User login
Assessment of Cardiovascular Disease Risk in Rheumatoid Arthritis
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
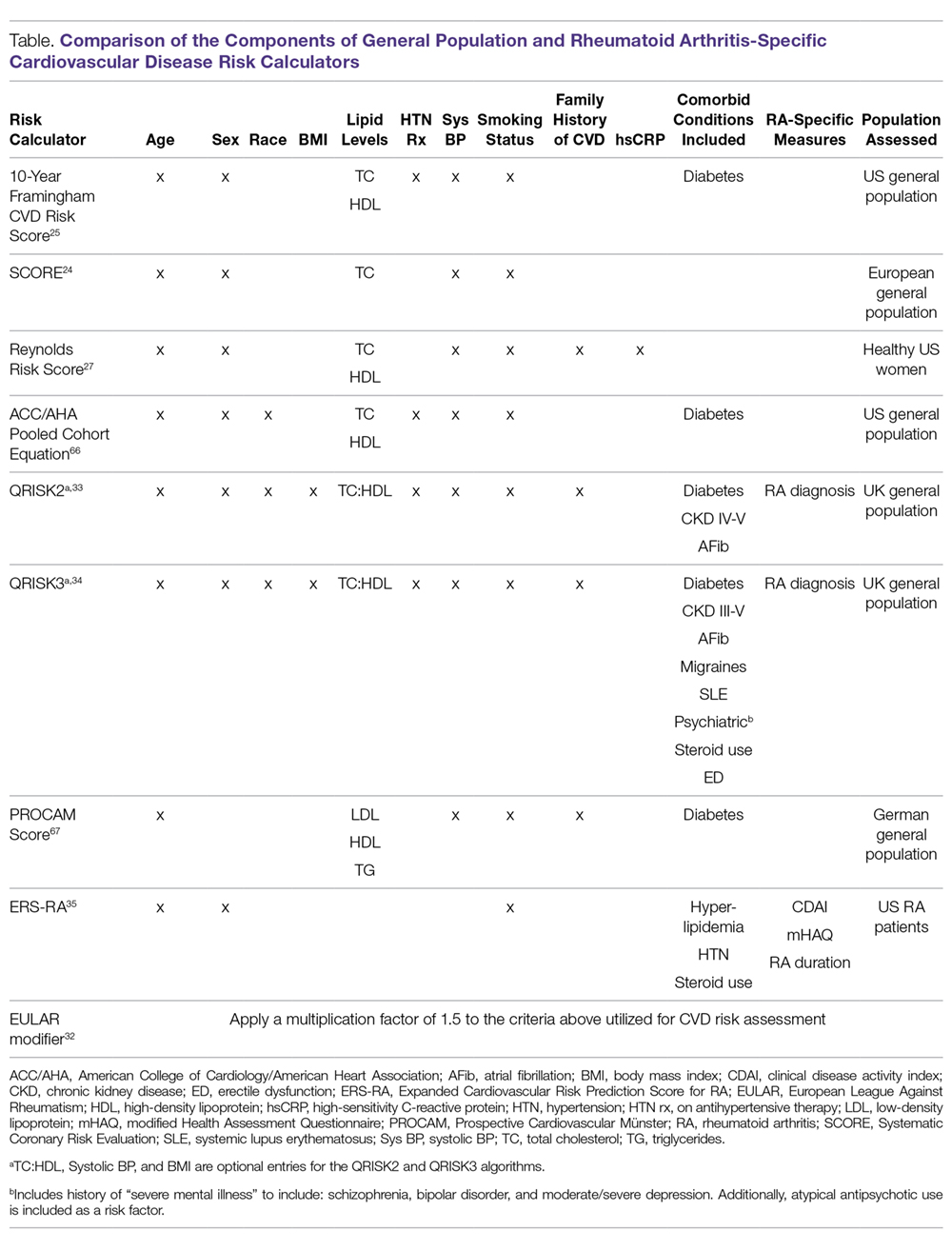
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30
Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30
Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
From the Division of Rheumatology & Immunology, University of Nebraska Medical Center, and Veterans Affairs Nebraska-Western Iowa Health Care System, Omaha, NE.
Abstract
- Objective: To review cardiovascular disease (CVD) risk assessment in patients with rheumatoid arthritis (RA).
- Methods: Literature review of the assessment of CVD risk in RA.
- Results: CVD is the leading cause of death among RA patients.
Because of the increased risk of CVD events and CVD mortality in patients with RA, regular assessment of CVD risk and aggressive management of CVD risk in these patients is crucial. CVD risk estimation typically centers on the use of well-established CVD risk calculators. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing, while novel RA-derived CVD risk scores that incorporate RA-related factors have had limited external validity testing. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. - Conclusion: Primary care and rheumatology providers must be aware of the increased risk of CVD in RA, a risk that approaches that of diabetic patients.
Routine assessment of CVD risk is an essential first step in minimizing CVD risk in this population. Until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Keywords: rheumatoid arthritis; cardiovascular disease; cardiovascular risk assessment.
Editor’s note: This article is part 1 of a 2-part article. “Management of Cardiovascular Disease Risk in Rheumatoid Arthritis” was published in the March/April 2019 issue.
Rheumatoid arthritis (RA) is a chronic, autoimmune inflammatory arthritis affecting up to 1% of the US population that can lead to joint damage, functional disability, and reduced quality of life.1 In addition to articular involvement, systemic inflammation accompanying RA may lead to extra-articular manifestations and increase the risk of premature death.2 Cardiovascular disease (CVD), accounting for nearly half of all deaths among RA patients, is now recognized as a critical extra-articular manifestation of RA.2,3 As such, assessment and management of CVD risk is essential to the comprehensive care of the RA patient. This article reviews the approach to assessing CVD risk in patients with RA; the management of both traditional and RA-specific risk factors is discussed in a separate article.
Scope of the Problem
In a large meta-analysis of observational studies that included more than 111,000 patients with RA, CVD-related mortality rates were 1.5 times higher among RA patients than among general population controls.4 The risk of overall CVD, including nonfatal events, is similar; a separate meta-analysis of observational studies that included more than 41,000 patients with RA calculated a pooled relative risk for incident CVD of 1.48.5 Individual analyses identified heightened risk of acute coronary syndrome (ACS), cerebrovascular accident, and congestive heart failure (CHF).5 Perhaps more illustrative of the magnitude of the problem, the risk of CVD in RA approaches that observed among individuals with diabetes mellitus.6,7
Coronary artery disease (CAD) accounts for a significant portion of the CVD risk in RA, but its presentation may be atypical in RA patients. RA patients are at higher risk of suffering unrecognized myocardial infarction (MI) and sudden cardiac death.8 The reasons for silent ischemia in RA are not fully known, but have been hypothesized to include imbalances of inflammatory cytokines, alterations in pain sensitization, or the female predominance of RA (with women more often presenting with atypical symptoms of myocardial ischemia).9 Alarmingly, a retrospective chart review study reported that RA patients admitted for an acute MI were less likely to receive appropriate reperfusion therapy as well as secondary prevention with beta-blockers and lipid-lowering agents.10 Even with appropriate therapy, long-term outcomes such as mortality and recurrent ischemic events are more likely to occur in RA patients after acute MI.11-13
Independent of ischemic heart disease, RA patients are at increased risk of CHF.14-16 RA patients are at particular risk for CHF with preserved ejection fraction,17 which may be a result of systemic inflammation causing left ventricular stiffening.18,19 Similar to CAD, patients with RA are less likely to present with typical CHF symptoms, are less likely to receive guideline-concordant care, and have higher mortality rates following presentation with CHF.17
Although accounting for a lower proportion of the excess CVD morbidity and mortality in RA, the risk of noncardiac vascular disease is also increased in RA patients. Large meta-analyses have identified positive associations between RA with both ischemic (odds ratio [OR], 1.64 [95% confidence interval {CI}, 1.32-2.05]) and hemorrhagic (OR, 1.68 [95% CI, 1.11-2.53]) stroke.20 Similarly, RA patients appear to have an approximately twofold higher risk of venous thromboembolic events.21 Less frequently studied than other forms of CVD, peripheral arterial disease may be increased in RA patients independent of other CVD and CVD risk factors.22,23
Assessing CVD Risk in RA
CVD Risk Scores
In order to identify patients who may benefit from primary prevention interventions, such as lipid-lowering therapy, CVD risk estimation typically centers on the use of well-established CVD risk calculators (Table). CVD risk scores such as the Framingham Risk Score (FRS), Systematic Coronary Risk Evaluation (SCORE), and American College of Cardiology/ American Heart Association (ACC/AHA) Pooled Cohort Equation incorporate traditional CVD risk factors, including age, sex, smoking status, blood pressure, lipid levels, and presence of diabetes mellitus.24,25 However, CVD risk in RA patients appears to be inadequately explained by traditional CVD risk factors,26 with disease activity and inflammation being associated with higher CVD risk. Recognizing that inflammation may contribute to CVD risk even among non-RA patients, the Reynolds Risk Score includes high-sensitivity C-reactive protein (hsCRP) in its calculation.27 In contrast to more robust performance in the general population, these well-established CVD risk scores have had variable predictive potential of incident CVD in RA patients.28-30
Several models, or adaptations to existing models, have been proposed to improve CVD risk assessment in RA populations (Table). In 2009, the European League Against Rheumatism (EULAR) task force suggested using a correction factor of 1.5 with traditional CVD risk models in RA patients with 2 of the following criteria: disease duration exceeding 10 years, rheumatoid factor or anti-cyclic citrullinated peptide (CCP) antibody positivity, or extra-articular manifestations of RA.31 An update to these recommendations in 2015 continued to propose the use of a 1.5 correction factor, but suggested applying this to all RA patients.32 QRISK2, a modification to QRISK1 which was developed to predict CVD in the UK general population, includes the diagnosis of RA as a risk factor, and in early validation efforts more accurately discriminated patients in the general population at increased risk of CVD compared to the FRS.33 Additional disease-specific risk factors such as systemic lupus, steroid use, severe mental illness, and steroid and atypical antipsychotic use were incorporated in the QRISK3 algorithm, with model performance similar to the QRISK2.34 The Expanded Cardiovascular Risk Prediction Score for RA (ERS-RA) was specifically developed to assess CVD risk in RA patients by including RA disease activity, level of physical disability, RA disease duration, and prednisone use.35 Despite efforts to develop “RA-specific” risk scores, these have not consistently outperformed traditional CVD risk calculators.36-38 In one study involving more than 1700 RA patients, the ERS-RA performed similarly to the FRS and Reynolds Risk Score, with a net reclassification index of just 2.3% versus the FRS.36
Imaging Modalities
Imaging modalities may assist in characterizing the increased risk of CVD in RA and the subclinical CVD manifestations that occur. For example, RA patients were shown to have more prevalent and unstable coronary plaque, higher carotid intima media thickness, and impaired myocardial function with computed tomography (CT) angiography and carotid ultrasound.39,40 However, studies harnessing noninvasive imaging to augment CVD risk assessment in RA patients are limited.
