User login
Pre-course focuses on perioperative care
Hospitalists packed the room on Monday for a pre-course brimming with information on how to better care for patients undergoing surgery – a category of care that can involve high-stakes and complex decisions before and after a procedure.
Topics covered in the wide-ranging talks included how to assess risk in those with ischemic heart disease, ways to manage anticoagulants in a variety of patients, the basics of anesthesia, and issues particular to patients with neurologic diseases.
“If you keep those things in mind then you will do a good job taking care of patients as long as you use good clinical sense,” said pre-course director Kurt Pfeifer, MD, FHM, professor of internal medicine at the Medical College of Wisconsin, Milwaukee.
Throughout the sessions, presenters posed audience-response questions to keep everyone engaged. In her discussion of perioperative considerations involving neurologic diseases, Rachel Thompson, MD, MPH, SFHM, associate professor of internal medicine at the University of Nebraska Medical Center, Omaha, asked whether it’s true or false that 1 in 10 patients with epilepsy will have a seizure on the day of surgery, even if they maintain their normal medication regimen.
In her talk on using anticoagulants, Barbara Slawski, MD, MS, SFHM, professor of medicine at the Medical College of Wisconsin, said it was important to understand the newest literature when using national guidelines, to consider clotting and bleeding risks when considering bridging anticoagulation therapy, and to make a specific plan for management for each patient.
She emphasized the team approach.
“It’s really important to listen to your surgical colleagues when they’re concerned about bleeding risk,” she said.
Dr. Pfeifer said the hospitalists’ involvement in surgical cases ranges from preoperative assessments, helping handle last-minutes changes in a care plan, managing patients afterward, and postdischarge follow-up.
“When you look at the perioperative continuum, there are a lot of places where we have a role to play – maybe more than anyone else in the equation.”
Hospitalists packed the room on Monday for a pre-course brimming with information on how to better care for patients undergoing surgery – a category of care that can involve high-stakes and complex decisions before and after a procedure.
Topics covered in the wide-ranging talks included how to assess risk in those with ischemic heart disease, ways to manage anticoagulants in a variety of patients, the basics of anesthesia, and issues particular to patients with neurologic diseases.
“If you keep those things in mind then you will do a good job taking care of patients as long as you use good clinical sense,” said pre-course director Kurt Pfeifer, MD, FHM, professor of internal medicine at the Medical College of Wisconsin, Milwaukee.
Throughout the sessions, presenters posed audience-response questions to keep everyone engaged. In her discussion of perioperative considerations involving neurologic diseases, Rachel Thompson, MD, MPH, SFHM, associate professor of internal medicine at the University of Nebraska Medical Center, Omaha, asked whether it’s true or false that 1 in 10 patients with epilepsy will have a seizure on the day of surgery, even if they maintain their normal medication regimen.
In her talk on using anticoagulants, Barbara Slawski, MD, MS, SFHM, professor of medicine at the Medical College of Wisconsin, said it was important to understand the newest literature when using national guidelines, to consider clotting and bleeding risks when considering bridging anticoagulation therapy, and to make a specific plan for management for each patient.
She emphasized the team approach.
“It’s really important to listen to your surgical colleagues when they’re concerned about bleeding risk,” she said.
Dr. Pfeifer said the hospitalists’ involvement in surgical cases ranges from preoperative assessments, helping handle last-minutes changes in a care plan, managing patients afterward, and postdischarge follow-up.
“When you look at the perioperative continuum, there are a lot of places where we have a role to play – maybe more than anyone else in the equation.”
Hospitalists packed the room on Monday for a pre-course brimming with information on how to better care for patients undergoing surgery – a category of care that can involve high-stakes and complex decisions before and after a procedure.
Topics covered in the wide-ranging talks included how to assess risk in those with ischemic heart disease, ways to manage anticoagulants in a variety of patients, the basics of anesthesia, and issues particular to patients with neurologic diseases.
“If you keep those things in mind then you will do a good job taking care of patients as long as you use good clinical sense,” said pre-course director Kurt Pfeifer, MD, FHM, professor of internal medicine at the Medical College of Wisconsin, Milwaukee.
