User login
Epilepsy surgery outcome prediction seeks to gain ground
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
BANGKOK –
She and her colleagues have created and validated an online risk prediction tool that clinicians can use to predict a patient’s individualized likelihood of complete freedom from seizures 2 and 5 years after undergoing resective brain surgery for drug-resistant epilepsy. The risk predictor, known as the Epilepsy Surgery Nomogram, uses a handful of simple clinical characteristics – patient gender, pathologic cause of the seizures, the proposed type of epilepsy surgery, the presence or absence of generalized tonic-clonic seizures, epilepsy duration, and preoperative seizure frequency – and spits out the patient’s predicted seizure outcome, she explained at the congress, sponsored by the International League Against Epilepsy.
“The point here is that every patient is an individual. And to give people predictions based on 500- or 600-patient Kaplan-Meier-derived curves that just provide the average outcome for the whole cohort isn’t really going to give them what they need as far as their individualized chance of becoming seizure free,” said Dr. Jehi, a neurologist at the Cleveland Clinic.
Similarly, reliance solely upon clinical judgment is a minefield. Multiple biases prevent physicians from making objective medical predictions, she continued.
“We think of the process of medical decision-making and outcome prediction as being a process that is logical and rational, where the accumulation of knowledge improves the decisions that we make, and where past experience improves judgment, and where collective decisions are more reliable. This is what intuitively we all think. That’s why we think we are invincible as physicians. And to that I say, really? There is a wealth of literature that actually disproves each one of these points,” Dr. Jehi declared.
Outcomes of brain surgery for drug-resistant epilepsy have remained static for more than half a century: Ten years after surgery, roughly half of treated patients remain completely seizure free. The inability of clinicians to use advanced statistics to inform potential surgical candidates about their individualized chance of becoming seizure free has probably contributed to underutilization of epilepsy surgery, she added.
The Epilepsy Surgery Nomogram was developed through detailed analysis of the records of 846 patients who underwent epilepsy surgery at the Cleveland Clinic. The resultant nomogram was then validated in a cohort of 604 patients who had resective surgery at the Mayo Clinic and epilepsy surgery centers in Brazil, Italy, and France. In the development cohort, the rate of complete freedom from seizures was 57% at 2 years and 40% at 5 years. In the validation study, the nomogram had a concordance statistic of 0.60 for complete freedom from seizures, which is considered better than chance, but well below the 0.80 threshold defined as strong concordance (Lancet Neurol. 2015 Mar;14[3]:283-90).
However, in an era when personalized medicine has become a catch phrase, the Epilepsy Surgery Nomogram has captured the attention of officials at the National Institutes of Health. Indeed, Dr. Jehi and her coworkers have received a $3.4 million, 5-year grant from the NIH to improve their risk prediction model by incorporating additional variables, including EEG data, MRI findings, family history, and genetic information. The enhanced risk calculator also will include a predictor of the likelihood that an individual will experience clinically meaningful improvement in quality of life in response to epilepsy surgery, since that’s an important outcome even in the absence of 100% freedom from seizures.
Recently, Dr. Jehi and coworkers have developed and then externally validated nomograms to predict the individualized risk of clinically relevant postoperative naming decline after temporal lobe epilepsy surgery in adults. A model based upon five variables – side of surgery, sex, education, age at epilepsy onset, and age at epilepsy surgery – performed very well, with a concordance statistic of 0.81. Moreover, a second nomogram predicting moderate to severe postoperative naming decline on the basis of just three variables – side of surgery, age at epilepsy onset, and preoperative score on the Boston Naming Test – had a concordance statistic of 0.84 (Neurology. 2018 Dec 4;91[23]:e2144-e2152. doi: 10.1212/WNL.0000000000006629).
“Our future hopefully is one where there will always be room for gut feelings and intuition because we definitely need them. We want to honor them. But hopefully it is one where algorithms can help our guesses be more educated and where the science of algorithms and predictive modeling can help inform our outcome predictions and decision-making process,” she said.
The original Epilepsy Surgery Nomogram project was funded by the Cleveland Clinic Epilepsy Center. The postoperative naming decline nomograms project was funded by the NIH.
REPORTING FROM IEC 2019
FDA update: Higher late mortality with paclitaxel-coated devices
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Product Update: Osphena’s NDA, new hysteroscope, TempSure RF technology, Resilient stirrup covers
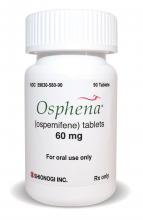
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
OSPHENA HAS NEW INDICATION
FOR MORE INFORMATION, VISIT: https://www.osphena.com/.
NEW 3-IN-1 HYSTEROSCOPE
FOR MORE INFORMATION, VISIT: https://gynsurgicalsolutions.com/product/omni-hysteroscope/.
SURGICAL RF TECHNOLOGY
FOR MORE INFORMATION, VISIT: https://www.cynosure.com/tempsure-platform.
PROFESSIONAL FOOT SUPPORTS
FOR MORE INFORMATION, VISIT: https://www.comenitymed.com.
Preoperative tramadol fails to improve function after knee surgery
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
chestphysiciannews@chestnet.org
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
FROM THE JOURNAL OF ARTHROPLASTY
Morcellation use in gynecologic surgery: Current clinical recommendations and cautions
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
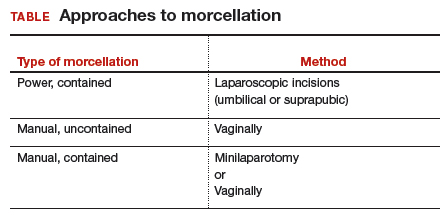
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7
Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
Morcellation of gynecologic surgical specimens became controversial after concerns arose about the potential for inadvertent spread of malignant cells throughout the abdomen and pelvis during tissue morcellation of suspected benign disease. In 2014, the US Food and Drug Administration (FDA) issued a warningagainst the use of laparoscopic power morcellation specifically for myomectomy or hysterectomy in the treatment of leiomyomas (fibroids) because of the risk of spreading undiagnosed malignancy throughout the abdomen and pelvis.1 This warning was issued after a high-profile case occurred in Boston in which an occult uterine sarcoma was morcellated during a supracervical robot-assisted hysterectomy for suspected benign fibroids.
Recently, the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion with updated recommendations for practice detailing the risks associated with morcellation and suggestions for patient counseling regarding morcellation.2
In this review, we summarize the techniques and risks of morcellation, the epidemiology of undiagnosed uterine malignancies, practice changes noted at our institution, and clinical recommendations moving forward. A case scenario illustrates keys steps in preoperative evaluation and counseling.
Morcellation uses—and risks
Morcellation is the surgical process of dividing a large tissue specimen into smaller pieces to facilitate their removal through the small incisions made in minimally invasive surgery. Morcellation may be performed with a power instrument or manually.
In power morcellation, an electromechanical instrument is used to cut or shave the specimen; in manual morcellation, the surgeon uses a knife to carve the specimen. Power morcellation is performed through a laparoscopic incision, while the manual technique is performed through a minilaparotomy or vaginally after hysterectomy (TABLE). Unlike uncontained morcellation, contained morcellation involves the use of a laparoscopic bag to hold the specimen and therefore prevent tissue dissemination in the abdomen and pelvis.
Morcellation has greatly expanded our ability to perform minimally invasive surgery—for example, in patients with specimens that cannot be extracted en bloc through the vagina after hysterectomy or, in the case of myomectomy or supracervical hysterectomy without a colpotomy, through small laparoscopic ports. Minimally invasive surgery improves patient care, as it is associated with lower rates of infection, blood loss, venous thromboembolism, wound and bowel complications, postoperative pain, and shorter overall recovery time and hospital stay versus traditional open surgery.3,4 Furthermore, laparoscopic hysterectomy has a 3-fold lower risk of mortality compared with open hysterectomy.4 For these reasons, ACOG recommends choosing a minimally invasive approach for all benign hysterectomies whenever feasible.3
With abundant data supporting the use of a minimally invasive approach, laparoscopic morcellation allowed procedures involving larger tissue specimens to be accomplished without the addition of a minilaparotomy for tissue extraction. However, disseminating potentially malignant tissue throughout the abdomen and pelvis during the morcellation process remains a risk. While tissue spread can occur with either power or manual morcellation, the case that drew media attention to the controversy used power morcellation, and thus intense scrutiny focused on this technique. Morcellation has additional risks, including direct injury to surrounding organs, disruption of the pathologic specimen, and distribution of benign tissue throughout the abdomen and pelvis, such as fibroid, endometriosis, and adenomyosis implants.5-7

