User login
FDA committee votes yes on romosozumab for osteoporosis
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
In an 18-1 vote, the FDA’s Bone, Reproductive, and Urologic Drugs Advisory Committee agreed that the risk-benefit profile of romosozumab, to be marketed by Amgen as Evenity, was favorable enough to support approval. Relative risk reductions of up to 75%, compared with placebo, and 36%, compared with alendronate, were seen in pivotal clinical trials.
A signal for increased major adverse cardiovascular events (MACE) among those receiving romosozumab had been seen in just one of the clinical trials, with a hazard ratio for MACE of 1.87 for those taking romosozumab, compared with those taking alendronate (95% confidence interval, 1.11-3.14).
Committee members were enthusiastic about the efficacy of the monoclonal antibody, which binds to sclerostin and prevents its inhibiting effect, allowing robust new bone formation. “I want to emphasize the remarkable skeletal efficacy of this drug; truly, it’s better than anything we’ve seen before,” said committee member Sundeep Khosla, MD, professor of medicine and physiology at the Mayo Clinic, Rochester, Minn.
In its application, the sponsor relied on two clinical trials. In the first, 7,180 women with osteoporosis aged 55-90 years were randomized 1:1 to receive romosozumab or placebo for 12 months in a double-blind trial. After this time, participants in each arm received follow-on treatment with denosumab (Prolia) for another 12 months. This study, dubbed Trial 337, followed morphometric vertebral fractures at 12 and 24 months. Morphometric fractures included both symptomatic and asymptomatic fractures.
Those treated with romosozumab had relative risk reductions of new vertebral fractures of 73% and 75%, compared with those given placebo at 12 and 24 months. Absolute risk reductions for vertebral fractures were 1.30% and 1.89% at 1 and 2 years (P less than .001 for both).
The second study, Trial 142, was a double-blind, active-controlled study that included 4,093 women aged 55-90 years with osteoporosis and a history of prior fragility fracture. Participants were randomized 1:1 to receive either romosozumab or alendronate for 12 months, with an additional variable period of alendronate follow-on of at least 12 months for both arms.
For Trial 142, one primary endpoint was morphometric vertebral fractures at month 24. An additional endpoint, clinical fracture, was a composite of symptomatic vertebral fractures and nonvertebral fractures. This second endpoint was assessed at the time of primary analysis, an event-driven cut point that occurred when at least 330 participants experienced a clinical fracture and all participants had completed the 24-month visit.
Hip fractures were less common among those given romosozumab, and bone mineral density increased significantly as well.
Vertebral fractures were reduced by 36% in the romosozumab group relative to the alendronate group, and clinical fractures by 27% (P less than .001 for both).
Overall, the number of adverse events for the more than 7,500 patients in the safety population was similar between those receiving romosozumab and either placebo or alendronate, said Scott Wasserman, MD, vice president of global development for Amgen.
However, in Trial 142, which included patients who were slightly older and on more cardiovascular medications at baseline than in Trial 337, MACE – defined as cardiovascular death, MI, and stroke – occurred more frequently among those taking romosozumab, driven primarily by increased cardiac and cerebral ischemic events occurring within the first 12 months of beginning the study drug. At 12 months, the romosozumab arm saw 41 instances of MACE, compared with 22 in the alendronate arm to produce the HR of 1.87 (2.0% vs. 1.1%).
With regard to the imbalance in MACE seen in Trial 142, both the FDA and presenters for the sponsor entertained the notion that alendronate may have been somewhat protective for cardiovascular events. Although there is some biologic plausibility for a cardioprotective event for bisphosphonates, alendronate is highly specific for bone activity and the preponderance of previous studies have not shown such cardioprotection, the FDA, sponsors, and committee members all agreed.
Marc Sabatine, MD, the Lewis Dexter, MD Distinguished Chair in Cardiovascular Medicine at Harvard Medical School, Boston, was available to answer questions on behalf of the study’s sponsor. He noted at several points during the meeting just how few total cardiovascular events were seen overall in the romosozumab trial. The overall small numbers, he said, made it very difficult to distinguish whether the smaller number of MACE seen in the alendronate arm of Trial 142 were a true safety signal or just “a play of chance.”
Almost all the committee’s questioning and discussion centered on this potential increased cardiovascular risk. Amgen, in discussion with the FDA, had agreed to a black box warning designed to wave off prescribing romosozumab to those at increased risk for cardiovascular disease, focusing on those with a history of MI or stroke.
Additionally, the sponsor proposed a postmarketing real-world observational study to track incidence of MACE in those receiving romosozumab, comparing them with those receiving standard of care for osteoporosis.
Several committee members pointed out a problem with the proposed safety mitigation scheme: By conducting a postmarketing observational study for a drug that has a black-box warning to exclude those at high risk for MACE, the chance of detecting an actual cardiovascular safety problem plummets. “This is the textbook example of when observational studies struggle or are virtually guaranteed to fail,” noted Tobias Gerhard, PhD, a pharmacoepidemiologist at Rutgers University, New Brunswick, N.J.
