User login
Stress, depression during pregnancy can harm child
New evidence points to the importance of helping mothers with their mental health during pregnancy.
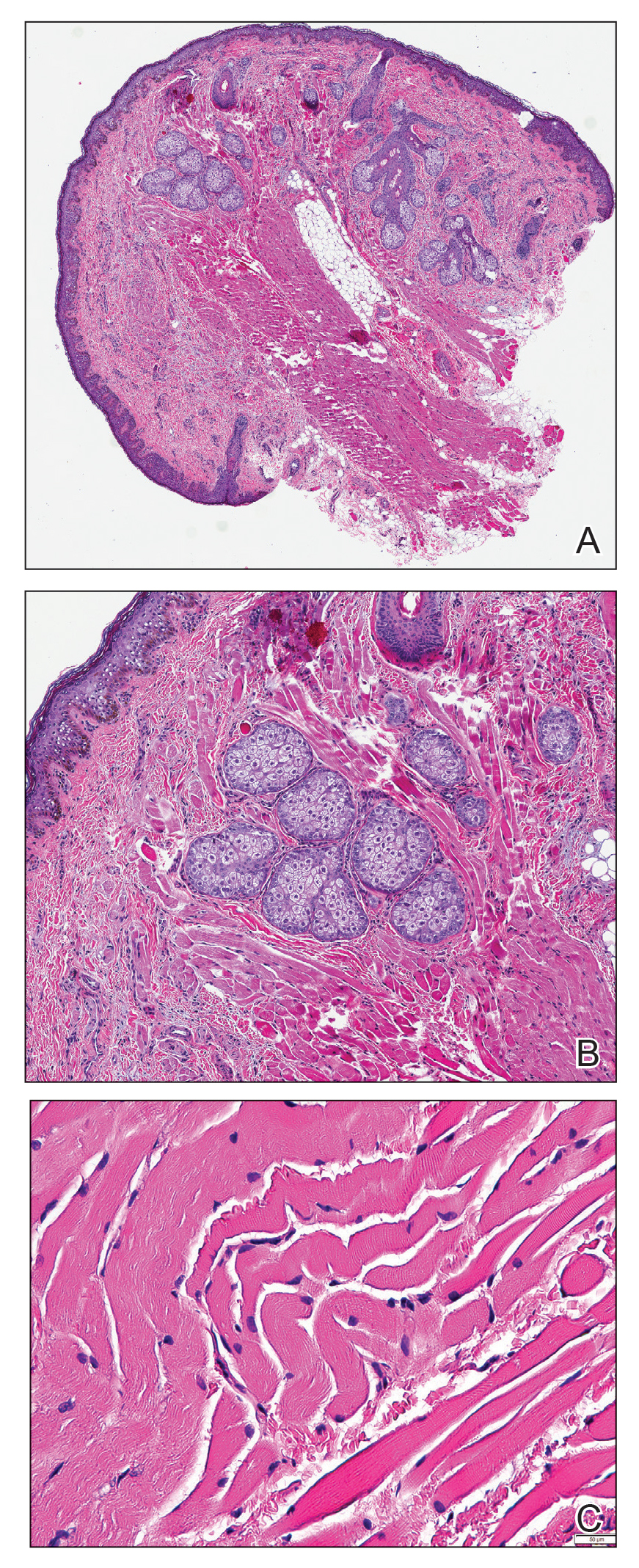
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
New evidence points to the importance of helping mothers with their mental health during pregnancy.
Researchers from the National Institutes of Health in Bethesda, Md., have found that feelings of stress or depression while pregnant are linked to changes in the placenta where the child is growing. The findings, published in Epigenomics, show these changes could alter gene activity.
Stress and depression are not uncommon among expectant women, with depression affecting an estimated 1 in 10 pregnancies, according to the American College of Obstetricians and Gynecologists.
And current evidence already suggests that depression during pregnancy can negatively affect a child later in life. For instance, one study found that depression during pregnancy was linked to behavioral and emotional disorders during childhood, and another found that it raised the risk of depression at age 18.
To investigate stress and depression during pregnancy, the NIH investigators evaluated 301 pregnant women from 12 clinics in the United States who had taken part in an earlier clinical study. The group was ethnically diverse, with 34% identifying as Hispanic, 26% as non-Hispanic White, 24% as non-Hispanic Black, and 17% as Asian or Pacific Islander.
At the start of the study, the women were asked to complete questionnaires routinely used to screen for stress and depression. They completed the questionnaire five more times during their pregnancies. Shortly after each woman gave birth, researchers took tissue samples from the placenta and analyzed the genetics.
The purpose of studying the placenta, according to lead researcher Markos Tesfaye, MD, a postdoctoral fellow at the NIH, is that chemical changes can regulate whether a nearby gene can be activated.
There is evidence that chemical modifications in the placenta can lead to changes in fetal tissues, such as the brain, he said. And the placenta is known for making neurotransmitters, which are needed for fetal brain development.
The team found 16 areas where changes to the exterior of placental DNA were linked to depression in the second or third trimester. They also found two areas where these changes were associated with stress in the third trimester.
“Maternal depression leaves signals in the placenta at genes critical for fetal brain programming,” said study author Fasil Tekola-Ayele, PhD, from the NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Two of the chemical changes linked to depression were near genes that are known to be involved with fetal brain development and neurologic and psychiatric illnesses.
“The findings illustrate that the developing fetus is sensitive to the mother›s condition during pregnancy, including maternal symptoms of low mood and perceived stress,” said Thalia K. Robakis, MD, from the women’s mental health program at Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study.
But Dr. Robakis cautioned that no clinical outcomes were measured among the babies born, meaning that the study could not document any effects of maternal depression and stress on fetal development. Rather, the work contributes to figuring out what mechanisms are involved.
“Pregnant women should continue to focus on optimizing their own physical and mental health,” Dr. Robakis said. “And they should know that a happy, healthy mother is the most important factor supporting the development of a happy, healthy baby.”
A version of this article first appeared on WebMD.com.
Why kids might reject sugar-free Halloween candy
Trick-or-treaters may not be so easily tricked into loving sugar-free treats, thanks to taste buds hard-wired to seek calorie-containing sweets, a new study suggests.
Taste isn’t all about choosing peanut butter cups over jelly beans. Since earliest humanity, our sense of taste has helped us detect salty, sweet, sour, savory, and bitter so that we can choose foods high in energy and low in poisons.
But these new findings suggest that our taste buds have another hidden talent: identifying foods that don’t give us any energy at all.
Scientists suspected this ability after research in mice showed that their taste buds could distinguish between sugar and calorie-free artificial sweeteners.
To test this possibility in humans, scientists asked people to drink a series of clear beverages and identify whether they were plain water or sweetened. The goal was to compare how people responded to glucose – a natural caloric sweetener in fruits, honey, and table sugar – and sucralose, a calorie-free artificial sweetener.
All participants wore nose plugs, ensuring that they would use only their taste buds and not their sense of smell for detection.
As expected, people could easily tell plain water from sweetened drinks, whether with glucose or sucralose, confirming that taste buds detect sweetness.
In a twist, researchers then mixed in flavorless chemicals that block taste buds from picking up sweetness. With these drinks, people could no longer distinguish sucralose-sweetened beverages from plain water. But they could still tell when they had a beverage sweetened with glucose.
This finding indicates that two separate pathways underlie the mouth’s response to sugar, researchers report in PLOS One. The first pathway identifies sweet flavors, and the second one detects foods that contain energy that can be used for fuel.
Scientists might one day come up with calorie-free sweets that trick taste buds into detecting the presence of calories, enhancing their appeal. But in the lab studies, the participants had no visual cues or smell to guide their reactions, meaning how these other sensory inputs would affect treat perception isn’t known.
A version of this article first appeared on WebMD.com.
Trick-or-treaters may not be so easily tricked into loving sugar-free treats, thanks to taste buds hard-wired to seek calorie-containing sweets, a new study suggests.
Taste isn’t all about choosing peanut butter cups over jelly beans. Since earliest humanity, our sense of taste has helped us detect salty, sweet, sour, savory, and bitter so that we can choose foods high in energy and low in poisons.
But these new findings suggest that our taste buds have another hidden talent: identifying foods that don’t give us any energy at all.
Scientists suspected this ability after research in mice showed that their taste buds could distinguish between sugar and calorie-free artificial sweeteners.
