User login
Be alert to deep SSI risk after knee surgery
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
Deep surgical-site infections (SSIs) and septic arthritis are not uncommon after the surgeries for periarticular knee fractures, a meta-analysis of existing research found.
A smaller analysis of 1,567 patients found that 2.4% had septic arthritis. “Surgeons managing periarticular knee fractures should be vigilant when wounds are not pristine,” the investigators recommended.
The report, which appeared in JAMA Network Open, was led by premed student Grayson R. Norris of High Point (N.C.) University.
The researchers noted that there are widely variable statistics regarding SSI after surgery for periarticular knee fractures. A better understanding of the risk would help orthopedic surgeons, given the mortality risk and extra costs associated with postoperative deep SSIs.
For the analysis, the researchers reviewed 117 studies with 11,432 patients who had fractures in the tibial plateau (61% of studies), distal femur (14%), proximal tibia (11%), patella (9%), and multiple sites (6%). More than two-thirds of the studies were retrospective.
Overall, 5.7% of patients suffered deep SSIs, with the highest percentage in the proximal tibia group (6.4%).
A total of 20 studies examined septic arthritis and found that 2.4% of patients in those studies suffered from the condition. Of 182 cases of deep SSIs with bacterial culture results, methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus were the most common bacteria.
“Considering that MRSA was the most common pathogen in our study and that this pathogen is increasing in prevalence, health care practitioners should revisit the use of specific and appropriate prophylactic antibiotics,” the researchers wrote. “Risk factors, such as open fractures, diabetes, smoking, and, most importantly, compartment syndrome, should alert the treating surgeon to an increased risk. Further work is needed to mitigate the association of these conditions with SSI risk in periarticular knee fractures.”
The researchers added that many of the studies in their analysis were of poor quality. “Authors in orthopedic traumatology should strive to conduct higher-quality research, such as randomized clinical trials and case-control or cohort studies,” they noted.
One author reported receiving grants from Zimmer Biomet and DePuy Synthes outside the submitted work. No other disclosures were reported. No study funding was reported.
SOURCE: Norris GR et al. JAMA Netw Open. 2019;2(8):e199951.
FROM JAMA NETWORK OPEN
Native tissue repair of POP: Apical suspension, anterior repair, and posterior repair



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes
Sensory feedback may smooth walking with a prosthetic leg
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
FROM NATURE MEDICINE
Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
Native tissue repair of POP: Surgical techniques to improve outcomes
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
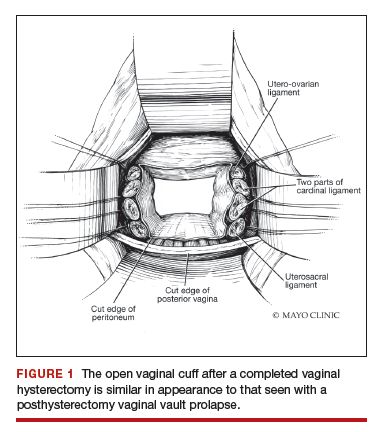
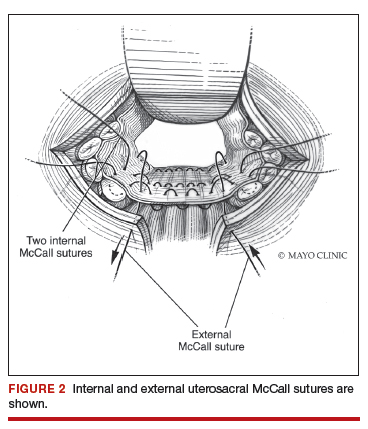
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.