On the other hand, noted several committee members, pausing to conduct a premarketing randomized, controlled trial would keep a beneficial drug away from a population in need. A postmarketing randomized, controlled trial, even a simple trial, still presents challenges, some of the committee acknowledged. Dr. Khosla voiced the opinion that “A randomized, controlled trial is virtually impossible.”
The committee, which was charged with discussing, but not voting on, what additional data should be obtained – and when – to sort out the cardiovascular safety question, was approximately evenly divided in the matter of whether an observational or registry-based trial, or a controlled trial, would be the best path forward.
Committee member Robert A. Adler, MD, put a realistic frame around the debate. “As an endocrinologist, I deal with nuances every day. I really think the kind of clinician who is going to be using this drug is used to dealing with benefits and risks and trying to tailor treatment to a given patient,” said Dr. Adler, professor of internal medicine and epidemiology at Virginia Commonwealth University, Richmond.
In its proposed indication, Amgen defined the population of menopausal women at high risk of fracture as those with a history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed or are intolerant to other available osteoporosis therapy. Romosozumab would be given as a once-monthly subcutaneous injection of 210 mg for a period of 12 months, to be followed by antiresorptive therapy.
Most of the participants in the clinical trials resided outside the United States, primarily because of the difficulty of recruiting clinical trial participants in the United States, Amgen officials said during their presentation. However, neither the time to the first positively adjudicated MACE nor bone mineral density responses at month 12 differed significantly across the various geographic regions where clinical trial sites were located, said Rachel Wagman, MD, the executive medical director of global clinical development for Amgen. Still, several committee members called for postmarketing data to focus on U.S. patients.
Romosozumab was approved for marketing in Japan on Jan. 8, 2019; approval is also being sought in Europe .
The FDA usually follows the recommendations of its advisory panels.
Treat-to-target approach for CVD risk factors decreased atherosclerosis in RA patients
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
It is well recognized that traditional cardiovascular disease (CVD) risk factors contribute to atherogenesis and CVD events in rheumatoid arthritis (RA) patients at least as much as inflammatory risk factors, and traditional risk factors and inflammatory risk factors may interact (i.e. inflammatory risk factors have a greater impact in the setting of higher number of traditional risk factors).
There is documented reluctance among many rheumatologists to take on the additional burden of screening and managing hyperlipidemia, hypertension, diabetes, weight management, diet, and so on in addition to all the other aspects of managing these patients that take a lot of time. Research is needed on how to efficiently streamline measurement and management of CVD risk in these patients to optimize outcomes.
Jon T. Giles, MD , is a rheumatologist in the division of rheumatology at Columbia University in New York. He was not involved in the study by Burggraaf et al. and reported no relevant conflicts of interest.
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
Researchers from the Netherlands found that a treat-to-target approach for cardiovascular risk factors in patients with rheumatoid arthritis was effective in reducing clinical and subclinical atherosclerosis; however, the researchers noted there was a “considerable” dropout in the study that could have limited the results, according to data recently published in Annals of the Rheumatic Diseases.
Benjamin Burggraaf, MD, and his associates at Franciscus Gasthuis & Vlietland Hospital in Rotterdam, the Netherlands, performed an open-label, randomized, controlled trial of 320 patients with rheumatoid arthritis (RA) who were younger than 70 years old (mean age, 52.4 years) without a prior history of cardiovascular disease (CVD) and diabetes mellitus. The patients received either usual care for traditional CVD risk factors or a treat-to-target approach using a prespecified protocol for treating hypertension, hyperlipidemia and hypertriglyceridemia, as well hemoglobin A1c greater than 48 mmol/mol. A total of 219 patients (68.4%) finished the study after 5 years. The two groups were similar at baseline, but the treat-to-target group had significantly more women (77.2%) compared with the usual care group (62.0%).
The researchers compared the progression of carotid intima media thickness (cIMT) at baseline and 5 years, with secondary outcomes of nonfatal myocardial infarction, nonfatal stroke, coronary artery bypass grafting, percutaneous coronary intervention, peripheral atherosclerotic arterial disease–related amputation, peripheral atherosclerotic arterial disease revascularization, and death due to cardiovascular causes.
“To account for the high dropout, all missing cIMT values after 5 years of follow-up were imputed with all available cIMT values (baseline, years 1-4) and treatment group as covariate,” the researchers said. “Assuming that unobserved cIMT values were missing at random, missing data were imputed with multiple imputation using the fully conditional specification method for seven cycles.”
