User login
Benefits, challenges emerge in evolution of rheumatology-dermatology clinics
MIAMI – Combined rheumatology-dermatology clinics to help people with psoriasis, psoriatic arthritis, and other relevant overlapping conditions continue to evolve, with evidence suggesting advantages and challenges for both patients and physicians.
Physicians like the improved communication and greater collaboration but still have reservations about billing and scheduling, according to preliminary results of a survey conducted by the Psoriasis and Psoriatic Arthritis Clinic Multicenter Advancement Network (PPACMAN). For patients, the advantages go beyond convenience, according to a 1-year study of outcomes at a Rhode Island Hospital–Brown University combined clinic in Providence.
“The goal of the combined clinics survey is to gauge strengths, barriers, and challenges to creating these models, to learn from one another to improve current clinical care, and to support propagation of these models,” said Joseph F. Merola, MD, at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Multiple clinic models
The clinic setups differ. In those settings where rheumatologists and dermatologists see patients on the same day, approximately three-quarters provide care together in the same room. The remaining clinics see patients through serial visits. Another 30% of respondents said dermatologists and rheumatologists generally see patients in a combined clinic on different days.
“Rheumatologists said it is a satisfying and rewarding endeavor, they form closer ties with colleagues, and it allows early, improved communication,” said Dr. Merola, a rheumatologist and dermatologist who is co-director of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston.
Some common benefits, each cited by more than 80% of respondents, include a prompt and accurate diagnosis of psoriatic arthritis, the ability of physicians to learn from each other, and multiple training opportunities for residents and fellows. In fact, 72% said their combined clinic has rheumatology fellows, 82% have dermatology residents, and 27% incorporate internal medicine trainees.
Other than rheumatologists and dermatologists, 31% said their clinic has dedicated nursing, 15% have a cardiologist, and 15% have a psychiatrist.
A majority of physicians (92%) said they bill through their own department. At a subsequent roundtable discussion, Dr. Merola noted that patients pay separate copays at the combined clinic. “We’ve had some complaints from patients. They don’t like having two copayments.”
“You have to tell patients in advance,” suggested Soumya M. Reddy, MD, a rheumatologist at NYU Langone Medical Center in New York and co-director of its Psoriasis and Psoriatic Arthritis Center.
Common conditions and challenges
Not surprisingly, psoriasis and psoriatic arthritis are the most common conditions treated in these clinics, followed by lupus and dermatomyositis. Most clinics see patients either once weekly or once monthly.
A major concern expressed by 75% surrounds scheduling. More than half, 58%, worry about demonstrating value to their institution. Only a minority, 17%, responded that achieving consensus on patient management is a challenge.
In general, rheumatology evaluations take more time than dermatology assessments, presenting a challenge for scheduling and maximizing physician time. “You don’t really want the dermatologist sitting there for 20 minutes not really doing a lot,” said Alison Ehrlich, MD, professor and chair of dermatology at George Washington University in Washington.
The solution at Brigham and Women’s Hospital is to staff the clinic with two dermatologists and one rheumatologist. The two dermatologists see about 20 patients each in a half-day; the rheumatologist probably treats about 6 to 8 of those 40 patients, Dr. Merola said. At George Washington, Dr. Ehrlich sees patients along with the rheumatologist, leaves the room to treat other patients, later consults with her colleague, and they go back in together as necessary.
“The patients are incredibly appreciative” of the combined clinic, Dr. Ehrlich said. “They really love it.”
The survey is ongoing. The preliminary responses above are based on 17 responses, a 52% response rate. Of the respondents, 10 are dermatologists, 6 are rheumatologists, and 1 is dual trained. Since the meeting, Dr. Merola indicated that another two dermatologists and three rheumatologists have responded to the survey.
The Brown University experience
Charis Gn, MD, and colleagues studied outcomes for 167 patients treated at a combined clinic at Rhode Island Hospital. The ultimate goal of the clinic is “to identify patients with psoriatic arthritis early on,” Dr. Gn said at a poster presentation at the GRAPPA annual meeting. “We wanted to see what kind of outcomes [we get] for patients who see rheumatology and dermatology.”
About one-third of patients left the clinic with changes in diagnosis. About 1 in 5 patients with psoriasis were diagnosed with psoriatic arthritis as well. For the psoriasis patients newly diagnosed with psoriatic arthritis, 80% had an escalation in treatment, said Dr. Gn, an internal medicine resident.
Of the 41 patients with psoriasis, 17 (41%) had a treatment escalation. The same was true for 15 (79%) of the 19 patients with psoriatic arthritis post-evaluation.
Regarding the combined clinic, “it’s shown to be very beneficial for patients with psoriatic arthritis,” Dr. Gn said.
The record review from July 2014 to February 2016 shows the clinic also serves patients with cutaneous lupus, dermatomyositis, pyoderma gangrenosum, vasculitis, and rheumatoid arthritis, among others.
The presenters had no relevant disclosures.
MIAMI – Combined rheumatology-dermatology clinics to help people with psoriasis, psoriatic arthritis, and other relevant overlapping conditions continue to evolve, with evidence suggesting advantages and challenges for both patients and physicians.
Physicians like the improved communication and greater collaboration but still have reservations about billing and scheduling, according to preliminary results of a survey conducted by the Psoriasis and Psoriatic Arthritis Clinic Multicenter Advancement Network (PPACMAN). For patients, the advantages go beyond convenience, according to a 1-year study of outcomes at a Rhode Island Hospital–Brown University combined clinic in Providence.
“The goal of the combined clinics survey is to gauge strengths, barriers, and challenges to creating these models, to learn from one another to improve current clinical care, and to support propagation of these models,” said Joseph F. Merola, MD, at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Multiple clinic models
The clinic setups differ. In those settings where rheumatologists and dermatologists see patients on the same day, approximately three-quarters provide care together in the same room. The remaining clinics see patients through serial visits. Another 30% of respondents said dermatologists and rheumatologists generally see patients in a combined clinic on different days.
“Rheumatologists said it is a satisfying and rewarding endeavor, they form closer ties with colleagues, and it allows early, improved communication,” said Dr. Merola, a rheumatologist and dermatologist who is co-director of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston.
Some common benefits, each cited by more than 80% of respondents, include a prompt and accurate diagnosis of psoriatic arthritis, the ability of physicians to learn from each other, and multiple training opportunities for residents and fellows. In fact, 72% said their combined clinic has rheumatology fellows, 82% have dermatology residents, and 27% incorporate internal medicine trainees.
Other than rheumatologists and dermatologists, 31% said their clinic has dedicated nursing, 15% have a cardiologist, and 15% have a psychiatrist.
A majority of physicians (92%) said they bill through their own department. At a subsequent roundtable discussion, Dr. Merola noted that patients pay separate copays at the combined clinic. “We’ve had some complaints from patients. They don’t like having two copayments.”
“You have to tell patients in advance,” suggested Soumya M. Reddy, MD, a rheumatologist at NYU Langone Medical Center in New York and co-director of its Psoriasis and Psoriatic Arthritis Center.
Common conditions and challenges
Not surprisingly, psoriasis and psoriatic arthritis are the most common conditions treated in these clinics, followed by lupus and dermatomyositis. Most clinics see patients either once weekly or once monthly.
A major concern expressed by 75% surrounds scheduling. More than half, 58%, worry about demonstrating value to their institution. Only a minority, 17%, responded that achieving consensus on patient management is a challenge.
In general, rheumatology evaluations take more time than dermatology assessments, presenting a challenge for scheduling and maximizing physician time. “You don’t really want the dermatologist sitting there for 20 minutes not really doing a lot,” said Alison Ehrlich, MD, professor and chair of dermatology at George Washington University in Washington.
The solution at Brigham and Women’s Hospital is to staff the clinic with two dermatologists and one rheumatologist. The two dermatologists see about 20 patients each in a half-day; the rheumatologist probably treats about 6 to 8 of those 40 patients, Dr. Merola said. At George Washington, Dr. Ehrlich sees patients along with the rheumatologist, leaves the room to treat other patients, later consults with her colleague, and they go back in together as necessary.
“The patients are incredibly appreciative” of the combined clinic, Dr. Ehrlich said. “They really love it.”
The survey is ongoing. The preliminary responses above are based on 17 responses, a 52% response rate. Of the respondents, 10 are dermatologists, 6 are rheumatologists, and 1 is dual trained. Since the meeting, Dr. Merola indicated that another two dermatologists and three rheumatologists have responded to the survey.
The Brown University experience
Charis Gn, MD, and colleagues studied outcomes for 167 patients treated at a combined clinic at Rhode Island Hospital. The ultimate goal of the clinic is “to identify patients with psoriatic arthritis early on,” Dr. Gn said at a poster presentation at the GRAPPA annual meeting. “We wanted to see what kind of outcomes [we get] for patients who see rheumatology and dermatology.”