Continue to: The challenge of leiomyosarcoma...
The challenge of leiomyosarcoma
The primary controversy surrounding morcellation of fibroid tissue specimens is the potential for undiagnosed malignancy, namely uterine leiomyosarcoma or endometrial stromal sarcoma. While other gynecologic malignancies, including cervical and endometrial cancers, are more common and potentially could be disseminated by morcellation, these cancers are more reliably diagnosed preoperatively with cervical and endometrial biopsies, and they do not tend to mimic benign diseases.
Epidemiology and risk factors. Uterine leiomyosarcoma is rare, with an estimated incidence of 0.36 per 100,000 woman-years.8 However, leiomyosarcoma can mimic the appearance and clinical course of benign fibroids, making preoperative diagnosis difficult. Risk factors for leiomyosarcoma include postmenopausal status, with a median age of 54 years at diagnosis, tamoxifen use longer than 5 years, black race, history of pelvic radiation, and certain hereditary cancer syndromes, such as Lynch syndrome.9-11 Because of these risk factors, preoperative evaluation is crucial to determine the most appropriate surgical method for removal of a large, fibroid uterus (see “Employ shared decision making”).
Estimated incidence at benign hysterectomy. The incidence of leiomyosarcoma diagnosed at the time of benign hysterectomy or myomectomy has been studied extensively since the FDA’s 2014 warning was released, with varying rates identified.11,12 The FDA’s analysis cited a risk of 1 in 498 for unsuspected leiomyosarcoma and 1 in 352 for uterine sarcoma.1 Notably, this analysis excluded studies of women undergoing surgery for presumed fibroids in which no leiomyosarcoma was found on pathology, likely inflating the quoted prevalence. The FDA and other entities subsequently performed further analyses, but a systematic literature review and meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) in 2017 is probably the most accurate. That review included 160 studies and reported a prevalence of less than 1 in 10,000 to 1 in 770, lower than the FDA-cited rate.13
Prognosis. The overall prognosis for women with leiomyosarcoma is poor. Studies indicate a 5-year survival rate of only 55.4%, even in stage 1 disease that is apparently confined to the uterus.9 Although evidence is limited linking morcellation to increased recurrence of leiomyosarcoma, data from small, single-center, retrospective studies cite a worse prognosis, higher risk of recurrence, and shorter progression-free survival after sarcoma morcellation compared with patients who underwent en bloc resection.12,14 Of note, these studies evaluated patients who underwent uncontained morcellation of specimens with unsuspected leiomyosarcoma.
CASE Woman with enlarged, irregular uterus and heavy bleeding
A 40-year-old woman (G2P2) with a history of 2 uncomplicated vaginal deliveries presents for evaluation of heavy uterine bleeding. She has regular periods, every 28 days, and she bleeds for 7 days, saturating 6 pads per day. She is currently taking only oral iron therapy as recommended by her primary care physician. Over the last 1 to 2 years she has felt that her abdomen has been getting larger and that her pants do not fit as well. She is otherwise in excellent health, exercises regularly, and has a full-time job. She has not been sexually active in several months.
The patient’s vitals are within normal limits and her body mass index (BMI) is 35 kg/m2.Pelvic examination reveals that she has an enlarged, irregular uterus with the fundus at the level of the umbilicus. The exam is otherwise unremarkable. On further questioning, the patient does not desire future fertility.
What next steps would you include in this patient’s workup, including imaging studies or lab tests? What surgical options would you give her? How would your management differ if this patient were 70 years old (postmenopausal)?
Continue to: Perform a thorough preoperative evaluation to optimize outcomes...
Perform a thorough preoperative evaluation to optimize outcomes
Women like this case patient who present with symptoms that may lead to treatment with myomectomy or hysterectomy should undergo appropriate preoperative testing to evaluate for malignancy.
According to ACOG guidance, patients should undergo a preoperative endometrial biopsy if they15:
- are older than 45 years with abnormal uterine bleeding
- are younger than 45 years with unopposed estrogen exposure (including obesity or polycystic ovary syndrome)
- have persistent bleeding, or
- failed medical management.
Our case patient is younger than 45 but is obese (BMI, 35) and therefore is a candidate for endometrial biopsy. Additionally, all patients should have up-to-date cervical cancer screening. ACOG also recommends appropriate use of imaging with ultrasonography or magnetic resonance imaging (MRI), although imaging is not recommended solely to evaluate for malignancy, as it cannot rule out the diagnosis of many gynecologic malignancies, including leiomyosarcoma.2
Currently, no tests are available to completely exclude a preoperative diagnosis of leiomyosarcoma. While studies have evaluated the use of MRI combined with lactate dehydrogenase isoenzyme testing, the evidence is weak, and this method is not recommended. Sarcoma is detected by endometrial sampling only 30% to 60% of the time, but it should be performed if the patient meets criteria for sampling or if she has other risk factors for malignancy.16 There are no data to support biopsy of presumed benign fibroids prior to surgical intervention. Patients should be evaluated with a careful history and physical examination for other uterine sarcoma risk factors.
Employ shared decision making
Clinicians should use shared decision making with patients to facilitate decisions on morcellation use in gynecologic surgeries for suspected benign fibroids. Informed consent must be obtained after thorough discussion and counseling regarding the literature on morcellation.17 For all patients, including the case patient described, this discussion should include alternative treatment options, surgical approach with associated risks, the use of morcellation, the incidence of leiomyosarcoma with presumed benign fibroids, leiomyosarcoma prognosis, and the risk of disseminating benign or undiagnosed cancerous tissue throughout the abdomen and pelvis.
Some would argue that the risks of laparotomy outweigh the possible risks associated with morcellation during a minimally invasive myomectomy or hysterectomy. However, this risk analysis is not uniform across all patients, and it is likely that in older women, because they have an a priori increased risk of malignancy in general, including leiomyosarcoma, the risks of power morcellation may outweigh the risks of open surgery.18 Younger women have a much lower risk of leiomyosarcoma, and thus discussion and consideration of the patient’s age should be a part of counseling. If the case patient described was 70 years of age, power morcellation might not be recommended, but these decisions require an in-depth discussion with the patient to make an informed decision and ensure patient autonomy.
The contained morcellation approach
Many surgeons who perform minimally invasive procedures use contained morcellation. In this approach, specimens are placed in a containment bag and morcellated with either power instruments or manually to ensure no dissemination of tissue. Manual contained morcellation can be done through a minilaparotomy or the vagina, depending on the procedure performed, while power contained morcellation is performed through a 15-mm laparoscopic incision.
Continue to: Currently, one containment bag has been...
Currently, one containment bag has been FDA approved for use in laparoscopic contained power morcellation.19 Use of a containment bag increases operative time by approximately 20 minutes, due to the additional steps required to accomplish the procedure.20 Its use, however, suggests a decrease in the risk of possible disease spread and it is feasible with appropriate surgeon training.
