User login
The latest treatments for urinary and fecal incontinence: Which hold water?
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
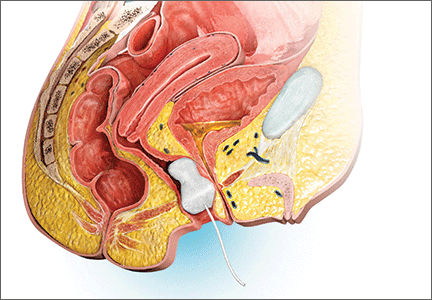
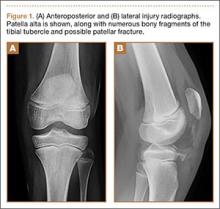
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
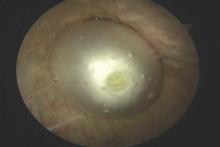
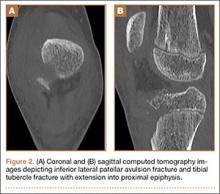
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
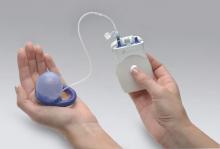
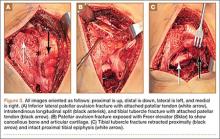
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
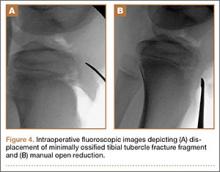
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Today, “normal” aging is no longer acceptable. From aesthetics to physical, mental, and sexual health, the maturing population seeks effective minimally invasive and practical methods to halt time and reverse its adverse effects. Nowhere is this more apparent than when dealing with urinary and fecal incontinence, conditions that can be not only embarrassing to patients but also debilitating, with potential crippling adverse affects on quality of life. As the US population ages, the prevalence of incontinence is increasing.
Patients commonly present with questions about their incontinence with preconceived notions on their available treatment options based on Internet searches and advertisements from magazines and television. Thus, as gynecologists, we have a pivotal role in educating women on their conditions and management options in a comprehensive, informative, and reassuring manner. By educating patients on the success rates and limitations of available treatments, patients can make informed decisions and reinforce their sense of autonomy. In this article we present the evidence on current, new, and investigative products available for the treatment of both stress urinary incontinence and overactive bladder, as well as fecal incontinence.
Case 1: Stress urinary incontinence
A 46-year-old woman (G2P2) presents with loss of urine with exercise, dancing, and sneezing that began after the birth of her last baby 5 years ago and is progressively becoming more frequent. She performs Kegel exercises occasionally and denies urinary urgency and/or urge incontinence. She reports a 20-lb weight gain in the past 3 years. Physical examination findings reveal normal pelvic examination with adequate pelvic organ support but weakened pelvic floor muscles during contraction. When you ask her to cough, you observe a small amount of urine loss from the urethral meatus. She has heard of “slings” before, but she is anxious about surgery.
Stress urinary incontinence (SUI) is the involuntary loss of urine with effort, physical exertion, sneezing, or coughing.1 It is the most common type of incontinence in younger women, with risk factors including increasing age, parity, and obesity.2,3 SUI treatment options, beginning from least to most invasive, include pelvic floor exercises, biofeedback and/or physical therapy, continence devices, off-label use of medications, urethral bulking agents, and surgical correction with slings. Midurethral tension-free slings are highly efficacious for the treatment of SUI. While a sling is a minimally invasive procedure, patients typically voice concerns regarding surgery and appropriately begin with conservative treatments.
A new FDA-approved OTC option for SUI
First-line conservative therapies offered to patients for SUI include pelvic floor muscle exercises and intravaginal continence devices. Disappointingly, such devices—including pessaries and the incontinence dish—have not been popular among patients for SUI. Authors of a randomized control trial evaluating incontinence pessaries versus behavioral therapy, including pelvic floor muscle training, found that, after 3 months, use of a pes‑ sary was not as effective as behavioral therapy in terms of patient satisfaction and improvement in bothersome urinary incontinence.4 In our experience, many patients wearing incontinence rings discontinue their use due to ineffectiveness or discomfort.
Patients now have an FDA-approved, over-the-counter option for SUI symptom management. The Poise Impressa is a disposable, nonabsorbent, flexible intravaginal device for patients with SUI (FIGURE 1). The device is comprised of a silicone core with a soft, nonwoven polypropylene fabric cover. It is inserted similar to a tampon, using an applicator, and provides nonobstructive support to the urethra to prevent stress urinary leakage. To find the proper fit, patients purchase the sizing kit, which includes 3 sizes. Patients are to insert size 1 first and monitor their comfort as well as improvement in leakage. Should size 1not sufficiently relieve leakage, the patient may try sizes 2 and 3 successively, with the goal of finding the most comfortable and effective insert. The insert is approved for up to 8 hours of wear in a 24-hour period, at which time the patient removes the device by pulling the string in a similar manner as removing a tampon.
Efficacy and quality of life data. Over 28 days, 85% of women with severe SUI confirmed on urodynamic testing achieved greater than 70% leakage reduction according to measured pad weights.5 Seventy percent of women reported 90% improvement in quality of life using validated questionnaires. In addition, 92% reported feeling dry with an improved perception of incontinence and greater confidence during strenuous activities.6 There were no serious adverse events, and the most common mild adverse events were discomfort, pain, and spotting.
As more patients become aware of the device through advertising and word of mouth, we expect patients to seek advice from their gynecologists on the safety and efficacy of the insert. In our experience, most patients report improvement in bothersome symptoms with the device and are overall satisfied. For patients who have discomfort with device placement, a water-based lubricant can be used. Patients using vaginal estrogen may apply the medication at night and wear the device during the day.
Office-based bladder control system in the pipeline
For SUI, options are limited for patients who would rather seek office-based procedures than invasive surgeries. Injections of urethral bulking agents can be performed in an office setting by injecting them transurethrally with a cystoscope slightly distal to the bladder neck. While bulking agents have a role in certain patients with SUI, especially those who are not interested in pursuing more invasive surgeries, only 43% have short-term (less than 6 months) cure and 75% report short-term improvement.7
A minimally invasive office-based procedure to treat SUI symptoms is under investigation in clinical trials currently. The Vesair Balloon bladder control system (Solace Therapeutics) is performed with cystoscopic guidance and is being tested at multiple sites throughout the United States (FIGURE 2).
The Vesair Balloon acts like a “shock absorber” to reduce momentary increases in bladder pressure due to external forces or stressors. The balloon is a small device, approximately the size of a quarter, and is implanted through the urethra via a specially designed applicator under cystoscopic guidance in the office setting. Pretreatment with pain medication usually is unnecessary. The VesairBalloon may be retained in situ for up to 12 months, at which time it is removed using a device-specific grasper under direct visualization with a cystoscope in the office.
Preliminary efficacy and safety data. In a single-blinded randomized controlled trial, 63% of women in the Vesair Balloon group had significant improvement in provocative pad weights and quality-of-life questionnaire scores at 3 months, compared with 31% in the control group.8 No serious adverse events were observed. Eleven of 63 patients (17%) withdrew from the study—most commonly for bladder irritation and dysuria.
We anxiously await the results of a second single-blinded randomized control trial currently being conducted.
