User login
Hydroxychloroquine use not linked to heart failure risk in patients with RA
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
Key clinical point: Use of hydroxychloroquine (HCQ) is not associated with increased risk of developing heart failure (HF) in patients with rheumatoid arthritis (RA).
Major finding: HCQ cumulative dose was not associated with HF (odds ratio [OR], 0.96 [95% confidence interval (CI), 0.90-1.03] per 100 g). No statistically significant association was found for patients with a cumulative dose of 300 g or more (OR, 0.92; 95% CI, 0.41-2.08). Duration of HCQ use prior to index was not associated with HF (OR, 0.98; 95% CI, 0.91-1.05).
Study details: The data come from a nested case-control study of 143 RA cases diagnosed with HF and 143 non-HF RA controls.
Disclosures: The study was funded by grants from the National Institutes of Health. The authors declared no conflicts of interest.
Source: Sorour AA et al. J Rheumatol. 2021 Jan 15. doi: 10.3899/jrheum.201180
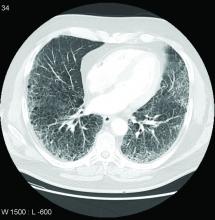
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: RA Jan 2021
The availability of biologic DMARDs such as TNF-alpha inhibitors has expanded the therapies available to patient with RA. However, these agents have been associated with immunogenicity and the development of anti-drug antibodies that can in turn be associated with allergic reactions as well as secondary non-response to the medication. This observational study analyzes the potential relationship between the presence of anti-nuclear antibodies before and after administration of different TNF-alpha inhibitors (infliximab (IFX), adalimumab (ADA), and etanercept (ADA)) with the development of anti-drug antibodies, with the aim of predicting treatment failure. Interestingly, patients who were ANA negative (by immunofluorescence) prior to initiation of therapy did not develop antibodies to ADA or IFX (antibodies to ETN were not measured). How this is related to treatment efficacy is not clear; more patients who had anti-drug antibodies discontinued therapy within 52 weeks and were classified as non-responders to therapy, but whether there was a secondary non-response was not examined in this study. As many patients with RA are also ANA positive and have SSA antibodies, these findings could potentially greatly alter treatment algorithms if proven in longer-term randomized trials and should be examined in other biologic DMARDs as well.
The Leiden Early Arthritis Cohort (EAC) is an inception cohort that was established to study inflammatory arthritis early in the disease state, with a particular interest in RA. This cross-sectional study evaluates the frequency of intermetatarsal and submetatarsal bursitis (IMB and SMB) in early RA. The presence of these forefoot disorders on MRI was evaluated in 441 consecutive patients presenting to the EAC and in 193 healthy controls. IMB and SMB were found more frequently in RA than in other inflammatory arthritides or healthy controls, with a specificity of 70-97%. Sensitivity was higher for IMB than SMB. While the study was not designed to evaluate MRI of the forefoot as a predictive test for development of RA, these findings raise the possibility that this could be used as a diagnostic test in early arthritis and would be worthwhile studying prospectively.
Another question with regard to prediction of response of RA to therapy is that of the possibility of maintaining sustained DMARD-free remission (SDFR). Another study from the Leiden EAC examined 772 RA patients treated with synthetic and biologic DMARDs who had achieved clinical remission (DAS < 2.4) without synovitis. Taper and discontinuation of therapy was attempted, and patients were followed to evaluate whether absence of synovitis on exam was sustained on followup for at least 1 year. Of these patients, 149 achieved SDFR within 7 years of followup; 130 of these patients were seronegative for ACPA. Baseline DAS was similar between patients who did and did not achieve SDFR, but a better early DAS response (larger decrease between baseline and 4 months) was associated an increased chance of SDFR. As the study is observational without a control, it is hard to evaluate how SDFR may proceed in the natural course of the disease. Although the importance of treatment response at 4 months places a premium on early reduction of inflammation, achievement of SDFR in these patients may also be related to other baseline characteristics of the group, especially as ACPA-positive patients less frequently achieved SDFR.
