User login
Lesbian, gay, bisexual youth miss out on health care
Youth identifying as lesbian, gay, or bisexual were significantly less likely than were their peers to communicate with a physician or utilize health care in the past 12 months, according to data from a cohort study of approximately 4,000 adolescents.
Disparities in physical and mental health outcomes for individuals who identify as lesbian, gay, or bisexual (LGB) persist in the United States, and emerge in adolescents and young adults, wrote Sari L. Reisner, ScD, of Boston Children’s Hospital, and colleagues.
“LGB adult research indicates substantial unmet medical needs, including needed care and preventive care,” for reasons including “reluctance to disclose sexual identity to clinicians, lower health insurance rates, lack of culturally appropriate preventive services, and lack of clinician LGB care competence,” they said.
However, health use trends by adolescents who identify as LGB have not been well studied, they noted.
In a study published in JAMA Network Open, the researchers analyzed data from 4,256 participants in the third wave (10th grade) of adolescents in Healthy Passages, a longitudinal, observational cohort study of diverse public school students in Birmingham, Ala.; Houston; and Los Angeles County. Data were collected in grades 5, 7, and 10.
The study population included 640 youth who identified as LGB, and 3,616 non-LGB youth. Sexual status was based on responses to questions in the grade 10 youth survey. Health care use was based on the responses to questions about routine care, such as a regular checkup, and other care, such as a sick visit. Data on delayed care were collected from parents and youth. At baseline, the average age of the study participants in fifth grade was 11 years, 48.9% were female, 44.5% were Hispanic or Latino, and 28.9% were Black.
Overall, more LGB youth reported not receiving needed medical care when they thought they needed it within the past 12 months compared with non-LGB youth (42.4% of LGB vs. 30.2% of non-LGB youth; adjusted odds ratio 1.68). The most common conditions for which LGB youth did not seek care were sexually transmitted infections, contraception, and substance use.
Overall, the main reason given for not seeking medical care was that they thought the problem would go away (approximately 26% for LGB and non-LGB). Approximately twice as many LGB youth as non-LGB youth said they avoided medical care because they did not want their parents to know (14.5% vs. 9.4%).
Significantly more LGB youth than non-LGB youth reported difficulty communicating with their physicians in the past 12 months (15.3% vs. 9.4%; aOR 1.71). The main reasons for not communicating with a clinician about a topic of concern were that the adolescent did not want parents to know (40.7% of LGB and 30.2% of non-LGB) and that they were too embarrassed to talk about the topic (37.5% of LGB and 25.9% of non-LGB).
The researchers were not surprised that “LGB youth self-reported greater difficulty communicating with a clinician about topics they wanted to discuss,” but they found no significant differences in reasons for communication difficulty based on sexual orientation.
Approximately two-thirds (65.8%) of LGB youth reported feeling “a little or not at all comfortable” talking to a health care clinician about their sexual attractions, compared with approximately one-third (37.8%) of non-LGB youth.
Only 12.5% of the LGB youth said that their clinicians knew their sexual orientation, the researchers noted. However, clinicians need to know youths’ sexual orientation to provide appropriate and comprehensive care, they said, especially in light of the known negative health consequences of LGB internalized stigma, as well as the pertinence of certain sexual behaviors to preventive care and screening.
The study findings were limited by several factors including the cross-sectional design and inability to show causality, and by the incongruence of different dimensions of sexual orientation, the researchers noted. Other limitations included the use only of English and Spanish language, and a lack of complete information on disclosure of sexual orientation to parents, the researchers noted.
The results were strengthened by the diverse demographics, although they may not be generalizable to a wider population, they added.
However, the data show that responsive health care is needed to reduce disparities for LGB youth, they emphasized. “Care should be sensitive and respectful to sexual orientation for all youth, with clinicians taking time to ask adolescents about their sexual identity, attractions, and behaviors, particularly in sexual and reproductive health,” they concluded.
Adolescents suffer barriers similar to those of adults
“We know that significant health disparities exist for LGBTQ adults and adolescents,” Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “LGBTQ adults often have had poor experiences during health care encounters – ranging from poor interactions with inadequately trained clinicians to frank discrimination,” she said. “These experiences can prevent individuals from seeking health care in the future or disclosing important information during a medical visit, both of which can contribute to worsened health outcomes,” she emphasized.
Prior to this study, data to confirm similar patterns of decreased health care utilization in LGB youth were limited, Dr. Curran said. “Identifying and understanding barriers to health care for LGBTQ youth are essential to help address the disparities in this population,” she said.
Dr. Curran said she was not surprised by the study findings for adolescents, which reflect patterns seen in LGBTQ adults.
Overcoming barriers to encourage LGB youth to seek regular medical care involves “training health care professionals about LGBTQ health, teaching the skill of taking a nonjudgmental, inclusive history, and making health care facilities welcoming and inclusive, such as displaying a pride flag in clinic, and using forms asking for pronouns,” Dr. Curran said.
Dr. Curran said she thinks the trends in decreased health care use are similar for transgender youth. “I suspect, if anything, that transgender youth will have even further decreased health care utilization when compared to cisgender heterosexual peers and LGB peers,” she noted.
Going forward, it will be important to understand the reasons behind decreased health care use among LGB youth, such as poor experiences, discrimination, or fears about confidentiality, said Dr. Curran. “Additionally, it would be important to understand if this decreased health utilization also occurs with transgender youth,” she said.