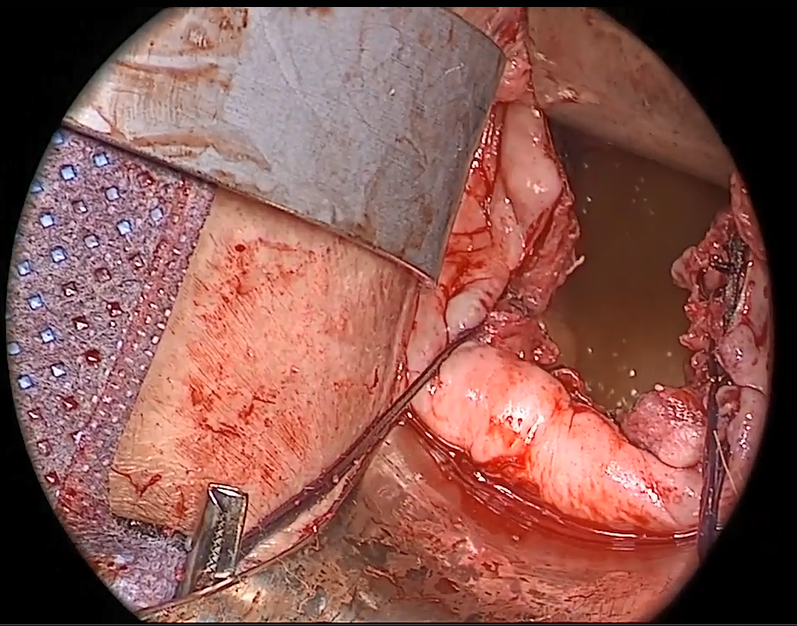
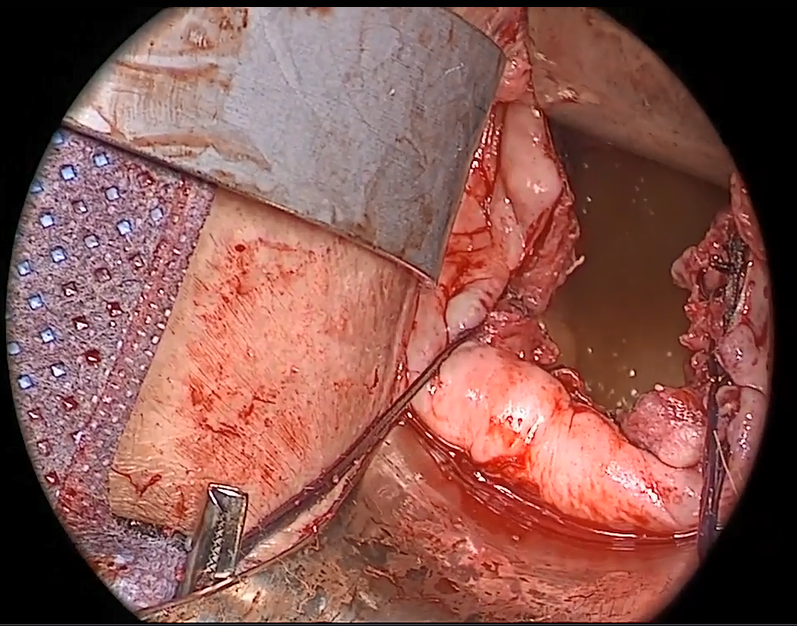
Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
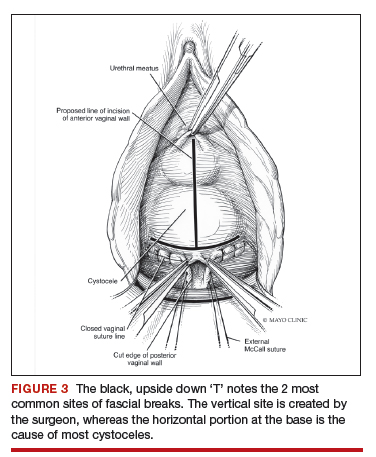
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.
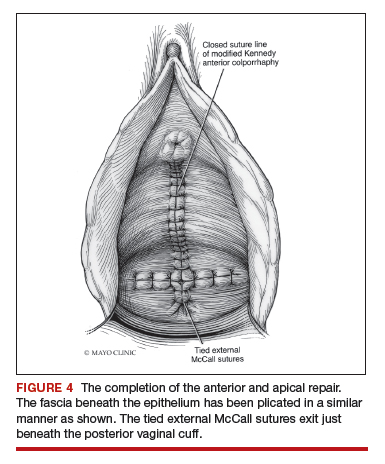
Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.
Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
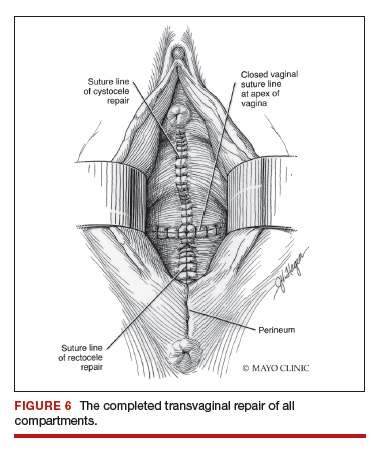
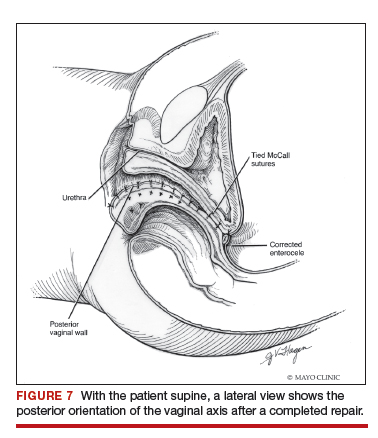
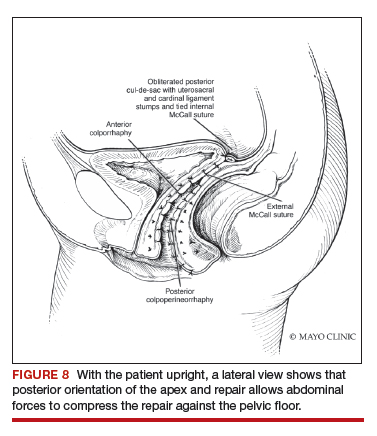
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.
Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
Weight loss surgery linked to lower CV event risk in diabetes
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
Despite a focus on reducing macrovascular events in individuals with type 2 diabetes, none of the major randomized controlled trials of glucose-lowering interventions that support current treatment guidelines have achieved this outcome. This study of bariatric surgery in obese patients with diabetes, however, does show reductions in major adverse cardiovascular events, although these outcomes should be interpreted with caution because of their observational nature and imprecise matching of the study groups.
Despite this, the many known benefits associated with bariatric surgery–induced weight loss suggest that for carefully selected, motivated patients with obesity and type 2 diabetes – who have been unable to lose weight by other means – this could be the preferred treatment option.
Dr. Edward H. Livingston is the deputy editor of JAMA and with the department of surgery at the University of California, Los Angeles. These comments are adapted from an accompanying editorial (JAMA 2019, Sept 2. DOI:10.1001/jama.2019.14577). No conflicts of interest were declared.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
, compared with nonsurgical management, according to data presented at the annual congress of the European Society of Cardiology.
The retrospective cohort study, simultaneously published in JAMA, looked at outcomes in 13,722 individuals with type 2 diabetes and obesity, 2,287 of whom underwent metabolic surgery and the rest of the matched cohort receiving usual care.
At 8 years of follow-up, the cumulative incidence of the primary endpoint – a composite of first occurrence of all-cause mortality, coronary artery events, cerebrovascular events, heart failure, nephropathy, and atrial fibrillation – was 30.8% in the weight loss–surgery group and 47.7% in the nonsurgical-control group, representing a 39% lower risk with weight loss surgery (P less than .001).
The analysis failed to find any interaction with sex, age, body mass index (BMI), HbA1c level, estimated glomerular filtration rate, or use of insulin, sulfonylureas, or lipid-lowering medications.
Metabolic surgery was also associated with a significantly lower cumulative incidence of myocardial infarction, ischemic stroke and mortality than usual care (17% vs. 27.6%).
