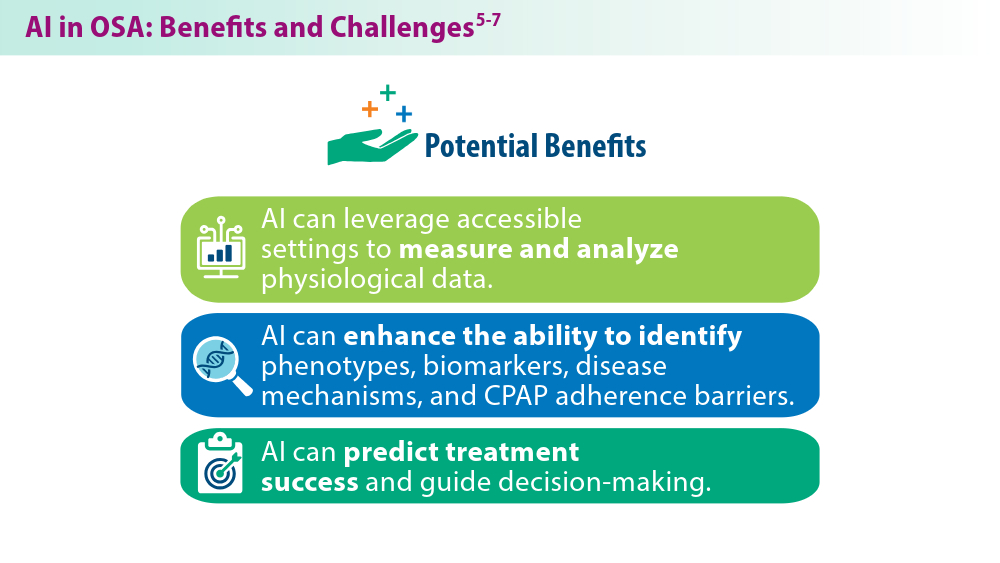
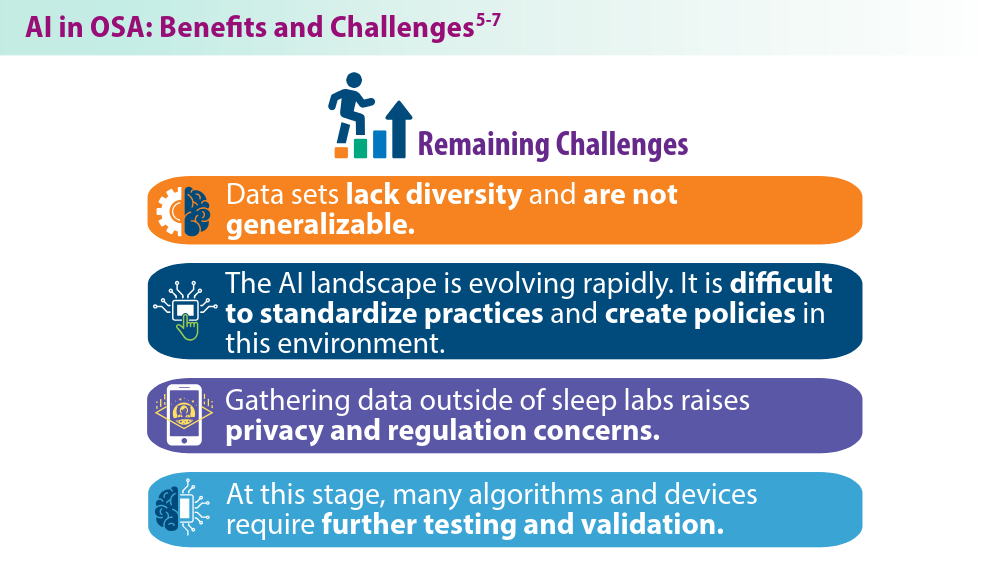
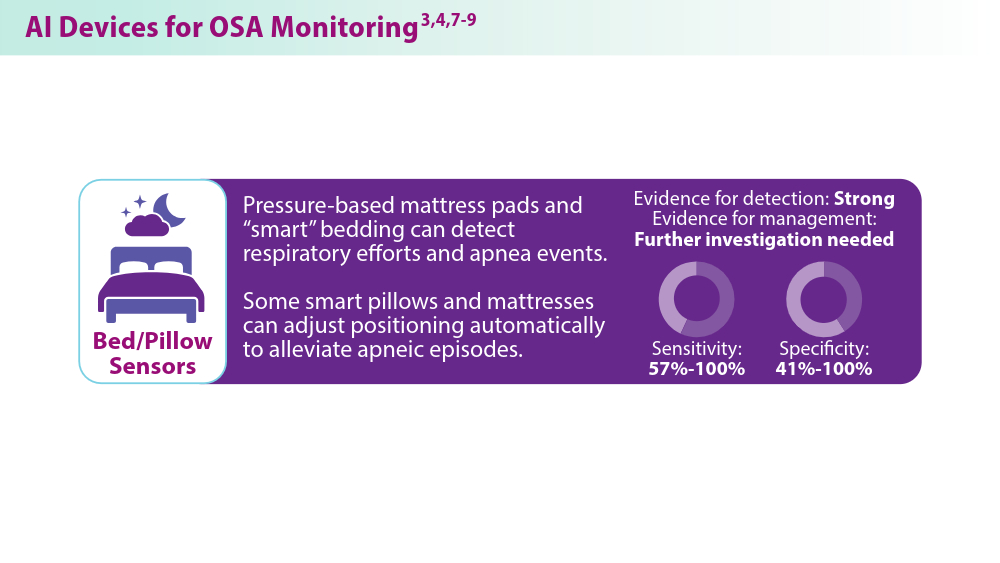
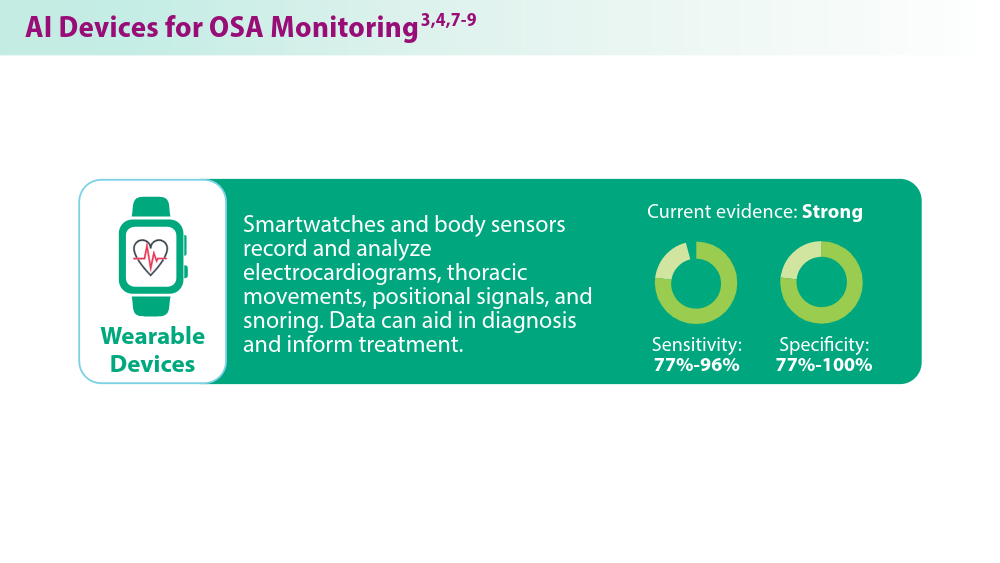
User login
Elinzanetant Shows Significant Improvement in Menopausal Vasomotor and Sleep Symptoms
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
CHICAGO — The nonhormonal investigational drug elinzanetant led to significant improvement in hot flashes as well as sleep disturbance and quality of life, according to data from three randomized controlled trials presented at The Menopause Society 2024 Annual Meeting in Chicago. Two phase 3 trials, OASIS 1 and 2, were also published in JAMA, and the longer-term OASIS 3 trial was presented as a poster at the conference.
Elinzanetant is a selective neurokinin (NK) receptor antagonist, similar to fezolinetant, the first drug in this class approved by the US Food and Drug Administration (FDA) for vasomotor symptoms in May 2023. This class of medications targets the estrogen-sensitive kisspeptin/NK B/dynorphin (KNDy) neurons thought to play a role in thermoregulation and hot flashes during menopause. While fezolinetant targets only the NK-3 receptor, elinzanetant is a dual NK receptor antagonist that targets both NK-1 and NK-3. Bayer submitted a New Drug Application for elinzanetant to the FDA on August 1.
For those in whom hormone therapy is contraindicated, “it’s always been difficult for women with really severe symptoms to have a safe and effective therapy,” lead author JoAnn Pinkerton, MD, a professor of ob.gyn. at the University of Virginia in Charlottesville, Virginia, told this news organization. “The nonhormonal therapies we’ve used mostly off-label — the antidepressants, gabapentin, clonidine, oxybutynin — do help the hot flashes, but they don’t work nearly as effectively as these new NK receptor antagonists, and having one that looks like it might have a broader use for hot flashes, night sweats, mood, and sleep is just really exciting.”
Dr. Pinkerton said approximately 80% of the women in the OASIS 1 and 2 studies had at least a 50% reduction in hot flashes. “It was a very strong, dramatic positive finding, but the improvements in sleep and mood have really encouraged us to go further,” she said.
Declining estrogen levels during and after menopause can cause hypertrophy and hyperactivity of the KNDy neurons, which has been linked to thermoregulation disruptions that may trigger hot flashes, James Simon, MD, a clinical professor of ob.gyn. at The George Washington University School of Medicine & Health Sciences and medical director of IntimMedicine in Washington, DC, told attendees. He presented pooled data from OASIS 1 and 2. The NK-1 receptor, targeted by elinzanetant but not fezolinetant, is also thought to play a role in insomnia and possibly in mood.
“Oftentimes the focus on a lot of these drugs is hot flashes, hot flashes, hot flashes, but we know hot flashes do not occur in isolation,” Chrisandra Shufelt, MD, professor and chair of general internal medicine and associate director of the Women’s Health Research Center at Mayo Clinic in Jacksonville, Florida, told this news organization. Elinzanetant is “an interesting compound because it actually works on sleep, and that was critical because sleep disturbance precedes” many other menopausal symptoms, said Dr. Shufelt, who was not involved in the study.
“I think it is an outstanding option for women who don’t have the opportunity to get hormones,” Dr. Shufelt said, and she was particularly pleased to see there were no safety concerns for the liver in the trial data. The FDA issued a warning on September 12 about the risk for rare liver injury with fezolinetant, but the early signals that had been seen in fezolinetant data were not seen in these elinzanetant data.
The OASIS 1 and 2 trials enrolled postmenopausal women, aged 40-65 years, who had at least 50 moderate to severe vasomotor occurrences per week.
“A moderate hot flash is a hot flash that is also associated with sweating, and a severe hot flash is a moderate hot flash that stops a woman in her tracks,” Dr. Simon said. “Namely, it’s severe enough with sweating and central nervous system effects that she is interrupted in whatever it is that she’s doing at the time.”