Carotid ultrasound has been the most extensively studied imaging modality for CVD risk assessment in RA. In a cohort of 599 RA patients with no history of ACS, rates of ACS were nearly 4 times higher in RA patients with bilateral carotid plaque on carotid ultrasound, and the association with ACS was independent of other traditional and RA-related risk factors.41 Presence of bilateral carotid plaques was similarly associated with an increased risk of overall CVD events (hazard ratio [HR], 3.34 [95% CI, 1.21-9.22]), ACS alone (HR, 6.31 [95% CI, 1.27-31.40]), and a lower mean CVD event-free survival (13.9 versus 15.2 years, P = 0.01) in a separate inception cohort of 105 RA patients with no prior history of CVD.42 The most useful application of carotid ultrasound may be in conjunction with clinical CVD risk models. Use of carotid ultrasound improved CVD risk stratification among RA patients who were considered at moderate risk by the EULAR-modified SCORE calculator.43 Beyond carotid ultrasound, measurement of arterial stiffness through ultrasound could also aid in CVD risk stratification. Aortic pulse wave velocity and augmentation index, measures of arterial stiffness, are predictive of CVD in the general population as well as RA patients and improve with reduction in RA disease activity.44,45 Peripheral arterial stiffness (brachial-ankle elasticity index) is impaired in RA patients and predictive of CVD morbidity and mortality in the general population.46,47
CT coronary angiography and coronary artery calcium (CAC) scores are reliable measures of coronary artery atherosclerosis and have been validated for CVD risk assessment in the general population.48-52 While the association between RA and CT-related findings of atherosclerosis is well established, assessment of CT-mediated evaluation as a prognostic tool for CVD in RA is limited. In one cohort study, CAC predicted higher rates of CVD events in Chinese patients with RA and systemic lupus erythematosus in a pooled analysis, although results were limited by low event rates and the absence of RA-only subanalyses.53
While the aforementioned imaging modalities have focused on enhancing the identification of atherosclerosis, echocardiography or cardiac magnetic resonance imaging (MRI) may be useful for detecting subclinical structural and/or functional abnormalities that predispose to CHF. Structural abnormalities including increased left ventricular mass and hypertrophy are more prevalent in RA patients and predict incident CHF in the general population.54-56 MRI measures of myocardial inflammation, including T1 mapping and extracellular volume, are associated with higher mortality rates and also appear to be elevated in RA patients.57,58 Whether identification of these imaging findings influences the cost-effective clinical management of RA patients needs further study.
Biomarkers
Serum biomarkers, such as the anti-CCP antibody, have become crucial to the evaluation of patients suspected to have RA. With the growing understanding of the role pro-inflammatory mediators play in CVD pathogenesis and the relative ease with which they can be measured, serum biomarkers have potential to inform CVD risk assessment. In the general population, hsCRP concentrations are predictive of CVD and are included in the Reynolds Risk Score.27 In RA, CRP concentrations are typically much higher than those observed among individuals in the general population solely at increased CVD risk, yet elevated levels remain predictive of CVD death independent of RA disease activity and traditional CVD risk factors.59 Several additional cytokines, chemokines, and adhesion molecules have been associated with surrogate markers of CVD in RA patients, although further study is needed to elucidate thresholds that signify increased CVD risk in a population characterized by the presence of systemic inflammation.60
Cardiac biomarkers used frequently in the general population may be useful to assess CVD risk in RA patients. N-terminal-pro brain natriuretic peptide (NT-pro BNP) is a biomarker typically used to evaluate CHF severity, but it may also predict long-term mortality in patients with coronary heart disease.61,62 Circulating NT-pro BNP concentrations are elevated in RA independent of prevalent CHF and may serve as a useful tool to identify subclinical cardiac disease in RA patients.63 High-sensitivity cardiac troponin I (HS-cTnI) assays are capable of detecting levels of cardiac troponin below the threshold typically used to diagnose ACS. HS-cTnI levels are increased in RA patients independent of additional CVD risk factors, and elevated levels (> 1.5 pg/mL) were associated with more severe CT angiography findings of coronary plaque as well as increased risk of CVD events.64,65
Clinical Application
A fully validated algorithm for CVD risk assessment in RA is lacking. Most CVD risk scores from the general population do not contain RA-related factors predictive of CVD but have had more extensive performance testing. In contrast, novel RA-derived CVD risk scores incorporate RA-related factors, but have had limited external validity testing. Additionally, RA-derived risk scores are less likely to be utilized and adopted by primary care providers and cardiologists involved in RA patients’ care. Neither set of risk scores incorporates novel imaging modalities or serum biomarkers, which are most likely to be helpful among individuals at intermediate risk. Therefore, until the performance of RA-specific CVD risk scores can be better established, we recommend the use of nationally endorsed CVD risk scores, with the frequency of reassessment based on CVD risk.
Conclusion
RA patients are at increased risk of CVD and CVD-related mortality relative to the general population. The disproportionate CVD burden seen in RA appears to be multifactorial, owing to the complex effects of systemic inflammation, endothelial dysfunction, and pro-atherogenic lipoprotein modifications. Additionally, many traditional CVD risk factors are more prevalent and suboptimally managed in RA patients. To mitigate the increased risk of CVD in RA, primary care and subspecialty providers alike must be aware of this heightened risk in RA, perform frequent assessment of CVD risk, and
Corresponding author: Bryant R. England, MD; 986270 Nebraska Medical Center, Omaha, NE 68198-6270; Bryant.england@unmc.edu.
Financial disclosures: Dr. England is supported by UNMC Internal Medicine Scientist Development Award, UNMC Physician-Scientist Training Program, the UNMC Mentored Scholars Program, and the Rheumatology Research Foundation Scientist Development Award. Dr. Mikuls is supported by a VA Merit Award (CX000896) and grants from the National Institutes of Health: National Institute of General Medical Sciences (U54GM115458), National Institute on Alcohol Abuse and Alcoholism (R25AA020818), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (2P50AR60772).
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
1. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. part I. Arthritis Rheum. 2008;58:15-25.
2. England BR, Sayles H, Michaud K, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68:36-45.
3. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26:S35-61.
4. Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690-1697.
5. Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524-1529.
6. van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: A cross-sectional study, the CARRE investigation. Ann Rheum Dis. 2009;68:1395-1400.
7. Peters MJ, van Halm VP, Voskuyl AE, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571-1579.
8. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52:402-411.
9. Cardiovascular disease in women--often silent and fatal. Lancet. 2011;378:200,6736(11)61108-61112.
10. Van Doornum S, Brand C, Sundararajan V, et al. Rheumatoid arthritis patients receive less frequent acute reperfusion and secondary prevention therapy after myocardial infarction compared with the general population. Arthritis Res Ther. 2010;12:R183.
11. Sodergren A, Stegmayr B, Lundberg V, et al. Increased incidence of and impaired prognosis after acute myocardial infarction among patients with seropositive rheumatoid arthritis. Ann Rheum Dis. 2007;66:263-266.
12. Douglas KM, Pace AV, Treharne GJ, et al. Excess recurrent cardiac events in rheumatoid arthritis patients with acute coronary syndrome. Ann Rheum Dis. 2006;65:348-353.
13. McCoy SS, Crowson CS, Maradit-Kremers H, et al. Long-term outcomes and treatment after myocardial infarction in patients with rheumatoid arthritis. J Rheumatol. 2013;40:605-610.
14. Mantel A, Holmqvist M, Andersson DC, et al. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275-1285.
15. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039-3044.
16. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005;52:412-420.
17. Davis JM,3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603-2611.
18. Arslan S, Bozkurt E, Sari RA, Erol MK. Diastolic function abnormalities in active rheumatoid arthritis evaluation by conventional doppler and tissue doppler: Relation with duration of disease. Clin Rheumatol. 2006;25:294-299.
19. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69:1665-1670.
20. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: A systematic review and meta-analysis. Stroke. 2016;47:943-950.
21. Ungprasert P, Srivali N, Spanuchart I, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol. 2014;33:297-304.
22. Stamatelopoulos KS, Kitas GD, Papamichael CM, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. 2010;212:305-309.
23. Chuang YW, Yu MC, Lin CL, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. 2016;115:439-445.
24. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in europe: The SCORE project. Eur Heart J. 2003;24:987-1003.
25. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743-753.
26. del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737-2745.
27. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA. 2007;297:611-619.
28. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74:668-674.
29. Crowson CS, Matteson EL, Roger VL, et al. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420-424.
30. Kawai VK, Chung CP, Solus JF, et al. The ability of the 2013 American College of Cardiology/American Heart Association cardiovascular risk score to identify rheumatoid arthritis patients with high coronary artery calcification scores. Arthritis Rheumatol. 2015;67:381-385.
31. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325-331.
32. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28.
33. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Predicting cardiovascular risk in England and Wales: Prospective derivation and validation of QRISK2. BMJ. 2008;336:1475-1482.
34. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ. 2017;357:j2099.
35. Solomon DH, Greenberg J, Curtis JR, et al. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A consortium of rheumatology researchers of north america registry study. Arthritis Rheumatol. 2015;67:1995-2003.
36. Crowson CS, Gabriel SE, Semb AG, et al. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology (Oxford). 2017;56:1102-1110.
37. Alemao E, Cawston H, Bourhis F, et al. Comparison of cardiovascular risk algorithms in patients with vs without rheumatoid arthritis and the role of C-reactive protein in predicting cardiovascular outcomes in rheumatoid arthritis. Rheumatology (Oxford). 2017;56:777-786.
38. Crowson CS, Rollefstad S, Kitas GD, et al. Challenges of developing a cardiovascular risk calculator for patients with rheumatoid arthritis. PLoS One. 2017;12: e0174656.
39. Karpouzas GA, Malpeso J, Choi TY, et al. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis. 2014;73:1797-1804.
40. van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: A meta-analysis. Semin Arthritis Rheum. 2011;40:3893-97.