One study demonstrated the safety and feasibility of power morcellation within an insufflated containment bag, and subsequent follow-up revealed negative intraperitoneal washings.21,22 In another study evaluating tissue dissemination with contained morcellation of tissue stained with dye, the authors noted actual spillage of tissue fragments in only one case.23 Although more information is needed to confirm prevention of tissue dissemination and the safety of contained tissue morcellation, these studies provide promising data supporting the use of tissue morcellation in appropriate cases in order to perform minimally invasive surgery with larger specimens.
CASE Next steps and treatment outcome
The patient has up-to-date and negative cervical cancer screening. The complete blood count is notable for a hemoglobin level of 11.0 g/dL (normal range, 12.1 to 15.1 g/dL). You perform an endometrial biopsy; results are negative for malignancy. You order pelvic ultrasonography to better characterize the location and size of the fibroids. It shows multiple leiomyomas throughout the myometrium, with the 2 largest fibroids (measuring 5 and 7 cm) located in the left anterior and right posterolateral aspects of the uterus, respectively. Several 3- to 4-cm fibroids appear to be disrupting the endometrial canal, and there is no evidence of an endometrial polyp. There do not appear to be any cervical or lower uterine segment fibroids, which may have further complicated the proposed surgery.
You discuss treatment options for abnormal uterine bleeding with the patient, including initiation of combined oral contraceptive pills, placement of a levonorgestrel-containing intrauterine device, endometrial ablation, uterine artery embolization, and hysterectomy. You discuss the risks and benefits of each approach, keeping in mind the fibroids that are disrupting the contour of the endometrial canal and causing her bulk symptoms.
The patient ultimately decides to undergo a hysterectomy and would like it to be performed with a minimally invasive procedure, if possible. Because of the size of her uterus, you discuss the use of contained power morcellation, including the risks and benefits. You have a thorough discussion about the risk of occult malignancy, although she is at lower risk because of her age, and she consents.
The patient undergoes an uncomplicated total laparoscopic hysterectomy with bilateral salpingectomy. The specimen is removed using contained power morcellation through the umbilical port site. She has an unremarkable immediate postoperative course and is discharged on postoperative Day 1.
You see the patient in the clinic 2 weeks later. She reports minimal pain or discomfort and has no other complaints. Her abdominal incisions are healing well. You review the final pathology report with her, which showed no evidence of malignancy.
Society guidance on clinical applications
In current clinical practice, many surgeons have converted to exclusively performing contained morcellation in appropriate patients with a low risk of uterine leiomyosarcoma. At our institution, uncontained morcellation has not been performed since the FDA’s 2014 warning.
ACOG and AAGL (formerly the American Association of Gynecologic Laparoscopists) recommend use of containment bags as a solution to continue minimally invasive surgery for large specimens without the risk of possible tissue dissemination, although more in-depth surgeon training is likely required for accurate technique.2,24 The Society of Gynecologic Oncology (SGO) states that power morcellation or any other techniques that divide the uterus in the abdomen are contraindicated in patients with documented or highly suspected malignancy.25
With the presented data of risks associated with uncontained morcellation and agreement of the ACOG, AAGL, and SGO professional societies, we recommend that all morcellation be performed in a contained fashion to prevent the dissemination of benign or undiagnosed malignant tissue throughout the abdomen and pelvis. Shared decision making and counseling on the risks, benefits, and alternatives are paramount for patients to make informed decisions about their medical care. Continued exploration of techniques and methods for safe tissue extraction is still needed to improve minimally invasive surgical options for all women.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
1. US Food and Drug Administration. Updated: Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. November 24, 2014; updated April 7, 2016. https://wayback.archiveit.org/7993/20170404182209/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm424443.htm. Accessed July 23, 2019.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 770: Uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
3. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 701: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017;129:1149-1150.
4. Wiser A, Holcroft CA, Tolandi T, et al. Abdominal versus laparoscopic hysterectomies for benign diseases: evaluation of morbidity and mortality among 465,798 cases. Gynecol Surg. 2013;10:117-122.
5. Winner B, Biest S. Uterine morcellation: fact and fiction surrounding the recent controversy. Mo Med. 2017;114:176-180.
6. Tulandi T, Leung A, Jan N. Nonmalignant sequelae of unconfined morcellation at laparoscopic hysterectomy or myomectomy. J Minim Invasive Gynecol. 2016;23:331-337.
7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486-491.
8. Toro JR, Travis LB, Wu HJ, et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the Surveillance, Epidemiology and End Results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer. 2006;119:2922-2930.
9. Seagle BL, Sobecki-Rausch J, Strohl AE, et al. Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol. 2017;145:61-70.
10. Ricci S, Stone RL, Fader AN. Uterine leiomyosarcoma: epidemiology, contemporary treatment strategies and the impact of uterine morcellation. Gynecol Oncol. 2017;145:208-216.
11. Leibsohn S, d’Ablaing G, Mishell DR Jr, et al. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968-974. Discussion 974-976.
12. Rowland M, Lesnock J, Edwards R, et al. Occult uterine cancer in patients undergoing laparoscopic hysterectomy with morcellation [abstract]. Gynecol Oncol. 2012;127:S29.
13. Hartmann KE, Fonnesbeck C, Surawicz T, et al. Management of uterine fibroids. Comparative effectiveness review no. 195. AHRQ Publication No. 17(18)-EHC028-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2017. https://effectivehealthcare.ahrq.gov/topics/uterine-fibroids /research-2017. Accessed July 23, 2019.
14. Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26-33.
15. American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin no. 128: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
16. Bansal N, Herzog TJ, Burke W, et al. The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol. 2008 Jul;110(1):43–48.
17. American College of Obstetricians and Gynecologists Committee on Ethics. ACOG committee opinion no. 439: Informed consent. Obstet Gynecol. 2009;114:401-408.
18. Wright JD, Cui RR, Wang A, et al. Economic and survival implications of use of electric power morcellation for hysterectomy for presumed benign gynecologic disease. J Natl Cancer Inst. 2015;107:djv251.
19. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients [press release]. April 7, 2016. https://www.fda.gov/NewsEvents /Newsroom/PressAnnouncements/ucm494650.htm. Accessed July 23, 2019.
20. Winner B, Porter A, Velloze S, et al. S. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol. 2015 Oct;126(4):834–8.
21. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491-497.
22. Cohen SL, Greenberg JA, Wang KC, et al. Risk of leakage and tissue dissemination with various contained tissue extraction (CTE) techniques: an in vitro pilot study. J Minim Invasive Gynecol. 2014;21:935-939.
23. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214(2):257. e1-257.e6.
24. AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
25. Society of Gynecologic Oncology. Position statement: morcellation. 2013. https://www.sgo.org/newsroom /position-statements-2/morcellation/.Accessed July 23, 2019.
Office hysteroscopic evaluation of postmenopausal bleeding
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.
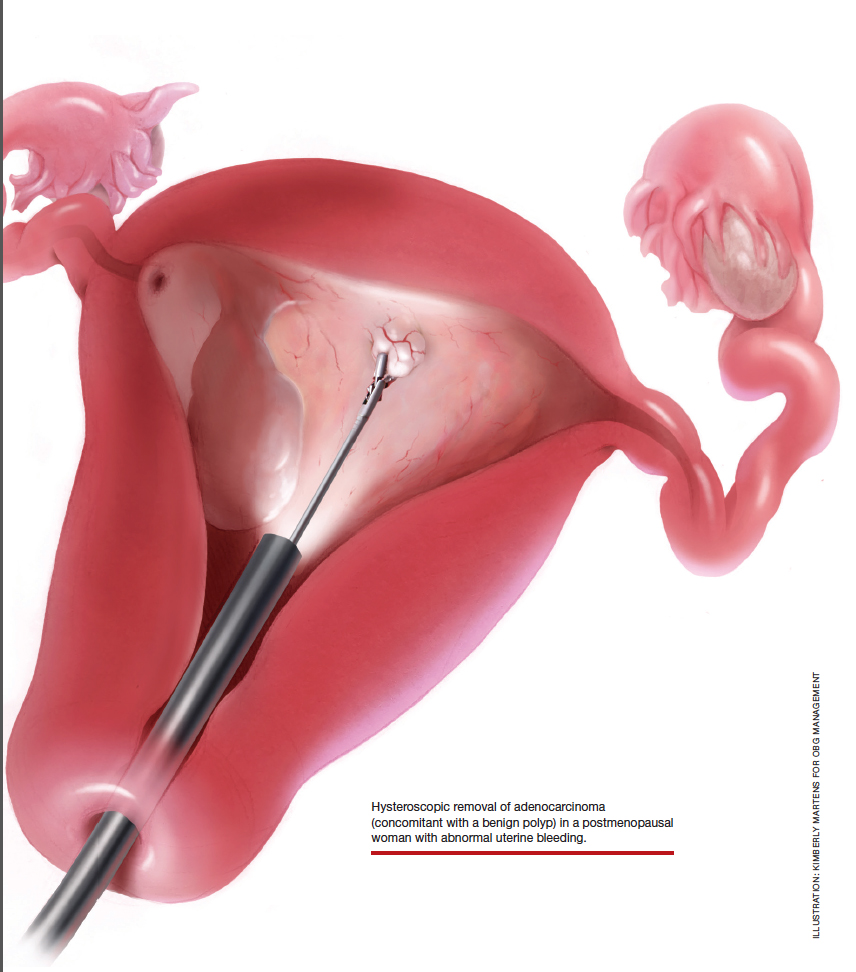
Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.
Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.
Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).
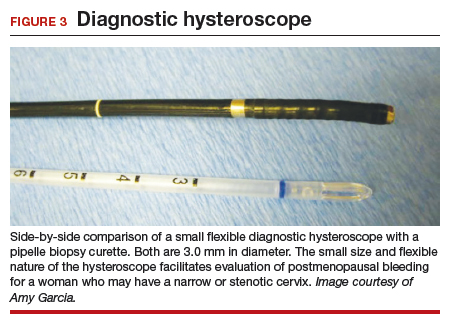
Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.
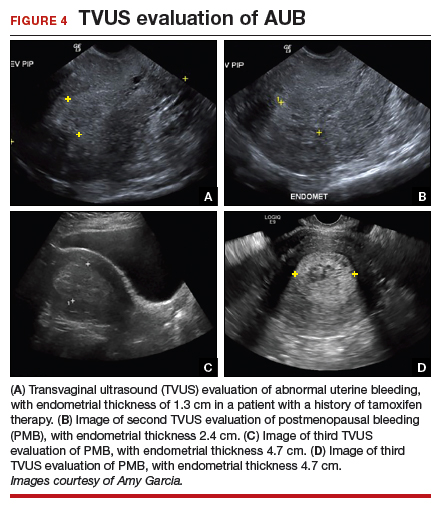
At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3