Dr. Burggraaf and his colleagues found the treat-to-target group had lower mean cIMT progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up than did the group that received usual care for CVD risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028). At 5 years, there were no significant differences between treat-to-target and usual care groups regarding mean systolic blood pressure (124.6 mm Hg vs. 124.7 mm Hg, respectively; P = .97), treat-to-target treatment targets for blood pressure (72.4% for usual care vs. 75.9% for treat-to-target; P = .56) and mean HbA1c (37.6 mmol/mol for treat-to-target vs. 37.0 mmol/mol for usual care; P = .39). The treat-to-target group also had fewer cardiovascular events (two events, 1.3%) compared with the usual care group (seven events, 4.7%; P = .048). There were five patients in the treat-to-target group who died during the study (4.7%), compared with seven patients (3.2%) in the usual care group (P = .51).
“Although the difference in cIMT progression between the groups was relatively small in absolute terms, the relative reduction in progression was almost 50% in favor of the treat-to-target group,” they noted. “In light of the reduction of cardiovascular events, these effects are, in our opinion, clinically relevant.”
Other limitations of the study included the use of cIMT as a “soft endpoint” for modern cardiovascular trials and the use of unblinded cIMT progression measurement. In addition, the researchers noted the study was underpowered, and they used data from a type 2 diabetes mellitus cohort to perform the power calculation, which carried a 50% reduction in CVD risk factors. “We had doubts whether this high risk would apply to patients with RA and therefore used a more conservative target of 20% reduction for cIMT progression,” the researchers said.
This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
SOURCE: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The treat-to-target group had greater mean carotid intima media thickness progression (0.023 mm; 95% confidence interval, 0.011-0.036) at 5-year follow-up compared with the group that received usual care for cardiovascular disease risk factors (0.045 mm; 95% CI, 0.030-0.059; P = .028).
Study details: An open-label, randomized, controlled trial of 320 RA patients who received a treat-to-target intervention or usual care for cardiovascular disease risk factors.
Disclosures: This study was funded by the Franciscus Gasthuis & Vlietland Hospital, the Foundation for Research and Development of the Department of Internal Medicine, and the Coolsingel Foundation, Rotterdam. One author reported being a consultant for and receiving lecture honoraria from Merck.
Source: Burggraaf B et al. Ann Rheum Dis. 2019 Jan 4. doi: 10.1136/annrheumdis-2018-214075.
Anti-TNFs slow SIJ damage in early axial spondyloarthritis
Long-term anti–tumor necrosis factor therapy seemed to slow progression of sacroiliac joint damage in early axial spondyloarthritis in a German study of 42 patients.
They were all part of the ESTHER trial, which found that in early axial spondyloarthritis (axSpA), active inflammatory lesions on MRI improved significantly more with etanercept (Enbrel) than with sulfasalazine-treated patients (Ann Rheum Dis. 2011 Apr 1;70[4]:590-6).
In the new work, published in the Arthritis & Rheumatology, investigators reviewed 42 ESTHER subjects who were on etanercept for up to 6 years and who also had baseline and at least one bilateral sacroiliac joint (SIJ) x-ray at 2-, 4-, or 6-year follow-up. Two blinded readers scored the x-rays, and a sacroiliitis sum score was assigned to each subject. The sum score ranged from 0, meaning no signs of sacroiliitis, to 8, meaning total ankylosis of both SIJs.
The mean progression in sacroiliitis sum score was 0.13 points from baseline to 2 years; followed by a regression of –0.27 points during years 2-4, and –0.09 points from years 4-6. There was no comparator group of untreated patients, but the authors noted that previous work would suggest a slight, but detectable, progression in joint damage over that time.
Elevated C-reactive protein and the presence of osteitis on MRI, meanwhile, were independently associated with progression.
“This is the first study to analyze the long-term (up to 6 years) progression of radiographic sacroiliitis in patients with axSpA treated with an anti-TNF agent, in this case with etanercept. Our results suggest that long-term treatment with a potent anti-inflammatory drug like a TNF inhibitor may influence the evolution of the disease by decelerating the radiographic progression of SIJ [damage] in patients with” early axSpA, wrote the investigators, led by Valeria Rios Rodriguez, MD, of the Charité Universitätsmedizin, Berlin.
Progression from nonradiographic to radiographic axSpA occurred only in the first 2 years of treatment, among 5 of 27 patients, while 2 of 15 patients who started with radiographic disease regressed to nonradiographic axSpA, yielding a net progression of 7.1%.
Although that’s higher than what’s been reported in previous studies, it might be related to greater inflammation in the SIJ at baseline in ESTHER, since active osteitis on MRI was an inclusion criteria. Also, “treatment with a TNF inhibitor ... might have triggered a faster bone repair visible on x-rays as a progression of structural damage,” the investigators said.
The results seem “congruent with the retardation of the spine progression seen in [ankylosing spondylitis] patients treated long-term with TNF inhibitors. ... Long-term treatment with TNF inhibitors could reduce ... new bone formation by preventing the development of new inflammatory lesions resulting in structural damage” of the SIJ, they said, which, in turn, “might have an impact on the functional status and spinal mobility in patients with axSpA, independently of structural damage of the spine.”
Two-thirds of the trial participants were men; the mean age was 34 years, and mean duration of symptoms 3.1 years at baseline. No information was reported on adverse events.
