User login
Is the WHO’s HPV vaccination target within reach?
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
The WHO’s goal is to have HPV vaccines delivered to 90% of all adolescent girls by 2030, part of the organization’s larger goal to “eliminate” cervical cancer, or reduce the annual incidence of cervical cancer to below 4 cases per 100,000 people globally.
Laia Bruni, MD, PhD, of Catalan Institute of Oncology in Barcelona, and colleagues outlined the progress made thus far toward reaching the WHO’s goals in an article published in Preventive Medicine.
The authors noted that cervical cancer caused by HPV is a “major public health problem, especially in low- and middle-income countries (LMIC).”
However, vaccines against HPV have been available since 2006 and have been recommended by the WHO since 2009.
HPV vaccines have been introduced into many national immunization schedules. Among the 194 WHO member states, 107 (55%) had introduced HPV vaccination as of June 2020, according to estimates from the WHO and the United Nations International Children’s Emergency Fund (UNICEF).
Still, vaccine introduction and coverages are suboptimal, according to several studies and international agencies.
In their article, Dr. Bruni and colleagues describe the mid-2020 status of HPV vaccine introduction, based on WHO/UNICEF estimates of national HPV immunization coverage from 2010 to 2019.
HPV vaccination by region
The Americas and Europe are by far the WHO regions with the highest rates of HPV vaccination, with 85% and 77% of their countries, respectively, having already introduced HPV vaccination, either partially or nationwide.
In 2019, a record number of introductions, 16, were reported, mostly in LMICs where access has been limited. In prior years, the average had been a relatively steady 7-8 introductions per year.
The percentage of high-income countries (HICs) that have introduced HPV vaccination exceeds 80%. LMICs started introducing HPV vaccination later and at a slower pace, compared with HICs. By the end of 2019, only 41% of LMICs had introduced vaccination. However, of the new introductions in 2019, 87% were in LMICs.
In 2019, the average performance coverage for HPV vaccination programs in 99 countries (both HICs and LMICs) was around 67% for the first vaccine dose and 53% for the final dose.
Median performance coverage was higher in LMICs than in HICs for the first dose (80% and 72%, respectively), but mean dropout rates were higher in LMICs than in HICs (18% and 11%, respectively).
Coverage of more than 90% was achieved for the last dose in only five countries (6%). Twenty-two countries (21%) achieved coverages of 75% or higher, while 35 countries (40%) had final dose coverages of 50% or less.
Global coverage of the final HPV vaccine dose (weighted by population size) was estimated at 15%. According to the authors, that low percentage can be explained by the fact that many of the most populous countries have either not yet introduced HPV vaccination or have low performance.
The countries with highest cervical cancer burden have had limited secondary prevention and have been less likely to provide access to vaccination, the authors noted. However, this trend appears to be reversing, with 14 new LMICs providing HPV vaccination in 2019.
HPV vaccination by sex
By 2019, almost a third of the 107 HPV vaccination programs (n = 33) were “gender neutral,” with girls and boys receiving HPV vaccines. Generally, LMICs targeted younger girls (9-10 years) compared with HICs (11-13 years).
Dr. Bruni and colleagues estimated that 15% of girls and 4% of boys were vaccinated globally with the full course of vaccine. At least one dose was received by 20% of girls and 5% of boys.
From 2010 to 2019, HPV vaccination rates in HICs rose from 42% in girls and 0% in boys to 88% and 44%, respectively. In LMICs, over the same period, rates rose from 4% in girls and 0% in boys to 40% and 5%, respectively.
Obstacles and the path forward
The COVID-19 pandemic has halted HPV vaccine delivery in the majority of countries, Dr. Bruni and colleagues noted. About 70 countries had reported program interruptions by August 2020, and delays to HPV vaccine introductions were anticipated for other countries.
An economic downturn could have further far-reaching effects on plans to introduce HPV vaccines, Dr. Bruni and colleagues observed.
While meeting the 2030 target will be challenging, the authors noted that, in every geographic area, some programs are meeting the 90% target.
“HPV national programs should aim to get 90+% of girls vaccinated before the age of 15,” Dr. Bruni said in an interview. “This is a feasible goal, and some countries have succeeded, such as Norway and Rwanda. Average performance, however, is around 55%, and that shows that it is not an easy task.”
Dr. Bruni underscored the four main actions that should be taken to achieve 90% coverage of HPV vaccination, as outlined in the WHO cervical cancer elimination strategy:
- Secure sufficient and affordable HPV vaccines.
- Increase the quality and coverage of vaccination.
- Improve communication and social mobilization.
- Innovate to improve efficiency of vaccine delivery.
“Addressing vaccine hesitancy adequately is one of the biggest challenges we face, especially for the HPV vaccine,” Dr. Bruni said. “As the WHO document states, understanding social, cultural, societal, and other barriers affecting acceptance and uptake of the vaccine will be critical for overcoming vaccine hesitancy and countering misinformation.”
This research was funded by a grant from Instituto de Salud Carlos III and various other grants. Dr. Bruni and coauthors said they have no relevant disclosures.
FROM PREVENTIVE MEDICINE
Some with long COVID see relief after vaccination
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Several weeks after getting his second dose of an mRNA vaccine, Aaron Goyang thinks his long bout with COVID-19 has finally come to an end.
Mr. Goyang, who is 33 and is a radiology technician in Austin, Tex., thinks he got COVID-19 from some of the coughing, gasping patients he treated last spring.
At the time, testing was scarce, and by the time he was tested – several weeks into his illness – it came back negative. He fought off the initial symptoms but experienced relapse a week later.
Mr. Goyang says that, for the next 8 or 9 months, he was on a roller coaster with extreme shortness of breath and chest tightness that could be so severe it would send him to the emergency department. He had to use an inhaler to get through his workdays.
“Even if I was just sitting around, it would come and take me,” he says. “It almost felt like someone was bear-hugging me constantly, and I just couldn’t get in a good enough breath.”
On his best days, he would walk around his neighborhood, being careful not to overdo it. He tried running once, and it nearly sent him to the hospital.
“Very honestly, I didn’t know if I would ever be able to do it again,” he says.
But Mr. Goyang says that, several weeks after getting the Pfizer vaccine, he was able to run a mile again with no problems. “I was very thankful for that,” he says.
Mr. Goyang is not alone. Some social media groups are dedicated to patients who are living with a condition that’s been known as long COVID and that was recently termed postacute sequelae of SARS-CoV-2 infection (PASC). These patients are sometimes referred to as long haulers.
On social media, patients with PASC are eagerly and anxiously quizzing each other about the vaccines and their effects.