The availability of biologic DMARDs such as TNF-alpha inhibitors has expanded the therapies available to patient with RA. However, these agents have been associated with immunogenicity and the development of anti-drug antibodies that can in turn be associated with allergic reactions as well as secondary non-response to the medication. This observational study analyzes the potential relationship between the presence of anti-nuclear antibodies before and after administration of different TNF-alpha inhibitors (infliximab (IFX), adalimumab (ADA), and etanercept (ADA)) with the development of anti-drug antibodies, with the aim of predicting treatment failure. Interestingly, patients who were ANA negative (by immunofluorescence) prior to initiation of therapy did not develop antibodies to ADA or IFX (antibodies to ETN were not measured). How this is related to treatment efficacy is not clear; more patients who had anti-drug antibodies discontinued therapy within 52 weeks and were classified as non-responders to therapy, but whether there was a secondary non-response was not examined in this study. As many patients with RA are also ANA positive and have SSA antibodies, these findings could potentially greatly alter treatment algorithms if proven in longer-term randomized trials and should be examined in other biologic DMARDs as well.
The Leiden Early Arthritis Cohort (EAC) is an inception cohort that was established to study inflammatory arthritis early in the disease state, with a particular interest in RA. This cross-sectional study evaluates the frequency of intermetatarsal and submetatarsal bursitis (IMB and SMB) in early RA. The presence of these forefoot disorders on MRI was evaluated in 441 consecutive patients presenting to the EAC and in 193 healthy controls. IMB and SMB were found more frequently in RA than in other inflammatory arthritides or healthy controls, with a specificity of 70-97%. Sensitivity was higher for IMB than SMB. While the study was not designed to evaluate MRI of the forefoot as a predictive test for development of RA, these findings raise the possibility that this could be used as a diagnostic test in early arthritis and would be worthwhile studying prospectively.
Another question with regard to prediction of response of RA to therapy is that of the possibility of maintaining sustained DMARD-free remission (SDFR). Another study from the Leiden EAC examined 772 RA patients treated with synthetic and biologic DMARDs who had achieved clinical remission (DAS < 2.4) without synovitis. Taper and discontinuation of therapy was attempted, and patients were followed to evaluate whether absence of synovitis on exam was sustained on followup for at least 1 year. Of these patients, 149 achieved SDFR within 7 years of followup; 130 of these patients were seronegative for ACPA. Baseline DAS was similar between patients who did and did not achieve SDFR, but a better early DAS response (larger decrease between baseline and 4 months) was associated an increased chance of SDFR. As the study is observational without a control, it is hard to evaluate how SDFR may proceed in the natural course of the disease. Although the importance of treatment response at 4 months places a premium on early reduction of inflammation, achievement of SDFR in these patients may also be related to other baseline characteristics of the group, especially as ACPA-positive patients less frequently achieved SDFR.
The availability of biologic DMARDs such as TNF-alpha inhibitors has expanded the therapies available to patient with RA. However, these agents have been associated with immunogenicity and the development of anti-drug antibodies that can in turn be associated with allergic reactions as well as secondary non-response to the medication. This observational study analyzes the potential relationship between the presence of anti-nuclear antibodies before and after administration of different TNF-alpha inhibitors (infliximab (IFX), adalimumab (ADA), and etanercept (ADA)) with the development of anti-drug antibodies, with the aim of predicting treatment failure. Interestingly, patients who were ANA negative (by immunofluorescence) prior to initiation of therapy did not develop antibodies to ADA or IFX (antibodies to ETN were not measured). How this is related to treatment efficacy is not clear; more patients who had anti-drug antibodies discontinued therapy within 52 weeks and were classified as non-responders to therapy, but whether there was a secondary non-response was not examined in this study. As many patients with RA are also ANA positive and have SSA antibodies, these findings could potentially greatly alter treatment algorithms if proven in longer-term randomized trials and should be examined in other biologic DMARDs as well.
The Leiden Early Arthritis Cohort (EAC) is an inception cohort that was established to study inflammatory arthritis early in the disease state, with a particular interest in RA. This cross-sectional study evaluates the frequency of intermetatarsal and submetatarsal bursitis (IMB and SMB) in early RA. The presence of these forefoot disorders on MRI was evaluated in 441 consecutive patients presenting to the EAC and in 193 healthy controls. IMB and SMB were found more frequently in RA than in other inflammatory arthritides or healthy controls, with a specificity of 70-97%. Sensitivity was higher for IMB than SMB. While the study was not designed to evaluate MRI of the forefoot as a predictive test for development of RA, these findings raise the possibility that this could be used as a diagnostic test in early arthritis and would be worthwhile studying prospectively.