The work was funded by Pfizer, a marketer of etanercept. Some of the investigators disclosed consulting and other payments from the company, as well as other companies involved in marketing and/or development of drugs for axSpA. One investigator was an employee of Pfizer.
SOURCE: Rios Rodriguez V et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40786
Long-term anti–tumor necrosis factor therapy seemed to slow progression of sacroiliac joint damage in early axial spondyloarthritis in a German study of 42 patients.
They were all part of the ESTHER trial, which found that in early axial spondyloarthritis (axSpA), active inflammatory lesions on MRI improved significantly more with etanercept (Enbrel) than with sulfasalazine-treated patients (Ann Rheum Dis. 2011 Apr 1;70[4]:590-6).
In the new work, published in the Arthritis & Rheumatology, investigators reviewed 42 ESTHER subjects who were on etanercept for up to 6 years and who also had baseline and at least one bilateral sacroiliac joint (SIJ) x-ray at 2-, 4-, or 6-year follow-up. Two blinded readers scored the x-rays, and a sacroiliitis sum score was assigned to each subject. The sum score ranged from 0, meaning no signs of sacroiliitis, to 8, meaning total ankylosis of both SIJs.
The mean progression in sacroiliitis sum score was 0.13 points from baseline to 2 years; followed by a regression of –0.27 points during years 2-4, and –0.09 points from years 4-6. There was no comparator group of untreated patients, but the authors noted that previous work would suggest a slight, but detectable, progression in joint damage over that time.
Elevated C-reactive protein and the presence of osteitis on MRI, meanwhile, were independently associated with progression.
“This is the first study to analyze the long-term (up to 6 years) progression of radiographic sacroiliitis in patients with axSpA treated with an anti-TNF agent, in this case with etanercept. Our results suggest that long-term treatment with a potent anti-inflammatory drug like a TNF inhibitor may influence the evolution of the disease by decelerating the radiographic progression of SIJ [damage] in patients with” early axSpA, wrote the investigators, led by Valeria Rios Rodriguez, MD, of the Charité Universitätsmedizin, Berlin.
Progression from nonradiographic to radiographic axSpA occurred only in the first 2 years of treatment, among 5 of 27 patients, while 2 of 15 patients who started with radiographic disease regressed to nonradiographic axSpA, yielding a net progression of 7.1%.
Although that’s higher than what’s been reported in previous studies, it might be related to greater inflammation in the SIJ at baseline in ESTHER, since active osteitis on MRI was an inclusion criteria. Also, “treatment with a TNF inhibitor ... might have triggered a faster bone repair visible on x-rays as a progression of structural damage,” the investigators said.
The results seem “congruent with the retardation of the spine progression seen in [ankylosing spondylitis] patients treated long-term with TNF inhibitors. ... Long-term treatment with TNF inhibitors could reduce ... new bone formation by preventing the development of new inflammatory lesions resulting in structural damage” of the SIJ, they said, which, in turn, “might have an impact on the functional status and spinal mobility in patients with axSpA, independently of structural damage of the spine.”
Two-thirds of the trial participants were men; the mean age was 34 years, and mean duration of symptoms 3.1 years at baseline. No information was reported on adverse events.
The work was funded by Pfizer, a marketer of etanercept. Some of the investigators disclosed consulting and other payments from the company, as well as other companies involved in marketing and/or development of drugs for axSpA. One investigator was an employee of Pfizer.
SOURCE: Rios Rodriguez V et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40786
Long-term anti–tumor necrosis factor therapy seemed to slow progression of sacroiliac joint damage in early axial spondyloarthritis in a German study of 42 patients.
They were all part of the ESTHER trial, which found that in early axial spondyloarthritis (axSpA), active inflammatory lesions on MRI improved significantly more with etanercept (Enbrel) than with sulfasalazine-treated patients (Ann Rheum Dis. 2011 Apr 1;70[4]:590-6).
In the new work, published in the Arthritis & Rheumatology, investigators reviewed 42 ESTHER subjects who were on etanercept for up to 6 years and who also had baseline and at least one bilateral sacroiliac joint (SIJ) x-ray at 2-, 4-, or 6-year follow-up. Two blinded readers scored the x-rays, and a sacroiliitis sum score was assigned to each subject. The sum score ranged from 0, meaning no signs of sacroiliitis, to 8, meaning total ankylosis of both SIJs.
The mean progression in sacroiliitis sum score was 0.13 points from baseline to 2 years; followed by a regression of –0.27 points during years 2-4, and –0.09 points from years 4-6. There was no comparator group of untreated patients, but the authors noted that previous work would suggest a slight, but detectable, progression in joint damage over that time.
Elevated C-reactive protein and the presence of osteitis on MRI, meanwhile, were independently associated with progression.