Best surgical options for SUI
Today, the standard surgical procedure for SUI is a midurethral sling. Midurethral slings may be placed through 3 routes: retropubic; transobturator; and single-incision, otherwise known as “mini-slings.” Subjective cure rates of retropubic versus transobturator slings are similar, with lower rates of bladder perforation, major vascular/visceral injury, and operative blood loss in the transobturator group.9 However, rates of groin pain are higher in the trans‑ obturator group.
Single-incision slings were developed in an effort to avoid the morbidity and pain with passing traditional sling trocars through the obturator space and skin of the groin. In a randomized controlled trial, the Miniarc single- incision sling (Astora Women’s Health) was found to be noninferior to the Monarc transobturator sling (Astora) at 12 and 36 months.10 There were no statistically significant differences between subjective and objective cure rates on cough stress tests. Postoperative pain and groin pain were significantly less in patients with the Miniarc sling, compared with the Monarc sling.
It is our opinion that as more data become available, single-incision slings will find their foothold in a subset of patients with SUI.
Case 2: Overactive bladder: Failed medication therapy
A healthy 63-year-old woman presents with a 9-month history of loss of urine with strong urges, urinating 4 times per night, and a feeling of urgency when she needs to urinate. She denies pain with urination, difficulty emptying her bladder fully, and pain with a full bladder. She has restricted her fluid intake to 4 glasses of water per day and has stopped drinking fluids 4 hours before bedtime.
She described her symptoms to her intern‑ ist, who prescribed oxybutynin. She took the medication for 3 months but stopped after she developed severe constipation and dry mouth. She states the medication did not help her urinary symptoms. You discuss with her trials of other medications including topical anticholinergics and mirabegron. She is frustrated with her symptoms and asks if there are any other options besides medications.
Overactive bladder (OAB) is present in up to 16% of the US population, with the percentage estimated to increase by 20% within the next 2 years.11,12 The drastic increase in prevalence, likely due to the aging population, may result in an increased counseling and management burden placed on general practitioners and gynecologists.
First-line management options for OAB are behavioral modifications and/or medications. Our patient in case 2 failed both first-line therapies. When a patient fails or is intolerant to an anticholinergic medication, we offer mirabegron, a beta-3 agonist (after excluding any contraindications to the medication). Beyond medications, the therapeutic options are rather limited.
Second-line OAB treatment options
In January 2013, the FDA expanded the approved use of onabotulinum toxin A (Botox, Allergan) for the treatment of OAB in those who are intolerant of or have failed treatment with anticholinergic medications. Using a cystoscope, 100 units of onabotulinum toxin A are injected into 20 sites within the bladder wall. Due to the risk of urinary retention in up to 6% of patients, it is recommended to administer onabotulinum toxin A to patients who are willing and capable of performing clean intermittent catheterization.13
Efficacy data. In a recent systematic review and meta-analysis, the authors concluded onabotulinum toxin A to be effective in the treatment of idiopathic OAB with a statistically significant reduction compared with baseline in the number of incontinence episodes per day (-2.77 in the treatment group vs -1.01 in the placebo group) and the number of voids per day (-1.61 in the treatment group vs -0.87 in the placebo group).14 Patients who received onabotulinum toxin A experienced a higher rate of adverse effects, such as urinary tract infections, and were more likely to require clean intermittent catheterization due to incomplete bladder emptying.13 Patients can expect symptom improvement for approximately 6 months or longer.15 Based on the manufacturers’ recommendations, patients are not to be reinjected sooner than 12 weeks from prior onabotulinum toxin A injection.
In women with refractory OAB, available second-line treatments include neuromodulation by sacral nerve or posterior tibial nerve stimulation (PTNS). The latter therapy is an office-based procedure that involves placement of a lead percutaneous to the medial aspect of the ankle near the tibial nerve. It is postulated that stimulation of the tibial nerve results in retrograde stimulation of the S3 sacral nerve plexus, resulting in OAB symptom relief in 54% to 70% of patients.16
Case 3: Fecal incontinence
A 57-year-old, otherwise healthy, multiparous woman presents with a 3-year history of fecal incontinence. She reports that it is embarrassing and distressing. She avoids certain social activities and is not currently sexually active due to the frequency of bowel leakage episodes.
In an effort to decrease her episodes of incontinence, she takes loperamide hydrochloride (Imodium) regularly with little improvement in the frequency of accidents. She has no history of gastrointestinal, rectal, or gynecologic surgery. She had 2 full-term vaginal deliveries that were uncomplicated. On review of systems, she also discloses occasional urinary incontinence.
Physical examination reveals normal vaginal anatomy with adequate pelvic organ support and no neurologic abnormalities. Rectal examination demonstrates normal tone and no evidence of rectal prolapse. Contractions of the pelvic floor muscles are weak. She is frustrated with her condition and seeks your guidance.
Fecal incontinence affects more than 20 million women in the United States, with only one-third of those with the condition disclosing their symptoms to their physician.17 Many etiologies for accidental bowel leakage exist, with some of the most common being advancing age and obstetric trauma. Up to one-third of women presenting for evaluation of urinary incontinence have fecal incontinence; therefore, one must be vigilant in screening for this potentially devastating condition.18
In case 3, the patient has tried medical therapies for fecal incontinence, including stool-bulking agents and motility regulators such as loperamide hydrochloride. Besides offering fiber supplements (or other stool-bulking agents) or physical therapy, nonsurgical options for this patient are limited.
Newly available: A vaginal insert for fecal incontinence
In 2015, the Eclipse System (Pelvalon) became the first FDA-approved vaginal insert for the treatment of fecal incontinence. The manufacturer recently was granted clearance for its second-generation device (FIGURE 3). The device consists of a silicone-coated stainless steel base with a posteriorly facing balloon and a pressure-regulated pump that allows the patient to control her bowel movements. After a patient is fitted with the device in the office setting, she is independently able to insert and remove it as well as deflate the balloon to allow for bowel movements and inflate the balloon to prevent accidental bowel leakage.
In a multicenter trial conducted by Richter and colleagues,19 78% of women successfully fitted with the device had a 50% mean reduction of fecal incontinence episodes. Two-week mean incontinence episodes decreased from 11 to 2 after 1 month of continued use of the insert. In addition, there was significant improvement in quality-of-life questionnaire scores.
Of the 110 patients fitted with the device, 32 (29%) withdrew due to unsatisfactory device fit or were unable to remove or insert the device themselves. Common adverse effects included pelvic cramping and discomfort during device fitting. One month after insertion, pelvic pain and cramping continued in up to 10% of patients. No serious adverse events related to the device were observed during the 1-month trial.19
In the approximate 70% of women successfully fitted with the vaginal insert, the system was highly efficacious in improving subjective and objective outcomes with no unexpected serious adverse events. Currently the device is available at investigative sites across the United States, and the company plans for sales to begin later this year.
Surgical options for fecal incontinence
In patients for whom conservative and medical therapies have failed, surgical treatments may be offered. Surgical options vary from minimally invasive procedures to colostomy. One of the minimally invasive procedures available is the InterStim procedure, or sacral nerve stimulation (SNS). An electrode is inserted percutaneously through the S3 foramen and is connected to an implanted battery under the skin of the buttocks. Low-voltage stimulation is applied to the leads that lie adjacent to the S3 sacral nerve roots.
