User login
HPV Positive Test: How to Address Patients’ Anxieties
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Faced with a positive human papillomavirus (HPV) test, patients are quickly overwhelmed by anxiety-inducing questions. It is crucial to provide them with adequate responses to reassure them, emphasized Jean-Louis Mergui, MD, president of the International Federation for Colposcopy, during the press conference of the Congress of the French Society of Colposcopy and Cervico-Vaginal Pathology.
“Do I have cancer? When did I catch this papillomavirus? Is it dangerous for my partner? How do I get rid of it?” “Not everyone is equipped to answer these four questions. However, it is extremely important that healthcare professionals provide correct answers to patients so that they stop worrying,” Dr. Mergui explained.
Papillomavirus and Cancer
One of the first instincts of patients who receive a positive HPV test is to turn to the Internet. There, they read about “high-risk HPV, which is potentially oncogenic,” and become completely panicked, said Dr. Mergui.
However, among women, the probability of having a high-grade CIN3 lesion or higher on the cervix when the HPV test is positive is about 7%, according to the ATHENA study. “About 93% of patients do not have a severe lesion on the cervix. That’s why colposcopy is not performed on all patients. They need to be reassured,” said Dr. Mergui. When the papillomavirus persists, there is a risk for a cervical lesion. After 11 years, between 20% and 30% of patients develop a high-grade lesion on the cervix. However, on average, a high-risk HPV is spontaneously eliminated within 1-2 years. “After 14 months, 50% of women will test negative for their papillomavirus,” Dr. Mergui noted.
“High-risk HPV does not mean there is a lesion; it means there is a risk of developing a lesion on the cervix one day. That’s why these patients need to be monitored and explored,” he added.
In practice, when a patient aged between 30 and 65 years has a positive HPV test, cytology is performed to look for lesions. Only in the case of an abnormal smear, ASC-US, is colposcopy recommended. In the absence of a lesion, a control HPV test is conducted 1 year later to monitor virus persistence.
It should be noted that patients who have been treated for a cervical lesion have a five times higher risk of developing invasive cervical, vaginal, or vulvar cancer. Therefore, treated patients must be monitored once every 3 years for life.
Time of Infection
Many patients ask, “When did I catch this papillomavirus?” In response, Dr. Mergui first emphasized that HPV infection is common. “Between ages 15 and 30 years, most of us are infected with a high-risk HPV. When we look at the incidence between ages 15 and 25 years, every year, 20% of all young girls are infected with HPV, including 17% with high-risk HPV. The virus is usually caught within the first 5 years of sexual activity, and typically disappears after about a year,” he explained.
However, the most disturbing scenario for patients is when their last examination was negative, and there is no apparent reason for having caught the virus since then. Suspicion often falls on the partner. Once again, the gynecologist seeks to reassure.
It is possible that the last time screening was conducted, the virus was not sought (HPV test), but rather cervical lesions were sought by smear. However, a normal smear does not mean that the papillomavirus is not present. A negative cytology does not mean a negative HPV test. As we have seen, the virus is not always associated with the presence of a lesion, explained Dr. Mergui.
Also, having had a negative HPV test a few years earlier does not mean that one was not already infected. The HPV test determines the quantity of virus. Therefore, it is possible that the virus was present in small quantities that were without clinical significance (hence, a negative test). However, a few years later, the virus may have multiplied, and the HPV test became positive.
“Sometimes, the virus re-emerges 40, 50 years after infection due to age-related immune decline,” said Dr. Mergui. “So, just because the smear was negative or the HPV test was negative at the last examination does not mean that one was infected between the two.” Moreover, only 15% of couples have the same virus present on the penis or vagina, he pointed out.
Protecting One’s Partner
Once the diagnosis is made, it is often too late to protect the partner because they have already been infected. “It is certain that the partner will be infected or has already been infected because when the patient comes to you with a positive HPV test, she has already had sexual intercourse. It is worth noting that the virus can be transmitted through digital touching, and condoms are not very effective in preventing virus transmission,” said Dr. Mergui.
The speaker further clarified that the risk for men is much lower than that for women. “In women, about 40,000 lesions linked to high-risk HPV types, precancerous or cancerous, are observed every year. In men, this number is 1900. So, this represents 20 times fewer neoplastic lesions in men. The problem in men is oropharyngeal lesions, which are three times more common than in women. However, there is no screening for oropharyngeal cancer.”
So, when should the partner consult? Dr. Mergui advised consulting when there are clinically visible lesions (small warts, bumps, or ear, nose, and throat symptoms). “I do not recommend systematic examination of male or female partners,” he added.
