371. doi:10.1016/s0736-4679(03)00237-3
3. Lammers RL, Gibson S, Kovacs D, Sears W, Strachan G. Comparison of test characteristics of urine dipstick and urinalysis at various test cutoff points. Ann Emerg Med. 2001;38(5):505-512. doi:10.1067/mem.2001.119427
4. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi:10.1093/cid/ciy1121
5. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi:10.1001/jamainternmed.2015.1878
6. Hartley S, Valley S, Kuhn L, et al. Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi:10.1017/ice.2014.73
7. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129(2):242-258. doi:10.1080/00325481.2017.1246055
8. Spivak ES, Burk M, Zhang R, et al. Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi:10.1093/cid/cix474
9. Kim EY, Patel U, Patel B, Suda KJ. Evaluation of bacteriuria treatment and follow-up initiated in the emergency department at a Veterans Affairs hospital. J Pharm Technol. 2017;33(5):183-188. doi:10.1177/8755122517718214
10. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290. doi:10.1086/646173
11. Fok C, Fitzgerald MP, Turk T, Mueller E, Dalaza L, Schreckenberger P. Reflex testing of male urine specimens misses few positive cultures may reduce unnecessary testing of normal specimens. Urology. 2010;75(1):74-76. doi:10.1016/j.urology.2009.08.071
12. Munigala S, Jackups RR Jr, Poirier RF, et al. Impact of order set design on urine culturing practices at an academic medical centre emergency department. BMJ Qual Saf. 2018;27(8):587-592. doi:10.1136/bmjqs-2017-006899
13. Jones CW, Culbreath KD, Mehrotra A, Gilligan PH. Reflect urine culture cancellation in the emergency department. J Emerg Med. 2014;46(1):71-76. doi:10.1016/j.jemermed.2013.08.042
14. Hertz JT, Lescallette RD, Barrett TW, Ward MJ, Self WH. External validation of an ED protocol for reflex urine culture cancelation. Am J Emerg Med. 2015;33(12):1838-1839. doi:10.1016/j.ajem.2015.09.026
15. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1B):53-58. doi:10.1016/0002-9343(83)90073-6
16. Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
17. Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2017;27:140-147. doi:10.1136/bmjqs-2016-006250
Analysis: Surgery may not be better than casting for some wrist fractures
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
There are around 100,000 adult distal radius fractures in the United Kingdom each year. Current National Health Service guidelines in the United Kingdom recommend using K-wires to stabilize wrist fractures when closed reduction is possible or there is no involvement of the articular surface. This is in contrast to fractures that require open reduction and internal fixation with a plate and screws to align the joint articular surface.
As a result, the use of K-wires for surgical fixation has been increasing since 2010 with a comparable decrease of the use of plates and screws.
Even though fixation with wires can provide reliable functional outcomes for patients after reduction of a displaced wrist fracture, surgery still carries risks for the patient and adds an additional expense. A well-molded plaster cast is a safer and cheaper intervention, but it is unclear if it could provide the same functional outcome as pinning.
Therefore, researchers in the United Kingdom conducted a multicenter, randomized trial among 36 hospitals within the NHS as part of the Distal Radius Acute Fracture Fixation Trial 2 (DRAFFT2). Investigators randomly assigned 500 patients aged 16 years and older with dorsally displaced distal radius fractures to manipulation followed by a molded cast or manipulation followed by surgical fixation with K-wires plus a cast.
The study was published online in BMJ.
At 1 year, there were no significant differences between the groups in Patient-Rated Wrist Evaluation (PRWE) scores centered on pain and function.
In an interview, Matthew Costa, PhD, professor of orthopaedic trauma at the University of Oxford (England) and the study’s lead author, said, “If a closed reduction of the fracture can be achieved, clinicians may consider the application of a molded plaster cast as a safe and cost-effective alternative to surgical fixation.”
However, in referencing the data his group published, he did find one thing surprising: “One in eight patients treated with a molded cast required later surgery for loss of fracture position in the first 6 weeks after their injury.”
Dr. Costa added, “This was indeed the key bit of information that patients need when making their decision about surgery. Initial feedback from our patient and public involvement group is that they would be happy to take this chance given that seven out of eight patients didn’t need any form of surgical fixation.”
Philip Blazar, MD, chief of the hand and upper extremity service, Brigham and Women’s Faulkner Hospital in Boston, commended the U.K. authors on completing a challenging randomized controlled trial.
Speaking to this news organization, Dr. Blazer observed a critical difference between U.K. and U.S. guidelines. “It is important to remember that a sizable number of these patients had surgery,” said Dr. Blazar, who was not involved with the study. “They had pins inserted under an anesthetic, and would not have [had] surgery compared to current practice as recommended by many authorities, including the American Academy of Orthopedic Surgeon’s Clinical Practice Guidelines on Distal Radius Fractures.”
Like Dr. Costa, Dr. Blazar expressed concerns about the secondary surgeries in the study group: “27% of patients had a second surgery: 13% in the first 6 weeks after manipulation for loss of reduction, and the remaining 14% had carpal tunnel releases, tendon transfers, tenolysis, and/or capsulectomy for limited range of motion.”
In addition, Dr. Blazar is worried that, although recovery is generally considered to be only 12 months for these type of injuries – the duration of follow-up time in the DRAFFT2 study – “the probable outcome is that in the second 12 months after the injury, there will continue to be more of these types of surgeries.”
Dr. Costa agreed that close follow-up is warranted, “It does suggest that patients treated in a molded cast do need to be followed up carefully to spot those that do need later surgery.”
Still, for Dr. Blazar, the largest takeaway of the study is that, “At 12 months, disability scores between these two groups are not different, but the group treated nonsurgically had 10 times the number of secondary surgeries (27% vs. 2%-3%).”
Moving forward, Dr. Blazar would like to see more specific indications for who would benefit from pinning. He told this news organization, “The greatest limitation is that this study provides no information on which patients with distal radius fractures where reduction is indicated would benefit from surgery. Looking at the details of this study, all patients with displaced fractures from age 16 to the elderly were treated as one indication. My impression is that most surgeons operate on patients taking into account radiographic and patient factors such as age, hand dominance, occupation, overall medical health, and activity level.”
The DRAFFT2 study was funded by the U.K. National Institute for Health Research Health Technology Assessment Programme and was supported by NIHR Oxford Biomedical Research Centre. Dr. Blazar and Dr. Costa have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ
Orthopedists rank third in malpractice suits, survey finds
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.
according to the Medscape Orthopedist Malpractice Report 2021.
Orthopedists ranked third among specialists most likely to be sued, surpassed only by plastic surgeons and general surgeons (both 83%). In comparison, just over half of physicians across all specialties (51%) reported being named in lawsuit. More than one-third of orthopedists (34%) said they had been individually named in a suit, whereas just 14% of all specialists were named individually.
More than half (54%) of orthopedists said they were sued over complications from treatment or surgery. The second-most common reason orthopedists were sued was poor outcome/disease progression (30%), followed by failure to diagnose/delayed diagnosis (21%), failure to treat/delayed treatment (13%), and abnormal injury (9%).
This new report was compiled from an online survey including more than 4,300 physicians from 29 specialties. The survey was available from May 21 to Aug. 28, 2021, and included 250 orthopedists and orthopedic surgeons. Most respondents (62%) had practiced orthopedics for more than 25 years and 60% were aged 60 years or older.
Orthopedists tended to pay more for malpractice insurance than do other specialists. Less than one-third of orthopedists (31%) reported a premium under $20,000 per year, compared with 52% of all specialists. The most common premium for orthopedists was $30,000 or more (29%), whereas only 11% of all specialists reported paying a similar premium.
Nearly 9 out of 10 (89%) of orthopedists said they were “very surprised” or “somewhat surprised” by the malpractice suit. In some of these cases, the physician never personally treated the patient. Wrote one respondent: “I was part of a group of physicians and got dragged into the suit.” The vast majority of orthopedists (82%) said the suit was not warranted, which was similar to responses for physicians as a whole (83%).
Most commonly, orthopedists said lawsuits were settled before trial (34%). The second-most common outcome was the judge and jury deciding in the respondent’s favor (16%), followed by the plaintiff voluntarily dismissing the suit prior to trial (8%), and the respondent being dismissed from the suit in the first few months (8%). Very few (2%) said the judge or jury ruled in the patient’s favor, and 9% of respondents said the case was ongoing.
Most orthopedists reported that cases lasted between 1 and 2 years (41%) and 29% said a lawsuit took 3-5 years. If the plaintiff did receive a monetary award, 42% of physicians reported paying under $100,000, and 30% paid less than $500,000. This is similar to reports from other specialties, though more patients in orthopedic cases received payments under $1 million, compared with other specialties (21% vs. 15%).
More than three-quarters of orthopedists (76%) said that the lawsuit did not negatively affect their career, and more than half (52%) said they did not undergo any attitude or career changes after the suit. More orthopedists than other specialists (31% vs. 24%) did say that they trusted patients less.
When asked if they would do anything differently, one-third (33%) of orthopedists said their actions would remain the same, compared with 43% of the general physician pool. One-quarter of orthopedists said they would have not taken on the patient in the first place, and 14% noted they would have referred to another physician.
A version of this article first appeared on Medscape.com.
We’re dying to tell you about fatigability
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
Using Telehealth Rehabilitation Therapy to Treat a Finger Flexor Tendon Repair During COVID-19
Telehealth-assisted finger rehabilitat ion therapy demonstrated good functional results following repair of a zone 2 flexor tendon laceration.
In 1948, Sterling Bunnell, MD, used the term no man’s land to describe the area between the A1 pulley at the volar aspect of the metacarpophalangeal joint and the insertion of the flexor digitorum superficialis tendons on the middle phalanx (zone 2).1 Bunnell’s description referenced the area of land in World War I between the trenches of opposing armies, and his goal was to emphasize the heightened risks of performing tendon repair in this area, as these repairs were notorious for poor outcomes. In lieu of tendon repair, Bunnell advocated treatment of tendon lacerations in this area with tendon excision and grafting.
It was not until the 1960s that researchers began to advocate for acute repair of tendons in this area.2,3 Since Verdan’s and Kleinart’s work, fastidious adherence to atraumatic technique and improvements in suture technique and rehabilitation protocols have allowed hand surgeons to repair tendons in this area with some level of success. Over the ensuing decades, acute repair of flexor tendon injuries within zone 2 has become the standard of care. The importance of meticulous technique during flexor tendon repair cannot be overemphasized; however, without appropriate hand therapy, even the most meticulous repair may fail.
COVID-19 has created significant barriers to patient care. Reducing travel and limiting face-to-face patient visits have been emphasized as methods that reduce spread of the virus, but these restrictions also prevent patients from easily accessing hand therapy. Recent adoption of telemedicine and videoconferencing technologies may help to reduce some of these barriers, but few previous studies have described the use of videoconferencing technology to supplant face-to-face hand therapy visits. This case describes the use of videoconferencing technology to provide hand therapy for a patient following repair of an acute flexor tendon laceration in zone 2.
Case Presentation
A patient aged < 50 years presented to a US Department of Veterans Affairs (VA) hand surgery clinic 2 days after sustaining a laceration to the flexor digitorum profundus (FDP) in zone 2 of the small finger while cleaning a knife. During the discussion of their treatment options and the recommended postoperative hand therapy protocol, the patient noted difficulty attending postoperative appointments due to COVID-19 as well as a lack of resources. Given these limitations and following discussion with our hand therapist, we discussed the potential for telehealth follow-up with videoconferencing. Four days following the injury, the patient underwent repair of the FDP. During surgery, the laceration was present at the level of the A3 pulley. The FDP was repaired using a 6-0 polypropylene synthetic suture for the epitendinous repair and 4-strand core suture repair using 3-0 Fiberwire suture in a modified cruciate fashion. The A2 and A4 pulleys were preserved, and venting of the pulleys was not required. At the time of surgery, the flexor digitorum superficialis and radial and ulnar digital neurovascular bundles were intact. Following surgical repair of the tendon, the patient was placed into a dorsal blocking splint with a plan for follow-up within 2 to 3 days.
The patient attended the first postoperative visit in person on postoperative day 2. During this visit, the postoperative splint and dressings were removed, and a forearm-based dorsal blocking orthosis was fabricated using thermoplastic. At this visit, the veteran relayed concerns regarding psychosocial and resource barriers in addition to concerns surrounding COVID-19 that would prevent travel to and from hand therapy appointments. Due to these concerns, a passive-motion protocol was initiated using the Indiana manual as a guide.4 The patient returned to the hand clinic at 2 weeks after surgery for evaluation by the operating surgeon and suture removal. All visits after the suture removal were conducted via either telehealth with videoconferencing or by telephone (Table 1).
