User login
Gabapentinoids associated with suicidal behavior, overdose
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
The findings by Molero et al. advance clinical knowledge about the drug class of gabapentinoids, wrote Derek K. Tracy, MB BCh. Though the study does not establish causality, it does rely on a solid, large dataset. The study shows the importance of uncoupling pregabalin and gabapentin. Both drugs are indeed gabapentinoids, but their use can lead to different outcomes, depending on the age of patients. For example, pregabalin – not gabapentin – appears tied to higher risks of harm. The demographic group that is most vulnerable is patients aged 15-24, the researchers found. Factors driving those age-related differences in risks tied to the drugs need to be understood.
Dr. Tracy is a consultant psychiatrist at Queen Mary’s Hospital in London. He is a trustee of the charity Mentor and has received honoraria from Janssen for delivering educational talks on novel psychoactive substances. His comments were adapted from an editorial (BMJ. 2019 Jun 12. doi: 10.1136/bmj.14021 ).
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
Young patients might be at increased risk of suicidal behavior, unintentional overdose, injuries, and traffic incidents during treatment periods with gabapentinoids, compared with periods without treatment with those medications, a cohort study of almost 200,000 people shows. Pregabalin is associated with higher hazards of those outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years, the researchers reported.
“If our findings are triangulated with other forms of evidence, ,” wrote Yasmina Molero, PhD, and associates. “Further restrictions for off-label prescription may need consideration.” The study was published in BMJ.
The use of gabapentinoids has risen in the United States (JAMA Intern Med. 2018;178[2]:292-4), and overdose deaths tied to gabapentin have led some states to explore reclassification of the drug as a controlled substance (Risk Manag Healthc Policy. 2018;11:109-16). In the United Kingdom, gabapentinoids are being reclassified as a class C controlled drug because of concerns about the risk of addiction, overdose, and safety, wrote Dr. Molero of the department of psychiatry at Warneford Hospital at the University of Oxford, England, and associates.
To study associations between gabapentinoids and adverse outcomes related to coordination, mental health, and criminality, Dr. Molero and her associates analyzed data from 191,973 people from the Swedish Prescribed Drug Register who collected prescriptions for pregabalin or gabapentin between 2006 and 2013. The researchers included patients aged 15 years and older in their analyses.
They examined suicidal behavior, unintentional overdoses, head or body injuries, road traffic incidents and offenses, and arrests for violent crime using the Swedish Patient Register and the National Crime Register. In addition, they defined suicidal behavior as emergency hospital visits attributable to self-injurious behavior or suicide attempt, or death by suicide. Unintentional overdoses were defined as emergency hospital visits or death attributable to poisoning by illicit drugs, medications, or biologic substances; accidental poisoning by noxious substances; or acute intoxications and overdoses by alcohol and illicit drugs, excluding intentional self-poisoning, wrote Dr. Molero, who is affiliated with the Karolinska Institute in Stockholm, and her associates.
Of the nearly 192,000 participants who collected prescriptions of gabapentinoids on at least two consecutive occasions, 120,664 received pregabalin, and 85,360 received gabapentin; 14,051 of the participants received both drugs. Fifty-nine percent were women, and most patients were aged 45 or older.
During the study period, 10,026 participants (5.2%) were treated for suicidal behavior or died from suicide, 17,144 participants (8.9%) experienced an unintentional overdose, and 12,070 participants (6.3%) had a road traffic incident or offense. In addition, 70,522 participants (36.7%) had head or body injuries, and 7,984 participants (4.1%) were arrested for a violent crime.
The study used a within-individual design that compared when a person was taking a gabapentinoid with when he or she was not. During treatment periods, participants were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13). Associations with arrests for violent crime were not significant.
Gabapentinoid treatment was associated with increased hazards of suicidal behavior in people young than 55 years, but hazards were reduced or not significant in participants aged 55 years and older. Participants aged 15-24 years had the highest hazards of suicidal behavior (1.67).