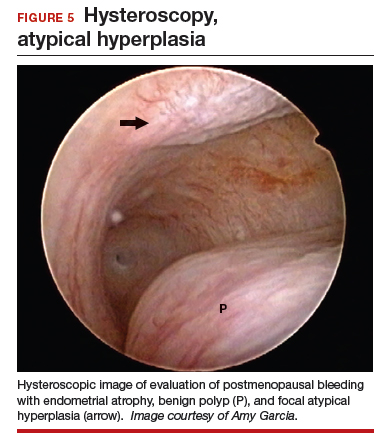
This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B

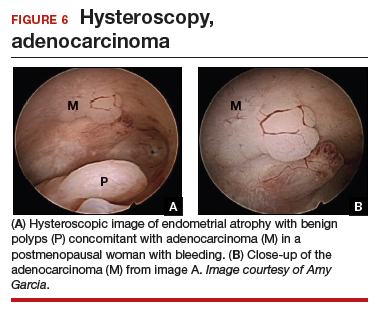
This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
Postmenopausal bleeding (PMB) is the presenting sign in most cases of endometrial carcinoma. Prompt evaluation of PMB can exclude, or diagnose, endometrial carcinoma.1 Although no general consensus exists for PMB evaluation, it involves endometrial assessment with transvaginal ultrasonography (TVUS) and subsequent endometrial biopsy when a thickened endometrium is found. When biopsy results reveal insufficient or scant tissue, further investigation into the etiology of PMB should include office hysteroscopy with possible directed biopsy. In this article I discuss the prevalence of PMB and steps for evaluation, providing clinical takeaways.