Patients with SNS experience fewer episodes of fecal incontinence, with over 80% maintaining a reduction in fecal incontinent episodes by greater than 50% up to 5 years after implantation.20,21
The transobturator postanal sling system (TOPAS, Astora) is a new investigational surgical device. It is inserted in a minimally invasive procedure and is currently undergoing a prospective, multicenter clinical trial (FIGURE 4). It consists of a polypropylene mesh sling placed perianally, with the mesh arms exiting through the obturator foramen bilaterally. It is intended to increase posterior pelvic support at the level of the anorectal junction. Efficacy and safety of the product have yet to be determined.
We need to stay up to date on new treatment options
As the prevalence increases for urinary and fecal incontinence, ObGyns are challenged to remain knowledgeable about the condition, the prognosis, and the success of interventions. Currently, patients have a range of options to manage their urinary and fecal incontinence symptoms, with the number of products and clinical data increasing over time. With the advent of novel products and the widespread availability of information via the Internet, physicians must remain the established source on new innovative treatments and up-to-date clinical data in order to provide competent and comprehensive care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189(2):428–434.
- Lensen EJ, Withagen MI, Kluivers KB, Milani AL, Vierhout ME. Urinary incontinence after surgery for pelvic organ prolapse. Neurourol Urodyn. 2013;32(5):455–459.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115(3):609–617.
- Ziv E, Stanton SL, Abarbanel J. Efficacy and safety of a novel disposable intravaginal device for treating stress urinary incontinence. Am J Obstet Gynecol. 2008;198(5):594.e1–e7.
- Ziv E, Stanton SL, Abarbanel J. Significant improvement in the quality of life in women treated with a novel disposable intravaginal device for stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(6):651–658.
- Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. Int Urogynecol J. 2013;24(1):27–36.
- Wyndaele JJ, De Wachter S, Tommaselli GA, et al. A randomized, controlled clinical trial of an intravesical pressure-attenuation balloon system for the treatment of stress urinary incontinence in females [published online ahead of print January 16, 2015]. Neurourol Urodyn. doi:10.1002/nau.22708.
- Ford AA, Rogerson L, Cody JD, Ogah J. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2015;7:CD006375.
- Lee JK, Rosamilia A, Dwyer PL, Lim YN, Muller R. Randomized trial of a single incision versus an outside-in transobturator midurethral sling in women with stress urinary incontinence: 12 month results. Am J Obstet Gynecol. 2015;213(1):35.e1–e9.
- Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132–1138.
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
- Nitti VW, Dmochowski R, Herschorn S, et al; EMBARK Study Group. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol. 2013;189(6):2186−2193.
- Cui Y, Zhou X, Zong H, Yan H, Zhang Y. The efficacy and safety of onabotulinumtoxinA in treating idiopathic OAB: A systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):413–419.
- Apostolidis A, Dasgupta P, Denys P, et al; European Consensus Panel. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- Johanson JF, Lafferty J. Epidemiology of fecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91(1):33–36.
- Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1997;89(3):423–427.
- Richter HE, Matthews CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125(3):540–547.
- Thaha MA, Abukar AA, Thin NN, Ramsanahie A, Knowles CH. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015;8:CD004464.
- Hull T, Giese C, Wexner SD, et al; SNS Study Group. Long-term durability of sacral nerve stimulation therapy for chronic fecal incontinence. Dis Colon Rectum. 2013;56(2):234–245.
In this Article
- New OTC option for SUI
- Second-line OAB treatments
- Promising vaginal insert for fecal incontinence
Failure to find breast cancer; later diagnosed at Stage 3
Stroke during delivery: $35.4M verdict
Stroke during delivery: $35.4M verdict
During delivery, a 25-year-old woman had a hemorrhagic stroke that left her unable to care for herself or her child.
Patient’s claim The patient’s neurologist failed to advise the ObGyn that the patient had a history of brain aneurysm and a venous varix, which increased the risk for stroke during labor and delivery. The patient had shared her history with the ObGyn, and she requested that her neurologist contact the ObGyn.
Neurologist’s defense There was no negligence. The entire medical file had been delivered to the ObGyn. Any negligence was on the part of the ObGyn for failure to educate herself as to the patient’s condition.
Verdict A $35.4 million Massachusetts verdict was returned against the neurologist, including $12.9 million for past and future pain and suffering, $4 million for past medical care, $11 million for future medical care, $4.5 million for the husband’s loss of consortium, $1.5 million for lost wages, and $1.5 million for the child’s loss of consortium.
IUGR detected but not immediately treated: $15.5M settlement
During a prenatal visit at 38 weeks’ gestation, a mother’s ObGyn saw signs of intrauterine growth restriction (IUGR) but did not order ultrasono- graphy to confirm the diagnosis or induce labor. When born 15 days later, the baby had a low birth weight and low Apgar scores. The child has permanent brain injury due to hypoxia.
Parents’ claim The ObGyn should have confirmed the presence of IUGR and appropriately managed the mother’s prenatal care. The child’s injuries could have been prevented if an earlier delivery had occurred.
Physician’s defense The case was settled during the trial.
Verdict A $15.5 million Illinois settlement was reached.
Ureter injured during total abdominal hysterectomy
A 40-year-old woman with uterine fibroids, excessive bleeding, and pelvic pain underwent total abdominal hysterectomy performed by her ObGyn.
Postoperatively, the patient reported abdominal pain, but she was discharged from the hospital. Two days later, she returned to the emergency department reporting continued and increasing abdominal pain and urine leakage. The ObGyn referred her to a urologist who diagnosed stricture of the left ureter with a ureterovaginal fistula. A nephrostomy procedure was performed. Three months later, left ureter reimplantation surgery was completed.
Patient’s claim The ObGyn was negligent in injuring the ureter during hysterectomy, in not identifying the injury during surgery, and in not diagnosing and treating the injury in a timely manner, despite the patient’s reports of increasing pain.
Physician’s defense The case was settled during the trial.
Verdict A $350,000 Virginia settlement was reached.
Breech presentation but cesarean not performed
When her water broke, a mother was admitted to the hospital. The ObGyn ordered induction of labor but deferred vaginal examination to avoid infection. After labor was induced, a nurse noticed the presence of meconium. She performed a vaginal examination and found that the baby was in breech position; she did not immediately contact the ObGyn. After several hours of labor, the baby was born limp and not breathing with a heart rate of 50 bpm. The baby was resuscitated but sustained severe brain damage.
Parents’ claim The mother should have been examined before induction of labor. When it was determined that the baby was in breech position, a cesarean delivery should have been ordered. Communication between the nurse and ObGyn was poor.
Defendants' defense There was no negligence; labor was managed according to the standard of care.
Verdict A New Jersey defense verdict was returned.
Stroke during delivery: $35.4M verdict
During delivery, a 25-year-old woman had a hemorrhagic stroke that left her unable to care for herself or her child.
Patient’s claim The patient’s neurologist failed to advise the ObGyn that the patient had a history of brain aneurysm and a venous varix, which increased the risk for stroke during labor and delivery. The patient had shared her history with the ObGyn, and she requested that her neurologist contact the ObGyn.
Neurologist’s defense There was no negligence. The entire medical file had been delivered to the ObGyn. Any negligence was on the part of the ObGyn for failure to educate herself as to the patient’s condition.