41. Evans MR, Escalante A, Battafarano DF, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211-1220.
42. Ajeganova S, de Faire U, Jogestrand T, et al. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis--an inception cohort study. J Rheumatol. 2012;39:1146-1154.
43. Corrales A, Gonzalez-Juanatey C, Peiro ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: Results of a population-based study. Ann Rheum Dis. 2014;73:722-727.
44. Ikdahl E, Rollefstad S, Wibetoe G, et al. Predictive value of arterial stiffness and subclinical carotid atherosclerosis for cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol. 2016;43:1622-1630.
45. Provan SA, Semb AG, Hisdal J, et al. Remission is the goal for cardiovascular risk management in patients with rheumatoid arthritis: A cross-sectional comparative study. Ann Rheum Dis. 2011;70:812-817.
46. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, et al. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556-562.
47. Ambrosino P, Tasso M, Lupoli R, et al. Non-invasive assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann Med. 2015;47:457-467.
48. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157-2162.
49. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336-1345.
50. Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003.
51. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935-2959.
52. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990-999.
53. Yiu KH, Mok MY, Wang S, et al. Prognostic role of coronary calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Rheumatol. 2012;30:345-350.
54. Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: A focus on heart failure. Heart Fail Clin. 2014;10:339-352.
55. Rudominer RL, Roman MJ, Devereux RB, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60:22-29.
56. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148-2155.
57. Ntusi NAB, Piechnik SK, Francis JM, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: Insights from CMR T1 mapping. JACC Cardiovasc Imaging. 2015;8:526-536.
58. Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216.
59. Goodson NJ, Symmons DP, Scott DG, et al. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005;52:2293-2299.
60. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: Differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). 2011;50:1944-1954.
61. Cardarelli R, Lumicao TG Jr. B-type natriuretic peptide: A review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract. 2003;16:327-333.
62. Kragelund C, Gronning B, Kober L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666-675.
63. Harney SM, Timperley J, Daly C, et al. Brain natriuretic peptide is a potentially useful screening tool for the detection of cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:136.
64. Bradham WS, Bian A, Oeser A, et al. High-sensitivity cardiac troponin-I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930.
65. Karpouzas GA, Estis J, Rezaeian P, et al. High-sensitivity cardiac troponin I is a biomarker for occult coronary plaque burden and cardiovascular events in patients with rheumatoid arthritis. Rheumatology (Oxford). 2018;57:1080-1088.
66. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889-2934.
67. Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (PROCAM) study. Circulation. 2002;105:310-315.
Findings in seropositive arthralgia patients may help to predict RA
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Key clinical point:
Major finding: 19 of 36 patients developed RA within 12 months, and 13 of those had no disease activity with treatment initiated at RA onset.
Study details: A study of 36 seropositive arthralgia patients.
Disclosures: Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
New SLE disease activity measure beats SLEDAI-2K
, according to investigators.
In terms of identifying clinically significant changes, the Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) was superior to the SLE Disease Activity Index 2000 (SLEDAI-2K), said the authors of a longitudinal cohort study comparing the two instruments.
The SLE-DAS maintained high specificity compared with the SLEDAI-2K and had a similar clinical workup time requirement, according to Diogo Jesus, MD, of the rheumatology department at Centro Hospitalar e Universitário de Coimbra, Portugal, and his colleagues.
“Such a performance can have major implications in the interpretation of clinical trials applying the disease activity as the primary endpoint, and in daily clinical practice, where SLE-DAS could provide robust guidance for treatment in the individual patient,” Dr. Jesus and his coauthors wrote in Annals of the Rheumatic Diseases.
The longitudinal cohort study by Dr. Jesus and his colleagues included 520 patients with SLE from tertiary care centers in Coimbra, Portugal, and Padova, Italy. These included a derivation cohort of 324 patients and an external validation cohort of 196 patients (the Padova Lupus Cohort).
A cutoff value of 1.72 for change in the 17-item SLE-DAS had significantly higher sensitivities to detect a clinical meaningful improvement in both the derivation (82.1%) and external validation (89.5%) cohorts, compared with a cutoff value of 4 or higher in the SLEDAI-2K at 44.8% and 47.4%, respectively, the investigators reported. Likewise, ability to detect clinically meaningful worsening was significantly higher with SLE-DAS, with sensitivities of 93.1% in the derivation cohort and 95.5% in the external validation group versus a respective 46.6% and 59.1% for SLEDAI-2K. Both clinical improvement and worsening were defined by a change in the Physician Global Assessment score of 0.3 or more.
Specificity was high for both instruments, with values around 98%-99% in both cohorts for clinically meaningful worsening and about 97%-100% for clinically meaningful improvement, the report shows.
Further analyses showed that disease activity tracked over time with SLE-DAS had a higher predictive value for damage accrual versus the SLEDAI-2K, according to the investigators.
The SLE-DAS formula, published in the journal by Dr. Jesus and his colleagues, includes a total of 17 weighted and mostly binary variables: presence or absence of alopecia, arthritis, cardiac/pulmonary involvement, generalized cutaneous rash, hemolytic anemia, hypocomplementemia, increased anti-dsDNA levels, leukopenia, localized cutaneous rash, mucocutaneous vasculitis, mucosal ulcers, myositis, neuropsychiatric involvement, proteinuria, serositis, systemic vasculitis, and thrombocytopenia.
Next, the researchers plan to define SLE-DAS cutoff values for remission, low disease activity, and moderate or high disease activity. An online calculator is also in the works, they said.
Dr. Jesus and his coauthors did not report any outside funding for the study and said they had no competing interests related to their research.
SOURCE: Jesus D et al. Ann Rheum Dis. 2019 Jan 9. doi: 10.1136/annrheumdis-2018-214502.
, according to investigators.
In terms of identifying clinically significant changes, the Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) was superior to the SLE Disease Activity Index 2000 (SLEDAI-2K), said the authors of a longitudinal cohort study comparing the two instruments.
The SLE-DAS maintained high specificity compared with the SLEDAI-2K and had a similar clinical workup time requirement, according to Diogo Jesus, MD, of the rheumatology department at Centro Hospitalar e Universitário de Coimbra, Portugal, and his colleagues.
“Such a performance can have major implications in the interpretation of clinical trials applying the disease activity as the primary endpoint, and in daily clinical practice, where SLE-DAS could provide robust guidance for treatment in the individual patient,” Dr. Jesus and his coauthors wrote in Annals of the Rheumatic Diseases.
The longitudinal cohort study by Dr. Jesus and his colleagues included 520 patients with SLE from tertiary care centers in Coimbra, Portugal, and Padova, Italy. These included a derivation cohort of 324 patients and an external validation cohort of 196 patients (the Padova Lupus Cohort).
A cutoff value of 1.72 for change in the 17-item SLE-DAS had significantly higher sensitivities to detect a clinical meaningful improvement in both the derivation (82.1%) and external validation (89.5%) cohorts, compared with a cutoff value of 4 or higher in the SLEDAI-2K at 44.8% and 47.4%, respectively, the investigators reported. Likewise, ability to detect clinically meaningful worsening was significantly higher with SLE-DAS, with sensitivities of 93.1% in the derivation cohort and 95.5% in the external validation group versus a respective 46.6% and 59.1% for SLEDAI-2K. Both clinical improvement and worsening were defined by a change in the Physician Global Assessment score of 0.3 or more.
Specificity was high for both instruments, with values around 98%-99% in both cohorts for clinically meaningful worsening and about 97%-100% for clinically meaningful improvement, the report shows.
Further analyses showed that disease activity tracked over time with SLE-DAS had a higher predictive value for damage accrual versus the SLEDAI-2K, according to the investigators.
The SLE-DAS formula, published in the journal by Dr. Jesus and his colleagues, includes a total of 17 weighted and mostly binary variables: presence or absence of alopecia, arthritis, cardiac/pulmonary involvement, generalized cutaneous rash, hemolytic anemia, hypocomplementemia, increased anti-dsDNA levels, leukopenia, localized cutaneous rash, mucocutaneous vasculitis, mucosal ulcers, myositis, neuropsychiatric involvement, proteinuria, serositis, systemic vasculitis, and thrombocytopenia.
Next, the researchers plan to define SLE-DAS cutoff values for remission, low disease activity, and moderate or high disease activity. An online calculator is also in the works, they said.
Dr. Jesus and his coauthors did not report any outside funding for the study and said they had no competing interests related to their research.
SOURCE: Jesus D et al. Ann Rheum Dis. 2019 Jan 9. doi: 10.1136/annrheumdis-2018-214502.
, according to investigators.
In terms of identifying clinically significant changes, the Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) was superior to the SLE Disease Activity Index 2000 (SLEDAI-2K), said the authors of a longitudinal cohort study comparing the two instruments.
The SLE-DAS maintained high specificity compared with the SLEDAI-2K and had a similar clinical workup time requirement, according to Diogo Jesus, MD, of the rheumatology department at Centro Hospitalar e Universitário de Coimbra, Portugal, and his colleagues.
“Such a performance can have major implications in the interpretation of clinical trials applying the disease activity as the primary endpoint, and in daily clinical practice, where SLE-DAS could provide robust guidance for treatment in the individual patient,” Dr. Jesus and his coauthors wrote in Annals of the Rheumatic Diseases.
The longitudinal cohort study by Dr. Jesus and his colleagues included 520 patients with SLE from tertiary care centers in Coimbra, Portugal, and Padova, Italy. These included a derivation cohort of 324 patients and an external validation cohort of 196 patients (the Padova Lupus Cohort).
A cutoff value of 1.72 for change in the 17-item SLE-DAS had significantly higher sensitivities to detect a clinical meaningful improvement in both the derivation (82.1%) and external validation (89.5%) cohorts, compared with a cutoff value of 4 or higher in the SLEDAI-2K at 44.8% and 47.4%, respectively, the investigators reported. Likewise, ability to detect clinically meaningful worsening was significantly higher with SLE-DAS, with sensitivities of 93.1% in the derivation cohort and 95.5% in the external validation group versus a respective 46.6% and 59.1% for SLEDAI-2K. Both clinical improvement and worsening were defined by a change in the Physician Global Assessment score of 0.3 or more.
Specificity was high for both instruments, with values around 98%-99% in both cohorts for clinically meaningful worsening and about 97%-100% for clinically meaningful improvement, the report shows.
Further analyses showed that disease activity tracked over time with SLE-DAS had a higher predictive value for damage accrual versus the SLEDAI-2K, according to the investigators.
