User login
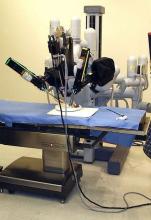
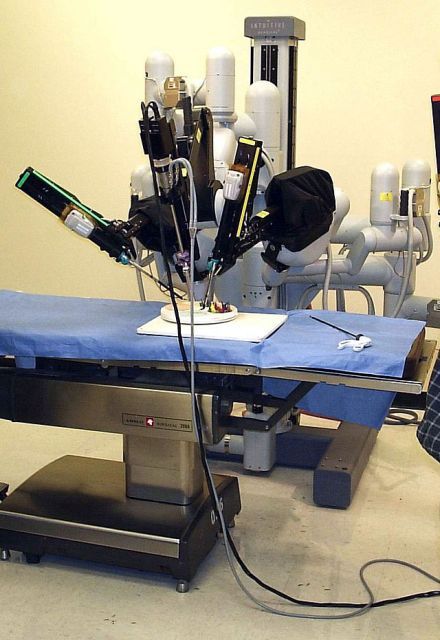
Robotic vs. conventional laparoscopic surgery for rectal cancer: No winner yet
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
Robot-assisted rectal surgery is gaining acceptance but, with some exceptions, outcomes are not significantly improved over the conventional laparoscopic approach, a meta-analysis has found.
Conducted by Katie Jones, MD, and her colleagues at Brighton and Sussex (England) University Hospital NHS Trust, the meta-analysis was designed as a follow-up to ROLARR (isrctn.org ID: ISRCTN80500123), a randomized clinical trial in which robot-assisted and. conventional laparoscopic surgery for rectal cancer were studied for risk of conversion to open surgery. That trial findings showed that robot-assisted laparoscopic surgery did not significantly reduce the risk of conversion. For other outcomes (pathology, complications, bladder, and sexual function), the differences between the two approaches were insignificant. But the two surgical approaches did differ on cost: The robot-assisted operation was significantly more expensive than the conventional laparoscopic procedure.
Dr. Jones and her colleagues analyzed data from ROLARR in the context of 27 other qualifying studies and confirmed many of the ROLARR findings. The 27 case control studies comprised 5,547 patients and had comparable outcomes data.
The outcomes of interest were duration of operation, conversion risk, blood loss, length of stay, oncological outcomes, time to first flatus, reoperation rate, postoperative morbidity, and postoperative mortality.
The investigators found that duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach, though this difference was not statistically significant (z = 1.28, P = .20), and blood loss, morbidity, and mortality were similar between the two groups. Oncological outcomes (risk of positive circumferential resection margins, lymph node yield, and length of distal resection margins) were similar for these two surgical approaches.
In contrast to the ROLARR findings, this meta-analysis found that the risk of conversion favored the robot-assisted procedure (z = 5.51, P = .00001). Hospital stay (z = 2.46, P = 01) and time to first flatus outcomes (z = 3.09, P = .002) favored the robot-assisted procedure. Postop morbidity and mortality and reoperation rate were similar in the two groups.
“Based upon the findings of this largest-ever series on the role of robotic surgery in rectal cancer resection, the [robot-assisted procedure] is certainly a feasible technique and oncologically safe surgical intervention but failed to demonstrate any superiority over [the conventional laparoscopic approach] for many surgical outcomes,” the investigators wrote. “Mere advantage of robotic surgery was noted in only three postoperative outcomes, that is early passage of flatus, lower risk of conversion, and shorter hospitalization.”
Dr. Jones and her colleagues declared they had no conflicts of interest.
SOURCE: Jones K et al. World J Gastroentrol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
FROM WORLD JOURNAL OF GASTROINTESTINAL ONCOLOGY
Key clinical point:
Major finding: Duration of the operation was longer for the robot-assisted procedure, compared with the conventional laparoscopic approach (z = 1.28, P = .20), but blood loss, morbidity, and mortality were similar between the two groups.
Study details: Meta-analysis of 27 studies and one RCT of patients who had robot-assisted laparoscopic surgery or conventional laparoscopic surgery for rectal cancer.
Disclosures: The investigators had no disclosures.
Source: Jones K. World J Gastrointest Oncol. 2018 Nov 15. doi: 10.4251/wjgo.v10.i11.449.
‘Organoid technology’ poised to transform cancer care
BOSTON– Imagine being able to .
The implications are nearly endless. To start, chemotherapy and radiation options could be screened in vitro, much like culture and sensitivity testing of bacteria, to find a patient’s best option. Tumor cultures could be banked for mass screening of new cytotoxic candidates.
It’s already beginning to happen in a few research labs around the world, and it might foretell a breakthrough in cancer treatment.
After decades of failure, the trick to growing tumor cells in culture has finally been figured out. When stem cells are fished out of healthy tissue – from the crypts of the gastrointestinal lining, for instance – and put into a three-dimensional matrix culture with growth factors, they grow into little replications of the organs they came from, called “organoids;” when stem cells are pulled from cancers, they replicate the primary tumor, growing into “tumoroids” ready to be tested against cytotoxic drugs and radiation.
Philip B. Paty, MD, FACS, a colorectal surgeon and organoid researcher at Memorial Sloan Kettering Cancer Center, New York, said he is certain that the person who led the team that figured out the right culture condition – Hans Clevers, MD, PhD, a molecular genetics professor at the University of Utrecht (the Netherlands) – is destined for a Nobel Prize.
