User login
Serious infections are increasing among psoriasis inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis is an independent risk factor for serious infections, and serious infections are increasing among inpatients with psoriasis.
Major finding: Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients in the United States with psoriasis between 2002 and 2012 (all P-values less than .05). In the United Kingdom during the same time period, patients with severe psoriasis had a 63% greater risk of serious infection than patients without psoriasis.
Data source: Analyses of data from the Nationwide Inpatient Sample for 2002 through 2012, and from The Health Improvement Network for 2003 through 2012.
Disclosures: The Nationwide Inpatient Sample analysis was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. The analysis of The Health Improvement Network was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
Make informed treatment decisions about biosimilars
In recent years federal laws have been enacted to help provide more treatment options and possibly lower costs to patients for therapeutic biological products. You have likely heard or read about “biosimilars” on the U.S. drug market. But are you ready to make informed treatment decisions and answer patients’ questions about these new drugs?
Biosimilars are not “generic biologics.” Although the shared goal of making biosimilars and generic drugs available is to provide more treatment options, there are important differences between the two approval pathways. For instance, unlike generic drugs, which are “bioequivalent” to their reference listed drug, biosimilar products can be either “biosimilar” to or “interchangeable” with their reference product. The differences may seem subtle based on the terminology, but the differences are important, and health care professionals who prescribe, dispense, or administer these products will need to understand them – as well as other aspects of these new therapies.
To help busy health care professionals better understand biosimilar and interchangeable products, the FDA has created a free 1.5-hour continuing education program titled, FDA Overview of Biosimilar Products. The course describes the important characteristics of biological products, particularly their complexity. For instance, a single molecule of a biological product, such as a monoclonal antibody, can easily be many hundreds of times larger and much more complex than that of a “small molecule” drug such as aspirin. Their increased complexity is one reason they are regulated differently than generics – and why, in the case of biosimilars, the law allows some differences from the reference product in clinically inactive components. The CE course discusses the “inherent variability” in the production of all biological products – both reference and biosimilar – and how the FDA accounts for these differences in assessing safety and efficacy of the products. The course also provides detailed definitions of important terms – such as “biosimilar” and “interchangeable” – and an overview of the standards the FDA has established for reviewing and approving biosimilar and interchangeable products. It also outlines the FDA review and approval standards for biosimilars and interchangeable products to help practitioners be assured that these products have been demonstrated to be safe and effective treatment options for their patients.
Some of our most important and expensive drugs are biological products used to treat patients who have serious medical conditions that are often life threatening and usually life altering. In April 2016, the Food and Drug Administration approved Inflectra (infliximab-dyyb) to help treat certain patients with certain gastrointestinal disorders, such as Crohn’s disease, and for other indications such as treatment of psoriasis and rheumatoid arthritis. The product is approved but not yet marketed in the United States. However, last year, Zarxio (filgrastim-sndz) became the first biosimilar actually available in the U.S. marketplace – approved to help boost white blood cell production for patients with severe neutropenia as well as for patients receiving various cancer therapies. These products are “biosimilar” to already-approved biological products called “reference products.” Inflectra is biosimilar to the reference product, Remicade (infliximab), and Zarxio is biosimilar to Neupogen (filgrastim).
Use of safe and effective biosimilar and interchangeable biological products can potentially make treatment more affordable for patients in need and improve public health. As patients begin to ask about the use of biosimilar and interchangeable products, the FDA hopes this course will help prepare health care practitioners to make informed decisions on behalf of their patients.
Dr. Christl is Associate Director for Therapeutic Biologics at the Office of New Drugs, Center for Drug Evaluation and Research, FDA.
The Food and Drug Administration’s recent approval of Inflectra (infliximab-dyyb) signals the advent of new therapeutic options in the treatment of patients with digestive diseases. However, there is still much to learn about biosimilars, their safety, and their efficacy. In the absence of sufficient clinical data, the American Gastroenterological Association has deferred taking a position on biosimilars. However, we recognize a critical need to educate gastroenterologists and their patients on this issue based on the information we have to date.
 |
Dr. Gary R. Lichtenstein |
In April 2016, the AGA Biosimilars Advisory Panel was created to determine key knowledge gaps regarding biosimilars, anticipate emerging issues around which to prepare AGA members, and recommend educational activities that address these gaps and issues. Upon review and approval by the AGA Institute Governing Board, the panel’s recommendations will be implemented by the AGA Center for Diagnostics and Therapeutics (CDT) in conjunction with other AGA committees. I am honored to chair this panel and look forward to working with my esteemed colleagues, all of whom are thought leaders in the field:
• Jean-Frederic Colombel, M.D., Mount Sinai Hospital
• Stephen B. Hanauer, M.D., AGAF, Northwestern University
• Sunanda V. Kane, M.D., MSPH, AGAF, Mayo Clinic
• Garrett Lawlor, M.D., Columbia University Medical Center
• James D. Lewis, M.D., MSCE, AGAF, University of Pennsylvania
• Loren A. Laine, M.D., AGAF, Yale University (CDT scientific advisory board liaison)
We hope to develop our initial recommendations in the next few months, recognizing that the field will continue to evolve quickly. AGA will also continue to work with the FDA and industry to ensure that the concerns of gastroenterologists are appropriately communicated and ensure that all of us are working together to improve the lives of patients with digestive diseases.
Dr. Gary R. Lichtenstein, AGAF, is chair, AGA Biosimilars Advisory Panel, and professor of medicine, Hospital of the University of Pennsylvania, Philadelphia.