Verdict A $35.4 million Massachusetts verdict was returned against the neurologist, including $12.9 million for past and future pain and suffering, $4 million for past medical care, $11 million for future medical care, $4.5 million for the husband’s loss of consortium, $1.5 million for lost wages, and $1.5 million for the child’s loss of consortium.
IUGR detected but not immediately treated: $15.5M settlement
During a prenatal visit at 38 weeks’ gestation, a mother’s ObGyn saw signs of intrauterine growth restriction (IUGR) but did not order ultrasono- graphy to confirm the diagnosis or induce labor. When born 15 days later, the baby had a low birth weight and low Apgar scores. The child has permanent brain injury due to hypoxia.
Parents’ claim The ObGyn should have confirmed the presence of IUGR and appropriately managed the mother’s prenatal care. The child’s injuries could have been prevented if an earlier delivery had occurred.
Physician’s defense The case was settled during the trial.
Verdict A $15.5 million Illinois settlement was reached.
Ureter injured during total abdominal hysterectomy
A 40-year-old woman with uterine fibroids, excessive bleeding, and pelvic pain underwent total abdominal hysterectomy performed by her ObGyn.
Postoperatively, the patient reported abdominal pain, but she was discharged from the hospital. Two days later, she returned to the emergency department reporting continued and increasing abdominal pain and urine leakage. The ObGyn referred her to a urologist who diagnosed stricture of the left ureter with a ureterovaginal fistula. A nephrostomy procedure was performed. Three months later, left ureter reimplantation surgery was completed.
Patient’s claim The ObGyn was negligent in injuring the ureter during hysterectomy, in not identifying the injury during surgery, and in not diagnosing and treating the injury in a timely manner, despite the patient’s reports of increasing pain.
Physician’s defense The case was settled during the trial.
Verdict A $350,000 Virginia settlement was reached.
Breech presentation but cesarean not performed
When her water broke, a mother was admitted to the hospital. The ObGyn ordered induction of labor but deferred vaginal examination to avoid infection. After labor was induced, a nurse noticed the presence of meconium. She performed a vaginal examination and found that the baby was in breech position; she did not immediately contact the ObGyn. After several hours of labor, the baby was born limp and not breathing with a heart rate of 50 bpm. The baby was resuscitated but sustained severe brain damage.
Parents’ claim The mother should have been examined before induction of labor. When it was determined that the baby was in breech position, a cesarean delivery should have been ordered. Communication between the nurse and ObGyn was poor.
Defendants' defense There was no negligence; labor was managed according to the standard of care.
Verdict A New Jersey defense verdict was returned.
Stroke during delivery: $35.4M verdict
During delivery, a 25-year-old woman had a hemorrhagic stroke that left her unable to care for herself or her child.
Patient’s claim The patient’s neurologist failed to advise the ObGyn that the patient had a history of brain aneurysm and a venous varix, which increased the risk for stroke during labor and delivery. The patient had shared her history with the ObGyn, and she requested that her neurologist contact the ObGyn.
Neurologist’s defense There was no negligence. The entire medical file had been delivered to the ObGyn. Any negligence was on the part of the ObGyn for failure to educate herself as to the patient’s condition.
Verdict A $35.4 million Massachusetts verdict was returned against the neurologist, including $12.9 million for past and future pain and suffering, $4 million for past medical care, $11 million for future medical care, $4.5 million for the husband’s loss of consortium, $1.5 million for lost wages, and $1.5 million for the child’s loss of consortium.
IUGR detected but not immediately treated: $15.5M settlement
During a prenatal visit at 38 weeks’ gestation, a mother’s ObGyn saw signs of intrauterine growth restriction (IUGR) but did not order ultrasono- graphy to confirm the diagnosis or induce labor. When born 15 days later, the baby had a low birth weight and low Apgar scores. The child has permanent brain injury due to hypoxia.
Parents’ claim The ObGyn should have confirmed the presence of IUGR and appropriately managed the mother’s prenatal care. The child’s injuries could have been prevented if an earlier delivery had occurred.
Physician’s defense The case was settled during the trial.
Verdict A $15.5 million Illinois settlement was reached.
Ureter injured during total abdominal hysterectomy
A 40-year-old woman with uterine fibroids, excessive bleeding, and pelvic pain underwent total abdominal hysterectomy performed by her ObGyn.
Postoperatively, the patient reported abdominal pain, but she was discharged from the hospital. Two days later, she returned to the emergency department reporting continued and increasing abdominal pain and urine leakage. The ObGyn referred her to a urologist who diagnosed stricture of the left ureter with a ureterovaginal fistula. A nephrostomy procedure was performed. Three months later, left ureter reimplantation surgery was completed.
Patient’s claim The ObGyn was negligent in injuring the ureter during hysterectomy, in not identifying the injury during surgery, and in not diagnosing and treating the injury in a timely manner, despite the patient’s reports of increasing pain.
Physician’s defense The case was settled during the trial.
Verdict A $350,000 Virginia settlement was reached.
Breech presentation but cesarean not performed
When her water broke, a mother was admitted to the hospital. The ObGyn ordered induction of labor but deferred vaginal examination to avoid infection. After labor was induced, a nurse noticed the presence of meconium. She performed a vaginal examination and found that the baby was in breech position; she did not immediately contact the ObGyn. After several hours of labor, the baby was born limp and not breathing with a heart rate of 50 bpm. The baby was resuscitated but sustained severe brain damage.
Parents’ claim The mother should have been examined before induction of labor. When it was determined that the baby was in breech position, a cesarean delivery should have been ordered. Communication between the nurse and ObGyn was poor.
Defendants' defense There was no negligence; labor was managed according to the standard of care.
Verdict A New Jersey defense verdict was returned.
Additional Medical Verdicts cases
Failure to find breast cancer; later diagnosed at Stage 3
Endometrial cancer after unopposed estrogen: $7.5M
Minimally invasive and abdominal hysterectomy yield similar results for endometrial cancer
Use of minimally invasive hysterectomy in patients with endometrial cancer, on the rise since 2007, has long-term survival rates comparable to those of abdominal hysterectomy, and a favorable morbidity profile.
Minimally invasive hysterectomy had similar overall mortality (hazard ratio[HR], 0.89; 95% CI, 0.75 to 1.04) and cancer-specific mortality (HR, 0.83; 95% CI, 0.59-1.16), compared with abdominal hysterectomy. Robot-assisted hysterectomy, compared with laparoscopic, had similar overall and cancer-specific mortality.
Adjuvant radiation was increased in women who underwent minimally invasive compared with abdominal hysterectomy (for pelvic radiation 34.3% vs. 31.3%; odds ratio[OR], 1.14; 95% CI, 1.04-1.26; for brachytherapy 33.6% vs. 31.0%; OR, 1.13; 95% CI, 1.03-1.24).
“The mechanism underlying the need for increased use of adjuvant therapy after minimally invasive hysterectomy remains unclear ... Particularly for women with large uteri, manipulation at the time of surgery or disruption or spillage of tumor from the uterine cavity may prompt use of radiation. This phenomenon warrants further investigation and careful monitoring,” wrote Dr. Jason Wright, chief of gynecologic oncology at Columbia University College of Physicians and Surgeons, New York, and colleagues (J Clin Oncol. 2016 Feb 1. doi: 10.1200/JCO.2015.65.3212).