Dr. Paty took a few minutes at the annual clinical congress of the American College of Surgeons to explain in an interview why, and what ‘organoid technology’ will likely mean for cancer treatment in a few years.
“The ability to grow and sustain cancer means that we now can start doing real science on human tissues. We could never do this before. We’ve been treating cancer without being able to grow tumors and study them.” The breakthrough opens the door to “clinical trials in a dish,” and will likely take personalized cancer treatment to a new level, he said.
“It remains to be proven that “organoid technology “can change outcomes for patients, but those studies are likely coming,” said Dr. Paty, who investigates tumoroid response to radiation in his own lab work.
BOSTON– Imagine being able to .
The implications are nearly endless. To start, chemotherapy and radiation options could be screened in vitro, much like culture and sensitivity testing of bacteria, to find a patient’s best option. Tumor cultures could be banked for mass screening of new cytotoxic candidates.
It’s already beginning to happen in a few research labs around the world, and it might foretell a breakthrough in cancer treatment.
After decades of failure, the trick to growing tumor cells in culture has finally been figured out. When stem cells are fished out of healthy tissue – from the crypts of the gastrointestinal lining, for instance – and put into a three-dimensional matrix culture with growth factors, they grow into little replications of the organs they came from, called “organoids;” when stem cells are pulled from cancers, they replicate the primary tumor, growing into “tumoroids” ready to be tested against cytotoxic drugs and radiation.
Philip B. Paty, MD, FACS, a colorectal surgeon and organoid researcher at Memorial Sloan Kettering Cancer Center, New York, said he is certain that the person who led the team that figured out the right culture condition – Hans Clevers, MD, PhD, a molecular genetics professor at the University of Utrecht (the Netherlands) – is destined for a Nobel Prize.
Dr. Paty took a few minutes at the annual clinical congress of the American College of Surgeons to explain in an interview why, and what ‘organoid technology’ will likely mean for cancer treatment in a few years.
“The ability to grow and sustain cancer means that we now can start doing real science on human tissues. We could never do this before. We’ve been treating cancer without being able to grow tumors and study them.” The breakthrough opens the door to “clinical trials in a dish,” and will likely take personalized cancer treatment to a new level, he said.
“It remains to be proven that “organoid technology “can change outcomes for patients, but those studies are likely coming,” said Dr. Paty, who investigates tumoroid response to radiation in his own lab work.
BOSTON– Imagine being able to .
The implications are nearly endless. To start, chemotherapy and radiation options could be screened in vitro, much like culture and sensitivity testing of bacteria, to find a patient’s best option. Tumor cultures could be banked for mass screening of new cytotoxic candidates.
It’s already beginning to happen in a few research labs around the world, and it might foretell a breakthrough in cancer treatment.
After decades of failure, the trick to growing tumor cells in culture has finally been figured out. When stem cells are fished out of healthy tissue – from the crypts of the gastrointestinal lining, for instance – and put into a three-dimensional matrix culture with growth factors, they grow into little replications of the organs they came from, called “organoids;” when stem cells are pulled from cancers, they replicate the primary tumor, growing into “tumoroids” ready to be tested against cytotoxic drugs and radiation.
Philip B. Paty, MD, FACS, a colorectal surgeon and organoid researcher at Memorial Sloan Kettering Cancer Center, New York, said he is certain that the person who led the team that figured out the right culture condition – Hans Clevers, MD, PhD, a molecular genetics professor at the University of Utrecht (the Netherlands) – is destined for a Nobel Prize.
Dr. Paty took a few minutes at the annual clinical congress of the American College of Surgeons to explain in an interview why, and what ‘organoid technology’ will likely mean for cancer treatment in a few years.
“The ability to grow and sustain cancer means that we now can start doing real science on human tissues. We could never do this before. We’ve been treating cancer without being able to grow tumors and study them.” The breakthrough opens the door to “clinical trials in a dish,” and will likely take personalized cancer treatment to a new level, he said.
“It remains to be proven that “organoid technology “can change outcomes for patients, but those studies are likely coming,” said Dr. Paty, who investigates tumoroid response to radiation in his own lab work.
REPORTING FROM THE ACS CLINICAL CONGRESS
SRS beats surgery in early control of brain mets, advantage fades with time
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
FROM JAMA ONCOLOGY
Key clinical point: Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than surgical resection, but this advantage fades with time.
Major finding: Patients treated with surgery were more likely to have local recurrence in the first 3 months following treatment, compared with patients treated with SRS (hazard ratio, 5.94).
Study details: An exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial. Analysis involved 268 patients with one to three brain metastases who underwent whole-brain radiotherapy or observation after SRS (n = 154) or complete surgical resection (n = 114).
Disclosures: The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. Dr. Handorf reported financial compensation from Pfizer, via her institution.
Source: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Nipple-sparing mastectomy safe in older patients
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point: Neoadjuvant chemotherapy and advancing age were not associated with increased rates of complications in women undergoing nipple-sparing mastectomy in two recent studies.
Major finding: The overall rate of complications was not significantly different for neoadjuvant chemotherapy vs. primary surgery (12.7% vs. 10.7%) or for age over 60 years vs. younger age (15.5% vs. 13.0%).
Study details: Retrospective studies of a nipple-sparing mastectomy registry including more than 3,000 breasts (neoadjuvant chemotherapy vs. primary surgery study) and a single-institution study of nearly 400 patients (older vs. younger study).