Another question with regard to prediction of response of RA to therapy is that of the possibility of maintaining sustained DMARD-free remission (SDFR). Another study from the Leiden EAC examined 772 RA patients treated with synthetic and biologic DMARDs who had achieved clinical remission (DAS < 2.4) without synovitis. Taper and discontinuation of therapy was attempted, and patients were followed to evaluate whether absence of synovitis on exam was sustained on followup for at least 1 year. Of these patients, 149 achieved SDFR within 7 years of followup; 130 of these patients were seronegative for ACPA. Baseline DAS was similar between patients who did and did not achieve SDFR, but a better early DAS response (larger decrease between baseline and 4 months) was associated an increased chance of SDFR. As the study is observational without a control, it is hard to evaluate how SDFR may proceed in the natural course of the disease. Although the importance of treatment response at 4 months places a premium on early reduction of inflammation, achievement of SDFR in these patients may also be related to other baseline characteristics of the group, especially as ACPA-positive patients less frequently achieved SDFR.
Inflammation of juxta-articular soft tissues could be an early feature of RA
Key clinical point: Intermetatarsal (IMB) and submetatarsal (SMB) bursitis are associated with and specific for early rheumatoid arthritis (RA), with IMB having the highest sensitivity.
Major finding: IMB (adjusted odds ratio [aOR], 4.5; P less than .001) and SMB (aOR, 2.2; P = .041) were associated with RA. Sensitivity for RA of IMB and SMB was 69% and 25%, respectively. Specificity of IMB vs. other arthritides and healthy controls was 70% and 84%, respectively. Specificity for SMB was 94% (vs. other arthritides) and 97% (vs. healthy controls).
Study details: The findings are based on a large case-controlled magnetic resonance imaging study of 157 patients presenting with early RA, 284 other arthritides, and 193 healthy controls.
Disclosures: The study was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program. The authors declared no conflicts of interest.
Source: Dakkak Y et al. Arthritis Res Ther. 2020 Nov 23. doi: 10.1186/s13075-020-02359-w.
Key clinical point: Intermetatarsal (IMB) and submetatarsal (SMB) bursitis are associated with and specific for early rheumatoid arthritis (RA), with IMB having the highest sensitivity.
Major finding: IMB (adjusted odds ratio [aOR], 4.5; P less than .001) and SMB (aOR, 2.2; P = .041) were associated with RA. Sensitivity for RA of IMB and SMB was 69% and 25%, respectively. Specificity of IMB vs. other arthritides and healthy controls was 70% and 84%, respectively. Specificity for SMB was 94% (vs. other arthritides) and 97% (vs. healthy controls).
Study details: The findings are based on a large case-controlled magnetic resonance imaging study of 157 patients presenting with early RA, 284 other arthritides, and 193 healthy controls.
Disclosures: The study was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program. The authors declared no conflicts of interest.
Source: Dakkak Y et al. Arthritis Res Ther. 2020 Nov 23. doi: 10.1186/s13075-020-02359-w.
Key clinical point: Intermetatarsal (IMB) and submetatarsal (SMB) bursitis are associated with and specific for early rheumatoid arthritis (RA), with IMB having the highest sensitivity.
Major finding: IMB (adjusted odds ratio [aOR], 4.5; P less than .001) and SMB (aOR, 2.2; P = .041) were associated with RA. Sensitivity for RA of IMB and SMB was 69% and 25%, respectively. Specificity of IMB vs. other arthritides and healthy controls was 70% and 84%, respectively. Specificity for SMB was 94% (vs. other arthritides) and 97% (vs. healthy controls).
Study details: The findings are based on a large case-controlled magnetic resonance imaging study of 157 patients presenting with early RA, 284 other arthritides, and 193 healthy controls.
Disclosures: The study was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program. The authors declared no conflicts of interest.
Source: Dakkak Y et al. Arthritis Res Ther. 2020 Nov 23. doi: 10.1186/s13075-020-02359-w.
RA: Depression tied to greater disease activity and pain
Key clinical point: Depression was associated with greater short-term disease activity and more severe and persistent pain in US veterans with early rheumatoid arthritis (RA) receiving methotrexate.