The Healthy Passages Study was funded by the Centers for Disease Control and Prevention. One of the study coauthors disclosed funding from the Agency for Healthcare Research and Quality as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Youth identifying as lesbian, gay, or bisexual were significantly less likely than were their peers to communicate with a physician or utilize health care in the past 12 months, according to data from a cohort study of approximately 4,000 adolescents.
Disparities in physical and mental health outcomes for individuals who identify as lesbian, gay, or bisexual (LGB) persist in the United States, and emerge in adolescents and young adults, wrote Sari L. Reisner, ScD, of Boston Children’s Hospital, and colleagues.
“LGB adult research indicates substantial unmet medical needs, including needed care and preventive care,” for reasons including “reluctance to disclose sexual identity to clinicians, lower health insurance rates, lack of culturally appropriate preventive services, and lack of clinician LGB care competence,” they said.
However, health use trends by adolescents who identify as LGB have not been well studied, they noted.
In a study published in JAMA Network Open, the researchers analyzed data from 4,256 participants in the third wave (10th grade) of adolescents in Healthy Passages, a longitudinal, observational cohort study of diverse public school students in Birmingham, Ala.; Houston; and Los Angeles County. Data were collected in grades 5, 7, and 10.
The study population included 640 youth who identified as LGB, and 3,616 non-LGB youth. Sexual status was based on responses to questions in the grade 10 youth survey. Health care use was based on the responses to questions about routine care, such as a regular checkup, and other care, such as a sick visit. Data on delayed care were collected from parents and youth. At baseline, the average age of the study participants in fifth grade was 11 years, 48.9% were female, 44.5% were Hispanic or Latino, and 28.9% were Black.
Overall, more LGB youth reported not receiving needed medical care when they thought they needed it within the past 12 months compared with non-LGB youth (42.4% of LGB vs. 30.2% of non-LGB youth; adjusted odds ratio 1.68). The most common conditions for which LGB youth did not seek care were sexually transmitted infections, contraception, and substance use.
Overall, the main reason given for not seeking medical care was that they thought the problem would go away (approximately 26% for LGB and non-LGB). Approximately twice as many LGB youth as non-LGB youth said they avoided medical care because they did not want their parents to know (14.5% vs. 9.4%).
Significantly more LGB youth than non-LGB youth reported difficulty communicating with their physicians in the past 12 months (15.3% vs. 9.4%; aOR 1.71). The main reasons for not communicating with a clinician about a topic of concern were that the adolescent did not want parents to know (40.7% of LGB and 30.2% of non-LGB) and that they were too embarrassed to talk about the topic (37.5% of LGB and 25.9% of non-LGB).
The researchers were not surprised that “LGB youth self-reported greater difficulty communicating with a clinician about topics they wanted to discuss,” but they found no significant differences in reasons for communication difficulty based on sexual orientation.
Approximately two-thirds (65.8%) of LGB youth reported feeling “a little or not at all comfortable” talking to a health care clinician about their sexual attractions, compared with approximately one-third (37.8%) of non-LGB youth.
Only 12.5% of the LGB youth said that their clinicians knew their sexual orientation, the researchers noted. However, clinicians need to know youths’ sexual orientation to provide appropriate and comprehensive care, they said, especially in light of the known negative health consequences of LGB internalized stigma, as well as the pertinence of certain sexual behaviors to preventive care and screening.
The study findings were limited by several factors including the cross-sectional design and inability to show causality, and by the incongruence of different dimensions of sexual orientation, the researchers noted. Other limitations included the use only of English and Spanish language, and a lack of complete information on disclosure of sexual orientation to parents, the researchers noted.
The results were strengthened by the diverse demographics, although they may not be generalizable to a wider population, they added.
However, the data show that responsive health care is needed to reduce disparities for LGB youth, they emphasized. “Care should be sensitive and respectful to sexual orientation for all youth, with clinicians taking time to ask adolescents about their sexual identity, attractions, and behaviors, particularly in sexual and reproductive health,” they concluded.
Adolescents suffer barriers similar to those of adults
“We know that significant health disparities exist for LGBTQ adults and adolescents,” Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “LGBTQ adults often have had poor experiences during health care encounters – ranging from poor interactions with inadequately trained clinicians to frank discrimination,” she said. “These experiences can prevent individuals from seeking health care in the future or disclosing important information during a medical visit, both of which can contribute to worsened health outcomes,” she emphasized.
Prior to this study, data to confirm similar patterns of decreased health care utilization in LGB youth were limited, Dr. Curran said. “Identifying and understanding barriers to health care for LGBTQ youth are essential to help address the disparities in this population,” she said.
Dr. Curran said she was not surprised by the study findings for adolescents, which reflect patterns seen in LGBTQ adults.
Overcoming barriers to encourage LGB youth to seek regular medical care involves “training health care professionals about LGBTQ health, teaching the skill of taking a nonjudgmental, inclusive history, and making health care facilities welcoming and inclusive, such as displaying a pride flag in clinic, and using forms asking for pronouns,” Dr. Curran said.
Dr. Curran said she thinks the trends in decreased health care use are similar for transgender youth. “I suspect, if anything, that transgender youth will have even further decreased health care utilization when compared to cisgender heterosexual peers and LGB peers,” she noted.
Going forward, it will be important to understand the reasons behind decreased health care use among LGB youth, such as poor experiences, discrimination, or fears about confidentiality, said Dr. Curran. “Additionally, it would be important to understand if this decreased health utilization also occurs with transgender youth,” she said.