Clearing the Virus
There are treatments for cervical lesions but not for papillomavirus infection.
“The only thing that can be suggested is quitting smoking, which increases viral clearance, thus reducing viral load. Also, the use of condoms helps improve viral clearance, but when women have a stable relationship, it seems unrealistic to think they will constantly use condoms. Finally, the prophylactic vaccine has been proposed, but it does not treat the infection. In fact, the real solution is to tell patients that they need to continue regular monitoring,” said Dr. Mergui.
“It should be noted that an ongoing study at the European level seems to show that when women who have undergone surgical treatment for a high-grade cervical lesion are vaccinated at the time of treatment or just after treatment, it reduces the risk of recurrence by 50%. So, the risk of recurrence is around 7%-8%. This strategy could be interesting, but for now, there is no official recommendation,” Dr. Mergui concluded.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
RNA Vaccines: Risk for Heavy Menstrual Bleeding Clarified
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Microbiome Impacts Vaccine Responses
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).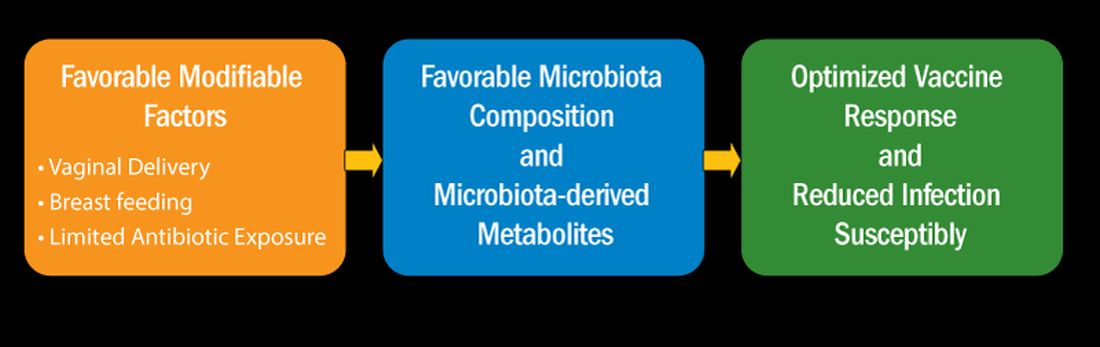
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
HPV Vaccine Shown to Be Highly Effective in Girls Years Later
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
Rubella Screening in Pregnancy No Longer Recommended in Italy
If a pregnant woman contracts rubella in the first 17 weeks of pregnancy, then the risk for congenital rubella in the newborn — which may entail spontaneous abortion, intrauterine death, or severe fetal malformations — is as high as 80%. This risk once frightened patients and clinicians in Italy. Thanks to widespread population vaccination, however, the World Health Organization declared the elimination of endemic transmission of rubella in Italy in 2021. The Italian National Institute of Health took note, and the recent update of the Guidelines for the Management of Physiological Pregnancy no longer recommends offering rubella screening to all pregnant women.
The Rubeo Test
The rubeo test, an analysis for detecting antibodies in the blood produced by vaccination or a past rubella infection, traditionally forms part of the examination package that every doctor prescribes to expectant patients at the beginning of pregnancy. If the test shows that the woman is not vaccinated and has never encountered the virus, making her susceptible to the risk for infection, according to the previous edition of the Guidelines, then the test should be repeated at 17 weeks of gestation. The purpose is to detect any rubella contracted during pregnancy and offer the woman multidisciplinary counseling in the case of a high risk for severe fetal damage. Infection contracted after the 17th week, however, poses only a minimal risk for congenital deafness. There is no treatment to prevent vertical transmission in case of infection during pregnancy.
For women at risk for infection, the old Guidelines also recommended planning vaccination postnatally, with the prospect of protecting future pregnancies. Rubella vaccination is contraindicated during pregnancy because the vaccine could be teratogenic.
Recommendation Update
In the early ‘90s, universal vaccination against rubella for newborns was introduced in Italy. It became one of the 10 mandatory pediatric vaccinations in 2017. In June 2022, the Ministry of Health reported a vaccination coverage of 93.8% among children aged 24 months, a coverage of 93.3% for the first dose, and a coverage of 89.0% for the second dose in the 2003 birth cohort.
“Rubella is a notifiable disease, and in 2013, the newly activated national surveillance system detected one case of congenital rubella per 100,000 newborns. From 2018 onward, no cases have been reported,” said Vittorio Basevi, a gynecologist of the Perinatal Technical-Scientific Advisory Commission in the Emilia Romagna Region and coordinator of the Technical-Scientific Committee that developed the updated Guidelines. “Thanks to extensive vaccination coverage, the infection no longer circulates in Italy. Based on these data, we decided not to offer screening to pregnant women anymore.”