“This is the first study to analyze the long-term (up to 6 years) progression of radiographic sacroiliitis in patients with axSpA treated with an anti-TNF agent, in this case with etanercept. Our results suggest that long-term treatment with a potent anti-inflammatory drug like a TNF inhibitor may influence the evolution of the disease by decelerating the radiographic progression of SIJ [damage] in patients with” early axSpA, wrote the investigators, led by Valeria Rios Rodriguez, MD, of the Charité Universitätsmedizin, Berlin.
Progression from nonradiographic to radiographic axSpA occurred only in the first 2 years of treatment, among 5 of 27 patients, while 2 of 15 patients who started with radiographic disease regressed to nonradiographic axSpA, yielding a net progression of 7.1%.
Although that’s higher than what’s been reported in previous studies, it might be related to greater inflammation in the SIJ at baseline in ESTHER, since active osteitis on MRI was an inclusion criteria. Also, “treatment with a TNF inhibitor ... might have triggered a faster bone repair visible on x-rays as a progression of structural damage,” the investigators said.
The results seem “congruent with the retardation of the spine progression seen in [ankylosing spondylitis] patients treated long-term with TNF inhibitors. ... Long-term treatment with TNF inhibitors could reduce ... new bone formation by preventing the development of new inflammatory lesions resulting in structural damage” of the SIJ, they said, which, in turn, “might have an impact on the functional status and spinal mobility in patients with axSpA, independently of structural damage of the spine.”
Two-thirds of the trial participants were men; the mean age was 34 years, and mean duration of symptoms 3.1 years at baseline. No information was reported on adverse events.
The work was funded by Pfizer, a marketer of etanercept. Some of the investigators disclosed consulting and other payments from the company, as well as other companies involved in marketing and/or development of drugs for axSpA. One investigator was an employee of Pfizer.
SOURCE: Rios Rodriguez V et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40786
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: The mean progression in sacroiliitis sum score was 0.13 points from baseline to 2 years; followed by a regression of –0.27 points during years 2-4, and –0.09 points from years 4-6.
Study details: Review of 42 patients with early disease.
Disclosures: The work was funded by Pfizer, a marketer of etanercept. Some of the investigators disclosed consulting and other payments from the company, as well as other companies involved in marketing and/or development of drugs for axSpA. One investigator was an employee of Pfizer.
Source: Rios Rodriguez V et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40786
No drop in gout prevalence, but no increase either
according to data from an ongoing, nationally representative survey.
Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
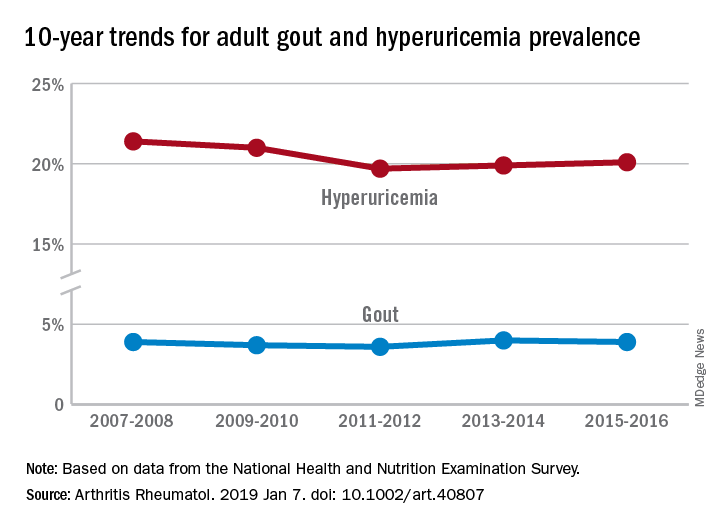
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
FROM ARTHRITIS & RHEUMATOLOGY
Back pain persists in one in five patients
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Back pain is a common health problem, and one in five Canadian adults reported persistent back pain.
Major finding: Approximately half (46%) of Canadian adults reported some type of back pain over a 16-year period.
Study details: The data come from a population-based study of 12,782 adults followed from 1994 to 2011.
Disclosures: The authors reported no relevant financial conflicts.