To test this possibility in humans, scientists asked people to drink a series of clear beverages and identify whether they were plain water or sweetened. The goal was to compare how people responded to glucose – a natural caloric sweetener in fruits, honey, and table sugar – and sucralose, a calorie-free artificial sweetener.
All participants wore nose plugs, ensuring that they would use only their taste buds and not their sense of smell for detection.
As expected, people could easily tell plain water from sweetened drinks, whether with glucose or sucralose, confirming that taste buds detect sweetness.
In a twist, researchers then mixed in flavorless chemicals that block taste buds from picking up sweetness. With these drinks, people could no longer distinguish sucralose-sweetened beverages from plain water. But they could still tell when they had a beverage sweetened with glucose.
This finding indicates that two separate pathways underlie the mouth’s response to sugar, researchers report in PLOS One. The first pathway identifies sweet flavors, and the second one detects foods that contain energy that can be used for fuel.
Scientists might one day come up with calorie-free sweets that trick taste buds into detecting the presence of calories, enhancing their appeal. But in the lab studies, the participants had no visual cues or smell to guide their reactions, meaning how these other sensory inputs would affect treat perception isn’t known.
A version of this article first appeared on WebMD.com.
Trick-or-treaters may not be so easily tricked into loving sugar-free treats, thanks to taste buds hard-wired to seek calorie-containing sweets, a new study suggests.
Taste isn’t all about choosing peanut butter cups over jelly beans. Since earliest humanity, our sense of taste has helped us detect salty, sweet, sour, savory, and bitter so that we can choose foods high in energy and low in poisons.
But these new findings suggest that our taste buds have another hidden talent: identifying foods that don’t give us any energy at all.
Scientists suspected this ability after research in mice showed that their taste buds could distinguish between sugar and calorie-free artificial sweeteners.
To test this possibility in humans, scientists asked people to drink a series of clear beverages and identify whether they were plain water or sweetened. The goal was to compare how people responded to glucose – a natural caloric sweetener in fruits, honey, and table sugar – and sucralose, a calorie-free artificial sweetener.
All participants wore nose plugs, ensuring that they would use only their taste buds and not their sense of smell for detection.
As expected, people could easily tell plain water from sweetened drinks, whether with glucose or sucralose, confirming that taste buds detect sweetness.
In a twist, researchers then mixed in flavorless chemicals that block taste buds from picking up sweetness. With these drinks, people could no longer distinguish sucralose-sweetened beverages from plain water. But they could still tell when they had a beverage sweetened with glucose.
This finding indicates that two separate pathways underlie the mouth’s response to sugar, researchers report in PLOS One. The first pathway identifies sweet flavors, and the second one detects foods that contain energy that can be used for fuel.
Scientists might one day come up with calorie-free sweets that trick taste buds into detecting the presence of calories, enhancing their appeal. But in the lab studies, the participants had no visual cues or smell to guide their reactions, meaning how these other sensory inputs would affect treat perception isn’t known.
A version of this article first appeared on WebMD.com.
‘Down to my last diaper’: The anxiety of parenting in poverty
For parents living in poverty, “diaper math” is a familiar and distressingly pressing daily calculation. Babies in the U.S. go through 6-10 disposable diapers a day, at an average cost of $70-$80 a month. Name-brand diapers with high-end absorption sell for as much as a half a dollar each, and can result in upwards of $120 a month in expenses.
One in every three American families cannot afford enough diapers to keep their infants and toddlers clean, dry, and healthy, according to the National Diaper Bank Network. For many parents, that leads to wrenching choices: diapers, food, or rent?
The COVID-19 pandemic has exacerbated the situation, both by expanding unemployment rolls and by causing supply chain disruptions that have triggered higher prices for a multitude of products, including diapers. Diaper banks – community-funded programs that offer free diapers to low-income families – distributed 86% more diapers on average in 2020 than in 2019, according to the National Diaper Bank Network. In some locations, distribution increased by as much as 800%.
Yet no federal program helps parents pay for this childhood essential. The government’s food assistance program does not cover diapers, nor do most state-level public aid programs.
California is the only state to directly fund diapers for families, but support is limited. CalWORKS, a financial assistance program for families with children, provides $30 per month to help families pay for diapers for children under age 3. Federal policy shifts also may be in the works: Democratic lawmakers are pushing to include $200 million for diaper distribution in the massive budget reconciliation package.
Without adequate resources, low-income parents are left scrambling for ways to get the most use out of each diaper. This stressful undertaking is the subject of a recent article in American Sociological Review by Jennifer Randles, PhD, professor of sociology at California State University–Fresno. In 2018, Randles conducted phone interviews with 70 mothers in California over nine months. She tried to recruit fathers as well, but only two men responded.
Dr. Randles spoke with KHN’s Jenny Gold about how the cost of diapers weighs on low-income moms, and the “inventive mothering” many low-income women adopt to shield their children from the harms of poverty. The conversation has been edited for length and clarity.
Q: How do diapers play into day-to-day anxieties for low-income mothers?
In my sample, half of the mothers told me that they worried more about diapers than they worried about food or housing.
I started to ask mothers, “Can you tell me how many diapers you have on hand right now?” Almost every one told me with exact specificity how many they had – 5 or 7 or 12. And they knew exactly how long that number of diapers would last, based on how often their children defecated and urinated, if their kid was sick, if they had a diaper rash at the time. So just all the emotional and cognitive labor that goes into keeping such careful track of diaper supplies.
They were worrying and figuring out, “OK, I’m down to almost my last diaper. What do I do now? Do I go find some cans [to sell]? Do I go sell some things in my house? Who in my social network might have some extra cash right now?” I talked to moms who sell blood plasma just to get their infants diapers.
Q: What coping strategies stood out to you?
Those of us who study diapers often call them diaper-stretching strategies. One was leaving on a diaper a little bit longer than someone might otherwise leave it on and letting it get completely full. Some mothers figured out if they bought a [more expensive] diaper that held more and leaked less, they could leave the diaper on longer.
They would also do things like letting the baby go diaperless, especially when they were at home and felt like they wouldn’t be judged for letting their baby go without a diaper. And they used every household good you can imagine to make makeshift diapers. Mothers are using cloth, sheets, and pillowcases. They’re using things that are disposable like paper towels with duct tape. They’re making diapers out their own period supplies or adult incontinence supplies when they can get a sample.
One of the questions I often get is, “Why don’t they just use cloth?” A lot of the mothers that I spoke with had tried cloth diapers and they found that they were very cost- and labor-prohibitive. If you pay for a full startup set of cloth diapers, you’re looking at anywhere from $500 to $1,000. And these moms never had that much money. Most of them didn’t have in-home washers and dryers. Some of them didn’t even have homes or consistent access to water, and it’s illegal in a lot of laundromats and public laundry facilities to wash your old diapers. So the same conditions that would prevent moms from being able to readily afford disposable diapers are the same conditions that keep them from being able to use cloth.
Q: You found that, for many women, the concept of being a good mother is wrapped up in diapering. Why is that?
Diapers and managing diapers was so fundamental to their identity as good moms. Most of the mothers in my sample went without their own food. They weren’t paying a cellphone bill or buying their own medicine or their own menstrual supplies, as a way of saving diaper money.
I talked to a lot of moms who said, when your baby is hungry, that’s horrible. Obviously, you do everything to prevent that. But there’s something about a diaper that covers this vulnerable part of a very young baby’s body, this very delicate skin. And being able to do something to meet this human need that we all have, and to maintain dignity and cleanliness.
A lot of the moms had been through the welfare system, and so they’re living in this constant fear [of losing their children]. This is especially true among mothers of color, who are much more likely to get wrapped up in the child welfare system. People can’t necessarily see when your baby’s hungry. But people can see a saggy diaper. That’s going to be one of the things that tags you as a bad mom.
Q: Was your work on diapers influenced by your experience as a parent?
When I was doing these interviews, my daughter was about 2 or 3. So still in diapers. When my daughter peed during a diaper change, I thought, “Oh, I can just toss that one. Here, let me get another clean one.” That’s a really easy choice. For me. That’s a crisis for the mothers I interviewed. Many of them told me they have an anxiety attack with every diaper change.
Q: Do you see a clear policy solution to diaper stress?
