User login
How to make the most of your time with psoriasis patients
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
In the clinical experience of George Han, MD, PhD, .
“They come in with bags of topical products to show you what they’ve tried,” Dr. Han, associate professor of dermatology at Hofstra University, Hempstead, N.Y., said during the ODAC Dermatology, Aesthetic & Surgical Conference. “And you’re supposed to see this patient, talk to them, and counsel them in about 10 minutes. How do you make time to conduct an efficient psoriasis visit?”
Patients have a long-term battle to get clear, and spending a little longer on the initial visit “pays a lot of dividends,” he said. “Some of these patients are the most thankful patients in our practices, and it truly is gratifying” to see how much they can improve.
Questions about diet
Dr. Han said that psoriasis patients often ask him if, what, or how much they’re eating affects their disease. “But how do you counsel patients about diet when we’re not dietitians? We can at least give some guidance based on available data.”
He referred to a nationwide study of psoriasis patient-reported outcomes and dietary behaviors, which found that the percentage of patients who reported skin improvement was greatest after reducing intake of alcohol (53.8%); gluten (53.4%); and nightshade vegetables, such as tomatoes, potatoes, and peppers (52.1%); and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). He noted that there is a threefold increased incidence of celiac disease in patients with psoriasis.
As for nightshade vegetables, intake leads to increased alkaloids, “which have been known to worsen bowel inflammation such as in IBD [inflammatory bowel disease], but there is a lack of controlled trials examining this in the overall psoriasis population,” Dr. Han said. The Mediterranean diet, he added, “is sensible, and adding olive oil to your diet seems to have a positive effect on ... PASI, while fish oil seems to reduce C-reactive protein.” The data on the effect of vitamin D supplements are mixed, he said.
A separate randomized study evaluated the impact of weight loss in overweight or obese patients with psoriasis, who had not achieved clearance after 4 weeks of systemic treatment. Significantly more of those in the dietary intervention arm reached the weight loss goal of 5% at 20 weeks, and patients in this arm had a median reduction in the Psoriasis Area and Severity Index (PASI) score of almost 50%, compared with almost 26% among those without an active dietary intervention.
Joint pain, PsA
For psoriasis patients who complain of joint pain, he recommends administering quick measures like the five-question Psoriasis Epidemiology Screening Test (PEST) to screen for psoriatic arthritis (PsA), which is available on the National Psoriasis Foundation web site. “I ask patients about swollen, tender joints – specifically hands, wrists, ankles, feet, and toes,” Dr. Han said. Joint stiffness in the morning is a “concerning finding,” which is “more indicative of psoriatic arthritis than vague knee or back pain that worsens with use. If you have a younger patient with back pain who has a reduced ability to flex their spine, think axial disease.”
Tumor necrosis factor (TNF)–alpha inhibitors are considered first- and second-line treatment for PsA, but interleukin (IL)–17 inhibitors are generally considered just as effective overall. “The IL-23 inhibitors have mixed signals,” said Dr. Han, who is also on the NPF’s medical board. “We know that guselkumab is effective against psoriatic arthritis, but there is no inhibition of joint progression at the approved dosage on the label – though it was pretty close.”
Risankizumab (Skyrizi), an IL-23 inhibitor, was approved in January 2022 for adults with PsA and while the American College of Rheumatology response data “look reasonably good, the results for inhibition of radiographic progression are quite far off and it’s not in the label,” he said. Tildrakizumab (Ilumya), an IL-23 inhibitor, “looks impressive in phase 2b trials. It will be interesting to see if there is differentiation between the IL-23 agents in treating joint disease going forward.”
Dr. Han considers biologic therapy a good option for patients with questionable joint involvement or very limited joint disease. “If the patient has some evidence of PsA, as long as it’s a medication that has approval for that, I’m OK with starting it,” he said. “However, for patients whose joint pain dominates over the skin, or [who] have severe joint disease at presentation, I would prioritize the TNF-alpha inhibitors and IL-17s and refer them to rheumatology for shared management.”
Topical, oral treatments
As for topical approaches to treating psoriasis, adding halobetasol propionate 0.01% to tazarotene 0.045% may have a synergistic effect, while tapinarof 1% cream holds promise, he said. Tapinarof, which is expected to be approved this year, is an investigative aryl hydrocarbon agonist that inhibits an array of proinflammatory cytokines, including interferon-gamma and TNF-alpha. “It has been shown to have inhibitory effects both on Th17 cytokines and Th2 cytokines,” Dr. Han said. “What’s nice about this is that patients still appear to have treatment effect 1-2 months after stopping the drug.”
Another topical agent now under FDA review for psoriasis, is roflumilast, a phosphodiesterase type 4 (PDE4) inhibitor, which has been shown to have a treatment efficacy of 30% or more. “We’ll see how this works into our treatment regimen for psoriasis,” he said, as strategies targeting PDE4 have already been reported to help treat psoriasis.
With regards to oral therapies, he said that there are concerns about the efficacy of the oral PDE4 inhibitor apremilast, approved for psoriasis, compared with other biologics. Deucravacitinib, an oral selective tyrosine kinase 2 (TYK2) inhibitor also under FDA review for psoriasis, “may fill this gap, because its efficacy seems much stronger and really capitalizes on blocking IL-23, which we know is a central pathway in the pathogenesis of psoriasis.”
Phototherapy is another treatment option. Home narrowband-UVB devices cost $3,000-$5,000, “which is a fraction of 1 year of biologic treatment,” Dr. Han said. Older data on phototherapy suggest that “lesions can clear within 2-3 months, depending on how often you do the phototherapy, while newer data suggest that 75% of patients can achieve clear or minimal disease” with phototherapy.