In particular, researchers saw a significant 41% reduction in the risk of death at eight years in the surgical group compared to usual care (10% vs. 17.8%), a 62% reduction in the risk of heart failure, a 31% reduction in the risk of coronary artery disease, and a 60% reduction in nephropathy risk. Metabolic surgery was also associated with a 33% reduction in cerebrovascular disease risk, and a 22% lower risk of atrial fibrillation.
In the group that underwent metabolic surgery, mean bodyweight at 8 years was reduced by 29.1 kg, compared with 8.7 kg in the control group. At baseline, 75% of the metabolic surgery group had a BMI of 40 kg/m2 or above, 20% had a BMI between 35-39.9, and 5% had a BMI between 30-34.9.
The surgery was also associated with significantly greater reductions in HbA1c, and in the use of noninsulin diabetes medications, insulin, antihypertensive medications, lipid-lowering therapies, and aspirin.
The most common surgical weight loss procedure was Roux-en-Y gastric bypass (63%), followed by sleeve gastrectomy (32%), and adjustable gastric banding (5%). Five patients underwent duodenal switch.
In the 90 days after surgery, 3% of patients experienced bleeding that required transfusion, 2.5% experienced pulmonary adverse events, 1% experienced venous thromboembolism, 0.7% experienced cardiac events, and 0.2% experienced renal failure that required dialysis. There were also 15 deaths (0.7%) in the surgical group, and 4.8% of patients required abdominal surgical intervention.
“We speculate that the lower rate of [major adverse cardiovascular events] after metabolic surgery observed in this study may be related to substantial and sustained weight loss with subsequent improvement in metabolic, structural, hemodynamic, and neurohormonal abnormalities,” wrote Ali Aminian, MD, of the Bariatric and Metabolic Institute at the Cleveland Clinic, and coauthors.
“Although large and sustained surgically induced weight loss has profound physiologic effects, a growing body of evidence indicates that some of the beneficial metabolic and neurohormonal changes that occur after metabolic surgical procedures are related to anatomical changes in the gastrointestinal tract that are partially independent of weight loss,” they wrote.
The authors, however, were also keen to point out that their study was observational, and should therefore be considered “hypothesis generating.” While the two study groups were matched on 37 baseline covariates, those in the surgical group did have a higher body weight, higher BMI, higher rates of dyslipidemia, and higher rates of hypertension.
“The findings from this observational study must be confirmed in randomized clinical trials,” they noted.
The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
SOURCE: Aminian A et al. JAMA 2019, Sept 2. DOI: 10.1001/jama.2019.14231.
AT THE ESC CONGRESS 2019
Key clinical point: Bariatric surgery may reduce the risk of cardiovascular events in people with type 2 diabetes.
Major finding: Bariatric surgery is associated with a 39% reduction in risk of major cardiovascular events.
Study details: Retrospective cohort study in 13,722 individuals with type 2 diabetes and obesity.
Disclosures: The study was partly funded by Medtronic, and one author was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Five authors declared funding and support from private industry, including from Medtronic, and one author declared institutional grants.
Source: Aminian A et al. JAMA 2019, September 2. DOI: 10.1001/jama.2019.14231.
Minimally invasive surgery for cervical cancer: Is surgeon volume a factor?
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
FDA’s low-risk TAVR okay set to propel case volume
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
With the Food and Drug Administration’s approval of two different pairs of transcatheter aortic valve replacement systems for patients at low surgical risk, U.S. case volume for the procedure should markedly rise given that patients at low surgical risk form the largest risk subgroup among patients with aortic stenosis severe enough to warrant valve replacement.
But even as transcatheter aortic valve replacement (TAVR) now becomes the predominant approach for fixing severely stenotic aortic valves regardless of a patient’s risk level, the procedure remains less optimal than surgical aortic valve replacement (SAVR) in selected patients, putting an onus on clinicians to identify and alert patients for whom the transcatheter approach is questionable.