What’s kind of ironic is how much physical, emotional, and cognitive labor goes into managing something that society and lawmakers don’t even recognize. Diapers are still not really recognized as a basic need, as evidenced by the fact that they’re still taxed in 35 states.
I think what California is doing is an excellent start. And I think diaper banks are a fabulous type of community-based organization that are filling a huge need that is not being filled by safety net policies. So, public support for diaper banks.
The direct cash aid part of the social safety net has been all but dismantled in the last 25 years. California is pretty generous. But there are some states where just the cost of diapers alone would use almost half of the average state TANF [Temporary Assistance for Needy Families] benefit for a family of three. I think we really do have to address the fact that the value of cash aid buys so much less than it used to.
Q: Your body of work on marriage and families is fascinating and unusual. Is there a single animating question behind your research?
The common thread is: How do our safety net policies support low-income families’ parenting goals? And do they equalize the conditions of parenting? I think of it as a reproductive justice issue. The ability to have a child or to not have a child, and then to parent that child in conditions where the child’s basic needs are met.
We like to say that we’re child and family friendly. The diaper issue is just one of many, many issues where we don’t really put our money or our policies where our mouth is, in terms of supporting families and supporting children. I think my work is trying to get people to think more collectively about having a social responsibility to all families and to each other. No country, but especially the richest country on the planet, should have one in three very young children not having one of their basic needs met.
I interviewed one dad who was incarcerated because he wrote a bad check. And as he described it to me, he had a certain amount of money, and they needed both diapers and milk for the baby. And I’ll never forget, he said, “I didn’t make a good choice, but I made the right one.”
These are not fancy shoes. These are not name-brand clothes. This was a dad needing both milk and diapers. I don’t think it gets much more basic than that.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
For parents living in poverty, “diaper math” is a familiar and distressingly pressing daily calculation. Babies in the U.S. go through 6-10 disposable diapers a day, at an average cost of $70-$80 a month. Name-brand diapers with high-end absorption sell for as much as a half a dollar each, and can result in upwards of $120 a month in expenses.
One in every three American families cannot afford enough diapers to keep their infants and toddlers clean, dry, and healthy, according to the National Diaper Bank Network. For many parents, that leads to wrenching choices: diapers, food, or rent?
The COVID-19 pandemic has exacerbated the situation, both by expanding unemployment rolls and by causing supply chain disruptions that have triggered higher prices for a multitude of products, including diapers. Diaper banks – community-funded programs that offer free diapers to low-income families – distributed 86% more diapers on average in 2020 than in 2019, according to the National Diaper Bank Network. In some locations, distribution increased by as much as 800%.
Yet no federal program helps parents pay for this childhood essential. The government’s food assistance program does not cover diapers, nor do most state-level public aid programs.
California is the only state to directly fund diapers for families, but support is limited. CalWORKS, a financial assistance program for families with children, provides $30 per month to help families pay for diapers for children under age 3. Federal policy shifts also may be in the works: Democratic lawmakers are pushing to include $200 million for diaper distribution in the massive budget reconciliation package.
Without adequate resources, low-income parents are left scrambling for ways to get the most use out of each diaper. This stressful undertaking is the subject of a recent article in American Sociological Review by Jennifer Randles, PhD, professor of sociology at California State University–Fresno. In 2018, Randles conducted phone interviews with 70 mothers in California over nine months. She tried to recruit fathers as well, but only two men responded.
Dr. Randles spoke with KHN’s Jenny Gold about how the cost of diapers weighs on low-income moms, and the “inventive mothering” many low-income women adopt to shield their children from the harms of poverty. The conversation has been edited for length and clarity.
Q: How do diapers play into day-to-day anxieties for low-income mothers?
In my sample, half of the mothers told me that they worried more about diapers than they worried about food or housing.
I started to ask mothers, “Can you tell me how many diapers you have on hand right now?” Almost every one told me with exact specificity how many they had – 5 or 7 or 12. And they knew exactly how long that number of diapers would last, based on how often their children defecated and urinated, if their kid was sick, if they had a diaper rash at the time. So just all the emotional and cognitive labor that goes into keeping such careful track of diaper supplies.
They were worrying and figuring out, “OK, I’m down to almost my last diaper. What do I do now? Do I go find some cans [to sell]? Do I go sell some things in my house? Who in my social network might have some extra cash right now?” I talked to moms who sell blood plasma just to get their infants diapers.
Q: What coping strategies stood out to you?
Those of us who study diapers often call them diaper-stretching strategies. One was leaving on a diaper a little bit longer than someone might otherwise leave it on and letting it get completely full. Some mothers figured out if they bought a [more expensive] diaper that held more and leaked less, they could leave the diaper on longer.
They would also do things like letting the baby go diaperless, especially when they were at home and felt like they wouldn’t be judged for letting their baby go without a diaper. And they used every household good you can imagine to make makeshift diapers. Mothers are using cloth, sheets, and pillowcases. They’re using things that are disposable like paper towels with duct tape. They’re making diapers out their own period supplies or adult incontinence supplies when they can get a sample.
One of the questions I often get is, “Why don’t they just use cloth?” A lot of the mothers that I spoke with had tried cloth diapers and they found that they were very cost- and labor-prohibitive. If you pay for a full startup set of cloth diapers, you’re looking at anywhere from $500 to $1,000. And these moms never had that much money. Most of them didn’t have in-home washers and dryers. Some of them didn’t even have homes or consistent access to water, and it’s illegal in a lot of laundromats and public laundry facilities to wash your old diapers. So the same conditions that would prevent moms from being able to readily afford disposable diapers are the same conditions that keep them from being able to use cloth.
Q: You found that, for many women, the concept of being a good mother is wrapped up in diapering. Why is that?
Diapers and managing diapers was so fundamental to their identity as good moms. Most of the mothers in my sample went without their own food. They weren’t paying a cellphone bill or buying their own medicine or their own menstrual supplies, as a way of saving diaper money.
I talked to a lot of moms who said, when your baby is hungry, that’s horrible. Obviously, you do everything to prevent that. But there’s something about a diaper that covers this vulnerable part of a very young baby’s body, this very delicate skin. And being able to do something to meet this human need that we all have, and to maintain dignity and cleanliness.
A lot of the moms had been through the welfare system, and so they’re living in this constant fear [of losing their children]. This is especially true among mothers of color, who are much more likely to get wrapped up in the child welfare system. People can’t necessarily see when your baby’s hungry. But people can see a saggy diaper. That’s going to be one of the things that tags you as a bad mom.
Q: Was your work on diapers influenced by your experience as a parent?
When I was doing these interviews, my daughter was about 2 or 3. So still in diapers. When my daughter peed during a diaper change, I thought, “Oh, I can just toss that one. Here, let me get another clean one.” That’s a really easy choice. For me. That’s a crisis for the mothers I interviewed. Many of them told me they have an anxiety attack with every diaper change.
Q: Do you see a clear policy solution to diaper stress?
What’s kind of ironic is how much physical, emotional, and cognitive labor goes into managing something that society and lawmakers don’t even recognize. Diapers are still not really recognized as a basic need, as evidenced by the fact that they’re still taxed in 35 states.
I think what California is doing is an excellent start. And I think diaper banks are a fabulous type of community-based organization that are filling a huge need that is not being filled by safety net policies. So, public support for diaper banks.
The direct cash aid part of the social safety net has been all but dismantled in the last 25 years. California is pretty generous. But there are some states where just the cost of diapers alone would use almost half of the average state TANF [Temporary Assistance for Needy Families] benefit for a family of three. I think we really do have to address the fact that the value of cash aid buys so much less than it used to.
Q: Your body of work on marriage and families is fascinating and unusual. Is there a single animating question behind your research?
The common thread is: How do our safety net policies support low-income families’ parenting goals? And do they equalize the conditions of parenting? I think of it as a reproductive justice issue. The ability to have a child or to not have a child, and then to parent that child in conditions where the child’s basic needs are met.
We like to say that we’re child and family friendly. The diaper issue is just one of many, many issues where we don’t really put our money or our policies where our mouth is, in terms of supporting families and supporting children. I think my work is trying to get people to think more collectively about having a social responsibility to all families and to each other. No country, but especially the richest country on the planet, should have one in three very young children not having one of their basic needs met.