The Food and Drug Administration’s recent approval of Inflectra (infliximab-dyyb) signals the advent of new therapeutic options in the treatment of patients with digestive diseases. However, there is still much to learn about biosimilars, their safety, and their efficacy. In the absence of sufficient clinical data, the American Gastroenterological Association has deferred taking a position on biosimilars. However, we recognize a critical need to educate gastroenterologists and their patients on this issue based on the information we have to date.
 |
Dr. Gary R. Lichtenstein |
In April 2016, the AGA Biosimilars Advisory Panel was created to determine key knowledge gaps regarding biosimilars, anticipate emerging issues around which to prepare AGA members, and recommend educational activities that address these gaps and issues. Upon review and approval by the AGA Institute Governing Board, the panel’s recommendations will be implemented by the AGA Center for Diagnostics and Therapeutics (CDT) in conjunction with other AGA committees. I am honored to chair this panel and look forward to working with my esteemed colleagues, all of whom are thought leaders in the field:
• Jean-Frederic Colombel, M.D., Mount Sinai Hospital
• Stephen B. Hanauer, M.D., AGAF, Northwestern University
• Sunanda V. Kane, M.D., MSPH, AGAF, Mayo Clinic
• Garrett Lawlor, M.D., Columbia University Medical Center
• James D. Lewis, M.D., MSCE, AGAF, University of Pennsylvania
• Loren A. Laine, M.D., AGAF, Yale University (CDT scientific advisory board liaison)
We hope to develop our initial recommendations in the next few months, recognizing that the field will continue to evolve quickly. AGA will also continue to work with the FDA and industry to ensure that the concerns of gastroenterologists are appropriately communicated and ensure that all of us are working together to improve the lives of patients with digestive diseases.
Dr. Gary R. Lichtenstein, AGAF, is chair, AGA Biosimilars Advisory Panel, and professor of medicine, Hospital of the University of Pennsylvania, Philadelphia.
The Food and Drug Administration’s recent approval of Inflectra (infliximab-dyyb) signals the advent of new therapeutic options in the treatment of patients with digestive diseases. However, there is still much to learn about biosimilars, their safety, and their efficacy. In the absence of sufficient clinical data, the American Gastroenterological Association has deferred taking a position on biosimilars. However, we recognize a critical need to educate gastroenterologists and their patients on this issue based on the information we have to date.
 |
Dr. Gary R. Lichtenstein |
In April 2016, the AGA Biosimilars Advisory Panel was created to determine key knowledge gaps regarding biosimilars, anticipate emerging issues around which to prepare AGA members, and recommend educational activities that address these gaps and issues. Upon review and approval by the AGA Institute Governing Board, the panel’s recommendations will be implemented by the AGA Center for Diagnostics and Therapeutics (CDT) in conjunction with other AGA committees. I am honored to chair this panel and look forward to working with my esteemed colleagues, all of whom are thought leaders in the field:
• Jean-Frederic Colombel, M.D., Mount Sinai Hospital
• Stephen B. Hanauer, M.D., AGAF, Northwestern University
• Sunanda V. Kane, M.D., MSPH, AGAF, Mayo Clinic
• Garrett Lawlor, M.D., Columbia University Medical Center
• James D. Lewis, M.D., MSCE, AGAF, University of Pennsylvania
• Loren A. Laine, M.D., AGAF, Yale University (CDT scientific advisory board liaison)
We hope to develop our initial recommendations in the next few months, recognizing that the field will continue to evolve quickly. AGA will also continue to work with the FDA and industry to ensure that the concerns of gastroenterologists are appropriately communicated and ensure that all of us are working together to improve the lives of patients with digestive diseases.
Dr. Gary R. Lichtenstein, AGAF, is chair, AGA Biosimilars Advisory Panel, and professor of medicine, Hospital of the University of Pennsylvania, Philadelphia.
In recent years federal laws have been enacted to help provide more treatment options and possibly lower costs to patients for therapeutic biological products. You have likely heard or read about “biosimilars” on the U.S. drug market. But are you ready to make informed treatment decisions and answer patients’ questions about these new drugs?
Biosimilars are not “generic biologics.” Although the shared goal of making biosimilars and generic drugs available is to provide more treatment options, there are important differences between the two approval pathways. For instance, unlike generic drugs, which are “bioequivalent” to their reference listed drug, biosimilar products can be either “biosimilar” to or “interchangeable” with their reference product. The differences may seem subtle based on the terminology, but the differences are important, and health care professionals who prescribe, dispense, or administer these products will need to understand them – as well as other aspects of these new therapies.
To help busy health care professionals better understand biosimilar and interchangeable products, the FDA has created a free 1.5-hour continuing education program titled, FDA Overview of Biosimilar Products. The course describes the important characteristics of biological products, particularly their complexity. For instance, a single molecule of a biological product, such as a monoclonal antibody, can easily be many hundreds of times larger and much more complex than that of a “small molecule” drug such as aspirin. Their increased complexity is one reason they are regulated differently than generics – and why, in the case of biosimilars, the law allows some differences from the reference product in clinically inactive components. The CE course discusses the “inherent variability” in the production of all biological products – both reference and biosimilar – and how the FDA accounts for these differences in assessing safety and efficacy of the products. The course also provides detailed definitions of important terms – such as “biosimilar” and “interchangeable” – and an overview of the standards the FDA has established for reviewing and approving biosimilar and interchangeable products. It also outlines the FDA review and approval standards for biosimilars and interchangeable products to help practitioners be assured that these products have been demonstrated to be safe and effective treatment options for their patients.
Some of our most important and expensive drugs are biological products used to treat patients who have serious medical conditions that are often life threatening and usually life altering. In April 2016, the Food and Drug Administration approved Inflectra (infliximab-dyyb) to help treat certain patients with certain gastrointestinal disorders, such as Crohn’s disease, and for other indications such as treatment of psoriasis and rheumatoid arthritis. The product is approved but not yet marketed in the United States. However, last year, Zarxio (filgrastim-sndz) became the first biosimilar actually available in the U.S. marketplace – approved to help boost white blood cell production for patients with severe neutropenia as well as for patients receiving various cancer therapies. These products are “biosimilar” to already-approved biological products called “reference products.” Inflectra is biosimilar to the reference product, Remicade (infliximab), and Zarxio is biosimilar to Neupogen (filgrastim).
Use of safe and effective biosimilar and interchangeable biological products can potentially make treatment more affordable for patients in need and improve public health. As patients begin to ask about the use of biosimilar and interchangeable products, the FDA hopes this course will help prepare health care practitioners to make informed decisions on behalf of their patients.
Dr. Christl is Associate Director for Therapeutic Biologics at the Office of New Drugs, Center for Drug Evaluation and Research, FDA.