Throughout the sessions, presenters posed audience-response questions to keep everyone engaged. In her discussion of perioperative considerations involving neurologic diseases, Rachel Thompson, MD, MPH, SFHM, associate professor of internal medicine at the University of Nebraska Medical Center, Omaha, asked whether it’s true or false that 1 in 10 patients with epilepsy will have a seizure on the day of surgery, even if they maintain their normal medication regimen.
In her talk on using anticoagulants, Barbara Slawski, MD, MS, SFHM, professor of medicine at the Medical College of Wisconsin, said it was important to understand the newest literature when using national guidelines, to consider clotting and bleeding risks when considering bridging anticoagulation therapy, and to make a specific plan for management for each patient.
She emphasized the team approach.
“It’s really important to listen to your surgical colleagues when they’re concerned about bleeding risk,” she said.
Dr. Pfeifer said the hospitalists’ involvement in surgical cases ranges from preoperative assessments, helping handle last-minutes changes in a care plan, managing patients afterward, and postdischarge follow-up.
“When you look at the perioperative continuum, there are a lot of places where we have a role to play – maybe more than anyone else in the equation.”
Arthroscopic Excision of Bipartite Patella With Preservation of Lateral Retinaculum in an Adolescent Ice Hockey Player
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
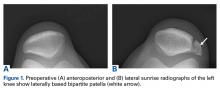
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
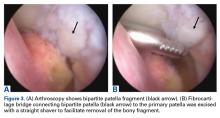
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
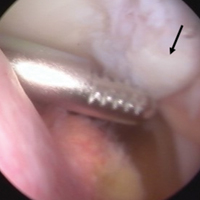
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
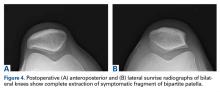
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Bipartite patella is an asymptomatic anatomical variant.
- Occasionally, some adolescent athletes can present with AKP, resulting in decreased participation and performance.
- Bipartite patella is classified in type I, inferior pole; type II, lateral margin; and type III, superior lateral pole, depending on where the accessory patellar fragment is.
- Nonoperative treatment is advocated first. If symptoms persist surgical treatment should be attempted.
In 2% to 3% of the general population, the finding of bipartite patella on knee radiographs is often incidental.1,2 During development, the patella normally originates in a primary ossification center. Occasionally, secondary ossification centers emerge around the margins of the primary center and typically join that center. In some cases, the secondary2 center remains separated, leading to patella partita and an accessory patellar fragment.3,4
The bipartite patella is connected to the primary patella by fibrocartilage. The fibrous attachment may become irritated or separated as a result of trauma, overuse, or strenuous activity.1,5-7 Saupe classification of bipartite patella is based on accessory patellar fragment location: type I, inferior pole; type II, lateral margin; and type III, superior lateral pole.8 When an individual with a bipartite patella becomes symptomatic, anterior knee pain (AKP) is the most common complaint—it has been described in adolescent athletes in numerous sports.7,9-11For most patients, first-line treatment is nonoperative management. A typical regimen includes reduced activity, use of nonsteroidal anti-inflammatory drugs, physical therapy, and isometric quadriceps-strengthening exercises.1,12 Other nonoperative approaches described in the literature are immobilization,5,10 steroid and anesthetic injection, and ultrasound therapy.13 If symptoms do not improve, surgical treatment should be considered. Surgical treatment options include open excision of fragment,3,9,12 arthroscopic excision of fragment,7,14,15 tension band wiring,5,16 open reduction and internal fixation,17 open or arthroscopic vastus lateralis release,18-20 and lateral retinacular release.21 However, the optimal surgical option remains controversial.
In this case report, we present a modification of an arthroscopic surgical technique for excising a symptomatic bipartite patella and report midterm clinical outcomes. The patient provided written informed consent for print and electronic publication of this report.
Case Report
A 16-year-old elite male ice hockey player presented to clinic with a 2-week history of left AKP. He could not recall a specific injury that triggered the symptoms. Radiographs were obtained at an outside institution, and knee patellar fracture was diagnosed. The patient, placed in a straight-leg immobilizer, later presented to a referral clinic for a second opinion and further evaluation. Physical examination revealed significant tenderness to palpation of the lateral aspect of the patella. Range of motion was symmetric and fully intact. Patellar mobility was excellent. However, the patient could not perform a straight-leg raise because of the pain.