Exclusion criteria for the trials included a history of arrhythmias, heart block, or QT prolongation; abnormal lab results; history of malignancy within the past 5 years; uncontrolled or treatment-resistant hypertension, hypothyroidism, or hyperthyroidism; unexplained postmenopausal bleeding; clinically relevant abnormal mammogram findings; or disordered proliferative endometrium, endometrial hyperplasia, polyp, or endometrial cancer.
The predominantly White (80%) women were an average 54 years old, with an average body mass index (BMI) of 27.8, and were an average 3.5 years from their last period. For the first 12 weeks of the trials, 399 women were assigned to receive 120 mg once daily of oral elinzanetant and 397 were assigned to once daily placebo. Then the women taking placebo switched to elinzanetant for the final 14 weeks of the study.
The endpoints included mean change in frequency and severity of vasomotor symptoms at weeks 1, 4, and 12 as well as change in sleep disturbance and quality of life at week 12. Sleep was assessed with the Patient-Reported Outcomes Measurement Information System Sleep Disturbance–Short Form score, which ranges from 28.9 to 76.5, with a higher number denoting greater sleep disturbance. The Menopause-Specific Quality-of-Life score ranges from 1 to 8, with a higher score indicating poorer quality of life.
Daily frequency of vasomotor symptoms was 14 per day at baseline in the elinzanetant group, decreasing by 4.8 per day at week 1, 8 per day at week 4, and 9.4 per day at week 12. In the placebo group, women had an average 15.2 occurrences per day at baseline, which decreased by 3.2 at week 1, 5.2 at week 4, and 6.4 at week 12. Comparing the groups at 12 weeks, those receiving elinzanetant had 3.2 fewer daily vasomotor symptoms than those receiving placebo (P < .0001).
The severity of vasomotor symptoms also improved more in the elinzanetant group than in the placebo group over 12 weeks, after which severity improved further in those who switched from placebo to elinzanetant (P < .0001).
Sleep disturbance scores, starting at a mean 61.5 in the elinzanetant group and 60.5 in the placebo group, fell 10.7 points in the elinzanetant group and 5.3 points in the placebo group at 12 weeks, for a difference of 4.9 points (P < .0001). Sleep then further improved in those who switched from placebo to elinzanetant. Quality-of-life scores improved 1.37 points (from 4.52 at baseline) in the elinzanetant group and 0.96 points (from 4.49 at baseline) in the placebo group, for a mean difference at 12 weeks of 0.36 (P < .0001).
Though no head-to-head data exist comparing elinzanetant and fezolinetant, Dr. Simon told this news organization the side effects with fezolinetant “tend to be gastrointestinal, whereas the side effects for elinzanetant tend to be central nervous system,” such as drowsiness and lethargy.
The women who are the best candidates for elinzanetant, Dr. Pinkerton told this news organization, include those who have had an estrogen-sensitive cancer, such as breast or endometrial cancer, or who have fear of it, a family history, or are otherwise high risk. Other ideal candidates include those with a history of venous thromboembolism, people who have migraine with aura (due to concerns about increased risk for stroke), and those who have endometriosis or large fibroids.
“Then the last group might be women who took hormone therapy in their 50s and want to continue, but they’re trying to go off, and they have a recurrence of their hot flashes or night sweats or sleep issues,” Dr. Pinkerton said. “This might be a great group to switch over.”
OASIS 3 assessed the drug for 1 year and “supported the results of OASIS 1 and 2, demonstrating efficacy over a longer study duration and in a population with a vasomotor symptom profile representative of that seen in clinical practice,” Nick Panay, BSc, MBBS, director of the Menopause & PMS Centre at Queen Charlotte’s Hospital & Imperial College London, London, England, and his colleague reported.
Among 628 postmenopausal women aged 40-65, the predominantly White (78.5%) women were an average 54 years old, with an average BMI of 27.6, and were an average 5 years past their last period. Half received 120 mg elinzanetant and half received a placebo for 52 weeks.
At 12 weeks, the women receiving elinzanetant reported an average 1.6 moderate to severe vasomotor symptoms per day, down from 6.7 at baseline. Daily average symptoms in the placebo group fell from 6.8 at baseline to 3.4 at 12 weeks, for a difference of 1.6 fewer occurrences per day in the elinzanetant group (P < .0001).
Sleep disturbances also improved, falling 9.4 points from a baseline 57.4 in the elinzanetant group and 5.7 points from a baseline 58 in the placebo group. Quality-of-life scores improved from 4.1 to 2.8 (−1.3 change) in the elinzanetant group and from 4.4 to 3.3 (−1.1 change) in the placebo group.
In addition to looking at treatment-emergent adverse events, the safety assessments also included endometrial biopsies; bone mineral density in the femoral neck, hip, and lumbar spine; weight; and labs. Adverse events related to the study drug occurred in 30.4% of those in the elinzanetant group and 14.6% of those in the placebo group. The most commonly reported adverse events were headache (9.6% elinzanetant vs 7% placebo), fatigue (7% vs 10.2%), and sleepiness (5.1% vs 1.3%). A higher proportion of women taking elinzanetant (12.5%) than those taking placebo (4.1%) discontinued the study.
No serious adverse events deemed to be treatment-related occurred in either group, and no endometrial hyperplasia or malignant neoplasm occurred in either group. Bone mineral density changes in both groups were within the expected range for the women’s age, and their weight remained stable over the 52 weeks.
Six women taking elinzanetant and four taking placebo met predefined criteria for close liver observation, but none showed hepatotoxicity or evidence of possible drug-induced liver injury.
The research was funded by Bayer. Dr. Pinkerton has run a trial funded by Bayer and is a consultant for Bayer and Pfizer. Dr. Shufelt had no disclosures. Dr. Simon had grant/research support, consulting/advisory board participation, and/or speaking disclosures with AbbVie, Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies, Camargo Pharmaceutical Services, Covance, Daré Bioscience, DEKA M.E.L.A S.r.l., Femasys, Ipsen, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mayne Pharma, Mitsubishi Tanabe Pharma Development America, Mylan/Viatris Inc, Myovant Sciences, ObsEva SA, Pfizer, Pharmavite, QUE Oncology, Scynexis, Sebela Pharmaceuticals, Sprout Pharmaceuticals, TherapeuticsMD, Vella Bioscience, and Viveve Medical, and he is a stockholder in Sermonix Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Epilepsy Drug May Reduce Symptoms of OSA
An epilepsy drug sold in Europe as Ospolot and also known as sulthiame showed promise in reducing sleep disordered breathing and other symptoms of obstructive sleep apnea (OSA), based on data from nearly 300 individuals presented in a late-breaking study at the annual congress of the European Respiratory Society.
“Current therapies are mechanical and based on the notion of an airway splint,” presenting author Jan Hedner, MD, professor of respiratory medicine at Sahlgrenska University Hospital and the University of Gothenburg, both in Sweden, said in an interview. “In other words, applying an airflow at elevated pressure [continuous positive airway pressure] or advancing the jaw with a dental device. Adherence to this type of therapy is limited. In the case of continuous positive airway pressure [CPAP], it is < 50% after 3-4 years of therapy.” Therefore, there is a need for a better-tolerated therapy, such as a drug, and possibly a combination of mechanical and pharmaceutical therapies.
The use of medication has emerged as a viable option for OSA, with a high rate of compliance and acceptable safety profile, Dr. Hedner said in his presentation.
“Modified carbonic anhydrase activity may be a pathophysiological mechanism in OSA,” said Dr. Hedner. Sulthiame, a carbonic anhydrase inhibitor, showed safety and effectiveness for improving OSA in a previous phase 2b trial.
In the current study, the researchers sought to determine the most effective dose of sulthiame for patients with OSA. They randomized 298 adults with OSA who could not accept or tolerate oral splints or CPAP to 100 mg, 200 mg, or 300 mg of sulthiame daily (74, 74, and 75 patients, respectively) or placebo (75 patients).
The mean age of the patients was 56 years, 26.2% were women, and the average apnea-hypopnea index (AHI3a) at baseline was 29 n/h. Patients were treated at centers in Spain, France, Belgium, Germany, and the Czech Republic. Baseline demographics and clinical characteristics were similar among the treatment groups.
The primary endpoint was the change in AHI3a from baseline to 15 weeks, and significant changes occurred in patients who received the 100-mg, 200-mg, and 300-mg doses, with decreases of 17.8%, 34.8%, and 39.9%, respectively.
Dr. Hedner said in his presentation.
Notably, in a post hoc analysis, apnea improved by 47.1% at a 300-mg dose when the AHI4 measure (apnea/hypopnea with ≥ 4% O2 desaturation) was used in a placebo-adjusted dose-dependent reduction, the researchers wrote. The changes in AHI4 from baseline in this analysis also were significant for 200 mg and 100 mg doses (36.8% and 26.2%, respectively).
Patients underwent polysomnography at baseline and at weeks 4 and 12.
Mean overnight oxygen saturation also improved significantly from baseline with doses of 200 mg and 300 mg, compared with placebo (P < .0001 for both).
In addition, scores on the Epworth Sleepiness Scale (ESS) improved from baseline to week 15 in all dosage groups, and the subgroup of patients with ESS scores of ≥ 11 at baseline showed even greater improvement in ESS, Dr. Hedner said in his presentation.
Total arousal index and sleep quality also improved from baseline compared with placebo, and no clinically relevant reduction in REM sleep was noted, Dr. Hedner added.
Treatment-emergent adverse events were in line with the known safety profile of sulthiame and included paresthesia, headache, fatigue, and nausea; these were mainly moderate and dose-dependent, with no evidence of cardiovascular safety issues, he said.
Although the study results were not surprising given previous research, the investigators were pleased with the potency of the therapy. “We are also happy about potential added values such as a blood pressure lowering effect, which is beneficial in this group of patients; however, we need to further study these mechanisms in detail,” Dr. Hedner noted.
The study findings were limited by the relatively small scale, and larger studies on long-term efficacy and tolerability are also needed, he said.
“The current study was a dose-finding study, and we now have useful information on most suitable dose,” he said.
However, the results support sulthiame as an effective, well-tolerated, and promising novel candidate for drug therapy in patients with OSA, worthy of phase 3 studies, Dr. Hedner said.
Oral Option Could Be Game-Changer, But Not Yet
The gold standard of treatment for OSA is a CPAP machine, but the effectiveness is limited by patient tolerance, Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, said in an interview.
“Presently, there are no effective pharmacological treatments for OSA — having a pill that treats OSA would be a total game changer and huge advance for the treatment of OSA and the field of sleep medicine,” said Dr. Shamim-Uzzaman, who was not involved in the study. “More patients may be able to obtain treatment for OSA and thereby reduce the potential complications of untreated OSA.
“Carbonic anhydrase inhibitors such as acetazolamide and sulthiame have been studied with limited success for the treatment of other forms of sleep disordered breathing such as central sleep apnea [CSA] but have shown less efficacy for OSA and are presently not recommended in the treatment of OSA by the American Academy of Sleep Medicine,” Dr. Shamim-Uzzaman said.