In recent years federal laws have been enacted to help provide more treatment options and possibly lower costs to patients for therapeutic biological products. You have likely heard or read about “biosimilars” on the U.S. drug market. But are you ready to make informed treatment decisions and answer patients’ questions about these new drugs?
Biosimilars are not “generic biologics.” Although the shared goal of making biosimilars and generic drugs available is to provide more treatment options, there are important differences between the two approval pathways. For instance, unlike generic drugs, which are “bioequivalent” to their reference listed drug, biosimilar products can be either “biosimilar” to or “interchangeable” with their reference product. The differences may seem subtle based on the terminology, but the differences are important, and health care professionals who prescribe, dispense, or administer these products will need to understand them – as well as other aspects of these new therapies.
To help busy health care professionals better understand biosimilar and interchangeable products, the FDA has created a free 1.5-hour continuing education program titled, FDA Overview of Biosimilar Products. The course describes the important characteristics of biological products, particularly their complexity. For instance, a single molecule of a biological product, such as a monoclonal antibody, can easily be many hundreds of times larger and much more complex than that of a “small molecule” drug such as aspirin. Their increased complexity is one reason they are regulated differently than generics – and why, in the case of biosimilars, the law allows some differences from the reference product in clinically inactive components. The CE course discusses the “inherent variability” in the production of all biological products – both reference and biosimilar – and how the FDA accounts for these differences in assessing safety and efficacy of the products. The course also provides detailed definitions of important terms – such as “biosimilar” and “interchangeable” – and an overview of the standards the FDA has established for reviewing and approving biosimilar and interchangeable products. It also outlines the FDA review and approval standards for biosimilars and interchangeable products to help practitioners be assured that these products have been demonstrated to be safe and effective treatment options for their patients.
Some of our most important and expensive drugs are biological products used to treat patients who have serious medical conditions that are often life threatening and usually life altering. In April 2016, the Food and Drug Administration approved Inflectra (infliximab-dyyb) to help treat certain patients with certain gastrointestinal disorders, such as Crohn’s disease, and for other indications such as treatment of psoriasis and rheumatoid arthritis. The product is approved but not yet marketed in the United States. However, last year, Zarxio (filgrastim-sndz) became the first biosimilar actually available in the U.S. marketplace – approved to help boost white blood cell production for patients with severe neutropenia as well as for patients receiving various cancer therapies. These products are “biosimilar” to already-approved biological products called “reference products.” Inflectra is biosimilar to the reference product, Remicade (infliximab), and Zarxio is biosimilar to Neupogen (filgrastim).
Use of safe and effective biosimilar and interchangeable biological products can potentially make treatment more affordable for patients in need and improve public health. As patients begin to ask about the use of biosimilar and interchangeable products, the FDA hopes this course will help prepare health care practitioners to make informed decisions on behalf of their patients.
Dr. Christl is Associate Director for Therapeutic Biologics at the Office of New Drugs, Center for Drug Evaluation and Research, FDA.
What Patients Need From Their Psoriasis Therapy
Psoriasis patients consider reduced scaling/flaking (64%), reduced itching (62%), and reduced redness and inflammation (52%) to be the top 3 most meaningful benefits when considering a new treatment, according to a patient poll during the US Food and Drug Administration (FDA) public meeting on patient-focused drug development for psoriasis. When asked to choose 1 benefit, 22% of patients indicated reduced scaling/flaking and reduced itching would be the most meaningful, with 18% of patients choosing reduction in the number of plaques, 16% reduced burning/stinging, and 11% reduced redness and inflammation.
The FDA held the meeting on March 17. According to Theresa Mullin, PhD, Director of the Office of Strategic Programs for the Center for Drug Evaluation and Research of the FDA, “Patient perspective helps inform [the FDA’s] understanding of the context for the assessment of benefit-risk and decision making for new drugs.” In addition to patient testimonials, the FDA posed a number of polls to learn about patient preferences. Their responses offer insight for dermatologists on the symptoms that are most bothersome to patients and the results patients want to see with treatment.
Of all the nonjoint symptoms of psoriasis, patients indicated that flaking/scaling (67%), itching (67%), dry cracked skin that may bleed (39%), pain or soreness (37%), and burning/stinging (25%) were the top 5 symptoms having the most significant impact on their daily life. The top 5 most bothersome impacts of psoriasis symptoms included emotional impacts (eg, self-esteem)(59%), limitations on activities (eg, work, school, sports, hobbies)(42%), stigma or embarrassment (41%), ability to fall asleep or stay asleep (39%), and impact on sexual intimacy (34%).
Of the drug therapies or medical devices used by patients to treat psoriasis, an overwhelming 96% of patients reported use of topical treatments, followed by oral or injected medications (76%), phototherapy (64%), and oral prescription medications (62%). Aside from drug therapies, patients indicated that they are managing their symptoms with over-the-counter products (eg, coal tar, salicylic acid, Epsom salt)(35%), complementary or alternative therapies (27%), other therapies not mentioned (18%), diet modifications (10%), no therapy (8%), or dietary and herbal supplements (2%).
The top ranking factors affecting the patients’ decisions about using treatments to help reduce or control the spread of psoriasis included whether the drug showed effectiveness for the specific benefit that is most meaningful for you (64%), access to treatment (eg, insurance coverage)(44%), and the possibility of rare but serious side effects (eg, blood disorders, certain cancers)(31%). The lowest ranking factor was how the medication is administered (4%).
In March 2015, Jeffrey M. Weinberg, MD, explored the topic of treatment refusal in his editorial, “First Refusal.” He reviewed the results from a study that sought to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal (J Dermatolog Treat. 2015;2:1-5). “The findings of this study indicate possible strategies to reduce patient refusal,” Dr. Weinberg wrote. “For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.” Focusing on the treatment of the patient’s most bothersome symptoms with therapies that will target these symptoms may help to strengthen the patient-physician relationship and ensure patient compliance to therapy.