The SLE-DAS formula, published in the journal by Dr. Jesus and his colleagues, includes a total of 17 weighted and mostly binary variables: presence or absence of alopecia, arthritis, cardiac/pulmonary involvement, generalized cutaneous rash, hemolytic anemia, hypocomplementemia, increased anti-dsDNA levels, leukopenia, localized cutaneous rash, mucocutaneous vasculitis, mucosal ulcers, myositis, neuropsychiatric involvement, proteinuria, serositis, systemic vasculitis, and thrombocytopenia.
Next, the researchers plan to define SLE-DAS cutoff values for remission, low disease activity, and moderate or high disease activity. An online calculator is also in the works, they said.
Dr. Jesus and his coauthors did not report any outside funding for the study and said they had no competing interests related to their research.
SOURCE: Jesus D et al. Ann Rheum Dis. 2019 Jan 9. doi: 10.1136/annrheumdis-2018-214502.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: The Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) had better performance in detecting clinically significant changes, compared with the commonly used SLE Disease Activity Index 2000 (SLEDAI-2K).
Major finding: In a validation cohort, the SLE-DAS (vs. SLEDAI-2K) had a significantly higher sensitivity to detect a clinical meaningful improvement (89.5% vs. 47.4%) and clinically meaningful worsening (95.5% vs. 59.1%), with comparably high specificity for both tools.
Study details: Longitudinal cohort study including 520 patients with SLE from two tertiary care centers.
Disclosures: The study authors did not report any outside funding for the study and said they had no competing interests related to the research.
Source: Jesus D et al. Ann Rheum Dis. 2019 Jan 9. doi: 10.1136/annrheumdis-2018-214502.
Experts cite different approaches to try for methotrexate-related nausea, fatigue
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Primary Sjögren’s syndrome: New research and new resources improve outlook
Recent findings, new classification criteria and treatment guidelines, and concerted efforts by various organizations to provide educational resources are among a number of factors improving the outlook for patients with primary Sjögren’s syndrome, according to Judith James, MD, PhD.
Additionally, the number of studies of primary Sjögren’s syndrome (pSS) is increasing, albeit slowly, and ongoing studies of biologics are showing promise, Dr. James said during a clinical update at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Classification criteria
The ACR in conjunction with the European League Against Rheumatism (EULAR) published new criteria for pSS classification in 2016 based on the available evidence and expert consensus. Inclusion criteria include daily, persistent, troublesome dry eyes for more than 3 months, recurrent sensation of sand or gravel in the eyes, use of tear substitutes more than three times each day, frequent drinking of liquids to aid in swallowing dry food, or at least one EULAR Sjögren’s syndrome disease activity index (ESSDAI) domain with a positive item. Exclusion criteria include prior head and neck radiation treatment, polymerase chain reaction–confirmed active hepatitis C infection, AIDS, sarcoidosis, amyloidosis, graft-versus-host disease, or IgG4-related disease, said Dr. James, professor and chair of the arthritis and clinical immunology research program at the University of Oklahoma, Oklahoma City (Arthritis Rheumatol. Jan 2017;69[1]:35-45).
- A score of 4 or higher in patients who meet the inclusion criteria and do not have any of the exclusion criteria leads to classification with pSS, based on the following findings:
- Labial salivary gland with focal lymphocytic sialadenitis and focus score of at least 1 foci/4 mm2 (weight/score = 3).
- Anti-SSA/Ro-positivity (weight/score = 3).
- Ocular Staining Score of at least 5, or van Bijsterveld score of at least 4, in at least one eye (weight/score = 1).
- Schirmer’s test of no more than 5 mm/5 min in at least one eye (weight/score = 1).
- Unstimulated whole saliva flow rate of no more than 0.1 mL/min (weight/score = 1).
Clinical pearls for detection and management
In Dr. James’ experience, the three symptoms (taken together) with the highest predictive value for diagnosing pSS are dry mouth, sore mouth/tongue, and dry eyes. About 25% of Sjögren’s patients may have no detectable salivary flow, she said.
Dry, cracked skin that can lead to secondary infections is another common issue affecting about 55% of patients.
“So we always have to talk to our Sjögren’s patients about skin,” she said. “We also have recurrent sinusitis, chronic cough, dyspepsia, constipation, and other symptoms.”
Concurrent autoimmune diseases are another concern in Sjögren’s patients, she said. One to particularly keep in mind, in addition to lupus and rheumatoid arthritis, is autoimmune thyroid disease.
Data suggest that up to 45% of Sjögren’s patients have thyroid dysfunction, and if you look at just those with autoimmune thyroiditis, their risk of Sjögren’s is increased 10-fold vs. those without autoimmune thyroiditis, she said.
Other conditions to keep in mind when it comes to diagnosing and managing patients, as has been shown in numerous studies over the years, include Raynaud’s phenomenon, which affects at least 13% of patients, and subclinical muscle inflammation, which affects more than 50% of patients, Dr. James said.
“Depression ... as well as anxiety, is quite common in Sjögren’s patients, and fatigue is profound,” she added, noting that fatigue is “the No. 1 issue” for many patients.
Another area of particular concern in Sjögren’s is the increased risk of lymphoma, she said.
Studies show varying rates of lymphoma in Sjögren’s, with one suggesting a 44-fold increased risk, but this is likely only among those at very high risk. Other studies suggest the increase is risk overall is in the range of 4- to 10-fold, she said.
Mortality in Sjögren’s patients
A 2015 study by Soledad Retamozo et al. showed that the presence of cryoglobulinemic vasculitis (CV) at diagnosis is associated with increased mortality risk.
Of 515 consecutive pSS patients with a mean follow-up of 110 months, 65 (12%) had cryoglobulins detected, and 21 of those (32%) fulfilled CV criteria. The patients with cryoglobulins had higher cumulative mean disease activity, 45 (9%) developed B-cell lymphoma, and 33 (6%) died (Arthritis Rheumatol. 2015;67[suppl 10]. Abstract 628).
Additionally, both CV-positive and CV-negative patients had higher risk of B-cell lymphoma, but the risk was greatest in the CV-positive group (hazard ratios, 7.47 and 2.56, respectively), and the CV-positive patients had a higher risk of death (HR, 11.68).
“This actually has changed practice in our Sjögren’s clinic because we didn’t used to do cryos on everybody unless they had leukocytoclastic vasculitis, because they also have a higher risk of death,” Dr. James said.
Systemic activity also predicts pSS mortality, according to findings published in 2016 by Pilar Brito-Zerón et al. Of 1,045 patients who were part of the Spanish Group of Autoimmune Disease-SS Study Group and who were followed for a mean of 117 months, mortality was 11%. Survival was 96% at 5 years, 90% at 10 years, 81% at 20 years, and 60% at 30 years (Ann Rheum Dis. 2016;75:348-55).
Baseline factors associated with increased mortality on multivariate analysis included male gender, cryoglobulins, and low complement levels; the strongest model for death included high activity in at least one ESSDAI domain, baseline ESSDAI of at least 14, more than one laboratory predictive marker such as lymphopenia, anti-La, monoclonal gammopathy, low C3, low C4, and/or cryoglobulins.
Predicting progression to pSS
Progress has also been made with respect to predicting progression to pSS among patients who present with some related symptoms but don’t meet Sjögren’s criteria, Dr. James noted.
A 2017 study by Caroline Shiboski et al. looked at 771 patients from the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry who had previously had objective measures of salivary hypofunction, dry eyes, focal lymphocytic sialadenitis or anti-Ro/anti-La. When these patients were recalled 2-3 years after their baseline evaluation, 28 (9%) of 308 patients who did not meet pSS criteria at baseline had then progressed to pSS (Arthritis Care Res. 2017;70[2]:284-94).
Those with baseline hypergammaglobulinemia were four times more likely to progress, and those with baseline low complement levels were six times more likely to progress.
Many patients will present with symptoms, but won’t ever develop Sjögren’s, but the subset of patients with these baseline characteristics may be at greater risk, she said.
Autoantibodies and pathogenesis
Up to 90% of pSS patients will have one or more of anti-Ro, anti-La, or rheumatoid factor, and many will have a positive antinuclear antibody (ANA) level of at least 1:320, Dr. James said, adding that anti-Ro and anti-La are linked with earlier disease onset, increased disease severity, longer disease duration, and extraglandular involvement.
Ro52, a target of Sjögren’s autoantibodies, may also confer more severe disease, and autoantibodies to muscarinic acetylcholine receptors appear to contribute to pathogenesis in Sjögren’s patients “above and beyond what we see with lymphocytic infiltrates and other things that are happening in the salivary gland,” she said.
Other exciting progress with respect to disease pathogenesis includes an increased focus on genetics and genetic predisposition beyond human leukocyte antigen associations.
Mutations in genes that overlap with Sjögren’s and lupus or Sjögren’s and RA, such as IRF5, Blk, and STAT4 appear to contribute to Sjögren’s syndrome development.
“And then there’s also some new genetics looking at Sjögren’s-specific genes, and these may help us as we think about new targetable pathways in this disorder,” she said.
Genomics and gene-environment interactions, such as interactions with viral infections or “other things that lead to molecular mimicry that get the disease process started,” are also getting increased attention; gene-expression profiling has shown overlap between Sjögren’s and lupus (shared genetics, autoantibodies, and similarly strong interferon signatures, for example), which isn’t surprising.
“But we’re also seeing NF-kB [NF-kappa B] activation, antigen presentation, and migration pathways that are being found in Sjögren’s that aren’t necessarily the ones that we see in lupus,” she added.
Clinical practice guidelines
A number of practice guidelines addressing various symptoms and issues associated with pSS have been released in the last few years, including several from EULAR, the United States, Brazil, and the United Kingdom, are summarized and reviewed in a recent paper by Vasco Romão et al. (RMD Open. 2018;4:e000789. doi: 10.1136/rmdopen-2018-000789).
The British Society of Rheumatology guideline, which came out about a year ago, has particularly practical guidance on the management of dryness and systemic disease, she said (Rheumatology. 2017;56[10]:1643-7).
The increasing focus on pSS research has important implications both for trial design and patient care, especially in light of the new classification criteria, practice guidelines, and educational resources provided by organizations such as the Sjögren’s Syndrome Foundation (including videos, health care provider information, and downloadable brochures and resource sheets) and the European Research Network’s ReCONNET Disease Info Toolbox for Sjögren’s, Dr. James concluded.
Dr. James reported having no disclosures.