Source: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
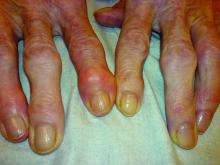
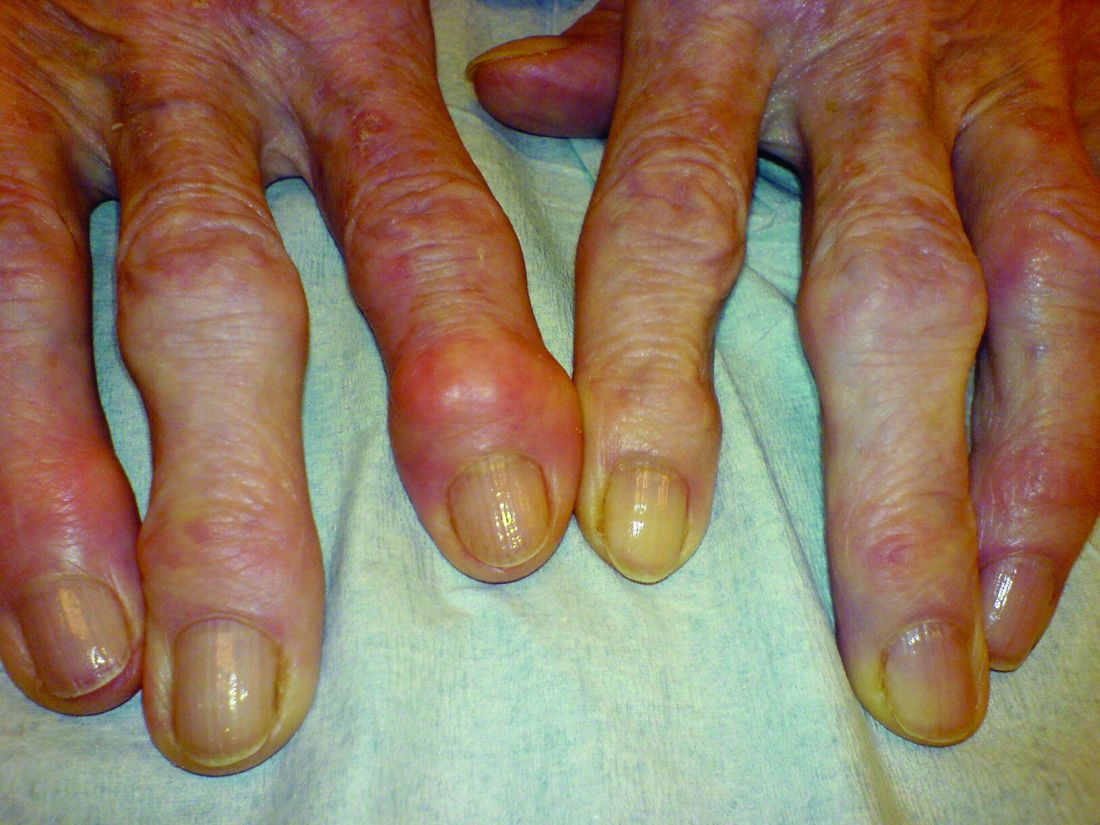
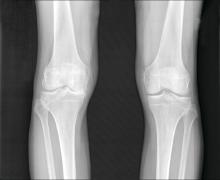
Heberden’s nodes linked to knee OA progression
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
according to a review of 575 participants in a substudy of the Osteoarthritis Initiative cohort.
After assessing Heberden’s nodes (HNs) – bony enlargements of the last finger joint – and knee MRI findings at baseline and 24 months, the investigators found that HNs were associated with periarticular bone area expansion in the knee. The investigators reported their findings in Arthritis & Rheumatology.
Comparing the 395 subjects with HNs with the 180 without, there was more periarticular bone area expansion among HN patients at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83), especially in the medial femur (aOR, 1.49; 95% CI, 1.05-2.13), lateral femur (aOR, 2.51; 95% CI, 1.58-3.97), femoral notch (aOR, 1.37; 95% CI, 1.02-1.84), and lateral trochlea (aOR, 1.44; 95% CI, 1.08-1.9). The comparisons were adjusted for age, sex, body mass index, and bone remodeling agent use.
“The presence of Heberden’s nodes in a physical examination is associated with a distinct pattern of worsening of osteoarthritis-related structural damage in the knee joint,” lead investigator Arya Haj-Mirzaian, MD, a radiologist and postdoctoral fellow at Johns Hopkins University, Baltimore, said in a press release.
However, HNs were also associated with less worsening of knee osteophytes, especially at the femoral end of the knee joint (aOR, 0.54; 95% CI, 0.31-0.95); the finding seemed to contradict the overall picture of worsening knee osteoarthritis with HNs.
“Although osteophytes are thought to be a late secondary sequel or compensatory repair mechanism in OA and indicator of advanced knee OA, less worsening in osteophytes’ score ... may propose that less ossification is involved in the pathophysiology of knee OA in the presence of HNs,” the investigators wrote. It’s a subject for future research.
Patients with HNs were older, more often female, and had a lower frequency for other knee OA risk factors, such as excessive body mass index and knee injury. Patients with gout were excluded.
There was no external funding, and the investigators reported no disclosures.
SOURCE: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Heberden’s nodes may be an indicator of knee OA progression.
Major finding: There was more periarticular bone area expansion among patients with Heberden’s nodes at 2 years in the knee joint (adjusted odds ratio, 1.39; 95% confidence interval, 1.06-1.83).
Study details: A substudy of 575 participants in the Osteoarthritis Initiative cohort
Disclosures: There was no external funding, and the investigators reported no disclosures.
Source: Haj-Mirzaian A et al. Arthritis Rheumatol. 2019 Jan 9. doi: 10.1002/art.40811.