About one-third of patients left the clinic with changes in diagnosis. About 1 in 5 patients with psoriasis were diagnosed with psoriatic arthritis as well. For the psoriasis patients newly diagnosed with psoriatic arthritis, 80% had an escalation in treatment, said Dr. Gn, an internal medicine resident.
Of the 41 patients with psoriasis, 17 (41%) had a treatment escalation. The same was true for 15 (79%) of the 19 patients with psoriatic arthritis post-evaluation.
Regarding the combined clinic, “it’s shown to be very beneficial for patients with psoriatic arthritis,” Dr. Gn said.
The record review from July 2014 to February 2016 shows the clinic also serves patients with cutaneous lupus, dermatomyositis, pyoderma gangrenosum, vasculitis, and rheumatoid arthritis, among others.
The presenters had no relevant disclosures.
MIAMI – Combined rheumatology-dermatology clinics to help people with psoriasis, psoriatic arthritis, and other relevant overlapping conditions continue to evolve, with evidence suggesting advantages and challenges for both patients and physicians.
Physicians like the improved communication and greater collaboration but still have reservations about billing and scheduling, according to preliminary results of a survey conducted by the Psoriasis and Psoriatic Arthritis Clinic Multicenter Advancement Network (PPACMAN). For patients, the advantages go beyond convenience, according to a 1-year study of outcomes at a Rhode Island Hospital–Brown University combined clinic in Providence.
“The goal of the combined clinics survey is to gauge strengths, barriers, and challenges to creating these models, to learn from one another to improve current clinical care, and to support propagation of these models,” said Joseph F. Merola, MD, at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Multiple clinic models
The clinic setups differ. In those settings where rheumatologists and dermatologists see patients on the same day, approximately three-quarters provide care together in the same room. The remaining clinics see patients through serial visits. Another 30% of respondents said dermatologists and rheumatologists generally see patients in a combined clinic on different days.
“Rheumatologists said it is a satisfying and rewarding endeavor, they form closer ties with colleagues, and it allows early, improved communication,” said Dr. Merola, a rheumatologist and dermatologist who is co-director of the Center for Skin and Related Musculoskeletal Diseases, a combined clinic at Brigham and Women’s Hospital in Boston.
Some common benefits, each cited by more than 80% of respondents, include a prompt and accurate diagnosis of psoriatic arthritis, the ability of physicians to learn from each other, and multiple training opportunities for residents and fellows. In fact, 72% said their combined clinic has rheumatology fellows, 82% have dermatology residents, and 27% incorporate internal medicine trainees.
Other than rheumatologists and dermatologists, 31% said their clinic has dedicated nursing, 15% have a cardiologist, and 15% have a psychiatrist.
A majority of physicians (92%) said they bill through their own department. At a subsequent roundtable discussion, Dr. Merola noted that patients pay separate copays at the combined clinic. “We’ve had some complaints from patients. They don’t like having two copayments.”
“You have to tell patients in advance,” suggested Soumya M. Reddy, MD, a rheumatologist at NYU Langone Medical Center in New York and co-director of its Psoriasis and Psoriatic Arthritis Center.
Common conditions and challenges
Not surprisingly, psoriasis and psoriatic arthritis are the most common conditions treated in these clinics, followed by lupus and dermatomyositis. Most clinics see patients either once weekly or once monthly.
A major concern expressed by 75% surrounds scheduling. More than half, 58%, worry about demonstrating value to their institution. Only a minority, 17%, responded that achieving consensus on patient management is a challenge.
In general, rheumatology evaluations take more time than dermatology assessments, presenting a challenge for scheduling and maximizing physician time. “You don’t really want the dermatologist sitting there for 20 minutes not really doing a lot,” said Alison Ehrlich, MD, professor and chair of dermatology at George Washington University in Washington.
The solution at Brigham and Women’s Hospital is to staff the clinic with two dermatologists and one rheumatologist. The two dermatologists see about 20 patients each in a half-day; the rheumatologist probably treats about 6 to 8 of those 40 patients, Dr. Merola said. At George Washington, Dr. Ehrlich sees patients along with the rheumatologist, leaves the room to treat other patients, later consults with her colleague, and they go back in together as necessary.
“The patients are incredibly appreciative” of the combined clinic, Dr. Ehrlich said. “They really love it.”
The survey is ongoing. The preliminary responses above are based on 17 responses, a 52% response rate. Of the respondents, 10 are dermatologists, 6 are rheumatologists, and 1 is dual trained. Since the meeting, Dr. Merola indicated that another two dermatologists and three rheumatologists have responded to the survey.
The Brown University experience
Charis Gn, MD, and colleagues studied outcomes for 167 patients treated at a combined clinic at Rhode Island Hospital. The ultimate goal of the clinic is “to identify patients with psoriatic arthritis early on,” Dr. Gn said at a poster presentation at the GRAPPA annual meeting. “We wanted to see what kind of outcomes [we get] for patients who see rheumatology and dermatology.”
About one-third of patients left the clinic with changes in diagnosis. About 1 in 5 patients with psoriasis were diagnosed with psoriatic arthritis as well. For the psoriasis patients newly diagnosed with psoriatic arthritis, 80% had an escalation in treatment, said Dr. Gn, an internal medicine resident.
Of the 41 patients with psoriasis, 17 (41%) had a treatment escalation. The same was true for 15 (79%) of the 19 patients with psoriatic arthritis post-evaluation.
Regarding the combined clinic, “it’s shown to be very beneficial for patients with psoriatic arthritis,” Dr. Gn said.
The record review from July 2014 to February 2016 shows the clinic also serves patients with cutaneous lupus, dermatomyositis, pyoderma gangrenosum, vasculitis, and rheumatoid arthritis, among others.
The presenters had no relevant disclosures.
AT 2016 GRAPPA ANNUAL MEETING
Psoriasiform eruptions in Kawasaki disease reveal distinct phenotype
A comparison of psoriasis-like eruptions in Kawasaki disease (KD) with classic psoriasis shows a distinct phenotype with greater remission, report Ellen S. Haddock, AB, MBA and coauthors from the School of Medicine at the University of California, San Diego.
Investigators performed a retrospective study of 11 KD cases with a psoriasiform eruption matched by gender, age, and ethnicity with psoriasis-only and KD-only controls. Genotyping was performed in 10 cases for a deletion of two late cornified envelope genes associated with pediatric-onset psoriasis.
KD-associated eruptions were similar to classic psoriasis in presentation, but with less frequent diaper area involvement, more crust, more serious exudate, and significantly higher remission (91% vs. 23%; P less than .001), the authors noted.
The findings indicate that despite similarities to classic psoriasis, “this appears to be a distinct phenotype with significantly greater propensity for remission,” the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
A comparison of psoriasis-like eruptions in Kawasaki disease (KD) with classic psoriasis shows a distinct phenotype with greater remission, report Ellen S. Haddock, AB, MBA and coauthors from the School of Medicine at the University of California, San Diego.
Investigators performed a retrospective study of 11 KD cases with a psoriasiform eruption matched by gender, age, and ethnicity with psoriasis-only and KD-only controls. Genotyping was performed in 10 cases for a deletion of two late cornified envelope genes associated with pediatric-onset psoriasis.
KD-associated eruptions were similar to classic psoriasis in presentation, but with less frequent diaper area involvement, more crust, more serious exudate, and significantly higher remission (91% vs. 23%; P less than .001), the authors noted.
The findings indicate that despite similarities to classic psoriasis, “this appears to be a distinct phenotype with significantly greater propensity for remission,” the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
A comparison of psoriasis-like eruptions in Kawasaki disease (KD) with classic psoriasis shows a distinct phenotype with greater remission, report Ellen S. Haddock, AB, MBA and coauthors from the School of Medicine at the University of California, San Diego.
Investigators performed a retrospective study of 11 KD cases with a psoriasiform eruption matched by gender, age, and ethnicity with psoriasis-only and KD-only controls. Genotyping was performed in 10 cases for a deletion of two late cornified envelope genes associated with pediatric-onset psoriasis.
KD-associated eruptions were similar to classic psoriasis in presentation, but with less frequent diaper area involvement, more crust, more serious exudate, and significantly higher remission (91% vs. 23%; P less than .001), the authors noted.
The findings indicate that despite similarities to classic psoriasis, “this appears to be a distinct phenotype with significantly greater propensity for remission,” the authors concluded.