The operative team evaluated the patient 5 times following surgery. Only 2 of these visits were in-person. The patient attended 6 hand therapy sessions with 2 in-person visits to occupational therapy (Figure 1). The remaining 4 visits were conducted using videoconferencing. The patient received therapy supplies by mail as needed, and their use was reviewed in telerehabilitation sessions with videoconferencing as needed. During their postoperative course, the patient experienced little edema or scar tissue formation, and recovery was uncomplicated. The patient developed a mild extensor lag for which a proximal interphalangeal joint spring extension orthosis was provided via mail (Figure 2). The patient admitted only partial adherence with this orthosis, and at discharge, a 10-degree extensor lag remained. The patient was not concerned by this extension deficit and did not experience any associated functional deficits, demonstrated by scores on the Quick Disabilities of the Arm, Shoulder and Hand questionnaire and Patient Specific Functional Scale (Table 2).
Discussion
Few studies have been published that address the efficacy of telerehabilitation after surgical management of traumatic injuries involving the upper extremity. One Australian study performed by Worboys and colleagues concluded that utilization of telehealth services for hand therapy visits may provide accurate patient assessment with favorable patient satisfaction.5 Another study performed in the UK by Gilbert and colleagues demonstrated that videoconferencing is well received by patients, as it may offer shorter wait times, improved convenience, and reduced travel cost.
The authors noted that although videoconferencing may not completely replace in-person therapy, it could act as an adjunct.6 While these in-person visits may be necessary, particularly to establish care, at least one study has demonstrated that patients may prefer follow-up via telehealth if provided the option.7 In a randomized, controlled study performed in Norway, patients were randomized to either an in-person or video consultation with an orthopedic outpatient clinic. Of patients randomized to the in-person clinic visit, 86% preferred to have follow-up via videoconferencing.7
Previous studies have demonstrated that telehealth may produce accurate patient assessment, with relatively high patient satisfaction. Given the COVID-19 pandemic and the limitations that this crisis has placed on in-person outpatient visits, clinics that previously may have been resistant to telehealth are adapting and using the technology to meet the needs of their population.8 The present case demonstrates that videoconferencing is feasible and may lead to successful results, even for cases requiring significant hand therapy follow-up, such as flexor tendon repairs.
Conclusions
Although in-person hand therapy remains the standard of care following flexor tendon repair of the hand, situations may exist in which hand therapy conducted via telehealth is better than no hand therapy at all. The present case study highlights the use of telehealth as an acceptable supplement to in-person postoperative visits.
In our case, use of a standardized protocol with an emphasis on hand function and patient satisfaction as opposed to strict range of motion measurements produced good results. Although a specific telehealth satisfaction measure was not used in this case, commonly used questionnaires may be integrated into future visits to improve telehealth implementation and patient experience. In this specific case, the veteran felt that hand function was regained and expressed general satisfaction with the telemedicine process at the conclusion of care. While telehealth was a useful adjunct in the treatment of the present patient, further study of videoconferencing should be conducted to determine whether hand therapy conducted via telehealth could be implemented more broadly following upper extremity surgery.
1. Hege JJ. History off-hand: Bunnell’s no-man’s land. Hand (NY). 2019;14(4):570-574. doi:10.1177/1558944717744337
2. Verdan C. Primary repair of flexor tendons. J Bone Joint Surg Am. 1960;42-A:647-657.
3. Kleinert HE, Kutz JE, Ashbell TS, et al. Primary repair of lacerated flexor tendon in no man’s land (abstract). J Bone Joint Surg. 1967;49A:577.
4. Cannon NM. Diagnosis and Treatment Manual for Physicians and Therapists: Upper Extremity Rehabilitation. 4th ed. Hand Rehabilitation Center of Indiana; 2001.
5. Worboys T, Brassington M, Ward EC, Cornwell PL. Delivering occupational therapy hand assessment and treatment sessions via telehealth. J Telemed Telecare. 2018;24(3):185-192. doi:10.1177/1357633X17691861
6. Gilbert AW, Jaggi A, May CR. What is the patient acceptability of real time 1:1 videoconferencing in an orthopaedics setting? A systematic review. Physiotherapy. 2018;104(2):178-186. doi:10.1016/j.physio.2017.11.217
7. Buvik A, Bugge E, Knutsen G, Smatresk A, Wilsgaard T. Patient reported outcomes with remote orthopaedic consultations by telemedicine: A randomised controlled trial. J Telemed Telecare. 2019;25(8):451-459. doi:10.1177/1357633X18783921
8. Loeb AE, Rao SS, Ficke JR, Morris CD, Riley LH 3rd, Levin AS. Departmental experience and lessons learned with accelerated introduction of telemedicine during the COVID-19 crisis. J Am Acad Orthop Surg. 2020;28(11):e469-e476. doi:10.5435/JAAOS-D-20-00380
Telehealth-assisted finger rehabilitat ion therapy demonstrated good functional results following repair of a zone 2 flexor tendon laceration.
Telehealth-assisted finger rehabilitat ion therapy demonstrated good functional results following repair of a zone 2 flexor tendon laceration.
In 1948, Sterling Bunnell, MD, used the term no man’s land to describe the area between the A1 pulley at the volar aspect of the metacarpophalangeal joint and the insertion of the flexor digitorum superficialis tendons on the middle phalanx (zone 2).1 Bunnell’s description referenced the area of land in World War I between the trenches of opposing armies, and his goal was to emphasize the heightened risks of performing tendon repair in this area, as these repairs were notorious for poor outcomes. In lieu of tendon repair, Bunnell advocated treatment of tendon lacerations in this area with tendon excision and grafting.
It was not until the 1960s that researchers began to advocate for acute repair of tendons in this area.2,3 Since Verdan’s and Kleinart’s work, fastidious adherence to atraumatic technique and improvements in suture technique and rehabilitation protocols have allowed hand surgeons to repair tendons in this area with some level of success. Over the ensuing decades, acute repair of flexor tendon injuries within zone 2 has become the standard of care. The importance of meticulous technique during flexor tendon repair cannot be overemphasized; however, without appropriate hand therapy, even the most meticulous repair may fail.
COVID-19 has created significant barriers to patient care. Reducing travel and limiting face-to-face patient visits have been emphasized as methods that reduce spread of the virus, but these restrictions also prevent patients from easily accessing hand therapy. Recent adoption of telemedicine and videoconferencing technologies may help to reduce some of these barriers, but few previous studies have described the use of videoconferencing technology to supplant face-to-face hand therapy visits. This case describes the use of videoconferencing technology to provide hand therapy for a patient following repair of an acute flexor tendon laceration in zone 2.
Case Presentation
A patient aged < 50 years presented to a US Department of Veterans Affairs (VA) hand surgery clinic 2 days after sustaining a laceration to the flexor digitorum profundus (FDP) in zone 2 of the small finger while cleaning a knife. During the discussion of their treatment options and the recommended postoperative hand therapy protocol, the patient noted difficulty attending postoperative appointments due to COVID-19 as well as a lack of resources. Given these limitations and following discussion with our hand therapist, we discussed the potential for telehealth follow-up with videoconferencing. Four days following the injury, the patient underwent repair of the FDP. During surgery, the laceration was present at the level of the A3 pulley. The FDP was repaired using a 6-0 polypropylene synthetic suture for the epitendinous repair and 4-strand core suture repair using 3-0 Fiberwire suture in a modified cruciate fashion. The A2 and A4 pulleys were preserved, and venting of the pulleys was not required. At the time of surgery, the flexor digitorum superficialis and radial and ulnar digital neurovascular bundles were intact. Following surgical repair of the tendon, the patient was placed into a dorsal blocking splint with a plan for follow-up within 2 to 3 days.
The patient attended the first postoperative visit in person on postoperative day 2. During this visit, the postoperative splint and dressings were removed, and a forearm-based dorsal blocking orthosis was fabricated using thermoplastic. At this visit, the veteran relayed concerns regarding psychosocial and resource barriers in addition to concerns surrounding COVID-19 that would prevent travel to and from hand therapy appointments. Due to these concerns, a passive-motion protocol was initiated using the Indiana manual as a guide.4 The patient returned to the hand clinic at 2 weeks after surgery for evaluation by the operating surgeon and suture removal. All visits after the suture removal were conducted via either telehealth with videoconferencing or by telephone (Table 1).
The operative team evaluated the patient 5 times following surgery. Only 2 of these visits were in-person. The patient attended 6 hand therapy sessions with 2 in-person visits to occupational therapy (Figure 1). The remaining 4 visits were conducted using videoconferencing. The patient received therapy supplies by mail as needed, and their use was reviewed in telerehabilitation sessions with videoconferencing as needed. During their postoperative course, the patient experienced little edema or scar tissue formation, and recovery was uncomplicated. The patient developed a mild extensor lag for which a proximal interphalangeal joint spring extension orthosis was provided via mail (Figure 2). The patient admitted only partial adherence with this orthosis, and at discharge, a 10-degree extensor lag remained. The patient was not concerned by this extension deficit and did not experience any associated functional deficits, demonstrated by scores on the Quick Disabilities of the Arm, Shoulder and Hand questionnaire and Patient Specific Functional Scale (Table 2).
Discussion
Few studies have been published that address the efficacy of telerehabilitation after surgical management of traumatic injuries involving the upper extremity. One Australian study performed by Worboys and colleagues concluded that utilization of telehealth services for hand therapy visits may provide accurate patient assessment with favorable patient satisfaction.5 Another study performed in the UK by Gilbert and colleagues demonstrated that videoconferencing is well received by patients, as it may offer shorter wait times, improved convenience, and reduced travel cost.
The authors noted that although videoconferencing may not completely replace in-person therapy, it could act as an adjunct.6 While these in-person visits may be necessary, particularly to establish care, at least one study has demonstrated that patients may prefer follow-up via telehealth if provided the option.7 In a randomized, controlled study performed in Norway, patients were randomized to either an in-person or video consultation with an orthopedic outpatient clinic. Of patients randomized to the in-person clinic visit, 86% preferred to have follow-up via videoconferencing.7
Previous studies have demonstrated that telehealth may produce accurate patient assessment, with relatively high patient satisfaction. Given the COVID-19 pandemic and the limitations that this crisis has placed on in-person outpatient visits, clinics that previously may have been resistant to telehealth are adapting and using the technology to meet the needs of their population.8 The present case demonstrates that videoconferencing is feasible and may lead to successful results, even for cases requiring significant hand therapy follow-up, such as flexor tendon repairs.
Conclusions
Although in-person hand therapy remains the standard of care following flexor tendon repair of the hand, situations may exist in which hand therapy conducted via telehealth is better than no hand therapy at all. The present case study highlights the use of telehealth as an acceptable supplement to in-person postoperative visits.
In our case, use of a standardized protocol with an emphasis on hand function and patient satisfaction as opposed to strict range of motion measurements produced good results. Although a specific telehealth satisfaction measure was not used in this case, commonly used questionnaires may be integrated into future visits to improve telehealth implementation and patient experience. In this specific case, the veteran felt that hand function was regained and expressed general satisfaction with the telemedicine process at the conclusion of care. While telehealth was a useful adjunct in the treatment of the present patient, further study of videoconferencing should be conducted to determine whether hand therapy conducted via telehealth could be implemented more broadly following upper extremity surgery.
In 1948, Sterling Bunnell, MD, used the term no man’s land to describe the area between the A1 pulley at the volar aspect of the metacarpophalangeal joint and the insertion of the flexor digitorum superficialis tendons on the middle phalanx (zone 2).1 Bunnell’s description referenced the area of land in World War I between the trenches of opposing armies, and his goal was to emphasize the heightened risks of performing tendon repair in this area, as these repairs were notorious for poor outcomes. In lieu of tendon repair, Bunnell advocated treatment of tendon lacerations in this area with tendon excision and grafting.
It was not until the 1960s that researchers began to advocate for acute repair of tendons in this area.2,3 Since Verdan’s and Kleinart’s work, fastidious adherence to atraumatic technique and improvements in suture technique and rehabilitation protocols have allowed hand surgeons to repair tendons in this area with some level of success. Over the ensuing decades, acute repair of flexor tendon injuries within zone 2 has become the standard of care. The importance of meticulous technique during flexor tendon repair cannot be overemphasized; however, without appropriate hand therapy, even the most meticulous repair may fail.
COVID-19 has created significant barriers to patient care. Reducing travel and limiting face-to-face patient visits have been emphasized as methods that reduce spread of the virus, but these restrictions also prevent patients from easily accessing hand therapy. Recent adoption of telemedicine and videoconferencing technologies may help to reduce some of these barriers, but few previous studies have described the use of videoconferencing technology to supplant face-to-face hand therapy visits. This case describes the use of videoconferencing technology to provide hand therapy for a patient following repair of an acute flexor tendon laceration in zone 2.