The Healthy Passages Study was funded by the Centers for Disease Control and Prevention. One of the study coauthors disclosed funding from the Agency for Healthcare Research and Quality as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Youth identifying as lesbian, gay, or bisexual were significantly less likely than were their peers to communicate with a physician or utilize health care in the past 12 months, according to data from a cohort study of approximately 4,000 adolescents.
Disparities in physical and mental health outcomes for individuals who identify as lesbian, gay, or bisexual (LGB) persist in the United States, and emerge in adolescents and young adults, wrote Sari L. Reisner, ScD, of Boston Children’s Hospital, and colleagues.
“LGB adult research indicates substantial unmet medical needs, including needed care and preventive care,” for reasons including “reluctance to disclose sexual identity to clinicians, lower health insurance rates, lack of culturally appropriate preventive services, and lack of clinician LGB care competence,” they said.
However, health use trends by adolescents who identify as LGB have not been well studied, they noted.
In a study published in JAMA Network Open, the researchers analyzed data from 4,256 participants in the third wave (10th grade) of adolescents in Healthy Passages, a longitudinal, observational cohort study of diverse public school students in Birmingham, Ala.; Houston; and Los Angeles County. Data were collected in grades 5, 7, and 10.
The study population included 640 youth who identified as LGB, and 3,616 non-LGB youth. Sexual status was based on responses to questions in the grade 10 youth survey. Health care use was based on the responses to questions about routine care, such as a regular checkup, and other care, such as a sick visit. Data on delayed care were collected from parents and youth. At baseline, the average age of the study participants in fifth grade was 11 years, 48.9% were female, 44.5% were Hispanic or Latino, and 28.9% were Black.
Overall, more LGB youth reported not receiving needed medical care when they thought they needed it within the past 12 months compared with non-LGB youth (42.4% of LGB vs. 30.2% of non-LGB youth; adjusted odds ratio 1.68). The most common conditions for which LGB youth did not seek care were sexually transmitted infections, contraception, and substance use.
Overall, the main reason given for not seeking medical care was that they thought the problem would go away (approximately 26% for LGB and non-LGB). Approximately twice as many LGB youth as non-LGB youth said they avoided medical care because they did not want their parents to know (14.5% vs. 9.4%).
Significantly more LGB youth than non-LGB youth reported difficulty communicating with their physicians in the past 12 months (15.3% vs. 9.4%; aOR 1.71). The main reasons for not communicating with a clinician about a topic of concern were that the adolescent did not want parents to know (40.7% of LGB and 30.2% of non-LGB) and that they were too embarrassed to talk about the topic (37.5% of LGB and 25.9% of non-LGB).
The researchers were not surprised that “LGB youth self-reported greater difficulty communicating with a clinician about topics they wanted to discuss,” but they found no significant differences in reasons for communication difficulty based on sexual orientation.
Approximately two-thirds (65.8%) of LGB youth reported feeling “a little or not at all comfortable” talking to a health care clinician about their sexual attractions, compared with approximately one-third (37.8%) of non-LGB youth.
Only 12.5% of the LGB youth said that their clinicians knew their sexual orientation, the researchers noted. However, clinicians need to know youths’ sexual orientation to provide appropriate and comprehensive care, they said, especially in light of the known negative health consequences of LGB internalized stigma, as well as the pertinence of certain sexual behaviors to preventive care and screening.
The study findings were limited by several factors including the cross-sectional design and inability to show causality, and by the incongruence of different dimensions of sexual orientation, the researchers noted. Other limitations included the use only of English and Spanish language, and a lack of complete information on disclosure of sexual orientation to parents, the researchers noted.
The results were strengthened by the diverse demographics, although they may not be generalizable to a wider population, they added.
However, the data show that responsive health care is needed to reduce disparities for LGB youth, they emphasized. “Care should be sensitive and respectful to sexual orientation for all youth, with clinicians taking time to ask adolescents about their sexual identity, attractions, and behaviors, particularly in sexual and reproductive health,” they concluded.
Adolescents suffer barriers similar to those of adults
“We know that significant health disparities exist for LGBTQ adults and adolescents,” Kelly Curran, MD, of the University of Oklahoma Health Sciences Center, Oklahoma City, said in an interview. “LGBTQ adults often have had poor experiences during health care encounters – ranging from poor interactions with inadequately trained clinicians to frank discrimination,” she said. “These experiences can prevent individuals from seeking health care in the future or disclosing important information during a medical visit, both of which can contribute to worsened health outcomes,” she emphasized.
Prior to this study, data to confirm similar patterns of decreased health care utilization in LGB youth were limited, Dr. Curran said. “Identifying and understanding barriers to health care for LGBTQ youth are essential to help address the disparities in this population,” she said.
Dr. Curran said she was not surprised by the study findings for adolescents, which reflect patterns seen in LGBTQ adults.
Overcoming barriers to encourage LGB youth to seek regular medical care involves “training health care professionals about LGBTQ health, teaching the skill of taking a nonjudgmental, inclusive history, and making health care facilities welcoming and inclusive, such as displaying a pride flag in clinic, and using forms asking for pronouns,” Dr. Curran said.
Dr. Curran said she thinks the trends in decreased health care use are similar for transgender youth. “I suspect, if anything, that transgender youth will have even further decreased health care utilization when compared to cisgender heterosexual peers and LGB peers,” she noted.
Going forward, it will be important to understand the reasons behind decreased health care use among LGB youth, such as poor experiences, discrimination, or fears about confidentiality, said Dr. Curran. “Additionally, it would be important to understand if this decreased health utilization also occurs with transgender youth,” she said.