The population-based analysis used SEER-Medicare data of 6,305 patients aged 65 years and greater with stage I-III uterine cancer who underwent hysterectomy from 2006 to 2011. In total, 4,139 (65.7%) underwent abdominal hysterectomy and 2,165 (34.3%) underwent minimally invasive surgery.
Results showed that the use of minimally invasive hysterectomy has increased from 9.3% in 2006 to 61.7% in 2011, and over 60% of the minimally invasive surgeries were robot-assisted.
Minimally invasive hysterectomy had fewer complications than did abdominal hysterectomy (22.7% vs. 39.7%; OR, 0.46; 95% CI, 0.41-0.51), including lower rates of surgical site complications, medical complications, transfusions, and perioperative mortality.
Robot-assisted hysterectomy had a slightly higher complication rate than did laparoscopic hysterectomy (23.7% vs. 19.5%; OR 1.28; 95% CI, 1.03-1.59), due to postoperative medical complications. Respiratory and renal failure were higher after robot-assisted surgery, as was bacteremia. The complications may be a result of longer operative times, as reported in previous studies.
Use of minimally invasive hysterectomy in patients with endometrial cancer, on the rise since 2007, has long-term survival rates comparable to those of abdominal hysterectomy, and a favorable morbidity profile.
Minimally invasive hysterectomy had similar overall mortality (hazard ratio[HR], 0.89; 95% CI, 0.75 to 1.04) and cancer-specific mortality (HR, 0.83; 95% CI, 0.59-1.16), compared with abdominal hysterectomy. Robot-assisted hysterectomy, compared with laparoscopic, had similar overall and cancer-specific mortality.
Adjuvant radiation was increased in women who underwent minimally invasive compared with abdominal hysterectomy (for pelvic radiation 34.3% vs. 31.3%; odds ratio[OR], 1.14; 95% CI, 1.04-1.26; for brachytherapy 33.6% vs. 31.0%; OR, 1.13; 95% CI, 1.03-1.24).
“The mechanism underlying the need for increased use of adjuvant therapy after minimally invasive hysterectomy remains unclear ... Particularly for women with large uteri, manipulation at the time of surgery or disruption or spillage of tumor from the uterine cavity may prompt use of radiation. This phenomenon warrants further investigation and careful monitoring,” wrote Dr. Jason Wright, chief of gynecologic oncology at Columbia University College of Physicians and Surgeons, New York, and colleagues (J Clin Oncol. 2016 Feb 1. doi: 10.1200/JCO.2015.65.3212).
The population-based analysis used SEER-Medicare data of 6,305 patients aged 65 years and greater with stage I-III uterine cancer who underwent hysterectomy from 2006 to 2011. In total, 4,139 (65.7%) underwent abdominal hysterectomy and 2,165 (34.3%) underwent minimally invasive surgery.
Results showed that the use of minimally invasive hysterectomy has increased from 9.3% in 2006 to 61.7% in 2011, and over 60% of the minimally invasive surgeries were robot-assisted.
Minimally invasive hysterectomy had fewer complications than did abdominal hysterectomy (22.7% vs. 39.7%; OR, 0.46; 95% CI, 0.41-0.51), including lower rates of surgical site complications, medical complications, transfusions, and perioperative mortality.
Robot-assisted hysterectomy had a slightly higher complication rate than did laparoscopic hysterectomy (23.7% vs. 19.5%; OR 1.28; 95% CI, 1.03-1.59), due to postoperative medical complications. Respiratory and renal failure were higher after robot-assisted surgery, as was bacteremia. The complications may be a result of longer operative times, as reported in previous studies.
Use of minimally invasive hysterectomy in patients with endometrial cancer, on the rise since 2007, has long-term survival rates comparable to those of abdominal hysterectomy, and a favorable morbidity profile.
Minimally invasive hysterectomy had similar overall mortality (hazard ratio[HR], 0.89; 95% CI, 0.75 to 1.04) and cancer-specific mortality (HR, 0.83; 95% CI, 0.59-1.16), compared with abdominal hysterectomy. Robot-assisted hysterectomy, compared with laparoscopic, had similar overall and cancer-specific mortality.
Adjuvant radiation was increased in women who underwent minimally invasive compared with abdominal hysterectomy (for pelvic radiation 34.3% vs. 31.3%; odds ratio[OR], 1.14; 95% CI, 1.04-1.26; for brachytherapy 33.6% vs. 31.0%; OR, 1.13; 95% CI, 1.03-1.24).
“The mechanism underlying the need for increased use of adjuvant therapy after minimally invasive hysterectomy remains unclear ... Particularly for women with large uteri, manipulation at the time of surgery or disruption or spillage of tumor from the uterine cavity may prompt use of radiation. This phenomenon warrants further investigation and careful monitoring,” wrote Dr. Jason Wright, chief of gynecologic oncology at Columbia University College of Physicians and Surgeons, New York, and colleagues (J Clin Oncol. 2016 Feb 1. doi: 10.1200/JCO.2015.65.3212).
The population-based analysis used SEER-Medicare data of 6,305 patients aged 65 years and greater with stage I-III uterine cancer who underwent hysterectomy from 2006 to 2011. In total, 4,139 (65.7%) underwent abdominal hysterectomy and 2,165 (34.3%) underwent minimally invasive surgery.
Results showed that the use of minimally invasive hysterectomy has increased from 9.3% in 2006 to 61.7% in 2011, and over 60% of the minimally invasive surgeries were robot-assisted.
Minimally invasive hysterectomy had fewer complications than did abdominal hysterectomy (22.7% vs. 39.7%; OR, 0.46; 95% CI, 0.41-0.51), including lower rates of surgical site complications, medical complications, transfusions, and perioperative mortality.
Robot-assisted hysterectomy had a slightly higher complication rate than did laparoscopic hysterectomy (23.7% vs. 19.5%; OR 1.28; 95% CI, 1.03-1.59), due to postoperative medical complications. Respiratory and renal failure were higher after robot-assisted surgery, as was bacteremia. The complications may be a result of longer operative times, as reported in previous studies.
Key clinical point: Robot-assisted and laparoscopic hysterectomy had long-term survival comparable to that of abdominal hysterectomy in patients with uterine cancer.
Major finding: Minimally invasive hysterectomy had similar overall mortality (hazard ratio[HR], 0.89; 95% CI, 0.75-1.04) and cancer-specific mortality (HR, 0.83; 95% CI, 0.59-1.16), compared with abdominal hysterectomy.
Data source: Population-based analysis using SEER-Medicare data on 6,305 patients who underwent hysterectomy (4,139 abdominal and 2,165 minimally invasive) from 2006 to 2011.
Disclosures: Dr. Wright reported having no disclosures. Two of his coauthors reported ties to industry.
Appendicitis, antibiotics, and surgery: An evolving trilogy
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Appendicitis is the most common surgical emergency in children. It is seen at all ages; however, it is less common in infants and toddlers younger than 4 years of age and peaks at an incidence of 25/100,000 in children 12- to 18-years-old. Fortunately, appendicitis is rarely fatal but can be associated with significant morbidity, especially in young children in whom the diagnosis is often delayed and perforation is more common. Reducing morbidity requires early diagnosis and optimizing management such that perforation and associated peritonitis are prevented.