The recommendation to offer rubella vaccination post partum to women without documentation of two doses or previous infection remains confirmed.
Patients Born Abroad
How should one handle the care of a pregnant woman born in a country where universal rubella vaccination is not provided? The likelihood that she is susceptible to infection is higher than the that of the general Italian population. “On the other hand, since the virus no longer circulates in our country, the probability of contracting the virus during pregnancy is negligible, unless she has recently traveled to her country of origin or come into contact with family members who recently arrived in Italy,” said Dr. Basevi. “The Guidelines refer to offering screening to all pregnant women. In specific cases, it is up to the treating physician to adopt the conduct they deem appropriate in science and conscience.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
If a pregnant woman contracts rubella in the first 17 weeks of pregnancy, then the risk for congenital rubella in the newborn — which may entail spontaneous abortion, intrauterine death, or severe fetal malformations — is as high as 80%. This risk once frightened patients and clinicians in Italy. Thanks to widespread population vaccination, however, the World Health Organization declared the elimination of endemic transmission of rubella in Italy in 2021. The Italian National Institute of Health took note, and the recent update of the Guidelines for the Management of Physiological Pregnancy no longer recommends offering rubella screening to all pregnant women.
The Rubeo Test
The rubeo test, an analysis for detecting antibodies in the blood produced by vaccination or a past rubella infection, traditionally forms part of the examination package that every doctor prescribes to expectant patients at the beginning of pregnancy. If the test shows that the woman is not vaccinated and has never encountered the virus, making her susceptible to the risk for infection, according to the previous edition of the Guidelines, then the test should be repeated at 17 weeks of gestation. The purpose is to detect any rubella contracted during pregnancy and offer the woman multidisciplinary counseling in the case of a high risk for severe fetal damage. Infection contracted after the 17th week, however, poses only a minimal risk for congenital deafness. There is no treatment to prevent vertical transmission in case of infection during pregnancy.
For women at risk for infection, the old Guidelines also recommended planning vaccination postnatally, with the prospect of protecting future pregnancies. Rubella vaccination is contraindicated during pregnancy because the vaccine could be teratogenic.
Recommendation Update
In the early ‘90s, universal vaccination against rubella for newborns was introduced in Italy. It became one of the 10 mandatory pediatric vaccinations in 2017. In June 2022, the Ministry of Health reported a vaccination coverage of 93.8% among children aged 24 months, a coverage of 93.3% for the first dose, and a coverage of 89.0% for the second dose in the 2003 birth cohort.
“Rubella is a notifiable disease, and in 2013, the newly activated national surveillance system detected one case of congenital rubella per 100,000 newborns. From 2018 onward, no cases have been reported,” said Vittorio Basevi, a gynecologist of the Perinatal Technical-Scientific Advisory Commission in the Emilia Romagna Region and coordinator of the Technical-Scientific Committee that developed the updated Guidelines. “Thanks to extensive vaccination coverage, the infection no longer circulates in Italy. Based on these data, we decided not to offer screening to pregnant women anymore.”
The recommendation to offer rubella vaccination post partum to women without documentation of two doses or previous infection remains confirmed.
Patients Born Abroad
How should one handle the care of a pregnant woman born in a country where universal rubella vaccination is not provided? The likelihood that she is susceptible to infection is higher than the that of the general Italian population. “On the other hand, since the virus no longer circulates in our country, the probability of contracting the virus during pregnancy is negligible, unless she has recently traveled to her country of origin or come into contact with family members who recently arrived in Italy,” said Dr. Basevi. “The Guidelines refer to offering screening to all pregnant women. In specific cases, it is up to the treating physician to adopt the conduct they deem appropriate in science and conscience.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
If a pregnant woman contracts rubella in the first 17 weeks of pregnancy, then the risk for congenital rubella in the newborn — which may entail spontaneous abortion, intrauterine death, or severe fetal malformations — is as high as 80%. This risk once frightened patients and clinicians in Italy. Thanks to widespread population vaccination, however, the World Health Organization declared the elimination of endemic transmission of rubella in Italy in 2021. The Italian National Institute of Health took note, and the recent update of the Guidelines for the Management of Physiological Pregnancy no longer recommends offering rubella screening to all pregnant women.