Disclosures: The authors reported no conflicts of interest.
Source: Cox S et al. and Bartholomew AJ et al. Session SF310 ACS Clinical Congress 2018.
'Liver first' for select stage IV colon cancer gaining traction
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
BOSTON –
It’s an alternative to usual care, meaning simultaneous bowel and liver resection or bowel resection with liver surgery later on.
Systemic chemotherapy comes first, followed by liver resection. If margins are microscopically negative, the patient gets another round of chemotherapy. If no additional lesions emerge, the primary tumor is taken out. The entire process can take up to a year.
The approach was developed in the Netherlands for rectal cancer with advanced liver metastases. The idea was to get the liver lesions out before they became unresectable, then remove the primary tumor later on. It’s gaining traction now for colon cancer, and beginning to trickle into the United States at a few academic medical centers.
It comes down to what’s more dangerous, the metastases or the primary tumor? Tumor science hasn’t answered that question yet. There’s general agreement that metastases are what kill people with cancer, but it’s not known if they come mostly from previous metastases or from the primary tumor. The liver-first approach assumes the former.
Liver-first is “extremely controversial. For older surgeons who are not in tertiary care centers, liver-first doesn’t make sense, and it doesn’t seem to make sense to patients. They wonder why you would go after the liver when they were diagnosed with a colon tumor,” said Janice Rafferty, MD, FACS, professor of surgery at the University of Cincinnati, at the annual clinical congress of the American College of Surgeons.
“Well, it’s because the primary tumor doesn’t limit your life,” she continued. “The life-limiting disease is in the liver, not the colon. If you explain it to them that way, it makes sense. If we cannot get an R0 resection on the liver, it doesn’t make sense to go after the primary, unless it’s symptomatic with obstruction, bleeding, or fistula.”
There have been about 10 attempts at a randomized trial of this approach versus usual care, but they were not successful because of the difficulty of recruiting patients. Patients – and no doubt, some surgeons – may have some resistance to the logic of going after metastases first.
Dr. Rafferty moderated a review of research from Yale University, New Haven, Conn., that attempted to plug the evidence gap. The Yale investigators “presented really interesting data that shows that liver-first has improved survival,” she said.
The Yale team used the National Cancer Database to compare 2010-2015 outcomes from liver-first patients with patients who had simultaneous or bowel-first resections, followed by later liver resections. The database didn’t allow them to tease out simultaneous from bowel-first cases, so they lumped them together as usual care. To avoid confounding, rectal carcinomas and metastases to the lung, brain, and other organs were excluded.
Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis. Among patients who completed their resections, median survival was 57 months among 140 liver-first patients versus 36 months with usual care in 3,988.
The benefit held after adjustment for patient and tumor characteristics (hazard ratio for death 0.77 in favor of liver first). When further adjusted for chemotherapy timing, there was a strong trend for liver-first but it was not statistically significant, suggesting that up-front chemotherapy contributed to the results (HR, 0.88; 95% confidence interval, 0.75-1.01; P = .09).
There were many caveats. The liver-first patients were younger, with over half under the age of 60 years versus just over 40% in usual care. They were also healthier based on Charlson comorbidity scores and more likely to have upfront chemotherapy and be treated at an academic center.
So, what should surgeons make of these findings? Lead investigator Vadim Kurbatov, MD, a Yale surgery resident, argued that, at the very least, they suggest that liver-first is a viable option for stage IV colon cancer with isolated liver metastases. Going further, they suggest that liver first may be the right way to go for younger, healthier patients at academic centers.
For sicker stage IV patients, however, the role of liver-first is unclear. “We really do need a randomized trial,” he said.
Dr. Kurbatov and Dr. Rafferty had no relevant disclosures to report. The work was funded in part by the National Institutes of Health.
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point: The liver-first approach may be appropriate for younger, healthier patients at academic centers.
Major finding: Median survival was 34 months among 358 liver-first patients versus 24 months among 18,042 usual care patients in an intention-to-treat analysis.
Study details: A review of over 18,000 patients in the National Cancer Database
Disclosures: The lead investigator had no disclosures to report. The work was funded in part by the National Institutes of Health.
Cervical cancer survival higher with open surgery in LACC trial
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Cervical cancer recurrence and survival rates were worse with minimally invasive vs. open surgery in a prospective study.
Major finding: Disease-free survival at 4.5 years was 86% with minimally invasive vs. 96.5% with open surgery.
Study details: The phase 3 LACC trial of more than 600 women with cervical cancer, and a population based study of nearly 2,500 women with cervical cancer.
Disclosures: Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani is a member of the OB.GYN. News editorial board, but reported having no other relevant disclosures.
Source: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
Hemithyroidectomy rates rose after guideline update
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
BOSTON – that position the procedure as equivalent to total thyroidectomy, an analysis of U.S. hospital data shows.
Patients undergoing hemithyroidectomy had fewer complications and shorter length of stay versus patients who received a bilateral procedure, with no corresponding increase in completion thyroidectomy, according to results of the retrospective analysis reported here at the annual clinical congress of the American College of Surgeons.
“We think this suggests that surgeons might be changing their practice at these hospitals, at least in part in response to the guidelines,” investigator Timothy M. Ullmann, MD, endocrine oncology research fellow in the department of surgery at New York Presbyterian Hospital–Weill Cornell Medical Center, New York.