Case Presentation
A patient aged < 50 years presented to a US Department of Veterans Affairs (VA) hand surgery clinic 2 days after sustaining a laceration to the flexor digitorum profundus (FDP) in zone 2 of the small finger while cleaning a knife. During the discussion of their treatment options and the recommended postoperative hand therapy protocol, the patient noted difficulty attending postoperative appointments due to COVID-19 as well as a lack of resources. Given these limitations and following discussion with our hand therapist, we discussed the potential for telehealth follow-up with videoconferencing. Four days following the injury, the patient underwent repair of the FDP. During surgery, the laceration was present at the level of the A3 pulley. The FDP was repaired using a 6-0 polypropylene synthetic suture for the epitendinous repair and 4-strand core suture repair using 3-0 Fiberwire suture in a modified cruciate fashion. The A2 and A4 pulleys were preserved, and venting of the pulleys was not required. At the time of surgery, the flexor digitorum superficialis and radial and ulnar digital neurovascular bundles were intact. Following surgical repair of the tendon, the patient was placed into a dorsal blocking splint with a plan for follow-up within 2 to 3 days.
The patient attended the first postoperative visit in person on postoperative day 2. During this visit, the postoperative splint and dressings were removed, and a forearm-based dorsal blocking orthosis was fabricated using thermoplastic. At this visit, the veteran relayed concerns regarding psychosocial and resource barriers in addition to concerns surrounding COVID-19 that would prevent travel to and from hand therapy appointments. Due to these concerns, a passive-motion protocol was initiated using the Indiana manual as a guide.4 The patient returned to the hand clinic at 2 weeks after surgery for evaluation by the operating surgeon and suture removal. All visits after the suture removal were conducted via either telehealth with videoconferencing or by telephone (Table 1).
The operative team evaluated the patient 5 times following surgery. Only 2 of these visits were in-person. The patient attended 6 hand therapy sessions with 2 in-person visits to occupational therapy (Figure 1). The remaining 4 visits were conducted using videoconferencing. The patient received therapy supplies by mail as needed, and their use was reviewed in telerehabilitation sessions with videoconferencing as needed. During their postoperative course, the patient experienced little edema or scar tissue formation, and recovery was uncomplicated. The patient developed a mild extensor lag for which a proximal interphalangeal joint spring extension orthosis was provided via mail (Figure 2). The patient admitted only partial adherence with this orthosis, and at discharge, a 10-degree extensor lag remained. The patient was not concerned by this extension deficit and did not experience any associated functional deficits, demonstrated by scores on the Quick Disabilities of the Arm, Shoulder and Hand questionnaire and Patient Specific Functional Scale (Table 2).
Discussion
Few studies have been published that address the efficacy of telerehabilitation after surgical management of traumatic injuries involving the upper extremity. One Australian study performed by Worboys and colleagues concluded that utilization of telehealth services for hand therapy visits may provide accurate patient assessment with favorable patient satisfaction.5 Another study performed in the UK by Gilbert and colleagues demonstrated that videoconferencing is well received by patients, as it may offer shorter wait times, improved convenience, and reduced travel cost.
The authors noted that although videoconferencing may not completely replace in-person therapy, it could act as an adjunct.6 While these in-person visits may be necessary, particularly to establish care, at least one study has demonstrated that patients may prefer follow-up via telehealth if provided the option.7 In a randomized, controlled study performed in Norway, patients were randomized to either an in-person or video consultation with an orthopedic outpatient clinic. Of patients randomized to the in-person clinic visit, 86% preferred to have follow-up via videoconferencing.7
Previous studies have demonstrated that telehealth may produce accurate patient assessment, with relatively high patient satisfaction. Given the COVID-19 pandemic and the limitations that this crisis has placed on in-person outpatient visits, clinics that previously may have been resistant to telehealth are adapting and using the technology to meet the needs of their population.8 The present case demonstrates that videoconferencing is feasible and may lead to successful results, even for cases requiring significant hand therapy follow-up, such as flexor tendon repairs.
Conclusions
Although in-person hand therapy remains the standard of care following flexor tendon repair of the hand, situations may exist in which hand therapy conducted via telehealth is better than no hand therapy at all. The present case study highlights the use of telehealth as an acceptable supplement to in-person postoperative visits.
In our case, use of a standardized protocol with an emphasis on hand function and patient satisfaction as opposed to strict range of motion measurements produced good results. Although a specific telehealth satisfaction measure was not used in this case, commonly used questionnaires may be integrated into future visits to improve telehealth implementation and patient experience. In this specific case, the veteran felt that hand function was regained and expressed general satisfaction with the telemedicine process at the conclusion of care. While telehealth was a useful adjunct in the treatment of the present patient, further study of videoconferencing should be conducted to determine whether hand therapy conducted via telehealth could be implemented more broadly following upper extremity surgery.
1. Hege JJ. History off-hand: Bunnell’s no-man’s land. Hand (NY). 2019;14(4):570-574. doi:10.1177/1558944717744337
2. Verdan C. Primary repair of flexor tendons. J Bone Joint Surg Am. 1960;42-A:647-657.
3. Kleinert HE, Kutz JE, Ashbell TS, et al. Primary repair of lacerated flexor tendon in no man’s land (abstract). J Bone Joint Surg. 1967;49A:577.
4. Cannon NM. Diagnosis and Treatment Manual for Physicians and Therapists: Upper Extremity Rehabilitation. 4th ed. Hand Rehabilitation Center of Indiana; 2001.
5. Worboys T, Brassington M, Ward EC, Cornwell PL. Delivering occupational therapy hand assessment and treatment sessions via telehealth. J Telemed Telecare. 2018;24(3):185-192. doi:10.1177/1357633X17691861
6. Gilbert AW, Jaggi A, May CR. What is the patient acceptability of real time 1:1 videoconferencing in an orthopaedics setting? A systematic review. Physiotherapy. 2018;104(2):178-186. doi:10.1016/j.physio.2017.11.217
7. Buvik A, Bugge E, Knutsen G, Smatresk A, Wilsgaard T. Patient reported outcomes with remote orthopaedic consultations by telemedicine: A randomised controlled trial. J Telemed Telecare. 2019;25(8):451-459. doi:10.1177/1357633X18783921
8. Loeb AE, Rao SS, Ficke JR, Morris CD, Riley LH 3rd, Levin AS. Departmental experience and lessons learned with accelerated introduction of telemedicine during the COVID-19 crisis. J Am Acad Orthop Surg. 2020;28(11):e469-e476. doi:10.5435/JAAOS-D-20-00380
1. Hege JJ. History off-hand: Bunnell’s no-man’s land. Hand (NY). 2019;14(4):570-574. doi:10.1177/1558944717744337
2. Verdan C. Primary repair of flexor tendons. J Bone Joint Surg Am. 1960;42-A:647-657.
3. Kleinert HE, Kutz JE, Ashbell TS, et al. Primary repair of lacerated flexor tendon in no man’s land (abstract). J Bone Joint Surg. 1967;49A:577.
4. Cannon NM. Diagnosis and Treatment Manual for Physicians and Therapists: Upper Extremity Rehabilitation. 4th ed. Hand Rehabilitation Center of Indiana; 2001.
5. Worboys T, Brassington M, Ward EC, Cornwell PL. Delivering occupational therapy hand assessment and treatment sessions via telehealth. J Telemed Telecare. 2018;24(3):185-192. doi:10.1177/1357633X17691861
6. Gilbert AW, Jaggi A, May CR. What is the patient acceptability of real time 1:1 videoconferencing in an orthopaedics setting? A systematic review. Physiotherapy. 2018;104(2):178-186. doi:10.1016/j.physio.2017.11.217
7. Buvik A, Bugge E, Knutsen G, Smatresk A, Wilsgaard T. Patient reported outcomes with remote orthopaedic consultations by telemedicine: A randomised controlled trial. J Telemed Telecare. 2019;25(8):451-459. doi:10.1177/1357633X18783921
8. Loeb AE, Rao SS, Ficke JR, Morris CD, Riley LH 3rd, Levin AS. Departmental experience and lessons learned with accelerated introduction of telemedicine during the COVID-19 crisis. J Am Acad Orthop Surg. 2020;28(11):e469-e476. doi:10.5435/JAAOS-D-20-00380
Review of Efficacy and Safety of Spinal Cord Stimulation in Veterans
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
Lower back pain (LBP) affects an estimated 9.4% of the global population and has resulted in more years lived with disability than any other health condition.1 LBP affects a wide range of populations, but US veterans have been shown to have significantly higher rates of back pain than nonveterans. The National Institutes of Health reports that 65.6% of veterans experience chronic pain; 9.1% of veterans experience severe, chronic pain.2 Chronic back pain is treated by a range of methods, including medications, surgery, physical therapy (PT), patient education, and behavioral therapy.3 However, chronic neuropathic back pain has been shown to have limited responsiveness to medication.4
Neuropathic pain is caused by lesions in the somatosensory nervous system, resulting in spontaneous pain and amplified pain responses to both painful and nonpainful stimuli.5 The most common location for neuropathic pain is the back and legs. Between 10% and 40% of people who undergo lumbosacral spine surgery to treat neuropathic radicular pain will experience further neuropathic pain.6 This condition is referred to as failed back surgery syndrome or postlaminectomy syndrome (PLS). While neuropathic back pain has had limited responsiveness to medication and repeated lumbosacral spine surgery, spinal cord stimulation (SCS) has shown promise as an effective form of pain treatment for those experiencing PLS and other spine disorders.7-10 In addition, SCS therapy has had a very low incidence of complications, which may be on the decline with recent technological advancements.11 Patients with a diagnosis of PLS, LBP, or complex regional pain syndrome (CRPS) who have not responded to medications, therapy, and/or injections for ≥ 6 months were eligible for a trial of SCS therapy. Trial leads were placed via the percutaneous route with the battery strapped to the waistline for 3 to 5 days and were removed in clinic. Patients who experienced > 60% pain relief and functional improvement received a SCS implant.
The effectiveness of SCS has been demonstrated in a nonveteran population, but it has not been studied in a veteran population.12 US Department of Veterans Affairs (VA) health care coverage is different from Medicare and private insurance in that it is classified as a benefit and not insurance. The goals of treatment at the VA may include considerations in addition to feeling better, and patient presentations may not align with those in the private sector.
We hypothesize that SCS is both a safe and beneficial treatment option for veterans with chronic intractable spine and/or extremity pain. The purpose of this study was to determine the efficacy and safety of SCS in a veteran population.
Methods
The efficacy and safety of SCS was determined via a retrospective study. Inclusion criteria for the study consisted of any Southeastern Louisiana Veterans Health Care System (SLVHCS) patient who had an SCS trial and/or implant from 2008 to 2020. Eligible veterans must have had chronic pain for at least 6 months and had previously tried multiple medications, PT, transcutaneous nerve stimulation, facet injections, epidural steroid injections, or surgery without success. For medication therapy to be considered unsuccessful, it must have included acetaminophen, nonsteroidal anti-inflammatory drugs, and ≥ 1 adjuvant medication (gabapentin, duloxetine, amitriptyline, lidocaine, and menthol). A diagnosis of chronic LBP, PLS, cervical or lumbar spondylosis with radiculopathy, complex regional pain syndrome, or chronic pain syndrome was required for eligibility. Patients whose pain decreased by > 60% and had functional improvement in a 3- to 5-day trial received SCS implantation with percutaneous leads by a pain physician or paddle lead by a neurosurgeon.
The SLVHCS Institutional Review Board approved this study. Electronic health records were reviewed to determine patient age, anthropometric data, and date of SCS implantation. Patients were then called and interviewed to complete a survey. After obtaining verbal consent to the study, subjects were surveyed regarding whether the patient would recommend the procedure to peers, adverse effects (AEs) or complications, and the ability to decrease opiates if applicable. A verbal Pain Outcome Questionnaire (POQ) assessment of activities of daily living also was given during the phone interview regarding pain levels before SCS and at the time of the phone interview.13 (eAppendix available at doi:10.12788/fp.0204) Following the survey, a chart review was performed to corroborate the given AEs or complications and opiate use information. Before and after results of the POQ were compared via a paired sample t test, and P values < .05 were considered significant. Analyses were performed by IBM SPSS, version 26.
The primary outcome measure for this study was whether veterans would recommend SCS to their peers; in our view, this categorical outcome measure seemed to be more valuable to share with future patients who might be candidates for SCS. Since VA health care coverage and goals of treatment may be different from a nonveteran population, we opted to use this primary measure to decrease the possibility of confounding variables.
Secondary outcome measures included changes in POC scores, improvements in activities of daily living, and decreases in use of opioid pain medications.
POQ responses were recorded during the telephone interviews (0 to 10 scale). A paired sample t test was conducted to compare pain levels before and after SCS implant. Pain levels were gathered in the single phone call. Patient opioid usage, if applicable, was assessed by converting medications to morphine milligram equivalent dosing (MMED). Since patients who were on chronic opioids took multiple formulations, we changed the total daily dose to all morphine; for this study, morphine was considered equivalent to hydrocodone, and oxycodone was 1.5x morphine.