The Healthy Passages Study was funded by the Centers for Disease Control and Prevention. One of the study coauthors disclosed funding from the Agency for Healthcare Research and Quality as part of the Harvard-wide Pediatric Health Services Research Fellowship Program. The researchers had no financial conflicts to disclose. Dr. Curran had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA NETWORK OPEN
HCV in pregnancy: One piece of a bigger problem
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
FROM JAMA HEALTH FORUM
Children and COVID: A look at the pace of vaccination
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?
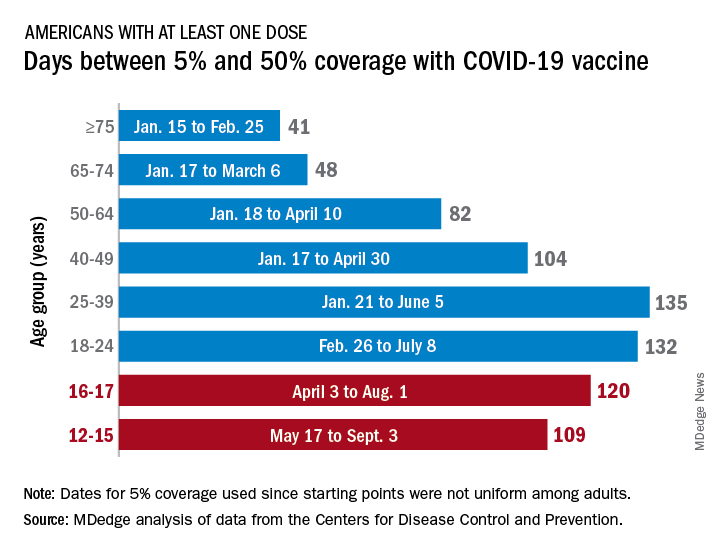
Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
The impact of modifiable risk factors such as diet and obesity in Pediatric MS patients
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
James Nicholas Brenton, M.D., is the director of the University of Virginia’s Pediatric and Young Adult MS and Related Disorders Clinic. He is also associate professor of neurology and pediatrics for clinical research and performs collaborative clinical research within the field of pediatric MS. His research focuses on pediatric demyelinating disease and autoimmune epilepsies.
As the director of a clinic focusing on pediatric and young adults MS and related disorders, how do modifiable risk factors such as obesity, smoking, et cetera, increase the risk of MS in general?
Dr. Brenton: There are several risk factors for pediatric-onset MS. When I say pediatric-onset, I'm referring to patients with clinical onset of MS prior to the age of 18 years. Some MS risk factors are not considered “modifiable,” such as genetic risks. The greatest genetic risk for MS is related to specific haplotypes in the HLA-DRB1 gene. Another risk factor that is less amenable to modification is early exposure to certain viruses, like the Epstein-Barr virus (Makhani, et al 2016).
On the other hand, there are several potentially modifiable risk factors for MS. This includes smoking - either first or second-hand smoke. In the case of pediatric MS patients, it is most often related to second-hand (or passive) smoke exposure (Lavery, et al 2019). Another example of a modifiable MS risk factor is vitamin D deficiency. Vitamin D levels are influenced significantly by duration and intensity of direct exposure to sunlight, which depends (in part) on the geographic location of where you grow up. For example, those who live at higher latitudes (e.g. live further away from the equator) have less exposure to direct sunlight than a child who lives at lower latitudes (e.g. closer to the equator) (Banwell, et al 2011).
Obesity during childhood or adolescence is another modifiable risk factor for MS. Obesity’s risk for MS (like smoking) is dose-dependent – meaning, the more obese that you are, the higher your overall risk for future development of MS. In fact, the BMI in children with MS is markedly higher than their non-MS peers, and begins in early childhood, years before the clinical onset of the disease (Brenton, et al 2019).
There is mixed evidence regarding the impact of certain perinatal factors on future risk for MS. For example, some literature suggests that Caesarean delivery increases the risk of MS (Maghzi, et al 2012). Our research has found that infantile breastfeeding is associated with a lower future risk of pediatric-onset MS (Brenton, et al 2017).
Children are two to three times more likely to experience MS relapses compared with adults. How likely is it for the childhood obesity epidemic to lead to increased morbidity from MS or CIS, particularly in adolescent girls?
Dr. Brenton: Obesity is a systemic disease that manifests as excessive or abnormal accumulation of body fat. We know that chronic obesity leads to higher overall morbidity, lower quality of life, and reduced life expectancy. There are several common co-morbidities associated with obesity - like cardiovascular disease, type II diabetes mellitus, hypertension, polycystic ovarian syndrome, dyslipidemia, infertility, and some cancers (Abdelaal, et al 2017). Certainly, all these implications for the general population would pertain to those with MS who exhibit chronic obesity.
While we have fairly good evidence that obesity is a causal risk factor for the development of MS, there actually is a paucity of literature that has studied the impact of persistent obesity on an already established MS disease state. Several recent studies show that obesity is associated with a pro-inflammatory state in the blood and cerebrospinal fluid of MS patients (Stampanoni, et al 2019). There are other studies that shown a direct association between MS-related neurologic disability and obesity – such that those with a greater waist circumference exhibit higher rates of neurologic disability (Fitzgerald, et al 2019).
Recent studies have assessed whether SNAP factors are associated with health outcomes. How does a modifiable SNAP risk score in people with multiple sclerosis impacts the likelihood of disability worsening??