Dr. Ullmann and colleagues queried the American College of Surgeons National Surgical Quality Improvement Program database for the 2014-2016 period to illustrate operative trends before and after release of the 2015 guidelines from the American Thyroid Association guidelines. They looked at a total of 26,562 procedures done before the guidelines were release, and 7,422 done after.
The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P less than .001), according to Dr. Ullmann. By contrast, the rates of completion thyroidectomy were 7.8% for the pre-guidelines period and 7.4% post-guidelines (P = .19).
The increase was gradual throughout the 2014-2016 period, though it was especially steep after the guideline introduction, according to co-investigator Toni Beninato, MD, of Weill Cornell Medicine.
“While we can’t say that the guidelines directly caused an increase, it’s a pretty good association,” Dr. Beninato said in a press conference. “I think going forward, we would expect this to continue to increase, because the vast majority of patients with thyroid cancer probably fit criteria to have a hemithyroidectomy rather than a total thyroidectomy.”
Patients treated by otolaryngologists were more likely to undergo hemithyroidectomies versus those treated by general surgeons, multivariate analysis of this data set suggested (odds ratio, 1.13, P less than .001). On the other hand, Hispanic patients and those with a higher operative risk classification were less likely to undergo a unilateral procedure.
Complications were less likely in the hemithyroidectomy patients, according to investigators. There were significantly fewer superficial surgical site infections, at 0.2% versus 0.4% for total thyroidectomy, and operative time was 91.6 minutes versus 141.1 minutes.
Hemithyroidectomy patients were less likely to be reintubated after surgery, had a shorter length of stay, and were more likely to be managed on an outpatient basis, they added at the press conference.
Prior ATA guidelines, in place since 2009, called for near-total or total thyroidectomy for cancers that were at least 1 cm in size in patients with no contraindications to the procedure. The 2015 update says the initial surgical procedure could also be a unilateral procedure, or lobectomy, in cancers greater than 1 cm, or smaller than 4 cm with no extrathyroidal extension.
Dr. Ullmann and Dr. Beninato had no relevant financial relationships with commercial interests pertaining to the content of their presentation.
SOURCE: Ullmann TM et al. Abstract SF121 presented at the American College of Surgeons Clinical Congress.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Hemithyroidectomy rates had a robust uptick following release of 2015 clinical practice guidelines, suggesting surgeons may be changing their practice in response.
Major finding: The rate of hemithyroidectomy increased from 15.6% before guidelines to 18.3% afterward (P < 0.001).
Study details: Analysis of the American College of Surgeons-NSQIP database from 2014 to 2016 including nearly 34,000 procedures.
Disclosures: Study authors reported no disclosures.
Source: Ullmann TM et al. Abstract SF125 presented at American College of Surgeons Clinical Congress
Post-mastectomy pain strategy allows for safe, same-day discharge
BOSTON – A multimodal pain regimen allowed for safe and effective same-day discharge of women undergoing mastectomy procedures, a recent study showed.
Women had little need for stronger oral narcotic use in the single center, retrospective study presented at the annual clinical congress of the American College of Surgeons.
The analysis included 72 consecutive mastectomies performed at a single center from November 2015 to July 2017. Most mastectomies were bilateral (61, or 84.7%) while 11 (15.3%) were unilateral.
Patients received a standardized pain regimen including 1 gram of IV acetaminophen interoperatively, combined with 30 mg of IV ketorolac and a 4-level intercostal nerve block with liposomal bupivacaine.
Liposomal bupivacaine has a longer half-life than other anesthetics, according to lead study author Radbeh Torabi, MD, a fifth-year plastic surgery resident at Louisiana State University (LSU) Health Science Center in New Orleans.
“That allows for prolonged pain control, especially during the time when the patient’s going to have the most amount of pain, which is the first day to two days postoperatively,” Dr. Torabi said in an interview.
All 72 patients were discharged home on the same day with just a 1-week prescription for acetaminophen with codeine.
Only 5 patients presented to the emergency room in the 30-day postoperative period, and of those, only 2 (2.8%) required readmission for reasons other than mastectomy-related pain, investigators said. The remaining 3 patients did present with pain, but did not require hospital admission.
Taken together, these findings suggest that this multimodal strategy offers excellent pain control and has the potential to minimize inpatient admissions while decreasing oral narcotic use, investigators said in an interview following their presentation.
“The main takeaway is reducing the amount of prescriptions we give,” Dr. Torabi said.
Study co-author Cameron T. Ward Coker, MD, a fourth-year general surgery resident at LSU, said the multimodal pain strategy used in this study could represent a step toward eliminating the risks associated with opioid prescribing.
“From the feedback we got from our lecture and the other surgeons in the room, it seems like that’s already becoming a widespread phenomenon,” Dr. Coker said.
Patients in the study had an average age of about 57 years and an average BMI of 30, according to the investigators.
Dr. Coker and Dr. Torabi had no disclosures related to the presentation.
SOURCE: Torabi R, et al. Scientific forum abstract at American College of Surgeons Clinical Congress. 2018 Oct 23.
BOSTON – A multimodal pain regimen allowed for safe and effective same-day discharge of women undergoing mastectomy procedures, a recent study showed.
Women had little need for stronger oral narcotic use in the single center, retrospective study presented at the annual clinical congress of the American College of Surgeons.
The analysis included 72 consecutive mastectomies performed at a single center from November 2015 to July 2017. Most mastectomies were bilateral (61, or 84.7%) while 11 (15.3%) were unilateral.