The anticipated surge in TAVR cases for low-risk patients after the FDA’s Aug. 16, 2019, decision will also likely lead to more hospitals offering TAVR. That development will test whether recently enacted rules from the Centers for Medicare & Medicaid Services on procedure-volume minimums for TAVR programs – at least 20 cases a year (or 40 within 2 years) at centers that also perform at least 300 percutaneous coronary interventions annually – lead to outcomes at lower-volume centers that come reasonably close to the outcomes at higher-volume programs for low-risk patients.
“The paradigm has definitely shifted from SAVR as the gold standard to TAVR as the primary treatment for aortic stenosis. This opens TAVR to the vast majority of patients with aortic stenosis,” roughly three-quarters of patients with aortic valve stenosis severe enough to need valve replacement, said Joseph C. Cleveland Jr., MD, a cardiothoracic surgeon and professor of surgery at the University of Colorado at Denver, Aurora.
The actual, immediate increase in TAVR patients may not be quite as large as this fraction suggests. That’s in part because many patients in the low-risk category based on their surgical risk score already have been judged to have higher-risk features by heart-valve teams that has allowed such patients to undergo TAVR, said John D. Carroll, MD, professor of medicine and director of interventional cardiology at the University of Colorado.
For several years, U.S. rates of TAVR have exceeded SAVR, he noted, and in 2018 U.S. programs performed roughly 58,000 TAVR procedures and about 25,000 SAVRs, according to data collected by the Transcatheter Valve Therapy (TVT) Registry run by the Society of Thoracic Surgeons and the American College of Cardiology. Dr. Carroll is vice chair of the steering committee for this registry, which was mandated by the FDA in 2011 when the agency first allowed TAVR onto the U.S. market and is designed to capture every TAVR case performed in routine U.S. practice.
Despite this caveat, “there will be substantial growth in TAVR. Going forward, there will be more of a shift from SAVR to TAVR. That is what the results of the low-risk trials did,” Dr. Carroll predicted. In addition, the coming growth in TAVR numbers will stem from more than just low-risk patients whom a month ago would have undergone SAVR but now undergo TAVR instead. The availability of TAVR as an option for a wider range of patients should help boost public awareness that a nonsurgical way exists to treat severe aortic stenosis, plus the aging of baby boomers is on the verge of generating a substantial wave of new patients, a wave so high that Dr. Carroll called it a looming “tsunami” of patients needing TAVR.
How will low-risk TAVR affect lower-volume sites?
More TAVR patients will inevitably mean more U.S. sites offering the procedure, experts agreed. “We anticipate more low-volume programs,” Dr. Carroll said.
“Approval of TAVR for low-risk patients will result in a significant increase in the number of programs offering it. Approximately 1,100 U.S. programs offer SAVR, and as of now about 600 of these programs also offer TAVR. Health systems face the risk of losing patients if they don’t offer TAVR now that low-risk patients can be treated,” observed Sreekanth Vemulapalli, MD, a cardiologist at Duke University, Durham, N.C. who has run several studies using TVT Registry data and serves as liaison between the registry and its analytic center at Duke.
One of these studies, published earlier in 2019, showed that, among more than 96,000 registry patients who underwent transfemoral TAVR during 2015-2017 at 554 U.S. centers, those treated at sites that fell into the bottom quartile for case volume had an adjusted 30-day mortality rate that was 21% higher relative to patients treated at centers in the top quartile, a statistically significant difference (N Engl J Med. 2019 Jun 27;380[26]:2541-50). The absolute difference in adjusted 30-day mortality between the lowest and highest quartiles was 0.54%, roughly 1 additional death for every 200 patients. The TAVR centers in the lowest-volume quartile performed 5-36 cases/year, averaging 27 TAVRs/year; those in the highest quartile performed 86-371 TAVRs annually with an overall quartile average of 143 procedures/year.
Dr. Vemulapalli and others cautioned that TAVR case volume is currently serving as a surrogate, and imperfect, marker for program quality until TAVR programs generate enough data to allow a directly measured, risk-adjusted, outcome-driven assessment of performance. In the study he and his associates published in June, the 140 TAVR programs in the lowest-volume quartile showed a “high” level of variability in their adjusted mortality rates. Despite this limitation, the prospect that new TAVR programs will soon open to meet growing TAVR demand from low-risk patients poses the question of how these programs will perform during their start-up days (and possibly beyond), when case volumes may be light, especially if sites open in more remote sections of the United States.