In a sensitivity analysis, the researchers examined participants who had a diagnosis of comorbid epilepsy, psychiatric disorders, or musculoskeletal disorders before the start of gabapentinoid treatment. Among patients with comorbid epilepsy, gabapentinoids were not significantly associated with suicidal behavior and were associated with reduced hazards for all other outcomes.
“In comorbid psychiatric disorders, gabapentinoids were associated with lower risk for all outcomes,” the researchers said. Among patients with comorbid musculoskeletal disorders, gabapentinoids were associated with reductions in head or body injuries, traffic incidents, and arrests for violent crime.
Dr. Molero and her associates noted that they lacked information about alcohol and drug use, as well as treatment adherence and the conditions for which gabapentinoids had been prescribed. Furthermore, differences in prescription practices and outcome rates might affect the generalizability of the results to other countries.
The different results for pregabalin and gabapentin “could be due to their different pharmacodynamic and pharmacokinetic profiles; pregabalin has a higher potency, greater bioavailability, and quicker absorption than gabapentin. Pregabalin also has been associated with withdrawal symptoms following rapid discontinuation, which could be related to suicidal behavior,” Dr. Molero and colleagues said. “The reduced hazards in older people could reflect pharmacodynamic differences related to age, less concurrent use of alcohol or drugs, different indications for treatment, or reduced symptom severity of underlying conditions.”
The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no disclosures relevant to the study. One author reported grants from Shire and Evolan and has served as a speaker for Shire.
SOURCE: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
FROM BMJ
Key clinical point: Patients might be at increased risk of suicidal behavior, unintentional overdose, head and body injuries, and traffic incidents during periods of treatment with gabapentinoids. Pregabalin is associated with higher hazards of these outcomes than is gabapentin, and the associations are strongest in patients aged 15-24 years.
Major finding: During treatment periods, patients were at increased risk of suicidal behavior or death from suicide (age-adjusted hazard ratio, 1.26), unintentional overdose (1.24), head or body injuries (1.22), and road traffic incidents or offenses (1.13).
Study details: An analysis of data from 191,973 people from the Swedish Prescribed Drug Register, which collected prescriptions for pregabalin or gabapentin between 2006 and 2013.
Disclosures: The Wellcome Trust, Swedish Research Council, and Karolinska Institute supported the study. The authors had no relevant disclosures. One author reported grants from Shire and Evolan, and has served as a speaker for Shire.
Source: Molero Y et al. BMJ. 2019 Jun 12. doi: 10.1136/bmj.l2147.
Psychiatry residents not getting training in treating chronic pain
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
SAN FRANCISCO –
Given the unique role of psychiatrists in helping chronic pain patients with coping strategies and managing comorbid psychiatric illness, this void is concerning, said Ali Ahsan Ali, MD, a resident psychiatrist at the Micah School of Medicine at Mount Sinai/Elmhurst Hospital Center in New York, in an interview at the annual meeting of the American Psychiatric Association.
In a video interview, Dr. Ali spoke with Ahmar M. Butt, MD, about how and why Dr. Ali and his colleagues conducted the survey of all 221 U.S. psychiatry residency programs in January 2019. They also discuss the implications of these trends for patients, particularly in light of the country’s opioid crisis.
Dr. Ali had no disclosures. Dr. Butt is board certified in general psychiatry, child and adolescent psychiatry, and preventive medicine, with a subspecialty in addiction medicine. Dr. Butt is interim program director of the psychiatry residency program at Broadlawns UnityPointe Health, Des Moines, Iowa. He had no disclosures.
REPORTING FROM APA 2019
Varicella vaccine delivers doubled benefit to children
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
The finding of a 78% lower incidence of zoster in varicella-vaccinated children is nothing short of “remarkable,” Anne A Gershon, MD, wrote in an accompanying editorial.
But the benefit could be in jeopardy, as parents question the safety and effectiveness of all vaccines, she wrote.