Patients received a standardized pain regimen including 1 gram of IV acetaminophen interoperatively, combined with 30 mg of IV ketorolac and a 4-level intercostal nerve block with liposomal bupivacaine.
Liposomal bupivacaine has a longer half-life than other anesthetics, according to lead study author Radbeh Torabi, MD, a fifth-year plastic surgery resident at Louisiana State University (LSU) Health Science Center in New Orleans.
“That allows for prolonged pain control, especially during the time when the patient’s going to have the most amount of pain, which is the first day to two days postoperatively,” Dr. Torabi said in an interview.
All 72 patients were discharged home on the same day with just a 1-week prescription for acetaminophen with codeine.
Only 5 patients presented to the emergency room in the 30-day postoperative period, and of those, only 2 (2.8%) required readmission for reasons other than mastectomy-related pain, investigators said. The remaining 3 patients did present with pain, but did not require hospital admission.
Taken together, these findings suggest that this multimodal strategy offers excellent pain control and has the potential to minimize inpatient admissions while decreasing oral narcotic use, investigators said in an interview following their presentation.
“The main takeaway is reducing the amount of prescriptions we give,” Dr. Torabi said.
Study co-author Cameron T. Ward Coker, MD, a fourth-year general surgery resident at LSU, said the multimodal pain strategy used in this study could represent a step toward eliminating the risks associated with opioid prescribing.
“From the feedback we got from our lecture and the other surgeons in the room, it seems like that’s already becoming a widespread phenomenon,” Dr. Coker said.
Patients in the study had an average age of about 57 years and an average BMI of 30, according to the investigators.
Dr. Coker and Dr. Torabi had no disclosures related to the presentation.
SOURCE: Torabi R, et al. Scientific forum abstract at American College of Surgeons Clinical Congress. 2018 Oct 23.
BOSTON – A multimodal pain regimen allowed for safe and effective same-day discharge of women undergoing mastectomy procedures, a recent study showed.
Women had little need for stronger oral narcotic use in the single center, retrospective study presented at the annual clinical congress of the American College of Surgeons.
The analysis included 72 consecutive mastectomies performed at a single center from November 2015 to July 2017. Most mastectomies were bilateral (61, or 84.7%) while 11 (15.3%) were unilateral.
Patients received a standardized pain regimen including 1 gram of IV acetaminophen interoperatively, combined with 30 mg of IV ketorolac and a 4-level intercostal nerve block with liposomal bupivacaine.
Liposomal bupivacaine has a longer half-life than other anesthetics, according to lead study author Radbeh Torabi, MD, a fifth-year plastic surgery resident at Louisiana State University (LSU) Health Science Center in New Orleans.
“That allows for prolonged pain control, especially during the time when the patient’s going to have the most amount of pain, which is the first day to two days postoperatively,” Dr. Torabi said in an interview.
All 72 patients were discharged home on the same day with just a 1-week prescription for acetaminophen with codeine.
Only 5 patients presented to the emergency room in the 30-day postoperative period, and of those, only 2 (2.8%) required readmission for reasons other than mastectomy-related pain, investigators said. The remaining 3 patients did present with pain, but did not require hospital admission.
Taken together, these findings suggest that this multimodal strategy offers excellent pain control and has the potential to minimize inpatient admissions while decreasing oral narcotic use, investigators said in an interview following their presentation.
“The main takeaway is reducing the amount of prescriptions we give,” Dr. Torabi said.
Study co-author Cameron T. Ward Coker, MD, a fourth-year general surgery resident at LSU, said the multimodal pain strategy used in this study could represent a step toward eliminating the risks associated with opioid prescribing.
“From the feedback we got from our lecture and the other surgeons in the room, it seems like that’s already becoming a widespread phenomenon,” Dr. Coker said.
Patients in the study had an average age of about 57 years and an average BMI of 30, according to the investigators.
Dr. Coker and Dr. Torabi had no disclosures related to the presentation.
SOURCE: Torabi R, et al. Scientific forum abstract at American College of Surgeons Clinical Congress. 2018 Oct 23.
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Of 72 women who had same-day discharge after mastectomy, only 3 presented for pain in the 30-day postoperative period.
Study details: A retrospective review of 72 consecutive mastectomies performed at a single surgical center.
Disclosures: The lead author had no disclosures related to the presentation.
Source: Torabi R, et al. Scientific forum abstract at American College of Surgeons Clinical Congress. 2018 Oct 23.
Opioid use cut nearly 50% for urologic oncology surgery patients
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
PHOENIX – Opioid use in urologic oncology patients dropped by 46% after one high-volume surgical center introduced changes to order sets and adopted new patient communication strategies, a researcher has reported.
The changes, which promoted opioid-sparing pain regimens, led to a substantial drop in postoperative opioid use with no compromise in pain control, according to Kerri Stevenson, a nurse practitioner with Stanford Health Care.
“Patients can be successfully managed with minimal opioid medication,” Ms. Stevenson said at a symposium on quality care sponsored by the American Society of Clinical Oncology.
However, “it takes a multidisciplinary team for effective change to occur – this cannot be done in silos,” she told attendees at the meeting.
Seeking to reduce their reliance on opioids to manage postoperative pain, Ms. Stevenson and her colleagues set out to reduce opioid use by 50%, from a baseline morphine equivalent daily dose (MEDD) of 95.1 in June to September 2017 to a target of 47.5 by March 2018.