Survivor Corps, which has a public Facebook group with 159,000 members, recently took a poll to see whether there was any substance to rumors that those with long COVID were feeling better after being vaccinated.
“Out of 400 people, 36% showed an improvement in symptoms, anywhere between a mild improvement to complete resolution of symptoms,” said Diana Berrent, a long-COVID patient who founded the group. Survivor Corps has become active in patient advocacy and is a resource for researchers studying the new condition.
Ms. Berrent has become such a trusted voice during the pandemic. She interviewed Anthony Fauci, MD, head of the National Institutes of Allergy and Infectious Diseases, last October.
“The implications are huge,” she says.
“Some of this damage is permanent damage. It’s not going to cure the scarring of your heart tissue, it’s not going to cure the irreparable damage to your lungs, but if it’s making people feel better, then that’s an indication there’s viral persistence going on,” says Ms. Berrent.
“I’ve been saying for months and months, we shouldn’t be calling this postacute anything,” she adds.
Patients report improvement
Daniel Griffin, MD, PhD, is equally excited. He’s an infectious disease specialist at Columbia University, New York. He says about one in five patients he treated for COVID-19 last year never got better. Many of them, such as Mr. Goyang, were health care workers.
“I don’t know if people actually catch this, but a lot of our coworkers are either permanently disabled or died,” Dr. Griffin says.
Health care workers were also among the first to be vaccinated. Dr. Griffin says many of his patients began reaching out to him about a week or two after being vaccinated “and saying, ‘You know, I actually feel better.’ And some of them were saying, ‘I feel all better,’ after being sick – a lot of them – for a year.”
Then he was getting calls and texts from other doctors, asking, “Hey, are you seeing this?”
The benefits of vaccination for some long-haulers came as a surprise. Dr. Griffin says that, before the vaccines came out, many of his patients were worried that getting vaccinated might overstimulate their immune systems and cause symptoms to get worse.
Indeed, a small percentage of people – about 3%-5%, based on informal polls on social media – report that they do experience worsening of symptoms after getting the shot. It’s not clear why.
Dr. Griffin estimates that between 30% and 50% of patients’ symptoms improve after they receive the mRNA vaccines. “I’m seeing this chunk of people – they tell me their brain fog has improved, their fatigue is gone, the fevers that wouldn’t resolve have now gone,” he says. “I’m seeing that personally, and I’m hearing it from my colleagues.”
Dr. Griffin says the observation has launched several studies and that there are several theories about how the vaccines might be affecting long COVID.
An immune system boost?
One possibility is that the virus continues to stimulate the immune system, which continues to fight the virus for months. If that is the case, Dr. Griffin says, the vaccine may be giving the immune system the boost it needs to finally clear the virus away.
Donna Farber, PhD, a professor of microbiology and immunology at Columbia University, has heard the stories, too.
“It is possible that the persisting virus in long COVID-19 may be at a low level – not enough to stimulate a potent immune response to clear the virus, but enough to cause symptoms. Activating the immune response therefore is therapeutic in directing viral clearance,” she says.
Dr. Farber explains that long COVID may be a bit like Lyme disease. Some patients with Lyme disease must take antibiotics for months before their symptoms disappear.
Dr. Griffin says there’s another possibility. Several studies have now shown that people with lingering COVID-19 symptoms develop autoantibodies. There’s a theory that SARS-CoV-2 may create an autoimmune condition that leads to long-term symptoms.
If that is the case, Dr. Griffin says, the vaccine may be helping the body to reset its tolerance to itself, “so maybe now you’re getting a healthy immune response.”
More studies are needed to know for sure.
Either way, the vaccines are a much-needed bit of hope for the long-COVID community, and Dr. Griffin tells his patients who are still worried that, at the very least, they’ll be protected from another SARS-CoV-2 infection.
A version of this article first appeared on Medscape.com.
Let’s apply the lessons from the AIDS crisis to the COVID-19 pandemic
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
In 2020, COVID-19 disrupted our medical system, and life in general. In the 1980s, the AIDS epidemic devastated communities and overwhelmed hospitals. There were lessons learned from the AIDS epidemic that can be applied to the current situation.
Patients with HIV-spectrum illness faced stigmatization and societal indifference, including rejection by family members, increased rates of suicide, fears of sexual and/or intrauterine transmission, substance abuse issues, and alterations of body image for those with wasting syndromes and disfiguring Kaposi lesions. AIDS prevention strategies such as the provision of condoms and needle exchange programs were controversial, and many caregivers exposed to contaminated fluids had to endure months of antiretroviral treatment.
Similar to the AIDS epidemic, the COVID-19 pandemic has had significant psychological implications for patients and caregivers. Patients with COVID-19 infections also face feelings of guilt over potentially exposing a family member to the virus; devastating socioeconomic issues; restrictive hospital visitation policies for family members; disease news oversaturation; and feelings of hopelessness. People with AIDS in the 1980s faced the possibility of dying alone, and there was initial skepticism about medications to treat HIV—just as some individuals are now uneasy about recently introduced coronavirus vaccines.
The similarities of both diseases allow us some foresight on how to deal with current COVID-19 issues. Looking back on the AIDS epidemic should teach us to prioritize attending to the mental health of sufferers and caregivers, creating advocacy and support groups for when a patient’s family is unavailable, instilling public confidence in treatment options, maintaining staff morale, addressing substance abuse (due to COVID-related stress), and depoliticizing prevention strategies. Addressing these issues is especially critical for minority populations.
As respected medical care leaders, we can provide and draw extra attention to the needs of patients’ family members and health care personnel during this COVID-19 pandemic. Hopefully, the distribution of vaccines will shorten some of our communal and professional distress.
Robert Frierson, MD
Steven Lippmann, MD
Louisville, KY
COVID-related immunization gaps portend return of preventable infections
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
pdnews@mdedge.com
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
pdnews@mdedge.com
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
pdnews@mdedge.com
FROM CLINICAL INFECTIOUS DISEASES
Federal Government Ramps Up COVID-19 Vaccination Programs
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
The Biden Administration launched the first phase of the Federally Qualified Health Center (FQHC) Program for COVID-19 Vaccination. Beginning February 15, FQHCs (including centers in the Urban Indian Health Program) began directly receiving vaccines.
The announcement coincided with a boost in vaccine supply for states, Tribes, and territories. In early February, the Biden Administration announced it would expand vaccine supply to 11 million doses nationwide, a 28% increase since January 20, when President Biden took office. According to a White House fact sheet, “The Administration is committing to maintaining this as the minimum supply level for the next three weeks, and we will continue to work with manufacturers in their efforts to ramp up supply.”