Psoriasis patients consider reduced scaling/flaking (64%), reduced itching (62%), and reduced redness and inflammation (52%) to be the top 3 most meaningful benefits when considering a new treatment, according to a patient poll during the US Food and Drug Administration (FDA) public meeting on patient-focused drug development for psoriasis. When asked to choose 1 benefit, 22% of patients indicated reduced scaling/flaking and reduced itching would be the most meaningful, with 18% of patients choosing reduction in the number of plaques, 16% reduced burning/stinging, and 11% reduced redness and inflammation.
The FDA held the meeting on March 17. According to Theresa Mullin, PhD, Director of the Office of Strategic Programs for the Center for Drug Evaluation and Research of the FDA, “Patient perspective helps inform [the FDA’s] understanding of the context for the assessment of benefit-risk and decision making for new drugs.” In addition to patient testimonials, the FDA posed a number of polls to learn about patient preferences. Their responses offer insight for dermatologists on the symptoms that are most bothersome to patients and the results patients want to see with treatment.
Of all the nonjoint symptoms of psoriasis, patients indicated that flaking/scaling (67%), itching (67%), dry cracked skin that may bleed (39%), pain or soreness (37%), and burning/stinging (25%) were the top 5 symptoms having the most significant impact on their daily life. The top 5 most bothersome impacts of psoriasis symptoms included emotional impacts (eg, self-esteem)(59%), limitations on activities (eg, work, school, sports, hobbies)(42%), stigma or embarrassment (41%), ability to fall asleep or stay asleep (39%), and impact on sexual intimacy (34%).
Of the drug therapies or medical devices used by patients to treat psoriasis, an overwhelming 96% of patients reported use of topical treatments, followed by oral or injected medications (76%), phototherapy (64%), and oral prescription medications (62%). Aside from drug therapies, patients indicated that they are managing their symptoms with over-the-counter products (eg, coal tar, salicylic acid, Epsom salt)(35%), complementary or alternative therapies (27%), other therapies not mentioned (18%), diet modifications (10%), no therapy (8%), or dietary and herbal supplements (2%).
The top ranking factors affecting the patients’ decisions about using treatments to help reduce or control the spread of psoriasis included whether the drug showed effectiveness for the specific benefit that is most meaningful for you (64%), access to treatment (eg, insurance coverage)(44%), and the possibility of rare but serious side effects (eg, blood disorders, certain cancers)(31%). The lowest ranking factor was how the medication is administered (4%).
In March 2015, Jeffrey M. Weinberg, MD, explored the topic of treatment refusal in his editorial, “First Refusal.” He reviewed the results from a study that sought to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal (J Dermatolog Treat. 2015;2:1-5). “The findings of this study indicate possible strategies to reduce patient refusal,” Dr. Weinberg wrote. “For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.” Focusing on the treatment of the patient’s most bothersome symptoms with therapies that will target these symptoms may help to strengthen the patient-physician relationship and ensure patient compliance to therapy.
Psoriasis patients consider reduced scaling/flaking (64%), reduced itching (62%), and reduced redness and inflammation (52%) to be the top 3 most meaningful benefits when considering a new treatment, according to a patient poll during the US Food and Drug Administration (FDA) public meeting on patient-focused drug development for psoriasis. When asked to choose 1 benefit, 22% of patients indicated reduced scaling/flaking and reduced itching would be the most meaningful, with 18% of patients choosing reduction in the number of plaques, 16% reduced burning/stinging, and 11% reduced redness and inflammation.
The FDA held the meeting on March 17. According to Theresa Mullin, PhD, Director of the Office of Strategic Programs for the Center for Drug Evaluation and Research of the FDA, “Patient perspective helps inform [the FDA’s] understanding of the context for the assessment of benefit-risk and decision making for new drugs.” In addition to patient testimonials, the FDA posed a number of polls to learn about patient preferences. Their responses offer insight for dermatologists on the symptoms that are most bothersome to patients and the results patients want to see with treatment.
Of all the nonjoint symptoms of psoriasis, patients indicated that flaking/scaling (67%), itching (67%), dry cracked skin that may bleed (39%), pain or soreness (37%), and burning/stinging (25%) were the top 5 symptoms having the most significant impact on their daily life. The top 5 most bothersome impacts of psoriasis symptoms included emotional impacts (eg, self-esteem)(59%), limitations on activities (eg, work, school, sports, hobbies)(42%), stigma or embarrassment (41%), ability to fall asleep or stay asleep (39%), and impact on sexual intimacy (34%).
Of the drug therapies or medical devices used by patients to treat psoriasis, an overwhelming 96% of patients reported use of topical treatments, followed by oral or injected medications (76%), phototherapy (64%), and oral prescription medications (62%). Aside from drug therapies, patients indicated that they are managing their symptoms with over-the-counter products (eg, coal tar, salicylic acid, Epsom salt)(35%), complementary or alternative therapies (27%), other therapies not mentioned (18%), diet modifications (10%), no therapy (8%), or dietary and herbal supplements (2%).
The top ranking factors affecting the patients’ decisions about using treatments to help reduce or control the spread of psoriasis included whether the drug showed effectiveness for the specific benefit that is most meaningful for you (64%), access to treatment (eg, insurance coverage)(44%), and the possibility of rare but serious side effects (eg, blood disorders, certain cancers)(31%). The lowest ranking factor was how the medication is administered (4%).
In March 2015, Jeffrey M. Weinberg, MD, explored the topic of treatment refusal in his editorial, “First Refusal.” He reviewed the results from a study that sought to investigate refusal of topical treatments by patients living with psoriasis in France as well as the factors that influence such refusal (J Dermatolog Treat. 2015;2:1-5). “The findings of this study indicate possible strategies to reduce patient refusal,” Dr. Weinberg wrote. “For example, enhanced education about the therapeutic options for psoriasis and their benefits could counter negative perceptions about these therapies. It also appears that increased focus on the physician-patient relationship may have a positive impact in this area.” Focusing on the treatment of the patient’s most bothersome symptoms with therapies that will target these symptoms may help to strengthen the patient-physician relationship and ensure patient compliance to therapy.
Brodalumab effective for rare, severe types of psoriasis
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
The investigational interleukin-17 inhibitor brodalumab was safe and effective in a small phase III Japanese study of adults with two rare and severe types of psoriasis, generalized pustular psoriasis (GPP) and psoriatic erythroderma (PsE). The results were published in the British Journal of Dermatology.