The Rubeo Test
The rubeo test, an analysis for detecting antibodies in the blood produced by vaccination or a past rubella infection, traditionally forms part of the examination package that every doctor prescribes to expectant patients at the beginning of pregnancy. If the test shows that the woman is not vaccinated and has never encountered the virus, making her susceptible to the risk for infection, according to the previous edition of the Guidelines, then the test should be repeated at 17 weeks of gestation. The purpose is to detect any rubella contracted during pregnancy and offer the woman multidisciplinary counseling in the case of a high risk for severe fetal damage. Infection contracted after the 17th week, however, poses only a minimal risk for congenital deafness. There is no treatment to prevent vertical transmission in case of infection during pregnancy.
For women at risk for infection, the old Guidelines also recommended planning vaccination postnatally, with the prospect of protecting future pregnancies. Rubella vaccination is contraindicated during pregnancy because the vaccine could be teratogenic.
Recommendation Update
In the early ‘90s, universal vaccination against rubella for newborns was introduced in Italy. It became one of the 10 mandatory pediatric vaccinations in 2017. In June 2022, the Ministry of Health reported a vaccination coverage of 93.8% among children aged 24 months, a coverage of 93.3% for the first dose, and a coverage of 89.0% for the second dose in the 2003 birth cohort.
“Rubella is a notifiable disease, and in 2013, the newly activated national surveillance system detected one case of congenital rubella per 100,000 newborns. From 2018 onward, no cases have been reported,” said Vittorio Basevi, a gynecologist of the Perinatal Technical-Scientific Advisory Commission in the Emilia Romagna Region and coordinator of the Technical-Scientific Committee that developed the updated Guidelines. “Thanks to extensive vaccination coverage, the infection no longer circulates in Italy. Based on these data, we decided not to offer screening to pregnant women anymore.”
The recommendation to offer rubella vaccination post partum to women without documentation of two doses or previous infection remains confirmed.
Patients Born Abroad
How should one handle the care of a pregnant woman born in a country where universal rubella vaccination is not provided? The likelihood that she is susceptible to infection is higher than the that of the general Italian population. “On the other hand, since the virus no longer circulates in our country, the probability of contracting the virus during pregnancy is negligible, unless she has recently traveled to her country of origin or come into contact with family members who recently arrived in Italy,” said Dr. Basevi. “The Guidelines refer to offering screening to all pregnant women. In specific cases, it is up to the treating physician to adopt the conduct they deem appropriate in science and conscience.”
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Coming Soon: The First mRNA Vaccine for Melanoma?
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Shingles Vaccine Offers 4 Years of Protection
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
The 2024 Adult Vaccine Schedule Changes Are Here
This transcript has been edited for clarity.
Sandra Fryhofer, MD, highlights .
The biggest change for 2024 is that you don’t need to wait till January 1, 2024, for these schedules go into effect. Both schedules were published and became available in November 2023 and became effective immediately. They include ACIP recommendations approved by the Centers for Disease Control and Prevention (CDC) director through October 23, 2023.
Subsequent recommendations (before publication of the 2025 schedule) will be added to the addendum, a new Step 5, Section 5 in the schedule. The addendum should make Affordable Care Act (ACA)–compliant insurance plans cover ACIP-recommended immunizations sooner.
This year’s schedule includes more vaccines with new recommendations and new color code keys for the schedule’s vaccine tables. The newest vaccine additions to the 2024 schedule include respiratory syncytial virus (RSV) vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of the updated COVID vaccine (both mRNA and protein-based adjuvanted versions).
These are listed on the cover page (in alphabetical order) by name, abbreviation, and trade name. Vaccine-specific details can be found in the (Step 3) Notes section, also organized alphabetically.
The Tables
Step 1 is Table 1: Vaccinations by Age. Step 2 is Table 2: Vaccinations by Medical Conditions or Other Indications. The table names haven’t changed. However, their color code legends have been adjusted and refined. Also, the legends for the some of the same colors are not the same for both tables.
The order of and conditions covered in the columns on Table 2 have been reorganized.
Even for vaccines whose recommendations have not changed, the color code keys reflecting the recommendations have changed. For this reason, the 2024 version of Table 2 looks very different from the 2023 version. Also, much of the wording on overlays has been removed, which means you have to rely more heavily on the Notes section.
The color brown has been introduced on Table 2 to spotlight groups and conditions that require recurrent revaccination:
- Give Tdap in each and every pregnancy at 27-36 weeks.
- Revaccinate people living with HIV with MenACWY every 5 years.
- Revaccinate those with asplenia and/or complement deficiency with MenACWY every 5 years and MenB every 2-3 years.
- Stem cell transplant recipients need three doses of Hib.
Vaccine order is the same on both tables.
