User login
Analysis supports daily folate for children with psoriasis on methotrexate
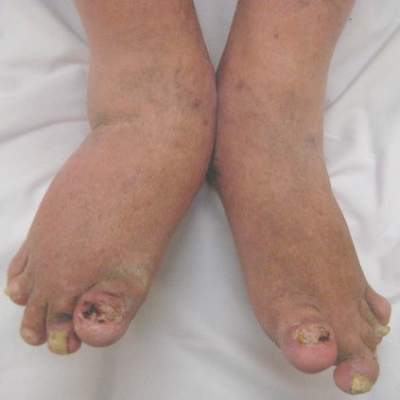
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Consider daily folate to reduce the likelihood of gastrointestinal side effects of methotrexate in children with psoriasis.
Major finding: The odds of gastrointestinal adverse effects were about 75% lower with daily folate, compared with weekly dosing or 6 days per week dosing that spared the methotrexate day (odds ratio, 0.25; P less than .001).
Data source: An international retrospective study of 446 children receiving phototherapy or systemic therapy for psoriasis.
Disclosures: The International Psoriasis Council funded the study. The investigators did not list disclosures.
Significant race and health care disparities exist among hospitalized psoriasis patients
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
Significant racial and health care disparities exist among hospitalized psoriasis patients, according to a recent study by Derek Y. Hsu of the Department of Dermatology at Northwestern University, Chicago, and his coauthors.
Researchers looked at a sample representative of 20% of all U.S. hospitalizations from 2002 to 2012, which showed racial and health care disparities for hospitalization of psoriasis cases. Hospitalization was associated with nonwhite race (Asian odds ratio, 2.08; 95% confidence interval, 1.55-2.78; black OR, 1.65; 95% CI, 1.43-1.89; and multiracial/other OR, 1.54; 95% CI, 1.13-2.11) and insurance status (Medicare OR, 1.33; 95% CI, 1.26-1.40; Medicaid OR, 1.32; 95% CI, 1.25-1.40; and uninsured OR, 1.94; 95% CI, 1.64-2.30). Additionally, length of stay was significantly prolonged for psoriasis patients, the investigators found.
“Patients who were admitted for psoriasis were significantly more likely to be Hispanic, Asian, or multiracial/other compared with Caucasians, less likely to be female and more likely to have Medicare, Medicaid, or be uninsured compared with private insurance,” the authors said in the report. “These patients also had higher odds of multiple chronic conditions.”
The results indicate a need for improved access to regular dermatological care for all patients with psoriasis, the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Obesity may attenuate anti-TNF response in psoriatic arthritis
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
LONDON – Patients with psoriatic arthritis appear less likely to achieve a good response to their first anti–tumor necrosis factor (anti-TNF) therapy if they are obese, according to data taken from two Nordic registries.
In a large observational cohort study, obese individuals with psoriatic arthritis (PsA) were significantly less likely than their nonobese counterparts to achieve a European League Against Rheumatism (EULAR) good or moderate response at 6 months (55% vs. 65%, P = .02). The overall odds ratio for achieving a good or moderate response was 0.47 when comparing obese with nonobese individuals.
The findings are potentially important because, with the exception of infliximab, anti-TNF therapy is not currently adjusted according to body weight, said presenting study author Pil Højgaard in an interview at the European Congress of Rheumatology.
Ms. Højgaard, who is an M.D. Ph.D. student at the department of rheumatology, Copenhagen University Hospital Gentofte, Rigshospitalet and the Parker Institute in Copenhagen, noted that obesity was a frequent comorbid condition in patients with PsA and that it is a known proinflammatory condition. As such, obesity could potentially affect immunologic processes, the pharmacokinetics of treatments, and ultimately patient outcomes.
Since TNF-alpha inhibitor (TNFi) treatment fails in around half of all patients with PsA treated in routine care, Ms. Højgaard noted that the aim of the cohort study was to investigate whether obesity could be having any influence on this.
Data on baseline characteristics, EULAR response rates, and drug adherence were obtained for 1,943 patients with PsA prescribed their anti-TNF therapy from two nationwide registries of disease-modifying therapies being used to treat rheumatic conditions in Denmark and Iceland, DANBIO (Rheumatology. 2011;50:69–77) and ICEBIO, respectively.