In February, President Biden and Vice President Harris travelled to Arizona and toured a vaccination site at State Farm Stadium in Glendale. Arizona, one of the first states to reach out for federal help from the new administration, has 15 counties and 22 Tribes with sovereign lands in the state. Those 37 entities work collaboratively with the Federal Emergency Management Agency (FEMA), said Major General Michael McGuire, head of the Arizona National Guard.
In his remarks during the tour, President Biden addressed equity, saying, “[I]t really does matter that we have access to the people who are most in need [and are] most affected by the COVID crisis, dying at faster rates, getting sick at faster rates, …but not being able to get into the mix. …Equity is a big thing.”
To that end, one of the programs under way is to stand up four vaccination centers for the Navajo Nation. Tammy Littrell, Acting Regional Administrator for FEMA, said the centers will help increase tribal members’ access to vaccination, as well as take the burden off from having to drive in “austere winter conditions.”
In addition to more vaccines, Indian Health Services (IHS) is allocating $1 billion it received to help with COVID-19 response. Of the $1 billion, $790 million will go to testing, contact tracing, containment, and mitigation, among other things. Another $210 million will support IHS, tribal, and urban Indian health programs for vaccine-related activities to ensure broad-based distribution, access, and vaccine coverage. The money is part of the fifth round of supplemental COVID-19 funding from the Coronavirus Response and Relief Supplemental Appropriations Act. The funds transferred so far amount to nearly $3 billion.
According to IHS, the money can be used to scale up testing by public health, academic, commercial, and hospital laboratories, as well as community-based testing sites, mobile testing units, healthcare facilities, and other entities engaged in COVID-19 testing. The funds are also legally available to lease or purchase non-federally owned facilities to improve COVID-19 preparedness and response capability.
Anticipating the care adolescents will need
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
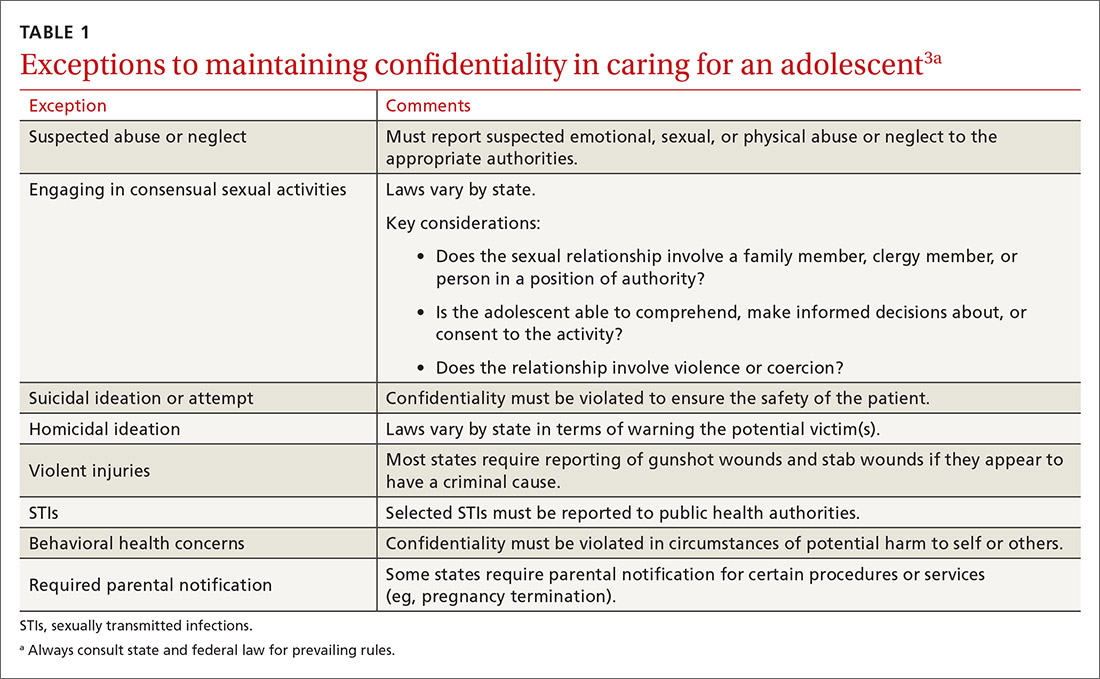
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; mstephens3@pennstatehealth.psu.edu.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; mstephens3@pennstatehealth.psu.edu.
Adolescents are an increasingly diverse population reflecting changes in the racial, ethnic, and geopolitical milieus of the United States. The World Health Organization classifies adolescence as ages 10 to 19 years.1 However, given the complexity of adolescent development physically, behaviorally, emotionally, and socially, others propose that adolescence may extend to age 24.2
Recognizing the specific challenges adolescents face is key to providing comprehensive longitudinal health care. Moreover, creating an environment of trust helps to ensure open 2-way communication that can facilitate anticipatory guidance.
Our review focuses on common adolescent issues, including injury from vehicles and firearms, tobacco and substance misuse, obesity, behavioral health, sexual health, and social media use. We discuss current trends and recommend strategies to maximize health and wellness.
Start by framing the visit
Confidentiality
Laws governing confidentiality in adolescent health care vary by state. Be aware of the laws pertaining to your practice setting. In addition, health care facilities may have their own policies regarding consent and confidentiality in adolescent care. Discuss confidentiality with both an adolescent and the parent/guardian at the initial visit. And, to help avoid potential misunderstandings, let them know in advance what will (and will not) be divulged.
The American Academy of Pediatrics has developed a useful tip sheet regarding confidentiality laws (www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/healthy-foster-care-america/Documents/Confidentiality_Laws.pdf). Examples of required (conditional) disclosure include abuse and suicidal or homicidal ideations. Patients should understand that sexually transmitted infections (STIs) are reportable to public health authorities and that potentially injurious behaviors to self or others (eg, excessive drinking prior to driving) may also warrant disclosure(TABLE 13).
Privacy and general visit structure
Create a safe atmosphere where adolescents can discuss personal issues without fear of repercussion or judgment. While parents may prefer to be present during the visit, allowing for time to visit independently with an adolescent offers the opportunity to reinforce issues of privacy and confidentiality. Also discuss your office policies regarding electronic communication, phone communication, and relaying test results.
A useful paradigm for organizing a visit for routine adolescent care is to use an expanded version of the HEADSS mnemonic (TABLE 24,5), which includes questions about an adolescent’s Home, Education, Activities, Drug and alcohol use, Sexual behavior, Suicidality and depression, and other topics. Other validated screening tools include RAAPS (Rapid Adolescent Prevention Screening)6 (www.possibilitiesforchange.com/raaps/); the Guidelines for Adolescent Preventive Services7; and the Bright Futures recommendations for preventive care from the American Academy of Pediatrics.8 Below, we consider important topics addressed with the HEADSS approach.