Recent findings, new classification criteria and treatment guidelines, and concerted efforts by various organizations to provide educational resources are among a number of factors improving the outlook for patients with primary Sjögren’s syndrome, according to Judith James, MD, PhD.
Additionally, the number of studies of primary Sjögren’s syndrome (pSS) is increasing, albeit slowly, and ongoing studies of biologics are showing promise, Dr. James said during a clinical update at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Classification criteria
The ACR in conjunction with the European League Against Rheumatism (EULAR) published new criteria for pSS classification in 2016 based on the available evidence and expert consensus. Inclusion criteria include daily, persistent, troublesome dry eyes for more than 3 months, recurrent sensation of sand or gravel in the eyes, use of tear substitutes more than three times each day, frequent drinking of liquids to aid in swallowing dry food, or at least one EULAR Sjögren’s syndrome disease activity index (ESSDAI) domain with a positive item. Exclusion criteria include prior head and neck radiation treatment, polymerase chain reaction–confirmed active hepatitis C infection, AIDS, sarcoidosis, amyloidosis, graft-versus-host disease, or IgG4-related disease, said Dr. James, professor and chair of the arthritis and clinical immunology research program at the University of Oklahoma, Oklahoma City (Arthritis Rheumatol. Jan 2017;69[1]:35-45).
- A score of 4 or higher in patients who meet the inclusion criteria and do not have any of the exclusion criteria leads to classification with pSS, based on the following findings:
- Labial salivary gland with focal lymphocytic sialadenitis and focus score of at least 1 foci/4 mm2 (weight/score = 3).
- Anti-SSA/Ro-positivity (weight/score = 3).
- Ocular Staining Score of at least 5, or van Bijsterveld score of at least 4, in at least one eye (weight/score = 1).
- Schirmer’s test of no more than 5 mm/5 min in at least one eye (weight/score = 1).
- Unstimulated whole saliva flow rate of no more than 0.1 mL/min (weight/score = 1).
Clinical pearls for detection and management
In Dr. James’ experience, the three symptoms (taken together) with the highest predictive value for diagnosing pSS are dry mouth, sore mouth/tongue, and dry eyes. About 25% of Sjögren’s patients may have no detectable salivary flow, she said.
Dry, cracked skin that can lead to secondary infections is another common issue affecting about 55% of patients.
“So we always have to talk to our Sjögren’s patients about skin,” she said. “We also have recurrent sinusitis, chronic cough, dyspepsia, constipation, and other symptoms.”
Concurrent autoimmune diseases are another concern in Sjögren’s patients, she said. One to particularly keep in mind, in addition to lupus and rheumatoid arthritis, is autoimmune thyroid disease.
Data suggest that up to 45% of Sjögren’s patients have thyroid dysfunction, and if you look at just those with autoimmune thyroiditis, their risk of Sjögren’s is increased 10-fold vs. those without autoimmune thyroiditis, she said.
Other conditions to keep in mind when it comes to diagnosing and managing patients, as has been shown in numerous studies over the years, include Raynaud’s phenomenon, which affects at least 13% of patients, and subclinical muscle inflammation, which affects more than 50% of patients, Dr. James said.
“Depression ... as well as anxiety, is quite common in Sjögren’s patients, and fatigue is profound,” she added, noting that fatigue is “the No. 1 issue” for many patients.
Another area of particular concern in Sjögren’s is the increased risk of lymphoma, she said.
Studies show varying rates of lymphoma in Sjögren’s, with one suggesting a 44-fold increased risk, but this is likely only among those at very high risk. Other studies suggest the increase is risk overall is in the range of 4- to 10-fold, she said.
Mortality in Sjögren’s patients
A 2015 study by Soledad Retamozo et al. showed that the presence of cryoglobulinemic vasculitis (CV) at diagnosis is associated with increased mortality risk.
Of 515 consecutive pSS patients with a mean follow-up of 110 months, 65 (12%) had cryoglobulins detected, and 21 of those (32%) fulfilled CV criteria. The patients with cryoglobulins had higher cumulative mean disease activity, 45 (9%) developed B-cell lymphoma, and 33 (6%) died (Arthritis Rheumatol. 2015;67[suppl 10]. Abstract 628).
Additionally, both CV-positive and CV-negative patients had higher risk of B-cell lymphoma, but the risk was greatest in the CV-positive group (hazard ratios, 7.47 and 2.56, respectively), and the CV-positive patients had a higher risk of death (HR, 11.68).
“This actually has changed practice in our Sjögren’s clinic because we didn’t used to do cryos on everybody unless they had leukocytoclastic vasculitis, because they also have a higher risk of death,” Dr. James said.
Systemic activity also predicts pSS mortality, according to findings published in 2016 by Pilar Brito-Zerón et al. Of 1,045 patients who were part of the Spanish Group of Autoimmune Disease-SS Study Group and who were followed for a mean of 117 months, mortality was 11%. Survival was 96% at 5 years, 90% at 10 years, 81% at 20 years, and 60% at 30 years (Ann Rheum Dis. 2016;75:348-55).
Baseline factors associated with increased mortality on multivariate analysis included male gender, cryoglobulins, and low complement levels; the strongest model for death included high activity in at least one ESSDAI domain, baseline ESSDAI of at least 14, more than one laboratory predictive marker such as lymphopenia, anti-La, monoclonal gammopathy, low C3, low C4, and/or cryoglobulins.
Predicting progression to pSS
Progress has also been made with respect to predicting progression to pSS among patients who present with some related symptoms but don’t meet Sjögren’s criteria, Dr. James noted.
A 2017 study by Caroline Shiboski et al. looked at 771 patients from the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry who had previously had objective measures of salivary hypofunction, dry eyes, focal lymphocytic sialadenitis or anti-Ro/anti-La. When these patients were recalled 2-3 years after their baseline evaluation, 28 (9%) of 308 patients who did not meet pSS criteria at baseline had then progressed to pSS (Arthritis Care Res. 2017;70[2]:284-94).
Those with baseline hypergammaglobulinemia were four times more likely to progress, and those with baseline low complement levels were six times more likely to progress.
Many patients will present with symptoms, but won’t ever develop Sjögren’s, but the subset of patients with these baseline characteristics may be at greater risk, she said.
Autoantibodies and pathogenesis
Up to 90% of pSS patients will have one or more of anti-Ro, anti-La, or rheumatoid factor, and many will have a positive antinuclear antibody (ANA) level of at least 1:320, Dr. James said, adding that anti-Ro and anti-La are linked with earlier disease onset, increased disease severity, longer disease duration, and extraglandular involvement.
Ro52, a target of Sjögren’s autoantibodies, may also confer more severe disease, and autoantibodies to muscarinic acetylcholine receptors appear to contribute to pathogenesis in Sjögren’s patients “above and beyond what we see with lymphocytic infiltrates and other things that are happening in the salivary gland,” she said.
Other exciting progress with respect to disease pathogenesis includes an increased focus on genetics and genetic predisposition beyond human leukocyte antigen associations.
Mutations in genes that overlap with Sjögren’s and lupus or Sjögren’s and RA, such as IRF5, Blk, and STAT4 appear to contribute to Sjögren’s syndrome development.
“And then there’s also some new genetics looking at Sjögren’s-specific genes, and these may help us as we think about new targetable pathways in this disorder,” she said.
Genomics and gene-environment interactions, such as interactions with viral infections or “other things that lead to molecular mimicry that get the disease process started,” are also getting increased attention; gene-expression profiling has shown overlap between Sjögren’s and lupus (shared genetics, autoantibodies, and similarly strong interferon signatures, for example), which isn’t surprising.
“But we’re also seeing NF-kB [NF-kappa B] activation, antigen presentation, and migration pathways that are being found in Sjögren’s that aren’t necessarily the ones that we see in lupus,” she added.
Clinical practice guidelines
A number of practice guidelines addressing various symptoms and issues associated with pSS have been released in the last few years, including several from EULAR, the United States, Brazil, and the United Kingdom, are summarized and reviewed in a recent paper by Vasco Romão et al. (RMD Open. 2018;4:e000789. doi: 10.1136/rmdopen-2018-000789).
The British Society of Rheumatology guideline, which came out about a year ago, has particularly practical guidance on the management of dryness and systemic disease, she said (Rheumatology. 2017;56[10]:1643-7).
The increasing focus on pSS research has important implications both for trial design and patient care, especially in light of the new classification criteria, practice guidelines, and educational resources provided by organizations such as the Sjögren’s Syndrome Foundation (including videos, health care provider information, and downloadable brochures and resource sheets) and the European Research Network’s ReCONNET Disease Info Toolbox for Sjögren’s, Dr. James concluded.
Dr. James reported having no disclosures.
Recent findings, new classification criteria and treatment guidelines, and concerted efforts by various organizations to provide educational resources are among a number of factors improving the outlook for patients with primary Sjögren’s syndrome, according to Judith James, MD, PhD.
Additionally, the number of studies of primary Sjögren’s syndrome (pSS) is increasing, albeit slowly, and ongoing studies of biologics are showing promise, Dr. James said during a clinical update at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Classification criteria
The ACR in conjunction with the European League Against Rheumatism (EULAR) published new criteria for pSS classification in 2016 based on the available evidence and expert consensus. Inclusion criteria include daily, persistent, troublesome dry eyes for more than 3 months, recurrent sensation of sand or gravel in the eyes, use of tear substitutes more than three times each day, frequent drinking of liquids to aid in swallowing dry food, or at least one EULAR Sjögren’s syndrome disease activity index (ESSDAI) domain with a positive item. Exclusion criteria include prior head and neck radiation treatment, polymerase chain reaction–confirmed active hepatitis C infection, AIDS, sarcoidosis, amyloidosis, graft-versus-host disease, or IgG4-related disease, said Dr. James, professor and chair of the arthritis and clinical immunology research program at the University of Oklahoma, Oklahoma City (Arthritis Rheumatol. Jan 2017;69[1]:35-45).
- A score of 4 or higher in patients who meet the inclusion criteria and do not have any of the exclusion criteria leads to classification with pSS, based on the following findings:
- Labial salivary gland with focal lymphocytic sialadenitis and focus score of at least 1 foci/4 mm2 (weight/score = 3).
- Anti-SSA/Ro-positivity (weight/score = 3).
- Ocular Staining Score of at least 5, or van Bijsterveld score of at least 4, in at least one eye (weight/score = 1).
- Schirmer’s test of no more than 5 mm/5 min in at least one eye (weight/score = 1).
- Unstimulated whole saliva flow rate of no more than 0.1 mL/min (weight/score = 1).