At baseline, body mass index (BMI) data were available for 1,271 patients and 408 (32%) of these had a BMI of 30 kg/m2 or more and were classed as being obese. The majority (39%) had received a first prescription for adalimumab, with around a quarter each prescribed etanercept (26%) or infliximab (24%), and the remainder prescribed golimumab (7%) and certolizumab (4%).
Compared to the 863 (68%) nonobese individuals, the obese patients were older (47 vs. 49 years, P = .01), less likely to smoke (30% vs. 23%, P = .01), and had higher disease activity measured on the Disease Activity Score 28 (DAS28) (4.4 vs. 4.6, P = .01). Health Assessment Questionnaire scores were also higher in obese than in nonobese individuals (1.1 vs. 0.9, P less than .01), and there were higher tender joint counts (6 vs. 5, P = .01), and higher pain levels assessed on a visual analog scale (VAS). Obese patients also had higher scores on a VAS patient global scale. The median follow-up time was 1.5 years.
Patients who were obese were found to adhere to TNFi treatment for shorter periods of time than nonobese patients, with median durations of 1.76 and 3.08 years, respectively (P less than .001). This discrepancy was most pronounced among men, a finding that may account for the fact that they were less likely to achieve a good EULAR response than their nonobese counterparts (OR = 0.5).
Being obese versus not being obese independently predicted TNFi withdrawal overall (hazard ratio, 1.6), especially in men (HR, 1.8; HR, 1.5 in women). TNFi withdrawal was more likely in obese than in nonobese patients even when individual treatments were considered; adalimumab: HR, 1.6; etanercept: HR, 2.0; infliximab: HR, 1.6.
An association between obesity and reduced response to anti-TNF therapy has also been observed in patients with rheumatoid arthritis, Ms. Højgaard acknowledged. There have also been a few studies of PsA and psoriasis “but to my knowledge, I think in the field of psoriatic arthritis, we are one of the few that have been looking at long-time drug survival,” she said. “We also include quite a lot of patients.”
“Of course this is not a randomized clinical study, so there could be residual confounding factors,” Ms. Højgaard cautioned. “It is always a bit difficult to say something about causality when it is a database study,” she added. “I think what we can see here is that there is an association, but in order to recommend weight loss we need some prospective studies.”
She noted that there was one published clinical study (Ann Rheum Dis. 2014;73:1157–62) that had looked at the benefit of a weight reduction program started at the same time as TNFi initiation in patients with PsA. This found there was a benefit of weight loss on response to TNFis, regardless of the type of diet.
DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Key clinical point: Obesity may reduce response and adherence to tumor necrosis factor–inhibitor therapy in patients with psoriatic arthritis.
Major finding: The EULAR good or moderate response rate at 6 months was 55% in obese vs. 65% in nonobese patients (P = .02).
Data source: Observational cohort study based on two Nordic registries of 1,943 patients with PsA prescribed their first TNFi.
Disclosures: DANBIO is supported by unrestricted grants from Abbott, Pfizer, MSD, Bristol-Myers Squibb, Roche, and UCB-Nordic. The sponsors have had no influence on data collection, analysis, or publication. ICEBIO is part of the electronic medical record system held by the University of Reykjavik and receives no industrial funding. Ms. Højgaard has received speaking fees from Celgene and UCB not related to this work.
Debunking Psoriasis Myths: Can Diet Clear Psoriasis?
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis Can Be Treated By Eating or Avoiding Certain Foods
Patients who Google “diet and psoriasis” are flooded with search results of diets claiming to cure psoriasis. This misinformation is dangerous for patients, as there is no scientific evidence that any specific psoriasis diet can treat the condition. Patients may wish to improve their diet to prevent comorbidities such as cardiovascular disease and metabolic syndrome. Even though it may not be a cure, encouraging patients to eat healthy is never a bad thing.
In a 2014 analysis of psoriasis, obesity, body mass index (BMI), and diet literature, an increased risk for psoriasis development in the setting of obesity was discussed. There is evidence suggesting that a BMI greater than 30 kg/m2 may potentially play a role in the ability to achieve a full therapeutic effect of psoriasis therapy. “This could be for two possible reasons,” Debbaneh et al reported. “It may be a consequence of decreased drug distribution into the body due to increased body mass, or it may be a consequence of increased pro-inflammatory cytokine release as a result of increased adipocyte count.” However, this finding may be treatment specific. For example, higher body weight was an independent predictor of response to ustekinumab, providing the rationale for offering 2 weight-based dosing regimens of the drug. Overweight and obese patients also were less likely to experience clearance with adalimumab. However, studies have found no association between BMI and biologic treatment.