The rows for 2023-2024 formulations of COVID and flu vaccines are at the top of both tables are coded yellow, meaning everyone needs a dose of both vaccines.
Both tables have added a row for RSV vaccines and mpox vaccines.
Notes Section
The notes have been edited for clarity and reveal who needs what and when and include special vaccine-specific sections for special circumstances.
COVID vaccines. The COVID vaccine note embraces the updated 2023-2024 formula. Everyone aged 6 months or older needs a dose of the updated COVID vaccine. Specifics of who needs what (and when) depend on what they have already received, as well as their immune status. Detailed recommendations for both mRNA and protein-based adjuvanted versions are included in the notes.
RSV vaccines. The notes also give vital details about RSV vaccines for pregnant people and for older adults. There are two RSV vaccines. Both are preF RSV vaccines. They’re identified by trade names for clarity. Arexvy contains an adjuvant. Abrysvo does not contain an adjuvant. The RSV vaccine note explains that only Abyrsvo (the vaccine without the adjuvant) can be given to pregnant people, only at 32-36 weeks, and only to those whose baby would be born during RSV season.
ACIP recommends a dose of either vaccine for adults aged 60 or older, under shared clinical decision-making (meaning you and your patients have to discuss and decide). The notes link to additional guidance for making that decision.
Mpox vaccines. For the mpox vaccine, all adults in any age group at increased risk of getting mpox should get a two-dose series of the vaccine. The mpox vaccine notes include a list of mpox risk factors.
Other Features of the 2024 Adult Immunization Schedule
The schedule has useful links to helpful information:
- Vaccine information statements
- Complete ACIP recommendations
- CDC’s General Best Practice Guidelines for Immunizations.
- VAERS (CDC’s Vaccine Adverse Event Reporting System)
A new “Additional Information” section in the Notes links to:
- Travel vaccination requirements
- Best practices guidelines for vaccinating persons with immunodeficiency
- The National Vaccine Injury Compensation program (for resolving any vaccine injury claims)
The cover page has links to:
- CDC’s vaccine app
- QR code to access the schedule online.
With all these tools literally at your fingertips, there’s no reason not to know which vaccines your patients need and when. The challenge now is making it happen: getting those needed vaccines into arms.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Sandra Fryhofer, MD, highlights .
The biggest change for 2024 is that you don’t need to wait till January 1, 2024, for these schedules go into effect. Both schedules were published and became available in November 2023 and became effective immediately. They include ACIP recommendations approved by the Centers for Disease Control and Prevention (CDC) director through October 23, 2023.
Subsequent recommendations (before publication of the 2025 schedule) will be added to the addendum, a new Step 5, Section 5 in the schedule. The addendum should make Affordable Care Act (ACA)–compliant insurance plans cover ACIP-recommended immunizations sooner.
This year’s schedule includes more vaccines with new recommendations and new color code keys for the schedule’s vaccine tables. The newest vaccine additions to the 2024 schedule include respiratory syncytial virus (RSV) vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of the updated COVID vaccine (both mRNA and protein-based adjuvanted versions).
These are listed on the cover page (in alphabetical order) by name, abbreviation, and trade name. Vaccine-specific details can be found in the (Step 3) Notes section, also organized alphabetically.
The Tables
Step 1 is Table 1: Vaccinations by Age. Step 2 is Table 2: Vaccinations by Medical Conditions or Other Indications. The table names haven’t changed. However, their color code legends have been adjusted and refined. Also, the legends for the some of the same colors are not the same for both tables.
The order of and conditions covered in the columns on Table 2 have been reorganized.
Even for vaccines whose recommendations have not changed, the color code keys reflecting the recommendations have changed. For this reason, the 2024 version of Table 2 looks very different from the 2023 version. Also, much of the wording on overlays has been removed, which means you have to rely more heavily on the Notes section.
The color brown has been introduced on Table 2 to spotlight groups and conditions that require recurrent revaccination:
- Give Tdap in each and every pregnancy at 27-36 weeks.
- Revaccinate people living with HIV with MenACWY every 5 years.
- Revaccinate those with asplenia and/or complement deficiency with MenACWY every 5 years and MenB every 2-3 years.
- Stem cell transplant recipients need three doses of Hib.
Vaccine order is the same on both tables.
The rows for 2023-2024 formulations of COVID and flu vaccines are at the top of both tables are coded yellow, meaning everyone needs a dose of both vaccines.
Both tables have added a row for RSV vaccines and mpox vaccines.
Notes Section
The notes have been edited for clarity and reveal who needs what and when and include special vaccine-specific sections for special circumstances.