Clinical pearls for detection and management
In Dr. James’ experience, the three symptoms (taken together) with the highest predictive value for diagnosing pSS are dry mouth, sore mouth/tongue, and dry eyes. About 25% of Sjögren’s patients may have no detectable salivary flow, she said.
Dry, cracked skin that can lead to secondary infections is another common issue affecting about 55% of patients.
“So we always have to talk to our Sjögren’s patients about skin,” she said. “We also have recurrent sinusitis, chronic cough, dyspepsia, constipation, and other symptoms.”
Concurrent autoimmune diseases are another concern in Sjögren’s patients, she said. One to particularly keep in mind, in addition to lupus and rheumatoid arthritis, is autoimmune thyroid disease.
Data suggest that up to 45% of Sjögren’s patients have thyroid dysfunction, and if you look at just those with autoimmune thyroiditis, their risk of Sjögren’s is increased 10-fold vs. those without autoimmune thyroiditis, she said.
Other conditions to keep in mind when it comes to diagnosing and managing patients, as has been shown in numerous studies over the years, include Raynaud’s phenomenon, which affects at least 13% of patients, and subclinical muscle inflammation, which affects more than 50% of patients, Dr. James said.
“Depression ... as well as anxiety, is quite common in Sjögren’s patients, and fatigue is profound,” she added, noting that fatigue is “the No. 1 issue” for many patients.
Another area of particular concern in Sjögren’s is the increased risk of lymphoma, she said.
Studies show varying rates of lymphoma in Sjögren’s, with one suggesting a 44-fold increased risk, but this is likely only among those at very high risk. Other studies suggest the increase is risk overall is in the range of 4- to 10-fold, she said.
Mortality in Sjögren’s patients
A 2015 study by Soledad Retamozo et al. showed that the presence of cryoglobulinemic vasculitis (CV) at diagnosis is associated with increased mortality risk.
Of 515 consecutive pSS patients with a mean follow-up of 110 months, 65 (12%) had cryoglobulins detected, and 21 of those (32%) fulfilled CV criteria. The patients with cryoglobulins had higher cumulative mean disease activity, 45 (9%) developed B-cell lymphoma, and 33 (6%) died (Arthritis Rheumatol. 2015;67[suppl 10]. Abstract 628).
Additionally, both CV-positive and CV-negative patients had higher risk of B-cell lymphoma, but the risk was greatest in the CV-positive group (hazard ratios, 7.47 and 2.56, respectively), and the CV-positive patients had a higher risk of death (HR, 11.68).
“This actually has changed practice in our Sjögren’s clinic because we didn’t used to do cryos on everybody unless they had leukocytoclastic vasculitis, because they also have a higher risk of death,” Dr. James said.
Systemic activity also predicts pSS mortality, according to findings published in 2016 by Pilar Brito-Zerón et al. Of 1,045 patients who were part of the Spanish Group of Autoimmune Disease-SS Study Group and who were followed for a mean of 117 months, mortality was 11%. Survival was 96% at 5 years, 90% at 10 years, 81% at 20 years, and 60% at 30 years (Ann Rheum Dis. 2016;75:348-55).
Baseline factors associated with increased mortality on multivariate analysis included male gender, cryoglobulins, and low complement levels; the strongest model for death included high activity in at least one ESSDAI domain, baseline ESSDAI of at least 14, more than one laboratory predictive marker such as lymphopenia, anti-La, monoclonal gammopathy, low C3, low C4, and/or cryoglobulins.
Predicting progression to pSS
Progress has also been made with respect to predicting progression to pSS among patients who present with some related symptoms but don’t meet Sjögren’s criteria, Dr. James noted.
A 2017 study by Caroline Shiboski et al. looked at 771 patients from the Sjögren’s International Collaborative Clinical Alliance (SICCA) registry who had previously had objective measures of salivary hypofunction, dry eyes, focal lymphocytic sialadenitis or anti-Ro/anti-La. When these patients were recalled 2-3 years after their baseline evaluation, 28 (9%) of 308 patients who did not meet pSS criteria at baseline had then progressed to pSS (Arthritis Care Res. 2017;70[2]:284-94).
Those with baseline hypergammaglobulinemia were four times more likely to progress, and those with baseline low complement levels were six times more likely to progress.
Many patients will present with symptoms, but won’t ever develop Sjögren’s, but the subset of patients with these baseline characteristics may be at greater risk, she said.
Autoantibodies and pathogenesis
Up to 90% of pSS patients will have one or more of anti-Ro, anti-La, or rheumatoid factor, and many will have a positive antinuclear antibody (ANA) level of at least 1:320, Dr. James said, adding that anti-Ro and anti-La are linked with earlier disease onset, increased disease severity, longer disease duration, and extraglandular involvement.
Ro52, a target of Sjögren’s autoantibodies, may also confer more severe disease, and autoantibodies to muscarinic acetylcholine receptors appear to contribute to pathogenesis in Sjögren’s patients “above and beyond what we see with lymphocytic infiltrates and other things that are happening in the salivary gland,” she said.
Other exciting progress with respect to disease pathogenesis includes an increased focus on genetics and genetic predisposition beyond human leukocyte antigen associations.
Mutations in genes that overlap with Sjögren’s and lupus or Sjögren’s and RA, such as IRF5, Blk, and STAT4 appear to contribute to Sjögren’s syndrome development.
“And then there’s also some new genetics looking at Sjögren’s-specific genes, and these may help us as we think about new targetable pathways in this disorder,” she said.
Genomics and gene-environment interactions, such as interactions with viral infections or “other things that lead to molecular mimicry that get the disease process started,” are also getting increased attention; gene-expression profiling has shown overlap between Sjögren’s and lupus (shared genetics, autoantibodies, and similarly strong interferon signatures, for example), which isn’t surprising.
“But we’re also seeing NF-kB [NF-kappa B] activation, antigen presentation, and migration pathways that are being found in Sjögren’s that aren’t necessarily the ones that we see in lupus,” she added.
Clinical practice guidelines
A number of practice guidelines addressing various symptoms and issues associated with pSS have been released in the last few years, including several from EULAR, the United States, Brazil, and the United Kingdom, are summarized and reviewed in a recent paper by Vasco Romão et al. (RMD Open. 2018;4:e000789. doi: 10.1136/rmdopen-2018-000789).
The British Society of Rheumatology guideline, which came out about a year ago, has particularly practical guidance on the management of dryness and systemic disease, she said (Rheumatology. 2017;56[10]:1643-7).
The increasing focus on pSS research has important implications both for trial design and patient care, especially in light of the new classification criteria, practice guidelines, and educational resources provided by organizations such as the Sjögren’s Syndrome Foundation (including videos, health care provider information, and downloadable brochures and resource sheets) and the European Research Network’s ReCONNET Disease Info Toolbox for Sjögren’s, Dr. James concluded.
Dr. James reported having no disclosures.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Stopping TNF inhibitors before 20 weeks’ gestation not linked to worsening RA, JIA
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: In contrast to a previous report,
Major finding: Patient Activity Scale (PAS) scores were stable over time in women who discontinued TNF inhibitors before gestational week 20. Those who continued past week 20 had improved PAS scores in the third trimester (univariate analysis; P = .02).
Study details: Analysis including 490 pregnant women in the United States or Canada who enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study.
Disclosures: Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
Source: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821.
Long-term opioid use substantial in elderly adults prior to total joint replacement
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
In elderly patients with osteoarthritis, long-term opioid use is highly prevalent and varies substantially by state, suggest the results of a large, observational cohort study.
Long term opioid use prior to total joint replacement (TJR) varied somewhat by access to primary care providers, but not by access to rheumatologists, according to authors of the study, led by Rishi J Desai, MS, PhD, of the department of medicine at Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
“These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states,” said Dr. Desai and his colleagues in a report on the study published in Arthritis & Rheumatology.
This study by Dr. Desai and his colleagues looked at long-term use of opioids, which was defined as at least 90 days of use in the year prior to TJR. They analyzed a total of 358,121 Medicare enrollees with advanced osteoarthritis, with a mean age of 74 years.
Geographic areas in the South tended to have higher proportions of long-term opioid users, while the Northeast and Midwest had lower proportions, according to investigators.
Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama, they reported. Beyond Alabama, the top 10 states included West Virginia, Georgia, Kentucky, Louisiana, Oklahoma, North Carolina, Virginia, Indiana, and Mississippi, with proportions of long-term opioid users ranging from 17% to 25%, the report shows.
Only modest associations were seen between provider density and opioid use, investigators said. There was a 1.4% mean difference (95% confidence interval, 0.8%-2.0%) in long-term opioid users between primary care service areas (PCSAs) with the highest concentrations of primary care providers versus those with the lowest, and there was just a 0.6% mean difference (95% CI, –0.1% to 1.3%) between PCSAs with the highest concentrations of rheumatologists and those with the lowest.
Among long-term opioid users, almost 20% were using an average daily dose of 50 or more morphine milligram equivalents, a range that potentially imparts a high risk of opioid-related harms, according to investigators.
Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Co-authors provided disclosures related to a number of pharmaceutical companies.
SOURCE: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Long-term opioid use is highly prevalent among older adults with osteoarthritis who underwent total joint replacement.
Major finding: Long-term use of opioids ranged from a low of 8.9% in Minnesota to 26.4% in Alabama.
Study details: An observational cohort study including 358,121 Medicare enrollees with advanced osteoarthritis.
Disclosures: Funding for the study came from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Desai reported disclosures related to Merck and Vertex. Coauthors provided disclosures related to a number of pharmaceutical companies.
Source: Desai RJ et al. Arthritis Rheumatol. 2019. doi: 10.1002/art.40834.
Comorbidities may cut effectiveness of psoriasis biologics
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
PARIS – in response to biologic therapy, according to the results of the prospective observational PSO-BIO-REAL study.
The clinical importance of this finding lies in the fact that comorbidities are highly prevalent among patients with moderate to severe psoriasis. Indeed, fully 64% of the 846 participants in PSO-BIO-REAL had at least one major comorbid condition at baseline, Finn Ziegler said at the annual congress of the European Academy of Dermatology and Venereology.
“I think this reflects a picture that has been seen in other studies,” noted Mr. Ziegler, director of global patient access at Leo Pharma in Ballerup, Denmark.