“That the varicella vaccine prevents not only varicella but zoster as well is an exciting dual benefit from the varicella vaccine, further improving the health of children by immunization,” Dr. Gershon said. “Additional studies will be necessary to show the mechanism for the protection against zoster (viral, immunologic, or both), how long this benefit lasts, and whether additional doses of some form of VZV [varicella-zoster virus] vaccine will be more useful.”
But, she suggested, in a time when cases of clinical varicella are dwindling, so is public awareness of the vaccine’s benefit. Clinical varicella is worse for adults than it is for children.
“Efforts to immunize all children against chickenpox must continue to be made to protect our population from wild-type VZV. Fortunately, antiviral therapy is also available for individuals who are unvaccinated and develop varicella or zoster, but immunization is, as usual, preferable,” Dr. Gershon concluded.
Dr. Gershon, a pediatric infectious disease specialist, is a professor of pediatrics at Columbia University, New York. She wrote a commentary to accompany the article by Weinmann et al. (Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-3561). Dr. Gershon had no relevant financial disclosures. The commentary was funded by the National Institutes of Health.
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
than those in unvaccinated children.
The benefit became largely apparent after children received the second vaccination in the recommended series, and persisted throughout childhood, Sheila Weinmann, PhD, of Kaiser Permanente Northern California, Oakland, and colleagues said.*
The analysis included 6.37 million children in the Kaiser Permanente database, 50% of whom were vaccinated for all or some of the study period stretching from 2003 to 2014. Overall, the crude lab-confirmed herpes zoster (HZ) incidence rate was 74/100,000 person-years. When stratified by vaccine status, the crude rate of HZ among vaccinated children was 78% lower than among unvaccinated children (38 vs. 170 cases per 100,000 person years).
Herpes zoster was more common among girls than boys and up to six times more common in immunosuppressed children than in nonimmunosuppressed children.
The authors also found that unvaccinated children benefited from the high rate of vaccination around them. Although the HZ rate was always lower among vaccinated children, the rate among unvaccinated children fell sharply after 2007.
“The trend of decreasing HZ incidence among children who were unvaccinated is likely due to a lack of primary VZV [varicella-zoster virus] infection resulting from herd immunity in a highly vaccinated population,” Dr. Weinmann and her associates said.
There was some variability among age groups, especially among the youngest who were not fully vaccinated.
“In the group aged 1-2 years, the confirmation-adjusted HZ rate among children who were vaccinated was 70% higher than among those who were unvaccinated,” the authors said. In the “older groups, HZ rates were significantly higher in children who were unvaccinated than in those who were vaccinated,” the researchers noted.
The highest incidence was among vaccinated 1-year-olds, who had a 140% higher risk of HZ than did unvaccinated 1-year-olds. But this risk elevation disappeared by age 2 years. For everyone else, aged 2-17 years, the rate of HZ remained significantly lower in vaccinated children.
“Among the small number of children vaccinated at 11 months of age (for whom the vaccine is not recommended), the HZ incidence rate was significantly higher than in children vaccinated at 1 year of age and older. Similarly, children who contract wild-type varicella infection at younger than 1 year of age also have a higher risk of HZ (relative risk, 13.5). The immature adaptive T-cell response in children less than 1 year of age appears less able to contain VZV as a latent infection, compared with older children.
“Our findings for 11-month-olds who were vaccinated should be interpreted with caution because this population included only three cases of HZ and could have included children participating in a prelicensure study with a vaccine formulation different from Varivax,” Dr. Weinmann and her associates said.
Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
SOURCE: Weinmann S et al. Pediatrics. 2019 Jun 10. doi: 10.1542/peds.2018-2917.
* This article was updated 6/14/2019
FROM PEDIATRICS
Key clinical point: Varicella vaccine is preventing pediatric zoster among children aged 2-17 years.
Major finding: Varicella-vaccinated children have a 78% lower incidence of pediatric zoster than do unvaccinated children.
Study details: The population-based cohort study included more than 6.3 million children.