CRP predicts anti-TNF response in ankylosing spondylitis
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
Patients with ankylosing spondylitis whose baseline C-reactive protein (CRP) levels were more than three times the upper limit of normal were significantly more likely to respond to 12 weeks of etanercept therapy than were patients with normal baseline CRP levels, investigators reported.
In a post hoc study of 867 patients who received etanercept during clinical trials, the adjusted odds of achieving 20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) at week 12 were 190% higher when CRP levels were “very high” rather than normal at baseline (odds ratio, 2.9; 95% confidence interval, 1.8-4.7). Very-high baseline CRP levels (more than three times the upper limit of normal) also significantly predicted week-12 ASAS50, a change in Ankylosing Spondylitis Disease Activity Score based on CRP (ASDAS-CRP) greater than 1.1 (clinically important improvement), and an ASDAS-CRP less than 1.3 (inactive disease), while a normalization of very-high CRP levels by 2, 4, or 8 weeks of treatment was a significant predictor of achieving week-12 ASDAS inactive disease. “Patient-reported outcomes were less consistent predictors of response,” Xenofon Baraliakos, MD, of Ruhr University Bochum in Herne, Germany, and his coinvestigators wrote in Seminars in Arthritis and Rheumatism.
While the advent of tumor necrosis factor (TNF) antagonists has greatly improved outcomes in ankylosing spondylitis, symptom ambiguity and a lack of objective response criteria still impede early diagnosis and radiographic interpretation. Elevated baseline CRP predicted clinical response to anti-TNF therapy by patients with ankylosing spondylitis in several prior post hoc studies. To further evaluate this finding, the researchers pooled and analyzed data from four randomized, placebo-controlled trials of etanercept in adults with ankylosing spondylitis. In all, 43% of patients had a normal baseline CRP level, 34% had a level that was elevated but did not exceed three times the upper limit of normal, and 23% had a very-high level.
Age of onset, disease duration, and baseline Bath Ankylosing Spondylitis Functional Index also predicted response to etanercept therapy. After controlling for these covariates, a very-high baseline CRP remained a significant predictor for all four week-12 outcomes. This finding points to the value of aggressive, early treatment to normalize CRP in patients with ankylosing spondylitis, the researchers wrote. “Bending the curve of inflammation in the early disease may alter the long-term trajectory of ankylosing spondylitis, which is an opportunity that may not exist in the later stages.”
Thus, CRP, in addition to patient-reported and clinical outcomes, might be useful to help monitor response to anti-TNF therapy, the investigators wrote. It remains unclear whether elevated CRP levels also predict future treatment response in patients who are currently clinically improved or stable.
Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
SOURCE: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Key clinical point:
Major finding: Compared with normal baseline CRP, a CRP level more than three times above the upper limit of normal correlated significantly with all four outcomes at week 12.
Study details: A post hoc analysis of data from 867 patients with ankylosing spondylitis who received etanercept during one of four clinical trials (NCT00421915, NCT00418548, NCT00247962, and NCT00356356).
Disclosures: Pfizer funded the post hoc analysis and acquired the company that had funded the trials (Wyeth). Dr. Baraliakos reported consultancy and speaker fees from Pfizer, AbbVie, Bristol-Myers Squibb, Celgene, Chugai, Janssen, Merck, Novartis, Sandoz, and UCB. Two coinvestigators were Pfizer employees. A third coinvestigator was contracted by Pfizer to provide statistical support.
Source: Baraliakos X et al. Semin Arthritis Rheum. 2018 Nov 2. doi: 10.1016/j.semarthrit.2018.10.019.
AAP guidance: How to ask about military service
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies, including multiple meniscal tears, predict accelerated OA
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Accelerated knee osteoarthritis is characterized by distinct features that include destabilizing meniscal tears in two or more areas as well as other pathologies, based on data from the Osteoarthritis Initiative.
The possibility of accelerated knee osteoarthritis (AKOA) as a unique subset of knee osteoarthritis has not been well studied, wrote Jeffrey B. Driban, PhD, of Tufts University, Boston, and his colleagues.
“If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies,” they wrote in their report, published in Arthritis & Rheumatology.
The researchers reviewed data from three groups of adults selected from participants in the Osteoarthritis Initiative, a cohort of 4,796 adults with KOA or at risk for symptomatic KOA who were recruited at four clinical sites in the United States. These groups included 125 with AKOA, 125 with typical knee osteoarthritis (KOA), and 125 without knee OA.
Overall, patients with AKOA were approximately seven times more likely than were patients with KOA to have destabilizing meniscal tears in two or more areas at the time of the index visit (42% vs. 14%); less than 5% of adults with no KOA experienced destabilizing meniscal tears. In addition, patients with AKOA were more than four times as likely to have miscellaneous pathology starting the year before the index visit, compared with those without AKOA.
Approximately 63% of the participants in each group were women, and the majority were overweight. The average age, weight, and global impact of arthritis were greater in the AKOA group when compared against the typical KOA and no-KOA groups.
Participants were assessed via MRI reviewed by radiologists who were blinded to the groups.