The purpose of the 12-month PSO-BIO-REAL (PSOriasis treated with BIOlogics in REAL life) study was to assess the effectiveness of a variety of biologic agents in a real-world population typical of patients encountered in routine clinical practice, as opposed to more restrictive format of often-cited randomized trials, which generally feature a lengthy list of exclusions. One-third of participants were from the United States, with the rest drawn from four Western European countries. Their mean age was 47 years, with an 18.4-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of 14.3.
Sixty percent of participants were starting treatment with a biologic agent for the first time. The other 40% had prior biologic experience. At physician discretion, 61% of enrollees were put on a tumor necrosis factor inhibitor, either etanercept (Enbrel), adalimumab (Humira), or infliximab (Remicade); 30% initiated treatment with the interleukin-12/23 inhibitor ustekinumab (Stelara); and 9% received secukinumab (Cosentyx), an interleukin-17 inhibitor.
The five most common comorbid conditions present at baseline were hypertension, present in 33.5% of participants; psoriatic arthritis (PsA), present in 28.1%; hyperlipidemia, 20.9%; diabetes, 13.9%, and depression, present in 13.7% of the psoriasis patients.
Baseline comorbidities were significantly more common among the biologic-experienced patients. For example, their prevalence of hypertension was 42%, compared with 28% in the biologic-naive group. PsA was present in 35% of the biologic-experienced and 23% of the biologic-naive patients. Nineteen percent of biologic-experienced patients had diabetes at baseline, as did 11% of the biologic-naive group.
During the 12-month study, 3.7% of patients developed a new comorbidity, the most common being anxiety, hypertension, PsA, depression, and hyperlipidemia.
The primary outcome in the study was the complete clearance rate – a PASI 100 response – at 6 months. It ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more. The results were similar at 12 months.
Conversely, an inadequate therapeutic response as defined by a PASI 50 or less at 6 months occurred in 15% of psoriasis patients with no baseline comorbidities, 27% with one, 35% with two comorbid conditions, and 28% with three or more.
The major caveat regarding this study is that the observed association between comorbid conditions and complete clearance rates doesn’t prove causality, Mr. Ziegler noted.
The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma. Mr. Ziegler is a Leo executive.
SOURCE: Ziegler F. EADV Congress, Abstract FC04.01.
REPORTING FROM THE EADV CONGRESS
Key clinical point: As the number of baseline comorbid conditions increases, the complete clearance rate in response to biologic agents for psoriasis falls.
Major finding: The complete clearance rate after 6 months of biologic therapy ranged from a high of 31% in patients with no baseline comorbid conditions to a low of 16.5% in those with three or more.
Study details: This multinational, prospective, observational, 12-month study included 846 patients initiating biologic therapy for moderate to severe psoriasis.
Disclosures: The PSO-BIO-REAL study was sponsored by Amgen, AstraZeneca, and Leo Pharma and was presented by a Leo executive.
Source: Ziegler F. EADV Congress, Abstract FC04.01.
Four distinct IgG4-related disease groups described in study
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
IgG4-related disease can be grouped into four distinct clusters based on the distribution of organs involved, according to researchers who analyzed a large, multicenter cohort of patients with this heterogeneous, autoimmune-mediated condition.
The four groups also varied by age, race, sex, time to diagnosis, and concentration of serum IgG4, according to the investigators, led by Zachary S. Wallace, MD, of the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“These phenotypes may be used by clinicians to improve recognition of IgG4-related disease,” Dr. Wallace and his coauthors wrote in a report on the study that appears in the Annals of the Rheumatic Diseases.
First described in a Japanese population, IgG4-related disease has been subsequently seen in all racial and ethnic groups, according to the researchers. It is associated with organ failure and can affect nearly any organ or anatomic site, most notably the lungs, kidneys, lymph nodes, salivary glands, pancreatobiliary structures, and retroperitoneum.
In the present study, Dr. Wallace and his coinvestigators used a novel cluster analysis method, called latent class analysis, to categorize 765 cases of IgG4-related disease submitted by 52 investigators from 17 countries. The investigators included 493 of those cases in a primary study population, and the remaining 272 in a smaller cohort used to replicate the results.
In the larger, primary study cohort, about 65% of cases were male, 58% were non-Asian and 40% were white, and the mean age at diagnosis was 59.5 years. The replication cohort had similar characteristics, according to the investigators.
The clustering analysis revealed four distinct subgroups, characterized by pancreato-hepatobiliary, accounting for 31% of cases; retroperitoneal fibrosis and/or aortitis in 24%; disease generally limited to head and neck structures in 24%, and head and neck disease consistent with Mikulicz syndrome plus systemic involvement in 22%.
The highest IgG4 concentrations were seen in the group of patients with Mikulicz syndrome and systemic involvement, according to Dr. Wallace and his coauthors. The serum concentration was 1,170 mg/dL in that group, compared with 445 mg/dL in the group of patients with head and neck-limited disease, 316 mg/dL in the pancreato-hepatobiliary group, and just 178 mg/dL in the retroperitoneal fibrosis/aorta group.
Female and Asian patients were overrepresented in the group characterized by head and neck involvement, investigators also found. Moreover, that group had a significantly lower mean age at diagnosis than did the other groups.
Those variations suggested differences in genetic or environmental risk factors between clusters, according to the investigators.
“Given the similar distribution of subspecialists among investigators in this study practicing in Asian and non-Asian countries, the observed differences are unlikely to be the result of detection or selection biases,” they said in their report.
The findings of this study help to inform subsequent investigations intended to evaluate those factors in more detail, they said.
Dr. Wallace and his coauthors reported no conflicts of interest related to their work, which was previously presented at the American College of Rheumatology annual meeting.
SOURCE: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The highest IgG4 concentrations (1,170 mg/dL) were seen in a group of patients with Mikulicz syndrome and systemic involvement. Females and Asian patients were overrepresented in a group characterized by head and neck involvement.
Study details: Two cross-sectional studies including a total of 765 cases of IgG4-related disease submitted by 52 investigators in 17 countries.
Disclosures: Authors reported no conflicts of interest.
Source: Wallace ZS et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214603.
MMF proves viable as alternative option in moderate ANCA-associated vasculitis
Mycophenolate mofetil was noninferior to pulsed cyclophosphamide for remission induction in ANCA-associated vasculitis, but there were more relapses, in a randomized, unblinded trial of 140 patients with non–life-threatening disease.
“Our results demonstrate that MMF [mycophenolate mofetil] represents an alternative to CYC [cyclophosphamide] for remission induction in AAV [ANCA-associated vasculitis],” said investigators led by Rachel B. Jones, MD, of the department of renal medicine at Addenbrooke’s Hospital, Cambridge, England.
“While treatment with MMF may be associated with a higher risk of relapse compared with pulsed CYC, this increased risk may be acceptable to avoid the potential adverse effects of CYC” – infections, malignancies, and infertility – “particularly when the baseline risk of relapse is low,” as with the elderly or myeloperoxidase (MPO)-ANCA disease, “or if rituximab is unavailable,” Dr. Jones and her colleagues wrote in Annals of the Rheumatic Diseases.
Since enrollment began in 2007, they also noted that “it has become common to use rituximab as an alternative to CYC induction therapy ... However, rituximab is expensive, and its use is restricted in many countries ... Alternative effective low-cost induction therapies” – such as MMF – “may be required in some cases.”
The work, which was done on behalf of the European Vasculitis Study Group, was the largest randomized trial to date of MMF for AAV remission induction, and the first to include children; patients were enrolled at 21 sites in Europe, Australia, and New Zealand.
They were randomly assigned to MMF or pulsed CYC, with 66 adults and 4 children ranging in age from 10 to 16 years in each group. In addition to those with life-threatening disease, patients younger than 6 years old and those on dialysis or with an estimated glomerular filtration rate below 15 mL/min/m2 were excluded. Following remission, subjects were switched to oral azathioprine and a prednisone taper.
The primary outcome was remission by 6 months, defined as the absence of disease activity on two consecutive occasions at least 1 month apart, plus adherence to the taper. Baseline ANCA subtype, disease activity, and organ involvement were similar between the groups.
Overall, 47 patients in the in the MMF group (67%), including 1 child, reached the primary endpoint, versus 43 patients (61%), again including 1 child, in the CYC group (risk difference = 5.7%; 90% confidence interval, –7.5% to 19%), which established noninferiority. The median time to remission was about 90 days in both arms. “Compliance was a contributory factor to the lower remission rate[s] in children,” the authors noted.
More relapses occurred in the MMF group (33% vs. 19% with CYC). Among MPO-ANCA patients, the relapse rate was 15% versus 12% with CYC. In proteinase 3 (PR3)-ANCA patients, almost half in the MMF group relapsed (48% versus 24%).
There were no significant differences in serious infections (26% with MMF versus 17% with CYC; P = .3), malignancies, thromboembolisms, or death (four CYC patients and five MMF patients at 6 months).
Adult patients in the MMF group received MMF 2 g/day, with dose increases to 3 g/day permitted for uncontrolled disease at 4 weeks. Patients younger than 17 years old were dosed according to body surface area. The CYC group received intravenous pulsed CYC 15 mg/kg every 2-3 weeks with reductions for age and renal function. A total of 58 CYC patients (83%) received at least six pulses.
After remission, azathioprine was dosed at 2 mg/kg per day. Oral prednisolone started at 1 mg/kg per day and was reduced to 5 mg/day by 6 months.
Plasma exchange or additional methylprednisolone were allowed at entry; there were no differences in their use between the groups.
The trial was sponsored by Cambridge University Hospitals NHS Foundation Trust. Vifor Pharma provided a research grant to cover the trial and MMF costs. Dr. Jones and the other investigators reported ties to several companies, including GlaxoSmithKline, Genentech/Roche, ChemoCentryx, and Genzyme/Sanofi. Vifor holds an equity stake in ChemoCentryx.
SOURCE: Jones RB et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214245.
We sometimes encounter patients for whom access to rituximab is a problem, for financial, logistical, or tolerability reasons. Also, some patients are really reluctant about an infusion, and there is still some skittishness about rituximab. I have had some patients who, despite my having strongly reiterated how rare progressive multifocal leukoencephalopathy is, have been unable to get past the fact that that’s in the package insert. It’s helpful to me as a clinician to know that mycophenolate is something we can turn to in these situations. As I discuss the ups and down of different potential strategies with patients, this is something that can be part of the discussion.
This was a really well done study, and they were honest about the limitations. They excluded patients with the most severe disease, so you are still not talking about your patients on dialysis getting mycophenolate. Also, there are questions in terms of it’s not being as effective at sustaining remission, but that’s a bridge you can cross once they are in remission, in terms of how vigilant you are about follow-up. You also can re-induce remission with mycophenolate if you have to.