Major finding: Depression was associated with significantly higher 28-joint count disease activity scores at 6 months (β, 0.345; P = .045) but not at 1- (P = .477) and 2- (P = .804) year follow-up. Moreover, depression was significantly associated with higher patient-reported pain at 6 months (β, 0.385; P = .029) and 1 year (β, 0.396; P = .028).
Study details: The study included 268 US veterans (89.2% males) with early RA (duration less than 2 years) prescribed methotrexate, identified from the Veterans Affairs Rheumatoid Arthritis Registry.
Disclosures: The study was supported by the Rheumatology Research Foundation’s Scientist Development Award, the National Institute on Ageing, VA Clinical Science Research and Development Service, and the National Institute of General Medical Sciences. The authors reported receiving grants from various organizations and/or pharmaceutical companies.
Source: Rathbun AM et al. J Rheumatol. 2020 Nov 15. doi: 10.3899/jrheum.200743.
Key clinical point: Depression was associated with greater short-term disease activity and more severe and persistent pain in US veterans with early rheumatoid arthritis (RA) receiving methotrexate.
Major finding: Depression was associated with significantly higher 28-joint count disease activity scores at 6 months (β, 0.345; P = .045) but not at 1- (P = .477) and 2- (P = .804) year follow-up. Moreover, depression was significantly associated with higher patient-reported pain at 6 months (β, 0.385; P = .029) and 1 year (β, 0.396; P = .028).
Study details: The study included 268 US veterans (89.2% males) with early RA (duration less than 2 years) prescribed methotrexate, identified from the Veterans Affairs Rheumatoid Arthritis Registry.
Disclosures: The study was supported by the Rheumatology Research Foundation’s Scientist Development Award, the National Institute on Ageing, VA Clinical Science Research and Development Service, and the National Institute of General Medical Sciences. The authors reported receiving grants from various organizations and/or pharmaceutical companies.
Source: Rathbun AM et al. J Rheumatol. 2020 Nov 15. doi: 10.3899/jrheum.200743.
Key clinical point: Depression was associated with greater short-term disease activity and more severe and persistent pain in US veterans with early rheumatoid arthritis (RA) receiving methotrexate.
Major finding: Depression was associated with significantly higher 28-joint count disease activity scores at 6 months (β, 0.345; P = .045) but not at 1- (P = .477) and 2- (P = .804) year follow-up. Moreover, depression was significantly associated with higher patient-reported pain at 6 months (β, 0.385; P = .029) and 1 year (β, 0.396; P = .028).
Study details: The study included 268 US veterans (89.2% males) with early RA (duration less than 2 years) prescribed methotrexate, identified from the Veterans Affairs Rheumatoid Arthritis Registry.
Disclosures: The study was supported by the Rheumatology Research Foundation’s Scientist Development Award, the National Institute on Ageing, VA Clinical Science Research and Development Service, and the National Institute of General Medical Sciences. The authors reported receiving grants from various organizations and/or pharmaceutical companies.
Source: Rathbun AM et al. J Rheumatol. 2020 Nov 15. doi: 10.3899/jrheum.200743.
Oral glucocorticoids plus PPIs raise osteoporotic fracture risk in patients with RA
Key clinical point: The concomitant use of oral glucocorticoids and proton pump inhibitors (PPIs) is associated with a higher risk of osteoporotic fractures in patients with rheumatoid arthritis (RA).
Major finding: The risk of osteoporotic fractures was significantly higher in concomitant users of oral glucocorticoids and PPIs (adjusted hazard ratio [aHR] 1.60; 95% confidence interval [CI] 1.35-1.89). Among current concomitant users, an increased risk was observed for fractures of the hip (aHR, 1.45; 95% CI, 1.11-1.91), clinical vertebrae (aHR, 2.84; 95% CI, 1.87-4.32), pelvis (aHR, 2.47; 95% CI, 1.41-4.34), and ribs (aHR, 4.03; 95% CI, 2.13-7.63).
Study details: The data come from a retrospective study of 12,351 patients with RA aged 50 years or older in the United Kingdom.
Disclosures: Two of the authors reported receiving research grants and speakers’ fees from various pharmaceutical companies. The others reported no conflicts of interest.