The 52-week open label studyevaluated the safety and efficacy of brodalumab in 30 Japanese adults (mean age 48 years) with GPP (12 patients) and PsE (18 patients). Brodalumab, a human monoclonal antibody against human IL-17RA that blocks the biologic activities of IL-17, was administered by subcutaneous injection. Efficacy was assessed via Clinical Global Impression of Improvement (CGI) scores, the primary endpoint (Br J Dermatol. 2016 April 23. doi: 10.1111/bjd.14702).
A high proportion of patients with either disease achieved “improved” or “remission” CGI scores at weeks 2, 12, and 52, reported Dr. Kenshi Yamasaki, of the department of dermatology at Tohoku University, Miyagi, Japan, and his associates
At week 52, almost 92% of those with GPP and 100% of those with PsE had achieved “improved” or “remission” scores. The most common adverse event was nasopharyngitis, which occurred in one-third of patients. Infection-related adverse events were grade 1 or 2, no adverse events were fatal, and none of the five serious adverse events noted were considered to be attributable to treatment, they added. Although anti-brodalumab neutralizing antibodies were not detected, one patient tested positive for anti-brodalumab binding antibodies.
Noting that treatment with brodalumab has been associated with significant improvements in patients with plaque psoriasis and psoriatic arthritis in phase II and III studies, “results from this study confirm that brodalumab can improve patient symptoms not long after treatment is initiated,” in patients with GPP and PsE, the authors concluded. While acknowledging the study limitations, including the open label design and a small sample size, they added, “IL-17RA blocking will be a promising therapeutic target in patients with GPP and PsE.”
The safety profile and low expression of anti-brodalumab antibodies indicated that brodalumab was suitable for long-term use, they said.
The study was funded by Kyowa Hakko Kirin. All authors disclosed ties to pharmaceutical companies, including the funding source; one author is an employee of the company.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The interluekin-17 inhibitor brodalumab significantly improved symptoms of generalized pustular psoriasis and psoriatic erythroderma in a small, Japanese open label study.
Major finding: Almost all brodalumab-treated patients with generalized pustular psoriasis (GPP) or psoriatic erythroderma (PsE) showed high levels of clinical improvement and low levels of adverse events.
Data sources: The phase III open-label multicenter study evaluated the safety and efficacy of brodalumab in 30 adults with GPP or PsE over 52 weeks.
Disclosures: Funding was provided by Kyowa Hakko Kirin. All authors disclosed ties to industry sources, including the funding source.
Effective psoriasis therapy may reduce coronary plaque burden
CHICAGO – Improvement in psoriasis was associated with a significant reduction in coronary plaque burden within 1 year in a pilot study conducted at the National Heart, Lung, and Blood Institute, Joseph B. Lerman reported at the annual meeting of the American College of Cardiology.
“If you look at psoriatic plaque on the skin, it’s spewing out cytokines such as tumor necrosis factor–alpha and interleukin-17 which are highly linked to atherosclerosis. What we’ve found is that if you treat those plaques and reduce the severity of psoriasis, we’ve noticed small but statistically significant regression in the early noncalcified plaque. It’s a very exciting observation,” said Mr. Lerman, a medical student at Mount Sinai School of Medicine, New York.
He presented an observational study involving 50 consecutive patients with mild to moderate psoriasis of roughly 20 years duration and a median baseline Framingham Risk Score of 4. They underwent measurement of coronary plaque burden by coronary CT angiography at baseline and 1 year later.
During the study year, 33 patients showed significant improvement in their psoriasis as reflected in a decline in their mean Psoriasis Area and Severity Index score from 5.6 to 3.1. Those patients also showed significant improvement in their total and noncalcified plaque burden, with total plaque burden adjusted for luminal attenuation declining from 126 mm2 to 117 mm2. The association remained significant even after adjustment for traditional cardiovascular risk factors, the use of statin therapy, body mass index, and the use of systemic psoriasis therapies, including biologic agents.
Importantly, the reduction in plaque burden appeared to be largely concentrated in the subgroup of 31 patients on methotrexate or a biologic. And while this was a naturalistic observational study, the investigators have followed up with a prospective study of psoriasis patients placed on tumor necrosis factor inhibitors and confirmed that they, too, experienced a reduction in coronary plaque as measured by coronary CT angiography.
The investigators plan to expand the size of the study in order to confirm the findings. Mr. Lerman said the next question they would like to address is, how early does a measurable reduction in coronary plaque burden occur in response to clinical improvement in psoriasis? In order to explore this, the investigators will have to obtain institutional approval of a new investigative protocol which permits more frequent use of coronary CT angiography. At present the imaging study can be conducted only once per year due to the radiation exposure.
Mr. Lerman was involved in the psoriasis study while participating in the National Institutes of Health Medical Research Scholars Program. Senior investigator in the pilot study was Dr. Nehal Mehta, chief of the Section of Inflammation and Metabolic Disease at NHLBI in Bethesda, Md.
Mr. Lerman reported having no financial conflicts of interest.
CHICAGO – Improvement in psoriasis was associated with a significant reduction in coronary plaque burden within 1 year in a pilot study conducted at the National Heart, Lung, and Blood Institute, Joseph B. Lerman reported at the annual meeting of the American College of Cardiology.
“If you look at psoriatic plaque on the skin, it’s spewing out cytokines such as tumor necrosis factor–alpha and interleukin-17 which are highly linked to atherosclerosis. What we’ve found is that if you treat those plaques and reduce the severity of psoriasis, we’ve noticed small but statistically significant regression in the early noncalcified plaque. It’s a very exciting observation,” said Mr. Lerman, a medical student at Mount Sinai School of Medicine, New York.
He presented an observational study involving 50 consecutive patients with mild to moderate psoriasis of roughly 20 years duration and a median baseline Framingham Risk Score of 4. They underwent measurement of coronary plaque burden by coronary CT angiography at baseline and 1 year later.