Biologic therapy
If patients meet criteria for treatment with a biologic, he begins the conversation by saying, “I don’t want to give you an immunosuppressant, but your psoriasis represents an overactivation of inflammation in your body, so in some way we have to bring that down. Ideally, we would target your immune system in a way that targets psoriasis very narrowly, while leaving it to do what it needs to: protecting against infections and neoplasia.”
XXXIL-17 inhibitors generally have the fastest onset of action, Dr. Han noted. Authors of a review paper found that achievement of Psoriasis Area and Severity Index (PASI) 50 was 1.8 weeks with brodalumab, 1.9 weeks for ixekizumab, 3 weeks for high-dose secukinumab, 3.5 weeks for adalimumab, 3.7 weeks for infliximab, 5.1 weeks for low-dose ustekinumab, 6.5 weeks for high-dose etanercept, and 10.9 weeks with low-dose etanercept, while achievement of PASI 50 was closer to 1 month for IL-23 inhibitors.
“The conversation I have with patients on IL-23 inhibitors is, ‘we’re in this for the long haul,’ otherwise they come in 2 months later,” he said. “They may have gotten clearer but we’re talking about getting well over half of our patients to PASI 100, or to clear or minimal disease, and they may not have gotten there yet. It helps to frame expectations.”
Dr. Han disclosed that he is a consultant to, a speaker for, or has received research support from Beiersdorf, CeraVe, Celgene, Janssen, Lilly, MC2, Pfizer, UCB, Boehringer Ingelheim, Bond Avillion, Athenex, Amgen, AbbVie, Regeneron/Sanofi, LEO Pharma, Ortho Dermatologics, BMS, Sun Pharma, Dermavant, Dermtech, MedX, Novartis, and Castle Biosciences.
FROM ODAC 2022
Severe Acute Systemic Reaction After the First Injections of Ixekizumab
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
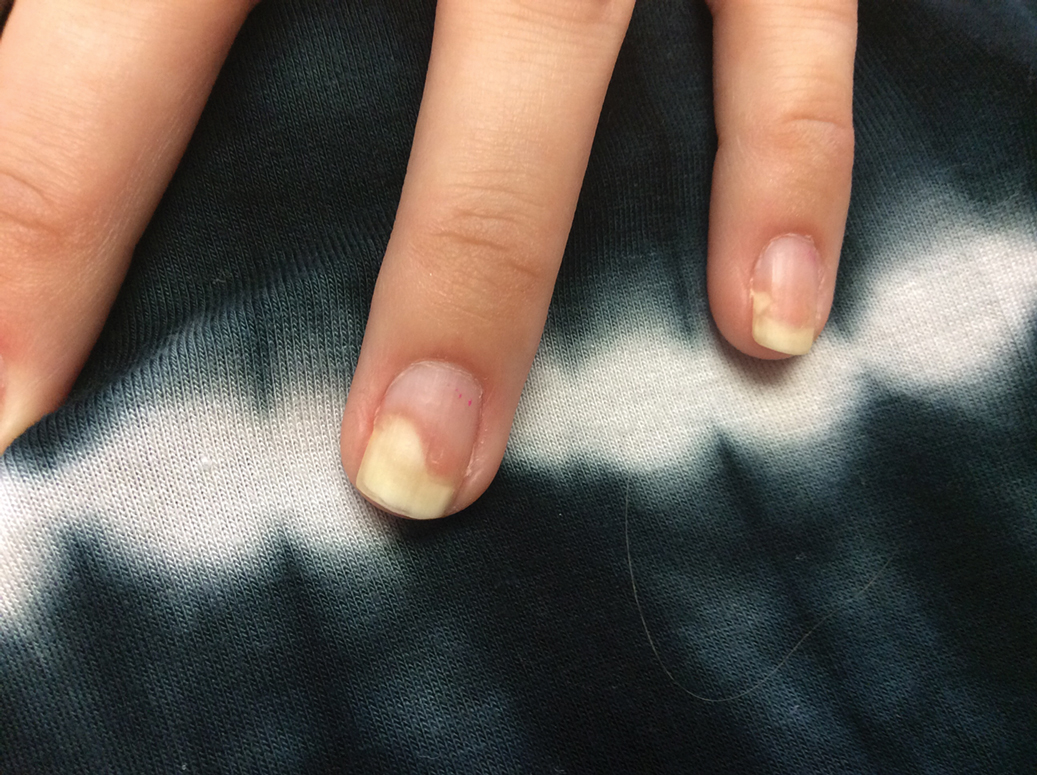
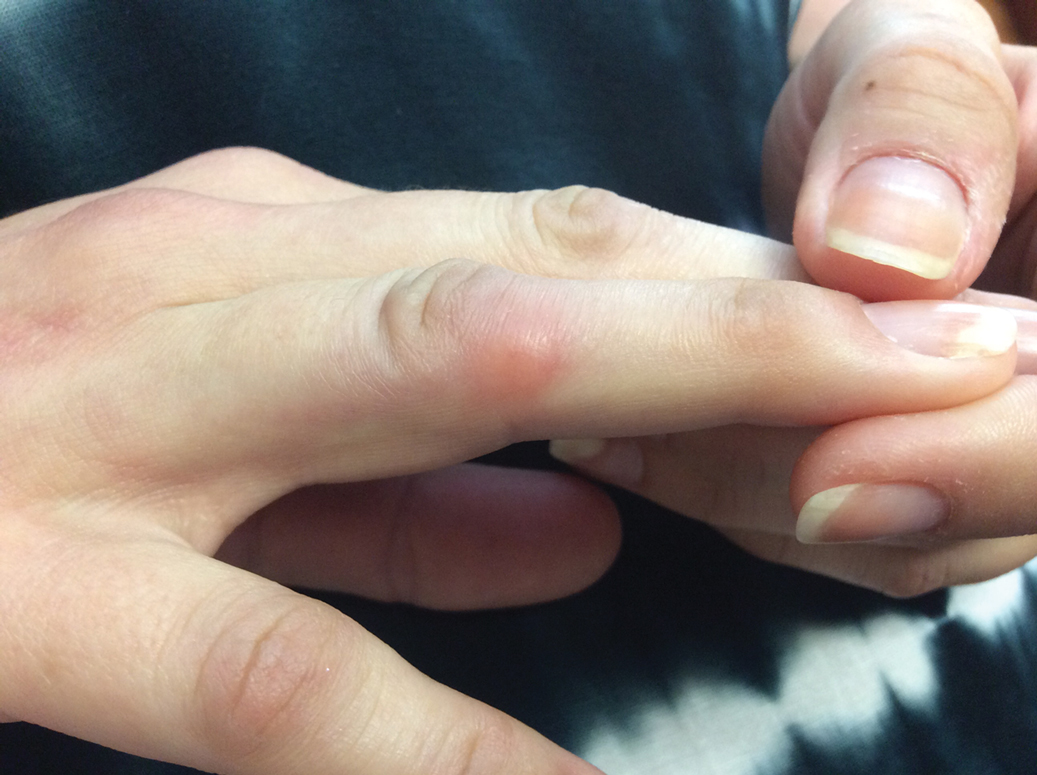
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Practice Points
- Psoriasis is an autoimmune disorder with a predominance of CD4+ and CD8+ T cells that release cytokines, such as tumor necrosis factor 11α and interleukins, which promote inflammation in the skin and joints and is associated with systemic inflammation predisposing patients to cardiovascular disease.
- Common adverse effects of most biologic medications for psoriasis include injection-site pain and rash, fever, malaise, back pain, urticaria and flushing, edema, dyspnea, and nausea.
- Ixekizumab is a humanized IL-17A antagonist intended for adults with moderate to severe psoriasis. Certain rare side effects specific to ixekizumab include inflammatory bowel disease, thrombocytopenia, severe injection-site reactions, and candidiasis.
- Acute onycholysis and acute exacerbation of arthritis/dactylitis are rare side effects of ixekizumab therapy.
A dermatologist-led model for CVD prevention in psoriasis may be feasible
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
A – may be feasible, given the positive perspectives expressed by both clinicians and patients in a set of electronic surveys, researchers say.