The actual MEDD at the end of the quality improvement project was 51.5, a 46% reduction that was just shy of that goal, she reported.
Factors fueling opioid use included patient expectations that they would be used and the belief that adjunct medications were not as effective as opioids, Dr. Stevenson found in a team survey.
“We decided to target those,” she said. “Our key drivers were really focused on appropriate prescriptions, increasing patient and provider awareness, standardizing our pathways, and setting expectations.”
To tackle the problem, they revised EMR order sets to default to selection of adjunct medications, educated providers, and introduced new patient communication strategies.
Instead of asking “Would you like me to bring you some oxycodone?” providers would instead start by asking about the patient’s current pain control medications and whether they were working well. When prescribed, opioids should be started at lower doses and escalated only if needed.
“Once we started our interventions, we noticed an immediate effect,” Ms. Stevenson.
The decreases were consistent across a range of surgery types. For example, the MEDD dropped to 55.1 with robotic prostatectomy, a procedure with a 1-day admission and very small incisions, and to 50.6 for open radical cystectomy, which involves a large incision and a stay of approximately 4 days, she said.
To address concerns that they might just be undertreating patients, investigators looked retrospectively at pain scores. They saw no differences pre- and post intervention in pain or anxiety scores within the first 24-48 hours post procedure, Ms. Stevenson reported.
Ms. Stevenson had no disclosures related to the presentation. Coauthor Jay Bakul Shah, MD of Stanford Health Care reported a consulting or advisory role with Pacira Pharmaceuticals.
SOURCE: Stevenson K et al. Quality Care Symposium, Abstract 269.
REPORTING FROM THE QUALITY CARE SYMPOSIUM
Key clinical point: Substantial reductions in postoperative opioid use might be achievable through strategies that promote opioid-sparing pain regimens.
Major finding: Postoperative opioid use dropped 46% for urologic oncology patients after changing default order sets, introducing new patient communication strategies, and educating providers.
Study details: An analysis of opioid prescribing before and after introduction of a quality improvement project at one high-volume surgical center.
Disclosures: One study coauthor reported a consulting or advisory role with Pacira Pharmaceuticals.
Source: Stevenson K et al. Quality Care Symposium, Abstract 269.
More frequent CT surveillance in NSCLC doesn’t improve survival
More even after researchers controlled for tumor histology and recurrence.
Compared with those followed every 3 months, the hazard ratio for 6-month follow-up with CT scanning was 1.16, and 1.06 for annual follow-up – a nonsignificant difference. Nor did more frequent imaging improve survival among the subgroup of patients who were cancer free 9 months after their surgery or among those who had recurrences, Timothy L. McMurry, PhD, and his colleagues reported in the Annals of Surgery. The paper was presented at the annual meeting of the American Surgical Association.
The results probably reflect the very poor survival rates of any patients who develop recurrent non–small cell lung cancer (NSCLC), wrote Dr. McMurry, a biostatistician at the University of Virginia, Charlottesville, and his coauthors.
“Surveillance recommendations need to be considered in the context of potential harms and benefits to patients and their caregivers,” they said. “Follow-up imaging and office visits increase cost and can lead to patient anxiety. Although it seems intuitive that earlier detection of asymptomatic recurrence could improve outcomes, patients with recurrent NSCLC do very poorly … poor survival after recurrence helps explain why more intense surveillance after surgical resection was not associated with improvement in overall survival.”
However, they noted, treatment advances for recurrent and metastatic disease may already be changing the outlook for these patients, “systemic therapy and targeted agents are demonstrating clinically significant survival benefits for small patient subgroups, which, in the future, may augment the benefits of early recurrence detection.”
The team undertook this retrospective study – the largest of its kind in NSCLC patients – in light of current follow-up recommendations that are based almost solely on expert consensus, with low-level data.
“Because there is a paucity of high-quality data on NSCLC surveillance, practice guidelines are based on small retrospective analyses and expert opinion. This results in wide variation in practice including both underuse and overuse of surveillance services.”
The study plumbed the National Cancer Database, extracting information on patients who underwent surgery for NSCLC stages I-III during 2006-2007. All had complete resection and negative margins. Patients were followed through 2012, or until they had a recurrence, a new primary cancer, or they died.
The cohort comprised 4,463 who were followed with CT imaging: 1,614 every 3 months, 1,999 every 6 months, and 850 annually. These intervals correspond to the three different major recommendations. The most common procedure was a lobectomy (about 80%). Patients with higher-stage cancers were significantly more likely to receive more frequent imaging. The regression model controlled for age, sex, comorbidities, tumor stage, and surgical procedure.
After 14 months, 3,552 patients (79.5%) were alive and cancer free. However, during the rest of the follow-up period, 11% developed a new primary cancer and 24% a recurrence of their lung cancer, with no between-group differences. The regression analysis showed no significant difference in recurrence related to surveillance interval, whether 6 months was compared with 3 months (hazard ratio, 1.16) or 1 year with 3 months (HR, 1.06).
Results were much the same for the subgroup of 3,165 who were alive and cancer free 9 months after surgery. In this group, 11% developed a new primary cancer and 29% a recurrence of their lung cancer, with similar numbers in each of the surveillance groups (HR, 1.12 for 6 months vs. 3 months).