During the study year, 33 patients showed significant improvement in their psoriasis as reflected in a decline in their mean Psoriasis Area and Severity Index score from 5.6 to 3.1. Those patients also showed significant improvement in their total and noncalcified plaque burden, with total plaque burden adjusted for luminal attenuation declining from 126 mm2 to 117 mm2. The association remained significant even after adjustment for traditional cardiovascular risk factors, the use of statin therapy, body mass index, and the use of systemic psoriasis therapies, including biologic agents.
Importantly, the reduction in plaque burden appeared to be largely concentrated in the subgroup of 31 patients on methotrexate or a biologic. And while this was a naturalistic observational study, the investigators have followed up with a prospective study of psoriasis patients placed on tumor necrosis factor inhibitors and confirmed that they, too, experienced a reduction in coronary plaque as measured by coronary CT angiography.
The investigators plan to expand the size of the study in order to confirm the findings. Mr. Lerman said the next question they would like to address is, how early does a measurable reduction in coronary plaque burden occur in response to clinical improvement in psoriasis? In order to explore this, the investigators will have to obtain institutional approval of a new investigative protocol which permits more frequent use of coronary CT angiography. At present the imaging study can be conducted only once per year due to the radiation exposure.
Mr. Lerman was involved in the psoriasis study while participating in the National Institutes of Health Medical Research Scholars Program. Senior investigator in the pilot study was Dr. Nehal Mehta, chief of the Section of Inflammation and Metabolic Disease at NHLBI in Bethesda, Md.
Mr. Lerman reported having no financial conflicts of interest.
CHICAGO – Improvement in psoriasis was associated with a significant reduction in coronary plaque burden within 1 year in a pilot study conducted at the National Heart, Lung, and Blood Institute, Joseph B. Lerman reported at the annual meeting of the American College of Cardiology.
“If you look at psoriatic plaque on the skin, it’s spewing out cytokines such as tumor necrosis factor–alpha and interleukin-17 which are highly linked to atherosclerosis. What we’ve found is that if you treat those plaques and reduce the severity of psoriasis, we’ve noticed small but statistically significant regression in the early noncalcified plaque. It’s a very exciting observation,” said Mr. Lerman, a medical student at Mount Sinai School of Medicine, New York.
He presented an observational study involving 50 consecutive patients with mild to moderate psoriasis of roughly 20 years duration and a median baseline Framingham Risk Score of 4. They underwent measurement of coronary plaque burden by coronary CT angiography at baseline and 1 year later.