I interviewed one dad who was incarcerated because he wrote a bad check. And as he described it to me, he had a certain amount of money, and they needed both diapers and milk for the baby. And I’ll never forget, he said, “I didn’t make a good choice, but I made the right one.”
These are not fancy shoes. These are not name-brand clothes. This was a dad needing both milk and diapers. I don’t think it gets much more basic than that.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
For parents living in poverty, “diaper math” is a familiar and distressingly pressing daily calculation. Babies in the U.S. go through 6-10 disposable diapers a day, at an average cost of $70-$80 a month. Name-brand diapers with high-end absorption sell for as much as a half a dollar each, and can result in upwards of $120 a month in expenses.
One in every three American families cannot afford enough diapers to keep their infants and toddlers clean, dry, and healthy, according to the National Diaper Bank Network. For many parents, that leads to wrenching choices: diapers, food, or rent?
The COVID-19 pandemic has exacerbated the situation, both by expanding unemployment rolls and by causing supply chain disruptions that have triggered higher prices for a multitude of products, including diapers. Diaper banks – community-funded programs that offer free diapers to low-income families – distributed 86% more diapers on average in 2020 than in 2019, according to the National Diaper Bank Network. In some locations, distribution increased by as much as 800%.
Yet no federal program helps parents pay for this childhood essential. The government’s food assistance program does not cover diapers, nor do most state-level public aid programs.
California is the only state to directly fund diapers for families, but support is limited. CalWORKS, a financial assistance program for families with children, provides $30 per month to help families pay for diapers for children under age 3. Federal policy shifts also may be in the works: Democratic lawmakers are pushing to include $200 million for diaper distribution in the massive budget reconciliation package.
Without adequate resources, low-income parents are left scrambling for ways to get the most use out of each diaper. This stressful undertaking is the subject of a recent article in American Sociological Review by Jennifer Randles, PhD, professor of sociology at California State University–Fresno. In 2018, Randles conducted phone interviews with 70 mothers in California over nine months. She tried to recruit fathers as well, but only two men responded.
Dr. Randles spoke with KHN’s Jenny Gold about how the cost of diapers weighs on low-income moms, and the “inventive mothering” many low-income women adopt to shield their children from the harms of poverty. The conversation has been edited for length and clarity.
Q: How do diapers play into day-to-day anxieties for low-income mothers?
In my sample, half of the mothers told me that they worried more about diapers than they worried about food or housing.
I started to ask mothers, “Can you tell me how many diapers you have on hand right now?” Almost every one told me with exact specificity how many they had – 5 or 7 or 12. And they knew exactly how long that number of diapers would last, based on how often their children defecated and urinated, if their kid was sick, if they had a diaper rash at the time. So just all the emotional and cognitive labor that goes into keeping such careful track of diaper supplies.
They were worrying and figuring out, “OK, I’m down to almost my last diaper. What do I do now? Do I go find some cans [to sell]? Do I go sell some things in my house? Who in my social network might have some extra cash right now?” I talked to moms who sell blood plasma just to get their infants diapers.
Q: What coping strategies stood out to you?
Those of us who study diapers often call them diaper-stretching strategies. One was leaving on a diaper a little bit longer than someone might otherwise leave it on and letting it get completely full. Some mothers figured out if they bought a [more expensive] diaper that held more and leaked less, they could leave the diaper on longer.
They would also do things like letting the baby go diaperless, especially when they were at home and felt like they wouldn’t be judged for letting their baby go without a diaper. And they used every household good you can imagine to make makeshift diapers. Mothers are using cloth, sheets, and pillowcases. They’re using things that are disposable like paper towels with duct tape. They’re making diapers out their own period supplies or adult incontinence supplies when they can get a sample.
One of the questions I often get is, “Why don’t they just use cloth?” A lot of the mothers that I spoke with had tried cloth diapers and they found that they were very cost- and labor-prohibitive. If you pay for a full startup set of cloth diapers, you’re looking at anywhere from $500 to $1,000. And these moms never had that much money. Most of them didn’t have in-home washers and dryers. Some of them didn’t even have homes or consistent access to water, and it’s illegal in a lot of laundromats and public laundry facilities to wash your old diapers. So the same conditions that would prevent moms from being able to readily afford disposable diapers are the same conditions that keep them from being able to use cloth.
Q: You found that, for many women, the concept of being a good mother is wrapped up in diapering. Why is that?
Diapers and managing diapers was so fundamental to their identity as good moms. Most of the mothers in my sample went without their own food. They weren’t paying a cellphone bill or buying their own medicine or their own menstrual supplies, as a way of saving diaper money.
I talked to a lot of moms who said, when your baby is hungry, that’s horrible. Obviously, you do everything to prevent that. But there’s something about a diaper that covers this vulnerable part of a very young baby’s body, this very delicate skin. And being able to do something to meet this human need that we all have, and to maintain dignity and cleanliness.
A lot of the moms had been through the welfare system, and so they’re living in this constant fear [of losing their children]. This is especially true among mothers of color, who are much more likely to get wrapped up in the child welfare system. People can’t necessarily see when your baby’s hungry. But people can see a saggy diaper. That’s going to be one of the things that tags you as a bad mom.
Q: Was your work on diapers influenced by your experience as a parent?
When I was doing these interviews, my daughter was about 2 or 3. So still in diapers. When my daughter peed during a diaper change, I thought, “Oh, I can just toss that one. Here, let me get another clean one.” That’s a really easy choice. For me. That’s a crisis for the mothers I interviewed. Many of them told me they have an anxiety attack with every diaper change.
Q: Do you see a clear policy solution to diaper stress?
What’s kind of ironic is how much physical, emotional, and cognitive labor goes into managing something that society and lawmakers don’t even recognize. Diapers are still not really recognized as a basic need, as evidenced by the fact that they’re still taxed in 35 states.
I think what California is doing is an excellent start. And I think diaper banks are a fabulous type of community-based organization that are filling a huge need that is not being filled by safety net policies. So, public support for diaper banks.
The direct cash aid part of the social safety net has been all but dismantled in the last 25 years. California is pretty generous. But there are some states where just the cost of diapers alone would use almost half of the average state TANF [Temporary Assistance for Needy Families] benefit for a family of three. I think we really do have to address the fact that the value of cash aid buys so much less than it used to.
Q: Your body of work on marriage and families is fascinating and unusual. Is there a single animating question behind your research?
The common thread is: How do our safety net policies support low-income families’ parenting goals? And do they equalize the conditions of parenting? I think of it as a reproductive justice issue. The ability to have a child or to not have a child, and then to parent that child in conditions where the child’s basic needs are met.
We like to say that we’re child and family friendly. The diaper issue is just one of many, many issues where we don’t really put our money or our policies where our mouth is, in terms of supporting families and supporting children. I think my work is trying to get people to think more collectively about having a social responsibility to all families and to each other. No country, but especially the richest country on the planet, should have one in three very young children not having one of their basic needs met.
I interviewed one dad who was incarcerated because he wrote a bad check. And as he described it to me, he had a certain amount of money, and they needed both diapers and milk for the baby. And I’ll never forget, he said, “I didn’t make a good choice, but I made the right one.”
These are not fancy shoes. These are not name-brand clothes. This was a dad needing both milk and diapers. I don’t think it gets much more basic than that.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Autism prevalence in children as high as 10% in some New Jersey communities
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
DMTs linked to better pediatric MS outcomes
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
An estimated 3%-10% of MS patients are diagnosed during childhood. These patients experience a higher relapse rate and have higher magnetic resonance imaging (MRI) activity than do adult-onset patients. They have a slower rate of progression, but they reach irreversible disability milestones at an early age, with more than 50% having secondary progressive disease by age 30.
Studies in adults suggest that use of high-efficacy DMTs is most effective when initiated during the early active phase of MS, but little is known about children. “Early recognition of predictors of faster disability in children is crucial for clinicians to make the treatment decisions at the earliest possible time,” Sifat Sharmin, PhD, said during her presentation of the study at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Dr. Sharmin is a statistician and research fellow at the University of Melbourne.
‘Reassuring’ data
“I think the most important observation that was made here is the protective factor of use of high efficacy disease modifying therapies,” said Bruce Cree, MD, PhD, who was asked to comment on the study.