Results
Of the 90 SLVHCS patients who received an SCS implant between 2008 and 2020, 76 were reached by telephone and 65 had their responses recorded in the study. Of the 11 patients who were not included, 5 had the SCS removed; it is unclear whether these veterans would have recommended the treatment. Four were unable to quantify pain and/or SCS effects, and 2 were excluded due to a dementia diagnosis years after the implant. The mean (SD) age of participants was 63.9 (10.3) years. Forty percent of patients had a diabetes mellitus diagnosis and 1 had prediabetes. Patients’ most common qualifying diagnosis for SCS was PLS (47.7%) followed by chronic LBP (26.2%). A percutaneous 2-lead technique was the most common type of SCS type used (60.0%) followed by 1-lead (21.5%). The most common SCS manufacturer was Boston Scientific (87.7%)(Table 1). Most veterans (76.9%) recommended SCS to their peers; 13.8% did not recommend SCS; 9.2% were undecided and stated that they were unable to recommend because they did not want to persuade a peer to get SCS (Figure).
There was a statistically significant decrease in opioid use for the 40 veterans for whom pain medication was converted (P < .001)(Table 2). Six patients reported using opioids at some point but could not remember their dose, and no records were found in their chart review, so they were not included in the MMED analysis. In that group, 4 patients reported using opioids before SCS but discontinued the opioid use after SCS implantation, and 2 patients noted using opioids before SCS and concomitantly. Eighteen subjects reported no opioid use at any point before or after SCS (Table 3).
There were few life-threatening complications of SCS. Three veterans developed skin dehiscence; 2 had dehiscence at the battery/generator site, and 1 had dehiscence at the lead anchor site. Two patients with dehiscence also had morbid obesity, and the third had postoperative malnourishment. The dehiscence occurred 3 and 8 months postoperation. All 3 patients with dehiscence had the SCS explanted, though they were eager to get a new SCS implanted as soon as possible because SCS was their most successful treatment to date.
Twenty of the 64 veterans surveyed reported other complications of SCS, including lead migration, lack of pain coverage, paresthesia and numbness, soreness around generator site, SCS shocking patient when performing full thoracic spine flexion, and shingles at the battery site (Table 4). There were 11 explants among the 76 veterans contacted. The primary reason for explant was lack of pain coverage.
Patient concerns included pain with sitting in chairs due to tenderness around the implant, SCS helping with physical pain but not mental pain, SCS only working during the day and not helping with sleep, and patients lacking education regarding possible complications of SCS.
Discussion
In this nonrandomized retrospective review, SCS was shown to be an effective treatment for intractable spine and/or extremity pain. Veterans’ pain levels were significantly reduced following SCS implantation, and more than three-fourths of veterans recommended SCS to their peers. We used the recommendation of SCS to peers as the most important metric regarding the effectiveness of SCS, as this measure was felt to be more valuable to share with future patients; furthermore, categorical analysis has been shown to be more valuable than ordinal pain scales to measure pain.14 In addition to wanting to expand the available research to the general public, we wanted a measure that we could easily relay to our patient population regarding SCS.
The explant rate of 14.5% among surveyed veterans falls at the higher end of the normal ranges found in previous studies of long-term SCS outcomes.15-17 One possible reason for the higher rate is that we did not differentiate based on the reason for the explant (ie, no benefit, further surgery needed for underlying medical condition, or SCS-specific complications). Another possible contributing factor to the higher than expected explant rate is the geographic location in the New Orleans metro area; New Orleans is considered to have one of the highest rates of obesity in the United States and obesity typically has other diseases associated with it such as hypertension and diabetes mellitus.
Limitations
Limitations of the study include the relatively low number of subjects, subjective nature of the interview questions, and the patients’ answers. Typically the POQ has been used as a prospective assessment of pain; whether it is valid in a retrospective analysis is not clear. While there was a statistically significant decrease of opioid use after getting SCS, this study can only show correlation, not causation. During the study period, there has been a drastic change in opioid prescribing patterns and efforts to decrease the amount of opioids prescribed.
Subjects also were asked to rate their pain and quality of life before SCS. Some subjects had SCS implantation up to 10 years prior to the phone interview. The variable amount of time between SCS implantation and interview likely affected subjects’ responses. Chronic pain is a moving target. Patients have good days and bad days that would likely change opinions on SCS benefits on a single phone interview. Some patients needed battery replacements at the time of the interview (battery life averaged about 3 to 5 years in our study population) and were asked to report current levels of pain from the perspective of when their batteries were still functional, further affecting results.
Conclusions
SCS was shown to improve the quality of life of US veterans at SLVHCS across a wide variety of metrics, including activities of daily living, as well as mental and physical health. For veterans with chronic intractable pain who have tried and failed more conservative treatments, SCS is a great treatment.
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634
1. Hoy DG, Smith E, Cross M, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982-989 doi:10.1136/annrheumdis-2013-204344
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
4. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. doi:10.1016/S1474-4422(14)70251-0
5. Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1-32. doi:10.1146/annurev.neuro.051508.135531
6. Wilkinson HA. The Failed Back Syndrome: Etiology and Therapy. 2nd ed. Harper & Row; 1991.
7. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1-2):179-188. doi:10.1016/j.pain.2007.07.028
8. North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-107. doi:10.1227/01.neu.0000144839.65524.e0
9. Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529. doi:10.1111/ner.12024
10. Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-5580. doi:10.1227/NEU.0b013e3182181e60
11. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148-153. doi:10.1111/j.1533-2500.2010.00407.x
12. Veizi E, Hayek SM, North J, et al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534-1548. doi:10.1093/pm/pnw286
13. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi:10.1016/j.jpain.2010.02.012
14. Kennedy DJ, Schneider B. Lies, damn lies, and statistic: a commentary. Pain Med. 2020;21(10):2052-2054. doi:10.1093/pm/pnaa287
15. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-649. doi:10.1111/ner.12642
16. Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. doi:10.1111/ner.12312
17. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation. 2017;20(6):543-552. doi:10.1111/ner.12634
Rotating Hinge Distal Femur Replacement: A Turn for the Worse
Preoperatively periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component is a rare event, and knowledge of this possible complication is important for arthroplasty surgeons.
The use of a rotating hinge distal femur replacement (DFR) for significant bone and soft tissue defects in the setting of total knee arthroplasty (TKA) revision has become increasingly more common. Although significant advancements have been made in modern DFR components, complications and failure rates remain high. The unanticipated early failure presented serves as the first reported case in the literature to our knowledge of a 180° rotation of a press-fit DFR.
Originally, DFRs were used primarily for oncology patients with substantial bone loss following large mass excisions. The utility of DFRs has grown to include massive bone loss in the setting of TKA revision, periprosthetic fractures, and periprosthetic joint infections.1-3 DFRs help restore the joint line in the setting of significant bone loss and contain a rotating hinge mechanism that provides functional movement despite the loss of soft tissue constraints around the knee.1-3
DFRs have been associated with early postoperative mobilization and decreased need for ambulatory devices at 1 year in revision TKA and periprosthetic and geriatric distal femur fractures.4-6 Advances in prosthetic design, biomechanics, and fixation technique have led to improved survival rates.3 Despite these improvements, the overall complication rate remains high at 30 to 40%.3-7 Commonly reported complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.3,4,7 Recent case studies also have reported on dislocation or disengagement of the rotating hinge.8-11
In this case report, we present a patient who had a DFR as the second stage of a 2-stage TKA revision due to a periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component. Although this is a rare event, knowledge of this possible complication is important for arthroplasty surgeons.
Case Presentation
A patient with a history of hypertension, osteopenia, and rheumatoid arthritis underwent a primary right TKA in 2007. Ten weeks postoperatively, the patient had a ground-level fall that resulted in a right periprosthetic supracondylar distal femur fracture that was treated with a distal femur locking plate. The patient healed, however, with a significant golf club deformity (Figure 1). The patient did well for more than a decade but in 2019 was admitted with pelvic inflammatory disease and adnexal abscess that was treated with broad-spectrum IV antibiotics. Shortly after this admission, the patient developed a right knee periprosthetic infection with cultures positive for Ureaplasma parvum.
The patient then underwent a 2-stage revision of the infected TKA. Stage 1 consisted of explant of the TKA components as well as removal of the distal femur plate and screws and placement of an articulating antibiotic cement spacer (Figure 2). The patient completed 6 weeks of IV antibiotics. Following completion of the antibiotic course, we obtained a serum erythrocyte sedimentation rate, C-reactive protein level, and white blood cell count, which were all within normal limits. A knee aspiration was performed and did not show signs of residual infection. Frozen histopathology was sent during the second stage of the revision and did not show infection. After the results of the frozen histopathology returned, the antibiotic spacer was removed, and the femoral canal was thoroughly debrided. Cement and fibrous tissue in the femoral canal were carefully removed. In the setting of significant bone loss and soft tissue compromise due to the previous infection and distal femur fracture, the Zimmer Biomet Orthopedic Salvage System (OSS) with porous coated press-fit elliptical femoral stem was utilized.
The femoral canal was reamed until good cortical chatter was obtained at 16 mm. Per the Biomet OSS guide, “For bowed (curved) long and short press-fit stems, the final flexible reamer shaft diameter may need to be larger than the definitive trial and implant diameter.” After trialing, size 15.5 mm was selected for implantation. Intraoperatively the final stem was noted to have good interference fit after insertion and was stable throughout knee range of motion and varus/valgus stress testing. The patient did well with mobilization while in the hospital postoperatively and was discharged home (Figure 3).
Five days after discharge, the patient kicked the repaired knee onto a chair for rest and elevation and experienced extreme pain and was unable to flex the knee. On presentation to the emergency department, the X-rays showed 180° rotation around the longitudinal axis of the femoral component without any other obvious component failure or fracture (Figure 4). The patient was taken back to surgery the following day. Intraoperatively, the femoral stem was found to be loose and rotated 180° (Figure 5). No failure or dislocation of the tibial or rotating hinge components were identified. The press-fit femoral stem was removed and replaced with a cemented stem (Figure 6).
The postoperative course after the revision surgery was uneventful, and the patient is doing well clinically with no pain, functional range of motion of 5 to 105°, and has returned to regular activities without difficulty.
Discussion
Despite advancements in DFRs and increasing use in the setting of revision TKA, the procedure remains high risk with respect to postoperative complications.3-7 Vertesich and colleagues demonstrated that 43.3% of patients who underwent DFR for failed TKA developed at least 1 postoperative complication that required a return to the operating room.7Physicians need to be aware of the high rate of complications and counsel patients appropriately preoperatively.
Complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.4,7 Soft tissue failures include insufficiency or rupture of the extensor mechanism and patella dislocation.4,7 Structural failures include fracture of the hinge mechanism, dissociation of the component from the stem, rotating hinge-bushing failure, and dislocation of the hinge.4,7 In the acute postoperative period, the most common complications are infection and rotating-hinge dislocation/failure.3,12 There are various component options available for DFRs, including straight vs curved, cemented vs cementless/press-fit, and long vs short stems.13 Studies have sought to elucidate the ideal implant to decrease the rate of complications. Lu and colleagues demonstrated that curved press-fit short stems provided a stable interface without loosening over the short term (2 years) in 42 patients.13 No implant failures or incidences of aseptic loosening occurred in their study.13
The implant used in this case was a curved press-fit short-stem DFR. It was thought that this patient was young and with good enough bone quality that a press-fit short stem would be best in preserving bone stock. Both the technique guide and literature support reaming 0 to 2 mm greater than the planned stem size to accommodate the implant curvature.13 In this case, the intramedullary canal was reamed 0.5 mm larger than the curved stem that was implanted (16 mm and 15.5 mm, respectively). Intraoperatively during the index DFR, the component was stable and seemed to have a good press-fit interface. Despite this, obvious loosening of the component occurred with a relatively low-energy mechanism when the patient kicked the leg onto a chair, causing just enough force and femoral rotation to result in 180° rotation of the component.
Conclusions
We present this case report to make surgeons aware of this rare but serious complication. Although the final implant is a porous and curved stem, careful attention should be made during trialing to use the best-fitting implant to prevent this complication. If an adequate interference fit cannot be obtained, cementing the component may be required to prevent its loosening and catastrophic failure.