Postmenopausal bleeding: Its risk for cancer
Abnormal uterine bleeding (AUB) in a postmenopausal woman is of particular concern to the gynecologist and the patient because of the increased possibility of endometrial carcinoma in this age group. AUB is present in more than 90% of postmenopausal women with endometrial carcinoma, which leads to diagnosis in the early stages of the disease. Approximately 3% to 7% of postmenopausal women with PMB will have endometrial carcinoma.2 Most women with PMB, however, experience bleeding secondary to atrophic changes of the vagina or endometrium and not to endometrial carcinoma. (FIGURE 1, VIDEO 1) In addition, women who take gonadal steroids for hormone replacement therapy (HRT) may experience breakthrough bleeding that leads to initial investigation with TVUS.
Video 1

The risk of malignancy in polyps in postmenopausal women over the age of 59 who present with PMB is approximately 12%, and hysteroscopic resection should routinely be performed. For asymptomatic patients, the risk of a malignant lesion is low—approximately 3%—and for these women intervention should be assessed individually for the risks of carcinoma and benefits of hysteroscopic removal.3
Clinical takeaway. The high possibility of endometrial carcinoma in postmenopausal women warrants that any patient who is symptomatic with PMB should be presumed to have endometrial cancer until the diagnostic evaluation process proves she does not.

Evaluation of postmenopausal bleeding
Transvaginal ultrasound
As mentioned, no general consensus exists for the evaluation of PMB; however, initial evaluation by TVUS is recommended. The American College of Obstetricians and Gynecologists (ACOG) concluded that when the endometrium measures ≤4 mm with TVUS, the likelihood that bleeding is secondary to endometrial carcinoma is less than 1% (negative predictive value 99%), and endometrial biopsy is not recommended.3 Endometrial sampling in this clinical scenario likely will result in insufficient tissue for evaluation, and it is reasonable to consider initial management for atrophy. A thickened endometrium on TVUS (>4 mm in a postmenopausal woman with PMB) warrants additional evaluation with endometrial sampling (FIGURE 2).
Clinical takeaway. A thickened endometrium on TVUS ≥4 mm in a postmenopausal woman with PMB warrants additional evaluation with endometrial sampling.

Endometrial biopsy
An endometrial biopsy is performed to determine whether endometrial cancer or precancer is present in women with AUB. ACOG recommends that endometrial biopsy be performed for women older than age 45. It is also appropriate in women younger than 45 years if they have risk factors for developing endometrial cancer, including unopposed estrogen exposure (obesity, ovulatory dysfunction), failed medical management of AUB, or persistence of AUB.4
Continue to: Endometrial biopsy has some...
Endometrial biopsy has some diagnostic shortcomings, however. In 2016 a systematic review and meta-analysis found that, in women with PMB, the specificity of endometrial biopsy was 98% to 100% (accurate diagnosis with a positive result). The sensitivity (ability to make an accurate diagnosis) of endometrial biopsy to identify endometrial pathology (carcinoma, atypical hyperplasia, and polyps) is lower than typically thought. These investigators found an endometrial biopsy failure rate of 11% (range, 1% to 53%) and rate of insufficient samples of 31% (range, 7% to 76%). In women with insufficient or failed samples, endometrial cancer or precancer was found in 7% (range, 0% to 18%).5 Therefore, a negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative. The results of endometrial biopsy are only an endpoint to the evaluation of PMB when atypical hyperplasia or endometrial cancer is identified.
Clinical takeaway. A negative tissue biopsy result in women with PMB is not considered to be an endpoint, and further evaluation with hysteroscopy to evaluate for focal disease is imperative.
Hysteroscopy
Hysteroscopy is the gold standard for evaluating the uterine cavity, diagnosing intrauterine pathology, and operative intervention for some causes of AUB. It also is easily performed in the office. This makes the hysteroscope an essential instrument for the gynecologist. Dr. Linda Bradley, a preeminent leader in hysteroscopic surgical education, has coined the phrase, “My hysteroscope is my stethoscope.”6 As gynecologists, we should be as adept at using a hysteroscope in the office as the cardiologist is at using a stethoscope.
It has been known for some time that hysteroscopy improves our diagnostic capabilities over blinded procedures such as endometrial biopsy and dilation and curettage (D&C). As far back as 1989, Dr. Frank Loffer reported the increased sensitivity (ability to make an accurate diagnosis) of hysteroscopy with directed biopsy over blinded D&C (98% vs 65%) in the evaluation of AUB.7 Evaluation of the endometrium with D&C is no longer recommended; yet today, few gynecologists perform hysteroscopic-directed biopsy for AUB evaluation instead of blinded tissue sampling despite the clinical superiority and in-office capabilities (FIGURE 3).

Continue to: Hysteroscopy and endometrial carcinoma...
Hysteroscopy and endometrial carcinoma
The most common type of gynecologic cancer in the United States is endometrial adenocarcinoma (type 1 endometrial cancer). There is some concern about the effect of hysteroscopy on endometrial cancer prognosis and the spread of cells to the peritoneum at the time of hysteroscopy. A large meta-analysis found that hysteroscopy performed in the presence of type 1 endometrial cancer statistically significantly increased the likelihood of positive intraperitoneal cytology; however, it did not alter the clinical outcome. It was recommended that hysteroscopy not be avoided for this reason and is helpful in the diagnosis of endometrial cancer, especially in the early stages of disease.8
For endometrial cancer type 2 (serous carcinoma, clear cell carcinoma, and carcinosarcoma), Chen and colleagues reported a statistically significant increase in positive peritoneal cytology for cancers evaluated by hysteroscopy versus D&C. The disease-specific survival for the hysteroscopy group was 60 months, compared with 71 months for the D&C group. While this finding was not statistically significant, it was clinically relevant, and the effect of hysteroscopy on prognosis with type 2 endometrial cancer is unclear.9
A common occurrence in the evaluation of postmenopausal bleeding (PMB) is an initial TVUS finding of an enlarged endometrium and an endometrial biopsy that is negative or reveals scant or insufficient tissue. Unfortunately, the diagnostic evaluation process often stops here, and a diagnosis for the PMB is never actually identified. Here are several clinical scenarios that highlight the need for hysteroscopy in the initial evaluation of PMB, especially when there is a discordance between transvaginal ultrasonography (TVUS) and endometrial biopsy findings.
Patient 1: Discordant TVUS and biopsy, with benign findings
The patient is a 52-year-old woman who presented to her gynecologist reporting abnormal uterine bleeding (AUB). She has a history of breast cancer, and she completed tamoxifen treatment. Pelvic ultrasonography was performed; an enlarged endometrial stripe of 1.3 cm was found (FIGURE 4A). Endometrial biopsy was performed, showing adequate tissue but with a negative result. The patient is told that she is likely perimenopausal, which is the reason for her bleeding.