At the index visit, 49% of adults with AKOA had either a destabilizing meniscal tear or miscellaneous pathology, compared with 15% of adults with KOA and 6% of adults without KOA.
Adults with AKOA also showed significantly greater cartilage loss prior to the index visit in comparison with typical KOA patients, and AKOA patients had less cartilage in the medial and lateral tibia and medial femur, compared with adults who had typical KOA or no KOA after the index visit.
Adults who developed AKOA showed a significantly higher bone marrow lesion volume when compared against the typical KOA and no-KOA groups at 1 year prior to the index visit, and their bone marrow lesion volume increased on average 13 times more compared with typical KOA patients over the 2 years before the index visit, the researchers noted (2.00 mL vs. 0.15 mL, respectively).
“These findings add to the evidence that AKOA is different [from] the typically perceived archetype of slow-progressing osteoarthritis” with a unique risk profile, the researchers said.
The study findings were limited by several factors, including the relatively small sample size, uncertain timing of disease onset, a potentially limited definition of a destabilizing meniscal tear (defined as a root tear, radial tear, or complex tear, which almost always featured a radial component), a lack of a universal AKOA pathology, and some missing MRI data, the researchers noted. However, the results support previous studies suggesting a link between meniscal pathology and increased risk for AKOA, they said.
“It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (2 mm or more within 1 year) and greater abnormal bone loss/destruction,” they noted.
“Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model,” they added.
The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
SOURCE: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: One year before the knee OA index visit, more than 75% of patients with accelerated knee OA had meniscal damage in at least two regions.
Study details: The data come from 375 adults with typical knee OA, accelerated knee OA, or no knee OA in the longitudinal Osteoarthritis Initiative cohort study.
Disclosures: The study was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and private funding included Merck, Novartis, GlaxoSmithKline, and Pfizer. The researchers had no financial conflicts to disclose.
Source: Driban JB et al. Arthritis Rheumatol. 2018 Dec 28. doi: 10.1002/art.40826.
Anticoagulation shows promise in concurrent lupus nephritis, thrombotic microangiopathy
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
The use of anticoagulation in patients with comorbid lupus nephritis and thrombotic microangiopathy was linked with a greater rate of clinical response after 12 months of therapy, according to results from a study published in Annals of the Rheumatic Diseases.
“The purpose of this multicenter retrospective study was to analyze the impact of anticoagulation (vitamin K antagonists and/or heparins), in addition to conventional immunosuppression on kidney outcomes, according to the Kidney Disease: Improving Global Outcomes guidelines,” wrote first author Savino Sciascia, MD, PhD, of the University of Turin (Italy), along with his colleagues.
The researchers analyzed data from 97 patients with biopsy-confirmed lupus nephritis (LN) and thrombotic microangiopathy (TMA) who were diagnosed during 2007-2017. The entire cohort was administered standard immunosuppressive agents, including corticosteroids, cyclophosphamide, and mycophenolate, among others. After 12 months of therapy, the patients were assessed for degree of clinical response, measured using complete, partial, or no response to therapy.
“Sixty-one patients (62.9%) were [antiphospholipid antibody] positive and 37 (38.1%) of these patients received anticoagulation with a vitamin K antagonist and/or heparins,” the investigators wrote. “Mean duration of anticoagulation therapy after TMA and LN diagnosis was 7.7 months,” they added.
After statistical analysis, the researchers found that patients treated with anticoagulation therapy experienced a greater rate of clinical response, compared with those not treated. The investigators saw a complete response to therapy in 22 (59.5%) patients given anticoagulation, compared with 15 (25.0%) patients without anticoagulation. Partial treatment responses were comparable, occurring in 7 (18.9%) with anticoagulation and 15 (25.0%) without. Without anticoagulation, 30 (50%) had no response to therapy, compared with 8 (21.6%) patients given anticoagulation.
“When limiting the analysis to patients with antiphospholipid antibodies, we observed a rate of any response (either complete response [or] partial response) as high as 66% in patients receiving anticoagulant treatment compared with those receiving immunosuppression alone (34%),” they added.
The authors acknowledged a major limitation of the study was the short duration of follow-up, which limited the ability to evaluate relapse rate.
“Despite its limitations, this study represents the largest available multicenter cohort of real-life systemic lupus erythematosus patients,” said Dr. Sciascia and his colleagues. “The use of anticoagulation appeared protective and warrants further investigation as a therapeutic tool,” they concluded.
The authors reported no conflicts of interest, and no specific study funding was declared.
SOURCE: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: A greater proportion of patients given anticoagulation achieved partial or complete response (78.4%) versus those not given anticoagulation (50.0%).
Study details: A retrospective analysis of 97 patients with biopsy-confirmed lupus nephritis and thrombotic microangiopathy.
Disclosures: The authors reported no conflicts of interest, and no specific study funding was declared.
Source: Sciascia S et al. Ann Rheum Dis. 2018 Dec 14. doi: 10.1136/annrheumdis-2018-214559.