Disclosures: Dr. Weinmann and her associates reported no relevant financial disclosures. The study was supported by the Centers for Disease Control and Prevention.
Source: Weinmann S et al. Pediatrics. 2019. doi: 10.1542/peds.2018-2917.
Less Is More When It Comes to Ketorolac for Pain
A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
Opioid prescriptions declined 33% over 5 years
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
Pain coping skills training doesn’t improve knee arthroplasty outcomes
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
TORONTO – A high level of pain catastrophizing prior to scheduled knee arthroplasty is not, as previously thought, a harbinger of poor outcomes, and affected patients don’t benefit from cognitive-behavioral therapy–based training in pain coping skills, Daniel L. Riddle, PhD, reported at the OARSI 2019 World Congress.
“The take-home message for us is knee arthroplasty is incredibly effective and there really is no reason to do pain coping skills training in these high–pain catastrophizing patients because the great majority of them have such good outcomes,” said Dr. Riddle, professor of physical therapy at Virginia Commonwealth University, Richmond.
“The other clear message from our trial is that, when you have pain-catastrophizing patients and you lower their pain, their catastrophizing is also lowered. So pain catastrophizing is clearly a response to pain and not a personality trait per se,” he said at the meeting sponsored by the Osteoarthritis Research Society International.
He presented the results of a 402-patient, randomized, three-arm, single-blind trial conducted at five U.S. medical centers. All participants were scheduled for knee arthroplasty for osteoarthritis, and all had moderate- to high-level pain catastrophizing as reflected in the group’s average Pain Catastrophizing Score of 30. They were assigned to an arthritis education active control group, usual care, or an intervention developed specifically for this study: a cognitive-behavioral therapy–based training program for pain coping skills. Similar pain coping skills training interventions have been shown to be beneficial in patients with medically treated knee OA but hadn’t previously been evaluated in surgically treated patients. The primary study endpoint was change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain Scale at 2, 6, and 12 months after surgery.
The improvement in WOMAC pain in the three study arms was virtually superimposable, going from an average pain score of about 12 preoperatively to 2 postoperatively.
“This was a clear no-effect trial,” Dr. Riddle observed. “These are patients we thought to be at increased risk for poor outcome, but indeed they’re not.”
Pain Catastrophizing Scores improved from 30 preoperatively to roughly 7 at 1 year. “We’ve never seen pain catastrophizing improvements of this magnitude,” the researcher commented.
The study participants typically had a large number of chronically painful areas, but only minimal change in pain scores occurred except in the surgically treated knee.
Of note, even with the impressively large improvements in knee pain, function, and other secondary endpoints in the study group as a whole, roughly 20% of study participants experienced essentially no improvement in their function-limiting knee pain during the first year after arthroplasty. These nonresponders were spread equally across all three study arms. Further research will be needed to develop interventions to help this challenging patient subgroup.
The pain coping skills training consisted of 8 weekly sessions, each an hour long, which began prior to surgery and continued afterward. The intervention was delivered by physical therapists who had been trained by pain psychologists with expertise in cognitive-behavioral therapy. The intervention was delivered by telephone and in face-to-face sessions. The trainers were tracked over the course of the study to make sure that the structured intervention was delivered as planned.
Dr. Riddle reported having no financial conflicts regarding the National Institutes of Health-funded study, the full details of which have been published (J Bone Joint Surg Am. 2019 Feb 6;101[3]:218-227).
REPORTING FROM OARSI 2019
Stewart Tepper: Emgality approval ‘very exciting’
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
The drug, a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP), is administered by self-injection in 300-mg doses.
Galcanezumab is the first medication for episodic cluster headache that reduces the frequency of attacks, the agency said in an announcement.
Cluster headache can be more intense than migraine. The pain is unilateral and occurs in the orbital, supraorbital, or temporal regions. It reaches its peak intensity within 5-10 minutes and generally lasts for 30-90 minutes. Symptoms include a burning sensation, conjunctival injection, rhinorrhea, and photosensitivity. Patients often have one to three of these headaches per day, and the headaches appear to be linked to the circadian rhythm. An episodic cluster cycle can last for weeks to months of daily or near daily attacks.