Continue to: Injury from vehicles and firearms
Injury from vehicles and firearms
Motor vehicle accidents and firearm wounds are the 2 leading causes of adolescent injury. In 2016, of the more than 20,000 deaths in children and adolescents (ages 1-19 years), 20% were due to motor vehicle accidents (4074) and 15% were a result of firearm-related injuries (3143). Among firearm-related deaths, 60% were homicides, 35% were suicides, and 4% were due to accidental discharge.9 The rate of firearm-related deaths among American teens is 36 times greater than that of any other developed nation.9 Currently, 1 of every 3 US households with children younger than 18 has a firearm. Data suggest that in 43% of these households, the firearm is loaded and kept in an unlocked location.10
To aid anticipatory guidance, ask adolescents about firearm and seat belt use, drinking and driving, and suicidal thoughts (TABLE 24,5). Advise them to always wear seat belts whether driving or riding as a passenger. They should never drink and drive (or get in a car with someone who has been drinking). Advise parents that if firearms are present in the household, they should be kept in a secure, locked location. Weapons should be separated from ammunition and safety mechanisms should be engaged on all devices.
Tobacco and substance misuse
Tobacco use, the leading preventable cause of death in the United States,11 is responsible for more deaths than alcohol, motor vehicle accidents, suicides, homicides, and HIV disease combined.12 Most tobacco-associated mortality occurs in individuals who began smoking before the age of 18.12 Individuals who start smoking early are also more likely to continue smoking through adulthood.
Encouragingly, tobacco use has declined significantly among adolescents over the past several decades. Roughly 1 in 25 high school seniors reports daily tobacco use.13 Adolescent smoking behaviors are also changing dramatically with the increasing popularity of electronic cigarettes (“vaping”). Currently, more adolescents vape than smoke cigarettes.13 Vaping has additional health risks including toxic lung injury.
Multiple resources can help combat tobacco and nicotine use in adolescents. The US Preventive Services Task Force recommends that primary care clinicians intervene through education or brief counselling to prevent initiation of tobacco use in school-aged children and adolescents.14 Ask teens about tobacco and electronic cigarette use and encourage them to quit when use is acknowledged. Other helpful office-based tools are the “Quit Line” 800-QUIT-NOW and texting “Quit” to 47848. Smokefree teen (https://teen.smokefree.gov/) is a website that reviews the risks of tobacco and nicotine use and provides age-appropriate cessation tools and tips (including a smartphone app and a live-chat feature). Other useful information is available in a report from the Surgeon General on preventing tobacco use among young adults.15
Continue to: Alcohol use
Alcohol use. Three in 5 high school students report ever having used alcohol.13 As with tobacco, adolescent alcohol use has declined over the past decade. However, binge drinking (≥ 5 drinks on 1 occasion for males; ≥ 4 drinks on 1 occasion for females) remains a common high-risk behavior among adolescents (particularly college students). Based on the Monitoring the Future Survey, 1 in 6 high school seniors reported binge drinking in the past 2 weeks.13 While historically more common among males, rates of binge drinking are now basically similar between male and female adolescents.13
The National Institute on Alcohol Abuse and Alcoholism has a screening and intervention guide specifically for adolescents.16
Illicit drug use. Half of adolescents report using an illicit drug by their senior year in high school.13 Marijuana is the most commonly used substance, and laws governing its use are rapidly changing across the United States. Marijuana is illegal in 10 states and legal in 10 states (and the District of Columbia). The remaining states have varying policies on the medical use of marijuana and the decriminalization of marijuana. In addition, cannabinoid (CBD) products are increasingly available. Frequent cannabis use in adolescence has an adverse impact on general executive function (compared with adult users) and learning.17 Marijuana may serve as a gateway drug in the abuse of other substances,18 and its use should be strongly discouraged in adolescents.
Of note, there has been a sharp rise in the illicit use of prescription drugs, particularly opioids, creating a public health emergency across the United States.19 In 2015, more than 4000 young people, ages 15 to 24, died from a drug-related overdose (> 50% of these attributable to opioids).20 Adolescents with a history of substance abuse and behavioral illness are at particular risk. Many adolescents who misuse opioids and other prescription drugs obtain them from friends and relatives.21
The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends universal screening of adolescents for substance abuse. This screening should be accompanied by a brief intervention to prevent, mitigate, or eliminate substance use, or a referral to appropriate treatment sources. This process of screening, brief intervention, and referral to treatment (SBIRT) is recommended as part of routine health care.22
Continue to: Obesity and physical activity
Obesity and physical activity
The percentage of overweight and obese adolescents in the United States has more than tripled over the past 40 years,23 and 1 in 5 US adolescents is obese.23 Obese teens are at higher risk for multiple chronic diseases, including type 2 diabetes, sleep apnea, and heart disease.24 They are also more likely to be bullied and to have poor self-esteem.25 Only 1 in 5 American high school students engages in 60 or more minutes of moderate-to-vigorous physical activity on 5 or more days per week.26
Regular physical activity is, of course, beneficial for cardiorespiratory fitness, bone health, weight control, and improved indices of behavioral health.26 Adolescents who are physically active consistently demonstrate better school attendance and grades.17 Higher levels of physical fitness are also associated with improved overall cognitive performance.24
General recommendations. The Department of Health and Human Services recommends that adolescents get at least 60 minutes of mostly moderate physical activity every day.26 Encourage adolescents to engage in vigorous physical activity (heavy breathing, sweating) at least 3 days a week. As part of their physical activity patterns, adolescents should also engage in muscle-strengthening and bone-strengthening activities on at least 3 days per week.