Recently, emerging evidence about different phenotypes of OSA suggests that nonanatomic features (such as high loop gain) may play a role in patients with OSA, not only in those with CSA, she said. Whether carbonic anhydrase inhibitors could play a greater role in treating sleep apnea in patients with predominantly nonanatomic pathophysiologic traits remains to be seen.
The sulthiame data are promising, but more research is needed, Dr. Shamim-Uzzaman said. Although patients in the highest dose group showed a reduction in AHI of nearly 40%, they still would have moderate OSA, and the OSA did not appear to decrease to a normal range in any of the treatment groups.
“More research is needed to identify which types of patients would be responders to this form of therapy, to understand if these effects are maintained long term (beyond 15 weeks), to evaluate patient-centered outcomes, especially in different sleep apnea subgroups (such as phenotypes with high loop gain vs those without), and to assess interactions with other therapies,” she said.
The study was supported by manufacturer Desitin. Dr. Hedner disclosed serving as a consultant to AstraZeneca, Bayer, CereusScience, Jazz Pharmaceuticals, MSD, Weinmann, Desitin, SomnoMed, and Itamar Medical; serving on the speakers’ bureau for Almirall, AstraZeneca, Jazz Pharmaceuticals, ResMed, Philips Respironics, and Weinmann; and receiving grants or research support from Bayer, ResMed, Philips Respironics, and SomnoMed. He also disclosed shared ownership of intellectual property related to sleep apnea therapy. Dr. Shamim-Uzzaman had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
An epilepsy drug sold in Europe as Ospolot and also known as sulthiame showed promise in reducing sleep disordered breathing and other symptoms of obstructive sleep apnea (OSA), based on data from nearly 300 individuals presented in a late-breaking study at the annual congress of the European Respiratory Society.
“Current therapies are mechanical and based on the notion of an airway splint,” presenting author Jan Hedner, MD, professor of respiratory medicine at Sahlgrenska University Hospital and the University of Gothenburg, both in Sweden, said in an interview. “In other words, applying an airflow at elevated pressure [continuous positive airway pressure] or advancing the jaw with a dental device. Adherence to this type of therapy is limited. In the case of continuous positive airway pressure [CPAP], it is < 50% after 3-4 years of therapy.” Therefore, there is a need for a better-tolerated therapy, such as a drug, and possibly a combination of mechanical and pharmaceutical therapies.
The use of medication has emerged as a viable option for OSA, with a high rate of compliance and acceptable safety profile, Dr. Hedner said in his presentation.
“Modified carbonic anhydrase activity may be a pathophysiological mechanism in OSA,” said Dr. Hedner. Sulthiame, a carbonic anhydrase inhibitor, showed safety and effectiveness for improving OSA in a previous phase 2b trial.
In the current study, the researchers sought to determine the most effective dose of sulthiame for patients with OSA. They randomized 298 adults with OSA who could not accept or tolerate oral splints or CPAP to 100 mg, 200 mg, or 300 mg of sulthiame daily (74, 74, and 75 patients, respectively) or placebo (75 patients).
The mean age of the patients was 56 years, 26.2% were women, and the average apnea-hypopnea index (AHI3a) at baseline was 29 n/h. Patients were treated at centers in Spain, France, Belgium, Germany, and the Czech Republic. Baseline demographics and clinical characteristics were similar among the treatment groups.
The primary endpoint was the change in AHI3a from baseline to 15 weeks, and significant changes occurred in patients who received the 100-mg, 200-mg, and 300-mg doses, with decreases of 17.8%, 34.8%, and 39.9%, respectively.
Dr. Hedner said in his presentation.
Notably, in a post hoc analysis, apnea improved by 47.1% at a 300-mg dose when the AHI4 measure (apnea/hypopnea with ≥ 4% O2 desaturation) was used in a placebo-adjusted dose-dependent reduction, the researchers wrote. The changes in AHI4 from baseline in this analysis also were significant for 200 mg and 100 mg doses (36.8% and 26.2%, respectively).
Patients underwent polysomnography at baseline and at weeks 4 and 12.
Mean overnight oxygen saturation also improved significantly from baseline with doses of 200 mg and 300 mg, compared with placebo (P < .0001 for both).
In addition, scores on the Epworth Sleepiness Scale (ESS) improved from baseline to week 15 in all dosage groups, and the subgroup of patients with ESS scores of ≥ 11 at baseline showed even greater improvement in ESS, Dr. Hedner said in his presentation.
Total arousal index and sleep quality also improved from baseline compared with placebo, and no clinically relevant reduction in REM sleep was noted, Dr. Hedner added.
Treatment-emergent adverse events were in line with the known safety profile of sulthiame and included paresthesia, headache, fatigue, and nausea; these were mainly moderate and dose-dependent, with no evidence of cardiovascular safety issues, he said.
Although the study results were not surprising given previous research, the investigators were pleased with the potency of the therapy. “We are also happy about potential added values such as a blood pressure lowering effect, which is beneficial in this group of patients; however, we need to further study these mechanisms in detail,” Dr. Hedner noted.
The study findings were limited by the relatively small scale, and larger studies on long-term efficacy and tolerability are also needed, he said.
“The current study was a dose-finding study, and we now have useful information on most suitable dose,” he said.
However, the results support sulthiame as an effective, well-tolerated, and promising novel candidate for drug therapy in patients with OSA, worthy of phase 3 studies, Dr. Hedner said.
Oral Option Could Be Game-Changer, But Not Yet
The gold standard of treatment for OSA is a CPAP machine, but the effectiveness is limited by patient tolerance, Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, said in an interview.
“Presently, there are no effective pharmacological treatments for OSA — having a pill that treats OSA would be a total game changer and huge advance for the treatment of OSA and the field of sleep medicine,” said Dr. Shamim-Uzzaman, who was not involved in the study. “More patients may be able to obtain treatment for OSA and thereby reduce the potential complications of untreated OSA.
“Carbonic anhydrase inhibitors such as acetazolamide and sulthiame have been studied with limited success for the treatment of other forms of sleep disordered breathing such as central sleep apnea [CSA] but have shown less efficacy for OSA and are presently not recommended in the treatment of OSA by the American Academy of Sleep Medicine,” Dr. Shamim-Uzzaman said.
Recently, emerging evidence about different phenotypes of OSA suggests that nonanatomic features (such as high loop gain) may play a role in patients with OSA, not only in those with CSA, she said. Whether carbonic anhydrase inhibitors could play a greater role in treating sleep apnea in patients with predominantly nonanatomic pathophysiologic traits remains to be seen.
The sulthiame data are promising, but more research is needed, Dr. Shamim-Uzzaman said. Although patients in the highest dose group showed a reduction in AHI of nearly 40%, they still would have moderate OSA, and the OSA did not appear to decrease to a normal range in any of the treatment groups.
“More research is needed to identify which types of patients would be responders to this form of therapy, to understand if these effects are maintained long term (beyond 15 weeks), to evaluate patient-centered outcomes, especially in different sleep apnea subgroups (such as phenotypes with high loop gain vs those without), and to assess interactions with other therapies,” she said.
The study was supported by manufacturer Desitin. Dr. Hedner disclosed serving as a consultant to AstraZeneca, Bayer, CereusScience, Jazz Pharmaceuticals, MSD, Weinmann, Desitin, SomnoMed, and Itamar Medical; serving on the speakers’ bureau for Almirall, AstraZeneca, Jazz Pharmaceuticals, ResMed, Philips Respironics, and Weinmann; and receiving grants or research support from Bayer, ResMed, Philips Respironics, and SomnoMed. He also disclosed shared ownership of intellectual property related to sleep apnea therapy. Dr. Shamim-Uzzaman had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
An epilepsy drug sold in Europe as Ospolot and also known as sulthiame showed promise in reducing sleep disordered breathing and other symptoms of obstructive sleep apnea (OSA), based on data from nearly 300 individuals presented in a late-breaking study at the annual congress of the European Respiratory Society.
“Current therapies are mechanical and based on the notion of an airway splint,” presenting author Jan Hedner, MD, professor of respiratory medicine at Sahlgrenska University Hospital and the University of Gothenburg, both in Sweden, said in an interview. “In other words, applying an airflow at elevated pressure [continuous positive airway pressure] or advancing the jaw with a dental device. Adherence to this type of therapy is limited. In the case of continuous positive airway pressure [CPAP], it is < 50% after 3-4 years of therapy.” Therefore, there is a need for a better-tolerated therapy, such as a drug, and possibly a combination of mechanical and pharmaceutical therapies.
The use of medication has emerged as a viable option for OSA, with a high rate of compliance and acceptable safety profile, Dr. Hedner said in his presentation.
“Modified carbonic anhydrase activity may be a pathophysiological mechanism in OSA,” said Dr. Hedner. Sulthiame, a carbonic anhydrase inhibitor, showed safety and effectiveness for improving OSA in a previous phase 2b trial.
In the current study, the researchers sought to determine the most effective dose of sulthiame for patients with OSA. They randomized 298 adults with OSA who could not accept or tolerate oral splints or CPAP to 100 mg, 200 mg, or 300 mg of sulthiame daily (74, 74, and 75 patients, respectively) or placebo (75 patients).
The mean age of the patients was 56 years, 26.2% were women, and the average apnea-hypopnea index (AHI3a) at baseline was 29 n/h. Patients were treated at centers in Spain, France, Belgium, Germany, and the Czech Republic. Baseline demographics and clinical characteristics were similar among the treatment groups.
The primary endpoint was the change in AHI3a from baseline to 15 weeks, and significant changes occurred in patients who received the 100-mg, 200-mg, and 300-mg doses, with decreases of 17.8%, 34.8%, and 39.9%, respectively.
Dr. Hedner said in his presentation.
Notably, in a post hoc analysis, apnea improved by 47.1% at a 300-mg dose when the AHI4 measure (apnea/hypopnea with ≥ 4% O2 desaturation) was used in a placebo-adjusted dose-dependent reduction, the researchers wrote. The changes in AHI4 from baseline in this analysis also were significant for 200 mg and 100 mg doses (36.8% and 26.2%, respectively).
Patients underwent polysomnography at baseline and at weeks 4 and 12.
Mean overnight oxygen saturation also improved significantly from baseline with doses of 200 mg and 300 mg, compared with placebo (P < .0001 for both).
In addition, scores on the Epworth Sleepiness Scale (ESS) improved from baseline to week 15 in all dosage groups, and the subgroup of patients with ESS scores of ≥ 11 at baseline showed even greater improvement in ESS, Dr. Hedner said in his presentation.
Total arousal index and sleep quality also improved from baseline compared with placebo, and no clinically relevant reduction in REM sleep was noted, Dr. Hedner added.
Treatment-emergent adverse events were in line with the known safety profile of sulthiame and included paresthesia, headache, fatigue, and nausea; these were mainly moderate and dose-dependent, with no evidence of cardiovascular safety issues, he said.
Although the study results were not surprising given previous research, the investigators were pleased with the potency of the therapy. “We are also happy about potential added values such as a blood pressure lowering effect, which is beneficial in this group of patients; however, we need to further study these mechanisms in detail,” Dr. Hedner noted.
The study findings were limited by the relatively small scale, and larger studies on long-term efficacy and tolerability are also needed, he said.