At the time of referral, the patient is evaluated with in-office hysteroscopy. Diagnosis of a 5 cm x 7 cm benign endometrial polyp is made. An uneventful hysteroscopic polypectomy is performed (VIDEO 2).
Video 2

This scenario illustrates the shortcoming of initial evaluation by not performing a hysteroscopy, especially in a woman with a thickened endometrium with previous tamoxifen therapy. Subsequent visits failed to correlate bleeding etiology with discordant TVUS and endometrial biopsy results with hysteroscopy, and no hysteroscopy was performed in the operating room at the time of D&C.
Patient 2: Discordant TVUS and biopsy, with premalignant findings
The patient is a 62-year-old woman who had incidental findings of a thickened endometrium on computed tomography scan of the pelvis. TVUS confirmed a thickened endometrium measuring 17 mm, and an endometrial biopsy showed scant tissue.
At the time of referral, a diagnostic hysteroscopy was performed in the office. Endometrial atrophy, a large benign appearing polyp, and focal abnormal appearing tissue were seen (FIGURE 5). A decision for polypectomy and directed biopsy was made. Histology findings confirmed benign polyp and atypical hyperplasia (VIDEO 3).
Video 3


This scenario illustrates that while the patient was asymptomatic, there was discordance between the TVUS and endometrial biopsy. Hysteroscopy identified a benign endometrial polyp, which is common in asymptomatic postmenopausal patients with a thickened endometrium and endometrial biopsy showing scant tissue. However, addition of the diagnostic hysteroscopy identified focal precancerous tissue, removed under directed biopsy.
Patient 3: Discordant TVUS and biopsy, with malignant findings
The patient is a 68-year-old woman with PMB. TVUS showed a thickened endometrium measuring 14 mm. An endometrial biopsy was negative, showing scant tissue. No additional diagnostic evaluation or management was offered.
Video 4A

At the time of referral, the patient was evaluated with in-office diagnostic hysteroscopy, and the patient was found to have endometrial atrophy, benign appearing polyps, and focal abnormal tissue (FIGURE 6). A decision for polypectomy and directed biopsy was made. Histology confirmed benign polyps and grade 1 adenocarcinoma (VIDEOS 4A, 4B, 4C).
Video 4B


This scenario illustrates the possibility of having multiple endometrial pathologies present at the time of discordant TVUS and endometrial biopsy. Hysteroscopy plays a critical role in additional evaluation and diagnosis of endometrial carcinoma with directed biopsy, especially in a symptomatic woman with PMB.
Video 4C