COVID vaccines. The COVID vaccine note embraces the updated 2023-2024 formula. Everyone aged 6 months or older needs a dose of the updated COVID vaccine. Specifics of who needs what (and when) depend on what they have already received, as well as their immune status. Detailed recommendations for both mRNA and protein-based adjuvanted versions are included in the notes.
RSV vaccines. The notes also give vital details about RSV vaccines for pregnant people and for older adults. There are two RSV vaccines. Both are preF RSV vaccines. They’re identified by trade names for clarity. Arexvy contains an adjuvant. Abrysvo does not contain an adjuvant. The RSV vaccine note explains that only Abyrsvo (the vaccine without the adjuvant) can be given to pregnant people, only at 32-36 weeks, and only to those whose baby would be born during RSV season.
ACIP recommends a dose of either vaccine for adults aged 60 or older, under shared clinical decision-making (meaning you and your patients have to discuss and decide). The notes link to additional guidance for making that decision.
Mpox vaccines. For the mpox vaccine, all adults in any age group at increased risk of getting mpox should get a two-dose series of the vaccine. The mpox vaccine notes include a list of mpox risk factors.
Other Features of the 2024 Adult Immunization Schedule
The schedule has useful links to helpful information:
- Vaccine information statements
- Complete ACIP recommendations
- CDC’s General Best Practice Guidelines for Immunizations.
- VAERS (CDC’s Vaccine Adverse Event Reporting System)
A new “Additional Information” section in the Notes links to:
- Travel vaccination requirements
- Best practices guidelines for vaccinating persons with immunodeficiency
- The National Vaccine Injury Compensation program (for resolving any vaccine injury claims)
The cover page has links to:
- CDC’s vaccine app
- QR code to access the schedule online.
With all these tools literally at your fingertips, there’s no reason not to know which vaccines your patients need and when. The challenge now is making it happen: getting those needed vaccines into arms.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Sandra Fryhofer, MD, highlights .
The biggest change for 2024 is that you don’t need to wait till January 1, 2024, for these schedules go into effect. Both schedules were published and became available in November 2023 and became effective immediately. They include ACIP recommendations approved by the Centers for Disease Control and Prevention (CDC) director through October 23, 2023.
Subsequent recommendations (before publication of the 2025 schedule) will be added to the addendum, a new Step 5, Section 5 in the schedule. The addendum should make Affordable Care Act (ACA)–compliant insurance plans cover ACIP-recommended immunizations sooner.
This year’s schedule includes more vaccines with new recommendations and new color code keys for the schedule’s vaccine tables. The newest vaccine additions to the 2024 schedule include respiratory syncytial virus (RSV) vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of the updated COVID vaccine (both mRNA and protein-based adjuvanted versions).
These are listed on the cover page (in alphabetical order) by name, abbreviation, and trade name. Vaccine-specific details can be found in the (Step 3) Notes section, also organized alphabetically.
The Tables
Step 1 is Table 1: Vaccinations by Age. Step 2 is Table 2: Vaccinations by Medical Conditions or Other Indications. The table names haven’t changed. However, their color code legends have been adjusted and refined. Also, the legends for the some of the same colors are not the same for both tables.
The order of and conditions covered in the columns on Table 2 have been reorganized.
Even for vaccines whose recommendations have not changed, the color code keys reflecting the recommendations have changed. For this reason, the 2024 version of Table 2 looks very different from the 2023 version. Also, much of the wording on overlays has been removed, which means you have to rely more heavily on the Notes section.
The color brown has been introduced on Table 2 to spotlight groups and conditions that require recurrent revaccination:
- Give Tdap in each and every pregnancy at 27-36 weeks.
- Revaccinate people living with HIV with MenACWY every 5 years.
- Revaccinate those with asplenia and/or complement deficiency with MenACWY every 5 years and MenB every 2-3 years.
- Stem cell transplant recipients need three doses of Hib.
Vaccine order is the same on both tables.
The rows for 2023-2024 formulations of COVID and flu vaccines are at the top of both tables are coded yellow, meaning everyone needs a dose of both vaccines.
Both tables have added a row for RSV vaccines and mpox vaccines.
Notes Section
The notes have been edited for clarity and reveal who needs what and when and include special vaccine-specific sections for special circumstances.
COVID vaccines. The COVID vaccine note embraces the updated 2023-2024 formula. Everyone aged 6 months or older needs a dose of the updated COVID vaccine. Specifics of who needs what (and when) depend on what they have already received, as well as their immune status. Detailed recommendations for both mRNA and protein-based adjuvanted versions are included in the notes.