“The current study was a dose-finding study, and we now have useful information on most suitable dose,” he said.
However, the results support sulthiame as an effective, well-tolerated, and promising novel candidate for drug therapy in patients with OSA, worthy of phase 3 studies, Dr. Hedner said.
Oral Option Could Be Game-Changer, But Not Yet
The gold standard of treatment for OSA is a CPAP machine, but the effectiveness is limited by patient tolerance, Q. Afifa Shamim-Uzzaman, MD, an associate professor and a sleep medicine specialist at the University of Michigan, Ann Arbor, said in an interview.
“Presently, there are no effective pharmacological treatments for OSA — having a pill that treats OSA would be a total game changer and huge advance for the treatment of OSA and the field of sleep medicine,” said Dr. Shamim-Uzzaman, who was not involved in the study. “More patients may be able to obtain treatment for OSA and thereby reduce the potential complications of untreated OSA.
“Carbonic anhydrase inhibitors such as acetazolamide and sulthiame have been studied with limited success for the treatment of other forms of sleep disordered breathing such as central sleep apnea [CSA] but have shown less efficacy for OSA and are presently not recommended in the treatment of OSA by the American Academy of Sleep Medicine,” Dr. Shamim-Uzzaman said.
Recently, emerging evidence about different phenotypes of OSA suggests that nonanatomic features (such as high loop gain) may play a role in patients with OSA, not only in those with CSA, she said. Whether carbonic anhydrase inhibitors could play a greater role in treating sleep apnea in patients with predominantly nonanatomic pathophysiologic traits remains to be seen.
The sulthiame data are promising, but more research is needed, Dr. Shamim-Uzzaman said. Although patients in the highest dose group showed a reduction in AHI of nearly 40%, they still would have moderate OSA, and the OSA did not appear to decrease to a normal range in any of the treatment groups.
“More research is needed to identify which types of patients would be responders to this form of therapy, to understand if these effects are maintained long term (beyond 15 weeks), to evaluate patient-centered outcomes, especially in different sleep apnea subgroups (such as phenotypes with high loop gain vs those without), and to assess interactions with other therapies,” she said.
The study was supported by manufacturer Desitin. Dr. Hedner disclosed serving as a consultant to AstraZeneca, Bayer, CereusScience, Jazz Pharmaceuticals, MSD, Weinmann, Desitin, SomnoMed, and Itamar Medical; serving on the speakers’ bureau for Almirall, AstraZeneca, Jazz Pharmaceuticals, ResMed, Philips Respironics, and Weinmann; and receiving grants or research support from Bayer, ResMed, Philips Respironics, and SomnoMed. He also disclosed shared ownership of intellectual property related to sleep apnea therapy. Dr. Shamim-Uzzaman had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ERS CONGRESS 2024
Night Owls May Be at Greater Risk for T2D, Beyond Lifestyle
MADRID — research presented at the annual meeting of the European Association for the Study of Diabetes suggested.
In the study, night owls were almost 50% more likely to develop T2D than those who went to sleep earlier.
“The magnitude of this risk was more than I expected, [although] residual confounding may have occurred,” said Jeroen van der Velde, PhD, Leiden University Medical Center in the Netherlands, who presented the study.
“Late chronotype has previously been associated with unhealthy lifestyle and overweight or obesity and, subsequently, cardiometabolic diseases,” he said in an interview. However, although the current study found that individuals with late chronotypes did indeed have larger waists and more visceral fat, “we (and others) believe that lifestyle cannot fully explain the relation between late chronotype and metabolic disorders.”
“In addition,” he noted, “previous studies that observed that late chronotype is associated with overweight or obesity mainly focused on body mass index [BMI]. However, BMI alone does not provide accurate information regarding fat distribution in the body. People with similar BMI may have different underlying fat distribution, and this may be more relevant than BMI for metabolic risk.”
The researchers examined associations between chronotype and BMI, waist circumference, visceral fat, liver fat, and the risk for T2D in a middle-aged population from the Netherlands Epidemiology of Obesity study. Among the 5026 participants, the mean age was 56 years, 54% were women, and mean BMI was 30.
Using data from the study, the study investigators calculated the midpoint of sleep (MPS) and divided participants into three chronotypes: Early MPS < 2:30 PM (20% of participants); intermediate MPS 2:30–4:00 PM (reference category; 60% of participants); and late MPS ≥ 4:00 PM (20% of participants). BMI and waist circumference were measured in all participants, and visceral fat and liver fat were measured in 1576 participants using MRI scans and MR spectroscopy, respectively.
During a median follow-up of 6.6 years, 225 participants were diagnosed with T2D. After adjustment for age, sex, education, physical activity, smoking, alcohol intake, diet quality, sleep quality and duration, and total body fat, participants with a late chronotype had a 46% increased risk for T2D.
Further, those with a late chronotype had 0.7 higher BMI, 1.9-cm larger waist circumference, 7 cm2 more visceral fat, and 14% more liver fat.
Body Clock Out of Sync?
“Late chronotype was associated with increased ectopic body fat and with an increased risk of T2D independent of lifestyle factors and is an emerging risk factor for metabolic diseases,” the researchers concluded.
“A likely explanation is that the circadian rhythm or body clock in late chronotypes is out of sync with the work and social schedules followed by society,” Dr. van der Velde suggested. “This can lead to circadian misalignment, which we know can lead to metabolic disturbances and ultimately type 2 diabetes.”
Might trying to adjust chronotype earlier in life have an effect on risk?
“Chronotype, as measured via midpoint of sleep, does change a lot in the first 30 years or so in life,” he said. “After that it seems to stabilize. I suppose that if you adapt an intermediate or early chronotype around the age of 30 years, this will help to maintain an earlier chronotype later in life, although we cannot answer this from our study.”
Nevertheless, with respect to T2D risk, “chronotype is likely only part of the puzzle,” he noted.
“People with late chronotypes typically eat late in the evening, and this has also been associated with adverse metabolic effects. At this stage, we do not know if a person changes his/her chronotype that this will also lead to metabolic improvements. More research is needed before we can make recommendations regarding chronotype and timing of other lifestyle behaviors.”
Commenting on the study, Gianluca Iacobellis, MD, PhD, director of the University of Miami Hospital Diabetes Service, Coral Gables, Florida, said: “Interesting data. Altering the physiological circadian rhythm can affect the complex hormonal system — including cortisol, ghrelin, leptin, and serotonin — that regulates insulin sensitivity, glucose, and blood pressure control. The night owl may become more insulin resistant and therefore at higher risk of developing diabetes.”
Like Dr. van der Velde, he noted that “late sleep may be associated with night binging that can cause weight gain and ultimately obesity, further increasing the risk of diabetes.”
Dr. Iacobellis’s group recently showed that vital exhaustion, which is characterized by fatigue and loss of vigor, is associated with a higher cardiovascular risk for and markers of visceral adiposity.
“Abnormal circadian rhythms can be easily associated with vital exhaustion,” he said. Therefore, night owls with more visceral than peripheral fat accumulation might also be at higher cardiometabolic risk through that mechanism.
“However environmental factors and family history can play an important role too,” he added.
Regardless of the mechanisms involved, “preventive actions should be taken to educate teenagers and individuals at higher risk to have healthy sleep habits,” Dr. Iacobellis concluded.
No information regarding funding was provided; Dr. van der Velde and Dr. Iacobellis reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
MADRID — research presented at the annual meeting of the European Association for the Study of Diabetes suggested.
In the study, night owls were almost 50% more likely to develop T2D than those who went to sleep earlier.
“The magnitude of this risk was more than I expected, [although] residual confounding may have occurred,” said Jeroen van der Velde, PhD, Leiden University Medical Center in the Netherlands, who presented the study.
“Late chronotype has previously been associated with unhealthy lifestyle and overweight or obesity and, subsequently, cardiometabolic diseases,” he said in an interview. However, although the current study found that individuals with late chronotypes did indeed have larger waists and more visceral fat, “we (and others) believe that lifestyle cannot fully explain the relation between late chronotype and metabolic disorders.”
“In addition,” he noted, “previous studies that observed that late chronotype is associated with overweight or obesity mainly focused on body mass index [BMI]. However, BMI alone does not provide accurate information regarding fat distribution in the body. People with similar BMI may have different underlying fat distribution, and this may be more relevant than BMI for metabolic risk.”
The researchers examined associations between chronotype and BMI, waist circumference, visceral fat, liver fat, and the risk for T2D in a middle-aged population from the Netherlands Epidemiology of Obesity study. Among the 5026 participants, the mean age was 56 years, 54% were women, and mean BMI was 30.
Using data from the study, the study investigators calculated the midpoint of sleep (MPS) and divided participants into three chronotypes: Early MPS < 2:30 PM (20% of participants); intermediate MPS 2:30–4:00 PM (reference category; 60% of participants); and late MPS ≥ 4:00 PM (20% of participants). BMI and waist circumference were measured in all participants, and visceral fat and liver fat were measured in 1576 participants using MRI scans and MR spectroscopy, respectively.
During a median follow-up of 6.6 years, 225 participants were diagnosed with T2D. After adjustment for age, sex, education, physical activity, smoking, alcohol intake, diet quality, sleep quality and duration, and total body fat, participants with a late chronotype had a 46% increased risk for T2D.
Further, those with a late chronotype had 0.7 higher BMI, 1.9-cm larger waist circumference, 7 cm2 more visceral fat, and 14% more liver fat.
Body Clock Out of Sync?
“Late chronotype was associated with increased ectopic body fat and with an increased risk of T2D independent of lifestyle factors and is an emerging risk factor for metabolic diseases,” the researchers concluded.
“A likely explanation is that the circadian rhythm or body clock in late chronotypes is out of sync with the work and social schedules followed by society,” Dr. van der Velde suggested. “This can lead to circadian misalignment, which we know can lead to metabolic disturbances and ultimately type 2 diabetes.”
Might trying to adjust chronotype earlier in life have an effect on risk?
“Chronotype, as measured via midpoint of sleep, does change a lot in the first 30 years or so in life,” he said. “After that it seems to stabilize. I suppose that if you adapt an intermediate or early chronotype around the age of 30 years, this will help to maintain an earlier chronotype later in life, although we cannot answer this from our study.”
Nevertheless, with respect to T2D risk, “chronotype is likely only part of the puzzle,” he noted.
“People with late chronotypes typically eat late in the evening, and this has also been associated with adverse metabolic effects. At this stage, we do not know if a person changes his/her chronotype that this will also lead to metabolic improvements. More research is needed before we can make recommendations regarding chronotype and timing of other lifestyle behaviors.”
Commenting on the study, Gianluca Iacobellis, MD, PhD, director of the University of Miami Hospital Diabetes Service, Coral Gables, Florida, said: “Interesting data. Altering the physiological circadian rhythm can affect the complex hormonal system — including cortisol, ghrelin, leptin, and serotonin — that regulates insulin sensitivity, glucose, and blood pressure control. The night owl may become more insulin resistant and therefore at higher risk of developing diabetes.”
Like Dr. van der Velde, he noted that “late sleep may be associated with night binging that can cause weight gain and ultimately obesity, further increasing the risk of diabetes.”