The classical signs and symptoms of appendicitis are periumbilical pain migrating to the right lower quadrant, nausea, and low-grade fever. Presentation may vary if the location of the appendix is atypical, but primarily is age associated. In young children, abdominal distension, hip pain with or without limp, and fever are commonplace. In older children, right lower quadrant abdominal pain that intensifies with coughing or movement is frequent. Localized tenderness also appears to be age related; right lower quadrant tenderness and rebound are more often found in older children and adolescents, whereas younger children have more diffuse signs.
When I started my career, abdominal x-rays would be performed in search of a fecalith. However, such studies were of low sensitivity, and clinical acumen had a primary role in the decision to take the child to the operating room. In the current era, ultrasound and CT scan provide reasonable sensitivity and specificity. Ultrasound criteria include a diameter greater than 6 mm, concentric rings (target sign), an appendicolith, high echogenicity, obstruction of the lumen, and fluid surrounding the appendix.
As the pathogenesis of appendicitis represents occlusion of the appendiceal lumen, followed by overgrowth or translocation of bowel flora resulting in inflammation of the wall of the appendix, anaerobes and gram-negative gut flora represent the most important pathogens. In advanced cases, necrosis and gangrene of the appendix result with progression to rupture and peritonitis.
The traditional management was early surgical intervention to reduce the risk of perforation and peritonitis with acceptance of high rates of negative abdominal explorations as an acceptable consequence. Today, the approach to management of appendicitis is undergoing reevaluation. Early antimicrobial treatment has become routine in the management of nonperforated, perforated, or abscessed appendicitis. However, the question being asked is, “Do all children with uncomplicated appendicitis need appendectomy, or is antibiotic management sufficient?”
P. Salminen et al. reported on the results of a randomized clinical trial in 530 patients aged 18-60 years, comparing antimicrobial treatment alone with early appendectomy. Among 273 patients in the surgical group, all but 1 underwent successful appendectomy, resulting in a success rate of 99.6% (95% CI, 98.0%-100.0%). In the antibiotic group, 186 of 256 patients (70%) treated with antibiotics did not require surgery; 70 (27%) underwent appendectomy within 1 year of initial presentation for appendicitis (JAMA. 2015 Jun 16;313[23]:2340-8). There were no intraabdominal abscesses or other major complications associated with delayed appendectomy in patients randomized to antibiotic treatment. The authors concluded that among patients with CT-proven, uncomplicated appendicitis, antibiotic treatment did not meet the prespecified criterion for noninferiority, compared with appendectomy. However, most patients randomized to antibiotics for uncomplicated appendicitis did not require appendectomy during the 1-year follow-up period.
J.A. Horst et al. reviewed published reports of medical management of appendicitis in children (Ann Emerg Med. 2015 Aug;66[2]:119-22). They concluded that medical management of uncomplicated appendicitis in a select low-risk pediatric population is safe and does not result in significant morbidity. The arguments against a nonoperative approach include the risk of recurrent appendicitis, including the anxiety associated with any recurrences of abdominal pain, the risk of antibiotic-related complications, the potential for increased duration of hospitalization, and the relatively low morbidity of appendectomy in children. Factors associated with failed antibiotic management included fecaliths, fluid collections, or an appendiceal diameter greater than 1.1 cm on CT scan. The investigators concluded such children are poor candidates for nonsurgical management.
The bottom line is that antimicrobial therapy, in the absence of surgery, can be effective. Certainly in remote settings where surgery is not readily available, antimicrobial therapy with fluid and electrolyte management and close observation can be used in children with uncomplicated appendicitis with few failures and relatively few children requiring subsequent appendectomy. In more complicated cases with evidence of fecalith, or appendiceal abscess or phlegm, initial antimicrobial therapy reduces the acute inflammation and urgent need for surgery, but persistent inflammation of the appendix is often observed and appendectomy, either acutely or after improvement following antimicrobial therapy, appears indicated. Many different antimicrobial regimens have proven effective; ceftriaxone and metronidazole are associated with low rates of complications, offer an opportunity for once-daily therapy, and are cost effective, compared with other once-daily regimens.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center.
Endometrial cancer after unopposed estrogen: $7.5M
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
Endometrial cancer after unopposed estrogen: $7.5M
A 42-year-old woman took unopposed estrogen as treatment for reported perimenopausal symptoms from October 2010 through October 2012, although she still had her uterus.
In December 2013, the patient was diagnosed with Stage 3 endometrial cancer. She underwent a radical hysterectomy followed by several rounds of chemotherapy. Despite treatment, the cancer metastasized, leaving the patient with a decreased life expectancy.
Patient’s claim Use of unopposed estrogens by a woman who still has her uterus significantly increases her risk of developing endometrial cancer. The gynecologist was negligent for prescribing the drug.
Physician’s defense The case was settled during the trial.
Verdict A $7.5 million Illinois settlement was reached
Preeclampsia treatment delayed because BP machine failed: $5M
At 31 4/7 weeks’ gestation, a woman went to the hospital. She reported burning pain in her chest, headache, and vomiting.
Two nurses cared for the mother: one who was completing her shift (Nurse A) and another who was beginning her shift (Nurse B). Nurse A stated that the blood pressure (BP) machine was not working correctly when she attempted to assess the patient at admission. When Nurse B took the patient’s BP, it indicated preeclampsia. Shortly thereafter, fetal heart-rate monitoring showed a concerning pattern. Nurse B notified the ObGyn 75 minutes after the mother’s arrival. The ObGyn ordered intervention to treat the baby’s concerning heart rate and, when those efforts failed, an emergency cesarean delivery was performed. The baby was found to have brain damage caused by hypoxia.
Parents’ claim Because the BP machine was not working properly when the mother arrived at the hospital, intervention for preeclampsia was delayed. Preeclampsia caused the baby’s injuries. An earlier cesarean delivery should have been performed.
Defendant’s defense The patient was adequately treated. The injury likely occurred before the mother arrived at the hospital. The case was settled during the trial.
Verdict A $5 million Massachusetts settlement was reached with the hospital and ObGyn.
Standard prenatal scan missed congenital syndrome: $3.75M
A woman receiving prenatal care at a medical center requested an ultrasonographic anatomical fetal scan. A limited ultrasound (US) was performed, and no abnormalities were detected.
Upon birth, the child was found to have Dandy Walker syndrome, a malformation of the brain affecting mobility. The child requires full-time nursing care.
Parents’ claim If a complete prenatal anatomical survey had been performed when requested, the abnormality would have been detected. The mother would have terminated the pregnancy.
Defendant’s defense It is the medical center’s policy to perform complete anatomical surveys only on women with high-risk pregnancies, which this was not. The woman switched health care providers during her pregnancy. A subsequent US performed by the new health care provider did not show a fetal abnormality. The case was settled during the trial.
Verdict A $3.75 million New Jersey settlement was reached with the medical center.
Should mother have been discharged? $700,000 settlement
Due to elevated fetal heart rate, a woman was admitted to the hospital for fetal heart-rate monitoring and then discharged a few hours later. After 2 days, the mother was readmitted for induction of labor, but she was discharged the following day. The next day, she was readmitted when she noticed lack of fetal movement. The infant was stillborn.