Read the full article in the Journal of the American Academy of Dermatology.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
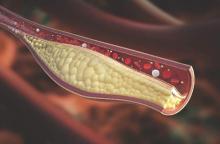
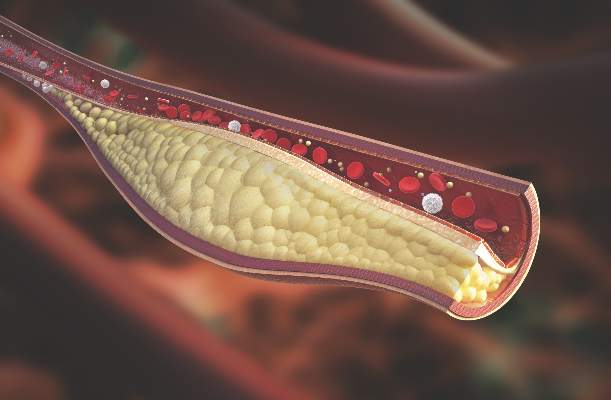
Psoriatic arthritis patients have elevated risk for coronary artery plaque
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
MIAMI – Patients with psoriatic arthritis had a higher prevalence and greater extent of coronary artery plaque in a pilot study comparison with healthy control patients that may point to increased risk independent of traditional cardiovascular risk factors.
In the study, coronary artery plaque as assessed by cardiac computed tomography angiography (CCTA) occurred in 39 (78%) of 50 patients with psoriatic arthritis, a significantly higher rate than that observed for healthy controls (11 of 25, 44%).
Investigators not only measured plaque volume, but also assessed the type of plaque: calcified, noncalcified, or mixed. Mixed plaque predominated. This could be important because “noncalcified and mixed carry higher risk for rupture and later cardiovascular events,” Agnes Szentpetery, MD, a research fellow at St. Vincent’s University Hospital in Dublin, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
She and her colleagues also found more clinically significant stenosis among the 50 participants with psoriatic arthritis, compared with 25 healthy controls matched for age, sex, smoking status, and presence of metabolic syndrome. “This pilot study is the first to assess coronary plaques in asymptomatic patients with psoriatic arthritis with CCTA,” Dr. Szentpetery said.
Total plaque volume was higher in the psoriatic arthritis group versus controls, and higher in the left main artery for psoriatic arthritis patients, both with and without metabolic syndrome.
The study points to increased risk independent of traditional cardiovascular risk factors. For example, CCTA revealed no difference in plaque volume between patients with and without metabolic disease. In addition, a previous study suggests “the burden of carotid artery plaques is higher in patients with psoriatic arthritis compared to those with psoriasis alone,” Dr. Szentpetery said, citing a cross-sectional study comparing 125 people with psoriasis to 114 others with psoriatic arthritis (Ann Rheum Dis. 2013 May;72[5]:715-20).
Perhaps not surprisingly, inflammation could be driving the association between psoriatic and cardiovascular disease risk. Other investigators suggest chronic, low-grade inflammation leads to atherosclerosis through a maladaptive immune response and altered lipid metabolism, for example (Nat Med. 2011 Nov;17[11]:1410-22).
In the current study, the patients with psoriatic arthritis had well-established disease, occurring for a mean duration of 19 years. Mean age was 58 years, and 54% were men. Approximately 60% were taking disease-modifying antirheumatic drugs, two-thirds were taking biologics, and about one-third were on combination treatment. Controls were similar demographically with a mean age of 57 years, and 52% were men.
Interestingly, Psoriasis Area and Severity Index (PASI) scores did not correlate with increased risk. During discussion after the presentation of the study, a researcher unaffiliated with the study offered an answer. “It could be their skin disease was controlled by the biologics. You had 67% on biologics,” said Nehal Mehta, MD, Clinical Research Scholar in the section of inflammation and cardiometabolic disease at the National Heart, Lung, and Blood Institute. “We at the NIH see a strong correlation between PASI and coronary artery disease risk.”
“We know methotrexate and anti-TNF agents can have a protective effect on atherosclerosis, but we did not look at this specifically,” Dr. Szentpetery said. Overall, PASI scores were relatively low in the study population, she added, which “may explain why we did not see the correlation with PASI scores.”
Dr. Szentpetery and Dr. Mehta had no relevant financial disclosures.
AT 2016 GRAPPA ANNUAL MEETING
Key clinical point:Imaging reveals a higher rate and greater extent of coronary plaque in people with psoriatic arthritis versus healthy controls.
Major finding: 78% of people with PsA had coronary artery plaque versus 44% of controls, a significant difference.
Data source: Comparison of 50 people with PsA versus 25 healthy controls undergoing CCTA.
Disclosures: Dr. Szentpetery and Dr. Mehta had no relevant disclosures.
Brodalumab approval recommended, despite possible suicide signal
A Food and Drug Administration advisory committee has unanimously supported approval of the monoclonal antibody brodalumab for the treatment of moderate to severe plaque psoriasis, with the majority recommending risk management options beyond labeling to address concerns about the six completed suicides among patients treated with brodalumab in clinical trials.
Though no members of the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee disputed the drug’s efficacy for plaque psoriasis, the possibility of a signal for suicidal ideation and behavior (SIB) among the brodalumab group prompted the committee’s chair, Michael Bigby, M.D., to remark, “The big problem is that you have six completed suicides. … I would say it’s a fairly big number for a randomized controlled trial. Patients and clinicians need to be aware of this as a fact.”
Dr. Bigby, professor of dermatology at Harvard Medical School, Boston, headed the 18-member panel, which included dermatologists, psychiatrists, and cardiologists. Fourteen members voted in favor of approval with risk management measures beyond labeling; four members voted for approval with labeling alone.
Brodalumab targets interleukin-17 receptors, inhibiting IL-17 uptake and thus blocking the pathway responsible for the cutaneous and systemic signs and symptoms of psoriasis. The pivotal phase III brodalumab studies, AMAGINE-1, -2, and -3, enrolled a total of 4,373 patients with moderate to severe plaque psoriasis. AMAGINE-1 enrolled 661 patients in a 1:1:1 ratio to a randomized, double-blind placebo-controlled study that included two doses of brodalumab: 210 mg and 140 mg. The placebo-controlled phase of the study ran for 12 weeks, followed by a withdrawal and retreatment phase, and after 52 weeks, by an open label long-term extension phase.
AMAGINE-2 (1,831 patients) and AMAGINE-3 (1,881 patients) were identically designed studies that pitted both doses of brodalumab against an active comparator, ustekinumab (Stelara), as well as placebo, in a 2:2:1:1 ratio. This double-blind phase of the study also lasted 12 weeks. During weeks 12-52, patients on the brodalumab arms continued, with an exploration of wider-dose intervals for the 140-mg dose. Patients also continued with ustekinumab, with a rescue option to transition to brodalumab 210 mg. The placebo group could continue on brodalumab 210 mg as well. These two studies also had an open label long-term extension phase.
At the 12-week mark in AMAGINE-1, 41.9% of patients achieved the coprimary endpoint of 100% reduction in the Psoriasis Area and Severity Index (PASI 100) score, compared with 0.5% of those on placebo (P less than .001). AMAGINE-2 and -3 saw 44.4% and 36.7% of the brodalumab 210-mg arms achieving PASI 100 at the 12-week mark, respectively. These figures were significantly higher than the 21.7% and 18.5% reaching PASI 100 on ustekinumab (P less than .001), as well as significantly higher than the less than 1% of patients in the placebo arm who reached that endpoint.
At the end of 52 weeks, 51% of those on brodalumab 210 mg had completely clear skin, compared with 28.1% of the patients receiving ustekinumab (P less than .001).
The safety analysis for brodalumab showed that the most common adverse events were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia; of these, the last two were considered adverse drug reactions. At 52 weeks, those on brodalumab had a slightly greater risk of having nonserious fungal infections than did those on ustekinumab. Overall, however, there was no difference between the brodalumab and ustekinumab arms at week 52 for treatment-emergent adverse events or for serious adverse events.
A cluster of SIB events occurred in 2013 and 2014 during the clinical trials, and the sponsor consulted the FDA. Additional monitoring for suicidal ideation and depression via the Columbia Suicide Severity Rating Scale (C-SSRS) and the Patient Health Questionnaire (PHQ) were implemented at a point where 84% of trial patients were already in the uncontrolled open label extension stage of the trial; in addition, a retrospective data review searched for suicidal ideation and attempts before the implementation of these two tools.
A total of six brodalumab patients completed suicide in all clinical trials, including those for psoriatic arthritis and RA. Suicides included two during the 12- to 52-week follow-up and four during the long-term follow-up phase. Another 29 patients attempted suicide, had intentional self-injury, or had suicidal ideation during these phases of the study, for a total of 35 SIB events. (SIB includes completed suicide, suicide attempt, suicide behavior, and suicide ideation.)*
The significance of the number of completed suicides was difficult to ascertain, given the increased baseline prevalence of depression and related illnesses among patients with psoriasis. Compared with the general population, individuals with psoriasis have hazard ratios of 1.39, 1.31, and 1.44 for depression, anxiety, and suicidality, according to a cohort study cited by Robert Levin, M.D. , director of the division of pharmacovigilance I at the FDA’s Center for Drug Evaluation and Research (CDER).