That result wasn’t unexpected, but it should provide reassurance. “For parents of children who are hesitant about use of high efficacy therapies, this study provides supporting evidence for use of these high efficacy therapies early on, to try and prevent irreversible disability from occurring,” said Dr. Cree, professor of clinical neurology and the George A. Zimmermann Endowed Professor in Multiple Sclerosis at the University of California at San Francisco UCSF Weill Institute for Neurosciences.
The study provides real-world data to back up findings from a phase 3 clinical trial that showed fewer relapses and fewer new lesions in pediatric patients with MS who were taking fingolimod versus interferon beta-1a.
“Given a large randomized, controlled trial, and now with this additional real-world data set showing the same thing, the only conclusion to reach is that if you’ve got a kid with MS, they should be treated with fingolimod,” said Dr. Cree. He noted that other DMTs such as natalizumab may also benefit pediatric patients, but fingolimod is the only drug that has been studied in randomized, controlled trials in children.
Real-world data
The researchers analyzed data from 672 patients drawn from the international MSBase Neuroimmunology Registry, who had undergone neurological assessment within 1 year of symptom onset and had at least two annual visits where the Expanded Disability Status Scale (EDSS) was recorded. They sought to identify predictors of Multiple Sclerosis Severity Score (MSSS). A secondary analysis looked at predictors of EDSS sustained worsening at 6 months, defined as an increase of 1.5 if EDSS baseline was 0, 1.0 or more if baseline EDSS was 1.0-5.5, or 0.5 if baseline EDSS was over 5.5.
The researchers also conducted a sensitivity analysis that looked at relapse phenotypes and relapse frequency in the first year, as well as a subgroup analysis of patients with available MRI data from the first year. The researchers adjusted for time on high-efficacy DMTs at each visit.
Among the study participants, 70% were female. The median age of onset was 16 years. The median EDSS score was 1.5 at inclusion, and the median score was 1.0 at follow-up of 3 years. At 6 months, 82 worsening events occurred in 57 patients.
A total of 76% of the patients were treated with DMTs. The most commonly prescribed DMTs were interferon beta (40.63%), natalizumab (8.48%), and fingolimod (6.40%). Seventy-eight percent of those who received DMTs started treatment before age 18. Twenty-seven percent received high-efficacy DMTs.
The analysis showed associations between disability and older age at onset [exp(beta), 1.09; 95% confidence interval, 1.03-1.16], maximum EDSS score during the first year of disease [exp(beta), 1.25; 95% CI, 1.13-1.36], or first-year pyramidal symptoms [exp(beta), 1.34; 95% CI, 1.13-1.58], visual symptoms [exp(beta), 1.28; 95% CI, 1.10-1.48], or cerebellum symptoms [exp(beta), 1.17; 95% CI, 1.00-1.39]. A greater amount of time on high-efficacy DMTs was associated with a lower probability of disability [exp(beta), 0.96; 95% CI, 0.93-0.99].
A complete recovery from the first relapse was associated with a lower probability of relapse, though this association did not reach statistical significance [exp(beta), 0.83; 95% CI, 0.68-1.03].
The secondary analyses found that the only predictor of 6-month EDSS worsening [exp(beta), 1.32; 95% CI, 1.21-1.45] was having a maximum EDSS score in the first year. Sensitivity analyses of complete and incomplete recovery from relapses found that a higher MSSS was associated incomplete recovery [exp(beta), 1.16; 95% CI, 1.02-1.32], and confirmed the primary finding that recovery from first relapse was associated with a lower probability of disability [exp(beta), 0.78; 95% CI, 0.63-0.96].
Among patients with MRI data, a new MRI lesion in year 1 was associated with a lower future MSSS score [exp(beta), 0.81; 95% CI, 0.66-0.99].
The study was funded by the National Health and Medical Research Council of Australia. The study authors disclosed ties with a wide range of pharmaceutical companies, including Biogen and Novartis. Dr. Cree has consulted for Biogen, Novartis, and other pharmaceutical companies.
FROM ECTRIMS 2021
Most infant formula trials lack transparency, carry high risk of bias: Systematic review
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.
Baby formula trials are not reliable, and have an “almost universal lack of transparency” which could undermine breastfeeding, according to the results of a systematic review published in BMJ. The findings underscore the need for significant change in the way such trials are conducted and reported, concluded lead author Bartosz Helfer, PhD, of the National Heart and Lung Institute at Imperial College London and the University of Wroclaw (Poland) Institute of Psychology and his coauthors. Citing a high risk of bias, selective reporting, and “almost universally favourable conclusions,” the international team of investigators suggested “some trials might have a marketing aim and no robust scientific aim,” concluding “much of the recent information generated about formula products might be misleading.”
The review included a detailed evaluation of 125 trials published since 2015, that compared at least two formula products in 23,757 children less than 3 years of age. The trials were evaluated for how they were conducted and reported, with specific attention paid to their risk of bias and risk of undermining breastfeeding.
Using the Cochrane risk-of-bias assessment 2.0 (ROB2), the analysis found that risk of bias was high in 80% of trials “usually because of inappropriate exclusions of participants from the analysis, and selective reporting,” the investigators noted. “This lack of transparency was complemented by favourable conclusions in more than 90% of recent trials, and evidence of publication bias in recent superiority trials.”
When conflict of interest was assessed, the analysis showed 84% of the trials received support from the formula milk industry, and of these, 77% had at least one author affiliated with a formula company. Overall, only 14% of trials had a low level of conflicts of interest according to the investigators’ definition “that the main source of funding had no commercial interest in the outcome of the trial and all of the authors of the study declared no financial ties to an entity with a commercial interest in the outcome of the trial.”
The investigators also noted that, by providing free formula to parents of breastfed or mixed-fed infants, many of the trials may have contravened the International Code of Marketing of Breast-milk Substitutes – an international agreement used to protect breastfeeding and limit the marketing of formula. “Claims arising from formula trials can contribute to formula marketing by narrowing the perceived benefits of breast milk over formula for consumers,” they wrote, calling for “improved oversight, conduct, and reporting of formula trials to ensure they provide a rigorous evidence base to inform nutrition in infants and young children.”
Asked to comment, Jennifer L. Pomeranz, JD, MPH, who was not involved in the study, told this publication the findings are “very concerning.” Ms. Pomeranz of New York University’s School of Global Public Health, recently reported similar issues in an analysis of baby formula websites. “Infant formula labels in the U.S. are adorned with a plethora of unsupported health and nutrition-related claims, including unregulated structure/function claims and breast milk comparison claims,” she said. “Moreover, infant formula marketing uses these claims to convince new parents that infant formula is necessary and even better for their infants than breast milk. Our research indicates that parents believe the popular claims made by formula companies and some even believe that infant formula is better for their child’s development than breast milk. If these claims are based on trials with no robust scientific basis, as the study suggests might be the case, then they are certainly false, deceptive, unfair, and misleading.”
Ms. Pomeranz called for the Food and Drug Administration’s regulation of infant formula labels, adding that “Congress should grant the FDA the explicit authority to require evidence to support structure/function claims on infant formula and prohibit breast milk comparison claims. ... The Federal Trade Commission and state attorneys general should bring actions against infant formula manufacturers for false and deceptive claims made in marketing materials,” she added.
Jack Newman, MD, another expert not involved in the study told this publication that the findings show how most formula studies “are essentially another marketing tool of the formula companies and are aimed at a very susceptible audience – health care professionals.” According to Dr. Newman, chief pediatrician and founder of the Newman Breastfeeding Clinic in Toronto and a former UNICEF consultant for the Baby Friendly Hospital Initiative, “health care professionals often like to believe they are immune to formula company marketing – yet this study shows that, even if they believed they were relying on scientific evidence, they were in fact being influenced toward formula feeding by studies that are biased, unreliable, and designed to promote formula to begin with.”
However, Stewart Forsyth, MD, honorary professor in child health, at the University of Dundee (Scotland) and retired consultant pediatrician and medical director at NHS Tayside, Scotland, cautioned that this is a delicate issue on all sides of the debate. The possibility of bias “is a potential issue with all aspects of research but is heightened in relation to infant feeding research because of the longstanding conflict involving the World Health Organisation, breastfeeding activist groups, and the infant formula industry, and as a consequence, all three of these organisations frequently resort to overinterpreting the data to favour their arguments,” he told this publication. An example is the suggestion that formula trials might contravene the International Code of Marketing of Breastmilk Substitutes because they provide free formula to participants. “Since when do participants in a research study have to pay for the intervention that is being studied?” he asked.