Weight loss through a low-calorie diet has been reported to achieve a greater reduction in psoriasis severity and a slower rebound of disease. “Interestingly, studies have shown that caloric restriction in obese subjects lowers the level of circulating inflammatory cytokines,” reported Debbaneh et al. “This may contribute to the observed beneficial effect in psoriatic disease.”
In patients whose disease has had a significant impact on quality of life, it is important that they are consulting resources online that will help them maintain a healthy lifestyle. The National Psoriasis Foundation provides useful information on diet and psoriasis, emphasizing that diet is not going to cure psoriatic disease but eating healthier can only help.
Expert Commentary
Many of my psoriasis patients ask me what should they avoid eating to prevent the psoriasis from worsening, or what did they eat to cause psoriasis to occur in the first place. I stress to patients that what they eat is not likely the cause of their psoriasis nor will avoiding certain foods prevent a flare. However, alcohol use may induce a psoriasis flare.
—Jashin J. Wu, MD (Los Angeles, California)
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Debbaneh M, Millsop JW, Bhatia BK, et al. Diet and psoriasis: part I. impact of weight loss interventions. J Am Acad Dermatol. 2014;71:133-140.
National Psoriasis Foundation. Diet and psoriasis. https://www.psoriasis.org/about-psoriasis/treatments/alternative/diet-supplements. Accessed June 20, 2016.
Debunking Psoriasis Myths: Do Biologics Cause Cancer?
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Biologics Cause Cancer
Biologics generally are safe and well-tolerated therapies; however, due to their immunosuppressive properties, the risk for lymphoma has been of potential concern, leading patients to believe that biologics cause cancer. The risk of some cancers, including some solid cancers, hematologic cancers, and skin cancers, appears to be increased in patients with psoriasis, possibly associated with chronic inflammation. Some psoriasis therapies may increase the risk for malignancy, including phototherapy with psoralen plus UVA, cyclosporine, and methotrexate.
Many studies have supported a favorable safety profile for biologics in terms of the risk for developing malignancy. In a 2015 analysis of 12,093 patients enrolled in PSOLAR (Psoriasis Longitudinal Assessment and Registry), none of the biologics were found to be associated with increased risk for malignancy.
In psoriasis patients with existing or prior malignancies, the benefits of biologic therapy to improve quality of life often outweigh the negligible risks for malignancy. However, coordinated care with oncology is recommended for psoriasis patients with a history of prior malignancy.
General recommendations from the American Academy of Dermatology indicate one should carefully consider the decision to use a tumor necrosis factor (TNF) antagonist in patients with a history of malignancy, particularly lymphoma. Short-term treatment with biologics (up to 4 years) appears to be safe with respect to lymphoma risk, especially with TNF-α inhibitors. The potential risk for melanoma, cutaneous T-cell lymphoma, and nonmelanoma skin cancer in patients treated with TNF inhibitors also has been raised.
Expert Commentary
OBSERVE-5 was a 5-year phase 4, prospective, multicenter surveillance registry of 2510 psoriasis patients with at least a baseline dose of etanercept (Kimball et al, 2015). There was no increased risk for cancer when compared to the Truven Health MarketScan database, which is a proxy for the general population.
ESPRIT is an ongoing, 10-year, international, prospective, observational registry of 6059 psoriasis patients with at least a baseline dose of adalimumab (Menter et al). There are no signals of increased risk for cancer.
We do not have enough numbers of psoriasis patients on secukinumab or ixekizumab yet, but their phase 3 trials also do not seem to indicate an increased risk for cancer.
—Jashin J. Wu, MD (Los Angeles, California)
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
American Academy of Dermatology. Psoriasis: TNF inhibitors general recommendations. https://www.aad.org/practice-tools/quality-care/clinical-guidelines/psoriasis/biologics/tnf-inhibitors-recommendations. Accessed June 14, 2016.
Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
Kimball AB, Rothman KJ, Kricorian G, et al. OBSERVE-5: Observational postmarketing safety surveillance registry of etanercept for the treatment of psoriasis final 5-year results. J Am Acad Dermatol. 2015;72:115-122.
Kimball AB, Schenfeld J, Accortt NA, et al. 5-year incidence rates of malignancies in psoriasis patients compared with the general US population. Poster presented at: Summer Meeting of the American Academy of Dermatology; August 6-10, 2014; Chicago, IL.