“Will the real-world results of TAVR in low-risk patients match the fantastic results in the two low-risk TAVR trials?” wondered Dr. Carroll, referring to the PARTNER 3 (N Engl J Med. 2019 May 2;380[18]:1695-1705) and Evolut Low-Risk Patients trial (N Engl J Med. 2019 May 2;380[18]:1706-15). “It’s unknown whether a site just starting to do TAVRs will get the same results. The sites that participated in the low-risk trials were mostly high-volume sites.” On the other hand, TVT Registry data have shown that patients with surgical risk that was judged prohibitive, high, or intermediate all have had overall real-world outcomes that match what was seen in the relevant TAVR trials.
In addition, some experts view a modest drop in 30-day survival among patients treated at lower-volume TAVR sites as a reasonable trade-off for easier access for patients seeking this life-changing treatment.
“We need to ensure that patients have access to this treatment option,” said Catherine M. Otto, MD, professor of medicine and director of the Heart Valve Clinic at the University of Washington, Seattle. The potentially better outcomes produced at larger TAVR programs “need to be balanced against having a greater number of programs to ensure access for more patients and allow patients to be treated closer to home,” she said in an interview. She suggested that the potential exists to use telemedicine to link larger and more experienced TAVR programs with smaller and newer programs to help boost their performance.
“There is no perfect solution or metric to ensure high quality while also allowing for adequate access. As indications for TAVR expand we need to maintain vigilance and accountability as the therapy is dispersed to more patients at more centers,” said Brian R. Lindman, MD, medical director of the Structural Heat and Valve Center at Vanderbilt University, Nashville, Tenn. “We also need to insure that certain groups of patients have adequate access to this therapy. Adequate access to TAVR and high-quality clinical outcomes are both important goals.”
Plus, “the volume relationship may be less important,” in lower-risk patients, suggested Dr. Cleveland in an interview. Low-risk patients are younger and have fewer comorbidities and less vascular disease. “Low-volume centers should be able to treat these patients,” he said. Despite that, he personally supported the higher volume minimum for TAVR of 50 cases/year that the ACC, STS, and other U.S. professional societies recommended to CMS during public comment on the proposed rules. “We’ll see whether the increased access is worth this volume minimum.”
Who still gets SAVR?
Given the inherent attraction TAVR holds over SAVR for patients, heart-valve teams will need to convey the right message to patients who may be better served with surgical replacement despite the added trauma and recovery time it produces.
“The decision to perform TAVR or SAVR should now be based on a patient’s expected longevity as well as patient preferences and values, and not on the patient’s estimated surgical risk, except for the highest-risk patients in whom TAVR is recommended,” said Dr. Otto. A patient’s age, comorbidities, and overall life expectancy now move to center stage when deciding the TAVR or SAVR question, along with individual anatomic considerations, the possible need for concurrent procedures, and of course what the patient prefers including their willingness and ability to remain on lifelong anticoagulation if they receive a durable mechanical valve. Dr. Otto outlined this new landscape of the heart-valve team’s decision making process in an editorial she recently published (N Engl J Med. 2019 May 2;380[18]: 1769-70) that accompanied publication of PARTNER 3 and the Evolut Low-Risk Patients trial.
“For some patients there will be clear benefit from one approach, but for many patients, particularly those at low surgical risk, both TAVR and SAVR are technically feasible. For these patients it’s essential that the heart-valve team provide unbiased information to guide patients,” Dr. Otto said. The ideal person to provide this unbiased presentation of the pros and cons would be a cardiologist experienced with valve disease but not actively involved in performing valve-replacement procedures.
A big issue younger patients must confront is what remains unknown about long-term durability of TAVR valves. Dr. Otto called this “the most important missing piece of information. We only have robust data out to about 5 years. If TAVR valve will be durable for 15-20 years, then TAVR will become preferred even in younger patients.”