Conclusion
Evaluation of PMB begins with a screening TVUS. Findings of an endometrium of ≤4 mm indicate a very low likelihood of the presence of endometrial cancer, and treatment for atrophy or changes to hormone replacement therapy regimen is reasonable first-line management; endometrial biopsy is not recommended. For patients with persistent PMB or thickened endometrium ≥4 mm on TVUS, biopsy sampling of the endometrium should be performed. If the endometrial biopsy does not explain the etiology of the PMB with atypical hyperplasia or endometrial cancer, then hysteroscopy should be performed to evaluate for focal endometrial disease and possible directed biopsy.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
- ACOG Committee Opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018;131:e124-e129.
- Goldstein SR. Appropriate evaluation of postmenopausal bleeding. Menopause. 2018;25:1476-1478.
- Bel S, Billard C, Godet J, et al. Risk of malignancy on suspicion of polyps in menopausal women. Eur J Obstet Gynecol Reprod Biol. 2017;216:138-142.
- Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- van Hanegem N, Prins MM, Bongers MY. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147-155.
- Embracing hysteroscopy. September 6, 2017. https://consultqd.clevelandclinic.org/embracing-hysteroscopy/. Accessed July 22, 2019.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Chang YN, Zhang Y, Wang LP, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Chen J, Clark LH, Kong WM, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12:e0174226.
Association of Nausea and Length of Stay with Carbohydrate Loading Prior to Total Joint Arthroplasty
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
From Stony Brook Medical Center, Stony Brook, NY (Dr. Blum), and NYU Winthrop Medical Center,
Abstract
- Background: Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response. One aspect of ERAS, carbohydrate loading, has been shown in multiple randomized controlled trials to result in postoperative benefits in patients undergoing colorectal surgery, but there appears to be insufficient data to make definitive recommendations for or against carbohydrate loading in joint replacement patients.
- Objective: To evaluate postoperative nausea and length of stay (LOS) after a preoperative carbohydrate loading protocol was initiated for patients undergoing total joint replacement.
- Design: Retrospective chart review.
- Setting and participants: 100 patients who underwent either total knee or hip arthroplasty at Winthrop University Hospital, Mineola, NY, in the past 4 years and either had (n = 50) or had not received preoperative carbohydrate supplements (n = 50).
- Methods: Using the total joint database, the medical record was reviewed for the patient’s demographics, LOS, documentation of postoperative nausea, and number of doses of antiemetic medication given to the patient.
- Results: The mean LOS for the carbohydrate-loading group and non-carbohydrate group was 1.9 days and 2.6 days. respectively, a difference of 0.70 days (P < 0.0001). The carbohydrate-loaded group received a total of 13 doses of antiemetic medications and the non-carbohydrate group received 21 doses. The average number of antiemetic doses given to a patient postoperatively was 0.26 for the carbohydrate-loaded group and 0.42 for the non-carbohydrate-loaded group. The difference was 0.16 doses (P < 0.7815).
- Conclusion: The implementation of carbohydrate loading decreased LOS for joint replacement patients by approximately 1 day. Additionally, there was a trend towards decreased antiemetic use and fewer documented cases of postoperative nausea after carbohydrate loading.
Keywords: carbohydrate loading, ERAS, joint arthroplasty, length of stay, nausea.
Enhanced Recovery After Surgery (ERAS) is a multimodal, standardized approach to the surgical patient that incorporates evidenced-based interventions designed to achieve rapid recovery after surgery by minimizing the patient’s stress response.1-4 The ERAS protocols have been shown to reduce complications, decrease length of stay (LOS), and improve patient outcomes.3-7 The program was originally designed to facilitate recovery after colorectal operative procedures by maintaining preoperative organ function and reducing the postoperative stress response. This was done through a coordinated program of preoperative counseling, optimizing nutritional status, standardizing analgesic regimens, and early mobilization.3
The principles of an ERAS program with standardized pre- and postoperative protocols appear ideally suited for the total joint arthroplasty patient.1,3-5 Prior studies have demonstrated ERAS to be effective in facilitating decreased LOS, with no apparent increase in readmission rates or complications for both colorectal and joint arthroplasty patients.1-7 The protocols have also been shown to be cost-effective, with decreased incidence of postoperative complications, including thromboembolic disease and infections.3,4,6
An important tenet of ERAS protocols is optimizing the nutritional status of the patient prior to surgery.6 This includes avoidance of preoperative fasting in conjunction with carbohydrate loading. ERAS protocols instruct the patient to ingest a carbohydrate-rich beverage 2 hours prior to surgery. The concept of allowing a patient to eat prior to surgery is based on the preference for the patient to present for surgery in an anabolic rather than a catabolic state.2,3,11 Patients in an anabolic state undergo less postoperative protein and nitrogen losses, which appears to facilitate wound healing.2,6,11
There have been multiple randomized controlled trials demonstrating the postoperative benefits of carbohydrate loading prior to colorectal surgery.2,6
Another potential benefit of preoperative carbohydrate loading is a decrease in postoperative nausea.1,5,12-14 A decrease in nausea in theory would allow for earlier mobilization with physical therapy and potentially a shorter LOS. Hence, the goal of this study was to examine the impact of preoperative carbohydrate loading on postoperative nausea directly, as well as on LOS, at a single institution in the setting of an ERAS protocol.
Methods
We retrospectively reviewed the records of 100 patients who underwent total hip or total knee replacement between 2014 and 2018 at NYU Winthrop University Hospital, Mineola, NY. Fifty patients had received preoperative carbohydrate supplements and 50 patients had not. The remainder of the total joint protocol was identical for the 2 groups.
Protocol
All patients attended preoperative educational classes. For patients receiving carbohydrate loading, written and oral instructions were given for the patient to drink Ensure Clear followed by 8 ounces of water before going to bed the night before surgery. They were also instructed to drink the Ensure Pre-Surgery Drink 2 hours prior to their operative procedure. Patients with diabetes were instructed to drink the Ensure Glucerna Clear drink the night before surgery. No carbohydrate drink was given on the day of surgery until a finger-stick glucose level was performed upon arrival at the hospital. Spinal anesthesia was utilized in all patients, with adductor canal block supplementation for patients undergoing total knee replacement. Orders were written to have physical therapy evaluate the patients in the PACU to facilitate ambulation. Pre- and postoperative pain protocols were identical for the 2 groups.
Data Collection
A chart review was performed using the patients’ medical record numbers from the joint replacement database at our institution. Exemption was obtained for the project from our institution’s Institutional Review Board (IRB).
Analysis
Descriptive statistics (mean, standard deviation, and median for continuous variables; frequencies and percentages for categorical variables) were calculated separately by group. The 2 groups were compared using the chi-square test or Fisher’s exact test, as deemed appropriate, for categorical variables, the 2-sample t-test for age, and the Mann-Whitney test for LOS and number of antiemetic doses given. A result was considered statistically significant at the P < 0.05 level of significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The carbohydrate-loading group (n = 50) and the non-carbohydrate-loading group (n = 50) were comparable for age, gender, type of arthroplasty, episodes of vomiting, diabetes, and nerve block (Table).
Discussion
In this study we explored whether carbohydrate loading prior to total joint replacement influenced postoperative nausea and LOS in a single institution. The 2 groups appeared similar in terms of demographics as well as the types of surgical procedures performed. After initiation of the carbohydrate-loading protocol, LOS decreased by approximately 1 day. There was also a trend toward decreased usage of antiemetics in the carbohydrate-loaded group, although the final values were not statistically significant. There were also fewer documented cases of postoperative nausea in the carbohydrate-loaded group.
The failure to find a statistical difference in postoperative antiemetic usage between carbohydrate-loaded and non-carbohydrate-loaded patients may be due to incomplete documentation (ie, not all patients who were nauseous having their symptoms documented in the chart). Due to the small number of antiemetic doses given to each patient, we may have lacked the necessary numbers to visualize the difference between the groups. We were unable to perform a post-hoc power calculation with our current data. Additionally, the decrease seen in LOS may not have been due solely to carbohydrate loading, since the data were collected over multiple years during implementation of the ERAS protocol. There is a possibility that the ERAS protocol, which is multimodal, was better implemented as time progressed, adding a confounding variable to our data. Despite these limitations, however, we were able to demonstrate a decreased LOS for patients who underwent total joint replacement with the initiation of a preoperative carbohydrate-loading ERAS protocol. Furthermore, there was a trend toward decreased documented postoperative nausea and decreased antiemetic use in the group that avoided fasting and received carbohydrate supplements.
This decrease in LOS by almost 1 day is consistent with multiple prior studies that demonstrated a similar decrease when implementing an ERAS protocol.3-5,7 The trend towards lower antiemetic use and less postoperative nausea in the carbohydrate-loading ERAS protocol gives merit to further research on this topic, with the goal of finding an optimal preoperative practice that allows patients to experience rapid mobilization, minimal postoperative nausea, and faster recovery overall.
Conclusion
Corresponding author: Christopher L. Blum, MD, Stony Brook Medical Center, Stony Brook, NY; blumc18@gmail.com.
Financial disclosures: None.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
1. Proudfoot S, Bennett B, Duff S, Palmer J. Implementation and effects of Enhanced Recovery After Surgery for hip and knee replacements and fractured neck of femur in New Zealand orthopaedic services. N Z Med J. 2017;130:77-90.
2. Geltzeiler CB, Rotramel A, Wilson C, et al. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg. 2014;149:955-961.
3. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl 3):iii62-iii72.
4. Stowers MD, Manuopangai L, Hill AG, et al. Enhanced Recovery After Surgery in elective hip and knee arthroplasty reduces length of hospital stay. ANZ J Surg. 2016;86:475-479.
5. Gwynne-Jones DP, Martin G, Crane C. Enhanced Recovery After Surgery for hip and knee replacements. Orthop Nurs. 2017;36:203-210.
6. Semerjian A, Milbar N, Kates M, et al. Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 2018;111:86-91.
7. Stambough JB, Nunley RM, Curry MC, et al. Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty. 2015;30:521-526.
8. Ljungqvist O, Soreide E. Preoperative fasting. Br J Surg. 2003;90:400-406.
9. Riis J, Lomholt B, Haxholdt O, et al. Immediate and long-term mental recovery from general versus epidural anesthesia in elderly patients. Acta Anaesthesiol Scand. 1983;27:44-49.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641.
11. Svanfeldt M, Thorell A, Hausel J, Soop M, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94:1342-1350.
12. Halaszynski TM, Juda R, Silverman DG. Optimizing postoperative outcomes with efficient preoperative assessment and management. Crit Care Med. 2004;32(4 suppl):S76-S86.
13. Aronsson A, Al-Ani NA, Brismar K, Hedstrom M. A carbohydrate-rich drink shortly before surgery affected IGF-I bioavailability after a total hip replacement. A double-blind placebo controlled study on 29 patients. Aging Clin Exp Res. 2009;21:97-101.
14. Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
How common is accelerated knee OA?
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
REPORTING FROM OARSI 2019
Lymphoma risk prompts FDA recall of Allergan’s textured breast implants
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
Diagnosis, treatment, and prevention of ovarian remnant syndrome
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.
Ovarian remnant syndrome (ORS) is an uncommon problem, but one that seems to be increasing in incidence and one that is important to diagnose and treat properly, as well as prevent. Retrospective cohort studies published in the past 15 years or so have improved our understanding of its presentation and the outcomes of surgical management – and recent literature has demonstrated that a minimally invasive surgical approach with either conventional laparoscopy or robot-assisted laparoscopy yields improved outcomes in a skilled surgeon’s hands.
Diagnosis is based on clinical history and should be further supported with imaging and laboratory evaluation. A definitive diagnosis of the disease comes through surgical intervention and pathological findings.
Surgery therefore is technically challenging, usually requiring complete ureterolysis, careful adhesiolysis (often enterolysis), and excision of much of the pelvic sidewall peritoneum with extirpation of the remnant and endometriosis. High ligation of the ovarian vasculature also often is required.
This complexity and the consequent risk of intraoperative injury to the bowel, bladder, and ureters requires careful preoperative preparation. When an ovarian remnant is suspected, it may be important to have other surgeons – such as gynecologic oncologists, urologists, colorectal surgeons, or general surgeons – either present or on standby during the surgical intervention. In expert hands, surgical intervention has been shown to resolve or improve pain in the majority of patients, with no recurrence of the syndrome.
Diagnosis of ORS