A study presented at the recent meeting of the American Academy of Neurology provided evidence of the drug’s efficacy in cluster headache. In this trial, researchers randomized 106 patients with episodic cluster headache to galcanezumab or placebo. The baseline cluster headache frequency was 17.3 attacks per week, and galcanezumab reduced this frequency to 9.1 attacks per week, compared with 12.1 attacks per week with placebo. The most common side effect reported in this and other clinical trials was injection-site reactions.
Galcanezumab entails a risk of hypersensitivity reactions, according to the FDA. These reactions may occur several days after administration and may be prolonged. “If a serious hypersensitivity reaction occurs, treatment should be discontinued,” the agency said.
“It’s a very exciting day. There had never been a drug approved for prevention of cluster headache,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth and director of the Dartmouth Headache Center, Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
It is difficult to achieve therapeutic concentrations of current preventive medications that do not have FDA approval for this indication, such as verapamil, lithium, or antiepileptic drugs. Galcanezumab, in contrast, works quickly. It is important to note that the approval was for preventive treatment of episodic cluster headache, not for prevention of chronic cluster headache, and not for acute treatment, Dr. Tepper said.
“It’s important to get optimal therapy for cluster headache. It is one of the most disabling, terrible disorders on Earth,” Dr. Tepper said. “The importance [of this approval] cannot be overestimated.”
When asked for comment, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, said “If this monoclonal antibody to the CGRP ligand works as well in real life as in the trial, it will be an important advance in the treatment of cluster headache.”
Prior to the approval of galcanezumab, noninvasive vagal nerve stimulation was approved in November 2018 for adjunctive use in the preventive treatment of cluster headache in adults.
The FDA granted the application for galcanezumab using a Priority Review and Breakthrough Therapy designation. The agency approved galcanezumab for the preventive treatment of migraine in adults in September 2018. The drug appears to have a similar safety profile in both patient populations. Eli Lilly, which is based in Indianapolis, Indiana, manufactures the drug.
This article was updated June 5, 2019.
Slow breathing: An effective, pragmatic analgesic technique?
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
MILWAUKEE – Mindfulness-based practices are effective in reducing pain perceptions, but a more easily taught breath control technique also showed efficacy in a recent study. Slow, rhythmic breathing alone, even without the additional attentional components of mindfulness, had significant analgesic effects in a human experimental model of pain.
“Slow breathing is much easier to perform” than mindfulness-based meditation, Fadel Zeidan, PhD, said at the scientific meeting of the American Pain Society. More research into the technique may offer a “clinically pragmatic” nonpharmacologic option for pain control, he said. And there may be some similarities between how the two techniques work: like mindfulness meditation, slow, rhythmic breathing’s analgesic properties are not dependent on the endogenous opioid system, said Dr. Zeidan, assistant professor of anesthesiology at the University of California, San Diego. His interests include mindfulness meditation–based pain relief.
In previous work, Dr. Zeidan and his collaborators had shown that the analgesic effect of mindfulness practices is not mediated by endogenous opioids. Participants in a study were trained in mindfulness meditation, and then exposed to a pain stimulus. Compared with a control group who listened to an audiobook rather than using mindfulness practices when exposed to pain, the meditators experienced a significant reduction in pain unpleasantness (J Neurosci. 16 March 2016;36[11]:3391-7).
In the experiment, both the meditation and the control group received first an intravenous saline solution, and then the opioid antagonist naloxone, which blocks endogenous opioids. When receiving naloxone, the meditators experienced reductions in the perceived unpleasantness of pain that were similar to what they experienced when they had received saline, showing that endogenous opioids weren’t responsible for meditation’s analgesic effects.
After verifying those findings, said Dr. Zeidan, he became interested in conducting a “graded analytical dissection of mindfulness,” to see exactly which components of the practice are nonopioidergic.