Behavioral health
As young people develop their sense of personal identity, they also strive for independence. It can be difficult, at times, to differentiate normal adolescent rebellion from true mental illness. An estimated 17% to 19% of adolescents meet criteria for mental illness, and about 7% have a severe psychiatric disorder.27 Only one-third of adolescents with mental illness receive any mental health services.28
Depression. The 1-year incidence of major depression in adolescents is 3% to 4%, and the lifetime prevalence of depressive symptoms is 25% in all high school students.27 Risk factors include ethnic minority status, poor self-esteem, poor health, recent personal crisis, insomnia, and alcohol/substance abuse. Depression in adolescent girls is correlated with becoming sexually active at a younger age, failure to use contraception, having an STI, and suicide attempts. Depressed boys are more likely to have unprotected intercourse and participate in physical fights.29 Depressed teens have a 2- to 3-fold greater risk for behavioral disorders, anxiety, and attention-deficit/hyperactivity disorder (ADHD).30
Continue to: Suicide
Suicide. Among individuals 15 to 29 years of age, suicide is the second leading cause of death globally, with an annual incidence of 11 to 15 per 100,000.31 Suicide attempts are 10 to 20 times more common than completed suicide.31 Males are more likely than females to die by suicide,32 and boys with a history of attempted suicide have a 30-fold increased risk of subsequent successful suicide.31 Hanging, drug poisoning, and firearms (particularly for males) are the most common means of suicide in adolescents. More than half of adolescents dying by suicide have coexisting depression.31
Characteristics associated with suicidal behaviors in adolescents include impulsivity, poor problem-solving skills, and dichotomous thinking.31 There may be a genetic component as well. In 1 of 5 teenage suicides, a precipitating life event such as the break-up of a relationship, cyber-bullying, or peer rejection is felt to contribute.31
ADHD. The prevalence of ADHD is 7% to 9% in US school-aged children.33 Boys more commonly exhibit hyperactive behaviors, while girls have more inattention. Hyperactivity often diminishes in teens, but inattention and impulsivity persist. Sequelae of ADHD include high-risk sexual behaviors, motor vehicle accidents, incarceration, and substance abuse.34 Poor self-esteem, suicidal ideation, smoking, and obesity are also increased.34 ADHD often persists into adulthood, with implications for social relationships and job performance.34
Eating disorders. The distribution of eating disorders is now known to increasingly include more minorities and males, the latter representing 5% to 10% of cases.35 Eating disorders show a strong genetic tendency and appear to be accelerated by puberty. The most common eating disorder (diagnosed in 0.8%-14% of teens) is eating disorder not otherwise specified (NOS).35 Anorexia nervosa is diagnosed in 0.5% of adolescent girls, and bulimia nervosa in 1% to 2%—particularly among athletes and performers.35 Unanticipated loss of weight, amenorrhea, excessive concern about weight, and deceleration in height/weight curves are potential indicators of an eating disorder. When identified, eating disorders are best managed by a trusted family physician, acting as a coordinator of a multidisciplinary team.
Sexual health
Girls begin to menstruate at an average age of 12, and it takes about 4 years for them to reach reproductive maturity.36 Puberty has been documented to start at younger ages over the past 30 years, likely due to an increase in average body mass index and a decrease in levels of physical activity.37 Girls with early maturation are often insecure and self-conscious, with higher levels of psychological distress.38 In boys, the average age for spermarche (first ejaculation) is 13.39 Boys who mature early tend to be taller, be more confident, and express a good body image.40 Those who have early puberty are more likely to be sexually active or participate in high-risk behaviors.41
Continue to: Pregnancy and contraception
Pregnancy and contraception
Over the past several decades, more US teens have been abstaining from sexual intercourse or have been using effective forms of birth control, particularly condoms and long-acting reversible contraceptives (LARCs).42 Teenage birth rates in girls ages 15 to 19 have declined significantly since the 1980s.42 Despite this, the teenage birth rate in the United States remains higher than in other industrialized nations, and most teen pregnancies are unintended.
There are numerous interventions to reduce teen pregnancy, including sex education, contraceptive counseling, the use of mobile apps that track a user’s monthly fertility cycle or issue reminders to take oral contraceptives,45 and the liberal distribution of contraceptives and condoms. The Contraceptive CHOICE Project shows that providing free (or low-cost) LARCs influences young women to choose these as their preferred contraceptive method.46 Other programs specifically empower girls to convince partners to use condoms and to resist unwanted sexual advances or intimate partner violence.
Adolescents prefer to have their health care providers address the topic of sexual health. Teens are more likely to share information with providers if asked directly about sexual behaviors.47TABLE 24,5 offers tips for anticipatory guidance and potential ways to frame questions with adolescents in this context. State laws vary with regard to the ability of minors to seek contraception, pregnancy testing, or care/screening for STIs without parental consent. Contraceptive counseling combined with effective screening decrease the incidence of STIs and pelvic inflammatory disease for sexually active teens.48
Sexually transmitted infections
Young adolescents often have a limited ability to imagine consequences related to specific actions. In general, there is also an increased desire to engage in experimental behaviors as an expression of developing autonomy, which may expose them to STIs. About half of all STIs contracted in the United States occur in individuals 15 to 24 years of age.49 Girls are at particular risk for the sequelae of these infections, including cervical dysplasia and infertility. Many teens erroneously believe that sexual activities other than intercourse decrease their risk of contracting an STI.50
Human papillomavirus (HPV) infection is the most common STI in adolescence.51 In most cases, HPV is transient and asymptomatic. Oncogenic strains may cause cervical cancer or cancers of the anogenital or oropharyngeal systems. Due to viral latency, it is not recommended to perform HPV typing in men or in women younger than 30 years of age; however, Pap tests are recommended every 3 years for women ages 21 to 29. Primary care providers are pivotal in the public health struggle to prevent HPV infection.
Continue to: Universal immunization of all children...
Universal immunization of all children older than 11 years of age against HPV is strongly advised as part of routine well-child care. Emphasize the proven role of HPV vaccination in preventing cervical52 and oropharyngeal53 cancers. And be prepared to address concerns raised by parents in the context of vaccine safety and the initiation of sexual behaviors (www.cdc.gov/hpv/hcp/answering-questions.html).