Even after TAVR became available to intermediate-risk patients in 2016, the median age of U.S. patients undergoing TAVR hardly budged, and has recently stood at about 81 years, Dr. Carroll noted. “With low-risk patients, we expect to see this change,” as more patients now who are in their 70s, 60s, and younger start to routinely undergo TAVR. As more younger patients with life expectancies on the order of 30 years consider TAVR, issues of valve durability “enter the discussion,” he said. “We need data to 10, 15 years,” and in its low-risk approval the FDA mandated manufacturers to follow these patients for at least 10 years. Although valve-in-valve replacement of failed TAVR valves is an option, it’s not always a smooth fix with the potential for prosthesis-patient mismatch (J Am Coll Cardiol. 2018 Dec 4;72[22]:2701-11) and resulting hemodynamic problems, Dr. Carroll said.
Bicuspid-valve replacement with TAVR is another big unknown, largely because these patients were excluded from the TAVR trials. A recently published analysis of the 2,726 patients with a bicuspid aortic valve who underwent TAVR anyway in routine U.S. practice between June 2015 and November 2018 and were in the TVT Registry (about 3% of all TAVR patients during this period) showed that these patients had similar mortality, compared with the tricuspid-valve patients, but a significantly increased stroke rate (JAMA. 2019 Jun 11;321[22]:2193-202). The authors concluded that a prospective, randomized study of TAVR, compared with SAVR, is needed for these patients, and many others in the field agree.
As availability of TAVR grows and public awareness increases, heart-valve teams may find it challenging sometimes to help patients understand the upsides of SAVR for their individual clinical needs when TAVR is superficially so much more attractive.
“The desire to avoid the prolonged hospitalization and recovery from SAVR is a huge driver of patient preference,” noted Dr. Carroll.
“It’s hard to tell a 55 year old to think about another procedure they may need when they are 65 or 70 if they undergo TAVR now rather than SAVR. They don’t want open-heart surgery; I hear that all the time,” Dr. Cleveland said. “If I were a 55-year-old aortic valve patient I’d strongly consider TAVR, too.”
Financial consideration at the site performing the interventions can also be a factor. “Differential costs and payments associated with SAVR and TAVR create different financial incentives for health systems between these two procedures,” noted Dr. Vemulapalli. “There likely needs to be a system that creates equal incentives to do SAVR or TAVR so that the decision between them can come down to just the patient and heart-valve team. We need further data and decision aids to help better define which patients will likely do better with SAVR and which with TAVR.”
What now?
Since the first large TAVR trials started in 2007, their main thrust has been to prove the efficacy and safety of TAVR in patients at sequentially less risk of undergoing SAVR. Now that this series of comparisons has ended, where will TAVR research turn its attention?
In addition to the big outstanding issues of TAVR-valve long-term durability, and the efficacy and safety of TAVR for replacing bicuspid valves, other big questions and issues loom. They include the optimal anticoagulant regimen for preventing leaflet thrombosis, reducing the need for pacemakers, reducing strokes, the applicability of TAVR to patients with less severe aortic stenosis, the impact of treating severe but asymptomatic aortic valve obstruction, optimizing valve-in-valve outcomes, and further improvements to valve design, hemodynamics, and delivery. In short, the question of TAVR’s suitability for patients regardless of their surgical risk may have now been answered, but many questions remain about the best way to use and to optimize this technology.
Dr. Cleveland and Dr. Carroll have participated in TAVR trials but had no personal financial disclosures. Dr. Otto had no disclosures. Dr. Vemulapalli has received personal fees from Janssen, Novella, Premiere, and Zafgen, and research funding from Boston Scientific and Abbott Vascular. Dr. Lindman has been a consultant to Medtronic, has served as an advisor to Roche, and has received research funding from Edwards Lifesciences.