With mindfulness meditation, participants engage in slow, rhythmic breathing, and they learn about observation and appraisal practices, which can briefly be described as “the awareness of arising sensory events without reaction,” Dr. Zeidan said.
Mere belief in meditation in combination with the slow rhythmic breathing might have an analgesic effect, he said. In effect, this is sham mindfulness.
To try to tease out the contributions of each component of mindfulness meditation, Dr. Zeidan and his colleagues devised an experiment that trained participants in one of three ways. Over the course of four 20-minute sessions, randomized participants were trained in slow breathing techniques, with a goal respiratory rate of 6 breaths per minute; in mindfulness meditation techniques; or in a sham mindfulness technique that did not teach specific mindfulness principles.
The randomized participants were subject to a painful heat stimulus before the training to establish a baseline.
After training, they returned for two further sessions. At each visit, they experienced the noxious stimulus with no medication. After a rest period, they then received either high-dose intravenous naloxone or saline. The allocation was randomized and administration of the study drug was double-blinded.
With naloxone or saline infusion ongoing, participants were then again subjected to the painful heat stimulus.
“All manipulations effectively reduced the respiration rate,” by 18%-21%, Dr. Zeidan said.
However, with the introduction of naloxone, both the slow-breathing group and the mindfulness group maintained reductions in pain unpleasantness, while those in the sham group had significant increases in pain unpleasantness. Reductions in pain unpleasantness ranged from 11% to 18% for these two groups, while the initial 8% reduction for the sham group climbed to a 13% increase in pain unpleasantness when this group received naloxone.
An unexpected finding was how effective slow breathing alone was as an analgesic. “There’s really something here,” said Dr. Zeidan, in reference to the analgesic effect of breath control. He explained that the slow breathing technique training was done with the aid of a device that emitted a blue glow that dimmed and brightened at the target respiratory rate.
Dr. Zeidan added that few participants were able to slow their breathing to 6 respirations per minute, but that the average rate did slow to about 12 from the normal 16 or so breaths per minute.
Dr. Zeidan reported no conflicts of interest. The National Institutes of Health funded the research.
REPORTING FROM APS 2019
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.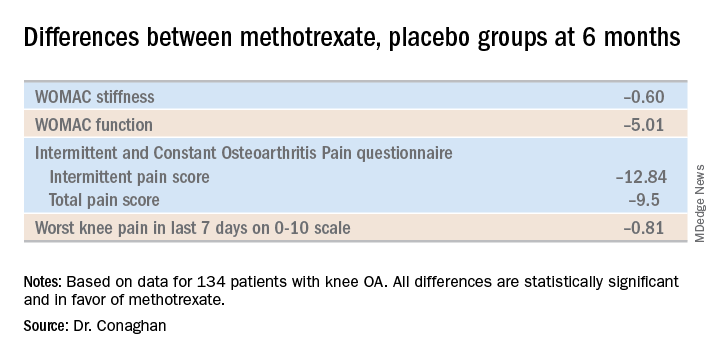
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Click for Credit: Biomarkers for VTE risk; Exercise & concussion recovery; more
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020
Here are 5 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Expert: There’s no single treatment for fibromyalgia
To take the posttest, go to: https://bit.ly/2EAI5v1
Expires February 3, 2020
2. Mood and behavior are different targets for irritability in children
To take the posttest, go to: https://bit.ly/2wpLS9X
Expires February 6, 2020
3. Biomarkers predict VTE risk with menopausal oral hormone therapy
To take the posttest, go to: https://bit.ly/2JKEQFC
Expires February 6, 2020
4. Mild aerobic exercise speeds sports concussion recovery
To take the posttest, go to: https://bit.ly/30RuYiE
Expires February 4, 2020
5. For CABG, multiple and single arterial grafts show no survival difference
To take the posttest, go to: https://bit.ly/2wtiCiF
Expires January 31, 2020