1. Sculco PK, Abdel MP, Hanssen AD, Lewallen DG. The management of bone loss in revision total knee arthroplasty: rebuild, reinforce, and augment. Bone Joint J. 2016;98-B(1 suppl A):120-124. doi:10.1302/0301-620X.98B1.36345
2. Harrison RJ Jr, Thacker MM, Pitcher JD, Temple HT, Scully SP. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop Relat Res. 2006;446:113-120. doi:10.1097/01.blo.0000214433.64774.1b
3. Smith EL, Shah A, Son SJ, et al. Survivorship of megaprostheses in revision hip and knee arthroplasty for septic and aseptic indications: a retrospective, multicenter study with minimum 2-year follow-up. Arthroplast Today. 2020;6(3):475-479. Published 2020 Jun 29. doi:10.1016/j.artd.2020.05.004
4. Wyles CC, Tibbo ME, Yuan BJ, Trousdale RT, Berry DJ, Abdel MP. Long-term results of total knee arthroplasty with contemporary distal femoral replacement. J Bone Joint Surg Am. 2020;102(1):45-51. doi:10.2106/JBJS.19.00489
5. Haidukewych GJ. Role of distal femoral replacement for periprosthetic fractures above a total knee arthroplasty: when and how?, J Orthop Trauma. 2019;33(suppl 6):S33-S35. doi:10.1097/BOT.0000000000001566
6. Hart GP, Kneisl JS, Springer BD, Patt JC, Karunakar MA. Open reduction vs distal femoral replacement arthroplasty for comminuted distal femur fractures in the patients 70 years and older: J Arthroplasty. 2017;32(1):202-206. doi:10.1016/j.arth.2016.06.006
7. Vertesich K, Puchner SE, Staats K, et al. Distal femoral reconstruction following failed total knee arthroplasty is accompanied with risk for complication and reduced joint function. BMC Musculoskelet Disord. 2019 Jan 31;20(1):47-54. doi:10.1186/s12891-019-2432-4
8. Biswas D, Haughom B, Mayle RE Jr, Della Valle CJ. Case report: Failure of rotating-hinge total knee prosthesis by disengagement of the hinge-post extension. Clin Orthop Relat Res. 2013;471(4):1389-1392. doi:10.1007/s11999-012-2736-2
9. Ward WG, Haight D, Ritchie P, Gordon S, Eckardt JJ. Dislocation of rotating hinge knee prostheses. A report of four cases. J Bone Joint Surg Am. 2005;87(5):1108-1112. doi:10.2106/JBJS.00837pp
10. Pacha-Vicente D, Malik A, Castellet-Feliu E, Nardi-Vilardaga J. Dislocation of rotating-hinge knee prostheses with antidislocation mechanism. J Arthroplasty. 2008;23(2):299-303. doi:10.1016/j.arth.2006.11.020
11. Manzano G, Schwarzkopf R. Posterior dislocation of the hinge-post extension in a rotating hinge total knee prosthesis. Case Rep Orthop. 2013;2013:756538. doi:10.1155/2013/756538
12. Vaishya R., Thapa, SS, Vaish A. Non-neoplastic indications and outcomes of the proximal and distal femur megaprosthesis: a critical review. Knee Surg Relat Res. 2020;32(1):18. Published 2020 Apr 9. doi:10.1186/s43019-020-00034-7
13. Lu M, Wang J, Xiao C, et al. Uncemented, curved, short endoprosthesis stem for distal femoral reconstruction: early follow-up outcomes. World J Surg Onc. 2018;16(1):183. doi:10.1186/s12957-018-1486-3
Preoperatively periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component is a rare event, and knowledge of this possible complication is important for arthroplasty surgeons.
Preoperatively periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component is a rare event, and knowledge of this possible complication is important for arthroplasty surgeons.
The use of a rotating hinge distal femur replacement (DFR) for significant bone and soft tissue defects in the setting of total knee arthroplasty (TKA) revision has become increasingly more common. Although significant advancements have been made in modern DFR components, complications and failure rates remain high. The unanticipated early failure presented serves as the first reported case in the literature to our knowledge of a 180° rotation of a press-fit DFR.
Originally, DFRs were used primarily for oncology patients with substantial bone loss following large mass excisions. The utility of DFRs has grown to include massive bone loss in the setting of TKA revision, periprosthetic fractures, and periprosthetic joint infections.1-3 DFRs help restore the joint line in the setting of significant bone loss and contain a rotating hinge mechanism that provides functional movement despite the loss of soft tissue constraints around the knee.1-3
DFRs have been associated with early postoperative mobilization and decreased need for ambulatory devices at 1 year in revision TKA and periprosthetic and geriatric distal femur fractures.4-6 Advances in prosthetic design, biomechanics, and fixation technique have led to improved survival rates.3 Despite these improvements, the overall complication rate remains high at 30 to 40%.3-7 Commonly reported complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.3,4,7 Recent case studies also have reported on dislocation or disengagement of the rotating hinge.8-11
In this case report, we present a patient who had a DFR as the second stage of a 2-stage TKA revision due to a periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component. Although this is a rare event, knowledge of this possible complication is important for arthroplasty surgeons.
Case Presentation
A patient with a history of hypertension, osteopenia, and rheumatoid arthritis underwent a primary right TKA in 2007. Ten weeks postoperatively, the patient had a ground-level fall that resulted in a right periprosthetic supracondylar distal femur fracture that was treated with a distal femur locking plate. The patient healed, however, with a significant golf club deformity (Figure 1). The patient did well for more than a decade but in 2019 was admitted with pelvic inflammatory disease and adnexal abscess that was treated with broad-spectrum IV antibiotics. Shortly after this admission, the patient developed a right knee periprosthetic infection with cultures positive for Ureaplasma parvum.
The patient then underwent a 2-stage revision of the infected TKA. Stage 1 consisted of explant of the TKA components as well as removal of the distal femur plate and screws and placement of an articulating antibiotic cement spacer (Figure 2). The patient completed 6 weeks of IV antibiotics. Following completion of the antibiotic course, we obtained a serum erythrocyte sedimentation rate, C-reactive protein level, and white blood cell count, which were all within normal limits. A knee aspiration was performed and did not show signs of residual infection. Frozen histopathology was sent during the second stage of the revision and did not show infection. After the results of the frozen histopathology returned, the antibiotic spacer was removed, and the femoral canal was thoroughly debrided. Cement and fibrous tissue in the femoral canal were carefully removed. In the setting of significant bone loss and soft tissue compromise due to the previous infection and distal femur fracture, the Zimmer Biomet Orthopedic Salvage System (OSS) with porous coated press-fit elliptical femoral stem was utilized.
The femoral canal was reamed until good cortical chatter was obtained at 16 mm. Per the Biomet OSS guide, “For bowed (curved) long and short press-fit stems, the final flexible reamer shaft diameter may need to be larger than the definitive trial and implant diameter.” After trialing, size 15.5 mm was selected for implantation. Intraoperatively the final stem was noted to have good interference fit after insertion and was stable throughout knee range of motion and varus/valgus stress testing. The patient did well with mobilization while in the hospital postoperatively and was discharged home (Figure 3).
Five days after discharge, the patient kicked the repaired knee onto a chair for rest and elevation and experienced extreme pain and was unable to flex the knee. On presentation to the emergency department, the X-rays showed 180° rotation around the longitudinal axis of the femoral component without any other obvious component failure or fracture (Figure 4). The patient was taken back to surgery the following day. Intraoperatively, the femoral stem was found to be loose and rotated 180° (Figure 5). No failure or dislocation of the tibial or rotating hinge components were identified. The press-fit femoral stem was removed and replaced with a cemented stem (Figure 6).
The postoperative course after the revision surgery was uneventful, and the patient is doing well clinically with no pain, functional range of motion of 5 to 105°, and has returned to regular activities without difficulty.
Discussion
Despite advancements in DFRs and increasing use in the setting of revision TKA, the procedure remains high risk with respect to postoperative complications.3-7 Vertesich and colleagues demonstrated that 43.3% of patients who underwent DFR for failed TKA developed at least 1 postoperative complication that required a return to the operating room.7Physicians need to be aware of the high rate of complications and counsel patients appropriately preoperatively.
Complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.4,7 Soft tissue failures include insufficiency or rupture of the extensor mechanism and patella dislocation.4,7 Structural failures include fracture of the hinge mechanism, dissociation of the component from the stem, rotating hinge-bushing failure, and dislocation of the hinge.4,7 In the acute postoperative period, the most common complications are infection and rotating-hinge dislocation/failure.3,12 There are various component options available for DFRs, including straight vs curved, cemented vs cementless/press-fit, and long vs short stems.13 Studies have sought to elucidate the ideal implant to decrease the rate of complications. Lu and colleagues demonstrated that curved press-fit short stems provided a stable interface without loosening over the short term (2 years) in 42 patients.13 No implant failures or incidences of aseptic loosening occurred in their study.13
The implant used in this case was a curved press-fit short-stem DFR. It was thought that this patient was young and with good enough bone quality that a press-fit short stem would be best in preserving bone stock. Both the technique guide and literature support reaming 0 to 2 mm greater than the planned stem size to accommodate the implant curvature.13 In this case, the intramedullary canal was reamed 0.5 mm larger than the curved stem that was implanted (16 mm and 15.5 mm, respectively). Intraoperatively during the index DFR, the component was stable and seemed to have a good press-fit interface. Despite this, obvious loosening of the component occurred with a relatively low-energy mechanism when the patient kicked the leg onto a chair, causing just enough force and femoral rotation to result in 180° rotation of the component.
Conclusions
We present this case report to make surgeons aware of this rare but serious complication. Although the final implant is a porous and curved stem, careful attention should be made during trialing to use the best-fitting implant to prevent this complication. If an adequate interference fit cannot be obtained, cementing the component may be required to prevent its loosening and catastrophic failure.
The use of a rotating hinge distal femur replacement (DFR) for significant bone and soft tissue defects in the setting of total knee arthroplasty (TKA) revision has become increasingly more common. Although significant advancements have been made in modern DFR components, complications and failure rates remain high. The unanticipated early failure presented serves as the first reported case in the literature to our knowledge of a 180° rotation of a press-fit DFR.
Originally, DFRs were used primarily for oncology patients with substantial bone loss following large mass excisions. The utility of DFRs has grown to include massive bone loss in the setting of TKA revision, periprosthetic fractures, and periprosthetic joint infections.1-3 DFRs help restore the joint line in the setting of significant bone loss and contain a rotating hinge mechanism that provides functional movement despite the loss of soft tissue constraints around the knee.1-3
DFRs have been associated with early postoperative mobilization and decreased need for ambulatory devices at 1 year in revision TKA and periprosthetic and geriatric distal femur fractures.4-6 Advances in prosthetic design, biomechanics, and fixation technique have led to improved survival rates.3 Despite these improvements, the overall complication rate remains high at 30 to 40%.3-7 Commonly reported complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.3,4,7 Recent case studies also have reported on dislocation or disengagement of the rotating hinge.8-11
In this case report, we present a patient who had a DFR as the second stage of a 2-stage TKA revision due to a periprosthetic joint infection with a postoperative complication of 180° rotation of the press-fit femoral component. Although this is a rare event, knowledge of this possible complication is important for arthroplasty surgeons.
Case Presentation
A patient with a history of hypertension, osteopenia, and rheumatoid arthritis underwent a primary right TKA in 2007. Ten weeks postoperatively, the patient had a ground-level fall that resulted in a right periprosthetic supracondylar distal femur fracture that was treated with a distal femur locking plate. The patient healed, however, with a significant golf club deformity (Figure 1). The patient did well for more than a decade but in 2019 was admitted with pelvic inflammatory disease and adnexal abscess that was treated with broad-spectrum IV antibiotics. Shortly after this admission, the patient developed a right knee periprosthetic infection with cultures positive for Ureaplasma parvum.
The patient then underwent a 2-stage revision of the infected TKA. Stage 1 consisted of explant of the TKA components as well as removal of the distal femur plate and screws and placement of an articulating antibiotic cement spacer (Figure 2). The patient completed 6 weeks of IV antibiotics. Following completion of the antibiotic course, we obtained a serum erythrocyte sedimentation rate, C-reactive protein level, and white blood cell count, which were all within normal limits. A knee aspiration was performed and did not show signs of residual infection. Frozen histopathology was sent during the second stage of the revision and did not show infection. After the results of the frozen histopathology returned, the antibiotic spacer was removed, and the femoral canal was thoroughly debrided. Cement and fibrous tissue in the femoral canal were carefully removed. In the setting of significant bone loss and soft tissue compromise due to the previous infection and distal femur fracture, the Zimmer Biomet Orthopedic Salvage System (OSS) with porous coated press-fit elliptical femoral stem was utilized.
The femoral canal was reamed until good cortical chatter was obtained at 16 mm. Per the Biomet OSS guide, “For bowed (curved) long and short press-fit stems, the final flexible reamer shaft diameter may need to be larger than the definitive trial and implant diameter.” After trialing, size 15.5 mm was selected for implantation. Intraoperatively the final stem was noted to have good interference fit after insertion and was stable throughout knee range of motion and varus/valgus stress testing. The patient did well with mobilization while in the hospital postoperatively and was discharged home (Figure 3).