Finally, a model including only those who had recurrence, new cancers, or were lost to follow-up within 14 months of surgery also showed no benefit for more frequent surveillance.
“More recent prerecurrence imaging was not associated with postrecurrence survival (HR, 1.02 per month since imaging), and patients who had gone more than 14 months without imaging were at no greater risk of death (HR, 1.01),” the investigators wrote.
The significant predictors of worse survival were nothing surprising, the authors noted. These included symptomatic recurrence (HR, 1.49), distant recurrence, age, male sex, congestive heart failure, coronary artery disease, and peripheral vascular disease.
The findings are not to say, however, that CT surveillance confers no benefit, the authors noted.
“Historically, 5-year survival for the earliest stage of lung cancer, stage IA, was only 70%. Increased use of CT scanning has, however, resulted in a decrease in the median tumor size of resected NSCLC and a shift toward earlier-stage disease. … The National Lung Screening Trial prospectively evaluated annual low-dose screening CT scans and demonstrated a 20% reduction in mortality from lung cancer. This enormous improvement in survival for NSCLC patients provides great promise for the future and is likely to increase the volume of lung cancer resections performed and the number of lung cancer survivors needing routine surveillance.”
However, the data do show that “at least annual CT surveillance is appropriate but that there is no benefit to more than biannual surveillance.”
The authors reported no financial conflicts.
SOURCE: McMurry TL et al. Ann Surg 2018 Jul 12. doi: 10.1097/SLA.0000000000002955.
More even after researchers controlled for tumor histology and recurrence.
Compared with those followed every 3 months, the hazard ratio for 6-month follow-up with CT scanning was 1.16, and 1.06 for annual follow-up – a nonsignificant difference. Nor did more frequent imaging improve survival among the subgroup of patients who were cancer free 9 months after their surgery or among those who had recurrences, Timothy L. McMurry, PhD, and his colleagues reported in the Annals of Surgery. The paper was presented at the annual meeting of the American Surgical Association.
The results probably reflect the very poor survival rates of any patients who develop recurrent non–small cell lung cancer (NSCLC), wrote Dr. McMurry, a biostatistician at the University of Virginia, Charlottesville, and his coauthors.
“Surveillance recommendations need to be considered in the context of potential harms and benefits to patients and their caregivers,” they said. “Follow-up imaging and office visits increase cost and can lead to patient anxiety. Although it seems intuitive that earlier detection of asymptomatic recurrence could improve outcomes, patients with recurrent NSCLC do very poorly … poor survival after recurrence helps explain why more intense surveillance after surgical resection was not associated with improvement in overall survival.”
However, they noted, treatment advances for recurrent and metastatic disease may already be changing the outlook for these patients, “systemic therapy and targeted agents are demonstrating clinically significant survival benefits for small patient subgroups, which, in the future, may augment the benefits of early recurrence detection.”
The team undertook this retrospective study – the largest of its kind in NSCLC patients – in light of current follow-up recommendations that are based almost solely on expert consensus, with low-level data.
“Because there is a paucity of high-quality data on NSCLC surveillance, practice guidelines are based on small retrospective analyses and expert opinion. This results in wide variation in practice including both underuse and overuse of surveillance services.”
The study plumbed the National Cancer Database, extracting information on patients who underwent surgery for NSCLC stages I-III during 2006-2007. All had complete resection and negative margins. Patients were followed through 2012, or until they had a recurrence, a new primary cancer, or they died.
The cohort comprised 4,463 who were followed with CT imaging: 1,614 every 3 months, 1,999 every 6 months, and 850 annually. These intervals correspond to the three different major recommendations. The most common procedure was a lobectomy (about 80%). Patients with higher-stage cancers were significantly more likely to receive more frequent imaging. The regression model controlled for age, sex, comorbidities, tumor stage, and surgical procedure.
After 14 months, 3,552 patients (79.5%) were alive and cancer free. However, during the rest of the follow-up period, 11% developed a new primary cancer and 24% a recurrence of their lung cancer, with no between-group differences. The regression analysis showed no significant difference in recurrence related to surveillance interval, whether 6 months was compared with 3 months (hazard ratio, 1.16) or 1 year with 3 months (HR, 1.06).
Results were much the same for the subgroup of 3,165 who were alive and cancer free 9 months after surgery. In this group, 11% developed a new primary cancer and 29% a recurrence of their lung cancer, with similar numbers in each of the surveillance groups (HR, 1.12 for 6 months vs. 3 months).
Finally, a model including only those who had recurrence, new cancers, or were lost to follow-up within 14 months of surgery also showed no benefit for more frequent surveillance.
“More recent prerecurrence imaging was not associated with postrecurrence survival (HR, 1.02 per month since imaging), and patients who had gone more than 14 months without imaging were at no greater risk of death (HR, 1.01),” the investigators wrote.
The significant predictors of worse survival were nothing surprising, the authors noted. These included symptomatic recurrence (HR, 1.49), distant recurrence, age, male sex, congestive heart failure, coronary artery disease, and peripheral vascular disease.
The findings are not to say, however, that CT surveillance confers no benefit, the authors noted.
“Historically, 5-year survival for the earliest stage of lung cancer, stage IA, was only 70%. Increased use of CT scanning has, however, resulted in a decrease in the median tumor size of resected NSCLC and a shift toward earlier-stage disease. … The National Lung Screening Trial prospectively evaluated annual low-dose screening CT scans and demonstrated a 20% reduction in mortality from lung cancer. This enormous improvement in survival for NSCLC patients provides great promise for the future and is likely to increase the volume of lung cancer resections performed and the number of lung cancer survivors needing routine surveillance.”