Dr. Brenton: SNAP factors may not be as well known to some people in this field. SNAP factors refer to smoking (“S”), poor nutrition (“N”), alcohol consumption (“A”) and insufficient physical activity (“P”). These four factors appear to be the most preventable causes of morbidity within the general population. SNAP factors are common in people with MS. The most common SNAP factors in MS patients are poor nutrition and insufficient physical activity. Cross-sectionally, these factors appear to be associated with worsening neurologic disability (Marck, et al 2019).
There is data suggesting that SNAP factors, particularly those that increase over time, can associate with worsening disability when followed over several years. Importantly, your baseline SNAP score does not appear to predict your future level of disability (Marck, et al 2019). Collective SNAP scores have not yet been well-studied in pediatric MS patients, but are important to study - particularly given that children with MS reach maximum neurologic disability at a younger age than adult-onset MS patients (Renoux, et al 2007).
What are some of the best practices MS health care providers can engage in to promote exercise and rehabilitative protocols to significantly impact the physical and cognitive performance of MS patients?
Dr. Brenton: Even though pediatric MS patients exhibit relatively low levels of physical neurologic disability early in their disease, the physical activity levels of youth with MS are quite low. These patients engage in less moderate and vigorous physical activity when you compare them to their non-MS peers (Grover, et al 2016), but we still don't fully understand why this is the case. In fact, it may be related to several different factors - including pain, fatigue, sleep quality, MS disease activity, and psychological factors (such as depression, social anxiety, and perceptions of self-efficacy). In order to truly provide patient-specific interventions that positively impact physical activity we need to better understand what factors to study and how these factors play into the individual patient. For example, if high levels of fatigue are inhibiting a patient from being physically active, the provider should explore sources of fatigue: “how are sleep patterns?”, “are they napping throughout the day?”, “does the fatigue occur only after a period of physical activity, or is it persistent despite how active they are?” These are examples of questions that may lead a neurologist to different approaches for managing reduced physical activity.
Generally speaking however, pediatric and adult MS providers would ideally provide healthy nutrition guidance and counseling to all patients, regardless of their weight. Though there is no particular proven “MS diet,” in general, we recommend a balanced diet that is lower in saturated fats and processed sugars and higher in fruits and vegetables. In the case of a pediatric MS patient, it's important to have the family on board with consuming a healthier diet, as parental involvement increases the likelihood of healthy behavioral changes in the child.
It is important to ask patients targeted questions about their physical activity and assist with goal setting toward achievable targets. If the patient is receptive, a provider can advise on the use of digital interventions, like apps or internet-based social groups that incorporate education, accountability, and self-monitoring. What we do not know yet, but hope to know soon, is if physical activity and/or reducing obesity/improving diet can serve as a modifier of disease in kids and adults with MS. My current research is focused on studying the role of obesity and diet on the clinical course of children with MS. Many others are studying the role of physical activity on the disease course of children with MS. Suffice to say, there is much more to learn on the role of diet, body composition, and physical activity in youth with MS.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
Lavery AM, Collins BN, Waldman AT, Hart CN, Bar-Or A, Marrie RA, Arnold D, O'Mahony J, Banwell B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult Scler. 2019 Apr;25(4):515-522.
Maghzi AH, Etemadifar M, Heshmat-Ghahdarijani K, Nonahal S, Minagar A, Moradi V. Cesarean delivery may increase the risk of multiple sclerosis. Mult Scler. 2012;18:468-471.
Marck CH, Aitken Z, Simpson S, Weiland TJ, Jelinek GA. Does a modifiable risk factor score predict disability worsening in people with multiple sclerosis? Mult Scler J Exp Transl Clin. 2019 Oct 11;5(4):2055217319881769.
Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2019:1352458519853473.
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
Fatal child poisonings linked to common cough and cold meds
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
A number of fatal child poisonings have been linked to common cough and cold medications, according to a report.
The Pediatric Cough and Cold Safety Surveillance System, which tracks fatal child poisonings, has identified 40 such deaths in recent years and raised particular concern about medications containing diphenhydramine, a common antihistamine that can be sedating.
“There is little evidence that cough and cold medicines make children feel better or reduce their symptoms, but there is evidence they can suffer harm,” says Kevin Osterhoudt, MD, medical director of the Poison Control Center at the Children’s Hospital of Philadelphia.
In recent years, the FDA has advised labeling changes and recommended that cough and cold medications not be given to children younger than 2. Drugmakers also voluntarily relabeled these products to state “do not use in children under 4 years of age.”
Compared to older children or adults, young children have a different physiology when they breathe, so any product containing antihistamines can be a danger to little kids, Dr. Osterhoudt says.
But a recent survey shows about half of American parents gave their child cough and cold medication the last time they were ill, Dr. Osterhoudt says. And the findings suggest that cough and cold medications are in homes where children might find them.
Using the new evidence from the national surveillance system, investigators set up an expert panel to review the results. They found that most of the deaths were in children under the age of 2. The results were reported in the October issue of Pediatrics.
In seven instances, death followed the intentional use of medication to sedate the child, reports lead investigator Laurie Seidel Halmo, MD, from Children’s Hospital Colorado, Aurora.
“It’s not uncommon for parents to use sedatives like diphenhydramine to make their child sleepy for activities like air travel,” Dr. Osterhoudt says.