Source: Abtahi S et al. Ann Rheum Dis. 2020 Dec 11. doi: 10.1136/annrheumdis-2020-218758.
Key clinical point: The concomitant use of oral glucocorticoids and proton pump inhibitors (PPIs) is associated with a higher risk of osteoporotic fractures in patients with rheumatoid arthritis (RA).
Major finding: The risk of osteoporotic fractures was significantly higher in concomitant users of oral glucocorticoids and PPIs (adjusted hazard ratio [aHR] 1.60; 95% confidence interval [CI] 1.35-1.89). Among current concomitant users, an increased risk was observed for fractures of the hip (aHR, 1.45; 95% CI, 1.11-1.91), clinical vertebrae (aHR, 2.84; 95% CI, 1.87-4.32), pelvis (aHR, 2.47; 95% CI, 1.41-4.34), and ribs (aHR, 4.03; 95% CI, 2.13-7.63).
Study details: The data come from a retrospective study of 12,351 patients with RA aged 50 years or older in the United Kingdom.
Disclosures: Two of the authors reported receiving research grants and speakers’ fees from various pharmaceutical companies. The others reported no conflicts of interest.
Source: Abtahi S et al. Ann Rheum Dis. 2020 Dec 11. doi: 10.1136/annrheumdis-2020-218758.
Key clinical point: The concomitant use of oral glucocorticoids and proton pump inhibitors (PPIs) is associated with a higher risk of osteoporotic fractures in patients with rheumatoid arthritis (RA).
Major finding: The risk of osteoporotic fractures was significantly higher in concomitant users of oral glucocorticoids and PPIs (adjusted hazard ratio [aHR] 1.60; 95% confidence interval [CI] 1.35-1.89). Among current concomitant users, an increased risk was observed for fractures of the hip (aHR, 1.45; 95% CI, 1.11-1.91), clinical vertebrae (aHR, 2.84; 95% CI, 1.87-4.32), pelvis (aHR, 2.47; 95% CI, 1.41-4.34), and ribs (aHR, 4.03; 95% CI, 2.13-7.63).
Study details: The data come from a retrospective study of 12,351 patients with RA aged 50 years or older in the United Kingdom.
Disclosures: Two of the authors reported receiving research grants and speakers’ fees from various pharmaceutical companies. The others reported no conflicts of interest.
Source: Abtahi S et al. Ann Rheum Dis. 2020 Dec 11. doi: 10.1136/annrheumdis-2020-218758.
Tocilizumab + methotrexate vs. tocilizumab alone for preventing radiographic progression in RA
Key clinical point: Tocilizumab (TCZ) and methotrexate (MTX) combination therapy was generally more effective than TCZ monotherapy in preventing radiographic progression in patients with early and established rheumatoid arthritis. However, effect modifiers were more joint damage or lower disease activity score (DAS) in early RA and longer disease duration in established RA.
Major finding: Overall, TCZ monotherapy was less effective in preventing radiographic progression (relative risk; 95% confidence interval) than TCZ+MTX combination therapy in patients with early (0.96; 0.90 to –1.03) and established (0.96; 0.87 to –1.07) RA. These effects were modified by baseline joint damage (P less than .01), DAS assessing 28 joints (P = .04) in early RA, and disease duration (P = .04) in established RA.
Study details: Analysis of individual patient data from randomized controlled trials comparing TCZ monotherapy and TCZ+MTX combination therapy in patients with early (n=1,089) and established (n=417) RA.
Disclosures: No study sponsor was identified. Some of the investigators reported ties with various pharmaceutical companies.
Source: Verhoeven MMA et al. Arthritis Care Res (Hoboken). 2020 Nov 30. doi: 10.1002/acr.24524.
Key clinical point: Tocilizumab (TCZ) and methotrexate (MTX) combination therapy was generally more effective than TCZ monotherapy in preventing radiographic progression in patients with early and established rheumatoid arthritis. However, effect modifiers were more joint damage or lower disease activity score (DAS) in early RA and longer disease duration in established RA.
Major finding: Overall, TCZ monotherapy was less effective in preventing radiographic progression (relative risk; 95% confidence interval) than TCZ+MTX combination therapy in patients with early (0.96; 0.90 to –1.03) and established (0.96; 0.87 to –1.07) RA. These effects were modified by baseline joint damage (P less than .01), DAS assessing 28 joints (P = .04) in early RA, and disease duration (P = .04) in established RA.