In addition, monitoring for suicidal ideation and attempts was stepped up during the course of the clinical trials. Brodalumab’s sponsor, Valeant Pharmaceuticals, noted that the rates of suicidal ideation and attempts increased after implementation of the C-SSRS, while other neuropsychiatric symptoms remained stable or declined during the course of the studies. “This makes us think this is ascertainment bias,” said Valeant consultant Lauren Marangell, M.D., a psychiatrist and president of Brain Health Consultants.
Dr. Marangell also reported that, after stratification for preexisting psychiatric comorbidities and risk factors, “there is no question that the rate of SIB is higher in both arms with patients with baseline risk factors.” The FDA’s data analysis showed an 18-fold increase in the rate of SIB for brodalumab arm participants with a prior history of suicidality.
During its presentation, Valeant also pointed out that patients with histories of drug and alcohol abuse, depression, and suicidality were not specifically excluded from the clinical trials. This differentiates the brodalumab program from other biologic studies, “making the studies more characteristic of the real world,” said R.K. Pillai, Ph.D., Valeant vice president.
Dr. Levin’s analysis, one of several independent analyses conducted by different FDA divisions, found that the data were inconclusive. “We currently can’t conclude whether or not these are drug-related risks,” he said. Considering the critical severity of suicide as an outcome, “we must consider the application and regulatory actions carefully.”
Jean Kim, M.D., medical officer in CDER’s division of psychiatry products (DPP), noted that the number of suicides seen in the brodalumab trials “is higher than typically seen in DPP’s large psychiatric drug trials, which involve populations with higher psychiatric morbidity than patients with psoriasis.”
Patients with psoriasis are also known to have a baseline elevated risk of cardiovascular disease, and no committee member felt the brodalumab data showed any additional signal for major adverse cardiovascular events (MACEs) beyond what would be suspected in this population. “I don’t right now see a safety signal for MACE,” said panelist Michael Blaha, M.D., a cardiologist and director of clinical research at Johns Hopkins Ciccarone Center for Prevention of Heart Disease, Baltimore. He noted, however, that research is ongoing to elucidate the relationship between cytokine levels and MACE. “This question about whether cytokines raise or reduce rates for [cardiovascular] events is an open one.”
One of the dermatologists on the committee, Lynn Drake, M.D., said that her decision making was influenced by the disease burden psoriasis inflicts on the patients she sees in her daily practice. “These patients are ill. People tend to think it’s just skin disease. It is not just skin disease. … I’d hate to see our patients deprived of a drug that might help alleviate their symptoms,” said Dr. Drake.
Though committee members voiced some disagreement about whether a patient registry should be established, and if it should be voluntary, they were in agreement that patients and prescribers both be aware of the potential risks of brodalumab, without making any risk management strategy so burdensome that appropriate patients would be deterred from considering the medication. Psoriasis, Dr. Drake said, “is a devastating disease. We need this in our armamentarium.”
The proposed dosing for brodalumab is 210 mg injected subcutaneously weekly for 3 weeks, followed by 210 mg every 2 weeks thereafter.
The FDA usually follows the recommendations of its advisory committees. The panelists had no relevant financial disclosures.
On Twitter @karioakes
*A previous version of this article misstated the number of patients who experienced SIB events.
A Food and Drug Administration advisory committee has unanimously supported approval of the monoclonal antibody brodalumab for the treatment of moderate to severe plaque psoriasis, with the majority recommending risk management options beyond labeling to address concerns about the six completed suicides among patients treated with brodalumab in clinical trials.
Though no members of the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee disputed the drug’s efficacy for plaque psoriasis, the possibility of a signal for suicidal ideation and behavior (SIB) among the brodalumab group prompted the committee’s chair, Michael Bigby, M.D., to remark, “The big problem is that you have six completed suicides. … I would say it’s a fairly big number for a randomized controlled trial. Patients and clinicians need to be aware of this as a fact.”
Dr. Bigby, professor of dermatology at Harvard Medical School, Boston, headed the 18-member panel, which included dermatologists, psychiatrists, and cardiologists. Fourteen members voted in favor of approval with risk management measures beyond labeling; four members voted for approval with labeling alone.
Brodalumab targets interleukin-17 receptors, inhibiting IL-17 uptake and thus blocking the pathway responsible for the cutaneous and systemic signs and symptoms of psoriasis. The pivotal phase III brodalumab studies, AMAGINE-1, -2, and -3, enrolled a total of 4,373 patients with moderate to severe plaque psoriasis. AMAGINE-1 enrolled 661 patients in a 1:1:1 ratio to a randomized, double-blind placebo-controlled study that included two doses of brodalumab: 210 mg and 140 mg. The placebo-controlled phase of the study ran for 12 weeks, followed by a withdrawal and retreatment phase, and after 52 weeks, by an open label long-term extension phase.
AMAGINE-2 (1,831 patients) and AMAGINE-3 (1,881 patients) were identically designed studies that pitted both doses of brodalumab against an active comparator, ustekinumab (Stelara), as well as placebo, in a 2:2:1:1 ratio. This double-blind phase of the study also lasted 12 weeks. During weeks 12-52, patients on the brodalumab arms continued, with an exploration of wider-dose intervals for the 140-mg dose. Patients also continued with ustekinumab, with a rescue option to transition to brodalumab 210 mg. The placebo group could continue on brodalumab 210 mg as well. These two studies also had an open label long-term extension phase.
At the 12-week mark in AMAGINE-1, 41.9% of patients achieved the coprimary endpoint of 100% reduction in the Psoriasis Area and Severity Index (PASI 100) score, compared with 0.5% of those on placebo (P less than .001). AMAGINE-2 and -3 saw 44.4% and 36.7% of the brodalumab 210-mg arms achieving PASI 100 at the 12-week mark, respectively. These figures were significantly higher than the 21.7% and 18.5% reaching PASI 100 on ustekinumab (P less than .001), as well as significantly higher than the less than 1% of patients in the placebo arm who reached that endpoint.
At the end of 52 weeks, 51% of those on brodalumab 210 mg had completely clear skin, compared with 28.1% of the patients receiving ustekinumab (P less than .001).
The safety analysis for brodalumab showed that the most common adverse events were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia; of these, the last two were considered adverse drug reactions. At 52 weeks, those on brodalumab had a slightly greater risk of having nonserious fungal infections than did those on ustekinumab. Overall, however, there was no difference between the brodalumab and ustekinumab arms at week 52 for treatment-emergent adverse events or for serious adverse events.
A cluster of SIB events occurred in 2013 and 2014 during the clinical trials, and the sponsor consulted the FDA. Additional monitoring for suicidal ideation and depression via the Columbia Suicide Severity Rating Scale (C-SSRS) and the Patient Health Questionnaire (PHQ) were implemented at a point where 84% of trial patients were already in the uncontrolled open label extension stage of the trial; in addition, a retrospective data review searched for suicidal ideation and attempts before the implementation of these two tools.
A total of six brodalumab patients completed suicide in all clinical trials, including those for psoriatic arthritis and RA. Suicides included two during the 12- to 52-week follow-up and four during the long-term follow-up phase. Another 29 patients attempted suicide, had intentional self-injury, or had suicidal ideation during these phases of the study, for a total of 35 SIB events. (SIB includes completed suicide, suicide attempt, suicide behavior, and suicide ideation.)*
The significance of the number of completed suicides was difficult to ascertain, given the increased baseline prevalence of depression and related illnesses among patients with psoriasis. Compared with the general population, individuals with psoriasis have hazard ratios of 1.39, 1.31, and 1.44 for depression, anxiety, and suicidality, according to a cohort study cited by Robert Levin, M.D. , director of the division of pharmacovigilance I at the FDA’s Center for Drug Evaluation and Research (CDER).
In addition, monitoring for suicidal ideation and attempts was stepped up during the course of the clinical trials. Brodalumab’s sponsor, Valeant Pharmaceuticals, noted that the rates of suicidal ideation and attempts increased after implementation of the C-SSRS, while other neuropsychiatric symptoms remained stable or declined during the course of the studies. “This makes us think this is ascertainment bias,” said Valeant consultant Lauren Marangell, M.D., a psychiatrist and president of Brain Health Consultants.
Dr. Marangell also reported that, after stratification for preexisting psychiatric comorbidities and risk factors, “there is no question that the rate of SIB is higher in both arms with patients with baseline risk factors.” The FDA’s data analysis showed an 18-fold increase in the rate of SIB for brodalumab arm participants with a prior history of suicidality.
During its presentation, Valeant also pointed out that patients with histories of drug and alcohol abuse, depression, and suicidality were not specifically excluded from the clinical trials. This differentiates the brodalumab program from other biologic studies, “making the studies more characteristic of the real world,” said R.K. Pillai, Ph.D., Valeant vice president.