We obtained anteroposterior and lateral radiographs (Figures 1A, 1B), which showed evidence of a Saupe type III bipartite patella with separation at the superolateral pole.
Two years later, the patient returned with left AKP, again localized to the lateral aspect of the patella, over the bipartite fragment. The pain was significant with compression. Given the patient’s history, arthroscopic excision of the bipartite patella was recommended. After discussing all treatment options, the patient elected to proceed with the surgery.
Surgical Technique
The patient was positioned supine on the operating table. Medial and lateral parapatellar arthroscopic portals were created. Menisci, cruciate ligaments, and tibiofemoral articular cartilage were arthroscopically visualized and determined to be normal. The bipartite patella was easily visualized, and notably loose when probed. Grade 2 chondromalacia was present diffusely throughout the bipartite patella and on the far lateral aspect of the patella, at the fragment interface.
Attention was then turned to arthroscopic removal of the accessory patellar fragment (Figures 3A, 3B).
Postoperative Rehabilitation
Rehabilitation focused on protection of the healing patella and accelerated rehabilitation for early return to play. Range-of-motion exercises and stationary bicycling were initiated on postoperative day 1. Weight-bearing was allowed as tolerated. Quadriceps sets, straight-leg raises, and ankle pumps were performed 5 times daily for 6 weeks. Six weeks after surgery, the patient was cleared, and he returned to full on-ice activities.
Outcomes
This study was approved by an Institutional Review Board. Preoperative and postoperative outcomes were obtained and stored in a data registry. The patient’s Lysholm score22 improved from 71 before surgery to 100 at 31-month follow-up. In addition, his subjective International Knee Documentation Committee score23 improved from 65.5 before surgery to 72.4 after surgery. At follow-up, patient satisfaction with outcome was 10/10. In addition, the patient had returned to playing hockey at a higher national level without functional limitation.
Discussion
The most important finding in this case is that arthroscopic excision of a bipartite patella with preservation of the lateral retinaculum in an elite adolescent hockey player resulted in improved subjective clinical outcomes scores and early return to competition. Arthroscopic excision was favored over open excision in this patient because of potential quicker recovery,14 less pain, and expedited return to competition. In addition, previous arthroscopic techniques were modified to shorten postoperative rehabilitation. The modified technique included preservation of the lateral retinaculum and total arthroscopic excision of the accessory bipartite patella fragment.
Although results of open techniques have been favorable,3,8,9 these procedures are far more invasive than arthroscopic techniques and may result in loss of quadriceps strength and prolonged rehabilitation.18 Weckström and colleagues12 followed 25 male military recruits for a minimum of 10 years after open excision of symptomatic bipartite patella. Mean Kujala score was 95 (range, 75-100), and median visual analog scale score for knee pain was 1.0 (range, 0.0-6.0). In a study by Bourne and Bianco,3 13 of 16 patients who were followed for an average of 7 years experienced complete pain relief with an average recovery time of 2 months.
Other studies have described the arthroscopic excision technique for symptomatic bipartite patella,7,14,15 but outcomes are underreported, especially for follow-ups longer than 2 years. Felli and colleagues7 described a case of arthroscopic excision and lateral release in a 23-year-old female professional volleyball player; at 1-year follow-up, the patient was symptom-free and back to full athletic participation. Azarbod and colleagues14 also reported on a patient who was symptom-free, 6 weeks after arthroscopic excision of bipartite patella. Carney and colleagues15 indicated that successful excision of bipartite patella was evident on 6-month radiographic follow-up. Our 31-month follow-up is the longest of any study on arthroscopic excision of bipartite patella. Clinical outcomes were excellent both in our patient’s case and in the earlier studies.
Our patient was a high-level hockey player who wanted to return to competition as quickly as possible. Conservative management, including physical therapy, initially resolved his symptoms and allowed him to resume on-ice activities after 6 weeks. In time, however, his symptoms returned and began limiting his on-ice performance. Arthroscopic removal of the bipartite patella accessory fragment allowed him to return to full on-ice activities after 6 weeks. His case provides evidence that arthroscopic management of bipartite patella with preservation of the vastus lateralis and lateral retinaculum may be an excellent treatment option for patients who want to return to athletics as quickly as possible.