In an analysis of survey responses from 183 dermatologists and 322 patients, John S. Barbieri, MD, MBA, and coinvestigators found that more than two-thirds of dermatologists (69.3%) agreed it “seems doable” to check lipids and calculate a 10-year cardiovascular risk score, and over one-third (36.1%) agreed they could prescribe statins when indicated.
The patient survey was distributed through the National Psoriasis Foundation to individuals who were seeing a dermatologist or rheumatologist for psoriatic disease; the clinician survey was distributed through the American Academy of Dermatology to dermatologists who reported caring for patients with psoriasis. (A survey of rheumatologists was similarly conducted, but the number of participants fell short of the needed sample size.)
Most patients surveyed indicated they would be receptive to their dermatologist (or rheumatologist) playing a larger role in screening and managing CVD risk, and that they would be similarly likely to follow recommendations regarding risk screening and management whether the advice came their dermatologist/rheumatologist or from their PCP.
The clinician survey focused on lipids and statin use, and did not address other elements of risk management. Still, the researchers see their findings as an early but promising step in finding better models to improve cardiovascular outcomes for patients with psoriatic disease, who too often do not engage with their PCPs despite their increased risk of CVD and a higher risk of premature mortality from CVD.
Fewer than half of commercially insured adults aged under 65 years visit a PCP each year, the researchers noted. And among the patients in their survey, approximately 20% did not have a PCP or had not seen their PCP in the past year.
Other research has shown that only a small minority of patients with psoriasis have an encounter with their PCP within a year of establishing care with their dermatologist, and that “over half of patients with psoriasis have undetected risk factors like dyslipidemia or hypertension,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said in an interview.
“There’s a gap here, a missing link in the chain of cardiovascular disease prevention,” he said. “What if the dermatologist or rheumatologist could be more engaged in [CV] risk protection? ... It’s the idea of meeting the patients where they are.”
The surveys
The clinician survey focused on statins because of their ease of use, efficacy and safety, and the need for minimal monitoring, Dr. Barbieri said in the interview. “On the spectrum of things you can do for cardiovascular disease prevention, it’s one of the easiest ones.”
In an accompanying editorial, cardiologists Michael S. Garshick, MD, MS, and Jeffrey S. Berger, MD, MS, both of the department of medicine, New York University, wrote that, “despite the well-described association between psoriasis and CVD, only 35% of patients with psoriasis diagnosed with hyperlipidemia are adequately treated with statin therapy.”
“For many of these patients, their dermatologist or rheumatologist may be their only source of contact with the health care system,” they added.
Most studies targeting CVD risk in psoriasis have focused on targeting psoriatic inflammation, and few studies have explored strategies to improve modifiable CVD risk factor control with pharmacological therapy, they said.