Robert F. Spiera, MD, is director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York. He had no relevant disclosures, and is on the editorial advisory board of MDedge Rheumatology.
We sometimes encounter patients for whom access to rituximab is a problem, for financial, logistical, or tolerability reasons. Also, some patients are really reluctant about an infusion, and there is still some skittishness about rituximab. I have had some patients who, despite my having strongly reiterated how rare progressive multifocal leukoencephalopathy is, have been unable to get past the fact that that’s in the package insert. It’s helpful to me as a clinician to know that mycophenolate is something we can turn to in these situations. As I discuss the ups and down of different potential strategies with patients, this is something that can be part of the discussion.
This was a really well done study, and they were honest about the limitations. They excluded patients with the most severe disease, so you are still not talking about your patients on dialysis getting mycophenolate. Also, there are questions in terms of it’s not being as effective at sustaining remission, but that’s a bridge you can cross once they are in remission, in terms of how vigilant you are about follow-up. You also can re-induce remission with mycophenolate if you have to.
Robert F. Spiera, MD, is director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York. He had no relevant disclosures, and is on the editorial advisory board of MDedge Rheumatology.
We sometimes encounter patients for whom access to rituximab is a problem, for financial, logistical, or tolerability reasons. Also, some patients are really reluctant about an infusion, and there is still some skittishness about rituximab. I have had some patients who, despite my having strongly reiterated how rare progressive multifocal leukoencephalopathy is, have been unable to get past the fact that that’s in the package insert. It’s helpful to me as a clinician to know that mycophenolate is something we can turn to in these situations. As I discuss the ups and down of different potential strategies with patients, this is something that can be part of the discussion.
This was a really well done study, and they were honest about the limitations. They excluded patients with the most severe disease, so you are still not talking about your patients on dialysis getting mycophenolate. Also, there are questions in terms of it’s not being as effective at sustaining remission, but that’s a bridge you can cross once they are in remission, in terms of how vigilant you are about follow-up. You also can re-induce remission with mycophenolate if you have to.
Robert F. Spiera, MD, is director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York. He had no relevant disclosures, and is on the editorial advisory board of MDedge Rheumatology.
Mycophenolate mofetil was noninferior to pulsed cyclophosphamide for remission induction in ANCA-associated vasculitis, but there were more relapses, in a randomized, unblinded trial of 140 patients with non–life-threatening disease.
“Our results demonstrate that MMF [mycophenolate mofetil] represents an alternative to CYC [cyclophosphamide] for remission induction in AAV [ANCA-associated vasculitis],” said investigators led by Rachel B. Jones, MD, of the department of renal medicine at Addenbrooke’s Hospital, Cambridge, England.
“While treatment with MMF may be associated with a higher risk of relapse compared with pulsed CYC, this increased risk may be acceptable to avoid the potential adverse effects of CYC” – infections, malignancies, and infertility – “particularly when the baseline risk of relapse is low,” as with the elderly or myeloperoxidase (MPO)-ANCA disease, “or if rituximab is unavailable,” Dr. Jones and her colleagues wrote in Annals of the Rheumatic Diseases.
Since enrollment began in 2007, they also noted that “it has become common to use rituximab as an alternative to CYC induction therapy ... However, rituximab is expensive, and its use is restricted in many countries ... Alternative effective low-cost induction therapies” – such as MMF – “may be required in some cases.”
The work, which was done on behalf of the European Vasculitis Study Group, was the largest randomized trial to date of MMF for AAV remission induction, and the first to include children; patients were enrolled at 21 sites in Europe, Australia, and New Zealand.
They were randomly assigned to MMF or pulsed CYC, with 66 adults and 4 children ranging in age from 10 to 16 years in each group. In addition to those with life-threatening disease, patients younger than 6 years old and those on dialysis or with an estimated glomerular filtration rate below 15 mL/min/m2 were excluded. Following remission, subjects were switched to oral azathioprine and a prednisone taper.
The primary outcome was remission by 6 months, defined as the absence of disease activity on two consecutive occasions at least 1 month apart, plus adherence to the taper. Baseline ANCA subtype, disease activity, and organ involvement were similar between the groups.
Overall, 47 patients in the in the MMF group (67%), including 1 child, reached the primary endpoint, versus 43 patients (61%), again including 1 child, in the CYC group (risk difference = 5.7%; 90% confidence interval, –7.5% to 19%), which established noninferiority. The median time to remission was about 90 days in both arms. “Compliance was a contributory factor to the lower remission rate[s] in children,” the authors noted.
More relapses occurred in the MMF group (33% vs. 19% with CYC). Among MPO-ANCA patients, the relapse rate was 15% versus 12% with CYC. In proteinase 3 (PR3)-ANCA patients, almost half in the MMF group relapsed (48% versus 24%).
There were no significant differences in serious infections (26% with MMF versus 17% with CYC; P = .3), malignancies, thromboembolisms, or death (four CYC patients and five MMF patients at 6 months).
Adult patients in the MMF group received MMF 2 g/day, with dose increases to 3 g/day permitted for uncontrolled disease at 4 weeks. Patients younger than 17 years old were dosed according to body surface area. The CYC group received intravenous pulsed CYC 15 mg/kg every 2-3 weeks with reductions for age and renal function. A total of 58 CYC patients (83%) received at least six pulses.
After remission, azathioprine was dosed at 2 mg/kg per day. Oral prednisolone started at 1 mg/kg per day and was reduced to 5 mg/day by 6 months.
Plasma exchange or additional methylprednisolone were allowed at entry; there were no differences in their use between the groups.
The trial was sponsored by Cambridge University Hospitals NHS Foundation Trust. Vifor Pharma provided a research grant to cover the trial and MMF costs. Dr. Jones and the other investigators reported ties to several companies, including GlaxoSmithKline, Genentech/Roche, ChemoCentryx, and Genzyme/Sanofi. Vifor holds an equity stake in ChemoCentryx.
SOURCE: Jones RB et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214245.
Mycophenolate mofetil was noninferior to pulsed cyclophosphamide for remission induction in ANCA-associated vasculitis, but there were more relapses, in a randomized, unblinded trial of 140 patients with non–life-threatening disease.
“Our results demonstrate that MMF [mycophenolate mofetil] represents an alternative to CYC [cyclophosphamide] for remission induction in AAV [ANCA-associated vasculitis],” said investigators led by Rachel B. Jones, MD, of the department of renal medicine at Addenbrooke’s Hospital, Cambridge, England.
“While treatment with MMF may be associated with a higher risk of relapse compared with pulsed CYC, this increased risk may be acceptable to avoid the potential adverse effects of CYC” – infections, malignancies, and infertility – “particularly when the baseline risk of relapse is low,” as with the elderly or myeloperoxidase (MPO)-ANCA disease, “or if rituximab is unavailable,” Dr. Jones and her colleagues wrote in Annals of the Rheumatic Diseases.
Since enrollment began in 2007, they also noted that “it has become common to use rituximab as an alternative to CYC induction therapy ... However, rituximab is expensive, and its use is restricted in many countries ... Alternative effective low-cost induction therapies” – such as MMF – “may be required in some cases.”
The work, which was done on behalf of the European Vasculitis Study Group, was the largest randomized trial to date of MMF for AAV remission induction, and the first to include children; patients were enrolled at 21 sites in Europe, Australia, and New Zealand.
They were randomly assigned to MMF or pulsed CYC, with 66 adults and 4 children ranging in age from 10 to 16 years in each group. In addition to those with life-threatening disease, patients younger than 6 years old and those on dialysis or with an estimated glomerular filtration rate below 15 mL/min/m2 were excluded. Following remission, subjects were switched to oral azathioprine and a prednisone taper.
The primary outcome was remission by 6 months, defined as the absence of disease activity on two consecutive occasions at least 1 month apart, plus adherence to the taper. Baseline ANCA subtype, disease activity, and organ involvement were similar between the groups.
Overall, 47 patients in the in the MMF group (67%), including 1 child, reached the primary endpoint, versus 43 patients (61%), again including 1 child, in the CYC group (risk difference = 5.7%; 90% confidence interval, –7.5% to 19%), which established noninferiority. The median time to remission was about 90 days in both arms. “Compliance was a contributory factor to the lower remission rate[s] in children,” the authors noted.
More relapses occurred in the MMF group (33% vs. 19% with CYC). Among MPO-ANCA patients, the relapse rate was 15% versus 12% with CYC. In proteinase 3 (PR3)-ANCA patients, almost half in the MMF group relapsed (48% versus 24%).
There were no significant differences in serious infections (26% with MMF versus 17% with CYC; P = .3), malignancies, thromboembolisms, or death (four CYC patients and five MMF patients at 6 months).
Adult patients in the MMF group received MMF 2 g/day, with dose increases to 3 g/day permitted for uncontrolled disease at 4 weeks. Patients younger than 17 years old were dosed according to body surface area. The CYC group received intravenous pulsed CYC 15 mg/kg every 2-3 weeks with reductions for age and renal function. A total of 58 CYC patients (83%) received at least six pulses.
After remission, azathioprine was dosed at 2 mg/kg per day. Oral prednisolone started at 1 mg/kg per day and was reduced to 5 mg/day by 6 months.
Plasma exchange or additional methylprednisolone were allowed at entry; there were no differences in their use between the groups.
The trial was sponsored by Cambridge University Hospitals NHS Foundation Trust. Vifor Pharma provided a research grant to cover the trial and MMF costs. Dr. Jones and the other investigators reported ties to several companies, including GlaxoSmithKline, Genentech/Roche, ChemoCentryx, and Genzyme/Sanofi. Vifor holds an equity stake in ChemoCentryx.
SOURCE: Jones RB et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214245.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Overall, 47 patients in the in the MMF group (67%), including 1 child, reached remission by 6 months, versus 43 subjects (61%), again including 1 child, in the CYC group (risk difference = 5.7%; 90% CI, –7.5% to 19%), which established noninferiority.
Study details: Randomized, multisite trial with 140 patients.
Disclosures: The Cambridge University Hospitals NHS Foundation Trust sponsored the study. Vifor Pharma provided a research grant to cover the trial and MMF costs. Dr. Jones and the other investigators reported ties to several companies, including GlaxoSmithKline, Genentech/Roche, and ChemoCentryx, which is owned in part by Vifor.
Source: Jones RB et al. Ann Rheum Dis. 2019 Jan 5. doi: 10.1136/annrheumdis-2018-214245.