Five days after discharge, the patient kicked the repaired knee onto a chair for rest and elevation and experienced extreme pain and was unable to flex the knee. On presentation to the emergency department, the X-rays showed 180° rotation around the longitudinal axis of the femoral component without any other obvious component failure or fracture (Figure 4). The patient was taken back to surgery the following day. Intraoperatively, the femoral stem was found to be loose and rotated 180° (Figure 5). No failure or dislocation of the tibial or rotating hinge components were identified. The press-fit femoral stem was removed and replaced with a cemented stem (Figure 6).
The postoperative course after the revision surgery was uneventful, and the patient is doing well clinically with no pain, functional range of motion of 5 to 105°, and has returned to regular activities without difficulty.
Discussion
Despite advancements in DFRs and increasing use in the setting of revision TKA, the procedure remains high risk with respect to postoperative complications.3-7 Vertesich and colleagues demonstrated that 43.3% of patients who underwent DFR for failed TKA developed at least 1 postoperative complication that required a return to the operating room.7Physicians need to be aware of the high rate of complications and counsel patients appropriately preoperatively.
Complications after DFR include infection, aseptic loosening, soft tissue failure, and structural failure.4,7 Soft tissue failures include insufficiency or rupture of the extensor mechanism and patella dislocation.4,7 Structural failures include fracture of the hinge mechanism, dissociation of the component from the stem, rotating hinge-bushing failure, and dislocation of the hinge.4,7 In the acute postoperative period, the most common complications are infection and rotating-hinge dislocation/failure.3,12 There are various component options available for DFRs, including straight vs curved, cemented vs cementless/press-fit, and long vs short stems.13 Studies have sought to elucidate the ideal implant to decrease the rate of complications. Lu and colleagues demonstrated that curved press-fit short stems provided a stable interface without loosening over the short term (2 years) in 42 patients.13 No implant failures or incidences of aseptic loosening occurred in their study.13
The implant used in this case was a curved press-fit short-stem DFR. It was thought that this patient was young and with good enough bone quality that a press-fit short stem would be best in preserving bone stock. Both the technique guide and literature support reaming 0 to 2 mm greater than the planned stem size to accommodate the implant curvature.13 In this case, the intramedullary canal was reamed 0.5 mm larger than the curved stem that was implanted (16 mm and 15.5 mm, respectively). Intraoperatively during the index DFR, the component was stable and seemed to have a good press-fit interface. Despite this, obvious loosening of the component occurred with a relatively low-energy mechanism when the patient kicked the leg onto a chair, causing just enough force and femoral rotation to result in 180° rotation of the component.
Conclusions
We present this case report to make surgeons aware of this rare but serious complication. Although the final implant is a porous and curved stem, careful attention should be made during trialing to use the best-fitting implant to prevent this complication. If an adequate interference fit cannot be obtained, cementing the component may be required to prevent its loosening and catastrophic failure.
1. Sculco PK, Abdel MP, Hanssen AD, Lewallen DG. The management of bone loss in revision total knee arthroplasty: rebuild, reinforce, and augment. Bone Joint J. 2016;98-B(1 suppl A):120-124. doi:10.1302/0301-620X.98B1.36345
2. Harrison RJ Jr, Thacker MM, Pitcher JD, Temple HT, Scully SP. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop Relat Res. 2006;446:113-120. doi:10.1097/01.blo.0000214433.64774.1b
3. Smith EL, Shah A, Son SJ, et al. Survivorship of megaprostheses in revision hip and knee arthroplasty for septic and aseptic indications: a retrospective, multicenter study with minimum 2-year follow-up. Arthroplast Today. 2020;6(3):475-479. Published 2020 Jun 29. doi:10.1016/j.artd.2020.05.004
4. Wyles CC, Tibbo ME, Yuan BJ, Trousdale RT, Berry DJ, Abdel MP. Long-term results of total knee arthroplasty with contemporary distal femoral replacement. J Bone Joint Surg Am. 2020;102(1):45-51. doi:10.2106/JBJS.19.00489
5. Haidukewych GJ. Role of distal femoral replacement for periprosthetic fractures above a total knee arthroplasty: when and how?, J Orthop Trauma. 2019;33(suppl 6):S33-S35. doi:10.1097/BOT.0000000000001566
6. Hart GP, Kneisl JS, Springer BD, Patt JC, Karunakar MA. Open reduction vs distal femoral replacement arthroplasty for comminuted distal femur fractures in the patients 70 years and older: J Arthroplasty. 2017;32(1):202-206. doi:10.1016/j.arth.2016.06.006
7. Vertesich K, Puchner SE, Staats K, et al. Distal femoral reconstruction following failed total knee arthroplasty is accompanied with risk for complication and reduced joint function. BMC Musculoskelet Disord. 2019 Jan 31;20(1):47-54. doi:10.1186/s12891-019-2432-4
8. Biswas D, Haughom B, Mayle RE Jr, Della Valle CJ. Case report: Failure of rotating-hinge total knee prosthesis by disengagement of the hinge-post extension. Clin Orthop Relat Res. 2013;471(4):1389-1392. doi:10.1007/s11999-012-2736-2
9. Ward WG, Haight D, Ritchie P, Gordon S, Eckardt JJ. Dislocation of rotating hinge knee prostheses. A report of four cases. J Bone Joint Surg Am. 2005;87(5):1108-1112. doi:10.2106/JBJS.00837pp
10. Pacha-Vicente D, Malik A, Castellet-Feliu E, Nardi-Vilardaga J. Dislocation of rotating-hinge knee prostheses with antidislocation mechanism. J Arthroplasty. 2008;23(2):299-303. doi:10.1016/j.arth.2006.11.020
11. Manzano G, Schwarzkopf R. Posterior dislocation of the hinge-post extension in a rotating hinge total knee prosthesis. Case Rep Orthop. 2013;2013:756538. doi:10.1155/2013/756538
12. Vaishya R., Thapa, SS, Vaish A. Non-neoplastic indications and outcomes of the proximal and distal femur megaprosthesis: a critical review. Knee Surg Relat Res. 2020;32(1):18. Published 2020 Apr 9. doi:10.1186/s43019-020-00034-7
13. Lu M, Wang J, Xiao C, et al. Uncemented, curved, short endoprosthesis stem for distal femoral reconstruction: early follow-up outcomes. World J Surg Onc. 2018;16(1):183. doi:10.1186/s12957-018-1486-3
1. Sculco PK, Abdel MP, Hanssen AD, Lewallen DG. The management of bone loss in revision total knee arthroplasty: rebuild, reinforce, and augment. Bone Joint J. 2016;98-B(1 suppl A):120-124. doi:10.1302/0301-620X.98B1.36345
2. Harrison RJ Jr, Thacker MM, Pitcher JD, Temple HT, Scully SP. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop Relat Res. 2006;446:113-120. doi:10.1097/01.blo.0000214433.64774.1b
3. Smith EL, Shah A, Son SJ, et al. Survivorship of megaprostheses in revision hip and knee arthroplasty for septic and aseptic indications: a retrospective, multicenter study with minimum 2-year follow-up. Arthroplast Today. 2020;6(3):475-479. Published 2020 Jun 29. doi:10.1016/j.artd.2020.05.004
4. Wyles CC, Tibbo ME, Yuan BJ, Trousdale RT, Berry DJ, Abdel MP. Long-term results of total knee arthroplasty with contemporary distal femoral replacement. J Bone Joint Surg Am. 2020;102(1):45-51. doi:10.2106/JBJS.19.00489
5. Haidukewych GJ. Role of distal femoral replacement for periprosthetic fractures above a total knee arthroplasty: when and how?, J Orthop Trauma. 2019;33(suppl 6):S33-S35. doi:10.1097/BOT.0000000000001566
6. Hart GP, Kneisl JS, Springer BD, Patt JC, Karunakar MA. Open reduction vs distal femoral replacement arthroplasty for comminuted distal femur fractures in the patients 70 years and older: J Arthroplasty. 2017;32(1):202-206. doi:10.1016/j.arth.2016.06.006
7. Vertesich K, Puchner SE, Staats K, et al. Distal femoral reconstruction following failed total knee arthroplasty is accompanied with risk for complication and reduced joint function. BMC Musculoskelet Disord. 2019 Jan 31;20(1):47-54. doi:10.1186/s12891-019-2432-4
8. Biswas D, Haughom B, Mayle RE Jr, Della Valle CJ. Case report: Failure of rotating-hinge total knee prosthesis by disengagement of the hinge-post extension. Clin Orthop Relat Res. 2013;471(4):1389-1392. doi:10.1007/s11999-012-2736-2
9. Ward WG, Haight D, Ritchie P, Gordon S, Eckardt JJ. Dislocation of rotating hinge knee prostheses. A report of four cases. J Bone Joint Surg Am. 2005;87(5):1108-1112. doi:10.2106/JBJS.00837pp
10. Pacha-Vicente D, Malik A, Castellet-Feliu E, Nardi-Vilardaga J. Dislocation of rotating-hinge knee prostheses with antidislocation mechanism. J Arthroplasty. 2008;23(2):299-303. doi:10.1016/j.arth.2006.11.020
11. Manzano G, Schwarzkopf R. Posterior dislocation of the hinge-post extension in a rotating hinge total knee prosthesis. Case Rep Orthop. 2013;2013:756538. doi:10.1155/2013/756538
12. Vaishya R., Thapa, SS, Vaish A. Non-neoplastic indications and outcomes of the proximal and distal femur megaprosthesis: a critical review. Knee Surg Relat Res. 2020;32(1):18. Published 2020 Apr 9. doi:10.1186/s43019-020-00034-7
13. Lu M, Wang J, Xiao C, et al. Uncemented, curved, short endoprosthesis stem for distal femoral reconstruction: early follow-up outcomes. World J Surg Onc. 2018;16(1):183. doi:10.1186/s12957-018-1486-3
Therapeutic aquatic exercise superior to physical therapy for back pain in study
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
“This is the first study to compare the efficacy of therapeutic aquatic exercise and physical therapy modalities in the treatment of chronic low back pain,” senior coauthors Pei-Jie Chen, PhD and Xue-Qiang Wang, PhD, both of the department of sport rehabilitation, Shanghai (China) University of Sport, wrote in JAMA Network Open. “Therapeutic aquatic exercise is a safe treatment for chronic low back pain and most participants who received it were willing to recommend it to other patients with chronic low back pain.”
As compared with individuals in the physical therapy modalities arm, the therapeutic aquatic exercise experienced greater relief of disability at all time points assessed: after the 3-month intervention, at the 6-month follow-up, and at the 12-month follow-up.
Commenting on the study, Linda Girgis, MD, FAAFP, a family physician in private practice in South River, N.J., agreed that aquatic therapy is a great tool for many chronic low back patients. “It helps them get active for one and do things that may exacerbate their symptoms doing the same exercises on land,” noted Dr. Girgis, who also is a clinical assistant professor at Robert Wood Johnson Medical School, New Brunswick.
She pointed out that access to a pool can be a problem. “But I have found a few physical therapy places in my area that do have access to a pool, and I refer appropriate patients there,” added Dr. Girgis, who was not involved with the study. “I have also found it works well for other types of pain, such as knee and hip pain. It is not for everyone but I have seen some patients get great benefit from it when they didn’t get any with traditional physical therapy.”
Aquatic therapy was more beneficial
Low back pain is a common condition, and clinical practice guidelines currently recommend therapeutic exercise and physical therapy modalities. Among the modalities that are available, therapeutic aquatic exercise is often prescribed for chronic low back pain, and it is becoming increasingly popular for treatment of chronic low back pain, the authors stated in their paper. The authors noted that water is an ideal environment for conducting an exercise program given its various properties, including buoyancy pressure, density, thermal capacity, and conductivity.
Two previously published systematic reviews have suggested that therapeutic aquatic exercise may be able to reduce the intensity of back pain and improve function in this population. But to date, evidence regarding long-term benefits in patients with chronic low back pain is very limited and there haven’t been any studies comparing the efficacy of therapeutic aquatic exercise and physical therapy modalities for chronic low back pain, according to the authors.
In this study, 113 individuals with chronic low back pain were randomized to either therapeutic aquatic exercise or to physical therapy, with an endpoint of efficacy regarding disability. This was measured using the Roland-Morris Disability Questionnaire.
Scores ranged from 0 to 24, with higher scores indicating more severe disability. Secondary endpoints included pain intensity, quality of life, sleep quality, and recommendation of intervention, and these were rated using various standardized tools.
Those randomized to the therapeutic aquatic exercise group had about an hour of therapy, beginning with a 10-minute active warm-up session to enhance neuromuscular activation, then an exercise session for 40 minutes followed by a 10-minute cooldown.
The physical therapy group received transcutaneous electrical nerve stimulation and infrared ray thermal therapy, also for 60 minutes. Both groups received these interventions twice a week for 3 months.