In addition to the questions about receptiveness to identifying and potentially treating CVD risk with statins, the dermatologist survey included a best-worst scaling choice experiment to assess preferences for implementation approaches. Dermatologists were asked to rank their preferences for eight implementation strategies that have been shown in published studies to help increase statin prescribing rates.
The three highest-ranked strategies among dermatologists were clinical decision support, physician educational outreach, and patient education materials. The lowest-ranked strategies were comparisons with peers, a pay-for-performance option, and a mobile app/texting service to remind patients to undergo CVD risk screening.
Of the 183 dermatologists in the survey, 28.4% were from academic settings, 11.5% were from multispecialty groups, and 45.4% were from dermatology groups. (A low response rate of 5.2% for dermatologists raises some questions about the generalizability of the findings, Dr. Garshick and Dr. Berger noted in their editorial.)
Where to go from here?
Asked to comment on the results, Jashin J. Wu, MD, founder and CEO of the Dermatology Research and Education Foundation, Irvine, Calif., who was not involved with the study, said that a larger role in CVD risk management is “not likely to find traction with everyday dermatologists.”
“It’s already a big ask for community dermatologists to go through the approval process to get biologics for patients, so I don’t think many would be willing to add more to their plate by taking a bigger role in CVD management,” he said in an interview. He generally has not prescribed statins, “as I don’t feel that is in my scope of work.”
In the interview, Dr. Barbieri said that a parallel qualitative study, not yet published, has looked at the facilitators and barriers – including time constraints and concern about scope of practice – to statin prescribing and other elements of cardiovascular risk reduction.
All told, he said, a centralized care coordinator model may be the best approach to engage the dermatologist more in CVD prevention, including lipid management, but to also “offload some of the management responsibility.”
In this model, which is partially described by Dr. Barbieri and colleagues, the dermatologist (or rheumatologist) would educate the patient, measure blood pressure and check a lipid panel, and refer the patient to a coordinator who would, in turn, collect more information and calculate a 10-year CVD risk score.
Using a protocol-driven clinical decision support approach, the care coordinator would provide counseling about diet, exercise, and smoking cessation, and about whether statin therapy or blood pressure management is indicated.
“That coordinator would be in a good position to help the patient work with their PCP, if they have one, to find a PCP if they don’t, or to use telemedicine or work with their dermatologist or rheumatologist,” Dr. Barbieri said.
The centralized care coordinator service could be funded through grants, charitable funds, and patient assistance funds so that it is free to patients, he said, and could possibly be “housed in the National Psoriasis Foundation.”
Dr. Barbieri said he and his colleagues plan to design a clinical trial to test whether such a model can be adopted in practice and whether it can improve outcomes associated with CVD risk management.
In their editorial, Dr. Garshick and Dr. Berger, who is director of NYU Langone’s Center for the Prevention of Cardiovascular Disease, wrote that many patients with psoriatic disease have or are at risk for cardiometabolic conditions, and that CVD risk reduction should extend beyond lipid management to include blood pressure, glucose lowering, obesity management, and antiplatelet therapy.
The joint AAD-NPF guidelines for the management and treatment of psoriasis with awareness and attention to comorbidities, published in 2019, were among the first to formally recognize the enhanced CVD risk of patients with psoriasis, they noted.
The guidelines call upon dermatologists to inform patients of the psoriasis-CVD association and ensure their patients are engaged with their PCP or cardiologist for appropriate screening. Now, the editorialists say, “moving the needle forward includes refining and developing modifiable CVD risk reduction strategies for patients with psoriasis, and collaboration between the fields of dermatology, rheumatology, and cardiology is key.”
Incorporating a preventive cardiologist into combined dermatology-rheumatology clinics, or partnering as a freestanding cardioinflammatory clinic, also have potential to improve CVD risk, they wrote.
The survey study was supported by a grant from the NPF Psoriasis Prevention Initiative. Dr. Barbieri reported no conflicts of interest. Several authors disclosed consulting fees and grants from numerous pharmaceutical companies. Dr. Berger reported receiving personal fees from Janssen and grants from AstraZeneca outside of the submitted work. Dr. Garshick reported receiving personal fees from AbbVie outside of the submitted work.
FROM JAMA DERMATOLOGY
FDA approves risankizumab (Skyrizi) for psoriatic arthritis
The Food and Drug Administration on Jan. 21 approved risankizumab-rzaa (Skyrizi) for a second indication – treating adults with active psoriatic arthritis (PsA) – making it the second anti–interleukin-23 monoclonal antibody available to treat PsA, according to an announcement from manufacturer AbbVie.
The agency previously approved risankizumab in April 2019 for adults with moderate to severe plaque psoriasis.
The dosing regimen for PsA is the same as it is for patients with moderate to severe plaque psoriasis: a single 150-mg subcutaneous injection four times a year (after two starter doses at weeks 0 and 4), and it can be administered alone or in combination with disease-modifying antirheumatic drugs (DMARDs).
Two phase 3 trials, KEEPsAKE 1 and KEEPsAKE 2, were the basis for the approval. These two trials tested the biologic agent in adults with active PsA, including those who had responded inadequately or were intolerant to biologic therapy and/or nonbiologic DMARDs. Fulfillment of the trials’ primary endpoint of at least a 20% improvement in American College of Rheumatology response criteria at 24 weeks occurred in 51.3%-57.3% of patients, compared with 26.5%-33.5% of placebo-treated patients.