Dr. Iacobellis’s group recently showed that vital exhaustion, which is characterized by fatigue and loss of vigor, is associated with a higher cardiovascular risk for and markers of visceral adiposity.
“Abnormal circadian rhythms can be easily associated with vital exhaustion,” he said. Therefore, night owls with more visceral than peripheral fat accumulation might also be at higher cardiometabolic risk through that mechanism.
“However environmental factors and family history can play an important role too,” he added.
Regardless of the mechanisms involved, “preventive actions should be taken to educate teenagers and individuals at higher risk to have healthy sleep habits,” Dr. Iacobellis concluded.
No information regarding funding was provided; Dr. van der Velde and Dr. Iacobellis reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
MADRID — research presented at the annual meeting of the European Association for the Study of Diabetes suggested.
In the study, night owls were almost 50% more likely to develop T2D than those who went to sleep earlier.
“The magnitude of this risk was more than I expected, [although] residual confounding may have occurred,” said Jeroen van der Velde, PhD, Leiden University Medical Center in the Netherlands, who presented the study.
“Late chronotype has previously been associated with unhealthy lifestyle and overweight or obesity and, subsequently, cardiometabolic diseases,” he said in an interview. However, although the current study found that individuals with late chronotypes did indeed have larger waists and more visceral fat, “we (and others) believe that lifestyle cannot fully explain the relation between late chronotype and metabolic disorders.”
“In addition,” he noted, “previous studies that observed that late chronotype is associated with overweight or obesity mainly focused on body mass index [BMI]. However, BMI alone does not provide accurate information regarding fat distribution in the body. People with similar BMI may have different underlying fat distribution, and this may be more relevant than BMI for metabolic risk.”
The researchers examined associations between chronotype and BMI, waist circumference, visceral fat, liver fat, and the risk for T2D in a middle-aged population from the Netherlands Epidemiology of Obesity study. Among the 5026 participants, the mean age was 56 years, 54% were women, and mean BMI was 30.
Using data from the study, the study investigators calculated the midpoint of sleep (MPS) and divided participants into three chronotypes: Early MPS < 2:30 PM (20% of participants); intermediate MPS 2:30–4:00 PM (reference category; 60% of participants); and late MPS ≥ 4:00 PM (20% of participants). BMI and waist circumference were measured in all participants, and visceral fat and liver fat were measured in 1576 participants using MRI scans and MR spectroscopy, respectively.
During a median follow-up of 6.6 years, 225 participants were diagnosed with T2D. After adjustment for age, sex, education, physical activity, smoking, alcohol intake, diet quality, sleep quality and duration, and total body fat, participants with a late chronotype had a 46% increased risk for T2D.
Further, those with a late chronotype had 0.7 higher BMI, 1.9-cm larger waist circumference, 7 cm2 more visceral fat, and 14% more liver fat.
Body Clock Out of Sync?
“Late chronotype was associated with increased ectopic body fat and with an increased risk of T2D independent of lifestyle factors and is an emerging risk factor for metabolic diseases,” the researchers concluded.
“A likely explanation is that the circadian rhythm or body clock in late chronotypes is out of sync with the work and social schedules followed by society,” Dr. van der Velde suggested. “This can lead to circadian misalignment, which we know can lead to metabolic disturbances and ultimately type 2 diabetes.”
Might trying to adjust chronotype earlier in life have an effect on risk?
“Chronotype, as measured via midpoint of sleep, does change a lot in the first 30 years or so in life,” he said. “After that it seems to stabilize. I suppose that if you adapt an intermediate or early chronotype around the age of 30 years, this will help to maintain an earlier chronotype later in life, although we cannot answer this from our study.”
Nevertheless, with respect to T2D risk, “chronotype is likely only part of the puzzle,” he noted.
“People with late chronotypes typically eat late in the evening, and this has also been associated with adverse metabolic effects. At this stage, we do not know if a person changes his/her chronotype that this will also lead to metabolic improvements. More research is needed before we can make recommendations regarding chronotype and timing of other lifestyle behaviors.”
Commenting on the study, Gianluca Iacobellis, MD, PhD, director of the University of Miami Hospital Diabetes Service, Coral Gables, Florida, said: “Interesting data. Altering the physiological circadian rhythm can affect the complex hormonal system — including cortisol, ghrelin, leptin, and serotonin — that regulates insulin sensitivity, glucose, and blood pressure control. The night owl may become more insulin resistant and therefore at higher risk of developing diabetes.”
Like Dr. van der Velde, he noted that “late sleep may be associated with night binging that can cause weight gain and ultimately obesity, further increasing the risk of diabetes.”
Dr. Iacobellis’s group recently showed that vital exhaustion, which is characterized by fatigue and loss of vigor, is associated with a higher cardiovascular risk for and markers of visceral adiposity.
“Abnormal circadian rhythms can be easily associated with vital exhaustion,” he said. Therefore, night owls with more visceral than peripheral fat accumulation might also be at higher cardiometabolic risk through that mechanism.
“However environmental factors and family history can play an important role too,” he added.
Regardless of the mechanisms involved, “preventive actions should be taken to educate teenagers and individuals at higher risk to have healthy sleep habits,” Dr. Iacobellis concluded.
No information regarding funding was provided; Dr. van der Velde and Dr. Iacobellis reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Cell Phone Use Linked to Higher Heart Disease Risk
“We found that a poor sleep pattern, psychological distress, and neuroticism significantly mediated the positive association between weekly mobile phone usage time and the risk for incident CVD, with a mediating proportion of 5.11%, 11.50%, and 2.25%, respectively,” said principal investigator Xianhui Qin, MD, professor of nephrology at Southern Medical University, Guangzhou, China.
Poor sleep patterns and poor mental health could disrupt circadian rhythms and endocrine and metabolic functions, as well as increase inflammation, he explained.
In addition, chronic exposure to radiofrequency electromagnetic fields (RF-EMF) emitted from cell phones could lead to oxidative stress and an inflammatory response. Combined with smoking and diabetes, this exposure “may have a synergistic effect in increasing CVD risk,” Dr. Qin suggested.
The study was published online in the Canadian Journal of Cardiology.
Risk Underestimated?
The researchers aimed to examine the association of regular cell phone use with incident CVD and explore the mediating effects of sleep and mental health using linked hospital and mortality records.
Their analysis included 444,027 participants (mean age, 56 years; 44% men) without a history of CVD from the UK Biobank. A total of 378,161 participants were regular cell phone users.
Regular cell phone use was defined as at least one call per week. Weekly use was self-reported as the average time of calls per week during the previous 3 months.
The primary outcome was incident CVD. Secondary outcomes were each component of CVD (ie, coronary heart disease, stroke, atrial fibrillation, and heart failure) and increased carotid intima media thickness (CIMT).
Compared with nonregular cell phone users, regular users were younger, had higher proportions of current smokers and urban residents, and had lower proportions of history of hypertension and diabetes. They also had higher income, Townsend deprivation index, and body mass index, and lower education levels.
During a median follow-up of 12.3 years, 56,181 participants developed incident CVD. Compared with nonregular cell phone users, regular users had a significantly higher risk for incident CVD (hazard ratio, 1.04) and increased CIMT (odds ratio, 1.11).
Among regular cell phone users, the duration of cell phone use and hands-free device/speakerphone use during calls was not significantly associated with incident CVD. Yet a significant and positive dose-response relationship was seen between weekly cell phone usage time and the risk for CVD. The positive association was stronger in current vs noncurrent smokers and people with vs without diabetes.
To different extents, sleep patterns (5.11%), psychologic distress (11.5%), and neuroticism (2.25%) mediated the relationship between weekly cell phone usage time and the risk for incident CVD.
“Our study suggests that despite the advantages of mobile phone use, we should also pay attention to the potential harm of mobile phone use to cardiovascular health,” Dr. Qin said. “Future studies to assess the risk-benefit balance will help promote mobile phone use patterns that are conducive to cardiovascular health.”
Meanwhile, he added, “We encourage measures to reduce time spent on mobile phones to promote the primary prevention of CVD. On the other hand, improving sleep and mental health status may help reduce the higher risk of CVD associated with mobile phone use.”
There are several limitations to the study in addition to its observational nature, which cannot show cause and effect. The questionnaires on cell phone use were restricted to phone calls; other use patterns of cell phones (eg, messaging, watching videos, and browsing the web) were not considered. Although the researchers adjusted for many potential confounders, unmeasured confounding bias (eg, the type of cell phone used and other sources of RF-EMF) cannot be eliminated.
Weak Link?
In a comment, Nicholas Grubic, MSc, a PhD student in epidemiology at the University of Toronto, Ontario, Canada, and coauthor of a related editorial, said, “I found it interesting that there was a connection observed between mobile phone use and CVD. However, it is crucial to understand that this link appeared to be much weaker compared with other well-known cardiovascular risk factors, such as smoking, diabetes, and high blood pressure. For now, mobile phone use should not be a major concern for most people.”
Nevertheless, clinicians should encourage patients to practice healthy habits around their screen time, he advised. “This could include limiting mobile phone use before bedtime and taking regular breaks to engage in activities that promote heart health, such as exercising or spending time outdoors.
“For the time being, we probably won’t see mobile phone use included in standard assessments for cardiovascular risk or as a focal point of cardiovascular health promotion initiatives,” he added. Instead, clinicians should “focus on established risk factors that have a stronger impact on patients’ cardiovascular health.”
Nieca Goldberg, MD, a clinical associate professor of medicine at NYU Grossman School of Medicine in New York City and American Heart Association volunteer expert, had a similar message. “You don’t have to go back to using a landline,” she said. “Instead, patients should be more mindful of how much phone use is taking away from their physical activity, keeping them from sleeping, and causing them stress.” Clinicians should also remember to counsel smokers on smoking cessation.
“It would be important for future studies to look at time spent on the phone and the type of activities patients are doing on their phones, such as social media, calls, texts, movies, or streaming TV shows,” she said. “It would be important to see how phone use is leading to a sedentary lifestyle” and what that means for a larger, more diverse population.
The study was supported by the National Key R&D Program, the National Natural Science Foundation of China, and the Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University. Dr. Qin, Dr. Grubic, and Dr. Goldberg reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We found that a poor sleep pattern, psychological distress, and neuroticism significantly mediated the positive association between weekly mobile phone usage time and the risk for incident CVD, with a mediating proportion of 5.11%, 11.50%, and 2.25%, respectively,” said principal investigator Xianhui Qin, MD, professor of nephrology at Southern Medical University, Guangzhou, China.
Poor sleep patterns and poor mental health could disrupt circadian rhythms and endocrine and metabolic functions, as well as increase inflammation, he explained.
In addition, chronic exposure to radiofrequency electromagnetic fields (RF-EMF) emitted from cell phones could lead to oxidative stress and an inflammatory response. Combined with smoking and diabetes, this exposure “may have a synergistic effect in increasing CVD risk,” Dr. Qin suggested.
The study was published online in the Canadian Journal of Cardiology.
Risk Underestimated?
The researchers aimed to examine the association of regular cell phone use with incident CVD and explore the mediating effects of sleep and mental health using linked hospital and mortality records.