Our technique of arthroscopic excision with preservation of lateral retinaculum is an excellent treatment option for symptomatic bipartite patella. This option, combined with an aggressive rehabilitation protocol, allows for pain relief and expedited return to competition.
Am J Orthop. 2017;46(3):135-138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
1. Atesok K, Doral MN, Lowe J, Finsterbush A. Symptomatic bipartite patella: treatment alternatives. J Am Acad Orthop Surg. 2008;16(8):455-461.
2. Insall J. Current concepts review: patellar pain. J Bone Joint Surg Am. 1982;64(1):147-152.
3. Bourne MH, Bianco AJ Jr. Bipartite patella in the adolescent: results of surgical excision. J Pediatr Orthop. 1990;10(1):69-73.
4. Oohashi Y, Koshino T, Oohashi Y. Clinical features and classification of bipartite or tripartite patella. Knee Surg Sports Traumatol Arthrosc. 2010;18(11):1465-1469.
5. Okuno H, Sugita T, Kawamata T, Ohnuma M, Yamada N, Yoshizumi Y. Traumatic separation of a type I bipartite patella: a report of four knees. Clin Orthop Relat Res. 2004;(420):257-260.
6. Yoo JH, Kim EH, Ryu HK. Arthroscopic removal of separated bipartite patella causing snapping knee syndrome. Orthopedics. 2008;31(7):717.
7. Felli L, Fiore M, Biglieni L. Arthroscopic treatment of symptomatic bipartite patella. Knee Surg Sports Traumatol Arthrosc. 2011;19(3):398-399.
8. Green WT Jr. Painful bipartite patellae. A report of three cases. Clin Orthop Relat Res. 1975;(110):197-200.
9. Ishikawa H, Sakurai A, Hirata S, et al. Painful bipartite patella in young athletes. The diagnostic value of skyline views taken in squatting position and the results of surgical excision. Clin Orthop Relat Res. 1994;(305):223-228.
10. Stocker RL, van Laer L. Injury of a bipartite patella in a young upcoming sportsman. Arch Orthop Trauma Surg. 2011;131(1):75-78.
11. Wong CK. Bipartite patella in a young athlete. J Orthop Sports Phys Ther. 2009;39(7):560.
12. Weckström M, Parviainen M, Pihlajamäki HK. Excision of painful bipartite patella: good long-term outcome in young adults. Clin Orthop Relat Res. 2008;466(11):2848-2855.
13. Kumahashi N, Uchio Y, Iwasa J, Kawasaki K, Adachi N, Ochi M. Bone union of painful bipartite patella after treatment with low-intensity pulsed ultrasound: report of two cases. Knee. 2008;15(1):50-53.
14. Azarbod P, Agar G, Patel V. Arthroscopic excision of a painful bipartite patella fragment. Arthroscopy. 2005;21(8):1006.
15. Carney J, Thompson D, O’Daniel J, Cassidy J. Arthroscopic excision of a painful bipartite patella fragment. Am J Orthop. 2010;39(1):40-43.
16. Tauber M, Matis N, Resch H. Traumatic separation of an uncommon bipartite patella type: a case report. Knee Surg Sports Traumatol Arthrosc. 2007;15(1):83-87.
17. Werner S, Durkan M, Jones J, Quilici S, Crawford D. Symptomatic bipartite patella: three subtypes, three representative cases. J Knee Surg. 2013;26(suppl 1):S72-S76.
18. Adachi N, Ochi M, Yamaguchi H, Uchio Y, Kuriwaka M. Vastus lateralis release for painful bipartite patella. Arthroscopy. 2002;18(4):404-411.
19. Maeno S, Hashimoto D, Otani T, Masumoto K, Hui C. The “coiling-up procedure”: a novel technique for extra-articular arthroscopy. Arthroscopy. 2010;26(11):1551-1555.
20. Ogata K. Painful bipartite patella. A new approach to operative treatment. J Bone Joint Surg Am. 1994;76(4):573-578.
21. Mori Y, Okumo H, Iketani H, Kuroki Y. Efficacy of lateral retinacular release for painful bipartite patella. Am J Sports Med. 1995;23(1):13-18.
22. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154
23. Grevnerts HT, Terwee CB, Kvist J. The measurement properties of the IKDC-subjective knee form. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3698-3706.
VIDEO: How to pick surgical margins with mixed breast lesions
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – According to recent guidelines, no ink on tumor is the right surgical margin for early stage invasive breast cancer and 2 mm is the right lumpectomy margin for ductal carcinoma in situ (DCIS) treated with whole breast radiation. But, what do you do when invasive carcinoma is associated with DCIS?
It’s a common question for breast surgeons. Monica Morrow, MD, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in New York City, explained how to handle the situation in a video interview at the annual meeting of the American Society of Breast Surgeons.
She was the senior author on the 2014 invasive breast cancer guidelines and the lead author on the 2016 DCIS guidelines, both of which were consensus statements on surgical margins from the Society of Surgical Oncology and other groups (Ann Surg Oncol. 2014 Mar;21[3]:704-16; Ann Surg Oncol. 2016 Nov;23[12]:3801-10).
Dr. Morrow explained the thinking behind the guidelines and how to apply them to mixed lesions and other clinical scenarios, as well as their limitations and what remains to be determined. A key point is that a margin less than 2 mm is not by itself an indication for mastectomy in DCIS.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
From ASBS 2017
Resection reduces inflammatory breast cancer lesion recurrence
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
LAS VEGAS – In patients with nonmetastatic inflammatory breast cancer (IBC), aggressive surgical resection can reduce local/regional recurrence rates to levels comparable to those seen in patients with noninflammatory breast cancer.
The key to the improved outcome is the presence of a plastic surgeon who can help close a tricky incision. “We have a multidisciplinary team that allows us to call upon the plastic surgeons if we can’t control the incision, and they help us with a flap closure,” said Kelly Rosso, MD, a breast surgical oncology fellow at the MD Anderson Cancer Center, Houston, who presented the study results at the annual meeting of the American Society of Breast Surgeons.
To see if their surgical interventions were producing better outcomes, the team analyzed data from 114 patients with nonmetastatic inflammatory breast cancer who had received trimodality therapy (neoadjuvant chemotherapy, surgery, and radiation therapy) from 2007 to 2015.
The median patient age was 52 years, and the median follow-up period was 3.6 years. In total, 55% of patients had N2 IBC, and 45% had N3 disease.
Nearly all (113 of 114) patients had negative surgical margins. A total of 29 patients died during the follow-up period, and the 5-year survival rate was 69%. The odds of a local/regional recurrence over 2 years was 3% (95% confidence interval, 1%-10%). The 2-year odds of recurrence or distant metastasis was 23% (95% CI, 16%-32%).
Dr. Lucci said that he suspects that many surgeons avoid resection of inflammatory breast cancer lesions under the assumption that the disease is metastatic in nature and fear that an attempt at local control would put the patient through unnecessary surgery with little benefit.
The new study challenges that belief. “I think this is a changing paradigm. We have to consider that those patients need to be thought of as still resectable and think about getting rid of that local disease, at the very least to improve their quality of life and hopefully to improve their overall outcome,” Dr. Lucci said.
The results should be readily achievable in other centers, he added. “If they have a plastic surgeon who’s willing to help them, they can resect to negative margins just as well [as in noninflammatory patients]. And, if they have a radiation therapist who’s dedicated to doing a more aggressive course, they can achieve the same results.”
Dr. Rosso and Dr. Lucci reported having no financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: The odds of a local/regional recurrence over 2 years were 3%.
Data source: A retrospective analysis of 114 patients.
Disclosures: Dr. Rosso and Dr. Lucci reported having no financial disclosures.
Don’t rely on MRI findings after DCIS needle biopsy
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
LAS VEGAS – MRI results after percutaneous biopsy for ductal carcinoma in situ (DCIS) are often unreliable, overestimating the extent of disease and leading to over treatment, investigators from the Mayo Clinic in Phoenix concluded after reviewing 54 cases.
Of the 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology, Barbara Pockaj, MD, senior investigator and surgical oncologist at the Mayo Clinic in Phoenix, reported at the annual meeting of the American Society of Breast Surgeons.