During the study year, 33 patients showed significant improvement in their psoriasis as reflected in a decline in their mean Psoriasis Area and Severity Index score from 5.6 to 3.1. Those patients also showed significant improvement in their total and noncalcified plaque burden, with total plaque burden adjusted for luminal attenuation declining from 126 mm2 to 117 mm2. The association remained significant even after adjustment for traditional cardiovascular risk factors, the use of statin therapy, body mass index, and the use of systemic psoriasis therapies, including biologic agents.
Importantly, the reduction in plaque burden appeared to be largely concentrated in the subgroup of 31 patients on methotrexate or a biologic. And while this was a naturalistic observational study, the investigators have followed up with a prospective study of psoriasis patients placed on tumor necrosis factor inhibitors and confirmed that they, too, experienced a reduction in coronary plaque as measured by coronary CT angiography.
The investigators plan to expand the size of the study in order to confirm the findings. Mr. Lerman said the next question they would like to address is, how early does a measurable reduction in coronary plaque burden occur in response to clinical improvement in psoriasis? In order to explore this, the investigators will have to obtain institutional approval of a new investigative protocol which permits more frequent use of coronary CT angiography. At present the imaging study can be conducted only once per year due to the radiation exposure.
Mr. Lerman was involved in the psoriasis study while participating in the National Institutes of Health Medical Research Scholars Program. Senior investigator in the pilot study was Dr. Nehal Mehta, chief of the Section of Inflammation and Metabolic Disease at NHLBI in Bethesda, Md.
Mr. Lerman reported having no financial conflicts of interest.
AT ACC 16
Key clinical point: Improved PASI scores were linked to regression of early noncalcified coronary plaque.
Major finding: Reduction in skin inflammation in psoriasis patients may cause regression of coronary plaque.
Data source: This prospective study of 50 patients with mild to moderate psoriasis featured precise measurements of coronary plaque burden at baseline and 1 year later.
Disclosures: The study was sponsored by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Pityriasis Lichenoides Chronica Presenting With Bilateral Palmoplantar Involvement
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
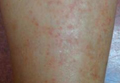
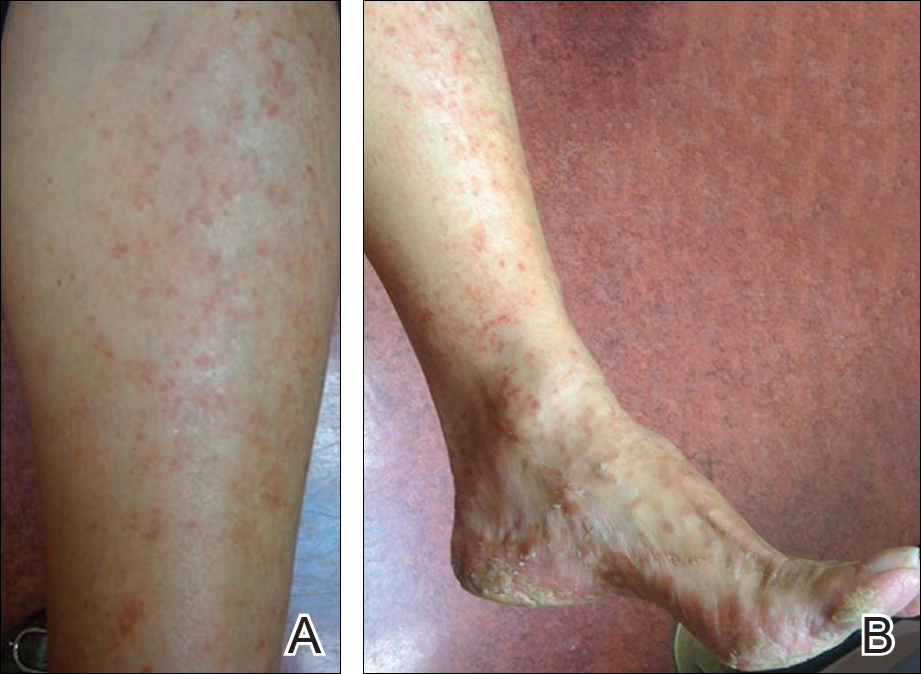
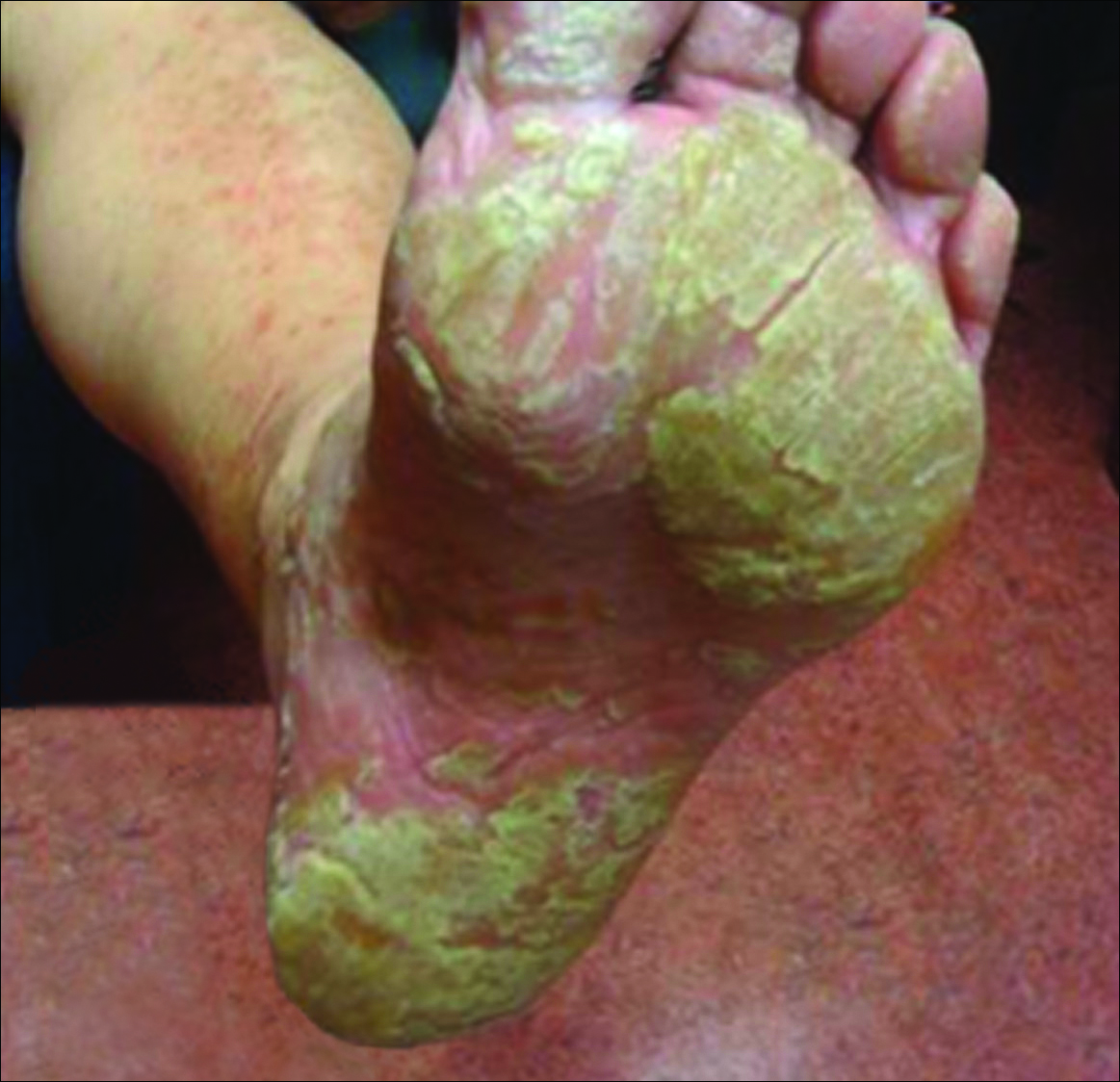
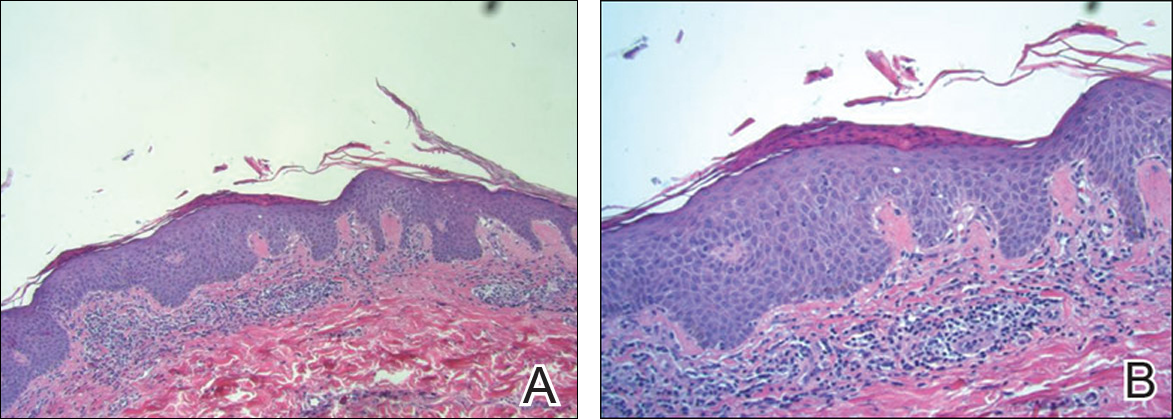
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).
The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Practice Points
- Diagnosis of pityriasis lichenoides may be difficult due to a wide spectrum of clinical presentations.
- Pityriasis lichenoides chronica (PLC) with palmoplantar involvement may mimic psoriasis.