Those on risankizumab also achieved significantly higher rates of ACR50 and ACR70 responses than those on placebo. In addition, patients with preexisting dactylitis and enthesitis experienced improvements in these PsA manifestations. Risankizumab was also associated with an improvement in physical function at 24 weeks on the Health Assessment Questionnaire–Disability Index, bettering placebo by a mean difference of 0.16-0.20 points in the two trials. A significantly higher percentage of patients who had psoriatic skin lesions experienced at least 90% improvement with risankizumab on the Psoriasis Area and Severity Index, compared with placebo.
AbbVie said that the safety profile of risankizumab in patients with PsA has been generally consistent with its effects in patients with plaque psoriasis.
The KEEPsAKE 1 and KEEPsAKE 2 studies are ongoing, and patients in the long-term extensions of the trials remain blinded to the original randomized allocation for the duration of the studies.
Phase 3 trials of risankizumab are also ongoing in patients with Crohn’s disease and ulcerative colitis.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Jan. 21 approved risankizumab-rzaa (Skyrizi) for a second indication – treating adults with active psoriatic arthritis (PsA) – making it the second anti–interleukin-23 monoclonal antibody available to treat PsA, according to an announcement from manufacturer AbbVie.
The agency previously approved risankizumab in April 2019 for adults with moderate to severe plaque psoriasis.
The dosing regimen for PsA is the same as it is for patients with moderate to severe plaque psoriasis: a single 150-mg subcutaneous injection four times a year (after two starter doses at weeks 0 and 4), and it can be administered alone or in combination with disease-modifying antirheumatic drugs (DMARDs).
Two phase 3 trials, KEEPsAKE 1 and KEEPsAKE 2, were the basis for the approval. These two trials tested the biologic agent in adults with active PsA, including those who had responded inadequately or were intolerant to biologic therapy and/or nonbiologic DMARDs. Fulfillment of the trials’ primary endpoint of at least a 20% improvement in American College of Rheumatology response criteria at 24 weeks occurred in 51.3%-57.3% of patients, compared with 26.5%-33.5% of placebo-treated patients.
Those on risankizumab also achieved significantly higher rates of ACR50 and ACR70 responses than those on placebo. In addition, patients with preexisting dactylitis and enthesitis experienced improvements in these PsA manifestations. Risankizumab was also associated with an improvement in physical function at 24 weeks on the Health Assessment Questionnaire–Disability Index, bettering placebo by a mean difference of 0.16-0.20 points in the two trials. A significantly higher percentage of patients who had psoriatic skin lesions experienced at least 90% improvement with risankizumab on the Psoriasis Area and Severity Index, compared with placebo.
AbbVie said that the safety profile of risankizumab in patients with PsA has been generally consistent with its effects in patients with plaque psoriasis.
The KEEPsAKE 1 and KEEPsAKE 2 studies are ongoing, and patients in the long-term extensions of the trials remain blinded to the original randomized allocation for the duration of the studies.
Phase 3 trials of risankizumab are also ongoing in patients with Crohn’s disease and ulcerative colitis.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Jan. 21 approved risankizumab-rzaa (Skyrizi) for a second indication – treating adults with active psoriatic arthritis (PsA) – making it the second anti–interleukin-23 monoclonal antibody available to treat PsA, according to an announcement from manufacturer AbbVie.
The agency previously approved risankizumab in April 2019 for adults with moderate to severe plaque psoriasis.
The dosing regimen for PsA is the same as it is for patients with moderate to severe plaque psoriasis: a single 150-mg subcutaneous injection four times a year (after two starter doses at weeks 0 and 4), and it can be administered alone or in combination with disease-modifying antirheumatic drugs (DMARDs).
Two phase 3 trials, KEEPsAKE 1 and KEEPsAKE 2, were the basis for the approval. These two trials tested the biologic agent in adults with active PsA, including those who had responded inadequately or were intolerant to biologic therapy and/or nonbiologic DMARDs. Fulfillment of the trials’ primary endpoint of at least a 20% improvement in American College of Rheumatology response criteria at 24 weeks occurred in 51.3%-57.3% of patients, compared with 26.5%-33.5% of placebo-treated patients.
Those on risankizumab also achieved significantly higher rates of ACR50 and ACR70 responses than those on placebo. In addition, patients with preexisting dactylitis and enthesitis experienced improvements in these PsA manifestations. Risankizumab was also associated with an improvement in physical function at 24 weeks on the Health Assessment Questionnaire–Disability Index, bettering placebo by a mean difference of 0.16-0.20 points in the two trials. A significantly higher percentage of patients who had psoriatic skin lesions experienced at least 90% improvement with risankizumab on the Psoriasis Area and Severity Index, compared with placebo.
AbbVie said that the safety profile of risankizumab in patients with PsA has been generally consistent with its effects in patients with plaque psoriasis.
The KEEPsAKE 1 and KEEPsAKE 2 studies are ongoing, and patients in the long-term extensions of the trials remain blinded to the original randomized allocation for the duration of the studies.
Phase 3 trials of risankizumab are also ongoing in patients with Crohn’s disease and ulcerative colitis.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: PsA February 2022
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
More frequent secukinumab dosing found to benefit overweight psoriasis patients
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
, results from a multicenter, double-blind, parallel-group trial showed.
The more frequent dosing was also associated with comparable safety, consistent with the established secukinumab safety profile.
“Weight may have an impact on pharmacokinetics and, therefore, on the clinical outcome of biologic treatment for psoriasis,” Matthias Augustin, MD, and colleagues wrote in the study, published recently in the British Journal of Dermatology. “Dose optimization may be highly beneficial for patients with higher body weight,” they noted, adding that their study supports previous study findings and pharmacokinetic/pharmacodynamic modelling data, showing that secukinumab dosed every 2 weeks “leads to a clinically and statistically significant advantage in PASI 90 response,” compared with standard dosing every 4 weeks in patients who weight 90 kg (about 198 pounds) or more, after 16 weeks of treatment, which was maintained until week 52.