Dr. Pockaj and the Mayo radiologists had a hunch that there was a problem and were uncomfortable interpreting MRI findings after needle biopsy, but there was nothing in the literature about it. The goal of the study was to quantify the problem and “bring this issue to light,” she said.
“I feel better, now that we have this data in hand, as I counsel patients,” who are sometimes so alarmed by MRI findings after needle biopsy that they opt for mastectomies. Now, Dr. Pockaj can tell them with certainty that MRI “may overestimate the extent of disease, so we have to take MRI findings with a grain of salt,” she said.
“Surgeons should be aware that, in patients with a postbiopsy MRI, tumor size may be different than anticipated” and may adversely “affect surgical decision-making and potentially cosmetic results. Percutaneous biopsy significantly limits the ability to accurately interpret the extent of DCIS on preoperative breast MRI by overestimating extent of disease,” the investigators concluded.
Postbiopsy MRIs were performed in 38 of the 54 women (70%), and 14 women (26%) had bigger surgeries because of their MRI results.
Three women had larger lumpectomies, and 11 had mastectomies. “Three really needed it, and one maybe half way,” but the remaining seven turned out on pathology to have only needed lumpectomies, Dr. Pockaj said.
Mean lesion size on preoperative MRI was 3.6 cm while mean lesion size on pathologic specimen was 1.6 cm. Postbiopsy MRI did not significantly correlate with the actual size of the tumor specimen (r = 0.028; P = 0.921).
If the core needle biopsy shows DCIS, in many cases, “I think you can go right to surgery,” Dr. Pockaj said. MRI might still be indicted for very large lesions, suspicions of invasive disease, or very dense breasts, but, even so, the results need to be put into context with the postbiopsy changes, she said.
Women in the study were a mean of 59 years old. Most had high- or intermediate-grade DCIS. Women upgraded to invasive disease at surgery were excluded from the analysis.
There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Of 54 women, 7 (13%) had mastectomies driven by postbiopsy MRI findings that were not indicated on final pathology.
Data source: Single-center review of 54 DCIS cases.
Disclosures: There was no industry funding for the work, and the investigators reported having no relevant financial disclosures.
Lumpectomy plus reconstruction outperforms mastectomy plus reconstruction
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
LAS VEGAS – Lumpectomy plus oncoplastic surgery has a total cost that is comparable to lumpectomy alone and that is less than mastectomy plus reconstructive surgery, according to an analysis of nearly 40,000 women.
While the complication rate was slightly higher than lumpectomy alone, the difference disappeared in the last year of data, possibly because surgical techniques had improved. The study looked at MarketScan Commercial Claims and Encounters Database data from 2000 to 2011 and confirms findings from an earlier study that looked at data from the MD Anderson Cancer Center (Ann Surg Oncol. 2016 Oct;23[10]:3190-8).
Lumpectomy plus oncoplastic surgery is increasingly being performed, but it still only represented about 2% of surgeries in women with invasive epithelial breast cancer, although this percentage had grown to 8.4% by 2011 and is likely higher now, according to Dr. Hwang, an associate professor of breast surgical oncology and surgical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Candidates for lumpectomy plus oncoplastic surgery include those with large volume disease, multifocal/centric disease, poorly located tumors, or macromastia. “If oncoplastic reconstruction was not available, these patients would oftentimes be undergoing total mastectomy with reconstruction,” Dr. Hwang said.
The study included records from 39,518 women who underwent breast surgery with or without reconstruction. It excluded patients who received postmastectomy radiation or neoadjuvant chemotherapy.
A total of 40% underwent lumpectomy plus whole breast irradiation (BCT), 2% received BCT plus oncoplastic reconstruction (BCT+R), 30% had total mastectomy (TM), and 29% had total mastectomy plus reconstruction (TM+R).
After adjusting for age, race, comorbidity, chemotherapy, axillary surgery, and nodal positivity, the complication rate was lowest in the TM group (25%; relative risk compared with BCT+R, 0.71; 95% confidence interval, 0.64-0.78; P less than .001), followed by BCT (29%; RR, 0.80; 95% CI, 0.72-0.88; P less than .001), BCT+R (37%), and TM+R (54%; RR, 1.49; 95% CI, 1.35-1.64; P less than .001).