Dr. Levin’s analysis, one of several independent analyses conducted by different FDA divisions, found that the data were inconclusive. “We currently can’t conclude whether or not these are drug-related risks,” he said. Considering the critical severity of suicide as an outcome, “we must consider the application and regulatory actions carefully.”
Jean Kim, M.D., medical officer in CDER’s division of psychiatry products (DPP), noted that the number of suicides seen in the brodalumab trials “is higher than typically seen in DPP’s large psychiatric drug trials, which involve populations with higher psychiatric morbidity than patients with psoriasis.”
Patients with psoriasis are also known to have a baseline elevated risk of cardiovascular disease, and no committee member felt the brodalumab data showed any additional signal for major adverse cardiovascular events (MACEs) beyond what would be suspected in this population. “I don’t right now see a safety signal for MACE,” said panelist Michael Blaha, M.D., a cardiologist and director of clinical research at Johns Hopkins Ciccarone Center for Prevention of Heart Disease, Baltimore. He noted, however, that research is ongoing to elucidate the relationship between cytokine levels and MACE. “This question about whether cytokines raise or reduce rates for [cardiovascular] events is an open one.”
One of the dermatologists on the committee, Lynn Drake, M.D., said that her decision making was influenced by the disease burden psoriasis inflicts on the patients she sees in her daily practice. “These patients are ill. People tend to think it’s just skin disease. It is not just skin disease. … I’d hate to see our patients deprived of a drug that might help alleviate their symptoms,” said Dr. Drake.
Though committee members voiced some disagreement about whether a patient registry should be established, and if it should be voluntary, they were in agreement that patients and prescribers both be aware of the potential risks of brodalumab, without making any risk management strategy so burdensome that appropriate patients would be deterred from considering the medication. Psoriasis, Dr. Drake said, “is a devastating disease. We need this in our armamentarium.”
The proposed dosing for brodalumab is 210 mg injected subcutaneously weekly for 3 weeks, followed by 210 mg every 2 weeks thereafter.
The FDA usually follows the recommendations of its advisory committees. The panelists had no relevant financial disclosures.
On Twitter @karioakes
*A previous version of this article misstated the number of patients who experienced SIB events.
A Food and Drug Administration advisory committee has unanimously supported approval of the monoclonal antibody brodalumab for the treatment of moderate to severe plaque psoriasis, with the majority recommending risk management options beyond labeling to address concerns about the six completed suicides among patients treated with brodalumab in clinical trials.
Though no members of the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee disputed the drug’s efficacy for plaque psoriasis, the possibility of a signal for suicidal ideation and behavior (SIB) among the brodalumab group prompted the committee’s chair, Michael Bigby, M.D., to remark, “The big problem is that you have six completed suicides. … I would say it’s a fairly big number for a randomized controlled trial. Patients and clinicians need to be aware of this as a fact.”
Dr. Bigby, professor of dermatology at Harvard Medical School, Boston, headed the 18-member panel, which included dermatologists, psychiatrists, and cardiologists. Fourteen members voted in favor of approval with risk management measures beyond labeling; four members voted for approval with labeling alone.
Brodalumab targets interleukin-17 receptors, inhibiting IL-17 uptake and thus blocking the pathway responsible for the cutaneous and systemic signs and symptoms of psoriasis. The pivotal phase III brodalumab studies, AMAGINE-1, -2, and -3, enrolled a total of 4,373 patients with moderate to severe plaque psoriasis. AMAGINE-1 enrolled 661 patients in a 1:1:1 ratio to a randomized, double-blind placebo-controlled study that included two doses of brodalumab: 210 mg and 140 mg. The placebo-controlled phase of the study ran for 12 weeks, followed by a withdrawal and retreatment phase, and after 52 weeks, by an open label long-term extension phase.
AMAGINE-2 (1,831 patients) and AMAGINE-3 (1,881 patients) were identically designed studies that pitted both doses of brodalumab against an active comparator, ustekinumab (Stelara), as well as placebo, in a 2:2:1:1 ratio. This double-blind phase of the study also lasted 12 weeks. During weeks 12-52, patients on the brodalumab arms continued, with an exploration of wider-dose intervals for the 140-mg dose. Patients also continued with ustekinumab, with a rescue option to transition to brodalumab 210 mg. The placebo group could continue on brodalumab 210 mg as well. These two studies also had an open label long-term extension phase.
At the 12-week mark in AMAGINE-1, 41.9% of patients achieved the coprimary endpoint of 100% reduction in the Psoriasis Area and Severity Index (PASI 100) score, compared with 0.5% of those on placebo (P less than .001). AMAGINE-2 and -3 saw 44.4% and 36.7% of the brodalumab 210-mg arms achieving PASI 100 at the 12-week mark, respectively. These figures were significantly higher than the 21.7% and 18.5% reaching PASI 100 on ustekinumab (P less than .001), as well as significantly higher than the less than 1% of patients in the placebo arm who reached that endpoint.
At the end of 52 weeks, 51% of those on brodalumab 210 mg had completely clear skin, compared with 28.1% of the patients receiving ustekinumab (P less than .001).
The safety analysis for brodalumab showed that the most common adverse events were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia; of these, the last two were considered adverse drug reactions. At 52 weeks, those on brodalumab had a slightly greater risk of having nonserious fungal infections than did those on ustekinumab. Overall, however, there was no difference between the brodalumab and ustekinumab arms at week 52 for treatment-emergent adverse events or for serious adverse events.
A cluster of SIB events occurred in 2013 and 2014 during the clinical trials, and the sponsor consulted the FDA. Additional monitoring for suicidal ideation and depression via the Columbia Suicide Severity Rating Scale (C-SSRS) and the Patient Health Questionnaire (PHQ) were implemented at a point where 84% of trial patients were already in the uncontrolled open label extension stage of the trial; in addition, a retrospective data review searched for suicidal ideation and attempts before the implementation of these two tools.
A total of six brodalumab patients completed suicide in all clinical trials, including those for psoriatic arthritis and RA. Suicides included two during the 12- to 52-week follow-up and four during the long-term follow-up phase. Another 29 patients attempted suicide, had intentional self-injury, or had suicidal ideation during these phases of the study, for a total of 35 SIB events. (SIB includes completed suicide, suicide attempt, suicide behavior, and suicide ideation.)*
The significance of the number of completed suicides was difficult to ascertain, given the increased baseline prevalence of depression and related illnesses among patients with psoriasis. Compared with the general population, individuals with psoriasis have hazard ratios of 1.39, 1.31, and 1.44 for depression, anxiety, and suicidality, according to a cohort study cited by Robert Levin, M.D. , director of the division of pharmacovigilance I at the FDA’s Center for Drug Evaluation and Research (CDER).
In addition, monitoring for suicidal ideation and attempts was stepped up during the course of the clinical trials. Brodalumab’s sponsor, Valeant Pharmaceuticals, noted that the rates of suicidal ideation and attempts increased after implementation of the C-SSRS, while other neuropsychiatric symptoms remained stable or declined during the course of the studies. “This makes us think this is ascertainment bias,” said Valeant consultant Lauren Marangell, M.D., a psychiatrist and president of Brain Health Consultants.
Dr. Marangell also reported that, after stratification for preexisting psychiatric comorbidities and risk factors, “there is no question that the rate of SIB is higher in both arms with patients with baseline risk factors.” The FDA’s data analysis showed an 18-fold increase in the rate of SIB for brodalumab arm participants with a prior history of suicidality.
During its presentation, Valeant also pointed out that patients with histories of drug and alcohol abuse, depression, and suicidality were not specifically excluded from the clinical trials. This differentiates the brodalumab program from other biologic studies, “making the studies more characteristic of the real world,” said R.K. Pillai, Ph.D., Valeant vice president.
Dr. Levin’s analysis, one of several independent analyses conducted by different FDA divisions, found that the data were inconclusive. “We currently can’t conclude whether or not these are drug-related risks,” he said. Considering the critical severity of suicide as an outcome, “we must consider the application and regulatory actions carefully.”
Jean Kim, M.D., medical officer in CDER’s division of psychiatry products (DPP), noted that the number of suicides seen in the brodalumab trials “is higher than typically seen in DPP’s large psychiatric drug trials, which involve populations with higher psychiatric morbidity than patients with psoriasis.”
Patients with psoriasis are also known to have a baseline elevated risk of cardiovascular disease, and no committee member felt the brodalumab data showed any additional signal for major adverse cardiovascular events (MACEs) beyond what would be suspected in this population. “I don’t right now see a safety signal for MACE,” said panelist Michael Blaha, M.D., a cardiologist and director of clinical research at Johns Hopkins Ciccarone Center for Prevention of Heart Disease, Baltimore. He noted, however, that research is ongoing to elucidate the relationship between cytokine levels and MACE. “This question about whether cytokines raise or reduce rates for [cardiovascular] events is an open one.”