Dr. Stewart advised three key considerations “to mitigate the damaging effects that this type of inappropriate and misleading information may have on policy, practice, and engagement with parents.” First, it must be acknowledged that there is need for “a product that will provide a safety net for infants who are not offered breast milk,” he said. “It has been argued that to determine optimum nutrient requirements in infants and young children collaboration with nutrition companies is required.” Second, “all researchers need to comply with regulations relating to scientific methods, ethical standards, and financial diligence.” And finally, “there needs to be more effective planning and coordination of research activities to ensure that lessons are learned from the many studies that have design and methodological deficiencies.”
The study was funded by Imperial Health Charity. Ms. Pomeranz and Dr. Newman reported no conflicts of interest. Dr. Forsyth has undertaken consultancy work with governments, health care institutions, academia, and industry and has received research grants and honoraria from governments, charitable organizations and industry, including infant formula companies.
Senior author Robert J. Boyle, MBChB, MRCP, PhD, received personal fees from Cochrane, DBV Technologies, and Prota Therapeutics, and from expert witness work in cases of food anaphylaxis and class actions related to infant formula health claims, outside the submitted work, and received personal fees from Public Health England as a member of the UK Nutrition and Health Claims Committee and the Maternal and Child Nutrition Subgroup of the Scientific Advisory Committee on Nutrition. Coauthor Jo Leonardi-Bee, MSc, PhD, received fees from Danone Nutricia Research and the Food Standards Agency, outside of the submitted work.
Beloved psychiatrist dies at 102
Respected psychiatrist and psychoanalyst Irwin Marcus, MD, died on October 3. He was 102. Dedicated to his profession, Dr. Marcus was seeing patients until earlier this year. His long and illustrious career included creating and founding programs and organizations wherever he saw a need.
Among his many professional accomplishments, Dr. Marcus helped found the child and adolescent psychiatry program at Tulane University School of Medicine, New Orleans, and was one of the founders and a past president of the New Orleans Psychoanalytic Institute.
Dr. Marcus was also former chairman of the psychiatric department at Touro Infirmary and clinical professor emeritus at Louisiana State University Medical School, both in New Orleans.
“He initiated a number of traditions that are still important to us – community outreach, treating underserved youth, and strong interdisciplinary relationships,” Charles H. Zeanah, Jr., MD, current Mary Peters Sellars-Polchow chair of psychiatry at Tulane, told this news organization.
Dr. Marcus also continued to treat adult patients by phone and at his home until mid-June of this year. He had also started writing a children’s book.
It was his “tremendous work ethic” and creativity that kept him working past the age of 100, his wife, Angela Hill, a former news anchor, said in an interview.
Even vision loss resulting from macular degeneration and long-standing hearing problems did not stop him, she noted.
“He was always thinking creatively; he was always thinking intellectually,” said Ms. Hill. “That was, to me, the marvel of him.”
Wartime service, brain-trauma clinic
Born in Chicago in 1919, Dr. Marcus studied first at the Illinois Institute of Technology before transferring to the University of Illinois School of Medicine.
Neurosurgery was an early interest, and Dr. Marcus undertook his medical residency at Cook County Hospital in Chicago. The day after the bombing of Pearl Harbor, he enlisted in the U.S. Army.
During World War II, Dr. Marcus served in the Army Medical Corps and treated brain injuries and other wounds before he was badly injured himself and had to return to the United States for treatment.
After his recovery, he worked at an army medical facility in El Paso, Texas. On the basis of his earlier experiences, he founded a clinic there to diagnose and treat brain trauma.
After the war, Dr. Marcus continued his studies at Columbia University’s College of Physicians and Surgeons, in New York. Soon, his focus became psychiatry, child psychiatry, and psychoanalysis.
In 1951, Dr. Marcus accepted a position at Tulane. He created the Family Study Unit there the following year. Dr. Zeanah noted that the original name was chosen out of concern over the stigma associated with the term “child psychiatry.”
However, the environment changed relatively quickly, and the unit soon became known as Tulane Child Psychiatry.
Research, books, helmet patent
Dr. Marcus received Tulane’s first research grant in child psychiatry from the National Institute of Mental Health to investigate the potential mechanisms behind accident-prone children. That interest was inspired by his own clinical experience.
The findings, which were published in Monographs of the Society for Research in Child Development, showed that being accident prone was a nonspecific response to stressors from multiple sources, including a temperamental disposition, parent-child conflict, and family conflict.
To provide care to young patients, Dr. Marcus collaborated with the Children’s Bureau, the Jewish Children’s Home, the German Protestant’s Orphan Asylum, and Associated Catholic Charities.
‘He saved my life’
In 2002, Dr. Marcus participated in the 50th anniversary celebration of Tulane’s child psychiatry program. He returned in 2009 for what would be his final grand rounds presentation, which included an inspiring interview with Dr. Zeanah.
“He talked about the early history of child psychiatry, the things that he’d been trying to do, and some of the challenges that he faced,” Dr. Zeanah said.
Dr. Marcus’s former patients often told Ms. Hill how much he had helped them, she said.
“A couple walked up at a restaurant, and both of them said, ‘He saved our family.’”
Throughout his professional life, Dr. Marcus continued to strive toward growth and providing aid, she added.
“That is the bottom line of Irwin Marcus: All of his work was to help,” said Ms. Hill.
A version of this article first appeared on Medscape.
Respected psychiatrist and psychoanalyst Irwin Marcus, MD, died on October 3. He was 102. Dedicated to his profession, Dr. Marcus was seeing patients until earlier this year. His long and illustrious career included creating and founding programs and organizations wherever he saw a need.
Among his many professional accomplishments, Dr. Marcus helped found the child and adolescent psychiatry program at Tulane University School of Medicine, New Orleans, and was one of the founders and a past president of the New Orleans Psychoanalytic Institute.
Dr. Marcus was also former chairman of the psychiatric department at Touro Infirmary and clinical professor emeritus at Louisiana State University Medical School, both in New Orleans.
“He initiated a number of traditions that are still important to us – community outreach, treating underserved youth, and strong interdisciplinary relationships,” Charles H. Zeanah, Jr., MD, current Mary Peters Sellars-Polchow chair of psychiatry at Tulane, told this news organization.
Dr. Marcus also continued to treat adult patients by phone and at his home until mid-June of this year. He had also started writing a children’s book.
It was his “tremendous work ethic” and creativity that kept him working past the age of 100, his wife, Angela Hill, a former news anchor, said in an interview.
Even vision loss resulting from macular degeneration and long-standing hearing problems did not stop him, she noted.
“He was always thinking creatively; he was always thinking intellectually,” said Ms. Hill. “That was, to me, the marvel of him.”
Wartime service, brain-trauma clinic
Born in Chicago in 1919, Dr. Marcus studied first at the Illinois Institute of Technology before transferring to the University of Illinois School of Medicine.
Neurosurgery was an early interest, and Dr. Marcus undertook his medical residency at Cook County Hospital in Chicago. The day after the bombing of Pearl Harbor, he enlisted in the U.S. Army.
During World War II, Dr. Marcus served in the Army Medical Corps and treated brain injuries and other wounds before he was badly injured himself and had to return to the United States for treatment.
After his recovery, he worked at an army medical facility in El Paso, Texas. On the basis of his earlier experiences, he founded a clinic there to diagnose and treat brain trauma.
After the war, Dr. Marcus continued his studies at Columbia University’s College of Physicians and Surgeons, in New York. Soon, his focus became psychiatry, child psychiatry, and psychoanalysis.
In 1951, Dr. Marcus accepted a position at Tulane. He created the Family Study Unit there the following year. Dr. Zeanah noted that the original name was chosen out of concern over the stigma associated with the term “child psychiatry.”
However, the environment changed relatively quickly, and the unit soon became known as Tulane Child Psychiatry.
Research, books, helmet patent
Dr. Marcus received Tulane’s first research grant in child psychiatry from the National Institute of Mental Health to investigate the potential mechanisms behind accident-prone children. That interest was inspired by his own clinical experience.
The findings, which were published in Monographs of the Society for Research in Child Development, showed that being accident prone was a nonspecific response to stressors from multiple sources, including a temperamental disposition, parent-child conflict, and family conflict.