Menter A, Thaçi D, Papp KA, et al. Five-year analysis from the ESPRIT 10-year postmarketing surveillance registry of adalimumab treatment for moderate to severe psoriasis. J Am Acad Dermatol. 2015;73:410-419.e6.
Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
Patel S, Patel T, Kerdel FA. The risk of malignancy or progression of existing malignancy in patients with psoriasis treated with biologics: case report and review of the literature. Int J Dermatol. 2016;55:487-493.
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
TB still a risk in psoriasis patients taking TNF blockers
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
FROM THE JOURNAL OF THE EADV
Key clinical point: Individuals with psoriasis treated with tumor necrosis factor inhibitors may still present with tuberculosis, despite guidelines to reduce the likelihood of developing the disease.
Major finding: Twelve individuals with psoriasis were diagnosed with TB while on anti-TNF therapy, although TB prevention guidelines had been employed.
Data source: The cases were identified from a national pharmacosurveillance database in France and from members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Disclosures: No funding or conflicts of interest were declared.
TNF blocker safety may differ in RA and psoriasis patients
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Rheumatoid arthritis patients on anti–tumor necrosis factor medications had approximately twice the rate of serious adverse events as did psoriasis patients on the same medications, based on data from a pair of prospective studies of about 4,000 adults.
The findings were published online in the British Journal of Dermatology.
“Current trends are to extrapolate the abundant literature existing on the safety of TNF antagonists in RA to define safety management for psoriasis,” wrote Dr. Ignacio García-Doval of the Fundación Academia Española de Dermatología y Venereología, Madrid, and colleagues. However, data on the similarity of risk associated with anti-TNF medications in RA and psoriasis are limited, the investigators said (BJD. 2016. doi: 10.1111/bjd.14776).
The researchers compared data from two similarly designed, overlapping prospective safety registry studies of anti-TNF medications in RA patients (the BIOBADASER study) and psoriasis patients (the BIOBADADERM study).
In the cohort of 3,171 RA patients, the researchers identified 1,248 serious or fatal adverse events during 16,230 person-years of follow-up; in the cohort of 946 psoriasis patients, they identified 124 serious or fatal adverse events during 2,760 person-years of follow-up. The resulting incidence rate ratio of psoriasis to RA was 0.6. The increased risk of serious adverse events for RA patients compared with psoriasis patients remained after the investigators controlled for confounding factors including age, sex, treatments, and comorbid conditions including hypertension, diabetes, hypercholesterolemia, and methotrexate therapy, for a hazard ratio of 0.54.
Moreover, the types of serious adverse events were different between the RA and psoriasis groups. Among those with RA, the rates of serious infections, cardiac disorders, respiratory disorders, and infusion reactions were significantly greater among those with RA, while psoriatic patients “had more skin and subcutaneous tissue disorders and hepatobiliary disorders,” the researchers noted.
By contrast, “rates of nonserious adverse events cannot be compared between the two cohorts,” because of differences in definitions, they pointed out. These differences resulted in a nonserious adverse event rate that was more than twice as high in the psoriasis group as in the RA group (582.2 events per 1,000 patient-years vs. 242.8 events per 1,000 patient-years).
Based on the findings, “published safety results of TNF-antagonists in RA cannot be fully extrapolated to psoriasis, as patients with RA have a higher risk and a different pattern of serious adverse events,” they concluded.
The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: TNF-antagonists provoke different adverse events in patients with RA than in those with psoriasis, and safety data should be extrapolated with caution.
Major finding: The risk of serious adverse events associated with anti-TNF therapy was approximately twice as high in RA patients as in psoriasis patients (hazard ratio, 0.54).
Data source: A pair of prospective studies including 4,117 adults with RA or psoriasis who received anti-TNF agents.
Disclosures: The BIOBADADERM project is promoted by the Fundación Academia Española de Dermatología y Venereología, which is supported by the Spanish Medicines and Health Products Agency and by multiple pharmaceutical companies. BIOBADASER received funding from Fundacion Española de Reumatología and the Spanish Medicines and Health Products Agency and grants from numerous pharmaceutical companies. Lead author Dr. Garcia-Doval disclosed travel grants for congresses from Merck/Schering-Plough Pharmaceuticals, Pfizer, and Janssen; the remaining two authors disclosed being a speaker and/or a consultant for several companies, including AbbVie.