Study details: Analysis of individual patient data from randomized controlled trials comparing TCZ monotherapy and TCZ+MTX combination therapy in patients with early (n=1,089) and established (n=417) RA.
Disclosures: No study sponsor was identified. Some of the investigators reported ties with various pharmaceutical companies.
Source: Verhoeven MMA et al. Arthritis Care Res (Hoboken). 2020 Nov 30. doi: 10.1002/acr.24524.
Key clinical point: Tocilizumab (TCZ) and methotrexate (MTX) combination therapy was generally more effective than TCZ monotherapy in preventing radiographic progression in patients with early and established rheumatoid arthritis. However, effect modifiers were more joint damage or lower disease activity score (DAS) in early RA and longer disease duration in established RA.
Major finding: Overall, TCZ monotherapy was less effective in preventing radiographic progression (relative risk; 95% confidence interval) than TCZ+MTX combination therapy in patients with early (0.96; 0.90 to –1.03) and established (0.96; 0.87 to –1.07) RA. These effects were modified by baseline joint damage (P less than .01), DAS assessing 28 joints (P = .04) in early RA, and disease duration (P = .04) in established RA.
Study details: Analysis of individual patient data from randomized controlled trials comparing TCZ monotherapy and TCZ+MTX combination therapy in patients with early (n=1,089) and established (n=417) RA.
Disclosures: No study sponsor was identified. Some of the investigators reported ties with various pharmaceutical companies.
Source: Verhoeven MMA et al. Arthritis Care Res (Hoboken). 2020 Nov 30. doi: 10.1002/acr.24524.
Prognostic factors for short-term mortality in RA patients admitted to ICU
Key clinical point: Nonuse of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), high updated Charlson’s comorbidity index (CCI), elevated acute physiology and chronic health evaluation (APACHE) II score, and coagulation abnormalities predicted poorer prognosis in patients with rheumatoid arthritis admitted to the intensive care unit (ICU).
Major finding: The 30-day, 90-day, and 1-year mortality rates were 22%, 27%, and 37%, respectively. Factors associated with an increased mortality risk after ICU admission were nonuse of csDMARDs (hazard ratio [HR], 0.413; P = .0229), elevated updated CCI (HR, 1.522; P = .0007), high APACHE II score (HR, 1.045; P = .0008), and extended prothrombin time-international normalized ratio (HR, 2.670; P = .0051). The liver (P = .0004) and renal (P = .0009) disease scores were significantly higher in nonsurvivors vs. survivors.
Study details: The findings are based on a single-center retrospective study of 67 patients (mean age at admission, 68±13 years) with RA (median duration, 14±15 years) admitted to the ICU.
Disclosures: The study was supported by grants from JSPS KAKENHI. The authors declared no conflicts of interest.
Source: Fujiwara T et al. BMC Rheumatol. 2020 Dec 4. doi: 10.1186/s41927-020-00164-1.
Key clinical point: Nonuse of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), high updated Charlson’s comorbidity index (CCI), elevated acute physiology and chronic health evaluation (APACHE) II score, and coagulation abnormalities predicted poorer prognosis in patients with rheumatoid arthritis admitted to the intensive care unit (ICU).
Major finding: The 30-day, 90-day, and 1-year mortality rates were 22%, 27%, and 37%, respectively. Factors associated with an increased mortality risk after ICU admission were nonuse of csDMARDs (hazard ratio [HR], 0.413; P = .0229), elevated updated CCI (HR, 1.522; P = .0007), high APACHE II score (HR, 1.045; P = .0008), and extended prothrombin time-international normalized ratio (HR, 2.670; P = .0051). The liver (P = .0004) and renal (P = .0009) disease scores were significantly higher in nonsurvivors vs. survivors.
Study details: The findings are based on a single-center retrospective study of 67 patients (mean age at admission, 68±13 years) with RA (median duration, 14±15 years) admitted to the ICU.
Disclosures: The study was supported by grants from JSPS KAKENHI. The authors declared no conflicts of interest.
Source: Fujiwara T et al. BMC Rheumatol. 2020 Dec 4. doi: 10.1186/s41927-020-00164-1.