Parents’ claim The mother and fetus were not properly monitored; she should not have been sent home after induction of labor. The hospital was negligent for not properly monitoring labor, for not assigning an ObGyn to care for the mother, and for not performing cesarean delivery.
Hospital’s defense The case was settled during the trial.
Verdict A $700,000 Illinois settlement was reached with the hospital.
ADDITIONAL MEDICAL VERDICTS CASES:
Stroke during delivery: $35.4M verdict: OBG Manag. 2016;28(2):46.
Failure to find breast cancer; later diagnosed at Stage 3: OBG Manag. 2016;28(2):48.
VIDEO: Prophylaxis for atopic dermatitis
GRAND CAYMAN – What simple prophylactic measures can you recommend to parents of children at risk of atopic dermatitis? In an interview at this year’s Caribbean Dermatology Symposium, Dr. Albert C. Yan, section chief of dermatology at the Children’s Hospital of Philadelphia and professor of pediatrics and dermatology, University of Pennsylvania, Philadelphia, discusses the evidence indicating that early use of moisturizers reduces the risk of atopic dermatitis in children at risk.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
On Twitter @whitneymcknight
GRAND CAYMAN – What simple prophylactic measures can you recommend to parents of children at risk of atopic dermatitis? In an interview at this year’s Caribbean Dermatology Symposium, Dr. Albert C. Yan, section chief of dermatology at the Children’s Hospital of Philadelphia and professor of pediatrics and dermatology, University of Pennsylvania, Philadelphia, discusses the evidence indicating that early use of moisturizers reduces the risk of atopic dermatitis in children at risk.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
On Twitter @whitneymcknight
GRAND CAYMAN – What simple prophylactic measures can you recommend to parents of children at risk of atopic dermatitis? In an interview at this year’s Caribbean Dermatology Symposium, Dr. Albert C. Yan, section chief of dermatology at the Children’s Hospital of Philadelphia and professor of pediatrics and dermatology, University of Pennsylvania, Philadelphia, discusses the evidence indicating that early use of moisturizers reduces the risk of atopic dermatitis in children at risk.
The meeting is provided by Global Academy for Medical Education. Global Academy and this news organization are owned by the same parent company.
On Twitter @whitneymcknight
AT THE CARIBBEAN DERMATOLOGY SYMPOSIUM
AAP says newborn pain management plans essential for all providers
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
Repeated exposure to physical pain can have both short-term and long-term adverse effects in newborns, yet providers too inconsistently assess and adequately manage pain in neonates, the American Academy of Pediatrics said in an updated policy statement on preventing and managing procedural pain in these infants.
“Neonates are frequently subjected to painful procedures, with the most immature infants receiving the highest number of painful events,” wrote the AAP Committee on Fetus and Newborn and the AAP Section on Anesthesiology and Pain Medicine. Premature infants also are at the highest risk for long-term sequelae, the committees noted. “These sequelae include physiologic instability; altered brain development; and abnormal neurodevelopment, somatosensory, and stress response systems, which can persist into childhood” (Pediatrics. 2016 Jan 25. doi: 10.1542/peds.2015-4271).
The statement nicely summarizes current evidence on the evaluation and management of pain in neonates, as well as potential short-term and long-term negative effects, said Dr. Clay T. Jones, a neonatal hospitalist at Newton-Wellesley Hospital in Newton, Mass., who was not involved in drafting the statement.
“It is a nice testament to how far we’ve come since the days of performing major surgeries in newborns without the use of analgesic medications, but there is still considerable room for improvement,” Dr. Jones said in an interview. “Although we’ve learned a lot about pain in this population over the past few decades, the authors go to great lengths to make it clear that the optimal way to treat pain hasn’t been established yet, and that the evaluation of risk versus benefit in regards to management using pharmaceutical agents is ongoing.”
The statement described the challenges of effective pain management, including foremost the newborn’s inability to communicate and the dearth of information about effective pain assessments and management in this population.
“Contextual factors such as gestational age and behavioral state may play a significant role in pain assessment and are beginning to be included in some assessment tools,” the committees wrote. “New and emerging technologies to measure pain responses, such as near-infrared spectroscopy, amplitude-integrated electroencephalography, functional MRI, skin conductance, and heart rate variability assessment, are being investigated.”
In the meantime, however, all facilities caring for newborns should implement a pain prevention program that takes advantage of what pain management strategies do exist. In addition to reviewing these strategies, the statement recommended all providers implement and use pain assessment and management plans that include both pharmacologic and nonpharmacologic therapies for major procedures and routine minor interventions that might still cause pain.
“Where this report might have the largest impact is in the outpatient offices of pediatric medical providers and in newborn nurseries,” Dr. Jones said. “It is not uncommon for young infants to undergo painful medical procedures, such as heel sticks and circumcisions, without a systematic approach to pain evaluation and inconsistent efforts to prevent or reduce pain.”
Yet there is no excuse for this lack of a consistent approach, he said.
“Nonpharmaceutical interventions are safe, easy to implement, [and] inexpensive, and there is good evidence that they reduce physiologic surrogate markers for pain such as blood pressure, heart rate, and respiratory rate,” Dr. Jones said.
The statement’s first recommendation is that all institutions have written guidelines for a stepwise plan of pain prevention and treatment in newborns. This plan also should include use of currently available and validated neonatal assessment tools “before, during, and after painful procedures to monitor the effectiveness of pain relief interventions.”
Another recommendation urged pediatricians and other neonatal health care providers and family members to receive ongoing education about recognizing and assessing pain in newborns and then managing it as much as current knowledge and evidence allow. The committees called for more research into pain assessment tools and pain prevention and amelioration strategies, including pharmacologic options.
The nonpharmacologic strategies shown to be effective for reducing pain during short-term mild or moderately painful procedures include facilitated tucking, sensorial stimulation, nonnutritive sucking, and breastfeeding or providing expressed human milk.
“Anything we can do to ease pain will improve a baby’s quality of life,” said Dr. Nathan Boonstra of Blank Children’s Hospital pediatric clinic in Des Moines, Iowa, who was not involved in writing the statement.
“Pediatricians should always be judicious when deciding to draw blood, but when we need to, we should reflexively think about what can keep the procedure’s pain at a minimum,” he said in an interview. “Many of my patients’ mothers instinctively want to breastfeed to help their newborns through something painful, and their instinct serves them well.”
The policy statement mentioned oral sucrose and/or glucose solutions as effective analgesic options for mild to moderately painful procedures, but recommended these be prescribed and tracked as medication.
This strategy carries risks and other drawbacks, however, Dr. Thomas M Seman, a pediatrician in group practice in Danvers, Mass., said in an interview
“Having sucrose or glucose solutions in the office can be dangerous because of the risk of overuse and hyperglycemia as well as the cost of these items,” said Dr. Seman, who was not involved with drafting the AAP statement. His office policy primarily focuses on the parents holding their children and talking, singing, or humming to them during procedures, followed by feeding and/or acetaminophen, he said.
“The other medications used are prohibitive for a number of reasons,” said Dr. Seman, although he added that most of the procedures described in the statement are performed on premature infants in a NICU with only a few done in private practices.