RSV vaccines. The notes also give vital details about RSV vaccines for pregnant people and for older adults. There are two RSV vaccines. Both are preF RSV vaccines. They’re identified by trade names for clarity. Arexvy contains an adjuvant. Abrysvo does not contain an adjuvant. The RSV vaccine note explains that only Abyrsvo (the vaccine without the adjuvant) can be given to pregnant people, only at 32-36 weeks, and only to those whose baby would be born during RSV season.
ACIP recommends a dose of either vaccine for adults aged 60 or older, under shared clinical decision-making (meaning you and your patients have to discuss and decide). The notes link to additional guidance for making that decision.
Mpox vaccines. For the mpox vaccine, all adults in any age group at increased risk of getting mpox should get a two-dose series of the vaccine. The mpox vaccine notes include a list of mpox risk factors.
Other Features of the 2024 Adult Immunization Schedule
The schedule has useful links to helpful information:
- Vaccine information statements
- Complete ACIP recommendations
- CDC’s General Best Practice Guidelines for Immunizations.
- VAERS (CDC’s Vaccine Adverse Event Reporting System)
A new “Additional Information” section in the Notes links to:
- Travel vaccination requirements
- Best practices guidelines for vaccinating persons with immunodeficiency
- The National Vaccine Injury Compensation program (for resolving any vaccine injury claims)
The cover page has links to:
- CDC’s vaccine app
- QR code to access the schedule online.
With all these tools literally at your fingertips, there’s no reason not to know which vaccines your patients need and when. The challenge now is making it happen: getting those needed vaccines into arms.
A version of this article first appeared on Medscape.com.
Some reasons to get off the fence about COVID booster
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
Though many people remain on the fence about getting the latest COVID vaccine booster, new research suggests a strong argument for getting the shot this winter: It sharply reduces the risk for COVID.
The risk reduction was 37% for those who received two doses. Experts say the research provides a strong argument for getting the vaccine, noting that about 10% of people infected with COVID go on to have long COVID, which can be debilitating for one quarter of those with long-lasting symptoms.
The data come from a systematic literature review and meta-analysis published in October in Antimicrobial Stewardship & Epidemiology. Researchers examined 32 studies published between December 2019 and June 2023, involving 775,931 adults. Twenty-four studies, encompassing 620,221 individuals, were included in the meta-analysis.
“The body of evidence from all these different studies converge on one single reality — that vaccines reduce the risk of long COVID, and people who keep up to date on their vaccinations also fared better than people who got it once or twice and didn’t follow up,” said Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University in St Louis.
Researchers have reported similar results for children. The National Institutes of Health RECOVER Initiative team found that vaccines are up to 42% effective in preventing long COVID in children, said Dr. Carlos Oliveira, MD, a pediatric infectious diseases specialist and Yale researcher who contributed to the study, which is in preprint.
Vaccines also protect children from multisystem inflammatory syndrome, a condition that can happen after COVID, as well as protect against other COVID-related problems, such as missed school days, Oliveira said. “Even if the vaccine doesn’t completely stop long COVID, it’s still good for kids to get vaccinated for all these other reasons.”
However, uptake for the latest boosters has been slow: the Centers for Disease Control and Prevention reported that by mid-November, less than 16% of people aged 18 years or older had received a shot. For children, the number was closer to 6%. A recent Kaiser Family Foundation survey found that booster rates for adults are similar to what it was 1 year ago.
The survey results suggest that people are no longer as worried about COVID, which is why there is less concerned about keeping up with boosters. Though the current mutation of the virus is not as debilitating as its predecessors, long COVID continues to be a problem: as of January 2023, 28% of people who had contracted the virus had experienced long-COVID symptoms. And though the mechanisms are still not fully understood, and researchers have yet to agree on a definition of long COVID, they are certain about this much: The best way to avoid it is to avoid getting infected to begin with.
The lack of a diagnostic test for long COVID and the fact that the symptoms mimic those of other diseases lead to inconsistency that can make studies hard to replicate. In the papers reviewed for the Antimicrobial Stewardship & Epidemiology study, long COVID was defined as having symptoms lasting from more than 4 weeks to more than 6 months. Alexandre Marra, MD, the lead author and a researcher at the Hospital Israelita Albert Einstein, in São Paulo, Brazil, and at the University of Iowa, said that a clear standard definition is needed to better understand the actual prevalence and evaluate vaccine effectiveness.
Al-Aly noted that there is a logical explanation for one finding in the paper: The percentage of individuals who had COVID and reported that long-COVID symptoms declined from 19% in June 2022 to 11% in January 2023.