The rate of complications in BCT+R fell over time, so that, by 2011, it was only slightly higher than those of BCT alone (31.5% vs 29.4%). That trend is “probably just additional experience with the technique,” Dr. Hwang said.
Total costs for TM+R was $89,187, compared with $48,767 for TM, $66,217 for BCT, and $69,781 for BCT+R. The cost difference between BCT and TM+R was about $23,000.
The findings are encouraging, said Judy Boughey, MD, professor of surgery at the Mayo Clinic, Rochester, Minn., who moderated the session. However, she called for caution in interpreting the results because the population of interest made up just 2% of the overall sample. “I think you’re going to have the widest variability of confidence intervals around that group,” Dr. Boughey said. “It’s eye-opening, but I would just view it with caution.”
Dr. Hwang and Dr. Boughey reported having no financial disclosures.
At ASBS 2017
Key clinical point:
Major finding: Mastectomy with reconstruction had a 49% higher complication rate and $20,000 higher cost, compared with lumpectomy plus reconstruction.
Data source: Nationwide retrospective analysis of 39,518 women.
Disclosures: Dr. Hwang and Dr. Boughey reported having no financial disclosures.
Multimodal breast cancer treatment linked with greater risk of lymphedema
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Chemotherapy, radiation, and higher body mass index are strongly associated with increased risk of lymphedema in breast cancer patients who have undergone sentinel node biopsy or axillary lymph node dissection, according to analysis of data from the Rochester Epidemiology Project.
Judy C. Boughey, MD, professor of surgery and vice chair of research at the Mayo Clinic, Rochester, Minn., and her associates performed a chart review of 1,794 patients diagnosed with a first breast cancer in Olmsted County, Minn., between 1990 and 2010. Patients’ median age at diagnosis was 60 years. About half (48%) were diagnosed at stage I, while 17% were diagnosed at Stage 0, 29% at Stage II, and 6% at Stage III.
At a median of 10 years of follow-up, 209 patients had been diagnosed with breast cancer–related lymphedema, with most diagnoses occurring within 5 years of surgery. No cases of lymphedema were found in patients who did not have axillary surgery.
There was no significant difference in the rate of lymphedema based on type of breast cancer surgery (mastectomy vs. lumpectomy with breast-conserving surgery); however, lymphedema occurred significantly more frequently in patients who received ALND as compared to those who received SLN (15.9% vs. 5.3%). Lymphedema rates did not differ based on type of axillary surgery (3.5% for ALND and 4.1% for SLN) in a subset of 453 patients who did not receive radiation or chemotherapy.
Almost a third (31.3%) of patients who had nodal radiation, with or without breast or chest wall radiation, developed lymphedema by 5 years, as compared with 5.9% of patients who did not receive radiation (P less than .001). Similarly, patients who received chemotherapy were significantly more likely to develop lymphedema. At 5 years, lymphedema was present in 27.2% of patients who received anthracylcline/cyclophosphamide (AC) with a taxane agent, 29.7% of those who received a taxane agent without AC , and 6.0% of those who did not get chemotherapy (P less than .001).
Five-year incidence of lymphedema also increased significantly with body mass index, occurring in 17.1% of patients with a BMI greater than 35 kg/m2, 13% of those with a BMI between 30-34.99, and 14.4% of those with a BMI between 25-29.99, as compared with 8% of those with a BMI below 25.
On univariate analysis, increased stage was associated with risk of lymphedema, Dr. Boughey said. However, on multivariate analysis, stage was no longer associated with risk of lymphedema and “the dominate factors were BMI, type of axillary surgery, use of radiation therapy and particularly nodal radiation, and the use of chemotherapy.”
The highest rate of lymphedema was seen in a patients with the most advanced disease who had ALND with nodal radiation and AC with a taxane agent, she said.
“We think this is primarily due to the modalities of treatment rather that the pure phenomenon of stage,” Dr. Boughey added. “This study can help identify patients at a higher risk of lymphedema so that we can individualize the surveillance of these patients to allow them to have earlier identification and earlier treatment of lymphedema.”
dfulton@frontlinemedcom.com
On Twitter @denisefulton
FROM ASBS 2017
Key clinical point:
Major finding: More than 30% of patients receiving nodal radiation developed lymphedema vs. 5.9% of those who did not. Similarly, lymphedema developed in just under 30% of patients who received a taxane vs. 6.9% of those who did not.