The overall mean age of the cohort was 31.0 years, and they were almost evenly divided by gender; 54 were men (47.8%), and 59 were women (52.2%).
As compared with the physical therapy group, individuals participating in therapeutic aquatic exercise group showed improvement in disability by an additional −1.77 points (95% confidence interval, −3.02 to −0.51) at the end of the 3-month intervention; at 6 months it was −2.42 points (95% CI, −4.13 to −0.70) and −3.61 points (95% CI, −5.63 to −1.58) at the 12-month follow-up (P < .001 for overall group x time interaction).
Functional improvement did not appear to be significantly affected by confounders that included age, sex, body mass index, low back pain duration, educational level, or pain level.
For secondary outcomes, those in the therapeutic aquatic exercise group demonstrated improvement in the most severe pain by an additional −0.79 points (95% CI, −1.31 to −0.27) after the 3-month intervention, −1.34 points (95% CI, −2.06 to −0.62) at 6 months, and −2.04 points (95% CI, −2.75 to −1.34) at the 12-month follow-up (P < .001 for overall group x time interaction), as compared with the physical therapy group. All pain scores differed significantly between the two groups at every time point.
In addition, individuals in the therapeutic aquatic exercise group showed more improvements on the 36-item Short-form Health Survey (overall group x time interaction, P = .003), Pittsburgh Sleep Quality Index (overall group x time interaction, P = .02), Tampa Scale for Kinesiophobia (overall group x time interaction, P < .001), and Fear-Avoidance Beliefs Questionnaire (physical activity subscale overall group x time interaction, P = .04), as compared with the physical therapy group. These improvements were also not influenced by confounders.
Finally, at the 12-month follow-up point, those in the aquatic therapy group had significantly greater improvements in the number of participants who met the minimal clinically important difference in pain (at least a 2-point improvement on the numeric rating scale).
More outside experts’ takes
“The current research evidence does suggest indeed that aquatic exercise therapy is suitable and often better than land exercise, passive relaxation, or other treatments for many people with low back pain,” commented Stelios Psycharakis PhD, senior lecturer in biomechanics, Institute for Sport, Physical Education and Health Sciences, University of Edinburgh.
He also noted that since low back pain is an issue affecting about 80% of all people at some stage of their life, it is “improbable that one could identify a single type of treatment or exercise therapy that would be suitable for every person with this problem.”
Dr. Psycharakis pointed out that there are also some contraindications for aquatic therapy, such as incontinence and skin conditions. “Other than that though, clinicians should definitely consider aquatic exercise therapy when advising people with chronic low back pain,” he said.
Justin M. Lantz, DPT, agreed that the study showed therapeutic aquatic exercise appears to be safe and beneficial in some patients with chronic low back pain, but he also shared limitations of the new research.
“The study has notable limitations as it did not include patients above 65 years old, pain levels were generally low for the subjects involved, and it did not include a treatment group with land therapeutic exercise – so it is difficult to determine if the beneficial effects reported were due to active exercise or because the exercises were performed in water,” said Dr. Lantz, director of the spine physical therapy fellowship program at the University of Southern California, Los Angeles, and an assistant professor of clinical physical therapy.
He also pointed out that, since active exercise has been shown to be beneficial and is advocated in multiple clinical practice guidelines for chronic low back pain, “it would be helpful to determine if the true effects on pain and disability were due to the water environment or the effect of active exercise itself.”
“Due to the significant positive long-term effects and limited adverse events reported, I believe this study supports the use of therapeutic aquatic exercise in select patient populations with chronic low back pain and should be considered as a part of a rehabilitation treatment plan if accessibility is feasible,” Dr. Lantz said.
The authors of the paper, Dr. Girgis, and Dr. Psycharakis had no conflicts of interest. Justin Lantz is a physical therapy consultant to SI-Bone.
FROM JAMA NETWORK OPEN
Multimodal Pain Management With Adductor Canal Block Decreases Opioid Consumption Following Total Knee Arthroplasty
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
Ease of access to opioids in the perioperative period is a risk factor for opioid misuse and has been identified as a strong risk factor for heroin use.1,2 Three-quarters of today’s heroin users were introduced to opioids through prescription medications.2 The United States accounts for about 80% of the global opioid supply consumption, and deaths from opioid overdose are increasing: 70,630 deaths in 2019 alone.3,4
The Centers for Disease Control and Prevention (CDC) has called for changes in opioid prescribing. The American Academy of Orthopaedic Surgeons (AAOS) also has published an information statement with strategies to decrease opioid misuse and abuse.5,6 Arthroplasty surgeons have recently focused on decreasing use of opioids in total knee arthroplasty (TKA), a procedure traditionally associated with high levels of opioid consumption and historical reliance on opioid monotherapy for postoperative analgesia.7,8 From a clinical perspective, prolonged postoperative opioid use contributes to poorer surgical outcomes due to increased risk of complications, including stiffness, infection, and revision TKA.9
Multimodal pain regimens are increasingly being used to control postoperative pain as data supports their efficacy.10,11 Previous studies have found that simultaneous modulation of multiple pain pathways decreases narcotics consumption and improves patient outcomes.12,13 Along with other adjuvant therapies, peripheral nerve blocks, such as adductor canal block (ACB) and femoral nerve block (FNB), have been used to decrease postoperative pain.14 Studies have shown that ACB has fewer complications and shorter functional recovery times compared with FNB.15,16 The distribution of the ACB excludes the femoral nerve, thus preserving greater quadriceps strength while providing equivalent levels of analgesia compared with FNB.15,17,18 The ACB has shown decreased near-fall events and improved balance scores in the immediate postoperative period.19
Our study analyzed opioid consumption patterns of TKA patients from a US Department of Veterans Affairs (VA) medical center before and after the institution of a multimodal analgesic protocol using ACB. The primary purpose of this study was to determine whether a protocol that included intraoperative spinal anesthesia with a postoperative multimodal analgesic regimen and ACB was associated with a decreased postoperative opioid requirement when compared with patients who received intraoperative general anesthesia and a traditional opioid regimen. Secondary outcomes included the effect of opioid consumption on range of motion on postoperative day (POD) 1 and number of opioid prescriptions written at the first postoperative clinic visit.
Methods
Approval for the study was obtained from the institutional review board at the Dayton Veterans Affairs Medical Center (DVAMC) in Ohio. A retrospective chart review was performed to collect data from all patients undergoing TKA at DVAMC from June 1, 2011, through December 31, 2015. Exclusion criteria included multiple surgeries in the study time frame, documented chronic pain, allergy to local anesthetics, daily preoperative use of opioids, and incomplete data in the health record.
All surgeries were performed by 2 staff arthroplasty surgeons at a single VAMC. All patients attended a preoperative visit where a history, physical, and anesthesia evaluation were performed, and watched an educational video detailing surgical indications and postoperative rehabilitation. All surgeries were performed with tourniquets and a periarticular injection was performed at the conclusion of each case. Surgeon 1 treatment of choice was 10 mL 0.5% bupivacaine, whereas surgeon 2 performed a posterior capsular injection of 30 mL 0.25% bupivacaine and a periarticular injection of 30 mg ketorolac in 10 mL 0.25% bupivacaine with epinephrine.
Prior to August 2014, general endotracheal anesthesia was used intraoperatively. A patient-controlled analgesia (PCA) pump of morphine or hydromorphone and additional oral oxycodone or hydrocodone was used for postoperative pain. PCA pumps were patient dependent. In the control group, 245 patients received the morphine PCA while 61 received the hydromorphone PCA. Morphine PCA dosing consisted of 1-mg doses every 10 minutes with potential baseline infusion rates of 0.5 to 1.0 mg/h and a 4-hour limit of 20 mg. Hydromorphone PCA dosing consisted of 0.2 to 0.4-mg doses with a potential continuous dose of 0.2 to 0.4 mg/h and a 4-hour limit of 4 mg.
In August 2014, a new analgesic protocol was adopted for TKA consisting of intraoperative spinal anesthesia (0.75% bupivacaine) with IV sedation (propofol), a postoperative multimodal analgesic regimen, an ACB performed in the postanesthesia care unit (PACU), and opioids as needed (protocol group). The ACB catheter was a 0.5% ropivo caine hydrochloride injection. It was attached to a local anesthetic fixed flow rate pump that administers 0.5% ropivacaine without epinephrine at 8 mL/h and was removed on POD 5 by the patient. The multimodal medication regimen included IV ketorolac 15 mg every 6 hours for 3 doses, gabapentin 300 mg every 8 hours, acetaminophen 975 mg every 8 hours, meloxicam 7.5 mg daily, tramadol 50 mg every 6 hours, oxycodone 5 mg 1 to 2 tabs every 4 hours as needed, and IV hydromorphone 0.5 mg every 4 hours as needed for breakthrough pain.
Preoperative demographic characteristics were collected (Table 1). Data on all IV and oral opioid requirements were collected for both groups, converted to morphine milligram equivalents (MME), and a total morphine equivalent dose (MED) was calculated.20,21
In April 2015, a separate protocol change occurred at the DVAMC with the goal of discharge on POD 1. To standardize outcomes before and after this change, data collection regarding opioid requirements was concluded at midnight on POD 1. If a patient was discharged before midnight on POD 1, opioid requirement through the time of discharge was collected. All surgeries were performed in the morning to early afternoon; however, specific surgical times were not collected. Patients were also evaluated by a physical therapist on POD 0, and maximal knee flexion and extension were measured on POD 1. Patients were discharged with prescriptions for oxycodone/acetaminophen and tramadol and were seen 3 weeks later for their first postoperative visit. Opioid refills at the first postoperative visit were recorded. All statistical analyses were performed in SAS 9.4 with significance set to α = 0.05. Between-groups differences in preoperative and perioperative characteristics as well as postoperative outcomes were analyzed using independent samples t tests for continuous variables and Fisher exact tests for dichotomous discrete variables. Where groups differed for a pre- or perioperative variable, linear mixed models analysis was used to determine whether IV, oral, and total MEDs were significantly affected by the interaction between the pre- or perioperative variable with analgesia group. For refills at the postoperative visit, the effects of pre- or perioperative differences were tested using χ2 tests. Effect sizes for outcome variables were estimated using Cohen d and probability of superiority (Δ) for continuous variables, and relative risk (RR) in the case of discrete variables.22
Results
During the study period from June 1, 2011, through December 31, 2015, 533 eligible TKAs were performed, 306 in the control group and 227 in the protocol group. The groups had similar sex distribution; body mass index; knee range of motion; diagnoses of diabetes mellitus, coronary artery disease, and chronic kidney disease; and history of deep vein thrombosis (DVT) or pulmonary embolism (P ≥ .05). The protocol group was significantly older (P = .04) and had a significantly higher rate of chronic obstructive pulmonary disease (COPD) (P = .002). There were no significant differences between number of procedures performed by surgeon (P = .48) or total tourniquet time (P = .13) (Table 2). Mean (SD) length of stay was significantly greater in the control group compared with the protocol group (2.5 [1.3] vs 1.4 [0.7] days, P < .001).
Figure 1 shows the distributions of each type of opioid used. Compared with the control group, the protocol group had a significantly lower mean (SD) IV opioid use: 178.2 (98.0) MED vs 12.0 (24.6) MED (P < .001; d = 2.19; Δ = 0.94) and mean (SD) total opioid use: 241.7 (120.1) MED vs 74.8 (42.7) MED (P < .001; d = 1.76; Δ = 0.89). Mean (SD) oral opioid use did not differ between groups (control, 63.6 [45.4] MED; protocol, 62.9 [31.4] MED; P = .85; d = 0.02; Δ = 0.51). A significantly lower percentage of patients in the protocol group received additional opioids at the 3-week follow-up when compared to the control group: 46.7% vs 61.3%, respectively (P < .001; RR, 0.76; 95% CI, 0.65-0.90).
There were no significant differences in postoperative mean (SD) maximum knee flexion (control, 67.2 [15.7]°; protocol, 67.8 [19.2]°; P = .72; d = 0.03; Δ = 0.51) or mean (SD) total flexion/extension arc (control, 66.2 [15.9]°; protocol, 67.9 [19.4]°; P = .32; d = 0.10; Δ = 0.53). Mean (SD) postoperative maximum knee extension was significantly higher in the protocol group compared with the control group (-0.1 [2.1]° vs 1.0 [3.7]°; P < .001; d = 0.35; Δ = 0.60). More patients in the protocol group (92.5%) were discharged to home compared with the control group (86.6%) (P = .02; RR, 1.07; 95% CI, 1.01-1.13).
Because age and rates of COPD differed between groups, sensitivity analyses were conducted to determine whether these variables influenced postoperative opioid use. The relationship between age and group was significant for IV (P < .001) and total opioid use (P < .001). Younger patients received higher MED doses than older patients within the control group, while dosages were fairly consistent regardless of age in the protocol group (Figure 2). There was no significance in age interaction effect with regard to oral opioids (P = .83) nor opioid refills at 3-week follow-up (P = .24).