Predictive model estimates likelihood of failing induction of labor in obese patients
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
FROM OBSTETRICS & GYNECOLOGY
Differential monocytic HLA-DR expression prognostically useful in PICU
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
LJUBLJANA, SLOVENIA – During their first 4 days in the pediatric ICU, critically ill children have significantly reduced human leukocyte antigen (HLA)–DR expression within all three major subsets of monocytes. The reductions are seen regardless of whether the children were admitted for sepsis, trauma, or after surgery, Navin Boeddha, MD, PhD, reported in his PIDJ Award Lecture at the annual meeting of the European Society for Paediatric Infectious Diseases.
The PIDJ Award is given annually by the editors of the Pediatric Infectious Disease Journal in recognition of what they deem the most important study published in the journal during the prior year. This one stood out because it identified promising potential laboratory markers that have been sought as a prerequisite to developing immunostimulatory therapies aimed at improving outcomes in severely immunosuppressed children.
Researchers are particularly eager to explore this investigative treatment strategy because the mortality and long-term morbidity of pediatric sepsis, in particular, remain unacceptably high. The hope now is that HLA-DR expression on monocyte subsets will be helpful in directing granulocyte-macrophage colony-stimulating factor, interferon-gamma, and other immunostimulatory therapies to the pediatric ICU patients with the most favorable benefit/risk ratio, according to Dr. Boeddha of Sophia Children’s Hospital and Erasmus University, Rotterdam, the Netherlands.
He reported on 37 critically ill children admitted to a pediatric ICU – 12 for sepsis, 11 post surgery, 10 for trauma, and 4 for other reasons – as well as 37 healthy controls. HLA-DR expression on monocyte subsets was measured by flow cytometry upon admission and again on each of the following 3 days.
The impetus for this study is that severe infection, major surgery, and severe trauma are often associated with immunosuppression. And while prior work in septic adults has concluded that decreased monocytic HLA-DR expression is a marker for immunosuppression – and that the lower the level of such expression, the greater the risk of nosocomial infection and death – this phenomenon hasn’t been well studied in critically ill children, he explained.
Dr. Boeddha and coinvestigators found that monocytic HLA-DR expression, which plays a major role in presenting antigens to T cells, decreased over time during the critically ill children’s first 4 days in the pediatric ICU. Moreover, it was lower than in controls at all four time points. This was true both for the percentage of HLA-DR–expressing monocytes of all subsets, as well as for HLA-DR mean fluorescence intensity.
In the critically ill study population as a whole, the percentage of classical monocytes – that is, CD14++ CD16– monocytes – was significantly greater at admission than in healthy controls by margins of 95% and 87%, while the percentage of nonclassical CD14+/-CD16++ monocytes was markedly lower at 2% than the 9% figure in controls.
The biggest discrepancy in monocyte subset distribution was seen in patients admitted for sepsis. Their percentage of classical monocytes was lower than in controls by a margin of 82% versus 87%; however, their proportion of intermediate monocytes (CD14++ CD16+) upon admission was twice that of controls, and it climbed further to 14% on day 2.
Among the key findings in the Rotterdam study: 13 of 37 critically ill patients experienced at least one nosocomial infection while in the pediatric ICU. Their day 2 percentage of HLA-DR–expressing classical monocytes was 42%, strikingly lower than the 78% figure in patients who didn’t develop an infection. Also, the 6 patients who died had only a 33% rate of HLA-DR–expressing classical monocytes on day 3 after pediatric ICU admission versus a 63% rate in survivors of their critical illness.
Thus, low HLA-DR expression on classical monocytes early during the course of a pediatric ICU stay may be the sought-after biomarker that identifies a particularly high-risk subgroup of critically ill children in whom immunostimulatory therapies should be studied. However, future confirmatory studies should monitor monocytic HLA-DR expression in a larger critically ill patient population for a longer period in order to establish the time to recovery of low expression and its impact on long-term complications, the physician said.
Dr. Boeddha reported having no financial conflicts regarding the award-winning study, supported by the European Union and Erasmus University.
SOURCE: Boeddha NP et al. Pediatr Infect Dis J. 2018 Oct;37(10):1034-40.
REPORTING FROM ESPID 2019