Chlamydia is the second most common STI in the United States, usually occurring in individuals younger than 24.54 The CDC estimates that more than 3 million new chlamydial infections occur yearly. These infections are often asymptomatic, particularly in females, but may cause urethritis, cervicitis, epididymitis, proctitis, or pelvic inflammatory disease. Indolent chlamydial infection is the leading cause of tubal infertility in women.54 Routine annual screening for chlamydia is recommended for all sexually active females ≤ 25 years (and for older women with specific risks).55 Annual screening is also recommended for men who have sex with men (MSM).55
Chlamydial infection may be diagnosed with first-catch urine sampling (men or women), urethral swab (men), endocervical swab (women), or self-collected vaginal swab. Nucleic acid amplification testing is the most sensitive test that is widely available.56 First-line treatment includes either azithromycin (1 g orally, single dose) or doxycycline (100 mg orally, twice daily for 7 days).56
Gonorrhea. In 2018, there were more than 500,000 annual cases of gonorrhea, with the majority occurring in those between 15 and 24 years of age.57 Gonorrhea may increase rates of HIV infection transmission up to 5-fold.57 As more adolescents practice oral sex, cases of pharyngeal gonorrhea (and oropharyngeal HPV) have increased. Symptoms of urethritis occur more frequently in men. Screening is recommended for all sexually active women younger than 25.56 Importantly, the organism Neisseria gonorrhoeae has developed significant antibiotic resistance over the past decade. The CDC currently recommends dual therapy for the treatment of gonorrhea using 250 mg of intramuscular ceftriaxone and 1 g of oral azithromycin.56
Syphilis. Rates of syphilis are increasing among individuals ages 15 to 24.51 Screening is particularly recommended for MSM and individuals infected with HIV. Benzathine penicillin G, 50,000 U/kg IM, remains the treatment of choice.56
Continue to: HIV
HIV. Globally, HIV impacts young people disproportionately. HIV infection also facilitates infection with other STIs. In the United States, the highest burden of HIV infection is borne by young MSM, with prevalence among those 18 to 24 years old varying between 26% to 30% (black) and 3% to 5.5% (non-Hispanic white).51 The use of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis (PrEP) has recently been approved for the prevention of HIV. PrEP reduces risk by up to 92% for MSM and transgender women.58
Sexual identity
One in 10 high school students self-identifies as “nonheterosexual,” and 1 in 15 reports same-sex sexual contact.59 The term LGBTQ+ includes the communities of lesbian, gay, bisexual, transgender, transsexual, queer, questioning, intersex, and asexual individuals. Developing a safe sense of sexual identity is fundamental to adolescent psychological development, and many adolescents struggle to develop a positive sexual identity. Suicide rates and self-harm behaviors among LGBTQ+ adolescents can be 4 times higher than among their heterosexual peers.60 Rates of mood disorders, substance abuse, and high-risk sexual behaviors are also increased in the LGBTQ+ population.61
The LGBTQ+ community often seeks health care advice and affirmation from primary care providers. Resources to enhance this care are available at www.lgbthealtheducation.org.
Social media
Adolescents today have more media exposure than any prior generation, with smartphone and computer use increasing exponentially. Most (95%) teens have access to a smartphone,62 45% describe themselves as constantly connected to the Internet, and 14% feel that social media is “addictive.”62 Most manage their social media portfolio on multiple sites. Patterns of adolescents' online activities show that boys prefer online gaming, while girls tend to spend more time on social networking.62
Whether extensive media use is psychologically beneficial or deleterious has been widely debated. Increased time online correlates with decreased levels of physical activity.63 And sleep disturbances have been associated with excessive screen time and the presence of mobile devices in the bedroom.64 The use of social media prior to bedtime also has an adverse impact on academic performance—particularly for girls. This adverse impact on academics persists after correcting for daytime sleepiness, body mass index, and number of hours spent on homework.64
Continue to: Due to growing concerns...
Due to growing concerns about the risks of social media in children and adolescents, the American Academy of Pediatrics has developed the Family Media Plan (www.healthychildren.org/English/media/Pages/default.aspx). Some specific questions that providers may ask are outlined in TABLE 3.64 The Family Media Plan can provide age-specific guidelines to assist parents or caregivers in answering these questions.
Cyber-bullying. One in 3 adolescents (primarily female) has been a victim of cyber-bullying.65 Sadly, 1 in 5 teens has received some form of electronic sexual solicitation.66 The likelihood of unsolicited stranger contact correlates with teens’ online habits and the amount of information disclosed. Predictors include female sex, visiting chat rooms, posting photos, and disclosing personal information. Restricting computer use to an area with parental supervision or installing monitoring programs does not seem to exert any protective influence on cyber-bullying or unsolicited stranger contact.65 While 63% of cyber-bullying victims feel upset, embarrassed, or stressed by these contacts,66 few events are actually reported. To address this, some states have adopted laws adding cyber-bullying to school disciplinary codes.
Negative health impacts associated with cyber-bullying include anxiety, sadness, and greater difficulty in concentrating on school work.65 Victims of bullying are more likely to have school disciplinary actions and depression and to be truant or to carry weapons to school.66 Cyber-bullying is uniquely destructive due to its ubiquitous presence. A sense of relative anonymity online may encourage perpetrators to act more cruelly, with less concern for punishment.
Young people are also more likely to share passwords as a sign of friendship. This may result in others assuming their identity online. Adolescents rarely disclose bullying to parents or other adults, fearing restriction of Internet access, and many of them think that adults may downplay the seriousness of the events.66
CORRESPONDENCE
Mark B. Stephens, MD, Penn State Health Medical Group, 1850 East Park Avenue, State College, PA 16803; mstephens3@pennstatehealth.psu.edu.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
1. World Health Organization. Adolescent health. Accessed February 23, 2021. www.who.int/maternal_child_adolescent/adolescence/en/
2. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223-228.
3. Pathak PR, Chou A. Confidential care for adoloscents in the U.S. healthcare system. J Patient Cent Res Rev. 2019;6:46-50.
4. AMA Journal of Ethics. HEADSS: the “review of systems” for adolescents. Accessed February 23, 2021. https://journalofethics.ama-assn.org/article/headss-review-systems-adolescents/2005-03
5. Cohen E, MacKenzie RG, Yates GL. HEADSS, a psychosocial risk assessment instrument: implications for designing effective intervention programs for runaway youth. J Adolesc Health. 1991;12:539-544.
6. Possibilities for Change. Rapid Adolescent Prevention Screening (RAAPS). Accessed February 23, 2021. www.possibilitiesforchange.com/raaps/
7. Elster AB, Kuznets NJ. AMA Guidelines for Adolescent Preventive Services (GAPS): Recommendations and Rationale. Williams & Wilkins; 1994.
8. AAP. Engaging patients and families - periodicity schedule. Accessed February 23, 2021. www.aap.org/en-us/professional-resources/practice-support/Pages/PeriodicitySchedule.aspx
9. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Eng J Med. 2018;379:2468-2475.
10. Schuster MA, Franke TM, Bastian AM, et al. Firearm storage patterns in US homes with children. Am J Public Health. 2000;90:588-594.
11. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States. JAMA. 2004;291:1238-1245.
12. HHS. Health consequences of smoking, surgeon general fact sheet. Accessed February 23, 2021. www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/consequences-smoking-factsheet/index.html
13. Johnston LD, Miech RA, O’Malley PM, et al. Monitoring the future: national survey results on drug use, 1975-2017. The University of Michigan. 2018. Accessed February 23, 2021. https://eric.ed.gov/?id=ED589762
14. US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
15. HHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta, GA: HHS, CDC, NCCDPHP, OSH; 2012. Accessed February 23, 2021. www.ncbi.nlm.nih.gov/books/NBK99237/
16. NIH. Alcohol screening and brief intervention for youth: a pocket guide. Accessed February 23, 2021. https://pubs.niaaa.nih.gov/publications/Practitioner/YouthGuide/YouthGuidePocket.pdf
17. Gorey C, Kuhns L, Smaragdi E, et al. Age-related differences in the impact of cannabis use on the brain and cognition: a systematic review. Eur Arch Psychiatry Clin Neurosci. 2019;269:37-58.
18. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, et al. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26:135-142.
19. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance—United States, 2017. MMWR Surveill Summ. 2018;67:1-114.
20. NIH. Drug overdoses in youth. How do drug overdoses happen?. Accessed February 23, 2021. https://teens.drugabuse.gov/drug-facts/drug-overdoses-youth
21. Branstetter SA, Low S, Furman W. The influence of parents and friends on adolescent substance use: a multidimensional approach. J Subst Use. 2011;162:150-160.
22. AAP. Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161210.
23. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief. 2017;288:1-8.
24. Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad Pediatr. 2013;13:6-13.
25. Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: a systematic review. Int J Pediatr Obes. 2010;5:282-304.
26. National Physical Activity Plan Alliance. The 2018 United States report card on physical activity for children and youth. Accessed February 23, 2021. http://physicalactivityplan.org/projects/PA/2018/2018%20US%20Report%20Card%20Full%20Version_WEB.PDF?pdf=page-link
27. HHS. NIMH. Child and adolescent mental health. Accessed February 23, 2021. www.nimh.nih.gov/health/topics/child-and-adolescent-mental-health/index.shtml
28. Yonek JC, Jordan N, Dunlop D, et al. Patient-centered medical home care for adolescents in need of mental health treatment. J Adolesc Health. 2018;63:172-180.
29. Brooks TL, Harris SK, Thrall JS, et al. Association of adolescent risk behaviors with mental health symptoms in high school students. |J Adolesc Health. 2002;31:240-246.
30. Weller BE, Blanford KL, Butler AM. Estimated prevalence of psychiatric comorbidities in US adolescents with depression by race/ethnicity, 2011-2012. J Adolesc Health. 2018;62:716-721.
31. Bilsen J. Suicide and youth: risk factors. Front Psychiatry. 2018;9:540.
32. Shain B, AAP Committee on Adolescence. Suicide and suicide attempts in adolescents. Pediatrics. 2016;138:e20161420.
33. Brahmbhatt K, Hilty DM, Hah M, et al. Diagnosis and treatment of attention deficit hyperactivity disorder during adolescence in the primary care setting: review and future directions. J Adolesc Health. 2016;59:135-143.
34. Bravender T. Attention-deficit/hyperactivity disorder and disordered eating. [editorial] J Adolesc Health. 2017;61:125-126.
35. Rosen DS, AAP Committee on Adolescence. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253.
36. Susman EJ, Houts RM, Steinberg L, et al. Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Arch Pediatr Adolesc Med. 2010;164:166-173.
37. Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(suppl 3):S208-S217.
38. Ge X, Conger RD, Elder GH. Coming of age too early: pubertal influences on girl’s vulnerability to psychologic distress. Child Dev. 1996;67:3386-3400.
39. Jørgensen M, Keiding N, Skakkebaek NE. Estimation of spermarche from longitudinal spermaturia data. Biometrics. 1991;47:177-193.
40. Kar SK, Choudhury A, Singh AP. Understanding normal development of adolescent sexuality: a bumpy ride. J Hum Reprod Sci. 2015;8:70-74.
41. Susman EJ, Dorn LD, Schiefelbein VL. Puberty, sexuality and health. In: Lerner MA, Easterbrooks MA, Mistry J (eds). Comprehensive Handbook of Psychology. Wiley; 2003.
42. Lindberg LD, Santelli JS, Desai S. Changing patterns of contraceptive use and the decline in rates of pregnancy and birth among U.S. adolescents, 2007-2014. J Adolesc Health. 2018;63:253-256.
43. Guttmacher Institute. Teen pregnancy. www.guttmacher.org/united-states/teens/teen-pregnancy. Accessed February 23, 2021.
44. CDC. Social determinants and eliminating disparities in teen pregnancy. Accessed February 23, 2021. www.cdc.gov/teenpregnancy/about/social-determinants-disparities-teen-pregnancy.htm
45. Widman L, Nesi J, Kamke K, et al. Technology-based interventions to reduce sexually transmitted infection and unintended pregnancy among youth. J Adolesc Health. 2018;62:651-660.
46. Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-115.e7.
47. Ham P, Allen C. Adolescent health screening and counseling. Am Fam Physician. 2012;86:1109-1116.
48. ACOG. Committee on Adolescent Health Care. Adolescent pregnancy, contraception and sexual activity. 2017. Accessed February 23, 2021. www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/adolescent-pregnancy-contraception-and-sexual-activity
49. Wangu Z, Burstein GR. Adolescent sexuality: updates to the sexually transmitted infection guidelines. Pediatr Clin N Am. 2017;64:389-411.
50. Holway GV, Hernandez SM. Oral sex and condom use in a U.S. national sample of adolescents and young adults. J Adolesc Health. 2018;62:402-410.
51. CDC. STDs in adults and adolescents. Accessed February 23, 2021. www.cdc.gov/std/stats17/adolescents.htm
52. McClung N, Gargano J, Bennett N, et al. Trends in human papillomavirus vaccine types 16 and 18 in cervical precancers, 2008-2014. Accessed February 23, 2021. https://cebp.aacrjournals.org/content/28/3/602
53. Timbang MR, Sim MW, Bewley AF, et al. HPV-related oropharyngeal cancer: a review on burden of the disease and opportunities for prevention and early detection. Hum Vaccin Immunother. 2019;15:1920-1928.
54. Carey AJ, Beagley KW. Chlamydia trachomatis, a hidden epidemic: effects on female reproduction and options for treatment. Am J Reprod Immunol. 2010;63:576-586.
55. USPSTF. Chlamydia and gonorrhea screening. Accessed February 23, 2021. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/chlamydia-and-gonorrhea-screening
56. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1-135.
57. CDC. Sexually transmitted disease surveillance 2018. Accessed February 23, 2021. www.cdc.gov/std/stats18/gonorrhea.htm
5
59. Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2015. MMWR Surveill Summ. 2016;65:1-174.
60. CDC. LGBT youth. Accessed February 23, 2021. www.cdc.gov/lgbthealth/youth.htm
61. Johns MM, Lowry R, Rasberry CN, et al. Violence victimization, substance use, and suicide risk among sexual minority high school students – United States, 2015-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1211-1215.