To provide care to young patients, Dr. Marcus collaborated with the Children’s Bureau, the Jewish Children’s Home, the German Protestant’s Orphan Asylum, and Associated Catholic Charities.
‘He saved my life’
In 2002, Dr. Marcus participated in the 50th anniversary celebration of Tulane’s child psychiatry program. He returned in 2009 for what would be his final grand rounds presentation, which included an inspiring interview with Dr. Zeanah.
“He talked about the early history of child psychiatry, the things that he’d been trying to do, and some of the challenges that he faced,” Dr. Zeanah said.
Dr. Marcus’s former patients often told Ms. Hill how much he had helped them, she said.
“A couple walked up at a restaurant, and both of them said, ‘He saved our family.’”
Throughout his professional life, Dr. Marcus continued to strive toward growth and providing aid, she added.
“That is the bottom line of Irwin Marcus: All of his work was to help,” said Ms. Hill.
A version of this article first appeared on Medscape.
Respected psychiatrist and psychoanalyst Irwin Marcus, MD, died on October 3. He was 102. Dedicated to his profession, Dr. Marcus was seeing patients until earlier this year. His long and illustrious career included creating and founding programs and organizations wherever he saw a need.
Among his many professional accomplishments, Dr. Marcus helped found the child and adolescent psychiatry program at Tulane University School of Medicine, New Orleans, and was one of the founders and a past president of the New Orleans Psychoanalytic Institute.
Dr. Marcus was also former chairman of the psychiatric department at Touro Infirmary and clinical professor emeritus at Louisiana State University Medical School, both in New Orleans.
“He initiated a number of traditions that are still important to us – community outreach, treating underserved youth, and strong interdisciplinary relationships,” Charles H. Zeanah, Jr., MD, current Mary Peters Sellars-Polchow chair of psychiatry at Tulane, told this news organization.
Dr. Marcus also continued to treat adult patients by phone and at his home until mid-June of this year. He had also started writing a children’s book.
It was his “tremendous work ethic” and creativity that kept him working past the age of 100, his wife, Angela Hill, a former news anchor, said in an interview.
Even vision loss resulting from macular degeneration and long-standing hearing problems did not stop him, she noted.
“He was always thinking creatively; he was always thinking intellectually,” said Ms. Hill. “That was, to me, the marvel of him.”
Wartime service, brain-trauma clinic
Born in Chicago in 1919, Dr. Marcus studied first at the Illinois Institute of Technology before transferring to the University of Illinois School of Medicine.
Neurosurgery was an early interest, and Dr. Marcus undertook his medical residency at Cook County Hospital in Chicago. The day after the bombing of Pearl Harbor, he enlisted in the U.S. Army.
During World War II, Dr. Marcus served in the Army Medical Corps and treated brain injuries and other wounds before he was badly injured himself and had to return to the United States for treatment.
After his recovery, he worked at an army medical facility in El Paso, Texas. On the basis of his earlier experiences, he founded a clinic there to diagnose and treat brain trauma.
After the war, Dr. Marcus continued his studies at Columbia University’s College of Physicians and Surgeons, in New York. Soon, his focus became psychiatry, child psychiatry, and psychoanalysis.
In 1951, Dr. Marcus accepted a position at Tulane. He created the Family Study Unit there the following year. Dr. Zeanah noted that the original name was chosen out of concern over the stigma associated with the term “child psychiatry.”
However, the environment changed relatively quickly, and the unit soon became known as Tulane Child Psychiatry.
Research, books, helmet patent
Dr. Marcus received Tulane’s first research grant in child psychiatry from the National Institute of Mental Health to investigate the potential mechanisms behind accident-prone children. That interest was inspired by his own clinical experience.
The findings, which were published in Monographs of the Society for Research in Child Development, showed that being accident prone was a nonspecific response to stressors from multiple sources, including a temperamental disposition, parent-child conflict, and family conflict.
To provide care to young patients, Dr. Marcus collaborated with the Children’s Bureau, the Jewish Children’s Home, the German Protestant’s Orphan Asylum, and Associated Catholic Charities.
‘He saved my life’
In 2002, Dr. Marcus participated in the 50th anniversary celebration of Tulane’s child psychiatry program. He returned in 2009 for what would be his final grand rounds presentation, which included an inspiring interview with Dr. Zeanah.
“He talked about the early history of child psychiatry, the things that he’d been trying to do, and some of the challenges that he faced,” Dr. Zeanah said.
Dr. Marcus’s former patients often told Ms. Hill how much he had helped them, she said.
“A couple walked up at a restaurant, and both of them said, ‘He saved our family.’”
Throughout his professional life, Dr. Marcus continued to strive toward growth and providing aid, she added.
“That is the bottom line of Irwin Marcus: All of his work was to help,” said Ms. Hill.
A version of this article first appeared on Medscape.
Flesh-Colored Papule in the Nose of a Child
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).
Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).
Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).
Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
A 4-year-old girl presented to our clinic with an asymptomatic flesh-colored papule in the left nostril. The lesion had been present since birth and grew in relation to the patient with no rapid changes. There had been no pigmentation changes and no bleeding, pain, or itching. The patient’s birth and developmental history were normal. Physical examination revealed a singular, 10×5-mm, flesh-colored, pedunculated mass on the left nasal sill. There were no additional lesions present. An excisional biopsy was performed and submitted for pathologic diagnosis.
How can doctors help kids recover from COVID-19 school disruptions?
Physicians may be able to help students get back on track after the pandemic derailed normal schooling, a developmental and behavioral pediatrician suggests.
The disruptions especially affected vulnerable students, such as those with disabilities and those affected by poverty. But academic setbacks occurred across grades and demographics.
“What we know is that, if it was bad before COVID, things are much worse now,” Eric Tridas, MD, said at the annual meeting of the American Academy of Pediatrics. “The pandemic disproportionately affected vulnerable populations. It exacerbated their learning and mental health problems to a high degree.”
In an effort to help kids catch up, pediatricians can provide information to parents about approaches to accelerated academic instruction, Dr. Tridas suggested. They also can monitor for depression and anxiety, and provide appropriate referrals and, if needed, medication, said Dr. Tridas, who is a member of the National Joint Committee on Learning Disabilities.
Doctors also can collaborate with educators to establish schoolwide plans to address mental health problems, he said.
Dr. Tridas focused on vulnerable populations, including students with neurodevelopmental disorders, as well as students of color, English language learners, and Indigenous populations. But other research presented at the AAP meeting focused on challenges that college students in general encountered during the pandemic.
Nelson Chow, a research intern at Cohen Children’s Medical Center in New Hyde Park, N.Y., and colleagues surveyed college students in June 2020 about academic barriers when their schools switched to virtual learning.
Nearly 80% of the 307 respondents had difficulties concentrating. Many students also agreed that responsibilities at home (57.6%), mental health issues (46.3%), family relationships (37.8%), financial hardships (31.5%), and limited Internet access (25.1%) were among the factors that posed academic barriers.
A larger proportion of Hispanic students reported that responsibilities at home were a challenge, compared with non-Hispanic students, the researchers found.
“It is especially important to have a particular awareness of the cultural and socioeconomic factors that may impact students’ outcomes,” Mr. Chow said in a news release highlighting the research.
Although studies indicate that the pandemic led to academic losses across the board in terms of students not learning as much as usual, these setbacks were more pronounced for vulnerable populations, Dr. Tridas said.
What can busy pediatricians do? “We can at least inquire about how the kids are doing educationally, and with mental health. That’s it. If we do that, we are doing an awful lot.”
Education
Dr. Tridas pointed meeting attendees to a report from the National Center for Learning Disabilities, “Promising Practices to Accelerate Learning for Students with Disabilities During COVID-19 and Beyond,” that he said could be a helpful resource for pediatricians, parents, and educators who want to learn more about accelerated learning approaches.
Research indicates that these strategies “may help in a situation like this,” Dr. Tridas said.
Accelerated approaches typically simplify the curriculum to focus on essential reading, writing, and math skills that most students should acquire by third grade, while capitalizing on students’ strengths and interests.
Despite vulnerable students having fallen farther behind academically, they likely are doing the same thing in school that they were doing before COVID-19, “which was not working to begin with,” he said. “That is why I try to provide parents and pediatricians with ways of ... recognizing when appropriate instruction is being provided.”