For example, opioids such as fentanyl and morphine are most frequently used for persistent pain, yet the data on appropriate dosing and long-term effects in the newborn period are “woefully lacking and/or conflicting,” the statement noted. The evidence for benzodiazepines, often used for sedation in the neonatal intensive care, shows little additional analgesic benefit, but these agents can potentiate the risk of respiratory depression and hypotension associated with opioid use. Caution should particularly be exercised before using methadone, ketamine, propofol, and dexmedetomidine because so little is known about safe and effective dosing of these medications in neonates. They also carry various potential risks ranging from neurotoxicity to bradycardia, desaturations, and prolonged hypotension.
While NSAIDs are not recommended at this young age, oral or intravenous acetaminophen has sufficient preliminary safety and efficacy to be considered for postoperative pain relief, according to the statement.
The AAP did not report disclosures for committee members. Dr. Boonstra, Dr. Jones, and Dr. Seman had no relevant financial disclosures.
FROM PEDIATRICS
Study: Robot-assisted hysterectomy as fast, safe as laparoscopic approach
LAS VEGAS – Robot-assisted laparoscopic hysterectomy is just as quick and safe as standard laparoscopic hysterectomy – at least in the hands of an experienced surgeon, according to a surgical trial of 144 women.
The first prospective, randomized trial comparing the two techniques found no differences in operative time, intraoperative complications, postoperative pain, length of stay, or 12-week complications, Dr. Timothy Deimling reported at a meeting sponsored by the AAGL.
He did caution, however, that the single surgeon who performed all of the procedures was highly experienced with robotic surgery, having performed more than 600 cases in his career.
The trial included 144 women who underwent hysterectomy for benign conditions. There were 72 women in each surgical group. They were consented at the time of consult, but not randomized until the morning of surgery, said Dr. Deimling of the Penn State Milton S. Hershey Medical Center, in Hershey, Pa.
There were no significant between-group differences in any of the baseline characteristics, nor in any of the indications for surgery, with one exception: More women in the laparoscopic group had undergone prior cesarean sections (44% vs. 23%).
The primary outcome was mean operative time, which was considered initial incision to closure. This was similar in the robot-assisted and laparoscopic groups (74 vs. 75 minutes).
Secondary outcomes were also similar, including pain, which was scored on a 1-10 scale. The mean score was 3.9 in the laparoscopic group and 3.8 in the robotic group. Likewise length of stay was similar (mean 19.6 vs. 22 hours).
There was one serious intraoperative complication. A patient in the laparoscopic group experienced a ureteral injury after the insertion of the first trocar, which resulted in termination of the surgery. She later had a successful laparoscopic hysterectomy. Postoperative complications were also similar in the two groups.
But it’s unclear if the results are generalizable. Dr. Deimling noted that the single surgeon who performed all the cases was highly experienced in robotic surgery. Additionally, all cases were assisted by a surgical team of nurses and technicians who were highly trained in both laparoscopic and robotic gynecologic surgery.
Dr. Deimling reported having no relevant financial disclosures.
LAS VEGAS – Robot-assisted laparoscopic hysterectomy is just as quick and safe as standard laparoscopic hysterectomy – at least in the hands of an experienced surgeon, according to a surgical trial of 144 women.
The first prospective, randomized trial comparing the two techniques found no differences in operative time, intraoperative complications, postoperative pain, length of stay, or 12-week complications, Dr. Timothy Deimling reported at a meeting sponsored by the AAGL.
He did caution, however, that the single surgeon who performed all of the procedures was highly experienced with robotic surgery, having performed more than 600 cases in his career.
The trial included 144 women who underwent hysterectomy for benign conditions. There were 72 women in each surgical group. They were consented at the time of consult, but not randomized until the morning of surgery, said Dr. Deimling of the Penn State Milton S. Hershey Medical Center, in Hershey, Pa.
There were no significant between-group differences in any of the baseline characteristics, nor in any of the indications for surgery, with one exception: More women in the laparoscopic group had undergone prior cesarean sections (44% vs. 23%).
The primary outcome was mean operative time, which was considered initial incision to closure. This was similar in the robot-assisted and laparoscopic groups (74 vs. 75 minutes).
Secondary outcomes were also similar, including pain, which was scored on a 1-10 scale. The mean score was 3.9 in the laparoscopic group and 3.8 in the robotic group. Likewise length of stay was similar (mean 19.6 vs. 22 hours).
There was one serious intraoperative complication. A patient in the laparoscopic group experienced a ureteral injury after the insertion of the first trocar, which resulted in termination of the surgery. She later had a successful laparoscopic hysterectomy. Postoperative complications were also similar in the two groups.
But it’s unclear if the results are generalizable. Dr. Deimling noted that the single surgeon who performed all the cases was highly experienced in robotic surgery. Additionally, all cases were assisted by a surgical team of nurses and technicians who were highly trained in both laparoscopic and robotic gynecologic surgery.
Dr. Deimling reported having no relevant financial disclosures.
LAS VEGAS – Robot-assisted laparoscopic hysterectomy is just as quick and safe as standard laparoscopic hysterectomy – at least in the hands of an experienced surgeon, according to a surgical trial of 144 women.
The first prospective, randomized trial comparing the two techniques found no differences in operative time, intraoperative complications, postoperative pain, length of stay, or 12-week complications, Dr. Timothy Deimling reported at a meeting sponsored by the AAGL.
He did caution, however, that the single surgeon who performed all of the procedures was highly experienced with robotic surgery, having performed more than 600 cases in his career.
The trial included 144 women who underwent hysterectomy for benign conditions. There were 72 women in each surgical group. They were consented at the time of consult, but not randomized until the morning of surgery, said Dr. Deimling of the Penn State Milton S. Hershey Medical Center, in Hershey, Pa.
There were no significant between-group differences in any of the baseline characteristics, nor in any of the indications for surgery, with one exception: More women in the laparoscopic group had undergone prior cesarean sections (44% vs. 23%).
The primary outcome was mean operative time, which was considered initial incision to closure. This was similar in the robot-assisted and laparoscopic groups (74 vs. 75 minutes).
Secondary outcomes were also similar, including pain, which was scored on a 1-10 scale. The mean score was 3.9 in the laparoscopic group and 3.8 in the robotic group. Likewise length of stay was similar (mean 19.6 vs. 22 hours).
There was one serious intraoperative complication. A patient in the laparoscopic group experienced a ureteral injury after the insertion of the first trocar, which resulted in termination of the surgery. She later had a successful laparoscopic hysterectomy. Postoperative complications were also similar in the two groups.
But it’s unclear if the results are generalizable. Dr. Deimling noted that the single surgeon who performed all the cases was highly experienced in robotic surgery. Additionally, all cases were assisted by a surgical team of nurses and technicians who were highly trained in both laparoscopic and robotic gynecologic surgery.
Dr. Deimling reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point: Robot-assisted minimally invasive hysterectomy was as quick and as safe as standard laparoscopic surgery.
Major finding: Mean operative time was similar in the robot-assisted hysterectomy and standard laparoscopic groups, at 74 and 75 minutes, respectively.
Data source: The randomized trial included 144 women who underwent hysterectomy for benign conditions.
Disclosures: Dr. Deimling reported having no relevant financial disclosures.
Combined Tibial Tubercle Avulsion Fracture and Patellar Avulsion Fracture: An Unusual Variant in an Adolescent Patient
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.