62. Pew Research Center. Teens, social media & technology 2018. . Accessed February 23, 2021. www.pewinternet.org/2018/05/31/teens-social-media-technology-2018/
63. Chassiakos YLR, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics. 2016;138:e20162593.
64. Arora T, Albahri A, Omar OM, et al. The prospective association between electronic device use before bedtime and academic attainment in adolescents. J Adolesc Health. 2018;63:451-458.
65. Mishna F, Saini M, Solomon S. Ongoing and online: children and youth’s perceptions of cyber bullying. Child Youth Serv Rev. 2009;31:1222-1228.
66. Sengupta A, Chaudhuri A. Are social networking sites a source of online harassment for teens? Evidence from survey data. Child Youth Serv Rev. 2011;33:284-290.
PRACTICE RECOMMENDATIONS
› Consider using a 2-question screening tool for adolescents that asks about personal use of alcohol and use of alcohol by friends; this resource offers a risk assessment with recommendations. C
› Consider using the American Academy of Pediatrics Family Media Plan to provide age-specific guidelines to help parents or caregivers establish rules for online activities. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
ACIP recommendations for COVID-19 vaccines—and more
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
AT PRESS TIME
The US Food and Drug Administration issued an Emergency Use Authorization for a third COVID-19 vaccine. The single-dose vaccine was developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. For more information, go to www.mdedge.com/familymedicine
Armpit swelling after COVID-19 vaccine may mimic breast cancer
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should therefore consider recent COVID-19 vaccination history in the differential diagnosis of patients who present with unilateral axillary adenopathy, according to a new article.
“We noticed an increasing number of patients with swollen lymph nodes on just one side/one underarm who presented for routine screening mammography or ultrasound, and some women who actually felt these swollen nodes,” said author Katerina Dodelzon, MD, assistant professor of clinical radiology at Weill Cornell Medicine, New York.
“Historically, swollen lymph nodes on just one side are relatively rare and are an uncommon occurrence on screening mammography – seen only 0.02%-0.04% of the time – and is a sign that alerts a radiologist to exclude the presence of breast malignancy on that side,” she added.
In an article published in Clinical Imaging, Dr. Dodelzon and colleagues described four cases involving women who received a COVID-19 vaccine and then sought breast screening. In describing these cases, the authors sought “to inform the medical community to consider this benign and self-resolving diagnosis in the setting of what can be alarming presentation of unilateral axillary adenopathy.”
They hope they will decrease unnecessary biopsies and help reassure patients.
Adenopathy has been reported in association with other vaccines, such as the bacille Calmette-Guérin vaccine, influenza vaccines, and the human papillomavirus vaccine, commented Jessica W. T. Leung, MD, president of the Society of Breast Imaging.
“It’s too early to say if there is something different about the COVID-19 vaccines,” said Dr. Leung, who is also professor of diagnostic radiology and deputy chair of breast imaging at the University of Texas MD Anderson Cancer Center, Houston.
“The two vaccines that are currently in use – Pfizer and Moderna – are both mRNA vaccines, and it is unknown if those will give a stronger immune response,” she said. “If the Johnson & Johnson and AstraZeneca vaccines do become available, it will be interesting to see if they elicit as strong a response, since they are not mRNA vaccines. At this time, we have no data to say one way or the other.”
Dr. Leung also noted that these latest vaccine reactions may be getting more attention because “it is COVID-19 related, and everything related to COVID-19 gets more attention.
“It may also be more noticeable because of the large number of people getting vaccinated within a short period of time in an effort to contain the pandemic, and this is not the case with the other vaccines,” she said.
New recommendations from SBI
The SBI recently issued recommendations to clinicians that women who experience axillary adenopathy and who have recently been vaccinated on the same side on which the adenopathy occurs be followed for a few weeks to see whether the lymph nodes return to normal, rather than undergo biopsy.
“Many practices are now routinely inquiring about history of recent vaccination and on which side it was given,” Dr. Dodelzon said. She emphasized that women should feel empowered to share that history if they are not asked.
“Letting your mammography technologist or breast imager know that you have recently been vaccinated, and on which side, will provide the breast imager more accurate context within which to interpret the results,” she said.
In addition, the SBI recommends that, if feasible, women schedule routine screening mammography either before the first dose of the COVID-19 vaccine or 4-6 weeks after the second dose to avoid a false-positive finding.
“We want to emphasize that screening mammography is very important, and if possible, to schedule it around the vaccine,” commented Dr. Leung. “But that may not be possible, as most of us don’t have a choice when to get the vaccine.”
If it is not possible to reschedule either the mammogram or the vaccine, Dr. Leung recommends that women inform the facility that they have recently received a COVID-19 vaccine. “Currently, we recommend a follow-up in 4-12 weeks,” she said. “The swelling could subside sooner, perhaps even within 1-2 weeks, but we generally recommend waiting at least 4 weeks to capture the majority of women.”
Differences between the vaccines?
The frequency with which axillary adenopathy occurs as a side effect differs with the two COVID-19 vaccines, according to reports from the Centers for Disease Control and Prevention.
For the Moderna vaccine, axillary adenopathy ipsilateral to the vaccination arm was the second most frequently reported local reaction, with 11.6% of recipients aged 18-64 years reporting it after the first dose, and 16.0% reporting it after the second. The average duration of this adenopathy was 1-2 days.
For the Pfizer-BioNTech COVID-19 vaccine, the CDC notes that reports of adenopathy were imbalanced between the vaccine and placebo groups and concluded that adenopathy was plausibly related to the vaccine.
The average duration of adenopathy was approximately 10 days.
Adenopathy was reported within 2-4 days after vaccination for both vaccine groups, the CDC noted.
However, details from the cases reported by Dr. Dodelzon and colleagues paint a somewhat different picture. For example, in case 1, the patient self-detected unilateral axillary adenopathy 9 days after receiving the first dose of the Pfizer-BioNTech vaccine. In case 3, the time between receiving the Moderna vaccine and detection of adenopathy was 13 days.
In both of these cases, the time was much longer than the average duration of 1-2 days noted by the CDC. The authors suggest that in taking the patient’s vaccination history, radiologists understand that the side effect may occur up to several weeks following the COVID-19 vaccination.
In cases 2 and 4, the axillary adenopathy was incidentally noted during mammography, so it is unclear when the onset of this reaction occurred after receiving the COVID-19 vaccine.
The authors and Dr. Leung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccination linked to less mechanical ventilation
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
FDA grants emergency use authorization to Johnson & Johnson COVID-19 vaccine
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.