Their analysis included 444,027 participants (mean age, 56 years; 44% men) without a history of CVD from the UK Biobank. A total of 378,161 participants were regular cell phone users.
Regular cell phone use was defined as at least one call per week. Weekly use was self-reported as the average time of calls per week during the previous 3 months.
The primary outcome was incident CVD. Secondary outcomes were each component of CVD (ie, coronary heart disease, stroke, atrial fibrillation, and heart failure) and increased carotid intima media thickness (CIMT).
Compared with nonregular cell phone users, regular users were younger, had higher proportions of current smokers and urban residents, and had lower proportions of history of hypertension and diabetes. They also had higher income, Townsend deprivation index, and body mass index, and lower education levels.
During a median follow-up of 12.3 years, 56,181 participants developed incident CVD. Compared with nonregular cell phone users, regular users had a significantly higher risk for incident CVD (hazard ratio, 1.04) and increased CIMT (odds ratio, 1.11).
Among regular cell phone users, the duration of cell phone use and hands-free device/speakerphone use during calls was not significantly associated with incident CVD. Yet a significant and positive dose-response relationship was seen between weekly cell phone usage time and the risk for CVD. The positive association was stronger in current vs noncurrent smokers and people with vs without diabetes.
To different extents, sleep patterns (5.11%), psychologic distress (11.5%), and neuroticism (2.25%) mediated the relationship between weekly cell phone usage time and the risk for incident CVD.
“Our study suggests that despite the advantages of mobile phone use, we should also pay attention to the potential harm of mobile phone use to cardiovascular health,” Dr. Qin said. “Future studies to assess the risk-benefit balance will help promote mobile phone use patterns that are conducive to cardiovascular health.”
Meanwhile, he added, “We encourage measures to reduce time spent on mobile phones to promote the primary prevention of CVD. On the other hand, improving sleep and mental health status may help reduce the higher risk of CVD associated with mobile phone use.”
There are several limitations to the study in addition to its observational nature, which cannot show cause and effect. The questionnaires on cell phone use were restricted to phone calls; other use patterns of cell phones (eg, messaging, watching videos, and browsing the web) were not considered. Although the researchers adjusted for many potential confounders, unmeasured confounding bias (eg, the type of cell phone used and other sources of RF-EMF) cannot be eliminated.
Weak Link?
In a comment, Nicholas Grubic, MSc, a PhD student in epidemiology at the University of Toronto, Ontario, Canada, and coauthor of a related editorial, said, “I found it interesting that there was a connection observed between mobile phone use and CVD. However, it is crucial to understand that this link appeared to be much weaker compared with other well-known cardiovascular risk factors, such as smoking, diabetes, and high blood pressure. For now, mobile phone use should not be a major concern for most people.”
Nevertheless, clinicians should encourage patients to practice healthy habits around their screen time, he advised. “This could include limiting mobile phone use before bedtime and taking regular breaks to engage in activities that promote heart health, such as exercising or spending time outdoors.
“For the time being, we probably won’t see mobile phone use included in standard assessments for cardiovascular risk or as a focal point of cardiovascular health promotion initiatives,” he added. Instead, clinicians should “focus on established risk factors that have a stronger impact on patients’ cardiovascular health.”
Nieca Goldberg, MD, a clinical associate professor of medicine at NYU Grossman School of Medicine in New York City and American Heart Association volunteer expert, had a similar message. “You don’t have to go back to using a landline,” she said. “Instead, patients should be more mindful of how much phone use is taking away from their physical activity, keeping them from sleeping, and causing them stress.” Clinicians should also remember to counsel smokers on smoking cessation.
“It would be important for future studies to look at time spent on the phone and the type of activities patients are doing on their phones, such as social media, calls, texts, movies, or streaming TV shows,” she said. “It would be important to see how phone use is leading to a sedentary lifestyle” and what that means for a larger, more diverse population.
The study was supported by the National Key R&D Program, the National Natural Science Foundation of China, and the Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University. Dr. Qin, Dr. Grubic, and Dr. Goldberg reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We found that a poor sleep pattern, psychological distress, and neuroticism significantly mediated the positive association between weekly mobile phone usage time and the risk for incident CVD, with a mediating proportion of 5.11%, 11.50%, and 2.25%, respectively,” said principal investigator Xianhui Qin, MD, professor of nephrology at Southern Medical University, Guangzhou, China.
Poor sleep patterns and poor mental health could disrupt circadian rhythms and endocrine and metabolic functions, as well as increase inflammation, he explained.
In addition, chronic exposure to radiofrequency electromagnetic fields (RF-EMF) emitted from cell phones could lead to oxidative stress and an inflammatory response. Combined with smoking and diabetes, this exposure “may have a synergistic effect in increasing CVD risk,” Dr. Qin suggested.
The study was published online in the Canadian Journal of Cardiology.
Risk Underestimated?
The researchers aimed to examine the association of regular cell phone use with incident CVD and explore the mediating effects of sleep and mental health using linked hospital and mortality records.
Their analysis included 444,027 participants (mean age, 56 years; 44% men) without a history of CVD from the UK Biobank. A total of 378,161 participants were regular cell phone users.
Regular cell phone use was defined as at least one call per week. Weekly use was self-reported as the average time of calls per week during the previous 3 months.
The primary outcome was incident CVD. Secondary outcomes were each component of CVD (ie, coronary heart disease, stroke, atrial fibrillation, and heart failure) and increased carotid intima media thickness (CIMT).
Compared with nonregular cell phone users, regular users were younger, had higher proportions of current smokers and urban residents, and had lower proportions of history of hypertension and diabetes. They also had higher income, Townsend deprivation index, and body mass index, and lower education levels.
During a median follow-up of 12.3 years, 56,181 participants developed incident CVD. Compared with nonregular cell phone users, regular users had a significantly higher risk for incident CVD (hazard ratio, 1.04) and increased CIMT (odds ratio, 1.11).
Among regular cell phone users, the duration of cell phone use and hands-free device/speakerphone use during calls was not significantly associated with incident CVD. Yet a significant and positive dose-response relationship was seen between weekly cell phone usage time and the risk for CVD. The positive association was stronger in current vs noncurrent smokers and people with vs without diabetes.
To different extents, sleep patterns (5.11%), psychologic distress (11.5%), and neuroticism (2.25%) mediated the relationship between weekly cell phone usage time and the risk for incident CVD.
“Our study suggests that despite the advantages of mobile phone use, we should also pay attention to the potential harm of mobile phone use to cardiovascular health,” Dr. Qin said. “Future studies to assess the risk-benefit balance will help promote mobile phone use patterns that are conducive to cardiovascular health.”
Meanwhile, he added, “We encourage measures to reduce time spent on mobile phones to promote the primary prevention of CVD. On the other hand, improving sleep and mental health status may help reduce the higher risk of CVD associated with mobile phone use.”
There are several limitations to the study in addition to its observational nature, which cannot show cause and effect. The questionnaires on cell phone use were restricted to phone calls; other use patterns of cell phones (eg, messaging, watching videos, and browsing the web) were not considered. Although the researchers adjusted for many potential confounders, unmeasured confounding bias (eg, the type of cell phone used and other sources of RF-EMF) cannot be eliminated.
Weak Link?
In a comment, Nicholas Grubic, MSc, a PhD student in epidemiology at the University of Toronto, Ontario, Canada, and coauthor of a related editorial, said, “I found it interesting that there was a connection observed between mobile phone use and CVD. However, it is crucial to understand that this link appeared to be much weaker compared with other well-known cardiovascular risk factors, such as smoking, diabetes, and high blood pressure. For now, mobile phone use should not be a major concern for most people.”
Nevertheless, clinicians should encourage patients to practice healthy habits around their screen time, he advised. “This could include limiting mobile phone use before bedtime and taking regular breaks to engage in activities that promote heart health, such as exercising or spending time outdoors.
“For the time being, we probably won’t see mobile phone use included in standard assessments for cardiovascular risk or as a focal point of cardiovascular health promotion initiatives,” he added. Instead, clinicians should “focus on established risk factors that have a stronger impact on patients’ cardiovascular health.”
Nieca Goldberg, MD, a clinical associate professor of medicine at NYU Grossman School of Medicine in New York City and American Heart Association volunteer expert, had a similar message. “You don’t have to go back to using a landline,” she said. “Instead, patients should be more mindful of how much phone use is taking away from their physical activity, keeping them from sleeping, and causing them stress.” Clinicians should also remember to counsel smokers on smoking cessation.
“It would be important for future studies to look at time spent on the phone and the type of activities patients are doing on their phones, such as social media, calls, texts, movies, or streaming TV shows,” she said. “It would be important to see how phone use is leading to a sedentary lifestyle” and what that means for a larger, more diverse population.
The study was supported by the National Key R&D Program, the National Natural Science Foundation of China, and the Outstanding Youth Development Scheme of Nanfang Hospital, Southern Medical University. Dr. Qin, Dr. Grubic, and Dr. Goldberg reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Nighttime Outdoor Light Pollution Linked to Alzheimer’s Risk
a new national study suggested.
Analyses of state and county light pollution data and Medicare claims showed that areas with higher average nighttime light intensity had a greater prevalence of Alzheimer’s disease.
Among people aged 65 years or older, Alzheimer’s disease prevalence was more strongly associated with nightly light pollution exposure than with alcohol misuse, chronic kidney disease, depression, or obesity.
In those younger than 65 years, greater nighttime light intensity had a stronger association with Alzheimer’s disease prevalence than any other risk factor included in the study.
“The results are pretty striking when you do these comparisons and it’s true for people of all ages,” said Robin Voigt-Zuwala, PhD, lead author and director, Circadian Rhythm Research Laboratory, Rush University, Chicago, Illinois.
The study was published online in Frontiers of Neuroscience.
Shining a Light
Exposure to artificial outdoor light at night has been associated with adverse health effects such as sleep disruption, obesity, atherosclerosis, and cancer, but this is the first study to look specifically at Alzheimer’s disease, investigators noted.
Two recent studies reported higher risks for mild cognitive impairment among Chinese veterans and late-onset dementia among Italian residents living in areas with brighter outdoor light at night.
For this study, Dr. Voigt-Zuwala and colleagues examined the relationship between Alzheimer’s disease prevalence and average nighttime light intensity in the lower 48 states using data from Medicare Part A and B, the Centers for Disease Control and Prevention, and NASA satellite–acquired radiance data.
The data were averaged for the years 2012-2018 and states divided into five groups based on average nighttime light intensity.
The darkest states were Montana, Wyoming, South Dakota, Idaho, Maine, New Mexico, Vermont, Oregon, Utah, and Nevada. The brightest states were Indiana, Illinois, Florida, Ohio, Massachusetts, Connecticut, Maryland, Delaware, Rhode Island, and New Jersey.
Analysis of variance revealed a significant difference in Alzheimer’s disease prevalence between state groups (P < .0001). Multiple comparisons testing also showed that states with the lowest average nighttime light had significantly different Alzheimer’s disease prevalence than those with higher intensity.
The same positive relationship was observed when each year was assessed individually and at the county level, using data from 45 counties and the District of Columbia.