One of the dermatologists on the committee, Lynn Drake, M.D., said that her decision making was influenced by the disease burden psoriasis inflicts on the patients she sees in her daily practice. “These patients are ill. People tend to think it’s just skin disease. It is not just skin disease. … I’d hate to see our patients deprived of a drug that might help alleviate their symptoms,” said Dr. Drake.
Though committee members voiced some disagreement about whether a patient registry should be established, and if it should be voluntary, they were in agreement that patients and prescribers both be aware of the potential risks of brodalumab, without making any risk management strategy so burdensome that appropriate patients would be deterred from considering the medication. Psoriasis, Dr. Drake said, “is a devastating disease. We need this in our armamentarium.”
The proposed dosing for brodalumab is 210 mg injected subcutaneously weekly for 3 weeks, followed by 210 mg every 2 weeks thereafter.
The FDA usually follows the recommendations of its advisory committees. The panelists had no relevant financial disclosures.
On Twitter @karioakes
*A previous version of this article misstated the number of patients who experienced SIB events.
FROM AN FDA ADVISORY COMMITTEE HEARING
Consider home phototherapy for some pediatric patients
MINNEAPOLIS, MINN. – For a select subset of pediatric dermatology patients, home phototherapy may represent a safe, effective, and even affordable alternative to office visits. Some families whose children are in treatment for vitiligo, psoriasis, and atopic dermatitis may find that the expense and learning curve of administering treatment at home are worthwhile, but dermatologists must select those families carefully.
Leslie Castelo-Soccio, MD, PhD, professor of pediatric dermatology at the Children’s Hospital of Philadelphia, gave an overview of medical phototherapy for childhood skin diseases at the annual meeting of the Society for Pediatric Dermatology.
For vitiligo, narrow-band UVB’s (NBUVB) effectiveness is maximized if treatment is begun relatively early, and if results are going to happen, they’ll show up fairly quickly. “If there’s no response after six months, stop the therapy,” Dr. Castelo-Soccio said.
Although the literature shows NBUVB to be effective in treating atopic dermatitis, Dr. Castelo-Soccio noted that most pediatric atopic dermatitis studies have been small and retrospective and conducted in a population with severe disease.
Regarding psoriasis in children, the literature shows “higher numbers of patients with near-complete or complete response,” she said.
The experience of NBUVB for pediatric dermatologic conditions at the Children’s Hospital of Philadelphia supports the idea that “the best responses are seen after at least 40 treatments,” and that 6 months is enough time to see whether a patient will respond. The best responders at her institution are children with facial vitiligo. “Of course, you get a better response with compliance,” she noted.
The experience of her patients falls in line with the data about side effects, in which the most common adverse events are reactivation of HSV and burning.
Families ask about cancer risk, but “there are no published data on the risk of skin cancer in long-term phototherapy in children,” she said. At this point, the best pediatric dermatologists can do is to extrapolate risk from data on phototherapy for neonatal jaundice, but even those data are inconclusive, she said.
Dr. Castelo-Soccio noted that it’s pretty common for families to request home treatment: “When you start talking to patients about phototherapy, the thing I always get questions about is, ‘Why can’t I do it at home?’ ” She prefers to initiate treatment in the clinic and then assess suitability for home therapy after a relationship has been established.
The ideal patient, said Dr. Castelo-Soccio, is one whose family has been diligent about coming to appointments and who otherwise demonstrates excellent compliance.
At first blush, the cost of acquiring a home device – often in the $2,000 range – might seem prohibitive for many families. The upfront cost may be worth it for some, since office visits involve copayments and lost time from school and work for multiple treatments weekly over a period of months. A big commute to the doctor’s office for treatment may further tip the scales toward home treatment. “I wouldn’t hesitate to offer this option to the right family,” she said.
Dr. Castelo-Soccio said she’s had some limited success getting insurance reimbursement for home phototherapy, especially if success has already been seen with office-based treatment.
NBUVB therapy has limitations, though. Some that have particular relevance for the pediatric population involve the challenges of safe delivery, including using appropriate eye wear and ensuring lack of movement. Each of these problems can be even more of a challenge at home, reinforcing the need to select appropriate patients for home phototherapy, she added.
Dr. Castelo-Soccio said she provides information about all of the various phototherapy devices to her patients and their parents, letting them make the choice. “All of the companies are really good about helping with paperwork” to apply for insurance reimbursement, she said. Options range from the bulkiest and most expensive – a full phototherapy box – to three-panel arrays, single panels, hand-foot devices, and even hand-held devices. The latter can be had for less than $1,000 and may be best suited for targeting smaller areas.
Features to look for in home phototherapy devices include a dosimeter accuracy sensor, which adjusts the treatment time to deliver the same dose, even if dust or aging lamps reduce output. User-friendly timers also are helpful for families, said Dr. Castelo-Soccio. A safety lock-out will allow only a certain number of treatments before the unit must be reset by the physician and is a reassuring feature. Each activation counts as a treatment, however, so families and physicians must be aware that if a hand-held unit is used to treat multiple small lesions in different body areas, a single treatment session will involve many device activations, each of which will be registered as a treatment.
Dr. Castelo-Soccio had no relevant financial disclosures.
On Twitter @karioakes
MINNEAPOLIS, MINN. – For a select subset of pediatric dermatology patients, home phototherapy may represent a safe, effective, and even affordable alternative to office visits. Some families whose children are in treatment for vitiligo, psoriasis, and atopic dermatitis may find that the expense and learning curve of administering treatment at home are worthwhile, but dermatologists must select those families carefully.
Leslie Castelo-Soccio, MD, PhD, professor of pediatric dermatology at the Children’s Hospital of Philadelphia, gave an overview of medical phototherapy for childhood skin diseases at the annual meeting of the Society for Pediatric Dermatology.
For vitiligo, narrow-band UVB’s (NBUVB) effectiveness is maximized if treatment is begun relatively early, and if results are going to happen, they’ll show up fairly quickly. “If there’s no response after six months, stop the therapy,” Dr. Castelo-Soccio said.
Although the literature shows NBUVB to be effective in treating atopic dermatitis, Dr. Castelo-Soccio noted that most pediatric atopic dermatitis studies have been small and retrospective and conducted in a population with severe disease.
Regarding psoriasis in children, the literature shows “higher numbers of patients with near-complete or complete response,” she said.
The experience of NBUVB for pediatric dermatologic conditions at the Children’s Hospital of Philadelphia supports the idea that “the best responses are seen after at least 40 treatments,” and that 6 months is enough time to see whether a patient will respond. The best responders at her institution are children with facial vitiligo. “Of course, you get a better response with compliance,” she noted.
The experience of her patients falls in line with the data about side effects, in which the most common adverse events are reactivation of HSV and burning.
Families ask about cancer risk, but “there are no published data on the risk of skin cancer in long-term phototherapy in children,” she said. At this point, the best pediatric dermatologists can do is to extrapolate risk from data on phototherapy for neonatal jaundice, but even those data are inconclusive, she said.
Dr. Castelo-Soccio noted that it’s pretty common for families to request home treatment: “When you start talking to patients about phototherapy, the thing I always get questions about is, ‘Why can’t I do it at home?’ ” She prefers to initiate treatment in the clinic and then assess suitability for home therapy after a relationship has been established.
The ideal patient, said Dr. Castelo-Soccio, is one whose family has been diligent about coming to appointments and who otherwise demonstrates excellent compliance.
At first blush, the cost of acquiring a home device – often in the $2,000 range – might seem prohibitive for many families. The upfront cost may be worth it for some, since office visits involve copayments and lost time from school and work for multiple treatments weekly over a period of months. A big commute to the doctor’s office for treatment may further tip the scales toward home treatment. “I wouldn’t hesitate to offer this option to the right family,” she said.
Dr. Castelo-Soccio said she’s had some limited success getting insurance reimbursement for home phototherapy, especially if success has already been seen with office-based treatment.
NBUVB therapy has limitations, though. Some that have particular relevance for the pediatric population involve the challenges of safe delivery, including using appropriate eye wear and ensuring lack of movement. Each of these problems can be even more of a challenge at home, reinforcing the need to select appropriate patients for home phototherapy, she added.
Dr. Castelo-Soccio said she provides information about all of the various phototherapy devices to her patients and their parents, letting them make the choice. “All of the companies are really good about helping with paperwork” to apply for insurance reimbursement, she said. Options range from the bulkiest and most expensive – a full phototherapy box – to three-panel arrays, single panels, hand-foot devices, and even hand-held devices. The latter can be had for less than $1,000 and may be best suited for targeting smaller areas.