Full resolution of psoriasis in half of ixekizumab patients
More than half of the patients treated with the interleukin-17A antagonist, ixekizumab, achieved full resolution of skin plaques after a year of 2- to 4-week dosing, according to the latest analysis of data from the three UNCOVER trials of almost 4,000 patients with moderate to severe psoriasis.
The results of the three randomized, double-blind placebo-controlled pivotal trials were published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1512711).
Ixekizumab was approved in March for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy; it is marketed as Taltz by Eli Lilly. Some of the UNCOVER data have been previously reported. The UNCOVER studies were conducted at more than 100 sites in 21 countries.
Patients in the UNCOVER trials were randomized to 80 mg of ixekizumab every 2 weeks after a starting dose of 160 mg, 80 mg every 4 weeks after the same starting dose, or subcutaneous placebo injections, for 12 weeks.
In the UNCOVER-2 and UNCOVER-3 trials, additional cohorts were also randomized to twice-weekly etanercept (50 mg). All three trials also included a long-term extension period to 60 weeks, which was randomly assigned in UNCOVER-3 (where patients received 80 mg of ixekizumab every 4 weeks), or was implemented for all patients who responded to ixekizumab in the other two trials (where patients were randomized to 80 mg every 4 or 12 weeks, or placebo).
In the UNCOVER-1 trial in 1,296 patients, 89.1% of patients in the 2-week dosing group and 82.6% of the 4-week dosing group had achieved a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75) by week 12, compared with 3.9% in the placebo group (P less than .0001 for all). Similarly, 35.3% of patients in the 2-week dosing group and 33.6% of those in the 4-week dosing group achieved a PASI score of 100 by week 12.
The high response rates seen during the induction period “were generally maintained during the long-term extension period in UNCOVER-3,” wrote Dr. Kenneth B. Gordon, professor of dermatology, Northwestern University, Chicago, and his coauthors. In the analysis of the long-term data from UNCOVER-3, the researchers reported that by week 60, 55% of patients receiving 80 mg of ixekizumab every 2 weeks during the induction period had achieved a PASI 100, and 52% of patients receiving ixekizumab every 4 weeks during the induction period had achieved a PASI 100.
Patients randomized to 80 mg of ixekizumab every 12 weeks after the first 12 weeks of treatment also showed significant long-term responses in the UNCOVER-1 and -2 trials. Nearly half (49.1%) of those who were initiated on 2-week dosing and 44.9% of those initiated on 4-week dosing achieved a PASI score of 75 on the 12-week dosing schedule, compared with 8%-9% of those randomized to placebo from week 12 through week 60.
The authors concluded that ixekizumab “provided high levels of clinical response at week 12 and through week 60,” adding, however, that “as with any treatment, the benefits need to be weighed against the adverse events, and the safety profile of longer-term treatment with ixekizumab should be examined.”
Although low serum IL-17 levels have previously been linked with cardiovascular disease, the study found no significant differences between the treatment and placebo groups in adverse cardiovascular events. Candidal infections were more common among those treated with ixekizumab, “a finding that is consistent with the role of IL-17A in the mucocutaneous defense against fungal infections,” they wrote.
There were 11 cases of inflammatory bowel disease reported among patients during treatment with ixekizumab and another three cases in patients receiving placebo during a randomized withdrawal period, who had received ixekizumab during the induction* period. These results “suggest that further evaluation is needed to understand the relationship between IL-17A inhibitors and inflammatory bowel disease,” the investigators said.
The authors of the study include Eli Lilly employees, several of whom also have stock options; the other authors declared a range of funding, advisory board positions, and speakers fees from pharmaceutical companies, including Eli Lilly. The UNCOVER studies were sponsored by Eli Lilly.
*A previous version of this article misstated the period during which three placebo patients received ixekizumab.
More than half of the patients treated with the interleukin-17A antagonist, ixekizumab, achieved full resolution of skin plaques after a year of 2- to 4-week dosing, according to the latest analysis of data from the three UNCOVER trials of almost 4,000 patients with moderate to severe psoriasis.
The results of the three randomized, double-blind placebo-controlled pivotal trials were published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1512711).
Ixekizumab was approved in March for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy; it is marketed as Taltz by Eli Lilly. Some of the UNCOVER data have been previously reported. The UNCOVER studies were conducted at more than 100 sites in 21 countries.
Patients in the UNCOVER trials were randomized to 80 mg of ixekizumab every 2 weeks after a starting dose of 160 mg, 80 mg every 4 weeks after the same starting dose, or subcutaneous placebo injections, for 12 weeks.