Strong Association
The investigators also found that state average nighttime light intensity is significantly associated with Alzheimer’s disease prevalence (P = .006). This effect was seen across all ages, sexes, and races except Asian Pacific Island, the latter possibly related to statistical power, the authors said.
When known or proposed risk factors for Alzheimer’s disease were added to the model, atrial fibrillation, diabetes, hyperlipidemia, hypertension, and stroke had a stronger association with Alzheimer’s disease than average nighttime light intensity.
Nighttime light intensity, however, was more strongly associated with Alzheimer’s disease prevalence than alcohol abuse, chronic kidney disease, depression, heart failure, and obesity.
Moreover, in people younger than 65 years, nighttime light pollution had a stronger association with Alzheimer’s disease prevalence than all other risk factors (P = .007).
The mechanism behind this increased vulnerability is unclear, but there may be an interplay between genetic susceptibility of an individual and how they respond to light, Dr. Voigt-Zuwala suggested.
“APOE4 is the genotype most highly associated with Alzheimer’s disease risk, and maybe the people who have that genotype are just more sensitive to the effects of light exposure at night, more sensitive to circadian rhythm disruption,” she said.
The authors noted that additional research is needed but suggested light pollution may also influence Alzheimer’s disease through sleep disruption, which can promote inflammation, activate microglia and astrocytes, and negatively alter the clearance of amyloid beta, and by decreasing the levels of brain-derived neurotrophic factor.
Are We Measuring the Right Light?
“It’s a good article and it’s got a good message, but I have some caveats to that,” said George C. Brainard, PhD, director, Light Research Program, Thomas Jefferson University in Philadelphia, Pennsylvania, and a pioneer in the study of how light affects biology including breast cancer in night-shift workers.
The biggest caveat, and one acknowledged by the authors, is that the study didn’t measure indoor light exposure and relied instead on satellite imaging.
“They’re very striking images, but they may not be particularly relevant. And here’s why: People don’t live outdoors all night,” Dr. Brainard said.
Instead, people spend much of their time at night indoors where they’re exposed to lighting in the home and from smartphones, laptops, and television screens.
“It doesn’t invalidate their work. It’s an important advancement, an important observation,” Dr. Brainard said. “But the important thing really is to find out what is the population exposed to that triggers this response, and it’s probably indoor lighting related to the amount and physical characteristics of indoor lighting. It doesn’t mean outdoor lighting can’t play a role. It certainly can.”
Reached for comment, Erik Musiek, MD, PhD, a professor of neurology whose lab at Washington University School of Medicine in St. Louis, Missouri, has extensively studied circadian clock disruption and Alzheimer’s disease pathology in the brain, said the study provides a 10,000-foot view of the issue.
For example, the study was not designed to detect whether people living in high light pollution areas are actually experiencing more outdoor light at night and if risk factors such as air pollution and low socioeconomic status may correlate with these areas.
“Most of what we worry about is do people have lights on in the house, do they have their TV on, their screens up to their face late at night? This can’t tell us about that,” Dr. Musiek said. “But on the other hand, this kind of light exposure is something that public policy can affect.”
“It’s hard to control people’s personal habits nor should we probably, but we can control what types of bulbs you put into streetlights, how bright they are, and where you put lighting in a public place,” he added. “So I do think there’s value there.”
At least 19 states, the District of Columbia, and Puerto Rico have laws in place to reduce light pollution, with the majority doing so to promote energy conservation, public safety, aesthetic interests, or astronomical research, according to the National Conference of State Legislatures.
To respond to some of the limitations in this study, Dr. Voigt-Zuwala is writing a grant application for a new project to look at both indoor and outdoor light exposure on an individual level.
“This is what I’ve been wanting to study for a long time, and this study is just sort of the stepping stone, the proof of concept that this is something we need to be investigating,” she said.
Dr. Voigt-Zuwala reported RO1 and R24 grants from the National Institutes of Health (NIH), one coauthor reported an NIH R24 grant; another reported having no conflicts of interest. Dr. Brainard reported having no relevant conflicts of interest. Dr. Musiek reported research funding from Eisai Pharmaceuticals.
A version of this article first appeared on Medscape.com.
a new national study suggested.
Analyses of state and county light pollution data and Medicare claims showed that areas with higher average nighttime light intensity had a greater prevalence of Alzheimer’s disease.
Among people aged 65 years or older, Alzheimer’s disease prevalence was more strongly associated with nightly light pollution exposure than with alcohol misuse, chronic kidney disease, depression, or obesity.
In those younger than 65 years, greater nighttime light intensity had a stronger association with Alzheimer’s disease prevalence than any other risk factor included in the study.
“The results are pretty striking when you do these comparisons and it’s true for people of all ages,” said Robin Voigt-Zuwala, PhD, lead author and director, Circadian Rhythm Research Laboratory, Rush University, Chicago, Illinois.
The study was published online in Frontiers of Neuroscience.
Shining a Light
Exposure to artificial outdoor light at night has been associated with adverse health effects such as sleep disruption, obesity, atherosclerosis, and cancer, but this is the first study to look specifically at Alzheimer’s disease, investigators noted.
Two recent studies reported higher risks for mild cognitive impairment among Chinese veterans and late-onset dementia among Italian residents living in areas with brighter outdoor light at night.
For this study, Dr. Voigt-Zuwala and colleagues examined the relationship between Alzheimer’s disease prevalence and average nighttime light intensity in the lower 48 states using data from Medicare Part A and B, the Centers for Disease Control and Prevention, and NASA satellite–acquired radiance data.
The data were averaged for the years 2012-2018 and states divided into five groups based on average nighttime light intensity.
The darkest states were Montana, Wyoming, South Dakota, Idaho, Maine, New Mexico, Vermont, Oregon, Utah, and Nevada. The brightest states were Indiana, Illinois, Florida, Ohio, Massachusetts, Connecticut, Maryland, Delaware, Rhode Island, and New Jersey.
Analysis of variance revealed a significant difference in Alzheimer’s disease prevalence between state groups (P < .0001). Multiple comparisons testing also showed that states with the lowest average nighttime light had significantly different Alzheimer’s disease prevalence than those with higher intensity.
The same positive relationship was observed when each year was assessed individually and at the county level, using data from 45 counties and the District of Columbia.
Strong Association
The investigators also found that state average nighttime light intensity is significantly associated with Alzheimer’s disease prevalence (P = .006). This effect was seen across all ages, sexes, and races except Asian Pacific Island, the latter possibly related to statistical power, the authors said.
When known or proposed risk factors for Alzheimer’s disease were added to the model, atrial fibrillation, diabetes, hyperlipidemia, hypertension, and stroke had a stronger association with Alzheimer’s disease than average nighttime light intensity.
Nighttime light intensity, however, was more strongly associated with Alzheimer’s disease prevalence than alcohol abuse, chronic kidney disease, depression, heart failure, and obesity.
Moreover, in people younger than 65 years, nighttime light pollution had a stronger association with Alzheimer’s disease prevalence than all other risk factors (P = .007).
The mechanism behind this increased vulnerability is unclear, but there may be an interplay between genetic susceptibility of an individual and how they respond to light, Dr. Voigt-Zuwala suggested.
“APOE4 is the genotype most highly associated with Alzheimer’s disease risk, and maybe the people who have that genotype are just more sensitive to the effects of light exposure at night, more sensitive to circadian rhythm disruption,” she said.
The authors noted that additional research is needed but suggested light pollution may also influence Alzheimer’s disease through sleep disruption, which can promote inflammation, activate microglia and astrocytes, and negatively alter the clearance of amyloid beta, and by decreasing the levels of brain-derived neurotrophic factor.
Are We Measuring the Right Light?
“It’s a good article and it’s got a good message, but I have some caveats to that,” said George C. Brainard, PhD, director, Light Research Program, Thomas Jefferson University in Philadelphia, Pennsylvania, and a pioneer in the study of how light affects biology including breast cancer in night-shift workers.
The biggest caveat, and one acknowledged by the authors, is that the study didn’t measure indoor light exposure and relied instead on satellite imaging.
“They’re very striking images, but they may not be particularly relevant. And here’s why: People don’t live outdoors all night,” Dr. Brainard said.
Instead, people spend much of their time at night indoors where they’re exposed to lighting in the home and from smartphones, laptops, and television screens.
“It doesn’t invalidate their work. It’s an important advancement, an important observation,” Dr. Brainard said. “But the important thing really is to find out what is the population exposed to that triggers this response, and it’s probably indoor lighting related to the amount and physical characteristics of indoor lighting. It doesn’t mean outdoor lighting can’t play a role. It certainly can.”
Reached for comment, Erik Musiek, MD, PhD, a professor of neurology whose lab at Washington University School of Medicine in St. Louis, Missouri, has extensively studied circadian clock disruption and Alzheimer’s disease pathology in the brain, said the study provides a 10,000-foot view of the issue.
For example, the study was not designed to detect whether people living in high light pollution areas are actually experiencing more outdoor light at night and if risk factors such as air pollution and low socioeconomic status may correlate with these areas.
“Most of what we worry about is do people have lights on in the house, do they have their TV on, their screens up to their face late at night? This can’t tell us about that,” Dr. Musiek said. “But on the other hand, this kind of light exposure is something that public policy can affect.”
“It’s hard to control people’s personal habits nor should we probably, but we can control what types of bulbs you put into streetlights, how bright they are, and where you put lighting in a public place,” he added. “So I do think there’s value there.”
At least 19 states, the District of Columbia, and Puerto Rico have laws in place to reduce light pollution, with the majority doing so to promote energy conservation, public safety, aesthetic interests, or astronomical research, according to the National Conference of State Legislatures.
To respond to some of the limitations in this study, Dr. Voigt-Zuwala is writing a grant application for a new project to look at both indoor and outdoor light exposure on an individual level.
“This is what I’ve been wanting to study for a long time, and this study is just sort of the stepping stone, the proof of concept that this is something we need to be investigating,” she said.
Dr. Voigt-Zuwala reported RO1 and R24 grants from the National Institutes of Health (NIH), one coauthor reported an NIH R24 grant; another reported having no conflicts of interest. Dr. Brainard reported having no relevant conflicts of interest. Dr. Musiek reported research funding from Eisai Pharmaceuticals.
A version of this article first appeared on Medscape.com.
a new national study suggested.
Analyses of state and county light pollution data and Medicare claims showed that areas with higher average nighttime light intensity had a greater prevalence of Alzheimer’s disease.
Among people aged 65 years or older, Alzheimer’s disease prevalence was more strongly associated with nightly light pollution exposure than with alcohol misuse, chronic kidney disease, depression, or obesity.
In those younger than 65 years, greater nighttime light intensity had a stronger association with Alzheimer’s disease prevalence than any other risk factor included in the study.
“The results are pretty striking when you do these comparisons and it’s true for people of all ages,” said Robin Voigt-Zuwala, PhD, lead author and director, Circadian Rhythm Research Laboratory, Rush University, Chicago, Illinois.
The study was published online in Frontiers of Neuroscience.