Features to look for in home phototherapy devices include a dosimeter accuracy sensor, which adjusts the treatment time to deliver the same dose, even if dust or aging lamps reduce output. User-friendly timers also are helpful for families, said Dr. Castelo-Soccio. A safety lock-out will allow only a certain number of treatments before the unit must be reset by the physician and is a reassuring feature. Each activation counts as a treatment, however, so families and physicians must be aware that if a hand-held unit is used to treat multiple small lesions in different body areas, a single treatment session will involve many device activations, each of which will be registered as a treatment.
Dr. Castelo-Soccio had no relevant financial disclosures.
On Twitter @karioakes
MINNEAPOLIS, MINN. – For a select subset of pediatric dermatology patients, home phototherapy may represent a safe, effective, and even affordable alternative to office visits. Some families whose children are in treatment for vitiligo, psoriasis, and atopic dermatitis may find that the expense and learning curve of administering treatment at home are worthwhile, but dermatologists must select those families carefully.
Leslie Castelo-Soccio, MD, PhD, professor of pediatric dermatology at the Children’s Hospital of Philadelphia, gave an overview of medical phototherapy for childhood skin diseases at the annual meeting of the Society for Pediatric Dermatology.
For vitiligo, narrow-band UVB’s (NBUVB) effectiveness is maximized if treatment is begun relatively early, and if results are going to happen, they’ll show up fairly quickly. “If there’s no response after six months, stop the therapy,” Dr. Castelo-Soccio said.
Although the literature shows NBUVB to be effective in treating atopic dermatitis, Dr. Castelo-Soccio noted that most pediatric atopic dermatitis studies have been small and retrospective and conducted in a population with severe disease.
Regarding psoriasis in children, the literature shows “higher numbers of patients with near-complete or complete response,” she said.
The experience of NBUVB for pediatric dermatologic conditions at the Children’s Hospital of Philadelphia supports the idea that “the best responses are seen after at least 40 treatments,” and that 6 months is enough time to see whether a patient will respond. The best responders at her institution are children with facial vitiligo. “Of course, you get a better response with compliance,” she noted.
The experience of her patients falls in line with the data about side effects, in which the most common adverse events are reactivation of HSV and burning.
Families ask about cancer risk, but “there are no published data on the risk of skin cancer in long-term phototherapy in children,” she said. At this point, the best pediatric dermatologists can do is to extrapolate risk from data on phototherapy for neonatal jaundice, but even those data are inconclusive, she said.
Dr. Castelo-Soccio noted that it’s pretty common for families to request home treatment: “When you start talking to patients about phototherapy, the thing I always get questions about is, ‘Why can’t I do it at home?’ ” She prefers to initiate treatment in the clinic and then assess suitability for home therapy after a relationship has been established.
The ideal patient, said Dr. Castelo-Soccio, is one whose family has been diligent about coming to appointments and who otherwise demonstrates excellent compliance.
At first blush, the cost of acquiring a home device – often in the $2,000 range – might seem prohibitive for many families. The upfront cost may be worth it for some, since office visits involve copayments and lost time from school and work for multiple treatments weekly over a period of months. A big commute to the doctor’s office for treatment may further tip the scales toward home treatment. “I wouldn’t hesitate to offer this option to the right family,” she said.
Dr. Castelo-Soccio said she’s had some limited success getting insurance reimbursement for home phototherapy, especially if success has already been seen with office-based treatment.
NBUVB therapy has limitations, though. Some that have particular relevance for the pediatric population involve the challenges of safe delivery, including using appropriate eye wear and ensuring lack of movement. Each of these problems can be even more of a challenge at home, reinforcing the need to select appropriate patients for home phototherapy, she added.
Dr. Castelo-Soccio said she provides information about all of the various phototherapy devices to her patients and their parents, letting them make the choice. “All of the companies are really good about helping with paperwork” to apply for insurance reimbursement, she said. Options range from the bulkiest and most expensive – a full phototherapy box – to three-panel arrays, single panels, hand-foot devices, and even hand-held devices. The latter can be had for less than $1,000 and may be best suited for targeting smaller areas.
Features to look for in home phototherapy devices include a dosimeter accuracy sensor, which adjusts the treatment time to deliver the same dose, even if dust or aging lamps reduce output. User-friendly timers also are helpful for families, said Dr. Castelo-Soccio. A safety lock-out will allow only a certain number of treatments before the unit must be reset by the physician and is a reassuring feature. Each activation counts as a treatment, however, so families and physicians must be aware that if a hand-held unit is used to treat multiple small lesions in different body areas, a single treatment session will involve many device activations, each of which will be registered as a treatment.
Dr. Castelo-Soccio had no relevant financial disclosures.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
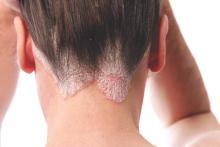
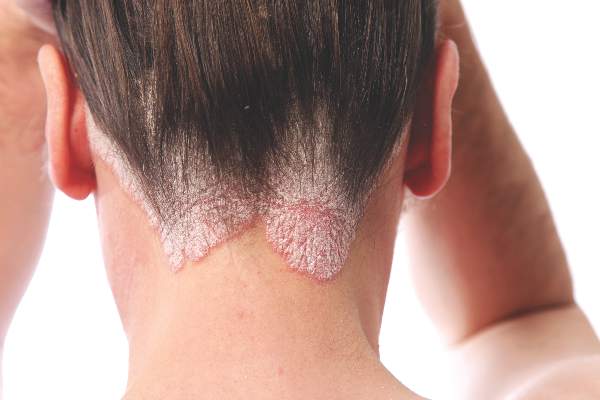
Cochrane review: Topical steroid–vitamin D combination best for scalp psoriasis
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
As a treatment for scalp psoriasis, the combination of a topical steroid and topical vitamin D was marginally better than topical steroids alone, but both approaches have a similar safety profile, according to a Cochrane review.
The systematic review included 59 randomized controlled trials of topical treatments for scalp psoriasis, representing a total of 11,561 participants of all ages, most of whom were followed for less than 6 months, according to Justin Schlager, MD, of the Charité – Universitätsmedizin Berlin, and coauthors (Cochrane Database of Systematic Reviews 2016. Issue 2. doi: 10.1002/14651858.CD009687.pub2).
In the analysis, topical steroid monotherapy was significantly better than topical vitamin D for clearance, as assessed by the Investigator’s Global Assessment of Disease Severity (risk ratio, 1.82; 95% confidence interval, 1.52-2.18). But the combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone (RR, 1.22; 95% CI, 1.08-1.36) and an even greater advantage over vitamin D alone (RR, 2.28; 95% CI, 1.87-2.78).
Similarly, for treatment response, the combination of a topical steroid and vitamin D showed the greatest benefit in terms of treatment response when compared with steroid monotherapy (RR, 1.15; 95% CI, 1.06-1.25) – an additional benefit the authors observed was small – and when compared with vitamin D alone (RR, 2.31; 95% CI, 1.75-3.04).
But for monotherapy, corticosteroids were more than twice as effective as vitamin D alone for treatment response (RR, 2.09; 95% CI, 1.80-2.41). The analysis also showed that corticosteroids of moderate, high, and very high potency were similarly effective.
Steroids were associated with a significantly lower risk of withdrawal because of adverse events than vitamin D (RR, 0.22; 95% CI, 0.11-0.42). Patients using topical steroids reported adverse events such as a burning sensation or irritation at the site of application, while patients taking vitamin D reported side effects such as pruritus, candidiasis, dermatitis, and erythema, although in both cases these were mostly limited to the application site.
The combination of vitamin D and topical steroid had a lower risk of withdrawals because of adverse events than vitamin D alone, but showed a similar rate to topical steroids alone.
“Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical corticosteroids may be fully acceptable for short-term therapy,” the authors wrote. The authors noted that data on patients’ quality of life was poor across all the studies included in the analysis and called for future trials to address this gap, and more long-term assessments.
“Regardless of the type of psoriasis, up to 79% of people with the condition present with scalp involvement, which has frequently been the first site to show symptoms of the disease,” they pointed out.
Thirty of the 59 studies were either conducted or sponsored by the manufacturer of the study medication, and the authors described the overall quality of the studies as “moderate.” Thirty-three studies were double blind, 14 were single blind, two had “third-party” blinding, six were open-label, and four did not report blinding information.
The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers’ fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
FDA advisory panel unanimously backs biosimilars for Humira, Enbrel
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
The Food and Drug Administration’s Arthritis Advisory Committee, together with an added complement of dermatologists and gastroenterologists, unanimously recommended during meetings on July 12 and 13 that the agency license a biosimilar Humira (adalimumab) that is made by Amgen and a biosimilar Enbrel (etanercept) that is made by Sandoz for many of the same indications held by the reference drugs.