Courtesy Dr. Charles E. Miller and Dr. Kirsten J. Sasaki
Patients with ORS have had previous oophorectomies with incomplete removal of ovarian tissue. Pelvic pain, either cyclical or most commonly chronic, is a common symptom. Other symptoms can include dyspareunia, dysuria and other urinary symptoms, and bowel symptoms. Ovarian remnants may have an expanding cystic structure – oftentimes secondary to endometriosis – that causes mass-like effects leading to pain and inflammation and to symptoms such as low back pain, constipation, and even urinary retention.
It also is important to discuss the patient’s history of menopausal symptoms, because the absence of these symptoms after oophorectomy may be a sign that ovarian tissue has been left behind. Menopausal symptoms do not exclude the diagnosis, however. Endometriosis, extensive surgical history, and other diseases that lead to significant adhesion formation – and a higher risk of incomplete removal of ovarian tissue, theoretically – also should be explored during history-taking.
Laboratory assessment of serum follicle-stimulating hormone (FSH) and estradiol can be helpful. Values that are indicative of ovarian function – FSH less than 30 mIU/mL and estradiol greater than 35 pg/mL – point towards ORS, but the absence of such premenopausal values should not rule out the possibility of an ovarian remnant.
The literature shows that FSH and estradiol levels are variable in women with ORS. A retrospective review published in 2005 by Paul M. Magtibay, MD, and colleagues at the Mayo Clinic, Scottsdale, Ariz., and Rochester, Minn., involved 186 patients treated surgically from 1985 to 2003 with a mean follow-up, via questionnaire, of 1.2 years. This is the largest series published thus far of patients with pathologically confirmed ORS. It reported premenopausal levels of FSH and estradiol in 69% and 63% of patients, respectively, who had preoperative hormonal evaluations.1
In another retrospective cohort study published in 2011 of 30 women – also with pathologically confirmed ovarian remnants – Deborah Arden, MD, and Ted Lee, MD, of the University of Pittsburgh Medical Center reported premenopausal levels of FSH and estradiol in 59% and 71%, respectively, of women whose concentrations were measured.2
ORS often involves a pelvic mass, and preoperative imaging is important in this regard. In Dr. Magtibay’s series, a pelvic mass was identified in 93%, 92%, and 78% of those who were imaged presurgically with ultrasonography, computed tomography, and magnetic resonance imaging, respectively.1 As with laboratory testing, however, a negative result does not rule out the presence of an ovarian remnant.
Some authors have advocated the use of clomiphene citrate stimulation before preoperative imaging – or before repeat imaging – to identify remnant ovarian tissue. Typically, clomiphene citrate 100 mg is administered for 10 days prior to imaging to potentially induce ovulation in patients with suspected ORS. Alternatively, at the Advanced Gynecologic Surgery Institute in Naperville and Park Ridge, Ill., ovarian stimulation is performed using FSH 300 IUs for 5 days. A finding of cystic structures consistent with ovarian follicles will help narrow the diagnosis.
Use of gonadotropins is superior in that an intact pituitary-ovarian axis is not required. Moreover, monitoring can be in real time; increasing estradiol levels and increasing mass size on ultrasound can be monitored as gonadotropin treatment is rendered. Again, however, negative findings should not necessarily rule out ORS. Unfortunately, there have been no clinical studies looking at the use of controlled ovarian stimulation as a definitive test.
The differential diagnosis includes supernumerary ovary (a rare gynecologic congenital anomaly) and residual ovary syndrome (a condition in which an ovary is intentionally or unintentionally left in place during a hysterectomy, as well as often an intended bilateral oophorectomy, and later causes pain). The latter occurs when surgical anatomy is poor and the surgery is consequently very difficult.
Surgical principles and approach
Previously, laparotomy was believed to be the best approach for minimizing intraoperative complications and achieving the extensive dissections necessary for effective treatment of ORS. In recent years, conventional laparoscopy and robot-assisted laparoscopy have been shown in retrospective reviews such as that by Arden et al.2 and a 2007 review by Rosanne M. Kho, MD,3 to be just as safe and effective provided that the same surgical principles – extensive retroperitoneal dissections and ureterolysis – are applied.
Good outcomes can be achieved with less blood loss, shorter operating room time, and less time in the hospital. The better visualization with greater magnification afforded by a minimally invasive approach offers a distinct advantage for such complex dissections.
A remnant of ovarian tissue can be located anywhere along the pelvic sidewall, which makes the surgical protocol largely individualized and based on the suspected location of the remnant.
Still, there are certain standard components of any surgical approach to ORS: The retroperitoneum should be entered at the level of the pelvic brim and the ureter must be clearly identified; usually, a partial or complete ureterolysis is necessary. Then, a window into the broad ligament inferior to the infundibulopelvic (IP) ligament is created, or the peritoneum of the broad ligament is removed, in order to completely isolate both the IP ligament and the ureter.
Once the ovarian remnant is isolated, a wide excision at least 2 cm from all ovarian tissue is performed. This wide surgical clearance is critical to prevent recurrence.
These standard components form the crux of the most basic and straightforward surgery for ORS. In some cases, more extensive dissections such as a cystectomy or even a bowel resection might be necessary. Ligation of the IP ligament as high because its connection to the aortic bifurcation also may be necessary – depending, again, on the location of the ovarian remnant.
The risk of intraoperative injury to the bowel, bladder, and ureters is not insignificant, but with careful planning and the involvement of other surgeons in the most complex cases, these risks can be minimized.
For patients who have a significant surgical history and do not want more surgery, pharmacologic therapy, such as leuprolide (Lupron) or danazol, is an option for ORS. It’s important to note, however, that no studies have been done to demonstrate that medical therapy is a curative option. In addition, one must consider the small risk that remnants may harbor or develop malignancy.
Malignancy has been reported in ovarian remnant tissue. While the risk is believed to be very small, 2 of the 20 patients in Dr. Kho’s cohort had malignancy in remnant tissue,3 and it is generally recommended that surgeons send frozen sections of suspected ovarian tissue to pathology. Frozen-section diagnosis of ovarian tissue is about 95% accurate.
Preventing ovarian remnants
Oophorectomy is a common procedure performed by gynecologic surgeons. While routine, it is imperative that it be performed correctly to prevent ovarian remnants from occurring. When performing a laparoscopic or robot-assisted laparoscopic oophorectomy, it is important to optimize visualization of the ovary and the IP ligament, and to account for the significant magnification provided by laparoscopic cameras.
Surgeons must make sure all adhesions are completely cleared in order to optimally transect the IP ligament. Furthermore, wide excision around ovarian tissue is critical. Accessory ovarian tissue has been found up to 1.4 cm away from the ovary itself, which is why we recommend that surgeons excise at least 2-3 cm away from the IP in order to safely ensure complete removal of ovarian tissue.
Dr. Kooperman completed the American Association of Gynecologic Laparoscopists (AAGL) Fellowship Program in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill., and will be starting practice at the Highland Park (Ill.) North Shore Hospital System in August 2019. He reported no relevant disclosures.
References
1. Am J Obstet Gynecol. 2005;193(6):2062-6.
2. J Minim Invasive Gynecol. 2011;18(2):194-9.
3. Fertil Steril. 2007;87(5):1005-9.