In the UNCOVER-2 and UNCOVER-3 trials, additional cohorts were also randomized to twice-weekly etanercept (50 mg). All three trials also included a long-term extension period to 60 weeks, which was randomly assigned in UNCOVER-3 (where patients received 80 mg of ixekizumab every 4 weeks), or was implemented for all patients who responded to ixekizumab in the other two trials (where patients were randomized to 80 mg every 4 or 12 weeks, or placebo).
In the UNCOVER-1 trial in 1,296 patients, 89.1% of patients in the 2-week dosing group and 82.6% of the 4-week dosing group had achieved a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75) by week 12, compared with 3.9% in the placebo group (P less than .0001 for all). Similarly, 35.3% of patients in the 2-week dosing group and 33.6% of those in the 4-week dosing group achieved a PASI score of 100 by week 12.
The high response rates seen during the induction period “were generally maintained during the long-term extension period in UNCOVER-3,” wrote Dr. Kenneth B. Gordon, professor of dermatology, Northwestern University, Chicago, and his coauthors. In the analysis of the long-term data from UNCOVER-3, the researchers reported that by week 60, 55% of patients receiving 80 mg of ixekizumab every 2 weeks during the induction period had achieved a PASI 100, and 52% of patients receiving ixekizumab every 4 weeks during the induction period had achieved a PASI 100.
Patients randomized to 80 mg of ixekizumab every 12 weeks after the first 12 weeks of treatment also showed significant long-term responses in the UNCOVER-1 and -2 trials. Nearly half (49.1%) of those who were initiated on 2-week dosing and 44.9% of those initiated on 4-week dosing achieved a PASI score of 75 on the 12-week dosing schedule, compared with 8%-9% of those randomized to placebo from week 12 through week 60.
The authors concluded that ixekizumab “provided high levels of clinical response at week 12 and through week 60,” adding, however, that “as with any treatment, the benefits need to be weighed against the adverse events, and the safety profile of longer-term treatment with ixekizumab should be examined.”
Although low serum IL-17 levels have previously been linked with cardiovascular disease, the study found no significant differences between the treatment and placebo groups in adverse cardiovascular events. Candidal infections were more common among those treated with ixekizumab, “a finding that is consistent with the role of IL-17A in the mucocutaneous defense against fungal infections,” they wrote.
There were 11 cases of inflammatory bowel disease reported among patients during treatment with ixekizumab and another three cases in patients receiving placebo during a randomized withdrawal period, who had received ixekizumab during the induction* period. These results “suggest that further evaluation is needed to understand the relationship between IL-17A inhibitors and inflammatory bowel disease,” the investigators said.
The authors of the study include Eli Lilly employees, several of whom also have stock options; the other authors declared a range of funding, advisory board positions, and speakers fees from pharmaceutical companies, including Eli Lilly. The UNCOVER studies were sponsored by Eli Lilly.
*A previous version of this article misstated the period during which three placebo patients received ixekizumab.
More than half of the patients treated with the interleukin-17A antagonist, ixekizumab, achieved full resolution of skin plaques after a year of 2- to 4-week dosing, according to the latest analysis of data from the three UNCOVER trials of almost 4,000 patients with moderate to severe psoriasis.
The results of the three randomized, double-blind placebo-controlled pivotal trials were published online in the New England Journal of Medicine (doi: 10.1056/NEJMoa1512711).
Ixekizumab was approved in March for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy; it is marketed as Taltz by Eli Lilly. Some of the UNCOVER data have been previously reported. The UNCOVER studies were conducted at more than 100 sites in 21 countries.
Patients in the UNCOVER trials were randomized to 80 mg of ixekizumab every 2 weeks after a starting dose of 160 mg, 80 mg every 4 weeks after the same starting dose, or subcutaneous placebo injections, for 12 weeks.
In the UNCOVER-2 and UNCOVER-3 trials, additional cohorts were also randomized to twice-weekly etanercept (50 mg). All three trials also included a long-term extension period to 60 weeks, which was randomly assigned in UNCOVER-3 (where patients received 80 mg of ixekizumab every 4 weeks), or was implemented for all patients who responded to ixekizumab in the other two trials (where patients were randomized to 80 mg every 4 or 12 weeks, or placebo).