For the study, Dr. Augustin, of the Institute for Health Services Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 331 patients with moderate to severe chronic plaque psoriasis who weighed 90 kg or more to receive secukinumab 300 mg every 2 weeks, or secukinumab 300 mg every 4 weeks. The mean age of the patients was 47 years, 75% were male, 92% were White, and their mean body weight was 111.1 kg, with a mean body mass index of 36.1 kg/m2.
Patients who did not achieve a Psoriasis Area and Severity Index (PASI) 90 at week 16 on the monthly regimen (Q4W) either remained on that regimen or were up-titrated to dosing every 2 weeks (Q2W). Of the 331 patients, 165 received Q2W dosing and 166 received Q4W dosing. The researchers found that, at 16 weeks, patients in the Q2W dosing group had significantly higher PASI 90 responses, compared with those in the Q4W group (73.2% vs. 55.5%, respectively; P = .0003; odds ratio estimate, 2.3).
At 52 weeks, a greater proportion of patients in the Q2W group maintained responses to several outcome measures, compared with those in the Q4W group, including PASI 75 (88.9% vs. 74.8%), PASI 90 (76.4% vs. 52.4%), and PASI 100 (46.7% vs. 27.3%) scores; Investigator’s Global Assessment score of 0 or 1 (75.9% vs. 55.6%); and Dermatology Life Quality Index scores of 0 or 1 (66.1% vs. 48.8%).
In addition, those who had not had a PASI 90 response at week 16 who were up-titrated to Q2W dosing demonstrated higher efficacy responses at week 32, compared with those who remained on the Q4W regimen, with PASI 90 scores of 37.7% versus 16.5%, respectively.
Both regimens were well-tolerated, consistent with the known secukinumab safety profile; safety was comparable in the treatment arms, and there was “no clear dose-response relationship seen” for the incidence of overall adverse events, serious AEs, and AEs leading to discontinuation of the study treatment, “or AEs related to the identified risks” of infections, hypersensitivity, neutropenia and potential risk of major adverse cardiovascular events, the authors wrote.
“Despite more frequent dosing, the incidence of Candida infections was numerically lower in the Q2W group versus the Q4W group,” although there were not many cases, three patients versus six patients, respectively.
Need for individualized treatment
“Despite a decades-long revolution in development of highly efficacious biologic treatments for psoriasis, we are only in the early stages of developing personalized clinical approaches,” said Raj Chovatiya, MD, PhD, a dermatologist at Northwestern University, Chicago, who was asked to comment on the study. “The need for individualized treatment in psoriasis is very real; not every patient may respond to therapy in the same way. Obesity is one important comorbidity of psoriasis, and increased body mass index may be associated with variable treatment outcomes with systemic therapy.”
The data from this study, he added, “suggest that dose optimization may be an important strategy to enhance psoriasis clearance in patients with suboptimal treatment outcomes on standard dosing, including those with increased weight. Future studies should examine optimal regimen of biologic therapy across a variety of patient factors.”
The study was funded by Novartis, the manufacturer of secukinumab (Cosentyx); several authors were company employees. Dr. Augustin disclosed that he has served as a consultant for or has been a paid speaker for clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LEO Pharma, Medac, Merck, MSD, Novartis, Pfizer, UCB, and Xenoport. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Ultrasound variables could help identify psoriasis patients at risk of progressing to PsA
Key clinical point: Patients with psoriasis who showed ultrasound abnormalities in hand, knee, toe joints, and few other anatomical sites are more likely to progress to psoriatic arthritis (PsA).
Major finding: Hand joint power Doppler (PD) signals (grade 0 and ≥1), knee joint PD signals (grade 0 and ≥1), toe joint PD signals (grade 0 and ≥1), quadriceps tendon and patellar tendon enthesitis (all P < .001), wrist joint synovial thickening (grade 1-3; P = .001), and Achilles tendon and plantar aponeurosis enthesitis (P = .007) were all significant risk predictors for PsA.
Study details: Findings are from a cross-sectional study including 852 patients with psoriasis but without PsA, 261 patients with PsA, and 86 healthy volunteers who underwent an ultrasound examination.
Disclosures: This study was funded by West China Hospital, West China Precision Medicine Industrial Technology Institutes, and Sichuan Provincial Science and Technology Project. The authors declared no conflict of interests.
Source: Wang Y et al. Dermatol Ther (Heidelb). 2021 (Dec 19). Doi: 10.1007/s13555-021-00663-0.
Key clinical point: Patients with psoriasis who showed ultrasound abnormalities in hand, knee, toe joints, and few other anatomical sites are more likely to progress to psoriatic arthritis (PsA).
Major finding: Hand joint power Doppler (PD) signals (grade 0 and ≥1), knee joint PD signals (grade 0 and ≥1), toe joint PD signals (grade 0 and ≥1), quadriceps tendon and patellar tendon enthesitis (all P < .001), wrist joint synovial thickening (grade 1-3; P = .001), and Achilles tendon and plantar aponeurosis enthesitis (P = .007) were all significant risk predictors for PsA.
Study details: Findings are from a cross-sectional study including 852 patients with psoriasis but without PsA, 261 patients with PsA, and 86 healthy volunteers who underwent an ultrasound examination.
Disclosures: This study was funded by West China Hospital, West China Precision Medicine Industrial Technology Institutes, and Sichuan Provincial Science and Technology Project. The authors declared no conflict of interests.