However, the data do show that “at least annual CT surveillance is appropriate but that there is no benefit to more than biannual surveillance.”
The authors reported no financial conflicts.
SOURCE: McMurry TL et al. Ann Surg 2018 Jul 12. doi: 10.1097/SLA.0000000000002955.
More even after researchers controlled for tumor histology and recurrence.
Compared with those followed every 3 months, the hazard ratio for 6-month follow-up with CT scanning was 1.16, and 1.06 for annual follow-up – a nonsignificant difference. Nor did more frequent imaging improve survival among the subgroup of patients who were cancer free 9 months after their surgery or among those who had recurrences, Timothy L. McMurry, PhD, and his colleagues reported in the Annals of Surgery. The paper was presented at the annual meeting of the American Surgical Association.
The results probably reflect the very poor survival rates of any patients who develop recurrent non–small cell lung cancer (NSCLC), wrote Dr. McMurry, a biostatistician at the University of Virginia, Charlottesville, and his coauthors.
“Surveillance recommendations need to be considered in the context of potential harms and benefits to patients and their caregivers,” they said. “Follow-up imaging and office visits increase cost and can lead to patient anxiety. Although it seems intuitive that earlier detection of asymptomatic recurrence could improve outcomes, patients with recurrent NSCLC do very poorly … poor survival after recurrence helps explain why more intense surveillance after surgical resection was not associated with improvement in overall survival.”
However, they noted, treatment advances for recurrent and metastatic disease may already be changing the outlook for these patients, “systemic therapy and targeted agents are demonstrating clinically significant survival benefits for small patient subgroups, which, in the future, may augment the benefits of early recurrence detection.”
The team undertook this retrospective study – the largest of its kind in NSCLC patients – in light of current follow-up recommendations that are based almost solely on expert consensus, with low-level data.
“Because there is a paucity of high-quality data on NSCLC surveillance, practice guidelines are based on small retrospective analyses and expert opinion. This results in wide variation in practice including both underuse and overuse of surveillance services.”
The study plumbed the National Cancer Database, extracting information on patients who underwent surgery for NSCLC stages I-III during 2006-2007. All had complete resection and negative margins. Patients were followed through 2012, or until they had a recurrence, a new primary cancer, or they died.
The cohort comprised 4,463 who were followed with CT imaging: 1,614 every 3 months, 1,999 every 6 months, and 850 annually. These intervals correspond to the three different major recommendations. The most common procedure was a lobectomy (about 80%). Patients with higher-stage cancers were significantly more likely to receive more frequent imaging. The regression model controlled for age, sex, comorbidities, tumor stage, and surgical procedure.
After 14 months, 3,552 patients (79.5%) were alive and cancer free. However, during the rest of the follow-up period, 11% developed a new primary cancer and 24% a recurrence of their lung cancer, with no between-group differences. The regression analysis showed no significant difference in recurrence related to surveillance interval, whether 6 months was compared with 3 months (hazard ratio, 1.16) or 1 year with 3 months (HR, 1.06).
Results were much the same for the subgroup of 3,165 who were alive and cancer free 9 months after surgery. In this group, 11% developed a new primary cancer and 29% a recurrence of their lung cancer, with similar numbers in each of the surveillance groups (HR, 1.12 for 6 months vs. 3 months).
Finally, a model including only those who had recurrence, new cancers, or were lost to follow-up within 14 months of surgery also showed no benefit for more frequent surveillance.
“More recent prerecurrence imaging was not associated with postrecurrence survival (HR, 1.02 per month since imaging), and patients who had gone more than 14 months without imaging were at no greater risk of death (HR, 1.01),” the investigators wrote.
The significant predictors of worse survival were nothing surprising, the authors noted. These included symptomatic recurrence (HR, 1.49), distant recurrence, age, male sex, congestive heart failure, coronary artery disease, and peripheral vascular disease.
The findings are not to say, however, that CT surveillance confers no benefit, the authors noted.
“Historically, 5-year survival for the earliest stage of lung cancer, stage IA, was only 70%. Increased use of CT scanning has, however, resulted in a decrease in the median tumor size of resected NSCLC and a shift toward earlier-stage disease. … The National Lung Screening Trial prospectively evaluated annual low-dose screening CT scans and demonstrated a 20% reduction in mortality from lung cancer. This enormous improvement in survival for NSCLC patients provides great promise for the future and is likely to increase the volume of lung cancer resections performed and the number of lung cancer survivors needing routine surveillance.”
However, the data do show that “at least annual CT surveillance is appropriate but that there is no benefit to more than biannual surveillance.”
The authors reported no financial conflicts.
SOURCE: McMurry TL et al. Ann Surg 2018 Jul 12. doi: 10.1097/SLA.0000000000002955.
FROM THE ANNALS OF SURGERY
Key clinical point: More frequent CT surveillance did not improve survival in patients with resected non–small cell lung cancer.
Major finding: Survival among those who got scans every 6 months was not significantly different than every 3 months (HR, 1.16)
Study details: The retrospective study comprised 4,463 patients.
Disclosures: The authors had no financial conflicts.
Source: McMurry TL et al. Ann Surg. 2018 Jul 12. doi: 10.1097/SLA.0000000000002955.