Data source: Analysis of all 1,794 cases of first breast cancers diagnosed in Olmsted County, Minn., 1990-2010.
Disclosures: The Rochester Epidemiology Project receives federal funding from agencies of the Health & Human Services Department. Dr. Boughey reported no relevant financial conflicts of interest.
How to reduce the open hysterectomy rate
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
SAN ANTONIO – About a decade ago, the Northern California Permanente Medical Group realized it had a problem: There were too many open hysterectomies being performed.
“We had a large number of low-volume surgeons doing a lot of open hysterectomies,” said Andrew Walter, MD, a gynecologic surgeon at the Permanente Medical Group (TPMG) campus in Roseville, Calif., part of Kaiser Permanente. Across 15 hospitals in the system, “our open rate was 64% in 2007.”
To address the problem, “our leadership basically set targets; we then provided surgical education and training in minimally invasive hysterectomy,” Dr. Walter said at the annual scientific meeting of the Society of Gynecologic Surgeons.
Kaiser Permanente is a capitated system, but even so, its experience in reducing open hysterectomy rates may be useful for other systems dealing with the same problem.
At first, Dr. Walter and his colleagues didn’t know how much of an improvement could be made. “In 2008, we said, ‘Okay, 60% seems doable’” as a minimally invasive target rate, Dr. Walter said. TPMG hit that target, and “the chiefs looked at the numbers and said we can do a little bit better.” With some more effort, the rate of minimally invasive hysterectomies hit 80%, and then 90%, about where it stands today, with a corresponding drop in open rates.
“It will be interesting to see if someone says, ‘Let’s try 95%,’ ” Dr. Walter said.
However, the process hasn’t been easy, and it hasn’t been entirely evidence based, he said. “We are working with hundreds of gynecologists, and very few of them have had advanced training. But ultimately, it worked.”
Surgeons trained with TPMG peers experienced in minimally invasive hysterectomy, and both surgeons were kept on full salary during the learning process. It took about 5-15 cases before learner surgeons were considered proficient. “Funded proctoring is the most important aspect of the program,” he said.
TPMG also reduced the number of physicians doing hysterectomies from 416 to 228 in 2015. “This was the hard part, deciding who is a surgeon and who is not,” Dr. Walter said. “I would like to tell you that these were Kumbaya moments, but there was consternation, and there remains consternation about the process.” A number of ob.gyns. voluntarily gave up their surgical privileges, saying, ‘Thank God I don’t have to operate anymore,’ ” he said.
For low-volume surgeons – those who performed 10 or fewer hysterectomies a year – who wanted stay in the operating room, “we either had to push them into this training or encourage them to give up their surgical practices.” The more than 3,600 hysterectomies performed annually at TPMG facilities are now mostly done by surgeons doing at least 11 of these procedures a year, and often more than 20.
TPMG also paid for training courses at outside institutions, and department chiefs were held accountable for performance.
“Obviously, there are unique processes within Kaiser Permanente that facilitated this, but some of them are not unique. Physician support by enhanced training – that’s something that can be done. The barriers are reimbursement, and deciding who is a surgeon,” he said.
The next target is vaginal hysterectomy. Rates have been stable lately at about 30%, but “we have found that many patients, when reviewed, are vaginal hysterectomy candidates. We’ve set a target of 40%.” The procedure needs to be incentivized, Dr. Walter said, but it’s unclear how to do that at this point.
Dr. Walter reported having no relevant financial disclosures.
* The meeting sponsor information was updated 6/9/2017.
EXPERT ANALYSIS FROM SGS 2017
Plazomicin beats gold standard antibiotics in complex, gram-negative bacterial infections
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Approaches to isolating the uterine artery at its origin from the internal iliac artery

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Advanced techniques in cystectomy for mature cystic teratomas
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Advanced techniques in cystectomy for mature cystic teratomas
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Advanced techniques in cystectomy for mature cystic teratomas
- Novel classification of labial anatomy and evaluation in the treatment of labial agglutination
- Strategies for prophylactic oophoropexy
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by