Source: Wang Y et al. Dermatol Ther (Heidelb). 2021 (Dec 19). Doi: 10.1007/s13555-021-00663-0.
Key clinical point: Patients with psoriasis who showed ultrasound abnormalities in hand, knee, toe joints, and few other anatomical sites are more likely to progress to psoriatic arthritis (PsA).
Major finding: Hand joint power Doppler (PD) signals (grade 0 and ≥1), knee joint PD signals (grade 0 and ≥1), toe joint PD signals (grade 0 and ≥1), quadriceps tendon and patellar tendon enthesitis (all P < .001), wrist joint synovial thickening (grade 1-3; P = .001), and Achilles tendon and plantar aponeurosis enthesitis (P = .007) were all significant risk predictors for PsA.
Study details: Findings are from a cross-sectional study including 852 patients with psoriasis but without PsA, 261 patients with PsA, and 86 healthy volunteers who underwent an ultrasound examination.
Disclosures: This study was funded by West China Hospital, West China Precision Medicine Industrial Technology Institutes, and Sichuan Provincial Science and Technology Project. The authors declared no conflict of interests.
Source: Wang Y et al. Dermatol Ther (Heidelb). 2021 (Dec 19). Doi: 10.1007/s13555-021-00663-0.
Generally mild/moderate nonserious adverse events in PsA patients treated with tofacitinib
Key clinical point: Patients with psoriatic arthritis (PsA) who received treatment with tofacitinib experienced mostly mild/moderate nonserious adverse events (AE), which persisted for a shorter duration and had a minimum effect on the continuation of tofacitinib treatment.
Major finding: In the first 3 months of treatment, the most frequent nonserious AEs were headache (incidence rate [IR] 16.9-39.2) and diarrhea (IR 15-17) and duration of such AEs was ≤4 weeks with none leading to permanent discontinuation of treatment.
Study details: Findings are from a post hoc analysis of phase 3 studies including 710 patients with active PsA who received 5 mg tofacitinib, 10 mg tofacitinib, or placebo and had a previous inadequate response to ≥1 conventional synthetic disease-modifying antirheumatic drug or ≥1 tumor necrosis factor inhibitor.
Disclosures: This study was funded by Pfizer. Five authors reported being employees and stockholders of AbbVie. The other authors reported ties with several sources including AbbVie.
Source: Dikranian A et al. Rheumatol Ther. 2021 (Dec 17). Doi: 10.1007/s40744-021-00405-w.
Key clinical point: Patients with psoriatic arthritis (PsA) who received treatment with tofacitinib experienced mostly mild/moderate nonserious adverse events (AE), which persisted for a shorter duration and had a minimum effect on the continuation of tofacitinib treatment.
Major finding: In the first 3 months of treatment, the most frequent nonserious AEs were headache (incidence rate [IR] 16.9-39.2) and diarrhea (IR 15-17) and duration of such AEs was ≤4 weeks with none leading to permanent discontinuation of treatment.
Study details: Findings are from a post hoc analysis of phase 3 studies including 710 patients with active PsA who received 5 mg tofacitinib, 10 mg tofacitinib, or placebo and had a previous inadequate response to ≥1 conventional synthetic disease-modifying antirheumatic drug or ≥1 tumor necrosis factor inhibitor.
Disclosures: This study was funded by Pfizer. Five authors reported being employees and stockholders of AbbVie. The other authors reported ties with several sources including AbbVie.
Source: Dikranian A et al. Rheumatol Ther. 2021 (Dec 17). Doi: 10.1007/s40744-021-00405-w.
Key clinical point: Patients with psoriatic arthritis (PsA) who received treatment with tofacitinib experienced mostly mild/moderate nonserious adverse events (AE), which persisted for a shorter duration and had a minimum effect on the continuation of tofacitinib treatment.
Major finding: In the first 3 months of treatment, the most frequent nonserious AEs were headache (incidence rate [IR] 16.9-39.2) and diarrhea (IR 15-17) and duration of such AEs was ≤4 weeks with none leading to permanent discontinuation of treatment.
Study details: Findings are from a post hoc analysis of phase 3 studies including 710 patients with active PsA who received 5 mg tofacitinib, 10 mg tofacitinib, or placebo and had a previous inadequate response to ≥1 conventional synthetic disease-modifying antirheumatic drug or ≥1 tumor necrosis factor inhibitor.
Disclosures: This study was funded by Pfizer. Five authors reported being employees and stockholders of AbbVie. The other authors reported ties with several sources including AbbVie.
Source: Dikranian A et al. Rheumatol Ther. 2021 (Dec 17). Doi: 10.1007/s40744-021-00405-w.
Ultrasound evaluation of entheses helps discriminate psoriatic arthritis from fibromyalgia
Key clinical point: Ultrasound assessment of entheses may help differentiate psoriatic arthritis (PsA) from fibromyalgia syndrome (FMS) as patients with PsA showed more frequent ultrasound changes both in gray scale (GS) and power Doppler (PD) mode than those with FMS.
Major finding: A higher proportion of patients with PsA vs. FMS was detected with ≥1 entheses in GS (P < .0001) and PD (P = .0033) mode, with GS and PD identifying changes in a higher proportion of PsA vs. FMS entheses (P < .0001 for both). Area under the curve values for GS and PD mode were 0.77 and 0.66, respectively, with 3.5 being the best cutoff GS score to discriminate PsA from FMS (sensitivity, 0.75; specificity, 0.63).