While antihistamines can be sedating, “an overdose of antihistamines like diphenhydramine can paradoxically become a stimulant,” having the opposite effect, he explains.
Adults and teens who take overdoses will sometimes become delirious, hallucinate, and have a racing heart.
But in young children, “if not careful with your dosing, you could actually give too much and create this stimulant activity,” Dr. Osterhoudt says.
In six other cases, the cough and cold medication was given to murder the child, the investigators reported.
The findings are “concerning,” especially with “more than one-half of nontherapeutic intent cases determined to be malicious in nature,” Michele Burns, MD, from Boston Children’s Hospital, and Madeline Renny, MD, from the Grossman School of Medicine in New York, wrote in a commentary with the report.
This important fatality review shows that despite safety efforts, young children remain at risk for death, they report.
The investigators point out that labeling changes do not seem to have protected vulnerable children, and they recommend that doctors educate parents and caregivers about the risk of cough and cold medications.
Dr. Halmo and her team also recommend that the medical community and child welfare advocates be on the lookout for medication use as a source of child abuse.
At home, preventing accidental ingestion could go along with other practices already ingrained in the minds of many, Dr. Osterhoudt says.
“We know to change the clocks in the spring and fall and make sure your smoke detector and carbon monoxide detector has fresh batteries, but maybe it’s also a good time to look at medicines in the house.”
In other words, after you change the clocks, it’s time to take inventory of medications around the house, and if they’re no longer in use, safely dispose of them.
The American Academy of Pediatrics offers guidelines on the safe home storage of medications to keep them out of reach of children and the use of protective caps on drugs.
A version of this article first appeared on WebMD.com.
Clinicians underprescribe behavior therapy for preschool ADHD
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
The majority of families of preschool children with a diagnosis of attention-deficit/hyperactivity disorder were not offered behavior therapy as a first-line treatment, according to data from nearly 200 children.
The American Academy of Pediatrics’ current clinical practice guidelines recommend parent training in behavior management (PTBM) as a first-line treatment for children aged 4-5 years diagnosed with attention-deficit/hyperactivity disorder (ADHD) or symptoms of ADHD such as hyperactivity or impulsivity, but data on how well primary care providers follow this recommendation in practice are lacking, wrote Yair Bannett, MD, of Stanford (Calif.) University, and colleagues.
To investigate the rates of PTBM recommendations, the researchers reviewed electronic health records for 22,714 children aged 48-71 months who had at least two visits to any 1 of 10 primary care practices in a California pediatric health network between Oct. 1, 2015, and Dec. 31, 2019. Children with an autism diagnosis were excluded; ADHD-related visits were identified via ADHD diagnosis codes or symptom-level diagnosis codes.
In the study, published in JAMA Pediatrics, 192 children (1%) had either an ADHD diagnosis or ADHD symptoms; of these, 21 (11%) received referrals for PTBM during ADHD-related primary care visits. Records showed an additional 55 patients (29%) had a mention of counseling on PTBM by a primary care provider, including handouts.
PCPs prescribed ADHD medications for 32 children; 9 of these had documented PTBM recommendations, and in 4 cases, the PCPs recommended PTBM before prescribing a first medication.
A majority (73%) of the children were male, 64% were privately insured, 56% had subspecialists involved in their care, and 17% were prescribed ADHD medications (88% of which were stimulants).
In a multivariate analysis, children with public insurance were significantly less likely to receive a PTBM recommendation than were those with private insurance (adjusted relative risk 0.87).
The most common recommendation overall was routine/habit modifications (for 79 children), such as reducing sugar or adding supplements to the diet; improving sleep hygiene; and limiting screen time.
The low rates of PTBM among publicly insured patients in particular highlight the need to identify factors behind disparities in recommended treatments, the researchers noted.
The study findings were limited by several factors including the reliance on primary care provider documentation during the study period and the inclusion only of medical record reviews with diagnostic codes for ADHD, the researchers noted. Further studies beyond a single health care system are needed to assess generalizability, they added.
However, the results present an opportunity for primary care providers to improve adherence to clinical practice guidelines and establish behavioral treatment at an early age to mitigate long-term morbidity, they concluded.
Low rates highlight barriers and opportunities
“We were surprised to find very low rates of documented recommendations for behavioral treatment mentioned by PCPs,” Dr. Bannett said in an interview. The researchers were surprised that recommendations for changes in daily routines and habits, such as reduced sugar intake, regular exercise, better sleep, and reduced screen time, were the most common recommendations for families of children presenting with symptoms of ADHD. “Though these are good recommendations that can support the general health of any young child, there is no evidence to support their benefit in alleviating symptoms of ADHD,” he said.
Dr. Bannett acknowledged the challenge for pediatricians to stay current on where and how families can access this type of behavioral treatment, but the evidence supports behavior therapy over medication in preschool children, he said.
“I think that it is important for primary care clinicians to know that there are options for parent training in behavioral management for both privately and publicly insured patients,” said Dr. Bannett. “In California, for example, parent training programs are offered through county mental health services. In some counties, there are other organizations that offer parent training for underserved populations and those with public insurance,” he said.
Dr. Bannett noted that online treatments, including behavioral treatments, may be possible for some families.
He cited Triple P, an evidence-based curriculum for parent training in behavior management, which offers an online course for parents at triplep-parenting.com, and an online parent training course offered through the CHADD website (chadd.org/parent-to-parent/).