Shining a Light
Exposure to artificial outdoor light at night has been associated with adverse health effects such as sleep disruption, obesity, atherosclerosis, and cancer, but this is the first study to look specifically at Alzheimer’s disease, investigators noted.
Two recent studies reported higher risks for mild cognitive impairment among Chinese veterans and late-onset dementia among Italian residents living in areas with brighter outdoor light at night.
For this study, Dr. Voigt-Zuwala and colleagues examined the relationship between Alzheimer’s disease prevalence and average nighttime light intensity in the lower 48 states using data from Medicare Part A and B, the Centers for Disease Control and Prevention, and NASA satellite–acquired radiance data.
The data were averaged for the years 2012-2018 and states divided into five groups based on average nighttime light intensity.
The darkest states were Montana, Wyoming, South Dakota, Idaho, Maine, New Mexico, Vermont, Oregon, Utah, and Nevada. The brightest states were Indiana, Illinois, Florida, Ohio, Massachusetts, Connecticut, Maryland, Delaware, Rhode Island, and New Jersey.
Analysis of variance revealed a significant difference in Alzheimer’s disease prevalence between state groups (P < .0001). Multiple comparisons testing also showed that states with the lowest average nighttime light had significantly different Alzheimer’s disease prevalence than those with higher intensity.
The same positive relationship was observed when each year was assessed individually and at the county level, using data from 45 counties and the District of Columbia.
Strong Association
The investigators also found that state average nighttime light intensity is significantly associated with Alzheimer’s disease prevalence (P = .006). This effect was seen across all ages, sexes, and races except Asian Pacific Island, the latter possibly related to statistical power, the authors said.
When known or proposed risk factors for Alzheimer’s disease were added to the model, atrial fibrillation, diabetes, hyperlipidemia, hypertension, and stroke had a stronger association with Alzheimer’s disease than average nighttime light intensity.
Nighttime light intensity, however, was more strongly associated with Alzheimer’s disease prevalence than alcohol abuse, chronic kidney disease, depression, heart failure, and obesity.
Moreover, in people younger than 65 years, nighttime light pollution had a stronger association with Alzheimer’s disease prevalence than all other risk factors (P = .007).
The mechanism behind this increased vulnerability is unclear, but there may be an interplay between genetic susceptibility of an individual and how they respond to light, Dr. Voigt-Zuwala suggested.
“APOE4 is the genotype most highly associated with Alzheimer’s disease risk, and maybe the people who have that genotype are just more sensitive to the effects of light exposure at night, more sensitive to circadian rhythm disruption,” she said.
The authors noted that additional research is needed but suggested light pollution may also influence Alzheimer’s disease through sleep disruption, which can promote inflammation, activate microglia and astrocytes, and negatively alter the clearance of amyloid beta, and by decreasing the levels of brain-derived neurotrophic factor.
Are We Measuring the Right Light?
“It’s a good article and it’s got a good message, but I have some caveats to that,” said George C. Brainard, PhD, director, Light Research Program, Thomas Jefferson University in Philadelphia, Pennsylvania, and a pioneer in the study of how light affects biology including breast cancer in night-shift workers.
The biggest caveat, and one acknowledged by the authors, is that the study didn’t measure indoor light exposure and relied instead on satellite imaging.
“They’re very striking images, but they may not be particularly relevant. And here’s why: People don’t live outdoors all night,” Dr. Brainard said.
Instead, people spend much of their time at night indoors where they’re exposed to lighting in the home and from smartphones, laptops, and television screens.
“It doesn’t invalidate their work. It’s an important advancement, an important observation,” Dr. Brainard said. “But the important thing really is to find out what is the population exposed to that triggers this response, and it’s probably indoor lighting related to the amount and physical characteristics of indoor lighting. It doesn’t mean outdoor lighting can’t play a role. It certainly can.”
Reached for comment, Erik Musiek, MD, PhD, a professor of neurology whose lab at Washington University School of Medicine in St. Louis, Missouri, has extensively studied circadian clock disruption and Alzheimer’s disease pathology in the brain, said the study provides a 10,000-foot view of the issue.
For example, the study was not designed to detect whether people living in high light pollution areas are actually experiencing more outdoor light at night and if risk factors such as air pollution and low socioeconomic status may correlate with these areas.
“Most of what we worry about is do people have lights on in the house, do they have their TV on, their screens up to their face late at night? This can’t tell us about that,” Dr. Musiek said. “But on the other hand, this kind of light exposure is something that public policy can affect.”
“It’s hard to control people’s personal habits nor should we probably, but we can control what types of bulbs you put into streetlights, how bright they are, and where you put lighting in a public place,” he added. “So I do think there’s value there.”
At least 19 states, the District of Columbia, and Puerto Rico have laws in place to reduce light pollution, with the majority doing so to promote energy conservation, public safety, aesthetic interests, or astronomical research, according to the National Conference of State Legislatures.
To respond to some of the limitations in this study, Dr. Voigt-Zuwala is writing a grant application for a new project to look at both indoor and outdoor light exposure on an individual level.
“This is what I’ve been wanting to study for a long time, and this study is just sort of the stepping stone, the proof of concept that this is something we need to be investigating,” she said.
Dr. Voigt-Zuwala reported RO1 and R24 grants from the National Institutes of Health (NIH), one coauthor reported an NIH R24 grant; another reported having no conflicts of interest. Dr. Brainard reported having no relevant conflicts of interest. Dr. Musiek reported research funding from Eisai Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS OF NEUROSCIENCE
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
SURMOUNT-OSA Results: ‘Impressive’ in Improving Sleep Apnea
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome. I’m Dr. Akshay Jain, an endocrinologist in Vancouver, Canada, and with me is a very special guest. Today we have Dr. James Kim, a primary care physician working in Calgary, Canada. Both Dr. Kim and I were fortunate to attend the recently concluded American Diabetes Association annual conference in Orlando in June.
We thought we could share with you some of the key learnings that we found very insightful and clinically quite relevant. We were hoping to bring our own conclusion regarding what these findings were, both from a primary care perspective and an endocrinology perspective.
There were so many different studies that, frankly, it was difficult to pick them, but we handpicked a few studies we felt we could do a bit of a deeper dive on, and we’ll talk about each of these studies.
Welcome, Dr. Kim, and thanks for joining us.
James W. Kim, MBBCh, PgDip, MScCH: Thank you so much, Dr Jain. It’s a pleasure to be here.
Dr. Jain: Probably the best place to start would be with the SURMOUNT-OSA study. This was highlighted at the American Diabetes Association conference. Essentially, it looked at people who are living with obesity who also had obstructive sleep apnea.
This was a randomized controlled trial where individuals tested either got tirzepatide (trade name, Mounjaro) or placebo treatment. They looked at the change in their apnea-hypopnea index at the end of the study.
This included both people who were using CPAP machines and those who were not using CPAP machines at baseline. We do know that many individuals with sleep apnea may not use these machines.
That was a big reduction.
Dr. Kim, what’s the relevance of this study in primary care?
Dr. Kim: Oh, it’s massive. Obstructive sleep apnea is probably one of the most underdiagnosed yet huge cardiac risk factors that we tend to overlook in primary care. We sometimes say, oh, it’s just sleep apnea; what’s the big deal? We know it’s a big problem. We know that more than 50% of people with type 2 diabetes have obstructive sleep apnea, and some studies have even quoted that 90% of their population cohorts had sleep apnea. This is a big deal.
What do we know so far? We know that obstructive sleep apnea, which I’m just going to call OSA, increases the risk for hypertension, bad cholesterol, and worsening blood glucose in terms of A1c and fasting glucose, which eventually leads to myocardial infarction, arrhythmia, stroke, and eventually cardiovascular death.
We also know that people with type 2 diabetes have an increased risk for OSA. There seems to be a bidirectional relationship between diabetes and OSA. It seems like weight plays the biggest role in terms of developing OSA, and numerous studies have shown this.
Also, thankfully, some of the studies showed that weight loss improves not just OSA but also blood pressure, cholesterol, blood glucose, and insulin sensitivities. These have been fascinating. We see these patients every single day. If you think about it in your population, for 50%-90% of the patients to have OSA is a large number. If you haven’t seen a person with OSA this week, you probably missed them, very likely.
Therefore, the SURMOUNT-OSA trial was quite fascinating with, as you mentioned, 50%-60% reduction in the severity of OSA, which is very impressive. Even more impressive, I think, is that for about 50% of the patients on tirzepatide, the OSA improves so much that they may not even need to be on CPAP machines.
Those who were on CPAP may not need to be on CPAP any longer. These are huge data, especially for primary care, because as you mentioned, we see these people every single day.
Dr. Jain: Thanks for pointing that out. Clearly, it’s very clinically relevant. I think the most important takeaway for me from this study was the correlation between weight loss and AHI improvement.
Clearly, it showed that placebo had about a 6% drop in AHI, whereas there was a 60% drop in the tirzepatide group, so you can see that it’s significantly different. The placebo group did not have any significant degree of weight loss, whereas the tirzepatide group had nearly 20% weight loss. This again goes to show that there is a very close correlation between weight loss and improvement in OSA.
What’s very important to note is that we’ve seen this in the past as well. We had seen some of these data with other GLP-1 agents, but the extent of improvement that we have seen in the SURMOUNT-OSA trial is significantly more than what we’ve seen in previous studies. There is a ray of hope now where we have medical management to offer people who are living with obesity and obstructive sleep apnea.
Dr. Kim: I want to add that, from a primary care perspective, this study also showed the improvement of the sleep apnea–related symptoms as well. The biggest problem with sleep apnea — or at least what patients’ spouses complain of, is the person snoring too much; it’s a symptom.
It’s the next-day symptoms that really do disturb people, like chronic fatigue. I have numerous patients who say that, once they’ve been treated for sleep apnea, they feel like a brand-new person. They have sudden bursts of energy that they never felt before, and over 50% of these people have huge improvements in the symptoms as well.
This is a huge trial. The only thing that I wish this study included were people with mild obstructive sleep apnea who were symptomatic. I do understand that, with other studies in this population, the data have been conflicting, but it would have been really awesome if they had those patients included. However, it is still a significant study for primary care.
Dr. Jain: That’s a really good point. Fatigue improves and overall quality of life improves. That’s very important from a primary care perspective.
From an endocrinology perspective, we know that management of sleep apnea can often lead to improvement in male hypogonadism, polycystic ovary syndrome, and insulin resistance. The amount of insulin required, or the number of medications needed for managing diabetes, can improve. Hypertension can improve as well. There are multiple benefits that you can get from appropriate management of sleep apnea.
Thanks, Dr. Kim. We really appreciate your insights on SURMOUNT-OSA.
Dr. Jain is a clinical instructor, Department of Endocrinology, University of British Columbia, Vancouver. Dr. Kim is a clinical assistant professor, Department of Family Medicine, University of Calgary in Alberta. Both disclosed conflicts of interest with numerous pharmaceutical companies.
A version of this article appeared on Medscape.com.
OSA in pregnancy: Who to test, how to screen, and does treatment help?
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
Artificial Intelligence in Sleep Apnea
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
New AFib Guidelines Address Underlying Illness, Comorbidities
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
FROM ESC 2024