Because a pandemic is a dynamic event, constantly producing different variants with different phenotypes, the prevalence of disease is naturally going to be affected. “People who got infected early in the pandemic may have a different long COVID profile and long COVID risk than people who got infected in the second or third year of the pandemic,” Al-Aly said.
Most of the studies reported data from before the Omicron-variant era. Only eight reported data during that era. Omicron was not as lethal as previous variants, and consequently, fewer patients developed long COVID during that time.
One of those who did is Yeng Chang, age 40 years, a family doctor who lives in Sherwood Park, Alberta, Canada. Chang developed long COVID during fall 2022 after getting the virus in June. By then, she’d been vaccinated three times, but she isn’t surprised that she got sick because each vaccine she had was developed before Omicron.
“When I had COVID I was really sick, but I was well enough to stay home,” she said. “I think if I didn’t have my immunizations, I might have been hospitalized, and I don’t know what would have happened.”
Long COVID has left Chang with brain fog, fatigue, and a lack of physical stamina that forced her to pause her medical practice. For the past year and a half, she’s spent more time as a patient than a physician.
Chang had her fifth COVID vaccination in the fall and recommends that others do the same. “The booster you got however many years ago was effective for the COVID of that time but there is a new COVID now. You can’t just say, ‘I had one and I’m fine forever.’”
A version of this article appeared on Medscape.com.
Global measles deaths increased by 43% in 2022
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.
The number of total reported cases rose by 18% over the same period, accounting for approximately 9 million cases and 136,000 deaths globally, mostly among children. This information comes from a new report by the World Health Organization (WHO), published in partnership with the US Centers for Disease Control and Prevention (CDC).
More Measles Outbreaks
The report also notes an increase in the number of countries experiencing significant measles outbreaks. There were 37 such countries in 2022, compared with 22 the previous year. The most affected continents were Africa and Asia.
“The rise in measles outbreaks and deaths is impressive but, unfortunately, not surprising, given the decline in vaccination rates in recent years,” said John Vertefeuille, PhD, director of the CDC’s Global Immunization Division.
Vertefeuille emphasized that measles cases anywhere in the world pose a risk to “countries and communities where people are undervaccinated.” In recent years, several regions have fallen short of their immunization targets.
Vaccination Trends
In 2022, there was a slight increase in measles vaccination after a decline exacerbated by the COVID-19 pandemic and its impact on global healthcare systems. However, 33 million children did not receive at least one dose of the vaccine last year: 22 million missed the first dose, and 11 million missed the second.
For communities to be considered protected against outbreaks, immunization coverage with the full vaccine cycle should be at least 95%. The global coverage rate for the first dose was 83%, and for the second, it was 74%.
Nevertheless, immunization recovery has not reached the poorest countries, where the immunization rate stands at 66%. Brazil is among the top 10 countries where more children missed the first dose in 2022. These nations account for over half of the 22 million unadministered vaccines. According to the report, half a million children did not receive the vaccine in Brazil.
Measles in Brazil
Brazil’s results highlight setbacks in vaccination efforts. In 2016, the country was certified to have eliminated measles, but after experiencing outbreaks in 2018, the certification was lost in 2019. In 2018, Brazil confirmed 9325 cases. The situation worsened in 2019 with 20,901 diagnoses. Since then, numbers have been decreasing: 8100 in 2020, 676 in 2021, and 44 in 2022.
Last year, four Brazilian states reported confirmed virus cases: Rio de Janeiro, Pará, São Paulo, and Amapá. Ministry of Health data indicated no confirmed measles cases in Brazil as of June 15, 2023.
Vaccination in Brazil
Vaccination coverage in Brazil, which once reached 95%, has sharply declined in recent years. The rate of patients receiving the full immunization scheme was 59% in 2021.
Globally, although the COVID-19 pandemic affected measles vaccination, measures like social isolation and mask use potentially contributed to reducing measles cases. The incidence of the disease decreased in 2020 and 2021 but is now rising again.
“From 2021 to 2022, reported measles cases increased by 67% worldwide, and the number of countries experiencing large or disruptive outbreaks increased by 68%,” the report stated.
Because of these data, the WHO and the CDC urge increased efforts for vaccination, along with improvements in epidemiological surveillance systems, especially in developing nations. “Children everywhere have the right to be protected by the lifesaving measles vaccine, no matter where they live,” said Kate O’Brien, MD, director of immunization, vaccines, and biologicals at the WHO.
“Measles is called the virus of inequality for a good reason. It is the disease that will find and attack those who are not protected.”
This article was translated from the Medscape Portuguese edition.