Dr. Bannett noted that the researchers are planning a follow-up study to investigate the reasons behind the low referral rates for PTBM. “A known barrier is the limited availability of therapists who can provide this type of therapy,” Dr. Bannett said. “Research is needed on the effectiveness of online versions of parent training, which can overcome some of the access barriers many families experience,” he added.
“Additionally, since behavioral treatment requires a significant effort on the part of the parents and caregivers, who often are not able to complete the therapy, there is a need for research on ways to enhance parent and family engagement and participation in these important evidence-based treatments,” as well as a need to research ways to increase adherence to evidence-based practices, said Dr. Bannett. “We are currently planning intervention studies that will enhance primary care clinicians’ knowledge and clinical practice; for example, decision support tools in the electronic health record, and up-to-date information about available resources and behavioral therapists in their community that they can share with families,” he said.
Barriers make it difficult to adhere to guidelines
The study authors missed a significant element of the AAP guidelines by failing to acknowledge the extensive accompanying section on barriers to adoption, which details why most pediatricians in clinical practice do not prescribe PTBM, Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“Academically, it is a wonderful article,” said Dr. Lessin, who was a member of the authoring committee of the AAP guidelines and a major contributor to the section on barriers. The AAP guidelines recommend PTBM because it is evidence based, but the barrier section is essential to understanding that this evidence-based recommendation is nearly impossible to follow in real-world clinical practice, he emphasized.
The American Academy of Pediatrics’ “Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents,” published in October of 2019 in Pediatrics, included a full subsection on barriers as to why the guidelines might not be followed in many cases in a real-world setting, and the study authors failed to acknowledge this section and its implications, said Dr. Lessin. Notably, the barriers section was originally published in Pediatrics under a Supplemental Data tab that might easily be overlooked by someone reviewing the main practice guideline recommendations, he said.
In most areas of the country, PTBM is simply unavailable, Dr. Lessin said.
There is a dearth of mental health providers in the United States in general, and “a monstrous shortage of mental health practitioners for young children,” he said. Children in underserved areas barely have access to a medical home, let alone mental health subspecialists, he added.
Even in areas where specialized behavior therapy may be available, it can be prohibitively expensive for all but the wealthiest patients, Dr. Lessin noted. Insurance does not cover this type of behavior therapy, and most mental health professionals don’t accept Medicaid, nor commercial insurance, he said.
“I don’t even bother with those referrals, because they are not available,” said Dr. Lessin. The take-home message is that most community-based pediatricians are not following the guidelines because the barriers are so enormous, he said.
The study was supported by a research grant from the Society of Developmental and Behavioral Pediatrics and salary support through the Instructor Support Program at the department of pediatrics, Lucile Packard Children’s Hospital Stanford, to Dr. Bannett. The researchers had no other financial conflicts to disclose. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Evaluations of novel approaches to treating NF-1 tumors are underway
In the clinical experience of R. Rox Anderson, MD, currently available treatment options for benign tumors caused by neurofibromatosis type 1 (NF-1) are not acceptable.
“Simply removing the tumors with surgery is not the answer,” Dr. Anderson, a dermatologist who is the director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We need a way to inhibit the cutaneous neurofibromatosis early in life and prevent disfigurement that occurs when kids become adults.
“Kids with NF-1 are born looking normal,” he said. “They have café au lait macules and Lisch nodules in their eye, but they’re normal-looking kids. By early adulthood, many will grow hundreds of tumors that are disfiguring.”
In patients with NF-1, surgical excision works for cutaneous tumors but is expensive and not widely available, and is usually not covered by health insurance. “Plus, you have these adults who have already been through a lot of trauma, with the disfigurement in their lives, who have to be put under general anesthesia to remove a large number of tumors,” Dr. Anderson said at the meeting, which was named What’s the Truth and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Cryotherapy is a minimally invasive way to treat cutaneous neurofibroma tumors, “but this destroys the overlying skin, so you get unwanted destruction,” he said. “I like the idea of selecting heating, but we don’t know yet by what method.”
Dr. Anderson and his colleagues just launched a comparative clinical They plan to perform one or more treatment methods per patient in a single treatment session, then follow up at least 6 months later. Baseline and untreated cutaneous NF lesions will serve as controls. The researchers plan to conduct three-dimensional imaging, clinical assessments, and evaluate pain and other subjective measures.
Use of deoxycholate in a pilot trial was well tolerated and induced tumor regression in adults with cutaneous NF, he said.
Dr. Anderson noted that other researchers are studying the potential role of topical or local mitogen-activated protein kinase (MEK) inhibitors for these tumors. “Systemic MEK inhibitors are effective for plexiform neuromas, but cause significant cutaneous side effects,” he said. A “soft” MEK inhibitor, NFX-179 is rapidly metabolized such that high drug levels are achieved in skin without systemic drug levels. However, Dr. Anderson said that it remains unclear if this approach will prevent cutaneous NF tumors from forming, arrest their growth, or induce their regression.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
Neurofibromatosis type 1 (NF1) is a common genodermatosis, associated with the development of neurofibromas derived from nerves, soft tissue, and skin. Cutaneous NFs often develop in later childhood onward and may be deforming, associated with pruritus, pain, and significant effect on quality of life. Dr. Anderson is a world leader in laser treatment, having developed the theories behind laser development for medical usage, as well as the laser technology used for vascular birthmarks and hair removal, laser and cooling techniques targeting fat, and “fractionating” laser energy, which has revolutionized scar management. We look forward to his group’s insights into better management of NF1 lesions!