The sensitivity analysis for COPD found that a diagnosis of COPD did not significantly influence utilization of IV opioids (P = .10), or total opioids (P = .68). There was a significant interaction effect for oral opioids (Figure 3). Patients in the control group with COPD required significantly higher mean (SD) oral opioids than patients without COPD (91.5 [123.9] MED and 62.0 [36.0] MED, respectively; P = .03). In the control group, the χ2 test was significant regarding opioid prescription refills at the 3-week visit (P = .004) with 62.4% of patients with COPD requiring refills vs 44.4% without COPD (P = .004). There was no difference in refills in the protocol group (46.4% vs 48.4%).
Finally, 2-sided independent samples t test evaluated total MED use between the 2 surgeons. There was no difference in total MED per patient for the surgeons. In the control group, mean (SD) total MED for surgeon 1 was 232.9 (118.7) MED vs 252.8 (121.5) MED for surgeon 2 (P = .18). In the protocol group, the mean (SD) total MED was 72.5 (43.2) and 77.4 (42.1) for surgeon 1 and surgeon 2, respectively (P = .39).
Discussion
Coordinated efforts with major medical organizations are being made to decrease opioid prescriptions and exposure.5,6 To our knowledge, no study has quantified a decrease in opioid requirement in a VA population after implementation of a protocol that includes intraoperative spinal anesthesia and a postoperative multimodal analgesic regimen including ACB after TKA. The analgesic protocol described in this study aligns with recommendations from both the CDC and the AAOS to decrease opioid use and misuse by maximizing nonopioid medications and limiting the size and number of opioid prescriptions. However, public and medical opinion of opioids as well as prescribing practices have changed over time with a trend toward lower opioid use. The interventions, as part of the described protocol, are a result of these changes and attempt to minimize opioid use while maximizing postoperative analgesia.
Our data showed a significant decrease in total opioid use through POD 1, IV opioid use, and opioid prescriptions provided at the first postoperative visit. The protocol group used only 6.7% of the IV opioids and 30.9% of the total opioids that were used by the control group. The substantial difference in IV opioid requirement, 166.2 MED, is equivalent to 8 mg of IV hydromorphone or 55 mg of IV morphine. The difference in total opioid requirement was similar at 166.9 MED, equivalent to 111 mg of oral oxycodone.
Decreasing opioid use has the additional benefit of improving outcomes, as higher doses of opioids have been associated with increased length of stay, greater rates of DVT, and postoperative infection.23 These complications occurred in a stepwise manner, suggesting a dose-response gradient that makes the sizable decrease noted in our data of greater relevance.23 While the adverse effects (AEs) of opioids are well known, there are limited data on opioid dosing and its effect on perioperative outcomes.23
A significant decrease in the percentage of patients receiving an opioid prescription at the first postoperative visit suggests a decrease in the number of patients on prolonged opioids after TKA with implementation of modern analgesic modalities. The duration of postoperative opioid use has been found to be the strongest predictor of misuse, and each postoperative refill increases the probability of misuse by 44%.24 In addition, opioid use for > 3 months after TKA is associated with increased risk of periprosthetic infection, increased overall revision rate, and stiffness at 1 year postoperatively.9 While not entirely under the control of the surgeon, measures to decrease the number of postoperative opioid refills may lead to a decrease in opioid misuse.
In the control group, older patients tended to receive less opioids. This is likely due to physiologic changes in opioid metabolism associated with aging, including decreased renal and hepatic opioid metabolism and alterations in overall body composition that increase relative potency and duration of action of opioids in a geriatric population.25,26 No difference in opioid use by age was found for the protocol group.
Patients in the protocol group demonstrated significantly greater maximal knee extension on POD 1 compared with the control group. No difference in maximal flexion was found. This difference in extension may partially be explained by the use of an ACB. One benefit of ACB is greater quadriceps strength and fewer near-fall events when compared with FNB.15,19
Our results corroborate the findings of similar studies. A randomized controlled trial comparing a multimodal analgesic regimen with a periarticular injection without a postoperative ACB to a hydromorphone PCA revealed a significant decrease in opioid use in the multimodal analgesic group.27 Along with lower opioid requirements, the multimodal analgesic group had lower visual analog scale pain scores, fewer AEs, faster progression to physical therapy milestones, and higher satisfaction.27 Recent guidelines from the French Society of Anaesthesia and Intensive Care Medicine recommend against the use of gabapentin as a method of postoperative pain control. However, this specifically refers to the preoperative administration of gabapentin. This same set of guidelines later cites a high level of evidence suggesting patients undergoing arthroplasty benefit more from gabapentinoids.28 Multiple analgesic protocols that include gabapentin as a part of a multimodal approach have been shown to have positive results.13,29
In our study, patients receiving the multimodal analgesic regimen were significantly more likely to be discharged home rather than to postacute care facilities, which have been associated with increased rates of major complications, 30-day readmission, and 30-day reoperation.30,31 In addition, discharge to an inpatient rehabilitation or skilled nursing facility has not been found to result in higher functional outcomes, despite $3.2 billion spent yearly on rehabilitation services after primary TKA.32,33
A component of our described analgesic protocol included spinal anesthesia intraoperatively. The differences between groups regarding anesthesia type can be attributed to this protocol change. A significantly greater percentage of patients in the protocol group received spinal anesthesia, while more patients in the control group received general anesthesia. While patients who received spinal anesthesia may have enhanced analgesia in the immediate postoperative period, no differences in opioid outcomes were seen based on anesthesia type. Known benefits of intraoperative spinal anesthesia include decreased perioperative blood loss and a smaller decrease in hemoglobin postoperatively, as well as lower rates of in-hospital complications, including pulmonary embolism, pneumonia, cerebrovascular events, and acute renal failure.34
Limitations
A number of limitations of this study should be noted. One was a protocol change regarding length of stay, which occurred during the study period and resulted in a significantly shorter length of stay in the protocol group. As a result, opioid use data were analyzed only through midnight at the end of POD 1. Patients who were discharged on POD 1 did not have opioid use data available for the full duration of the first POD, which may exaggerate the decrease in opioid requirements, as opioids used after discharge but prior to midnight on POD 1 were not recorded. However, opioids taken at home are oral with a low MME compared with IV opioids received by hospitalized patients in the control group. In addition, if taken as prescribed, patients at home would only have enough time to take a few doses of opioids prior to the midnight cutoff. We do not believe this difference in time of opioid use meaningfully affected the data. An additional limitation includes the variability between periarticular injections between surgeons. While the percentage of patients that received injections from surgeon 1 vs surgeon 2 were similar, it cannot be ruled out as a potential confounding factor. Other limitations include a lack of pain scores to compare subjective pain ratings, the retrospective nature of the study, and a largely homogenous male VA population.
Conclusions
Ease of access to opioids is a risk factor for opioid abuse, which itself is a risk factor for subsequent heroin use.1,2 The CDC and AAOS have thus published recommendations regarding opioid prescribing practices to decrease opioid use and abuse.5,6 Our described protocol, which aligns with these recommendations, resulted in a significant decrease in IV opioid requirement, total opioid requirement, and lower rates of opioid prescriptions provided at the first postoperative visit. These promising findings demonstrate a lower percentage of patients on long-term opioids after TKA and a significantly decreased cumulative opioid exposure.
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003
1. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44. doi:10.1016/j.drugpo.2011.05.014
2. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100. doi:10.1016/j.drugalcdep.2013.01.007
3. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11(suppl 2):S63-S88.
4. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants - United States, 2015-2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349-358. Published 2018 Mar 30. doi:10.15585/mmwr.mm6712a1
5. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
6. American Academy of Orthopaedic Surgeons. Information statement: opioid use, misuse, and abuse in orthopaedic practice. Published October 2015. Accessed November 12, 2021. https://aaos.org/globalassets/about /bylaws-library/information-statements/1045-opioid-use -misuse-and-abuse-in-practice.pdf
7. Hernandez NM, Parry JA, Taunton MJ. Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty. 2017;32(8):2395-2398. doi:10.1016/j.arth.2017.02.060
8. Gerner P, Poeran J, Cozowicz C, Mörwald EE, Zubizarreta N, Mazumdar M, Memtsoudis SG, Multimodal pain management in total hip and knee arthroplasty: trends over the last 10 years. Abstract presented at: American Society of Anesthesiologists Annual Meeting; October 21, 2017; Boston, MA.
9. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
10. Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60-73. doi:10.5435/JAAOS-D-14-00259
11. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.doi:10.1001/jamasurg.2017.0898
12. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthoplasty. 2014;29(2):329-334. doi:10.1016/j.arth.2013.06.005
13. Golladay GJ, Balch KR, Dalury DF, Satpathy J, Jiranek WA. Oral multimodal analgesia for total joint arthroplasty. J Arthroplasty. 2017;32(9S):S69-S73. doi:10.1016/j.arth.2017.05.002
14. Ardon AE, Clendenen SR, Porter SB, Robards CB, Greengrass RA. Opioid consumption in total knee arthroplasty patients: a retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J Clin Anesth. 2016;31:19-26. doi:10.1016/j.jclinane.2015.12.014
15. Li D, Ma GG. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(8):2614-2619. doi:10.1007/s00167-015-3874-3
16. Li D, Yang Z, Xie X, Zhao J, Kang P. Adductor canal block provides better performance after total knee arthroplasty compared with femoral nerve block: a systematic review and meta-analysis. Int Orthop. 2016;40(5):925-933. doi:10.1007/s00264-015-2998-x
17. Horner G, Dellon AL. Innervation of the human knee joint and implications for surgery. Clin Orthop Relat Res. 1994;(301):221-226.
18. Kim DH, Lin Y, Goytizolo EA, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a prospective, randomized, controlled trial. Anesthesiology. 2014;120(3):540-550. doi:10.1097/ALN.0000000000000119
19. Thacher RR, Hickernell TR, Grosso MJ, et al. Decreased risk of knee buckling with adductor canal block versus femoral nerve block in total knee arthroplasty: a retrospective cohort study. Arthroplasty Today. 2017;3(4):281-285. Published 2017 Apr 15. doi:10.1016/j.artd.2017.02.008
20. Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain [published correction appears in Clin J Pain. 2014 Sep;30(9):830. Korff, Michael Von [corrected to Von Korff, Michael]]. Clin J Pain. 2008;24(6):521-527. doi:10.1097/AJP.0b013e318169d03b
21. Kishner S. Opioid equivalents and conversions: overview. Published January 29, 2018. Accessed November 12, 2021. https://emedicine.medscape.com/article/2138678 -overview#a1
22. Ruscio J, Mullen T. Confidence intervals for the probability of superiority effect size measure and the area under a receiver operating characteristic curve. Multivariate Behav Res. 2012;47(2):201-223. doi:10.1080/00273171.2012.658329
23. Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic orthopedic surgery. Pain. 2017;158(12):2422-2430. doi:10.1097/j.pain.0000000000001047
24. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. Published 2018 Jan 17. doi:10.1136/bmj.j5790
25. Tegeder I, Lötsch J, Geisslinger G. Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet. 1999;37(1):17- 40. doi:10.2165/00003088-199937010-00002
26. Linnebur SA, O’Connell MB, Wessell AM, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396-1430. doi:10.1592/phco.2005.25.10.1396
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329- 334. doi:10.1016/j.arth.2013.06.005
28. Aubrun F, Nouette-Gaulain K, Fletcher D, et al. Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi:10.1016/j.accpm.2019.02.011
29. Han C, Li XD, Jiang HQ, Ma JX, Ma XL. The use of gabapentin in the management of postoperative pain after total knee arthroplasty: A PRISMA-compliant metaanalysis of randomized controlled trials [published correction appears in Medicine (Baltimore). 2016 Jul 18;95(28):e0916]. Medicine (Baltimore). 2016;95(23):e3883. doi:10.1097/MD.0000000000003883
30. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day postdischarge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32(9S):S113-S118. doi:10.1016/j.arth.2017.01.039
31. Pelt CE, Gililland JM, Erickson JA, Trimble DE, Anderson MB, Peters CL. Improving value in total joint arthroplasty: a comprehensive patient education and management program decreases discharge to post-acute care facilities and post-operative complications. J Arthroplasty. 2018;33(1):14-18. doi:10.1016/j.arth.2017.08.003
32. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33(6):1663-1667. doi:10.1016/j.arth.2017.12.033
33. Lavernia CJ, D’Apuzzo MR, Hernandez VH, Lee DJ, Rossi MD. Postdischarge costs in arthroplasty surgery. J Arthroplasty. 2006;21(6 Suppl 2):144-150. doi:10.1016/j.arth.2006.05.003