Sharing this information does not necessarily mean that schools will implement those strategies, or that schools are not applying them already. Still, making parents aware of these approaches can help, he said.
Emotional health
Social isolation, loss of routine and structure, more screen time, and changes in sleeping and eating patterns during the pandemic are factors that may have exacerbated mental health problems in students.
Vulnerable populations are at higher risk for these issues, and it will be important to monitor these kids for suicidal ideation and depression, especially in middle school and high school, Dr. Tridas said.
Doctors should establish alliances with mental health providers in their communities if they are not able to provide cognitive-behavioral therapy or medication management in their own practices.
And at home and at school, children should have structure and consistency, positive enforcement of appropriate conduct, and a safe environment that allows them to fail and try again, Dr. Tridas said.
Dr. Tridas and Mr. Chow had no relevant financial disclosures.
Physicians may be able to help students get back on track after the pandemic derailed normal schooling, a developmental and behavioral pediatrician suggests.
The disruptions especially affected vulnerable students, such as those with disabilities and those affected by poverty. But academic setbacks occurred across grades and demographics.
“What we know is that, if it was bad before COVID, things are much worse now,” Eric Tridas, MD, said at the annual meeting of the American Academy of Pediatrics. “The pandemic disproportionately affected vulnerable populations. It exacerbated their learning and mental health problems to a high degree.”
In an effort to help kids catch up, pediatricians can provide information to parents about approaches to accelerated academic instruction, Dr. Tridas suggested. They also can monitor for depression and anxiety, and provide appropriate referrals and, if needed, medication, said Dr. Tridas, who is a member of the National Joint Committee on Learning Disabilities.
Doctors also can collaborate with educators to establish schoolwide plans to address mental health problems, he said.
Dr. Tridas focused on vulnerable populations, including students with neurodevelopmental disorders, as well as students of color, English language learners, and Indigenous populations. But other research presented at the AAP meeting focused on challenges that college students in general encountered during the pandemic.
Nelson Chow, a research intern at Cohen Children’s Medical Center in New Hyde Park, N.Y., and colleagues surveyed college students in June 2020 about academic barriers when their schools switched to virtual learning.
Nearly 80% of the 307 respondents had difficulties concentrating. Many students also agreed that responsibilities at home (57.6%), mental health issues (46.3%), family relationships (37.8%), financial hardships (31.5%), and limited Internet access (25.1%) were among the factors that posed academic barriers.
A larger proportion of Hispanic students reported that responsibilities at home were a challenge, compared with non-Hispanic students, the researchers found.
“It is especially important to have a particular awareness of the cultural and socioeconomic factors that may impact students’ outcomes,” Mr. Chow said in a news release highlighting the research.
Although studies indicate that the pandemic led to academic losses across the board in terms of students not learning as much as usual, these setbacks were more pronounced for vulnerable populations, Dr. Tridas said.
What can busy pediatricians do? “We can at least inquire about how the kids are doing educationally, and with mental health. That’s it. If we do that, we are doing an awful lot.”
Education
Dr. Tridas pointed meeting attendees to a report from the National Center for Learning Disabilities, “Promising Practices to Accelerate Learning for Students with Disabilities During COVID-19 and Beyond,” that he said could be a helpful resource for pediatricians, parents, and educators who want to learn more about accelerated learning approaches.
Research indicates that these strategies “may help in a situation like this,” Dr. Tridas said.
Accelerated approaches typically simplify the curriculum to focus on essential reading, writing, and math skills that most students should acquire by third grade, while capitalizing on students’ strengths and interests.
Despite vulnerable students having fallen farther behind academically, they likely are doing the same thing in school that they were doing before COVID-19, “which was not working to begin with,” he said. “That is why I try to provide parents and pediatricians with ways of ... recognizing when appropriate instruction is being provided.”
Sharing this information does not necessarily mean that schools will implement those strategies, or that schools are not applying them already. Still, making parents aware of these approaches can help, he said.
Emotional health
Social isolation, loss of routine and structure, more screen time, and changes in sleeping and eating patterns during the pandemic are factors that may have exacerbated mental health problems in students.
Vulnerable populations are at higher risk for these issues, and it will be important to monitor these kids for suicidal ideation and depression, especially in middle school and high school, Dr. Tridas said.
Doctors should establish alliances with mental health providers in their communities if they are not able to provide cognitive-behavioral therapy or medication management in their own practices.
And at home and at school, children should have structure and consistency, positive enforcement of appropriate conduct, and a safe environment that allows them to fail and try again, Dr. Tridas said.
Dr. Tridas and Mr. Chow had no relevant financial disclosures.
Physicians may be able to help students get back on track after the pandemic derailed normal schooling, a developmental and behavioral pediatrician suggests.
The disruptions especially affected vulnerable students, such as those with disabilities and those affected by poverty. But academic setbacks occurred across grades and demographics.
“What we know is that, if it was bad before COVID, things are much worse now,” Eric Tridas, MD, said at the annual meeting of the American Academy of Pediatrics. “The pandemic disproportionately affected vulnerable populations. It exacerbated their learning and mental health problems to a high degree.”
In an effort to help kids catch up, pediatricians can provide information to parents about approaches to accelerated academic instruction, Dr. Tridas suggested. They also can monitor for depression and anxiety, and provide appropriate referrals and, if needed, medication, said Dr. Tridas, who is a member of the National Joint Committee on Learning Disabilities.
Doctors also can collaborate with educators to establish schoolwide plans to address mental health problems, he said.
Dr. Tridas focused on vulnerable populations, including students with neurodevelopmental disorders, as well as students of color, English language learners, and Indigenous populations. But other research presented at the AAP meeting focused on challenges that college students in general encountered during the pandemic.
Nelson Chow, a research intern at Cohen Children’s Medical Center in New Hyde Park, N.Y., and colleagues surveyed college students in June 2020 about academic barriers when their schools switched to virtual learning.
Nearly 80% of the 307 respondents had difficulties concentrating. Many students also agreed that responsibilities at home (57.6%), mental health issues (46.3%), family relationships (37.8%), financial hardships (31.5%), and limited Internet access (25.1%) were among the factors that posed academic barriers.
A larger proportion of Hispanic students reported that responsibilities at home were a challenge, compared with non-Hispanic students, the researchers found.
“It is especially important to have a particular awareness of the cultural and socioeconomic factors that may impact students’ outcomes,” Mr. Chow said in a news release highlighting the research.
Although studies indicate that the pandemic led to academic losses across the board in terms of students not learning as much as usual, these setbacks were more pronounced for vulnerable populations, Dr. Tridas said.
What can busy pediatricians do? “We can at least inquire about how the kids are doing educationally, and with mental health. That’s it. If we do that, we are doing an awful lot.”
Education
Dr. Tridas pointed meeting attendees to a report from the National Center for Learning Disabilities, “Promising Practices to Accelerate Learning for Students with Disabilities During COVID-19 and Beyond,” that he said could be a helpful resource for pediatricians, parents, and educators who want to learn more about accelerated learning approaches.
Research indicates that these strategies “may help in a situation like this,” Dr. Tridas said.
Accelerated approaches typically simplify the curriculum to focus on essential reading, writing, and math skills that most students should acquire by third grade, while capitalizing on students’ strengths and interests.
Despite vulnerable students having fallen farther behind academically, they likely are doing the same thing in school that they were doing before COVID-19, “which was not working to begin with,” he said. “That is why I try to provide parents and pediatricians with ways of ... recognizing when appropriate instruction is being provided.”
Sharing this information does not necessarily mean that schools will implement those strategies, or that schools are not applying them already. Still, making parents aware of these approaches can help, he said.
Emotional health
Social isolation, loss of routine and structure, more screen time, and changes in sleeping and eating patterns during the pandemic are factors that may have exacerbated mental health problems in students.
Vulnerable populations are at higher risk for these issues, and it will be important to monitor these kids for suicidal ideation and depression, especially in middle school and high school, Dr. Tridas said.
Doctors should establish alliances with mental health providers in their communities if they are not able to provide cognitive-behavioral therapy or medication management in their own practices.
And at home and at school, children should have structure and consistency, positive enforcement of appropriate conduct, and a safe environment that allows them to fail and try again, Dr. Tridas said.
Dr. Tridas and Mr. Chow had no relevant financial disclosures.
FROM AAP 2021