Key clinical point: Nonuse of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), high updated Charlson’s comorbidity index (CCI), elevated acute physiology and chronic health evaluation (APACHE) II score, and coagulation abnormalities predicted poorer prognosis in patients with rheumatoid arthritis admitted to the intensive care unit (ICU).
Major finding: The 30-day, 90-day, and 1-year mortality rates were 22%, 27%, and 37%, respectively. Factors associated with an increased mortality risk after ICU admission were nonuse of csDMARDs (hazard ratio [HR], 0.413; P = .0229), elevated updated CCI (HR, 1.522; P = .0007), high APACHE II score (HR, 1.045; P = .0008), and extended prothrombin time-international normalized ratio (HR, 2.670; P = .0051). The liver (P = .0004) and renal (P = .0009) disease scores were significantly higher in nonsurvivors vs. survivors.
Study details: The findings are based on a single-center retrospective study of 67 patients (mean age at admission, 68±13 years) with RA (median duration, 14±15 years) admitted to the ICU.
Disclosures: The study was supported by grants from JSPS KAKENHI. The authors declared no conflicts of interest.
Source: Fujiwara T et al. BMC Rheumatol. 2020 Dec 4. doi: 10.1186/s41927-020-00164-1.
Gut microbiome influences response to methotrexate in new-onset RA patients
Key clinical point: Nonresponse to methotrexate (MTX) therapy can be predicted based on the gut microbiome of an individual patient newly diagnosed with rheumatoid arthritis.
Major finding: A model developed using machine learning predicted 83.3% of patients who did not respond to MTX and 78% of MTX responders.
Study details: An analysis of DNA from fecal samples obtained from a training cohort of 26 patients with new-onset RA (NORA), a validation cohort of 21 patients with NORA, and a control group of 20 patients with RA.
Disclosures: This study was funded by the National Institutes of Health, the Rheumatology Research Foundation, the Searle Scholars Program, various funds from the Spanish government, the UCSF Breakthrough Program for Rheumatoid Arthritis-related Research, and the Arthritis Foundation Center for Excellence. Four authors report consultancies and memberships on scientific advisory boards with pharmaceutical and biotechnology companies that do not overlap with the current study.
Source: Artacho A et al. Arthritis Rheumatol. 2020 Dec 13. doi: 10.1002/art.41622.
Key clinical point: Nonresponse to methotrexate (MTX) therapy can be predicted based on the gut microbiome of an individual patient newly diagnosed with rheumatoid arthritis.
Major finding: A model developed using machine learning predicted 83.3% of patients who did not respond to MTX and 78% of MTX responders.
Study details: An analysis of DNA from fecal samples obtained from a training cohort of 26 patients with new-onset RA (NORA), a validation cohort of 21 patients with NORA, and a control group of 20 patients with RA.
Disclosures: This study was funded by the National Institutes of Health, the Rheumatology Research Foundation, the Searle Scholars Program, various funds from the Spanish government, the UCSF Breakthrough Program for Rheumatoid Arthritis-related Research, and the Arthritis Foundation Center for Excellence. Four authors report consultancies and memberships on scientific advisory boards with pharmaceutical and biotechnology companies that do not overlap with the current study.
Source: Artacho A et al. Arthritis Rheumatol. 2020 Dec 13. doi: 10.1002/art.41622.
Key clinical point: Nonresponse to methotrexate (MTX) therapy can be predicted based on the gut microbiome of an individual patient newly diagnosed with rheumatoid arthritis.
Major finding: A model developed using machine learning predicted 83.3% of patients who did not respond to MTX and 78% of MTX responders.
Study details: An analysis of DNA from fecal samples obtained from a training cohort of 26 patients with new-onset RA (NORA), a validation cohort of 21 patients with NORA, and a control group of 20 patients with RA.
Disclosures: This study was funded by the National Institutes of Health, the Rheumatology Research Foundation, the Searle Scholars Program, various funds from the Spanish government, the UCSF Breakthrough Program for Rheumatoid Arthritis-related Research, and the Arthritis Foundation Center for Excellence. Four authors report consultancies and memberships on scientific advisory boards with pharmaceutical and biotechnology companies that do not overlap with the current study.
Source: Artacho A et al. Arthritis Rheumatol. 2020 Dec 13. doi: 10.1002/art.41622.