- Screening for infections is not recommended in patients with PLC unless the patient has other symptoms pointing to a specific infection.
- Phototherapy currently is the most effective treatment modality for PLC.
Study of twins quantifies diabetes, obesity link with psoriasis
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.
 |
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.
 |
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
Given the association of psoriasis – particularly more severe disease – and increases in BMI, most of our patients would be recommended for diabetes screening based on standard recommendations.
 |
Dr. Joel M. Gelfand |
Therefore, dermatologists have the opportunity to educate patients with psoriasis and initiate appropriate screenings (or refer them to primary care physicians), which can result in better health outcomes through evidence-based interventions.
Dr. Joel M. Gelfand is from the department of dermatology and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania Perelman School of Medicine. He made this comments in an accompanying editorial (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2016.0670). Dr. Gelfand declared consultancies for Abbvie, AstraZeneca, Celgene, Coherus, Eli Lilly, Janssen Biologics (formerly Centocor), Sanofi, Merck, Novartis, Endo, Valeant, and Pfizer; research grant support from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Regeneron, and Pfizer (to the trustees of the University of Pennsylvania); and having received payment for continuing medical education work related to psoriasis. He is is a co–patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
The statistically significant association between psoriasis and both obesity and type 2 diabetes comes down to genetics, according to a large population-based study of twins.
Across the entire cohort of 33,588 Danish twins, researchers found the prevalence of psoriasis was 53% higher in individuals with type 2 diabetes, while the prevalence of psoriasis was 81% higher in individuals with a body mass index of at least 35, after adjustment for confounders such as sex, age, and smoking (JAMA Dermatol. 2016 April 27. doi:10.1001/jamadermatol.2015.6262).
Among the 449 twin pairs discordant for psoriasis, the risk for obesity was more than double in the twin with psoriasis compared to the unaffected twin, although this was significant only among dizygotic pairs, not monozygotic pairs.
The twin analysis also found that the risk of type 2 diabetes was the same between twins with and without psoriasis.
“Increased plasma levels of tumor necrosis factor, tumor necrosis factor receptors, and interleukin 6, which have important roles in the pathogenesis of psoriasis, have been found to be linked with obesity,” wrote Dr. Ann Sophie Lønnberg of the department of dermato-allergology, Gentofte Hospital, University of Copenhagen, and her associates.
“The association between type 2 diabetes mellitus and psoriasis also might be owing to increased tumor necrosis factor production from psoriatic inflammation and low-grade obesity inflammation, because it contributes to insulin resistance,” they wrote.
While obesity and type 2 diabetes are known comorbidities of psoriasis, Dr. Lønnberg and colleagues said this was the first study, to their knowledge, to explore the contribution of genetic and environmental factors to this interaction, and they suggested that future studies could look for genes and epigenetic factors that might underlie these associations.
Their analysis showed the genetic correlation between psoriasis and type 2 diabetes was 0.13, while the environmental correlation was 0.10. Similarly, the genetic correlation between psoriasis and BMI was 0.12, while environmental correlation was –0.05.
No conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: The prevalence of both type 2 diabetes and psoriasis is significantly higher in individuals with psoriasis.
Major finding: The risk of obesity is twofold higher in a twin with psoriasis compared to the co-twin without psoriasis.
Data source: Cross-sectional, population-based study of 33,588 Danish twins, including 449 pairs discordant for psoriasis.
Disclosures: No conflicts of interest were declared.
Psoriasis and Erectile Dysfunction

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?
Psoriasis tied to abdominal aortic aneurysm in nationwide study
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Psoriasis predicted abdominal aortic aneurysm in a large, population-based study.
Major finding: The adjusted risk of abdominal aortic aneurysm was 1.67 times greater among patients with severe psoriasis than in the reference population.
Data source: A retrospective cohort study of 5.5 million Danish adults, including 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis.
Disclosures: The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Inflectra becomes first FDA-approved biosimilar for inflammatory diseases
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.
A biosimilar version of the anti–tumor necrosis factor–alpha agent Remicade has been approved by the Food and Drug Administration, making it the first biosimilar drug approved by the agency for inflammatory diseases and just the second biosimilar it has approved.
The agency said in its April 5 announcement that the biosimilar drug, to be marketed as Inflectra, will have the same indications as Remicade: moderately to severely active Crohn’s disease in patients aged 6 years and older who have had an inadequate response to conventional therapy; moderately to severely active ulcerative colitis that has inadequately responded to conventional therapy; moderately to severely active rheumatoid arthritis in combination with methotrexate; active ankylosing spondylitis; active psoriatic arthritis; and chronic, severe plaque psoriasis.
The drug, given the generic name of infliximab-dyyb under the agency’s nomenclature for biosimilar products, earned its approval as a biosimilar by showing it has no clinically meaningful differences in terms of safety and effectiveness from Remicade. According to FDA regulations, biosimilar products can have only minor differences in clinically inactive components and must have the same mechanism(s) of action (to the extent that it is known) and route(s) of administration, dosage form(s), and strength(s) as the reference product; and can be approved only for the indication(s) and condition(s) of use that have been approved for the reference product.
Inflectra’s approval is only as a biosimilar, not as an interchangeable product. The agency has yet to define the regulatory requirements for interchangeability that are necessary to meet the requirements of the Biologics Price Competition and Innovation Act of 2009. That Act states that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” A statement about implementation of the Act on the FDA website explains that for interchangeability, “a sponsor must demonstrate that the biosimilar product can be expected to produce the same clinical result as the reference product in any given patient and, for a biological product that is administered more than once, that the risk of alternating or switching between use of the biosimilar product and the reference product is not greater than the risk of maintaining the patient on the reference product.”
Like Remicade, Inflectra will come with a boxed warning and a Medication Guide that describes important information about its uses and risks, which include serious infections (tuberculosis, bacterial sepsis, invasive fungal infections, and others), lymphoma and other malignancies, liver injury, blood problems, lupuslike syndrome, psoriasis, and in rare cases, nervous system disorders.
Inflectra is manufactured by Celltrion, based in South Korea, for Illinois-based Hospira. Inflectra’s label can be found here.