Study details: Findings are from a post hoc analysis of the cross‐sectional ULISSE study including 140 and 51 patients with PsA and FMS, respectively.
Disclosures: This study was funded by AbbVie Srl. Three authors declared being employees and shareholders of AbbVie, and some of the authors declared receiving consultancy fees and research support from several sources.
Source: Marchesoni A et al. J Clin Med. 2021;11(1):180 (Dec 29). Doi: 10.3390/jcm11010180.
Key clinical point: Ultrasound assessment of entheses may help differentiate psoriatic arthritis (PsA) from fibromyalgia syndrome (FMS) as patients with PsA showed more frequent ultrasound changes both in gray scale (GS) and power Doppler (PD) mode than those with FMS.
Major finding: A higher proportion of patients with PsA vs. FMS was detected with ≥1 entheses in GS (P < .0001) and PD (P = .0033) mode, with GS and PD identifying changes in a higher proportion of PsA vs. FMS entheses (P < .0001 for both). Area under the curve values for GS and PD mode were 0.77 and 0.66, respectively, with 3.5 being the best cutoff GS score to discriminate PsA from FMS (sensitivity, 0.75; specificity, 0.63).
Study details: Findings are from a post hoc analysis of the cross‐sectional ULISSE study including 140 and 51 patients with PsA and FMS, respectively.
Disclosures: This study was funded by AbbVie Srl. Three authors declared being employees and shareholders of AbbVie, and some of the authors declared receiving consultancy fees and research support from several sources.
Source: Marchesoni A et al. J Clin Med. 2021;11(1):180 (Dec 29). Doi: 10.3390/jcm11010180.
Key clinical point: Ultrasound assessment of entheses may help differentiate psoriatic arthritis (PsA) from fibromyalgia syndrome (FMS) as patients with PsA showed more frequent ultrasound changes both in gray scale (GS) and power Doppler (PD) mode than those with FMS.
Major finding: A higher proportion of patients with PsA vs. FMS was detected with ≥1 entheses in GS (P < .0001) and PD (P = .0033) mode, with GS and PD identifying changes in a higher proportion of PsA vs. FMS entheses (P < .0001 for both). Area under the curve values for GS and PD mode were 0.77 and 0.66, respectively, with 3.5 being the best cutoff GS score to discriminate PsA from FMS (sensitivity, 0.75; specificity, 0.63).
Study details: Findings are from a post hoc analysis of the cross‐sectional ULISSE study including 140 and 51 patients with PsA and FMS, respectively.
Disclosures: This study was funded by AbbVie Srl. Three authors declared being employees and shareholders of AbbVie, and some of the authors declared receiving consultancy fees and research support from several sources.
Source: Marchesoni A et al. J Clin Med. 2021;11(1):180 (Dec 29). Doi: 10.3390/jcm11010180.
Real-world efficacy and safety of apremilast in patients with psoriatic arthritis
Key clinical point: This real-world study confirms sustained improvements in signs and symptoms of psoriatic arthritis (PsA) with apremilast along with a tolerable safety profile.
Major finding: Overall, 43.5% of patients who received apremilast within 30 days of participating in the study and completed ≥150 days of treatment achieved PsA Response Criteria. In detail, 26.8% and 41.8% of patients with 68-tender joint count >0 and 66-swollen joint count >0 at baseline, respectively, achieved complete joint count resolution at month 6. No new adverse events were reported.
Study details: Findings are from the prospective, observational APOLO study including 107 patients with active PsA, of which 106 patients received ≥1 dose of apremilast.
Disclosures: This study was funded by Celgene. Some of the authors declared receiving research grants and consultancy and speaker fees from Celgene and other sources.
Source: Vlam KD et al. Adv Ther. 2022 (Jan 3). Doi: 10.1007/s12325-021-02016-x.
Key clinical point: This real-world study confirms sustained improvements in signs and symptoms of psoriatic arthritis (PsA) with apremilast along with a tolerable safety profile.
Major finding: Overall, 43.5% of patients who received apremilast within 30 days of participating in the study and completed ≥150 days of treatment achieved PsA Response Criteria. In detail, 26.8% and 41.8% of patients with 68-tender joint count >0 and 66-swollen joint count >0 at baseline, respectively, achieved complete joint count resolution at month 6. No new adverse events were reported.
Study details: Findings are from the prospective, observational APOLO study including 107 patients with active PsA, of which 106 patients received ≥1 dose of apremilast.
Disclosures: This study was funded by Celgene. Some of the authors declared receiving research grants and consultancy and speaker fees from Celgene and other sources.
Source: Vlam KD et al. Adv Ther. 2022 (Jan 3). Doi: 10.1007/s12325-021-02016-x.
Key clinical point: This real-world study confirms sustained improvements in signs and symptoms of psoriatic arthritis (PsA) with apremilast along with a tolerable safety profile.
Major finding: Overall, 43.5% of patients who received apremilast within 30 days of participating in the study and completed ≥150 days of treatment achieved PsA Response Criteria. In detail, 26.8% and 41.8% of patients with 68-tender joint count >0 and 66-swollen joint count >0 at baseline, respectively, achieved complete joint count resolution at month 6. No new adverse events were reported.
Study details: Findings are from the prospective, observational APOLO study including 107 patients with active PsA, of which 106 patients received ≥1 dose of apremilast.
Disclosures: This study was funded by Celgene. Some of the authors declared receiving research grants and consultancy and speaker fees from Celgene and other sources.
Source: Vlam KD et al. Adv Ther. 2022 (Jan 3). Doi: 10.1007/s12325-021-02016-x.