The FDA advisory panel that endorsed biosimilar Humira recommended the agent’s approval in a 26-0 vote for many, but not all of the indications currently assigned to Humira itself: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in patients at least 4 years old, plaque psoriasis, adult Crohn’s disease, and adult ulcerative colitis.
A slightly different group of 20 advisory panel members (without any gastroenterologists) voted 20-0 in favor of the FDA granting biosimilar Enbrel all five of the indications now held by Enbrel: rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, and plaque psoriasis.
The biosimilar Humira and the biosimilar Enbrel are, respectively, the third and fourth candidate biosimilars to emerge from the FDA’s development program and receive advisory committee scrutiny and support. The first agent through the process, biosimilar filgrastim (Zarxio) received FDA approval in 2015 and is available in the United States. Although the second biosimilar through the process, the tumor necrosis factor inhibitor Inflectra that is biosimilar Remicade (infliximab), received FDA approval in April of this year, it has not yet become available for sale, although a spokeswoman for the company that will market it, Pfizer, said that the company expects to start U.S. sales of Inflectra before the end of 2016.
While the Arthritis Advisory Committee ended each of its daylong deliberations for each of the two candidate biosimilars with unanimous support, the panelists’ discussions among themselves and with FDA staffers reflected some uncertainty with the biosimilar concept, especially during the first day when they focused on biosimilar Humira. The major sticking point revolved around the regulatory pathway to approval first established by the Biologics Price Competition and Innovation Act of 2009 and subsequently refined by the FDA that allows a candidate biosimilar to establish its biosimilarity primarily though the results of analytical studies that establish that the candidate molecule is highly similar to the reference molecule. This approval scheme uses clinical trials in a confirmatory role to establish biosimilarity rather than as the linchpin of approval.
It also means that the FDA can grant clinical indications to the biosimilar drug based not on the results from clinical trials, but based entirely on what have already been demonstrated as safe and effective clinical applications for the reference drug. For example, the biosimilar Humira underwent testing in two clinical studies showing similar efficacy and safety as Humira in patients with rheumatoid arthritis and in patients with plaque psoriasis, but received endorsements based on extrapolations for an additional five indications. Biosimilar Enbrel was compared with Enbrel in patients with plaque psoriasis only and still received extrapolated indications for the additional four rheumatologic conditions.
“This is a new level of extrapolation, across indications,” noted Sarah E. Streett, MD, a gastroenterologist at Stanford (Calif.) University, one of several panelists who initially voiced uncertainty about the concept.
But FDA staffer Nikolay P. Nikolov, MD, who led the agency’s presentation, assured the panelists that the concept of extrapolation was at the heart of biosimilar development and regulatory assessment.
“We have confidence from the data that the two molecules [the reference drug and biosimilar drug] are so similar that we can rely on the safety and efficacy of the reference product. The premise of our approach to biosimilars is that this is not a new molecule that we know nothing about.”
The other uncertainty about biosimilar Humira and biosimilar Enbrel that raised concerns of many panelists were the prospects for nonmedical switching once these drugs reach the market. Nonmedical switching refers to when an insurance company or pharmacy benefit manager substitutes a biosimilar for a reference drug without approval from or even the knowledge of the prescribing physician or the patient. Many of the people who spoke during the public forum period on both days of hearings voiced their concerns about this prospect.
“Nonmedical switching is a major concern of clinicians and policy makers, and we need greater clarification from the FDA,” said committee chair Daniel H. Solomon, MD, a rheumatologist and professor of medicine at Harvard Medical School in Boston.
“I see a remarkable disconnect between the public’s concerns [about nonmedical switching] and the charge to the committee. These are essential issues that need a forum to be aired out,” said panelist Steven F. Solga, MD, chief of gastroenterology at St. Luke’s Hospital in Bethlehem, Pa.
Dr. Nikolov assured committee members that the FDA recognized this concern and was working on it. “We appreciate the disconnect between the charge and the concerns of the community. I assure you that the issues brought up will be part of our discussions so we can get this [biosimilar pathway] implemented the right way.”
According to the FDA’s regulations, a biosimilar designation does not allow for nonmedical switching, something that could only happen under a related but distinct designation known as interchangeability. During the committee meeting on July 13, a FDA staffer said that the agency is currently developing guidance for an “interchangeable” designation and plans to have it available before then end of 2016.
On Twitter @mitchelzoler
Debunking Psoriasis Myths: Is Psoriasis Contagious?
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Myth: Psoriasis is contagious
One of the challenges patients with psoriasis face is misinformation among their peers, especially about the cause of psoriasis. Results from a survey conducted in France (N=1005) regarding knowledge of psoriasis and perceptions toward psoriasis patients indicated that approximately 16.5% of respondents believed that psoriasis is contagious; 6.8% believed that the disease is related to personal hygiene and 3.2% believed that it affects more people with low personal hygiene (Halioua et al). Moreover, approximately 62.4% of respondents recognized a lack of information about psoriasis and 19.7% noted that they have misconceptions about the disease.
Another study from Poland on the feelings of stigmatization among psoriasis patients (N=102) found that 72 psoriasis patients reported that they felt other people think their skin disease is contagious; only 30 psoriasis patients indicated that they did not feel this way (Hrehorów et al).
These findings underscore the need for more information on psoriasis in the general public worldwide. It is important to spread the message that psoriasis is a chronic inflammatory disease of the immune system that affects approximately 7.5 million individuals in the United States. One cannot "catch" psoriasis; psoriasis starts or worsens because of a variety of triggers (eg, infections, injury to the skin, stress, cold weather, smoking, heavy alcohol consumption, certain medications). The National Institute of Arthritis and Musculoskeletal and Skin Diseases offers a simple but revealing explanation for the cause of psoriasis: "Normally, T cells help protect the body against infection and disease. In the case of psoriasis, T cells are put into action by mistake and become so active that they trigger other immune responses, which lead to inflammation and to rapid turnover of skin cells." Genetics may play a role in the development of psoriasis, along with environmental factors.
With several new therapies available, psoriasis has gained media attention. Dermatologists can circulate more information about the causes of psoriasis, which may work toward helping patients with psoriasis cope with the social impact of the condition.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
Hrehorów E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
Psoriasis. American Academy of Dermatology website. https://www.aad.org/media/stats/conditions/psoriasis. Accessed July 7, 2016.
Psoriasis causes. Mayo Clinic website. http://www.mayoclinic.org/diseases-conditions/psoriasis/basics/causes/con-20030838. Published June 17, 2015. Accessed July 7, 2016.
Questions and answers about psoriasis. National Institute of Arthritis and Musculoskeletal and Skin Diseases website. http://www.niams.nih.gov/Health_Info/Psoriasis/default.asp. Published October 2013. Accessed July 7, 2016.
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
Subclinical Joint Disease
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?
We are aware of the clinical importance of diagnosing psoriatic arthritis (PsA) as early as possible to initiate appropriate therapy. Because psoriasis precedes PsA in the majority of cases, it is incumbent on clinicians to seek any evidence of joint involvement at each clinical encounter.
In a study published online on February 25 in Annals of the Rheumatic Diseases. Faustini et al reported that patients with psoriasis but without PsA experience structural joint changes at the entheses. Therefore, evidence for structural joint alterations may already exist at the time of apparently exclusive skin involvement in psoriatic disease.
In the analysis, 85 participants without arthritis, including 55 with psoriasis and 30 healthy controls, received high-field magnetic resonance imaging (MRI) of the hand. These scans were scored for synovitis, osteitis, tenosynovitis, and periarticular inflammation. Participants with psoriasis also received complete clinical investigation as well as high-resolution peripheral quantitative computed tomography for detecting erosions and enthesiophytes. All participants were followed for at least 1 year to evaluate for the development of PsA.
Magnetic resonance imaging evaluation showed that 47% (26/55) of participants with psoriasis possessed at least 1 inflammatory lesion. Synovitis was the most prevalent inflammatory lesion (38% [21/55]), while osteitis (11% [6/55]), tenosynovitis (4% [2/55]), and periarticular inflammation (4% [2/55]) were less frequent.
The incidence of enthesiophytes and bone erosions did not differ between patients with psoriasis, with or without inflammatory changes on MRI. The risk for developing PsA was as high as 60% in those with subclinical synovitis and symptoms related to arthralgia. However, the risk was only 13% if the patients had normal MRIs and did not report arthralgia. Faustini et al concluded that the prevalence of subclinical inflammatory lesions is high in patients with cutaneous psoriasis. Specifically, arthralgia in conjunction with MRI synovitis constitutes a high-risk constellation for the development of PsA.
What’s the issue?
These findings are critical, as they indicate the nature of the potential genesis of PsA in many patients. If the data are confirmed in future investigations, it may change the way we evaluate or treat early PsA. How will these findings affect your workup for early PsA?