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
In the clinical experience of R. Rox Anderson, MD, currently available treatment options for benign tumors caused by neurofibromatosis type 1 (NF-1) are not acceptable.
“Simply removing the tumors with surgery is not the answer,” Dr. Anderson, a dermatologist who is the director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We need a way to inhibit the cutaneous neurofibromatosis early in life and prevent disfigurement that occurs when kids become adults.
“Kids with NF-1 are born looking normal,” he said. “They have café au lait macules and Lisch nodules in their eye, but they’re normal-looking kids. By early adulthood, many will grow hundreds of tumors that are disfiguring.”
In patients with NF-1, surgical excision works for cutaneous tumors but is expensive and not widely available, and is usually not covered by health insurance. “Plus, you have these adults who have already been through a lot of trauma, with the disfigurement in their lives, who have to be put under general anesthesia to remove a large number of tumors,” Dr. Anderson said at the meeting, which was named What’s the Truth and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Cryotherapy is a minimally invasive way to treat cutaneous neurofibroma tumors, “but this destroys the overlying skin, so you get unwanted destruction,” he said. “I like the idea of selecting heating, but we don’t know yet by what method.”
Dr. Anderson and his colleagues just launched a comparative clinical They plan to perform one or more treatment methods per patient in a single treatment session, then follow up at least 6 months later. Baseline and untreated cutaneous NF lesions will serve as controls. The researchers plan to conduct three-dimensional imaging, clinical assessments, and evaluate pain and other subjective measures.
Use of deoxycholate in a pilot trial was well tolerated and induced tumor regression in adults with cutaneous NF, he said.
Dr. Anderson noted that other researchers are studying the potential role of topical or local mitogen-activated protein kinase (MEK) inhibitors for these tumors. “Systemic MEK inhibitors are effective for plexiform neuromas, but cause significant cutaneous side effects,” he said. A “soft” MEK inhibitor, NFX-179 is rapidly metabolized such that high drug levels are achieved in skin without systemic drug levels. However, Dr. Anderson said that it remains unclear if this approach will prevent cutaneous NF tumors from forming, arrest their growth, or induce their regression.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
Neurofibromatosis type 1 (NF1) is a common genodermatosis, associated with the development of neurofibromas derived from nerves, soft tissue, and skin. Cutaneous NFs often develop in later childhood onward and may be deforming, associated with pruritus, pain, and significant effect on quality of life. Dr. Anderson is a world leader in laser treatment, having developed the theories behind laser development for medical usage, as well as the laser technology used for vascular birthmarks and hair removal, laser and cooling techniques targeting fat, and “fractionating” laser energy, which has revolutionized scar management. We look forward to his group’s insights into better management of NF1 lesions!
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
In the clinical experience of R. Rox Anderson, MD, currently available treatment options for benign tumors caused by neurofibromatosis type 1 (NF-1) are not acceptable.
“Simply removing the tumors with surgery is not the answer,” Dr. Anderson, a dermatologist who is the director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We need a way to inhibit the cutaneous neurofibromatosis early in life and prevent disfigurement that occurs when kids become adults.
“Kids with NF-1 are born looking normal,” he said. “They have café au lait macules and Lisch nodules in their eye, but they’re normal-looking kids. By early adulthood, many will grow hundreds of tumors that are disfiguring.”
In patients with NF-1, surgical excision works for cutaneous tumors but is expensive and not widely available, and is usually not covered by health insurance. “Plus, you have these adults who have already been through a lot of trauma, with the disfigurement in their lives, who have to be put under general anesthesia to remove a large number of tumors,” Dr. Anderson said at the meeting, which was named What’s the Truth and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. Cryotherapy is a minimally invasive way to treat cutaneous neurofibroma tumors, “but this destroys the overlying skin, so you get unwanted destruction,” he said. “I like the idea of selecting heating, but we don’t know yet by what method.”
Dr. Anderson and his colleagues just launched a comparative clinical They plan to perform one or more treatment methods per patient in a single treatment session, then follow up at least 6 months later. Baseline and untreated cutaneous NF lesions will serve as controls. The researchers plan to conduct three-dimensional imaging, clinical assessments, and evaluate pain and other subjective measures.
Use of deoxycholate in a pilot trial was well tolerated and induced tumor regression in adults with cutaneous NF, he said.
Dr. Anderson noted that other researchers are studying the potential role of topical or local mitogen-activated protein kinase (MEK) inhibitors for these tumors. “Systemic MEK inhibitors are effective for plexiform neuromas, but cause significant cutaneous side effects,” he said. A “soft” MEK inhibitor, NFX-179 is rapidly metabolized such that high drug levels are achieved in skin without systemic drug levels. However, Dr. Anderson said that it remains unclear if this approach will prevent cutaneous NF tumors from forming, arrest their growth, or induce their regression.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
Neurofibromatosis type 1 (NF1) is a common genodermatosis, associated with the development of neurofibromas derived from nerves, soft tissue, and skin. Cutaneous NFs often develop in later childhood onward and may be deforming, associated with pruritus, pain, and significant effect on quality of life. Dr. Anderson is a world leader in laser treatment, having developed the theories behind laser development for medical usage, as well as the laser technology used for vascular birthmarks and hair removal, laser and cooling techniques targeting fat, and “fractionating” laser energy, which has revolutionized scar management. We look forward to his group’s insights into better management of NF1 lesions!
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Managing simple febrile seizures without lumbar puncture safe: 15-year study
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
FROM PEDIATRICS
CDC: Urgency remains to vaccinate children
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.