In the UNCOVER-1 trial in 1,296 patients, 89.1% of patients in the 2-week dosing group and 82.6% of the 4-week dosing group had achieved a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75) by week 12, compared with 3.9% in the placebo group (P less than .0001 for all). Similarly, 35.3% of patients in the 2-week dosing group and 33.6% of those in the 4-week dosing group achieved a PASI score of 100 by week 12.
The high response rates seen during the induction period “were generally maintained during the long-term extension period in UNCOVER-3,” wrote Dr. Kenneth B. Gordon, professor of dermatology, Northwestern University, Chicago, and his coauthors. In the analysis of the long-term data from UNCOVER-3, the researchers reported that by week 60, 55% of patients receiving 80 mg of ixekizumab every 2 weeks during the induction period had achieved a PASI 100, and 52% of patients receiving ixekizumab every 4 weeks during the induction period had achieved a PASI 100.
Patients randomized to 80 mg of ixekizumab every 12 weeks after the first 12 weeks of treatment also showed significant long-term responses in the UNCOVER-1 and -2 trials. Nearly half (49.1%) of those who were initiated on 2-week dosing and 44.9% of those initiated on 4-week dosing achieved a PASI score of 75 on the 12-week dosing schedule, compared with 8%-9% of those randomized to placebo from week 12 through week 60.
The authors concluded that ixekizumab “provided high levels of clinical response at week 12 and through week 60,” adding, however, that “as with any treatment, the benefits need to be weighed against the adverse events, and the safety profile of longer-term treatment with ixekizumab should be examined.”
Although low serum IL-17 levels have previously been linked with cardiovascular disease, the study found no significant differences between the treatment and placebo groups in adverse cardiovascular events. Candidal infections were more common among those treated with ixekizumab, “a finding that is consistent with the role of IL-17A in the mucocutaneous defense against fungal infections,” they wrote.
There were 11 cases of inflammatory bowel disease reported among patients during treatment with ixekizumab and another three cases in patients receiving placebo during a randomized withdrawal period, who had received ixekizumab during the induction* period. These results “suggest that further evaluation is needed to understand the relationship between IL-17A inhibitors and inflammatory bowel disease,” the investigators said.
The authors of the study include Eli Lilly employees, several of whom also have stock options; the other authors declared a range of funding, advisory board positions, and speakers fees from pharmaceutical companies, including Eli Lilly. The UNCOVER studies were sponsored by Eli Lilly.
*A previous version of this article misstated the period during which three placebo patients received ixekizumab.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Half of patients treated with the IL-17A–neutralizing ixekizumab achieve full resolution of psoriasis plaques after 1 year.
Major finding: Among psoriasis patients treated with two weekly or four weekly doses of ixekizumab, 55% and 52%, respectively, achieve full resolution of plaques at 60 weeks.
Data source: Randomized, double-blind placebo-controlled trials (UNCOVER-1, -2, and -3) in 3,866 patients with psoriasis.
Disclosures: The studies were supported by Eli Lilly. The authors included employees of Eli Lilly, some of whom have stock options, while the other authors all declared a range of funding, advisory board positions, and speakers fees from pharmaceutical companies, including Eli Lilly.
Abdominal Aortic Aneurysm in Psoriasis Patients
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?
In a study published online on April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology, Khalid et al evaluated the risk for AAA in patients with psoriasis in a nationwide cohort study in Denmark. The study participants were Danish residents 18 years and older who were observed from January 1, 1997 until diagnosis of AAA; December 31, 2011; migration; or death. Incidence rates for AAA were calculated, and incidence rate ratios were adjusted for age, sex, comorbidity, medications, socioeconomic status, and smoking.
A total of 5,495,203 individuals were eligible for this study. Of them, Khalid et al identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis. The overall incidence rates of AAA were 3.72, 7.30, and 9.87 per 10,000 person-years for the reference population (23,696 cases), mild psoriasis (240 cases), and severe psoriasis (50 cases), respectively. The corresponding adjusted incidence rate ratios for AAA were increased in patients with psoriasis with incidence rate ratios of 1.20 (95% CI, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32) for individuals with mild and severe disease, respectively.
Khalid et al concluded that psoriasis was associated with a disease severity–dependent increased risk for AAA; however, the mechanisms and consequences of this novel finding require further investigation.
What’s the issue?
Another example of an association of a comorbidity with psoriasis, this finding emphasizes the need for cardiovascular referral in psoriasis patients with risk factors such as hypertension and diabetes mellitus. How will these data influence your evaluation of psoriasis patients?